Abstract
Objective
Radical prostatectomy is the recommended treatment for localized prostate cancer; however, it is an invasive procedure that can leave serious morbidity. Robot-assisted radical prostatectomy was introduced with the aim of reducing postoperative morbidity and facilitating rapid recovery compared to the traditional Walsh's open radical retropubic prostatectomy. Therefore, a protocol was developed to perform an open prostatectomy comparable to that performed by robotics, but without involving novel instrumentation.
Methods
A total of 220 patients diagnosed with localized prostate cancer underwent radical prostatectomy. They were divided into two groups: anterograde technique (115 patients) and the retrograde method (105 patients). The study outcomes were observed 3 months after surgery.
Results
No differences were found in terms of surgical time, hospital stay, and suction drainage. However, reduced bleeding was observed in the anterograde technique (p=0.0003), with rapid anastomosis duration (p=0.005). Among the patients, 60.9% undergoing the anterograde technique were continent 3 months after surgery compared to 42.9% treated by the retrograde method (p=0.007). Additionally, fewer complications in terms of the number (p=0.007) and severity (p=0.0006) were observed in the anterograde technique.
Conclusion
The anterograde method displayed increased efficiency in reducing complications, compared to the retrograde technique.
Keywords: Prostatectomy, Localized prostate cancer, Vesicourethral anastomosis, Continence
1. Introduction
Prostate cancer (PCa) is the second most frequent malignancy in adult men and the fifth leading cause of cancer deaths worldwide. Radical prostatectomy (RP) is the recommended treatment for localized PCa; however, it is an invasive procedure that can leave serious morbidity, blood loss, prolonged hospitalization, urinary incontinence, and sexual dysfunction in its wake [1].
Robot-assisted RP (RARP) was introduced with the aim of reducing postoperative morbidity and facilitating rapid recovery compared to the traditional Walsh's open retrograde radical retropubic prostatectomy (ORP) [2,3]. The progression in the RARP technique comprises a modification of the anterograde technique described by Campbell in 1959 [2,[4], [5], [6]].
When studies compare RARP with OPR, they are comparing not only an open surgery with a laparoscopic robotic technique, but also an anterograde with retrograde route [1,[7], [8], [9]]. We hypothesized that if an open prostatectomy was performed in an anterograde way, that would obtain better results than Walsh's ORP [3] even without RARP technology. We developed a technique based on the recommendations of the Pasadena Consensus Panel that critically assesses current surgical technique and generates best practice guidelines to perform robotic anterograde prostatectomy [2]. The technique was developed to reproduce robotic prostatectomy by an open procedure without the employment of novel instruments. This was termed the “open anterograde anatomic radical retropubic prostatectomy (AORP)” [2,10,11].
Thus, we carried out a pilot study to evaluate this technique with ten patients and obtained good results, which motivated us to carry out a prospective, controlled, randomized trial comparing AORP with ORP [10,11].
The aim of the present study was to evaluate the surgical and early postoperative results of 220 patients as part of a trial by an open procedure “open anterograde radical prostatectomy vs. retrograde technique”.
2. Patients and methods
Two hundred and forty patients with localized PCa confirmed by biopsy and indication for nerve-sparing prostatectomy were selected at the urology service of Pedro Ernesto University Hospital of the State University of Rio de Janeiro, Brazil.
To calculate the sample size, it was assumed, without loss of generality, that the difference between the groups is not large (effect size about 0.20). According to the G∗Power program version 3.1.9.2 (Heinrich-Heine-University Dusseldorf 2014, Germany), the minimum number of patients is 99 patients for each group. Assuming a drop out percentage of up to 20%, we reached 240 initial patients for the study. Patients were randomized into two arms according to the kind of technique, and in three sets according to the three different main surgeons. Randomization was performed by software on the site “www.randomizer.org/” on November 17, 2015. The first patient underwent surgery on March 1, 2016, and the last on February 27, 2019.
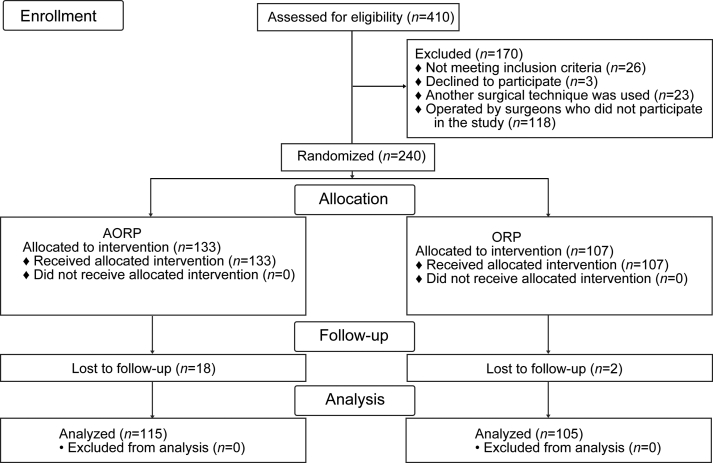
Inclusion criteria were clinically localized PCa in adults over 18 years of age and estimated life expectancy of 10 years or more. Exclusion criteria were evidence of clinically non-localized PCa, prostate-specific antigen (PSA) >20 ng/mL, previous pelvic radiotherapy or extensive surgery, and malignancy within the past 5 years (except non-melanoma skin cancer). Among these 240 patients, 220 completed the 3-month follow-up: one hundred and fifteen underwent AORP, and 105 underwent ORP. Twenty patients were excluded due to failed randomization, loss of follow-up, and withdrawal of informed consent (Fig. 1).
Figure 1.
Consort flow diagram. AORP, open anterograde anatomic radical retropubic prostatectomy; ORP, open retrograde radical retropubic prostatectomy.
Ethical approval was obtained from the Research Ethics Committee of the State University of Rio de Janeiro in November 2015 (Number 1.335.683), and informed consents were taken from all individual participants prior to enrolling them in the study. The present study was registered in Plataforma Brasil CAAE:41908815.9.0000.5259 and in ClinicalTrials.gov identifier (NCT02687308).
According to the State University of Rio de Janeiro urologic service provision routine for this kind of surgery, the patients were operated on by second-year residents of the urological program, aided by one of our three expert surgeons (Carrerette FB, Filho RTF, and Lara CC) that participated in the study. Each of them had over 10 years of experience and more than 200 prostatectomies performed. Anesthesia for all the procedures consisted of a spinal block with or without venous sedatives.
The essential AORP surgical steps are in accordance with the recommendations of the 2012 Pasadena Consensus Panel for Robotic Surgery [2]. These were modified by the first author of this paper (Carrerette FB) to adapt to open retropubic surgery: anterograde dissection with ligation of the dorsal vascular complex without division, preservation of the bladder neck, anterograde nerve sparing, preservation of the posterior layer of Denonvilliers’ fascia remaining on the rectum and the abdominal urethra, as well as the vesicourethral anastomosis with a running suture. Surgical technique of AORP was described in detail in previous publications [10,11].
Primary outcomes were monitored by evaluations up to 3 months following surgery and focused on three outcome groups:
-
(1)
Technical aspects: time to complete surgery and vesicourethral anastomosis; estimated blood loss; percentage of nerve sparing perceived by the surgeon; intra- and post-operative complications; length of hospital stay; drainage and duration of urethral catheter requirement.
-
(2)
Oncological outcomes: positive surgical margin (PSM) status and biochemical control; serum PSA measurement to evaluate biochemical recurrence (BCR), as per the trial protocol as a PSA of 0.2 ng/mL or higher 90 days after surgery.
-
(3)
Functional control was evaluated by urinary control and sexual outcomes. Urine leakage was assessed based on clinical history regarding involuntary urinary loss and pad use, following the removal of the urinary catheter at 1 month and 3 months postoperatively. Patients were considered continent if they answered that they never leaked urine and required no pads. Erectile function recovery was defined as the ability to achieve penetration and maintain a significant erection, according to the International Index of Erectile Function-5 survey, 3 months after surgery.
All patients were evaluated in postoperative consultations, AORP group at 7 days and ORP at 14 days, if there had been no complications that prevented for the removal of the urethral catheter or skin stitches. During subsequent follow-ups at 1 month and 3 months, patients were evaluated for serum PSA concentration, urinary continence, sexual function, and treated for surgical or medical complications.
Categorical variables were expressed as number and percentage, and continuous variables were indicated as median and interquartile range. Patient demographics and preoperative and perioperative outcomes were analyzed using the Mann-Whitney U test. The Chi-square or Fisher's exact test were used to analyze the surgical and postoperative outcomes and complications, BCR, the continence rates, and the erectile function recovery rates at 3 months of follow-up. SPSS version 22 (SPSS Inc., IBM, Armonk, NY, USA) software was used for all analyses. Bivariate and multivariate analyzes were performed, according to binary logistic regression, to identify possible predictors related to urinary continence.
3. Results
3.1. Patients' characteristics
The two groups, ORP and AORP, were homogeneous, and no significant differences were found between them in terms of the patients' demographic data and disease features, such as age, prostate volume, preoperative PSA, and the biopsy Gleason and D'Amico risk classifications (Table 1).
Table 1.
Preoperative characteristics of cases in ORP group and AORP group.
| Characteristic | ORP (n=105) | AORP (n=115) | p-Value |
|---|---|---|---|
| Age, median (IQR), year | 64 (60–68) | 64 (59–68) | 0.90a |
| PSA, median (IQR), ng/mL | 8.43 (6.33–11.90) | 8.70 (5.92–12.30) | 0.57a |
| Prostate, median (IQR), cm3 | 41 (32–54) | 40 (31–48) | 0.29a |
| Biopsy Gleason score, n (%) | 0.48b | ||
| 6 | 43 (41.0) | 38 (33.0) | |
| 7 | 53 (50.5) | 66 (57.4) | |
| 8 or 9 | 9 (8.6) | 11 (9.6) | |
| D'Amico, n (%) | 0.44b | ||
| Low | 20 (19.0) | 19 (16.5) | |
| Intermediate | 73 (69.5) | 76 (66.1) | |
| High | 12 (11.4) | 20 (17.4) |
PSA, prostate-specific antigen; ORP, open retrograde radical retropubic prostatectomy; AORP, open anterograde anatomic radical retropubic prostatectomy; IQR, interquartile range.
Mann-Whitney U test.
Chi-square or Fisher's exact test.
3.2. Perioperative and postoperative outcomes and complications
Although both procedures were completed as per protocol and had identical duration, certain technical aspects of operative parameters were found significantly better in AORP. In particular, the median estimated blood loss was lower in AORP (p=0.0003), with a urethrovesical anastomosis that was significantly more rapid (p=0.005). The surgeon's perception of the nerve-sparing occurred in 101 (87.8%) and 71 (67.6%) members of the AORP and ORP groups, respectively (p=0.0009). The indwelling vesical catheterization was required for a shorter duration in the AORP group (p<0.0001). Complications occurred more frequently (p=0.007) and severely (Clavien-Dindo grades II and III; p=0.0006) in patients in the ORP group compared to AORP (Table 2).
Table 2.
Perioperative and postoperative outcomes and complications, oncological and functional control in ORP group and AORP group.
| Characteristic | ORP (n=105) | AORP (n=115) | p-Value |
|---|---|---|---|
| Operative time median (IQR), min | 150 (120–180) | 140 (120–150) | 0.14a |
| Anastomosis time, median (IQR), min | 25 (20–30) | 20 (15–30) | 0.005a |
| Estimated blood loss, median (IQR), mL | 500 (300–600) | 300 (200–500) | 0.0003a |
| Hospitalization, median (IQR), day | 3 (3–4) | 3 (3–4) | 0.16a |
| Days with drain, median (IQR) | 3 (2–4) | 3 (2–4) | 0.09a |
| Days with indwelling bladder catheter, median (IQR) | 14 (14–15) | 7 (7–7) | <0.0001a |
| Nerve sparing, n (%) | 0.0009b | ||
| Absent | 34 (32.4) | 14 (12.2) | |
| Unilateral | 46 (43.8) | 59 (51.3) | |
| Bilateral | 25 (23.8) | 42 (36.5) | |
| Surgical complication, n (%) | 0.007b | ||
| Absent | 76 (72.4) | 100 (87.0) | |
| Present | 29 (27.6) | 15 (13.0) | |
| Clavien-Dindo classification, n (%) | 0.0006b | ||
| Grade I | 2 (1.9) | 7 (6.1) | |
| Grade II | 18 (17.1) | 3 (2.6) | |
| Grade III | 9 (8.6) | 5 (4.3) | |
| Gleason score, n (%) | 0.11b | ||
| 6 | 15 (14.3) | 22 (19.1) | |
| 7 | 79 (75.2) | 72 (62.6) | |
| 8 or 9 | 11 (10.5) | 21 (18.3) | |
| Pathologic stage, n (%) | 0.22b | ||
| pT2a | 4 (3.8) | 11 (9.6) | |
| pT2b | 12 (11.4) | 17 (14.8) | |
| pT2c | 68 (64.8) | 60 (52.2) | |
| pT3a | 7 (6.7) | 13 (11.3) | |
| pT3b | 14 (13.3) | 14 (12.2) | |
| Lymph node, n (%) | 0.28b | ||
| Negative | 65 (98.5) | 82 (95.3) | |
| Positive | 1 (1.5) | 4 (4.7) | |
| Surgical margin, n (%) | |||
| T2 negative | 64 (76.2) | 66 (75.0) | 0.85b |
| T2 positive | 20 (23.8) | 22 (25.0) | |
| T3 negative | 12 (57.1) | 19 (70.4) | 0.34b |
| T3 positive | 9 (42.9) | 8 (29.6) | |
| Biochemical recurrence, n (%) | 0.69b | ||
| Negative | 94 (89.5) | 101 (87.8) | |
| Positive | 11 (10.5) | 14 (12.2) | |
| Continence, n (%) | 0.007b | ||
| Absent | 60 (57.1) | 45 (39.1) | |
| Present | 45 (42.9) | 70 (60.9) | |
| Sexual potency, n (%) | 0.34b | ||
| Absent | 94 (89.5) | 98 (85.2) | |
| Present | 11 (10.5) | 17 (14.8) |
IQR, interquartile range; ORP, open retrograde radical retropubic prostatectomy; AORP, open anterograde anatomic radical retropubic prostatectomy.
Mann-Whitney U test.
Chi-square or Fisher's exact test.
3.3. Perioperative and postoperative oncological control
No intergroup differences were observed with respect to cancer control, pathological stage, Gleason score, or lymph node involvement. The PSM rates in T2 (p=0.85), PSM rates in T3 (p=0.34), and 3-month BCR (p=0.69) were very similar in the two groups (Table 2).
3.4. Perioperative and postoperative functional control
A larger number of patients achieved early continence through the AORP compared to ORP technique; in the bivariate analysis only AORP was a predictor of urinary continence (relative risk [RR]=2.07; p=0.007). Multivariate logistic regression showed that only AORP (RR=1.91; p=0.023) was an independent predictor of urinary continence. In both methods, other variables showed no significant individual relationship. Recovery of sexual potency was observed in 14.8% of patients who underwent AORP against 10.5% with ORP, but this was not statistically significant (p=0.34) (Table 2).
4. Discussion
The present study demonstrates the feasibility of performing AORP without the increased costs of novel technology. Literature has been published regarding anterograde RP; however, the step-by-step procedure following the robotic technique recommended by the 2012 Pasadena Consensus Panel remains to be described [2,[12], [13], [14]]. Our study was the first to employ and demonstrate this open anterograde prostatectomy technique [10,11]. The AORP surgical time was similar to the time required for the ORP technique, and lower than the time described in the literature for video laparoscopic and robotic surgery [15].
Nevertheless, the AORP displayed improvement in certain aspects over ORP, maintaining the same oncological control, which were only found in studies comparing RARP with ORP: reduced total blood loss, rapid anastomosis, reduced duration of indwelling vesical catheterization, diminished number and severity of complications, and earlier achievement of urinary continence [1,[7], [8], [9],16].
Reduced bleeding can become a significant factor in RP; it may also facilitate an enhanced dissection technique by improving the visualization of structures and anatomical planes. The estimated total blood loss in robotic and laparoscopic surgery had a significantly reduced contrasted with open surgery [1]. We found similar results that the AORP had a significantly reduced blood loss contrasted with ORP. Therefore, decreased bleeding is suspected to be related to the dissection technique rather than technological progress.
Greater surgeon perception of nerve preservation occurred in AORP compared to ORP (87.8% vs. 67.6%; p=0.0009), which reinforces the technical superiority of anterograde dissection. The AORP procedure is initiated in the bladder neck, placing the prostate in front of the dissection plane and not between it and the surgeon; this avoids the dorsal vein plexus manipulation, incision of the endopelvic fascia, and the division of the puboprostatic ligament, preservation of structures adjoining the urethral sphincter aids in nerve preservation, in addition to reduction in bleeding; much of hemorrhage during surgery is from the plexus of the dorsal vein, the urethra, and the endopelvic fascia [17].
Vesicourethral anastomosis with an impermeable running suture, after ensuring precise approximation of the preserved bladder neck and urethra, is a crucial step in RP; it has a direct implication on the duration of indwelling vesical catheterization, possibly affecting the timely recovery of continence [8,9,16,[18], [19], [20], [21], [22], [23]]. An elevated rate of early continence was observed in the present study following the removal of the indwelling catheter, within 3 months in 60.9% of patients who underwent AORP compared to 42.9% in patients treated by ORP (p=0.007).
Urinary continence is a major marker of surgical success; it is one of the most critical factors affecting the patient's quality of life, even more than erectile function [2]. These improvements in recovery-related outcomes may be due to technical aspects. Initiating prostate dissection through the anterograde approach facilitates the preservation of the bladder neck, the nerve bundle, and Denonvilliers' fascia, prevents possible injury to nerve fibers, and creates a structural bed to support vesicourethral anastomosis. This technique facilitates the dissection of the apex of the prostate by preserving the nerve bundle adjoining the urethra; an enhanced extension of the abdominal urethra facilitates the hermetic Van Velthoven running suture anastomosis technique [1,10,11,[17], [18], [19],23].
Studies have demonstrated the superiority of the continuous suture used in AORP compared to the interrupted technique in ORP, maintaining identical completion time; this reflects the viability of the technique, as well as improvement in the functional results, such as reduced catheterization time and improved earlier continence [[19], [20], [21], [22], [23]]. The present study proved that AORP running suture is better than interrupted suture of the ORP technique and demonstrated the ease of performance and the negligible complications, reflecting how quickly it is learnt. Complications such as urinary retention requiring vesical catheterization and bladder neck stenosis were less frequent in AORP.
Two systematic reviews have demonstrated improved continence outcomes among patients who underwent RARP compared to ORP [1,7]. Ficarra et al. [9] reported a 97% continence rate at the 1-year follow-up in the RARP group compared to 88% with ORP; the study also observed earlier continence in the RARP group, 25 days versus 75 days in the retropubic RP group.
Several studies have also attempted developing improved methods to preserve continence following RP; however, we are not sure which factors are responsible for improving this outcome in RP, which appears to be multifactorial [3,[19], [20], [21], [22], [23], [24]]. However, anterograde dissection with bladder neck preservation, nerve sparing, non-opening of the endopelvic fascia, preservation of the abdominal urethra, and vesicourethral anastomosis with an impermeable running suture may all be factors that improve early continence recovery after RP, regardless of whether it is done by robotics or by open method.
We observed a similar proportion of PSM for both techniques (26.1% and 27.6% for AORP and ORP, respectively) and no significant association between the techniques and PSM (p=0.85 for T2 stage and p=0.34 for T3 stage). These results are consistent with literature; in a systematic review and meta-analysis, the overall PSM rate of RARP and laparoscopy RP was 22.3% (1098 of 4929 cases), similar to ORP, where it was 28.6% (965 of 3370 cases) [1]. In a systematic review and meta-analysis, Tewari et al. [24] compared retropubic, laparoscopic, and robotic prostatectomies; the overall PSM rates were demonstrated to be 24.2% for ORP, 20.4% for laparoscopy RP, and 16.2% for RARP. In terms of BCR an elevated rate was demonstrated in the study (10.5% and 12.25%, in AORP and ORP, respectively); however, no differences were observed between the two techniques. These values are similar to those found in the systematic review and meta-analysis with evaluation 3 months after surgery, ranging from 2.7% to 12.0% [1]. PSM and BCR rates for RP vary with the surgeon's experience, as well as cancer stage, prostate volume, and grade. The hypothesis for this high index is accounted for by the fact that the surgeries were performed by residents with less surgical experience and the most of patients had more extensive disease.
The limitation of this study was that the surgeries were performed by residents; there were 12 surgeons with low surgical volume and low frequency of surgery over a long period of 3 years. However, this reflects the reality of the study location and does not affect the methodology, since the same surgeons performed both types of surgery and with a balanced number of procedures among them.
5. Conclusion
AORP was superior to ORP in critical parameters, such as estimated blood loss, urethrovesical anastomosis, shorter duration of indwelling vesical catheterization, nerve sparing, and urinary continence; these resulted in fewer complications with similar oncological control. This allows patients without access to robotic technology to be similarly operated with an improved procedure. Furthermore, the AORP method was reproducible by low-volume PCa surgeons; therefore, it may assist inexperienced surgeons to develop valuable skills for future training with robotic techniques.
Author contributions
Study concept and design: Fabricio B. Carrerette, Ronaldo Damião.
Data acquisition: Fabricio B. Carrerette, Rui T. F. Filho, Celso C. Lara.
Data analysis: Fabricio B. Carrerette, Daniela B. Rodeiro, Rui T. F. Filho, Paulo A. Santos, Ronaldo Damião.
Drafting of manuscript: Fabricio B. Carrerette, Daniela B. Rodeiro, Rui T. F. Filho, Paulo A. Santos, Ronaldo Damião.
Critical revision of the manuscript: Fabricio B. Carrerette, Daniela B. Rodeiro, Rui T. F. Filho, Paulo A. Santos, Ronaldo Damião.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Cao L., Yang Z., Qi L., Chen M. Robot-assisted and laparoscopic vs. open radical prostatectomy in clinically localized prostate cancer: perioperative, functional, and oncological outcomes: a systematic review and meta-analysis. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montorsi F., Wilson T.G., Rosen R.C., Ahlering T.E., Artibani W., Carroll P.R., et al. Best practices in robot-assisted radical prostatectomy: recommendations of the Pasadena Consensus Panel. Eur Urol. 2012;62:368–381. doi: 10.1016/j.eururo.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Walsh P.C., Lepor H., Eggleston J.C. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–485. doi: 10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 4.Guazzoni G., Cestari A., Naspro R., Riva M., Centemero A., Zanoni M., et al. Intra- and peri-operative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol. 2006;50:98–104. doi: 10.1016/j.eururo.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay C., Pickard R., Robertson C., Close A., Vale L., Armstrong N., et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16:1–313. doi: 10.3310/hta16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell E.W. Total prostatectomy with preliminary ligation of the vascular pedicles. J Urol. 1959;81:464–467. doi: 10.1016/S0022-5347(17)66044-0. [DOI] [PubMed] [Google Scholar]
- 7.Ilic D., Evans S.M., Allan C.A., Jung J.H., Murphy D., Frydenberg M. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev. 2017;9:CD009625. doi: 10.1002/14651858.CD009625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 9.Ficarra V., Novara G., Fracalanza S., D'Elia C., Secco S., Iafrate M., et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–539. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 10.Carrerette F.B., Damião R., da Silva E.A., Figueiredo R.T., Lara C.C., Perroni F., et al. Description of the open anterograde anatomic radical retropubic prostatectomy technique. Surg Curr Res. 2017;7 doi: 10.4172/2161-1076.1000304. [DOI] [Google Scholar]
- 11.Carrerette F.B., Carvalho E., Machado H., Freire F.C., Damião R. Open anterograde anatomic radical retropubic prostatectomy technique: description of the first fifty-five procedures. Int Braz J Urol. 2019;45:1071–1072. doi: 10.1590/S1677-5538.IBJU.2018.0421. [DOI] [PubMed] [Google Scholar]
- 12.Sciarra A., Cristini C., Von Heland M., Salciccia S., Gentile V. Randomized trial comparing an anterograde versus a retrograde approach to open radical prostatectomy: results in terms of positive margin rate. Can Urol Assoc J. 2010;4:192–198. doi: 10.5489/cuaj.09089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciarra A., Gentile V., De Matteis A., Dattilo C., Autran Gomez A.M., Salciccia S., et al. Long-term experience with an anatomical anterograde approach to radical prostatectomy: results in terms of positive margin rate. Urol Int. 2008;80:151–156. doi: 10.1159/000112605. [DOI] [PubMed] [Google Scholar]
- 14.Kwon S.Y., Lee J.N., Ha Y.S., Choi S.H., Kim T.H., Kwon T.G. Open radical prostatectomy reproducing robot-assisted radical prostatectomy: involving antegrade nerve sparing and continuous anastomosis. IBJU. 2017;43:1043–1051. doi: 10.1590/S1677-5538.IBJU.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frota R., Turna B., Barros R., Gill I.S. Comparison of radical prostatectomy techniques: open, laparoscopic and robotic assisted. Int Braz J Urol. 2008;34:259–269. doi: 10.1590/s1677-55382008000300002. [DOI] [PubMed] [Google Scholar]
- 16.Lowrance W.T., Tarin T.V., Shariat S.F. Evidence-based comparison of robotic and open radical prostatectomy. ScientificWorldJournal. 2010;10:2228–2237. doi: 10.1100/tsw.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tewari A., Takenaka A., Mtui E., Horninger W., Peschel R., Bartsch G., et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006;98:314–323. doi: 10.1111/j.1464-410X.2006.06266.x. [DOI] [PubMed] [Google Scholar]
- 18.Brunocilla E., Pultrone C., Pernetti R., Schiavina R., Martorana G. Preservation of the smooth muscular internal (vesical) sphincter and of the proximal urethra during retropubic radical prostatectomy: description of the technique. Int J Urol. 2012;19:783–785. doi: 10.1111/j.1442-2042.2012.03028.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Velthoven R.F., Ahlering T.E., Peltier A., Skarecky D.W., Clayman R.V. Technique for laparoscopic running urethrovesical anastomosis: the single knot method. Urology. 2003;61:699–702. doi: 10.1016/s0090-4295(02)02543-8. [DOI] [PubMed] [Google Scholar]
- 20.Ozu C., Hagiuda J., Nakagami Y., Hamada R., Horiguchi Y., Yoshioka K., et al. Radical retropubic prostatectomy with running vesicourethral anastomosis and early catheter removal: our experience. Int J Urol. 2009;16:487–492. doi: 10.1111/j.1442-2042.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsuyama H., Matsumoto H., Nagao K., Harada N., Hara T., Sakano S. Running suture versus interrupted suture for vesicourethral anastomosis in retropubic radical prostatectomy: a randomized study. Int J Urol. 2015;22:271–277. doi: 10.1111/iju.12667. [DOI] [PubMed] [Google Scholar]
- 22.Lim J.H., Park C.M., Kim H.K., Park J.Y. Comparison of perioperative outcomes between running versus interrupted vesicourethral anastomosis in open radical prostatectomy: a single-surgeon experience. Kor J Urol. 2015;56:443–448. doi: 10.4111/kju.2015.56.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridhar A.N., Abozaid M., Rajan P., Sooriakumaran P., Shaw G., Nathan S., et al. Surgical techniques to optimize early urinary continence recovery post robot assisted radical prostatectomy for prostate cancer. Curr Urol Rep. 2017;18:71. doi: 10.1007/s11934-017-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewari A., Sooriakumaran P., Bloch D.A., Seshadri-Kreaden U., Hebert A.E., Wiklund P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]