Abstract
Calcification in prosthetic vascular conduits is a major challenge in cardiac and vascular surgery that compromises the long-term performance of these devices. Significant research efforts have been made to understand the etiology of calcification in the cardiovascular system and to combat calcification in various cardiovascular devices. Novel biomaterial design and tissue engineering strategies have shown promise in preventing or delaying calcification in prosthetic vascular grafts. In this review, we highlight recent advancements in the development of acellular prosthetic vascular grafts with preclinical success in attenuating calcification through advanced biomaterial design. We also discuss the mechanisms of action involved in the designs that will contribute to the further understanding of cardiovascular calcification. Lastly, recent insights into the etiology of vascular calcification will guide the design of future prosthetic vascular grafts with greater potential for translational sucess.
Keywords: Cardiovascular Disease, Vascular Graft, Vascular Calcification, Biomaterial Design
1. Introduction
Cardiovascular disease (CVD)-related mortalities have risen from 12.1 million in 1990 to 18.6 million in 2019 (Roth et al., 2020). The majority stems from narrowing or blockage of vital blood vessels, resulting in end- organ and tissue ischemia due to interrupted blood supply. To re-establish the blood supply, more than 300,000 coronary artery bypass surgeries (Benjamin et al., 2019) and 375,000 lower extremity bypass surgeries (Goodney et al., 2009) are performed in the United States each year. Autologous vein or artery remains the conduit of choice for bypass surgeries; however, poor quality and limited availability have led to the use of synthetic materials such as polytetrafluoroethylene (PTFE), polyurethane (PU) and silicone (Park et al., 2001). The global vascular grafts market is currently dominated by synthetic grafts (e.g., GoreTex and Dacron), with an estimated value of $2.01 billion USD in 2018 (Gupta & Mandal, 2021). Unfortunately, current prosthetic vascular conduits have unacceptably high failure rates: saphenous vein grafts used in coronary artery bypass fail at rates of ~40% in 10 years (Goldman et al., 2004). Grafts for peripheral artery bypass experience failure rates of ~35% at just one year, and over 70% of lower extremity grafts eventually require re-intervention (Conte et al., 2006). Thus, these devices often require future hospital visits for graft replacement or endovascular manipulation to restore patency, introducing further costs and risks to the patient.
Interstitial calcification is frequently found within synthetic vascular grafts and is associated with vascular graft failure. In a 2011 report, Mehta et al. examined 40 clinically failed PTFE bypass grafts and found 68% of the cases presented calcifications either within or adjacent to the prosthetic conduits, some as early as 1 month after implantation. Calcification is characterized by ectopic mineral formation in the form of calcium phosphate or other calcium salts, which can cause clinical device failure due to mechanical dysfunction. Additionally, calcification can also increase the risk of heart attack, stroke and amputation for patients due to vascular obstruction and embolization of calcific deposits (Levy et al., 1991). Therefore, there is a need to design novel vascular graft materials that are resistant to developing calcification.
Calcification within prosthetic vascular conduits closely resembles vascular calcification in atherosclerotic arteries, which is a highly prevalent feature of aging, chronic kidney disease (CKD), and diabetes (Ho & Shanahan, 2016). Once considered a passive process of mineral precipitation, it is now clear that vascular calcification is a consequence of a tightly regulated process that resembles skeletal bone formation (Demer & Tintut, 2008). During the process, vascular smooth muscle cells (VSMC) undergo osteo-chondrogenic transdifferentiation, developing ectopic mineralization in the vascular walls (Johnson et al., 2006). Although prevailing perspective on vascular calcification has evolved significantly in the past two decades, the specific mechanism of its pathogenesis remains elusive. A variety of factors have been linked to the pathogenesis of vascular calcification, including matrix remodeling, endoplasmic reticulum stress (ERS), apoptosis, inflammation, and reactive oxygen species (ROS), which have been previously reviewed by others (Figure 1) (Durham et al., 2018; El-Abbadi & Giachelli, 2007; Ho & Shanahan, 2016; Leopold, 2015).
Figure 1.
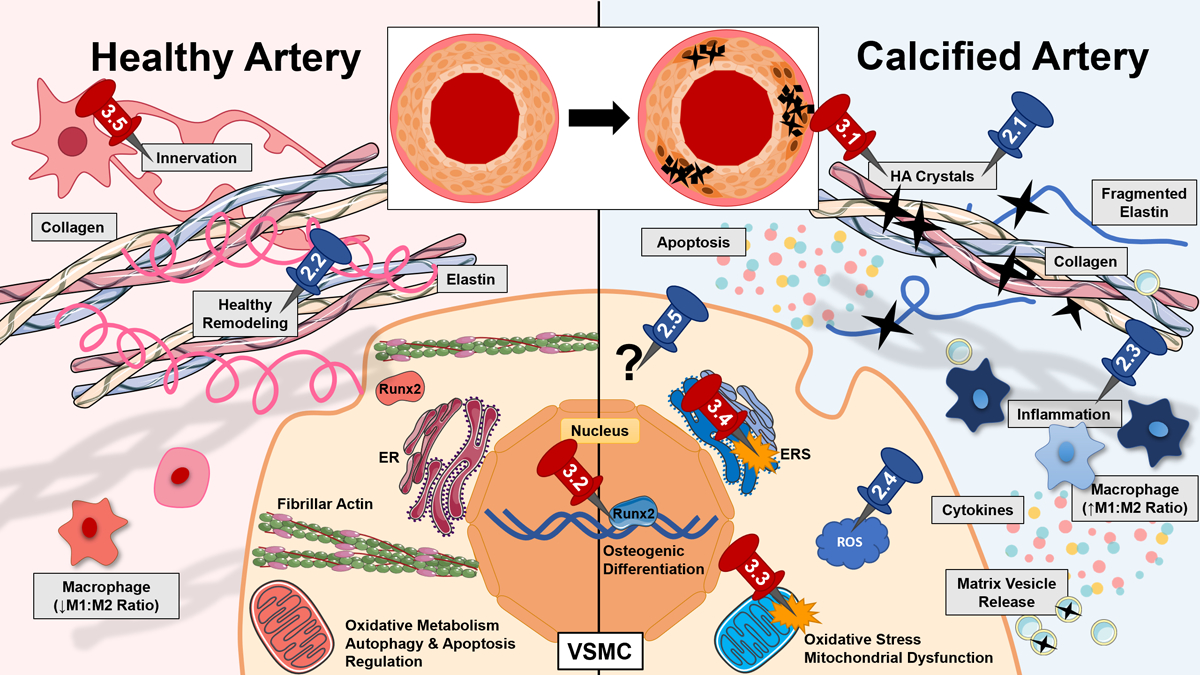
Vascular calcification in native vessels and vascular grafts is a multifactorial disease with complex etiology (Durham et al., 2018; El-Abbadi & Giachelli, 2007; Ho & Shanahan, 2016; Leopold, 2015). Here, differences in healthy (left) and calcified (right) vessels are outlined, and pins represent sections of the article discussing current (blue) and future (red) targets of anti-calcific grafts. In disease development, calcium deposition onto a biomaterial surface or elastic lamina occurs as calcium (Ca2+) and phosphate (Pi) ions combine to form hydroxyapatite (HA) crystals (Back & Michel, 2021). VSMCs transdifferentiate into osteogenic cells via transcription factors such as runt-related transcription factor 2 (RUNX2) (Chen et al., 2021; Hortells et al., 2018). These cells secrete extracellular vesicles (EV) that can bind calcium directly or stimulate further osteogenic transdifferentiation (Schurgers et al., 2018). Phenotype changes are also driven by high levels of ROS (Byon et al., 2008) (in part from dysfunctional mitochondria (Phadwal et al., 2021)) and growth factor sequestration and stiffness of the extracellular matrix (ECM) (Ngai et al., 2018). Endoplasmic reticulum (ER) stress (ERS) in vascular cells mediates apoptosis (Duan et al., 2009). Persistent inflammation of the vessel involves cytokine release and upregulation of inflammatory (M1) versus anti-inflammatory (M2) macrophages (Zhang & King, 2022). Additional mechanisms may contribute both to pathology and unexplained graft successes in the field, such as vascular innervation. Some cellular components were imported images from Servier Medical Art by Servier (licensed under Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)).
In this review, we highlight recent bioengineering advances from the past 30 years (with a heavy emphasis on the past 10 years) in the design of acellular prosthetic vascular conduits to combat device calcification. To the authors’ knowledge, there has been no comprehensive update of calcification in cardiovascular implants since Levy et al. published their review in 1991. This new summary includes efforts to specifically target biological processes contributing to calcification, especially as the field’s understanding of calcification etiology has improved greatly in the past three decades. Vascular grafts that demonstrated a reduction in calcification with non-specific mechanisms of action are also discussed through the lens of future opportunities to better understand calcification pathology and inhibition. Cell-seeded vascular grafts and other graft performance outcomes (including neointimal hyperplasia and thrombosis results) have been extensively reviewed elsewhere (Naegeli et al., 2022; Pashneh-Tala et al., 2016; Saito et al., 2021) and are beyond the scope of this review. Most efforts are still in early stages of development and have been evaluated preclinically. The translational perspective of those materials are discussed. Additionally, we propose a number of bioengineering strategies for future vascular conduit design that utilize the current understanding of vascular calcification pathogenesis. Improvements in prosthetic graft technology to combat calcification will have a far-reaching impact on many different fields.
2. Bioengineering Strategies to Combat Vascular Graft Calcification
A number of bioengineering strategies have been explored to prevent and reduce calcification in prosthetic vascular grafts, including the development of novel materials, the incorporation of therapeutics, and the fabrication of vascular scaffolds with different microstructures. Those vascular graft designs were evaluated in different preclinical animal models for various periods of time for signs of calcification, along with other parameters such as neointimal hyperplasia, inflammation, and endothelialization. Due to the heterogenity of the study designs, it is difficult to compare vascular grafts made from different materials and claim certain materials as anti-calcific. Instead, we aim to summarize strategies that could be generally applicable to a number of materials for vascular applications. The approaches with their mechanisms of action are highlighted in Figure 2 and summarized in Table 1. Structures of polymers used in anti-calcific grants are provided in Figure 3.
Figure 2.
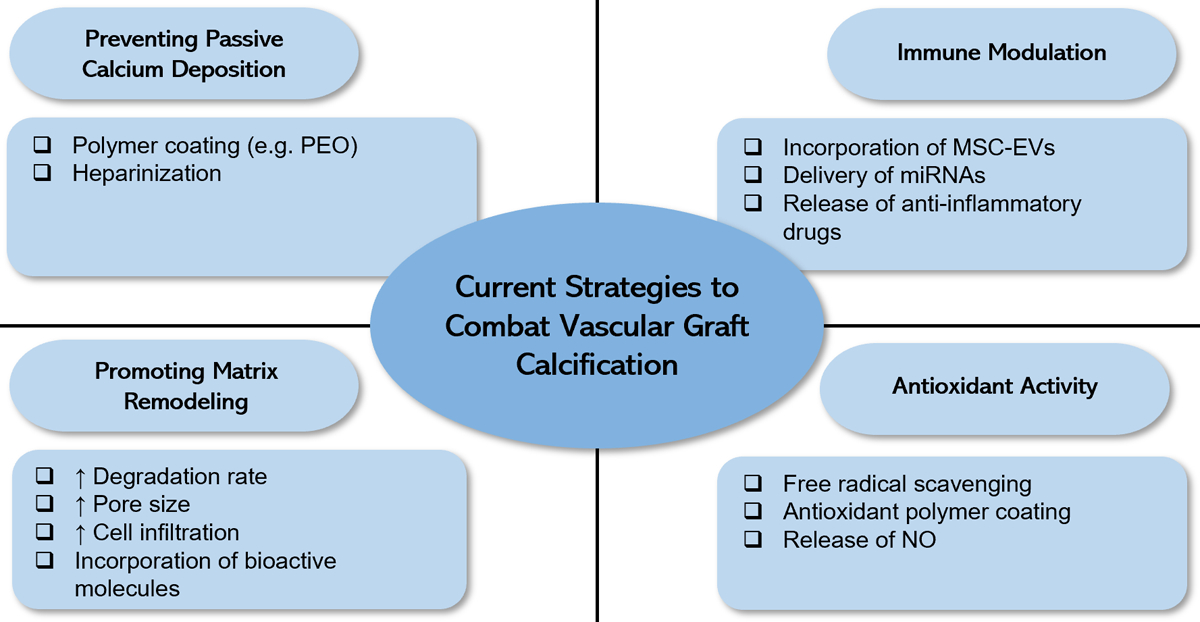
Current strategies to combat calcification in vascular grafts when evaluated in preclinical animal models.
Table 1.
Currently Existing Vascular Graft Technologies for Anti-Calcification
| Mechanism of Action | Base Material | Modifications | Models for Evaluation | Primary Results | Reference |
|---|---|---|---|---|---|
| Calcium Deposition Prevention | PU | Coated with PU-PEO-SO3 | Canine RV-PA shunt model | Calcium deposition was reduced on PU-PEO-SO3 coated grafts in comparison to untreated PU grafts after 39-day transplantation | (Han et al., 1993) |
| Porcine or rabbit vessel | Heparin coupling | Rat subcutaneous implantation | Reduced calcium content at 5 months in heparin-coupled grafts but poor host cell interactions, showed calcification occurs along elastin fibers of bioprosthetic grafts. | (Chanda et al., 1999) | |
| Cell Recruitment & Matrix Remodeling | 50:50 PLCL copolymer | PLA nanofiber outer layer (slow degrading) or PLA/PGA nanofiber outer layer (fast degrading) | Mouse infrarenal aortic interposition | No evidence of calcification was present in fast degrading grafts 8 weeks after implantation, while 7/12 slow degradation grafts showed calcification by von Kossa staining. Fast degrading grafts showed more cellular infiltration in outer graft layer compared to slow degrading grafts. | (Sugiura et al., 2017) |
| Fast degrading PDO/PLCL (9:1) | N/A | Rat abdominal aortic interposition | Fast degrading grafts showed no calcification at 1 month in contrast to slow degrading grafts (lower PDO/PLCL ratio) that showed calcification in von Kossa staining and polarized light microscopy. | (Fukunishi et al., 2021) | |
| Large-pore PLA | PLCL coating | Mouse infrarenal aortic interposition | Large-pore PLA-PLCL grafts showed significantly less calcification than small-pore PLA grafts after 12 months of implantation by Alizarin red staining. PLA-PLCL grafts showed more VSMC infiltration (expressing both osteoblast and osteoclast factors) and less macrophage infiltration. | (Tara et al., 2014) | |
| Electrospun PCL/fibrin | N/A | Rat abdominal aorta interposition | The PCL/fibrin grafts showed higher level of expression of SMC contractile protein, higher microvessel density, and significantly less calcification (by von Kossa staining) than PCL controls at 1, 3, and 9 months. | (Zhao et al., 2021) | |
| Macro-porous electrospun PCL | N/A | Rat abdominal aorta interposition | No evidence of calcification was shown by von Kossa staining 1 year after implantation. | (Wu et al., 2018) | |
| Melt-spun/heat treated PCL fiber skeleton | Autologous fibrotic biotube (formed by subcutaneous implantation) | Canine peripheral arterial replacement Sheep arteriovenous graft |
Grafts stained negative for calcification with von Kossa stain (along with ePTFE controls) after 7 months in canine study and 3 months in sheep. | (Zhi et al., 2022) | |
| Heparin-coated porous PGS tube with mesh electrospun PCL sheath | N/A | Rat abdominal aorta interposition | Neoarteries demonstrate rapid remodeling and minimal foreign materials at 3 months without signs of calcification while resembling function, mechanical properties, and ECM organization of native arteries | (Wu et al., 2012) | |
| Braided PGA | PGS coating | Rat infrarenal abdominal aorta interposition | PGS coating significantly decreased the calcification area in the graft compared to the PGA graft alone at 1, 3, and 6 months after implantation quantified from von Kossa staining. | (Fukunishi et al., 2019) | |
| Microporous PGS tube reinforced with PCL nanofibers | N/A | Rat abdominal aorta interposition | Patent vessels at 1 year after implantation showed no evidence of calcification by von Kossa staining and some recovered physiological function. Innervation was demonstrated in the adventitial space with similar morphology to native arteries. | (Allen et al., 2014) | |
| PGS tube reinforced with PCL nanofibers | N/A | Rat carotid artery interposition | PGS/PCL grafts showed no evidence of calcification at 1 year compared to vein grafts which showed sparse mineralization. PGS/PCL grafts also demonstrated innervation and improved contraction function. | (Yang et al., 2019) | |
| Decellularized rat aorta | Fibronectin (adventitia) and SDF1α (intima) coating | Rat infrarenal implantation | SDF1α-coated grafts improved intimal recellularization at 2 weeks and reduced medial calcification and neointimal hyperplasia at 8 weeks compared to grafts coated with only fibronectin. | (Sugimura et al., 2020) | |
| Immunomodulation | Electrospun PCL | Heparin coating, MSC sEV loading | Rat abdominal aorta interposition (hyperlipidemia model) | Loading with sEV prevented calcification due to heparinization in the PCL graft for 3 months, shown by von Kossa staining and micro-CT. | (Wei et al., 2019) |
| PELCL and PELCL-REDV in inner layer, PELCL in middle, and PCL in outer | miR-145 incorporated in middle layer and miR-126 incorporated in inner layer | Rat abdominal aorta interposition | Grafts with miRNA showed no calcification in von Kossa staining at 4 weeks while PELCL/PCL grafts showed extensive calcification. Trilayer grafts with miRNA showed higher M2 and lower M1 expression than PELCL/PCL grafts alone. | (Wen et al., 2020) | |
| PGS core with PCL/collagen nanofibrous sheath | Rapamycin loading in PCL sheath | Rat abdominal aorta interposition | Rapamycin-loaded grafts showed higher myogenic differentiation of vascular progenitor cells and a reduction in calcification by Alizarin red staining at 6 months in comparison to PBS-treated control or autophagy inhibitor-loaded grafts which demonstrate calcification colocalized with residual PCL. | (Chen et al., 2022) | |
| Antioxidant Activity | Decellularized artery | POCC coating | Rat abdominal aorta interposition | Polymer-ECM grafts showed highest levels of antioxidant activity and lowest levels of calcification by von Kossa and Alizarin red staining (3 months) compared to polymer-ECM-heparin grafts or ECM grafts which showed lower antioxidant activity. | (Jiang et al., 2017) |
| Electrospun nitrate-modified PCL | N/A | Rat abdominal aorta interposition | PCL/NO grafts improved cell infiltration and reduced calcification area in von Kossa staining for 3 months compared to PCL. | (Yang et al., 2021) | |
| Mixed Mechanisms | Decellularized swim bladder | GA crosslinking | Rat abdominal aorta interposition | Swim bladder grafts showed no calcification (by von Kossa staining) after 4 weeks of implantation, and in vitro and subcutaneous implantation studies showed lower calcification than bovine pericardium. | (Liu et al., 2020) |
| Decellularized fibrotic conduits (autologous) | Heparin chemical conjugation | Rat common carotid artery interposition | Autograft fibrotic conduits showed more M2 macrophages than allografts and no calcification by von Kossa staining at 3 or 6 months after implantation. Both autograft and allograft fibrotic conduits demonstrated better cellular infiltration than decellularized arteries. | (Qiu et al., 2021) |
Figure 3.
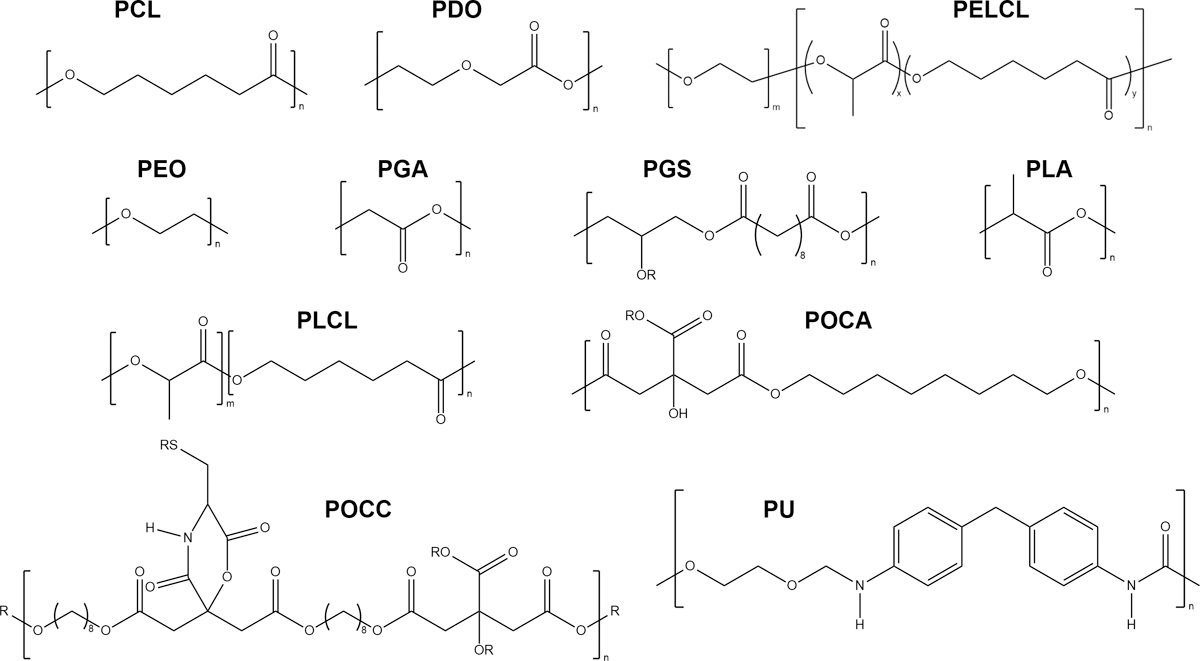
Polymers employed in the designs of novel vascular grafts, arranged alphabetically. For additional R groups, the reader is directed to the original publications. PCL: polycaprolactone, PDO: polydioxanone, PELCL: poly(ethylene glycol)-b-poly(L-lactide-co-εcaprolactone) (Wen et al., 2020), PEO: poly(ethylene oxide) [also referenced as PEG: poly(ethylene glycol)], PGA: polyglycolic acid, PGS: poly(glycerol sebacate) (Wang et al., 2002), PLA: poly (L-lactic acid), PLCL: poly(L-lactic-co-ε-caprolactone), POCA: poly(1,8-octanediol-co-citrate-co-ascorbate) (Tran et al., 2015), POCC: poly(1, 8-octamethylene-citrate-co-cysteine) (Yang et al., 2009), PU: polyurethane.
2.1. Surface Modification that Prevents Calcium Deposition
Calcification in the vascular system was once considered a passive process of mineral deposition. Thus, early studies in the field mostly focused on surface modification of prosthetic vascular grafts to prevent passive calcium deposition. One such strategy was sulfonated poly(ethylene oxide) (PEO) coating to prevent calcium binding (Han et al., 1993; Park et al., 1997). Han et al. (1993) used PEO coating on a porous PU graft in a canine right ventricle-pulmonary artery shunt system in an attempt to improve hemocompatibility, biostability, and anti-calcification properties. In addition to a reduction in platelet adhesion, dissolved calcium from the coated portion of each graft was lower than the control side of the graft as determined by inductively coupled plasma atomic emission spectrometer. The group proposed that the reduction in calcium deposition was due to the non-adhesive and mobile characteristics of PEO and the negative charge of the sulfonate acid groups. However, limitations in this study include the short duration of the animals’ survival (up to 39 days) and the small sample size (n=3). The authors noted that the extent of calcium deposition increased over time even in PEO treated samples, suggesting that the coating slows calcification rather than prevents it in the long-term. Others have used heparin coating for its anti-thrombotic and anti-calcific activities. For example, Chanda et al. (1999) used a heparin coupling technique to reduce the amount of calcification in bioprosthetic vascular grafts made of glutaraldehyde-cross-linked segments of arteries and veins. In a rat subcutaneous model, heparin-coupled bioprosthetic vascular grafts significantly reduced calcium content compared to controls without heparin coating at 5 months after implantation. However, as recent studies have indicated that vascular calcification is an active process that resembles bone formation, prevention of passive calcium deposition is expected to have limited effect on the long-term prevention of calcification.
2.2. Biomaterial Design that Favors Cell Recruitment and Matrix Remodeling
One of the major criteria to consider during prosthetic vascular graft design is the host remodeling capability. Prompt remodeling of prosthetic vascular grafts from biomaterials to neo-arteries is important to reduce the duration of host exposure to foreign materials, thus reducing the chance of calcification due to chronic foreign body reaction. Rapid degradation of synthetic materials and improvment of cell recruitment in the prosthetic grafts to accelerate the process of extracellular matrix (ECM) remodeling has resulted in the prevention of calcification as summarized below.
A variety of bioresorbable polymers have been developed for vascular tissue engineering applications, such as poly(L-lactic-co-ε-caprolactone) (PLCL), poly (L-lactic acid) (PLA) and polyglycolic acid (PGA) (Toong et al., 2020). These polymers can be fabricated into vascular scaffolds with various dimensions, pore size, and tunable degradation rate, resulting in differences in material degradation, cell infiltration and matrix remodeling rate. From several studies carried out by different research groups, vascular grafts with higher rates of cellular infiltration consistently led to significant reduction in calcification development. For example, Sugiura et al. (2017) demonstrated that vascular grafts made with a fast-degrading PLA and PGA nanofiber outer layer exhibited significantly lower calcification than those with a slow-degrading PLA nanofiber outer layer when evaluated in a murine abdominal aorta model for 8 weeks. This was attributed to the fast cellular infiltration within the fast-degrading material. Another study conducted by Fukunishi et al. (2021) used fast-degrading engineered vascular grafts with high polydioxanone (PDO):PLCL ratio, which showed formation of monolayer of endothelial cells, mechanical properties acting like native arteries, well-organized ECM, and no calcification after one month of implantation in the abdominal aorta of female rats. In contrast, slow degrading grafts with low PDO:PLCL ratio exhibited more vascular stiffness, less collagen and elastin, and evidence of vascular calcification. This study indicated that controlling degradation rate through polymer ratios can influence the development of calcification through regulation of cell infiltration. Tara et al. (2014) demonstrated that bioresorbable PLA-PLCL grafts fabricated with larger pore size led to higher VSMC infiltration, lower macrophage density, and significantly less calcification after 12 months of implantation compared to small pore PLA grafts. This suggests that the pore size of the material is important in regulating the cell type involved during tissue remodeling. All three of these studies together demonstrate that the related effects of faster degradation rates, higher cell infiltration, and reduced foreign body presence confer anti-calcific properties.
Polycaprolactone (PCL), a biodegrading polymer, has been frequently used in vascular applications due to its excellent mechanical properties, slow degradation rate, and good biocompatibility. To fabricate PCL into vascular grafts, electrospinning is often used to produce polymeric micro and nano-fibers, forming porous scaffolds. However, calcification is common in PCL-based vascular grafts, especially when evaluated in the long-term. For example, de Valence et al. (2012) implanted electrospun PCL micro and nano-fiber based vascular grafts in a rat abdominal aorta replacement model and found chondroid metaplasia and calcification occurring in the intimal hyperplasia layers starting at 6 weeks after implantation, which spread over time in both volume and extent. After 18 months, calcification in the form of compact bone with viable osteocytes can be found, with 86 ± 5% of the graft length and 14.4 ± 1.9% of the graft volume calcified. In another study, Zhao et al. (2021) also used electrospinning technology to prepare tubular vascular grafts made with PCL with and without fibrin. The incorporation of fibrin improved cell infiltration and proliferation which led to higher microvessel density and less calcification when compared to PCL control at 3 months after implantation in the rat abdominal aorta. The PCL/fibrin graft also induced the regeneration of neo-arteries which stimulated faster endothelialization and ECM deposition, with higher level of expression of smooth muscle contractile protein at 9 months after implantation compared to PCL graft. This shows that inclusion of fibrin to PCL vascular grafts can induce the host to perform functional reconstruction and vascular regeneration, which may be attributed to the increased cell infiltration. In addition, Wu et al. examined the long-term performance of a macro-porous PCL graft. Previously, the group had shown that using fibers 5–6 microns in diameter with resulting pores of around 30 microns allowed for increased cell infiltration, increased ECM production, and the modulation of macrophages to their immunomodulatory phenotype (Wang et al., 2014). The PCL graft showed no calcification after 1 year of implantation in the rat abdominal artery. Moreover, neo-vessels were reconstructed on the luminal surface of the graft forming a complete layer of endothelial cells and some layers of smooth muscle cells (Wu et al., 2018). This demonstrated that using macro-porous vascular grafts improved the regeneration of the neo-vessel which mirror the structure and function of the native arteries. More recently, Zhi et al. (2022) created PCL-based vascular grafts inspired by architectural engineering. Specifically, modeled after architectural design of steel fiber reinforcement of concrete for tunnel construction, PCL fiber skeletons were fabricated by melt-spinning and heat treatment before subcutaneous embedding for the deposition of cells and ECM. The biotubes were then implanted in a rat abdominal aorta replacement model (1 month), canine peripheral bypass model (7 months), and a sheep arteriovenous graft model (3 months) and demonstrated long-term patency without neointimal hyperplasia or calcification. Therefore, modification of PCL-based vascular grafts with bioactive components or structure that favor cell recruitment and matrix remodeling can reduce calcification.
Poly(glycerol sebacate) (PGS) is another biodegradable polymer that has been studied extensively for various biomedical applications. The degradation of PGS was found to be based on surface erosion rather than bulk degradation, which reduces the formation of fibrotic tissue (Wang et al., 2002; Wang et al., 2003). Due to its biodegradability, non-thrombogenic properties and mechanical properties closely resembling native vessels, PGS has been frequently selected for vascular graft design by different groups. Vascular grafts made with PGS have demonstrated anti-calcific properties. For example, Wu et al., designed a rapid degrading vascular graft made with heparin-coated porous PGS tube wrapped with an electrospun PCL sheath. This cell-free biodegradable elastomeric graft degraded rapidly to yield neoarteries nearly free of foreign materials 3 months after interposition grafting in rat abdominal aorta without signs of calcification (Wu et al., 2012). This design helped the neo-arteries to mirror the structure of the native arteries in terms of regular, strong, and synchronous pulsation, layers of endothelium and contractile VSMCs expression of elastin, collagen, and glycosaminoglycan with desirable mechanical properties. In another study, Fukunishi et al. (2019) developed a braided PGA textile graft and coated with PGS. The PGA textile graft alone was insufficient to stop calcification when implanted in a rat infrarenal abdominal aorta interposition model. However, with PGS coating, calcification area of the media was reduced significantly at 1, 3, and 6 months. Additionally, Allen et al. (2014) and Yang et al. (2019) both independently demonstrated the lack of calcification in fast-degrading PGS-PCL grafts at 1 year in abdominal aorta interposition and carotid artery bypass surgeries, respectively. These results suggest PGS is a promising anti-calcific material for prosthetic vascular grafts, owing to its fast-degrading and matrix remodeling properties.
In addition to the synthetic vascular grafts, others have demonstrated the incorporation of bioactive molecules in ECM-based vascular grafts can also promote cell recruitment and prevent calcification. For instance, fibronectin is an ECM protein known to promote cell adhesion and recellularization of vascular implants. Stromal cell-derived factor 1 (SDF1) is known to stimulate the migration of both smooth muscle progenitor cells (Psaltis & Simari, 2015) and endothelial progenitor cells (Moore et al., 2001). Sugimura et al. (2020) designed vascular conduits with decellularized rat aortas coated with SDF1α and fibronectin on the lumen and adventitial sides, respectively. The engineered aortic grafts were infrarenally implanted in rats and followed up for up to 8 weeks. Luminal coating of SDF1α significantly reduced medial calcification throughout the graft in comparison to aortic grafts with only adventitial fibronectin coating. The authors predict that cellular recruitment from the bloodstream, overall cell infiltration, and immune regulation may all play a role in the reduced calcification. This differential coating between sides of the graft also represents a useful technique for future combinations of coating or molecule release.
2.3. Vascular Conduits with Immunomodulatory Capability
Inflammation and adverse foreign body reaction is another contributing factor for pathological calcification in cardiovascular devices (Simionescu et al., 2011). Traditional vascular grafts (e.g. PTFE and Dacron) were designed to be bio-inert so as to not induce a strong immune response from the host. However, as more is understood about foreign body responses towards implanted biomaterials, bioactive materials and molecules have been incorporated into vascular conduit design to reduce inflammation and prevent calcification. Specifically, efforts have been made to modulate the phenotypic switching of macrophages, which have been categorized into non-activated (M0), pro-inflammatory (M1), or anti-inflammatory and anti-osteogenic (M2) phenotype (Martin & Garcia, 2021; Zhang & King, 2022). A progression from M1 to M2 dominance in the environment enables optimal remodeling and regeneration (Zhang & King, 2022). Vascular conduits that can appropriately modulate macrophages into the anti-inflammatory and anti-osteogenic M2 phenotype can therefore reduce inflammation-associated calcification formation. While macrophages are not the only immune cells relevant to graft implantation and vascular remodeling, regulation of their phenotype has been shown to be a promising approach to improve graft outcomes. A variety of strategies have been used to induce the M2 phenotype and attenuate inflammation, including the incorporation of stem cells, their secretomes, or anti-inflammatory pharmacological agents.
The immunomodulatory effects of mesenchymal stem cells (MSCs) have been well documented for various applications (English, 2013). Importantly, in vitro and in vivo studies have revealed MSCs modulate inflammation with paracrine effects via secretion of EVs containing immunomodulatory factors (Borger et al., 2017; Zhou et al., 2019). Therefore, incorporation of MSC-derived EVs into vascular graft design can potentially reduce calcification by attenuating inflammation. Wei et al. (2019) incorporated MSC-derived small extracellular vesicles (sEVs) into a heparinized, electrospun PCL graft. They demonstrated that MSC-derived sEVs were successful in inhibiting calcification in a hyperlipidemia rat aortic replacement model. Specifically, at 3 months after the surgery, MSC-derived sEVs prevented calcification by triggering the transition of macrophages from M1 to M2 phenotype. This is attributed to the various bioactive molecules contained in the MSC-derived sEVs, such as microRNAs (miRNAs) and cytokines, which play an important role in immune modulation. The immunomodulatory effect of miRNAs could also be harnessed directly to attenuate vascular graft calcification. Wen et al. (2020) created a trilayered electrospun polymer graft (including poly(ethylene glycol)-b-poly(L-lactide-co-εcaprolactone) (PELCL) and PCL) that releases both miR-145 and miR-126 with unique release profiles to modulate VSMC and macrophage phenotype. When implanted in a rat abdominal aorta interpositional model, grafts containing either one or both types of miRNAs showed no calcification at 4 weeks. In contrast, the control graft without miRNA showed high levels of calcification between the graft and neointimal layer. The authors propose that these factors promote contractile VSMC phenotype and the anti-inflammatory M2 phenotype to contribute to beneficial tissue growth in the graft.
Incorporation of anti-inflammatory pharmacological agents into vascular grafts could be another strategy to attenuate inflammation-induced calcification. Rapamycin, a mechanistic target of rapamycin kinase (mTOR) inhibitor (sold under the name Sirolimus), is an immunosuppressant agent that could be taken orally or used to coat vascular devices. Sirolimus-eluting stents reduce restenosis occurrence after percutaneous coronary revascularization in comparison to bare metal stents (Moses et al., 2003). Chen et al. (2022) demonstrated that rapamycin released from a PCL sheath around a PGS-PCL vascular graft can polarize macrophages to the M2 phenotype. This graft was implanted interpositionally in the rat abdominal aorta. Neoarteries formed from the rapamycin-loaded PGS-PCL grafts showed reduced Alizarin red staining compared to control PGS-PCL grafts loaded only with PBS, while those same grafts loaded with the autophagy inhibitor 3-methyladenine showed extensive positive staining. Thus, the authors propose that activation of macrophage autophagy and M2 phenotype plays a role in the anti-calcification effects of the graft.
2.4. Vascular Grafts with Antioxidant Activity
An equal balance between the levels of ROS and antioxidants is crucial for normal cellular function (Senoner & Dichtl, 2019). A disruption in this balance can lead to the pathology of arterial mineralization through oxidative stress, which triggers transdifferentiation of the VSMCs to osteoblasts (Byon et al., 2008; Chao et al., 2019). This behavior has also been seen in vascular grafts where oxidative stress induces graft calcification and transdifferentiation of VSMCs into osteoblasts (Jiang et al., 2017). Although the exact signaling pathways by which ROS stimulate the progression of calcification are not fully understood at this time, many antioxidant compounds capable of scavenging free radicals and balancing intracellular ROS have been studied for the treatment of vascular calcification, as summarized by Chao et al. in 2019. In this section we review vascular grafts designed with antioxidant acitivies as a potential strategy to combat calcification.
A variety of citric-based elastomers have been designed by Ameer and Yang et al. with antioxidant activities for vascular applications (Tran et al., 2015). For instance, poly(1,8-octanediol-co-citrate-co-ascorbate) (POCA), a citric-acid based biodegradable elastomer with native, intrinsic antioxidant properties was used to coat ePTFE vascular grafts (van Lith et al., 2014). The POCA-coated ePTFE grafts reduced intimal hyperplasia with no sign of calcification when implanted into an aortic interposition model in guinea pigs for 4 weeks. Another study from the same group used poly(1, 8-octamethylene-citrate-co-cysteine) (POCC) as an antioxidant polymer coating on decellularized rat ECM grafts with and without heparin functionalization (Jiang et al., 2017). Those materials were implanted in a rat abdominal aortic interposition and evaluated at 3 months. POCC-modified ECM grafts exhibited elevated antioxidant activity compared to the control ECM grafts, resulting in a significant reduction in calcification. In contrast, further modification of the ECM-POCC with heparin lowered the material antioxidant activity due to inactivation of the thiol groups from cysteine, resulting an increase in calcification compared to the ECM grafts. This is in contradiction to the work by Chanda et al. (1999), where coating glutaraldehyde-fixed grafts with a heparin coupling technique led to less calcification during subcutaneous implantation of vascular grafts for five months. This contradiction may result from the differences in animal models employed, as the more recent study by Jiang et al. (2017) used the material in an rat interposition model where the material could contact the blood and experience relevant fluid dynamics. Thus, heparin modification alone may be insufficient to prevent calcification, while antioxidant activity has a dominant effect when the graft is implanted in the vasculature.
Nitric oxide (NO) is a well-known gasotransmitter and antioxidant produced by the endothelium that has important roles in cardiovascular hemostasis and vascular tissue regeneration. Yang et al. (2021) aimed to improve the long-term success of artificial small diameter vascular grafts by locally delivering NO from electrospun nitrate-functionalized PCL scaffolds. PCL/NO demonstrated enhanced cellular infiltration and greater VSMC organization compared to unmodified PCL grafts 3 months after implantation in the abdominal aorta of rats. Delivery of NO inhibited the differentiation of vascular progenitor cells into osteopontin-positive cells and reduced vascular calcification formation. This is consistent with a previous study, where Kanno et al. (2008) showed that NO plays an important role in regulating the osteoblastic transdifferentiation of VSMCs in vitro by interfering with TGF-β signaling. NO is therefore an antioxidant moelcule with multiple mechanisms of action for the reduction of calcification in vascular grafts.
2.5. Novel Anti-Calcific Vascular Conduits with Mixed Mechanisms
In addition to the promising graft designs reviewed above, a number of vascular grafts showed good anti-calcification properties with unclear/mixed mechanisms. Here we review the design of the vascular grafts with properties to reduce or prevent calcification. Liu et al. (2020) used decellularized fish swim bladder to create small diameter vascular grafts by rolling. The material is mainly composed of collagen I, glycosaminoglycan and elastin, with good cytocompatibility, hemocompatibility, and enzymatic stability. When implanted as a replacement for the rat abdominal aorta, the material showed a reduction in calcification compared to acellular rat blood vessel control grafts, shown by von Kossa staining. The swim bladder grafts also showed a reduction in cytotoxicity and CD68+ macrophages with more cell ingrowth than acellular rat blood vessels in vivo, which the authors predicted was due to higher pore diameter. The use of decellularized rat vessels as a control was useful to compare acellular grafts; going forward, comparison to harvested rat allograft may also improve clinical relevancy. Although the material may not be a sustainable choice for producing grafts on a market-level scale, this study shows promising results from the combination of immunomodulatory and remodeling behavior.
The use of fibrotic conduits as vascular grafts has been one way to create naturally produced ECM structures. They are created by implanting mandrels into an animal and allowing the host body to form a fibrotic capsule around the implant. This capsule can be harvested, decellularized, and implanted as a graft in the first animal (autograft) or a different animal (allograft). Cells interact both to initially form the material and to react to it in the animal in which it is implanted. Qiu et al. (2021) investigated how macrophages play a role in remodeling these conduits and demonstrated that their polarization varies depending on if the graft used is an autograft or allograft. When implanted into a carotid interposition model, autografts showed no sign of calcification for up to 180 days and recruited more anti-inflammatory macrophages (while allografts recruited more T cells and pro-inflammatory macrophages). Allografts would be more applicable for understanding options for human patients, as using a fibrotic conduit autograft would be prohibitively invasive (two additional surgeries in comparison to one for a vein or artery autograft). This work re-emphasizes how macrophage phenotype regulation plays a role in successfully remodeling vascular grafts.
3. Future Strategies for Engineering Prosthetic Vascular Conduits
With rapidly evolving mechanistic understanding on the pathogenesis of calcification in the cardiovascular system, efforts have been made to develop therapeutic treatments that could attenuate ectopic cardiovascular calcification. Many of the approaches could be incorporated into future vascular conduit design. We highlight a number of innovative strategies with potential for translational success. The approaches with their mechanisms of action are highlighted in Figure 4.
Figure 4.
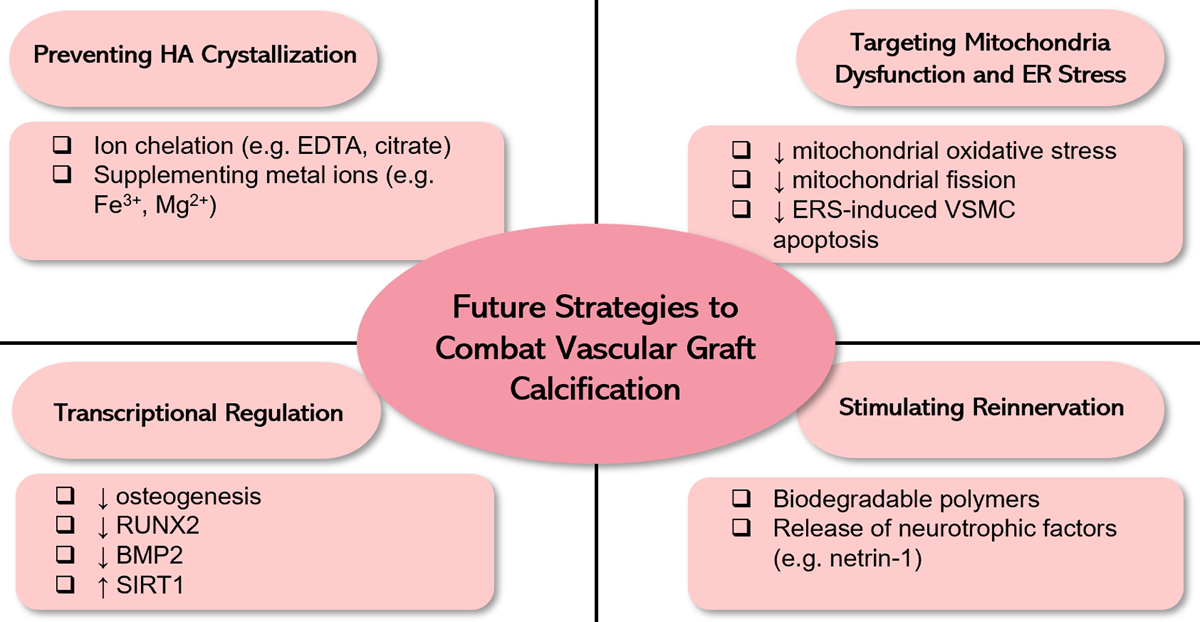
Future strategies to combat vascular graft calcification based on the current understanding on the etiology of vascular calcification.
3.1. Preventing HA Crystallization
The formation and growth of HA crystals within arterial or vascular graft walls is the hallmark of vascular calcification. Moreover, HA was also found to accelerate vascular calcification via lysosome impairment and autophagy dysfunction in VSMCs, thus setting up a vicious cycle that exacerbates vascular calcification (Liu et al., 2022). Therefore, strategies to inhibit HA crystallization in vascular grafts can potentially prevent calcification. Ethylene diamine tetraacetic acid (EDTA), a chelating agent, can resorbe HA crystal formation by chelating and removing Ca2+. Lei et al. (2014) demonstrated EDTA-loaded albumin nanoparticles designed to target calcified elastic lamina can regress arterial calcification without causing systemic side effects when administrated by intravenous injection. This targeted EDTA chelation therapy was also later demonstrated to successfully reverse medial arterial calcification in an adenine-induced rat model of CKD (Karamched et al., 2019). Therefore, incorporation of ion chelators such as EDTA into prosthetic vascular grafts or the use of targeted EDTA chelation therapy could potentially prevent or reverse calcification in vascular devices.
Interestingly, several metal ions such as iron (Fe3+) and magnesium (Mg2+) have also demonstrated anti-calcific activities in several studies by preventing the formation and growth of HA crystals. For example, Singh and Wang explored metal ions as an anti-calcification agent in PU implants in in vitro study using metal ion-loaded nanofiber matrices to inhibit calcification. The inclusion of either Fe3+ or Mg2+ ions in the PU mesh significantly inhibited calcification depositions after calcium solution incubation for 60 days compared to the unloaded PU mesh control sample (Singh & Wang, 2017). Iron citrate is another compound that can protect against calcification. Ciceri et al. (2016) showed that iron citrate treatment can protect VSMCs from high-phosphate-induced calcification in vitro. In a second study by the same group, aortic rings treated with high phosphate and iron citrate showed reduced elastin degradation, lower matrix vesicle production, and even partially reverted fibrosis in comparison to groups only treated with high levels of phosphate (Ciceri et al., 2019). In addition to the anti-calcific effect of Fe3+, citrate is also a known chelator of Ca2+. Therefore, iron citrate may exhibit dual effect on HA crystallization to prevent calcification.
3.2. Transcriptional Regulation
The osteogenic trandifferentiation of VSMCs is a tightly regulated biological process that is regulated by multiple transcptional pathways. Interventions that interfere with a single or multiple transcriptional pathways invovled in osteogenesis can potentially prevent calcification formation by preventing the osteogenic transdifferentiation of VSMCs. Incorporation of therapeutics to regulate transcription into future vascular graft designs can potentially provide anti-calcific effect locally without causing systemic effects. For example, bromodomain and extraterminal (BET) proteins cooperate with several transcription factors to promote VSMC transdifferentiation and calcification. Apabetalone, a BET inhibitor, has been demonstrated to prevent calcification of VSMCs by an epigenetic mechanism involving specific transcription factors, such as RUNX2 (Gilham et al., 2019). Incorporation of apabetalone into prospthetic vascular grafts can potentially reduce calcification by preventing VSMC transdifferentiation through inhibition of BET. Another example is sirtuin-1 (SIRT1), an NAD-dependent deacetylase, which has been found to prevent hyperphosphatemia-induced arterial calcification via inhibition of osteogenic transdifferentiation (Takemura et al., 2011). Downregulation of SIRT1 by miR-34a promoted VSMC calcification both in vitro and in vivo (Badi et al., 2018). Therefore, strategies to promote SIRT1 expression or activation can potentially prevent calcification and can be incorporated to future vascular graft design. Transcription factor specificity protein 1 (Sp1) can target and bind part of the bone morphogenetic protein 2 (BMP2) promoter to induce its expression and increase calcification. In a Vitamin D3/nicotine-induced calcification rat model, Sp1 was knocked down by the administration of an adenovirus containing DNA for its knockdown gene (Ad-Sp1). Zhang et al. (2018) showed that calcification in the thoracic aortas was reduced in rats that had received Ad-Sp1. Sp1 was upregulated in both human and rat calcified tissue, and Sp1 silencing was correlated with less VSMC transdifferentiation (as shown by western blot analysis of BMP2, RUNX2, α-smooth muscle actin, and calponin) (Zhang et al., 2018). Sp1 inhibitors such as plicamycin (also known as mithramycin or trade name Mithracin (Choi et al., 2014; Sleiman et al., 2011)) and miR-375-3p (Xu et al., 2019) can be incorporated into future vascular graft designs to inhibit Sp1 promoted calcification.
3.3. Targeting Oxidative Stress and Mitochondrial Dysfunction
Given the important role of oxidative stress in vascular calcification development, incorporation of antioxidant compounds into vascular grafts has been used as a strategy by many groups to prevent calcification, including gallic acid (Das et al., 2021), proanthocyanidins (Chen et al., 2020), chitosan and carrageenan (Campelo et al., 2016). For example, proanthocyanidins are a class of polyphenols with antioxidant and anti-calcification activities (Castellan et al., 2010; Han et al., 2003; Zhai et al., 2009; Zhai et al., 2014). These properties were used to design a novel material scaffold by Chen et al. (2020) Proanthocyanidins, collagen, and konjac glucomannan chains were combined and self-assembled into fibril scaffolds. The incorporation of proanthocyanidins prevented calcification in vitro after incubation with simulated body fluid for 30 days. When subcutaneously implanted in rats, these scaffolds showed minimal signs of calcification at 20 weeks. Additionally, the proanthocyanidin-incorporated scaffolds exhibited radical scavenging ability and improved endothelial cell proliferation, supporting their potential vascular applications. However, without in vivo studies with relevant animal models, it is difficult to predict how long the antioxidant activities will last in the vascular grafts to successfully prevent calcification in the long-term. Another possible route of antioxidant delivery is through dietary supplementation, which has long been suggested to have cardiovascular protective effects. However, concrete clinical evidence supporting their beneficial effects on preventing calcification is still lacking, most likely due to the non-specific distribution and inadequate accumulation to the target cell and tissues.
Mitochondria, the organelles responsible for energy production, are the major source of ROS production in mammalian cells. Moreover, ROS also induces modifications to mitochondrial components, including mtDNA, proteins, and lipids, which further exacerbate ROS production and set-up a vicious cycle that contributes to vascular calcification (Wang et al., 2020). Therefore, mitochondria represent an important therapeutic target for calcification in the cardiovascular system. Several strategies aimed at therapeutically restoring mitochondrial function are emerging, with potential applications in preventing calcification in arteries and prosthetic vascular grafts. For example, the mitochondria-targeted antioxidant, mitoquinone (MitoQ), has been shown to reduce mitochondrial oxidative stress and prevent calcification both in vitro and in vivo. Specifically, Cui et al. (2020) demonstrated that MitoQ inhibits oxidative stress and apoptosis through Keap1/Nrf2 pathway, thereby reducing vascular calcification in an adenine-induced aortic calcification rat model and in a Pi-induced VSMC calcification model. Mitochondria-targeted antioxidants such as MitoQ can cross the mitochondrial phospholipid bilayer and eliminate mitochondrial ROS, thus improving mitochondrial function and preventing VSMC apoptosis and calcification. Another strategy to target mitochondrial dysfunction is by preventing mitochondrial fission. Dynamin-Related Protein 1 (DRP1), a key regulator of mitochondrial fission, was found enriched in calcified regions of human carotid arteries and in VSMCs under osteogenic differentiation by Rogers et al. (2017). Moreover, inhibition of DRP1 by a small molecule inhibitor Mitochondrial Division Inhibitor 1 (mdivi-1) or siRNA attenuated calcification in VSMCs and primary human valve interstitial cells. Therefore, incorporation of mitochondrial therapeutics such as MitoQ and mdivi-1 into future vascular graft designs can potentially inhibit calcification.
3.4. ERS Mitigation
Previous studies suggested that ERS induced-apoptosis promoted vascular calcification, especially in the cases of CKD and diabetes. Therefore, therapeutics that target ERS can potentially reduce calcification in vascular grafts used those patients. Pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist and anti-diabetic drug, reduces ERS in multiple cell types (Hong et al., 2018; Yoon et al., 2019; Zou et al., 2019). In a small animal streptozotocin model of diabetes, diet administration of pioglitazone resulted in significantly less vascular calcification at 12 weeks than the control group alone, which experienced extensive crystal formation in the medial layer (Katahira et al., 2021). The drug-treated group also showed reduced expression of RUNX2 and osteopontin, both markers of osteogenic transdifferentiation. Pioglitazone could thus be investigated as a possible prescription to be given after graft implantation or as a molecule to be released from an engineered vascular graft for anti-ERS and anti-calcific activities. Erythropoietin (EPO), an endogenous glycoprotein, exerts multiple tissue protective effects by inhibiting ERS and apoptosis. Chang et al. (2020) demonstrated that EPO treatment both reduced calcification and restored VSMC markers (smoothelin and calponin) in VSMC cultures and CKD rats. The authors proposed that EPO effects were caused by interacting with its receptor to inhibit ERS-mediated apoptosis. However, the effect of EPO on vascular calcification seems to be dose-dependent, as another study showed that EPO can increase vascular calcification in VSMC cultures and CKD rats when used at higher concentrations (He et al., 2019). Thus, further studies with EPO are warranted, including studies with modified EPOs.
3.5. Promoting Innervation
Sympathetic innervation of arteries play an important role in arterial development and maturation (Eichmann & Brunet, 2014). Therefore, lack of innervation to the prosthetic vascular grafts could play a role in pathological remodeling such as calcification. Yang et al. (2019) demonstrated the establishment of innervation of a biodegradable elastomeric graft made of PGS-PCL when implanted in an interpositional rat carotid artery model, with no calcification in comparison to the vein graft at 12 months. Allen et al. (2014) also showed non-specific nerve regeneration around PGS-PCL graft neoarteries at the same time point in a rat abdominal aortic interposition model. Their vessel showed no evidence of calcification and responded to vasomotor agents in a similar manner to native tissue. Additionally, a small diameter TEVG by Li et al. (2022) was designed to release netrin-1 (a molecule that stimulates vascular sympathetic innervation during development (Brunet et al., 2014)) from a thin hydrogel graft coating upon interaction with the ROS environment commonly found in diabetic vasculature. After 30 days of implantation to replace the carotid artery in a diabetic rat model, they showed that nerve fibers had grown into the graft, which then remained patent with no sign of neointimal hyperplasia or VSMC pathological phenotype at 90 days (Li et al., 2022). These studies suggest that innervated vascular grafts are less likely to develop calcification. However, since most studies do not assess either histological presence of sympathetic nerves or functional performance of grafts in response to neurotransmitters, it is difficult to conclude the extent to which sympathetic innervation contributes to the mitigation of calcification development. Therefore, future work in the area should consider innervation as a potential metric of function. Strategies used in neuro-regeneration can also be adapted to promote reinnervation of vascular grafts to potentially prevent calcification.
4. Challenges and Translational Perspective
At this time, there is still no clinically-approved prosthetic vascular conduit that can successfully prevent calcification in the long-term. This is largely due to the lack of fundamental knowledge to pinpoint the specific causes of calcification in the cardiovascular system. Although prevailing perspective on vascular calcification has evolved significantly in the past two decades, the specific mechanisms of its pathogenesis remain elusive. As such, no effective pharmacotherapies exist to prevent, reverse, or slow calcification formation in arteries or vascular devices. Current state-of-the-art research in the field of vascular bioengineering to develop functional vascular replacement has been focused on advances in biomaterial design, tissue fabrication and stem cell engineering to reconstruct the autologous vessels with the vascular cell types. Despite encouraging results in preclinical studies, only a handful of bioengineered vascular grafts have been tested in patients. Gupta and Mandal (2021) summarized the clinical trials as of 2021 involving biodegradable prosthetic vascular grafts. One major challenge is that many clinical trials do not explicitly evaluate calcification as one of the primary or secondary endpoints, though it may be captured in general adverse events evaluation in a secondary endpoint. Since calcification can be detrimental to the long-term performance of a vascular graft, it is important to include calcification as another endpoint for their clinical evaluation.
Additionally, the different patient population and their clinical conditions pose distinct challenges and opportunities when translating the technological advancements into clincial use. For example, many patients at risk of developing calcification in the cardiovascular system have comorbidities such as diabetes and CKD. Patients with CKD are more likely to have high levels of circulating phosphates, thus strategies that disrupt crystal propagation and passive calcification are likely more effective for these patients. Patients with diabetes and CKD are also prone to develop ERS, thus therapeutics that target ERS can potentially prevent calcification with higher efficacy in those patients. However, very few preclinical studies evaluated prosthetic vascular devices in an animal model with these relevant clinical conditions. With increasing demand for personalized medicine, the design of vascular grafts by “one-size-fits-all” approach will likely become outdated. Future vascular device devleopment needs be tailored in their design and evaluation to take into consideration the clinical conditions of the target patient population, such as age, comorbidities and life styles.
Although most current strategies utilize a single approach to prevent calcification, convergence of diverse approaches to achieve multifunctional effects are likely needed for more effective treatment. For example, therapeutics incorporated into vascular grafts for localized delivery may only temorarily prevent calcification until the prosthetic vascular conduits are remodeled into native tissues. If taken orally as medication or dietary supplementation, patient compliance and targeted therapeutic delivery may become an issue. An integrated approach with bioengineered vascular graft design in conjunction with oral medications/dietary supplements can potentially provide long-term benefit for the patients.
5. Conclusion
In this review, we provide the state-of-the-art in the design of prosthetic vascular conduits to combat device calcification. Effective strategies discovered in preclinical animal studies include surface modification to reduce calcium deposition, high graft remodeling capacity for favorable ECM composition and cellular infiltration, regulation of immune response from highly inflammatory states toward remodeling states, and improvement of antioxidant activity to reduce oxidative stress. With increased fundamental understanding of the pathogenesis of vascular calcification, more advanced designs are expected to be developed to target single or multiple cellular pathways to prevent the active process of osteogenesis. The long-term performance of future vascular grafts will need to be carefully evaluated in clinically-relevant animal models to provide valuable lessons on the promises and challenges of bioengineered designs.
Acknowledgements
Conceptualization: TKB and BJ. Manuscript drafting: TKB, SA, and BJ. Manuscript edits and revision: TKB, SA, KJH, and BJ.
TKB is funded by a training grant from the National Institutes of Biomedical Imaging and Bioengineering (T32-EB031527) through the Center for Advanced Regenerative Engineering (CARE) at Northwestern University.
References
- Allen RA, Wu W, Yao M, Dutta D, Duan X, Bachman TN, Champion HC, Stolz DB, Robertson AM, Kim K, Isenberg JS, & Wang Y (2014). Nerve regeneration and elastin formation within poly(glycerol sebacate)-based synthetic arterial grafts one-year post-implantation in a rat model. Biomaterials, 35(1), 165–173. 10.1016/j.biomaterials.2013.09.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back M, & Michel JB (2021). From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovascular Research, 117(9), 2016–2029. 10.1093/cvr/cvab038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, Milano G, Brambilla F, Saccu C, Bianchi ME, Pompilio G, Capogrossi MC, & Raucci A (2018). miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arteriosclerosis, Thrombosis, and Vascular Biology, 38(9), 2079–2090. 10.1161/ATVBAHA.118.311298 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, … Subcomm SS (2019). Heart Disease and Stroke Statistics-2019 Update A Report From the American Heart Association. Circulation, 139(10), E56–E528. 10.1161/Cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, & Giebel B (2017). Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. International Journal of Molecular Sciences, 18(7), 1450. 10.3390/ijms18071450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet I, Gordon E, Han J, Cristofaro B, Broqueres-You D, Liu C, Bouvree K, Zhang J, del Toro R, Mathivet T, Larrivee B, Jagu J, Pibouin-Fragner L, Pardanaud L, Machado MJ, Kennedy TE, Zhuang Z, Simons M, Levy BI, … Eichmann A (2014). Netrin-1 controls sympathetic arterial innervation. Journal of Clinical Investigation, 124(7), 3230–3240. 10.1172/JCI75181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, & Chen Y (2008). Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. Journal of Biological Chemistry, 283(22), 15319–15327. 10.1074/jbc.M800021200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo CS, Lima LD, Rebelo LM, Mantovani D, Beppu MM, & Vieira RS (2016). In vitro evaluation of anti-calcification and anti-coagulation on sulfonated chitosan and carrageenan surfaces. Materials Science and Engineering C, 59, 241–248. 10.1016/j.msec.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, & Bedran-Russo AK (2010). Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dental Materials, 26(10), 968–973. 10.1016/j.dental.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda J, Kuribayashi R, & Abe T (1999). Heparin coupling in inhibition of calcification of vascular bioprostheses. Biomaterials, 20(19), 1753–1757. 10.1016/s0142-9612(98)00078-7 [DOI] [PubMed] [Google Scholar]
- Chang JR, Sun N, Liu Y, Wei M, Zhao Y, Gan L, Zhu JX, & Su XL (2020). Erythropoietin attenuates vascular calcification by inhibiting endoplasmic reticulum stress in rats with chronic kidney disease. Peptides, 123, 170181. 10.1016/j.peptides.2019.170181 [DOI] [PubMed] [Google Scholar]
- Chao CT, Yeh HY, Tsai YT, Chuang PH, Yuan TH, Huang JW, & Chen HW (2019). Natural and non-natural antioxidative compounds: potential candidates for treatment of vascular calcification. Cell Death Discovery, 5, 145. 10.1038/s41420-019-0225-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cai Z, Wei Q, Wang D, Wu J, Tan Y, Lu J, & Ai H (2020). Proanthocyanidin-crosslinked collagen/konjac glucomannan hydrogel with improved mechanical properties and MRI trackable biodegradation for potential tissue engineering scaffolds. Journal of Materials Chemistry B, 8(2), 316–331. 10.1039/c9tb02053e [DOI] [PubMed] [Google Scholar]
- Chen W, Xiao W, Liu X, Yuan P, Zhang S, Wang Y, & Wu W (2022). Pharmacological manipulation of macrophage autophagy effectively rejuvenates the regenerative potential of biodegrading vascular graft in aging body. Bioactive Materials, 11, 283–299. 10.1016/j.bioactmat.2021.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao X, & Wu H (2021). Transcriptional Programming in Arteriosclerotic Disease: A Multifaceted Function of the Runx2 (Runt-Related Transcription Factor 2). Arteriosclerosis, Thrombosis, and Vascular Biology, 41(1), 20–34. 10.1161/ATVBAHA.120.313791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ES, Nam JS, Jung JY, Cho NP, & Cho SD (2014). Modulation of specificity protein 1 by mithramycin A as a novel therapeutic strategy for cervical cancer. Scientific Reports, 4, 7162. 10.1038/srep07162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri P, Elli F, Braidotti P, Falleni M, Tosi D, Bulfamante G, Block GA, & Cozzolino M (2016). Iron citrate reduces high phosphate-induced vascular calcification by inhibiting apoptosis. Atherosclerosis, 254, 93–101. 10.1016/j.atherosclerosis.2016.09.071 [DOI] [PubMed] [Google Scholar]
- Ciceri P, Falleni M, Tosi D, Martinelli C, Bulfamante G, Block GA, Messa P, & Cozzolino M (2019). High-phosphate induced vascular calcification is reduced by iron citrate through inhibition of extracellular matrix osteo-chondrogenic shift in VSMCs. International Journal of Cardiology, 297, 94–103. 10.1016/j.ijcard.2019.09.068 [DOI] [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS, & Investigators PI (2006). Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. Journal of Vascular Surgery, 43(4), 742–751; discussion 751. 10.1016/j.jvs.2005.12.058 [DOI] [PubMed] [Google Scholar]
- Cui L, Zhou Q, Zheng X, Sun B, & Zhao S (2020). Mitoquinone attenuates vascular calcification by suppressing oxidative stress and reducing apoptosis of vascular smooth muscle cells via the Keap1/Nrf2 pathway. Free Radical Biology and Medicine, 161, 23–31. 10.1016/j.freeradbiomed.2020.09.028 [DOI] [PubMed] [Google Scholar]
- Das A, Ahmad Shiekh P, & Kumar A (2021). A coaxially structured trilayered gallic acid-based antioxidant vascular graft for treating coronary artery disease. European Polymer Journal, 143, 110203. 10.1016/j.eurpolymj.2020.110203 [DOI] [Google Scholar]
- de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Moller M, & Walpoth BH (2012). Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials, 33(1), 38–47. 10.1016/j.biomaterials.2011.09.024 [DOI] [PubMed] [Google Scholar]
- Demer LL, & Tintut Y (2008). Vascular calcification: pathobiology of a multifaceted disease. Circulation, 117(22), 2938–2948. 10.1161/CIRCULATIONAHA.107.743161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Zhou Y, Teng X, Tang C, & Qi Y (2009). Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochemical and Biophysical Research Communications, 387(4), 694–699. 10.1016/j.bbrc.2009.07.085 [DOI] [PubMed] [Google Scholar]
- Durham AL, Speer MY, Scatena M, Giachelli CM, & Shanahan CM (2018). Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovascular Research, 114(4), 590–600. 10.1093/cvr/cvy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann A, & Brunet I (2014). Arterial Innervation in Development and Disease. Science Translational Medicine, 6(252), 252ps259. 10.1126/scitranslmed.3008910 [DOI] [PubMed] [Google Scholar]
- El-Abbadi M, & Giachelli CM (2007). Mechanisms of vascular calcification. Advances in Chronic Kidney Disease, 14(1), 54–66. 10.1053/j.ackd.2006.10.007 [DOI] [PubMed] [Google Scholar]
- English K (2013). Mechanisms of mesenchymal stromal cell immunomodulation. Immunology and Cell Biology, 91(1), 19–26. 10.1038/icb.2012.56 [DOI] [PubMed] [Google Scholar]
- Fukunishi T, Ong CS, He YJ, Inoue T, Zhang H, Steppan J, Matsushita H, Johnson J, Santhanam L, & Hibino N (2021). Fast-Degrading Tissue-Engineered Vascular Grafts Lead to Increased Extracellular Matrix Cross-Linking Enzyme Expression. Tissue Engineering: Part A, 27(21–22), 1368–1375. 10.1089/ten.TEA.2020.0266 [DOI] [PubMed] [Google Scholar]
- Fukunishi T, Ong CS, Lui C, Pitaktong I, Smoot C, Harris J, Gabriele P, Vricella L, Santhanam L, Lu S, & Hibino N (2019). Formation of Neoarteries with Optimal Remodeling Using Rapidly Degrading Textile Vascular Grafts. Tissue Engineering: Part A, 25(7–8), 632–641. 10.1089/ten.TEA.2018.0167 [DOI] [PubMed] [Google Scholar]
- Gilham D, Tsujikawa LM, Sarsons CD, Halliday C, Wasiak S, Stotz SC, Jahagirdar R, Sweeney M, Johansson JO, Wong NCW, Kalantar-Zadeh K, & Kulikowski E (2019). Apabetalone downregulates factors and pathways associated with vascular calcification. Atherosclerosis, 280, 75–84. 10.1016/j.atherosclerosis.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, & Group VACS (2004). Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. Journal of the American College of Cardiology, 44(11), 2149–2156. 10.1016/j.jacc.2004.08.064 [DOI] [PubMed] [Google Scholar]
- Goodney PP, Beck AW, Nagle J, Welch HG, & Zwolak RM (2009). National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. Journal of Vascular Surgery, 50(1), 54–60. 10.1016/j.jvs.2009.01.035 [DOI] [PubMed] [Google Scholar]
- Gupta P, & Mandal BB (2021). Tissue.2009.01.035 & Zwolak, R. M. (2009). National trends in lgies. Advanced Functional Materials, 31(33), 2100027, Article 2100027. 10.1002/adfm.202100027 [DOI] [Google Scholar]
- Han B, Jaurequi J, Tang BW, & Nimni ME (2003). Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. Journal of Biomedical Materials Research, Part A, 65A(1), 118–124. 10.1002/jbm.a.10460 [DOI] [PubMed] [Google Scholar]
- Han DK, Lee KB, Park KD, Kim CS, Jeong SY, Kim YH, Kim HM, & Min BG (1993). In vivo canine studies of a Sinkhole valve and vascular graft coated with biocompatible PU-PEO-SO3. ASAIO Journal, 39(3), M537–M541. https://www.ncbi.nlm.nih.gov/pubmed/8268593 [PubMed] [Google Scholar]
- He J, Zhong X, Zhao L, & Gan H (2019). JAK2/STAT3/BMP-2 axis and NF-kappaB pathway are involved in erythropoietin-induced calcification in rat vascular smooth muscle cells. Clinical and Experimental Nephrology, 23(4), 501–512. 10.1007/s10157-018-1666-z [DOI] [PubMed] [Google Scholar]
- Ho CY, & Shanahan CM (2016). Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(8), 1475–1482. 10.1161/ATVBAHA.116.306717 [DOI] [PubMed] [Google Scholar]
- Hong SW, Lee J, Cho JH, Kwon H, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, & Lee WY (2018). Pioglitazone Attenuates Palmitate-Induced Inflammation and Endoplasmic Reticulum Stress in Pancreatic beta-Cells. Endocrinology and Metabolism, 33(1), 105–113. 10.3803/EnM.2018.33.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortells L, Sur S, & St Hilaire C (2018). Cell Phenotype Transitions in Cardiovascular Calcification. Frontiers in Cardiovascular Medicine, 5, 27. 10.3389/fcvm.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Suen R, Wang JJ, Zhang ZJ, Wertheim JA, & Ameer GA (2017). Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials, 144, 166–175. 10.1016/j.biomaterials.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Leopold JA, & Loscalzo J (2006). Vascular calcification: pathobiological mechanisms and clinical implications. Circulation Research, 99(10), 1044–1059. 10.1161/01.RES.0000249379.55535.21 [DOI] [PubMed] [Google Scholar]
- Kanno Y, Into T, Lowenstein CJ, & Matsushita K (2008). Nitric oxide regulates vascular calcification by interfering with TGF- signalling. Cardiovascular Research, 77(1), 221–230. 10.1093/cvr/cvm049 [DOI] [PubMed] [Google Scholar]
- Karamched SR, Nosoudi N, Moreland HE, Chowdhury A, & Vyavahare NR (2019). Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Scientific Reports, 9(1), 2629. 10.1038/s41598-019-39639-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira S, Sugimura Y, Grupp S, Doepp R, Selig JI, Barth M, Lichtenberg A, & Akhyari P (2021). PPAR-Gamma Activation May Inhibit the In Vivo Degeneration of Bioprosthetic Aortic and Aortic Valve Grafts under Diabetic Conditions. International Journal of Molecular Sciences, 22(20), 11081. 10.3390/ijms222011081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Nosoudi N, & Vyavahare N (2014). Targeted chelation therapy with EDTA-loaded albumin nanoparticles regresses arterial calcification without causing systemic side effects. Journal of Controlled Release, 196, 79–86. 10.1016/j.jconrel.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold JA (2015). Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends in Cardiovascular Medicine, 25(4), 267–274. 10.1016/j.tcm.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RJ, Schoen FJ, Anderson HC, Harasaki H, Koch TH, Brown W, Lian JB, Cumming R, & Gavin JB (1991). Cardiovascular implant calcification: a survey and update. Biomaterials, 12, 707–714. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Xue F, Feng X, Ba Z, Chen J, Zhou Z, Wang Y, Guan G, Yang G, Xi Z, Tian H, Liu Y, Tan J, Li G, Chen X, Yang M, Chen W, Zhu C, & Zeng W (2022). Programmable dual responsive system reconstructing nerve interaction with small-diameter tissue-engineered vascular grafts and inhibiting intimal hyperplasia in diabetes. Bioactive Materials, 7, 466–477. 10.1016/j.bioactmat.2021.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li B, Jing H, Wu Y, Kong D, Leng X, & Wang Z (2020). Swim Bladder as a Novel Biomaterial for Cardiovascular Materials with Anti-Calcification Properties. Advanced Healthcare Materials, 9(2), e1901154. 10.1002/adhm.201901154 [DOI] [PubMed] [Google Scholar]
- Liu Q, Luo Y, Zhao Y, Xiang P, Zhu J, Jing W, Jin W, Chen M, Tang R, & Yu H (2022). Nano-hydroxyapatite accelerates vascular calcification via lysosome impairment and autophagy dysfunction in smooth muscle cells. Bioactive Materials, 8, 478–493. 10.1016/j.bioactmat.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KE, & Garcia AJ (2021). Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomaterialia, 133, 4–16. 10.1016/j.actbio.2021.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RI, Mukherjee AK, Patterson TD, & Fishbein MC (2011). Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovascular Pathology, 20(4), 213–221. 10.1016/j.carpath.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Moore MAS, Hattori K, Heissig B, Shieh J-H, Dias S, Crystal RG, & Rafii S (2001). Mobilization of Endothelial and Hematopoietic Stem and Progenitor Cells by Adenovector-Mediated Elevation of Serum Levels of SDF-1, VEGF, and Angiopoietin-1. Annals of the New York Academy of Sciences, 938, 36–47. 10.1111/j.1749-6632.2001.tb03572.x [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, & Kuntz RE (2003). Sirolimus-Eluting Stents versus Standard Stents in Patients with Stenosis in a Native Coronary Artery. New England Journal of Medicine, 349(14), 1315–1323. 10.1056/NEJMoa035071 [DOI] [PubMed] [Google Scholar]
- Naegeli KM, Kural MH, Li Y, Wang J, Hugentobler EA, & Niklason LE (2022). Bioengineering Human Tissues and the Future of Vascular Replacement. Circulation Research, 131(1), 109–126. 10.1161/CIRCRESAHA.121.319984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai D, Lino M, & Bendeck MP (2018). Cell-Matrix Interactions and Matricrine Signaling in the Pathogenesis of Vascular Calcification. Frontiers in Cardiovascular Medicine, 5, 174. 10.3389/fcvm.2018.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-C, Song MJ, Hwang Y-S, & Suh H (2001). Calcification Comparison of Polymers for Vascular Graft. Yonsei Medical Journal, 42(3), 304–310. 10.3349/ymj.2001.42.3.304 [DOI] [PubMed] [Google Scholar]
- Park KD, Lee WK, Yun JY, Han DK, Kim SH, Kim YH, Kim HM, & Kim KT (1997). Novel anti-calcification treatment of biological tissues by grafting of sulphonated poly(ethylene oxide). Biomaterials, 18(1), 47–51. 10.1016/s0142-9612(96)00096-8 [DOI] [PubMed] [Google Scholar]
- Pashneh-Tala S, MacNeil S, & Claeyssens F (2016). The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Engineering: Part B, 22(1), 68–100. 10.1089/ten.teb.2015.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadwal K, Vrahnas C, Ganley IG, & MacRae VE (2021). Mitochondrial Dysfunction: Cause or Consequence of Vascular Calcification? Frontiers in Cell and Developmental Biology, 9, 611922. 10.3389/fcell.2021.611922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltis PJ, & Simari RD (2015). Vascular wall progenitor cells in health and disease. Circulation Research, 116(8), 1392–1412. 10.1161/CIRCRESAHA.116.305368 [DOI] [PubMed] [Google Scholar]
- Qiu X, Lee BL, Wong SY, Ding X, Xu K, Zhao W, Wang D, Sochol R, Dong N, & Li S (2021). Cellular remodeling of fibrotic conduit as vascular graft. Biomaterials, 268, 120565. 10.1016/j.biomaterials.2020.120565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Maldonado N, Hutcheson JD, Goettsch C, Goto S, Yamada I, Faits T, Sesaki H, Aikawa M, & Aikawa E (2017). Dynamin-Related Protein 1 Inhibition Attenuates Cardiovascular Calcification in the Presence of Oxidative Stress. Circulation Research, 121(3), 220–233. 10.1161/CIRCRESAHA.116.310293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, … Group, G.-N.-J. G. B. o. C. D. W. (2020). Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. Journal of the American College of Cardiology, 76(25), 2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito J, Kaneko M, Ishikawa Y, & Yokoyama U (2021). Challenges and Possibilities of Cell-Based Tissue-Engineered Vascular Grafts. Cyborg and Bionic Systems, 2021, 1532103. 10.34133/2021/1532103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurgers LJ, Akbulut AC, Kaczor DM, Halder M, Koenen RR, & Kramann R (2018). Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles. Frontiers in Cardiovascular Medicine, 5, 36. 10.3389/fcvm.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoner T, & Dichtl W (2019). Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients, 11(9), 2090. 10.3390/nu11092090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu A, Schulte JB, Fercana G, & Simionescu DT (2011). Inflammation in cardiovascular tissue engineering: the challenge to a promise: a minireview. International Journal of Inflammation, 2011, 958247. 10.4061/2011/958247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C, & Wang X (2017). Metal Ion-Loaded Nanofibre Matrices for Calcification Inhibition in Polyurethane Implants. Journal of Functional Biomaterials, 8(3), 22. 10.3390/jfb8030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, Kharel MK, Guo H, Marsh JL, Thompson LM, Mahishi L, Ahuja P, MacLellan WR, Geschwind DH, Coppola G, Rohr J, & Ratan RR (2011). Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. Journal of Neuroscience, 31(18), 6858–6870. 10.1523/JNEUROSCI.0710-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Chekhoeva A, Oyama K, Nakanishi S, Toshmatova M, Miyahara S, Barth M, Assmann AK, Lichtenberg A, Assmann A, & Akhyari P (2020). Controlled autologous recellularization and inhibited degeneration of decellularized vascular implants by side-specific coating with stromal cell-derived factor 1alpha and fibronectin. Biomedical Materials, 15(3), 035013. 10.1088/1748-605X/ab54e3 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tara S, Nakayama H, Yi T, Lee YU, Shoji T, Breuer CK, & Shinoka T (2017). Fast-degrading bioresorbable arterial vascular graft with high cellular infiltration inhibits calcification of the graft. Journal of Vascular Surgery, 66(1), 243–250. 10.1016/j.jvs.2016.05.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, & Ouchi Y (2011). Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(9), 2054–2062. 10.1161/ATVBAHA.110.216739 [DOI] [PubMed] [Google Scholar]
- Tara S, Kurobe H, Rocco KA, Maxfield MW, Best CA, Yi T, Naito Y, Breuer CK, & Shinoka T (2014). Well-organized neointima of large-pore poly(L-lactic acid) vascular graft coated with poly(L-lactic-co-epsilon-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(L-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis, 237(2), 684–691. 10.1016/j.atherosclerosis.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toong DWY, Toh HW, Ng JCK, Wong PEH, Leo HL, Venkatraman S, Tan LP, Ang HY, & Huang Y (2020). Bioresorbable Polymeric Scaffold in Cardiovascular Applications. International Journal of Molecular Sciences, 21(10), 3444. 10.3390/ijms21103444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran RT, Yang J, & Ameer GA (2015). Citrate-Based Biomaterials and Their Applications in Regenerative Engineering. Annual Review of Materials Research, 45, 277–310. 10.1146/annurev-matsci-070214-020815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lith R, Gregory EK, Yang J, Kibbe MR, & Ameer GA (2014). Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues. Biomaterials, 35(28), 8113–8122. 10.1016/j.biomaterials.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang N, Wu B, Wu S, Zhang Y, & Sun Y (2020). The Role of Mitochondria in Vascular Calcification. Journal of Translational Internal Medicine, 8(2), 80–90. 10.2478/jtim-2020-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, & Langer R (2002). A tough biodegradable elastomer. Nature Biotechnology, 20(6), 602–606. 10.1038/nbt0602-602 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim YM, & Langer R (2003). In vivo degradation characteristics of poly(glycerol sebacate). Journal of Biomedical Materials Research, 66A(1), 192–197. 10.1002/jbm.a.10534 [DOI] [PubMed] [Google Scholar]
- Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng XL, Zhu Y, Kong D, & Zhao Q (2014). The effect of thick fibers and large pores of electrospun poly(epsilon-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials, 35(22), 5700–5710. 10.1016/j.biomaterials.2014.03.078 [DOI] [PubMed] [Google Scholar]
- Wei Y, Wu Y, Zhao R, Zhang K, Midgley AC, Kong D, Li Z, & Zhao Q (2019). MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials, 204, 13–24. 10.1016/j.biomaterials.2019.01.049 [DOI] [PubMed] [Google Scholar]
- Wen M, Zhi D, Wang L, Cui C, Huang Z, Zhao Y, Wang K, Kong D, & Yuan X (2020). Local Delivery of Dual MicroRNAs in Trilayered Electrospun Grafts for Vascular Regeneration. ACS Applied Materials & Interfaces, 12(6), 6863–6875. 10.1021/acsami.9b19452 [DOI] [PubMed] [Google Scholar]
- Wu W, Allen RA, & Wang Y (2012). Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nature Medicine, 18(7), 1148–1153. 10.1038/nm.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Qin Y, Wang Z, Wang J, Zhang C, Li C, & Kong D (2018). The regeneration of macro-porous electrospun poly(varepsilon-caprolactone) vascular graft during long-term in situ implantation. Journal of Biomedical Materials Research, Part B: Applied Biomaterials, 106(4), 1618–1627. 10.1002/jbm.b.33967 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen X, Xu M, Liu X, Pan B, Qin J, Xu T, Zeng K, Pan Y, He B, Sun H, Sun L, & Wang S (2019). miR-375–3p suppresses tumorigenesis and partially reverses chemoresistance by targeting YAP1 and SP1 in colorectal cancer cells. Aging, 11(18), 7357–7385. 10.18632/aging.102214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, & Tang, a. L. (2009). Development of aliphatic biodegradable photoluminescent polymers. PNAS, 106(25), 10086–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zheng X, Qian M, Wang H, Wang F, Wei Y, Midgley AC, He J, Tian H, & Zhao Q (2021). Nitrate-Functionalized poly(epsilon-Caprolactone) Small-Diameter Vascular Grafts Enhance Vascular Regeneration via Sustained Release of Nitric Oxide. Frontiers in Bioengineering and Biotechnology, 9, 770121. 10.3389/fbioe.2021.770121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gao Z, Liu H, & Wu W (2019). Biodegrading highly porous elastomeric graft regenerates muscular and innervated carotid artery-Comparative study with vein graft. Journal of Tissue Engineering and Regenerative Medicine, 13(7), 1095–1108. 10.1002/term.2856 [DOI] [PubMed] [Google Scholar]
- Yoon YM, Lee JH, Yun CW, & Lee SH (2019). Pioglitazone Improves the Function of Human Mesenchymal Stem Cells in Chronic Kidney Disease Patients. International Journal of Molecular Sciences, 20(9), 2314. 10.3390/ijms20092314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai W, Chang J, Lu X, & Wang Z (2009). Procyanidins-crosslinked heart valve matrix: anticalcification effect. Journal of Biomedical Materials Research, Part B: Applied Biomaterials, 90(2), 913–921. 10.1002/jbm.b.31363 [DOI] [PubMed] [Google Scholar]
- Zhai W, Zhang H, Wu C, Zhang J, Sun X, Zhang H, Zhu Z, & Chang J (2014). Crosslinking of saphenous vein ECM by procyanidins for small diameter blood vessel replacement. Journal of Biomedical Materials Research, Part B: Applied Biomaterials, 102(6), 1190–1198. 10.1002/jbm.b.33102 [DOI] [PubMed] [Google Scholar]
- Zhang F, & King MW (2022). Immunomodulation Strategies for the Successful Regeneration of a Tissue-Engineered Vascular Graft. Advanced Healthcare Materials, 11(12), e2200045, Article 2200045. 10.1002/adhm.202200045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li R, Qin X, Wang L, Xiao J, Song Y, Sheng X, Guo M, & Ji X (2018). Sp1 Plays an Important Role in Vascular Calcification Both In Vivo and In Vitro. Journal of the American Heart Association, 7(6), e007555. 10.1161/JAHA.117.007555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li X, Yang L, Sun L, Mu S, Zong H, Li Q, Wang F, Song S, Yang C, Zhao C, Chen H, Zhang R, Wang S, Dong Y, & Zhang Q (2021). Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Materials Science and Engineering C, 118, 111441. 10.1016/j.msec.2020.111441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi D, Cheng Q, Midgley AC, Zhang Q, Wei T, Li Y, Wang T, Ma T, Rafique M, Xia S, Cao Y, Li Y, Li J, Che Y, Zhu M, Wang K, & Kong D (2022). Mechanically reinforced biotubes for arterial replacement and arteriovenous grafting inspired by architectural engineering. Science Advances, 8(eabl3888), eabl3888. 10.1126/sciadv.abl3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yamamoto Y, Xiao Z, & Ochiya T (2019). The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. Journal of Clinical Medicine, 8(7), 1025. 10.3390/jcm8071025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Zhou Z, Tu Y, Wang W, Chen T, & Hu H (2019). Pioglitazone Attenuates Reoxygenation Injury in Renal Tubular NRK-52E Cells Exposed to High Glucose via Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress. Frontiers in Pharmacology, 10, 1607. 10.3389/fphar.2019.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]


