Abstract
Introduction:
Ovarian cancer (OC) is associated with the highest gynecologic cancer mortality. The development of novel, effective combinations of targeted therapeutics remains an unmet medical need. We evaluated the preclinical efficacy of the of the Poly (ADP-ribose) polymerase (PARP) inhibitor (olaparib) and the pan-ErbB inhibitor (neratinib) as single agents and in combination in ovarian cancer cell lines and xenografts with variable HER2 expression.
Methods:
In vitro cell viability with olaparib, neratinib, and their combination was assessed using flow-cytometry based assays against a panel of OC primary cell lines with variable HER2 expression. Immunoblotting experiments were performed to elucidate the mechanism of activity and synergism. The in vivo antitumor activity of the olaparib/neratinib combination versus single agents was tested in HER2 positive xenograft OC models.
Results:
HER2 + OC cell lines demonstrated higher sensitivity to olaparib and neratinib when compared to HER2 negative tumors (i.e., IC50: 2.06±0.33μM vs. 39.28±30.51μM, p=0.0035 for olaparib and 19.42±2.63nM vs. 235.0±165.0nM, p=0.0035 for neratinib). The combination of olaparib with neratinib was more potent when compared to single-agent olaparib or neratinib both in vitro and in vivo, and demonstrated synergy in all primary HER2 + OC models. Western blot experiments showed neratinib decreased pHER2/neu while increased Poly(ADP-ribose) (PAR) enzymatic activity; olaparib increased pHER2/Neu expression and blocked PAR activatio. Olaparib/neratinib in combination decreased both pHER2/Neu as well as PAR activation.
Conclusion:
The combination of olaparib and neratinib is synergistic and endowed with remarkable preclinical activity against HER2+ ovarian cancers. This combination may represent a novel therapeutic option for ovarian cancer patients with HER2+, homologous recombination-proficient tumors resistant to chemotherapy.
Keywords: PARP inhibitors, Olaparib, HER2, Neratinib, Ovarian cancer
1. Introduction
The American Cancer Society estimates that 19,880 new ovarian cancer cases and 12,810 ovarian cancer-related deaths will occur in the United States in 2022 [1]. Of all gynecologic cancers in the western world, epithelial ovarian cancer (EOC) is associated with the highest case-fatality ratio. Standard therapy includes maximal cytoreductive surgery and platinum-based chemotherapy. Although the majority of patients respond to initial therapy, most will develop recurrence which is invariably fatal [2]. The development of novel, effective therapies for patients with recurrent, chemotherapy-resistant ovarian cancer remains an unmet medical need.
Poly (ADP-ribose) polymerase inhibitors (PARPi) target PARP family members, mostly PARP-1 and PARP-2. PARP enzymatic activity is essential for the repair of single-strand breaks (SSB) through the base excision repair (BER) pathway. Once BER is impaired, unrepaired SSBs can result in continuous and lethal DNA damage [3]. Importantly, PARPi prevent repair of single-strand breaks, in turn causing DNA destabilization and eventual double-strand breaks. Cancer cells with deficient double-strand repair pathways (HRD) are particularly sensitive to PARPi for this reason. Accordingly, PARPi have emerged as promising agents for both BRCA-mutated and BRCA wild-type ovarian cancer, with a more modest response in BRCA wild-type. [4–6]. Based on preclinical and clinical results, olaparib has received FDA approval as first line maintenance therapy following platinum-based chemotherapy for advanced stage, high grade ovarian cancer with BRCA mutations; Niraparib has received approval as maintenance therapy for patients with any HRD status. In the second line or greater maintenance setting with platinum-sensitive disease, in addition to niraparib (for BRCA) and olaparib (any HRD), rucaparib is also approved for use in patients with deleterious BRCA mutations. However, recent survival data has prompted important FDA withdrawals for PARPi as single agent therapy in the recurrent setting [7–9], demonstrating the ongoing unmet need for effective treatments, especially in our recurrent platinum-resistant patient population.
The Human Epidermal Growth Factor Type II receptor (i.e., HER2, encoded by the c-ErbB2 gene) is a transmembrane receptor protein which includes an extracellular ligand-binding domain, a membrane spanning region, and an intracellular tyrosine kinase domain. HER2 functions as a preferred partner for heterodimerization with members of the EGF receptor family (HER1, HER3 and HER4), and thus plays an important role in coordination of the complex c-ErbB2 signaling network responsible for regulating cell growth and differentiation. HER2 overexpression is thought to result in constitutive activation of the tyrosine kinase domain, causing activation of downstream protein pathways (such as the PIK3CA/AKT/mTOR and RAS/RAF/MAPK), and thus dysregulated gene transcription [10]. Amplification of the HER2 gene (ERBB2) has been reported in several human malignancies including breast, colon, gastric, uterus, and ovary [11–14], and has a reported prevalence of 8 to 66% in ovarian cancers [11]. Importantly, amplification of ERBB2 and overexpression of HER2 have been associated with more aggressive disease and worse prognosis in multiple human tumors including ovarian cancer [12, 14, 15].
The impact of HER2/neu overexpression on PARPi activity has been most thoroughly studied in breast cancer [16–19]. These investigations demonstrate that high HER2 expression may act as an alternative “BRCAness” mechanism, resulting in sensitization of breast cancer to PARPi regardless of BRCA status [17, 18]. In a HER2 overexpressing (+) breast cancer study, lapatinib, a dual tyrosine kinase inhibitor against HER1/HER2, significantly reduced homologous recombination (HR) mediated repair capacity [19]. Another study in breast cancer showed that targeting downstream of the HER2 receptor impaired BRCA1/2 and increased PARP activity, thereby leading to sensitization of the tumor to PARPi [20]. Lastly, one in vitro only study evaluating the combination of niraparib and neratinib demonstrated that the combination had a synergistic effect against ovarian cancer cells through convergent DNA damage mechanisms [21].
Based on these previous studies, we hypothesized that the combination of neratinib (ERBB1/2/4 inhibitor) and olaparib (PARP 1 inhibitor) would demonstrate increased cytotoxicity in EOC with HER2 overexpression. Accordingly, in this study we evaluated the preclinical activity of neratinib and olaparib against multiple primary ovarian cancer cell lines with variable HER2 expression in vitro and in vivo in xenograft models.
2. Methods and Materials
2.1. Establishment of cell lines and HER2 expression analysis
Approval for this study was obtained through the institutional review board. All patients were consented before tissue collection per institutional guidelines. Cancer cell lines were established from fresh tumor biopsy samples as previously described [22, 23]. Briefly, solid tumors were mechanically disrupted to portions no larger than 3mm3 in an enzymatic solution of 0.14% collagenase type I and 0.01% DNAse (2000KU/mg) in RPMI 1640 (Gibco Life Technologies, Carlsbad, CA). The minced samples were then incubated in the same solution in a magnetic stirring apparatus for 1 hour at room temperature. Enzymatically dissociated tumor was then washed twice in RPMI 1640 with 10% fetal bovine serum (FBS, Gemini, Calabasas, CA) and plated in Petri dishes using RPMI 1640, 10% FBS, 1% penicillin with streptomycin (Mediatech, Manassas, VA), and 1% amphotericin (Life Technologies, Carlsbad, CA). The cell lines were kept in an incubator at 37°C with 5% CO2 and continually monitored for growth. The experiments were performed with primary cell lines, all with limited passages (i.e., <50). Tumors were staged per the International Federation of Gynecology and Obstetrics (FIGO) staging system. HER2 surface expression of cell blocks of primary EOC was evaluated by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH), as had been reported previously [24, 25]. HER2 expression of cell lines was also evaluated with flow-cytometry (FACSCalibur, Beckton-Dickinson, San Jose, CA). Table 1 shows the HER2 expression of ovarian cancer cell lines used in this study.
Table 1.
Characteristics and demographic data of ovarian cancer cell lines
| Cell line | Age | Race | FIGOa | Histology | HR status | HER2 expression |
||
|---|---|---|---|---|---|---|---|---|
| IHCb cell block | FISHc | Flow cytometry | ||||||
|
| ||||||||
| OVA3 | 53 | W | IIIA | Serous | HRPd | 0 | Not amplified | 0 |
| OVA4 | 64 | W | IIIC | Serous | HRP | 0 | Not amplified | 0 |
| OVA10 | 51 | W | IIC | Clear cell | HRP | 3+ | Amplified | 3+ |
| OVA11 | 79 | W | IC | Clear cell | HRD | 3+ | Amplified | 3+ |
| OVA12 | 32 | W | IC | Clear cell | HRP | 0 | Not amplified | 0 |
| OVA13 | 42 | W | IIIC | Clear cell | HRP | 3+ | Amplified | 2+ |
| KRCH31 | 69 | W | IV | Serous | HRP | 3+ | Amplified | 3+ |
FIGO, International Federation of Gynecology and Obstetrics
IHC, immunohistochemistry
FISH, fluorescent in situ hybridization
HRP/HRD, homologous recombination proficient/deficient
2.2. Drug
Neratinib was obtained from Puma Biotechnology (Los Angeles, CA) through an MTA. It was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) as a 10mM stock solution for the in vitro experiment. For the in vivo experiments, sterile water with 0.5% methylcellulose (Sigma Life Science, St. Louis, MO) and 0.4% of Tween 80 (Fisher Bioreagents) was used to dissolve neratinib to a concentration of 4mg/mL to ensure a dose of 20mg/kg in mice with 100 μL oral gavage volume. Olaparib was obtained from AstraZeneca (Cambridge, United Kingdom). It was prepared in the same way as neratinib for the in vitro study. For the in vivo experiment, it was dissolved in 50% Kleptose (Roquette-Pharma) and sterile water to reach a concentration of 10mg/mL to ensure a dose of 50 mg/kg in mice in 100 μL oral gavage volume.
2.3. Cell viability assay and synergism
Ovarian cancer cell lines were plated in six-well tissue culture plates at a density of 20,000 – 40,000 cells in RPMI 1640 media supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% amphotericin. Cells were incubated at 37°C, 5% CO2 for 24 hours. After this brief incubation, cells were treated with olaparib at concentrations of 2.5, 10, 20, 40, and 100 μM (2.5, 10, 100, 200, and 400 μM for olaparib resistant cells). The six-well plates were then allowed to incubate for 72 hours. After 72 hours, well contents were harvested in their entirety, centrifuged, and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS). The viable cells were then quantified using flow-cytometry as a mean ± standard error of mean (SEM) relative to untreated cells as 100% viable controls. A minimum of 3 independent experiments per cell line was performed to determine the IC50 of olaparib in cancer cell lines. The IC50 of neratinib was determined in the same way as olaparib. The concentrations of neratinib used for cell viability assay were 2.5, 10, 20, 40, and 100 nM (2.5, 10, 100, 200, and 400 nM for neratinib resistance cells). After having determined the IC50 of olaparib and neratinib, the synergistic effect was assessed by the combination index (CI), according to the method of Chou and Talalay using CompuSyn (CompuSyn, Inc.) as previously described [26].
2.4. Immunoblotting
A representative cancer cell line was plated in cell culture dishes. The cells were treated with olaparib (0.6μM), neratinib (6nM), or the combination of both drugs (olaparib 0.6μM + neratinib 6ƞM) after 24 hours incubation. Next, the treated cells were incubated for an additional 48–72 hours. After 48–72 hours of incubation, cells were collected and lysed with radioimmunoprecipitation assay (RIPA) buffer (50mmol/L Tris-HCl, pH 8, 150mmol/L NaCl, 1% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, 5mmol/L MgCl in H2O) supplemented with protease inhibitors (Thermo Scientific, Rockford, IL). Lysates were cleared by centrifugation at 13,000 rpm for 10 minutes at 4°C, and supernatants were removed and assayed for protein concentration using BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). An equal amount of protein was loaded for SDS-PAGE using 4% to 20% acrylamide precast gel (Bio-Rad), followed by transfer to polyvinylidene difluoride membranes (Bio-Rad). The antibodies used for western blotting were PAR (#4336, Trevigen), PARP (#9532, Cell Signaling Technology, Inc.), pHER2 (#2247, Cell Signaling Technology, Inc.), ERBB2/HER2 (#06-562, Millipore, Inc.), and GAPDH (#2118, Cell Signaling Technology, Inc.). The membranes were incubated with primary antibody overnight in 3% BSA-Tween at 4°C. The next morning, the membranes were washed three times with 1% milk in PBS-Tween at room temperature and incubated with an HRP-linked secondary antibody (#7074, Cell Signaling Technology, Inc.) in 5% milk PBS-Tween for 1 hour. After a 1-hour incubation, the membranes were washed four times in 1% milk PBS-Tween. Signals were detected with Western blotting detection reagent (Thermo Scientific, Rockford, IL), and bands were visualized using an enhanced chemiluminescent system (GEL Logic 1500, Carestream Health, Rochester, NY).
2.5. Primary cell lines mutational signatures
Whole exome sequencing (WES) data from all 7 primary ovarian cancer cell lines were analyzed for mutational signatures as described by Alexandrov et al. [27]. Briefly, mutational signatures were extracted using base substitutions and additionally included information on the sequence context of each mutation. Since there are six classes of base substitution C>A, C>G, C>T, T>A, T>C, T>G (all substitutions are referred to by the pyrimidine of the mutated Watson-Crick base pair) and since information on the bases immediately 5′ and 3′ to each mutated base is incorporated in this analysis, there are 96 possible mutations in this classification. In published studies, applying this approach to multiple human cancer types revealed over 30 distinct validated mutational signatures. Importantly, signature 3 was strongly associated with BRCA1/2 mutations within the ovarian, breast and prostate cancer types (i.e., HRD-related) [28].
2.6. In vivo experiments
To conduct the in vivo experiments, we selected the KrCH31 cancer cell line, as it has high HER2 expression and has a high grade serous histology (i.e., the most common histologic type of ovarian cancer) and it has previously shown to be able to consistently grow as xenografts in SCID mice [29, 30]. The cell line was injected into 5–8-week-old SCID mice subcutaneously (Harlan Laboratories, Indianapolis, IN). Each mouse was injected with 6 million cells of ovarian cancer cells suspended in approximately 200 μL of a 1:1 solution of sterile PBS-containing cells and Matrigel (BD Biosciences). After the tumor volume reached 0.2cm3, the mice were randomized into four groups (6 mice/group): control, olaparib, neratinib, and the combination of olaparib and neratinib. Olaparib was given orally at doses of 50mg/kg twice/day. Neratinib was given orally at doses of 20mg/kg, once/day, and 5 days/week. Tumor volume was measured twice weekly. Tumor volume was determined using the formula (A2 * B)/2, where B represented the largest tumor diameter size and A was the smaller perpendicular tumor diameter. Animal care and euthanasia were carried out according to the rules and regulations set forth by the Institutional Animal Care and Use Committee.
2.7. Statistical analysis
Statistical analysis was performed using Graph Pad Prism 7 (GraphPad Software, Inc. San Diego, CA). One-way ANOVA with Bonferroni post-test was used to determine the statistical significance of the effects of combination treatment on the different cell lines in vitro when compared to control and single agent treatments. Tumor volume differences at specific time points were compared using an unpaired t-test. Overall survival data were analyzed using the Kaplan-Meier method. Survival curves were compared using the log-rank test. A two-sided p-value of <0.05 was considered to be significant.
3. Results
In vitro activity of olaparib, neratinib, and Olaparib/neratinib combinations
Table 1 shows the demographic data of patients and the characteristics of the ovarian cancer cell lines used in this study. When we analyzed the tumor mutational signatures of the 7 primary fully sequenced ovarian cancer cell lines, as described by Alexandrov et al. [27], only one cell line (i.e., OVA-11) was found to harbor a dominant HRD-related signature (i.e., signature-3). The IC50s of olaparib and neratinib for each ovarian cancer cell line tested was determined as described and are shown in Table 2. After exposure to olaparib, HER2 + ovarian cancer cells had significantly lower IC50 than HER2 non-expressing (−) cancer cells (IC50: 2.06 ± 0.33 μM vs. 39.28 ± 30.51 μM, p= 0.0035). Similarly, HER2+ cell lines were significantly more sensitive to neratinib when compared to HER2− cell lines 19.42 ± 2.63 nM vs. 235.0± 165.0 nM, p= 0.0035, excluding OVA-4). OVA-4, one of the HER2− cell lines, was sensitive to neratinib exposure (Table 2). OVA-4 WES results demonstrated a somatic mutation of HER2 at exon7; c.A776G, p.N259S. This mutation is in close proximity to the pro-oncotic S310A/F/Y mutation in the Furin-like domain of the ErbB [31, 32], a “hot spot” know to confer sensitivity to pan-ErbB inhibitors [33]. We next studied the combination of the two agents in HER2+ cell lines. We found that olaparib and neratinib caused a significantly higher cell growth inhibition when compared to single agent therapy (Figure 1). By testing combination treatment at multiple paired concentrations, we demonstrated that olaparib and neratinib to have additive as well as synergistic activity in all cell lines (Figure 1). The synergistic activity (CI values) at each affected fraction (Fa) of 0.50, 0.75, 0.85, 0.90, and 0.95 are shown in Table S1. OVA11 and OVA13 cell lines showed synergistic and additive effects in all various Fa’s tested. In OVA 10 and KrCH31 the synergistic and additive effects were shown at multiple Fa’s up to Fa of 0.85 (Table S1).
Table 2.
IC50 of olaparib and neratinib of ovarian cancer cell lines
| Cell line | IC50 of olaparib (μM) | IC50 of neratinib (nM) |
|---|---|---|
|
| ||
| OVA3 | 14.12 | > 400§ |
| OVA4 | >100§ | 14.39 |
| OVA10 | 2.57 | 19.35 |
| OVA11 | 1.31 | 13.04 |
| OVA12 | 3.72 | 69.94 |
| OVA13 | 1.71 | 25.93 |
| KRCH31 | 2.64 | 19.35 |
Represents maximal dose of the agent during cytotoxic analyses.
Figure 1.
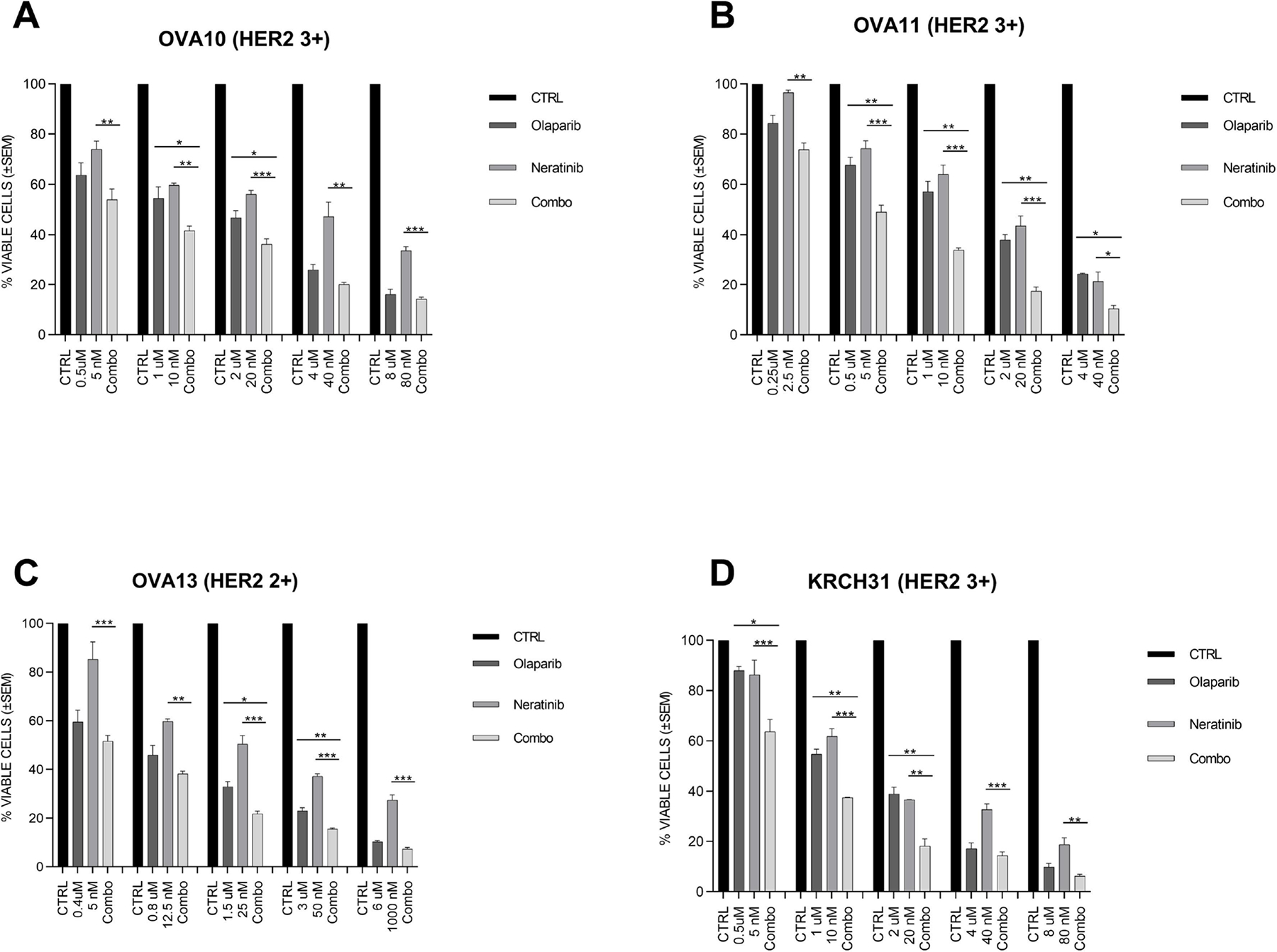
Cell viability assay of the four epithelial ovarian cancer cell lines with high HER2 expression. Four cell lines with high HER2 expression were treated with olaparib, neratinib, and the combination of both at the indicated concentration for 72 hours. Cell viability was analyzed by flow cytometry and was normalized to the mean of the control group receiving no drug. One-way ANOVA was used to determine the statistical significance of the effects of the combination treatment on ovarian cancer cell lines in vitro when compared to the two single-agent treatment. The one-way ANOVA was corrected with the Bonferroni’s statistic for comparing each group with each other. (*p < 0.05, **p < 0.01, and ***p < 0.005)
3.2. Immunoblotting analysis
Western blotting was performed on tumor cells after 48–72 hours of treatment with olaparib, neratinib, or the combination of the two at the selected concentrations described in the material and methods section. A representative cell line with HER2+ expression was used for the western blot analysis. We found that the total HER2 level remained unchanged with olaparib, neratinib, and the combination of both drugs (Figure 2A), while the phosphorylated (i.e., active) HER2 decreased after neratinib exposure (Figure 2B). Neratinib also induced an increase in PAR levels at 48 hours of neratinib treatment (Figure 2D). Olaparib caused minimal changes in PARP levels (Figure 2C) and caused a mild increase in pHER2 after 48–72 hours. The combination of olaparib/neratinib caused a notable decrease in PAR enzymatic activity and pHER2 (Figure 2D).
Figure 2.
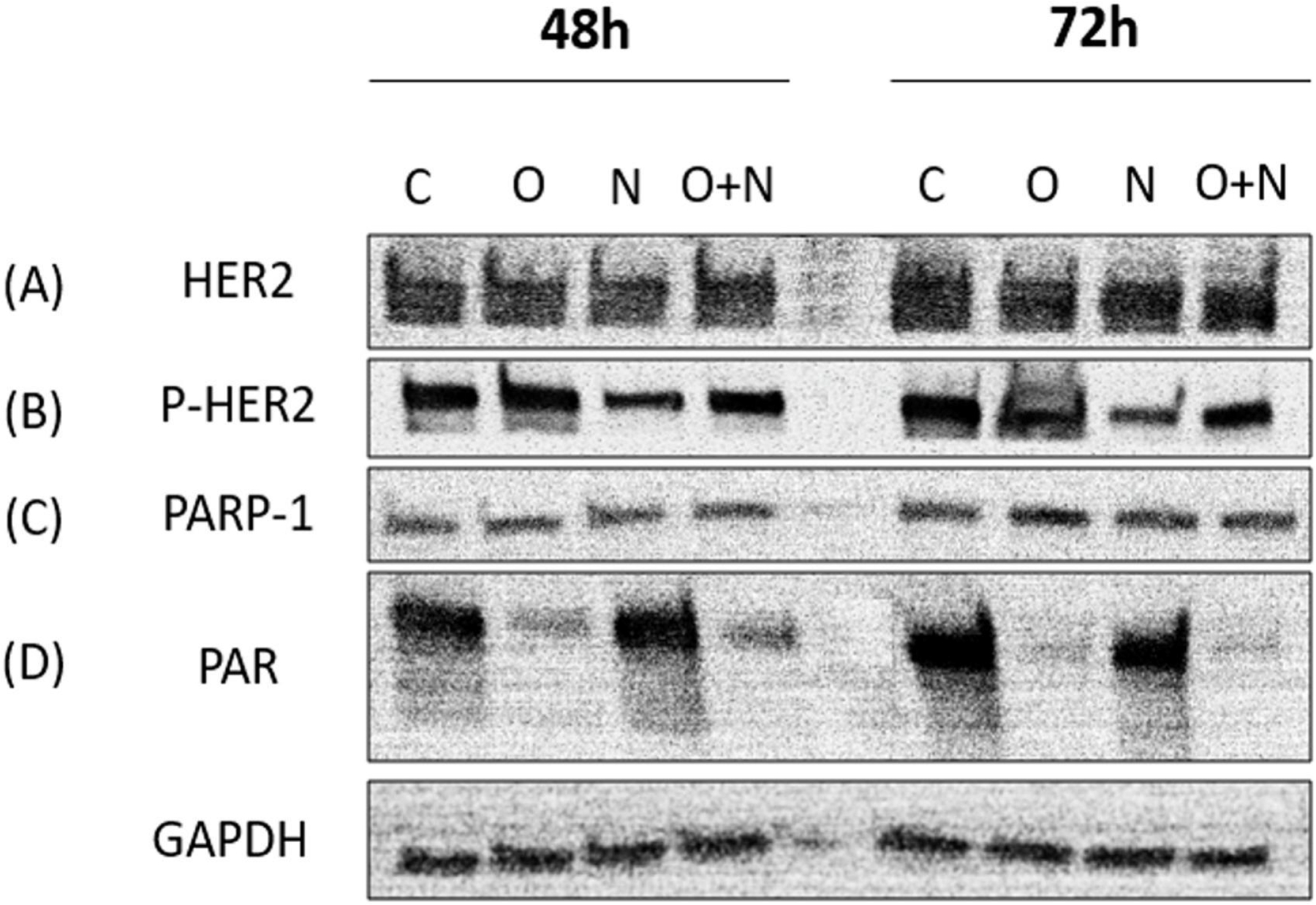
Western blotting analyses after the treatment with olaparib, neratinib, or the combination of olaparib and neratinib. A representative cell line was treated with olaparib (0.6 μM), neratinib (6nM), and the combination of olaparib (0.6 μM) and neratinib (6nM) after 48–72 hours.
3.3. Anti-tumor activity of the combination of olaparib and neratinib in vivo.
The therapeutic effect of the single agents and the combination treatment was tested in HER2+ ovarian cancer xenografts. As demonstrated in Figure 3A, the efficacy of the combination of olaparib and neratinib seen in vitro was also demonstrated in our in vivo experiments. A statistically significant difference in tumor growth inhibition of the combination (olaparib + neratinib) compared to olaparib single-agent treatment was detected on day 22 and beyond (p = 0.035 on day 22). A statistically significant difference in tumor growth inhibition of the combination compared to neratinib was detectable on day 36 and continued to increase in significance until day 47 (p = 0.0463 on day 36 and p = 0.0001 on day 47) (Figure 3A). The mice treated with the combination of olaparib and neratinib had a significantly longer overall survival when compared to control mice (p = 0.0005), mice treated with olaparib (p = 0.0005), and mice treated with neratinib single agents (p = 0.0013) (Figure 3B). Mice treated with the single agents, or the combination tolerated the treatment well without major weight change (Figure 3C).
Figure 3.
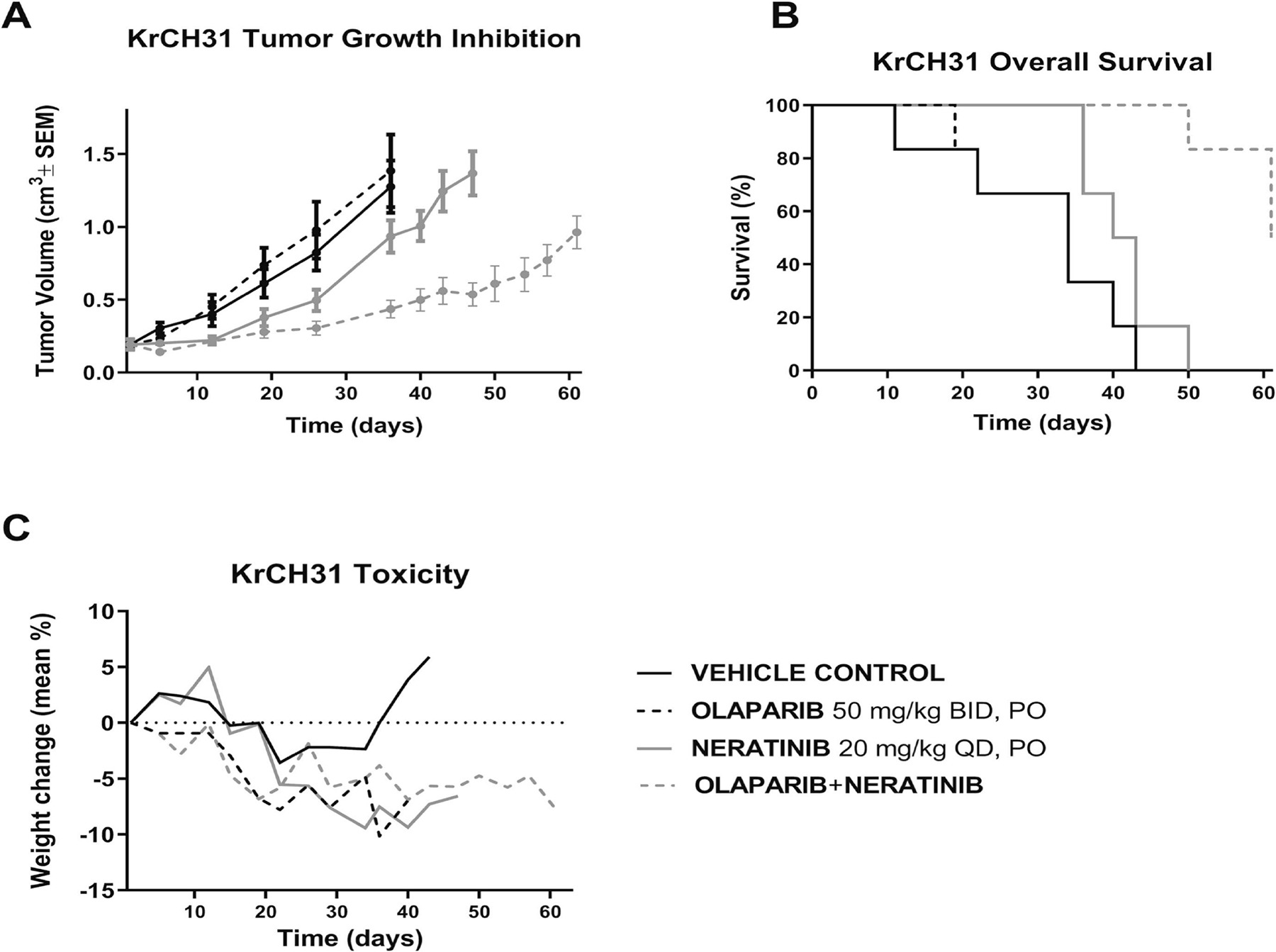
In vivo antitumor activity of the combination of olaparib and neratinib compared to single-agent olaparib and neratinib. Mice were treated with olaparib (50mg/kg), neratinib (20mg/kg) or the combination of olaparib and neratinib for 60days. The mice treated with combination showed a statistically significant difference in tumor growth inhibition compared to the mice treated with single-agent olaparib or neratinib (A). A statistically significant difference in tumor growth inhibition of the combination (olaparib + neratinib) compared to olaparib single-agent treatment was detected on day 22 and beyond. (p = 0.0350 on day 22). A statistically significant difference in tumor growth inhibition of the combination compared to neratinib began on day 36 and continued as in vivo experiment continued until day 47 (p = 0.0463 on day 36 and p = 0.0001 on day 47). The mice treated with the combination of olaparib and neratinib had a significantly longer overall survival when compared to control mice (p = 0.0005), mice treated with olaparib (p = 0.0005), mice treated with neratinib (p = 0.0013) (B). The mice treated with the combination treatment tolerated the treatment well (C).
Discussion
Despite the use of aggressive cytoreductive surgery and platinum-based chemotherapy combinations, treatment of ovarian cancer remains challenging. The development of novel, effective targeted agents such as PARPi in ovarian cancer has recently transformed treatment guidelines for patients harboring specific DNA repair defects which cause deficiency in the cell HR repair system (i.e., BRCA1/BRCA2 mutations) [6, 34]. Accordingly, some PARPi approval has been expanded to maintenance therapy for patients with platinum-sensitive relapsed ovarian cancer, who responded to their second line regimen, regardless of BRCA1 or BRCA2 mutation status, [9, 35, 36] even though most recent data has led to removal of PARPi approval as single agent therapy in the recurrent setting [7, 8]. This broader use of PARPi stems from the evidence that tumors that share molecular features with BRCA-mutant tumors also exhibit different levels of defective HR DNA repair, and therefore respond to PARP inhibition [37]. Indeed, the cytotoxic effects of PARPi are reported to be mediated by catalytic inhibition of PAR and trapping of PARP-DNA complexes [38]. Taken together these studies suggest that multiple molecular events may potentially make ovarian tumors sensitive to PARPi. Accordingly, in this study we tested the novel combination of olaparib, a PARP1 inhibitor, which derives its cytotoxic effect from inhibition of BER, trapping of PARP on damaged DNA, disruption of BRCA1 recruitment to damaged DNA, and activation of non-homologous end-joining [39, 40], and neratinib, an irreversible pan-inhibitor of ERBB1, HER2, and HER4 tyrosine kinase with promising activity against HER2-overexpressing cancers [41], against multiple biologically aggressive, HR-proficient ovarian cancer cell lines overexpressing HER2.
Overexpression of the HER2 (ERBB2) gene has been reported in various human malignancies including ovarian cancer and found to be associated with aggressive biologic behavior [12, 14, 15]. This biologc aggressiveness is partly explained by the activation of NF-κB by HER2 which it has been shown to be PARP-dependent [42] and requires the activation of IKKα which increases cytokine and chemokine expression, as well as invasiveness [43]. HER2 expression affecting DNA repair capacity has been reported in breast cancer studies [17–20]. Exquisite susceptibility of HER2+ breast cancer cells to PARPi was observed independently from an inherent HR-deficiency in breast cancers [17]. Lapatinib, a dual tyrosine kinase inhibitor against HER1/HER2, significantly reduced HR-mediated repair capacity in HER2+ breast cancer cells [19] and thereby increased the sensitivity to PARPi. Suppression of PI3K, a downstream in the HER2 pathway, resulted in impaired BRCA 1/2, increased PARP activity, and sensitization of breast cancers to PARPi [20].
Our preclinical study with olaparib against multiple HER2+ and HER2− ovarian cancer cell lines demonstrated that HER2+ ovarian cancer cells (OVA10, OVA11, OVA13, and KRCH31) were significantly more sensitive (i.e., lower olaparib IC50) when compared to HER2− ovarian cancer cells (OVA3, OVA4, and OVA12). These data are therefore consistent with previous results obtained using HER2+ breast cancer cell lines where the sensitivity to PARPi was demonstrated to be secondary to attenuation of NF-κB signaling by PARPi [17]. As expected, HER2− ovarian cancer cells had a higher IC50 of neratinib compared to HER2+ ovarian cancer cells. The exception in this study was OVA4, which surprisingly showed an equivalent sensitivity to neratinib as HER2+ ovarian cancer cell lines. Importantly, when we reviewed WES results in OVA4 we found a mutation in the Furin-like domain of the ErbB gene (g.chr17:37866609A>G, p.N259S) in a location close to a known “hot-spot” (i.e., S310A/F/Y) able to activate the downstream of HER2 and leading to sensitivity to neratinib [33]. Indeed, mutations in this area of the gene have been reported to cause dimerization of HER2 and kinase activation even in cancers without HER2 amplification [31, 32]. Consistent with this view, clinical trials with neratinib showed a 36% clinical response rate in a heavily treated patient with HER2-mutated non-amplified breast cancers [44]. Given the close geographic relation of N259S to S310A/F/Y, further investigation into the effect of this N259S mutation on HER2 dimerization and kinase activation is warranted.
There are a paucity of studies evaluating the interaction of PARPi and HER2 targeting agents in ovarian cancers. Our investigation found that olaparib is more active against HER2+ vs. HER2− ovarian cancer cell lines, while the combination of olaparib and neratinib is synergistic and significantly more effective than the single agent against HRP ovarian tumors with both serous and clear cell histology. The effectivity in clear cell histology is especially promising given its historically poor response to standard chemotherapy regimens [45]. The finding of increased PARP activity in neratinib-treated cancer cells is consistent with previous breast cancer studies showing increased PARP activity when targets downstream of HER2 are suppressed [20]. The encouraging results from these in vitro experiments were confirmed in vivo using HER2+ xenografts. We demonstrate significantly improved overall survival in mice treated with the combined medications than in control mice, mice treated with olaparib, and mice treated with neratinib (p = 0.0005, p = 0.0005, and p = 0.0013, respectively), without significant side effects from this combination. Reports of HER2 expression in epithelial ovarian cancer vary from 8–66%, however there is evidence that conventional immunohistochemical analysis greatly underestimates the true frequency of HER2 expression[46]. Our study demonstrates that olaparib/neratinib in combination may represent a novel therapeutic option for patients with HER2+, HR-proficient ovarian tumors. While this population may not represent most patients with ovarian cancer, it is a population that carries a significantly worse prognosis [15] and stands to benefit greatly from novel, targeted, and effective therapies. Of note, the iNNOVATE trial (NCT04502602) is currently accruing up to 12 patients for a phase1b expansion arm that will evaluate anticancer activity of niraparib plus neratinib in patients with BRCA wild type platinum resistant ovarian cancer with any HER2 tumor status. We hope this exciting clinical work will analyze anticancer activity of this combination by HER2 status and look forward to the results. Future clinical trials testing olaparib/neratinib in HER2+ HR-proficient ovarian cancer patients are warranted.
Supplementary Material
Table S1. Combination index values HER2+ ovarian cancer cell lines
PARP inhibitor olaparib and pan-c-erb inhibitor neratinib show preclinical activity against HER2+ ovarian cancers
Olaparib and neratinib in combination showed synergistic effects both in vitro and in vivo among HER2+ tumors
In vivo, combination of olaparib/neratinib increased survival in HER2+ PDX mouse models
Tumor cells exposed to neratinib showed decreased phosphorylated-HER2/neu and increased PAR enzymatic activity on western blot
Tumor cells exposed to olaparib showed increased phosphorylated-HER2/neu and blocked PAR activation in HER2+ cell lines on western blot
Financial support:
This work was supported in part by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley, the Domenic Cicchetti, the Discovery to Cure Foundations and the Guido Berlucchi Foundations to AS. This investigation was also supported by NIH Research Grant CA-16359 from NCI and Standup-to-cancer (SU2C) convergence grant 2.0 to AS.
Footnotes
Conflict of interest statement
Dr Santin declares grants from PUMA, grants from IMMUNOMEDICS, grants from GILEAD, grants from SYNTHON, grants and personal fees from MERCK, grants from BOEHINGER-INGELHEIM, grants from GENENTECH, grants and personal fees from TESARO and grants and personal fees from EISAI and R-PHARM-US. The other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. [DOI] [PubMed] [Google Scholar]
- [3].Lord CJ, Garrett MD, Ashworth A. Targeting the double-strand DNA break repair pathway as a therapeutic strategy. Clin Cancer Res. 2006;12:4463–8. [DOI] [PubMed] [Google Scholar]
- [4].Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. [DOI] [PubMed] [Google Scholar]
- [5].Ledermann J, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. LBA40_PRARIEL3: A phase 3, randomised, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma (OC). Annals of Oncology. 2017;28:mdx440.034–mdx440.034. [Google Scholar]
- [6].Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- [7].Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, de Melo AC, Shparyk Y, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465–78. [DOI] [PubMed] [Google Scholar]
- [8].Penson RT VR, Colombo N, et al. Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1 and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer. 2022 SGO Annual Meeting on Women’s Cancer. Phoenix, Arizona. [Google Scholar]
- [9].Tew WP, Lacchetti C, Kohn EC. Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update. J Clin Oncol. 2022;40:3878–81. [DOI] [PubMed] [Google Scholar]
- [10].Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. [DOI] [PubMed] [Google Scholar]
- [11].Tuefferd M, Couturier J, Penault-Llorca F, Vincent-Salomon A, Broet P, Guastalla JP, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2:e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–4. [DOI] [PubMed] [Google Scholar]
- [13].Geng Y, Chen X, Qiu J, Zhou Y, Wang J, Liu L, et al. Human epidermal growth factor receptor-2 expression in primary and metastatic gastric cancer. Int J Clin Oncol. 2014;19:303–11. [DOI] [PubMed] [Google Scholar]
- [14].Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013;26:1605–12. [DOI] [PubMed] [Google Scholar]
- [15].Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–91. [PubMed] [Google Scholar]
- [16].Garcia-Parra J, Dalmases A, Morancho B, Arpi O, Menendez S, Sabbaghi M, et al. Poly (ADP-ribose) polymerase inhibition enhances trastuzumab antitumour activity in HER2 overexpressing breast cancer. Eur J Cancer. 2014;50:2725–34. [DOI] [PubMed] [Google Scholar]
- [17].Nowsheen S, Cooper T, Bonner JA, LoBuglio AF, Yang ES. HER2 overexpression renders human breast cancers sensitive to PARP inhibition independently of any defect in homologous recombination DNA repair. Cancer Res. 2012;72:4796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pierce A, McGowan PM, Cotter M, Mullooly M, O’Donovan N, Rani S, et al. Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines. Cancer Biol Ther. 2013;14:537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Booth L, Roberts JL, Samuel P, Avogadri-Connors F, Cutler RE, Lalani AS, et al. The irreversible ERBB1/2/4 inhibitor neratinib interacts with the PARP1 inhibitor niraparib to kill ovarian cancer cells. Cancer Biol Ther. 2018;19:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bellone S, Siegel ER, Cocco E, Cargnelutti M, Silasi DA, Azodi M, et al. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer. 2009;19:860–6. [DOI] [PubMed] [Google Scholar]
- [23].Santin AD, Rose GS, Hiserodt JC, Fruehauf J, Eck LM, Garcia RI, et al. Effects of cytokines combined with high-dose gamma irradiation on the expression of major histocompatibility complex molecules and intercellular adhesion molecule-1 in human ovarian cancers. Int J Cancer. 1996;65:688–94. [DOI] [PubMed] [Google Scholar]
- [24].Menderes G, Bonazzoli E, Bellone S, Black J, Altwerger G, Masserdotti A, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecol Oncol. 2017;146:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Menderes G, Bonazzoli E, Bellone S, Altwerger G, Black JD, Dugan K, et al. Superior in vitro and in vivo activity of trastuzumab-emtansine (T-DM1) in comparison to trastuzumab, pertuzumab and their combination in epithelial ovarian carcinoma with high HER2/neu expression. Gynecol Oncol. 2017;147:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. [DOI] [PubMed] [Google Scholar]
- [27].Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tymon-Rosario JR, Manara P, Manavella DD, Bellone S, Hartwich TMP, Harold J, et al. Homologous recombination deficiency (HRD) signature-3 in ovarian and uterine carcinosarcomas correlates with preclinical sensitivity to Olaparib, a poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor. Gynecologic Oncology. 2022;166:117–25. [DOI] [PubMed] [Google Scholar]
- [29].Menderes G, Bonazzoli E, Bellone S, Black JD, Lopez S, Pettinella F, et al. Efficacy of neratinib in the treatment of HER2/neu-amplified epithelial ovarian carcinoma in vitro and in vivo. Med Oncol. 2017;34:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li C, Bonazzoli E, Bellone S, Choi J, Dong W, Menderes G, et al. Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proceedings of the National Academy of Sciences. 2019;116:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109:14476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cocco E, Lopez S, Santin AD, Scaltriti M. Prevalence and role of HER2 mutations in cancer. Pharmacol Ther. 2019;199:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. [DOI] [PubMed] [Google Scholar]
- [35].Miller RE, Ledermann JA. The status of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in ovarian cancer, part 2: extending the scope beyond olaparib and BRCA1/2 mutations. Clin Adv Hematol Oncol. 2016;14:704–11. [PubMed] [Google Scholar]
- [36].Sonnenblick A, de Azambuja E, Azim HA Jr., Piccart M. An update on PARP inhibitors--moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27–41. [DOI] [PubMed] [Google Scholar]
- [37].Gadducci A, Guerrieri ME. PARP inhibitors alone and in combination with other biological agents in homologous recombination deficient epithelial ovarian cancer: From the basic research to the clinic. Crit Rev Oncol Hematol. 2017;114:153–65. [DOI] [PubMed] [Google Scholar]
- [38].Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Konecny GE, Kristeleit RS. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: current practice and future directions. Br J Cancer. 2016;115:1157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. [DOI] [PubMed] [Google Scholar]
- [42].Stilmann M, Hinz M, Arslan SC, Zimmer A, Schreiber V, Scheidereit C. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IkappaB kinase activation. Mol Cell. 2009;36:365–78. [DOI] [PubMed] [Google Scholar]
- [43].Merkhofer EC, Cogswell P, Baldwin AS. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ma CX, Bose R, Gao F, Freedman RA, Pegram MD, Blackwell K, et al. Phase II trial of neratinib for HER2 mutated, non-amplified metastatic breast cancer (HER2mut MBC). Journal of Clinical Oncology. 2016;34:516-.26729434 [Google Scholar]
- [45].Gadducci A, Multinu F, Cosio S, Carinelli S, Ghioni M, Aletti GD. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol Oncol. 2021;162:741–50. [DOI] [PubMed] [Google Scholar]
- [46].Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, et al. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS One. 2012;7:e49829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Combination index values HER2+ ovarian cancer cell lines


