Abstract
There are numerous clinical reports that youth with cerebral palsy (CP) have proprioceptive, stereognosis and tactile discrimination deficits. The growing consensus is that the altered perceptions in this population are attributable to aberrant somatosensory cortical activity seen during stimulus processing. It has been inferred from these results that youth with CP likely do not adequately process ongoing sensory feedback during motor performance. However, this conjecture has not been tested. Herein, we address this knowledge gap using magnetoencephalographic (MEG) brain imaging by applying electrical stimulation to the median nerve of youth with CP (N=15, Age = 15.8 ± 0.83 yrs, Males = 12, MACS levels I-III) and neurotypical (NT) controls (N=18, Age = 14.1 ± 2.4 yrs, Males = 9) while at rest (i.e., passive) and during a haptic exploration task. The results illustrated that somatosensory cortical activity was reduced in the group with CP compared to controls during the passive and haptic conditions. Furthermore, the strength of the somatosensory cortical responses during the passive condition were positively associated with the strength of somatosensory cortical responses during the haptic condition (r = 0.75, P = 0.004). This indicates that the aberrant somatosensory cortical responses seen in youth with CP during rest are a good predictor of the extent of somatosensory cortical dysfunction during the performance of motor actions. These data provide novel evidence that aberrations in somatosensory cortical function in youth with CP likely contribute to the difficulties in sensorimotor integration and the ability to effectively plan and execute motor actions.
Keywords: Hand, MEG, sensorimotor integration, sensory feedback, motor
Introduction
Cerebral palsy (CP) results from an insult to the developing brain, and it is one of the most prevalent and costly pediatric movement disorders in the United States (Strauss, Brooks et al. 2008, Kirby, Wingate et al. 2011, Christensen, Van Naarden Braun et al. 2014). CP is often accompanied by a range of upper extremity motor impairments, as well as proprioceptive, stereognosis, and tactile discrimination deficits (Cooper, Majnemer et al. 1995, Clayton, Fleming et al. 2003, Rosenbaum, Paneth et al. 2007, Sanger and Kukke 2007, Wingert, Burton et al. 2008). The need to identify the neurophysiological mechanisms that underlie these alterations in somatosensory processing has gained interest over the past decade, as they are presumed to be tightly linked with the altered motor actions seen in this patient population.
Several magnetoencephalography (MEG) and electroencephalography (EEG) studies have illustrated that the somatosensory-evoked cortical activity is reduced and sometimes latent when the hands and feet of youth with CP are stimulated (Kulak, Sobaniec et al. 2006, Kurz and Wilson 2011, Teflioudi, Zafeiriou et al. 2011, Maitre, Barnett et al. 2012, Papadelis, Ahtam et al. 2014), and this altered activity appears to follow an aberrant developmental trajectory (Trevarrow, Kleinsmith et al. 2020). Furthermore, several investigations have identified altered oscillatory activity within the theta/alpha and beta bands in response to peripheral stimulation (Guo, Xiang et al. 2012, Kurz, Heinrichs-Graham et al. 2014, Kurz, Becker et al. 2015, Kurz, Heinrichs-Graham et al. 2015). Paired-pulse stimulation paradigms have also demonstrated that somatosensory cortical activity is hyper-gated in both youth and adults with CP when two identical sensory stimuli are presented sequentially (Kurz, Wiesman et al. 2018, Trevarrow, Lew et al. 2021), indicating that increased noise within the somatosensory system may result in excessive filtering of incoming information. Such filtering may contribute to difficulties with sensorimotor integration and lead to inadequate processing of ongoing sensory feedback during the performance of motor actions. This notion is supported by our previous results that have demonstrated that youth with CP who have weaker somatosensory cortical activity also tend to have more severe disruptions in their gait and muscular force production (Kurz, Heinrichs-Graham et al. 2014, Kurz, Heinrichs-Graham et al. 2015). Further supporting this premise is the abundant data showing that youth with CP who have more severe sensory impairments also tend to have more severe motor impairments and difficulty in learning new motor skills (Gordon and Duff 1999, Sakzewski, Ziviani et al. 2010, Auld, Boyd et al. 2012, Robert, Guberek et al. 2013, Zarkou, Lee et al. 2020). Hence, there is mounting evidence that somatosensory cortical dysfunction is likely a key contributor to the altered motor actions seen in youth with CP.
Several behavioral studies with neurotypical (NT) controls have shown that the perception of somatosensations is reduced during movement (Angel and Malenka 1982, Milne, Aniss et al. 1988, Gori, Squeri et al. 2012, Holst-Wolf, Yeh et al. 2016). Such altered perception is presumed to be partially attributable to a suppression of the somatosensory evoked potentials during the ongoing movement (Papakostopoulos, Cooper et al. 1975, Milne, Aniss et al. 1988, Gori, Squeri et al. 2012, Holst-Wolf, Yeh et al. 2016). Along a similar line, our prior MEG investigation with NT controls has also shown that high-frequency somatosensory cortical oscillations (>6–24 Hz) are completely gated while performing a haptic motor task (Kurz, Wiesman et al. 2018). Altogether these imaging results suggest that somatosensory cortical responses are modulated during motor performance, however, whether similar movement-related gating occurs in those with CP remains unknown.
It is evident that the altered somatosensory perceptions reported clinically in persons with CP are related to diminished somatosensory cortical activity (Kurz, Heinrichs-Graham et al. 2014, Kurz, Heinrichs-Graham et al. 2015). However, the primary data supporting this view is based on studies that evaluated somatosensory cortical activity at rest and not during movement. Thus, it is possible that somatosensory cortical activity in those with CP might become even more aberrant during movement, which would further illuminate the potential link between altered somatosensory processing and the uncharacteristic motor actions in this population. To this end, we used MEG brain imaging to address this knowledge gap by applying an electrical stimulation to the median nerve during rest (i.e., passive) and a haptic ball exploration task in those with CP and demographically-matched NT controls. Our primary hypothesis was that somatosensory responses would be weaker in those with CP across both tasks. Secondarily, we hypothesized that the strength of somatosensory cortical activity during the passive condition (i.e., no movement) would predict somatosensory cortical responses during the haptic condition. Support for this hypothesis would indicate that somatosensory cortical activity measured at rest is a good surrogate for somatosensory processing seen in youth during the performance of a wide repertoire of movements.
Materials and Methods
Participants
Fifteen youth with CP (Males = 12; Age = 15.8 ± 3.2 yrs.) with Manual Ability Classification System (MACS) levels between I-III participated in this investigation. Seven of the youth with CP were more affected on the right side, and eight were more affected on the left side. An additional eighteen NT youth (Males = 9; Age = 14.1 ± 2.4 yrs.) served as a control group. The youth with CP were excluded if they had an orthopedic surgery or anti-spasticity treatments within the last six-months, had a prior history of epileptic seizures, or if they had undergone a dorsal rhizotomy. In addition, youth that had discernable volume loss on their structural MRI were excluded, as such loss could affect the integrity of the somatosensory cortices. The Institutional Review Board reviewed and approved this investigation. Informed consent was acquired from the parents, and the youth assented to participate in the experiment.
MEG Data Acquisition and Experimental Paradigm
Throughout the somatosensory experiment, participants were seated in a non-magnetic chair with their head positioned within the MEG helmet-shaped sensor array while focusing on a fixation cross. A single pulse, unilateral electrical stimulation was applied using electrodes that were affixed to the skin overlying the left or right median nerve. We stimulated the median nerve on the more affected side in those with CP. We chose to stimulate the non-dominant side in the NT controls because it is less utilized than the dominant side. Thus, we compared the less utilized side in the persons with CP and NT controls to avoid inflating the results. The intensity of stimulation was set to the individual’s motor threshold to control for impedance differences among participants. During the experiment, stimulation was applied every 2 s for 4 min per block, yielding a total of 120 trials per block. Each participant underwent stimulation during rest (i.e., a passive condition) and during a haptic movement condition, and the order of blocks was counterbalanced across participants. During the passive condition, individuals were instructed to look at a fixation cross and remain still while being stimulated. During the haptic condition, individuals were instructed to look at the same fixation cross while moving a ball around within their left-hand while being stimulated (Figure 1).
Figure 1.
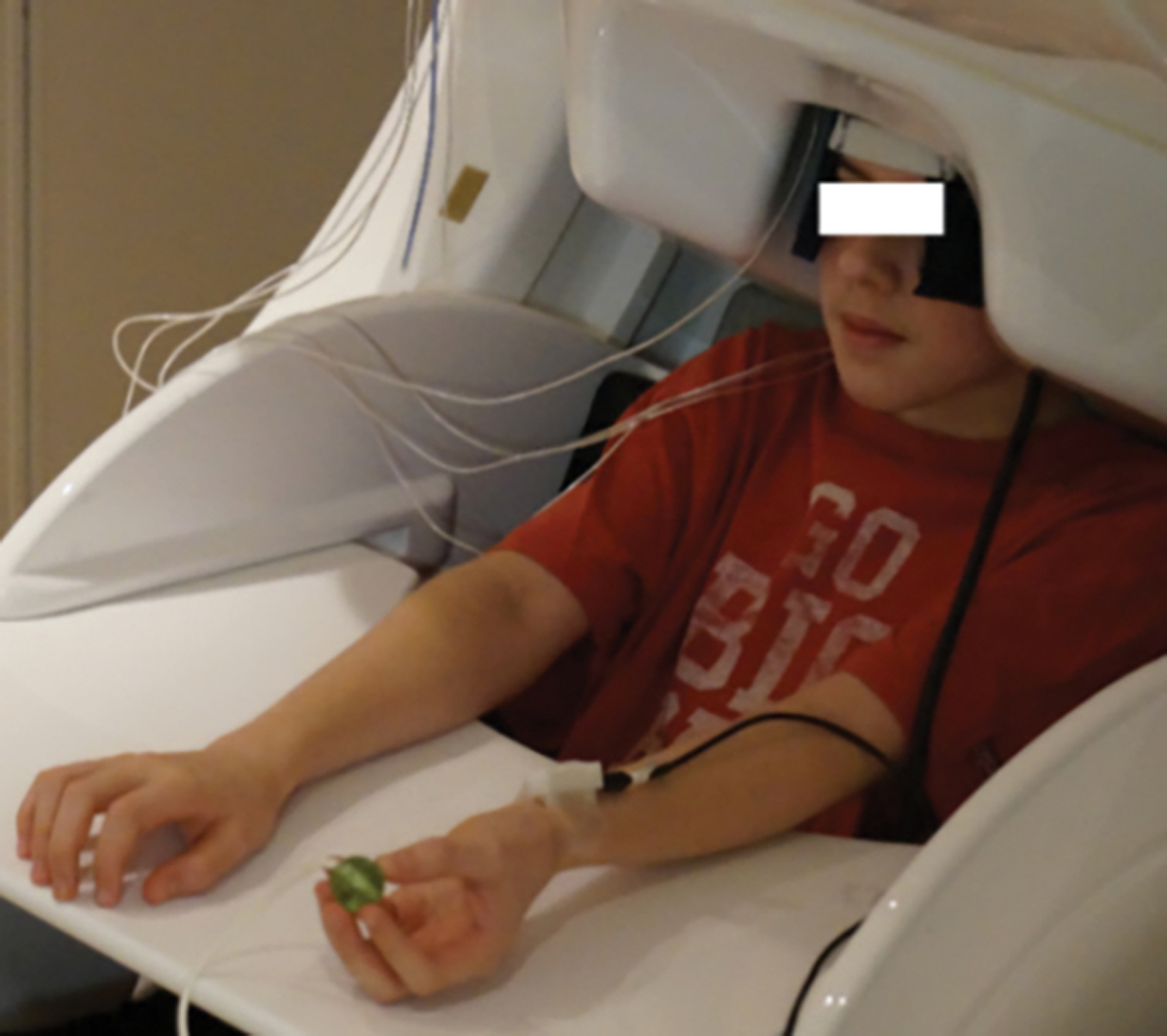
Depiction of the haptic condition where the youth is seated with their head in the MEG and moving the ball within the fingers of the left hand. Note that the electrical stimulator is positioned proximal to the wrist and near the left median nerve.
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio-video feeds from inside the shielded room. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using a MEGIN/Elekta MEG system (Helsinki, Finland) with 306 sensors, including 204 planar gradiometers and 102 magnetometers. Each MEG data set was individually corrected for head motion during the task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola 2006).
MEG co-registration and structural MRI processing
Four coils were affixed to the head of the participant and were used for continuous head localization during the experiment. Prior to the experiment, the location of these coils, three fiducial points and the scalp surface were digitized to determine their three-dimensional position (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for the MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data was co-registered with a structural T1-weighted MRI data prior to source reconstruction. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into a standardized space. Structural MRI data were acquired using a Philips Achieva 3T scanner. High-resolution T1-weighted sagittal images were obtained with an eight-channel head coil using a 3D fast field echo sequence with the following parameters: FOV: 24 cm, 1 mm slice thickness, no gap, in-plane resolution of 1.0 × 1.0 mm and sense factor of 2.0.
MEG Preprocessing
The raw MEG recordings were initially filtered with a 200 Hz zero order low pass digital filter, and a 0.5 zero order high pass digital filter. Additionally, a notch filter was applied to remove the 60 Hz line noise. Cardiac artifacts were subsequently removed from the data using signalspace projection, which was accounted for during source reconstruction (Uusitalo and Ilmoniemi 1997). The continuous magnetic time series was divided into epochs of 1750 ms duration, from −600 ms to 1150 ms with the baseline being defined as −600 ms to −200 ms and 0.0 ms being stimulation onset. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, etc.) were rejected based on a fixed-threshold method using individual amplitude and gradient thresholds, supplemented with visual inspection.
An independent samples t-test revealed that the number of trials accepted between groups was not significantly different (CP = 102.75 ± 10.36, NT = 106.28 ± 15.68, P = 0.452), and a paired samples t-test revealed no differences in trials accepted between the haptic and passive conditions (Haptic = 105.91 ± 17.61, Passive = 102.62 ± 10.46, P = 0.354).
Sensor Level Analysis
The artifact-free epochs were averaged across trials to generate a mean time series per sensor and participant, and the specific time windows used for subsequent source imaging were determined by statistical analysis of the sensor-level time series across all participants and conditions using the entire array of gradiometers. Each data point in the time series was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, paired-sample t tests were conducted to test for differences from baseline at each data point and the output time series of t values was thresholded at P < 0.05 to define time bins containing potentially significant phase-locked activity across all participants. In stage two, the time points that survived the threshold were clustered with temporally and/or spatially neighboring time points that were also above the threshold (P < 0.05), and a cluster value was derived by summing all of the t values of all data points in the cluster. Non-parametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Maris and Oostenveld 2007). For each comparison, 1000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time windows that contained significant phase-locked events across all participants were used to guide subsequent time-domain source level analysis.
MEG Source Imaging (sLORETA)
Time domain source images were computed using standardized low-resolution brain electromagnetic tomography (sLORETA) (Pascual-Marqui 2002). The resulting whole-brain maps were 4-dimensional estimates of current density per voxel, per time sample across the experimental epoch. These data were normalized to the sum of the noise covariance and theoretical signal covariance, and thus the units are arbitrary. These maps were then averaged temporally over the time windows identified in the sensor-level analysis. As the stimulation was applied to the more affected/non-dominant hand, we identified the peak voxel of activity on an individual basis. From this peak voxel, the sLORETA units were extracted to derive estimates of the time-domain response amplitude for each participant per condition. All imaging procedures were done with the Brain Electrical Source Analysis (BESA) software (BESA v7.0; Grafelfing, Germany). For additional methodological detail, please see our recent paper (Wiesman and Wilson 2020).
Statistical Analysis
A two-way mixed model ANOVA was utilized to investigate the differences in somatosensory-evoked cortical activity between groups (CP and NT) and conditions (Passive and Haptic). Linear regression models were used to assess whether the somatosensory cortical activity in the passive condition predicted that observed in the haptic condition. Shapiro Wilke tests were used to test normality. All statistical analyses were performed at the 0.05 alpha level.
Results
Three participants with CP and one NT control were excluded from the final analysis due to excessive motion and/or other MEG artifacts. Thus, 12 individuals with CP (16.08 ± 0.77 yrs) and 17 NT controls (14.35 ± 0.55 yrs) remained and were included in the final analysis. Robust somatosensory cortical responses were observed across a wide array of sensors within the frontal and parietal cortices, with the strongest activity in the lateral sensors near the contralateral hand region of the sensorimotor cortices. Permutation testing of the sensor-level data revealed that the somatosensory cortical response was significantly different from baseline during the 28 – 168 ms time window. Thus, source activity estimates were averaged across this window. The resulting sLORETA data revealed that the peak neural response in each condition, per group and hand stimulated, emanated from the somatosensory cortices (Figure 2). Note that the image scale bars shown in Figure 2 are different across the displayed images. This was done to enable visualization of the precise anatomical location of each response across the respective groups and conditions. Hence, the magnitude of the scale bars should be used when interpreting whether the source activity was weaker or stronger. We used this visualization approach because the responses were much stronger in the controls; essentially, if we displayed the participants with CP at the threshold used in controls there would be no discernable response, and conversely if we used the threshold of participants with CP on the control data the response would extend across most of the hemisphere and the peak would not be discernable. Qualitative inspection of these images suggests that the somatosensory cortical activity was stronger during the passive stimulation condition when compared with the haptic condition in both groups. Further, the images indicate that the somatosensory cortical activity was overall weaker for the participants with CP when compared with the NT controls.
Figure 2:
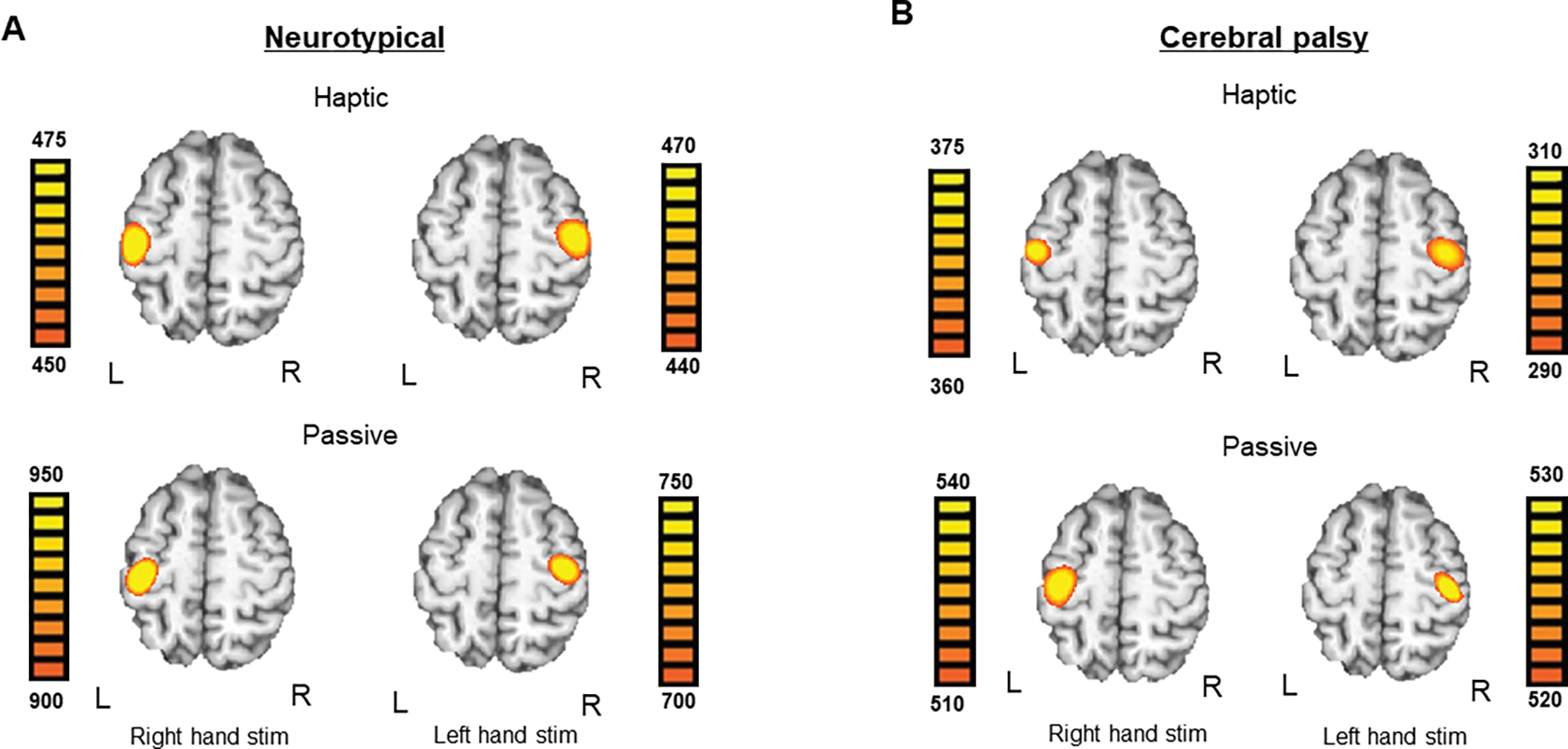
Brain images averaged within group for neurotypical (NT) controls and persons with cerebral palsy (CP), separated by condition and side of stimulation. In each panel, neural data for stimulation of the right hand is shown on the left and stimulation of the left hand is shown on the right. A.) Brain images averaged across NT controls on the left and right side during the haptic condition (top) and passive condition (bottom) B.) Brain images averaged across persons with CP on the left and right side during the haptic condition (top) and passive condition (bottom). Note that the image scale bars are different across the respective images. This was done to enable the anatomical location of the peak response to be accurately visualized in each map. Hence, the magnitude of the scale bars should be used when interpreting if the neural responses were weaker or stronger per condition, side, and group. See text for further explanation.
We subsequently extracted the amplitude of the response for each condition per participant from the peak voxel for our statistical analyses. Note that the units were extracted from each individual’s peak voxel as the stimulation was applied to the participant’s more affected/nondominant side. To assess for group (CP vs. NT controls) and conditional (passive vs. haptic) differences, we conducted a 2×2 mixed model ANOVA, which revealed significant main effects of group (NT = 743.16 ± 43.90 AU; CP = 515.36 ± 44.68 AU, F(1, 27) = 12.75; P = 0.001) and condition (Haptic = 493.39 ± 29.56 AU; Passive = 782.20 ± 51.58 AU, F(1, 27) = 59.57; P < 0.001; Figure 3). The main effect of group confirmed that those with CP had significantly weaker somatosensory cortical responses than their NT peers across both conditions, while the conditional effect indicated that somatosensory cortical activity was weaker across all participants during the haptic condition. The interaction between group and condition was not significant (P = 0.167). To visualize these statistically significant differences, we created group and condition-wise average images and neural time courses (Figure 3). Once again, it should be noted that the scale bars shown in Figure 3 are different across the respective brain images. This was done to highlight the precise anatomical location of the neural responses in the group and condition-wise images. Hence, the magnitude of the scale bars should be used when interpreting if the neural responses were weaker or stronger across groups and conditions. Qualitative inspection of the brain images and neural time courses supports our statistical observation that the youth with CP tended to have weaker somatosensory cortical responses and that across both groups’ somatosensory activity was notably stronger during the Passive condition. Brain images and time series for representative subjects are also provided in Supplementary Figure 1.
Figure 3.
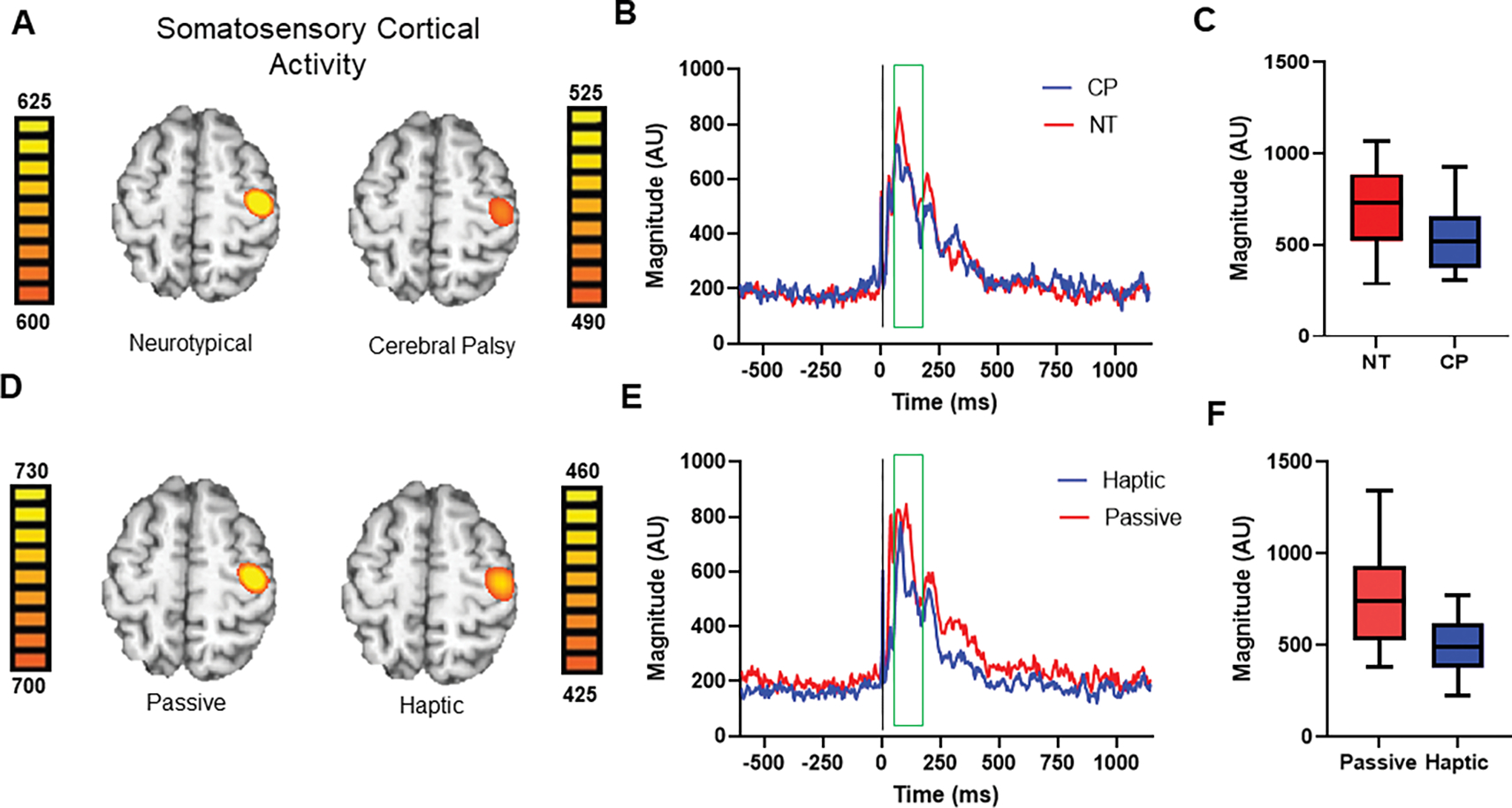
A.) Somatosensory cortical activity in the neurotypical (NT) controls and the youth with cerebral palsy (CP) averaged across the two conditions. For illustration purposes, persons with stimulation on the left side are shown. Note that the image scale bars are different across the respective images. This was done to highlight the precise anatomical location of the neural responses. Hence, the magnitude of the scale bars should be used when interpreting if the source activity was weaker or stronger across groups. B.) Neural time course representing the somatosensory cortical activity averaged across all NT controls (red) and youth with CP (dark blue). The green box denotes the time window analyzed (28 – 168 ms), with the stimulation onset being at time zero, represented by a vertical black line. Note that time series were extracted from individual peaks. C.) Box and whisker plot representing the difference in somatosensory cortical activity between the youth with CP and NT controls. The box represents the median, 25th and 75th percentiles, and the whiskers represent the 5th and 95th percentile. As depicted, the youth with CP had a much weaker response (P = 0.014). D.) The somatosensory cortical activity from the haptic and passive conditions averaged across all participants. For illustration purposes, persons with stimulation on the left side are shown. Note that the image scale bars are different across the respective images. This was done to highlight the precise anatomical location of the neural responses. Hence, the magnitude of the scale bars should be used when interpreting if the source activity was weaker or stronger across conditions. E.) Neural time course representing the somatosensory cortical activity averaged by condition across all participants, with the haptic data shown in dark blue and the passive data shown in red. The green box denotes the time window analyzed, and the stimulation onset is at time zero, represented by a vertical black line. F.) Box and whisker plot representing the difference in somatosensory cortical activity between the haptic and passive conditions. The box represents the median, 25th and 75th percentiles, and the whiskers represent the 5th and 95th percentile. As depicted, the somatosensory cortical activity evoked during the passive condition was much stronger than the cortical activity evoked during haptic condition (P < 0.001).
Linear Regression
The strength of somatosensory cortical responses during the passive condition predicted the strength of such responses during the haptic condition across all participants (F (1, 27) = 24.00, P < 0.001), implying that individuals who had weaker somatosensory cortical activity at rest also tended to have weaker somatosensory cortical activity during the haptic condition. This held true when the regression was separated by group. The strength of the somatosensory cortical responses during the passive condition predicted the strength of such responses during the haptic condition in persons with CP (F (1, 10) = 13.23, P = 0.005) and NT controls (F (1, 15) = 5.26, P = 0.037) (Figure 4). Overall, our regression analysis implies that the aberrant somatosensory cortical responses seen in youth with CP at rest are an adequate predictor of the degree of somatosensory dysfunction observed during movement.
Figure 4.
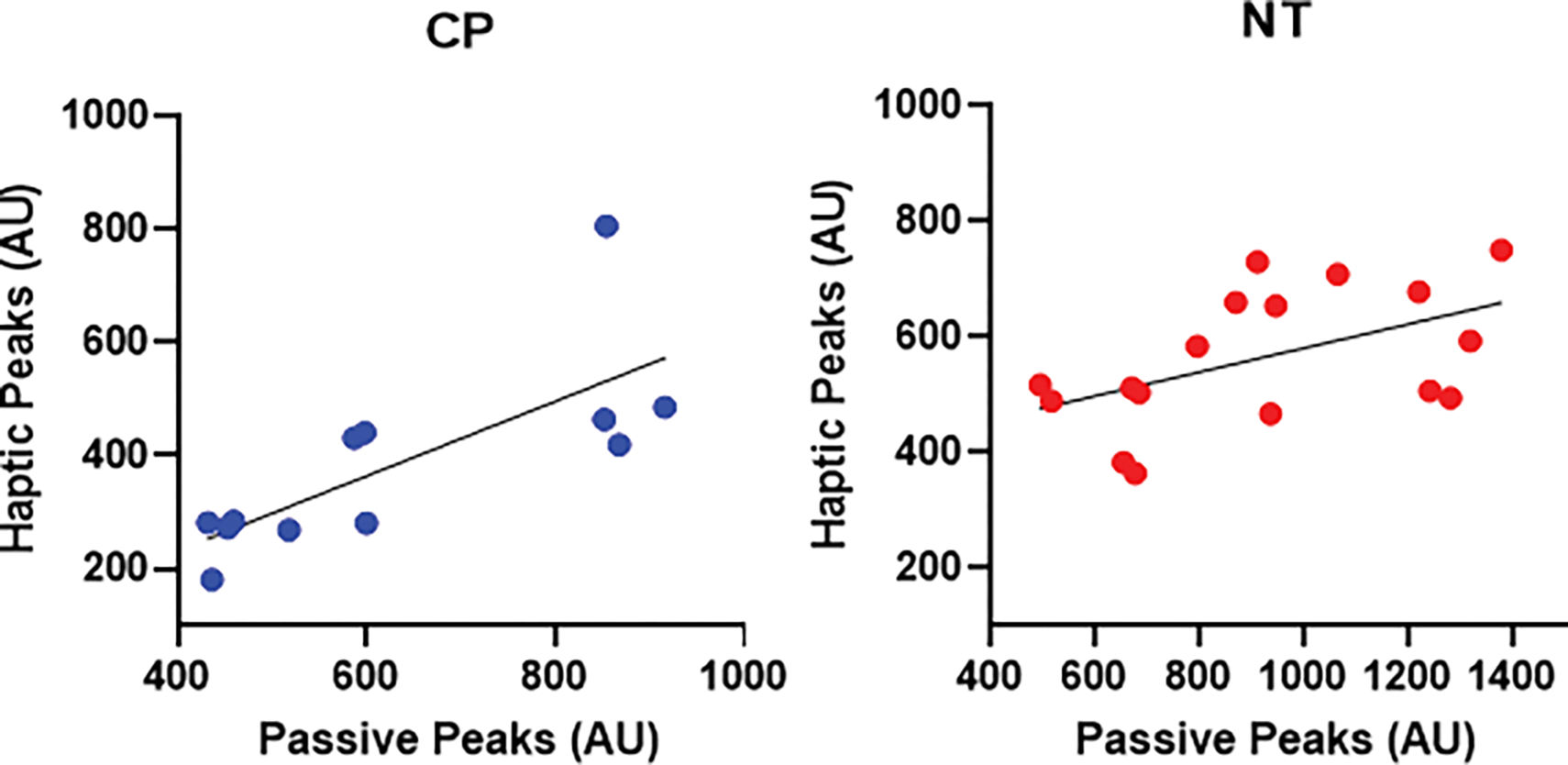
Scatterplots depicting the relationship between the strength of somatosensory cortical responses during the haptic condition (y-axis) and the passive condition (x-axis) in persons with cerebral palsy (CP) (left) and neurotypical (NT) controls (right). The respective scatter plots show that the individuals who had weaker somatosensory cortical activity during the passive condition also tended to have weaker cortical activity during the haptic condition (P’s < 0.05).
Discussion
In the current experiment, we sought to identify whether the somatosensory cortical activity was aberrant when youth with CP performed a haptic movement task. Our novel experimental results illustrated that the somatosensory-evoked cortical activity was weaker in response to median nerve stimulation during passive stimulation, as well as during performance of a haptic hand movement task in youth with CP compared to a demographically-matched group of their NT peers. Furthermore, we found that the somatosensory cortical activity was attenuated during the haptic condition relative to the passive condition across all participants. Finally, both groups showed a significant association between somatosensory cortical responses during the passive condition and during the haptic movement task. These results imply that the altered somatosensory cortical activity seen in youth with CP may continue to be abnormal during the execution of hand motor actions. The implications of these results are discussed further below.
Our MEG brain imaging results were in agreement with an abundance of literature demonstrating that somatosensory cortical activity is weaker in youth with CP (Riquelme and Montoya 2010, Kurz and Wilson 2011, Papadelis, Ahtam et al. 2014, Papadelis, Butler et al. 2018, Trevarrow, Kleinsmith et al. 2020), as well as numerous studies that have shown alterations in the oscillatory activity induced by peripheral stimulations in those with CP (Guo, Xiang et al. 2012, Kurz, Heinrichs-Graham et al. 2014, Kurz, Heinrichs-Graham et al. 2015, Kurz, Wiesman et al. 2018). Our previous work has also demonstrated that somatosensory activity in youth with CP follows an aberrant developmental trajectory (Trevarrow, Kleinsmith et al. 2020), and that this may at least partially be attributable to reduced cortical thickness within the postcentral gyri (Trevarrow, Lew et al. 2021). Ultimately, it is becoming increasingly evident that alterations in cortical processing of incoming sensory information likely plays a major role in the clinical deficits seen in somatosensory processing (Cooper, Majnemer et al. 1995, Clayton, Fleming et al. 2003, Sanger and Kukke 2007, Wingert, Burton et al. 2008, Hoon, Stashinko et al. 2009, Wingert, Burton et al. 2009). Such dampened ability to adequately integrate mechanoreceptive feedback from the fingers likely contributes to core deficits in sensorimotor integration, which may result in a reduced capacity to properly discriminate between tactile stimuli and different objects, contributing to the deficits in stereognosis commonly noted in the clinic (Wingert, Burton et al. 2008). This interpretation is well aligned with prior fMRI studies that have shown that somatosensory cortical activity during haptic tasks is associated with the localization of objects across the hand (Marangon, Kubiak et al. 2015).
Another noteworthy finding from the current study was that across all individuals the somatosensory cortical activity was reduced during movement in comparison with the passive condition. This aligns with numerous EEG and animal studies demonstrating somatosensory activity to be reduced during movement (Papakostopoulos, Cooper et al. 1975, Jones, Halonen et al. 1989, Seki, Perlmutter et al. 2003, Houdayer, Labyt et al. 2006, Seki and Fetz 2012). Somatosensory-evoked cortical activity can be regulated by top-down processing, such that the response is stronger when attention is paid to the stimulus (Dockstader, Cheyne et al. 2010). Thus, the diminished somatosensory responses during the haptic movement condition may at least partially be attributable to less attention being put toward the stimulation and more focus on performing the motor task. Alternatively, the attenuated response during the haptic movement condition could be a result of gating instigated by the efferent signals driven by the motor command (Jones, Halonen et al. 1989, Wasaka, Hoshiyama et al. 2003, Saradjian 2015). In either case, we did not find a significant interaction between group and condition, indicating that both the individuals with CP and NT controls similarly attenuated the somatosensory cortical activity during the haptic movement condition. However, given that individuals with CP also exhibited reduced somatosensory cortical responses while at rest (Kurz and Wilson 2011, Papadelis, Ahtam et al. 2014, Papadelis, Butler et al. 2018, Trevarrow, Kleinsmith et al. 2020), we found that their somatosensory cortical activity was also relatively weaker in the haptic condition, with their responses during the passive condition being strongly predictive of the somatosensory cortical response strength observed during the haptic hand motor action. This implies that youth with CP who have reduced somatosensory cortical response amplitudes at rest also tend to have aberrant somatosensory cortical processing during hand motor actions. Thus, our conjecture is that the further attenuation of somatosensory responses during movement in youth with CP likely impedes their ability to effectively update their internal model to accurately plan and execute motor actions. In summary, the underlying neurophysiological changes contributing to the altered somatosensory cortical processing that individuals with CP exhibit at rest likely persistent during movement.
Before closing, it is important to acknowledge a few limitations. First, the persons with CP included in this study were all youth. It is well known that persons with CP have mobility and other functional declines during the transition into adulthood (Haak, Lenski et al. 2009, Riquelme, Cifre et al. 2011, Van Der Slot, Nieuwenhuijsen et al. 2012, Opheim, McGinley et al. 2013, Lundh, Nasic et al. 2018), and future work could benefit from assessing how the processing of sensory feedback during movement may be related to the functional declines noted within the clinic during the transition into adulthood. Second, as there were persons with both right and left hemiplegic CP included within this study, individual peaks were used to extract the magnitude of the somatosensory cortical responses. Thus, the cortical responses were not measured from the exact same anatomical location across individuals. However, the responses were each extracted from the sensorimotor cortex, and extracting individual peaks may enhance the sensitivity of identifying groupwise differences in the strength of the somatosensory cortical response by accounting for the spatial variability of the cortical responses. Third, it should be noted that the somatosensory processing deficits noted within this study are at the group level, thus it is possible that some persons with CP may have less severe (or no) somatosensory processing deficits. Lastly, as prior research has shown that a stronger motor command is associated with greater sensory attenuation, one could presume that the reduced somatosensory response seen for the participants with CP might be partially connected with their muscular performance (i.e., cocontraction or more movement). However, we think this is unlikely because previous findings have consistently demonstrated that the motor command is weaker in persons with CP. For example, neuromuscular activation—which is the maximum contraction one can perform voluntarily relative to the maximum that the muscle can be activated by electrical stimulation—is decreased in persons with CP (Rose and McGill 2005). This implies that the total number of alpha motor neurons involved in the muscular contraction is decreased in persons with CP. Furthermore, other studies have demonstrated that increases in muscle contraction result in less change to motor-evoked potentials in persons with CP compared with NT controls (Condliffe, Jeffery et al. 2019). These findings further support the notion that the number of alpha motor neurons active during a muscular contraction are decreased in CP. Therefore, a larger motor command is likely not directly contributing to the weaker somatosensory cortical activity in persons with CP. Rather, we suggest that the reduction in somatosensory cortical activity places them at a disadvantage for processing incoming sensations during movement. This line of reasoning is supported by the correlation between the somatosensory cortical activity during the passive condition and during movement that we uncovered. Despite these noted limitations, the finding presented in this investigation provide new insights into the underlying neurological mechanisms that may contribute to the altered sensorimotor integration that can ultimately contribute to the altered hand motor actions seen in persons with CP.
Supplementary Material
Highlights.
Individuals with cerebral palsy have reduced somatosensory cortical activity during movement
The reduced somatosensory cortical activity during movement is related to reduced somatosensory cortical activity during rest
The reduced somatosensory cortical activity during movement may contribute to clinical deficits in motor actions
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (R01-HD086245, R01-HD101833, R21-HD096390, P20-GM144641).
Footnotes
Competing Interests
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angel RW and Malenka RC (1982). “Velocity-dependent suppression of cutaneous sensitivity during movement.” Exp Neurol 77(2): 266–274. [DOI] [PubMed] [Google Scholar]
- Auld ML, Boyd RN, Moseley GL, Ware RS and Johnston LM (2012). “Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy.” Arch Phys Med Rehabil 93(4): 696–702. [DOI] [PubMed] [Google Scholar]
- Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R and Yeargin-Allsopp M (2014). “Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008.” Dev Med Child Neurol 56(1): 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Fleming JM and Copley J (2003). “Behavioral responses to tactile stimuli in children with cerebral palsy.” Phys Occup Ther Pediatr 23(1): 43–62. [PubMed] [Google Scholar]
- Condliffe EG, Jeffery DT, Emery DJ, Treit S, Beaulieu C and Gorassini MA (2019). “Full Activation Profiles and Integrity of Corticospinal Pathways in Adults With Bilateral Spastic Cerebral Palsy.” Neurorehabil Neural Repair 33(1): 59–69. [DOI] [PubMed] [Google Scholar]
- Cooper J, Majnemer A, Rosenblatt B and Birnbaum R (1995). “The determination of sensory deficits in children with hemiplegic cerebral palsy.” J Child Neurol 10(4): 300–309. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Cheyne D and Tannock R (2010). “Cortical dynamics of selective attention to somatosensory events.” Neuroimage 49(2): 1777–1785. [DOI] [PubMed] [Google Scholar]
- Gordon AM and Duff SV (1999). “Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy.” Dev Med Child Neurol 41(9): 586–591. [DOI] [PubMed] [Google Scholar]
- Gori M, Squeri V, Sciutti A, Masia L, Sandini G and Konczak J (2012). “Motor commands in children interfere with their haptic perception of objects.” Exp Brain Res 223(1): 149–157. [DOI] [PubMed] [Google Scholar]
- Guo X, Xiang J, Mun-Bryce S, Bryce M, Huang S, Huo X, Wang Y, Rose D, Degrauw T, Gartner K, Song T, Schmit J and Vargus-Adams J (2012). “Aberrant high-gamma oscillations in the somatosensory cortex of children with cerebral palsy: a meg study.” Brain Dev 34(7): 576–583. [DOI] [PubMed] [Google Scholar]
- Haak P, Lenski M, Hidecker MJ, Li M and Paneth N (2009). “Cerebral palsy and aging.” Dev Med Child Neurol 51 Suppl 4: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst-Wolf JM, Yeh IL and Konczak J (2016). “Development of Proprioceptive Acuity in Typically Developing Children: Normative Data on Forearm Position Sense.” Front Hum Neurosci 10: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S and Johnston MV (2009). “Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways.” Dev Med Child Neurol 51(9): 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL and Derambure P (2006). “Relationship between event-related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations.” Clin Neurophysiol 117(3): 628–636. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Halonen JP and Shawkat F (1989). “Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement.” Electroencephalogr Clin Neurophysiol 74(1): 36–45. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Wingate MS, Van Naarden Braun K, Doernberg NS, Arneson CL, Benedict RE, Mulvihill B, Durkin MS, Fitzgerald RT, Maenner MJ, Patz JA and Yeargin-Allsopp M (2011). “Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network.” Res Dev Disabil 32(2): 462–469. [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Solowiej E and Bockowski L (2006). “Somatosensory and visual evoked potentials in children with cerebral palsy: correlations and discrepancies with MRI findings and clinical picture.” Pediatr Rehabil 9(3): 201–209. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E and Wilson TW (2015). “Children with cerebral palsy have uncharacteristic somatosensory cortical oscillations after stimulation of the hand mechanoreceptors.” Neuroscience 305: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM and Wilson TW (2014). “Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy.” J Neurophysiol 111(3): 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Becker KM and Wilson TW (2015). “The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy.” J Neurophysiol 113(9): 3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM and Wilson TW (2018). “Children with Cerebral Palsy Hyper-Gate Somatosensory Stimulations of the Foot.” Cereb Cortex 28(7): 2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM and Wilson TW (2018). “Haptic exploration attenuates and alters somatosensory cortical oscillations.” J Physiol 596(20): 5051–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ and Wilson TW (2011). “Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy.” Neurosci Lett 490(1): 1–5. [DOI] [PubMed] [Google Scholar]
- Lundh S, Nasic S and Riad J (2018). “Fatigue, quality of life and walking ability in adults with cerebral palsy.” Gait Posture 61: 1–6. [DOI] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP and Key AP (2012). “Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy.” J Child Neurol 27(10): 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon M, Kubiak A and Króliczak G (2015). “Haptically Guided Grasping. fMRI Shows Right-Hemisphere Parietal Stimulus Encoding, and Bilateral Dorso-Ventral Parietal Gradients of Object- and Action-Related Processing during Grasp Execution.” Front Hum Neurosci 9: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E and Oostenveld R (2007). “Nonparametric statistical testing of EEG- and MEG-data.” J Neurosci Methods 164(1): 177–190. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE and Gandevia SC (1988). “Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study.” Exp Brain Res 70(3): 569–576. [DOI] [PubMed] [Google Scholar]
- Opheim A, McGinley JL, Olsson E, Stanghelle JK and Jahnsen R (2013). “Walking deterioration and gait analysis in adults with spastic bilateral cerebral palsy.” Gait Posture 37(2): 165–171. [DOI] [PubMed] [Google Scholar]
- Papadelis C, Ahtam B, Nazarova M, Nimec D, Snyder B, Grant PE and Okada Y (2014). “Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study.” Front Hum Neurosci 8: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Butler EE, Rubenstein M, Sun L, Zollei L, Nimec D, Snyder B and Grant PE (2018). “Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts.” Neuroimage Clin 17: 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostopoulos D, Cooper R and Crow HJ (1975). “Inhibition of cortical evoked potentials and sensation by self-initiated movement in man.” Nature 258(5533): 321–324. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. Spain. 24 Suppl D: 5–12. [PubMed] [Google Scholar]
- Riquelme I, Cifre I and Montoya P (2011). “Age-related changes of pain experience in cerebral palsy and healthy individuals.” Pain Med 12(4): 535–545. [DOI] [PubMed] [Google Scholar]
- Riquelme I and Montoya P (2010). “Developmental changes in somatosensory processing in cerebral palsy and healthy individuals.” Clin Neurophysiol 121(8): 1314–1320. [DOI] [PubMed] [Google Scholar]
- Robert MT, Guberek R, Sveistrup H and Levin MF (2013). “Motor learning in children with hemiplegic cerebral palsy and the role of sensation in short-term motor training of goal-directed reaching.” Dev Med Child Neurol 55(12): 1121–1128. [DOI] [PubMed] [Google Scholar]
- Rose J and McGill KC (2005). “Neuromuscular activation and motor-unit firing characteristics in cerebral palsy.” Dev Med Child Neurol 47(5): 329–336. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B and Jacobsson B (2007). “A report: the definition and classification of cerebral palsy April 2006.” Dev Med Child Neurol Suppl 109: 8–14. [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J and Boyd R (2010). “The relationship between unimanual capacity and bimanual performance in children with congenital hemiplegia.” Dev Med Child Neurol 52(9): 811–816. [DOI] [PubMed] [Google Scholar]
- Sanger TD and Kukke SN (2007). “Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy.” J Child Neurol 22(3): 289–293. [DOI] [PubMed] [Google Scholar]
- Saradjian AH (2015). “Sensory modulation of movement, posture and locomotion.” Neurophysiol Clin 45(4–5): 255–267. [DOI] [PubMed] [Google Scholar]
- Seki K and Fetz EE (2012). “Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement.” J Neurosci 32(3): 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI and Fetz EE (2003). “Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement.” Nat Neurosci 6(12): 1309–1316. [DOI] [PubMed] [Google Scholar]
- Strauss D, Brooks J, Rosenbloom L and Shavelle R (2008). “Life expectancy in cerebral palsy: an update.” Dev Med Child Neurol 50(7): 487–493. [DOI] [PubMed] [Google Scholar]
- Taulu S and Simola J (2006). “Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements.” Phys Med Biol 51(7): 1759–1768. [DOI] [PubMed] [Google Scholar]
- Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E and Tsikoulas I (2011). “Somatosensory evoked potentials in children with bilateral spastic cerebral palsy.” Pediatr Neurol 44(3): 177–182. [DOI] [PubMed] [Google Scholar]
- Trevarrow MP, Kleinsmith J, Taylor BK, Wilson TW and Kurz MJ (2020). “The somatosensory cortical activity in individuals with cerebral palsy displays an aberrant developmental trajectory.” J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow MP, Lew BJ, Hoffman RM, Taylor BK, Wilson TW and Kurz MJ (2021). “Altered Somatosensory Cortical Activity Is Associated with Cortical Thickness in Adults with Cerebral Palsy: Multimodal Evidence from MEG/sMRI.” Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA and Ilmoniemi RJ (1997). “Signal-space projection method for separating MEG or EEG into components.” Med Biol Eng Comput 35(2): 135–140. [DOI] [PubMed] [Google Scholar]
- Van Der Slot WM, Nieuwenhuijsen C, Van Den Berg-Emons RJ, Bergen MP, Hilberink SR, Stam HJ and Roebroeck ME (2012). “Chronic pain, fatigue, and depressive symptoms in adults with spastic bilateral cerebral palsy.” Dev Med Child Neurol 54(9): 836–842. [DOI] [PubMed] [Google Scholar]
- Wasaka T, Hoshiyama M, Nakata H, Nishihira Y and Kakigi R (2003). “Gating of somatosensory evoked magnetic fields during the preparatory period of self-initiated finger movement.” Neuroimage 20(3): 1830–1838. [DOI] [PubMed] [Google Scholar]
- Wiesman AI and Wilson TW (2020). “Attention modulates the gating of primary somatosensory oscillations.” Neuroimage 211: 116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE and Damiano DL (2008). “Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination.” Dev Med Child Neurol 50(11): 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE and Damiano DL (2009). “Joint-position sense and kinesthesia in cerebral palsy.” Arch Phys Med Rehabil 90(3): 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkou A, Lee SCK, Prosser LA and Jeka JJ (2020). “Foot and Ankle Somatosensory Deficits Affect Balance and Motor Function in Children With Cerebral Palsy.” Front Hum Neurosci 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


