Abstract
A subset of functional regions within large RNAs fold into complex structures able to bind small-molecule ligands with high affinity and specificity. Fragment-based ligand discovery (FBLD) offers notable opportunities for discovery and design of potent small molecules that bind pockets in RNA. Here we share an integrated analysis of recent innovations in FBLD, emphasizing opportunities resulting from fragment elaboration via both linking and growing. Analysis of elaborated fragments emphasizes that high-quality interactions form with complex tertiary structures in RNA. FBLD-inspired small molecules have been shown to modulate RNA functions by competitively inhibiting protein binding and by stabilizing dynamic RNA states. FBLD is creating a foundation to interrogate the relatively unknown structural space for RNA ligands and for RNA-targeted therapeutics discovery.
Keywords: fragment-based ligand discovery, pockets, elaboration, linking and growing, RNA therapeutics
Graphical Abstract
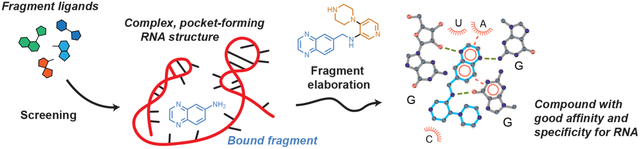
Overview
RNA lies upstream of nearly all biology. In principle, it is therefore possible to modulate diverse downstream cellular functions by targeting RNA. Certain regions in large RNA fold to form well-defined pockets capable of specific and high-affinity recognition of small-molecule ligands, supporting an enormous potential to manipulate RNA function [1,2]. Small-molecule-mediated modulation of RNA function could make possible targeting of difficult-to-drug, disease-implicated proteins and is now an intense focus of ongoing discovery efforts by pharmaceutical and academic groups [3,1,4–9]. Notable challenges must be overcome, however, before scalable, specific, and functional targeting of RNA with small-molecule ligands is routinely successful. Fragment-based ligand discovery (FBLD), well validated for clinical drugs that target proteins [10], is an attractive strategy to overcome these challenges in the design of small molecules that engage functional RNAs [11,12]. Here, we focus on recent innovations in RNA-targeted FBLD with an emphasis on elaborated fragment hits and high-quality fragment-inspired and fragment-related small molecule-RNA interactions.
Disclaimer: RNA-targeted FBLD is in its infancy
Although the promise of RNA-targeted drug discovery is expansive, the number of human designed or discovered classes of small molecules that bind RNA and alter biology is modest. Known RNA targeting molecules include a diverse set of natural products (many of which are too toxic for human use), the linezolid class of (highly successful) antibiotics, several splicing modulators (which likely function as molecular glues linking suboptimal splice sites to the spliceosome), and a handful of preclinical humandesigned molecules. In this challenging scenario, FBLD has strong potential for creating novel chemical matter targeting RNA. FBLD leverages small libraries comprised of simple, chemically diverse molecules (<300 molecular weight) that carry functional groups capable of forming high-quality interactions with the target molecule [10,12]. Our current understanding of FBLD as applied to RNA is limited. First, FBLD has been applied to RNA but only a handful of elaborated molecules, based on initial fragment hits, have been reported [13–21]. Second, no fragment-inspired lead molecule has yielded a highly potent pre-clinical molecule. Third, the druggable landscape of cellular RNA structures is poorly defined, as transcriptome-wide screening technologies are at intriguing, but very early, stages [22,23]. As a result, FBLD is far from creating a clinical drug that targets RNA. In this review, we will focus on what can be learned from critical analyses of published fragment-focused studies and will also extend our analysis to include a few “honorary” fragment-like small molecules. We highlight impactful examples where the initial potency of a molecule was enhanced through fragment elaboration, showcase high-quality fragment-inspired interactions with complex RNA structures, and outline therapeutic mechanisms for modulating RNA function with small-molecules ligands.
Brief introduction to FBLD
FBLD of RNA is, broadly, a two-step process: first, initial (low affinity) fragments that form high-quality contacts with RNA are identified, and second, these fragment hits are elaborated to yield more complex, potent, and specific small molecules that modulate RNA function. Robust and sensitive biophysical methods are required to detect low-affinity fragments that engage RNA. Many screening strategies have proven successful in RNA-targeted FBLD and have been expertly reviewed [12]. Identified fragment hits can be elaborated into more potent RNA binders by either linking or growing. If two fragments are identified that occupy the same RNA pocket, or pockets that are close in space, these fragments can be linked synthetically using a flexible or rigid chemical linker (Fig. 1A). Alternatively, a single fragment found to bind an RNA pocket can be synthetically grown into adjacent pocket space by adding functional groups or other fragment moieties (Fig. 1B). To date, there have been many examples of initial fragment hits that engage RNA [12], and two very recent studies show that it is possible to obtain high-affinity fragment-like hits for RNA [24,25]. Going forward, FBLD – with a focus on fragment elaboration – is well suited to fill current knowledge gaps in small molecule-RNA interactions, as FBLD efficiently and simultaneously interrogates both small molecule chemical space and RNA structural space.
Figure 1.
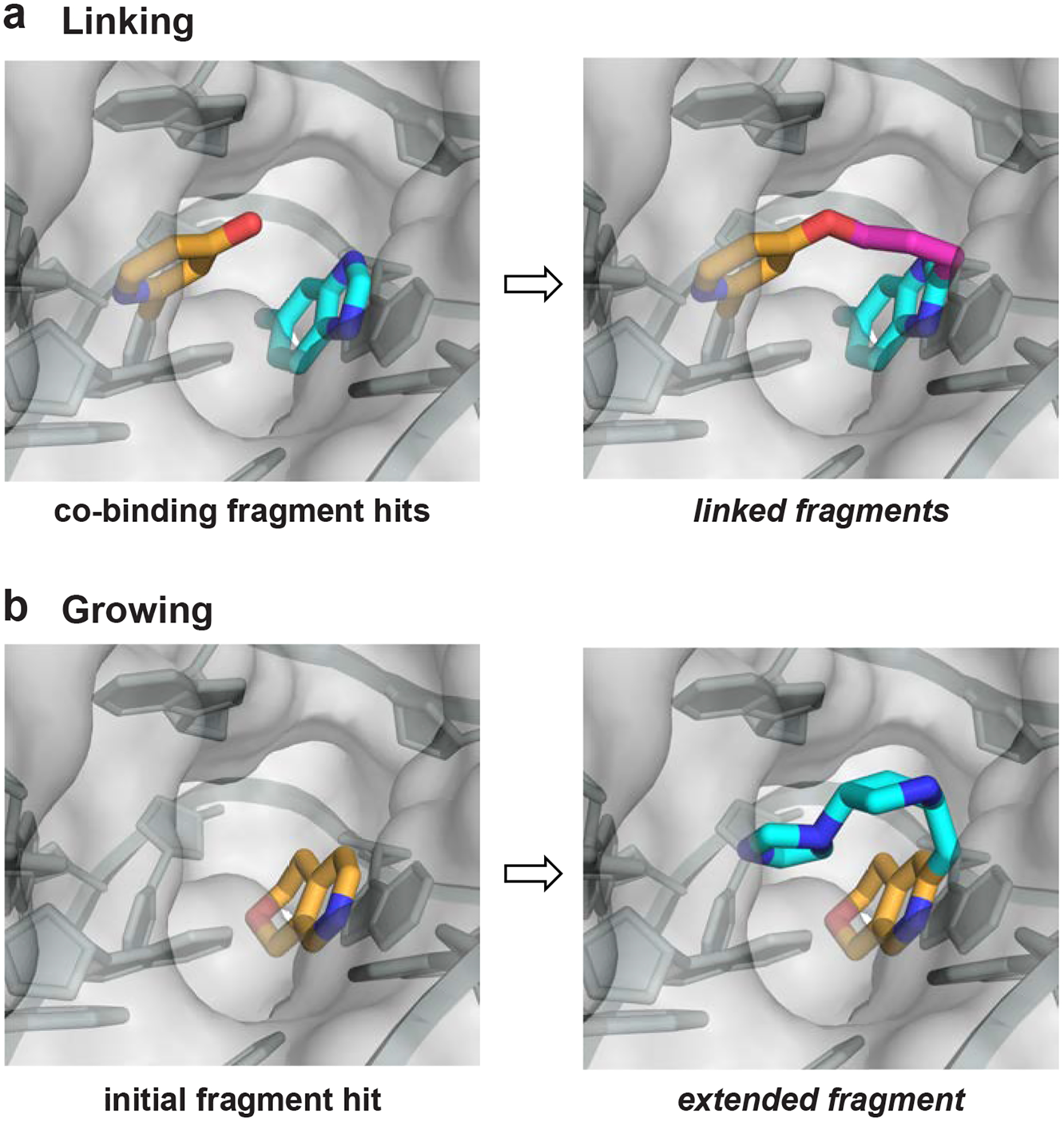
Strategies for elaboration of fragments that bind RNA. (a) Linking of co-binding fragments via a synthetic bridging group. (b) Growing a fragment into neighboring space of an RNA pocket by adding functional groups.
Fragment linking
The earliest reported example of fragment elaboration and optimization toward an RNA target used a strategy called structure-activity relationships by mass spectroscopy (SAR by MS) to identify two fragments, 1 and 2, that bind with low affinity to a segment of the bacterial 23S ribosomal RNA (Fig. 2A) [13]. These fragments were linked using a rigid furan (a flexible linker resulted in weaker binding). The linked fragments yielded compound 3 with low micromolar affinity and functional activity in cells (Fig. 2A). Binding measurements for fragment analogs informed linking of the fragment motifs, demonstrating that high-resolution structural information is not a requirement for successful FBLD toward RNA. A ligand that binds the D-arm of tRNALys3 was created by linking two independently binding fragments (4 and 5, with mM affinities), identified by NMR, creating low micromolar affinity compound 6, and corresponding to an impressive >1000-fold improvement in Kd upon fragment linking (Fig. 2A) [15].
Figure 2.
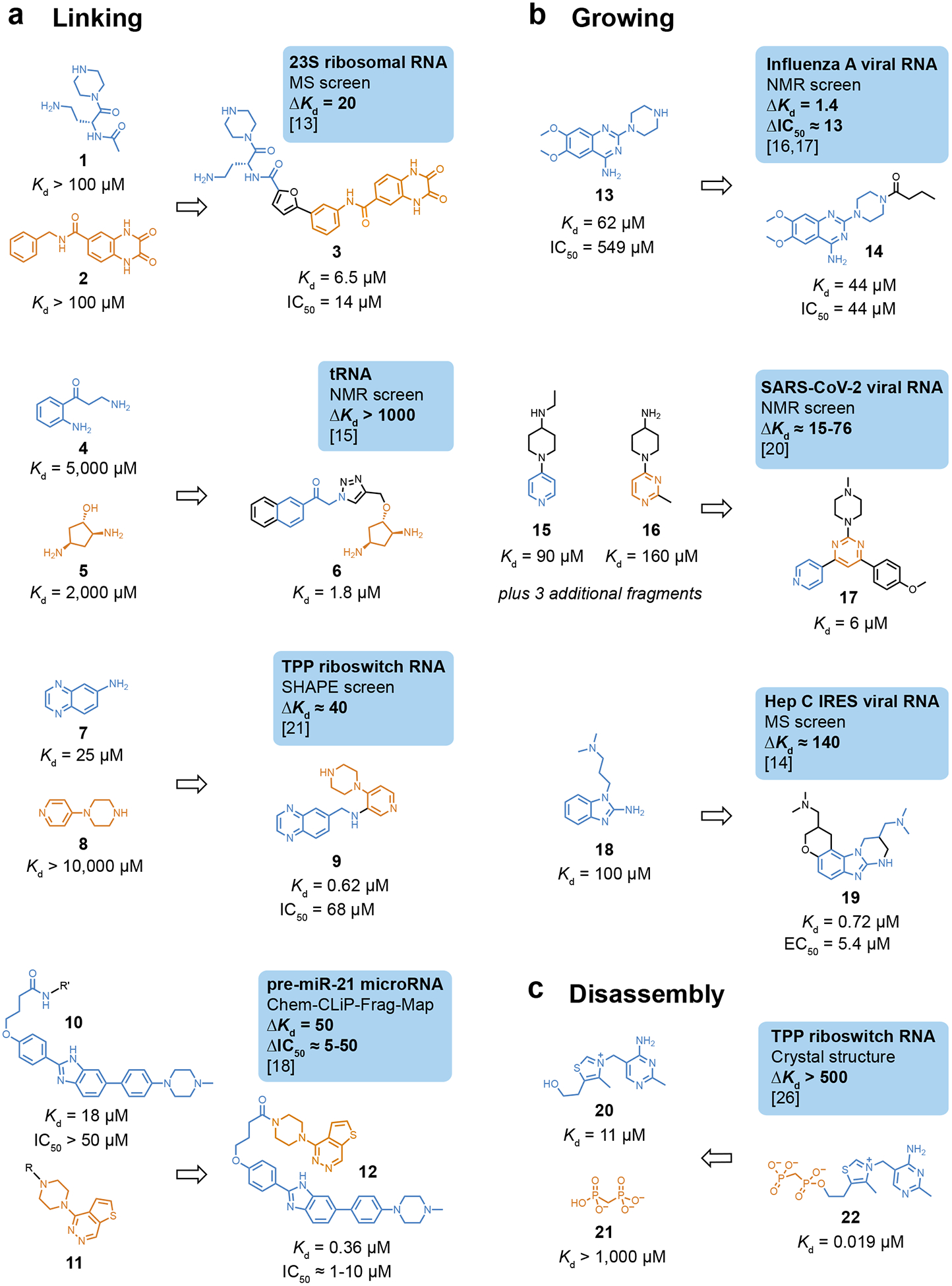
Examples of elaboration strategies for fragments targeting RNA. (a) Linking of co-binding fragments. (b) Growing an initially identified fragment. (c) Use of fragment disassembly to illustrate energetics of elaboration. Within a category, compounds are listed in order of increasing reported affinity. Boxes highlight the target, screening method, change in affinity upon elaboration, and reference; ΔKd and ΔIC50 are ratios, corresponding to fold-change. R, sites of photocrosslinking groups.
Two recent studies further emphasize that elaboration can be successful in the absence of high-resolution structural information. Two fragments were identified by SHAPE chemical probing (7, Kd 25 μM, and 8, Kd >10 mM) as cooperative co-binders to the thiamine pyrophosphate (TPP) riboswitch, and were linked to yield a compound with high nanomolar affinity (compound 9, Fig. 2A) [21]. This work emphasized that pernucleotide chemical probing information can guide compound elaboration. Compound 9 is one of the most druglike compounds identified as an RNA binder and induces conformational switching of the TPP riboswitch in a transcriptional assay (IC50 = 68 μM). Photocrosslinking with diazirine-linked fragments was used to identify compound 11 as a binder to a pre-miR-21 RNA [18]. This compound, when appended to known binder 10, furnished 12 as a mid-nanomolar binder to the pre-micro-RNA (Fig. 2A). Compound 12 has an IC50 of 1–10 μM in cellular assays of microRNA function. Here, the linkage to a photoreactive group also defined an accessible handle (R in Fig. 2A), facilitating efficient fragment linking to form 12.
Fragment growing and disassembly
Elaborations of initial fragments that broadly resemble fragment growing have been reported. A weak binding fragment to an RNA promoter element in the influenza A viral RNA (compound 13) was initially identified by NMR (Fig. 2B) [16,17]. The secondary amine on the piperazine moiety in 13 was subsequently modified to increase interactions with RNA. Compound 14, with a butyl amide at this position, showed a small effect on affinity but is a 12-fold better replication inhibitor, providing a reminder that biophysically-measured Kd should not be the sole focus for modulating RNA function with small molecules. In a second example, an NMR screen of a pseudoknot structure in the frameshifting element of SARS-CoV-2 viral RNA identified a family of five fragments with similar chemotypes, including compounds 15 and 16 (Fig. 2B) [20]. Compound 17 was selected using SAR by catalog as an “elaborated” molecule. The 6 μM Kd of compound 17 is a 15-fold improvement in affinity compared to initially selected fragment 15. Finally, MS was used to identify fragment hit 18, which binds weakly to the hepatitis C virus IRES element (Fig. 2B) [14]. Based on SAR by MS information from additional analogs, the compound was elaborated into 19, which has high nanomolar affinity for the RNA target and an EC50 of 5.4 μM in a cellular replicon assay.
Many natural (often metabolite) ligands bind RNA in pockets consisting of distinct subsites, including for the TPP riboswitch. TPP was disassembled into thiamine and a soluble (methylene-bridged) analog of pyrophosphate that independently engage distinct subsites (Fig. 2C) [26]. The “honorary” fragments 20 and 21 bind with micromolar to millimolar affinities and their re-linking yields 22, which binds with 20 nM affinity. This deconstruction experiment emphasizes that potent ligands can be created from relatively weakly binding starting compounds that bind subsites in a well-defined RNA pocket.
In sum, these examples highlight the ability of FBLD linking and growing strategies to deliver intriguing small-molecule leads against functional motifs in RNA.
Visualization of fragment and fragment-like interactions with RNA
Non-covalent interactions between RNA and small-molecule ligands consist primarily of π stacking and hydrogen bonding [27]. We analyzed available high-resolution models of fragment-based and fragment-like small-molecules bound with their target RNA. One group of these compounds binds simple stem-loop motifs in RNA (Fig. 3A–C). Compound 13 binds to a viral stem-loop structure, widens the major groove of the helix, and disrupts base pairing by forming two hydrogen bonds with neighboring cytosine bases (Fig. 3A) [16,17]. Fragment-like ligands 23 and 24 bind a hairpin that contains a bulged adenosine near a splice junction in the pre-mRNA encoding the Tau protein. Compound 23 binds in the major groove and forms two hydrogen bonds with the RNA backbone, involving non-bridging oxygen and 2’-hydroxyl groups; 24 intercalates into the helix and forms a direct hydrogen bond with a nearby adenosine (Fig. 3B and 3C) [28].
Figure 3.
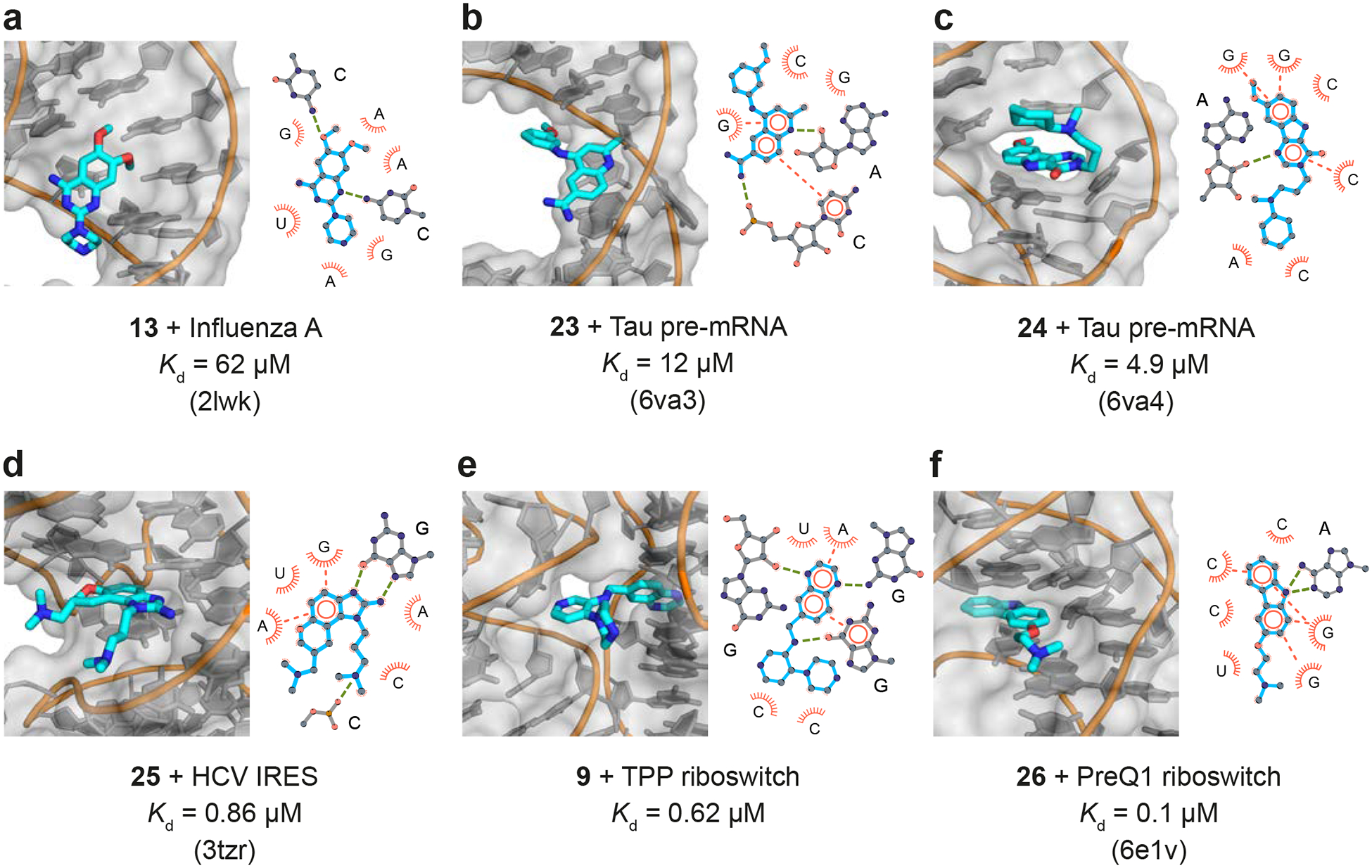
Three-dimensional structures (left) and interaction maps (right) for ligands that interact (a-c) with simple RNA motifs and (d-f) with complex RNA structures. In the interaction maps, hydrogen bonds are shown with green dashed lines; π stacking is illustrated with orange circles and dashes. For NMR structures (panels a-c), interpretations of average structures are shown. Structures and interaction maps were composed with PyMOL and LigPlot+, respectively.
Other ligands interact with complex RNA structures (Fig. 3D–F). Compound 25 (closely related to 19, see Fig. 2B) induces a conformational switch in the HCV IRES by intercalating in a hydrophobic pocket. The benzimidazole scaffold forms hydrogen bonds with the Hoogsteen face of a nearby guanosine nucleotide (Fig. 3D) [29]. The quinoxaline moiety of elaborated fragment 9 binds in the native thiamine-binding pocket of the TPP riboswitch, forming multiple hydrogen bonds, and the piperazine moiety appears to protrude into an electrostatic pocket normally occupied by a metal ion (Fig. 3E) [21]. Finally, the fragment-like ligand 26 binds to the PreQ1 riboswitch in the same overall site as the native ligand via multiple π-stacking and hydrogen-bonding interactions. A change in heteroatom in the central 5-membered ring, from oxygen to nitrogen, results in a 1-Å shift inside the pocket and formation of two hydrogen bonds (Fig. 3F) [30].
Potency reflects high-quality fragment contacts with RNA
For the RNA ligands with known structures discussed here, there is a roughly linear correlation between the total number of non-covalent stacking and hydrogen-bonding interactions formed with the RNA target and the free energy change due to binding (Fig. 4). The three ligands that form the fewest interactions (and also have the weakest affinities) interact with simpler base-paired RNA structures. In these structures of 13, 23 and 24 with their RNA targets, canonical base pairing is prevalent, and the nucleobases are only partially accessible in the grooves of the helices. In contrast, the three ligands that interact with more complex structures (in increasing order of affinity: 25, 9 and 26) bind with sub-micromolar affinity to their RNA targets, and each forms extensive stacking interactions and multiple hydrogen bonds with well-structured but non-base-paired nucleotides. These features are likely to be core requirements for specific and potent recognition of RNA by small molecules [1,2,24].
Figure 4.
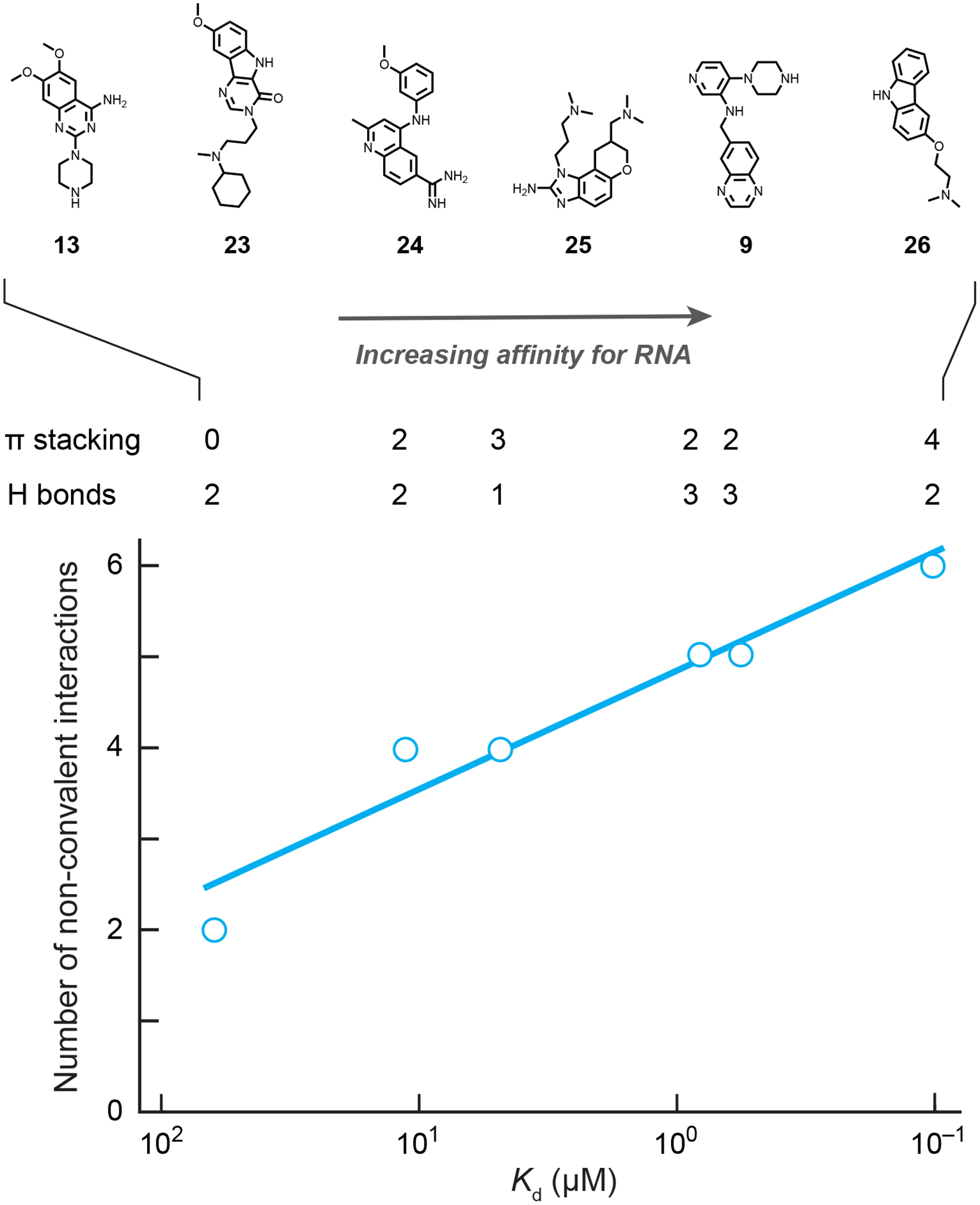
Qualitative correlation between binding affinity (Kd) and intermolecular hydrogen bonding and π-stacking interactions for fragment-like ligands that engage RNA. Note: plotting Kd values on a logarithmic scale yields a relationship proportional to ΔG. Complexes are the same as shown in Fig. 3.
Mechanisms for modulating RNA function
Fragment-derived compounds have been shown to modulate RNA-based functions via two primary mechanisms: through competitive binding to a protein-recognition site or through stabilization of an RNA structural motif (Fig. 5) The former mechanism has been exploited to disrupt viral replication (compound 14) [16,17] and to prevent microRNA maturation (compound 12) [18]. For compounds that act by structural stabilization, ligand binding induces a change in the relative populations of RNA conformational states. This mechanism has been exploited to stabilize specific states for viral frameshift and IRES elements (compounds 17, 19, 25) [29,20], to alter splicing (compounds 23, 24) [28], and to stabilize the bound states of riboswitches (compounds 9, 26) [11,30,21]. Ultimately, fragment-based ligands should be capable of modulating RNA-mediated function by any extant mechanism [3,6,7,9], including many not (quite yet) reduced to practice.
Figure 5.
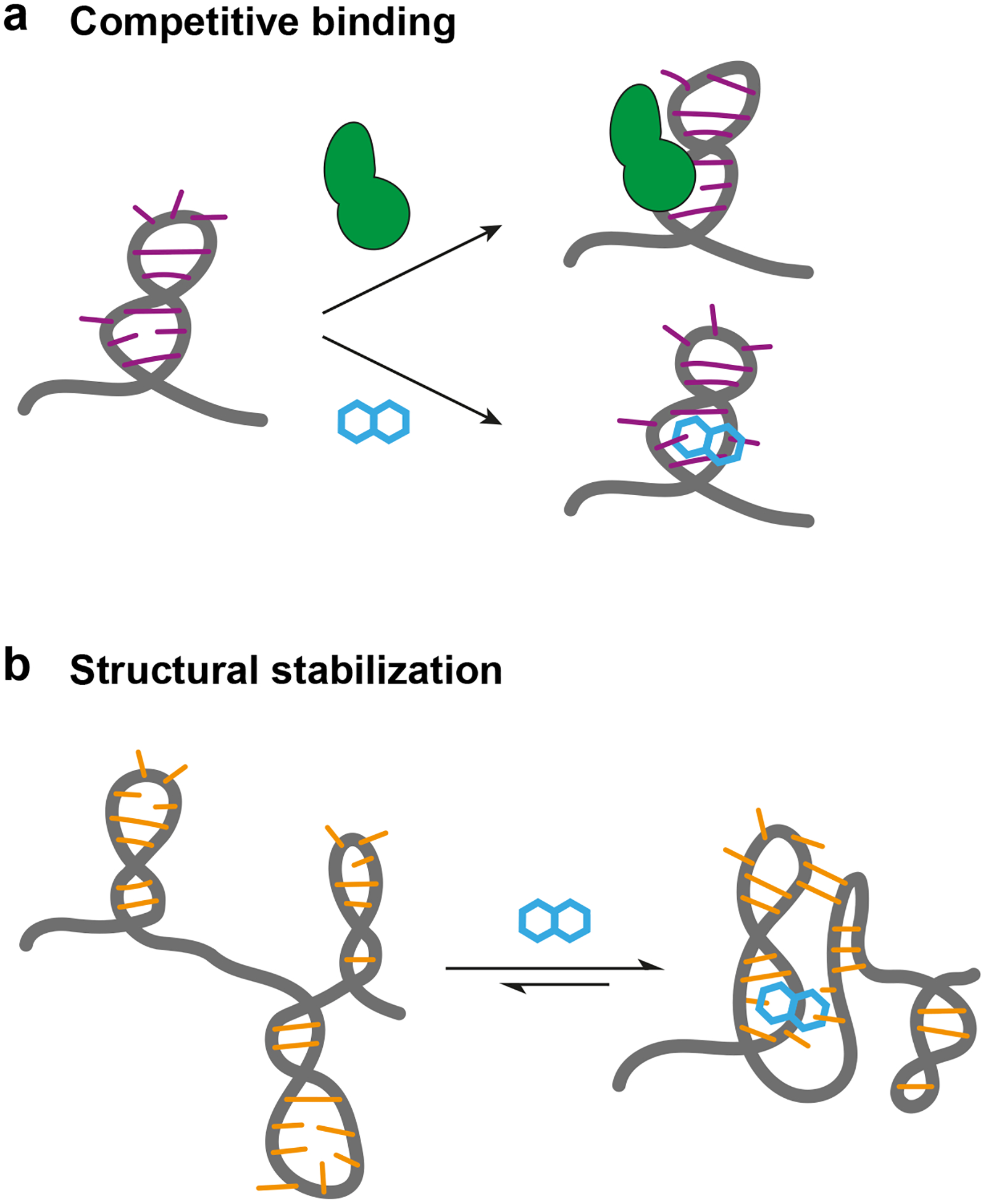
Mechanisms by which known fragment-based small molecules modulate RNA function. (a) Competition with RNA-binding proteins. (b) Stabilization of an RNA structure.
Summary
FBLD for RNA targets is at an early stage but holds enormous promise. We infer three key lessons from studies reported to date.
First, obtaining a fragment hit is just a first step. Fragment elaboration, either by linking or growing, is essential. Using conventional medicinal chemistry strategies, it appears relatively straightforward to elaborate fragments into sub-micromolar small-molecule ligands that engage RNA with good specificity. Intriguingly, multiple examples show elaboration can be successful in the absence of initial high-resolution structural information.
Second, the quality of the RNA target matters. Complex RNA tertiary structures form more and higher-quality interactions with fragment hits and their elaborated versions than do simple structures such as RNA stem-loop motifs. Target selection should focus on both RNA structural complexity and the potential mechanism by which ligand binding will influence RNA-based cellular function.
Finally, FBLD provides an opportunity to understand fundamental principles underlying the chemical space of elaborated ligands, the physicochemical RNA space they bind, and their mechanisms for modulating biological processes. The major obstacle to the realization of FBLD targeted toward RNA lies in the efficient identification of clinically validated RNA structures whose cellular function can be altered by ligand binding.
Highlights.
Fragment-based ligand discovery holds substantial promise for RNA-targeted therapeutics.
Fragment elaboration, either by linking or growing, is essential.
Elaboration can be successful without initial high-resolution RNA structural information.
Complex RNA motifs form more and higher-quality interactions with small-molecule ligands.
Modestly elaborated ligands engage RNA with sub-micromolar affinity and good specificity.
Acknowledgements
Work in our laboratory is supported by the US National Institutes of Health, the National Science Foundation, and industry partners. J.T.K. is a UNC Lineberger Integrated Training in Cancer Model Systems Fellow (T32CA009156) and an NIH Kirschstein Postdoctoral Fellow (F32GM143863).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
K.M.W. is an advisor to and holds equity in Ribometrix. Other authors declare no conflict of interest.
References
• of special interest
•• of outstanding interest
- 1.Warner KD, Hajdin CE, Weeks KM: Principles for targeting RNA with drug-like small molecules. Nat Rev Drug Discov 2018, 17:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt WM, Calabrese DR, Schneekloth JS: Evidence for ligandable sites in structured RNA throughout the Protein Data Bank. Bioorg Med Chem 2019, 27:2253–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connelly CM, Moon MH, Schneekloth JS: The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chemical Biology 2016, 23:1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umuhire Juru A, Patwardhan NN, Hargrove AE: Understanding the Contributions of Conformational Changes, Thermodynamics, and Kinetics of RNA–Small Molecule Interactions. ACS Chem Biol 2019, 14:824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giorgio AD, Duca M: Synthetic small-molecule RNA ligands: future prospects as therapeutic agents. Med Chem Commun 2019, 10:1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sztuba-Solinska J, Chavez-Calvillo G, Cline SE: Unveiling the druggable RNA targets and small molecule therapeutics. Bioorganic & Medicinal Chemistry 2019, 27:2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu A-M, Choi YH, Tu M-J: RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharmacol Rev 2020, 72:862–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamani F, Suzuki T: Synthetic RNA Modulators in Drug Discovery. J Med Chem 2021, 64:7110–7155. [DOI] [PubMed] [Google Scholar]
- 9.Childs-Disney JL, Yang X, Gibaut QMR, Tong Y, Batey RT, Disney MD: Targeting RNA structures with small molecules. Nat Rev Drug Discov 2022, 21:736–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlanson DA, Fesik SW, Hubbard RE, Jahnke W, Jhoti H: Twenty years on: the impact of fragments on drug discovery. Nat Rev Drug Discov 2016, 15:605–619. [DOI] [PubMed] [Google Scholar]
- 11.Warner KD, Homan P, Weeks KM, Smith AG, Abell C, Ferré-D’Amaré AR: Validating Fragment-Based Drug Discovery for Biological RNAs: Lead Fragments Bind and Remodel the TPP Riboswitch Specifically. Chemistry & Biology 2014, 21:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundquist KP, Panchal V, Gotfredsen CH, Brenk R, Clausen MH: Fragment-Based Drug Discovery for RNA Targets. ChemMedChem 2021, 16:2588–2603. [DOI] [PubMed] [Google Scholar]
- 13.Swayze EE, Jefferson EA, Sannes-Lowery KA, Blyn LB, Risen LM, Arakawa S, Osgood SA, Hofstadler SA, Griffey RH: SAR by MS: A Ligand Based Technique for Drug Lead Discovery Against Structured RNA Targets. J Med Chem 2002, 45:3816–3819. [DOI] [PubMed] [Google Scholar]
- 14.Seth PP, Miyaji A, Jefferson EA, Sannes-Lowery KA, Osgood SA, Propp SS, Ranken R, Massire C, Sampath R, Ecker DJ, et al. : SAR by MS: Discovery of a New Class of RNA-Binding Small Molecules for the Hepatitis C Virus: Internal Ribosome Entry Site IIA Subdomain. J Med Chem 2005, 48:7099–7102. [DOI] [PubMed] [Google Scholar]
- 15.Chung F, Tisné C, Lecourt T, Dardel F, Micouin L: NMR-Guided Fragment-Based Approach for the Design of tRNALys3 Ligands. Angewandte Chemie International Edition 2007, 46:4489–4491. [DOI] [PubMed] [Google Scholar]
- 16.Lee M-K, Bottini A, Kim M, Bardaro MF, Zhang Z, Pellecchia M, Choi B-S, Varani G: A novel small-molecule binds to the influenza A virus RNA promoter and inhibits viral replication. Chem Commun 2013, 50:368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottini A, De SK, Wu B, Tang C, Varani G, Pellecchia M: Targeting Influenza A Virus RNA Promoter. Chemical Biology & Drug Design 2015, 86:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh BM, Li W, Zhang P, Wang KW, Yildirim I, Parker CG, Disney MD: A general fragment-based approach to identify and optimize bioactive ligands targeting RNA. Proceedings of the National Academy of Sciences 2020, 117:33197–33203. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Functionalized, photoreactive fragments enabled capture of ligand binding to a microRNA and provided guidance for fragment linking. Linking to a known binder created a compound with mid nanomolar affinity and low micromolar biological activity.
- 19.Binas O, de Jesus V, Landgraf T, Völklein AE, Martins J, Hymon D, Kaur Bains J, Berg H, Biedenbänder T, Fürtig B, et al. : 19F NMR-Based Fragment Screening for 14 Different Biologically Active RNAs and 10 DNA and Protein Counter-Screens. ChemBioChem 2021, 22:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreeramulu S, Richter C, Berg H, Wirtz Martin MA, Ceylan B, Matzel T, Adam J, Altincekic N, Azzaoui K, Bains JK, et al. : Exploring the Druggability of Conserved RNA Regulatory Elements in the SARS-CoV-2 Genome. Angewandte Chemie International Edition 2021, 60:19191–19200. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A high-impact resource and tour de force for the FBLD field. Fifteen simple structures and 5 larger RNAs, derived from motifs in the SARS-CoV-2 viral RNA, were screened against a 768-member fragment library. Specific, promiscuous, and non-binding fragments were identified and characterized in the context of diverse RNA targets.
- 21.Zeller MJ, Favorov O, Li K, Nuthanakanti A, Hussein D, Michaud A, Lafontaine DA, Busan S, Serganov A, Aubé J, et al. : SHAPE-enabled fragment-based ligand discovery for RNA. Proceedings of the National Academy of Sciences 2022, 119:e2122660119. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• SHAPE RNA structure probing is used to identify co-binding fragment hits and inform elaboration via linking to create a fully non-native ligand that engages the TPP riboswitch with high nanomolar affinity. The resulting molecule has high ligand efficiency and druglikeness, and modulates RNA conformation during co-transcriptional folding.
- 22.Mukherjee H, Blain JC, Vandivier LE, Chin DN, Friedman JE, Liu F, Maillet A, Fang C, Kaplan JB, Li J, et al. : PEARL-seq: A Photoaffinity Platform for the Analysis of Small Molecule-RNA Interactions. ACS Chem Biol 2020, 15:2374–2381. [DOI] [PubMed] [Google Scholar]
- 23.Tong Y, Gibaut QMR, Rouse W, Childs-Disney JL, Suresh BM, Abegg D, Choudhary S, Akahori Y, Adibekian A, Moss WN, et al. : Transcriptome-Wide Mapping of Small-Molecule RNA-Binding Sites in Cells Informs an Isoform-Specific Degrader of QSOX1 mRNA. Journal of the American Chemical Society 2022, doi: 10.1021/jacs.2c01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menichelli E, Lam BJ, Wang Y, Wang VS, Shaffer J, Tjhung KF, Bursulaya B, Nguyen TN, Vo T, Alper PB, et al. : Discovery of small molecules that target a tertiary-structured RNA. Proceedings of the National Academy of Sciences 2022, 119:e2213117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suresh BM, Akahori Y, Taghavi A, Crynen G, Gibaut QMR, Li Y, Disney MD: Low-Molecular Weight Small Molecules Can Potently Bind RNA and Affect Oncogenic Pathways in Cells. J Am Chem Soc 2022, 144:20815–20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeller MJ, Nuthanakanti A, Li K, Aubé J, Serganov A, Weeks KM: Subsite Ligand Recognition and Cooperativity in the TPP Riboswitch: Implications for Fragment-Linking in RNA Ligand Discovery. ACS Chem Biol 2022, 17:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Proof-of-concept analysis of fragment binding, linking, and cooperativity by disassembling a known riboswitch-binding ligand into “honorary”, weak-binding fragments. Emphasizes the role of subsite binding in pockets of complex RNAs.
- 27.Padroni G, N. Patwardhan N, Schapira M, E. Hargrove A: Systematic analysis of the interactions driving small molecule–RNA recognition. RSC Medicinal Chemistry 2020, 11:802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JL, Zhang P, Abe M, Aikawa H, Zhang L, Frank AJ, Zembryski T, Hubbs C, Park H, Withka J, et al. : Design, Optimization, and Study of Small Molecules That Target Tau Pre-mRNA and Affect Splicing. J Am Chem Soc 2020, 142:8706–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dibrov SM, Ding K, Brunn ND, Parker MA, Bergdahl BM, Wyles DL, Hermann T: Structure of a hepatitis C virus RNA domain in complex with a translation inhibitor reveals a binding mode reminiscent of riboswitches. Proceedings of the National Academy of Sciences 2012, 109:5223–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connelly CM, Numata T, Boer RE, Moon MH, Sinniah RS, Barchi JJ, Ferré-D’Amaré AR, Schneekloth JS: Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure. Nat Commun 2019, 10:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Impactful example of structural characterization of a fragment-like molecule, applied to a riboswitch RNA. Changing a single heteroatom from O to N, induces a shift in binding pose and increases binding affinity.


