Abstract
Dendritic cells (DCs) present internalized antigens to CD8 T cells through cross-presentation by major histocompatibility complex class I (MHC-I) molecules. While conventional cDC1 excel at cross-presentation, cDC2 can be licensed to cross-present during infection by signals from inflammatory receptors, most prominently Toll-like receptors (TLRs). At the core of the regulation of cross-presentation by TLRs is the control of subcellular MHC-I traffic. Within DCs, MHC-I are enriched within endosomal recycling compartments (ERC) and traffic to microbe-carrying phagosomes under the control of phagosome-compartmentalized TLR signals to favor CD8 T cell cross-priming to microbial antigens. Viral blockade of the transporter associated with antigen processing (TAP), known to inhibit the classic MHC-I presentation of cytoplasmic protein-derived peptides, depletes the ERC stores of MHC-I to simultaneously also block TLR-regulated cross-presentation. DCs counter this impairment in the two major pathways of MHC-I presentation to CD8 T cells by mobilizing noncanonical cross-presentation, which delivers MHC-I to phagosomes from a new location in the ER-Golgi intermediate compartment (ERGIC) where MHC-I abnormally accumulate upon TAP blockade. Noncanonical cross-presentation thus rescues MHC-I presentation and cross-primes TAP-independent CD8 T cells best-matched against target cells infected with immune evasive viruses. Because noncanonical cross-presentation relies on a phagosome delivery route of MHC-I that is not under TLR control, it risks potential cross-presentation of self-antigens during infection. Here I review these findings to illustrate how the subcellular route of MHC-I to phagosomes critically impacts the regulation of cross-presentation and the nature of the CD8 T cell response to infection and cancer. I highlight important and novel implications to CD8 T cell vaccines and immunotherapy.
Keywords: Dendritic cells, cross-presentation, Toll-like receptors, phagosomes, vesicular traffic, noncanonical cross-presentation, CD8 T cells
Introduction
Major histocompatibility complex class I (MHC-I) molecules present peptides at the cell surface to CD8 T cells [1]. Classic MHC-I presentation relies on shuttling cytosolic peptides into the endoplasmic reticulum (ER) lumen by the transporter associated with antigen processing (TAP) [1]. The cytosolic peptides are generated via the proteasome and derived from either cellular proteins or viruses and bacteria that infect the cells. TAP is a heterodimer of TAP1 and TAP2 transmembrane ER proteins of the ABC transporter family, and it shuttles cytosolic proteasome-generated peptides across ER membranes [2]. Cryo-electron microscopy recently revealed the 3-dimensional organization of TAP within a macromolecular peptide loading complex comprised of the chaperones tapasin and calreticulin and the oxidoreductase ERp57, and tasked with loading and folding of MHC-I heavy-chain and β2-microglobulin heterodimers with peptides [3–7].
Contrasting with classic MHC-I presentation of endogenous cellular peptides, cross-presentation enables MHC-I presentation of exogenous antigens in the form of soluble proteins and viruses internalized through endocytosis or bacteria and dying cells internalized through phagocytosis [1,8–11]. Cross-presentation by dendritic cells (DCs) initiates CD8 T cell immunity against viruses, bacteria and tumors, and induces peripheral tolerance [1,8–13]. Its role is particularly important in the immune response against cancer or during infection with tissue-tropic viruses that do not infect hematopoietic antigen-presenting cells (APCs), such that priming CD8 T cell responses relies on cross-presentation by DCs that internalize dying cancer cells or infected cells [9,14]. A number of pathways and subcellular locations have been described where cross-presentation might occur [13,15]. Internalized proteins can be translocated to the cytosol and degraded by the constitutive proteasome and the immunoproteasome after which they are transported via TAP either back into phagosomes and endosomes for loading vacuolar MHC-I [13] or into the ER for loading nascent MHC-I (cytosolic pathway) [13]. Alternatively, internalized proteins are degraded by endosomal or phagosomal proteases and resultant peptides loaded onto MHC-I independently of immuno-proteasomal degradation and TAP function (vacuolar pathway) [13]. Delivery of the MHC-I peptide loading complex from a sub-compartment of the ER, the ER-Golgi intermediate compartment (ERGIC), to phagosomes and endosomes via the SNARE protein Sec22b, has provided a mechanism for MHC-I loading within phagosomes and endosomes [16].
Extensive investigations into the mechanisms of cross-presentation have been facilitated by a source of DCs that can be cultured at large numbers to enable detailed investigations at the cell biological, genetic, and biochemical level. Bone marrow derived DCs (BMDCs) have served this function well laying the foundation for understanding the biology, activation, and function of DCs. Original protocols entailed growing bone marrow progenitors in GM-CSF, but later improvements included growth in Flt3L, supplementation of Flt3L with GM-CSF, or coculture with feeder OP-9 cells expressing Delta-like 1 (DLL1) [17,18]. These improvements have enabled these cultures to faithfully represent cDC subsets found in vivo. BMDCs continue to provide a blueprint for understanding DC functions, including those of human DCs, under different conditions. Studies on BMDCs have also informed therapeutic harnessing of DCs for cancer immunotherapy [19–24].
In studying cross-presentation, the choice of ‘particulate’ versus ‘soluble’ antigens determines the internalization route into DCs [25–27]. The mode of antigen internalization is a large determinant of the regulatory mechanisms of cross-presentation. ‘Particulate’ antigens (typically >0.5 μm), including those from bacteria or dying cells, are optimally internalized by phagocytosis [28]. The contribution of phagocytosis steadily decreases with decreasing particle diameter such that endocytosis is considered to favor the internalization of particles <0.5 μm [29–34]. Viruses (20–400 nm), soluble proteins (4–6 nm) and subunit vaccines comprised of purified or recombinant proteins (e.g. Pertussis, influenza, hepatitis B, hepatitis A, meningococcal and pneumococcal vaccines) are internalized by endocytosis –also possibly internalized by micropinocytosis. Virus-like particle-based human papillomavirus vaccines, outer membrane vesicle- or liposome nanoparticle-based vaccines, which all range 25–100 nm, are also endocytosed and engage cross-presentation [35]. Other vaccines are based on targeting DC receptors which undergo endocytosis [36]. The route of internalization is an important factor to consider when studying the regulation of cross-presentation because it dictates the type of cellular receptors engaged, subcellular compartments antigens are delivered to, and the nature of subcellular vesicular trafficking mobilized to internalized antigens to mediate cross-presentation [25–27]. These differences necessitate tailored regulation. Therefore, mechanisms that regulate the cross-presentation of phagocytic antigens are anticipated to be different than those regulating the cross-presentation of endocytic antigens. A thorough dissection of these mechanisms has not been conducted to date. Careful deciphering of the regulation of cross-presentation taking the routes of internalization and receptors engaged into consideration is important because it directly impacts the design of direly needed CD8 T cell vaccines [37]. Understanding the mechanisms that regulate DC cross-presentation is critical for designing novel adjuvants that elicit strong CD8 T cell immunity. This review will discuss the current understanding of the regulation of cross-presentation focusing on the vesicular trafficking routes of MHC-I molecules to sites of internalized antigen.
1. The positive regulation of cross-presentation by Toll-like receptors
1.1. Cross-presentation can be licensed by inflammatory signals
Cross-presentation is a specialty of conventional DCs, classified into cDC1 and cDC2 [38]. cDC1, comprised of CD8α+ and CD103+ DC, are generally considered superior cross-presenting DC, largely based on their constitutive ability to do so [8–10,38–45]. As such, they are also thought to predominate cross-presentation during infection and inflammation. For example, in pulmonary influenza A virus infection, lymph node (LN) resident cDC1 cross-prime CD8 T cells to influenza virus after migratory tissue DCs carry infected dying cells from the lungs to the LNs and transfer the antigen to CD8α+ DCs for cross-presentation to CD8 T cells [46]. Migrant airway-derived or skin-derived CD103+ DCs in the inflamed draining LNs can also cross-present influenza or herpes simplex virus type 1 antigens, respectively, to CD8 T cells [47,48]. While cDC1s occupy center stage in cross-presentation, they are not the only cross-presenting DCs [49]. Voluminous work by many groups has shown that other murine and human DCs can acquire cross-presentation under inflammatory conditions, e.g. upon vaccination or upon ligation of inflammatory receptors on DCs [39,46,49–58]. These DCs include cDC2 and inflammatory DCs, the latter now validated to comprise CD14+ human DC3 or murine cDC2B, and which arise from monocytes recruited into inflamed tissues and comprise the most abundant DC population during inflammation [59–62]. In fact, the earliest studies by Michael Bevan’s group had shown that both the CD8α+ cDC1 and CD8α− cDC2 subset of murine splenic DCs cross-present equally well, with the difference being that the CD8α+ cDC1s cross-present constitutively while the CD8α− DCs cross-present upon activation, in this case when FcγR was engaged and activated during antigen-immune complex internalization [39]. Neonatal Fc receptor for IgG (FcRn) drives cross-presentation in CD8−CD11b+ inflammatory DCs and induces a proinflammatory T cell response in a model of chronic intestinal inflammation [54]. Work from the late Nobel Laureate Ralph Steinman has shown that CD14 is required alongside TLR4 in mobilizing inflammatory monocyte-derived DCs into LNs by LPS or Gram-negative bacteria, and these DCs are as active as cDCs in cross-presentation of proteins and live Gram-negative bacteria [62]. During a fungal infection, induction of a CD8 T cell response to fungal-derived antigen was restricted to CD8α− DCs [50]. In influenza A virus infection, CD103−CD11bhi cDC2 in the submucosa and lung parenchyma, can capture exogenous virus-derived antigen in the lung and migrate to the mediastinal LN to directly cross-prime CD8 T cells [46]. Antigens complexed to antibodies are cross-presented by both cDC1 and cDC2 [39]. Cross-presentation by inflammatory DCs mediates efficacy of anti-tumor chemotherapy or immunostimulatory agents [63]. Adjuvant-dependent tissue accumulation of inflammatory cDC2 is responsible for cross-priming CD8 T cells after immunization via the skin or mucosal routes [53]. Similarly, a subset of F4/80hi cDC2 of monocytic origin in the small intestine, lung, spleen, mesenteric and mediastinal LNs, and strongly enriched in tumors, can cross-present efficiently [53]. During experimental autoimmune encephalomyelitis, inflammatory TNF and nitric oxide releasing ‘Tip’ DCs accumulate in the central nervous system and cross-present myelin basic protein to activate naive and effector CD8 T cells [59,60]. Inflammatory human CD14+ DC3 cDC2 acquire a multitude of pro-inflammatory functions including cross-presentation. All human BDCA3+CD141+ (cDC1), BDCA1+CD1c+ (cDC2), and plasmacytoid DCs, have the ability to cross-present extracellular antigens [10,49,64,65]. The mechanisms that license DC cross-presentation function during infection and inflammation are not well understood.
Recent comparative transcriptomic and functional studies on human and murine DCs revealed relatively homogenous cDC1, but extensive heterogeneity in cDC2 [66–69]. Within cDC2, a novel subset of inflammatory DCs was found that expressed CD14 and was named ‘DC3’ in humans and ‘cDC2B’ in mice [66–69]. The primary and consistent characteristic of CD14+ cDC2 in humans and mice, across multiple high-dimensional single cell approaches, was their pro-inflammatory profile, accumulation in inflamed tissues, and enrichment in transcripts associated with antigen presentation, compared to other cDC2 and cDC1 [66–69]. Importantly, among DCs, inflammatory human CD14+ DC3 expressed high levels of TLR2 and TLR4 [66]. Similarly, compared to cDC1 and other cDC2, murine CD14+ cDC2B expressed the highest levels of transcripts involved in antigen processing and presentation [69]. While cDC1 expressed TLRs 3, 11 and 12, cDC2 expressed TLRs 1, 2, 4, 5, 6, 7, 8 and 9 [69]. Inflammatory human DCs (comprised of CD14+ DC3 subset of cDC2 [66]), are efficient at cross-presentation and induction of cytotoxic CD8 T cells [70].
This collective body of work provides strong support to the concept that inflammatory signals have the potential to license cross-presentation by cDC2, including human CD14+ DC3 and murine cDC2B. Acquisition of cross-presentation capacity by cDC2 during infection and inflammation is consistent with regulation of cross-presentation by inflammatory signals such as those from TLRs which alert the immune system to a microbial infection [8,15,25–27,71–77].
1.2. Endocytic recycling of MHC-I molecules is intricately linked to cross-presentation
The study of cross-presentation has primarily been focused on the cytosolic and vacuolar pathways of liberating peptides for MHC-I presentation and has resulted in the classification of cross-presentation into either vacuolar or cytosolic [78–84]. The subcellular sources of MHC-I and the signals that control their trafficking to sites of internalized antigen have received less attention despite the central role of MHC-I molecules in cross-presentation [8,72,85]. Confocal imaging of murine BMDCs stained with a conformation dependent antibody, AF6-88.5, which detects fully assembled MHC-I molecules [86], shed important insight into the subcellular locations of MHC-I within DCs. Murine BMDCs, CD8α+ splenic DCs, and DC2.4 or JAWS-II DC lines, as well as human monocyte-derived DCs, all harbor rich stores of MHC-I within endosomal recycling compartments (ERC) [75,77,87,88]. Plasma membrane MHC-I undergo clathrin-independent endocytosis (CIE) and recycle through either the rapid route through early endosomes or the slow route through the ERC [8,72,85,89]. In DC2.4 cells, MHC-I were internalized into Rab3b/3c positive endosomes that were implicated in rapid recycling and colocalized with other Rabs specific to early endosomes and recycling endosomal tubules such as Rab5, Rab8, Rab10, and Rab35, and whose knockdown during a siRNA-mediated screen impaired cross-presentation [90]. Interestingly, a fraction of Rab3b/c colocalized near bacteria-carrying phagosomes with Rab27a [90], which contributes to phagosomal assembly of the NADPH oxidase NOX2 to mediate phagosome alkalinization and promote cross-presentation [91–94].
The CIE recycling pathway is regulated by the GTPase ARF6, which was originally described to be associated with tubular endosomes emanating from the ERC [89]. Although silencing ARF6 in murine BMDCs was associated with a 30% increase in surface MHC-I levels, it did not impact MHC-I entry into early endosomes or localization to the ERC [87]. These findings were taken to suggest an unidentified source of ERC-resident MHC-I other than the plasma membrane in DCs [87]. Alternatively, the lack of impact of ARF6 silencing on the ERC stores of MHC-I in DCs could have been due to the function of ARF6 specifically in the step of recycling CIE cargo back to the plasma membrane [89].
The ERC is maintained by the activity of the small GTPases Rab11a and is enriched for vesicle-associated membrane protein (VAMP)8/endobrevin and VAMP3/cellubrevin [8,75]. Concordantly, the subcellular ERC localization of MHC-I in DCs is dependent on the activity of Rab11a [75], and disrupting ERC formation by silencing Rab11a abrogated TLR-dependent cross-presentation of phagocytic antigen [75]. Silencing the small GTPase Rab22a, which is important for traffic from early endosomes to the ERC but also from the ERC back to the plasma membrane [89], led to the disappearance of a perinuclear pool of MHC-I (presumably in the ERC) and also impaired cross-presentation [88]. Silencing ARF6, on the other hand, which did not impact the ERC stores of MHC-I [87], had no effect on the cross-presentation of soluble, mannose-receptor targeted, or yeast-associated antigen [87,95], except notably the cross-presentation of Fc receptor-targeted immune complexes [87]. These studies collectively supported a critical role for ERC-resident MHC-I in cross-presentation, although importantly, there may be nuances to the role of the ERC depending on the internalization of cross-presented antigens by phagocytosis or endocytosis. Concordant with this possibility, cross-presentation of soluble antigens, which are internalized by endocytosis, remained intact when either Rab11a or Rab22a function was disrupted [95].
1.3. Control of MHC-I vesicular traffic is at the core of TLR regulation of cross-presentation
TLR engagement in phagosomes carrying microbial ligands transduces a signal through the TLR signaling adaptor MyD88 and the downstream kinase IKK2 to phosphorylate the synaptosomal associated protein 23 (SNAP-23), which flags those phagosomes for MHC-I recruitment from the ERC [75]. Phosphorylated SNAP23 stabilizes SNARE interactions between phagosomal syntaxins and ERC VAMP3 or VAMP8, which result in delivery of ERC-resident MHC-I specifically to phagosomes carrying microbial antigens [75]. Mobilization of ERC-to-phagosome vesicular traffic under the control of TLR signals ensures the availability of large numbers of MHC-I molecules to meet the increased demands of cross-presentation during infection. These findings were made mostly in the context of TLR4 engagement and MyD88 signaling during phagocytosis. TRIF signaling was not involved in the TLR regulation of phagocytic antigen cross-presentation [75]. Whether signals from other MyD88-dependent TLRs such as endosomal TLRs 7 and 9 also license vesicular traffic from the ERC to their respective ligand-carrying phagosomes remains to be tested directly.
Regulated ERC-to-phagosome delivery of MHC-I is a necessary but not sufficient step for completing the process of phagocytic antigen cross-presentation [75]. A parallel ERGIC-to-phagosome pathway of vesicular traffic must also be mobilized to deliver several components of the MHC-I peptide-loading complex and presumably facilitate MHC-I loading in phagosomes [96–98]. ERGIC-to-phagosome directed traffic relies on the pairing of ER SNARE Sec22b with phagosomal syntaxins [16,99], and is not subject to TLR regulation unlike ERC-to-phagosome traffic [75]. Thus, at least two pathways of vesicular traffic converge on the cross-presenting phagosome to deliver different components necessary for phagocytic antigen cross-presentation (Figure 1) [8]: the TLR-regulated ERC-to-phagosome pathway which delivers the centerpiece MHC-I molecules, and the TLR-independent ERGIC-to-phagosome pathway which delivers the accessory molecules potentially needed to both aid peptide generation and peptide loading of MHC-I. It is conceivable that other pathways of vesicular traffic aid cross-presentation by delivering additional components such as the replenishment of NOX2 components from lysosome related organelles mediated by Rab27a [93].
Figure 1. Subcellular localization and traffic of MHC-I molecules during TLR-regulated cross-presentation.
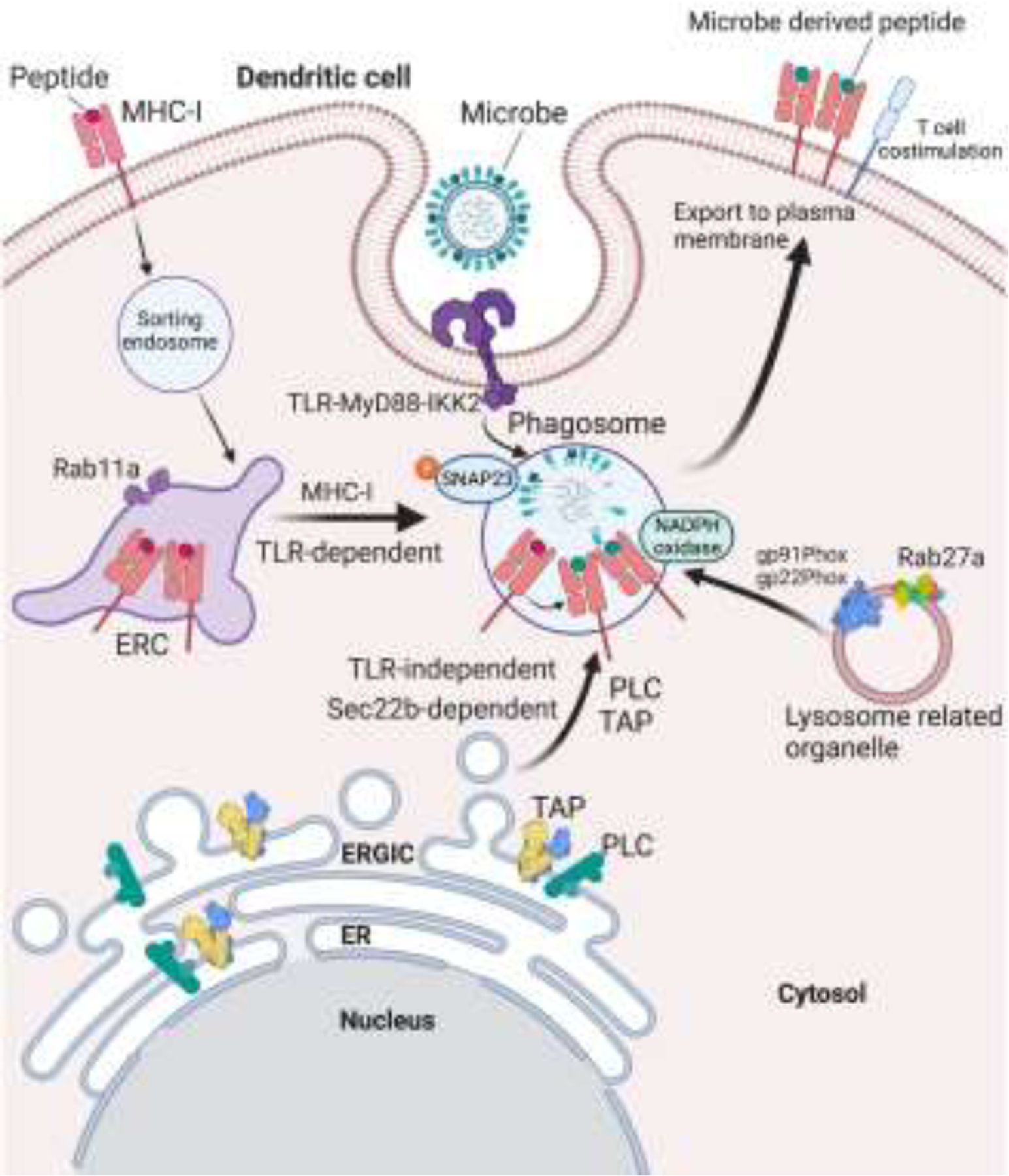
DC phagocytosis of microbes engages TLR-MyD88 signaling. A pathway of vesicular traffic is mobilized from MHC-I-rich ERC to TLR ligand (microbe)-carrying phagosomes. ERC-to-phagosome traffic is controlled by TLR-MyD88 signaling. Activation of IKK2 downstream of TLR-MyD88 signaling phosphorylates phagosomal SNAP-23 and stabilizes interactions of ERC R-SNAREs Endobrevin (VAMP8) and Cellubrevin (VAMP3) with phagosomal syntaxin 4 to mediate ERC-phagosome fusion. As a result, MHC-I molecules are delivered specifically to phagosomes carrying the microbial antigens which warrant cross-presentation during infection. This TLR-regulated pathway of cross-presentation favors the presentation of microbial antigens to CD8 T cells in the context of T cell costimulation during infection. Two other pathways of vesicular traffic emanating from the ERGIC and lysosome related organelles contribute additional components necessary for cross-presentation. See text for more details.
2. Emergency noncanonical cross-presentation during infection with immune evasive viruses
The proteasome-TAP pathway is considered as the conventional processing route for classic MHC-I presentation. Given the crucial role of TAP in translocating peptides to nascent MHC-I molecules within the ER, many clinically important human viruses such as Herpesviridae and Poxviridae have evolved strategies to block TAP and evade host CD8 T cell responses [7,100,101]. Virally encoded proteins that inhibit TAP mediated peptide transport include Infected Cell Protein 47 (ICP47) encoded by herpes simplex virus (HSV)-1 and HSV-2, US6 encoded by human cytomegalovirus (HCMV), and CPXV012 encoded by cowpox virus [7,100] The ability of cowpox to block TAP is particularly noteworthy as it was used by Edward Jenner in 1796 to effectively vaccinate against smallpox leading to the eventual global eradication of smallpox [102]. Pioneering work by Peter Cresswell and Victor Engelhard showed that TAP-deficient cells can present signal-sequence derived peptides by classical MHC-I [103,104]. Others extended these findings showing presentation of a selective peptide repertoire by TAP-deficient murine and human cells, varying in length, amino acid composition, and binding to both classical MHC-1a and nonclassical MHC-Ib [105–109]. In absence of the dominant proteasome-TAP pathway, peptides are liberated by alternate processing pathways which operate alongside the proteasome as evidenced by the presentation of TAP-independent peptides, albeit at low density, when TAP is functional [103,104,110,111]. Alternate processing of endogenous proteins can be mediated by furin, endopeptidases, aminopeptidases, ER signal peptidase, signal peptide peptidase [112,113] and autophagy [114,115].
2.1. Cross-presentation by bystander DC compensates for viral blockade of DC MHC-I presentation
Besides the alternate processing pathways of endogenous proteins that operate independently of TAP function, processing of exogenous proteins in DC during cross-presentation also compensates for TAP inhibition, and can take place in vacuolar compartments by cathepsins S and D [82,112,116], and by recently reported proteasomes within the phagosome lumen [117,118]. Thus, cross-presentation is an important pathway that overcomes impairment in classic MHC-I presentation [9]. When TAP function is compromised in infected tissues or when hematopoietic APCs lack the ability to present antigens through classic MHC-I presentation (for instance owing to compromised TAP function), cross-presentation by bystander or uninfected DCs can prime virus specific CD8 T cells [14,119–123]. Furthermore, hematopoietic deficiency in TAP compromises the ability to generate CD8 T cell responses to cancers [123] or to tissue tropic viral infections that do not infect DCs [121]. These reports have collectively supported the notion that only TAP-sufficient DCs can cross-present and prime CD8 T cells [9,121].
2.2. DC-intrinsic noncanonical cross-presentation in the face of viral interference of MHC-I presentation
The current paradigm suggests that CD8 T cell priming is impaired in the absence of TAP function in DCs [9,121]. Instead, as discussed above, virus-specific CD8 T cells are primed through cross-presentation by TAP-sufficient bystander DCs, which internalize virus-infected cells [8,9,13,14,119–124]. However, the dilemma is that CD8 T cells cross-primed by TAP-sufficient DCs would be unlikely to find their cognate epitopes on tissues infected with immune evasive viruses (Figure 2A). It has been suggested that TAP blockade, reduces but does not completely eliminate MHC-I presentation, which suffices for recognition by already primed effector TAP-dependent CD8 T cells [9]. While residual antigen presentation might allow for some control over infection, immune evasive viruses establish chronic infection and recrudescence causes significant health problems [125–127]. Furthermore, cells infected with immune evasive viruses would present peptides liberated independently of the TAP-proteasome processing pathway [111,112,118,128,129]. TAP blockade in infected tissues thus creates a diminished target or a mismatched target for TAP-dependent CD8 T cells. Can CD8 T cells be primed independently of TAP and if so, how? The answer may lie in noncanonical cross-presentation mobilized as discussed below, when TAP function is compromised (Figure 2B).
Figure 2. Model for cross-priming CD8 T cells to immune evasive viruses through noncanonical cross-presentation.
A. It is widely believed that priming a CD8 T cell response against immune evasive viruses such as Herpesviridae and Poxviridae that block TAP, can only be achieved by uninfected DCs which acquire viral peptides from internalized infected dying cells and present them to T cells. This solution accommodates the paradigm that CD8 T cell immunity is impaired without TAP. However, the presentation of TAP-dependent peptides is severely reduced on tissues infected with immune evasive viruses or in cancers that downmodulate TAP, and instead those tissues or cancers present peptides independently of TAP. Thus, the MHC-I peptidome on these tissues or cancers presents either a diminished or a mismatched target for TAP-dependent CD8 T cells primed by TAP-sufficient DCs leading to inefficient CD8 T cell targeting of infected or cancer cells. B. Noncanonical cross-presentation intrinsic to DCs that have compromised TAP function enables the cross-presentation of epitopes generated in the absence of TAP function to CD8 T cells. The resultant TAP-independent CD8 T cell response is best matched for targeting TAP-independent epitopes presented by tissues infected with immune evasive viruses or in cancers that downmodulate TAP. Mobilization of noncanonical cross-presentation can thus have critical impact on the design of CD8 T cell vaccines to immune evasive viruses and successful immunotherapy for immune evasive cancers.
The debated involvement of TAP in cross-presentation has ranged from necessary to dispensable, and likely dependent on the nature of the antigen, its route of delivery into DCs, and as highlighted in this review, the subcellular localization of MHC-I molecules [130]. Contrary to expectations of the current paradigm, TAP blockade in DCs does not abrogate their ability to prime CD8 T cells [77]. DC cross-presentation of phagocytic antigens can still occur in the absence of TAP function, but it has unique characteristics determined by the following set of events.
2.2.1. TAP blockade alters the location of MHC-I in DCs from the ERC to the ERGIC
Normally, nascent MHC-I molecules in the ER are newly loaded with peptides, exit the ER, and traffic via COPII-coated vesicles to the ERGIC where rigorous quality control ensures proper conformational flexibility and folding [8]. In resting murine DCs, fully assembled MHC-I molecules do not normally colocalize with calreticulin, calnexin, TAP2 or the ERGIC-resident lectin ERGIC-53, consistent with successful export to the plasma membrane once MHC-I molecules have passed ERGIC quality control [75,131,132]. Unstable, empty, and sub-optimally loaded MHC-I heavy-chain/β2-microglobulin dimers, which fail quality control, are prevented from reaching the plasma membrane [1]. This is in fact what happens in the absence of peptides with high-affinity binding to MHC-I when TAP is no longer functional [133–135]. TAP blockade depletes the ERC stores of MHC-I in BMDCs derived from TAP deficient mice, or upon TAP blockade in human monocyte-derived DCs upon infection with HCMV or treatment with ICP47 [77]. Instead of enrichment in the ERC, the majority of MHC-I in DCs are retained within the ERGIC as a consequence of their failure to bind high-affinity peptides and to pass quality control for export to the plasma membrane [77]. Thus, by blocking TAP function, viruses severely compromise not only the surface levels of MHC-I but also the intracellular pools of ERC-resident MHC-I. As such, these viruses can inhibit not only classic MHC-I presentation, but also ERC-dependent TLR-regulated cross-presentation by the DCs they infect.
2.2.2. Alternate MHC-I delivery from the ERGIC to phagosomes for noncanonical cross-presentation
Despite the MHC-I depletion of the ERC and the altered ERGIC localization of MHC-I in the absence of TAP function, the recruitment of MHC-I to phagosomes in DCs remains intact and is independent of Rab11a. Although ERC-to-phagosome vesicular traffic continues to be mobilized upon phagocytosis of TLR ligand-containing particulates, this traffic does not deliver MHC-I because MHC-I are no longer present within the ERC [77]. In the absence of TAP function, MHC-I delivery to phagosomes is sensitive to Sec22b inhibition, which disrupts vesicular traffic between the ERGIC and phagosomes [75,77,99]. Because of the altered MHC-I localization to the ERGIC instead of the ERC, Sec22b-dependent ERGIC-to-phagosome vesicular traffic becomes the dominant route of trafficking MHC-I molecules [77]. Thus, the subcellular source of MHC-I for cross-presentation switches from the ERC to the ERGIC in the absence of TAP function. These data show that when two major pathways of MHC-I presentation, classic MHC-I presentation and the Rab11a-dependent ERC pathway of TLR-regulated cross-presentation are impaired in the face of TAP blockade, DC mobilize a Sec22b-dependent ERGIC pathway of cross-presentation that rescues MHC-I presentation and restores CD8 T cell priming. We have termed this pathway as noncanonical cross-presentation (Figure 3A).
Figure 3. Subcellular localization and traffic of MHC-I molecules during noncanonical cross-presentation of microbial or self antigens.
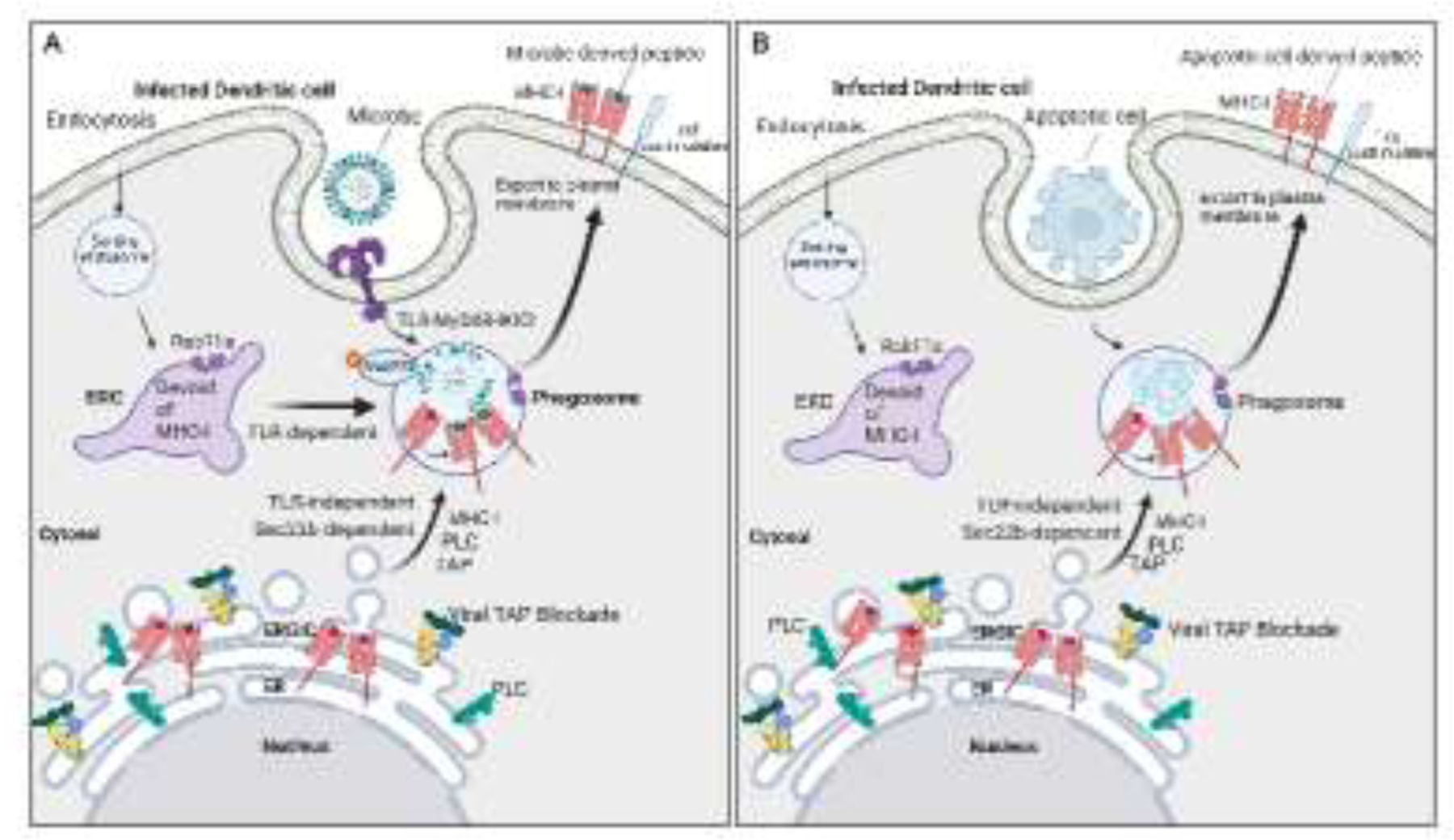
A. The ERC, which normally harbors large numbers of MHC-I molecules in DCs, becomes depleted of MHC-I molecules upon blockade of TAP. While TLRs continue to mobilize ERC-to-phagosome traffic, this traffic can no longer deliver MHC-I to phagosomes negatively impacting TLR-regulated cross-presentation. Under these conditions, DCs traffic MHC-I to phagosomes from the ERGIC instead of the ERC, in a manner dependent on the ER-SNARE protein Sec22b, to restore the MHC-I presentation of exogenously derived microbial peptides and rescue CD8 T cell priming. Because of the reliance on abnormal traffic of MHC-I, this pathway of antigen presentation is named as noncanonical cross-presentation. B. Noncanonical cross-presentation escapes regulation by TLR signals because it relies on trafficking MHC-I to phagosomes from the ERGIC, where they abnormally accumulate upon TAP blockade. Because TLRs do not control Sec22b-mediated traffic from the ERGIC to phagosomes, noncanonical cross-presentation carries the risk of cross-presenting peptides derived from phagocytosed apoptotic cells to self-reactive CD8 T cells in the context of infection-induced T cell costimulation.
By generating bone marrow chimeric mice where all hematopoietic cells are deficient for TAP, insight could be gained into the physiological relevance of a CD8 T cell response primed by DCs lacking TAP function. TAP-deficient mice have greatly reduced numbers of CD8 T cells because of the central role of MHC-I in thymic CD8 T cell selection [136], but this is not the case in TAP deficient chimeric mice that retain TAP-sufficient radioresistant and non-hematopoietic thymic epithelial cells. Infection of TAP deficient chimeric mice with influenza A virus was associated with an increase in activated CD8 T cells, and revealed that the mice were not particularly susceptible to infection or lethal challenge over a month later [77]. TAP deficient chimeric mice recovered from infection similarly to wild-type chimeric mice, and despite their inability to mount a TAP-dependent CD8 T cell response. These findings were surprising in light of the fact that all DC within these mice lack TAP, including DC that are infected directly with influenza virus as well as the bystander uninfected DC reported to conduct cross-presentation. Virally infected non-hematopoietic cells that retain TAP expression in either wild-type or TAP deficient chimeric mice are unable to directly prime CD8 T cells [121]. Presumably, parallel cross-presentation by uninfected DC and direct presentation by infected DC primes the TAP-dependent CD8 T cell response in wild type chimeric mice [9,14]. In contrast, TAP-dependent CD8 T cell responses to two immunodominant influenza A virus epitopes which require proteasome degradation, were absent in TAP deficient chimeric mice, consistent with the global TAP deficiency in all hematopoietic cells and the absence of residual TAP-sufficient APC persisting post lethal irradiation [77]. Importantly, despite the absence of TAP-dependent CD8 T cells in TAP deficient chimeric mice, their protection against a lethal challenge of influenza virus was nevertheless dependent on CD8 T cells. Indeed, systemic depletion of CD8 T cells significantly compromised the ability of TAP deficient chimeric mice to survive the infection [77], and in effect phenocopied the previously reported susceptibility of TAP deficient mice, which lack CD8 T cells [137], to infection with the intracellular pathogen Mycobacterium tuberculosis [138].
2.2.3. The ERGIC-to-phagosome route of MHC-I traffic is not regulated by TLR signals
Because TLRs control ERC-to-phagosome but not ERGIC-to-phagosome traffic, TLR signals can no longer control TAP-independent phagocytic antigen cross-presentation [75]. DCs derived from TAP deficient mice were shown to efficiently cross-present phagocytic antigen derived from apoptotic cells or antigen-conjugated microspheres in the absence of TLR signals and despite the provision of T cell costimulation. This was unlike the requirement of TLR signaling for cross-presentation of the same antigens by DCs derived from wild-type mice. Cross-presentation by TAP deficient DCs was independent of IKK2 activity [77], which under normal conditions of functional TAP would be necessary to license the traffic of MHC-I molecules to phagosomes from the ERC [75]. This finding was also consistent with ERC depletion of MHC-I in the absence of TAP function in both human and murine DCs [77].
Despite the advantages to anti-viral immunity, noncanonical cross-presentation comes at the cost of bypassing the control that TLRs exert over cross-presentation [77,130] (Figure 3B). It is tempting to speculate that the symptoms of chronic inflammation presented in individuals with genetic TAP1 and TAP2 deficiency [139,140], and perhaps cases of autoimmunity reported following HCMV infections [141] might be triggered by unregulated MHC-I presentation of apoptotic cell antigens as a result of noncanonical cross-presentation. Several single nucleotide polymorphisms (SNPs) in TAP1 and TAP2 genes exist within the human population with no defined effect on peptide transport [142]. Although TAP1 and TAP2 genes are located within the genomic region harboring MHC class II genes that confer high risk for autoimmune diseases, SNPs in TAP2 have been significantly associated with systemic lupus erythematosus (SLE) and autonomously from the HLA allele disease associations [143]. SNPs in TAP1 have shown positive and negative associations with Grave’s disease not explained by linkage disequilibrium between TAP and the HLA-DQ alleles known to confer predisposition to this autoimmune disorder [144]. Interestingly, downregulation of TAP transcripts has been observed in several MHC-I-linked autoimmune diseases including type I diabetes mellitus, Sjögren’s syndrome, Graves’ disease, and Hashimoto’s disease [144], and whether this disrupts the regulatory control of self versus non-self antigen cross-presentation is an important question for future investigations.
The observations discussed above collectively paint a picture of a DC cell-intrinsic pathway of cross-presentation that overcomes the block in both classical MHC-I presentation and conventional cross-presentation in the face of TAP inhibition by immune evasive viruses. This emergency cross-presentation is termed noncanonical cross-presentation because it traffics MHC-I to antigen-containing phagosomes from a new location and via an unconventional route. In this manner, noncanonical cross-presentation results in continued MHC-I presentation of viral antigens by DCs where TAP function is compromised to ensure the priming of an anti-viral CD8 T cell response. The trade-off, however, is the indiscriminate cross-presentation of self and non-self antigens because the alternate route of trafficking MHC-I from the ERGIC to phagosomes is not controlled by TLR signals [77] (Figure 3B).
3. Implications of noncanonical cross-presentation to tumor immunity
Like viruses, many tumors evade cytotoxic CD8 T cells by lowering surface MHC-I expression. This can occur through reduced expression or activity of NLRC5, a master transcriptional coactivator of the genes involved in MHC-I presentation including TAP, immunoproteasome subunits, and β2-microglobulin [130,145]. TAP genes are frequently lost or epigenetically silenced in cancer [146]. Resistance to checkpoint blockade therapy has also been associated with loss of TAP1/2 and MHC-I expression as a functional consequence to mutations in JAK1/JAK2 and lack of responsiveness to IFN-γ in patients with metastatic melanoma [147,148]. CD8 T cells mobilized by vaccines based on TAP-dependent peptides are unlikely to recognize the peptides presented on the surface of TAP-deficient tumor cells [149]. It has been shown that the peptides presented by MHC-I on the surface of TAP-deficient tumor cells are not expressed by normal cells and as such comprise neoantigens [110,150,151]. Designing strategies that mobilize non-canonical cross-presentation in DC should in theory enable the TAP-independent presentation of peptides derived from phagocytosed tumor cells and the priming of TAP-independent CD8 T cells that effectively target immune escape tumor variants. CD8 T cells specific to alternate peptides generated upon the downregulation of TAP have been identified and shown to target a broad range of murine and human tumor cells [106,152].
Conclusions
Studying the vesicular traffic of MHC-I molecules within DCs under different functional states has revealed the existence of two routes of MHC-I delivery to antigen carrying phagosomes: the ERC route and the ERGIC route. During infection, MHC-I molecules are recruited from rich intracellular stores of MHC-I within the ERC to phagosomes carrying microbial antigen. During conditions of TAP dysfunction, modeled by genetic TAP deficiency in murine DCs or infection of human DCs with viruses that encode proteins which block TAP function to escape CD8 T cell immunity, DCs mobilize a cell-intrinsic noncanonical pathway of cross-presentation that relies on an alternate route of MHC-I traffic from the ERGIC –where MHC-I abnormally accumulate upon TAP dysfunction– to antigen carrying phagosomes. Noncanonical cross-presentation operates only when the two major pathways of MHC-I presentation in DCs, classic MHC-I presentation and cross-presentation, are blocked. MHC-I traffic from the ERC to phagosomes is regulated by TLRs, and serves to augment cross-presentation and ensure robust priming of a microbe-specific CD8 T cell response. The ERGIC route of MHC-I traffic operates upon infection with viruses that actively impair the major pathways of MHC-I presentation in DCs; classical MHC-I presentation and cross-presentation. To ensure delivery of MHC-I molecules to phagosomes for cross-presentation, DCs bypass TLR control by mobilizing MHC-I traffic from the ERGIC to phagosomes to restore MHC-I presentation in DCs through noncanonical cross-presentation which rescues CD8 T cell priming. Noncanonical cross-presentation has important implications to immunity and immunotherapy against immune evasive viruses and tumors.
Highlights.
Control of MHC-I traffic is at the heart of TLR regulation of cross-presentation
ERC-to-phagosome MHC-I delivery is critical for phagocytic antigen cross-presentation
TAP blockade inhibits classic MHC-presentation and TLR-regulated cross-presentation
ERGIC-to-phagosome MHC-I delivery upon TAP blockade rescues MHC-I presentation
DC-intrinsic noncanonical cross-presentation cross-primes TAP-independent CD8 T cells
Acknowledgments
The author is grateful to current and former Blander lab members and particularly to Dr. Priyanka Nair-Gupta, Dr. Gaetan Barbet, and Dr. Kristel Yee Mon who have contributed significantly to understanding the cell biology, regulation, immune consequences, and therapeutic harnessing of cross-presentation. Work on cross-presentation in the Blander Lab has been supported by NIH grants AI073899, AI123284, AI159772 and AI170897 to J.M.B. The author declares no financial conflict. Figures were created using BioRender.
Abbreviations:
- MHC-I
major histocompatibility complex class I
- TAP
transporter associated with antigen processing
- DCs
dendritic cells
- APCs
antigen-presenting cells
- BMDCs
bone marrow-derived dendritic cells
- ER
endoplasmic reticulum
- ERGIC
ER-Golgi intermediate compartment
- ERC
endosomal recycling compartment
- DLL1
Delta-like
- ICP47
Infected Cell Protein 47
- HSV
herpes simplex virus
- HCMV
human cytomegalovirus
- LN
lymph node
- TLR
Toll-like receptor
- IKK2
inhibitor of nuclear factor-κB kinase 2
- SNAP-23
synaptosomal associated protein-23
- VAMP
vesicle-associated membrane protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blum JS, Wearsch PA, Cresswell P, Pathways of antigen processing, Annual review of immunology 31 (2013) 443–473. 10.1146/annurev-immunol-032712-095910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas C, Tampe R, Multifaceted structures and mechanisms of ABC transport systems in health and disease, Curr Opin Struct Biol 51 (2018) 116–128. 10.1016/j.sbi.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 3.Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, Moeller A, Tampe R, Structure of the human MHC-I peptide-loading complex, Nature 551 (2017) 525–528. 10.1038/nature24627 [DOI] [PubMed] [Google Scholar]
- 4.Hofmann S, Januliene D, Mehdipour AR, Thomas C, Stefan E, Bruchert S, Kuhn BT, Geertsma ER, Hummer G, Tampe R, Moeller A, Conformation space of a heterodimeric ABC exporter under turnover conditions, Nature 571 (2019) 580–583. 10.1038/s41586-019-1391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehnert E, Tampe R, Structure and Dynamics of Antigenic Peptides in Complex with TAP, Front Immunol 8 (2017) 10. 10.3389/fimmu.2017.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldham ML, Hite RK, Steffen AM, Damko E, Li Z, Walz T, Chen J, A mechanism of viral immune evasion revealed by cryo-EM analysis of the TAP transporter, Nature 529 (2016) 537–540. 10.1038/nature16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Praest P, Liaci AM, Forster F, Wiertz E, New insights into the structure of the MHC class I peptide-loading complex and mechanisms of TAP inhibition by viral immune evasion proteins, Mol Immunol 113 (2019) 103–114. 10.1016/j.molimm.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 8.Blander JM, Regulation of the Cell Biology of Antigen Cross-Presentation, Annu Rev Immunol 36 (2018) 717–753. 10.1146/annurev-immunol-041015-055523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz FM, Colbert JD, Merino E, Kriegsman BA, Rock KL, The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules, Annu Rev Immunol 35 (2017) 149–176. 10.1146/annurev-immunol-041015-055254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segura E, Amigorena S, Cross-Presentation in Mouse and Human Dendritic Cells, Adv Immunol 127 (2015) 1–31. 10.1016/bs.ai.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Pishesha N, Harmand TJ, Ploegh HL, A guide to antigen processing and presentation, Nat Rev Immunol (2022). 10.1038/s41577-022-00707-2 [DOI] [PubMed]
- 12.Kurts C, Robinson BW, Knolle PA, Cross-priming in health and disease, Nat Rev Immunol 10 (2010) 403–414. 10.1038/nri2780 nri2780 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Joffre OP, Segura E, Savina A, Amigorena S, Cross-presentation by dendritic cells, Nat Rev Immunol 12 (2012) 557–569. 10.1038/nri3254 nri3254 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC, Saveanu L, Guermonprez P, Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets, Front Immunol 6 (2015) 363. 10.3389/fimmu.2015.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair P, Amsen D, Blander JM, Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T cells, Traffic 12 (2011) 1669–1676. 10.1111/j.1600-0854.2011.01251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, Enninga J, Moita LF, Amigorena S, Savina A, Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells, Cell 147 (2011) 1355–1368. S0092–8674(11)01359–6 [pii] 10.1016/j.cell.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 17.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis ESC, Dendritic Cells Revisited, Annu Rev Immunol 39 (2021) 131–166. 10.1146/annurev-immunol-061020-053707 [DOI] [PubMed] [Google Scholar]
- 18.Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, Siebel CW, Salmon H, Merad M, Tsirigos A, Collin M, Bigley V, Reizis B, Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells, Cell Rep 23 (2018) 3658–3672 e3656. 10.1016/j.celrep.2018.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flinsenberg TW, Compeer EB, Boelens JJ, Boes M, Antigen cross-presentation: extending recent laboratory findings to therapeutic intervention, Clin Exp Immunol 165 (2011) 8–18. 10.1111/j.1365-2249.2011.04411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palucka K, Banchereau J, Dendritic-cell-based therapeutic cancer vaccines, Immunity 39 (2013) 38–48. 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena M, Bhardwaj N, Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment, Trends Cancer 4 (2018) 119–137. 10.1016/j.trecan.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez CR, De Palma M, Engineering dendritic cell vaccines to improve cancer immunotherapy, Nat Commun 10 (2019) 5408. 10.1038/s41467-019-13368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner A, de Mingo Pulido A, Ruffell B, Dendritic Cells and Their Role in Immunotherapy, Front Immunol 11 (2020) 924. 10.3389/fimmu.2020.00924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filin IY, Kitaeva KV, Rutland CS, Rizvanov AA, Solovyeva VV, Recent Advances in Experimental Dendritic Cell Vaccines for Cancer, Front Oncol 11 (2021) 730824. 10.3389/fonc.2021.730824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blander JM, Signalling and phagocytosis in the orchestration of host defence, Cellular microbiology 9 (2007) 290–299. 10.1111/j.1462-5822.2006.00864.x [DOI] [PubMed] [Google Scholar]
- 26.Blander JM, Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation, Trends in immunology 28 (2007) 19–25. 10.1016/j.it.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Blander JM, Phagocytosis and antigen presentation: a partnership initiated by Toll-like receptors, Annals of the rheumatic diseases 67 Suppl 3 (2008) iii44–49. 10.1136/ard.2008.097964 [DOI] [PubMed] [Google Scholar]
- 28.Aderem A, Underhill DM, Mechanisms of phagocytosis in macrophages, Annu Rev Immunol 17 (1999) 593–623. 10.1146/annurev.immunol.17.1.593 [DOI] [PubMed] [Google Scholar]
- 29.Champion JA, Walker A, Mitragotri S, Role of particle size in phagocytosis of polymeric microspheres, Pharm Res 25 (2008) 1815–1821. 10.1007/s11095-008-9562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH, Size of IgG-opsonized particles determines macrophage response during internalization, Exp Cell Res 242 (1998) 265–273. 10.1006/excr.1998.4110 [DOI] [PubMed] [Google Scholar]
- 31.Paul D, Achouri S, Yoon YZ, Herre J, Bryant CE, Cicuta P, Phagocytosis dynamics depends on target shape, Biophys J 105 (2013) 1143–1150. 10.1016/j.bpj.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratten MK, Lloyd JB, Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro, Biochim Biophys Acta 881 (1986) 307–313. 10.1016/0304-4165(86)90020-6 [DOI] [PubMed] [Google Scholar]
- 33.Prietl B, Meindl C, Roblegg E, Pieber TR, Lanzer G, Frohlich E, Nano-sized and micro-sized polystyrene particles affect phagocyte function, Cell Biol Toxicol 30 (2014) 1–16. 10.1007/s10565-013-9265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabata Y, Ikada Y, Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage, Biomaterials 9 (1988) 356–362. 10.1016/0142-9612(88)90033-6 [DOI] [PubMed] [Google Scholar]
- 35.Pollard AJ, Bijker EM, A guide to vaccinology: from basic principles to new developments, Nat Rev Immunol 21 (2021) 83–100. 10.1038/s41577-020-00479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastenmuller W, Kastenmuller K, Kurts C, Seder RA, Dendritic cell-targeted vaccines--hope or hype?, Nat Rev Immunol 14 (2014) 705–711. 10.1038/nri3727 [DOI] [PubMed] [Google Scholar]
- 37.Ho NI, Huis LGM In ‘t Veld, Raaijmakers TK, Adema GJ, Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines?, Front Immunol 9 (2018) 2874. 10.3389/fimmu.2018.02874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S, Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny, Nat Rev Immunol 14 (2014) 571–578. 10.1038/nri3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Haan JM, Bevan MJ, Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo, J Exp Med 196 (2002) 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H, Constitutive class I-restricted exogenous presentation of self antigens in vivo, J Exp Med 184 (1996) 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T, Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo, Immunity 28 (2008) 521–532. S1074–7613(08)00114–3 [pii] 10.1016/j.immuni.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 42.Wilson NS, El-Sukkari D, Villadangos JA, Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis, Blood 103 (2004) 2187–2195. 10.1182/blood-2003-08-2729 2003–08-2729 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Cancel JC, Crozat K, Dalod M, Mattiuz R, Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How?, Front Immunol 10 (2019) 9. 10.3389/fimmu.2019.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM, Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity, Science 322 (2008) 1097–1100. 10.1126/science.1164206 322/5904/1097 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, Iizuka A, Sato K, Tanaka T, Hoshino K, Kaisho T, Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1, J Immunol 190 (2013) 6071–6082. 10.4049/jimmunol.1202798 [DOI] [PubMed] [Google Scholar]
- 46.Ballesteros-Tato A, Leon B, Lund FE, Randall TD, Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza, Nat Immunol 11 (2010) 216–224. 10.1038/ni.1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR, Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells, Nat Immunol 10 (2009) 488–495. ni.1724 [pii] 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- 48.Kim TS, Braciale TJ, Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses, PLoS One 4 (2009) e4204. 10.1371/journal.pone.0004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nierkens S, Tel J, Janssen E, Adema GJ, Antigen cross-presentation by dendritic cell subsets: one general or all sergeants?, Trends Immunol 34 (2013) 361–370. 10.1016/j.it.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backer R, van Leeuwen F, Kraal G, den Haan JM, CD8-dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens, Eur J Immunol 38 (2008) 370–380. 10.1002/eji.200737647 [DOI] [PubMed] [Google Scholar]
- 51.Embgenbroich M, Burgdorf S, Current Concepts of Antigen Cross-Presentation, Front Immunol 9 (2018) 1643. 10.3389/fimmu.2018.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji Q, Castelli L, Goverman JM, MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells, Nat Immunol 14 (2013) 254–261. 10.1038/ni.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheng J, Chen Q, Soncin I, Ng SL, Karjalainen K, Ruedl C, A Discrete Subset of Monocyte-Derived Cells among Typical Conventional Type 2 Dendritic Cells Can Efficiently Cross-Present, Cell Rep 21 (2017) 1203–1214. 10.1016/j.celrep.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 54.Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS, Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells, Proc Natl Acad Sci U S A 108 (2011) 9927–9932. 10.1073/pnas.1019037108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yrlid U, Wick MJ, Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter, J Immunol 169 (2002) 108–116. 10.4049/jimmunol.169.1.108 [DOI] [PubMed] [Google Scholar]
- 56.den Brok MH, Bull C, Wassink M, de Graaf AM, Wagenaars JA, Minderman M, Thakur M, Amigorena S, Rijke EO, Schrier CC, Adema GJ, Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation, Nat Commun 7 (2016) 13324. 10.1038/ncomms13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, Couto S, Drane D, Boyle J, Belz GT, Ashkenazi A, Maraskovsky E, ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway, Immunol Cell Biol 90 (2012) 540–552. 10.1038/icb.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, Lambeck AJ, Figdor CG, de Vries IJ, Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets, Blood 121 (2013) 459–467. 10.1182/blood-2012-06-435644 [DOI] [PubMed] [Google Scholar]
- 59.Schlitzer A, McGovern N, Ginhoux F, Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems, Semin Cell Dev Biol 41 (2015) 9–22. 10.1016/j.semcdb.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 60.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG, TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection, Immunity 19 (2003) 59–70. 10.1016/s1074-7613(03)00171-7 [DOI] [PubMed] [Google Scholar]
- 61.Shi C, Pamer EG, Monocyte recruitment during infection and inflammation, Nat Rev Immunol 11 (2011) 762–774. 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM, Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas, Cell 143 (2010) 416–429. 10.1016/j.cell.2010.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G, Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells, Immunity 38 (2013) 729–741. 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 64.Segura E, Durand M, Amigorena S, Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells, J Exp Med 210 (2013) 1035–1047. 10.1084/jem.20121103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet JG, Gannage M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Maranon C, Antigen crosspresentation by human plasmacytoid dendritic cells, Immunity 27 (2007) 481–492. 10.1016/j.immuni.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 66.Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, Chevrier M, Zhang XM, Yong PJA, Koh G, Lum J, Howland SW, Mok E, Chen J, Larbi A, Tan HKK, Lim TKH, Karagianni P, Tzioufas AG, Malleret B, Brody J, Albani S, van Roon J, Radstake T, Newell EW, Ginhoux F, Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells, Immunity 51 (2019) 573–589 e578. 10.1016/j.immuni.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 67.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N, Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors, Science 356 (2017). 10.1126/science.aah4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alcantara-Hernandez M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, Idoyaga J, High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization, Immunity 47 (2017) 1037–1050 e1036. 10.1016/j.immuni.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallee VP, Mendoza A, Fromme R, Mazutis L, Ariyan C, Leslie C, Pe’er D, Rudensky AY, Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity, Cell 179 (2019) 846–863 e824. 10.1016/j.cell.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang-Huau TL, Gueguen P, Goudot C, Durand M, Bohec M, Baulande S, Pasquier B, Amigorena S, Segura E, Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway, Nat Commun 9 (2018) 2570. 10.1038/s41467-018-04985-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann E, Kotsias F, Visentin G, Bruhns P, Savina A, Amigorena S, Autonomous phagosomal degradation and antigen presentation in dendritic cells, Proc Natl Acad Sci U S A 109 (2012) 14556–14561. 10.1073/pnas.1203912109 1203912109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blander JM, The comings and goings of MHC class I molecules herald a new dawn in cross-presentation, Immunol Rev 272 (2016) 65–79. 10.1111/imr.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blander JM, Medzhitov R, On regulation of phagosome maturation and antigen presentation, Nature immunology 7 (2006) 1029–1035. 10.1038/ni1006-1029 [DOI] [PubMed] [Google Scholar]
- 74.Blander JM, Medzhitov R, Toll-dependent selection of microbial antigens for presentation by dendritic cells, Nature 440 (2006) 808–812. 10.1038/nature04596 [DOI] [PubMed] [Google Scholar]
- 75.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM, TLR Signals Induce Phagosomal MHC-I Delivery from the Endosomal Recycling Compartment to Allow Cross-Presentation, Cell 158 (2014) 506–521. 10.1016/j.cell.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair-Gupta P, Blander JM, An Updated View of the Intracellular Mechanisms Regulating Cross-Presentation, Front Immunol 4 (2013) 401. 10.3389/fimmu.2013.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbet G, Nair-Gupta P, Schotsaert M, Yeung ST, Moretti J, Seyffer F, Metreveli G, Gardner T, Choi A, Tortorella D, Tampe R, Khanna KM, Garcia-Sastre A, Blander JM, TAP dysfunction in dendritic cells enables noncanonical cross-presentation for T cell priming, Nat Immunol 22 (2021) 497–509. 10.1038/s41590-021-00903-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackerman AL, Kyritsis C, Tampe R, Cresswell P, Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells, Nat Immunol 6 (2005) 107–113. 10.1038/ni1147 [DOI] [PubMed] [Google Scholar]
- 79.Albert ML, Sauter B, Bhardwaj N, Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs, Nature 392 (1998) 86–89. 10.1038/32183 [DOI] [PubMed] [Google Scholar]
- 80.Burgdorf S, Lukacs-Kornek V, Kurts C, The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation, J Immunol 176 (2006) 6770–6776. 176/11/6770 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Kovacsovics-Bankowski M, Rock KL, A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules, Science 267 (1995) 243–246. [DOI] [PubMed] [Google Scholar]
- 82.Shen L, Sigal LJ, Boes M, Rock KL, Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo, Immunity 21 (2004) 155–165. 10.1016/j.immuni.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 83.Song R, Harding CV, Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway, J Immunol 156 (1996) 4182–4190. [PubMed] [Google Scholar]
- 84.Gros M, Amigorena S, Regulation of Antigen Export to the Cytosol During Cross-Presentation, Front Immunol 10 (2019) 41. 10.3389/fimmu.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montealegre S, van Endert PM, Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells, Front Immunol 9 (2018) 3098. 10.3389/fimmu.2018.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuhns ST, Pease LR, A region of conformational variability outside the peptide-binding site of a class I MHC molecule, J Immunol 161 (1998) 6745–6750. [PubMed] [Google Scholar]
- 87.Montealegre S, Abramova A, Manceau V, de Kanter AF, van Endert P, The role of MHC class I recycling and Arf6 in cross-presentation by murine dendritic cells, Life Sci Alliance 2 (2019). 10.26508/lsa.201900464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cebrian I, Croce C, Guerrero NA, Blanchard N, Mayorga LS, Rab22a controls MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells, EMBO Rep 17 (2016) 1753–1765. 10.15252/embr.201642358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grant BD, Donaldson JG, Pathways and mechanisms of endocytic recycling, Nat Rev Mol Cell Biol 10 (2009) 597–608. 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou L, Zhou J, Zhang J, Li J, Liu N, Chai L, Li N, Liu T, Li L, Xie Z, Liu H, Wan Y, Wu Y, The GTPase Rab3b/3c-positive recycling vesicles are involved in cross-presentation in dendritic cells, Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 15801–15806. 10.1073/pnas.0905684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S, NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells, Blood 112 (2008) 4712–4722. 10.1182/blood-2008-01-134791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S, NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells, Cell 126 (2006) 205–218. S0092–8674(06)00761–6 [pii] 10.1016/j.cell.2006.05.035 [DOI] [PubMed] [Google Scholar]
- 93.Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, Amigorena S, Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes, Nature cell biology 9 (2007) 367–378. 10.1038/ncb1552 [DOI] [PubMed] [Google Scholar]
- 94.Dingjan I, Paardekooper LM, Verboogen DRJ, von Mollard GF, Ter Beest M, van den Bogaart G, VAMP8-mediated NOX2 recruitment to endosomes is necessary for antigen release, Eur J Cell Biol 96 (2017) 705–714. 10.1016/j.ejcb.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rong H, Shi Y, Disruption of key GTPase regulators of endocytic recycling compartment does not interfere with soluble antigen crosspresentation in dendritic cells, Cell Mol Immunol 13 (2016) 554–556. 10.1038/cmi.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ackerman AL, Kyritsis C, Tampe R, Cresswell P, Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens, Proc Natl Acad Sci U S A 100 (2003) 12889–12894. 10.1073/pnas.1735556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S, ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells, Nature 425 (2003) 397–402. 10.1038/nature01911 [DOI] [PubMed] [Google Scholar]
- 98.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M, Phagosomes are competent organelles for antigen cross-presentation, Nature 425 (2003) 402–406. 10.1038/nature01912 nature01912 [pii] [DOI] [PubMed] [Google Scholar]
- 99.Alloatti A, Rookhuizen DC, Joannas L, Carpier JM, Iborra S, Magalhaes JG, Yatim N, Kozik P, Sancho D, Albert ML, Amigorena S, Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity, J Exp Med 214 (2017) 2231–2241. 10.1084/jem.20170229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayerhofer PU, Tampe R, Antigen translocation machineries in adaptive immunity and viral immune evasion, J Mol Biol 427 (2015) 1102–1118. 10.1016/j.jmb.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 101.Ressing ME, Luteijn RD, Horst D, Wiertz EJ, Viral interference with antigen presentation: trapping TAP, Mol Immunol 55 (2013) 139–142. 10.1016/j.molimm.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 102.Wilkinson GW, Lehner PJ, Jenner’s irony: cowpox taps into T cell evasion, Cell Host Microbe 6 (2009) 395–397. 10.1016/j.chom.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 103.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH, HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation, Science 255 (1992) 1264–1266. [DOI] [PubMed] [Google Scholar]
- 104.Wei ML, Cresswell P, HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides, Nature 356 (1992) 443–446. 10.1038/356443a0 [DOI] [PubMed] [Google Scholar]
- 105.Durgeau A, El Hage F, Vergnon I, Validire P, de Montpreville V, Besse B, Soria JC, van Hall T, Mami-Chouaib F, Different expression levels of the TAP peptide transporter lead to recognition of different antigenic peptides by tumor-specific CTL, J Immunol 187 (2011) 5532–5539. 10.4049/jimmunol.1102060 [DOI] [PubMed] [Google Scholar]
- 106.van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, de Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R, Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants, Nat Med 12 (2006) 417–424. 10.1038/nm1381 [DOI] [PubMed] [Google Scholar]
- 107.Oliveira CC, van Veelen PA, Querido B, de Ru A, Sluijter M, Laban S, Drijfhout JW, van der Burg SH, Offringa R, van Hall T, The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects, J Exp Med 207 (2010) 207–221. 10.1084/jem.20091429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lampen MH, Verweij MC, Querido B, van der Burg SH, Wiertz EJ, van Hall T, CD8+ T cell responses against TAP-inhibited cells are readily detected in the human population, J Immunol 185 (2010) 6508–6517. 10.4049/jimmunol.1001774 [DOI] [PubMed] [Google Scholar]
- 109.Lopez D, Lorente E, Barriga A, Johnstone C, Mir C, Vaccination and the TAP-independent antigen processing pathways, Expert Rev Vaccines 12 (2013) 1077–1083. 10.1586/14760584.2013.825447 [DOI] [PubMed] [Google Scholar]
- 110.Oliveira CC, Querido B, Sluijter M, Derbinski J, van der Burg SH, van Hall T, Peptide transporter TAP mediates between competing antigen sources generating distinct surface MHC class I peptide repertoires, Eur J Immunol 41 (2011) 3114–3124. 10.1002/eji.201141836 [DOI] [PubMed] [Google Scholar]
- 111.Oliveira CC, van Hall T, Alternative Antigen Processing for MHC Class I: Multiple Roads Lead to Rome, Front Immunol 6 (2015) 298. 10.3389/fimmu.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lazaro S, Gamarra D, Del Val M, Proteolytic enzymes involved in MHC class I antigen processing: A guerrilla army that partners with the proteasome, Mol Immunol 68 (2015) 72–76. 10.1016/j.molimm.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 113.Oliveira CC, van Hall T, Importance of TAP-independent processing pathways, Mol Immunol 55 (2013) 113–116. 10.1016/j.molimm.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 114.Tey SK, Khanna R, Autophagy mediates transporter associated with antigen processing-independent presentation of viral epitopes through MHC class I pathway, Blood 120 (2012) 994–1004. 10.1182/blood-2012-01-402404 [DOI] [PubMed] [Google Scholar]
- 115.Vyas JM, Van der Veen AG, Ploegh HL, The known unknowns of antigen processing and presentation, Nat Rev Immunol 8 (2008) 607–618. 10.1038/nri2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merzougui N, Kratzer R, Saveanu L, van Endert P, A proteasome-dependent, TAP-independent pathway for cross-presentation of phagocytosed antigen, EMBO Rep 12 (2011) 1257–1264. 10.1038/embor.2011.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vigneron N, Ferrari V, Van den Eynde BJ, Cresswell P, Leonhardt RM, Cytosolic Processing Governs TAP-Independent Presentation of a Critical Melanoma Antigen, J Immunol 201 (2018) 1875–1888. 10.4049/jimmunol.1701479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sengupta D, Graham M, Liu X, Cresswell P, Proteasomal degradation within endocytic organelles mediates antigen cross-presentation, EMBO J 38 (2019) e99266. 10.15252/embj.201899266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, Murphy KM, Garcia-Sastre A, Merad M, Cross-presenting CD103+ dendritic cells are protected from influenza virus infection, J Clin Invest 122 (2012) 4037–4047. 10.1172/JCI60659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silvin A, Yu CI, Lahaye X, Imperatore F, Brault JB, Cardinaud S, Becker C, Kwan WH, Conrad C, Maurin M, Goudot C, Marques-Ladeira S, Wang Y, Pascual V, Anguiano E, Albrecht RA, Iannacone M, Garcia-Sastre A, Goud B, Dalod M, Moris A, Merad M, Palucka AK, Manel N, Constitutive resistance to viral infection in human CD141(+) dendritic cells, Sci Immunol 2 (2017). 10.1126/sciimmunol.aai8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sigal LJ, Crotty S, Andino R, Rock KL, Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen, Nature 398 (1999) 77–80. 10.1038/18038 [DOI] [PubMed] [Google Scholar]
- 122.Whitney PG, Makhlouf C, MacLeod B, Ma JZ, Gressier E, Greyer M, Hochheiser K, Bachem A, Zaid A, Voehringer D, Heath WR, Wagle MV, Parish I, Russell TA, Smith SA, Tscharke DC, Gebhardt T, Bedoui S, Effective Priming of Herpes Simplex Virus-Specific CD8(+) T Cells In Vivo Does Not Require Infected Dendritic Cells, J Virol 92 (2018). 10.1128/JVI.01508-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang AY, Bruce AT, Pardoll DM, Levitsky HI, In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter, Immunity 4 (1996) 349–355. 10.1016/s1074-7613(00)80248-4 [DOI] [PubMed] [Google Scholar]
- 124.Heath WR, Carbone FR, Cross-presentation in viral immunity and self-tolerance, Nat Rev Immunol 1 (2001) 126–134. 10.1038/35100512 [DOI] [PubMed] [Google Scholar]
- 125.Stern L, Withers B, Avdic S, Gottlieb D, Abendroth A, Blyth E, Slobedman B, Human Cytomegalovirus Latency and Reactivation in Allogeneic Hematopoietic Stem Cell Transplant Recipients, Front Microbiol 10 (2019) 1186. 10.3389/fmicb.2019.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Burg SH, Arens R, Melief CJ, Immunotherapy for persistent viral infections and associated disease, Trends Immunol 32 (2011) 97–103. 10.1016/j.it.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 127.Odumade OA, Hogquist KA, Balfour HH Jr., Progress and problems in understanding and managing primary Epstein-Barr virus infections, Clin Microbiol Rev 24 (2011) 193–209. 10.1128/CMR.00044-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rock KL, Shen L, Cross-presentation: underlying mechanisms and role in immune surveillance, Immunological reviews 207 (2005) 166–183. 10.1111/j.0105-2896.2005.00301.x [DOI] [PubMed] [Google Scholar]
- 129.Lorente E, Palomo C, Barnea E, Mir C, Del Val M, Admon A, Lopez D, Natural Spleen Cell Ligandome in Transporter Antigen Processing-Deficient Mice, J Proteome Res 18 (2019) 3512–3520. 10.1021/acs.jproteome.9b00416 [DOI] [PubMed] [Google Scholar]
- 130.Mantel I, Sadiq BA, Blander JM, Spotlight on TAP and its vital role in antigen presentation and cross-presentation, Mol Immunol 142 (2022) 105–119. 10.1016/j.molimm.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ackerman AL, Cresswell P, Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1, J Immunol 170 (2003) 4178–4188. [DOI] [PubMed] [Google Scholar]
- 132.Delamarre L, Holcombe H, Mellman I, Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation, J Exp Med 198 (2003) 111–122. 10.1084/jem.20021542 jem.20021542 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Donaldson JG, Williams DB, Intracellular assembly and trafficking of MHC class I molecules, Traffic 10 (2009) 1745–1752. 10.1111/j.1600-0854.2009.00979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raposo G, van Santen HM, Leijendekker R, Geuze HJ, Ploegh HL, Misfolded major histocompatibility complex class I molecules accumulate in an expanded ER-Golgi intermediate compartment, J Cell Biol 131 (1995) 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Hateren A, James E, Bailey A, Phillips A, Dalchau N, Elliott T, The cell biology of major histocompatibility complex class I assembly: towards a molecular understanding, Tissue Antigens 76 (2010) 259–275. 10.1111/j.1399-0039.2010.01550.x [DOI] [PubMed] [Google Scholar]
- 136.Aldrich CJ, Ljunggren HG, Van Kaer L, Ashton-Rickardt PG, Tonegawa S, Forman J, Positive selection of self- and alloreactive CD8+ T cells in Tap-1 mutant mice, Proc Natl Acad Sci U S A 91 (1994) 6525–6528. 10.1073/pnas.91.14.6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S, TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells, Cell 71 (1992) 1205–1214. S0092–8674(05)80068–6 [pii] [DOI] [PubMed] [Google Scholar]
- 138.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB, Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis, J Exp Med 189 (1999) 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cerundolo V, de la Salle H, Description of HLA class I- and CD8-deficient patients: Insights into the function of cytotoxic T lymphocytes and NK cells in host defense, Semin Immunol 18 (2006) 330–336. 10.1016/j.smim.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 140.Gadola SD, Moins-Teisserenc HT, Trowsdale J, Gross WL, Cerundolo V, TAP deficiency syndrome, Clin Exp Immunol 121 (2000) 173–178. 10.1046/j.1365-2249.2000.01264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Halenius A, Hengel H, Human cytomegalovirus and autoimmune disease, Biomed Res Int 2014 (2014) 472978. 10.1155/2014/472978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Praest P, Luteijn RD, Brak-Boer IGJ, Lanfermeijer J, Hoelen H, Ijgosse L, Costa AI, Gorham RD Jr., Lebbink RJ, Wiertz E, The influence of TAP1 and TAP2 gene polymorphisms on TAP function and its inhibition by viral immune evasion proteins, Mol Immunol 101 (2018) 55–64. 10.1016/j.molimm.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 143.Ramos PS, Langefeld CD, Bera LA, Gaffney PM, Noble JA, Moser KL, Variation in the ATP-binding cassette transporter 2 gene is a separate risk factor for systemic lupus erythematosus within the MHC, Genes Immun 10 (2009) 350–355. 10.1038/gene.2009.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rau H, Nicolay A, Usadel KH, Finke R, Donner H, Walfish PG, Badenhoop K, Polymorphisms of TAP1 and TAP2 genes in Graves’ disease, Tissue Antigens 49 (1997) 16–22. 10.1111/j.1399-0039.1997.tb02704.x [DOI] [PubMed] [Google Scholar]
- 145.Yoshihama S, Vijayan S, Sidiq T, Kobayashi KS, NLRC5/CITA: A Key Player in Cancer Immune Surveillance, Trends Cancer 3 (2017) 28–38. 10.1016/j.trecan.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campoli M, Ferrone S, HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance, Oncogene 27 (2008) 5869–5885. 10.1038/onc.2008.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P, Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy, Cell 167 (2016) 397–404 e399. 10.1016/j.cell.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A, Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma, N Engl J Med 375 (2016) 819–829. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ, Vaccines for established cancer: overcoming the challenges posed by immune evasion, Nat Rev Cancer 16 (2016) 219–233. 10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
- 150.Seidel UJ, Oliveira CC, Lampen MH, Hall T, A novel category of antigens enabling CTL immunity to tumor escape variants: Cinderella antigens, Cancer Immunol Immunother 61 (2012) 119–125. 10.1007/s00262-011-1160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shastri N, Nagarajan N, Lind KC, Kanaseki T, Monitoring peptide processing for MHC class I molecules in the endoplasmic reticulum, Curr Opin Immunol 26 (2014) 123–127. 10.1016/j.coi.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garrido G, Schrand B, Rabasa A, Levay A, D’Eramo F, Berezhnoy A, Modi S, Gefen T, Marijt K, Doorduijn E, Dudeja V, van Hall T, Gilboa E, Tumor-targeted silencing of the peptide transporter TAP induces potent antitumor immunity, Nat Commun 10 (2019) 3773. 10.1038/s41467-019-11728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


