Abstract
Background:
Depression and substance use (SU) disorders are prevalent among people living with HIV (PLWH) and impact health outcomes despite successful antiretroviral therapy (ART). We explored quality of life, functional ability and associated factors among PLWH screened positive for depression and/or SU.
Methods:
This cross-sectional study recruited adult PLWH during routine follow-up at five HIV clinical sites in the Asia-Pacific region. Participants were screened for depression using PHQ-9 and SU using ASSIST. Quality of life (QoL) was assessed with WHOQOL-HIV BREF and functional ability with WHODAS 2.0. Factors associated with mean QoL and disability scores were analysed using linear regression.
Results:
Of 864 PLWH enrolled, 753 screened positive for depression or SU. The median (interquartile range, IQR) age was 38 (31–47) years and 97% were on ART. Overall mean WHOQOL-HIV BREF and WHODAS scores indicated greater impairment with increasing depressive symptom severity and SU risk. In multivariate analysis, PLWH reporting previous trauma/stress (difference = 2.7, 95% CI 1.5 to 3.9, p<0.001) and past mental health diagnosis (difference =5.0, 95% CI 2.9 to 7.1, p<0.001) were associated with greater disability and poorer QoL scores across multiple domains (p<0.01 for all). Higher CD4 T-cell counts was also associated with better QoL scores and functional ability.
Conclusion:
PLWH with depression/SU experienced poorer QoL and function despite routine engagement in HIV care. Efforts to integrate mental health services and interventions addressing disability into HIV management should be prioritised in the region.
Keywords: Quality of life, HIV, functional ability, WHODAS, depression, substance use, Asia
Introduction
Despite well suppressed viral replication on antiretroviral therapy (ART), a significant proportion of people living with HIV (PLWH) continue to experience poor health-related outcomes [1, 2]. Quality of life is the subjective assessment of an individual’s well-being, while functionality, assesses an individual’s ability to participate and perform daily physical, social and occupational activities. Functional ability is influenced by both extrinsic factors (example stigma experience, social support) and intrinsic factors (frailty, multimorbidity) which in the context of PLWH may be exacerbated [3, 4]. Both measures help chart an individual’s overall health status and there is growing consensus for these measures to be included as part of routine HIV management, redefining the focus of HIV care beyond HIV RNA and CD4 T-cell count [5, 6].
A significant comorbidity which contributes to poor quality of life and disability among PLWH are mental health (MH) disorders including substance use (SU) which occur at rates significantly higher than that reported in the general population [7, 8]. Addressing MH and SU issues through the integration of MH into routine HIV care is one of the key priority actions mooted in the Global AIDS Strategy 2021–2026 [9] as it has been shown to improve mental and overall health outcomes among PLWH [10]. However, the adoption of such programs has been slow in the LMIC setting [11]. In the Asia-Pacific region, the provision of MH care assessments for PLWH are among the lowest globally [12] and efforts to improve such services are often met with multiple challenges. These include the lack of trained MH specialists to meet even the basic MH needs of the general population, poor coordination in planning of MH and HIV services which are done almost exclusively of one another and a high-level of stigma in the healthcare setting against people living and at risk of HIV impacting both health-seeking behaviour among PLWH and the willingness of providers to engage in services for PLWH [13, 14].
To date, there is limited data describing the extent of disability and health-related quality of life experienced by PLWH with MH issues undergoing routine HIV care in Asia. This understanding is especially important as health systems in the region work to develop person-centered, integrated care to address the growing MH and SU burden among PLWH. Understanding factors which negatively impact quality of life and functioning in individuals with pre-existing MH issues can help streamline limited MH care resources and facilitate more timely linkage to specialist care. In this multisite-regional study, we aimed to explore the overall functional ability and QoL experienced by PLWH who screened positive for depression/SU during their routine HIV clinic follow-up, describe the specific QoL domains impacted in these individuals and assess individual and HIV/treatment-related factors associated with QoL domains and overall function.
Methods
Study population
The study population included PLWH returning for routine follow-up between July 2019 and June 2020 at five urban HIV treatment centres in Hong Kong SAR, China, Philippines, Malaysia, South Korea and Thailand. All individuals aged 18 years and above with documented HIV infection were eligible to participate.
All participants provided written informed consent and the study protocol was approved by the institutional ethics board at each recruiting centre, the study coordinating centre (TREAT Asia, amfAR/The Foundation for AIDS Research, Thailand) and the data management centre (The Kirby Institute, University of New South Wales, Australia).
Study measurements
All consenting participants answered a standardised study questionnaire which included information on demographic and socio-economic characteristics, HIV disclosure status, recent traumatic events, medical and family MH history. Medical records were also accessed to extract data on HIV-related parameters.
All participants completed a series of self-administered screening tools with study personnel on standby to assist, if needed. Locally validated tools were utilised where available, and if not, study screening tools were professionally translated and reviewed for face validity by investigators with clinical and research experience. Training was conducted virtually for all study staff prior to study initiation to ensure standardised execution of the questionnaires. Site staff also had access to training videos for periodic review. Screening for depression in the past 2 weeks was done using the Patient Health Questionnaire-9 (PHQ-9) and substance use with the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST v3.1).
Additionally, participants were assessed for quality of life and functional ability using the World Health Organisation Quality of Life - HIV (WHOQOL-HIV BREF) and World Health Organisation Disability Assessment Schedule 2.0 (WHODAS 2.0), respectively. The WHOQOL-HIV BREF is a 31-item validated tool which assesses an individual’s perception of quality of life (QoL) in six domains; physical, psychological, level of independence, social relationships, environment and spirituality/self-belief as well as an overall rating of QoL (Question 1, Q1). Items were rated on a 5-point scale with 1 indicating low, negative perception and 5 high, positive perception. Mean scores for each domain were computed with resulting scores ranging between 4 and 20, where higher scores indicated better QoL. The version of WHODAS 2.0 utilised in this study was the short 12-item questionnaire which assesses the overall profile of functioning and disability from a series of questions in the domains of cognition, self-care, getting along, life activities (household and work) and participation. The tool rates level of functioning across a 5-point scale with 1 indicating no disability and 5 indicating extreme disability. A simple scoring approach was used to score functional ability where sums of scores of the 12-items were assigned to each participant (total scores ranging between 12 to 60) with higher scores indicating a greater level of disability.
Statistical analysis
The WHOQOL-HIV BREF and WHODAS 2.0 scores were reported descriptively in all participants and in the sub-population with mild to severe depressive symptoms (PHQ-9 scores ≥5) or those reporting low to high risk use of any substance according to the ASSIST scores of 0–10 (lower risk), 11–26 (moderate risk), and ≥27 (high risk) for alcohol and 0–3 (lower risk), 4–26 (moderate risk), and ≥27 (high risk) for other substances. [15]. We assessed factors associated with mean WHOQOL-HIV BREF scores (Q1 scores and each of the six domain scores) and mean WHODAS 2.0 scores in this sub-population using linear regression. Missing questionnaire responses were imputed using the “hot deck” imputation method where missing values are replaced by random values from the same variable [4].
World Bank country income grouping was adjusted a priori in all analyses to account for heterogeneity across sites. Covariates included were patient characteristics, as well as socio-economic risk factors from the study-specific questionnaire. Regression analyses were fitted using backward stepwise selection process. Covariates with p<0.10 in the univariate analysis were included in the multivariate model. Covariates with p<0.05 in the multivariate regression model were considered statistically significant.
Data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata software version 16.1 (Stata Corp., College Station, TX, USA).
Results
A total of 864 participants were recruited, of which 753 (87%) screened positive for either depression or SU. The clinical characteristics and disposition of those screened positive for depression or SU are summarised in Table 1 and Fig S1 (supplementary data), respectively. The median age in participants who screened positive for depression/SU was 38 (interquartile range, IQR 31–47) years and 90% were males. A total of 97% were on antiretroviral therapy (ART). Of those with HIV RNA (n=533) and CD4 T-cell count (n=528) measures available in the prior 6 months, 92% had HIV RNA <1000 copies/ml and the median CD4 T-cell count was 518 (324–725) cells/ul. The majority (53%) reported experiencing a traumatic or stressful event in the past 5 years and 8% had a prior diagnosis of MH. The demographic, socio-behavioural and clinical parameters of participants who screened positive for depression/SU and those without are summarised in Supplementary table 1.
Table 1:
Demographic, socio-behavioural and clinical characteristics of participants screened positive for depression or substance use (n=753)
| Characteristics | N (%) |
|---|---|
| Total | 753 (100) |
| Age at survey (years) | Median = 38, IQR (31–47) |
| ≤30 | 188 (25) |
| 31–40 | 239 (32) |
| 41–50 | 216 (29) |
| >50 | 110 (15) |
| Sex | |
| Male | 678 (90) |
| Female | 75 (10) |
| HIV mode of exposure | |
| Heterosexual contact | 233 (31) |
| MSM | 406 (54) |
| Injecting drug use | 13 (2) |
| Other/Unknown | 101 (13) |
| Viral load at recruitment (copies/mL) | Median = 33, IQR (19–39) |
| <50 | 455 (60) |
| 50–399 | 32 (4) |
| 400–999 | 3 (0) |
| ≥1000 | 43 (6) |
| Not tested | 220 (29) |
| CD4 count at recruitment (cells/μL) | Median = 518, IQR (324–725) |
| ≤200 | 63 (8) |
| 201–350 | 86 (11) |
| 351–500 | 103 (14) |
| >500 | 276 (37) |
| Not tested | 225 (30) |
| Current ART | |
| NRTI+NNRTI | 387 (51) |
| NRTI+PI | 51 (7) |
| INSTI | 270 (36) |
| Other | 24 (3) |
| None/unknown | 21 (3) |
| Hepatitis B co-infection | |
| Negative | 250 (33) |
| Positive | 27 (4) |
| Not tested | 476 (63) |
| Hepatitis C co-infection | |
| Negative | 343 (46) |
| Positive | 26 (3) |
| Not tested | 384 (51) |
| Prior AIDS Diagnosis | |
| No | 476 (63) |
| Yes | 176 (23) |
| Not reported | 101 (13) |
| House hold income (USD) per month | |
| ≤$500 | 184 (14) |
| $501-$2000 | 215 (29) |
| >$2000 | 233 (31) |
| Not reported/unknown | 121 (16) |
| Employment | |
| No | 159 (21) |
| Full time | 426 (57) |
| Part time | 113 (15) |
| Not reported/unknown | 55 (7) |
| Highest education level | |
| No education | 3 (0) |
| Primary to high school | 235 (31) |
| College to university | 497 (66) |
| Not reported/unknown | 18 (2) |
| HIV disclosure status | |
| Fully | 37 (5) |
| Partially | 538 (71) |
| Not disclosed | 140 (19) |
| Not reported/unknown | 38 (5) |
| Previous stressors | |
| No | 301 (40) |
| Yes | 399 (53) |
| Not reported/unknown | 53 (7) |
| Family history of mental health disorder | |
| No | 637 (85) |
| Yes | 32 (4) |
| Not reported/unknown | 84 (11) |
| Comorbidities | |
| No | 302 (40) |
| Yes | 130 (17) |
| Not reported/unknown | 321 (43) |
| Previous mental health disorder | |
| No | 539 (72) |
| Yes | 61 (8) |
| Not reported/unknown | 153 (20) |
| History of STIs in the past 5 years | |
| No | 339 (45) |
| Yes | 237 (31) |
| Not reported/unknown | 177 (24) |
| Year of ART initiation | |
| <2010 | 199 (26) |
| 2010–2012 | 98 (13) |
| 2013–2015 | 160 (21) |
| 2016–2020 | 286 (38) |
| No ART/unknown | 10 (1) |
| ART adverse events in the previous year | |
| No | 510 (68) |
| Yes | 80 (11) |
| Not reported/unknown | 163 (22) |
| ART adherence in the previous year | |
| ≥95 | 479 (64) |
| <95 | 52 (7) |
| Not reported/unknown | 222 (29) |
| World Bank country income grouping | |
| High | 292 (39) |
| Upper middle and lower middle | 461 (61) |
Abbreviations: MSM-men who have sex with men; ART-antiretroviral therapy; NRTI-non-nucleoside reverse transcriptase inhibitors; NNRTI-non-nucleoside reverse transcriptase inhibitors; PI-protease inhibitors; INSTI-integrase strand transfer inhibitors; STIs-sexually transmitted infections.
Functional ability and quality of life scores in PLWH with depression and substance use issues.
The mean overall WHOQOL-HIV BREF score, defined as mean score for Question 1, for the full study cohort was 3.44 (standard deviation (SD) =0.89). For the different sub-population groups, the mean QOL scores were: 3.12 (SD=0.88) for depression sub-group; 3.43 (SD=0.87) for SU sub-group; 3.40 (SD=0.89) for depression or SU sub-group; 3.13 (SD=0.86) for depression and SU sub-group and 3.77 (SD=0.83) for no depression or SU sub-group (Fig 1A). The mean WHODAS 2.0 scores were 20.24 (SD=8.91) for the study cohort; 24.25 (SD=9.31) for the depression sub-group; 20.31 (SD=8.90) for the SU sub-group; 20.78 (SD=9.02) for the depression or SU sub-group; 24.04 (SD=9.38) for the depression and SU sub-group and 16.59 (SD=7.16) for no depression and SU sub-group. Participants screening positive for depression demonstrated greater impairment in mean QoL and WHODAS scores than PLWH screening positive for SU. There was also a clear trend of decline in mean overall QoL scores and a corresponding increase in mean WHODAS 2.0 scores indicating greater disability with increasing severity of depressive symptoms (Fig 1B). These findings indicate a strong, consistent inverse relationship between indicators of health outcomes and MH symptoms. Mean scores for overall QoL and WHODAS 2.0 were varied depending on the type of substance used. Among PLWH reporting moderate to high risk use, alcohol and tobacco users reported lesser functional impairment compared to users of cocaine and opioids (Fig 2). In general, overall mean QoL and WHODAS 2.0 scores displayed a trend of greater impairment among high compared to low risk users for all substances.
Figure 1: Distribution of PLWH screened positive for depression and/or substance use and mean scores for WHODAS 2.0 and overall Quality of Life (QoL).
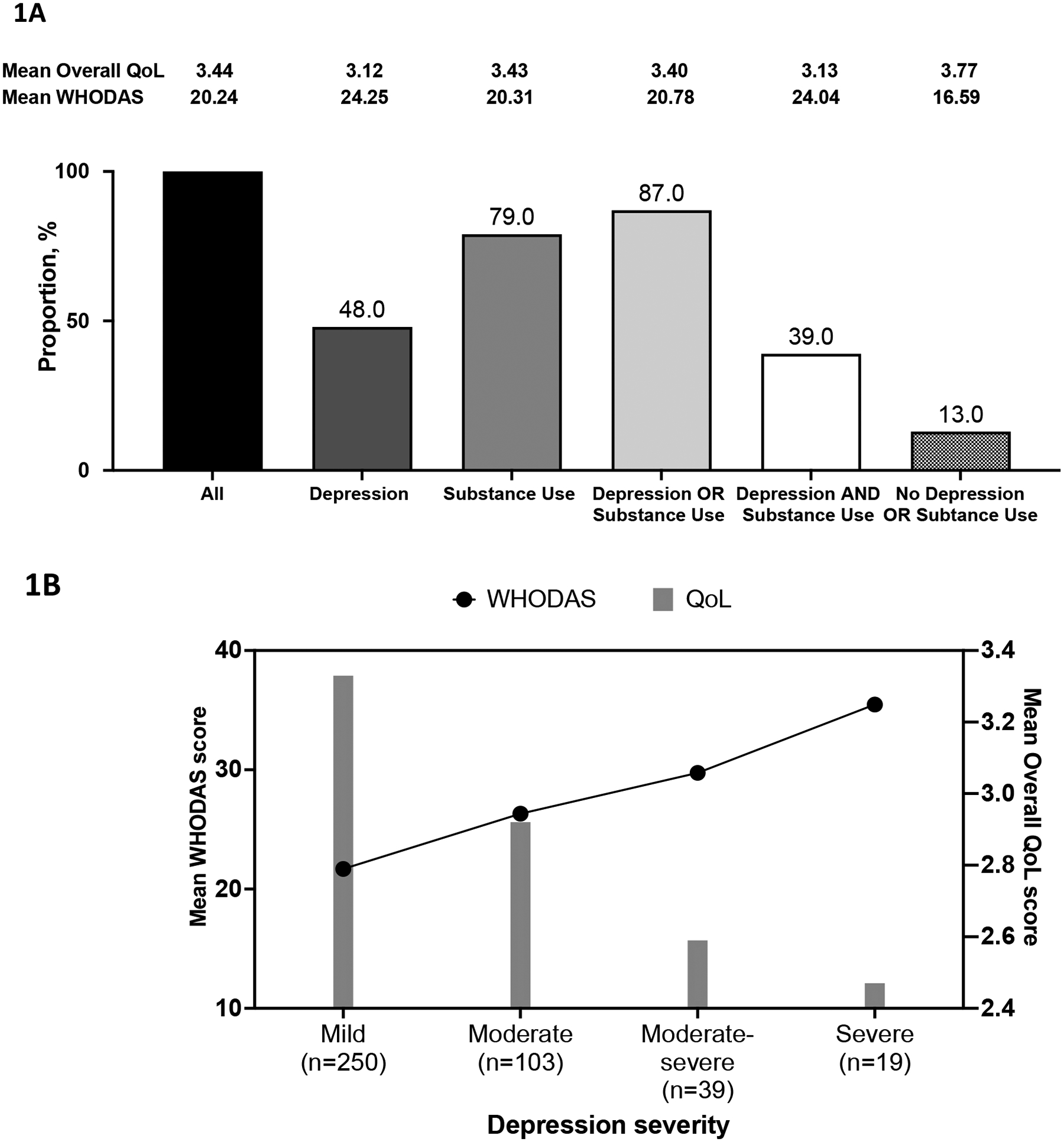
(A) Distribution of participants screened positive for depression and/or substance use from all participants recruited in the study (n=864) and corresponding mean WHODAS 2.0 and overall QoL (Q1 of WHOQOL-HIV BREF) scores in each group; (B) Trends in mean WHODAS 2.0 (black line) and overall QoL scores (grey bars) by depression symptom severity assessed using the PHQ-9 in PLWH screened positive for depression in the cohort (n=411). The number of participants for each severity group is indicated in the x-axis.
Figure 2: Heat plots describing (A) mean overall QoL and (B) WHODAS 2.0 scores among PLWH screened positive for substance use categorised by type and extent (low, moderate, high) of substance use reported (n=681).
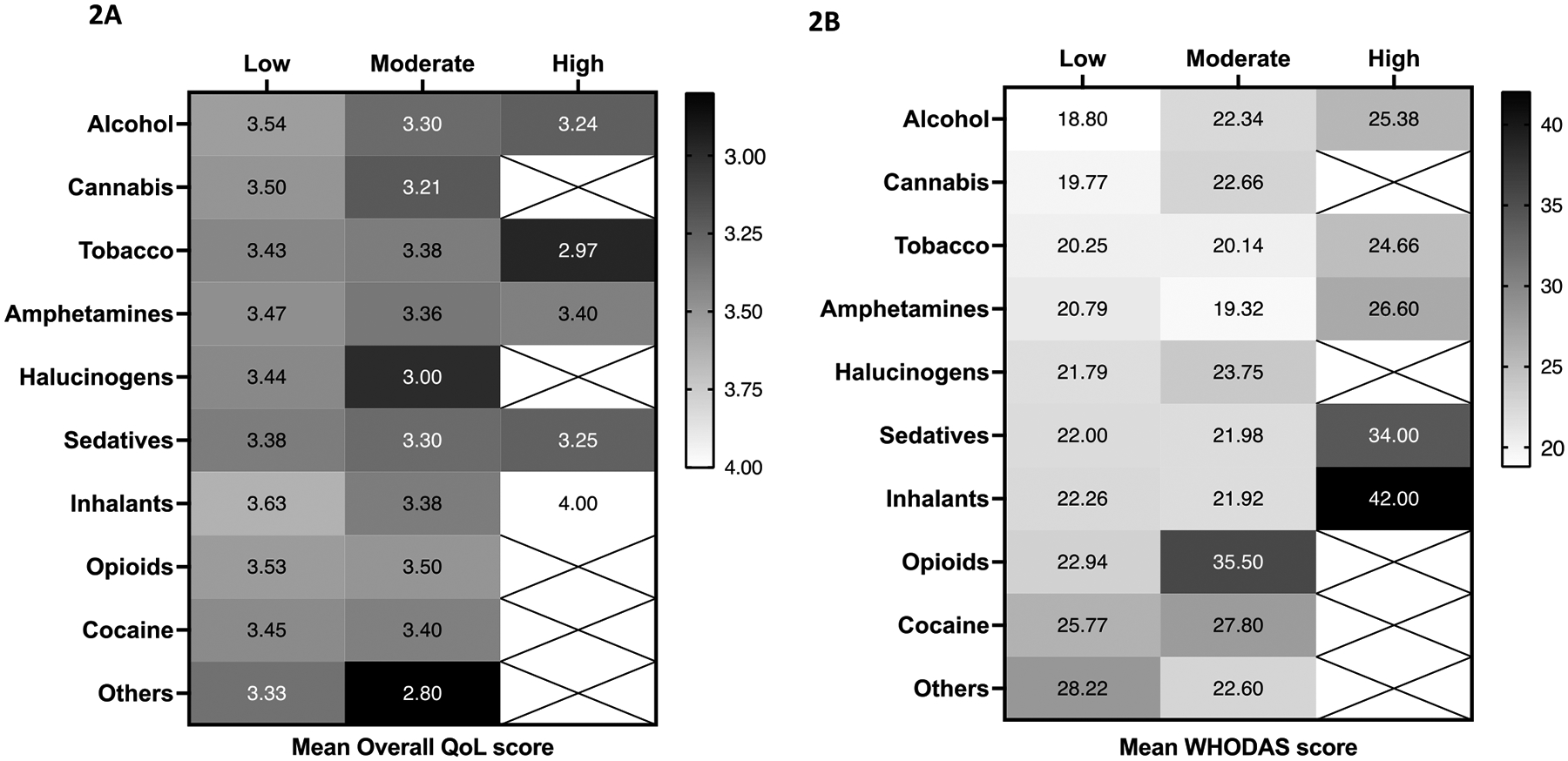
Mean QoL and WHODAS 2.0 scores are displayed in each box for each type of substance (vertical scale) and the extent of its use (low to high-risk) (horizontal scale). Darker shades indicate poorer QoL and greater disability which corresponds with lower mean QoL and higher WHODAS scores, respectively.
Quality of life domains impacted in PLWH with depression/SU and factors associated with QoL domain scores
When assessing each of the WHOQOL HIV-BREF domains, PLWH screened positive for depression/SU had significantly lower mean scores compared to those without depression/SU for all domains except for environment (Fig S2).
In multivariate analysis assessing factors associated with each QoL domain score, aspects encompassing HIV/treatment-related factors, socio-economic factors, psychosocial and physical well-being were all found to be associated with specific domains (Table 2). The majority of factors identified had an overlapping influence on multiple domains. Older age was associated with better QoL scores in the domains of physical health (p = 0.003), independence (p = 0.015) and spiritual/personal beliefs (p = 0.028). Higher CD4 T-cell counts were associated with better QoL in the domains of physical health (p <0.001), psychological health (p = 0.013), independence (p <0.001) and environment (p = 0.009). Females (compared to males) were associated with better QoL scores in the domains of independence (p = 0.024) and social relationship (p = 0.016). Individuals experiencing previous traumatic/stressful events in the past 5 years were associated with lower QoL scores in the domains of physical (p <0.001), psychological (p = 0.004), independence (p = 0.029) and spiritual/personal beliefs (p = 0.003). A previous diagnosis of MH or a family history of MH were associated with lower QoL scores in all domains except physical health and spiritual/personal beliefs.
Table 2:
Findings from multivariate analyses of risk factors associated with each WHOQOL-HIV BREF domain in individuals who screened positive for depression or substance use (n=753)
| Overall | Physical | Psychological | Independence | Social relationships | Environment | Spirituality / personal beliefs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean score | Diff | Mean score | Diff | Mean score | Diff | Mean score | Diff | Mean score | Diff | Mean score | Diff | Mean score | Diff |
| Total | 3.4 | 14.4 | 13.8 | 14.9 | 13.5 | 13.9 | 13.6 | |||||||
| Age at survey (years) | ||||||||||||||
| ≤30 | 3.4 | 13.9 | Ref | 13.5 | 14.4 | Ref | 13.6 | 13.7 | 13.3 | Ref | ||||
| 31–40 | 3.4 | 14.3 | + | 13.8 | 14.8 | + | 13.5 | 14.0 | 13.3 | + | ||||
| 41–50 | 3.4 | 14.7 | + | 14.3 | 15.5 | + | 13.7 | 14.2 | 14.0 | + | ||||
| >50 | 3.2 | 14.5 | + | 13.5 | 14.8 | + | 13.2 | 13.4 | 13.9 | + | ||||
| Sex | ||||||||||||||
| Male | 3.4 | 14.3 | 13.7 | 14.8 | Ref | 13.4 | Ref | 13.9 | 13.5 | |||||
| Female | 3.5 | 15.0 | 14.7 | 15.8 | + | 14.4 | + | 14.2 | 14.6 | |||||
| HIV mode of exposure | ||||||||||||||
| Heterosexual contact | 3.3 | 14.3 | 14.1 | 14.9 | 13.5 | 13.7 | 14.1 | Ref | ||||||
| MSM | 3.5 | 14.5 | 13.8 | 15.1 | 13.6 | 14.1 | 13.3 | − | ||||||
| Injecting drug use | 3.3 | 13.8 | 13.3 | 14.3 | 13.1 | 13.4 | 11.6 | − | ||||||
| Other/Unknown | 3.2 | 14.0 | 13.3 | 14.4 | 13.5 | 13.5 | 13.7 | − | ||||||
| Viral Load at survey (copies/mL) | ||||||||||||||
| <1000 | 3.4 | 14.6 | 13.8 | 15.2 | 13.7 | 14.0 | 13.6 | |||||||
| ≥1000 | 3.3 | 13.2 | 13.2 | 13.9 | 13.0 | 13.4 | 13.3 | |||||||
| Not tested | 3.5 | 14.2 | 14.0 | 14.5 | 13.4 | 13.7 | 13.6 | |||||||
| CD4 at survey (cells/μL) | ||||||||||||||
| ≤200 | 3.4 | 12.4 | Ref | 13.1 | Ref | 13.3 | Ref | 13.2 | 13.4 | + | 13.4 | |||
| 201–350 | 3.2 | 13.9 | + | 13.1 | + | 14.2 | + | 12.7 | 13.0 | Ref | 13.3 | |||
| 351–500 | 3.3 | 14.5 | + | 13.6 | + | 14.9 | + | 13.3 | 13.9 | + | 12.9 | |||
| >500 | 3.5 | 15.0 | + | 14.1 | + | 15.7 | + | 13.9 | 14.3 | + | 13.9 | |||
| Not tested | 3.4 | 14.3 | 14.0 | 14.7 | 13.6 | 13.9 | 13.7 | |||||||
| Current ART | ||||||||||||||
| NRTI+NNRTI | 3.5 | 14.7 | 14.3 | 15.1 | 13.8 | 14.1 | 13.8 | |||||||
| NRTI+PI | 3.4 | 14.5 | 14.1 | 14.5 | 13.3 | 14.0 | 13.8 | |||||||
| INSTI | 3.3 | 14.1 | 13.2 | 15.1 | 13.3 | 13.8 | 13.2 | |||||||
| Other | 3.0 | 13.7 | 13.1 | 14.3 | 14.0 | 13.5 | 14.3 | |||||||
| None/unknown | 3.2 | 12.6 | 12.6 | 12.6 | 12.3 | 13.0 | 12.0 | |||||||
| Hepatitis B co-infection | ||||||||||||||
| Negative | 3.5 | 14.7 | 13.7 | 15.6 | 13.6 | 14.2 | 13.6 | |||||||
| Positive | 3.6 | 15.8 | 15.1 | 16.4 | 14.7 | 14.7 | 14.3 | |||||||
| Not tested | 3.3 | 14.1 | 13.8 | 14.5 | 13.4 | 13.7 | 13.5 | |||||||
| Hepatitis C co-infection | ||||||||||||||
| Negative | 3.5 | 15.0 | 14.3 | 15.9 | 14.0 | 14.4 | 13.8 | |||||||
| Positive | 3.7 | 15.2 | 14.4 | 16.3 | 15.0 | 15.2 | 14.5 | |||||||
| Not tested | 3.3 | 13.7 | 13.3 | 14.0 | 13.0 | 13.4 | 13.3 | |||||||
| Prior AIDS Diagnosis | ||||||||||||||
| No | 3.4 | 14.7 | 13.8 | 15.4 | 13.8 | 14.2 | 13.4 | |||||||
| Yes | 3.4 | 14.3 | 13.9 | 14.7 | 13.3 | 13.8 | 13.7 | |||||||
| Not reported | 3.2 | 12.9 | 13.6 | 13.1 | 12.9 | 12.8 | 14.1 | |||||||
| Household income (USD) per month | ||||||||||||||
| ≤$500 | 3.2 | Ref | 13.7 | Ref | 13.8 | 14.0 | Ref | 13.1 | 13.2 | Ref | 13.6 | |||
| $501-$2000 | 3.5 | + | 15.0 | + | 14.3 | 15.4 | + | 14.1 | 14.5 | + | 13.8 | |||
| >$2000 | 3.6 | + | 14.7 | + | 13.8 | 15.7 | + | 13.7 | 14.5 | + | 13.6 | |||
| Not reported/unknown | 3.1 | 13.8 | 13.1 | 14.0 | 12.8 | 12.9 | 13.3 | |||||||
| Employment | ||||||||||||||
| No | 3.1 | 13.5 | 13.2 | 13.8 | 12.9 | 13.1 | 13.5 | − | ||||||
| Full time | 3.6 | 14.9 | 14.2 | 15.6 | 13.9 | 14.4 | 13.9 | Ref | ||||||
| Part time | 3.3 | 13.8 | 13.4 | 14.5 | 13.2 | 13.4 | 12.8 | − | ||||||
| Not reported/unknown | 3.3 | 14.2 | 13.4 | 14.1 | 13.3 | 13.7 | 13.4 | |||||||
| Highest education level | ||||||||||||||
| No education | 3.3 | 17.7 | 14.7 | 17.0 | 13.0 | − | 13.7 | + | 14.3 | |||||
| Primary to high school | 3.2 | 14.1 | 13.4 | 14.6 | 12.9 | − | 13.3 | − | 13.2 | |||||
| College to university | 3.5 | 14.5 | 14.0 | 15.1 | 13.8 | Ref | 14.2 | Ref | 13.7 | |||||
| Not reported/unknown | 3.0 | 14.3 | 14.2 | 14.7 | 13.5 | 13.7 | 14.1 | |||||||
| HIV disclosure status | ||||||||||||||
| Fully | 3.4 | 13.9 | 14.5 | 14.1 | 14.5 | Ref | 13.9 | 15.1 | Ref | |||||
| Partially | 3.4 | 14.4 | 13.7 | 15.0 | 13.6 | − | 13.9 | 13.6 | − | |||||
| Not disclosed | 3.4 | 14.5 | 14.1 | 15.2 | 12.9 | − | 13.9 | 12.9 | − | |||||
| Not reported/unknown | 3.2 | 14.4 | 13.8 | 14.5 | 13.7 | 13.6 | 14.1 | |||||||
| Previous stressors | ||||||||||||||
| No | 3.6 | Ref | 15.4 | Ref | 14.6 | Ref | 15.8 | Ref | 14.1 | 14.5 | 14.4 | Ref | ||
| Yes | 3.3 | − | 13.7 | − | 13.3 | − | 14.4 | − | 13.2 | 13.6 | 13.1 | − | ||
| Not reported/unknown | 3.2 | 13.7 | 13.1 | 14.1 | 12.9 | 13.2 | 12.8 | |||||||
| Family history of mental health disorder | ||||||||||||||
| No | 3.4 | 14.6 | 14.1 | Ref | 15.1 | 13.7 | Ref | 14.1 | Ref | 13.8 | ||||
| Yes | 3.4 | 13.6 | 12.4 | − | 13.8 | 12.3 | − | 13.0 | − | 12.9 | ||||
| Not reported/unknown | 3.1 | 13.2 | 12.1 | 13.9 | 12.9 | 13.0 | 12.4 | |||||||
| Comorbidities | ||||||||||||||
| No | 3.3 | 14.0 | 13.0 | 14.8 | 13.2 | 13.8 | 13.2 | |||||||
| Yes | 3.4 | 14.3 | 13.5 | 14.6 | 13.2 | 13.9 | 13.2 | |||||||
| Not reported/unknown | 3.5 | 14.7 | 14.7 | 15.2 | 14.0 | 14.0 | 14.1 | |||||||
| Previous mental health disorder | ||||||||||||||
| No | 3.5 | Ref | 14.8 | 14.1 | Ref | 15.6 | Ref | 13.8 | Ref | 14.2 | 13.7 | |||
| Yes | 2.9 | − | 12.9 | 11.8 | − | 13.4 | − | 12.2 | − | 13.1 | 12.0 | |||
| Not reported/unknown | 3.2 | 13.3 | 13.7 | 13.3 | 13.0 | 13.0 | 13.9 | |||||||
| History of STIs in the past 5 years | ||||||||||||||
| No | 3.4 | 14.5 | 13.8 | 15.4 | 13.6 | 14.1 | 13.3 | |||||||
| Yes | 3.5 | 14.7 | 13.8 | 15.4 | 13.8 | 14.3 | 13.7 | |||||||
| Not reported/unknown | 3.3 | 13.6 | 13.7 | 13.6 | 13.1 | 13.1 | 13.9 | |||||||
| Year of ART initiation | ||||||||||||||
| <2010 | 3.4 | 14.8 | 14.2 | 15.5 | 13.6 | 13.9 | 14.0 | |||||||
| 2010–2012 | 3.5 | 15.2 | 14.2 | 15.8 | 13.9 | 14.6 | 13.6 | |||||||
| 2013–2015 | 3.4 | 14.4 | 13.9 | 14.7 | 13.6 | 13.9 | 13.5 | |||||||
| 2016–2020 | 3.3 | 13.9 | 13.4 | 14.4 | 13.3 | 13.7 | 13.4 | |||||||
| No ART/unknown | 3.3 | 13.0 | 13.0 | 13.3 | 13.9 | 13.7 | 12.6 | |||||||
| ART adverse events in the previous year | ||||||||||||||
| No | 3.4 | 14.7 | 13.9 | 15.4 | 13.7 | 14.1 | 13.6 | |||||||
| Yes | 3.5 | 14.3 | 13.9 | 15.4 | 13.9 | 14.5 | 13.0 | |||||||
| Not reported/unknown | 3.2 | 13.3 | 13.6 | 13.3 | 13.0 | 13.0 | 13.9 | |||||||
| ART adherence in the previous year | ||||||||||||||
| ≥95 | 3.5 | 14.7 | 13.9 | 15.5 | 13.7 | 14.2 | 13.6 | Ref | ||||||
| <95 | 3.3 | 13.9 | 12.8 | 14.5 | 12.8 | 13.5 | 12.5 | − | ||||||
| Not reported/unknown | 3.2 | 13.7 | 13.8 | 13.9 | 13.3 | 13.3 | 13.9 | |||||||
| WHODAS score | ||||||||||||||
| ≤17 | 3.7 | Ref | 15.9 | Ref | 15.0 | Ref | 16.6 | Ref | 14.7 | Ref | 15.0 | Ref | 14.8 | Ref |
| >17 | 3.1 | − | 12.8 | − | 12.6 | − | 13.3 | − | 12.3 | − | 12.8 | − | 12.3 | − |
| World Bank country income grouping | ||||||||||||||
| High | 3.3 | Ref | 13.9 | Ref | 12.9 | Ref | 14.8 | Ref | 13.0 | Ref | 13.5 | Ref | 13.2 | Ref |
| Upper-middle and lower-middle | 3.5 | + | 14.7 | + | 14.4 | + | 15.0 | + | 13.9 | + | 14.1 | + | 13.8 | + |
Not reported values were included in the analysis as a separate category but were excluded from test for heterogeneity.
Global p-value for age, viral load, CD4, household income were test for trend.
Abbreviations: Ref-reference group; CI-confidence interval; Diff-difference; MSM-men who have sex with men; ART-antiretroviral therapy; NRTI-non-nucleoside reverse transcriptase inhibitors; NNRTI-non-nucleoside reverse transcriptase inhibitors; PI-protease inhibitors; INSTI-integrase strand transfer inhibitors; STIs-sexually transmitted infections; WHODAS-World Health Organization Disability Assessment Schedule
+ indicates higher mean scores compared to the reference group, − indicates lower mean scores to the reference group.
Bold fonts indicate statistical significance at a level of p<0.05
Refer to Supplementary Table 3 and Supplementary Table 4 for details of differences in mean scores across variable
Socio-economic factors including higher household income and higher education levels were associated with better QoL scores in physical and environmental domains. PLWH residing in the LMIC setting reported better QoL scores in all domains compared to those residing in high-income settings.
Factors associated with functional ability in PLWH screened positive for depression/SU
Factors associated with lower WHODAS 2.0 scores (better functional ability, Table 3) were CD4 T-cell counts >500 cells/μL (difference = −3.0, 95% CI −5.3 to −0.7, p=0.010) compared to CD4 ≤200 cells/μL; currently on INSTI-based regimen (difference = −2.4, 95% CI −4.2 to −0.5, p=0.013) compared to NRTI+NNRTI-regimen; higher household income of $501-$2000 (difference = −2.2, 95% CI −3.8 to −0.5, p=0.010) and >$2000 (difference = −4.0, 95% CI −5.9 to −2.2, p<0.001) compared to ≤$500 per month; and being from upper-middle or lower-middle income countries (difference = −3.6, 95% CI −5.5 to −1.7, p=0.001) compare to high-income countries. The reversed association of country-income grouping in the multivariate model compared to the univariate model could possibly be due to the presence of the Simpson’s Paradox where the trend observed in the aggregated data is reversed when the data are separated [16]. Conversely, factors associated with higher WHODAS 2.0 scores (poorer functional ability) were not currently on ART (difference = 4.6, 95% CI 1.0 to 8.2, p=0.013) compared to receiving NRTI+NNRTI-based regimen; having had previous stressors (difference = 2.7, 95% CI 1.4 to 3.9, p<0.001) compared to none; and having had a previous MH disorder (difference = 5.0, 95% CI 2.9 to 7.1, p<0.001) compared to none.
Table 3:
Multivariate analyses of risk factors associated with WHODAS 2.0 scores in individuals who screened positive for depression or substance use (n=753)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Total patients | Mean WHODAS score | Difference (95% CI) | p | Difference (95% CI) | p |
| Total | 753 | 20.8 | ||||
| Age at survey (years) | 0.766 | - | - | |||
| ≤30 | 188 | 21.2 | Ref | - | - | |
| 31–40 | 239 | 20.9 | −0.3 (−2.0, 1.4) | 0.737 | - | - |
| 41–50 | 216 | 19.5 | −1.7 (−3.5, 0.0) | 0.057 | - | - |
| >50 | 110 | 22.1 | 0.8 (−1.3, 2.9) | 0.448 | - | - |
| Sex | - | - | ||||
| Male | 678 | 20.9 | Ref | - | - | |
| Female | 75 | 19.7 | −1.3 (−3.4, 0.9) | 0.255 | - | - |
| HIV mode of exposure | 0.002 | - | - | |||
| Heterosexual contact | 233 | 21.8 | Ref | - | - | |
| MSM | 406 | 19.7 | −2.1 (−3.6, −0.7) | 0.004 | - | - |
| Injecting drug use | 13 | 21.4 | −0.4 (−5.4, 4.6) | 0.870 | - | - |
| Other/Unknown | 101 | 22.9 | 1.0 (−1.0, 3.1) | 0.325 | - | - |
| Viral Load at survey (copies/mL) | - | - | ||||
| <1000 | 490 | 19.7 | Ref | - | - | |
| ≥1000 | 43 | 22.5 | 2.8 (0.0, 5.6) | 0.046 | - | - |
| Not tested | 220 | 22.9 | - | - | ||
| CD4 at survey (cells/μL) | <0.001 | 0.001 | ||||
| ≤200 | 63 | 25.1 | Ref | Ref | ||
| 201–350 | 86 | 22.2 | −2.9 (−5.8, −0.1) | 0.045 | −1.4 (−4.0, 1.2) | 0.284 |
| 351–500 | 103 | 19.9 | −5.2 (−7.9, −2.4) | <0.001 | −2.2 (−4.7, 0.4) | 0.094 |
| >500 | 276 | 18.5 | −6.6 (−9.0, −4.2) | <0.001 | −3.0 (−5.3, −0.7) | 0.010 |
| Not tested | 225 | 22.3 | ||||
| Current ART | <0.001 | 0.003 | ||||
| NRTI+NNRTI | 387 | 21.5 | Ref | Ref | ||
| NRTI+PI | 51 | 22.0 | 0.5 (−2.1, 3.0) | 0.729 | −0.5 (−2.9, 1.8) | 0.668 |
| INSTI | 270 | 18.7 | −2.8 (−4.2, −1.4) | <0.001 | −2.4 (−4.2, −0.5) | 0.013 |
| Other | 24 | 22.2 | 0.6 (−3.0, 4.3) | 0.730 | 1.2 (−2.2, 4.6) | 0.487 |
| None | 21 | 28.8 | 7.3 (3.4, 11.2) | <0.001 | 4.6 (1.0, 8.2) | 0.013 |
| Hepatitis B co-infection | 0.609 | - | - | |||
| Negative | 250 | 17.9 | Ref | - | - | |
| Positive | 27 | 17.0 | −0.9 (−4.4, 2.6) | - | - | |
| Not tested | 476 | 22.5 | - | - | ||
| Hepatitis C co-infection | 0.318 | - | - | |||
| Negative | 343 | 17.8 | Ref | - | - | |
| Positive | 26 | 16.1 | −1.7 (−5.1, 1.7) | - | - | |
| Not tested | 384 | 23.8 | - | - | ||
| Prior AIDS Diagnosis | 0.023 | - | - | |||
| No | 476 | 19.3 | Ref | - | - | |
| Yes | 176 | 21.0 | 1.7 (0.2, 3.2) | - | - | |
| Not reported | 101 | 27.5 | - | - | ||
| House hold income (USD) per month | <0.001 | <0.001 | ||||
| ≤$500 | 184 | 24.0 | Ref | Ref | ||
| $501–$2000 | 215 | 19.7 | −4.3 (−6.0, −2.6) | <0.001 | −2.2 (−3.8, −0.5) | 0.010 |
| >$2000 | 233 | 17.8 | −6.2 (−7.8, −4.5) | <0.001 | −4.0 (−5.9, −2.2) | <0.001 |
| Not reported/unknown | 121 | 23.5 | ||||
| Employment | <0.001 | - | - | |||
| No | 159 | 24.0 | 4.9 (3.3, 6.5) | - | - | |
| Full time | 426 | 19.1 | Ref | - | - | |
| Part time | 113 | 22.3 | 3.2 (1.4, 5.1) | - | - | |
| Not reported/unknown | 55 | 21.3 | - | - | ||
| Highest education level | 0.172 | - | - | |||
| No education | 3 | 15.3 | −5.1 (−15.3, 5.2) | 0.332 | - | - |
| Primary to high school | 235 | 21.5 | 1.1 (−0.3, 2.5) | 0.118 | - | - |
| College to university | 497 | 20.4 | Ref | - | - | |
| Not reported/unknown | 18 | 22.6 | - | - | ||
| HIV disclosure status | 0.062 | - | - | |||
| Fully | 37 | 24.1 | Ref | - | - | |
| Partially | 538 | 20.5 | −3.6 (−6.6, −0.6) | 0.018 | - | - |
| Not disclosed | 140 | 20.7 | −3.4 (−6.7, −0.2) | 0.040 | - | - |
| Not reported/unknown | 38 | 21.9 | - | - | ||
| Previous stressors | <0.001 | <0.001 | ||||
| No | 301 | 17.9 | Ref | Ref | ||
| Yes | 399 | 22.4 | 4.5 (3.2, 5.8) | 2.7 (1.4, 3.9) | ||
| Not reported/unknown | 53 | 25.0 | ||||
| Family history of mental health disorder | 0.012 | <0.001 | ||||
| No | 637 | 20.2 | Ref | Ref | ||
| Yes | 32 | 24.3 | 4.1 (0.9, 7.2) | 5.0 (2.9 – 7.1) | ||
| Not reported/unknown | 84 | 24.1 | - | - | ||
| Comorbidities | 0.159 | - | - | |||
| No | 302 | 19.7 | Ref | - | - | |
| Yes | 130 | 21.1 | 1.3 (−0.5, 3.2) | - | - | |
| Not reported/unknown | 321 | 21.6 | - | - | ||
| Previous mental health disorder | <0.001 | - | - | |||
| No | 539 | 18.6 | Ref | - | - | |
| Yes | 61 | 24.2 | 5.6 (3.4, 7.8) | - | - | |
| Not reported/unknown | 153 | 27.1 | - | - | ||
| History of STIs in the past 5 years | 0.769 | - | - | |||
| No | 339 | 19.3 | Ref | - | - | |
| Yes | 237 | 19.1 | −0.2 (−1.6, 1.2) | - | - | |
| Not reported/unknown | 177 | 25.9 | - | - | ||
| Year of ART initiation | 0.213 | - | - | |||
| <2010 | 199 | 20.2 | Ref | - | - | |
| 2010–2012 | 98 | 19.5 | −0.7 (−2.9, 1.4) | 0.508 | - | - |
| 2013–2015 | 160 | 20.9 | 0.7 (−1.2, 2.6) | 0.472 | - | - |
| 2016–2020 | 286 | 21.4 | 1.2 (−0.4, 2.9) | 0.136 | - | - |
| No ART | 10 | 24.1 | 3.9 (−1.8, 9.6) | 0.183 | - | - |
| ART adverse events in the previous year | 0.494 | - | - | |||
| No | 510 | 18.9 | Ref | - | - | |
| Yes | 80 | 19.6 | 0.7 (−1.3, 2.7) | - | - | |
| Not reported/unknown | 163 | 27.2 | ||||
| ART adherence in the previous year | 0.460 | - | - | |||
| ≥95 | 479 | 19.0 | Ref | - | - | |
| <95 | 52 | 20.0 | 0.9 (−1.5, 3.4) | - | - | |
| Not reported/unknown | 222 | 24.7 | - | - | ||
| World Bank country income grouping | 0.009 | <0.001 | ||||
| High | 292 | 19.7 | Ref | Ref | ||
| Upper-middle and lower-middle | 461 | 21.5 | 1.8 (0.4, 3.1) | −3.6 (−5.5, −1.7) | ||
Abbreviations: Ref-reference group; CI-confidence interval; MSM-men who have sex with men; ART-antiretroviral therapy; NRTI-non-nucleoside reverse transcriptase inhibitors; NNRTI-non-nucleoside reverse transcriptase inhibitors; PI-protease inhibitors; INSTI-integrase strand transfer inhibitors; STIs-sexually transmitted infections; WHODAS-World Health Organization Disability Assessment Schedule.
Discussion
To our knowledge, this is the first study to assess functional ability and health-related quality of life among PLWH screened positive for MH issues in the ambulatory care setting in Asia. The majority of PLWH attending routine HIV follow-up screened positive for depression and/or SU, with those positive for depression demonstrating poorer QoL and greater disability compared to those positive for SU. Both QoL and WHODAS scores demonstrated a strong consistent correlation with depressive symptom severity, demonstrating the utility of these patient-reported outcome measures (PROMs) to reflect clinical symptom burden in an out-patient HIV care setting. We found many of the same factors encompassing an individuals’ socio-economic and psycho-social environment to independently influence QoL scores across multiple domains, as well as functional ability, highlighting a profile of PLWH with depression/SU issues who may benefit from additional interventions to improve their overall health outcomes. Higher CD4 T-cell counts was associated with both higher QoL scores across multiple domains and better functional ability, underscoring that early ART initiation should be prioritized in all PLWH including those with issues of depression and SU.
Numerous country-specific studies have explored QoL among PLWH in Asia [17–19] but few have offered a regional perspective and none have specifically explored functional limitations in the context of depression and SU in the region. Mental well-being and mental distress lie along a continuum and most symptoms associated with mental disorders exist on a spectrum, with many present to some degree even in the “normal” population [20]. These symptoms become a disability when it impacts an individual’s ability to function. In our study, the overall scores for QoL and functional ability among PLWH in the region were significantly higher compared to the sub-group of PLWH reporting no issues of MH/SU. With close to 50% of PLWH reporting mild or worse depressive symptoms and 80% reporting ever using substance, the higher overall scores potentially reflect the major, insidious role these comorbidities have on health outcomes in PLWH in the region. These findings are also consistent with the lack of MH focus in the majority of HIV programs in the Asia-Pacific region as previously reported [12]. Despite a higher proportion of PLWH screening positive for SU compared to depression, QoL and functional ability scores indicated greater impairment in individuals with depression. Of note the majority screening positive for SU in our cohort were for low to moderate use of alcohol and tobacco and our study did not discern the use of substances for sexual activity which may be episodic and have less impact on daily functioning. Both QoL and functional ability demonstrated a consistent, incremental impairment with greater depressive symptom severity. Prior studies have also suggested that MH symptom severity accounts for a greater variance in perceptions of health and physical functioning above that predicted by HIV-related factors alone [21, 22]. These findings suggest that these PROMs may be used as a proxy for assessing MH in settings where MH stigma is a barrier to screening uptake.
PLWH reporting a stressful event in the past 5 years, a prior MH disorder or a family history of MH were all associated with poorer QoL scores with each factor impacting at least three of the six QoL domains assessed. A history of stressors and a prior history of MH were also associated with greater disability. Of note, a history of MH disorder had the biggest impact in adjusted disability scores (5 points) compared to all the other factors found to independently influence this outcome. The co-occurrence of traumatic stressful events and MH disorders is well described in the literature [23, 24] and people living or at risk of HIV, especially MSM, have reported significantly higher rates than the general population (reviewed in [25]). These same factors have also been associated with increased healthcare utilisation in PLWH and HIV disease progression [22, 26]. Our findings suggest that the cumulative burden of past trauma and MH identify a profile of PLWH who may be more vulnerable to functional deficits and poor quality of life and could potentially benefit from more intense monitoring and MH intervention even when prevalent depressive symptoms or reported SU are mild [21].
Prior studies have found socio-economic factors including lower household income and lower education levels to be associated with poorer QoL domain scores which was consistent with our study [17, 27]. However, we also found PLWH residing in LMIC settings was associated with better QoL scores across all domains and better functional ability compared to those residing in HI settings when controlling for all other significant covariates, a phenomenon which has also been reported in other multi-site studies spanning different socio-economic settings [28, 29]. This could potentially be attributed to an array of inter-setting/country differences which were not measured including lived experiences, perceptions of hardship, access to health resources, social and family support networks, resilience and health policy among others. For example, PLWH in LMIC settings may be more accepting and able to deal with and normalise their depressive symptoms from years of hardship, leading to it having less impact on their QoL and daily functioning compared to PLWH in HI settings. Social support among PLWH specifically has been shown to influence health outcomes differently across different income and/or culture settings [30, 31]. Individuals from HI/westernised settings tend to value independence more than LMIC settings which value inter-dependence. The influences and interactions of these factors are likely to be complex but could potentially be dissected through further qualitative studies to identify unique country/setting-specific mediators of QoL and disability in PLWH.
We additionally observed a number of HIV and treatment-related factors to positively impact functional ability including CD4 T-cell counts>500 cells/ul and receiving a INSTI-based vs an NNRTI-based regimen while not receiving ART was associated with greater disability, underscoring the extended benefits of initiating treatment early as has been highlighted in other studies [17, 28, 32]. We also found higher CD4 T-cell counts were consistently associated with better QoL scores across multiple domains as described in other studies [17, 33]. The findings of less severe disability in PLWH on INSTI- vs NNRTI-based regimen has not been previously described and we speculate that this could potentially be associated with drug-related effects including disturbances in sleep and concentration which have been reported to be more frequent with NNRTIs compared to INSTIs, even in the setting of stable disease [34].
There were several limitations in this study. First, we did not explore the influence of physical symptom burden especially chronic pain which has been reported to strongly impact outcomes of QoL and disability [35]. This omission could contribute to the variance in the disability and QoL scores measured in this cohort. Second, we cannot exclude the potential influence of cultural differences on the perception of ability or disability that may have influenced responses in the different countries the study was performed as previously described [28]. Third, as this was a cross sectional study design, we cannot infer any causation for the factors identified to be associated with QoL and disability. Finally, we used the 12-item version of WHODAS 2.0 and did not have data to assess specific domains of function with greater detail nor able to determine clinically significant thresholds of disability scores as we lacked normative data for this measure for PLWH in the region. This should be an area for future research.
In conclusion, we found PLWH screened positive for depression and SU to experience poorer quality of life and functional ability compared to those not screening positive, with greater impairment observed among those with more severe depressive symptoms and high risk substance use. PLWH experiencing a prior stressful event, previous diagnosis of MH or a family history of MH were associated with greater disability and poorer quality of life across multiple domains, suggesting a profile of PLWH who may benefit from greater support services during their routine HIV care. Higher CD4 T-cell counts was also associated with better QoL scores across multiple domains suggesting the extended benefits of early treatment even in PLWH with depression and SU issues. Further implementation studies are needed to develop integrated MH and HIV services to address the poor health-related outcomes associated with the high burden of MH and SU among PLWH in the Asia-Pacific region.
Supplementary Material
Acknowledgements
We would like to thank the patients at each study site who participated in this study.
The S2D2 Study Group: MP Lee, I Chan, YT Chan, SM Au, Queen Elizabeth Hospital, Hong Kong SAR; JY Choi, Na S, JH Kim, JM Kim, Yonsei University College of Medicine, Seoul, South Korea; I Azwa, R Rajasuriar, ML Chong, JY Ong, University Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco, MI Melgar, ES Gomez, Research Institute for Tropical Medicine, Muntinlupa City, Philippines; A Avihingsanon, C Padungpol, J Jamthong, S Thammasala, S Phonphithak, P Chaiyahong, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; AH Sohn, JL Ross, B Petersen, C Chansilpa, TREAT Asia, amfAR, Bangkok, Thailand; MG Law, A Jiamsakul, The Kirby Institute, UNSW Sydney, Australia.
This study was funded through a grant to amfAR, The Foundation for AIDS Research from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions above.
Footnotes
Competing interest
The authors declare no competing interests.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Langebeek N, Kooij KW, Wit FW, Stolte IG, Sprangers MAG, Reiss P, et al. Impact of comorbidity and ageing on health-related quality of life in HIV-positive and HIV-negative individuals. Aids 2017; 31(10):1471–1481. [DOI] [PubMed] [Google Scholar]
- 2.Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. The lancet HIV 2014; 1(1):e32–40. [DOI] [PubMed] [Google Scholar]
- 3.Group TW. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Social science & medicine (1982) 1995; 41(10):1403–1409. [DOI] [PubMed] [Google Scholar]
- 4.Ustün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010; 88(11):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarus JV, Safreed-Harmon K, Kamarulzaman A, Anderson J, Leite RB, Behrens G, et al. Consensus statement on the role of health systems in advancing the long-term well-being of people living with HIV. Nature Communications 2021; 12(1):4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kall M, Marcellin F, Harding R, Lazarus JV, Carrieri P. Patient-reported outcomes to enhance person-centred HIV care. The lancet HIV 2020; 7(1):e59–e68. [DOI] [PubMed] [Google Scholar]
- 7.Gooden TE, Gardner M, Wang J, Chandan JS, Beane A, Haniffa R, et al. The risk of mental illness in people living with HIV in the UK: a propensity score-matched cohort study. The lancet HIV 2022; 9(3):e172–e181. [DOI] [PubMed] [Google Scholar]
- 8.Hoare J, Sevenoaks T, Mtukushe B, Williams T, Heany S, Phillips N. Global Systematic Review of Common Mental Health Disorders in Adults Living with HIV. Curr HIV/AIDS Rep 2021; 18(6):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.End inequalities. End AIDS. Global AIDS strategy 2021–2026.. In. Geneva: Joint United Nations Programme on HIV/AIDS; 2021. [Google Scholar]
- 10.Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS 2019; 33(9):1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuah FLH, Haldane VE, Cervero-Liceras F, Ong SE, Sigfrid LA, Murphy G, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan 2017; 32(suppl_4):iv27–iv47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parcesepe AM, Mugglin C, Nalugoda F, Bernard C, Yunihastuti E, Althoff K, et al. Screening and management of mental health and substance use disorders in HIV treatment settings in low- and middle-income countries within the global IeDEA consortium. J Int AIDS Soc 2018; 21(3):e25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohn AH, Ross J, Wainberg ML. Barriers to mental healthcare and treatment for people living with HIV in the Asia-Pacific. J Int AIDS Soc 2018; 21(10):e25189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Integration of mental health and HIV interventions. Key considerations.. In. Geneva: Joint United Nations Programme on HIV/AIDS and the World Health Organization; 2022. [Google Scholar]
- 15.Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro M. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): manual for use in primary care. In. Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Simpson EH. The Interpretation of Interaction in Contingency Tables. Journal of the Royal Statistical Society: Series B (Methodological) 1951; 13:238–241. [Google Scholar]
- 17.Tran BX. Quality of life outcomes of antiretroviral treatment for HIV/AIDS patients in Vietnam. PLoS One 2012; 7(7):e41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasanah CI, Zaliha AR, Mahiran M. Factors influencing the quality of life in patients with HIV in Malaysia. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2011; 20(1):91–100. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A, Mohan A, Nandi PK, Rajeshwari K. Perceived social support, depression and their impact on quality of life of people living with HIV in India. AIDS care 2021; 33(10):1329–1334. [DOI] [PubMed] [Google Scholar]
- 20.Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018; 392(10157):1553–1598. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan MC, Wirtz MR, McKetchnie SM, Hart TA, Fitch C, Lazkani S, et al. The impact of depression and post-traumatic stress symptoms on physical health perceptions and functional impairment among sexual minority men living with HIV with histories of trauma. AIDS care 2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Cleirigh C, Skeer M, Mayer KH, Safren SA. Functional impairment and health care utilization among HIV-infected men who have sex with men: the relationship with depression and post-traumatic stress. J Behav Med 2009; 32(5):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng J, Tang C, Xiao X, Välimäki M, Wang H. Co-occurrence Pattern of Posttraumatic Stress Disorder and Depression in People Living With HIV: A Latent Profile Analysis. Front Psychol 2021; 12:666766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress 2013; 26(3):299–309. [DOI] [PubMed] [Google Scholar]
- 25.Brezing C, Ferrara M, Freudenreich O. The syndemic illness of HIV and trauma: implications for a trauma-informed model of care. Psychosomatics 2015; 56(2):107–118. [DOI] [PubMed] [Google Scholar]
- 26.Boarts JM, Sledjeski EM, Bogart LM, Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav 2006; 10(3):253–261. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro F, Canavarro MC, Pereira M. Factors associated with quality of life in middle-aged and older patients living with HIV. AIDS care 2016; 28 Suppl 1(sup1):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kietrys D, Myezwa H, Galantino ML, Parrott JS, Davis T, Levin T, et al. Functional Limitations and Disability in Persons Living with HIV in South Africa and United States: Similarities and Differences. Journal of the International Association of Providers of AIDS Care 2019; 18:2325958219850558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida Y, Kitayama S. Happiness and unhappiness in east and west: themes and variations. Emotion 2009; 9(4):441–456. [DOI] [PubMed] [Google Scholar]
- 30.Khamarko K, Myers JJ. The influence of social support on the lives of HIV-infected individuals in low- and middle-income countries. In: World Health Organisation; 2013.
- 31.Taylor SE. Social support. New York: Oxford University Press; 2007. [Google Scholar]
- 32.Myezwa H, Hanass-Hancock J, Ajidahun AT, Carpenter B. Disability and health outcomes - from a cohort of people on long-term anti-retroviral therapy. SAHARA J 2018; 15(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liping M, Peng X, Haijiang L, Lahong J, Fan L. Quality of Life of People Living with HIV/AIDS: A Cross-Sectional Study in Zhejiang Province, China. PLoS One 2015; 10(8):e0135705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twimukye A, Laker M, Odongpiny EAL, Ajok F, Onen H, Kalule I, et al. Patient experiences of switching from Efavirenz- to Dolutegravir-based antiretroviral therapy: a qualitative study in Uganda. BMC Infect Dis 2021; 21(1):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slawek DE. People living with HIV and the emerging field of chronic pain-what is known about epidemiology, etiology, and management. Curr HIV/AIDS Rep 2021; 18(5):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


