Abstract
Background:
Invasive hemodynamic variables obtained from right heart catheterization have been used for risk-stratifying patients with advanced heart failure. However, there is a paucity of data on the prognostic value of invasive hemodynamic variables in patients with left ventricular assist devices (LVAD). We hypothesized that cardiac power output (CPO), cardiac power efficiency (CPE), and left ventricular stroke work index (LVSWI) can serve as prognostic markers in patients with LVADs.
Methods:
Baseline hemodynamic data from patients who had LVAD ramp studies at our institution from 4/2014 to 7/2018 were prospectively collected, from which advanced hemodynamic variables (CPO, CPE, and LVSWI) were retrospectively analyzed. Univariate and multivariable analyses were performed for hemocompatibility-related adverse events (HRAE), HF admissions, and mortality.
Results:
Ninety-one participants (age 61 ± 11 years, 34% women, 40% Black or African American, and 38% ischemic cardiomyopathy) were analyzed. Low CPE was significantly associated with mortality (HR 2.42, 95% CI 1.02–5.74, p = 0.045) in univariate analysis and Kaplan–Meier analysis (p = 0.04). Low LVSWI was significantly associated with mortality (HR 2.13, 95% CI 1.09–4.17, p = 0.03) in univariate analysis and Kaplan–Meier analysis (p = 0.02). CPO was not associated with mortality. CPO, CPE, and LVSWI were not associated with HRAE or HF admissions.
Conclusions:
Advanced hemodynamic variables can serve as prognostic indicators for patients with LVADs. Low CPE and LVSWI are prognostic for higher mortality, but no variables were associated with HF admissions or HRAEs.
Keywords: advanced hemodynamic variables, cardiac power efficiency, cardiac power output, heart failure, left ventricular assist device, left ventricular stroke work index
1 |. INTRODUCTION
Invasive hemodynamic variables derived from measurements obtained from right heart catheterization (RHC) have been used in clinical practice to guide the management of patients with advanced heart failure (HF) and cardiogenic shock.1,2 Their utility for prognostication in patients who have already received left ventricular assist devices (LVAD) has not yet been adequately explored. Recently, there has been a renewed interest in the prognostic power of advanced hemodynamic metrics that incorporate a combination of intracardiac pressures together with either cardiac output or a surrogate for output to better quantify cardiac performance. Cardiac power output (CPO) has been shown to significantly predict mortality in patients with cardiogenic shock due to acute myocardial infarction.3 Cardiac power efficiency (CPE) was identified as a predictor of patients in cardiogenic shock who may not survive medical therapy alone.4 Left ventricular stroke work index (LVSWI) assesses left ventricular stroke work and energetics, and when measured noninvasively, predicts mortality in critical patients.5
There is a unique relationship between individual patient hemodynamics and adverse clinical outcomes in patients supported by LVAD therapy. Hemocompatibility-related adverse events (HRAE) are thought to be largely driven by the pump–patient interaction. HRAEs include device malfunction due to confirmed or suspected pump thrombosis, neurological events, nonsurgical bleeding, thromboembolic events, and death related to one of these events. These complications do not uniformly affect all patients with LVADs. Specifically, there is a greater propensity for HRAEs in HeartWare HVAD, (HVAD, Medtronic Inc., Minneapolis MN) and HeartMate II (HMII, Abbott Laboratories, Abbott Park IL) systems than in the HeartMate 3 (HM3, Abbott Laboratories, Abbott Park IL).6–8 The purpose of this study is to identify the advanced hemodynamic variables that best reflect patients’ contribution to the pump–patient interaction with LVAD support. We hypothesized that greater native left ventricular contribution to flow will result in an improved pump–patient interaction and result in less morbidity and mortality.
2 |. METHODS
2.1 |. Patient selection
The study cohort was comprised of adult patients supported by an LVAD at a tertiary care center. All patients were clinically stable outpatients at the time of enrollment and hemodynamic assessment. Patients with suspected device thrombosis or malfunction were not included. The study protocol was approved by our center’s Institutional Review Board. All patients provided written informed consent and were prospectively enrolled in the hemodynamic ramp test database between April 2014 and July 2018. We then performed a retrospective analysis of this prospectively collected data.
2.2 |. Data collection
We prospectively collected the baseline hemodynamic data from patients with LVADs who had undergone an invasive hemodynamic ramp study at our institution between April 2014 and July 2018 with a minimum follow-up time of 1 year. A ramp study has performed a minimum of 90 days after LVAD implantation. The advanced hemodynamic variables of CPO, CPE, and LVSWI (Table 1) were calculated from right heart catheterization hemodynamics at the patients’ baseline LVAD speed at the beginning of the ramp study (Table 2). Patients were divided into groups of high and low CPO, CPE, and LVSWI, using receiver operator characteristic (ROC) derived cutoff values optimized for mortality. A previously described hemocompatibility score (HCS) uses a tiered approach to individual HRAEs on the basis of increasing clinical severity.8,9 The composite HCS served as a single end-point representing the totality of HRAEs for each patient supported by LVAD therapy. We followed the hierarchal system as described by Mehra et al.,9 where (a) Tier I (mild, 1 point per event) – ≤2 bleeding episodes, or a suspected pump thrombosis that requires hospitalization (successfully medically treated), or nonstroke-related neurological events, or arterial thromboembolism without organ loss (b) Tier II (Moderate, 2 points per event) – >2 bleeding episodes, or a nondisabling stroke, or arterial thromboembolism with organ loss, and (c) Tier III (moderately severe, 3 points per event) – pump malfunction due to pump thrombosis that leads to reoperation or (severe, 4 points per event) – a disabling stroke or death due to hemocompatibility-related causes.
TABLE 1.
Advanced hemodynamic variable equations
| Cardiac power output (W) | |
| Cardiac power efficiency (W*mm Hg/m2) | |
| Left ventricular stroke work index (g/m2/beat) | 13.6 * (cardiac index/heart rate) * (mean arterial pressure − pulmonary capillary wedge pressure) |
TABLE 2.
Baseline characteristics
| Low CPE (≤0.0554 W*mm Hg/m2) (N = 68) | High CPE (>0.0554 W*mm Hg/m2) (N = 23) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 62 (55, 71) | 60 (54, 68) | 0.602 |
| Female | 22 (32%) | 9 (39%) | 0.735 |
| Ethnicity | 0.364 | ||
| White | 37 (54%) | 13 (57%) | |
| Black/African merican | 27 (40%) | 9 (39%) | |
| Hispanic/Latino | 0 (0%) | 1 (4%) | |
| Asian | 2 (3%) | 0 (0%) | |
| Other | 2 (3%) | 0 (0%) | |
| BMI | 29.3 (24.0, 34.6) | 28.1 (24.1, 33.2) | 0.535 |
| Ischemic etiology | 24 (35%) | 11 (48%) | 0.412 |
| LVAD type | 0.341 | ||
| HVAD | 17 (25%) | 9 (39%) | |
| HMII | 41 (60%) | 10 (44%) | |
| HM3 | 10 (15%) | 4 (17%) | |
| Comorbidities | |||
| Diabetes mellitus | 28 (41%) | 7 (30%) | 0.504 |
| Atrial fibrillation | 20 (29%) | 10 (44%) | 0.325 |
| History of stroke | 6 (9%) | 4 (17%) | 0.453 |
| Hypertension | 39 (57%) | 16 (70%) | 0.430 |
| Chronic kidney disease | 23 (34%) | 7 (30%) | 0.966 |
| Medications at ramp study | |||
| Beta blocker | 43 (62%) | 18 (78%) | 0.285 |
| ACEi/ARB/ARNI | 39 (57%) | 19 (83%) | 0.054 |
| MRA | 34 (50%) | 12 (52%) | 1.000 |
| Diuretic | 58 (85%) | 19 (83%) | 1.000 |
| Anticoagulation | 66 (97%) | 23 (100%) | 0.993 |
| Time from implant to ramp study (days) | 259 (119, 698) | 142 (90, 275) | 0.038* |
| Baseline hemodynamics before ramp study | |||
| LVEDD (cm) | 6.2 ± 1.2 | 5.4 ± 0.71 | 0.002* |
| LVESD (cm) | 5.9 ± 1.2 | 5.0 ± 0.8 | 0.002* |
| Flow | 4.7 (4.2, 5.3) | 4.6 (4.3, 5.5) | 0.915 |
| CVP (mm Hg) | 10.0 (6.75, 15.0) | 5.0 (3.0, 7.0) | <0.001* |
| PCWP (mm Hg) | 16.6 ± 4.8 | 6.9 ± 2.8 | <0.001* |
| CI (L/min/m2) | 2.5 (2.1, 2.9) | 2.8 (2.6, 3.2) | 0.003* |
| MAP (mm Hg) | 87.4 ± 12.0 | 90.0 ± 11.4 | 0.361 |
| HR (bpm) | 80.7 ± 12.1 | 85.1 ± 9.1 | 0.115 |
| LVAD speed | |||
| Baseline LVAD speed (rpm) | |||
| HVAD | 2640 (2520, 2800) | 2660 (2560, 2860) | 0.516 |
| HMII | 9370 (9000, 9400) | 9000 (8845, 9200) | 0.063 |
| HM3 | 5200 (5100, 5200) | 5250 (5200, 5350) | 0.120 |
| Ramp LVAD set speed (rpm) | |||
| HVAD | 2800 (2610, 2840) | 2570 (215, 2695) | 0.217 |
| HMII | 9600 (9200, 9600) | 8990 (8800, 9000) | <0.001* |
| HM3 | 5300 (5300, 5400) | 5350 (5275, 5400) | 0.942 |
Abbreviations: BMI, body mass index; CI, cardiac index; CPE, cardiac power efficiency; CVP, central venous pressure; HMII, HeartMate II; HM3, HeartMate 3; HR, heart rate; HVAD, HeartWare assist device; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; PCWP, pulmonary capillary wedge pressure; MAP, mean arterial pressure; rmp, revolutions per minute.
2.3 |. Ramp test protocol
RHC was performed for patients presenting for hemodynamic and echocardiographic LVAD ramp studies. Intracardiac pressures were measured over a range of LVAD speeds as previously described.10 Hemodynamic parameters including right atrial pressure (RAP), right ventricular systolic and diastolic pressures, pulmonary artery systolic, diastolic, and mean pressures, and pulmonary capillary wedge pressure (PCWP) were measured. Cardiac output and index were calculated by the Fick method. Echocardiographic parameters including left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) were recorded, as described previously.11 The device was set to the speed that yielded optimal hemodynamic measurements, with RAP and PCWP closest to normal limits.
2.4 |. Statistical analysis
For the statistical analysis, IBM SPSS Statistics v. 26.0 (IBM, Armonk, NY) was used. Two-sided p values of <0.05 were considered statistically significant. Continuous data are reported as mean and SD if normally distributed or median and interquartile range if skewed. Continuous variables were compared using Mann–Whitney U test. Categorical variables are expressed as the number and percentage of patients and were compared using Fisher’s exact test. Univariate Cox Regression was performed to identify potential risk factors for adverse events and mortality. Univariate and multivariable logistic regression analyses were performed to determine the association between CPO, CPE, and LVSWI with primary endpoints of HRAE, HF admissions, and mortality. Multivariable analysis adjusted for LVAD type and pulmonary artery pulsatility index (PAPI), calculated as follows: ([PA systolic pressure – PA diastolic pressure]/RAP) was performed. Multivariable analysis was performed on variables with a p value of <0.05 to identify significant independent predictors. The proportional hazards assumption was verified using the Schoenfeld residuals.
3 |. RESULTS
3.1 |. Baseline characteristics
A total of 91 participants were included in the analysis. The average age at the time of procedure was 61 ± 11 years, 34% were women, 40% were Black or African American, and 38% had ischemic cardiomyopathy. There were 26 (29%) HVADs, 52 (57%) HMIIs, and 13 (14%) HM3s (Table 2). Both HMIIs and HVADs had lower freedom from HRAE compared with HM3s on Kaplan–Meier analysis (p = 0.027; Figure 1), with a hazard ratio of 4.09 (1.25–13.37, p = 0.020) for HMIIs and a hazard ratio of 4.61 (1.36–15.63, p = 0.014) for HVADs. Invasive hemodynamic ramp studies were performed between 2014 and 2018 with a minimum follow-up for HRAEs of 1 year.
FIGURE 1.
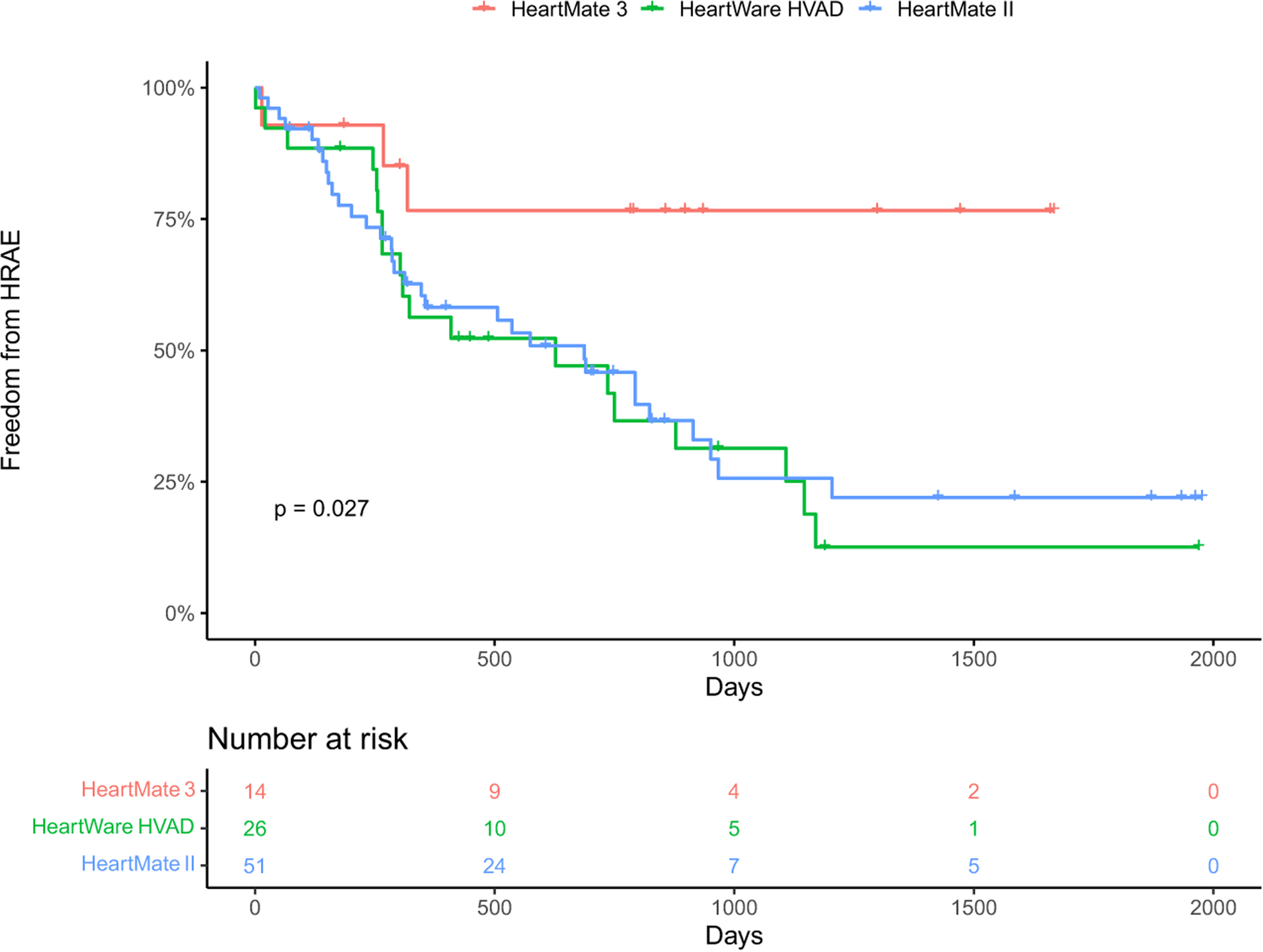
Kaplan–Meier analysis of freedom from hemocompatibility-related adverse events by left ventricular assist device type (HeartMate 3, HeartWare HVAD, and HeartMate II; p = 0.027).
3.2 |. Cardiac power output
ROC analysis yielded an optimal CPO cutoff point of 0.86 W. Low CPO (≤0.86 W) was not significantly associated with higher rates of HRAE (HR 1.28, 95% CI 0.69–2.40, p = 0.43) in univariate analysis, or in multivariable analysis after adjustment for PAPI and LVAD type. Low CPO was not significantly associated with mortality (HR 1.01, 95% CI 0.46–2.2, p = 0.98) or HF admission (HR 1.35, 95% CI 0.63–2.91, p = 0.44) in univariate analysis, or in multivariable analysis when adjusted for PAPI and LVAD type.
3.3 |. Cardiac power efficiency
ROC analysis yielded an optimal CPE cutoff point of 0.055. Low CPE (≤0.0554 W*mm Hg/m2) was not significantly associated with HRAE (HR 0.85, 95% CI 0.48–1.51, p = 0.58) in univariate analysis, or in multivariable analysis when adjusted for PAPI and LVAD type. There was a significant association between low CPE and mortality (HR 2.42, 95% CI 1.02–5.74, p = 0.045) in univariate analysis. After adjusting for PAPI and LVAD in multivariable analysis, there was no significant association. Low CPE had significantly higher mortality than high CPE on Kaplan–Meier analysis (p = 0.04; Figure 2). Low CPE was not associated with HF admissions (HR 1.58, 95% CI 0.69–3.65, p = 0.01) in univariate analysis, however, when adjusted for LVAD type in multivariable analysis, low CPE was associated with HF admissions in HM3 (HR 0.12, 95% CI 0.01–0.91, p = 0.04) but not HMIIs or HVADs.
FIGURE 2.
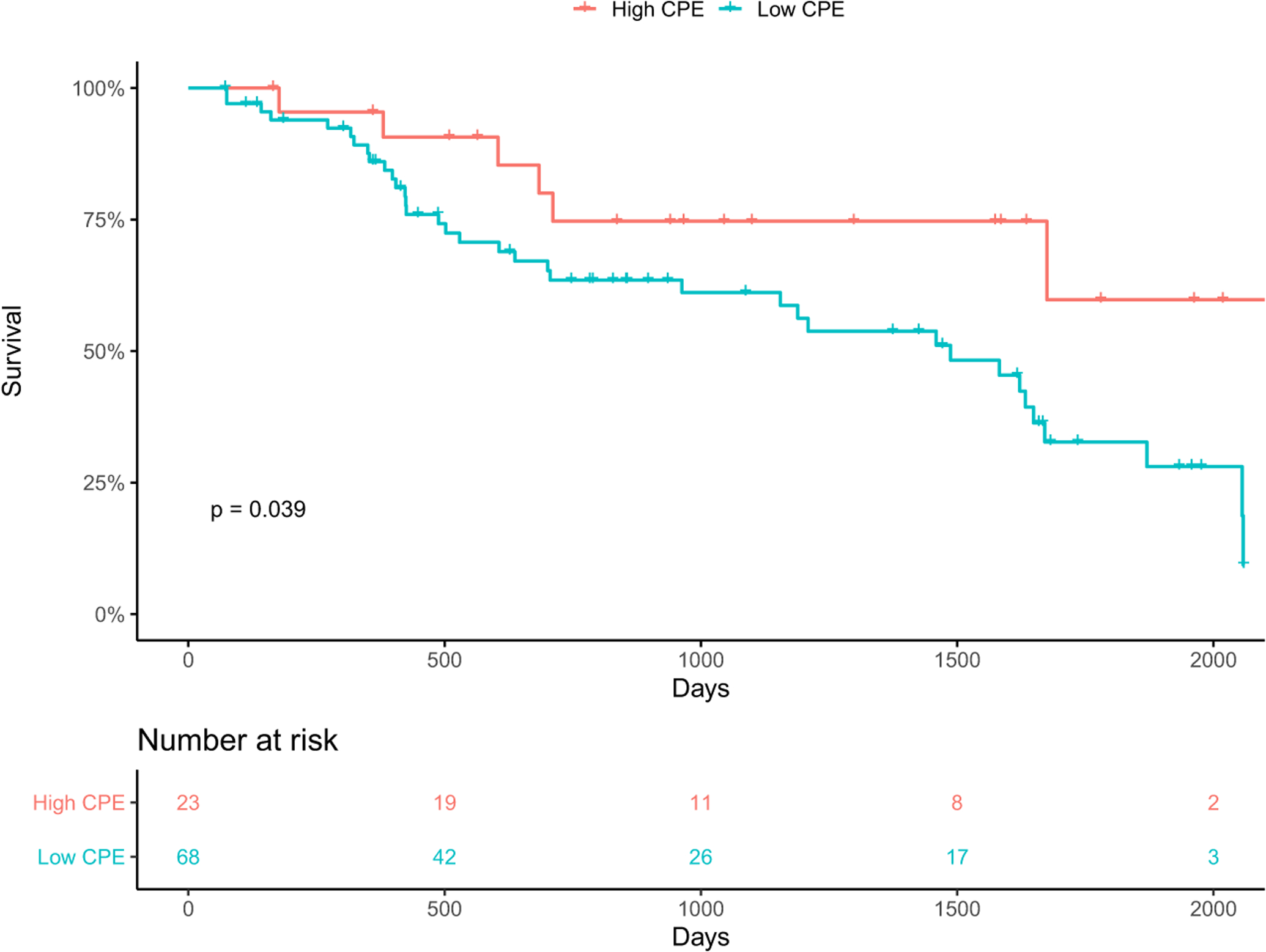
Kaplan–Meier analysis of cardiac power efficiency (CPE) and mortality. Low CPE (≤0.055 W*mm Hg/m2) had significantly higher mortality than high CPE (p = 0.04).
3.4 |. Left ventricular stroke work index
ROC analysis yielded an optimal LVSWI cutoff point of 33.56 g/m2/beat. Low LVSWI (≤33.56 g/m2/beat) was not significantly associated with HRAE (HR 1.06, 95% CI 0.62–1.82, p = 0.83) in univariate analysis, or in multivariable analysis when adjusted for PAPI and LVAD type. Low LVSWI was significantly associated with mortality (HR 2.13, 95% CI 1.09–4.17, p = 0.03) in univariate analysis. However, after adjusting for PAPI and LVAD in multivariable analysis, there was no significant association. Low LVSWI had significantly higher mortality than high LVSWI on Kaplan–Meier analysis (p = 0.02; Figure 3). Low LVSWI was not significantly associated with HF admission (HR 1.75, 95% CI 0.86–3.57, p = 0.13) in univariate analysis, however when adjusted for LVAD type in multivariable analysis, low LVSWI was associated with HF admissions in HM3 (HR 0.10, 95% CI 0.01–0.78, p = 0.03) but not HMIIs or HVADs.
FIGURE 3.
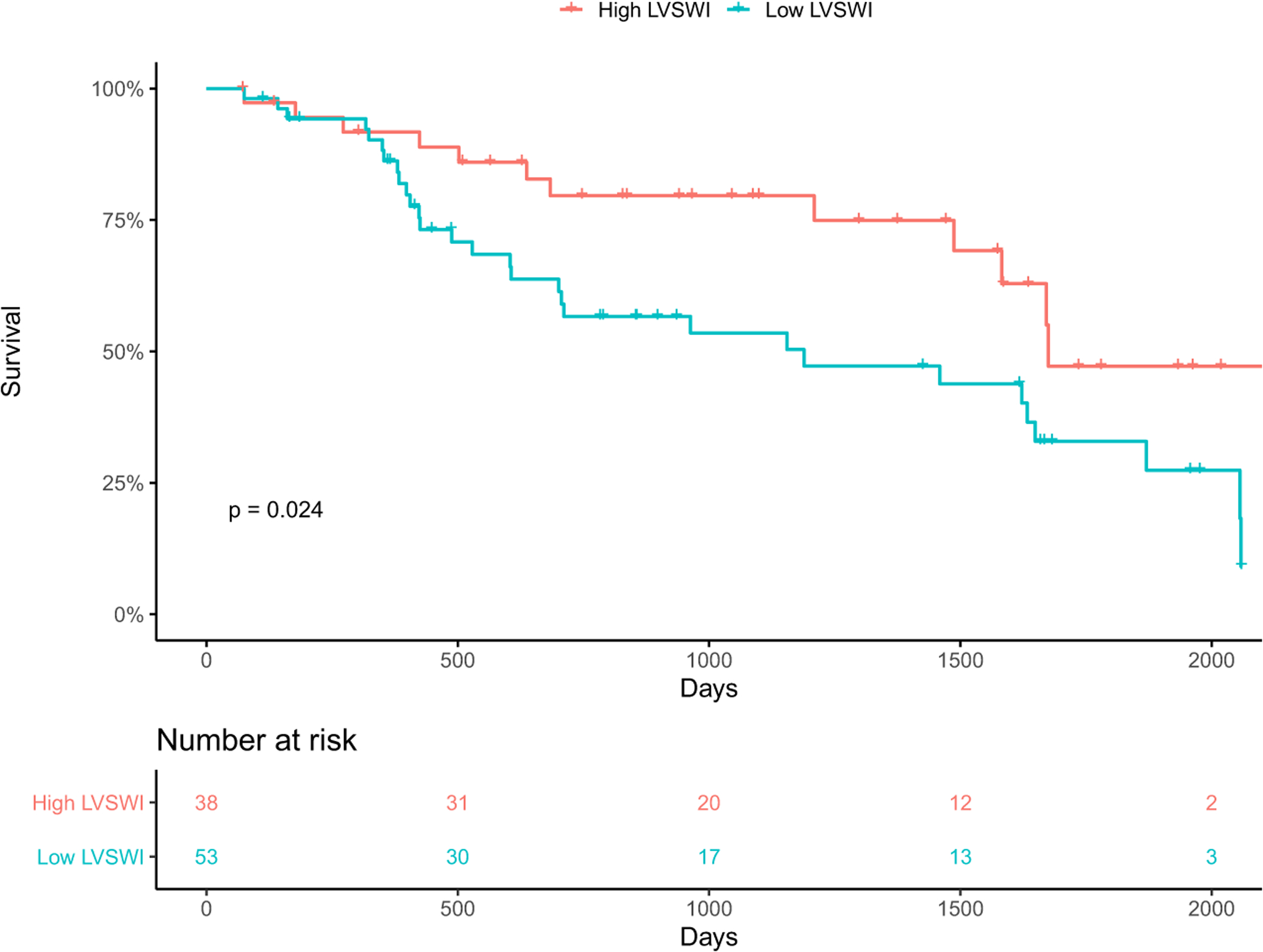
Kaplan–Meier analysis of left ventricular stroke work index (LVSWI) and mortality. Low LVSWI (≤33.56 g/m2/beat) had significantly higher mortality than high LVSWI (p = 0.02).
4 |. DISCUSSION
Invasive hemodynamics has had an extensive role in risk stratification and prognostication of patients with advanced HF. Yet, its prognostic role has been limited in patients with LVADs. This analysis presents evidence for additional risk stratification using these advanced hemodynamic variables in patients with LVAD therapy. Our study demonstrates that (1) low CPE is associated with higher mortality, (2) low LVSWI is also associated with higher mortality, and (3) CPO, CPE, and LVSWI are not associated with HF admissions or HRAEs in our cohort.
Our analysis exploring advanced hemodynamic variables revealed that low CPE and low LVSWI, both surrogates of cardiac pump performance, were significantly associated with higher mortality. This coincides with our hypothesis that a decrease in native LV contribution will lead to worse outcomes, despite LVAD therapy. Studies have shown that there is an association between reverse cardiac remodeling and neurohormonal blockade with guideline-directed medical therapy and improved outcomes (morbidity and mortality) when used in the background of LVAD therapy.12,13 This supports the concept that native left ventricular function is crucial to improving morbidity in patients with LVADs. Prognostic markers like CPE and LVSWI can help determine which patients need optimization of their LVADs, via either medical therapy or hemodynamic optimization, to augment their native cardiac function.
HRAEs are the chief drivers of morbidity in patients with LVADs. These events, manifesting as nonsurgical bleeding, thromboembolism, neurological events, and death, are related to the interaction between the patient and their pump. In his editorial, Mehra notes one of the five key mechanisms for HRAEs in LVADs was a low pulsatile state. Low pulsatile states were associated with higher angiogenic factors, elevated muscle sympathetic nerve activity, and increased vascular stiffness.9 Also, two recent studies by Imamura et al. found that optimized right- and left-sided filling pressures (i.e., CVP < 12 mm Hg, PCWP <18 mm Hg) along with higher cardiac function (cardiac index >2.2 L/min/m2) was associated with fewer HRAEs and HF readmissions.14,15 Based on these findings, we would have expected to find a significant association between these advanced hemodynamic metrics, which represent both cardiac filling pressures and function, and HRAEs in this study population. However, it is possible this null result was due to the hemodynamic optimization that these patients underwent in the RAMP study, during which pump–patient interaction was optimized and HRAEs and HF admissions were likely reduced.14,15
Furthermore, given the inherent physiological differences between the three LVADs, the effects on HRAEs are different between pumps. Our results demonstrated that both HMIIs and HVADs had lower freedom from HRAE compared with HM3s, consistent with other studies. For example, HM3s (fully magnetically levitated centrifugal flow devices) are known to have fewer HRAEs such as pump thrombosis and stroke, than HMIIs (axial continuous flow pumps) as seen in the MOMENTUM 3 trial.8 This is likely due to the different pump mechanisms: (a) HMII – axial flow, mechanical bearing, with restrictive side gaps, whereas (b) HM3 – centrifugal flow, fully magnetically levitated, with permissive side gaps.9 Separately, HVADs are centrifugal flow, partially magnetic with hydrodynamic lift, and restrictive side gaps. All three pumps have similarly high rates of gastrointestinal bleeding rates. While our current understanding of the pump–patient interaction is limited, new research with computational fluid dynamics allows us to simulate the unique flow patterns between the different pumps that influence blood stagnation, shear stress, and platelet activation.16 Computational fluid dynamics can provide an immense amount of insight into HRAEs with the current LVADs as well as future devices. Due to the small sample size of each pump in our study, potential significant findings for HRAEs may have been obscured.
4.1 |. Limitations
The study utilizes data obtained from baseline hemodynamics prior to a ramp study, where all patients received a similar intervention of optimizing hemodynamics by changing LVAD speeds. Our study includes clinically stable patients with LVADs at a single center and may not be generalizable to all patients on LVAD therapy. Last, our analysis includes a mix of three LVAD systems with varying hemocompatibility profiles complicating the uniformity of our data. There was limited power to detect differences by LVAD type and a multi-center prospective study is needed, however, future prospective studies will only have LVAD therapy with HM3 models, which supports the importance of a retrospective study on older generation LVAD models.
5 |. CONCLUSION
The use of advanced hemodynamic variables can serve as prognostic indicators for patients with LVADs. Low CPE and LVSWI are prognostic for higher mortality, but no advanced hemodynamic metric was associated with HF admissions or HRAEs.
Footnotes
CONFLICT OF INTEREST
Dr. Salerno is a consultant to Medtronic and Abbott; educator for Abbott. Dr. Jeevanandam is an advisory board member for Abbott. Dr. Pinney is a consultant to Abbott, Medtronic, and Procyrion. Dr. Grinstein is a speaker for Abbott. All other authors have no relevant disclosures.
REFERENCES
- 1.Coordinators* TEIaES. Evaluation study of congestive heart failure and pulmonary artery catheterization Effectiveness The ESCAPE trial. JAMA. 2005;294:1625–33. [DOI] [PubMed] [Google Scholar]
- 2.Furer A, Wessler J, Burkhoff D. Hemodynamics of cardiogenic shock. Interv Cardiol Clin. 2017;6:359–71. [DOI] [PubMed] [Google Scholar]
- 3.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340–8. [DOI] [PubMed] [Google Scholar]
- 4.Symalla T, Narang N, Jeevanandam V. Cardiac power efficiency as a hemodynamic predictor of outcomes in congestive heart failure. J Heart Lung Transplant. 2020;39:S53. [Google Scholar]
- 5.Jentzer JC, Anavekar NS, Burstein BJ, Borlaug BA, Oh JK. Noninvasive echocardiographic left ventricular stroke work index predicts mortality in cardiac intensive care unit patients. Circ Cardiovasc Imaging. 2020;13:e011642. [DOI] [PubMed] [Google Scholar]
- 6.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–60. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device — final report. N Engl J Med. 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- 8.Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135:2003–12. [DOI] [PubMed] [Google Scholar]
- 9.Mehra MR. The burden of haemocompatibility with left ventricular assist systems: a complex weave. Eur Heart J. 2019;40:673–7. [DOI] [PubMed] [Google Scholar]
- 10.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, et al. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–17. [DOI] [PubMed] [Google Scholar]
- 11.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullough M, Caraballo C, Ravindra NG, Miller PE, Mezzacappa C, Levin A, et al. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol. 2020;5:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah P, Psotka M, Taleb I, Alharethi R, Shams MA, Wever-Pinzon O, et al. Framework to classify reverse cardiac remodeling with mechanical circulatory support: the Utah-Inova stages. Circ Heart Fail. 2021;14:e007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura T, Nguyen A, Kim G, Raikhelkar J, Sarswat N, Kalantari S, et al. Optimal haemodynamics during left ventricular assist device support are associated with reduced haemocompatibility-related adverse events. Eur J Heart Fail. 2019;21:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura T, Jeevanandam V, Kim G, Raikhelkar J, Sarswat N, Kalantari S, et al. Optimal hemodynamics during left ventricular assist device support are associated with reduced readmission rates. Circ Heart Fail. 2019;12:e005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinstein J, Torii R, Bourantas CV, Garcia-Garcia HM. Left ventricular assist device flow pattern analysis using a novel model incorporating left ventricular Pulsatility. ASAIO J. 2021;67:724–32. [DOI] [PubMed] [Google Scholar]


