Abstract
Pannexin channels play fundamental roles in regulating inflammation and have been implicated in many diseases including hypertension, stroke and neuropathic pain. Thus, the ability to pharmacologically block these channels is a vital component of several therapeutic approaches. Pharmacologic interrogation of model systems also provides a means to discover new roles for pannexins in cell physiology. Here, we review the state of the art for agents that can be used to block pannexin channels, with a focus on chemical pharmaceuticals and peptide mimetics that act on pannexin 1. Guidance on interpreting results obtained with pannexin pharmacologics in experimental systems is discussed, as well as strengths and caveats of different agents, including specificity and feasibility of clinical application.
Keywords: pannexin, connexin, peptide mimetic, inflammation, hypertension
Introduction
Pannexins (Panx) are proteins that form large-pore, high conductance membrane channels. There are three pannexin isoforms, Panx1, Panx2 and Panx3 [1]. Of these, Panx1 is the most ubiquitously expressed and is the focus of this review. Pannexin channels play central roles in paracrine and autocrine signaling and are thus critical to the control of several physiologic processes, including inflammation [2,3], blood pressure [4,5], pain [6], tumorigenesis [7] and function of the central nervous system [8,9]. Considering the diverse roles for pannexins in regulating health and disease, they represent an appealing pharmacologic target. On the other hand, this range of pannexin channel functions also represents one of the challenges in identifying specific pharmacologic strategies to regulate their function.
Pannexin channels are highly permeable and facilitate the diffusion of a broad array of substrates. Classically, Panx1 is most closely associated with ATP secretion and is generally considered anion selective [10], although this is not absolute. For instance, Panx1 channels have been shown to mediate transport of other biologically active molecules and ions, including glutamine, spermidine and possibly calcium, suggesting that they enable general permeation for substrates smaller than1 kDa [11,12]. In this respect, pannexin channels are functionally equivalent to hemichannels formed by connexin family gap junction proteins, however, pannexins and connexins are structurally distinct [1]. Given the functional similarity of pannexin channels and connexin hemichannels, it is important to use agents with the ability to specifically inhibit pannexins or connexins [13].
To date, no intrinsic extracellular ligands have been identified that regulate Panx1 permeability. Instead, Panx1 activity is generally controlled by ionotropic and metabotropic co-receptors that recognize different ligands [14]. For instance, ATP secretion by Panx1 can be stimulated by P2X7 purinergic receptors [15], alpha-adrenoreceptors [16] or TNF-alpha receptors [17,18]. Panx1 has also been associated with N-methyl-d-aspartate (NMDA) receptors in the CNS [19]. Because so many different stimuli can induce Panx1 channel activity, examining the effect of a pharmacologic agent on an output variable in a native system, such as ATP secretion or uptake of fluorescent dyes into cells, does not necessarily distinguish between an interaction with the co-receptor and a direct interaction with the pannexin channel [15]. Given this, heterologous expression systems, such as Xenopus oocytes microinjected with mRNA, have been used to measure the effect of agents on pannexin function, using depolarization as a channel opening stimulus [12,20,21].
Chemical agents
There are a number of chemical agents being used to block Panx1 channels (Figure 1; Table 1). A common theme in the initial discovery of pannexin channel inhibitors was to screen previously known ion channel inhibitors, such as chloride channel inhibitors, for the ability to inhibit Panx1 [10,22,23]. While this has proven to identify Panx1 inhibitors, these agents also will have off target effects that need to be considered.
Figure 1. Chemical pannexin inhibitors.
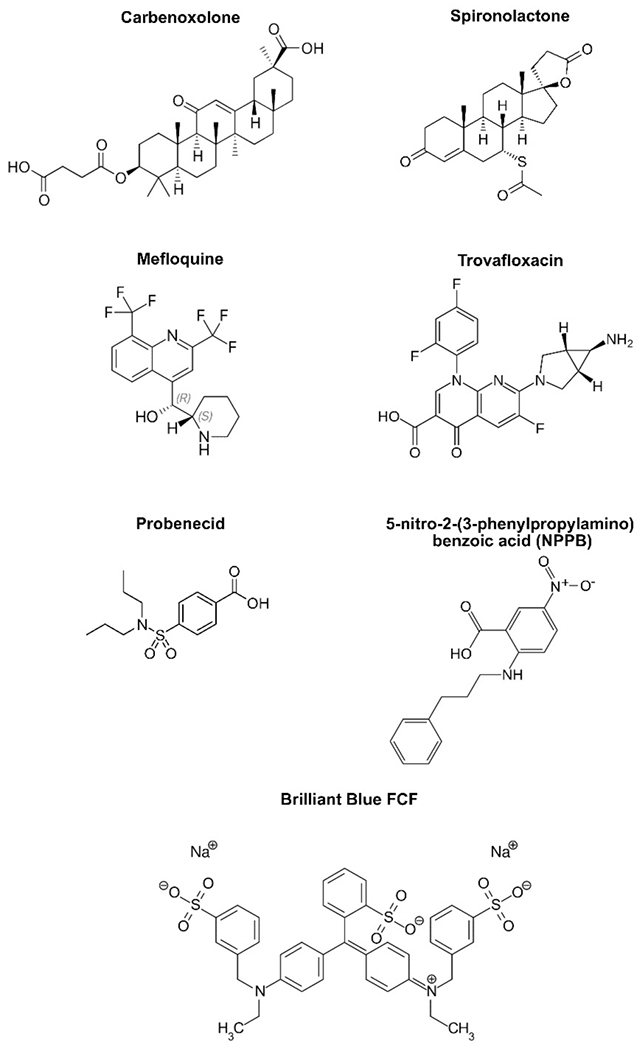
Shown are the structures of representative pannexin channel inhibitors. The structure of trovafloxacin was used under a CC BY-SA 3.0 license (https://commons.wikimedia.org/wiki/File:Trovafloxacin.svg), all other structures were from public domain images.
Table 1. Current described Pannexin channel inhibitors.
| Chemical agents | IC50 | First shown to inhibit Pannexin1 |
|---|---|---|
| Probenecid | 150 - 360 μM | Silverman, et al., 2008 [21] |
| NPPB | 21 μM | Silverman, et al., 2008 [21] |
| Spironolactone | 10 μM | Good, et al., 2018 [33] |
| Carbenoxolone | 5 μM | Bruzzone, et al., 2005 [20] |
| Trovafloxacin | 4 μM | Poon, et al., 2014 [32] |
| Mefloquine | 0.05 μM (erythro) 0.8 μM (threo) |
Iglesias, et al., 2008 [15] |
| Brilliant Blue G | 3 μM | Qiu and Dahl, 2009 [60] |
| Brilliant Blue FCF | 0.27 μM | Wang, et al., 2013 [59] |
| Peptide mimetics | Working concentration | First shown to inhibit Pannexin1 |
| 10Panx1 74-WRQAAFVDSY-83 | 10 μM | Pelegrin and Surprenant, 2006 [63] |
| PxIL2P 191-KYPIVEQYLK-200 | 3-20 μM | Billaud, et al., 2015 [16] |
| Panx308 306-LKVYEILPTFDVLH-319 | 1-10 μM | Weilinger, et al., 2016 [68]. |
Probenecid
Probenecid is commonly prescribed to prevent gout, promoting uric acid excretion by blocking its reabsorption by transporters present in renal tubules [24]. It was first demonstrated to inhibit Panx1 channels by using electrophysiologic analysis of Xenopus oocytes expressing mammalian Panx1 [21]. By contrast, probenecid has little effect on connexin hemichannels, which increases its utility for the study of Panx1 channel function [25–28]. While often used as an agent to inhibit transport activity in Panx1 channels in vitro, probenecid is known to broadly inhibit other organic anion transport channels as well [29], which can be a confounding factor in interpreting experiments using probenecid.
Another limitation regarding the use of probenecid is the high dose (in the mM range) required for significant inhibition of Panx1, which undermines its potential for therapeutic use in humans. The exact mechanism of channel inhibition via probenecid has not yet been fully resolved, however biochemical studies have determined the first extracellular loop (ECL1) as a key region required for probenecid to inhibit Panx1 by a gating mechanism [30].
NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) is a chloride channel blocker that was also demonstrated to inhibit ATP secretion [31]. Panx1 mRNA microinjected into Xenopus oocytes were used to confirm that NPPB directly inhibits Panx1 channels, at a lower concentration than probenecid [21]. The effects of NPPB and probenecid were not additive, suggesting that they may be competing for the same Panx1 binding sites.
Another limitation of NPPB is that it also has the capacity to inhibit connexin hemichannels (e.g. Cx46 and a Cx43/32 chimera), so unlike probenecid, it is not able to be used to distinguish between connexin and pannexin channels [21].
Spironolactone
Spironolactone was discovered using a non-biased, flow cytometry-based screen for Panx1 inhibitors [32,33]. Spironolactone has been used for decades to treat hypertension, and known to act as a mineralocorticoid receptor (MR) antagonist [34]. Since Panx1 has a demonstrated role in regulation of vasoconstriction [16], it seemed likely that Panx1 inhibition could also be part of the mechanism of action for spironolactone in the control of blood pressure. In fact, smooth muscle cell (SMC) specific MR-deficient mice showed decreased blood pressure in response to spironolactone, whereas SMC specific Panx1 deficient mice were unaffected [33]. This was further confirmed in a model of acute hypoxic pulmonary vasoconstriction [35] and spironolactone was also demonstrated to inhibit Panx1 expressed by vascular endothelial cells [18] and in melanoma cells [36]. These studies do not rule out a role for spironolactone in regulating hypertension by attenuating renal MR function [37]. Interestingly, spironolactone metabolites that more specifically target MR are less potent as pannexin channel inhibitors [33], suggesting the potential to produce spironolactone derivatives that more specifically target pannexins.
Carbenoxolone
Carbenoxolone is a derivative of glycyrrhetinic acid, which is known for its anti-inflammatory capacity [38] as well as being a gap junction inhibitor [39]. Bruzonne et al. first showed the micromolar sensitivity of Panx1 and Panx2 to carbenoxolone treatment with mRNA microinjected into Xenopus oocytes [20], results that have been confirmed in multiple different experimental systems [26,40–42]. More recently, Michalski et al. identified a putative binding site for carbenoxolone in a region also targeted by probenecid [30]. They then used Cryo-EM to resolve frog Panx1 and determined that the binding site for carbenoxolone is between ECLs 1 and 2, which locks the channel in a closed conformation [43]. The discovery of this binding site suggests that pore blocking is the main mechanism of inhibition of pannexins by carbenoxolone [44]. Although carbenoxolone inhibits both connexin and pannexin channels, the dose responses for these effects are different [45], which may enable carbenoxolone to be used to distinguish between these two classes of channels [46].
Trovafloxacin
As was the case for spironolactone, trovafloxacin also was discovered using a non-biased screen for pannexin channel inhibitors [32]. Trovafloxacin is specific for Panx1 channels and does not inhibit Panx2 channels or Cx43 gap junctions [32], suggesting utility for this agent as an experimental tool [47]. However, trovafloxacin is known to have serious side effects, including hepatotoxicity [48], which preclude its therapeutic use as a pannexin channel inhibitor.
Mefloquine
Mefloquine was initially discovered through a screening assay of quinine analogs for potential malaria treatments [49]. Its utility as an anti-malarial has diminished due to Plasmodium resistance as well as potential neurological complications following mefloquine treatment [50]. Nonetheless, it is still employed in the study of gap junctions and pannexins [51–53]. Nanomolar doses of the racemic erythro form of mefloquine are sufficient for significant inhibition of Panx1 [54], whereas gap junction channels (e.g., Cx36 and Cx50) are inhibited with μM to mM doses [55]. Given this difference in IC50, mefloquine as a high affinity pannexin inhibitor has allowed researchers to distinguish the importance of Panx1 in the context of neurological disorders such as epilepsy and opiate withdrawal [56-58].
Brilliant Blue FCF
Brilliant Blue FCF is a food additive used to color several consumer goods, including processed foods, drinks, and medications. Using the Xenopus model system, it has been shown that Brilliant Blue FCF inhibits Panx1 with very high affinity and lacks an effect on connexin hemichannels [59]. Critically, Brilliant Blue FCF does not directly inhibit P2X7 receptors, in contrast to the parent compound Brilliant Blue G which acts on both P2X7 and Panx1 [60]. Although Brilliant Blue FCF has high affinity for the ability to inhibit Panx1, the dark blue color has the potential to interfere with some types of experiments. Also, since Brilliant Blue FCF is a strong pannexin inhibitor, it is not an inert food additive. Given this, efforts are underway to find natural compounds that can be used in consumer products instead of Brilliant Blue FCF [61].
Peptide mimetics
In contrast to chemical agents, peptide mimetics have the potential to specifically target proteins by recognizing specific motifs (e.g., Figure 2; Table 1). This approach has successfully been used to manipulate connexins [13,62]. By analogy, peptides have also been developed that have the capacity to interfere with pannexin function.
Figure 2. Pannexin domains corresponding to peptide mimetics used to block active Panx1 channels.
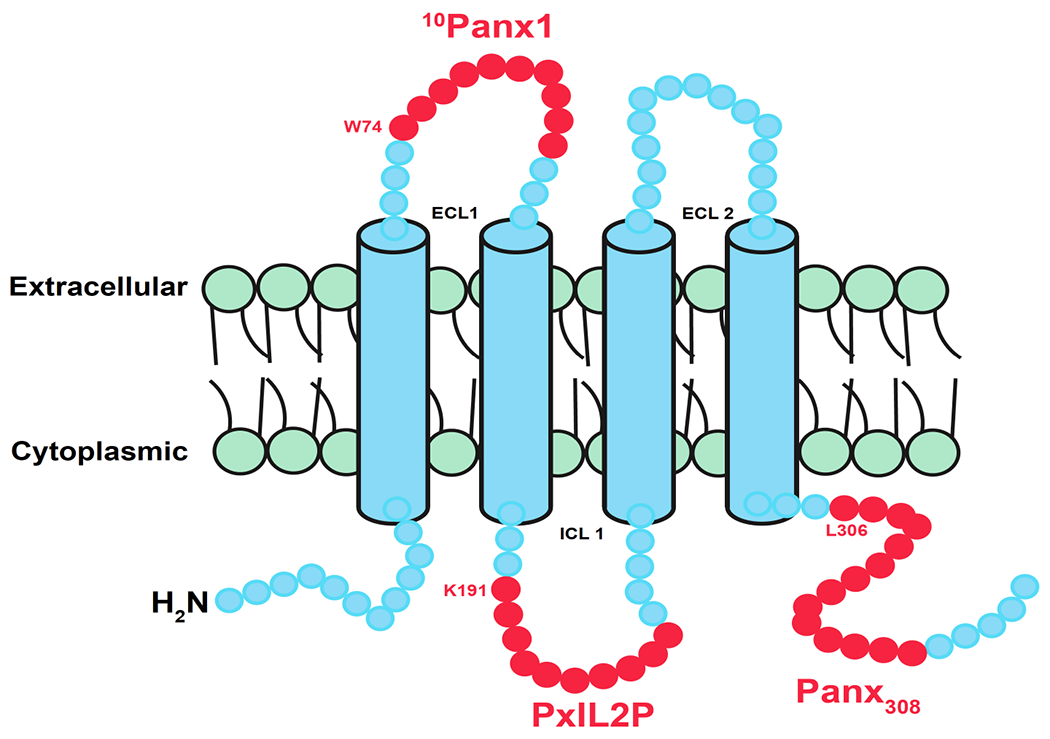
The schematic represents one monomer from the heptameric Panx1 channel in blue. Red represents the number of amino acids composing a mimetic peptide made in that region, with the starting amino acid and position noted. ICL is intracellular loop, and ECL is extracellular loop. Note that each circle does not correspond to an individual amino acid.
10Panx1
The synthetic peptide 10Panx1 was first introduced in 2006 by Pelegrin and Surprenant [63], in which Panx1 was shown to be the functional link between P2X7 receptor large pore formation and the caspase 1 cascade [63]. The short synthetic 10Panx1 peptide works by blocking amino acid residues 74-83 present in the extracellular loop 1 (ECL1) domain of Panx1. As discussed previously, ECL1, and more specifically the W74 residue in each of the Panx1 subunits, is an essential region for channel function. To date, 10Panx1 has been used in micromolar doses to investigate effects of this ECL1 blockade in a wide variety of cell types including neurons, erythrocytes, T cells, as well as in vivo [19,64-66]. Although the peptide sequence is specific to Panx1, current through Cx46 channels was shown to be moderately inhibited by 10Panx1 [67], comparable to the cross reactivity of the connexin mimetic peptides gap26 and gap27 with the ability to block pannexin channels [67]. The cross-reactivity of these peptides underscores the need to interpret studies using 10Panx1 with caution, and the need to use complementary methods to ensure that pannexin channels are involved.
PxIL2P and Panx308
In contrast to peptides made against the Panx1 extracellular loop, both PxIL2P and Panx308 were made against regulatory regions of Panx1 on intracellular Panx1 domains. Given this, both PxIL2P and Panx308 require modifications to be cell permeable. This has been accomplished by creating peptide chimeras where the N terminus is conjugated to the cell penetrating TAT (GRKKRRQRRRPQ) sequence. Both PxIL2P and Panx308 fused to TAT were shown to enter cells without any other chemical modification or assistance [16,68].
Interestingly, both peptides cover tyrosine residues that were shown to be important in Panx1 channel opening depending on the tissue, PxIL2P targeting the intracellular loop (ICL) domain [16], and Panx308 targeting the COOH-tail [68]. PxIL2P covers 10 amino acids starting at K191 and was first described by Billaud et al in a screening for peptides that block alpha-adrenergic constriction of arteries [16]. After multiple peptides were mapped to different intracellular regions of Panx1, PxIL2P was found to provide significant inhibition of vasoconstriction analogous to genetic deletion of Panx1 from smooth muscle cells [16]. The peptide was also found to block Panx1 current and ATP release in a heterologous system, with the Y198 amino acid being of critical importance to the Panx1 channel opening [16]. Further work determined that this effect was due to Src dependent phosphorylation of Y198 [69]. PxIL2P has been used to block Panx1 channel function in multiple cell types [11,33,70].
The Panx308 peptide covers 14 amino acids starting at L306 on the COOH tail of the Panx1 monomer [68]. As was the case for Y198, the Y308 residue of Panx1 is also a target for Src phosphorylation, although it is induced in response to neuronal NMDA activation after stroke [68]. Whether Src phosphorylation of Y308 is sufficient to activate Panx1 opening is an open question. Of note, Panx1 is phosphorylated immediately upon NMDA stimulation, however, Panx1 channel activity only occurs after a 10 minute latent period [68]. This suggests a model where Y308 phosphorylation may render Panx1 to be more sensitized to a second stimulus that is required for complete channel opening. Also, the common theme of Src phosphorylation in regulating these two distinct Panx1 domains suggests that both of the PxIL2P and Panx308 peptides might act as competitive inhibitors that will prevent phosphorylation of both Y198 and Y308. Whether this is the case has not been directly tested although it could be determined through the use of phospho-specific anti-Panx1 antibodies.
Conclusion and perspectives
Panx1 has been the focus of this review due to its ubiquitous expression and function. Less is known about the pharmacology of Panx2, which is predominantly expressed in the central nervous system or Panx3 which has been found in bone, cartilage and, potentially, blood vessels [71,72]. To date, there have not been any pharmacologic agents developed that have been shown to specifically block Panx2 or Panx3 and not Panx1. Another caveat related to the pharmacologic manipulation of pannexins is that it has been demonstrated that there are several subconductance states for Panx1 that can be revealed by dose response studies [10,47,73,74] and which are likely to reflect different functional conformations. Panx2 and Panx3 are likely to also have subconductance states and they may differ from those observed for Panx1. Finally, there is a lack of agonists with the ability to stimulate pannexin channel activity. The ability to enhance channel function would certainly have experimental value and could also have therapeutic application.
Thus, considerable caution should be taken when using pharmacologic approaches to assign a functional role for pannexins. Specifically, using a single inhibitor is not sufficient, especially if the experiment does not render definitive inhibition. This problem is compounded in whole animals where Panx1 expression is ubiquitous. For example, Panx1 inhibition in renin secreting cells causes a large release of renin which will increase blood pressure [70], whereas inhibition of Panx1 on smooth muscle cells lowers blood pressure through adrenergic activity [16]. These two processes could offset and give an erroneously negative finding. This also underscores the importance of transgenic mouse models to assist in interpreting pharmacologic studies, especially the use of tissue targeted gene knockouts.
Although the agents described here all have caveats, when their limitations are considered, they can provide insights into roles for pannexins in physiologic processes. We suggest as a best practice to always use multiple complementary pannexin inhibitors (e.g. spironolactone and PxIL2P) to assign a role for pannexin channels in a physiologic process. If the aggregate data (with genetic knockout in cells or animals) still supports an effect of Panx1 channel inhibition, then this provides a strong accumulation of evidence in support of pannexin channels having a functional role.
Acknowledgements
Supported by NIH grant R01-HL137112 (BEI and MK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esseltine JL, Laird DW: Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol 2016, 26:944–955. [DOI] [PubMed] [Google Scholar]
- 2.Koval M, Cwiek A, Carr T, Good ME, Lohman AW, Isakson BE: Pannexin 1 as a driver of inflammation and ischemia-reperfusion injury. Purinergic Signal 2021, 17:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou KQ, Green CR, Bennet L, Gunn AJ, Davidson JO: The Role of Connexin and Pannexin Channels in Perinatal Brain Injury and Inflammation. Front Physiol 2019, 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molica F, Figueroa XF, Kwak BR, Isakson BE, Gibbins JM: Connexins and Pannexins in Vascular Function and Disease. Int J Mol Sci 2018, 19:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Good ME, Begandt D, DeLalio LJ, Keller AS, Billaud M, Isakson BE: Emerging concepts regarding pannexin 1 in the vasculature. Biochem Soc Trans 2015, 43:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz MF, Griffith TN, Contreras JE: Mechanisms of ATP release in pain: role of pannexin and connexin channels. Purinergic Signal 2021, 17:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laird DW, Penuela S: Pannexin biology and emerging linkages to cancer. Trends Cancer 2021, 7:1119–1131. [DOI] [PubMed] [Google Scholar]
- 8.Lohman AW, Weilinger NL, Santos SM, Bialecki J, Werner AC, Anderson CL, Thompson RJ: Regulation of pannexin channels in the central nervous system by Src family kinases. Neurosci Lett 2019, 695:65–70. [DOI] [PubMed] [Google Scholar]
- 9.Giaume C, Naus CC, Saez JC, Leybaert L: Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol Rev 2021, 101:93–145. [DOI] [PubMed] [Google Scholar]
- 10.Ma W, Hui H, Pelegrin P, Surprenant A: Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 2009, 328:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Delalio LJ, Best AK, Macal E, Milstein J, Donnelly I, Miller AM, McBride M, Shu X, Koval M, et al. : Endothelial Pannexin 1 Channels Control Inflammation by Regulating Intracellular Calcium. J Immunol 2020, 204:2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Study detailing a signaling loop between Panx1 and Nfκb regulation of IL-1B release from endothelial cells.
- 12.Narahari AK, Kreutzberger AJ, Gaete PS, Chiu YH, Leonhardt SA, Medina CB, Jin X, Oleniacz PW, Kiessling V, Barrett PQ, et al. : ATP and large signaling metabolites flux through caspase-activated Pannexin 1 channels. Elife 2021, 10:e64787. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Study demonstrating that a wide range of molecules are transported through pannexin channels and that caspase cleavage increases their permeability. Pannexin-1 channels were significantly more permselective for anionic molecules than cationic molecules.
- 13.King DR, Sedovy MW, Leng X, Xue J, Lamouille S, Koval M, Isakson BE, Johnstone SR: Mechanisms of Connexin Regulating Peptides. Int J Mol Sci 2021, 22:10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isakson BE, Thompson RJ: Pannexin-1 as a potentiator of ligand-gated receptor signaling. Channels (Austin) 2014, 8:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E: P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 2008, 295:C752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, et al. : A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal 2015, 8:ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, et al. : Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun 2015, 6:7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier-Begandt D, Comstra HS, Molina SA, Kruger N, Ruddiman CA, Chen YL, Chen X, Biwer LA, Johnstone SR, Lohman AW, et al. : A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal 2021, 14:eaba2940. [DOI] [PMC free article] [PubMed] [Google Scholar]; **First study showing veins are more sensitive to inflammation induced leak than arteries. ATP secreted by Pannexin-1 in response to TNFalpha causes venous specific vascular leak through a pathway requiring hydrolysis to adenosine, adenosine receptor stimulation and TRPV4 channel function.
- 19.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA: Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 2008, 322:1555–1559. [DOI] [PubMed] [Google Scholar]
- 20.Bruzzone R, Barbe MT, Jakob NJ, Monyer H: Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 2005, 92:1033–1043. [DOI] [PubMed] [Google Scholar]
- 21.Silverman W, Locovei S, Dahl G: Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 2008, 295:C761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl G, Qiu F, Wang J: The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 2013, 75:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navis KE, Fan CY, Trang T, Thompson RJ, Derksen DJ: Pannexin 1 Channels as a Therapeutic Target: Structure, Inhibition, and Outlook. ACS Chem Neurosci 2020, 11:2163–2172. [DOI] [PubMed] [Google Scholar]
- 24.Steele TH: Control of uric acid excretion. N Engl J Med 1971, 284:1193–1196. [DOI] [PubMed] [Google Scholar]
- 25.Nyberg M, Piil P, Kiehn OT, Maagaard C, Jorgensen TS, Egelund J, Isakson BE, Nielsen MS, Gliemann L, Hellsten Y: Probenecid Inhibits alpha-Adrenergic Receptor-Mediated Vasoconstriction in the Human Leg Vasculature. Hypertension 2018, 71:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao BA, Lai CP, Naus CC, Morgan JR: Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton. J Biol Chem 2012, 287:8407–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopatar J, Dale N, Frenguelli BG: Pannexin-1-mediated ATP release from area CA3 drives mGlu5-dependent neuronal oscillations. Neuropharmacology 2015, 93:219–228. [DOI] [PubMed] [Google Scholar]
- 28.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, et al. : Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 2018, 315:L301–L312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham RF, Israili ZH, Dayton PG: Clinical pharmacokinetics of probenecid. Clin Pharmacokinet 1981, 6:135–151. [DOI] [PubMed] [Google Scholar]
- 30.Michalski K, Kawate T: Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J Gen Physiol 2016, 147:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM: A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci U S A 1998, 95:7174–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS: Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 2014, 507:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, et al. : Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ Res 2018, 122:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagart J, Hillisch A, Huyet J, Barfacker L, Fay M, Pleiss U, Pook E, Schafer S, Rafestin-Oblin ME, Kolkhof P: A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem 2010, 285:29932–29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimmer B, Krauszman A, Hu X, Kabir G, Connelly KA, Li M, Grune J, Madry C, Isakson BE, Kuebler WM: Pannexin 1: a novel regulator of acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 2022, 118:2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayedyahossein S, Huang K, Li Z, Zhang C, Kozlov AM, Johnston D, Nouri-Nejad D, Dagnino L, Betts DH, Sacks DB, et al. : Pannexin 1 binds beta-catenin to modulate melanoma cell growth and metabolism. J Biol Chem 2021, 296:100478. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Beta-catenin is a critical transcription factor that shuttles between intercellular junctions and the nucleus and is also involved in Wnt signaling. The authors provide the first demonstration that beta-catenin directly interacts with pannexin-1 in a channel-dependent manner to regulate cell proliferation.
- 37.Watson K, Kukin A, Wasik AK, Shulenberger CE: Nonsteroidal Mineralocorticoid Receptor Antagonists: Exploring Role in Cardiovascular Disease. J Cardiovasc Pharmacol 2021, 77:685–698. [DOI] [PubMed] [Google Scholar]
- 38.Leite CDS, Bonafe GA, Carvalho Santos J, Martinez CAR, Ortega MM, Ribeiro ML: The Anti-Inflammatory Properties of Licorice (Glycyrrhiza glabra)-Derived Compounds in Intestinal Disorders. Int J Mol Sci 2022, 23:4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson JS, Baumgarten IM, Harley EH: Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun 1986, 134:29–36. [DOI] [PubMed] [Google Scholar]
- 40.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD: The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A 2007, 104:6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godecke S, Roderigo C, Rose CR, Rauch BH, Godecke A, Schrader J: Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol 2012, 302:C915–923. [DOI] [PubMed] [Google Scholar]
- 42.Alhouayek M, Sorti R, Gilthorpe JD, Fowler CJ: Role of pannexin-1 in the cellular uptake, release and hydrolysis of anandamide by T84 colon cancer cells. Sci Rep 2019, 9:7622. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Pannexins have previously been shown to act as endocannibinoid transporters in the hippocampus. Using T84 colon cells, the authors show that anandamide and other related compounds are taken up by a pannexin-independent mechanism, indicating a unique role for pannexins in endocannibinoid metabolism.
- 43.Michalski K, Syrjanen JL, Henze E, Kumpf J, Furukawa H, Kawate T: The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 2020, 9:e54670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan Z, Orozco IJ, Du J, Lu W: Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 2020, 584:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]; *An in-depth analysis of the Pan1 heptamer via CryoEM demonstrating the channel’s strong selectivity for anionic molecules.
- 45.Hansen DB, Ye ZC, Calloe K, Braunstein TH, Hofgaard JP, Ransom BR, Nielsen MS, MacAulay N: Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. J Biol Chem 2014, 289:26058–26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figueroa VA, Jara O, Oliva CA, Ezquer M, Ezquer F, Retamal MA, Martinez AD, Altenberg GA, Vargas AA: Contribution of Connexin Hemichannels to the Decreases in Cell Viability Induced by Linoleic Acid in the Human Lens Epithelial Cells (HLE-B3). Front Physiol 2019, 10:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu YH, Jin X, Medina CB, Leonhardt SA, Kiessling V, Bennett BC, Shu S, Tamm LK, Yeager M, Ravichandran KS, et al. : A quantized mechanism for activation of pannexin channels. Nat Commun 2017, 8:14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw PJ, Ganey PE, Roth RA: Idiosyncratic drug-induced liver injury and the role of inflammatory stress with an emphasis on an animal model of trovafloxacin hepatotoxicity. Toxicol Sci 2010, 118:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trenholme CM, Williams RL, Desjardins RE, Frischer H, Carson PE, Rieckmann KH, Canfield CJ: Mefloquine (WR 142,490) in the treatment of human malaria. Science 1975, 190:792–794. [DOI] [PubMed] [Google Scholar]
- 50.Dow GS, Koenig ML, Wolf L, Gerena L, Lopez-Sanchez M, Hudson TH, Bhattacharjee AK: The antimalarial potential of 4-quinolinecarbinolamines may be limited due to neurotoxicity and cross-resistance in mefloquine-resistant Plasmodium falciparum strains. Antimicrob Agents Chemother 2004, 48:2624–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negoro H, Lutz SE, Liou LS, Kanematsu A, Ogawa O, Scemes E, Suadicani SO: Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model. Sci Rep 2013, 3:2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voss LJ, Gauffin E, Ringqvist A, Sleigh JW: Investigation into the role of gap junction modulation of intracortical connectivity in mouse neocortical brain slices. Brain Res 2014, 1553:24–30. [DOI] [PubMed] [Google Scholar]
- 53.Seemann N, Welling A, Rustenbeck I: The inhibitor of connexin Cx36 channels, mefloquine, inhibits voltage-dependent Ca(2+) channels and insulin secretion. Mol Cell Endocrinol 2018, 472:97–106. [DOI] [PubMed] [Google Scholar]
- 54.Iglesias R, Spray DC, Scemes E: Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes 2009, 16:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M: Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A 2004, 101:12364–12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dossi E, Blauwblomme T, Moulard J, Chever O, Vasile F, Guinard E, Le Bert M, Couillin I, Pallud J, Capelle L, et al. : Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci Transl Med 2018, 10:eaar3796. [DOI] [PubMed] [Google Scholar]
- 57.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E: Targeting pannexin1 improves seizure outcome. PLoS One 2011, 6:e25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, et al. : Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 2017, 23:355–360. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Jackson DG, Dahl G: The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J Gen Physiol 2013, 141:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu F, Dahl G: A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol 2009, 296:C250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denish PR, Fenger JA, Powers R, Sigurdson GT, Grisanti L, Guggenheim KG, Laporte S, Li J, Kondo T, Magistrato A, et al. : Discovery of a natural cyan blue: A unique food-sourced anthocyanin could replace synthetic brilliant blue. Sci Adv 2021, 7:eabe7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans WH, Boitano S: Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 2001, 29:606–612. [DOI] [PubMed] [Google Scholar]
- 63.Pelegrin P, Surprenant A: Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 2006, 25:5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ: Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 2011, 286:38397–38407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shoji KF, Saez PJ, Harcha PA, Aguila HL, Saez JC: Pannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell death. Channels (Austin) 2014, 8:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan M, Huang YA, Kuo CY, Lin T, Lu CH, Chen LC, Kuo ML: Blocking pannexin1 reduces airway inflammation in a murine model of asthma. Am J Transl Res 2020, 12:4074–4083. [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Ma M, Locovei S, Keane RW, Dahl G: Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 2007, 293:C1112–1119. [DOI] [PubMed] [Google Scholar]
- 68.Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, et al. : Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 2016, 19:432–442. [DOI] [PubMed] [Google Scholar]
- 69.DeLalio LJ, Billaud M, Ruddiman CA, Johnstone SR, Butcher JT, Wolpe AG, Jin X, Keller TCSt, Keller AS, Riviere T, et al. : Constitutive SRC-mediated phosphorylation of pannexin 1 at tyrosine 198 occurs at the plasma membrane. J Biol Chem 2019, 294:6940–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Manuscript describes in detail the tyrosine-based phosphorylation of Y198 by Src-kinase on the intracellular loop of Panx1 and how this regulates channel function.
- 70.DeLalio LJ, Masati E, Mendu S, Ruddiman CA, Yang Y, Johnstone SR, Milstein JA, Keller TCSt, Weaver RB, Guagliardo NA, et al. : Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis. Kidney Int 2020, 98:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bond SR, Naus CC: The pannexins: past and present. Front Physiol 2014, 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penuela S, Harland L, Simek J, Laird DW: Pannexin channels and their links to human disease. Biochem J 2014, 461:371–381. [DOI] [PubMed] [Google Scholar]
- 73.Chiu YH, Schappe MS, Desai BN, Bayliss DA: Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 2018, 150:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS: The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci 2012, 125:5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]


