Abstract
Children with sickle cell disease commonly experience vaso-occlusive pain episodes due to sickling of erythrocytes, which often requires care in the emergency department. Our objective was to assess the use and impact of intranasal fentanyl for the treatment of children with SCD-VOE on discharge from the emergency department in a multicenter study. We conducted a cross-sectional study at 20 academic pediatric emergency departments in the United States and Canada. We used logistic regression to test bivariable and multivariable associations between the outcome of discharge from the emergency department and candidate variables theoretically associated with discharge. The study included 400 patients; 215 (54%) were female. The median age was 14.6 (interquartile range 9.8, 17.6) years. Nineteen percent (n=75) received intranasal fentanyl in the emergency department. Children who received intranasal fentanyl had nearly nine-fold greater adjusted odds of discharge from the emergency department compared to those who did not (adjusted odds ratio 8.99, 95% CI 2.81-30.56, P<0.001). The rapid onset of action and ease of delivery without IV access offered by intranasal fentanyl make it a feasible initial parenteral analgesic in the treatment of children with sickle cell disease presenting with vaso-occlusive pain episodes in the acute-care setting. Further study is needed to determine potential causality of the association between intranasal fentanyl and discharge from the emergency department observed in this multicenter study.
Keywords: sickle cell disease, children; vaso-occlusive pain crisis, intranasal fentanyl
Introduction
Multiple novel therapies have failed to demonstrate efficacy in reducing pain from sickle cell disease (SCD) vaso-occlusive episodes (VOE),1-3 making opioid medications the primary therapy for moderate-to-severe pain. SCD-VOE is a medical emergency and helping patients achieve timely relief from VOE-related pain is paramount. Results from one study suggest that timely administration of intravenous (IV) opioids for SCD-VOE may be associated with reduced odds of hospital admission from the emergency department (ED),4 though other studies have not shown the same association.5
Intranasal fentanyl is a safe and effective analgesic in children6-8 that may circumvent challenges associated with the administration of other initial parenteral opioids9 and may reduce the time to administration of initial opioid treatment among children presenting to the ED with pain.10-12 Furthermore, intranasal fentanyl has been associated with shorter times to determining a patient’s disposition (i.e., discharge vs. hospital admission) and shorter overall ED length of stay.13 Prior single-center studies suggest a potential association between intranasal fentanyl administration and discharge from the ED.10,12
A multicenter study assessing the impact of intranasal fentanyl on patient disposition among children presenting with SCD-VOE is lacking but has the potential to inform an important outcome for children and potentially change practice. To this end, our objective was to assess the use and impact of intranasal fentanyl for the treatment of children with SCD-VOE on patient disposition in a multicenter study in academic pediatric EDs.
Methods
Study Design
We conducted a planned secondary analysis of a cross-sectional study which was conducted as an aim of an R34 Clinical Trials Planning Grant (NHLBI R34-HL122557). Clinical charts of encounters for SCD-VOE were reviewed at 20 academic pediatric EDs in the United States and Canada from 2015 to 2016. After training to establish uniformity in the approach to data extraction at each site, investigators at each site reviewed charts of 20 consecutive children aged 3-21 years who presented with SCD-VOE.
Study Setting
This study was conducted at 20 academic pediatric EDs in the United States (n=19) and Canada (n=1). Fourteen of the EDs included in this study were part of the Pediatric Emergency Care Applied Research Network (PECARN). The annual volume for patients with SCD-VOE at these EDs ranged from 80-700.5
Inclusion and Exclusion Criteria
In order to include children with more severe forms of SCD, those with hemoglobin SS disease or hemoglobin Sβ° Thalassemia who presented to one of the study EDs for VOE and received treatment for pain were included. We included children and adolescents aged 3-21 years. No exclusion was made for children with SCD who presented with fever if the patient was treated for VOE. Intranasal fentanyl is available at a concentration of 50 mcg/ml and was delivered via atomizer at a maximum of 1 ml to each nostril (maximum 100 mcg). Its use is contraindicated if the patient has a current upper respiratory tract infection, if there is concern for stroke, altered state of consciousness, or any form of head injury. We excluded encounters for acute chest syndrome.
Variables
Our primary outcome was discharge to home from the ED. We extracted the following variables from each patient’s chart: age, sex, hemoglobin genotype in the electronic medical record, time of ED presentation, opioids administered in the ED, dose and route of administered opioids (mg/kg and mg/kg/hour of morphine equivalents), time of opioid administration, non-steroidal anti-inflammatory drugs (NSAIDs) administered in the ED, use of IV fluids (i.e., bolus and maintenance fluids), time of day the patient presented to the ED, time from ED arrival or triage to the administration of the first parenteral opioid (and second parenteral opioid in patients receiving >1 dose), and patient disposition from the ED (i.e., discharge vs. hospital admission).
Statistical Analyses
We calculated descriptive statistics for patient demographics, therapies provided in the ED, and patient disposition from the ED. We conducted an exploratory analysis using logistic regression to test bivariable associations between the outcomes of discharge from the ED and candidate variables theoretically associated with ED discharge as well as time from ED presentation to opioid administration. Candidate variables included patient age, sex, time from triage to first parenteral opioid administration, intranasal fentanyl use, dose of total parenteral opioid morphine equivalents, ketorolac use, administration of oral opioids, administration of bolus IV fluids, time of day of ED presentation, and site. All associations with an overall P value <0.1 were included in a multivariable model to determine variables associated with ED discharge. Since we found higher odds of discharge from the ED among children with SCD who received intranasal fentanyl in our primary model, we conducted post hoc sensitivity analyses restricted to sites that used intranasal fentanyl regularly. A P value ≤0.05 was considered statistically significant. We conducted all analyses using SAS (version 9.4).
Results
The study included 400 patients, of whom 215 (54%) were female. The median age was 14.6 (interquartile range [IQR] 9.8, 17.6) years, 92% (n=367) had hemoglobin SS disease, and 8% (n=33) had hemoglobin Sβ° Thalassemia. The overall hospital admission rate was 67% (n=268). Hospital admission rates ranged from 45-90% across sites.
Therapies Administered
Of the 400 included patients, 91% (n=365) received IV opioids, 66% (n=265) received IV ketorolac, 19% (n=75) received intranasal fentanyl, and 25% (n=98) received oral opioids. Eighty four percent (n=335) received IV fluids (66%, n=263 received IV fluids in the form of a bolus and 44%, n=174 received them in the form of maintenance fluids).15
Intranasal fentanyl was available at 75% of sites (n=15/20) and was used at 50% of the sites (n=10/20) in the included population. Among the 75 children who received intranasal fentanyl, 69% (n=52) also received IV opioids including morphine and hydromorphone. Children who received intranasal fentanyl received a mean total IV opioid dose of 0.2 mg/kg compared to 0.2 mg/kg in those who did not receive intranasal fentanyl (P=0.069). There was no difference in the mean change in pain scores among children who received intranasal fentanyl (mean −2.2, SD 2.54) compared to those who did not (mean −2.5, SD 3.28) (P=0.68). Children who received intranasal fentanyl received higher overall total parenteral opioid morphine equivalents (mean 0.36 mg/kg, SD 0.14) than those who did not (mean 0.22 mg/kg, SD 0.25) (P<0.001). However, there was no difference in the mean total IV opioid morphine equivalents administered to those who received intranasal fentanyl (0.2 mg/kg, SD 0.12) compared to those who did not (0.2 mg/kg, SD 0.25) (P=0.07). Among children who received parenteral opioids ≤60 minutes from ED arrival 34% (n=65) received intranasal fentanyl and the other 66% (n=123) received IV opioids including morphine and hydromorphone.
Time from ED Presentation to Parenteral Opioid Administration
Children who received intranasal fentanyl (n=75) were more likely to receive their first parenteral opioid ≤30 minutes after presentation to the ED compared to children who did not receive intranasal fentanyl (OR 9.38, 95% CI 5.22-16.83, P<0.001). Similarly, children who received intranasal fentanyl were more likely to receive their first parenteral opioid ≤60 minutes after presentation to the ED compared to children who did not receive intranasal fentanyl (OR 9.83, 95% CI 4.87-19.85, P<0.001).
When restricted to the 10 sites that used intranasal fentanyl, the same patterns emerged of greater likelihood of receipt of parenteral opioids ≤30 minutes after presentation to the ED compared to children who did not receive intranasal fentanyl (OR 15.02, 95% CI 6.44-35.05, P<0.001). Additionally, children who received intranasal fentanyl were more likely to receive their first parenteral opioid ≤60 minutes after presentation to the ED (OR 9.13, 95% CI 4.29-19.41, P<0.001) compared to children who did not receive intranasal fentanyl at the 10 sites that used intranasal fentanyl.
Factors Associated with Discharge from the ED
Univariable and multivariable analyses of factors associated with discharge from the ED after presentation with SCD-VOE are summarized in Table 1. In unadjusted analyses, children aged ≥12 years, those who had smaller decrease in mean pain scores in the ED, those with higher final pain scores, those who received the first parenteral opioid >60 minutes, those who received a higher mean dose of morphine equivalents, and those who received bolus IV fluids had lower odds of discharge from the ED (Table 1). Children who received intranasal fentanyl or oral opioids had greater odds of ED discharge than those who did not in unadjusted analyses.
Table 1.
Factors associated with emergency department discharge among children with sickle cell disease presenting with vaso-occlusive pain episodes.*
| Discharged (N=129), n (%) |
Admitted (N=268), n (%) |
Odds Ratio (95% CI) |
P value | Adjusted Odds Ratio (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤12 years | 53 (41.1%) | 83 (31.0%) | Referent | 0.048 | Referent | 0.327 |
| >12 years | 76 (58.9%) | 185 (69.0%) | 0.64 (0.42, 1.00) | 1.44 (0.70, 3.07) | ||
| Sex | ||||||
| Male | 62 (48.1%) | 120 (44.8%) | Referent | 0.539 | ||
| Female | 67 (51.9%) | 148 (55.2%) | 0.88 (0.57, 1.34) | |||
| Presenting pain score, mean (SD) 1 | 7.7 (2.11) | 8.1 (2.16) | 0.91 (0.83, 1.00) | 0.062 | 0.71 (0.60, 0.83) | <0.001 |
| Change in pain score, mean (SD) 1 | −4.0 (3.04) | −1.7 (2.95) | 0.79 (0.73, 0.85) | <0.001 | 0.64 (0.55, 0.72) | <0.001 |
| Final pain score, mean (SD) 1 ** | 3.7 (3.04) | 6.4 (3.01) | 0.76 (0.71, 0.82) | <0.001 | ||
| Intranasal fentanyl administered | 38 (29.5%) | 36 (13.4%) | 2.69 (1.61, 4.52) | <0.001 | 8.99 (2.81, 30.56) | <0.001 |
| Time from ED arrival to first parenteral opioid 2 | ||||||
| ≤60 minutes | 68 (52.7%) | 120 (44.8%) | Referent | 0.046 | Referent | 0.377 |
| >60 minutes | 53 (41.1%) | 145 (54.1%) | 0.65 (0.42, 0.99) | 0.71 (0.33, 1.51) | ||
| Time from ED arrival to first IV opioid 3 | ||||||
| ≤60 minutes | 37 (28.7%) | 96 (35.8%) | Referent | 0.878 | ||
| >60 minutes | 62 (48.1%) | 167 (62.3%) | 0.96 (0.60, 1.56) | |||
| Time from first to second IV opioid 4 | ||||||
| ≤60 minutes | 18 (14.0%) | 89 (33.2%) | Referent | 0.258 | ||
| >60 minutes | 34 (26.4%) | 117 (43.7%) | 1.44 (0.77, 2.75) | |||
| Total parenteral opioid morphine equivalents (mg/kg), mean (SD) 5 | 0.18 (0.13) | 0.28 (0.27) | 0.97 (0.95, 0.98) | <0.001 | 0.93 (0.90, 0.96) | <0.001 |
| Total parenteral opioid morphine equivalents (mg/kg/hr), mean (SD) | 0.05 (0.04) | 0.06 (0.07) | 0.97 (0.92, 1.01) | 0.114 | ||
| Ketorolac administered | 81 (62.8%) | 182 (67.9%) | 0.80 (0.51, 1.24) | 0.314 | ||
| Oral opioids administered | 46 (35.7%) | 52 (19.4%) | 2.30 (1.44, 3.69) | <0.001 | 3.03 (1.43, 6.60) | 0.004 |
| Fever present (defined as ≥ 38C) | 13 (10.0%) | 28 (10.4%) | 0.96 (0.47, 1.89) | 0.909 | ||
| Bolus IV fluids administered | 73 (56.6%) | 187 (69.8%) | 0.56 (0.37, 0.87) | 0.010 | 0.75 (0.33, 1.65) | 0.470 |
| Time of day for ED presentation | ||||||
| Day shift | 56 (43.4%) | 107 (39.9%) | 0.91 (0.51, 1.63) | |||
| Evening shift | 47 (36.4%) | 116 (43.3%) | 0.70 (0.39, 1.27) | |||
| Overnight shift | 26 (20.2%) | 45 (16.8%) | Referent | 0.404 |
Excluding 5 patients who did not have documented initial pain score.
Excluding 11 patients who did not receive parenteral opioids.
Excluding 35 patients who did not receive IV opioids.
Limited to patients who received a second IV opioid.
Limited to patients with documented parenteral opioid dosages.
All analyses were adjusted for site. Three children were excluded from the regression analyses because they were not discharged or admitted (i.e., 2 were transferred elsewhere and 1 left against medical advice).
Final pain score was left out of the multivariable model since it is a linear combination of first pain score and change in pain score.
The adjusted model demonstrated that children with SCD-VOE who presented with lower mean pain scores, those who had greater decrease in mean pain score in the ED, and those who received lower mean total morphine equivalents were more likely to be discharged from the ED.
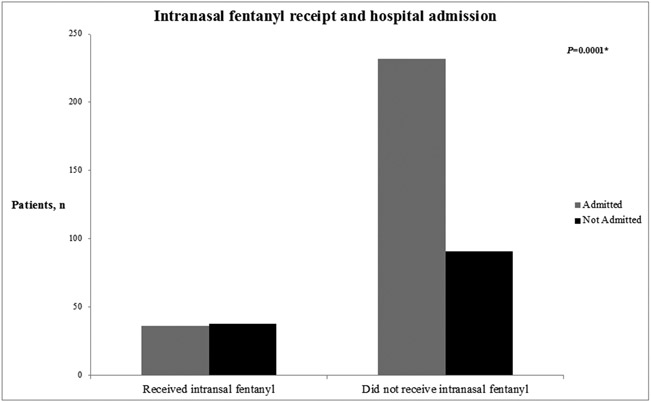
Children who received intranasal fentanyl were less likely to be admitted (Figure 1) and had nearly nine-fold greater adjusted odds of discharge from the ED compared to those who did not. Additionally, children who received oral opioids had three-fold greater adjusted odds of discharge from the ED than those who did not. The same associations between intranasal fentanyl and oral opioids were demonstrated in a sensitivity analysis restricted to sites that used intranasal fentanyl regularly (Supplemental Table 1). In fact, children who received intranasal fentanyl at sites that used intranasal fentanyl had nearly 40-fold greater odds of discharge from the ED compared to those who did not at these sites (aOR 39.25, 95% CI 7.23-271.63, P<0.001).
Figure 1.
Number of children requiring admission from the emergency department based on the receipt of intranasal fentanyl (INF) for the treatment of sickle cell disease vaso-occlusive pain crisis.
*Chi square test
The median ED length of stay from arrival to disposition order placement was 3 hours (IQR 2, 5) and there was no difference among children treated with intranasal fentanyl (median 4 hours, IQR 2, 6) and those who did not receive intranasal fentanyl (median 3 hours, IQR 2, 5) (P=0.554). The median ED length of stay did not differ between institutions that used INF (median 3 hours, IQR 2, 5) and those that did not (median 3 hours, IQR 2, 6) (P=0.790).
Discussion
Our multicenter study including 20 academic, pediatric EDs demonstrated an association between the administration of intranasal fentanyl and discharge from the ED among children with SCD who presented to the ED with VOE. Additionally, children who had lower mean presenting pain scores, greater decrease in pain scores during the ED visit, and who received lower overall morphine equivalent dosages of opioids had greater odds of discharge from the ED. Children with SCD who received oral opioids had greater odds of discharge from the ED than those who did not.
Children with SCD who received intranasal fentanyl had the highest odds of discharge from the ED when adjusting for pain scores during the ED visit as well as other key factors. The odds of ED discharge after intranasal fentanyl administration were nearly 40-fold greater when excluding sites that did not use intranasal fentanyl. Our findings support the observation by Kavanagh and colleagues that demonstrated a 32% to 48% increase in the proportion of children with SCD-VOE who were discharged from the ED in a quality improvement initiative that focused on an initial dose of intranasal fentanyl, standardizing care, implementing a pain medication calculator, and education of providers and caregivers.12 Akinsola and colleagues found a significantly higher discharge rate in patients receiving intranasal fentanyl compared to those receiving IV opioids only (48% vs. 71%, P=0.004) in a prospective quality improvement initiative,10 while results from other single center studies have differed.4,16 It is possible that the other interventions in the study by Kavanagh et al. may have also contributed to greater rates of ED discharge and not the intranasal fentanyl alone. However, the use of a population from 20 sites in the United States and Canada adds further evidence in support of the use of intranasal fentanyl. Using multiple sites supersedes confounders present in prior single-site studies. A randomized controlled trial to establish the potential impact of intranasal fentanyl on discharge from the ED is warranted. A protocol paper for such a trial was published in 2012, but the results have yet to be reported.17 Moreover, further studies are needed to elucidate potential mechanisms that may lead to greater rates of ED discharge among children with SCD-VOE treated with intranasal fentanyl.
Despite guidelines from the National Heart, Lung, and Blood Institute (NHLBI) recommending parenteral opioids be administered in ≤30 minutes from triage or ≤60 minutes from registration in the ED,18 prior studies have shown significant delays in the administration of IV opioid medications to children presenting to the ED with SCD-VOE.5,19-21 While the timing of opioid administration is considered a quality indicator for pain episodes in SCD,20 ED crowding, staffing shortages, and delays in obtaining IV access may contribute to delays in administering IV opioids to children with SCD-VOE.22,23 We have previously reported a median time to IV placement from ED arrival of 52 minutes (IQR 33, 81),5 which represents an important bottleneck to rapid delivery of IV opioids. This report adds to an expanding list of studies demonstrating that the use of intranasal fentanyl improves time to analgesic administration for patients with SCD-VOE.5,10-14 Our results suggest that the use of intranasal fentanyl may be an excellent approach to rapidly treat for SCD-VOE pain as recommended by recent NHLBI, American College of Emergency Physicians, and American Society for Hematology guidelines.18,24,25 Additionally, intranasal fentanyl has rapid onset of action, is rapidly absorbed via the nasal mucosa, and its analgesic properties have efficacy similar to intravenous opioids.6 Our results suggest a beneficial aspect of intranasal fentanyl therapy not related to rapid delivery of pain medication. Given common delays that have been demonstrated in the time to placement of an intravenous line for patients with SCD-VOE,5,26 intranasal fentanyl may be a good first-line parenteral opioid. Early studies utilizing delivery of longer acting opioids including diamorphine and hydromorphone are promising and warrant further investigation.27,28
Children who presented with lower initial pain scores, who had larger decreases in their pain scores during their ED visit, and those who received lower overall morphine equivalent dosages of opioids had higher odds of ED discharge. These may reflect less severe pain episodes as reported by children and their caregivers through pain scores. The higher overall morphine equivalent dosages of opioids in children requiring admission likely reflect the need for ongoing pain management in the inpatient setting. Though the administration of intranasal fentanyl was most strongly associated with discharge from the ED in our study, children who received oral opioids before delivery of their parenteral opioids also had greater odds of ED discharge than those who did not receive oral opioids. This association between oral opioid administration and ED discharge remained when adjusting for pain severity. Though not measured in this study, this may have been due to patients who did not receive sufficient oral opioids at home to manage VOE, since oral opioids are often given in the ED to patients who have not received an oral opioid within four hours of presentation. This is another area to target when educating families about outpatient pain management.
Limitations
This retrospective study of 400 pediatric patients with SCD is subject to several limitations. First, these data were collected between 2015 and 2016 and may not account for possible secular trends in the emergent management of SCD-VOE for children, although there have not been significant changes to ED care for VOE in children over this timeframe that would significantly confound or alter these results. Children who presented with less pain may have been preferentially given intranasal fentanyl, which may have impacted the association we observed with ED discharge. However, sites with an intranasal protocol would offer intranasal fentanyl to all patients with moderate-to-severe pain. We did not, however, assess reasons that patients or caregivers refused intranasal fentanyl. Future studies assessing potential barriers to use of intranasal fentanyl from the perspective of patients and caregivers are warranted. We also adjusted for initial pain scores and changes in pain scores in our multivariable model. There is also variation in the use of intranasal fentanyl across the sites included in our study, which may have impacted our findings. However, our sensitivity analyses restricted to those sites that used intranasal fentanyl, though limited to a relatively small numbers of outcomes, demonstrated the same association with greater odds of discharge from the ED. Additionally, our results are limited to children who had hemoglobin SS disease or hemoglobin Sβ° Thalassemia and thus may not apply to all genotypes for SCD. Our findings may be limited to the experience of academic pediatric EDs and may not be generalizable to non-academic centers. Since intranasal fentanyl was associated strongly with opioid administration in <60 minutes as the first parenteral opioid, INF could be simply a marker of cases that occurred when the ED was less busy and more able to focus on delivering SCD pain management. We did not account for any opioids that may have been taken prior to ED presentation, which may have influenced our findings. However, those receiving oral opioids prior to ED arrival would not be treated with oral opioids upon ED arrival. Although this study included children and young adults up to 21 years old, the efficacy of its administration in older adults cannot be extrapolated from this study as dosing volume restricted to 1 ml per nare may limit its benefits in adult populations. Finally, causality of intranasal fentanyl use on ED discharge rates cannot be shown by this non-randomized retrospective study and would require a prospective randomized, controlled trial.
Conclusions
Children with SCD who received intranasal fentanyl for VOE had greater odds of being discharged from the ED than those who did not receive it. The rapid onset of action and ease of delivery without IV access offered by intranasal fentanyl make it a feasible initial parenteral analgesic in the treatment of children with SCD presenting with VOE in the acute care setting. Further study is needed to determine potential causality of the association between intranasal fentanyl and discharge from the ED observed in this multicenter observational study.
Supplementary Material
Acknowledgements
We would like to thank the patients and their families and the emergency department staff who provided the clinical care provided to the patients included in this study. Drs. Morris and Casper and Rachel Richards had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the data collection. Dr. Casper and Ms. Richards were responsible for all statistical analyses. Drs. Rees and Morris were responsible for the manuscript preparation. All authors assisted with data interpretation and revised and critically reviewed the manuscript. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS, or the U.S. Government.
Funding Statement
This study was supported by the NIH/NHLBI under Award Number R34HL122557 (to CRM), and in part by NIH/NCCIH K24AT009893 (to CRM) and the Pediatric Emergency Care Applied Research Network (PECARN), supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS), in the Maternal and Child Health Bureau (MCHB), under the Emergency Medical Services for Children (EMSC) program through the following cooperative agreements: DCC-University of Utah, GLEMSCRN-Nationwide Children’s Hospital, HOMERUN-Cincinnati Children’s Hospital Medical Center, PEMNEWS-Columbia University Medical Center, PRIME-University of California at Davis Medical Center, CHaMP node-State University of New York at Buffalo, WPEMR-Seattle Children's Hospital, and SPARC-Rhode Island Hospital/Hasbro Children's Hospital. NB received funding from the NIH/NHLBI under award number 1K23HL140142 and 1K23HL140142-03S1, from the Doris Duke Charitable Foundation COVID19 Fund to Retain Clinical Scientists-PeRSEVERE Program at Emory University School of Medicine, and the Georgia Clinical and Translational Science Alliance under award UL1-TR002378. The funders had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation, review, approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosure: No authors have potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of their manuscript.
Ethics Approval Statement: This study was approved by the institutional review boards at each participating center prior to data collection.
Patient Consent Statement: A waiver of consent was granted because all data were retrospectively extracted.
Data Availability Statement
The data included in this study may be made available upon reasonable request to the corresponding author.
References
- 1.Gladwin MT, Kato GJ, Weiner D, et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 2011;305:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brousseau DC, Scott JP, Badaki-Makun O, et al. A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children. Blood 2015;126:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casella JF, Barton BA, Kanter J, et al. Effect of Poloxamer 188 vs Placebo on Painful Vaso-Occlusive Episodes in Children and Adults With Sickle Cell Disease: A Randomized Clinical Trial. JAMA 2021;325:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muslu CS, Kopetsky M, Nimmer M, et al. The association between timely opioid administration and hospitalization in children with sickle cell disease presenting to the emergency department in acute pain. Pediatr Blood Cancer 2020;67:e28268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees CA, Brousseau DC, Fahd A, et al. Adherence to NHLBI Guidelines for the Emergent Management of Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Multicenter Perspective. Am J Hematol 2022;97:E412–E415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy A, O’Sullivan R, Wakai A, et al. Intranasal fentanyl for the management of acute pain in children. Cochrane Database Syst Rev 2014;2014:CD009942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borland M, Jacobs I, King B, O'Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med 2007;49:335–340. [DOI] [PubMed] [Google Scholar]
- 8.Borland ML, Jacobs I, Geelhoed G. Intranasal fentanyl reduces acute pain in children in the emergency department: a safety and efficacy study. Emerg Med 2002;14:275–280. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer JA, Mlekoday TJ. Time to opioid administration after implementation of an intranasal fentanyl protocol. Am J Emerg Med 2015;33:1805–1807. [DOI] [PubMed] [Google Scholar]
- 10.Akinsola B, Hagbom R, Zmitrovich A, et al. Impact of Intranasal Fentanyl in Nurse Initiated Protocols for Sickle Cell Vaso-occlusive Pain Episodes in a Pediatric Emergency Department. Am J Hematol 2018;93:E205–E207. [DOI] [PubMed] [Google Scholar]
- 11.Holdgate A, Cao A, Lo KM. The implementation of intranasal fentanyl for children in a mixed adult and pediatric emergency department reduces time to analgesic administration. Acad Emerg Med 2010;17:214–217. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh PL, Sprinz PG, Wolfgang TL, et al. Improving the management of vaso-occlusive episodes in the pediatric emergency department. Pediatrics 2015;136:e1016–e1025. [DOI] [PubMed] [Google Scholar]
- 13.Kelly GS, Stewart RW, Strouse JJ, Anders J. Intranasal fentanyl improves time to analgesic delivery in sickle cell pain crises. Am J Emerg Med 2018;36:1305–1307. [DOI] [PubMed] [Google Scholar]
- 14.Fein DM, Avner JR, Scharbach K, Manwani D, Khine H. Intranasal fentanyl for initial treatment of vaso-occlusive crisis in sickle cell disease. Pediatr Blood Cancer 2017;64:6. [DOI] [PubMed] [Google Scholar]
- 15.Carden MA, Brousseau DC, Ahmad FA, et al. Normal Saline Bolus Use is Associated with Worse Pain Control in Children with Sickle Cell Disease and Vaso-occlusive Pain Episodes: A Multicenter Experience. Am J Hematol 2019;94:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquin H, Trottier ED, Pastore Y, et al. Evaluation of a clinical protocol using intranasal fentanyl for treatment of vaso-occlusive crisis in sickle cell patients in the emergency department. Paediatr Child Heal 2020;25:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett MJ, Cronin J, Murphy A, et al. Intranasal fentanyl versus intravenous morphine in the emergency department treatment of severe painful sickle cell crises in children: study protocol for a randomised controlled trial. Trials 2012;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014. https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/evidence-based-management-sickle-cell-disease-expert-0. Published 2014. Accessed July 23, 2021.
- 19.Treadwell MJ, Bell M, Leibovich SA, et al. A Quality Improvement Initiative to Improve Emergency Department Care for Pediatric Patients with Sickle Cell Disease. J Clin Outcomes Manag 2014;21:62–70. [PMC free article] [PubMed] [Google Scholar]
- 20.Mathias MD, McCavit TL. Timing of opioid administration as a quality indicator for pain crises in sickle cell disease. Pediatrics 2015;135:475–482. [DOI] [PubMed] [Google Scholar]
- 21.Lin SM, Strouse JJ, Whiteman LN, Anders J, Stewart RW. Improving quality of care for sickle cell patients in the pediatric emergency department. Pediatr Emerg Care 2016;32:14–16. [DOI] [PubMed] [Google Scholar]
- 22.Michelson KA, Bachur RG, Levy JA. The impact of critically ill children on paediatric ED medication timeliness. Emerg Med J 2017;34:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenoi R, Ma L, Syblik D, Yusuf S. Emergency department crowding and analgesic delay in pediatric sickle cell pain crises. Pediatr Emerg Care 2011;27:911–917. [DOI] [PubMed] [Google Scholar]
- 24.Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv 2020;4:2656–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Emergency Physicians. Managing Sickle Cell Disease in the ED. 2021. Available at https://www.acep.org/patient-care/sickle-cell/. Accessed September 26, 2022.
- 26.Lazio MP, Costello HH, Courtney DM, Martinovich Z, Myers R, Zosel A TP. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain 2010;26:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telfer P, Criddle J, Sandell J, Davies F, Morrison I, Challands J. Intranasal diamorphine for acute sickle cell pain. Arch Dis Child 2009;94:979–80. [DOI] [PubMed] [Google Scholar]
- 28.Tsze DS, Pan SS, DePeter KC, Wagh AM, Gordon SL, Dayan PS. Intranasal hydromorphone for treatment of acute pain in children: A pilot study. Am J Emerg Med 2019;37:1128–1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data included in this study may be made available upon reasonable request to the corresponding author.