Abstract
Cardiovascular disease and cancer are two of the leading causes of death worldwide. While improvements in outcomes have been noted for both disease entities, the success of cancer therapies has come at the cost of at times very impactful adverse events such as cardiovascular events. Hypertension has been noted as both, a side effect as well as a risk factor for the cardiotoxicity of cancer therapies. Some of these dynamics are in keeping with the role of hypertension as a cardiovascular risk factor not only for heart failure, but also for the development of coronary and cerebrovascular disease, and kidney disease and its association with a higher morbidity and mortality overall. Other aspects, such as the molecular mechanisms underlying the amplification of acute and long-term cardiotoxicity risk of anthracyclines and increase in blood pressure with various cancer therapeutics remain to be elucidated. In this review, we cover the latest clinical data regarding the risk of hypertension across a spectrum of novel anti-cancer therapies as well as the underlying known or postulated pathophysiological mechanisms. Further, we review the acute and long-term implications for the amplification of the development cardiotoxicity with drugs not commonly associated with hypertension such as anthracyclines. An outline of management strategies, including pharmacological and lifestyle interventions as well as models of care aimed to facilitate early detection and more timely management of hypertension in cancer patients and survivors concludes this review, which overall aims to improve both cardiovascular and cancer-specific outcomes.
Keywords: Cardio-oncology, Hypertension, Cancer therapy, Epidemiology, Etiology, Mechanisms, Management
Introduction
There has been a rapid emergence of (next generation) anti-neoplastic treatments over the last few decades, which (combined with early detection strategies and population education) have markedly extended overall patient survival across almost all cancer types. Whilst the benefits of these therapies have been clearly established, the cardiovascular adverse events (CVAE) associated with these therapies have also emerged and can impact the quality of life and even the life expectancy of patients. The significance of this issue is magnified by the fact that cardiovascular disease (CVD) and cancer are the two most common causes of morbidity and mortality in developed nations.1
CVD and cancer not infrequently coexist, and mortality and morbidity from CVD is significantly higher amongst cancer patients.2–4 The association is complex and not explained by one single (shared) risk factor such as age, smoking status, diabetes, alcohol intake, diet, obesity, chronic inflammatory states and genetic risk. Rather, it can be postulated that a combination of risk factors with pathophysiologic changes induced by cancer and its treatment generates an exaggerated risk.5 One such factor is hypertension; in fact, numerous studies have confirmed hypertension to be a key factor for the risk of cardiac dysfunction and heart failure in patients exposed to anthracycline therapy.6 Of further note, hypertension is not only a major risk factor for the development of CVAE, but can by itself be a form of CVAE. Indeed, multiple anti-cancer therapies have been linked to new onset or worsening hypertension (Table 1 and Figure 1).7–10 This is on the background of an already ~20% higher prevalence of hypertension in cancer patients than in matched non-cancer control populations.4
Table 1:
Overview of anti-cancer agents associated with development/exacerbation of hypertension
| Class | Drug (Brand) | Mechanism(s) | Reference |
|---|---|---|---|
| VEGF inhibitors | Aflibercept Bevacizumab Ramucirumab |
|
19,73–75,83 |
| Tyrosine inhibitors | Axitinib Cabozantinib Cediranib Intedanib Lenvatinib Nintedanib Pazopanib Regorafenib Sorafenib Sunitinib Tivozanib Vandetanib Vatalanib |
|
13,19,73–75,80,84,289 |
| Phosphatidylinositol 3-Kinase (PI3K) inhibitor | Alpelisib Copanlisib Duvelisib Idelalisib |
|
131,290 |
| Burton tyrosine kinase inhibitors | Ibrutinib Acalabrutinib Zanubrutinib |
|
8,84,117,272,291 |
| Alkylating agents | Cyclophosphamide Ifosfamide Busulfan Cisplatin |
|
19,74,255,292 |
| Immune checkpoint inhibitors |
PD-1 inhibitors: Cemiplimab Nivolumab Pembrolizumab PD-L1 inhibitors: Atezolizumab Avelumab Durvalumab CTLA-4 inhibitors: Ipilimumab |
|
13,293–295 |
| Vinca Alkaloids | Vincristine Vinblastine |
|
13,296,297 |
| Glucocorticoids | Prednisone Methylpredisolone |
|
298 |
| Calcineurin inhibitors | Cyclosporine Tacrolimus |
|
299,300 |
| Anti-androgens | Abiraterone Enzalutamide (mechanism unknown |
|
8,301,302 |
| Taxanes | Paclitaxel |
|
13,303 |
Figure 1. Pathophysiological changes associated with hypertension induced by new and emerging anti-cancer agents.
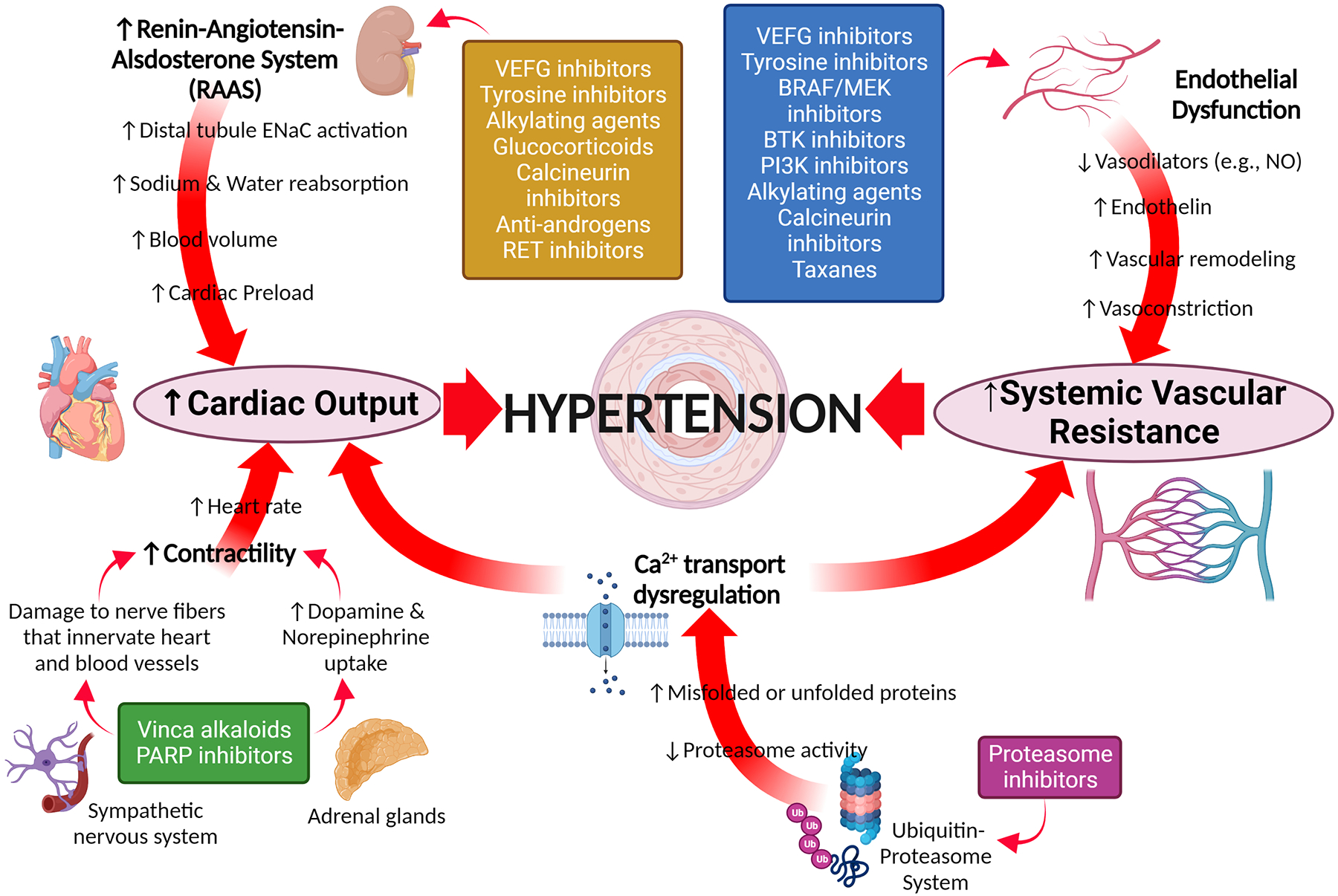
BRAF - B1 homolog v-raf murine sarcoma viral kinase oncogene; BTK - Bruton tyrosine kinase; ENaC -; MEK - mitogen-activated protein kinase; NO – nitric oxide; PARP - Poly(ADP-ribose) polymerases; PI3K - phosphatidylinositol 3-kinase; VEGF - Vascular endothelial growth factor.
Interest in this area among nephrologists, cardiologists, generalists, and oncologists has led to the emergence of “onco-hypertension” as a field within and beyond cardio-oncology and onco-nephrology. Onco-hypertension takes a broader approach to the understanding and management of hypertension, whilst acknowledging the gaps in evidence and the complexity of integrated factors including comorbidities, the choice of anti-cancer treatments, the cancer type, and patient factors.11–13 Recent developments in this area include a harmonized definition for hypertension in cancer patients, which we will discuss herein. We will furthermore reflect on blood pressure surveillance and management strategies for patients undergoing cancer therapies, including new models of care such as remote monitoring. This review will also cover the role hypertension plays in the development of cardiotoxicity with drugs commonly not associated with hypertension as a side effect. This article will commence though with a summary of clinical data linking new and emerging anti-cancer treatments with hypertension and underlying established or putative mechanisms for this side effect profile.
VEGF Inhibitors
Vascular endothelial growth factor (VEGF) inhibitors are one of the classic targeted anti-cancer therapies, still in common use globally. VEGF-A is a potent angiogenesis growth factor, part of a VEGF family of ligands. VEGF’s regulate angiogenesis via their effects on vascular endothelial cells affecting musculoskeletal growth, embryogenesis, reproductive function and importantly tumour genesis and growth.14,15 VEGF-A has been the most effective target for anti-angiogenesis treatment primarily due to its overexpression across a vast range of solid tumour types, including colorectal cancer (CRC), non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), peritoneal cancer, glioblastoma, cervical and ovarian cancers.15,16
Since approval of Bevacizumab, the first anti-VEGF-A recombinant humanised monoclonal antibody (mAb), there has been a steady growth of newer therapies within this class such as Ramucirumab, a VEGFR2 mAb, and Aflibercept, a novel fusion protein that has several targets (VEGF-A, VEGF-B, and PLGF),15,17 now in use in advanced gastro-oesophageal adenocarcinomas, metastatic gastric cancer, NSCLC, CRC, and hepatocellular carcinoma.17
The success of these specific VEGF inhibitors (VEGFi) led to the development and use of targeted Tyrosine Kinase inhibitors (TKI) against VEGF receptors and other downstream pathways. The oral bioavailability of these small molecule therapies offered a significant benefit over first generation VEGFi whilst also providing significant downstream kinase pathway effects further enhancing anti-tumor effects. There is now an array of VEGFi’s and VEGF-TKIs targeting a variety of downstream effector pathways available for treatment of many cancer types (Table 2).
Table 2:
FDA approved VEGF inhibitor anti-neoplastic therapies.
| Drug (Type) | Target | FDA-approved Indications | References |
|---|---|---|---|
| Bevacizumab (IgG1) |
|
|
304,305 |
| Sorafenib (TKI) |
|
|
306–308 |
| Lenalidomide (Immunomodulatory) |
|
|
309 |
| Sunitinib (TKI) |
|
|
310 |
| Thalidomide (TKI) |
|
|
311 |
| Pazopanib (TKI) |
|
|
74,312 |
| Vandetanib (TKI) |
|
|
313,314 |
| Axitinib (TKI) |
|
|
315 |
| Aflibercept (Fusion protein) |
|
|
316 |
| Regorafenib (TKI) |
|
|
317 |
| Cabozantinib (TKI) |
|
|
74,318,319 |
| Ponatinib (TKI) |
|
|
74 |
| Pomalidomide (TKI) |
|
|
320 |
| Ramucirumab (IgG1) |
|
|
321,322 |
| Nintedanib (TKI) |
|
|
323 |
| Lenvatinib (TKI) |
|
|
324 |
Crucial role of VEGF signalling in vascular homeostasis, vascular neo-angiogenesis and the maintenance of endothelial cell function likely accounts for VEGF-inhibitor-related CVAE such as arterial thromboembolism, cardiac dysfunction, QT interval prolongation, arrythmia and most commonly hypertension.18,19 There have also been reports of an increased risk of aneurysm and aortic dissection resulting from changes in the vascular wall which are compounded by the hypertensive effects of the treatment.20 Many of the CVAE including hypertension have been demonstrated across both the extracellular VEGF-mAb and the intracellular VEGF-TKIs.19
Clinical and epidemiological evidence for VEGF inhibitor induced hypertension
Hypertension as a CVAE of VEGFi has been variably reported to range from 30% to 80% in both clinical trials and real world cohorts with a majority of patients experiencing some form of blood pressure increase (grade 1 or 2) generally occurring early, however the true incidence may be much higher given significant underreporting of low grade events.19,21–23 More severe hypertension (grade 3 or 4) has been reported with the frequency of 6–40% in different clinical trials.19
A meta-analysis from Totzeck et al included over 10 000 bevacizumab-treated patients across a variety of cancers demonstrated 4.73-fold relative risk increase of all-grade hypertension with bevacizumab treatment in a dose-dependent manner.24 Comparatively in the most recent review of 105 RCTs with over 65000 cancer patients’ treatment with VEGFi mAbs was associated with a 3.22-fold increased risk of all-grade hypertension and a 6.15-fold increased risk of high-grade hypertension. While individual VEGFi mAb types conferred differential hypertensive risk, factors such as cancer type, prior treatment and duration did not.25
Multiple systematic reviews, collectively including 100 VEGF-TKI RCTs, have also confirmed their significant hypertensive effects, with relative risk increase of all-grade hypertension of between 3.46 and 3.85 fold, whilst grade 3 or above (high grade) hypertension risk was up to four times higher in treated patients.26–28 Subgroup analyses from one large metanalysis revealed marked variability in hypertension depending on tumour type, VEGFR-TKI used, control therapy, and chemotherapy regimens: breast cancer patients had the greatest risk for any-grade hypertension, while the largest proportion of high grade hypertension was seen in prostate cancer patients treated with VEGF-TKIs.27 Age, obesity and pre-existing hypertension are the key risk factors for development/worsening of anti-VEGF treatment-associated hypertension.29,30
The elevated hypertensive risk has also been demonstrated with newer multi-target (including VEGF) TKI’s such as fruquintinib, anlotinib and apatinib: fruquintinib and alotinib were associated with 5–21% of grade 3 or above and 13–67% of any-grade hypertension.31 32 In the REALITY RCT published this year, apatinib was associated with an even higher (34%) incidence of grade 3 or above hypertension.33 Similarly Anlotinib, a novel TKI with multiple targets including VEGF, has also been strongly associated with hypertension with an incidence ranging between 13%−67% across clinical trials with severe hypertension reported between 4% and 16%.32
Clinical trials to date have highlighted the importance of early identification and treatment of hypertension along with tailored dosage regimens to reduce severe hypertension and discontinuation.
Comparative head-to-head data of treatment-related hypertensive rates between different VEGFi mAbs and VEGF-TKIs is limited. A subgroup analysis of a large systematic review of 77 phase III and IV randomised control trials between 1990 and 2014 showed no significant differences for incident hypertension and for most CVAE.18 In contrast, a recent comparative network meta-analysis of over 20000 patients from 45 RCTs of nine VEGF-TKIs found that lenvatinib was the most likely to induce hypertension closely followed by vandetanib, cabozantinib, axitinib, pazopanib, sorafenib, sunitinib, regorafenib and nintedanib with no significant difference found for severe CVAE and severe hypertension.34 Unsurprisingly hypertension is magnified with a combination of targeted anti-VEGF therapies as was demonstrated between axitinib and crizotinib.35 A 2022 real-world Australian population study looking broadly at vascular signalling pathway inhibitor treatments, reported the incidence of new-onset and aggravated hypertension during treatment was similar, at 24% and 25% respectively, with a combined overall incidence of almost 50%, similar to clinical trial populations.30 Similarly a 2022 review of both VEGFi oral TKI’s and bevacizumab reported a median onset of 47 days for development of hypertension and importantly no difference in treatment interruption or discontinuation between treated groups due to hypertension.36
Building upon clinical effectiveness of VEGF inhibitors there has been an expansion of anti-angiogenic drugs in combination with other anti-neoplastic therapies such as with chemotherapy, and other targeted biological and immunotherapies. These combinations reduce treatment resistance and improve efficacy with promising results already realised in several cancers including melanoma, CRC, RCC, NSCLC, and glioblastoma.37 These treatment combinations (e.g. Lenvatinib combined with EGFR targeted TKI gefitinib or pembrolizumab) have demonstrated improved anti-cancer efficacy compared with hypertension incidence comparable to monotherapy alone.38–40 Similarly, trials of conventional chemotherapy combined with anti-angiogenic treatments (e.g. combination of etoposide with apatinib and docetaxel, epirubicin and cyclophosphamide with Apatinib in a recent 2022 publication) have also shown renewed treatment efficacy without significant unexpected hypertensive toxicity, with data predominantly showing higher rates of lower grade 1 and 2 hypertension.41–44
Interestingly, there is mounting evidence that VEGFi-induced hypertension, which is an “on-target” treatment response, may predict favourable cancer outcomes in patients and possibly represent a prognostic biomarker.8,29,36,45–60 In SELECT trial of patients with advanced thyroid cancer, there was a significant association between Lenvatinib-induced hypertension and overall survival.55 While similar findings have also been demonstrated with some other novel agents, eg anlotinib,32 other studies have failed to corroborate this association, leading to knowledge gap.51,61
Predicting VEGFi induced hypertension has been an identified are of interest, there are currently no proven predictive serum biomarkers available in clinical practice to guide physicians when assessing their patients for these treatments.62 Exploration has already begun into potential novel biomarkers such as VEGF-A and VCAM-1 which may utilised to predict hypertension in VEGFi treated patients. Most recently a study by Quintanilha et al found lower levels of these markers to be significant predictors of hypertension in colorectal patients treated with Regorafenib. Although not available in clinical use currently markers such as these may potentially path the way for tailored dosage regimens for patients and better help clinicians assess hypertensive risk and in doing so reduce treatment discontinuation or interruption.63 There has been recent interest as to whether genetics may prove key to this issue and help both prediction of both treatment efficacy and hypertensive effects. Examples have been demonstrated in breast cancer patients treated with bevacizumab whereby certain genetic polymorphisms were associated with improved survival whilst others associated with lower severe hypertensive effects.64 Similarly studies across a variety of tumours treated with bevacizumab have identified various single nucleotide polymorphisms (SNPs) of genes (WNK1, KLKB1, GRK4, SLC29A1 and HSP90AB1) which are involved in a wide array of mechanisms related to blood pressure regulation, these SNPs have been associated with the development of significant VEGFi related hypertension.65,66 Further research into genetic polymorphisms associated with the development of hypertension in novel VEGFi treatments is slowly emerging, for example VEGFA and eNOS polymorphisms and sunitinib associated hypertension.67 These associations will likely lead to a better understanding of the mechanistic influences underpinning the development of VEGFi hypertension and may one day lead to serum biomarkers to predict patient risk and treatment efficacy however larger clinical studies are required to validate findings such as these.
Recent research interest in the management of VEGFi induced hypertension and its effective management is evident by the diverse range of recent and ongoing clinical trials (CHA-RISMA (NCT04467021), NCT03709771, UNICO (NCT03882580) and TITAN (NCT01621659))62 some of which aim to assess the clinical outcomes of VEGFRi treated patients whilst others have been specifically designed to assess the ideal BP targets for treated cancer patients to better guide clinical practice. Recent 2022 publication demonstrated in animal models (Wistar rats) treated with Axitinib that losartan, an established angiotensin receptor blocker, can be used to effectively reduce hypertensive effects without effect on antitumor activity.68 A 2022 publication by Ren et al found that in metastatic CRC patients with bevacizumab related hypertension, treatment with renin-angiotensin inhibitors showed significant survival benefit over patients treated with calcium channel blockers or no treatment.69 Interestingly Mice studies using lisinopril further demonstrated a positive synergistic anti-tumour effect with increased 5-fluorouracil (5-Fu) tissue penetration and decrease both collagen and hyaluronic acid (HA) deposition whilst significantly downregulating the expression of TGF-β1 and downstream SMAD signalling which may also improve anti-tumour effect.69 These findings may suggest ACE-I as a class may be a favoured first line anti-hypertensive therapy in selective cancer patient populations. Efforts have been made to explore novel treatments for VEGFi hypertension utilising preclinical animal models and pluripotent stem cell models70, for example the use of rodent models to explore Endothelin blockade therapies71 and targeted treatments (Sildenafil) to utilise the NO regulatory pathway.72
Mechanisms of VEGFi induced hypertension
Our current knowledge of VEGFi-induced hypertension largely stems from an established understanding of chemotherapy-induced hypertension which revolves around two major mechanisms: endothelial dysfunction and microvascular rarefaction.8,13,19,45,73–76 VEGF signalling pathway disruption/inhibition is a critical factor leading to endothelial dysfunction in numerous chemotherapies. Despite their established use, a complete understanding of the molecular processes driving VEGFi-induced hypertension and vascular toxicities remains unclear. The key underlying mechanisms likely include inhibition of endothelial nitric oxide synthase (eNOS), diminished nitric oxide (NO) production, oxidative stress, activation of the endothelin-1 system promoting vasoconstriction, and rarefaction (Figures 1 and 2).19,32,75,76
Figure 2. Potential mechanisms involved in the onset of hypertension by established and emerging cancer therapies.
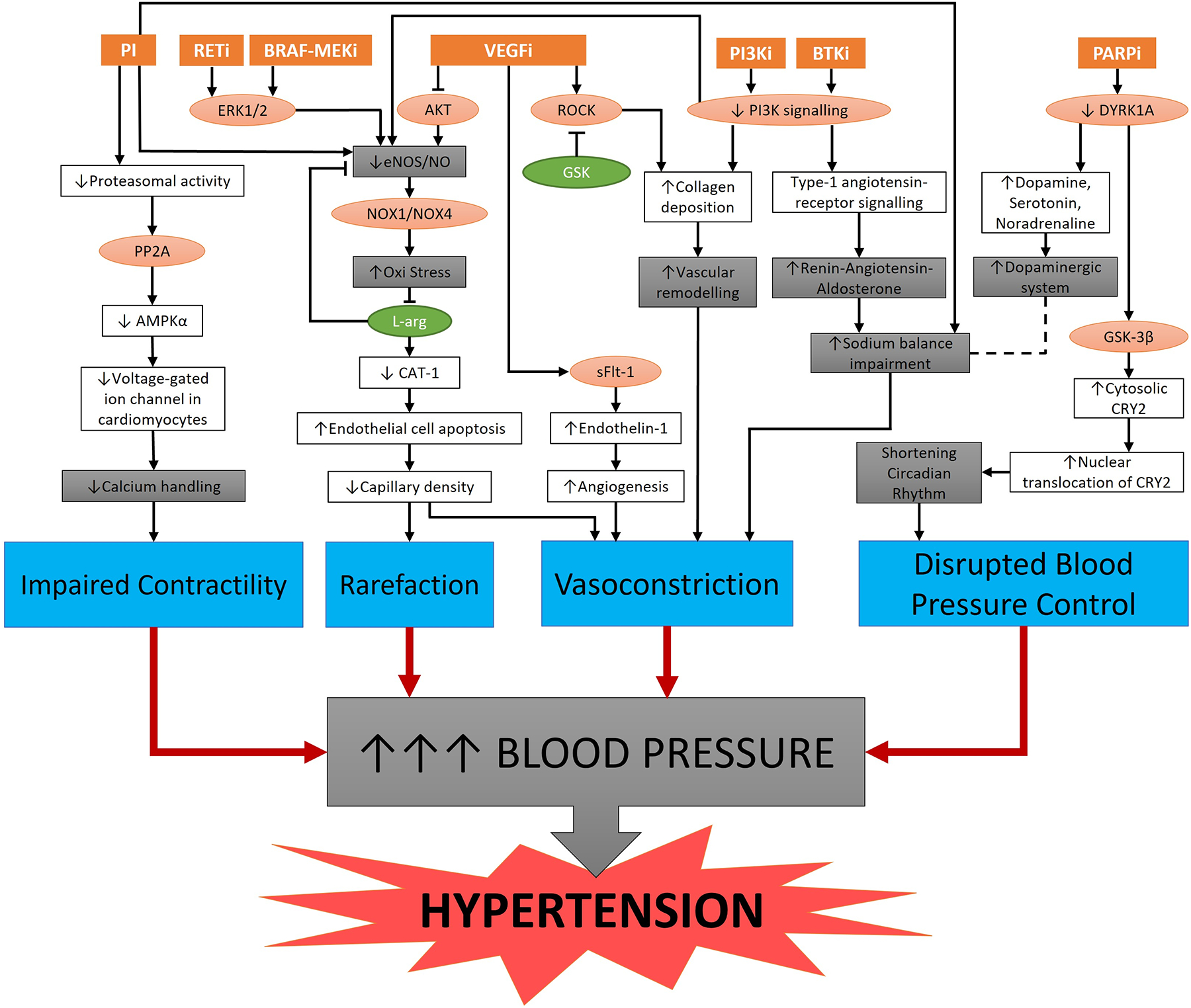
Schematic representations of major pathways affected by cancer therapies (orange boxes): Proteasome inhibitors (PI), RET inhibitors (RETi), BRAF-MEK inhibitors (BRAF-MEKi), Vascular endothelial growth factor inhibitors (VEGFi), Phosphatidylinositol 3-kinase inhibitors (PI3Ki), Bruton tyrosine kinase inhibitors (BTKi), and Poly (ADP Ribose) Polymerase inhibitors (PARPi). Lines with arrowheads and flatheads at the end represent “activation” and “inhibition,” respectively. Dashed line represents a plausible pathway. The red circles are key signalling molecules whilst green circles are potential anti-hypertensive therapeutic targets. The grey boxes represent the major mechanisms whilst the blue boxes indicate the common pathogenesis underpinning the onset of elevated blood pressure and, consequently, the development of hypertension. ERK = extracellular signal–regulated kinases; AKT = Protein Kinase B; ROCK = Rho-associated protein kinase; PI3K = Phosphoinositide 3-kinases; DYRK1A = Dual-specificity tyrosine phosphorylation-regulated kinase; eNOS = endothelial nitric oxide synthase; NO = nitric oxide; Y-27632 = ROCK Inhibitor; PP2A = Protein phosphatase 2; NOX = NADPH Oxidase; AMPKα = AMP-activated protein kinase; L-arg = L-arginine; GSK-3β = Glycogen synthase kinase-3 beta; CAT-1 = Cationic amino acid transporter-1; sFlt-1 = Soluble fms-like tyrosine kinase-1; and CRY2 = Cryptochrome circadian regulator 2.
Microvascular rarefaction may occur when antiangiogenic therapy reduces the microvascular surface area whilst increasing vascular resistance and blood pressure.77 It was thought that chronic VEGF depletion leads to reduced microvascular endothelial cell survival and consequently reduced tissue microvascular density.77 Formation of local thrombosis leads to a further decrease in vascular perfusion and exacerbates endothelial cell apoptosis and microvascular obliteration. These cumulative effects lead to increased systemic vascular resistance, resulting in a further increase in blood pressure. However, the role of microvascular rarefaction in antiangiogenic therapy-induced hypertension remains conflicting.78 Preclinical studies in mice treated with small molecule VEGF inhibitors showed that up to 30% of capillary networks regress by 21 days of therapy and reverse with therapy discontinuation. In humans, the capillary density in patients receiving Bevacizumab was only reduced by ~10% after 6 months of treatment and was associated with the increased blood pressure,79 while another study reported ~20% reduction in capillary density after 5 weeks of therapy with VEGF-TKI Telatinib.80 However, the time course for capillary rarefaction of several days to weeks does not match the rapid rise in blood pressure observed in patients who started antiangiogenic therapy. It is likely that capillary rarefaction does play a role in antiangiogenic therapy-induced hypertension but is not the sole factor in its development.
Animal studies have provided key insights into the mechanisms of antineoplastic treatment related hypertension.81 A landmark study by Facemire et al demonstrated that mice receiving anti-VEGFR-2 antibody rapidly developed hypertension.82 The NO synthesis inhibitor L-NAME administration abolished the difference in blood pressure between the vehicle- and anti-VEGFR2-treated groups. Thus, this suggested that VEGFi-induced hypertension is mediated by NO inhibition.82 Another study in C57BL/6 mice showed that administration of a single-dose aflibercept led to a rapid and dose-dependent elevation in systolic blood pressure associated with NOX1/NOX4-mediated ROS accumulation, impaired AKT/eNOS/NO signalling and EDR, reduced intracellular levels of L-arg, and decreased expression of CAT-1.83 L-arg supplement significantly inhibited aflibercept-induced hypertension once again highlighting the role of NO signalling VEGFi-induced hypertension and potential therapeutic utility of L-arg in this setting.83
Additional postulated mechanisms for TKI-induced hypertension relate to interruption of downstream intracellular VEGF signalling and include decreased renal NO bioavailability via downregulation of soluble guanylate cyclase activity, inhibition of intrarenal NOS activity, activation of the renin-angiotensin-aldosterone system, and decreased fractional sodium excretion.84 Other mechanisms suggested for VEGFi-induced hypertension include impaired sodium balance resulting in salt-sensitive hypertension23 due to impaired buffering of salt in the skin and the development of vascular stiffness which may occur within the first few weeks of treatment with sunitinib85 and sorafenib86. Soluble Fms-like tyrosine kinase 1 (sFlt-1) was recently shown to inhibit angiogenesis and lead to cardiac toxicity.87 Similarities between the pathophysiology of VEGFi-induced hypertension and the role of VEGF, sFlt-1 and endothelin-1 in the development of preeclampsia88,89 have also been drawn and this remains an area of interest which may provide further insight and understanding of VEGFi-induced hypertension and vascular toxicity.90
Interestingly, a recent study showed that Apatinib treatment increased blood pressure in Wistar–Kyoto rats by causing a marked increase in intralaminar distances and collagen deposition in mid-aorta tissues.91 These Apatinib-induced vascular remodelling were reversed following the treatment with Y27632, a nonspecific RhoA/Rho kinase (ROCK) inhibitor.91 Therefore, the ROCK signalling pathway could be an important mechanism of hypertension while ROCK inhibitors have emerged to be attractive anti-hypertensive drugs.
BRAF-MEK Inhibitors
Targeted B1 homolog v-raf murine sarcoma viral kinase oncogene (BRAF) and mitogen-activated protein kinase (MEK) inhibition disrupts the crucial RAS-RAF-MEK-ERK signalling pathway which drives cell differentiation, proliferation and provides resistance to apoptosis. In certain types of malignancy activating somatic point mutations in BRAF or downstream along the signalling pathway lead to upregulated and unbalanced cell propagation and survival.92
BRAF inhibitor use has shown significant improvements in progression-free and overall survival across a range of malignancies with greatest effect in metastatic melanoma, hepatocellular carcinoma, thyroid cancer, renal cell carcinoma, gastrointestinal stromal tumours, colon cancer and non-small cell lung cancer.8,93 BRAF resistance through reactivation of the MAPK pathway94 has emerged as a limiting step in the use of these agents in clinical practice, leading to the development of combination BRAF inhibitors with other therapies targeting downstream signalling pathways.93,95 MEK inhibition is one of the major downstream targeted strategies to overcome BRAF resistance, with several approved combination BRAF/MEK inhibitors (vemurafenib [BRAF]/cobimetinib [MEK], dabrafenib [BRAF]/trametinib [MEK], and encorafenib [BRAF]/binimetinib [MEK]) which have shown improved antineoplastic efficacy particularly in the metastatic setting.95–97
BRAF and BRAF-MEK inhibition is associated with a range of significant cardiac adverse events including cardiac failure with reduced ejection fraction, atrial fibrillation, QT prolongation, cardiac hypertrophy and arterial hypertension.97,98 Both BRAF-specific therapies (vemurafenib and dabrafenib) as well as multi-targeted BRAF-inhibiting TKIs (sorafenib and regorafenib) can induce hypertension which predisposes patients to further cardiovascular toxicity.98
Clinical and epidemiological evidence for BRAF-MEK inhibitor-induced hypertension
A review of BRAF inhibitor (Sorafenib, vemurafenib, Regorafenib and Dabrafenib) clinical trials highlighted the potential cardiovascular toxicity of BRAF inhibition as a class, with Regorafenib having the highest mean occurrence of all-grade hypertension (37.5%).99 MEK inhibitors are also associated with significantly increased occurrence of hypertension: in METRIC trial of trametinib compared with standard chemotherapy in metastatic melanoma, the incidence of all-grade and grade 3 hypertension was 15% and 12%, respectively in trametinib treatment arm compared to 7% and 3%, respectively in the chemotherapy treatment arm.100 A meta-analysis reported a relative risk increase of 1.5 for hypertension and 1.85 for high-grade hypertension in patients treated with MEK inhibitors compared to control patients, with no significant difference in hypertensive risk between individual agents.101
Over the last decade, there have been multiple trials (COMBI, coBRIM and COLUMBUS) and meta-analyses which have consistently demonstrated a significantly higher risk of hypertension with combination BRAF-MEK inhibitors relative to single agent BRAF therapies (Table 3).96,98,102 In the largest review to date across two national registries, the overall combination therapy incident hypertension was significantly higher (ROR 1.75) compared with BRAF inhibitor monotherapy with a difference appreciated within the first 6 months of treatment.98 Further assessment of combination treatments with Encorafenib and Binimetinib are underway with preliminary results expected to be published late in 2022. Of note, an important exclusion criteria of most BRAF-MEK inhibitor trials was patients with pre-existing clinically significant cardiac disease or cardiac dysfunction,103 which could potentially underestimate the real world incidence of CVAE.
TABLE 3:
Hypertension Incidence in major BRAF-MEK inhibitor trials adapted from Glen et al, 2022.97
| Treatment and Cancer | Grade of AE and Hypertension Result | Study and References |
|---|---|---|
| Dabrafenib and placebo (n=54) in metastatic melanoma BRAF V600 mutant. | All grade: 4% (2) Grade ≥3: 0 |
|
| Dabrafenib and placebo (n=212) unresectable stage IIIc or IV melanoma BRAF V600 mutant | All grade: 14% (29) Grade ≥3: 5% (10) |
|
| Vemurafenib (n=352) Metastatic melanoma BRAF V600 mutant | All grade: 24% (84) Grade ≥3: 9% (32) |
|
| Placebo (n=432) Stage III melanoma with resection and BRAF V600 mutant | All grade: 8% (35) Grade ≥3: 2% (8) |
|
| Vemurafenib and placebo (n=248) unresectable stage IIIc or IV melanoma BRAF V600 mutant | All grade: 8% (20) Grade ≥3: 3% (7) |
|
| Vemurafenib (n=191) unresectable stage IIIc or IV melanoma BRAF V600 mutant | All grade: 11% (21) Grade ≥3: 3% (6) |
The expanding development and application of BRAF-MEK inhibitors has led to trials of “triplet” treatment combinations such as with immune checkpoint inhibitors104,105, PARP inhibitors and EGFR inhibitors106 which may improve resistance to BRAF-MEK available treatments and efficacy for patients. While limited, there are data from trials in advanced BRAF-mutated melanoma, which compared addition of pembrolizumab or placebo to a combination of dabrafenib and trametinib, of higher incidence of grade 3 hypertension (8.3%) in the “triplet” therapy group compared with the placebo group (3.3%).107 This highlights the potential for even greater incidence of hypertension and other CVAE with multi-combinations therapies.
Mechanisms of BRAF-MEKi-induced hypertension
The RAS-RAF-MEK-ERK pathway is important in cell repair, proliferation and survival and has been shown to be involved in pathogenesis of hypertension and other CVD.108–111 Although the exact mechanism of BRAF-MEK inhibitor-induced hypertension remains unclear; indirect inhibition of MAPK pathway has been postulated to be involved in the pathological development of cardiac hypertrophy and dysfunction.93,97 It has been proposed that BRAF/MEK inhibitors lead to CD47 upregulation in cells via rebound ERK activation,112 which results in the inhibition of both NO bioavailability and soluble guanylate cyclase activation propagating endothelial dysfunction, vasoconstriction and the development of hypertension.102,113 Yet, inhibition of ERK1/2 activation in development of cardiotoxicity have yielded discordant results: trametinib is thought to lead to cardiotoxicity via inhibition of ERK1/2 activation,114 while dabrafenib, was shown to be cardioprotective in vitro, via inhibition of Raf kinase pathway, disrupting ERK1/2 signaling.115
Bruton tyrosine kinase inhibitors (BTKi)
Bruton tyrosine kinase (BTK) inhibitors (BTKi) act by selectively targeting and irreversibly disrupting the BTK downstream of the B cell receptor preventing chemokine-induced adhesion and migration.84,116 Overall BTKi have shown dramatically better efficacy across a range of hematological malignancies than traditional chemoimmunotherapies (CITs) and are well tolerated, however accumulating data have revealed multiple adverse events, including CVAE.117
Clinical and epidemiological evidence for BTKi-induced hypertension
A pooled analysis of 424 patients from three phase III trials on ibrutinib therapy for CLL reported incident hypertension in 18% of patients with severe grade hypertension occurring in 6%, with similar results reported in an extended phase 3 RESONATE-2 clinical trial.118,119 A recent meta-analysis of 8 randomised control trials reported a risk ratio of 2.85 for development of hypertension on ibrutinib.120 In a detailed review of 562 patients treated with ibrutinib over a 7 year period, 78% patients developed new or worsening hypertension, with incident hypertension in 71% and high grade hypertension (>160/100 mm Hg) 17% of those with pre-existing hypertension.121 The true incidence of hypertension was suggested to be even higher during real world experience.122 Reassuringly treatment for hypertension reduced the risk of MACE (hazard ratio 0.4) regardless of antihypertensive drug selection.121
In addition, ibrutinib has been associated with other cardiovascular toxicities including atrial fibrillation and bleeding, however next generation, more selective BTKi (acalabrutinib and zanubrutinib) seem to have an overall lower cardiovascular toxicity, including hypertension.117 Like ibrutinib, acalabrutinib (second generation BTKi), was also trialled in refractory CLL patients and similarly demonstrated a treatment related hypertensive incidence of 7%.123 However, more recent experience with acalabrutinib from the ELEVATE-TN trial124 and also the ASCEND trial125 suggests lower rates of hypertension, with comparable rates of all grade and severe grade hypertension as control treatment groups.124,125 Similarly, evidence from the ASPEN trial showed a comparatively lower incidence of hypertension and a significantly lower hazard ratio of 0.59 amongst patients treated with the novel agent zanubrutinib compared with ibrutinib.126 While these data are encouraging, the most recent assessment from a 2022 publication of 280 patients treated with Acalabrutinib found that almost half developed or experienced worsening hypertension over 41 months with 53% developing new hypertension at one year with 1.7% having severe hypertension (≥grade 3).127 Moreover there was a significant correlation between the degree of SBP rise following initiation and predicted MACE risk suggestive of an adverse class effect from BTKi and opposing recent opinion that newer generation BTKi may cause less hypertension.127 Importantly the study demonstrated there was no single anti-hypertensive treatment which was found to prevent this hypertensive effect.127 These opposing data presented support the need for further larger clinical trials and real world studies to properly ascertain the true hypertensive effects of the newer generation BTKi treatments.
Mechanism of BTKi-induced hypertension
Evidence of the underlying mechanism by which BTKi are associated with the development and worsening of hypertension is limited. However, it has been suggested that a decrease in downstream heat shock protein 70 (hsp70) may be associated along with decreased phosphatidylinositol 3-kinase (PI3K) signalling resulting in downstream depletion of NO production and reduced VEGF propagation with eventual vascular remodelling, and endothelial cell dysfunction.13,121,128,129
Phosphatidylinositol 3-kinase inhibitors (PI3Ki)
PI3K inhibitors (PI3Ki), including the pan-PI3Ki copanlisib and isoform specific PI3Ki alpelisib, idelalisib, umbralisib and duvelisib, work via inhibition of the PI3K enzyme signalling pathway which is essential for a diverse range of cell functions including cell survival, metabolism, immune function and cell division.130,131 Upregulation through mutation within one of the four isoforms of the class 1 PI3K enzymes often found within immune cells and has been implicated in malignancies such as lymphoma, glioblastoma, ovarian, endometrial, breast, colorectal, gastric and prostate cancers.131,132 A major challenge for this class of treatments has been to overcome significant non-CV toxicities including hyperglycaemia, multiple gastrointestinal side and myelosuppression.131,133 Hypertension has been less commonly recognised as a significant adverse reaction of PI3Ki, however does need to be considered by treating clinicians.
Clinical and epidemiological evidence for PI3Ki-induced hypertension
CHRONOS-1 study of indolent B cell lymphoma with intravenous copanlisib reported any-grade hypertension incidence of 29.6% and grade 3 or above hypertension incidence of 23.2%.132 Hypertensive effects of copanlisib appear to be rapid in onset, within 2 hrs of starting treatment, and do not appear to be lasting, however the mechanism of this transient effect is unknown.134 Copanlisib combinations with MEK inhibitor Refamentinib135 or HER2 inhibitor trastuzumab136 have been associated with an even higher incidence of grade 3+ hypertension of 26% and 33%, respectively.
Hypertension does not appear to be class effect of all .PI3Ki131,137 Isoform specificity of certain PI3K inhibitors such as idelaslisib, umbralisib, duvelisib, parsaclisib138 and dactolisib139 have resulted in a different adverse effect profile with lower overall rates of hypertension,131 compared to pan-PI3Ki copanlisib.140,141 This difference may also be in part attributed to drug delivery method (intermittent intravenous infusion opposed to regular oral dosing).13,133
Mechanism of PI3Ki-induced hypertension
Although the pathophysiology behind PI3Ki-induced hypertension is not yet characterised it is postulated that PI3K plays a role in the endothelial cell function, whereby inhibition results in endothelial dysfunction and vasoconstriction.142 Furthermore, PI3K isoforms may help regulate blood pressure via type-1 angiotensin-receptor signalling which has led to exploration of the therapeutic anti-hypertensive utility of this pathway.134 PI3K pathway may also play a significant role in pathologic remodelling, hypertrophy and contractility in the heart which should encourage a degree of caution for cardiovascular toxicities particularly amongst the elderly whilst taking these medications.143 Interestingly, disruption of this cascade pathway via PI3K isoform inhibition has been investigated both in-vivo and various animal models with promising results in the prevention and progression of pulmonary hypertension.144 This has led to recent increasing interest in the inhibition of the PI3K-AKT pathways as a novel therapeutic target for the treatment of evolving and established pulmonary hypertension, which carries significant morbidity and mortality for patients.145
RET kinase inhibitors (RETi)
RET is a transmembrane receptor tyrosine kinase, critical for the development of multiple tissues.146 RET activation, either wild-type or through mutations, promotes tumour growth and contributes to development of NSCLC, papillary thyroid cancers, and other cancers.146,147 This led to the development of both multikinase inhibitors with RET inhibitor (RETi) activity (Vandetanib and Cabozantinib) and selective RETi (Pralsetinib and Selpercatinib) that are highly potent with a better toxicity profile.147
Clinical and epidemiological evidence for RETi-induced hypertension
Hypertension has been associated with RETi, which has only recently been demonstrated in several trials of Selpercatinib and Pralsetinib, as well as retrospective real world safety analysis of Selpercatinib, with any-grade hypertension rate of 31–43%, and severe grade hypertension rate of 14–21%.148–151
Mechanism of RETi-induced hypertension
Similar to most other kinase inhibitors, the specific mechanism by which RETi cause hypertension remains unclear. However, given that RET kinase plays a role in the RAS-RAF-MEK-ERK signalling cascade, it has been proposed that the mechanism is similar to that seen with BRAF-MEKi, i.e. rebound ERK activation and CD47 upregulation leading downstream hypertensive effects,152 as discussed in the BRAF-MEKi section above. This is supported by the in-vitro studies which identified upregulated K-RAS protein in RETi-treated patients, which may be a sign of rebound ERK activation and potential treatment resistance;153 however, this remains speculative. Furthermore, the selective RETi Pralsetinib and Selpercatinib have greater intracranial efficacy and improved penetration into the central nervous system compared to the previous multikinase TKIs.154,155 Thus, long-term monitoring will be crucial to identify any potential adverse effects on the development and function of neurons following suppression of RET activity.156
Proteasome inhibitors
Proteasome inhibitors (PI) have a defined role both as single and combination therapy in the treatment of lymphoma and multiple myeloma with their efficacy related to the regulation of protein degradation.157 Proteasomes are important for normal cell function, and also particularly for susceptible malignant cells, as they help maintain protein homeostasis via clearance of cytotoxic or misfolded proteins, which would otherwise threaten cell survival and propagation.157 Currently there are three PI in clinical use – first generation reversible PI Bortezomib, and two second generation irreversible PI – Carfilzomib and Ixazomib.
Clinical and epidemiological evidence for PI-induced hypertension
Bortezomib and carfilzomib are both effectively used in the treatment of multiple myeloma. Both have been implicated in chemotherapy-induced hypertension, with carfilzomib having a more significant pro-hypertensive effect than bortezomib.158 In Phase III ENDEAVOR trial, hypertension (16% of patients on carfilzomib and 6% on bortezomib) was reported as one of the most frequent grade-3+ CVAE.159 Hypertension was also reported in 14.3% of PI-treated patients in one of 4 phase II studies.160 Recently, data from the SEER-Medicare dataset inclusive of 815 carfilzomib treated patients, found a hazard ratio of 1.41 for all CVAE, with hypertension reported in 27.6% patients with a hazard ratio of 3.33 compared to non-carfilzomib patients.161 A meta-analysis suggested that hypertension, the commonest reported CVAE, may be more frequent with carfilzomib as opposed to other PI’s, and also with combination therapy, especially with immunomodulatory agents, than PI monotherapy.162 Orally-bioavailable Ixazomib is reported to have an improved toxicity profile, especially peripheral neuropathy, however, cardiovascular toxicity remains a recognised ongoing issue,163 with incidence rates of severe grade 3–4 hypertension between 5–20%.164,165
Mechanisms of PI-induced hypertension
The underlying mechanism of PI-induced hypertension remains unknown. Given the PI mechanism of action, it was postulated that it could be associated with abnormal accumulation of ubiquitinated or misfolded proteins from proteasome inhibition.166 Carfilzomib decreases proteasomal activity in-vitro and in-vivo with subsequent increased PP2A activity, decreased AMPKα phosphorylation, and upregulated eNOS, Bip, Raptor, and enhanced LC3B-dependent autophagy.166 Chronic proteasome inhibition with another PI (MLN-273) has been associated with increased oxidatively (4-hydroxy-2-nonenal [4-HNE])-modified proteins, NOX subunit p47phox, and eNOS levels in coronary arteries.167 Furthermore, MLN-273 also impaired coronary endothelium-dependent vasorelaxation and intimal thickening, resembling and aggravating the vascular effects of traditional cardiovascular risk factors such as hypercholesterolemia.167
Interestingly, Bortezomib treatment was reported to attenuate hypoxia-induced elevation of Ca2+ and alleviate pulmonary hypertension in hypoxia- and monocrotaline-induced rat pulmonary hypertension models.168 In contrast, Carfilzomib treatment impairs Ca2+ transients and contractility of cardiomyocytes in conjunction with decreased expression of genes associated with Ca2+ handling (e.g., SLC8A1 [solute carrier family 8 member A1], RYR2 [ryanodine receptor 2], CASQ2 [calsequestrin 2], and ATP2A2 [ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2]).169 These recent results indicate a potential role of Ca2+ handling in PI-induced hypertension and may, in part, explain the differential toxicity profile of PIs.
Poly (ADP Ribose) Polymerase inhibitors (PARPi)
Poly(ADP-ribose) polymerases (PARPs) play a crucial role in the normal DNA repair of single-strand breaks, along with several other diverse functions. PARP inhibition (PARPi) is a novel and effective therapeutic strategy for targeting malignancy,170 especially for breast, fallopian, peritoneal and ovarian cancers expressing BRCA1 and BRCA2 germline mutations170 and for pancreatic and metastatic castrate resistant prostate cancers with mutations leading to impaired homologous recombinant repair.171 PARPi are used both as monotherapy and in combination with other agents, including immune checkpoint inhibitors, VEGFi, TKI and traditional chemotherapy to improve patient outcomes.171,172
Clinical and epidemiological evidence for PARPi-induced hypertension
As PARPi have been introduced into the oncological armamentarium, clinical studies suggest a differential effect of Niraparib versus other PARPi on blood pressure.173 Concerns for hypertension stem from the ENGOT-IVA16/NOVA clinical trial, which assessed Niraparib maintenance therapy in recurrent ovarian cancer, and reported any-grade hypertension and grade 3/4 hypertension in 19% and 8% of patients the treatment arm, respectively compared with 4% and 2% of patients in the placebo arm, respectively.174 The recently published (NCT03404960) trial with ongoing follow up assessing niraparib plus either nivolumab or ipilimumab in patients with advanced pancreatic cancer showed ≥ grade 3 hypertension in approximately 8% and 9% respectively in both treatment groups containing niraparib, a result in keeping with previous clinical trial data.175 Other reported associations between PARPi and incident hypertension have come from smaller clinical trials combining VEGFi, chemotherapies with PARPi therapies,176,177 likely confounded by the known association between VEGFi and hypertension.
However, other PARPi including olaparib, rucaparib, and talazoparib are generally not implicated in development of hypertension. It may be the case that some PARPi may even reduce the risk of hypertension. This was supported by the results from the PAOLA-1 trial, which showed that fewer participants in the olaparib combination with bevacizumab group experienced hypertension compared to the placebo and bevacizumab combination group.178 This concept that PARPi may play a cardioprotective role had also been identified in earlier preclinical animal models.179 The underlying protective mechanism of PARPi may be explained by the suggested role PARP-1 activation has in the development of atherosclerotic lesions, myocardial dysfunction and hypertension180, possibly driven by angiotensin-II signalling. Ongoing investigations into the use of PARPi in prevention of fibrosis181 may yield a better understanding of PARP-associated hypertension, and the potential protective cardiovascular effects of PARPi.
Mechanisms of PARPi-induced hypertension
To date, the mechanism(s) underlying the pro-hypertensive effect of Niraparib remain unknown. Given that Niraparib can interacts with various neurotransmitter transporters, it has been speculated that an off-target effects on vasoactive receptor binding of transports for dopamine, norepinephrine and serotonin, may result in overall reduced cellular uptake of dopamine and norepinephrine, which may contribute to hypertension.173 Hypertension associated with Niraparib may be explained via the inhibition of DYRK1A which could in turn see increased levels of the previously mentioned neurotransmitters in turn promoting inotropic increase and hypertension.182,183 Niraparib has the distinct pharmacological action to inhibit dual-specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A) and potentially contribute to hypertension.182,184 DYRK1A has been shown to play an important role in regulating the turnover of monoamine neurotransmitters (i.e., dopamine, serotonin and noradrenaline), whereby studies showed a strong relationship between DYRK1A expression and the dopaminergic system.182 DYRK1A overexpression induced dramatic deficits in the levels of dopamine, serotonin and noradrenaline in certain regions of the brain.182 Therefore, the inhibition of DYRK1A by Niraparib may have inotropic effects on the heart and potentially causes high blood pressure (hypertension). Interestingly, previous studies also showed that DYRK1A also plays a role in circadian rhythm.185,186 By cooperating with glycogen synthase kinase 3β (GSK-3β), DYRK1A regulates the rhythmic Ser557 phosphorylation-triggered degradation of cryptochrome-2 (CRY2).186 Knockdown of DYRK1A consequently led to abnormal accumulation of cytosolic CRY2, advancing the timing of a nuclear increase of CRY2, and shortened the period length of the cellular circadian rhythm.186 Emerging evidence are suggesting that the molecular circadian clock plays a crucial role in blood pressure control187–189, thus disruption of the circadian rhythm may potentially cause hypertension. These findings could be important for the selection of PARPi for cancer patients.
With the increasing use of more novel anti-angiogenic therapies alone or in combinations with, there must be a balanced appreciation of severe adverse side effects and cardiovascular risk which has been previously cautioned within the literature and needs to be diligently assessed and managed.190
Figure 2 summarizes the known mechanistic signalling cascades implicated in development of hypertension across all the anti-cancer medication classes described above.
The long-term outcomes of cancer therapy-related hypertension
There is evolving interest in gaining insight into the true ramifications which cardiovascular diseases such as hypertension and in particular cancer treatment-related hypertension may have on both long term patient morbidity and mortality.191 The data for long term outcomes of therapy-related hypertension is extremely limited and in general long term CVD for cancer patients has often been extrapolated from childhood cancer survivor cohorts however these data may not be accurate given the exponential growth in available novel anti-cancer therapies over the last decade. Nevertheless, poorly controlled hypertension is a clear risk factor of development of heart failure during treatment with anthracyclines, ibrutinib and VEGFi.117,192–194 The National Cancer Institute has previously identified CVD as a key clinical manifestation of aging in cancer survivors195 and a factor that crucially can be identified early and either prevented or efficiently managed making a strong and valid point that more needs to be done in the way of screening and managing the long term CVD risk of these cancer survivors.196
Similar to historic data, recent evidence from the analysis of large cohort reviews of several different populations has shown that cancer and cancer-therapy amongst cancer survivors is associated with significant increase in cardiovascular disease risk.197,198 An observational analysis of a US population inclusive of over 1.2 million cancer patients with 28 different cancer types identified approximately 11% of cancer patients died from CVDs, 76% of these were due to heart disease. The mortality risk was greatest in cancer patients diagnosed at a younger age (<35), with the highest CVD mortality in the first 12 months from diagnosis.199 The most recent data from a 2022 publication of retrospective cohort Canadian population data inclusive of over 4.5 million patients with a new cancer diagnosis compared with a non-cancer cohort over an 11 year period demonstrated hazard ratio of 1.33 (95% CI: 1.29–1.37) for CVD mortality. The data conveyed that comparatively, cancer patients had approximately a 60% increase in the relative risk of cardiac failure, 44% increased risk of stroke and over three times the risk of pulmonary embolus. Concerningly the prevalence of almost all cardiovascular risk factors was significantly elevated within the cancer population cohort (diabetes 10% vs 3%, severe obesity 17% vs 11%, dyslipidaemia 24% vs 22% and atrial fibrillation 3% vs 1%) with a striking difference in hypertension rates between the cancer and non-cancer cohorts of 31.7% vs 10.7%.200 The results convincingly demonstrate that a new cancer diagnosis correlated with both CVD morbidity and mortality risk even after adjustment for baseline CV risk. Furthermore this CVD risk for several measured outcomes was consistently demonstrated out to 10 years similar to previous evidence from both large observational population studies199 and smaller targeted studies such as CAROLE (Cardiac-Related Oncologic Late Effects)201 further strengthening the concept of persistent cancer and cancer therapy-related CVD risk. In keeping with these findings previously reported evidence from the breast cancer SEER dataset has suggested that cancer may be an risk factor for CVD independent of cancer treatments such as chemotherapy or radiation therapy.202
Much of the long term CVD outcome data has arisen from well-established chemotherapeutic agents such as anthracycline and platinum based chemotherapy and from patients treated with radiation therapy.203–205 In an era of rapid evolution of targeted cancer therapies there is an obvious void in available long term population data about the specific long term CVD risks associated and the absolute impact novel therapies will have on both CV morbidity and mortality. It is important to recognise that although effective management and early intervention for CVD related to cancer treatment has been instituted into clinical practice in many oncology and cardio-oncology centres around the world the implementation of CV screening and management of disease risk is often not continued as routine practice in long-term survivors.191
An optimistic outlook can be had that we will see a more complete understanding of the long-term cardiovascular consequences of pro-hypertensive cancer therapies given the pace of progress within the field of cardio-oncology itself. However, this will require several important changes such as:
Increasing the scope of CVD screening and the duration of follow up of cardio-oncology patients beyond there discharge from active treatment.
Improving the communication and data sharing between all practitioners involved in cancer patients care and encouraging the education and awareness of silent and often overlooked cardiovascular sequalae of treatment such as treatment related hypertension which may not be apparent until several months or even years following treatment.
Improved uptake and active participation of multiple collaborations and cardio-oncology centres in data sharing of patient outcomes related to treatments.
Capturing important data points which are currently lacking including therapy-related CVD complication data with a focus on both old and the new and emerging cancer therapies in clinical practice.
Most importantly re-innovation and institution of improved clinical trial design with the aim of more extended follow-up periods and improved patient retention allowing for the capture of these important long term data points.
Hypertension as a risk factor for cardiotoxicity
Hypertension is the most prevalent modifiable risk factor for the development of cardiovascular disease206 and is an issue that persists following acute cancer treatment, plaguing both adult207 and childhood cancer survivors later in their lives. Rates of hypertension in cancer survivors are as high as two and a half times that of the adjusted general population,208 further adding to an already increased CVD risk and CVD mortality in cancer survivors.209–211 Recently published observational cohort data demonstrated childhood cancer survivors had higher rates underdiagnosed CVD risk factors, most concerningly these patients were significantly undertreated compared with the standard matched American population (21.0% versus 13.9%, P=0.007) with hypertension being the most prevalent undertreated risk factor at 18.9%. This may in part be due to lower rates of health-related self-efficacy which crucially highlights a need for focused care from cardiologists and general care practitioners to address cardiovascular risk modification and improve cancer patient self-efficacy by effectively engaging them with responsibility in their own care decisions and management.212
CVD mortality in cancer patients can be described as a “multiple-hit” paradigm, with hypertension and CVD compounding the mortality risk in a complex interplay of overlapping risk factors including direct treatment toxicity, premorbid CV disease and lifestyle and behavioural risk factors.213 Similarly in most recently published literature a “multiple strike theory” conveys the complex interplay of genetic predisposition for common cardiovascular disease and cancer risk factors (genes such as TTN, Tet2, PHTF1 and DDR) and exogenous factors (smoking, radiation, age, metabolic syndrome and environmental factors). These elements which taken in combination with anti-cancer therapies and treatments for CVD perpetuate poor short and long term outcomes for cancer patients.214
Furthermore several factors often overlooked when assessing both hypertension and CVD risk within cancer patient populations such as advances in cancer and cardiovascular disease screening, increased public awareness and engagement with their own health, improved therapies and imaging techniques, prolonged survival post treatment and of most significance an escalating aging population could indicate we may be on the verge of an even larger expansion era for cardio-oncology and its treatment related issues.215,216
An overt deficiency of standardised CV risk assessment tools established and validated for use in cancer patients and survivors, highlights a significant deficit in care within this population. An explanation for this may be that most cardio-oncology and cancer patient trials include younger, typically low-risk populations and the application of CVD risk assessment/predictive tools may falsely underestimate cardiovascular risk and events which may not be reflective of real world practice and therefore incorrectly guide treatment decisions. Furthermore limited sample sizes, CV event rates, lack of accurately captured CV data, vast heterogeneity amongst cancer therapy trial populations and short trial follow up durations make risk assessment tools and cardioprotective strategies difficult to accurately assess and validate.217
Efforts have been made by the European Society of Cardiology (ESC) in collaboration with the International Cardio Oncology Society (ICOS) to develop risk assessment tools to guide screening and decision making for cancer patients in clinical practice,218 but these have not yet achieved widespread use.
Defining cancer therapy-related hypertension
Despite the established use of many anti-cancer therapy classes covered in this review, optimal blood pressure (BP) targets and therapeutic strategies for hypertension are largely based on broader general hypertension recommendations, which have not been widely validated in cancer populations.219–224 Furthermore, hypertension guidelines are commonly generalised both to a variety of cancers and their respective treatments, which fails to account for the complexity and heterogeneity of different cancers, the vast array of anti-neoplastic treatment mechanisms and the individual patients. This poses the question: “does one size fits all approach is still appropriate within growing cancer populations?”.
Treatment-related hypertension can be of various grades and can be defined as systolic and/or diastolic BP increase following initiation of cancer therapies without other contributing changes.225 There have been considerable efforts from various collaborations (CTCAE version 5226, ACC/AHA 2017224, ESC 2018220, ISH-2020223) to define parameters around hypertensive toxicity and treatment related hypertension amongst cancer patients97,121,227–229. In an effort to formalise and simplify such recommendations a recently published IC-OS 2021 consensus statement has been released which defines normal SBP ≤130 mmHg and DBP ≤80 mmHg within the cancer population cohort.225 Treatment initiation is recommended with antihypertensive therapy for patients prior, during or post cancer therapy above theses office/practice BP cut-offs if CVD risk ≥ 10% otherwise treatment should be initiated when BP exceeds SBP ≥140mmHg or DBP ≥ 90mmHg.225 A rise in SBP>20mmHG or mean arterial BP >15mmHg are considered exaggerated responses and also prompt the consideration of therapy.225 Importantly cancer therapy should be withheld with SBP≥180mmHg and DBP≥110mmHg and patients with a hypertensive emergency response causing end organ damage need immediate intervention in the particularly vulnerable cancer patient population.225 Recently published first international cardio-oncology guidelines, developed jointly by the ESC, ICOS, European Hematology Association (EHA) and the European Society for Therapeutic Radiology and Oncology (ESTRO), provide a “sliding scale” thresholds for treatment of cancer therapy-associated hypertension based on the cancer metastatic state and prognosis (Figure S1).230
Screening and management of cancer-therapy associated hypertension
All physicians involved in the care of cancer patients face challenges in balancing adequate oncological treatment with acute and chronic cardiotoxicity associated with cancer therapies. Cardiotoxicities, including hypertension, may influence decisions around the type and duration of cancer therapy, which may ultimately impact on the overall survival outcomes for patients.73 The goal of management of these patients is to provide individualised care by prevention, mitigation and appropriate management to minimise cardiovascular risk without disruption to cancer treatment throughout patient’s cancer journey.225,231
Screening for Hypertension and therapy-related Hypertension in the cancer patient population
Hypertension screening and monitoring in cancer patients requires a detailed CVD and CV risk factor history, diligent cardiovascular exam for end-organ involvement, and most importantly, accurate BP assessment and monitoring, ideally with out of office methods (ambulatory or home BP monitoring)227, given the considerable inaccuracy within the office setting232.19,228,233. However, it is important to acknowledge the limited evidence for ambulatory BP monitoring within cancer populations, and a need for further research to establish the optimal approach. Different proposed pharmacologic management pathways for hypertension in cancer patients have been documented in recent literature and are not the focus of this discussion. However, a well summarised and up-to-date pathway for management has just been published as part of the first international cardio-oncology guidelines, developed jointly by the ESC, ICOS, EHA and ESTRO (Figure S2).230
Further investigation to compliment Hypertensive and CVD screening within the cardio-oncology setting may also encompass a multi-marker approach to assess for cardiac dysfunction and improve initiation of cardio-protective measures.217,219,234 This may include the use of transthoracic echocardiography with Global Longitudinal Strain (GLS), 3-D Left Ventricular Ejection Fraction (LVEF) assessment and cardiac MRI imaging.217,235,236 The use of Troponin, Naturetic Peptide and D-Dimer biomarkers have an established role in cardio-oncological workup for cardio-protective screening237 particularly in the setting of cancer therapies known to cause cardiac dysfunction however there use as a marker for hypertensive heart disease has not been described in the literature.
Lifestyle interventions
Addressing the care needs and gaps for cancer patients/survivors will require improved efforts to appropriately manage modifiable CVD risk factors,238 including lifestyle factors contributing to hypertensive risk.239 Our increasing understanding of hypertension in cancer patients has unveiled the complexity of the multiple environmental and patient factors contributing to its development, including the role of polygenic risk,240 while the roles of physical factors such as diet, obesity, sedentary lifestyle, smoking, excessive alcohol intake, diabetes and metabolic syndrome, kidney diseases, sleep apnoea, recreational drug use and non-physical attributes such as psychological stress and cancer-related pain are less well studied in cancer patient populations.213
In general population, major lifestyle interventions such as regular exercise, minimisation of alcohol intake, reduction in sodium intake and improved dietary habits as well as less traditional strategies targeting stress reduction and improving sleep hygiene can promote antihypertensive effects and may have a preventive role in the development of hypertension.220,223,224,241 Proven lifestyle interventions such as these may add significant benefit to patients with resistant hypertension requiring multiple anti-hypertensive agents as was demonstrated in the recently published TRIUMPH trial. However the applicability of the trial results given the intensive interventions patients received in the trial and the prolonged effect of these interventions on patient outcomes are unknown.242 Cancer guidelines around treatment and prevention of hypertension often mirror recommendations from general population guidelines with respect to lifestyle changes however it’s important to appreciate the differences within cancer patient populations and acknowledge that recommendations need to be tailored to the patients current clinical status, prognosis and quality of life.243
For example the reduction in salt (sodium chloride; NaCl) intake has been a proven to reduce hypertension and its comorbid manifestations in the general population and is featured in the most recent hypertensive guidelines244, most recent evidence has even shown that partial substitution of intake with potassium chloride (KCl) reduces cardiovascular disease risk and cerebral vascular events.245. However, this may not be appropriate in certain cancer patients such as those at risk of electrolyte imbalance and hyponatraemia from commonly used medications classes such as diuretics, ACE-Inhibitor/Angiotensin-receptor blockers (ARB), antidepressants, and antipsychotics. Furthermore, several randomized controlled trials suggested that increasing cumulative exposure to ARBs may inadvertently increase the risk of cancer, specifically lung cancer.246 Several chemotherapeutic agents have been causally associated syndrome of inappropriate anti-diuretic hormone (SIADH) such as vincristine, carboplatin, cisplatin and cyclophosphamide and more novel immunotherapy agents can seldom cause significant immune related complications such as colitis and adrenalitis resulting in gastrointestinal losses and adrenal insufficiency which can significantly disrupt sodium balance within the body.
Dietary lifestyle interventions have been shown to improve obesity rates in cancer patients and have a modest effect on improving quality of life.247 However despite the crucial role of diet as a preventative lifestyle treatment for hypertension in the general population, and its proven role in lowering the risk of cancers such as CRC248, evidence of diet interventions reducing hypertension in cancer populations is lagging.
CV fitness and exercise capacity are commonly reduced in cancer patients and there is strong evidence exercise/fitness training offers significant CV, cancer-related and general health benefits in cancer populations particularly in long term cancer survivors.249–253 Exercise has been shown to decrease Reactive Oxygen Species (ROS) formation and both improve endothelial function whilst decreasing cardiac intracellular anthracycline levels.254,255 It is plausible that exercise may cause a reduction in hypertension specifically, and more studies to ascertain this are crucial. Currently evidence for the positive impact exercise can have specifically on hypertension is drawn from non-cancer populations.256 The American College of Sports Medicine have published a consensus statement regarding exercise safety for cancer patient groups including cancer survivors, confirming exercise’s overall safety and efficacy.257
There is evidence for the use of cognitive behavioural therapies to promote lifestyle changes which can have a positive impact on hypertension in non-cancer patients224, this may represent a novel strategy for the treatment of hypertension in cardio-oncology practice within the future as part of multidisciplinary approach to individualised management of hypertension.
Lifestyle factor modification is a dynamic process requiring lasting adherence and ongoing patient education and support. One method of addressing this with proven effectiveness will be with implementation of multi-disciplinary teams inclusive of both clinical practitioners, specialist nursing staff and allied health teams.6,258
Cancer therapy-related hypertension and its pharmacological management
Guidelines currently reflect that low dose combination antihypertensive medications are an appropriate starting point for hypertension in non-cancer patients. This has also been reflected in the QUARTET trial which found combination therapy superior to monotherapy treatments.259 The recent publication of the STEP trial also demonstrated significant reductions in stroke, ACS and heart failure in the patients treated in the intensive therapy group.260 Strategies such as these may also be true for cancer treatment-related hypertension by which there are multiple poorly defined pro-hypertensive driving mechanisms facilitating often difficult to control BP. However, caution must be given to multi-drug pharmacokinetic and pharmacodynamic interactions with cancer therapies and consideration for the higher risk of adverse events with more rigorous therapy in the cancer patient cohort.
An example of the difficulties faced when navigating clinical guidelines can be demonstrated with the choice of the initial antihypertensive pharmacological agent. Renin-angiotensin-aldosterone inhibitors may be preferred first line agents on the basis of their established efficacy in hypertension, primary and secondary prevention of CVD in general population, and an association between their use with significantly improved disease free survival outcomes in multiple malignancies261, including breast, pancreatic, prostate, CRC and NSCLC, as well as in patients treated with immune-checkpoint inhibitors.262 On the other hand, a recent study found that some HCC patients experienced tumour progression by ACE-inhibitor Captopril administration for the treatment of proteinuria caused by Apatinib.263 A follow up study using tumour-bearing mouse models showed that the combination of ACE-inhibitor and anti-angiogenesis treatments may exacerbate the production of kidney-derived erythropoietin (EPO) and, in turn, reduce the anticancer efficacy of anti-angiogenesis treatments.263
With the rapid development and approval of many varying classes of cancer treatments with dynamic and often poorly understood mechanisms of actions, consideration of drug safety and important interactions when selecting anti-hypertensive therapy must routinely be considered. A well-documented adverse interaction is between non-dihydropyridine CCBs (verapamil and diltiazem) and sunitinib or sorafenib via CYP3A4 pathway inhibition.264 The American Heart Association has recently published a guide for important pharmacokinetic drug interactions for hypertensive management in cancer patients, with many other relevant examples.265
The application of new emerging therapeutic options for the management of hypertension may also present both prospective beneficial treatment alternatives as well as challenges for individualised cancer therapy-related hypertension management in the future. More recently evidence from post hoc analysis of the PARAGON trial showed that the novel cardio-protective heart failure medication sacubitril/valsartan had a significant BP lowering effect in patients with defined resistant hypertension (patients optimised on three BP agents CCB, ARB and diuretic).266 This is currently not reflected as a first line option in antihypertensive guidelines nor in patients without cardiac-failure and there is limited trail evidence for the use of these medications in cancer populations however given the relationship between many cancer therapies and the development of both hypertension and heart-failure and the evolving understanding of how treatment-related hypertension may proceed diastolic dysfunction and eventually heart-failure, treatment strategies such as these may play a pivotal role in the future of individualised antihypertensive management in this specific population group.
Similarly the adoption of interventional treatments such renal artery denervation (RDN) via thermal or chemical ablation to reduce sympathetic nervous activity around the renal arteries, which has evidence in non-cancer populations as a minimally invasive option for difficult to control arterial hypertension267,268 could provide an abstract way to manage cancer treatment-related hypertension and circumnavigate issues with medication compliance, drug and therapy interactions and possible adverse effects for patients sensitive to effects of antihypertensive medications269 however this would need to be a carefully individualised given associated risks with any interventional procedure and the lack of evidence in this area which is needed to guide future practice.
A lot of current uncertainties stem from limitations of clinical trials’ data in cancer patients, which are often inadequately powered to assess screening protocols, devise optimal BP targets, and ideal hypertensive drug choice, and from challenges both logistically and economically of following patients for extended periods to document long-term risks and detailed outcomes to guide assessment and management in survivors. Compounding this issue is the variability in the reporting of lower grade hypertensive adverse events, which masks the true incidence within the trial population along with selection bias in trials which have historically excluded patients with pre-existing CVD and hypertension.270,271 These issues may pose challenges for clinicians in deciding on the most appropriate management for their patients and may potentially lead to a hesitancy to treat hypertension, and instead adopt a more passive approach. This could in part explain a concerning report that over 60% of patients treated with ibrutinib, who were hypertensive at follow-up did not receive an increase or any additional BP medications.272
The consensus statement by the International Cardio-Oncology Society is an example of progress made to better adapt and modify recommendations for management of hypertension whilst incorporating CVD risk, BP thresholds for withholding anti-cancer treatment, and consideration of abrupt treatment-related hypertensive changes which may trigger initiation or escalation of treatment.225 However, there remain inconsistencies between various guidelines and recommendations for optimal assessment and pharmacological management of hypertension, and there is growing need for large scale clinical trials to further guide optimal individualised cancer and therapy specific treatment pathways.11,233 Jointly developed by the ESC, ICOS, EHA and ESTRO cardio-oncology guidelines published in 2022 provide evidence-based treatment framework for the cancer therapy-associated hypertension (Figure S2).230
Navigating the complexity of decisions around anti-hypertensive treatment selection with consideration of drug pharmacokinetics and pharmacodynamics, with the appreciation of both potential side effects and individual patient comorbidities requires a multidisciplinary approach and supports a shift in our practice of CVD management in cancer patients towards an “individualised approach” within the growing concept of onco-hypertension.11,13,213,225
Multidisciplinary models of care
In combination with established pharmacological treatment, a multidisciplinary approach to the treatment of hypertension in general population is now mandated in guidelines and is an accepted practice in many centres.220,223,273–275 Multidisciplinary models of care have established efficacy in the management of a range of CVD including, hypertension, heart failure and atrial fibrillation,276–279 as well as most forms of cancer.280 These models of care have shown to be safe, improve patient care and clinical outcomes, improve quality of care, improvement medication adherence, and have shown to be cost effective.281 Importantly, these teams can be embedded in a variety of settings including in hospital settings, outpatient clinics, home based care and now the emergence of telehealth options has increased post discharge follow up support.
The need for ongoing cardiovascular care in cancer patients is clear,218,219,282 however the challenges of local availability of staff, differing geography, funding arrangements and different health systems, a “one size fits all approach” remains a challenge. Clearly defined, evidenced based care exists separately within CVD and cancer fields to assess, monitor and educate patients from diagnosis to long term follow up. There exists a great opportunity for merging these multidisciplinary models of care to enhance the immediate and long-term care of the cancer patients to improve their cardiovascular outcomes.
Ways to implement a multidisciplinary approach to preventative cardio-oncology whilst incorporating all of the patient lifestyle factors, risk factor screening, management and surveillance are poorly defined however technology such as artificial intelligence may provide an answer in the future to tackle these complex multifactorial issues and assist in development of validated patient screening tools and individualised treatment plans.283
Challenges and future directions
There remain notable gaps in our understanding of hypertension within cancer populations and the mechanisms by which cancer therapies promote hypertension. A significant proportion of cancer treatment-related data comes from oncological clinical trials designed to assess for anti-cancer drug efficacy and prognostic cancer outcomes: these trials have limited collection of CV data are not powered enough to detect a difference in the latter. This highlights a pressing need for larger, high-quality trials with a focus on specific CV outcomes such as hypertension dedicated trials of anti-hypertensive therapies in cancer patients and survivors, which in turn can inform clinical guidelines and recommendations for screening and management within this unique cohort. Additionally, as cancer patients are living longer and often pass the conclusion of important clinical trials, an opportunity is often lost to extract accurate data from long term cohorts to properly assess the hypertensive and CV risk and outcomes in these patients.
To gain a better mechanistic understanding of therapy related hypertension, we also need more and better preclinical models and studies which assess the underlying pathophysiological processes on a molecular, cellular and even genomic level.284 We rely heavily on pre-clinical animal and cellular models to improve our understanding of these mechanisms. However, pre-clinical models are limited due to common overlapping risk factors for hypertension and cancer patients and accounting for this complexity is seldom possible.285 Furthermore pre-clinical toxicity data for novel drugs is often extracted from in vivo and in vitro studies in the absence of cancer which does not replicate the important effect tumours may have on the development of hypertension as an adverse outcome.19
Genomic and transcription studies have highlighted we are in our infancy of understanding of the impact of variable gene expression within cancer populations and pharmacogenomic interactions between cancer treatments and antihypertensive therapies. Further studies assessing large patient cohorts may provide new insights on the underlying mechanisms and identify common signalling pathways (biosignatures) involved in the onset of hypertension in cancer patients. These biosignatures can then serve as potential targets and allow for discovery of blood-based biomarkers that may help prevent and predict adverse hypertension. These biomarkers would be also useful and beneficial for precision individualised management for cancer patients in the future.283,286 The emergence of novel nanoparticle-based therapy may provide a platform in the future for cell based drug delivery and targeted gene expression modification.287 Development of plasma therapeutic drug monitoring (TDM) for agents such as VEGF-TKI could potentially guide dose adjustment to reduce hypertensive adverse effects, however like many future projects requires more studies to assess application within clinical practice.288
Addressing the adverse cardiovascular outcomes of cancer treatment remains of critical importance in both the acute phase, and the survivorship phase. While development of and exploration of pre-clinical models and conduct of dedicated clinical trials is still some time away, in the interim we can focus on the delivery of best possible care that is within our knowledge. Implementing multidisciplinary models of joint cardiovascular and cancer care will help deliver more equitable care and improving both CV and cancer outcomes in cancer patients.
There are many opportunities that exist and can be taken advantage of to improve detection and management of hypertension and other CVD throughout patient’s journey (Figure 3). These require better communication and collaboration between cancer and cardiovascular clinicians, primary care providers, allied health pretensioners, and most important centred upon the needs of individual patients and their carers.
Figure 3.
Avenues for the detection and management of hypertension in cancer patients
Overall, only though better integration of care, collaboration between researchers and clinicians, involvement of relevant stakeholders and breaking down silos of “cardiovascular” versus “cancer” care, can we achieve the ultimate goal of improving outcomes for our patients living with and beyond cancer.
Supplementary Material
Sources of Funding
DTMN is supported by the National Heart Foundation Future Leader Fellowship (Award ID 104814), ALS is supported by the National Heart Foundation Future Leader Fellowships (Award ID 106025), TW is supported by the HNE Clinical and Health Service Research Fellowship. This work is supported in part by the NSW Health Cardiovascular Capacity Building Grant (ALS), Hunter Cancer Research Alliance New Strategic Initiatives Grant (TJH, DTMN and ALS), and John Hunter Charitable Trust Grants (TJH, TW, DTMN and ALS). S.M. Herrmann is supported by National Institutes of Health (NIH) Grant No. K08 DK118120 from the National Institute of Diabetes and Digestive and Kidney Diseases. J. Herrmann is supported by National Institutes of Health (NIH) Grant No. CA233610 from the National Cancer Institute.
Disclosures
ALS reports research grants from Biotronik, RACE Oncology, Bristol Myer Squibb, Novartis Australia, Roche Diagnostics, and Vifor; and consultancy fees/speaking honoraria from Novartis, Bayer, Bristol Myer Squibb, AstraZeneca, and Boehringer Ingelheim.
References
- 1.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah L, Abdela J, Abdelalim A, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: Doi 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 3.Lancellotti P, Nguyen Trung M-L, Oury C, Moonen M. Cancer and cardiovascular mortality risk: is the die cast? European Heart Journal. 2021;42:110–112. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DI, Wiebe N, Cheung WY, Mackey JR, Pituskin E, Reiman A, Tonelli M, Network AKD. Incident Cardiovascular Disease Among Adults With Cancer A Population-Based Cohort Study. Jacc-Cardiooncol. 2022;4:85–94. doi: 10.1016/j.jaccao.2022.01.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). European heart journal. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 7.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ, Lang NN. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ Res. 2021;128:1040–1061. doi: 10.1161/CIRCRESAHA.121.318051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malyszko J, Kozlowska K, Kozlowski L, Malyszko J. Nephrotoxicity of anticancer treatment. Nephrol Dial Transplant. 2017;32:924–936. doi: 10.1093/ndt/gfw338 [DOI] [PubMed] [Google Scholar]
- 10.Katsi V, Magkas N, Georgiopoulos G, Athanasiadi E, Virdis A, Masi S, Kliridis P, Hatziyanni A, Tsioufis C, Tousoulis D. Arterial hypertension in patients under antineoplastic therapy: a systematic review. J Hypertens. 2019;37:884–901. doi: 10.1097/HJH.0000000000002006 [DOI] [PubMed] [Google Scholar]
- 11.Kidoguchi S, Sugano N, Tokudome G, Yokoo T, Yano Y, Hatake K, Nishiyama A. New Concept of Onco-Hypertension and Future Perspectives. Hypertension. 2021;77:16–27. doi: 10.1161/HYPERTENSIONAHA.120.16044 [DOI] [PubMed] [Google Scholar]
- 12.Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, Cohen P, Groarke JD, Herrmann J, Reilly CM, et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e579–e602. doi: 10.1161/CIR.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruf R, Yarandi N, Ortiz-Melo DI, Sparks MA. Onco-hypertension: Overview of hypertension with anti-cancer agents. Journal of Onco-Nephrology. 2021;5:57–69. [Google Scholar]
- 14.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403 [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483 [DOI] [PubMed] [Google Scholar]
- 16.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0 [DOI] [PubMed] [Google Scholar]
- 17.Zirlik K, Duyster J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol Res Treat. 2018;41:166–171. doi: 10.1159/000488087 [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–127. doi: 10.1016/j.ctrv.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN. Cardiotoxic effects of angiogenesis inhibitors. Clin Sci (Lond). 2021;135:71–100. doi: 10.1042/CS20200305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyon J, Gouverneur A, Maumus-Robert S, Bérard X, Pariente A, Bikfalvi A, Noize P. Association Between Antiangiogenic Drugs Used for Cancer Treatment and Artery Dissections or Aneurysms. JAMA oncology. 2021;7:775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallerio P, Orenti A, Tosi F, Maistrello M, Palazzini M, Cingarlini S, Colombo P, Bertuzzi M, Spina F, Amatu A, et al. Major adverse cardiovascular events associated with VEGF-targeted anticancer tyrosine kinase inhibitors: a real-life study and proposed algorithm for proactive management. ESMO Open. 2022;7:100338. doi: 10.1016/j.esmoop.2021.100338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cignarella A, Fadini GP, Bolego C, Trevisi L, Boscaro C, Sanga V, Seccia TM, Rosato A, Rossi GP, Barton M. Clinical efficacy and safety of angiogenesis inhibitors: sex differences and current challenges. Cardiovasc Res. 2022;118:988–1003. doi: 10.1093/cvr/cvab096 [DOI] [PubMed] [Google Scholar]
- 23.Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension. 2018;71:e1–e8. doi: 10.1161/HYPERTENSIONAHA.117.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. Journal of the American Heart Association. 2017;6:e006278. doi: ARTN e006278 10.1161/JAHA.117.006278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin G, Zhao L. Risk of hypertension with anti-VEGF monoclonal antibodies in cancer patients: a systematic review and meta-analysis of 105 phase II/III randomized controlled trials. J Chemother. 2022;34:221–234. doi: 10.1080/1120009X.2021.1947022 [DOI] [PubMed] [Google Scholar]
- 26.Faruque LI, Lin M, Battistella M, Wiebe N, Reiman T, Hemmelgarn B, Thomas C, Tonelli M. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS One. 2014;9:e101145. doi: 10.1371/journal.pone.0101145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Ding F, Liu Y, Xiong G, Lin T, He D, Zhang Y, Zhang D, Wei G. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget. 2016;7:67661–67673. doi: 10.18632/oncotarget.11813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: A meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25:482–494. doi: 10.1177/2047487318755193 [DOI] [PubMed] [Google Scholar]
- 29.Hamnvik OP, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, Kaymakcalan MD, Williams JS. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121:311–319. doi: 10.1002/cncr.28972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S, Daniels B, van Leeuwen MT, Pearson SA, Vajdic CM. Incidence and risk factors of hypertension therapy in Australian cancer patients treated with vascular signalling pathway inhibitors. Discov Oncol. 2022;13:6. doi: 10.1007/s12672-022-00468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu S, Chen G, Sun Y, Sun S, Chang J, Yao Y, Chen Z, Ye F, Lu J, Shi J, et al. A Phase III, randomized, double-blind, placebo-controlled, multicenter study of fruquintinib in Chinese patients with advanced nonsquamous non-small-cell lung cancer - The FALUCA study. Lung Cancer. 2020;146:252–262. doi: 10.1016/j.lungcan.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 32.Lv B, Chen J, Liu XL. Anlotinib-Induced Hypertension: Current Concepts and Future Prospects. Curr Pharm Des. 2022;28:216–224. doi: 10.2174/1381612827666211006145141 [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Qin S, Li Z, Yang H, Fu W, Li S, Chen W, Gao Z, Miao W, Xu H, et al. Apatinib vs Placebo in Patients With Locally Advanced or Metastatic, Radioactive Iodine-Refractory Differentiated Thyroid Cancer: The REALITY Randomized Clinical Trial. JAMA Oncol. 2022;8:242–250. doi: 10.1001/jamaoncol.2021.6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou WT, Ding MF, Li XH, Zhou XH, Zhu Q, Varela-Ramirez A, Yi C. Comparative evaluation of cardiovascular risks among nine FDA-approved VEGFR-TKIs in patients with solid tumors: a Bayesian network analysis of randomized controlled trials. J Cancer Res Clin. 2021;147:2407–2420. doi: 10.1007/s00432-021-03521-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelson MD, Gupta S, Agarwal N, Szmulewitz R, Powles T, Pili R, Bruce JY, Vaishampayan U, Larkin J, Rosbrook B, et al. A Phase Ib Study of Axitinib in Combination with Crizotinib in Patients with Metastatic Renal Cell Cancer or Other Advanced Solid Tumors. Oncologist. 2019;24:1151–e1817. doi: 10.1634/theoncologist.2018-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duco MR, Murdock JL, Reeves DJ. Vascular endothelial growth factor inhibitor induced hypertension: Retrospective analysis of the impact of blood pressure elevations on outcomes. J Oncol Pharm Pract. 2022;28:265–273. doi: 10.1177/1078155220985915 [DOI] [PubMed] [Google Scholar]
- 37.Vafopoulou P, Kourti M. Anti-angiogenic drugs in cancer therapeutics: a review of the latest preclinical and clinical studies of anti-angiogenic agents with anticancer potential. Journal of Cancer Metastasis and Treatment. 2022;8:18. [Google Scholar]
- 38.Jin H, Shi Y, Lv Y, Yuan S, Ramirez CFA, Lieftink C, Wang L, Wang S, Wang C, Dias MH, et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature. 2021;595:730–734. doi: 10.1038/s41586-021-03741-7 [DOI] [PubMed] [Google Scholar]
- 39.Powles T, Plimack ER, Soulieres D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563–1573. doi: 10.1016/S1470-2045(20)30436-8 [DOI] [PubMed] [Google Scholar]
- 40.Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, Stepan DE, Shumaker RC, Motzer RJ. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol. 2020;38:1154–1163. doi: 10.1200/JCO.19.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, Zheng M, Zhang YN, Feng YL, Liu Q, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19:1239–1246. doi: 10.1016/S1470-2045(18)30349-8 [DOI] [PubMed] [Google Scholar]
- 42.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 43.Palazzo A, Dellapasqua S, Munzone E, Bagnardi V, Mazza M, Cancello G, Ghisini R, Iorfida M, Montagna E, Goldhirsch A, et al. Phase II Trial of Bevacizumab Plus Weekly Paclitaxel, Carboplatin, and Metronomic Cyclophosphamide With or Without Trastuzumab and Endocrine Therapy as Preoperative Treatment of Inflammatory Breast Cancer. Clin Breast Cancer. 2018;18:328–335. doi: 10.1016/j.clbc.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 44.Yang C, Zhang J, Zhang Y, Ji F, Chen Y, Zhu T, Zhang L, Gao H, Yang M, Li J, et al. Low-dose apatinib combined with neoadjuvant chemotherapy in the treatment of early-stage triple-negative breast cancer (LANCET): a single-center, single-arm, phase II trial. Ther Adv Med Oncol. 2022;14:17588359221118053. doi: 10.1177/17588359221118053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Paper/Poster presented at: Seminars in nephrology; 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y, Xiao J, Fang W, Lu P, Fan Q, Shu Y, Feng J, Zhang S, Ba Y, Zhao Y. The relationship between treatment-induced hypertension and efficacy of anlotinib in recurrent or metastatic esophageal squamous cell carcinoma. Cancer biology & medicine. 2021;18:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of Sunitinib activity. Ann Oncol. 2007;18:1117–1117. doi: 10.1093/annonc/mdm184 [DOI] [PubMed] [Google Scholar]
- 48.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637 [DOI] [PubMed] [Google Scholar]
- 49.Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–954. doi: 10.1200/JCO.2009.25.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Annals of Oncology. 2012;23:3180–3187. doi: 10.1093/annonc/mds179 [DOI] [PubMed] [Google Scholar]
- 51.Hurwitz HI, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA, Chen D, Rosen O. Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist. 2013;18:273–280. doi: 10.1634/theoncologist.2012-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita S, Uehara K, Nakayama G, Shibata T, Oguri T, Inada-Inoue M, Shimokata T, Sugishita M, Mitsuma A, Ando Y. Association between bevacizumab-related hypertension and vascular endothelial growth factor (VEGF) gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemoth Pharm. 2013;71:405–411. doi: 10.1007/s00280-012-2028-2 [DOI] [PubMed] [Google Scholar]
- 53.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik O-PR, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK. Angiotensin System Inhibitors and Survival Outcomes in Patients with Metastatic Renal Cell CarcinomaAngiotensin System Inhibitors in Renal Cell Carcinoma. Clinical Cancer Research. 2015;21:2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Österlund P, Soveri L, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. British journal of cancer. 2011;104:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wirth LJ, Tahara M, Robinson B, Francis S, Brose MS, Habra MA, Newbold K, Kiyota N, Dutcus CE, Mathias E, et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124:2365–2372. doi: 10.1002/cncr.31344 [DOI] [PubMed] [Google Scholar]
- 56.Ptinopoulou AG, Sprangers B. Tyrosine kinase inhibitor-induced hypertension—marker of anti-tumour treatment efficacy or cardiovascular risk factor? In: Oxford University Press; 2021:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong J, Ali AN, Voloschin AD, Liu Y, Curran WJ, Crocker IR, Shu HKG. Bevacizumab-Induced Hypertension Is a Predictive Marker for Improved Outcomes in Patients With Recurrent Glioblastoma Treated With Bevacizumab. Cancer. 2015;121:1456–1462. doi: 10.1002/cncr.29234 [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Zhou L, Chen YT, Liao BH, Ye DH, Wang KJ, Li H. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. Bmc Urology. 2019;19:1–13. doi: ARTN 49 10.1186/s12894-019-0481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez Montes A, Martinez Lago N, Covela Rua M, de la Camara Gomez J, Gonzalez Villaroel P, Mendez Mendez JC, Jorge Fernandez M, Salgado Fernandez M, Reboredo Lopez M, Quintero Aldana G, et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: Prognostic and predictive markers. Cancer Med. 2019;8:882–889. doi: 10.1002/cam4.1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang CJ, Zhang SY, Zhang CD, Lin CR, Li XY, Li QY, Yu HT. Usefulness of bevacizumab-induced hypertension in patients with metastatic colorectal cancer: an updated meta-analysis. Aging (Albany NY). 2018;10:1424–1441. doi: 10.18632/aging.101478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim HH, Hopkins AM, Rowland A, Yuen HY, Karapetis CS, Sorich MJ. Effect of Early Adverse Events on Survival Outcomes of Patients with Metastatic Colorectal Cancer Treated with Ramucirumab. Target Oncol. 2019;14:743–748. doi: 10.1007/s11523-019-00683-z [DOI] [PubMed] [Google Scholar]
- 62.Camarda N, Travers R, Yang VK, London C, Jaffe IZ. VEGF Receptor Inhibitor-Induced Hypertension: Emerging Mechanisms and Clinical Implications. Curr Oncol Rep. 2022;24:463–474. doi: 10.1007/s11912-022-01224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintanilha JCF, Hammond K, Liu YM, Marmorino F, Borelli B, Cremolini C, Nixon AB, Innocenti F. Plasma levels of VEGF-A and VCAM-1 as predictors of drug-induced hypertension in patients treated with VEGF pathway inhibitors. British Journal of Clinical Pharmacology. 2022;88:4171–4179. doi: 10.1111/bcp.15356 [DOI] [PubMed] [Google Scholar]
- 64.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frey MK, Dao F, Olvera N, Konner JA, Dickler MN, Levine DA. Genetic predisposition to bevacizumab-induced hypertension. Gynecol Oncol. 2017;147:621–625. doi: 10.1016/j.ygyno.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 66.Li M, Mulkey F, Jiang C, O’Neil BH, Schneider BP, Shen F, Friedman PN, Momozawa Y, Kubo M, Niedzwiecki D, et al. Identification of a Genomic Region between SLC29A1 and HSP90AB1 Associated with Risk of Bevacizumab-Induced Hypertension: CALGB 80405 (Alliance). Clinical Cancer Research. 2018;24:4734–4744. doi: 10.1158/1078-0432.Ccr-17-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eechoute K, van der Veldt AA, Oosting S, Kappers MH, Wessels JA, Gelderblom H, Guchelaar HJ, Reyners AK, van Herpen CM, Haanen JB, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92:503–510. doi: 10.1038/clpt.2012.136 [DOI] [PubMed] [Google Scholar]
- 68.Fu Y, Saxu R, Ridwan KA, Yao J, Chen X, Xu X, Zheng W, Yu P, Teng Y. Losartan Alleviates the Side Effects and Maintains the Anticancer Activity of Axitinib. Molecules. 2022;27:2764. doi: 10.3390/molecules27092764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren T, Jia H, Wu Q, Zhang Y, Ma Q, Yao D, Gao X, Xie D, Xu Z, Zhao Q, et al. Inhibition of Angiogenesis and Extracellular Matrix Remodeling: Synergistic Effect of Renin-Angiotensin System Inhibitors and Bevacizumab. Front Oncol. 2022;12:829059. doi: 10.3389/fonc.2022.829059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmstrom A, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017;9:eaaf2584. doi: 10.1126/scitranslmed.aaf2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirabito Colafella KM, Neves KB, Montezano AC, Garrelds IM, van Veghel R, de Vries R, Uijl E, Baelde HJ, van den Meiracker AH, Touyz RM, et al. Selective ETA vs. dual ETA/B receptor blockade for the prevention of sunitinib-induced hypertension and albuminuria in WKY rats. Cardiovasc Res. 2020;116:1779–1790. doi: 10.1093/cvr/cvz260 [DOI] [PubMed] [Google Scholar]
- 72.Dabiré H, Dramé F, Cita N, Ghaleh B. The hypertensive effect of sorafenib is abolished by sildenafil. Cardio-Oncology. 2020;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohammed T, Singh M, Tiu JG, Kim AS. Etiology and management of hypertension in patients with cancer. Cardiooncology. 2021;7:14. doi: 10.1186/s40959-021-00101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12:409–425. doi: 10.1016/j.jash.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Versmissen J, Mirabito Colafella KM, Koolen SLW, Danser AHJ. Vascular Cardio-Oncology: Vascular Endothelial Growth Factor inhibitors and hypertension. Cardiovasc Res. 2019;115:904–914. doi: 10.1093/cvr/cvz022 [DOI] [PubMed] [Google Scholar]
- 76.Maki-Petaja KM, McGeoch A, Yang LL, Hubsch A, McEniery CM, Meyer PAR, Mir F, Gajendragadkar P, Ramenatte N, Anandappa G, et al. Mechanisms Underlying Vascular Endothelial Growth Factor Receptor Inhibition-Induced Hypertension: The HYPAZ Trial. Hypertension. 2021;77:1591–1599. doi: 10.1161/HYPERTENSIONAHA.120.16454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coschignano MA, De Ciuceis C, Agabiti-Rosei C, Brami V, Rossini C, Chiarini G, Malerba P, Fama F, Cosentini D, Muiesan ML, et al. Microvascular Structural Alterations in Cancer Patients Treated With Antiangiogenic Drugs. Front Cardiovasc Med. 2021;8:651594. doi: 10.3389/fcvm.2021.651594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–559. doi: 10.1152/ajpheart.00616.2005 [DOI] [PubMed] [Google Scholar]
- 79.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550 [DOI] [PubMed] [Google Scholar]
- 80.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. doi: 10.1158/1078-0432.CCR-07-5050 [DOI] [PubMed] [Google Scholar]
- 81.Jama HA, Muralitharan RR, Xu C, O’Donnell JA, Bertagnolli M, Broughton BRS, Head GA, Marques FZ. Rodent models of hypertension. Br J Pharmacol. 2022;179:918–937. doi: 10.1111/bph.15650 [DOI] [PubMed] [Google Scholar]
- 82.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong ZC, Wu MM, Zhang YL, Wang QS, Liang C, Yan X, Zou LX, Chen C, Han X, Zhang B, et al. The vascular endothelial growth factor trap aflibercept induces vascular dysfunction and hypertension via attenuation of eNOS/NO signaling in mice. Acta Pharmacol Sin. 2021;42:1437–1448. doi: 10.1038/s41401-020-00569-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Budolfsen C, Faber J, Grimm D, Kruger M, Bauer J, Wehland M, Infanger M, Magnusson NE. Tyrosine Kinase Inhibitor-Induced Hypertension: Role of Hypertension as a Biomarker in Cancer Treatment. Curr Vasc Pharmacol. 2019;17:618–634. doi: 10.2174/1570161117666190130165810 [DOI] [PubMed] [Google Scholar]
- 85.Catino AB, Hubbard RA, Chirinos JA, Townsend R, Keefe S, Haas NB, Puzanov I, Fang JC, Agarwal N, Hyman D, et al. Longitudinal Assessment of Vascular Function With Sunitinib in Patients With Metastatic Renal Cell Carcinoma. Circ Heart Fail. 2018;11:e004408. doi: 10.1161/CIRCHEARTFAILURE.117.004408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O’Dwyer PJ. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503 [DOI] [PubMed] [Google Scholar]
- 87.Berardi C, Bluemke DA, Houston BA, Kolb TM, Lima JA, Pezel T, Tedford RJ, Rayner SG, Cheng RK, Leary PJ. Association of soluble Flt-1 with heart failure and cardiac morphology: The MESA angiogenesis study. J Heart Lung Transplant. 2022;41:619–625. doi: 10.1016/j.healun.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verlohren S, Brennecke SP, Galindo A, Karumanchi SA, Mirkovic LB, Schlembach D, Stepan H, Vatish M, Zeisler H, Rana S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42–50. doi: 10.1016/j.preghy.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 89.Saleh L, Vergouwe Y, van den Meiracker AH, Verdonk K, Russcher H, Bremer HA, Versendaal HJ, Steegers EAP, Danser AHJ, Visser W. Angiogenic Markers Predict Pregnancy Complications and Prolongation in Preeclampsia: Continuous Versus Cutoff Values. Hypertension. 2017;70:1025–1033. doi: 10.1161/HYPERTENSIONAHA.117.09913 [DOI] [PubMed] [Google Scholar]
- 90.Kahramanoglu O, Schiattarella A, Demirci O, Sisti G, Ammaturo FP, Trotta C, Ferrari F, Rapisarda AMC. Preeclampsia: state of art and future perspectives. A special focus on possible preventions. J Obstet Gynaecol. 2022;42:766–777. doi: 10.1080/01443615.2022.2048810 [DOI] [PubMed] [Google Scholar]
- 91.Li C, Ma L, Wang Q, Shao X, Guo L, Chen J, Wang W, Yu J. Rho kinase inhibition ameliorates vascular remodeling and blood pressure elevations in a rat model of apatinib-induced hypertension. J Hypertens. 2022;40:675–684. doi: 10.1097/HJH.0000000000003060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 93.Poulikakos PI, Sullivan RJ, Yaeger R. Molecular Pathways and Mechanisms of BRAF in Cancer Therapy. Clin Cancer Res. 2022:OF1–OF11. doi: 10.1158/1078-0432.CCR-21-2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luebker SA, Koepsell SA. Diverse Mechanisms of BRAF Inhibitor Resistance in Melanoma Identified in Clinical and Preclinical Studies. Front Oncol. 2019;9:268. doi: 10.3389/fonc.2019.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subbiah V, Baik C, Kirkwood JM. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer. 2020;6:797–810. doi: 10.1016/j.trecan.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 96.Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, Totzeck M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2:e198890. doi: 10.1001/jamanetworkopen.2019.8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glen C, Tan YY, Waterston A, Evans TRJ, Jones R, Petrie MC, Lang N. Cardiovascular toxicity of BRAF and MEK inhibitors in patients with cancer: mechanistic and clinical overview. Jacc-Cardiooncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guha A, Jain P, Fradley MG, Lenihan D, Gutierrez JM, Jain C, de Lima M, Barnholtz-Sloan JS, Oliveira GH, Dowlati A, et al. Cardiovascular adverse events associated with BRAF versus BRAF/MEK inhibitor: Cross-sectional and longitudinal analysis using two large national registries. Cancer Medicine. 2021;10:3862–3872. doi: 10.1002/cam4.3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bronte E, Bronte G, Novo G, Bronte F, Bavetta MG, Re GL, Brancatelli G, Bazan V, Natoli C, Novo S. What links BRAF to the heart function? New insights from the cardiotoxicity of BRAF inhibitors in cancer treatment. Oncotarget. 2015;6:35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421 [DOI] [PubMed] [Google Scholar]
- 101.Abdel-Rahman O, ElHalawani H, Ahmed H. Risk of Selected Cardiovascular Toxicities in Patients With Cancer Treated With MEK Inhibitors: A Comparative Systematic Review and Meta-Analysis. J Glob Oncol. 2015;1:73–82. doi: 10.1200/JGO.2015.000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bronte E, Bronte G, Novo G, Rinaldi G, Bronte F, Passiglia F, Russo A. Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Pharmacol Ther. 2018;192:65–73. doi: 10.1016/j.pharmthera.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 103.Riely GJ, Ahn M-J, Felip E, Ramalingam SS, Smit EF, Tsao AS, Alcasid A, Usari T, Wissel PS, Wilner KD. Encorafenib plus binimetinib in patients with BRAF V600-mutant non-small cell lung cancer: phase II PHAROS study design. Future Oncology. 2022;18:781–791. [DOI] [PubMed] [Google Scholar]
- 104.Arangalage D, Degrauwe N, Michielin O, Monney P, Ozdemir BC. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat Rev. 2021;100:102282. doi: 10.1016/j.ctrv.2021.102282 [DOI] [PubMed] [Google Scholar]
- 105.Ferrucci PF, Lens M, Cocorocchio E. Combined BRAF-Targeted Therapy with Immunotherapy in BRAF-Mutated Advanced Melanoma Patients. Curr Oncol Rep. 2021;23:138. doi: 10.1007/s11912-021-01134-7 [DOI] [PubMed] [Google Scholar]
- 106.Yeh JH, Tsai HL, Chen YC, Li CC, Huang CW, Chang TK, Su WC, Chen PJ, Liu YP, Wang JY. BRAF, MEK, and EGFR Triplet Inhibitors as Salvage Therapy in BRAF-Mutated Metastatic Colorectal Cancer-A Case Series Study Target Therapy of BRAF-Mutated mCRC. Medicina-Lithuania. 2021;57:1339. doi: ARTN 1339 10.3390/medicina57121339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, Queirolo P, Long GV, Di Giacomo AM, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019;25:941–946. doi: 10.1038/s41591-019-0448-9 [DOI] [PubMed] [Google Scholar]
- 108.Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277:23065–23075. doi: 10.1074/jbc.M200328200 [DOI] [PubMed] [Google Scholar]
- 109.Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272:5783–5791. doi: 10.1074/jbc.272.9.5783 [DOI] [PubMed] [Google Scholar]
- 110.Ramirez MT, Sah VP, Zhao X-L, Hunter JJ, Chien KR, Brown JH. The MEKK-JNK pathway is stimulated by α1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. Journal of Biological Chemistry. 1997;272:14057–14061. [DOI] [PubMed] [Google Scholar]
- 111.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893 [DOI] [PubMed] [Google Scholar]
- 112.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. P Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu F, Jiang CC, Yan XG, Tseng HY, Wang CY, Zhang YY, Yari H, La T, Farrelly M, Guo ST, et al. BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget. 2017;8:69477–69492. doi: 10.18632/oncotarget.17704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol. 2017;17:487–493. doi: 10.1007/s12012-017-9425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meijles DN, Cull JJ, Cooper ST, Markou T, Hardyman MA, Fuller SJ, Alharbi HO, Haines ZH, Alcantara-Alonso V, Glennon PE. The anti-cancer drug dabrafenib is not cardiotoxic and inhibits cardiac remodelling and fibrosis in a murine model of hypertension. Clinical Science. 2021;135:1631–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35:312–332. doi: 10.1038/s41375-020-01072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Awan FT, Addison D, Alfraih F, Baratta SJ, Campos RN, Cugliari MS, Goh YT, Ionin VA, Mundnich S, Sverdlov AL, et al. International consensus statement on the management of cardiovascular risk of Bruton’s tyrosine kinase inhibitors in CLL. Blood Adv. 2022;6:5516–5525. doi: 10.1182/bloodadvances.2022007938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coutre SE, Byrd JC, Hillmen P, Barrientos JC, Barr PM, Devereux S, Robak T, Kipps TJ, Schuh A, Moreno C, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3:1799–1807. doi: 10.1182/bloodadvances.2018028761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL, Burger JA, Hillmen P, Coutre S, Devereux S, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103:1502–1510. doi: 10.3324/haematol.2018.192328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One. 2019;14:e0211228. doi: 10.1371/journal.pone.0211228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, Guha A, Rogers KA, Bhat S, Byrd JC, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. doi: 10.1182/blood.2019000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roeker LE, Sarraf Yazdy M, Rhodes J, Goodfriend J, Narkhede M, Carver J, Mato A. Hypertension in Patients Treated With Ibrutinib for Chronic Lymphocytic Leukemia. JAMA Netw Open. 2019;2:e1916326. doi: 10.1001/jamanetworkopen.2019.16326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, Hillmen P, Martin P, Awan FT, Stephens DM, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204–1213. doi: 10.1182/blood.2018884940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, Kamdar M, Munir T, Walewska R, Corbett G. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial. The Lancet. 2020;395:1278–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, Kaplan P, Kraychok I, Illes A, de la Serna J, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol. 2020;38:2849–2861. doi: 10.1200/JCO.19.03355 [DOI] [PubMed] [Google Scholar]
- 126.Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, Owen RG, Marlton P, Wahlin BE, Sanz RG, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–2050. doi: 10.1182/blood.2020006844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen ST, Azali L, Rosen L, Zhao Q, Wiczer T, Palettas M, Gambril J, Kola-Kehinde O, Ruz P, Kalathoor S, et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J Hematol Oncol. 2022;15:92. doi: 10.1186/s13045-022-01302-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kappers MHW, van Esch JHM, Sluiter W, Sleijfer S, Danser AHJ, van den Meiracker AH. Hypertension Induced by the Tyrosine Kinase Inhibitor Sunitinib Is Associated With Increased Circulating Endothelin-1 Levels. Hypertension. 2010;56:675–U216. doi: 10.1161/Hypertensionaha.109.149690 [DOI] [PubMed] [Google Scholar]
- 129.Sestier M, Hillis C, Fraser G, Leong D. Bruton’s tyrosine kinase Inhibitors and Cardiotoxicity: More Than Just Atrial Fibrillation. Current Oncology Reports. 2021;23:1–12. doi: ARTN 113 10.1007/s11912-021-01102-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int J Mol Sci. 2021;22:3464. doi: 10.3390/ijms22073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dreyling M, Santoro A, Mollica L, Leppa S, Follows G, Lenz G, Kim WS, Nagler A, Dimou M, Demeter J, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol. 2020;95:362–371. doi: 10.1002/ajh.25711 [DOI] [PubMed] [Google Scholar]
- 133.Hanker AB, Kaklamani V, Arteaga CL. Challenges for the Clinical Development of PI3K Inhibitors: Strategies to Improve Their Impact in Solid Tumors. Cancer Discov. 2019;9:482–491. doi: 10.1158/2159-8290.Cd-18-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perrotta M, Lembo G, Carnevale D. The Multifaceted Roles of PI3Kgamma in Hypertension, Vascular Biology, and Inflammation. Int J Mol Sci. 2016;17:1858. doi: 10.3390/ijms17111858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramanathan RK, Von Hoff DD, Eskens F, Blumenschein G, Richards D, Genvresse I, Reschke S, Granvil C, Skubala A, Peña C. Phase Ib trial of the PI3K inhibitor copanlisib combined with the allosteric MEK inhibitor refametinib in patients with advanced cancer. Targeted oncology. 2020;15:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keegan NM, Furney SJ, Walshe JM, Gullo G, Kennedy MJ, Smith D, McCaffrey J, Kelly CM, Egan K, Kerr J, et al. Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”. Cancers (Basel). 2021;13:1225. doi: 10.3390/cancers13061225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shouse G, Danilova OV, Danilov AV. Current status of phosphoinotiside-3 kinase inhibitors in blood cancers. Curr Opin Oncol. 2022;34:540–545. doi: 10.1097/CCO.0000000000000871 [DOI] [PubMed] [Google Scholar]
- 138.Coleman M, Belada D, Casasnovas RO, Gressin R, Lee HP, Mehta A, Munoz J, Verhoef G, Corrado C, DeMarini DJ, et al. Phase 2 study of parsaclisib (INCB050465), a highly selective, next-generation PI3Kdelta inhibitor, in relapsed or refractory diffuse large B-cell lymphoma (CITADEL-202). Leuk Lymphoma. 2021;62:368–376. doi: 10.1080/10428194.2020.1832660 [DOI] [PubMed] [Google Scholar]
- 139.Seront E, Rottey S, Filleul B, Glorieux P, Goeminne JC, Verschaeve V, Vandenbulcke JM, Sautois B, Boegner P, Gillain A, et al. Phase II study of dual phosphoinositol-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor BEZ235 in patients with locally advanced or metastatic transitional cell carcinoma. Bju Int. 2016;118:408–415. doi: 10.1111/bju.13415 [DOI] [PubMed] [Google Scholar]
- 140.Cheson BD, O’Brien S, Ewer MS, Goncalves MD, Farooki A, Lenz G, Yu A, Fisher RI, Zinzani PL, Dreyling M. Optimal Management of Adverse Events From Copanlisib in the Treatment of Patients With Non-Hodgkin Lymphomas. Clin Lymphoma Myeloma Leuk. 2019;19:135–141. doi: 10.1016/j.clml.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, Kim WS, Nagler A, Panayiotidis P, Demeter J, et al. Phosphatidylinositol 3-Kinase Inhibition by Copanlisib in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2017;35:3898–3905. doi: 10.1200/jco.2017.75.4648 [DOI] [PubMed] [Google Scholar]
- 142.Morschhauser F, Machiels JP, Salles G, Rottey S, Rule SAJ, Cunningham D, Peyrade F, Fruchart C, Arkenau HT, Genvresse I, et al. On-Target Pharmacodynamic Activity of the PI3K Inhibitor Copanlisib in Paired Biopsies from Patients with Malignant Lymphoma and Advanced Solid Tumors. Mol Cancer Ther. 2020;19:468–478. doi: 10.1158/1535-7163.MCT-19-0466 [DOI] [PubMed] [Google Scholar]
- 143.McLean BA, Zhabyeyev P, Pituskin E, Paterson I, Haykowsky MJ, Oudit GY. PI3K inhibitors as novel cancer therapies: implications for cardiovascular medicine. Journal of cardiac failure. 2013;19:268–282. [DOI] [PubMed] [Google Scholar]
- 144.Berghausen EM, Janssen W, Vantler M, Gnatzy-Feik LL, Krause M, Behringer A, Joseph C, Zierden M, Freyhaus HT, Klinke A, et al. Disrupted PI3K subunit p110alpha signaling protects against pulmonary hypertension and reverses established disease in rodents. J Clin Invest. 2021;131. doi: 10.1172/JCI136939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hsieh MW, Wang WT, Yeh JL, Lin CY, Kuo YR, Lee SS, Hou MF, Wu YC. The Potential Application and Promising Role of Targeted Therapy in Pulmonary Arterial Hypertension. Biomedicines. 2022;10:1415. doi: 10.3390/biomedicines10061415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takahashi M RET receptor signaling: Function in development, metabolic disease, and cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2022;98:112–125. doi: 10.2183/pjab.98.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J Clin Oncol. 2020;38:1209–1221. doi: 10.1200/JCO.19.02551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Illini O, Hochmair MJ, Fabikan H, Weinlinger C, Tufman A, Swalduz A, Lamberg K, Hashemi SMS, Huemer F, Vikstrom A, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Therapeutic Advances in Medical Oncology. 2021;13:17588359211019675. doi: Artn 17588359211019675 10.1177/17588359211019675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Subbiah V, Cassier PA, Siena S, Alonso G, Paz-Ares LG, Garrido P, Nadal E, Curigliano G, Vuky J, Lopes G, et al. Clinical activity and safety of the RET inhibitor pralsetinib in patients with RET fusion-positive solid tumors: Update from the ARROW trial. Journal of Clinical Oncology. 2021;39:86. doi: 10.1200/JCO.2021.39.15_suppl.3079 [DOI] [Google Scholar]
- 151.Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med. 2020;383:825–835. doi: 10.1056/NEJMoa2005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer Discov. 2020;10:498–505. doi: 10.1158/2159-8290.CD-19-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin JJ, Liu SV, McCoach CE, Zhu VW, Tan AC, Yoda S, Peterson J, Do A, Prutisto-Chang K, Dagogo-Jack I, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol. 2020;31:1725–1733. doi: 10.1016/j.annonc.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gainor JF, Lee DH, Curigliano G, Doebele RC, Kim DW, Baik CS, Tan DSW, Lopes G, Gadgeel SM, Cassier PA, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion plus non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2019;37. [Google Scholar]
- 155.Oxnard G, Subbiah V, Park K, Bauer T, Wirth L, Velcheti V, Shah M, Besse B, Boni V, Reckamp K. OA12. 07 clinical activity of LOXO-292, a highly selective RET inhibitor, in patients with RET Fusion+ non-small cell lung cancer. Journal of Thoracic Oncology. 2018;13:S349–S350. [Google Scholar]
- 156.Regua AT, Najjar M, Lo HW. RET signaling pathway and RET inhibitors in human cancer. Front Oncol. 2022;12:932353. doi: 10.3389/fonc.2022.932353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10:2034–2042. doi: 10.1158/1535-7163.MCT-11-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laubach JP, Moslehi JJ, Francis SA, San Miguel JF, Sonneveld P, Orlowski RZ, Moreau P, Rosinol L, Faber EA Jr., Voorhees P, et al. A retrospective analysis of 3954 patients in phase 2/3 trials of bortezomib for the treatment of multiple myeloma: towards providing a benchmark for the cardiac safety profile of proteasome inhibition in multiple myeloma. Br J Haematol. 2017;178:547–560. doi: 10.1111/bjh.14708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng W-J, Oriol A, Orlowski RZ, Ludwig H, Facon T, Hajek R. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. The lancet oncology. 2017;18:1327–1337. [DOI] [PubMed] [Google Scholar]
- 160.Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, Badros AZ, Jagannath S, McCulloch L, Rajangam K, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753–1761. doi: 10.3324/haematol.2013.089334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bishnoi R, Xie Z, Shah C, Bian J, Murthy HS, Wingard JR, Farhadfar N. Real-world experience of carfilzomib-associated cardiovascular adverse events: SEER-Medicare data set analysis. Cancer Med. 2021;10:70–78. doi: 10.1002/cam4.3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shah C, Bishnoi R, Jain A, Bejjanki H, Xiong S, Wang Y, Zou F, Moreb JS. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;59:2557–2569. doi: 10.1080/10428194.2018.1437269 [DOI] [PubMed] [Google Scholar]
- 163.Cole DC, Frishman WH. Cardiovascular Complications of Proteasome Inhibitors Used in Multiple Myeloma. Cardiol Rev. 2018;26:122–129. doi: 10.1097/Crd.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 164.Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, Stewart AK, Hari P, Roy V, Vescio R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–1512. doi: 10.1016/S1470-2045(14)71125-8 [DOI] [PubMed] [Google Scholar]
- 165.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282 [DOI] [PubMed] [Google Scholar]
- 166.Efentakis P, Psarakou G, Varela A, Papanagnou ED, Chatzistefanou M, Nikolaou PE, Davos CH, Gavriatopoulou M, Trougakos IP, Dimopoulos MA, et al. Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin. Int J Mol Sci. 2021;22:10956. doi: 10.3390/ijms222010956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Herrmann J, Saguner AM, Versari D, Peterson TE, Chade A, Olson M, Lerman LO, Lerman A. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ Res. 2007;101:865–874. doi: 10.1161/CIRCRESAHA.107.152959 [DOI] [PubMed] [Google Scholar]
- 168.Zhang J, Lu W, Chen Y, Jiang Q, Yang K, Li M, Wang Z, Duan X, Xu L, Tang H, et al. Bortezomib alleviates experimental pulmonary hypertension by regulating intracellular calcium homeostasis in PASMCs. Am J Physiol Cell Physiol. 2016;311:C482–497. doi: 10.1152/ajpcell.00324.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Forghani P, Rashid A, Sun F, Liu R, Li D, Lee MR, Hwang H, Maxwell JT, Mandawat A, Wu R, et al. Carfilzomib Treatment Causes Molecular and Functional Alterations of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J Am Heart Assoc. 2021;10:e022247. doi: 10.1161/JAHA.121.022247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Przybycinski J, Nalewajska M, Marchelek-Mysliwiec M, Dziedziejko V, Pawlik A. Poly-ADP-ribose polymerases (PARPs) as a therapeutic target in the treatment of selected cancers. Expert Opin Ther Tar. 2019;23:773–785. doi: 10.1080/14728222.2019.1654458 [DOI] [PubMed] [Google Scholar]
- 171.Genta S, Martorana F, Stathis A, Colombo I. Targeting the DNA damage response: PARP inhibitors and new perspectives in the landscape of cancer treatment. Crit Rev Oncol Hematol. 2021;168:103539. doi: 10.1016/j.critrevonc.2021.103539 [DOI] [PubMed] [Google Scholar]
- 172.Nero C, Ciccarone F, Pietragalla A, Duranti S, Daniele G, Salutari V, Carbone MV, Scambia G, Lorusso D. Ovarian Cancer Treatments Strategy: Focus on PARP Inhibitors and Immune Check Point Inhibitors. Cancers (Basel). 2021;13:1298. doi: 10.3390/cancers13061298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:E15–E28. doi: Doi 10.1016/S1470-2045(18)30786-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 175.Reiss KA, Mick R, Teitelbaum U, O’Hara M, Schneider C, Massa R, Karasic T, Tondon R, Onyiah C, Gosselin MK, et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. 2022;23:1009–1020. doi: 10.1016/S1470-2045(22)00369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dumbrava EE, Shapiro G, Bendell JC, Yap TA, Jeselsohn R, Lepley DM, Hurley S, Lin KK, Liao M, Habeck J. Phase 1b/2 SEASTAR trial: Safety, pharmacokinetics, and preliminary efficacy of the poly (ADP)-ribose polymerase (PARP) inhibitor rucaparib and angiogenesis inhibitor lucitanib in patients with advanced solid tumors. In: Wolters Kluwer Health; 2021. [Google Scholar]
- 177.Landrum LM, Brady WE, Armstrong DK, Moore KN, DiSilvestro PA, O’Malley DM, Tenney ME, Rose PG, Fracasso PM. A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2016;140:204–209. doi: 10.1016/j.ygyno.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, Fujiwara K, Vergote I, Colombo N, Maenpaa J, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. New England Journal of Medicine. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 179.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Perrotta I, Brunelli E, Sciangula A, Conforti F, Perrotta E, Tripepi S, Donato G, Cassese M. iNOS induction and PARP-1 activation in human atherosclerotic lesions: an immunohistochemical and ultrastructural approach. Cardiovasc Pathol. 2011;20:195–203. doi: 10.1016/j.carpath.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 181.Rao PD, Sankrityayan H, Srivastava A, Kulkarni YA, Mulay SR, Gaikwad AB. ‘PARP’ing fibrosis: repurposing poly (ADP ribose) polymerase (PARP) inhibitors. Drug Discov Today. 2020;25:1253–1261. doi: 10.1016/j.drudis.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 182.London J, Rouch C, Bui LC, Assayag E, Souchet B, Daubigney F, Medjaoui H, Luquet S, Magnan C, Delabar JM, et al. Overexpression of the DYRK1A Gene (Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A) Induces Alterations of the Serotoninergic and Dopaminergic Processing in Murine Brain Tissues. Mol Neurobiol. 2018;55:3822–3831. doi: 10.1007/s12035-017-0591-6 [DOI] [PubMed] [Google Scholar]
- 183.Sandhu D, Antolin AA, Cox AR, Jones AM. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. British Journal of Clinical Pharmacology. 2022;88:742–752. [DOI] [PubMed] [Google Scholar]
- 184.Sandhu D, Antolin AA, Cox AR, Jones AM. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Br J Clin Pharmacol. 2022;88:742–752. doi: 10.1111/bcp.15015 [DOI] [PubMed] [Google Scholar]
- 185.Kinoshita C, Okamoto Y, Aoyama K, Nakaki T. MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks Sleep. 2020;2:282–307. doi: 10.3390/clockssleep2030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kurabayashi N, Hirota T, Sakai M, Sanada K, Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol Cell Biol. 2010;30:1757–1768. doi: 10.1128/MCB.01047-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 2018;119:108–114. doi: 10.1016/j.freeradbiomed.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Costello HM, Gumz ML. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension. 2021;78:1185–1196. doi: 10.1161/HYPERTENSIONAHA.121.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang J, Sun R, Jiang T, Yang G, Chen L. Circadian Blood Pressure Rhythm in Cardiovascular and Renal Health and Disease. Biomolecules. 2021;11. doi: 10.3390/biom11060868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neves KB, Montezano AC, Lang NN, Touyz RM. Vascular toxicity associated with anti-angiogenic drugs. Clin Sci (Lond). 2020;134:2503–2520. doi: 10.1042/CS20200308 [DOI] [PubMed] [Google Scholar]
- 191.Muhandiramge J, Zalcberg JR, van Londen G, Warner ET, Carr PR, Haydon A, Orchard SG. Cardiovascular Disease in Adult Cancer Survivors: a Review of Current Evidence, Strategies for Prevention and Management, and Future Directions for Cardio-oncology. Current Oncology Reports. 2022:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wilk M, Waśko-Grabowska A, Skoneczna I, Szmit S. Angiotensin System Inhibitors May Improve Outcomes of Patients With Castration-Resistant Prostate Cancer During Abiraterone Acetate Treatment-A Cardio-Oncology Study. Front Oncol. 2021;11:664741. doi: 10.3389/fonc.2021.664741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Szmit S, Jurczak W, Zaucha JM, Drozd-Sokołowska J, Spychałowicz W, Joks M, Długosz-Danecka M, Torbicki A. Pre-existing arterial hypertension as a risk factor for early left ventricular systolic dysfunction following (R)-CHOP chemotherapy in patients with lymphoma. J Am Soc Hypertens. 2014;8:791–799. doi: 10.1016/j.jash.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 194.Di Lorenzo G, Autorino R, Bruni G, Cartenì G, Ricevuto E, Tudini M, Ficorella C, Romano C, Aieta M, Giordano A, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009;20:1535–1542. doi: 10.1093/annonc/mdp025 [DOI] [PubMed] [Google Scholar]
- 195.Guida JL, Ahles TA, Belsky D, Campisi J, Cohen HJ, DeGregori J, Fuldner R, Ferrucci L, Gallicchio L, Gavrilov L. Measuring aging and identifying aging phenotypes in cancer survivors. JNCI: Journal of the National Cancer Institute. 2019;111:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guida JL, Agurs-Collins T, Ahles TA, Campisi J, Dale W, Demark-Wahnefried W, Dietrich J, Fuldner R, Gallicchio L, Green PA. Strategies to prevent or remediate cancer and treatment-related aging. JNCI: Journal of the National Cancer Institute. 2021;113:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, dos-Santos-Silva I, Smeeth L, Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. The Lancet. 2019;394:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schoormans D, Vissers PA, van Herk-Sukel MP, Denollet J, Pedersen SS, Dalton SO, Rottmann N, van de Poll-Franse L. Incidence of cardiovascular disease up to 13 year after cancer diagnosis: a matched cohort study among 32 757 cancer survivors. Cancer medicine. 2018;7:4952–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. European heart journal. 2019;40:3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paterson DI, Wiebe N, Cheung WY, Mackey JR, Pituskin E, Reiman A, Tonelli M, Network AKD. Incident cardiovascular disease among adults with cancer: a population-based cohort study. Cardio Oncology. 2022;4:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Puckett LL, Saba SG, Henry S, Rosen S, Rooney E, Filosa SL, Gilbo P, Pappas K, Laxer A, Eacobacci K. Cardiotoxicity screening of long-term, breast cancer survivors—The CAROLE (Cardiac-Related Oncologic Late Effects) Study. Cancer medicine. 2021;10:5051–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guan X, Wei R, Yang R, Lu Z, Liu E, Zhao Z, Chen H, Yang M, Liu Z, Jiang Z. Risk and prognosis of secondary bladder cancer after radiation therapy for rectal cancer: a large population-based cohort study. Frontiers in oncology. 2021;10:586401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stoltzfus KC, Zhang Y, Sturgeon K, Sinoway LI, Trifiletti DM, Chinchilli VM, Zaorsky NG. Fatal heart disease among cancer patients. Nature communications. 2020;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang R, Zhou Y, Hu S, Ren G, Cui F, Zhou P-K. Radiotherapy exposure in cancer patients and subsequent risk of stroke: a systematic review and meta-analysis. Frontiers in neurology. 2019;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang L, Wang F, Chen L, Geng Y, Yu S, Chen Z. Long-term cardiovascular disease mortality among 160 834 5-year survivors of adolescent and young adult cancer: an American population-based cohort study. European Heart Journal. 2021;42:101–109. [DOI] [PubMed] [Google Scholar]
- 206.Feigin V, Collaborators GCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Armenian SH, Xu LF, Ky B, Sun CL, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. Journal of Clinical Oncology. 2016;34:1122–+. doi: 10.1200/Jco.2015.64.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gibson TM, Li Z, Green DM, Armstrong GT, Mulrooney DA, Srivastava D, Bhakta N, Ness KK, Hudson MM, Robison LL. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26:1705–1713. doi: 10.1158/1055-9965.EPI-17-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chow EJ, Baker KS, Lee SJ, Flowers ME, Cushing-Haugen KL, Inamoto Y, Khera N, Leisenring WM, Syrjala KL, Martin PJ. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A. SEER cancer statistics review, 1975–2014. Bethesda, MD: National Cancer Institute. 2017;2018. [Google Scholar]
- 211.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chow EJ, Chen Y, Armstrong GT, Baldwin LM, Cai CR, Gibson TM, Hudson MM, McDonald A, Nathan PC, Olgin JE, et al. Underdiagnosis and Undertreatment of Modifiable Cardiovascular Risk Factors Among Survivors of Childhood Cancer. Journal of the American Heart Association. 2022;11:e024735. doi: ARTN e024735 10.1161/JAHA.121.024735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gudsoorkar P, Ruf R, Adnani H, Safdar K, Sparks MA. Onco-hypertension: An Emerging Specialty. Adv Chronic Kidney Dis. 2021;28:477–489 e471. doi: 10.1053/j.ackd.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 214.Yinghui W, Yonggang W, Xiao M, Zhaoyang C, Jian S, Xiaorong H, Cheng L, Jin Z, Adhikari BK. Cardio-Oncology: A myriad of relationship between cardiovascular disease and cancer. Frontiers in Cardiovascular Medicine. 2022:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ba Z, Xiao Y, He M, Liu D, Wang H, Liang H, Yuan J. Risk Factors for the Comorbidity of Hypertension and Renal Cell Carcinoma in the Cardio-Oncologic Era and Treatment for Tumor-Induced Hypertension. Front Cardiovasc Med. 2022;9:810262. doi: 10.3389/fcvm.2022.810262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kourek C, Touloupaki M, Rempakos A, Loritis K, Tsougkos E, Paraskevaidis I, Briasoulis A. Cardioprotective Strategies from Cardiotoxicity in Cancer Patients: A Comprehensive Review. J Cardiovasc Dev Dis. 2022;9:259. doi: 10.3390/jcdd9080259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler-Klein J. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the C ardio-O ncology S tudy G roup of the H eart F ailure A ssociation of the E uropean S ociety of C ardiology in collaboration with the I nternational C ardio-O ncology S ociety. European journal of heart failure. 2020;22:1945–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, De Simone G, Dominiczak A. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European heart journal. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 221.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A. 2013 ESH/ESC practice guidelines for the management of arterial hypertension: ESH-ESC the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood pressure. 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 222.Virani SA, Dent S, Brezden-Masley C, Clarke B, Davis MK, Jassal DS, Johnson C, Lemieux J, Paterson I, Sebag IA, et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can J Cardiol. 2016;32:831–841. doi: 10.1016/j.cjca.2016.02.078 [DOI] [PubMed] [Google Scholar]
- 223.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 224.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 225.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifilogr. 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 227.Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol. 2019;1:238–251. doi: 10.1016/j.jaccao.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gomez JA. Vascular endothelial growth factor-tyrosine kinase inhibitors: Novel mechanisms/predictors of hypertension; management strategies. American Heart Journal Plus: Cardiology Research and Practice. 2022:100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 231.Lancellotti P, Suter TM, Lopez-Fernandez T, Galderisi M, Lyon AR, Van der Meer P, Solal AC, Zamorano JL, Jerusalem G, Moonen M, et al. Cardio-Oncology Services: rationale, organization, and implementation A report from the ESC Cardio-Oncology council. European Heart Journal. 2019;40:1756–1763. doi: 10.1093/eurheartj/ehy453 [DOI] [PubMed] [Google Scholar]
- 232.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–97. doi: 10.1056/NEJMc072330 [DOI] [PubMed] [Google Scholar]
- 233.Rao VU, Reeves DJ, Chugh AR, O’Quinn R, Fradley MG, Raghavendra M, Dent S, Barac A, Lenihan D. Clinical Approach to Cardiovascular Toxicity of Oral Antineoplastic Agents: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:2693–2716. doi: 10.1016/j.jacc.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem JE, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J Am Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lambert J, Lamacie M, Thampinathan B, Altaha MA, Esmaeilzadeh M, Nolan M, Fresno CU, Somerset E, Amir E, Marwick TH, et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. 2020;106:817–823. doi: 10.1136/heartjnl-2019-316297 [DOI] [PubMed] [Google Scholar]
- 236.Kourek C, Touloupaki M, Rempakos A, Loritis K, Tsougkos E, Paraskevaidis I, Briasoulis A. Cardioprotective Strategies from Cardiotoxicity in Cancer Patients: A Comprehensive Review. Journal of Cardiovascular Development and Disease. 2022;9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Semeraro GC, Cipolla CM, Cardinale DM. Role of Cardiac Biomarkers in Cancer Patients. Cancers (Basel). 2021;13:5426. doi: 10.3390/cancers13215426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand J-B, Ewer M. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 239.Graffagnino J, Kondapalli L, Arora G, Hawi R, Lenneman CG. Strategies to Prevent Cardiotoxicity. Curr Treat Options Oncol. 2020;21:32. doi: 10.1007/s11864-020-0722-6 [DOI] [PubMed] [Google Scholar]
- 240.Sapkota Y, Li N, Pierzynski J, Mulrooney DA, Ness KK, Morton LM, Michael JR, Zhang JH, Bhatia S, Armstrong GT, et al. Contribution of Polygenic Risk to Hypertension Among Long-Term Survivors of Childhood Cancer. Jacc-Cardiooncol. 2021;3:76–84. doi: 10.1016/j.jaccao.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Valenzuela PL, Carrera-Bastos P, Galvez BG, Ruiz-Hurtado G, Ordovas JM, Ruilope LM, Lucia A. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. 2021;18:251–275. doi: 10.1038/s41569-020-00437-9 [DOI] [PubMed] [Google Scholar]
- 242.Blumenthal JA, Hinderliter AL, Smith PJ, Mabe S, Watkins LL, Craighead L, Ingle K, Tyson C, Lin PH, Kraus WE, et al. Effects of Lifestyle Modification on Patients With Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation. 2021;144:1212–1226. doi: 10.1161/CIRCULATIONAHA.121.055329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Essa H, Dobson R, Wright D, Lip GYH. Hypertension management in cardio-oncology. J Hum Hypertens. 2020;34:673–681. doi: 10.1038/s41371-020-0391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, Biffi A, Boavida JM, Capodanno D, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). European Heart Journal. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 245.Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, Zhang J, Tian M, Huang L, Li Z, et al. Effect of Salt Substitution on Cardiovascular Events and Death. N Engl J Med. 2021;385:1067–1077. doi: 10.1056/NEJMoa2105675 [DOI] [PubMed] [Google Scholar]
- 246.Sipahi I Risk of cancer with angiotensin-receptor blockers increases with increasing cumulative exposure: Meta-regression analysis of randomized trials. PLoS One. 2022;17:e0263461. doi: 10.1371/journal.pone.0263461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–178. doi: 10.3109/0284186X.2010.529822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) Diet with the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Nutr Cancer. 2020;72:778–790. doi: 10.1080/01635581.2019.1651880 [DOI] [PubMed] [Google Scholar]
- 249.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cormie P, Zopf EM, Zhang X, Schmitz KH. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017;39:71–92. doi: 10.1093/epirev/mxx007 [DOI] [PubMed] [Google Scholar]
- 251.Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 253.Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, et al. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol. 2005;289:H722–731. doi: 10.1152/ajpheart.01249.2004 [DOI] [PubMed] [Google Scholar]
- 255.Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, Papadimitriou C. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38:473–483. doi: 10.1016/j.ctrv.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 256.Emery J, Butow P, Lai-Kwon J, Nekhlyudov L, Rynderman M, Jefford M. Management of common clinical problems experienced by survivors of cancer. Lancet. 2022;399:1537–1550. doi: 10.1016/S0140-6736(22)00242-2 [DOI] [PubMed] [Google Scholar]
- 257.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 258.Mahmood S, Shah KU, Khan TM, Nawaz S, Rashid H, Baqar SWA, Kamran S. Non-pharmacological management of hypertension: in the light of current research. Ir J Med Sci. 2019;188:437–452. doi: 10.1007/s11845-018-1889-8 [DOI] [PubMed] [Google Scholar]
- 259.Chow CK, Atkins ER, Hillis GS, Nelson MR, Reid CM, Schlaich MP, Hay P, Rogers K, Billot L, Burke M, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398:1043–1052. doi: 10.1016/S0140-6736(21)01922-X [DOI] [PubMed] [Google Scholar]
- 260.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, Yang J, Jiang Y, Xu X, Wang TD, et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N Engl J Med. 2021;385:1268–1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 261.Song T, Choi CH, Kim MK, Kim ML, Yun BS, Seong SJ. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: a meta-analysis. Eur J Cancer Prev. 2017;26:78–85. doi: 10.1097/CEJ.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 262.Drobni ZD, Michielin O, Quinaglia T, Zlotoff DA, Zubiri L, Gilman HK, Supraja S, Merkely B, Muller V, Sullivan RJ, et al. Renin-angiotensin-aldosterone system inhibitors and survival in patients with hypertension treated with immune checkpoint inhibitors. Eur J Cancer. 2022;163:108–118. doi: 10.1016/j.ejca.2021.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang S, Cao MQ, Hou ZY, Gu XY, Chen YZ, Chen L, Luo Y, Chen LW, Liu DM, Zhou HY, et al. Angiotensin-converting enzyme inhibitors have adverse effects in anti-angiogenesis therapy for hepatocellular carcinoma. Cancer Lett. 2021;501:147–161. doi: 10.1016/j.canlet.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 264.Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation. 2011;124:1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230 [DOI] [PubMed] [Google Scholar]
- 265.Beavers CJ, Rodgers JE, Bagnola AJ, Beckie TM, Campia U, Di Palo KE, Okwuosa TM, Przespolewski ER, Dent S, American Heart Association Clinical Pharmacology C, et al. Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e811–e838. doi: 10.1161/CIR.0000000000001056 [DOI] [PubMed] [Google Scholar]
- 266.Jackson AM, Jhund PS, Anand IS, Dungen HD, Lam CSP, Lefkowitz MP, Linssen G, Lund LH, Maggioni AP, Pfeffer MA, et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. European Heart Journal. 2021;42:3741–+. doi: 10.1093/eurheartj/ehab499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 268.Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, Rump LC, Persu A, Basile J, Bloch MJ, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 269.Schmieder RE, Mahfoud F, Mancia G, Azizi M, Bohm M, Dimitriadis K, Kario K, Kroon AA, M DL, Ott C, et al. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39:1733–1741. doi: 10.1097/HJH.0000000000002933 [DOI] [PubMed] [Google Scholar]
- 270.Seruga B, Templeton AJ, Badillo FE, Ocana A, Amir E, Tannock IF. Under-reporting of harm in clinical trials. Lancet Oncol. 2016;17:e209–219. doi: 10.1016/S1470-2045(16)00152-2 [DOI] [PubMed] [Google Scholar]
- 271.Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- 272.Lee DH, Hawk F, Seok K, Gliksman M, Emole J, Rhea IB, Viganego F, Welter-Frost A, Armanious M, Shah B. Association between ibrutinib treatment and hypertension. Heart. 2022;108:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Patel S, Dushenkov A, Jungsuwadee P, Krishnaswami A, Barac A. Team-Based Approach to Management of Hypertension Associated with Angiogenesis Inhibitors. J Cardiovasc Transl Res. 2020;13:463–477. doi: 10.1007/s12265-020-10024-5 [DOI] [PubMed] [Google Scholar]
- 274.Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the Role of Nurses to Improve Hypertension Care and Control Globally. Ann Glob Health. 2016;82:243–253. doi: 10.1016/j.aogh.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 275.Brush JE Jr., Handberg EM, Biga C, Birtcher KK, Bove AA, Casale PN, Clark MG, Garson A Jr., Hines JL, Linderbaum JA, et al. 2015 ACC Health Policy Statement on Cardiovascular Team-Based Care and the Role of Advanced Practice Providers. J Am Coll Cardiol. 2015;65:2118–2136. doi: 10.1016/j.jacc.2015.03.550 [DOI] [PubMed] [Google Scholar]
- 276.Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R, Pison LA, Blaauw Y, Tieleman RG. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–2699. doi: 10.1093/eurheartj/ehs071 [DOI] [PubMed] [Google Scholar]
- 277.Stewart S, Wiley JF, Ball J, Chan YK, Ahamed Y, Thompson DR, Carrington MJ. Impact of Nurse-Led, Multidisciplinary Home-Based Intervention on Event-Free Survival Across the Spectrum of Chronic Heart Disease: Composite Analysis of Health Outcomes in 1226 Patients From 3 Randomized Trials. Circulation. 2016;133:1867–1877. doi: 10.1161/CIRCULATIONAHA.116.020730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC (vol 42, pg 373, 2021). European Heart Journal. 2021;42:4194–4194. doi: 10.1093/eurheartj/ehab648 [DOI] [PubMed] [Google Scholar]
- 279.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:4901. doi: 10.1093/eurheartj/ehab670 [DOI] [PubMed] [Google Scholar]
- 280.Taberna M, Gil Moncayo F, Jane-Salas E, Antonio M, Arribas L, Vilajosana E, Peralvez Torres E, Mesia R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front Oncol. 2020;10:85. doi: 10.3389/fonc.2020.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Berra K, Miller NH, Jennings C. Nurse-based models for cardiovascular disease prevention from research to clinical practice. European Journal of Cardiovascular Nursing. 2011;10:S42–S50. [DOI] [PubMed] [Google Scholar]
- 282.Zamorano J An ESC position paper on cardio-oncology. Eur Heart J. 2016;37:2739–2740. doi: 10.1093/eurheartj/ehw359 [DOI] [PubMed] [Google Scholar]
- 283.Iacopo F, Branch M, Cardinale D, Middeldorp M, Sanders P, Cohen JB, Achirica MC, Jaiswal S, Brown S-A. Preventive Cardio-Oncology: Cardiovascular Disease Prevention in Cancer Patients and Survivors. Current Treatment Options in Cardiovascular Medicine. 2021;23:1–23. [Google Scholar]
- 284.Asnani A, Moslehi JJ, Adhikari BB, Baik AH, Beyer AM, de Boer RA, Ghigo A, Grumbach IM, Jain S, Zhu H, et al. Preclinical Models of Cancer Therapy-Associated Cardiovascular Toxicity: A Scientific Statement From the American Heart Association. Circ Res. 2021;129:e21–e34. doi: 10.1161/RES.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gerber HP, Wu XM, Yu LL, Wiesmann C, Liang XH, Lee CV, Fuh G, Olsson C, Damico L, Xie D, et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. P Natl Acad Sci USA. 2007;104:3478–3483. doi: 10.1073/pnas.0611492104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sionakidis A, McCallum L, Padmanabhan S. Unravelling the tangled web of hypertension and cancer. Clin Sci (Lond). 2021;135:1609–1625. doi: 10.1042/CS20200307 [DOI] [PubMed] [Google Scholar]
- 287.de la Torre P, Pérez-Lorenzo MJ, Alcázar-Garrido Á, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: Lessons from anti-angiogenesis treatments. Molecules. 2020;25:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin Pharmacol Ther. 2017;102:765–776. doi: 10.1002/cpt.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Agarwal M, Thareja N, Benjamin M, Akhondi A, Mitchell GD. Tyrosine Kinase Inhibitor-Induced Hypertension. Curr Oncol Rep. 2018;20:65. doi: 10.1007/s11912-018-0708-8 [DOI] [PubMed] [Google Scholar]
- 290.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ping LY, Ding N, Shi YF, Feng LX, Li J, Liu YL, Lin YF, Shi CZ, Wang X, Pan ZY, et al. The Bruton’s tyrosine kinase inhibitor ibrutinib exerts immunomodulatory effects through regulation of tumor-infiltrating macrophages. Oncotarget. 2017;8:39218–39229. doi: 10.18632/oncotarget.16836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nuver J, Smit A, Sleijfer DT, Van Gessel A, Van Roon A, Van Der Meer J, Van Den Berg M, Burgerhof J, Hoekstra H, Sluiter W. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. European Journal of Cancer. 2004;40:701–706. [DOI] [PubMed] [Google Scholar]
- 293.Patel RP, Parikh R, Gunturu KS, Tariq RZ, Dani SS, Ganatra S, Nohria A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr Oncol Rep. 2021;23:79. doi: 10.1007/s11912-021-01070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Glick M, Baxter C, Lopez D, Mufti K, Sawada S, Lahm T. Releasing the brakes: a case report of pulmonary arterial hypertension induced by immune checkpoint inhibitor therapy. Pulm Circ. 2020;10:2045894020960967. doi: 10.1177/2045894020960967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fournel L, Arrondeau J, Boudou-Rouquette P, Girault F, Revel M-P, Mansuet-Lupo A, Roche N, Damotte D, Goldwasser F, Alifano M. Effect of nivolumab therapy on pulmonary artery. In: American Society of Clinical Oncology; 2017. [Google Scholar]
- 296.Batra A, Patel B, Addison D, Baldassarre LA, Desai N, Weintraub N, Deswal A, Hussain Z, Brown SA, Ganatra S, et al. Cardiovascular safety profile of taxanes and vinca alkaloids: 30 years FDA registry experience. Open Heart. 2021;8. doi: 10.1136/openhrt-2021-001849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Herradon E, Gonzalez C, Gonzalez A, Uranga JA, Lopez-Miranda V. Cardiovascular Toxicity Induced by Chronic Vincristine Treatment. Front Pharmacol. 2021;12:692970. doi: 10.3389/fphar.2021.692970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059–1066. doi: 10.1007/s00467-011-1928-4 [DOI] [PubMed] [Google Scholar]
- 299.Morales JM. Influence of the new immunosuppressive combinations on arterial hypertension after renal transplantation. Kidney Int Suppl. 2002;62:S81–87. doi: 10.1046/j.1523-1755.62.s82.16.x [DOI] [PubMed] [Google Scholar]
- 300.Bursztyn M, Zelig O, Or R, Nagler A. Isradipine for the prevention of cyclosporine-induced hypertension in allogeneic bone marrow transplant recipients: a randomized, double-blind study. Transplantation. 1997;63:1034–1036. doi: 10.1097/00007890-199704150-00025 [DOI] [PubMed] [Google Scholar]
- 301.Zhu XL, Wu SH. Risk of hypertension in cancer patients treated with abiraterone: a meta-analysis. Clin Hypertens. 2019;25:1–9. doi: ARTN 12 10.1186/s40885-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin Genitourin Cancer. 2018;16:e645–e653. doi: 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 303.Osman M, Elkady M. A Prospective Study to Evaluate the Effect of Paclitaxel on Cardiac Ejection Fraction. Breast Care (Basel). 2017;12:255–259. doi: 10.1159/000471759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 305.Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010;23:460–468. doi: 10.1038/ajh.2010.25 [DOI] [PubMed] [Google Scholar]
- 306.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43–9006, Nexavar (R)), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Method Enzymol. 2006;407:597–+. doi: 10.1016/S0076-6879(05)07047-3 [DOI] [PubMed] [Google Scholar]
- 307.White PT, Cohen MS. The discovery and development of sorafenib for the treatment of thyroid cancer. Expert Opin Drug Discov. 2015;10:427–439. doi: 10.1517/17460441.2015.1006194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Funakoshi T, Latif A, Galsky MD. Risk of hypertension in cancer patients treated with sorafenib: an updated systematic review and meta-analysis. J Hum Hypertens. 2013;27:601–611. doi: 10.1038/jhh.2013.30 [DOI] [PubMed] [Google Scholar]
- 309.Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015;126:2366–2369. doi: 10.1182/blood-2015-07-567958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hao Z, Sadek I. Sunitinib: the antiangiogenic effects and beyond. Onco Targets Ther. 2016;9:5495–5505. doi: 10.2147/OTT.S112242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sherbet GV. Therapeutic Potential of Thalidomide and Its Analogues in the Treatment of Cancer. Anticancer Res. 2015;35:5767–5772. [PubMed] [Google Scholar]
- 312.Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2 [DOI] [PubMed] [Google Scholar]
- 313.Deshpande H, Roman S, Thumar J, Sosa JA. Vandetanib (ZD6474) in the Treatment of Medullary Thyroid Cancer. Clin Med Insights Oncol. 2011;5:213–221. doi: 10.4137/CMO.S6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Qi WX, Shen Z, Lin F, Sun YJ, Min DL, Tang LN, He AN, Yao Y. Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol. 2013;75:919–930. doi: 10.1111/j.1365-2125.2012.04417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76:348–357. doi: 10.1111/bcp.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Patel A, Sun W. Ziv-aflibercept in metastatic colorectal cancer. Biologics. 2014;8:13–25. doi: 10.2147/BTT.S39360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Arai H, Battaglin F, Wang J, Lo JH, Soni S, Zhang W, Lenz HJ. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev. 2019;81:101912. doi: 10.1016/j.ctrv.2019.101912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. Journal of Clinical Oncology. 2017;35:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ribatti D, Vacca A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int J Mol Sci. 2018;19:2031. doi: 10.3390/ijms19072031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fala L. Cyramza (Ramucirumab) Approved for the Treatment of Advanced Gastric Cancer and Metastatic Non-Small-Cell Lung Cancer. Am Health Drug Benefits. 2015;8:49–53. [PMC free article] [PubMed] [Google Scholar]
- 322.Roviello G, Pacifico C, Corona P, Generali D. Risk of hypertension with ramucirumab-based therapy in solid tumors: data from a literature based meta-analysis. Invest New Drugs. 2017;35:518–523. doi: 10.1007/s10637-017-0452-1 [DOI] [PubMed] [Google Scholar]
- 323.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K, Xiao Y. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget. 2016;7:44545–44557. doi: 10.18632/oncotarget.10019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Long GV, Stroyakovsky DL, Gogas H, Levchenko E, de Braud F, Larkin JM, Garbe C, Jouary T, Hauschild A, Grob JJ. COMBI-d: a randomized, double-blinded, Phase III study comparing the combination of dabrafenib and trametinib to dabrafenib and trametinib placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAFV600E/K mutation-positive cutaneous melanoma. In: American Society of Clinical Oncology; 2014. [Google Scholar]
- 327.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. New England Journal of Medicine. 2015;372:30–39. doi: 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 328.Long GV, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 329.Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. The lancet oncology. 2016;17:1248–1260. [DOI] [PubMed] [Google Scholar]
- 330.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology. 2018;19:603–615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


