Abstract
Objective:
The Lifestyle Intervention for oVarian cancer Enhanced Survival (LIVES) is a national study of a combined diet and physical activity intervention for stage II-IV ovarian cancer survival, an under-represented cancer in lifestyle behavioral intervention research. Here, we present the data on recruitment, retention, and baseline demographic, clinical and lifestyle behavior characteristics of the LIVES study participants.
Methods:
The LIVES study (NRG Oncology/GOG 0225) is a Phase III diet plus physical activity intervention trial testing the hypothesis that ovarian cancer survivors in the lifestyle intervention will demonstrate better progression-free survival than those in the control condition. Study interventions were delivered via centralized telephone-based health coaching. Baseline descriptive statistics were computed for demographic, clinical, and lifestyle behavior characteristics.
Results:
The LIVES study exceeded its recruitment goals, enrolling 1205 ovarian cancer survivors from 195 NRG/NCORP-affiliated oncology practices across 49 states from 2012–2018. The mean age of enrollees was 59.6 years; the majority (69.4%) with stage III disease; 89% White, 5.5% Hispanic; 64% overweight/obese. Baseline self-reported diet showed a mean daily intake of 6.6 servings of fruit and vegetables, 62.7 fat grams, and 21.7 grams of fiber. Physical activity averaged 13.0 MET-hours/week of moderate to vigorous physical activity; 50.9 hours/week of sedentary time. Retention rates exceeded 88%.
Conclusion:
The LIVES study demonstrates efficiency in recruiting and retaining ovarian cancer survivors in a 24-month study of diet and physical activity intervention with a primary endpoint of progression free survival that will be reported.
Keywords: Ovarian cancer, cancer survivorship, lifestyle, diet, physical activity, progression-free survival, nutrition, exercise, vegetables, fruit, fiber, fat, steps
INTRODUCTION
Ovarian cancer is considered a recalcitrant disease with poor overall survival (OS), partially related to advanced stage at diagnosis given the lack of reliable or valid screening tests. Approximately 21,410 U.S. women will be diagnosed with ovarian cancer in 2021 and an estimated 13,770 will die of the disease [1]. While recent advances in therapy, including the use of targeted therapies, hold promise to enhance survival, 75% of women diagnosed with invasive epithelial stage III disease, the most common presentation, will succumb to the disease within 5 years; overall 5-year survival rates are 48.6% [2]. Currently, an estimated 233,364 ovarian cancer survivors reside in the U.S. Efforts to identify effective strategies to promote survival in these women are needed.
Of the more promising strategies to impact ovarian cancer outcomes are modification of lifestyle factors, such as diet and physical activity, which have been associated with ovarian cancer risk and survival in epidemiological studies [3–5]. In a 2014 analysis from the Women’s Health Initiative (WHI), higher diet quality, a pattern of higher fruit, vegetable and fiber intake, and lower fat and alcohol consumption was associated with a 37% lower all-cause mortality among 600 women diagnosed with ovarian cancer [6]. These findings were corroborated by epidemiological analyses from Australia wherein higher diet quality after ovarian cancer was associated with a 39% higher survival [7] as well as in a recent study by Sasmoto among 1003 ovarian cancer survivors where a pro-inflammatory diet score was associated with higher ovarian cancer specific mortality [8]. Yet, an analysis among 650 ovarian cancer survivors showed no protective effect of improvements in diet quality from pre to post ovarian cancer diagnosis [9], and in an analysis of 635 ovarian cancer survivors, protective associations of diet and physical activity were not consistently demonstrated [10], suggesting this question remains unresolved. Data from the WHI indicate that pre-diagnosis moderate-to-vigorous physical activity was associated with an estimated 25% lower risk for ovarian cancer-specific and all-cause mortality [11]. These findings are supported by a 2015 review showing a protective effect of recreational physical activity on epithelial ovarian cancer risk, with the majority of studies demonstrating significant risk reductions in the most active women [12]. Further, sedentary time, identified with prolonged sitting, screen time and/or rest, has been explored as an exposure driving ovarian cancer mortality. A recent epidemiological analysis in the Ovarian Cancer Association Consortium estimated a 22 to 34% higher mortality risk among inactive women without or with residual disease, respectively [13]. While this evidence is somewhat compelling, there remains a paucity of well-designed, randomized, controlled trials testing hypotheses related to the survival-driving effects of diet and physical activity after an ovarian cancer diagnosis [14]. Further, diet and physical activity are topics of high interest to ovarian cancer survivors, with 69% of survivors attributing a healthy lifestyle to improved survival [15]. Many survivors report adopting a healthier diet and increased physical activity after diagnosis [16, 17].
The Lifestyle Intervention for oVarian cancer Enhanced Survival (LIVES – NRG/GOG0225) trial was designed to test the hypothesis that dietary factors and physical activity could effectively modify ovarian cancer progression-free survival. The protocol has previously been described [18]. Here we present the data on recruitment, retention, and baseline demographic, clinical and lifestyle behavior characteristics of the LIVES study participants.
MATERIALS AND METHODS
Overview
The LIVES study is a randomized, controlled Phase III lifestyle (diet and physical activity modifications) intervention delivered remotely by trained health coaches to stage II-IV ovarian cancer survivors (cancer in ovary, fallopian tube or peritoneum) enrolled nationally across the NRG Oncology academic and community National Cancer Institute (NCI) Community Oncology Research Program (NCORP) sites (Figure 1. Study Design). Eligibility criteria included enrollment between six weeks to six and a half months post primary chemotherapy and/or consolidation therapy (cancer treatment given after induction therapy to consolidate gains, reduce cancer cells and enhance likelihood of durable complete remission) completion, under care at an NRG study site that had completed study training. To be eligible, patients had to have complete clinical remission of ovarian cancer with no evidence of disease and no other invasive malignancies in the previous 10 years. Participants had a Gynecological Oncology Group Performance Grade of 0–2, were over 18 years of age, English- or Spanish-speaking, and free of chronic disease that would preclude study participation (i.e. required a specialized diet). Patients with a life expectancy of less than one year, body mass index indicating underweight status (BMI < 20 kg/m2), on a therapeutically restricted diet, reported a history of disordered eating, or those who reported engaging in endurance-related physical activity (marathons, triathlons) were not eligible to participate. Participants completed the consent process at their local oncology treatment clinic and provided signed institutional review board approved and protocol-specific informed consent before enrollment. The study is registered on ClinicalTrials.gov (NCT NCT00719303).
Figure 1.
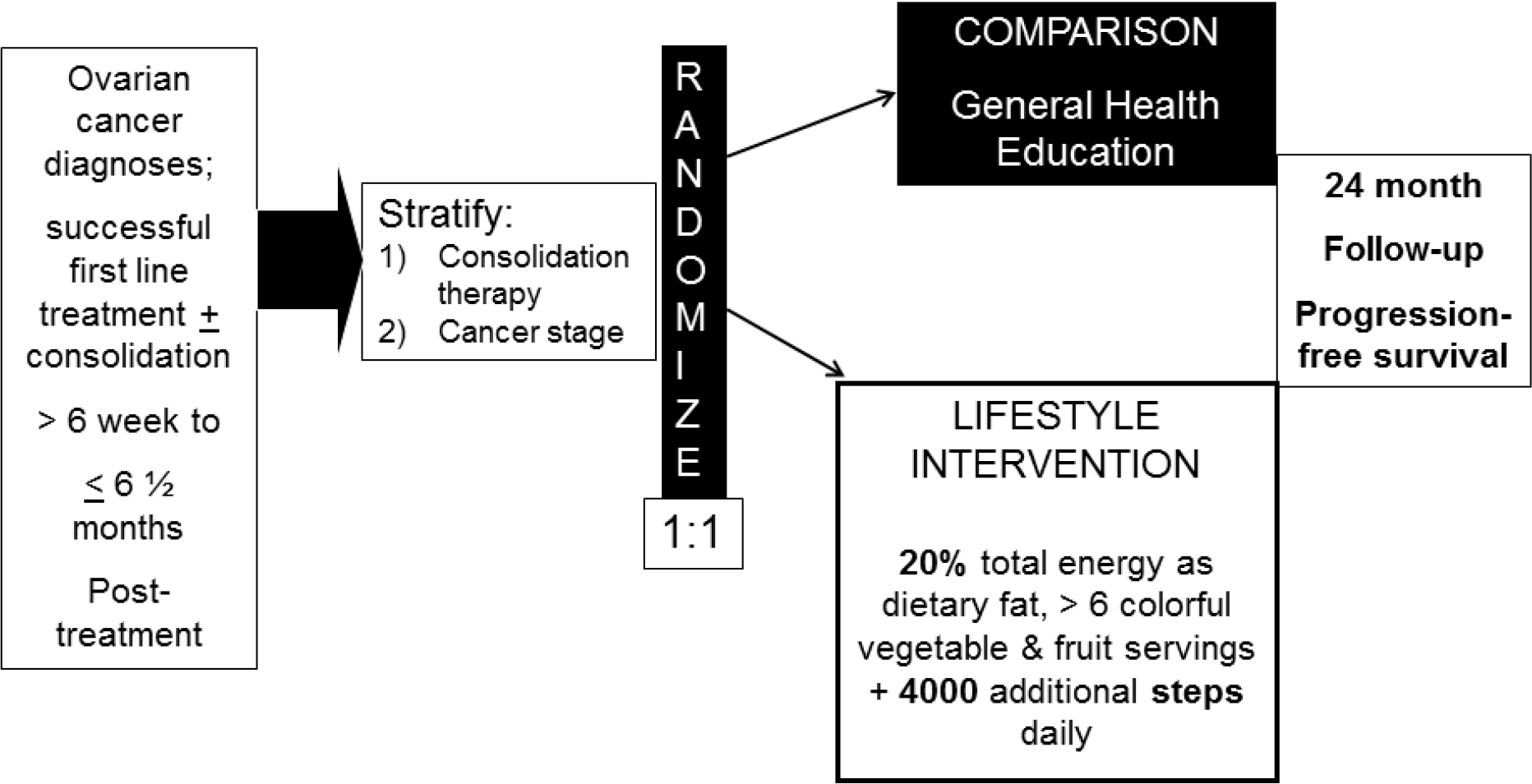
NRG/GOG 0225 LIVES Study schematic
Study Design
For the LIVES study participating medical provider clinics were blinded to the intervention assignment (Figure 1). Women were randomized 1:1 in permuted blocks into the lifestyle intervention or attention control conditions. The randomization was stratified for prior consolidation therapy (yes vs no) and stage of disease (II vs III vs IV). Randomization was conducted using centralized computer software programming implemented through the National Clinical Trials Network (NCTN).
Study Conditions
Intervention Condition
Dietary goals for the intervention condition included 4 servings of vegetables and 2 servings of fruit daily, targeting a daily intake of cruciferous, dark green, and orange vegetables and citrus fruit. Additional dietary goals included low fat intake, with a goal of 20% of daily energy intake as fat, and high fiber intake with a goal of at least 30 grams of fiber per day. For physical activity, women aimed to achieve an increase of 4000 steps daily above baseline levels and to reduce daily sedentary time.
Health coaches for the intervention group were competitively selected from a pool of college juniors and seniors majoring in nutrition science or dietetics. Coaches completed 6 weeks of intensive training in health coaching including evidence and behavior theory-based strategies for promoting behavior change and the application of motivational interviewing [19–21]. Intervention coaches completed a 6-week training course on Motivational Interviewing (MI), survivorship, and protocol adherence, including a minimum of six practice calls with experienced coaches and the study coordinator. All practice calls were recorded and reviewed with the coach in between each call to focus on areas for skill improvement. A final call with the PI was required to obtain approval for the coach to begin working with participants. The behavioral intervention was based in on Social Cognitive Theory, with integration on the Theory of Planned Behavior. Motivational interviewing, self-monitoring and tailored health messaging guided the behavior modification. Women in the intervention group were provided support for lifestyle behavior reinforcement using optional text messaging and/or email communications. Text messages to support behavior change to study goals were sent twice a week. Women in the intervention arm received about 33 telephone coaching sessions over 24 months.
Attention Control Condition
Women randomized to the attention control group received about 22 telephone sessions over 24 months. The attention control condition was delivered by trained, college juniors and seniors majoring in public health. Control coaches were trained over a 6-week period on ovarian cancer and survivorship and approved general health education topics. All practice calls were recorded and reviewed with the coach to focus on areas for skill improvement; a minimum of 6 practice calls were necessary prior to completing a final “certification” call. A final call with the PI was required to obtain approval for the coach to begin working with participants. The control group call content focused on general health topics other than diet and physical activity such as sun safety, hydration or skin care. The attention control group received optional general health information text messages twice weekly and/or email communications.
Coach competence was monitored during the study through weekly collaborative team call reviews and one-on-one call reviews between the coach and study coordinator (at minimum quarterly) for both study arms. Intervention calls were assessed for adherence to MI using the MITI 3.0 scoring rubric, and overall fidelity was assessed using a protocol-specified performance checklist. Control calls were assessed for protocol adherence, with particular attention to assure coaches were not discussing study related diet and physical activity topics with control participants. The program fidelity has been validated (in press) [22] [23].
Retention Efforts
To promote participant retention throughout the 24-month intervention period, a priori retention efforts were identified and implemented for all women, regardless of group assignment. All retention efforts were tailored for study arm assignment and several were tailored for the individual participant. Upon study entry, all enrollees received an introductory welcome letter, a participant binder specific to randomization assignment, and a pedometer. Every quarter, women received treatment arm-specific print newsletters and treatment arm-specific incentives to promote participation and reduced recidivism (Supplemental Table 3). The incentives for Intervention group participants were selected to support diet and physical activity goals, including fat gram counter handbooks, water bottles, measuring cups, and an insulated lunch bag. Each December, participants received a holiday photo card of the LIVES study team, signed by all study personnel. Participants also received a personalized, handcrafted birthday card each year and additional cards when life events were reported (i.e., weddings, births, promotions, new employment, illness, retirement, etc.). Further, participants received a one-year congratulations card and a signed LIVES study certificate upon completing 24 months of intervention.
Primary and Secondary Outcomes
The primary outcome for the trial is progression-free survival defined as the number of months between study enrollment and documentation of disease progression (radiologic image, histological biopsy report, or two repeated measures of CA-125 at or above 2-fold normal values) or death from any cause. These outcomes are collected during regularly scheduled clinic visits, generally occurring every three months, over the 24-month intervention period by the treating oncologist per study protocol and using the centralized NRG data collection form and system. Further follow-up and events are needed before analyzing and reporting this endpoint. Secondary outcomes include quality of life as assessed by the RAND-36 and bowel health (Gastrointestinal Symptom Scale). Lifestyle behavior goal adherence, defined by an a priori score derived from factors supporting study participation and behavior change, was an exploratory aim. Finally, fasting blood samples were collected on a subsample of 600 participants at baseline, 6, 12, and 24 months to ascertain disease-associated cardiometabolic biomarkers including serum lipids, insulin, glucose, high-sensitivity C-reactive protein, and serum carotenoid concentrations as a biomarker of vegetable and fruit intake.
Exploratory Assessments
Participants were asked to complete questionnaires describing existing conditions, behaviors, and other factors expected to influence their progression-free and OS prognoses. Existing conditions were assessed by a comprehensive demographic, health behavior (e.g., smoking, alcohol use), and medical-clinical questionnaire that was completed before randomization. Other questionnaires collected during the study are described in detail in the protocol publication [18] and included the Arizona Food Frequency Questionnaire (AFFQ) [24], the Arizona Physical Activity Questionnaire (APAQ) [25], and the Pittsburgh Sleep Quality Index (PSQI) [26]. Anthropometric measurements were collected in the oncology clinics at randomization and 6-, 12-, and 24-months using standardized protocols. A sub-sample of women (n= 560 of 580 available) wore accelerometers for objective measures of physical activity at the four designated study time-points and also reported cancer-therapy related symptoms [27].
Statistics
For this report, descriptive statistics are reported for baseline demographics, clinical characteristics, health behaviors, and quality of life. Diet data derived from the AFFQ excluded responses <600 kcal/day or >5000 kcal from reporting. Fruit and vegetable servings estimates included 100% juice and excluded potatoes. Metabolic equivalents (METs) of ≥3 for recreational activities were used for moderate to vigorous physical activity (MVPA). Sedentary time included activities during wake time, excluding work, that were <1.5 METs. Total METs derived from the APAQ were adjusted for sleep and occupation as standardly applied. Statistical analyses were performed using STATA 16.1 (StataCorp, LLC, College Station, TX, USA) or SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Supplemental Figure 2 shows the enrollment over time, by calendar month. The study enrolled 1205 women, 601 to the intervention and 604 to the control condition, over the six years between August 2012 and August 2018. After a gradual start-up, enrollment progressed as projected, except for a slight drop in 2017 when a competing NRG trial began recruiting from the same patient population. Recruitment included women from 49 states, with the largest number recruited from California (Supplemental Figure 3). Given the procedures for enrollment in cooperative group trials, no data are available to inform on the proportion of women approached who consented to participate. Overall retention, defined as completing clinic based assessments, across the sample was 88.5%.
The baseline characteristics of the study sample are shown in Table 1. Overall, the mean age at enrollment was 59.4 years; 32.8% were over age 65 years, and 70.4% were married and 55.6% were college graduates. Over 30% were obese, and almost 60% had a waist circumference of 88 cm or more. The majority were originally diagnosed with stage III epithelial ovarian cancer; 32.2% had no family history of cancer, while a small percentage (10.9%) reported known positive BCRA carrier status. Co-morbidities were commonly reported with 67.1% reporting some chronic disease (e.g. osteoporosis, hyper or hypo-thyroid, diabetes, etc.).
Table 1.
Baseline Demographic, Anthropometric and Clinical Characteristics of the LIVES Trial Participants (N= 1205)
| Characteristics | Number (%) of total sample enrolled |
|---|---|
| Demographics | |
| Age (years) [mean (SD)] | 59.57 (9.7) |
| Race / ethnicity | |
| White | 1018 (89.0) |
| Black | 57 (5.0) |
| American Indian/ Alaskan Native / Native Hawaiian | 14 (1.2) |
| Asian | 27 (2.4) |
| Other/ More than 1 race selected | 28 (2.5) |
| Ethnicity | |
| Non-Hispanic | 1077 (94.5) |
| Hispanic | 63 (5.5) |
| Education | |
| Less than college | 185 (16.2) |
| Some college | 324 (28.3) |
| College graduate | 636 (55.6) |
| Marital Status | |
| Married | 807 (7.4) |
| Separated | 174 (15.2) |
| Widowed | 73 (6.4) |
| Never married | 92 (8.0) |
| Clinical | |
| Body Mass Index | |
| Normal (≤24.9 kg/m2) | 437 (36.4) |
| Overweight (25.0–29.9 kg/m2) | 401 (33.4) |
| Obese (≥30.0 kg/m2) | 363 (30.2) |
| Waist Circumference | |
| <88 cm | 483 (40.3) |
| ≥88 cm | 715 (59.7) |
| Stage of disease | |
| II | 188 (15.6) |
| III | 836 (69.4) |
| IV | 181 (15.0) |
| Disease Site | |
| Ovary | 894 (74.9) |
| Fallopian Tube | 118 (3.9) |
| Peritoneum | 111 (9.3) |
| Pathology | |
| Serous Adenocarcinoma | 991 (82.2) |
| Endometrioid Adenocarcinoma | 64 (5.3) |
| Clear Cell Carcinoma | 56 (4.7) |
| Adenocarcinoma, unspecified | 43 (3.6) |
| Mixed Epithelial Carcinoma | 30 (2.5) |
| Other | 21 (1.7) |
| Histology | |
| Well differentiated | 54 (4.8) |
| Moderately differentiated | 62 (6.0) |
| Poorly differentiated | 918 (88.8) |
| Consolidation therapy (% ) | 272 (22.6) |
| Hormone therapy (%) | 336 (29.6) |
| Family history of cancer (%) | 776 (67.8) |
| BRCA mutation carrier (%) | 125 (10.9) |
| Medical History | |
| Number live births [mean (SD)] | 2.00 (1.4) |
| Infertility | 74 (6.5) |
| Diabetes | 80 (7.0) |
| Hypertension | 395 (34.5) |
| Hyperthyroidism | 65 (5.8) |
| Hypothyroidism | 213 (18.8) |
| Hyperlipidemia | 407 (35.6) |
| Myocardial infarction or stroke | 29 (2.5) |
| Osteoporosis | 129 (11.3) |
| Medication use | |
| Oral contraceptives | 731 (63.8) |
| Hormone therapy | 336 (29.6) |
| Aspirin | 319 (27.9) |
| Bisphosphonate | 102 (8.9) |
| Tamoxifen | 27 (2.4) |
Missing data <10%
Baseline diet and physical activity are displayed in Table 2. Diet, as estimated using self-reported responses from the validated AFFQ, demonstrated intakes of 3.3 and 3.3 servings of vegetables and fruit per day, respectively. Fiber intake was estimated at 21.7 grams/day while fat intake composed 32.5% of energy intake or 63 grams/day. Using self-reported data from the APAQ, weekly moderate-to-vigorous physical activity was estimated at 13.0 MET-hrs/week, on average, with less than half of the women meeting current American College of Sports Medicine and the Centers for Disease Control and Prevention activity recommendations. Self-reported daily steps were collected among women randomized to the intervention arm only; for these women steps averaged 5,773 per day at baseline. Less than 10% of women reported current tobacco use; 27% were former smokers.
Table 2.
Baseline Behavior for Intervention-specific Behavioral Targets (diet, physical activity) (N =1,157)
| Behavior | Mean (SD) | Study Goal | n (%) Below |
|---|---|---|---|
| Diet | |||
| Total Fruit (servings/day) | 3.3 (2.7) | 2 | 444 (39.43) |
| Citrus fruit (servings/day) | 0.6 (0.8) | 1 | 880 (78.15) |
| Other fruit (servings/day) | 2.6 (2.3) | 1 | 246 (21.85) |
| Total Vegetable (servings/day) | 3.3 (2.8) | 4 | 797 (70.78) |
| Dark green vegetable (servings/day) | 0.6 (0.7) | 1 | 912 (80.99) |
| Orange vegetable (servings/day) | 0.4(0.5) | 1 | 1054 (93.61) |
| Cruciferous vegetable (servings/day) | 0.4(0.5) | 1 | 1018 (90.41) |
| Other vegetable | 1.9 (1.7) | 1 | 406 (36.06) |
| Fat (grams/day) | 62.7 (31.3) | N/A | |
| Fat (percent kcal/day) | 32.5 (6.5) | 20% | 1109 (98.49) |
| Fiber (grams/day) | 21.7 (12.1) | 30 | 903 (80.20) |
| HEI-2015 | 63.7 (9.7) | N/A | |
| Energy intake (kcal/day) | 1730.1 (751.4) | N/A | |
| Physical Activity * | |||
| MVPA (MET-hrs/week) | 13.0 (17.0) | 8.3 | 613 (53.82) |
| Sedentary time (hr/week) | 50.9 (18.0) | N/A | |
| Steps/ day | 5773(2819) | N/A | |
Goal is 4000 additional steps from baseline; self-reported steps for intervention women only reported at first coaching call n=539.
Completion rates for coaching calls (33 in intervention and 22 calls in control) among ovarian cancer survivors participating in the trial was 28/33 and 19/22 over 24 months, in women assigned to the intervention and control arms, respectively. An analysis of intervention fidelity, using a study-specified checklist, suggested over 87% of individual calls met fidelity requirement [22, 23].
Serum cardiometabolic and carotenoid concentrations were analyzed on a subsample of 605 women. The majority of women had elevated total cholesterol and hsCRP. Serum cardiometabolic biomarkers and carotenoid concentrations are detailed in Supplemental Table 4. Total serum carotenoid values were in the highest quartile as compared to sampling of U.S. adult women for which this level suggested a potential survival advantage [28].
DISCUSSION
To date, LIVES is the largest randomized, controlled lifestyle intervention study for ovarian cancer survivors and the to test the effect of a diet and physical activity lifestyle behavior intervention on clinical outcomes in ovarian cancer survivors. The recruitment and retention data suggest that a national study across NCORP, wherein both academic and community sites actively recruit participants, is feasible and efficient in terms of enrolling ovarian cancer survivors in lifestyle behavior trials. Furthermore, the centralized delivery of the lifestyle intervention to ensure fidelity will facilitate the future robust testing of hypotheses relating lifestyle behavior change to ovarian cancer prognosis.
Accrual into the trial was completed over 6.0 years and slightly exceeded the recruitment goal of 1200 women with 601 assigned to the intervention and 604 to the attention control condition. While recruitment was slower during the first 6 months, once clinic staff were afforded opportunities at the biannual Gynecological Oncology Group/NRG Oncology meetings to complete in-person training on the trial protocol, and the training was expanded for web-based delivery, recruitment generally proceeded at a monthly pace that exceeded estimated rates. A slight reduction in the 17 per month accrual target was shown in 2017–2018, likely attributable to competing enrollment with poly (ADP-ribose) polymerase proliferator-activator receptor (PARP) inhibitor trials introduced at the time. Importantly, accrual included women from 49 states across the U.S. and provided broad regional representation, with Western (California; n=102), Northern (Pennsylvania; n=66) and Southern (North Carolina; n=62) states accruing the highest number of study participants.
The sample of participating women is representative of the disease in terms of race/ethnic disease incidence, with 5.53% reporting Hispanic ethnicity and 5% reporting Black race. Study participants were slightly younger than the average age of diagnosis reported in national data [29]. As has been described for this disease, women are more likely to be well-educated (55.6% have attained a college degree) and were White (89.0%). Clinically, the majority, or 69.4%, presented with Stage III disease, with the remainder of women equally distributed between Stage II and Stage IV disease. The majority were diagnosed with disease of the ovary (74.9%), most were treated for serous adenocarcinoma (82.2%) and a significant majority presented with poorly differentiated disease (88.8%). Only 11% reported BRCA mutations. The LIVES study invited women with stage II-IV disease to represent the range of disease severity. These common disease and tumor characteristics have been shown to be major drivers of mortality in ovarian cancer, potentially overshadowing more subtle effects of anthropometrics, lifestyle or reproductive history [10].
Ovarian cancer, primarily non-serous, is described as one of 13 obesity-related cancers [30]. In terms of overall clinical health, 30.2% of the LIVES study participants were obese, well below the 42.4% national average [31] while 60% demonstrated elevated waist circumferences, indicative of greater visceral/central adiposity. Only 7% of women reported a prior history of diabetes, despite 170 women or 28% of the participants demonstrating fasting glucose levels above 100 mg/dLa cut-point associated with the diagnosis of diabetes. Baseline metabolic blood panels suggested mean serum cholesterol level of 208 mg/dL, with an estimated 55% showing levels above the clinically recommended cut-point of 200 mg/dL. Further, 69.4% demonstrated an elevation in hs-CRP above 3 mg/dL. The recruited sample did represent higher rates of serous tumors, a potential contributor factor in relation to the elevated metabolic and inflammatory measures. Overall, these anthropometric and biochemical indices suggest a significant proportion of study participants had elevated metabolic biomarkers at baseline that may respond to the healthy diet and physical activity intervention being tested in the LIVES study even independent of a weight loss goal.
In relation to diet and physical activity, the LIVES participants reported daily vegetable and fruit intake averaging 6.6 servings/day. This amount of intake exceeds that of non-cancer patients [32] and suggests the women enrolled in the LIVES study, on average, are meeting the American Cancer Society guidance for vegetable and fruit intake for survivorship. However, related to study specific fruit and vegetable goals, the average intake was below the study goals for cruciferous, dark green and orange vegetables as well as citrus fruit. While dietary fiber intake is above national averages reported for US adult women [33], average intake was below current recommendations for cancer survivors as suggested by ACS [34, 35] and baseline intake of dietary fat exceeded the protocol-specified 20% of energy from fat. In terms of physical activity, 54% of women reported activity below 8.75 MET-hr/ week, reflective of activity below the 150 minutes/week moderate-to-vigorous activity recommended for cancer survivors by the American College of Sports Medicine 2019 [36]. Overall, these self-reported behaviors suggest that a notable percentage of participants reflect “healthy volunteers” who may have had motivation to change health behaviors given the recalcitrant nature of ovarian cancer. This reality occurred despite purposeful encouragement of study staff to present the study to any and all eligible participants with an intention to enroll a more representative sample in relation to lifestyle behaviors.
A key outcome of the early stages of this study is the demonstrated feasibility to deliver a high fidelity, theory-based lifestyle behavior intervention (vs attention control contact) to over 1200 ovarian cancer survivors across the U.S. In fact, GOG/NRG 0225 is the first Cancer Prevention and Control Committee lifestyle behavior study to be delivered within the NCORP infrastructure that relied on a centralized intervention delivery approach. Centralizing the behavioral intervention was essential to assure treatment fidelity and to avoid undue burden on clinic staff. Further, this approach allowed for extensive, protocol-specific training of all health coaches with demonstrated competence in intervention (or control) delivery prior to any study participant contact. It also supported a reduction in bias that would be introduced related to unblinding if personnel delivering the intervention were also providing oncology care. Logistically this centralized approach also offered an ongoing opportunity to evaluate recorded coaching and communications calls for treatment fidelity and enhanced training. Relevant for the longer term is the high retention rate, estimated at 88.5%, which exceeded protocol-specified estimates thus assuring the statistical power of the study to test our primary hypothesis related to improved progression-free survival. Should beneficial effects on progression free survival or OS and/or quality of life and/or metabolic markers be demonstrated, there is a realistic opportunity to disseminate this centralized intervention to the larger population of ovarian cancer survivors.
Lessons Learned
As the first large-scale, national lifestyle intervention trial to test hypotheses relevant to ovarian cancer survival, LIVES has afforded several opportunities to advance knowledge to date, even before the outcomes are evaluated. First and foremost, the LIVES study has demonstrated that ovarian cancer survivors are interested in participating in lifestyle behavioral studies in that recruitment was both timely and efficient. These findings are supported by sparse but informative published evidence suggesting ovarian cancer survivors see lifestyle as an attributable factor in their survival [15, 16]. Baseline health behaviors of recruited survivors suggested greater attainment of guideline-specified diet and physical activity behaviors than was anticipated, particularly given targeted efforts to exclude women on vegan diets as well as those with reported history of regular, vigorous exercise such as training for running events. Future studies may need to be even more purposeful in this regard in order to reduce the healthy volunteer effect and support greater change, and thus effect size, across treatment conditions. The study provides evidence that a centralized health coaching approach is feasible and can be used to deliver high fidelity interventions to ovarian cancer survivors. While participants demonstrated coaching call completion rates of 85% across the two groups, a completion rate above the a priori protocol threshold of 80%, a more in-depth analysis of engagement over time, including behavioral goal completion, will be needed to understand the effectiveness of the coaching engagement.
Despite modest limitations identified, the LIVES study is pioneering in its attention to ovarian cancer, national recruitment, implementation of high fidelity student health coaching and the translational science endpoints that afford numerous opportunities to expand the evidence base informing on the impact of diet and physical activity on ovarian cancer survival. Importantly, several other health-related, modifiable cardiometabolic biomarkers will be interrogated for relevance to ovarian cancer survival, further informing clinical care. Given the recalcitrant nature of this disease, there is a significant need to identify low cost, low toxicity approaches to improve health after ovarian cancer. The LIVES intervention holds significant promise in this regard.
Supplementary Material
Research Highlights.
Ovarian cancer has suboptimal survival and lifestyle factors -diet and physical activity- may improve survival.
The NRG-GOG0225 randomized controlled trial in 1200+ ovarian cancer survivors will be the first to test this hypothesis.
Rigorous design and protocols as well as national recruitment and centralized telephonic behavioral intervention delivery drive the trial’s impact.
ACKNOWLEDGEMENTS
The research described in this publication was supported by Up the Volume, West Valley Ovarian Cancer Alliance, and the National Ovarian Cancer Coalition for financial donations in support of implementation of the standardized coaching program and participant retention for this study. We recognize the valuable contribution of the LIVES coaches and support staff for delivery of this intervention. We would like to acknowledge the individual clinic staff supporting participant recruitment and clinical measurements as well as the support of NRG Oncology/GOG staff including Sam DiBernardo, Nick Celender, Linda Gedeon, Marion Piedmonte, and Heather Lankes and the Gynecologic Oncology Group from which this trial began.
The authors wish to thank all the work and efforts of the staff and the undergraduate and graduate student research team who worked on this project from its inception: Angela Yung, Sarah Freylersythe, Amanda Younger and all the LIVES coaches. We also thank the 1205 ovarian cancer survivors who dedicated their time and provided data for this research.
This work was supported by the National Cancer Institute grants to NRG Oncology (U10 CA 180822), NRG operations (U10 CA180868), and UG1CA189867 (NCORP). Additional funding for this research was provided by the NRG Grant (R305B160003), the National Institutes of Health National Cancer Institute (1R01 CA186700), the BMISR at the UACC Support Grant (P30 CA023074), and the West Valley Ovarian Cancer Alliance.
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in the primary treatment studies: Roswell Park Comprehensive Cancer Center, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital-Asplundh Cancer Pavilions, Walter Reed National Military Medical Center, Wayne State University/Karmanos Cancer Institute, University of Minnesota Medical Center-Fairview, Northwestern University, University of Mississippi Medical Center, University of Colorado Cancer Center-Anschutz Cancer Pavilion, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, Abramson Cancer Center of the University of Pennsylvania, University of Cincinnati, University of North Carolina at Chapel Hill, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Wake Forst University Health Sciences, University of California Medical Center at Irvine-Orange Campus, Rush University Medical Center, University of Pittsburgh Cancer Institute, University of Kentucky, University of New Mexico, Cleveland Clinic Foundation, Stany Brook University Medical Center, Washington University School of Medicine, Cooper Hospital University Medical Center, Ohio State University Comprehensive Cancer Center, MD Anderson Cancer Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, Women’s Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago, Mayo Clinic, Case Western Reserve University, Yale University, University of Wisconsin Hospital and Clinics, Women and Infants Hospital, The Hospital of Central Connecticut, Georgia Center for Oncology Research and Education (CORE), Gynecologic Oncology of West Michigan PLLC, Saint Joseph’s Hospital and Medical Center, Carolinas Medical Center/Levine Cancer Institute, Froedhert and the Medical College of Wisconsin, University of Arkansas for Medical Sciences, City of Hope, UC San Diego Moores Cancer Center, UF Cancer Center at Orlando Health, University of Kansas Medical Center, Norton Hospital Pavillion and Medical Campus, Lahey Hospital and Medical Center, Greater Baltimore Medical Center, Rutgers Cancer Insitute of New Jersey, Autlman Health Foundation, Thomas Jefferson University Hospital, Medical University of South Carolina, The Methodist Hospital System, University Medical Center Brackenridge, University of Michigan Health System-Cancer Center, Dartmouth-Hitchcock Medical Center, Huntsman Cancer Institute/University of Utah, Dana-Farber Cancer Institute, University of Southern California, University of California Davis-Cancer Center, Oncology Alliance-Glendale, Alleghany General Hospital, Hartford Hospital, Maine Medical Center – Scarborough Campus, Kaiser Permanente-San Diego Mission, Saint Luke’s Mountain States Tumor Institute, WelSpan Health-York Hospital, University of Tennessee-Knoxville, Sutter Cancer Research Consortium, Saint Peters Hospital-Albany, Northwestern Medicine Cancer Center Warrenville, University of Arizona Medical Center-University Campus, Cedars-Sinai Medical Center, Kansas City CCOP, Wisconsin NCI Community Oncology Research Program, University of Hawaii, Carle Cancer Center, Cancer Research Consortium of West Michigan NCORP, Metr-Minnesota CCOP, Central Illinois CCOP, Delaware/Christiana Care CCOP, Northern Indiana Cancer Research Consortium, Geisinger Medical Center, Virginia Commonwealth University, Virginia Mason CCOP, Saint Vincent Hospital, Colorado Cancer Research Program NCORP, Wichita CCOP, Greenville Health System Cancer Institute/Greenville CCOP, Sanford NCI Community Oncology Research Program of the North Central Plains, Florida Hospital Cancer Institute CCOP, Tulane University MBCCOP, Columbus NCI Community Oncology Research Program, Illinois Oncology Research Association CCOP, University of New Mexico, North Shore – LIJ Health System CCOP, Northwest NCI Community Oncology Research Program, Southeast Cancer Control Consortium CCOP, Pacific Cancer Research Consortium, Georgia Cares Minority Underserved NCORP, Northside Hospital and Catholic Health Initiatives NCORP.
Footnotes
CONFLICTS OF INTEREST
Dr. Austin Miller reports that his institution received grants from the following: Vascular Biogenics LTD, Advaxis, Abbvie. Dr. Miller also reports participating on the Steering Committee for AstraZeneca as well as receiving institutional support from both Regeneron and GSK.
Dr. Michael Gold received honoraria from ASCCP as an Educational Course Speaker.
Dr. Matthew Powell received support from the NIH/NIH to NCTN NRG Oncology Network. Dr. Powell also reports participating on Advisory Boards for the following entities: GSK/Tesaro, Clovis Oncology, Merck, AstraZeneca, Eisai and SeaGen.
Dr. Kristin Bixel reports receiving intuitive unrestricted research grant to the GOG for funding of randomized controlled trial (ROCC). Additionally, Dr. Bixel reports serving on Advisory Boards for Intuitive and Merck and receiving honoraria from both of these entities.
Dr. Ratner reports receiving consulting fees from Aspire and has stock in same.
Dr. Warner Huh reports receiving consulting fees from AstraZeneca and receiving payment for expert testimony from Shook, Hardy & Bacon LLP.
Dr. Basen-Engquist reports MPI, R01CA186700, Study of biomarkers in ovarian cancer: Modulation by activity & diet intervention, MD Anderson subcontract from University of Arizona. She also receives grant from NCI, R21CA239079, Reducing breast cancer risk through modifying boy composition and decreasing inflammation in normal weight women. Dr. Basen-Engquist also reports pilot study grant from NCI, R21 CA215410 as well as receiving funding from Cancer Prevention & Research Institute of Texas: PP170023 & PP200028, Active Living After Cancer: Combining a physical activity program with survivor navigation and she received honoraria for Speaking from the both NCI as well as the University of New Mexico Cancer Center, Gynecologic Oncology Group and Yale University for Faculty TREC training program. Dr. Basen-Engquist also reports receiving support for travel from the National Cancer Policy Forum, National Academies of Science, Engineering, and Medicine and Yale University. She also participated as an Independent Safety Monitor for Boston University, digital health supported weight management intervention delivered by community health workers among public housing residents (R01CA238335). Dr. Basen-Engquist reports serving as President of the American Society of Preventive Oncology.
Dr. Walker reports serving in a leadership role for NCORP.
Dr. Lisa Kachnic reports NCI NCORP Funding and serving as Associate NCORP PI Support and Chair of Cancer Control Support.
All other authors have no conflict of interest related to this manuscript.
Trial registration: ClinicalTrials.gov NCT00719303.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Henley J, Ward E, Scott S, Ma J, Anderson R, Firth A, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Services UDoHaH. Cancer Stat Facts: Ovarian Cancer: National Cancer Institute; 2021. [Available from: https://seer.cancer.gov/statfacts/html/ovary.html. [Google Scholar]
- 3.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99(20):1534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA. Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(2):255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorman PG, Jones LW, Akushevich L, Schildkraut JM. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol. 2011;21(3):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CA, T EC, Wertheim BC, Neuhouser ML, Li W, Snetselaar LG, et al. Diet quality and survival after ovarian cancer: results from the Women’s Health Initiative. J Natl Cancer Inst. 2014;106(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen JM, Nagle CM, Ibiebele TI, Grant PT, Obermair A, Friedlander ML, et al. A healthy lifestyle and survival among women with ovarian cancer. Int J Cancer. 2020;147(12):3361–9. [DOI] [PubMed] [Google Scholar]
- 8.Sasamoto N, Wang T, Townsend MK, Eliassen AH, Tabung FK, Giovannucci EL, et al. Pre-diagnosis and post-diagnosis dietary patterns and survival in women with ovarian cancer. Br J Cancer. 2022;127(6):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Ramadhani RM, Nagle CM, Ibiebele TI, Grant P, Friedlander M, DeFazio A, et al. Pre- and Post-Diagnosis Diet Quality and Ovarian Cancer Survival. Cancer Epidemiol Biomarkers Prev. 2021;30(1):229–32. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Klint A, Lambe M, Bellocco R, Riman T, Bergfeldt K, et al. Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer. 2008;123(3):672–9. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannioto RA, Moysich KB. Epithelial ovarian cancer and recreational physical activity: A review of the epidemiological literature and implications for exercise prescription. Gynecol Oncol. 2015;137(3):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannioto RA, LaMonte MJ, Kelemen LE, Risch HA, Eng KH, Minlikeeva AN, et al. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. Br J Cancer. 2016;115(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole EM, Konstantinopoulos PA, Terry KL. Prognostic implications of reproductive and lifestyle factors in ovarian cancer. Gynecol Oncol. 2016;142(3):574–87. [DOI] [PubMed] [Google Scholar]
- 15.Stewart DE, Duff S, Wong F, Melancon C, Cheung AM. The views of ovarian cancer survivors on its cause, prevention, and recurrence. Medscape Womens Health. 2001;6(5):5. [PubMed] [Google Scholar]
- 16.Alimujiang A, Khoja L, Wiensch A, Pike MC, Webb PM, Chenevix-Trench G, et al. “I am not a statistic” ovarian cancer survivors’ views of factors that influenced their long-term survival. Gynecol Oncol. 2019;155(3):461–7. [DOI] [PubMed] [Google Scholar]
- 17.Jones T, Sandler C, Vagenas D, Janda M, Obermair A, Hayes S. Physical activity levels among ovarian cancer survivors: a prospective longitudinal cohort study. Int J Gynecol Cancer. 2021. [DOI] [PubMed] [Google Scholar]
- 18.Thomson CA, Crane TE, Miller A, Garcia DO, Basen-Engquist K, Alberts DS. A randomized trial of diet and physical activity in women treated for stage II-IV ovarian cancer: Rationale and design of the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES): An NRG Oncology/Gynecologic Oncology Group (GOG-225) Study. Contemporary clinical trials. 2016;49:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spahn JM, Reeves RS, Keim KS, Laquatra I, Kellogg M, Jortberg B, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010;110(6):879–91. [DOI] [PubMed] [Google Scholar]
- 20.Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099–105. [DOI] [PubMed] [Google Scholar]
- 21.Tahan HA, Sminkey PV. Motivational interviewing: building rapport with clients to encourage desirable behavioral and lifestyle changes. Prof Case Manag. 2012;17(4):164–72; quiz 73–4. [DOI] [PubMed] [Google Scholar]
- 22.Yung TC AK, Loescher LJ, Younger AM, Bingham LM, Thomson CA, editor A New Model to Support Lifestyle Intervention in Cancer Survivors: Undergraduate Students and Multimodal Software Program Society of Behavioral Medicine; 2017; San Diego, CA.
- 23.Werts MS SJ, Freyersythe SJ, Thomson CA, Crane TE, editor Coaching Fidelity in a Lifestyle Intervention for Ovarian Cancer Survivors. American Society for Preventive Oncology; 2021; online due to COVID-19. [Google Scholar]
- 24.Martinez ME, Marshall JR, Graver E, Whitacre RC, Woolf K, Ritenbaugh C, et al. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol Biomarkers Prev. 1999;8(10):941–6. [PubMed] [Google Scholar]
- 25.Staten LK, Taren DL, Howell WH, Tobar M, Poehlman ET, Hill A, et al. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33(11):1959–67. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 27.Crane TE, Skiba MB, Miller A, Garcia DO, Thomson CA. Development and Evaluation of an Accelerometer-Based Protocol for Measuring Physical Activity Levels in Cancer Survivors: Development and Usability Study. JMIR Mhealth Uhealth. 2020;8(9):e18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res. 2011;31(3):178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Society AC. Cancer Facts & Figures 2021 2021. [Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf.
- 30.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales CM, Carroll MD, Fryar CD, CL O Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360: National Center for Health Statistics; 2020. [Available from: https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf. [PubMed] [Google Scholar]
- 32.Control CfD. Only 1 in 10 Adults Get Enough Fruits or Vegetables 2021. [Available from: https://www.cdc.gov/media/releases/2017/p1116-fruit-vegetable-consumption.html.
- 33.Hoy MK, Goldman J. Fiber Intake of the U.S. population: What We Eat in America, NHANES 2009–2010 2014. [Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/12_fiber_intake_0910.pdf#:~:text=Fiber%20intake%20of%20the%20U.S.%20population%20What%20We,15%20grams%20per%20day%2C%20respectively%20%28data%20not%20shown%29. [PubMed]
- 34.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 36.Medicine ACoS. Effects of Exercise on Health-Related Outcomes in Those with Cancer 2019. [Available from: https://www.acsm.org/blog-detail/acsm-certified-blog/2019/11/25/acsm-guidelines-exercise-cancer-download.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


