Abstract
Background:
The purpose of this study was to compare the clinical outcomes of adults with uncomplicated streptococcal bacteremia who received either oral stepdown (PO) or continued intravenous (IV) therapy.
Methods:
This was a retrospective, single-center, cohort study, including adults admitted with Streptococcal bloodstream infection between January 1, 2013, and December 31, 2020. Only patients with uncomplicated Streptococcal bloodstream infections were included. Patients transitioned to oral therapy within 5 days from bacteremia onset were compared to patients receiving continued IV therapy. The primary outcome was clinical failure, defined by either 90-day hospital readmission or mortality. Secondary outcomes included hospital length of stay (LOS) and antibiotic-related adverse events (AAEs).
Results:
Of the 264 patients included, 42% were transitioned to oral step-down therapy. Group B Streptococcus (22.7%) was the most common isolate. The most common sources of infection were skin and soft tissue (35%) and pulmonary (25%). Intensive care unit stay was more common in the continued IV therapy group (22.2%) compared to the step-down PO group (5.4%). The frequency of clinical failure was similar in continued IV therapy and step-down PO (24.2% vs 18.0%, p= 0.23). The IV group had longer LOS (median, [IQR]) compared to the PO group (7 [5-13.5] vs 4 [3-5] days, p< 0.001). AAEs were similar between the two groups (1.3% vs 1.8%, p=0.74).
Conclusion:
Stepdown to oral therapy may be appropriate for the treatment of uncomplicated Streptococcal bacteremia with consideration of factors such as patient comorbidities, type of infection, source control and clinical progress.
Keywords: Streptococcal infection, bloodstream infection, antimicrobial stewardship, Gram positive cocci, oral stepdown
Introduction
Streptococcus species are Gram-positive aerobic organisms frequently encountered in invasive infections, and significantly contribute to global morbidity and mortality. 1,2 Due to concerns related to efficacy, bioavailability, absorption, and adherence, patients with Streptococcal bloodstream infections frequently complete antibiotic courses via intravenous (IV) route. 3 However, there is increasing interest in transitioning to oral (PO) stepdown therapy in bloodstream infection given the lower cost, convenience, reduced risk of line-related complications, and potential shorter hospital length of stay (LOS). 4 The transition to PO antibiotic is appropriate in patients demonstrating clinical response and achieve source control, even for severe infections caused by other organisms. Oral step-down therapy for Gram-negative bloodstream infection, is considered a standard practice as many studies have demonstrated its efficacy without compromise of safety. 5 The most frequently studied PO agents for bloodstream infection have historically been fluoroquinolones in Gram-negative infections because of high bioavailability. 6,7 Switching to PO therapy has also been examined for severe Gram-positive bloodstream infections (i.e. Staphylococcus aureus) 8,9 however, this is still not considered standard of care in the United States. 10
The use of PO antibiotic therapy as step-down therapy for uncomplicated Streptococcal bloodstream infections remains controversial, likely due to the diversity of streptococcal species, varying virulence profiles, and the heterogeneous types of infections they may cause. 6,11 This has led to differences in prescription practices. A recently published retrospective cohort study on uncomplicated streptococcal bloodstream infections showed no difference in clinical outcomes in patient treated with step-down oral antibiotic therapy compared to continued IV treatment. 12
The purpose of this study was to compare PO and IV therapies for adult patients with uncomplicated streptococcal bloodstream infections. We hypothesized that early switch to PO therapy would not have higher clinical failure compared to continued IV treatment. Length of in hospital stay and antibiotic-associated adverse effects were also compared between the two groups.
Material and methods
Study participants
This single-center retrospective cohort study was conducted at a tertiary teaching hospital in Portland, Maine, USA. The study was reviewed as exempt by the MaineHealth Institutional Review Board (1707684-1).
All patients aged ≥18 years admitted with a positive blood culture growing Streptococcus spp. drawn between January 1, 2013, and December 31, 2020, and initially treated with IV antibiotics were potentially eligible for inclusion in the study. Patients with polymicrobial bloodstream infections, unknown 90-day follow up outcome, complicated streptococcal bloodstream infections (endocarditis, bone, joint, spine, empyema, central nervous system, unattainable source control) and/or with antibiotics administered more than 24 hours of the time that the first positive blood culture was collected were excluded. Patients with blood cultures that were presumed to be contaminants by treating clinician or transitioned to hospice care before completion of antibiotic therapy, as well as those transitioned to oral antibiotic therapy >5 days after a positive blood culture were also excluded. Subjects receiving an entire course of IV antibiotic therapy for treatment of Streptococcal bloodstream infection were assigned to the IV-only group. Patients transitioned from IV to oral antibiotic therapy within five days of onset of bloodstream infection were included in the oral PO-stepdown group.
Data Collection
An automated report was generated from the electronic health record to identify patients with positive blood cultures for streptococci. For eligible patients, data were collected from the electronic medical record for demographic and clinical variables, microbiology results, antibiotic regimens, and outcome measures. Our primary outcome measure (clinical failure) was a composite of all-cause 90-day hospital readmission and 90-day mortality; secondary outcomes measures were hospital LOS and antibiotic-related adverse events (AAEs). Data were entered into and maintained in a REDCap® database.
Definitions
Stepdown was defined as a switch to an active PO antibiotic therapy following empirical IV antibiotics within ≤5 days from onset of bloodstream infection, whilst patients in IV group were not converted to any PO therapy during the course of treatment.
Total duration of therapy was calculated using the day of the first negative follow-up blood culture as day one, and including planned days of therapy post-discharge, if applicable. Repeat positive culture growing the same organism was defined as a blood culture drawn >24 hours after the index culture. Patients without repeat blood cultures were excluded from calculation of total duration of therapy. Source control was defined as completion of a procedure that eliminated infectious foci prior to completion of antimicrobial therapy. AAEs were assessed up to 90 days following discharge. Clostridioides difficile infection was defined by a positive C. difficile polymerase chain reaction test and provider documentation of diarrhea. Other AAEs were reported as documented by provider documentation. Documentation of intervention by an infectious diseases (ID) physician for antibiotic selection was considered an ID consultation.
Data Analysis
Data were summarized using descriptive statistics; continuous data are shown as mean (standard deviation) or median [interquartile range], as appropriate, and categorical data are shown as frequency (n, %), both overall and after stratification by IV and PO subgroups and by the primary outcome measure (clinical failure). Differences between subgroups were analyzed using Chi Square Test (with or without continuity correction) or Fisher’s exact test (categorical variables) or two-sided Student’s T test or Mann-Whitney U test (continuous variables), as appropriate. Variables that differed between primary outcome subgroups in univariate analysis (p<0.1) were entered either separately (unadjusted analysis) or together (adjusted analysis) as covariates into a multivariable logistic regression model to identify independent predictors of the composite adverse outcome. To confirm any independent associations with the primary outcomes and PO-stepdown therapy, an inverse probability treatment weight (IPTW) was created in a multivariable regression model based on covariates potentially associated with either the primary outcome or assignment to a treatment arm. Results were presented as odds ratios (OR) and adjusted odds ratios (aOR) with 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS statistical software, version 28 (IBM SPSS Inc, Armonk, NY).
Results
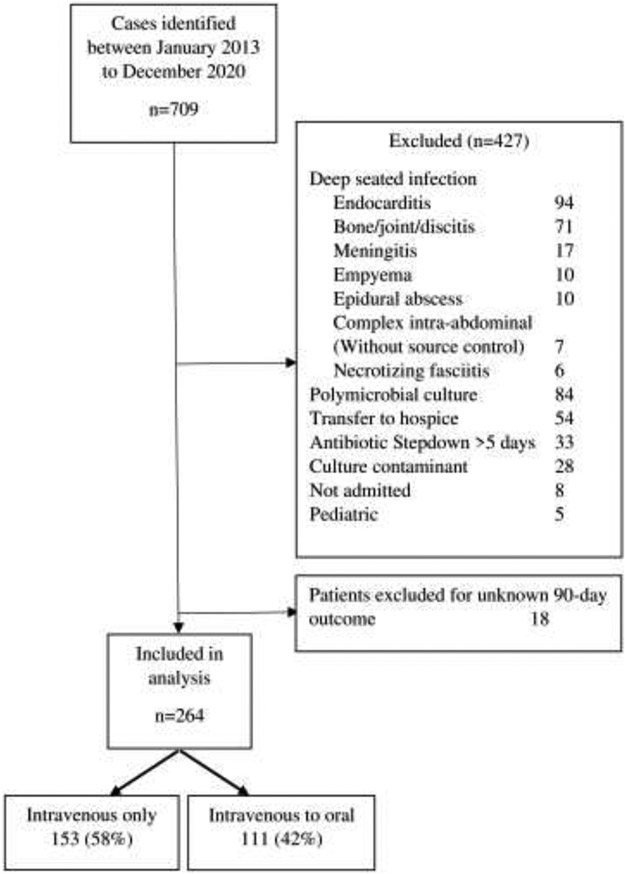
Between January 1, 2013, to December 31, 2020, 709 patients were admitted for Streptococcal bloodstream infection, among whom 264 were eligible for inclusion in the study (Figure 1). Deep seated infection (215/427, 50.4%) was the most frequent reason for exclusion. The majority of eligible patients (58%) were treated with continued IV antibiotics. The most frequent isolates identified were Group B Streptococcus (22.7%), Streptococcus pneumoniae (17%) and alpha-hemolytic Streptococci (17%). Group C/F/G Streptococcus were combined as well as group A Streptococcus accounted for 27% and 13% of cases, respectively.
Figure 1:
Study inclusion and exclusion
The mean (SD) patient age was 64.4± 17.2 years and 62.5% were male (Table 1). The rate of intensive care unit (ICU) admission was 15.2%. Hypertension (59.8%), diabetes (34.5%) and cancer (14.4%) were frequent comorbidities. The quick sepsis related organ failure assessment (qSOFA) score was <2 in 84.8% of cases at the time of diagnosis with bacteremia. The most common sources of infection were skin and soft tissue (35%), pulmonary (25%) and gastro-intestinal (13.6%). Among patients in the PO-stepdown group, 54/111(48.6%) were changed within 3 days and 57/111 (51.4%) patients were deescalated between day 4 to 5 of the initial positive blood culture. While demographic characteristics were similar between the PO-stepdown and IV-only groups, there was variation among clinical characteristics. Patients in the IV-only group were significantly more likely to have ICU admission (34/153 (22.2%) versus 6/111 (5.4%), p<0.001), hypertension (101/153 (66.6%) versus 57/111 (51.4%), p=0.016), or presence of intravascular device (17/153 (11.1%) versus 2/111 (1.8%), p=0.003), while those in the PO stepdown treatment group were more likely to have received outpatient steroids (15/111 (13.5%) versus 8/153 (5.2%), p=0.02). Gastrointestinal infections were significantly more frequent in the IV-only group (27/153 (17.6%) versus 9/111 (8.1%), p=0.025).
Table 1:
Demographic and Clinical Characteristics
| Variable1 | Overall | IV only | PO stepdown | P value |
|---|---|---|---|---|
| N | 264 | 153 | 111 | |
| Age at admission (years) | 64.4 ± 17.2 | 63.8 ± 16.4 | 65.1 ± 18.3 | 0.542 |
| Body mass index (kg/m2) | 28.4 [23.9-34.9] | 28.5 [24.0-34.0] | 28.1 [23.8-35.9] | - |
| Male sex | 165 (62.5) | 95 (62.1) | 70 (63.1) | 0.873 |
| Admitted to ICU | 40 (15.2) | 34 (22.2) | 6 (5.4) | <0.0013 |
| Comorbidities | ||||
| Diabetes | 91 (34.5) | 59 (38.6) | 32 (28.8) | 0.103 |
| Cancer (active) | 38 (14.4) | 26 (17.0) | 12 (10.8) | 0.163 |
| Neutropenia (ANC<500 at admit) | 9 (3.4) | 8 (5.2) | 1 (0.9) | 0.084 |
| Dialysis | 7 (2.7) | 5 (3.3) | 2 (1.8) | 0.74 |
| IV drug use | 6 (2.3) | 0 (0.0) | 6 (5.4) | - |
| Transplant | 6 (2.3) | 3 (2.0)5 | 3 (2.7)6 | 0.74 |
| Hypertension | 158 (59.8) | 101 (66.0) | 57 (51.4) | 0.0163 |
| Prior procedures | ||||
| Heart valve replacement history | 18 (6.8) | 14 (9.2) | 4 (3.6) | 0.0884 |
| Intravascular device | 19 (7.2) | 17 (11.1) | 2 (1.8) | 0.0034 |
| PICC line placement | 93 (35.2) | 92 (60.1) | 1 (1.1) | - |
| qSOFA score: | ||||
| At the time of diagnosis with bacteremia | ||||
| 0 | 135 (51.1) | 75 (49.0) | 60 (54.1) | 0.423 |
| 1 | 89 (33.7) | 48 (31.4) | 41 (36.9) | 0.343 |
| 2 | 36 (13.6) | 26 (17.0) | 10 (9.0) | - |
| 3 | 4 (1.5) | 4 (2.6) | 0 (0.0) | - |
| Steroid use | 23 (8.7) | 8 (5.2) | 15 (13.5) | 0.024 |
| Outpatient immunosuppression treatment 7 | 25 (9.5) | 17 (11.1) | 8 (7.2) | 0.394 |
| Serum Lactate at admission (mmol/L) | 2.3 [1.7-3.4] (n=210) | 2.4 [1.7-3.6] (n=118) | 2.2 [1.6-2.9] (n=92) | - |
| Source of infection identified | ||||
| Cellulitis/wound | 93 (35.2) | 51 (33.3) | 42 (37.8) | 0.453 |
| Respiratory | 66 (25.0) | 29 (19.0) | 37 (33.3) | 0.083 |
| Gastrointestinal | 36 (13.6) | 27 (17.6) | 9 (8.1) | 0.0253 |
| Dental | 11 (4.2) | 9 (5.9) | 2 (1.8) | 0.124 |
| Urinary tract | 8 (3.0) | 3 (2.0) | 5 (4.5) | 0.284 |
| Catheter-associated | 3 (1.1) | 3 (2.0) | 0 (0.0) | - |
| Other | 11 (4.2) | 4 (2.6) | 7 (6.3) | - |
Abbreviations: ANC, absolute neutrophil count; ICU, Intensive care unit; IV, Intravenous; N, number of patients; PO, per os; QSOFA, quick sequential organ failure assessment
Data presented as n (%), mean ± standard deviation, or as median (interquartile range).
t test,
Chi square test with continuity correction,
Fisher’s exact test,
Includes: n=2 heart, n=1 liver,
Includes n=2 kidney, n=1 heart and lung
Includes biologies and calcineurin inhibitors
Compared with those in the PO-stepdown group, patients in the IV-only group were more likely to undergo a transthoracic echocardiography (TTE) (108/153 (70.6%) versus 40/111 (36%), p<0.001) or a transesophageal echocardiography (TEE) (43/153 (28.1%) versus 4/111 (3.6%), p<0.001), as well as to receive a consult from an infectious disease specialist (89/153 (58.2%) vs 11/111 (9.9%), p<0.001) (Table 2). The median (IQR) duration of inpatient antibiotic treatment was 6 (4-10) days for the IV-only group, compared to 3 (3-4) days for the PO-stepdown group (p<0.001). Estimated total duration of antibiotic therapy was comparable in the two groups of IV-only versus PO-stepdown therapy (14 (13-14) versus 13 (10-14) days).
Table 2:
Management of Patients with Streptococcal Infection, Stratified by Treatment with IV Only versus PO Stepdown Antibiotics.
| Variable1 | Overall (n=264) |
IV only (n=153) |
PO stepdown (n=111) |
P value |
|---|---|---|---|---|
| TTE done | 148 (56.1) | 108 (70.6) | 40 (36.0) | < 0.0012 |
| Time from admit to TTE (days) | 1.0 (1-3) | 1 (1-3) | 1 (1-2) | - |
| TEE done | 47 (17.8) | 43 (28.1) | 4 (3.6) | < 0.0012 |
| Time from admit to TEE (days) | 4 (3-5) | 4 (3-5) | (2.5-18.2) | - |
| ID consult ordered by primary team | 100 (37.9) | 89 (58.2) | 11 (9.9) | < 0.0012 |
| Time to ID consult (days) | 2 (1-4) | 2 (1-4) | 1 (1-2) | - |
| Number of positive bottles of blood culture prior to starting antibiotic | ||||
| 1 | 97 (36.7) | 51 (33.3) | 46 (41.4) | 0.362 |
| >1 | 167 (63.3) | 102 (66.7) | 65 (58.6) | 0.182 |
| Number of patients with repeat blood cultures | 254 (96) | 151 (98) | 103 (92) | - |
| Duration of bacteremia (hours) 3 | 27.6 (21.8-42.6) | 28.7 (22.6-42.7) | 26.8 (21.7-42.5) | - |
| Duration of inpatient antibiotics (days) | 4 (3-7) | 6 (4-10) | 3 (3-4) | - |
| Total duration of antibiotics (days) | 14 (11-14) | 14 (3-14) | 13 (10-14) | - |
Abbreviations: ID, infectious disease; IV, intravenous; N, number of patients; PO, per os; Strep, streptococcus; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography
Data presented as n (%) or as median (interquartile range).
Chi square test with continuity correction
Calculated based on 254 patients where repeat blood culture data was available
The IV-only group was more likely to complete their antibiotic course in the hospital (41/153, 26.8% versus 2/111 (1.8%) respectively, p<0.001). Consistent with this, patients in the PO-stepdown group were more likely to be discharged with antibiotic than those in the IV-only group (109/111, 98.2% versus 112/153, 73.2%, respectively, p<0.001). The most commonly used targeted antibiotics in the outpatient setting among those discharged with IV antibiotics was ceftriaxone (92/112, 82.1%). For those in the PO group, the most frequent targeted PO antibiotics at discharge were cefdinir (27/109, 24.7%) and amoxicillin (25/109, 22.9%). (Supplementary Tables)
There was no significant difference in clinical failure between the IV-only and PO-stepdown treatment groups (37/153 (24.2%) versus 20/111 (18.0%), respectively, p=0.23) or its components (Table 3). Median (IQR) LOS was longer in the IV-only group compared with the PO-stepdown group (7 [5-13.5] days versus 4 [3-5] days, respectively, p< 0.001) and discharge to home was more frequent in the PO-stepdown group (94/111 (84.7%) versus 98/153 (64.1%), respectively, p =0.001).
Table 3:
Clinical Outcome of Patients with Streptococcal Infection, Stratified by Treatment with IV Only versus PO Stepdown Antibiotics.
| Variable1 | Overall (n=244) |
IV only (n=153) |
PO stepdown (n=111) |
P value |
|---|---|---|---|---|
| Primary outcome | ||||
| Clinical failure 2 | 57 (21.6) | 37 (24.2) | 20 (18.0) | 0.233 |
| 90-day mortality | 7/262 (2.7) | 4/152 (2.6) | 3/110 (2.7) | 1.003 |
| Readmission within 90 days | 53 (20.1) | 35 (22.9) | 18 (16.2) | 0.183 |
| Readmission due to recurrent strep bacteremia | 6/53 (11.3) | 5/35 (14.3) | 1/18 (5.6) | 0.653 |
| Secondary outcomes | ||||
| Length of stay (days) | 5 (4-9) | 7 (5-13.5) | 4 (3-5) | < 0.0014 |
| Discharge home | 192 (72.7) | 98 (64.1) | 94 (84.7) | 0.0013,5 |
| Antibiotic-associated adverse events 6 | 4 (1.5) | 2 (1.3) | 2 (1.8) | 0.743 |
Abbreviations: IV, intravenous; N, number of patients; PO, per os
Data presented as n (%) or as median (interquartile range).
Composite of 90-day mortality and/or 90-day readmission
Chi square test with continuity correction,
Mann Whitney U test
Comparison used between home versus skilled nursing plus rehabilitation facility, plus self-directed discharge,
Types of antibiotic associated with adverse events included Clostridium difficile infection (n=2), leukopenia (n=1), and skin rash (n=1).
After adjusting for covariates associated with clinical failure in the univariate analysis (see Supplementary Tables), only receipt of dialysis was independently associated with clinical failure (adjusted OR (95% CI), 11.5 [2.1-61.9], p=0.004) (Table 4). Compared with the IV-only group, treatment with PO-stepdown was not associated with clinical failure in the unadjusted (OR (95% CI), 0.7 [0.4-1.3], p=0.23), covariate-adjusted (OR (95% CI), 0.8 [0.4-1.6], p= 0.54) (Table 4), or IPTW adjusted (aOR (95% CI), 0.6 [0.3-1.1], p=0.10) analyses.
Table 4:
Independent predictors of 90-day any-cause death or readmission among 264 patients admitted with uncomplicated streptococcal infection
| Variable | Reference | OR (95%CI) for primary outcome)1 | |||
|---|---|---|---|---|---|
| Unadjusted | p-value | Adjusted1 | p-value | ||
| PO Stepdown | IV group | 07 (0.4-1.3) | 0.23 | 0.8 (0.4-1.6) | 0.54 |
| Cancer | none | 2.8 (1.4-5.9) | 0.005 | 2.0 (0.84.8) | 0.14 |
| Dialysis | none | 9.8 (1.8-52.2) | 0.007 | 11.5 (2.1-61.9) | 0.004 |
| Neutropenia | none | 8.0 (1.9-33.1) | 0.004 | 3.3 (0.6-18.1) | 0.17 |
| >1 positive BC sets | 1 positive BC set | 1.6 (0.9-3.1) | 0.13 | 1.6 (0.8-3.1) | 0.19 |
| Steroid/immunosuppressant | None | 2.6 (1.2-5.5) | 0.01 | 1.6 (0.74.0) | 0.30 |
Abbreviations: BC, blood culture; CI, confidence interval; IV, intravenous; OR, odds ratio; PO, per os
Variables were entered into the logistic regression model as covariates if they were associated (p≤0.1) with the primary outcome measure in univariate analysis. IV vs stepdown was also included in the model, to explore whether adjustment altered its relationship with 90-day any-cause death or readmission. N=264 cases had complete data for all variables and were included in both the adjusted and unadjusted analysis.
Discussion
In this study, there was no association between clinical failure and PO-stepdown treatment for uncomplicated Streptococcal bloodstream infections. The two groups had similar rates of 90-day mortality, hospital readmission, recurrence, and AAEs. Readmission due to recurrent Streptococcal bacteremia was overall low in this study, reflecting the low risk of treatment failure in uncomplicated Streptococcal bloodstream cases. The LOS was shorter in the PO-stepdown group compared to the IV-only group.
Recent studies have demonstrated the safety of PO antibiotic therapies for treatment of certain bloodstream infections. In a retrospective cohort study published by Tamma el al., patients with uncomplicated Enterobacterales bloodstream infection that were switched to PO-stepdown therapy within five days of initiation of parenteral therapy had similar 30-day mortality and recurrence rates compared to continuation of IV therapy for the duration of the course. 5 A meta-analysis compared outcomes in patients transitioned to either PO fluoroquinolones or trimethoprim-sulfamethoxazole versus ß-lactams after an initial IV course for Gram-negative bloodstream infection; most commonly used ß-lactam antibiotic regimens were amoxicillin/clavulanic acid, amoxicillin, and cephalexin. 13 While there was no difference in mortality, patients transitioned to PO ß-lactams had higher rates of infection recurrences compared to fluoroquinolones. The authors explained these findings by the lower bioavailability of some ß-lactam antibiotics, concentration of antibiotic necessary to inhibit an organism, and frequent dosing required for ß-lactam antibiotics, which could have led to lower compliance. 13 In our study, the most common PO-stepdown agents were also ß-lactams (81.6%) mostly comprised of cefdinir and amoxicillin. While serum concentration of these oral beta-lactams does not reach those of their IV counterparts, such as ceftriaxone and ampicillin, the wild-type MIC distributions of ß-streptococci and S. pneumoniae likely remain attainable in respect to reaching concentrations at target sites. 14,15
A common practice of treatment of Gram-positive bloodstream infection is parenteral antibiotics. A large, randomized trial examining treatment-related outcomes in endocarditis (consisting of mostly streptococci) found those with PO stepdown (dual agents) therapy after a two-week IV lead-in had similar outcomes to IV-only. 16 Ghandi et al assessed 308 patient treated for non-Staphylococcal Gram-positive bloodstream infections and demonstrated that PO antibiotic step-down therapy was non-inferior to IV antibiotic therapy. 17 Some additional recent studies showed conflicting data about the safety of switching to PO therapies in Staphylococcus aureus bloodstream infections. 18-20
While streptococci and enterococci are also Gram-positive bacterium, data for PO-stepdown in S. aureus infections may not be applicable, for a number of reasons. Each Streptococcal species has different virulence factors and may cause different types of infections. 21,22 Prevalence of infective endocarditis in Streptococcal bloodstream infections is dependent on Streptococcal species, with S mutans, S gordonii, S sanguinis, S gallolyticus, and S mitis/oralis being most common. 23 This shows the spectrum of diversity of Streptococcal infections. Specific Streptococcal spp. was unable to be determined based on diagnostic platforms utilized at our institution. In addition, bloodstream infections with S aureus are associated with higher rates of ICU admission, as well as persistently positive blood cultures for several days on targeted antibiotics compared to Streptococcus bloodstream infections. 24 The findings in our study may not be generalizable for all adult patients with uncomplicated Streptococcal bloodstream infections.
Two retrospective cohorts demonstrated the safety of treating uncomplicated Streptococcal bloodstream infection with PO antibiotics. Kang et al assessed 244 patients and concluded that patients treated with PO-stepdown had significantly shorter LOS compared with continued IV therapy with similar 30-day recurrence of bloodstream infection, 30-day readmission, 30-day all-cause mortality, and catheter-related or drug-related adverse events. 12 Ramos-Otero et al included 98 patients and resulted in similar outcomes in IV-to-PO compared to IV-only antibiotic therapy for treatment of uncomplicated Streptococcal bloodstream infection. 25 Our study has comparable findings. However, these publications included slightly different definitions of clinical success and only a 30-day follow up period. In addition, exclusion criteria, and outcomes differed significantly. The overall rate of hospital readmission was 20.1% in our study, which is comparable to the rate report by Kang et al. 12 This is likely caused by higher patient comorbidities. Hospital LOS was shorter in the PO-stepdown group compared to IV-only group, which is a common finding in the previous studies. 12,25
The optimal timing to switch to PO stepdown antibiotic therapy for uncomplicated Streptococcal bloodstream infections remains unclear. This study demonstrated that switching to PO antibiotic as early as 3 to 5 days from start of parenteral therapy did not compromise the clinical outcome. We chose five days as the cut off for maximum duration of IV antibiotic therapy in the PO-stepdown group because seven days of antibiotics may be sufficient treatment in some bloodstream infections. 26 Some studies have evaluated the switch from IV to PO antibiotic therapy in patients with clinical stability and have reported no compromise in clinical outcomes. 3,27 The decision to switching from IV to PO antibiotic therapy may be reasonable in patients with uncomplicated Streptococcal bloodstream infection, depending on the patient, causative pathogen, and the PO antibiotic. 6
Antibiotic associated adverse events were rarely reported in this study. No complications from IV therapy were reported such as infection or thrombosis. This is comparable to a previous similar study by Kang et al. 12
This study has several limitations. This is a single center, retrospective cohort study with data collected from chart review. This limits the ability to assess clinical outcomes in a homogeneous way or evaluate cause and effect. Strict exclusion criteria as well as qSOFA scores were used to account for selection bias. The baseline demographic analysis confirms that the two groups of patients had comparable comorbidities. However, the IV-only group of patients had more ICU stays, ID consult involvement, TTE/TEE performed and completion of antibiotic courses in the hospital compared to the PO group of patients, indicating a possible sicker population. In attempt to mitigate these differences in the populations, multivariable regression and IPTW analyses were applied. The only variable associated independently with the primary outcome was dialysis. This could also be refuted by the small dialysis population included and the wide confidence interval, although patients with end-stage renal disease are generally at high risk for mortality and readmission. 28 Outpatient antibiotic adherence was not feasible to assess and AAEs were only identified when documented in the EMR. Readmissions to other hospitals beyond the capacity of the health system EMR would not have been captured. Patients without known clinical outcome were not included in the analysis because the 90-day follow up outcome could not be determined. This could have excluded some patient who had the outcome during the follow up period. Patients transferred to hospice care during antibiotic treatment were excluded from this study, which may have contributed to exclusion of sicker patients. Moreover, antibiotic adverse events were collected from patients’ charts as documented by the providers in the EMR. There may have been AAEs that were not documented. This study excluded complicated Streptococcal bloodstream infection, and the clinical failure rate was expected to be similar in the two groups of IV versus PO stepdown therapy given the low risk of failure. The results of this study cannot be generalized to more complicated or deep-seated Streptococcal bloodstream infections.
Conclusion
In conclusion, this study showed no difference in clinical failure rates in adult patients receiving PO-stepdown antibiotic therapy compared to full course IV therapy for uncomplicated Streptococcal bloodstream infection. LOS was shorter in the PO group compared to the IV group. Although randomized prospective studies are needed to confirm the results of this study, stepdown to PO therapy for the treatment of uncomplicated Streptococcal bloodstream infection may be a reasonable option with appropriate consideration of factors such as patient comorbidities, type of infection, source control, and clinical response.
Supplementary Material
Highlights:
The frequency of clinical failure was similar in continued IV therapy and step-down PO (24.2% vs 18.0%, p= 0.23).
The IV group had longer length of stay compared to the PO group (7 [5-13.5] vs 4 [3-5] days, p<001).
Antibiotic adverse events were similar between the two groups (1.3% vs 1.8%, p=0.74).
Funding:
This research project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. This work was also supported in part by the Northern New England Clinical and Translational Research grant U54GM115516.
Glossary
- PO
Oral stepdown
- IV
intravenous
- LOS
length of stay
- AAEs
antibiotic-related adverse events
- ID
infectious diseases
- IPTW
inverse probability treatment weight
- OR
odds ratios
- aOR
adjusted odds ratios
- CI
confidence intervals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: None
Ethical Approval: The study was reviewed as exempt by the MaineHealth Institutional Review Board (1707684-1).
Credit authorship contribution statement:
Rami Waked: Writing - original draft, Methodology, Investigation
Wendy Y. Craig: Methodology, data analysis, Writing - Review & Editing
Nicholas J. Mercuro: data analysis, Writing - Review & Editing
Minkey Wungwattana: Conceptualization, Methodology, Writing - Review & Editing
Emily Wood: Methodology, Writing - Review & Editing
Kristina E. Rokas: Conceptualization, Methodology, Writing - Review & Editing
References
- 1.Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–509. doi: 10.1111/1469-0691.12195 [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664. doi: 10.1128/CMR.00002-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hospenthal DR, Waters CD, Beekmann SE, Polgreen PM. Practice Patterns of Infectious Diseases Physicians in Transitioning From Intravenous to Oral Therapy in Patients With Bacteremia. Open Forum Infect Dis. 2020;7(12):ofz386. doi: 10.1093/ofid/ofz386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. New England Journal of Medicine. 2019;380(5):425–436. doi: 10.1056/NEJMoa1710926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-Day Mortality With Oral Step-Down vs Continued Intravenous Therapy in Patients Hospitalized With Enterobacteriaceae Bacteremia. JAMA Intern Med. 2019;179(3):316–323. doi: 10.1001/jamainternmed.2018.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale AJ, Snyder GM, Ahem JW, Eliopoulos G, Ricotta D, Alston WK. When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. J Hosp Med. 2018; 13(5):328–335. doi: 10.12788/jhm.2949 [DOI] [PubMed] [Google Scholar]
- 7.Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents. 2018;51(5):687–692. doi: 10.1016/j.ijantimicag.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 8.Bupha-Intr O, Blackmore T, Bloomfield M. Efficacy of Early Oral Switch with β-Lactams for Low-Risk Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother. 2020;64(7):e02345–19. doi: 10.1128/AAC.02345-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen SCJ, Lagnf AM, Bhatia S, Shamim MD, Rybak MJ. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother. 2019;74(2):489–498. doi: 10.1093/jac/dky452 [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clinical Infectious Diseases. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 11.Li HK, Agweyu A, English M, Bejon P. An Unsupported Preference for Intravenous Antibiotics. PLOS Medicine. 2015;12(5):e1001825. doi: 10.1371/journal.pmed.1001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang A, Beuttler R, Minejima E. Evaluation of step-down oral antibiotic therapy for uncomplicated streptococcal bloodstream infections on clinical outcomes. Therapeutic Advances in Infection. 2022;9:20499361211073250. doi: 10.1177/20499361211073248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punjabi C, Tien V, Meng L, Deresinski S, Holubar M. Oral Fluoroquinolone or Trimethoprim-sulfamethoxazole vs. ß-lactams as Step-Down Therapy for Enterobacteriaceae Bacteremia: Systematic Review and Meta-analysis. Open Forum Infect Dis. Published online August 14, 2019:ofz364. doi: 10.1093/ofid/ofz364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook PJ, Andrews JM, Wise R, Honeybourne D. Distribution of cefdinir, a third generation cephalosporin antibiotic, in serum and pulmonary compartments. J Antimicrob Chemother. 1996;37(2):331–339. doi: 10.1093/jac/37.2.331 [DOI] [PubMed] [Google Scholar]
- 15.Mogle BT, Beccari MV, Steele JM, Fazili T, Kufel WD. Clinical considerations for oral beta-lactams as step-down therapy for Enterobacteriaceae bloodstream infections. Expert Opin Pharmacother. 2019;20(8):903–907. doi: 10.1080/14656566.2019.1594774 [DOI] [PubMed] [Google Scholar]
- 16.Iversen K, Ihlemann N, Gill SU, et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. New England Journal of Medicine. 2019;380(5):415–424. doi: 10.1056/NEJMoa1808312 [DOI] [PubMed] [Google Scholar]
- 17.Gandhi K, Wrzesinski M, Bunnell K, et al. 191. Oral Antibiotic Step-Down Therapy for Non- Staphylococcal Gram-Positive Bloodstream Infections. Open Forum Infectious Diseases. 2021;8(Supplement_1):S203. doi: 10.1093/ofid/ofab466.393 [DOI] [Google Scholar]
- 18.Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. doi: 10.1136/bmj.h2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh N, Hadano Y, Saito S, Myokai M, Nakamura Y, Kurai H. Intravenous to oral switch therapy in cancer patients with catheter-related bloodstream infection due to methicillin-sensitive Staphylococcus aureus: A single-center retrospective observational study. PLoS One. 2018;13(11):e0207413. doi: 10.1371/journal.pone.0207413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early Oral Switch to Linezolid for Low-risk Patients With Staphylococcus aureus Bloodstream Infections: A Propensity-matched Cohort Study. Clin Infect Dis. 2019;69(3):381–387. doi: 10.1093/cid/ciy916 [DOI] [PubMed] [Google Scholar]
- 21.Terao Y The virulence factors and pathogenic mechanisms of Streptococcus pyogenes. Journal of Oral Biosciences. 2012;54(2):96–100. doi: 10.1016/j.job.2012.02.004 [DOI] [Google Scholar]
- 22.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301. doi : 10.1038/nrmicro1871 [DOI] [PubMed] [Google Scholar]
- 23.Chamat-Hedemand S, Dahl A, Østergaard L, et al. Prevalence of Infective Endocarditis in Streptococcal Bloodstream Infections Is Dependent on Streptococcal Species. Circulation. 2020;142(8):720–730. doi: 10.1161/CIRCULATIONAHA.120.046723 [DOI] [PubMed] [Google Scholar]
- 24.Vos FJ, Kullberg BJ, Sturm PD, et al. Metastatic infectious disease and clinical outcome in Staphylococcus aureus and Streptococcus species bacteremia. Medicine (Baltimore). 2012;91(2):86–94. doi: 10.1097/MD.0b013e31824d7ed2 [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Otero GP, Sarangarm P, Walraven C. A Retrospective Analysis of Intravenous vs Oral Antibiotic Step-Down Therapy for the Treatment of Uncomplicated Streptococcal Bloodstream Infections. J Clin Pharmacol. Published online June 5, 2022. doi: 10.1002/jcph.2097 [DOI] [PubMed] [Google Scholar]
- 26.Yahav D, Franceschini E, Koppel F, et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin Infect Dis. 2019;69(7):1091–1098. doi: 10.1093/cid/ciy1054 [DOI] [PubMed] [Google Scholar]
- 27.Ramirez JA, Bordon J. Early Switch From Intravenous to Oral Antibiotics in Hospitalized Patients With Bacteremic Community-Acquired Streptococcus pneumoniae Pneumonia. Arch Intern Med. 2001;161(6):848–850. doi: 10.1001/archinte.161.6.848 [DOI] [PubMed] [Google Scholar]
- 28.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57(3): 1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.