Abstract
Objectives:
Voluntary medical male circumcision (VMMC) is an important component of combination HIV prevention. Inclusion of traditionally circumcised HIV negative men in VMMC uptake campaigns may be important if traditional male circumcision is less protective against HIV acquisition than VMMC.
Methods:
We used data from the HPTN 071 (PopART) study. This cluster-randomized trial assessed the impact of a combination prevention package on population-level HIV incidence in 21 study communities in Zambia and South Africa.We evaluated uptake of VMMC, using a two-stage analysis approach and used discrete-time survival analysis to evaluate the association between the types of male circumcision and HIV incidence.
Results:
10,803 HIV-negative men with self-reported circumcision status were included in this study. At baseline, 56% reported being uncircumcised, 26% traditionally circumcised and 18% were medically circumcised. During the PopART intervention, 11% of uncircumcised men reported uptake of medical male circumcision. We found no significant difference in the uptake of VMMC in communities receiving the PopART intervention package and standard of care (adj rate ratio=1·10 (95% CI 0·82, 1·50, P=0·48)). The rate of HIV acquisition for medically circumcised men was 70% lower than for those who were uncircumcised adjHR=0·30 (95% CI 0·16 to 0·55; p<0·0001). There was no difference in rate of HIV acquisition for traditionally circumcised men compared to those uncircumcised adjHR= 0·84 (95% CI 0·54, 1·31; P=0·45).
Conclusions:
Household-based delivery of HIV testing followed by referral for medical male circumcision did not result in substantial VMMC uptake. Traditional circumcision is not associated with lower risk of HIV acquisition.
Keywords: HIV incidence, circumcision, male, prevention, prevalence, South Africa, Zambia
Introduction
Male circumcision is practiced in many parts of the world for social, cultural, and medical reasons[1]. There is substantial evidence that Medical male circumcision (MMC) is protective against HIV infection·Randomized controlled trials have shown that MMC reduces the risk of HIV acquisition by approximately 60% amongst heterosexual men[2-4]. As result, in 2007 the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) recommend voluntary MMC (VMMC) as a key component of combination HIV prevention in countries with generalized epidemics and low prevalence of male circumcision[5]. Fifteen counties, including South Africa and Zambia, have been identified as priority countries with a target to expand medical male circumcision coverage among eligible population to 80%.[6]
VMMC scale-up has been challenging· Despite a decade of effort to increase uptake of VMMC in men, through multiple different implementation strategies, such as pop-up surgery campaigns[7], deployment of non-surgical devices[8], and infant circumcision[9],most priority countries are still working towards achieving the 80% target. Nevertheless, VMMC remains an important component of combination prevention strategies that implement multiple known effective interventions to reduce the risk of HIV infection. In the HPTN 071 (PopART) combination prevention intervention, a comprehensive HIV prevention package was delivered house-to-house including HIV testing, referral to HIV prevention and treatment services, and referral for VMMC for men testing HIV-negative.
VMMC coverage remains far below the WHO/UNAIDS 80% target in both Zambia and South Africa[10]. Nevertheless, traditional circumcision (TMC) is widely practiced in some communities in Zambia and South Africa [11,12]. These forms of male circumcision are predominantly a cultural practice, particularly as a rite of passage to adulthood in many Southern African communities[12]. TMC is usually performed in a non-clinical setting by a traditional practitioner without formal medical training [13,14]. Although previous studies have shown that TMC, as practiced in many regions, can be protective [15,16] compared to no circumcision, there is a substantial variation across cultures in the tools used and the proportion of foreskin removed. The biological mechanism of protection for male circumcision is thought to be the reduction of HIV target cells including Langerhans’ cells in the foreskin, although other factors may also play a part[17]. However, unlike VMMC, part or all of the foreskin may be left intact during TMC, depending on how the TMC is performed[1]. It is therefore an open question as to whether TMC is as effective as VMMC for reducing the risk of HIV acquisition, or whether VMMC should be encouraged irrespective of TMC.
In this study we investigated two questions about male circumcision and HIV, using data from men followed in a large-scale cluster-randomized trial (HPTN 071/PopART) conducted in 21 communities in Zambia and South Africa. First, we evaluated the impact of the combination prevention package, which included referrals for VMMC, on the uptake of VMMC in HIV-negative men. Second, we examined associations between TMC, VMMC, and no circumcision and risk of HIV infection in men.
Method
Study design:
We used data from the HPTN 071 study of the Population Effects of Antiretroviral therapy to Reduce HIV Transmission (PopART) intervention. This cluster-randomized trial assessed the impact of a combination prevention package on population-level HIV incidence in Zambia and South Africa. A total of 21 urban study communities (12 in Zambia and 9 in South Africa) were matched into 7 matched triplets (4 triplets in Zambia and 3 in South Africa) based on geographic location and HIV prevalence. In each triplet, one community was randomly selected to receive the full PopART intervention (Arm A), a second community received the full intervention except that ART was offered according to current local guidelines (Arm B) and the third continued with the current standard of care (Arm C). The intervention in Arms A and B included at least annual house-to-house visits by HIV community care providers, with an offer of in-home HIV testing, referrals, and follow-up for local provision of VMMC, STI treatment, and support for linkage to ART at the local clinic. Condoms were also distributed. To measure the impact of the intervention, a research cohort called the Population Cohort, consisting of adults aged 18–44 years, were recruited from randomly selected household in the population in each of the 21 communities (an overall total of 48,302 across all communities) and was followed up once a year for three years to measure HIV incidence and other outcomes. At each follow-up visit, demographics, socioeconomic, and behavioral data as well as data related to HIV prevention, diagnosis, and treatment were collected. Questions for men included self-report of whether they were circumcised (Yes/No/Don’t know/Prefer not to answer) and who carried out the circumcision (medical worker/traditional practitioner). No clinical examination occurred. Blood was collected from participants for laboratory-based HIV testing at each visit; testing was performed in central laboratories in South Africa and Zambia and additional testing to confirm seroconversions occurred at the HPTN Laboratory Center (Baltimore).
Ethical considerations:
Review of the trial protocol was carried out by the ethics committees of the University of Zambia, Stellenbosch University, and London School of Hygiene and Tropical Medicine. All participants provided written informed consent.
Study population:
For the analysis of the association between circumcision and HIV incidence, we included male participants who were HIV-negative at enrollment with at least one reported circumcision status in follow-up visits. To evaluate the impact of the intervention on uptake of medical male circumcision, we restricted the population to those who were HIV negative and self-reporting uncircumcised baseline. In a sensitivity analysis, we assessed the intervention impact among those uncircumcised or traditionally circumcised at baseline.
Statistical Methods:
Male circumcision was defined as uncircumcised, medical (VMMC, by a medical worker at a government clinic, NGO, or doctor’s office), or traditional (TMC, by a traditional practitioner). Missing circumcision status was selectively imputed as follows: if a participant reported VMMC at a visit, VMMC was imputed if missing at subsequent visits; if a participant reported not circumcised at a visit, not circumcised was imputed if missing for earlier visits. We excluded men with discordant circumcision status (e.g. report uncircumcised status after prior report of VMMC or TMC, or report TMC status after prior VMMC report) from the analysis. As a sensitivity analysis, for participants with discordant circumcision report, we included their data up to the visit where discordant circumcision status was reported.
To evaluate the effect of the community level PopART intervention on uptake of voluntary medical male circumcision, we used a two-stage analysis approach, recommended for cluster-randomized trials with fewer than 15 clusters per group[18]. Intervention effects are estimated within matched triplets, which were matched for urban/rural in addition to HIV epidemic characteristics (HIV prevalence, ART uptake). As the implementation of referrals for VMMC was the same in Arms A and B, intervention effects were averaged across both intervention arms. In the first stage, Poisson regression was used to predict the rate of medical male circumcision, adjusted for age and baseline prevalence of medical male circumcision, from which adjusted rate ratios of observed versus predicted were calculated for each community. In the second stage, two-way ANOVA of the log adjusted rate ratios by triplet and arm estimated the intervention effect; confidence intervals were computed using the MSE from the two-way ANOVA to compare arms A and B combined against arm C. Analyses were conducted using R software 4.0 and Stata version 15 (Stata Corp, College Station, TX).
Discrete-time survival analysis was used to evaluate the association between circumcision status reported at the previous visit and HIV incidence in the following year. For seroconverters, HIV infection was assumed to occur at the midpoint between the last HIV-negative sample and the first HIV-positive sample, or at a visit in which acute infection was identified. We adjusted for age at the previous visit as a fixed effect and community as a random effect.
Results
Approximately 14,000 men were enrolled in the population cohort of HPTN 071 (PopART). After excluding those who were HIV positive or unknown HIV status at enrollment (N=2062), missing circumcision status at every study visit (N=202), and those with discordant circumcision reports (N=955), 10,803 HIV-negative men with self-reported circumcision status were included in this study. Discordant report of circumcision status was equally distributed among treatment arms. Before imputation, 30% of visits were missing circumcision status and after imputation, circumcision status was missing from 14% of visits. A similar number of men were recruited in each study arm, with approximately 55% from 12 communities in Zambia, and 45% from 9 communities in South Africa; 51% were below the age of 25 (Table 1).
Table 1.
Demographic characteristics of HIV-negative men at enrollment.
| Characteristics | Overall (N=10,803) |
Not circumcised (N=5,974) |
Medical (N=1,889) |
Traditional (N=2,713) |
|---|---|---|---|---|
| Randomization Arm | ||||
| PopART: Arm A | 3606 (33%) | 1915 (32%) | 630 (33%) | 974 (36%) |
| PopART: Arm B | 3472 (32%) | 1897 (32%) | 585 (31%) | 921 (34%) |
| PopART: Arm C | 3725 (35%) | 2162 (36%) | 674 (36%) | 818 (30%) |
| Country | ||||
| Zambia | 5914 (55%) | 3981 (67%) | 1575 (83%) | 267 (10%) |
| South Africa | 4889 (45%) | 1993 (33%) | 314 (17%) | 2446 (90%) |
| Age (years) | ||||
| 25+ | 5278 (49%) | 2811 (47%) | 653 (35%) | 1687 (62%) |
| 18-24 | 5525 (51%) | 3163 (53%) | 1236 (65%) | 1026 (38%) |
| Marital Status | ||||
| Married or living as married | 2495 (23%) | 1593 (27%) | 366 (19%) | 495 (18%) |
| Never married | 7755 (72%) | 4053 (68%) | 1445 (76%) | 2128 (78%) |
| Divorced, separated or Widowed | 466 (4%) | 302 (5%) | 75 (4%) | 84 (3%) |
| Number of sexual partners (past 12 months) | ||||
| 0-1 | 8839 (82%) | 5068 (85%) | 1552 (82%) | 2082 (77%) |
| 2+ | 1347 (13%) | 608 (10%) | 270 (14%) | 453 (17%) |
| Condom use during last sex event (past 12 months) | ||||
| Yes | 3356 (31%) | 2009 (34%) | 590 (31%) | 709 (26%) |
| No | 6920 (64%) | 3672 (62%) | 1237 (66%) | 1900 (70%) |
At baseline, 56% were uncircumcised, 26% were traditionally circumcised and 18% were medically circumcised. The prevalence of TMC and VMMC was different between South Africa and Zambia; 83% of men who reported being medically circumcised were from Zambia, 90% of men who reported traditional circumcision were from South Africa. These disparities also existed across communities: prevalence of traditional male circumcision was higher than 50% in six out of the nine communities in South Africa (these 6 communities comprised two matched triplets and the vast majority of residents were from the same ethnic group). In Zambia, the prevalence of traditional male circumcision ranged from 3% to 13%. The prevalence of medical male circumcision at baseline in communities in South Africa ranged from 4% to 23% while in Zambia the prevalence of medical male circumcision ranged from 15% to 42% [supplemental Figure 1].
Effect of the intervention on medical male circumcision uptake
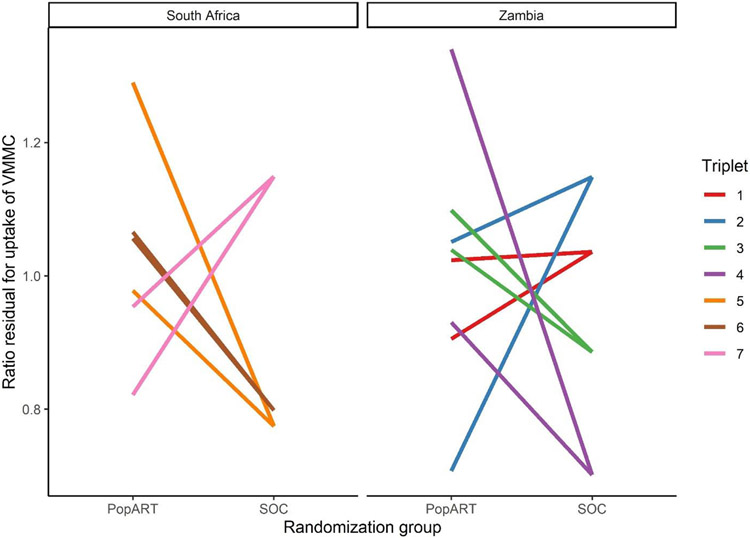
Among 5,974 HIV negative men who were uncircumcised at baseline, 676 (11%) reported uptake of medical male circumcision during follow-up (61% in Zambia vs 39% in South Africa), with similar uptake in PopART communities compared to the standard of care (Table 2): the adjusted rate ratio in PopART communities compared to standard of care was 1·10 (95% CI 0·82, 1·50, P=0·48), indicating a non-significant 10% higher uptake of VMMC in communities randomized to the PopART intervention. VMMC uptake was higher in PopART than the standard of care communities in four triplets (Figure 1). Similar results were seen when including traditionally circumcised men: among 8687 men, 854 (9·8%) reported uptake of VMMC, and the adjusted rate ratio was 0·92 (95% CI 0·71-1·18). The results of the sensitivity analysis also indicate similar uptake of VMMC among PopART communities compared to the standard of care [Supplemental Table 1]
Table 2:
The effect of PopART intervention on medical male circumcision among HIV-negative men who were not medically circumcised at enrollment.
| Arm | VMMC uptake | Unadjusted rate ratio (95% CI) |
Adjusted rate ratio (95% CI)1 |
P value |
|---|---|---|---|---|
| Among uncircumcised men (n = 5974) | ||||
| PopART communities | 418/3812 (10·5%) | 1·08 (0·97-1·29) | 1·10 (0·82-1·50) | 0·48 |
| Standard of care communities | 258/2162 (11·9 %) | Ref. | Ref. | |
| Among uncircumcised and traditionally circumcised men (n = 8687) | ||||
| PopART | 527/5707 (9·2%) | 0·89 (0·75-1·06) | 0·92 (0·71-1·18) | 0·46 |
| Standard of care | 327/2980 (11·0%) | Ref. | Ref. | |
Adjusted for age, baseline VMMC prevalence and triplet
Figure 1:
Observed relative change in VMMC among uncircumcised men at baseline as a result of the PopART intervention within each triplet. Ratio residuals (ratio of observed to expected medical male circumcision) are adjusted for age and baseline medical male circumcision prevalence. Two PopART intervention communities are shown compared with the single standard of care (SOC) community in each triplet. A ratio residual of 1·0 corresponds to no increase relative to the community means.
Effect of male circumcision on HIV incidence:
During the three-year follow-up period, 142 seroconversions were observed in men. The HIV incidence among those who were medically circumcised was 0·31 per 100 person-years (PY), among those traditionally circumcised was 0·94/100 PY and among uncircumcised was 0·97/100 PY (Table 3). The rate of HIV for medically circumcised men was 70% lower than for those who were uncircumcised adjHR=0·30 (95% confidence interval [CI], 0·16 to 0·55; p<0·0001). There was no difference in rate of HIV for traditionally circumcised compared to those uncircumcised adjHR= 0·84 (95% CI 0·54, 1·31; P=0·45).
Table 3:
Male circumcision status and HIV incidence during HPTN 071 follow-up.
| Circumcision status |
Incidence HIV infections (rate per 100 person-yr) |
Adjusted hazard ratios1 (95% CI) |
P value |
|---|---|---|---|
| Medical | 11/3458 (0·31) | 0·30 (0·16, 0·55) | <0·0001 |
| Traditional | 39/4166 (0·94) | 0·84 (0·54, 1·31) | 0·45 |
| Uncircumcised | 92/9402 (0·97) | Ref. | Ref. |
Adjusted for community and age.
Discussion:
In this large-scale population-based study in Zambia and South Africa, we found that traditional circumcision was not associated with a protective effect for HIV acquisition, with very similar rates of infection occurring in traditionally circumcised and uncircumcised men. Medical male circumcision was associated with a 70% reduction in risk of new HIV infection, consistent with the 60% reduction observed in the RCTs demonstrating the strong protective effect of VMMC[2-4]. The communities randomized to the PopART combination prevention intervention did not have a significant increase in uptake of VMMC compared to the standard of care communities.
Our findings complement and strengthen the evidence from previous studies in South Africa[19] and Lesotho[20], where analysis of cross-sectional data found no association between HIV status and whether the participant had been traditionally circumcised or was uncircumcised. However, our results differ from studies in Eastern Africa, which found TMC to be associated with lower risk of HIV compared to no circumcision[15,21], possibly emphasizing the need to take into account the type of traditional circumcision carried out. Unlike VMMC, the amount of foreskin removed during a traditional male circumcision can vary between cultures, some may remove it entirely while others remove a small amount of foreskin or leave it intact[22,23]. Additionally, previous studies conducted in Southern African countries have found that for those who self-reported being circumcised by traditional practitioners in those countries, only a small percentage had sufficient removal of the foreskin for HIV prevention[23]. The lack of protective effect of TMC against HIV acquisition among communities in Zambia and South Africa could be due to the variation in the amount of foreskin removed during circumcision.
Medical male circumcision is known to substantially reduce the risk of HIV acquisition in men and could contribute to epidemic control in high HIV burden southern African countries. However, achieving high uptake has been challenging, despite substantial implementation efforts [24]. Uptake of medical male circumcision was modest in our study, with 11% of uncircumcised men and 6% of traditionally circumcised men reporting medical circumcision by the end of the 3 years follow-up time; the PopART house-to-house intervention did not have a significant impact on uptake. Imperfect implementation of the VMMC referral as well as prioritization of other elements of the HIV combination prevention package might have contributed to low VMMC uptake in our study. In addition, the low uptake of VMMC could potentially be explained by relatively high background traditional circumcision rates in some communities. Previous studies have shown that going against cultural beliefs and associated anticipated stigma can deter men living in communities with a high rate of traditional male circumcision from opting for VMMC[25]. Additionally, in many settings VMMC is regarded as less culturally acceptable or prestigious than TMC[25,26]. To improve community support for VMMC, intervention efforts should engage religious and tribal leaders[27]. Engagement needs to respect cultural traditions [28], and messaging needs to be tailored to specific communities, and not only focused on HIV prevention[27]. VMMC is a cost-effective [29,30] and a onetime intervention that offers HIV protection and VMMC scale-up should a high priority.
This work highlights the need to differentiate between VMMC and TMC, and to understand the clinical, and protective outcomes of different types of traditional circumcision. Current DHS surveys include a single question: “Are you circumcised?” with possible responses “yes”, “no” and “don’t know”[31]. Prevalence measures of circumcision are usually derived from DHS data, and these may be misinterpreted as ‘protected’ in the context of HIV. These data form the basis for inputs into mathematical modeling such as the EPP/SPECTRUM model[30],which are in turn used to guide national and global HIV intervention efforts. In addition, countries with high levels of TMC that may not be effective in decreasing HIV acquisition risk may need to be added to the countries prioritized by UNAIDS and WHO for VMMC scale-up. In future studies examining circumcision, a clinical examination should ideally be carried out, but even a small amendment to existing questions to differentiate “full” and “partial” circumcision, possibly using pictures to help guide respondents, may be informative regarding the extent to which they are protected against HIV[12].
Limitations:
We acknowledge that our study has several limitations. Data on circumcision status were self-reported, and a previous study showed that men may report being circumcised with no clinical evidence[22]. Circumcision is a sensitive topic for disclosure, and we cannot rule out the potential effect of social desirability bias, although it is not clear whether men would be more inclined to report one status over another. This is especially true for the South African data where traditionally talking about circumcision with a woman is highly taboo; many of study field staff were women. We excluded about 8% of eligible participants from this analysis for discordant reporting of circumcision. Additionally, 14% of visits included in the analysis had missing circumcision status as a result of missing self-report. Thus, we cannot rule out the potential for selection bias. We adjusted for the community to control for differences in male circumcision types across communities. However, it is possible that the adjustment might not be complete and there might be residual confounding that could have weakened the association reported for TMC. Despite these limitations, this represents a large study involving men in the general population aged 18-44 in sub-Saharan Africa incorporating information on circumcision, differentiating between traditional and medical, with direct measurement of HIV incidence.
Conclusions
Evidence from this large community-based study showed that traditional male circumcision as practiced in Southern Africa is not protective against HIV acquisition. These findings have implications for the importance of better characterization of male circumcision in questionnaires and /or clinical assessments, and future determining targets for VMMC programs, and for education and advocacy programs, to ensure correct messaging. Household-based delivery of HIV testing followed by referral for medical male circumcision did not result in substantial MMC uptake; implementation of male circumcision remains a challenging but an important prevention goal.
Supplementary Material
Acknowledgment:
We wish to acknowledge partners in South Africa including PEPFAR partners (Kheth’Impilo, ANOVA Healthcare and the SACTWU Worker Health Program) and City of Cape Town and Western Cape Government department of health colleagues who have worked to implement the HPTN 071 (PopART) trial activities, as well as partners in Zambia including the Zambian Ministry of Health, CIDRZ, ZPCT II and JSI. The team acknowledges the work of the administrative and support teams at the institutions involved in this trial and the hundreds of field staff who delivered the intervention and collected the research data, all the communities and participants which took part in the study without whom the work would not have been possible. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIMH, NIDA, PEPFAR, 3ie, or the Bill & Melinda Gates Foundation.
Funding:
HPTN 071 (PopART) was funded by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR). Additional funding is provided by the International Initiative for Impact Evaluation (3ie) with support from the Bill & Melinda Gates Foundation,
RH, SFl, KSa are jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, which is also part of the EDCTP2 programme supported by the European Union. Grant Ref: MR/R010161/1
SFi acknowledges funding from the Imperial College National Institute for Health Research Biomedical Research Centre
Footnotes
Declaration of interests:
CF reports payments from the Public Health Company and the University of Oxford.GH has funding from the London School of Tropical Medicine. NM reports the HPTN Scholars grant. All other authors declare no competing interests.
Contributor Information
Kidist Zewdie, University of Washington, Department of Epidemiology, Seattle, Washington USA.
Michael Pickles, Medical Research Council Centre for Global Infectious Disease Analysis, School of Public Health, Imperial College London, London, UK.
Sian Floyd, Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, UK.
Sarah Fidler, Department of Medicine, Imperial College London, UK.
Helen Ayles, ZAMBART, University of Zambia, School of Medicine, Ridgeway Campus, Lusaka, Zambia; Department of Clinical Research, London School of Hygiene and Tropical Medicine, UK.
Peter Bock, Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa.
Graeme Hoddinott, Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa.
Nomtha Mandla, Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa.
Kwame Shanaube, ZAMBART, University of Zambia, School of Medicine, Ridgeway Campus, Lusaka, Zambia.
Musonda Simwinga, ZAMBART, University of Zambia, School of Medicine, Ridgeway Campus, Lusaka, Zambia.
Christophe Fraser, Big Data Institute, Nuffield Department of Medicine, University of Oxford, Oxford, UK..
Janet Seeley, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, UK.
Estelle Piwowar-Manning, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland USA..
Richard Hayes, Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, UK.
Deborah Donnell, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Data sharing:
The data used in the analysis are available from the corresponding author upon request.
References:
- 1.Weiss H, World Health Organization, Joint United Nations Programme on HIV/AIDS, London School of Hygiene and Tropical Medicine, editors. Male circumcision: global trends and determinants of prevalence, safety, and acceptability. Geneva: World Health Organization : UNAIDS; 2008. [Google Scholar]
- 2.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLoS Med 2005; 2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. The Lancet 2007; 369:657–666. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet Lond Engl 2007; 369:643–656. [DOI] [PubMed] [Google Scholar]
- 5.WHO ∣ New data on male circumcision and HIV prevention: policy and programme implications. WHO. https://www.who.int/hiv/pub/meetingreports/mc_montreux_march07/en/ (accessed 8 Jul2021). [Google Scholar]
- 6.Remarkable progress in the scale up of voluntary medical male circumcision as an HIV prevention intervention in 15 ESA countries. https://www.who.int/publications-detail-redirect/voluntary-medical-male-circumcision-progress-brief-2019 (accessed 24 Aug2021). [Google Scholar]
- 7.Atkins K, Yeh PT, Kennedy CE, Fonner VA, Sweat MD, O’Reilly KR, et al. Service delivery interventions to increase uptake of voluntary medical male circumcision for HIV prevention: A systematic review. PLoS ONE 2020; 15:e0227755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone MA, Li PS, Awori QD, Lee R, Goldstein M. Clinical trials using the Shang Ring device for male circumcision in Africa: a review. Transl Androl Urol 2014; 3:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neonatal and child male circumcision: a global review. https://www.who.int/hiv/pub/malecircumcision/neonatal_child_MC_UNAIDS.pdf (accessed 28 Sep2021).
- 10.Cork MA, Wilson KF, Perkins S, Collison ML, Deshpande A, Eaton JW, et al. Mapping male circumcision for HIV prevention efforts in sub-Saharan Africa. BMC Med 2020; 18:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garenne M, Matthews A. Voluntary medical male circumcision and HIV in Zambia: expectations and observations. J Biosoc Sci 2020; 52:560–572. [DOI] [PubMed] [Google Scholar]
- 12.Maughan-Brown B, Venkataramani AS, Nattrass N, Seekings J, Whiteside AW. A Cut Above the Rest: Traditional Male Circumcision and HIV Risk Among Xhosa Men in Cape Town, South Africa. JAIDS J Acquir Immune Defic Syndr 2011; 58:499–505. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RC, Egesah O, Rosenberg S. Male circumcision for HIV prevention: a prospective study of complications in clinical and traditional settings in Bungoma, Kenya. Bull World Health Organ 2008; 86:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcken A, Keil T, Dick B. Traditional male circumcision in eastern and southern Africa: a systematic review of prevalence and complications. Bull World Health Organ 2010; 88:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer DN, Bautista CT, Sateren WB, Sawe FK, Kiplangat SC, Miruka AO, et al. The Protective Effect of Circumcision on HIV Incidence in Rural Low-Risk Men Circumcised Predominantly by Traditional Circumcisers in Kenya: Two-Year Follow-Up of the Kericho HIV Cohort Study. JAIDS J Acquir Immune Defic Syndr 2007; 45:371–379. [DOI] [PubMed] [Google Scholar]
- 16.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS Lond Engl 2000; 14:2361–2370. [DOI] [PubMed] [Google Scholar]
- 17.Jayathunge PHM, McBride WJH, MacLaren D, Kaldor J, Vallely A, Turville S. Male Circumcision and HIV Transmission; What Do We Know? Open AIDS J 2014; 8:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes RJ, Moulton LH. Cluster randomised trials, second edition. CRC Press; 2017. doi: 10.4324/9781315370286 [DOI] [Google Scholar]
- 19.Connolly C, Simbayi LC, Shanmugam R, Nqeketo A. Male circumcision and its relationship to HIV infection in South Africa: results of a national survey in 2002. South Afr Med J Suid-Afr Tydskr Vir Geneeskd 2008; 98:789–794. [PubMed] [Google Scholar]
- 20.Maffioli EM. Is traditional male circumcision effective as an HIV prevention strategy? Evidence from Lesotho. PLoS ONE 2017; 12:e0177076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urassa M, Todd J, Boerma JT, Hayes R, Isingo R. Male circumcision and susceptibility to HIV infection among men in Tanzania. AIDS Lond Engl 1997; 11:73–79. [DOI] [PubMed] [Google Scholar]
- 22.Thomas AG, Tran BR, Cranston M, Brown MC, Kumar R, Tlelai M. Voluntary medical male circumcision: a cross-sectional study comparing circumcision self-report and physical examination findings in Lesotho. PloS One 2011; 6:e27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltzer K, Nqeketo A, Petros G, Kanta X. Traditional circumcision during manhood initiation rituals in the Eastern Cape, South Africa: a pre-post intervention evaluation. BMC Public Health 2008; 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2020 Global AIDS Update — Seizing the moment — Tackling entrenched inequalities to end epidemics. 2020; :384. [Google Scholar]
- 25.Peltzer K, Kanta X. Medical circumcision and manhood initiation rituals in the Eastern Cape, South Africa: a post intervention evaluation. Cult Health Sex 2009; 11:83–97. [DOI] [PubMed] [Google Scholar]
- 26.Mark D, Middelkoop K, Black S, Roux S, Fleurs L, Wood R, et al. Low acceptability of medical male circumcision as an HIV/AIDS prevention intervention within a South African community that practises traditional circumcision. South Afr Med J Suid-Afr Tydskr Vir Geneeskd 2012; 102:571–573. [DOI] [PubMed] [Google Scholar]
- 27.Sgaier SK, Baer J, Rutz DC, Njeuhmeli E, Seifert-Ahanda K, Basinga P, et al. Toward a Systematic Approach to Generating Demand for Voluntary Medical Male Circumcision: Insights and Results From Field Studies. Glob Health Sci Pract 2015; 3:209–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khumalo-Sakutukwa G, Lane T, van-Rooyen H, Chingono A, Humphries H, Timbe A, et al. Understanding and Addressing Socio-Cultural Barriers to Medical Male Circumcision in Traditionally Non-Circumcising Rural Communities in Sub-Saharan Africa. Cult Health Sex 2013; 15: 10.1080/13691058.2013.807519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn JG, Marseille E, Auvert B. Cost-Effectiveness of Male Circumcision for HIV Prevention in a South African Setting. PLOS Med 2006; 3:e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njeuhmeli E, Forsythe S, Reed J, Opuni M, Bollinger L, Heard N, et al. Voluntary Medical Male Circumcision: Modeling the Impact and Cost of Expanding Male Circumcision for HIV Prevention in Eastern and Southern Africa. PLOS Med 2011; 8:e1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The DHS Program - Research Topics - Male Circumcision. https://dhsprogram.com/topics/male-circumcision.cfm (accessed 21 Jul2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the analysis are available from the corresponding author upon request.