Abstract
Introduction
Ailanthus altissima is an indigenous plant known for various remedial properties. The present study aimed to evaluate the neuroprotective potential of methanolic extract Ailanthus altissima (AA) bark as current scientific trend is searching plant for neurodegenerative diseases, worldwide.
Methodology
In in-vitro experiments, the AA was analyzed for phenols, flavonoids, antioxidative and cholinesterase inhibitory properties with subsequent detailed characterization for secondary metabolites. The in-vivo neurological effects were evaluated in rats through behavioral assessment for anxiety and memory after chronic administration (28 days) of 50–200 mg/kg of AA. At the end of behavior studies, isolated brains were biochemically tested to determine antioxidant enzyme activity.
Results
AA was found rich in phenols/flavonoids and active in radical scavenging with the presence of 13 secondary metabolites in UHPLC-MS analysis. The AA yielded anxiolytic effects dose-dependently in the open field, light/dark and elevated-plus maze tests as animals significantly (P < 0.05 vs control group) preferred open arena, illuminated zone and exposed arms of maze. Similarly, the animals treated with AA showed significant (P < 0.05 vs amnesic group) increase in spontaneous alternation, discrimination index in y-maze, novel object recognition tests. Further, AA.Cr treated rats showed noticeably shorter escape latencies in Morris water maze tests.In biochemical analysis, the dissected brains AA treated rats showed reduced levels of AChE and malondialdehyde with increased levels of first-line antioxidant enzymes i.e. glutathione peroxidase and superoxide dismutase. These observed biological effects might be attributed to phenols and flavonoids constituents owned by AA. -The in-silico studies showed thatconessine and lophirone J phytocompounds have good blood–brain barrier permeability and interaction with AChE.
Conclusion
The outcomes of this study validate that bark of Ailanthus altissima might work as a source of bioactive phytochemicals of neuroprotective potential.
Keywords: Ailanthus altissima, Memory, Anxiety, Open field test, Morris water Maze
1. Introduction
With the increasing life expectancy, various neurological ailments especially memory disorders are becoming dominant in elderly people (Tarawneh and Holtzman, 2012). Memory is the individual’s capability to retain information from certain stimuli or events for a shorter or longer duration and to recall that information when needed. Dementia is considered an epidemic as approximately the number of sufferers has crossed 46.8 million worldwide and the number is predicted to get double every 20 years (Arvanitakis et al., 2019). The growing socioeconomic burden resulting from the increasing prevalence of this disease appeals to a huge scientific investment to look for better therapeutic options (Lleo, 2007). Alzheimer's disease (AD), the topmost dementia precipitator, involves neuronal loss and memory impairment as gradually progressing irreversible deterioration of cognitive function affects memory, language, and problem-solving abilities (Miedel et al., 2017). There is sufficient evidence from preclinical, clinical and post-mortem studies reporting the association of reduced acetylcholine levels with intellectual disability, diverting the researcher’s attention to developing some novel prophylactic and therapeutic options capable to tackle central cholinergic impairments (John et al., 2022).
A broad range of medicinally active compounds of plant origin is globally popular, especially in low-income countries that provide the justification to look forward to novel therapeutic options from natural resources (Anand et al., 2019). Anticholinesterases are the drugs used to treat dementia in the early stages. In the nucleus basalis of Meynert, deterioration of cholinergic neurons contributes to impaired cognition in AD (Mufson et al., 2008). By inhibiting the acetylcholinesterase (AChE), prolongation of acetylcholine (Ach) activity takes place which plays a role in treating dementia (Griffin et al., 2003). A naturally found alkaloid, Galantamine obtained from Galanthus species, is an anticholinesterase drug presently prescribed for AD (Sharifi-Rad et al., 2020). Naringin and crocin are few other secondary metabolites reported to restore the cognitive deficit by inhibiting the AChE and increasing the cholinergic neurotransmission in the rodent AD model (Costa et al., 2016, Zang et al., 2018).
Globally, the experimentation with plants for natural and novel therapeutic agents is attracting researcher’s attention. Ailanthus altissima, unanimously called the tree of heaven, is known as an ornamental plant belonging to the family Simaroubaceae. This multipurpose plant is distributed in Japan and China, but has been introduced and cultivated successfully throughout the world (Albouchi et al., 2013, Albright et al., 2010). Beyond ornamental purposes, the plant has been known for its medicinal benefits in various gastrointestinal ailments (Rashid et al., 2015). Rashed et al. worked on the bark extracts of Ailanthus altissima and reported its dose-dependent cytoprotective and antiulcerogenic effects in mice (Rashed et al., 2012). Jin et al. reported that the methanolic extract of its leaves exerted inhibitory effects on cyclooxygenases and lipoxygenases in mouse bone marrow-derived mast cells suggesting the anti-inflammatory potential (Jin et al., 2006). The leaves of Ailanthus altissima have been reported to own phytoconstituents with antioxidant, antimicrobial and phytotoxic properties. The chemical characterization of Ailanthus altissima reveals the presence of hundreds of chemical constituents including and alkaloids with the basic structure of indole, β-carboline, canthine 6-one-3 N-oxide and Canthine-6-one (Kim et al., 2016).
The bark of Ailanthus altissima is reported to possess a plethora of structurally diverse chemical compounds comprising 32 alkaloids, 40 quassinoids, 103 phenylpropanoids, 23 triterpenes. Beside these, the presence of fatty acids, olefins, and esters have also been reported (Li et al., 2021). The bark of the plant has been anciently used for multiple benefits in ophthalmic diseases, bleeding, asthma and spermatorrhea (Kang et al., 2010, Kim et al., 2016, Yang et al., 2014). However, the dearth of evidence about its secondary metabolites and their neuropharmacological potential emphasized designing the current study. The present study aims to provide scientific evidence of the neuropharmacological importance of Ailanthus altissima by analyzing the aqueous-methanolic extract of its bark in a set of in-vitro phytochemical analysis and in-vivo neurobehavioral experiments further supported with biochemical and molecular docking studies.
2. Materials and methods
2.1. Chemicals
The drugs and chemicals of research grade were used in this study. Scopolamine hydrobromide trihydrate (S1875) and Piracetam (P5295) were purchased from Sigma-Aldrich and Diazepam (F1126) was procured from Roche Pharma. These drugs were dispensed freshly on the day of the experiment.
2.2. Preparation of plant extract
After gathering the fresh bark of Ailanthus altissima from the premises of Bahauddin Zakariya University, Mulan, Pakistan, the voucher (R.R. Stewart 607) was allotted after identification and verification by a skilled taxonomist. The whole collected bark was passed through shade-dried, coarse grinding and maceration (70 % methanol). The macerate was shaken intermittently for seven days and filtrated. The filtrate was evaporated on a rotary evaporator unless a product of semisolid consistency (AA) is yielded.
2.3. In-vitro studies
2.3.1. Total phenols and flavonoids
The AA was examined for total phenols (TP) by using the Folin-Ciocalteau method (Chandra et al., 2014). The 0.5 ml of AA or standard gallic acid(398225; Sigma Aldrich) was mixed with 0.1 ml of Folin-Ciocalteau reagent (0.5 N) (39520; Sisco research Lab). 2.5 ml of sodium carbonate (13419; Sigma Aldrich) was added to this solution and incubated for 0.5 h. The absorbance of this mixture was measured at 760 nm and the standard curve method was used to determine the TP, expressed as mg gallic acid equivalents (GAE) per gram of the extract.
The aluminum chloride method was used to analyze total flavonoids (TF) (Javaid et al., 2021). The 1 mg/ml of plant extract was mixed with 1 ml of 10 % AlCl3 (801081; Merck). After incubation for 30 min, the absorbance was checked at 415 nm and results were expressed as mg quercetin equivalent (QE) unit per gram of the extract.
2.3.2. Antioxidant activity in DPPH method
The AA was tested for antioxidant potential by using DPPH (2,2-diphenyl-1-picrylhydrazyl) method (Rabelo et al., 2018). 0.125 ml of DPPH (D4313; TCI ltd.) and 0.375 ml of methanol were mixed with 0.5 ml of AA used in the concentration of 0, 100, 200, 400, 800 and 1000 µg/mL and every mixture was incubated for 0.5 h. The absorbance was noted at 517 nm using ascorbic acid (A4403; Sigma Aldrich) as a positive control.
2.3.3. Antioxidant activity in DPPH method
The antioxidant potential of the test extract was further validated by another method (Adebiyi et al., 2017). The solutions comprising 7 mmol/L of ABTS (21530042–1; Bioplus chemicals) and 2.45 mmol/L of potassium persulfate (216224; Sigma Aldrich) were prepared. Both solutions in equal quantities were incubated together for 16 h in darkness. The resulting reaction solution was further diluted before use. 195 µL of this solution was mixed with 5 µL of extract in the concentration of 0, 100, 200, 400, 800 and 1000 µg/mL or standard i.e. Ascorbic acid solutions and incubated for 5 min at 25 °C. Absorbance at 734 nm was noted taking 95 % of ethanol as a blank by a 96-well microplate reader. The radical scavenging potential of the plant was calculated by the given formula and outcomes were presented as IC50:
2.3.4. Anticholinesterase (AChE) activity
The 25–400 µL of plant extract, 20 µL of 10 mM DTNB and 10 µL of 6.67 U/ml AChE were mixed in 1710 µL of 50 mM Tris-HCL buffer (pH 8.0) (17–1212-500; BioEditing). After that, 10 µL of 200 mM acetylthiocholine chloride (ATC) (01480; Sigma Aldrich) was added to the reaction and absorbance was determined at 412 nm by employing galantamine as a positive control (Ellman et al., 1961). Any change in absorbance was monitored for 5 min. The % inhibition of AChE was evaluated for every concentration by using the following formula and outcomes were presented as IC50:
2.3.5. Secondary metabolites analysis by UHPLC-MS
The AA was examined for secondary metabolites through ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) analysis. For this study, Agilent 1290 Infinity LC system along with Agilent 6520 Accurate-Mass Q-TOF mass spectrometer with dual ESI source (Agilent technology, USA) was used. The column used for UHPLC analysis was Agilent Zorbax Eclipse XDB-C18, narrow-bore 2.1 × 150 mm, 3.5 µm. The temperature was regulated at 25 °C for the column while at 4 °C for auto-sampler. The 1.0 µL of the sample was injected and its flow was maintained at 0.5 ml/minute. The sample run time was 25 min and the post-run time was 5 min. The mobile phases used in the analysis were formic acid (0.1 %) (A) and in acetonitrile (B). Full scan MS analysis was done using negative electrospray ionization mode, over a range of m/z 100–1000. Nitrogen was supplied at flow rates of 25 and 600 L/hour as nebulizing and drying gas, respectively and the drying gas temperature was kept at 350 °C. The capillary voltage was set to 3500 V while the fragmentation voltage was optimized to 125 V. Obtained data was examined through Agilent Mass Hunter software (B.05.00) for qualitative analysis (Method: Metabolomics-2017–00004.m) and the detected compounds were identified through Search Database (METLIN_AM_PCDL-N- 170502.cdb).
2.4. In-vivo studies
2.4.1. Animals
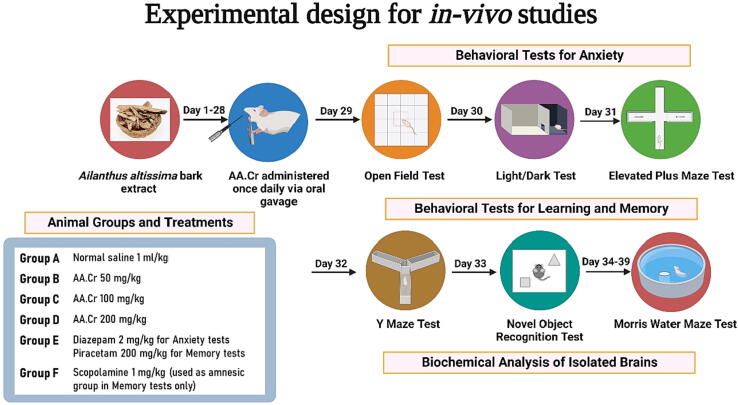
The male Sprague Dawley rats of 6–8 weeks and weighing 200–300 g were purchased from the National Institute of Health, Islamabad, Pakistan and later inbred at the animal house of Faculty of Pharmacy, B.Z.U, Multan. The hygienic housing conditions were regulated at 25 °C and rats were fed with a rodent diet and water. The ethical permission for the study was granted by the Departmental Ethical Committee of B.Z.U Multan vide letter number 09/PEC/2015. All adopted procedures were in agreement with the guidelines directed by the Institute of Laboratory Animal Resources (ILAR), a directive on Life Sciences, National Research Council (NRC, 1996). Before behavioral experimentation, the animals were familiarized with the experimenter’s handling for 2–4 days. All behavior experiments were arranged in a day-wise series from the least aversive to the most aversive set of experiments and carried out during the daytime. To eliminate the risk of biases in experimental design, the behavioral experiments were analyzed by an individual who was kept unaware of the respective treatments. Further, all behavioral test trials were video recorded by Logitech camera and evaluated by ANY-maze software version 6.1, Stoelting Co., Wood Dale, IL, USA.
2.4.2. Grouping of animals
The randomly chosen rats were separated into six groups (n = 8). The animals of Group A were designated as healthy control and administered with normal saline (1 ml/kg) once daily. The animals of Groups B, C and D received a dose of AA 50 (Group B), 100 (Group C) and 200 mg/kg (Group D). Animals of A,B,C and D groups were treated with a once-daily dose of their respective treatments through oral gavage for 28 consecutive days before commencement of the first behavioral experiment (open field test) and these treatments were continued till completion of behavioral tests and extraction of brains for biochemical assays.
During the behavioral experiments for anxiety, another set of animals, Group E, was included as positive control who received diazepam (2 mg/kg, i.p), 1 h before the test. For behavioral tests for memory, these positive control animals received piracetam (200 mg/kg) intraperitoneally for seven days before the start of the y-maze. Another set of animals, Group F, was included in memory tests and the animals of this group received scopolamine (1 mg/kg) through the intraperitoneal route 30 min before the test. For each behavioral experiment for learning and memory, the experimental amnesia was induced in animals of Groups B-F by administration of scopolamine (1 mg/kg, i.p.) and the results were compared with Group F (amnesic rats). The details of animal grouping with respective treatments are given in Fig. 1.
Fig. 1.
The details of animals grouping (n = 8) and behavioral studies for anxiety and memory. Created with BioRender.com under the agreement number PF24OEO8MB (Date: 22 November 2022).
2.4.3. Open field test (OFT)
OFT is one of the most relied-on paradigms to investigate anxiety-related performance in rats. The open field maze consists of a black rectangular poly-acrylate box (80 × 80 × 45 cm) with its top typically left uncovered. On the 29th day, the rats were placed individually in the central arena and movements were recorded for 5 min (Shakeel et al., 2020). Every rat explored the arena once only and prior to the testing of the next animal, 70 % isopropyl alcohol was used to clean the maze arena to avoid any interference from the residual scent of the preceding session. The animal’s preference for central arena was estimated by monitoring. their entries and time spent in the central zone. The impact of chronic administration of plant extract on animal’s locomotor activity was also assessed by monitoring the number of rearings and line crossings during 5 min of maze exploration. All behavioral parameters were evaluated by using any-maze software (Stoelting Co., USA) version 6.1.
2.4.4. Light and dark (L/D) test
This behavioral experiment is based on the innate tendency of the rats to avoid brightly lit open areas and the spontaneous urge to explore dark environments. This experiment has been broadly used by researchers to determine the impact of plant extracts on rodents’ anxiety levels. On the 30th day, the rats were placed individually in a chamber (40 × 40 × 20 cm) segregated into a light and dark compartment connected through an entrance (10 × 10 cm) (Malik et al., 2020). After introduction into the light zone, the animals moved freely between two chambers for 5 min to quantify the time spent in both chambers of the apparatus. The rodent’s preference for light zone indicates lesser anxiety-like behavior.
2.4.5. Elevated plus-maze (EPM) test
EPM is used as another tool to assess anxiety-like behavior in animal models of CNS disorders. The test was carried out in a plus-shaped maze having two bare (50 × 10 cm) and two walled arms (50 × 10 × 30 cm) joined by a central platform (10 cm). The rats innately tend to stay in closed areas but venture out into open arms as well. In anxiety, rats prefer a closed arms of maze as compared to open arms to avoid their exposure to anxiogenic open areas. On the 31st day, after placing in the center facing toward exposed arms, all animals were tested for 5 min for their spontaneous exploratory behavior (Imran et al., 2021). The parameters such as entries and time in open arms were analyzed to evaluate the anxiolytic-like effect of plant extract.
2.4.6. Y-maze (spontaneous alteration) test
Y-maze evaluates the spatial memory in rodents by testing them in a trio-arm maze (55 × 10 × 15 cm) arranged at 120°. Normal rats tend to prefer different arms of the maze by remembering the arm visited on their previous entry. On the 32nd day, each rat was allowed to freely move in all arms (A,B and C) for 5 min and their spontaneous preferences for each arm were recorded (Sajjad Haider et al., 2021). The sequence of their entries in each arm was monitored to calculate the alteration behavior to check the effect of AA on scopolamine-amnesic rodents memory. The spontaneous alteration (SAP) was the sign of better remembrance and was calculated as follows:
2.4.7. Novel object recognition (NOR) test
This experiment is based on the rodent’s innate inclination towards new objects. The animals with efficient memory have remembrance of familiarized items and tend to explore the newly introduced ones in the arena of the open maze (80 × 80 × 45 cm). The test comprised of the training and test sessions (each of 5 min duration). On the 33rd day, every rat was familiarized with two geometrically similar objects for 5 min duration (training session). Immediately after the training session, one object was changed with a newer one and test session was carried out to allow the exploration for another 5 min (Javaid et al., 2021). On the basis of observation of the test session, the discrimination index (DI) for every individual rat was calculated as:
where T1 = time with the previously explored object and T2 = time with a novel object.
2.4.8. Morris water maze (MWM) test
In MWM, rodents are skilled to detect the platform disguised beneath the surface of opacified water to quantify their learning capability and long-term spatial memory. In a water maze, the performance of animals depends on their hippocampal cells (Illouz et al., 2016, Liu et al., 2018). MWM test was carried out in a spherical tank filled with water (150 × 50 cm) with a square-shaped (10 × 10 cm) platform placed 2 cm below the water. The tank was filled with water opacified with any milk or non-toxic dye to the level of 32 cm. To offer the spatial orientation points to rats, various proximal and distal visual cues were arranged on the inner sides of the tank together with the mounted wooden standees in the surroundings. This test comprised six consecutive days subdivided into two days for training, three days for testing and one day for probe testing (Rahman et al., 2021).
In the training phase, animals were given four trials per day, each trial of 2 min in which animals were trained to reach the platform placed visibly. During training days, animals were not treated with scopolamine and guided towards the platform if unable to escape within 120 s. In this case, the rats were kept on the platform for 10 s only for memory development (Rehman et al., 2022). During experimental trials for the next three days, 30 min after scopolamine administration, each rat was tested individually for 2 min to locate the submerged platform to note their escape latencies were recorded. The scopolamine-treated rats have the characteristics of forgetfulness and remain thigmotaxic in the water maze. Their amnesia is depicted by their longer escape latencies which if ameliorated by AA could be an indication of the memory-enhancing property of the test extract. On day 6 named probe day, the platform was removed from a tank and animals were tested for 120 sec to observe entries and duration of swimming in the targeted platform quadrant to assess long-term spatial remembrance of earlier platform position.
2.5. Biochemical studies
Soon after MWM probe day testing, all rats (n = 8) from each group were decapitated through cervical dislocation for the isolation of brains. To be investigated for biochemical evaluation, 0.4 g of each dissected brain was weighed and 4 ml of phosphate buffer (pH, 7.4) (P1000; Solarbio Lifesciences) was added. The mixture was homogenized and centrifuged for 20 min at 12000 rpm under reduced temperature (Imran et al., 2021). The supernatant was removed and subjected to enzymatic evaluation according to the previously reported methods.
2.5.1. AChE assay
A previously reported method was used to evaluate AChE levels in isolated brains (Ellman et al., 1961). In detail, 2.6 ml of 0.1 M phosphate buffer and 100 µL of DTNB (27025260; Molekula) and were added to 400 µL of brain homogenate. The absorbance was taken at 412 nm followed by the addition of 5.2 µL of ATC in the solution. The resulting solution was checked for a change in absorbance for 10 min.
2.5.2. Malondialdehyde (MDA) assay
An increase in the level of free radicals causes increased levels of MDA, the end product of lipid peroxidation, which provides an idea about brain oxidative stress. To quantify the MDA content in isolated brains, 1:1 ratio of trichloroacetic acid (TCA) (T59648-4I; Uni-Chem)and thiobarbituric acid (TBA) (T32050-3E; Uni-Chem) was added to 3 ml of brain homogenate. The mixture was boiled for 15 min, cooled, and centrifuged (3500 rpm) for 10 min (Chow and Tappel, 1972). The absorbance was observed at 532 nm.
2.5.3. Superoxide dismutase (SOD) assay
For SOD content determination, a reaction mixture was prepared by mixing brain tissue homogenate (0.5 ml) with 50 mM solution of sodium carbonate (1 ml) 0.1 mM EDTA (0.2 ml) (27285) and nitro blue tetrazolium (0.4 ml) (13727374; Molekula) (Naskar et al., 2009). To initiate a reaction, 1 mM solution of hydroxylamine hydrochloride (0.4 ml) was introduced, and absorbance was noted at 570 nm.
2.5.4. Glutathione peroxidase (GPx) assay
To estimate the activity of GPx, 1 mM hydrogen peroxide (0.1 ml) (216763; Sigma Aldrich), 0.4 M phosphate buffer of pH 7.0 (0.3 ml), 2 mM of reduced glutathione (0.2 ml) (213215; Oakwood chemicals) and 10 mM sodium azide (0.1 ml) was added to the brain supernatant (0.2 ml) (Flohé and Günzler, 1984). Incubation was carried out at 37 °C for 15 min and then 10 % TCA (0.5 ml) was inoculated followed by subsequent centrifugation (1500 rpm) for 5 min. 0.1 ml of this supernatant was then combined with 0.3 mM Phosphate buffer (0.2 ml) and Ellman’s reagent (0.7 ml) and absorbance was monitored spectrophotometrically at 420 nm.
2.6. In-silico studies
2.6.1. ADME analysis
SwissADME (https://www.swissadme.ch) an online web server was used to calculate the drug-likeness, physiochemical and pharmacokinetic properties of the secondary metabolites of Ailanthus altissima. The compounds were converted into SMILES notations and subjected to SwissADME for the evaluation of the aforementioned properties.
2.6.2. Molecular docking studies
The choice of docking protocol was made on the basis of the success of the redocking experiment so that the capacity of the docking algorithm to replicate the co-crystallized pose of the cognate ligand could be determined. For the control docking experiment, the cognate ligand in the PDB 5NAP was extracted, prepared and re-docked into the active site of the target protein using MOE v.2019 (Caliandro et al., 2018). To evaluate the deviation between the crystal pose and the redock pose, Root Mean Square Deviation (RMSD) was calculated which serves as a validation metric for docking protocol.
To rationalize the memory-enhancing effects of the isolated secondary metabolites of Ailanthus altissima, the compounds were docked with the crystal structure of AChE in complex with donepezil-like analog (PDB ID 5NAP). All the compounds were sketched in MOE v.2019 using the Builder module and subsequently subjected to structure correction, protonation and energy minimization using MMFF94x force field. The crystal structure of the target protein was retrieved from RCSB Protein Data Bank and subjected to structure preparation. During preparation, hydrogens were placed and ionization states were assigned to the molecular system followed by minimization which calculates atomic coordinates that are local minima of a potential energy function. A docking grid was generated, centered on the coordinates of the cognate ligand and the compounds were docked into the defined grid. During docking, receptor and compounds were kept flexible by using Induce Fit protocol while Triangular Matcher was used as a placement method. The resulting poses of the compounds were scored using London dG and the toped ranked poses were analyzed. All the graphics were rendered using Chimera software (Pettersen et al., 2004).
2.7. Statistical analysis
After testing the data for normality by D'Agostino, Shapiro-Wilk and Kolmogorov-Smirnov tests, parametric one-way ANOVA followed by Dunnet’s test analyzed all behavioral and biochemical parameters but, escape latencies recorded for 3 days in MWM were evaluated by two-way ANOVA with Tukey’s test using Graph pad prism (version 8). For behavioral and biochemical tests in this study, P ≤ 0.05 was considered significant for the data depicted as mean ± SD while Brown-Forsythe and Barlett’s tests were used for the equality of group variances.
3. Results
3.1. In-vitro experiments
3.1.1. 1Total phenols and flavonoids content in AA
The methanolic extract of bark of Ailanthus altissima (AA) was analyzed for the assessment of total phenols and flavonoids. It was found that one gram of AA comprised 56.15 ± 3.3 mg GAE and 29.45 ± 4.8 mg QE of phenols and flavonoids, respectively (Table 1).
Table 1.
Total phenols (TP), total flavonoids (TF), antioxidant (ABTS and DPPH) and AChE inhibitory activities of crude extract of Ailanthus altissima.
| Test sample |
TP mg GAE/g |
TF mg QE/g |
ABTS IC50 (µg/mL) |
DPPH IC50 (µg/mL) |
AChE IC50 (µg/mL) |
|---|---|---|---|---|---|
| AA | 56.15 ± 3.3 | 29.45 ± 4.8 | 643.21 ± 24.9 | 741.74 ± 19.31 |
|
The data are presented as mean ± SEM (n = 3).
3.1.2. Antioxidant activity of AA
The antioxidant assays of plant extract analyzed by DPPH and ABTS tests depicted IC50 values of 741.74 ± 19.31 µg/mL and 643.21 ± 24.9 µg/mL, respectively (Table 1).
3.1.3. Acetylcholinesterase inhibitory activity in AA
The analysis of acetylcholinesterase inhibitory activity using galantamine as standard showed concentration-dependent inhibition of acetylcholinesterase by AA with an IC50 value of 211.31 ± 16.31 µg/mL as compared to galantamine having an IC50 value of 5.3 ± 0.19 µg/mL (Table 1).
3.1.4. Identification of secondary metabolites in AA by UHPLC-MS
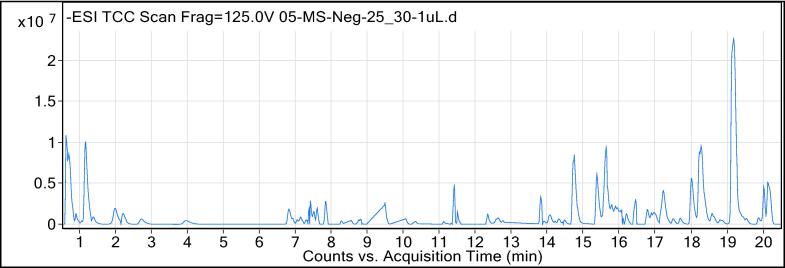
The AA was chemically characterized through UHPLC-MS where analysis was performed in the negative ionization mode and outcomes were represented as total ion chromatogram (Fig. 2). The outcomes of analysis discovered the occurrence of 13 secondary metabolites belonging to diverse phytochemical classes (Table 2).
Fig. 2.
Total ion chromatogram (TIC) of AA (negative ionization mode).
Table 2.
The secondary metabolites identified in AA by UHPLC-MS analysis.
| S. No | Phytocompounds | RT (minutes) | Mol. Wt. | Mol. Formula | Phytochemical class | B. peak (m/z) |
|---|---|---|---|---|---|---|
| 1 | Sphalleroside A | 0.642 | 342.1314 | C16 H22 O8 | Phenol | 341.1314 |
| 2 | Verbenalin | 0.652 | 388.1358 | C17 H24 O10 | Iridoid glucoside | 387.1358 |
| 3 | 4′-O-Methyldavidioside | 2.243 | 434.1581 | C22 H26 O9 | Glucoside | 433.1581 |
| 4 | 2′,4′-Dihydroxy-2,3′,6′-trimethoxychalcone | 2.744 | 330.1104 | C18 H18 O6 | Chalcone | 329.1104 |
| 5 | Heteroflavanone A | 3.99 | 360.1223 | C19 H20 O7 | Flavonoid | 359.1223 |
| 6 | Kanokoside A | 7.213 | 476.1874 | C21 H32 O12 | Glycoside | 475.1874 |
| 7 | Lophirone J | 7.312 | 400.1321 | C25 H20 O5 | Flavonoid | 399.1321 |
| 8 | (E)-Squamosamide | 7.619 | 465.18 | C26 H27 N O7 | Stilbene | 464.18 |
| 9 | Lonchocarpenin | 7.619 | 448.1874 | C27 H28 O6 | Coumarin | 447.1874 |
| 10 | Cyclotricuspidogenin C | 14.055 | 536.3733 | C31 H52 O7 | Phyto | 535.3733 |
| 11 | 12-oxo-10Z-octadecenoic acid | 15.644 | 296.2334 | C18 H32 O3 | Fatty acid | 295.2334 |
| 12 | Cyclopassifloic acid B | 19.23 | 520.3732 | C31 H52 O6 | Triterpene | 519.3732 |
| 13 | Conessine | 20.017 | 356.3207 | C24 H40 N2 | Alkaloid | 355.3207 |
RT: retention time; Mol. Wt.: molecular weight; Mol. Formula: molecular formula; B.peak; base peak.
3.2. In-vivo experiments
3.2.1. Anxiolytic effects of AA in open field test
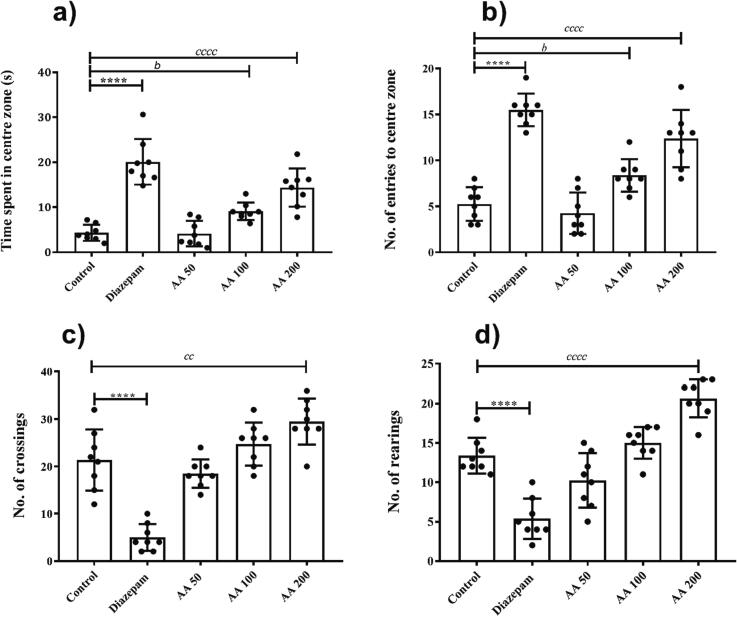
The animals were behaviorally examined in an open field for 5 min to evaluate their anxiety toward open areas. The rats of the control group showed increased anxiety after exposure to open arena which was evidenced by their lesser preference for central zones of the maze. A significant inter-group variations for the duration of central zone exploration (F (4,35) = 31.92, P < 0.0001) were noted. In detail, the post-hoc multiple comparison tests indicated that time spent in the center by diazepam-treated rats was higher (P < 0.0001 vs control group). A significant anxiety reduction was observed by AA at a dose of 100 (P < 0.05) and 200 mg/kg (P < 0.0001) in comparison to control rats (Fig. 3a). Similarly, the statistically significant disparity was evident for entries to the centre zone of maze (F (4, 35) = 37.12, P < 0.0001). The control rats showed the least interest to visit the central zone, but these observations were significantly reversed by the 100 (P < 0.05) and 200 mg/kg (P < 0.0001) of AA like diazepam (P < 0.0001) (Fig. 3b).
Fig. 3.
Anxiolytic effects of AA in open field test: The rats (n = 8) were tested in open field for 5 min to assess the dose-dependent impact of of AA effects on anxiety by monitoring the (a) time spent by rats in the centre zone, (b) entries in the centre, (c) line crossings and (d) rearings for 5 min in the open field test. *P < 0.05, bP < 0.05, cP < 0.05 were considered significant statistically for diazepam vs control, AA 100 vs control and AA 200 vs control, respectively.
The influence of prolonged treatment of rats with AA on their locomotor activities was studied by analyzing the line crossings and rearings. In comparison to control animals, both parameters of locomotion were notably increased with AA 200 (P < 0.01) (Fig. 3c and d). However, these parameters remained unaffected at lower doses of AA.
3.2.2. Anxiolytic effects of AA in light and dark test
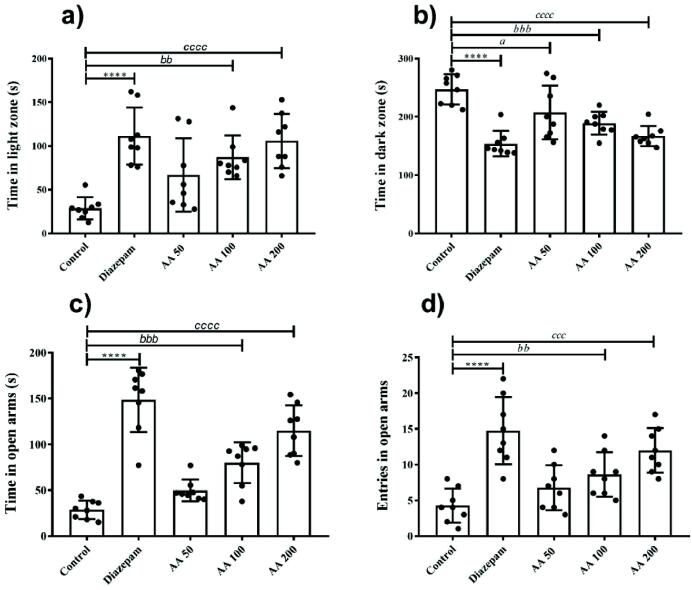
The effects of AA on animal’s anxiety for the illuminated zone was estimated by monitoring the animal’s duration of stay in the light zone of light and dark box. The one-way ANOVA revealed that in light and dark box test, animals with different treatments varied significantly for time spent in light (F (4, 35) = 10.24, P < 0.0001) and dark (F (4, 35) = 13.91, P < 0.0001). The post-hoc Dunnet’s test showed that a significant increment in time spent in the light zone was observed in animals of groups treated with AA 100 (P < 0.001) and AA 200 (P < 0.0001), in comparison to control group animals (Fig. 4a). Likewise, a significant reduction in dark zone preference was also observed by the animals exposed to AA 100 (P < 0.001) and AA 200 (P < 0.0001) treatments as compared to control (Fig. 4b). But no significant results were evident after the administration of AA at 50 mg/kg.
Fig. 4.
Anxiolytic effects of AA in light and dark and elevated plus maze tests: The rats (n = 8) chronically treated with AA showed dose-dependent anxiolytic effects in light and dark chamber test and elevated plus maze. The effects were noted by monitoring the rats for (a) time in the light zone and (b) time in dark zone (c) time in the open arm and (d) entries in open arms for 5 min. *P < 0.05, bP < 0.05, cP < 0.05 were considered significant statistically for diazepam vs control, AA 100 vs control and AA 200 vs control, respectively.
3.2.3. Anxiolytic effects of AA in elevated plus maze test
The elevated plus maze test was used to assess the animal’s anxiety which was depicted by their fearlessness to explore the open arms of the elevated maze. The outcomes of EPM showed a significant group-wise variation for time spent in open arms (F (4, 35) = 35.21, P < 0.0001) and a number of entries there (F (4, 35) = 12.14, P < 0.0001). In detail, the animals treated with AA 100 and AA 200 mg/kg spent more time in open arms with P < 0.001 and P < 0.0001 as compared to the control group (Fig. 4c). Similarly, the animals exposed to AA showed an increased number of entries in the open arms of the maze in a dose-dependent manner. The animals administered with 100 and 200 mg/kg were less anxious as their number of open arms visits were noticeably increased with P < 0.01 and P < 0.0001, respectively (Fig. 4d) but animals of AA 50 mg/kg did not show any significant changes.
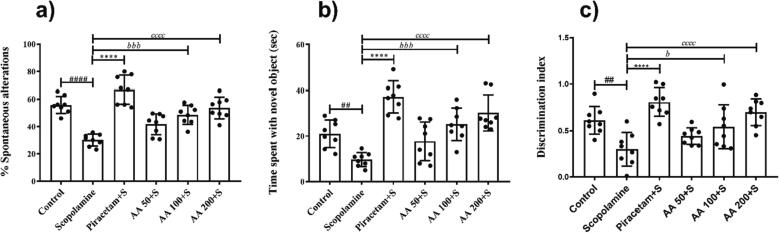
3.2.4. Effects of AA on spontaneous alterations in Y-maze test
The rats were allowed to freely explore all three arms of y-maze for 5 min and their spontaneous alterations were compared to estimate the impact of AA on the animal’s recalling capacity. The statistical analysis indicated a noteworthy change for spontaneous alterations between differently treated rats (F (5, 42) = 22.45, P < 0.0001). The scopolamine-induced amnesic rats showed decreased spontaneous alterations (P < 0.0001) revealing that scopolamine administration led to poor memory retention of the recently visited maze arms in those animals. This memory impairment was improved by AA as these animals preferred to visit novel arms instead of already visited ones. The prolonged pre-treatment of animals with AA at 100 and 200 mg/kg resulted in increased spontaneous alteration scores with P < 0.001 and P < 0.0001, respectively as compared to scopolamine-amnesic rats and the same was done by piracetam (P < 0.0001) as shown in Fig. 5a.
Fig. 5.
Effects of AA on learning and memory in y-maze and novel object recognition tests: The dose-dependent impact of AA was examined on the learning and memory of rats (n = 8) by testing for 5 min in y-maze and novel object recognition tests. The outcomes were estimated by observing the(a) spontaneous alteration, (b) time spent with a novel object and (c) discrimination index. On experiment day, the animals were administered with AA 60 min before followed by administration of scopolamine 30 min before experiment. #P < 0.05, *P < 0.05, bP < 0.05 and cP < 0.05 were considered as significant for control, piracetam, AA 100 and AA 200 compared to scopolamine-treated amnesic rats, respectively.
3.2.5. Effects of AA on memory in novel object recognition test
In this test, rats were presented with familiar objects one of which was replaced by a newer one to test their ability to identify, recall and differentiate the new item from the familiarized one. There were significant inter-treatment differences for duration of novel object exploration (F (5, 42) = 15.79, P < 0.0001) and discrimination index (F (5, 42) = 9.626, P < 0.0001). The administration of scopolamine showed the development of amnesic behavior in rats as they interacted with the novel object for a shorter duration (P < 0.01). But AA treatment improved the recalling capability of the animals as they explored the novel object for long and pronounced effects were noted at doses of 100 and 200 mg/kg with P < 0.001 and P < 0.0001 as compared to the scopolamine-amnesic group (Fig. 5b).
Similarly, groups of rats treated with higher doses of AA (100 and 200) exhibited a significantly increased discrimination index with P < 0.05 and P < 0.0001, respectively (Fig. 5c). But, the outcomes remained negligible at AA 50 mg/kg revealing a dose-dependent memory-improving potential of AA.
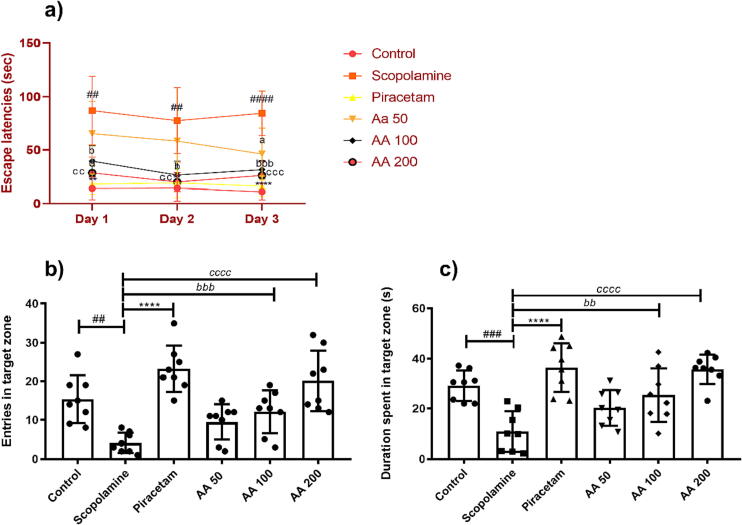
3.2.6. Effects of AA on learning and memory in Morris water maze test
In Morris water maze test, the rats were chronically treated with AA and tested for their memory by locating the hidden platform in test trials carried out over three days. The two-way ANOVA revealed statistically significant variation in all differently treated groups for escape latencies [F (5,42) = 35.59, P < 0.0001] during three days of the experiment. The escape latencies in scopolamine-treated rats showed noticeable difference for escape latencies as compared to healthy rats [F (1,42) = 127.5, P < 0.0001]. In detail, the animals treated with scopolamine constantly spin in the water maze and took a long time to reach the hidden platform with P < 0.01, as compared to the healthy control. However, these amnesic signs were dose-dependently reduced by AA in comparison to scopolamine-treated rats with [F (3,84) = 37.69, P < 0.0001]. The animals treated with 100 and 200 mg/kg of AA took less time to locate the platform revealing improved memory. The thigmotaxic behavior and escape latencies of AA 100 and AA 200 treated rats were significantly less with P < 0.05 and P < 0.01, in comparison to amnesic rats and outcomes were comparable with piracetam (P < 0.01) as shown in Fig. 6a.
Fig. 6.
Effects of AA on learning and memory in Morris water maze test: The dose-dependent impact of AA on learning and memory was evaluated by testing the rats (n = 8) to locate the disguised platform for 3 test days and the duration to reach the platform were noted as (a) escape latencies. The long-term memory was examined by testing the rats on probe day for their (b) entries in the platform zone and (c) duration spent in the platform zone. During three test days and one probe day, the animals were treated with AA 60 min before their testing in MWM test followed by administration of scopolamine 30 min before experiment. The effects of AA on learning and memory of animals were determined by monitoring their All data were expressed as mean ± SD and escape latencies were evaluated by two-way ANOVA with Tukey's test while entries and duration in target quadrant were evaluated by one-way ANOVA followed by Dunnet’s test. #P < 0.05, *P < 0.05, bP < 0.05 and cP < 0.05 were considered as significant for control, piracetam, AA 100 and AA 200 compared to scopolamine-treated amnesic rats, respectively.
During a probe trial of 120 s on day 6th of MWM, the rats were evaluated for their learning capabilities and memory consolidation via remembering the target quadrant. Their memory for the target quadrant was quantified by estimating the number of entries and time spent in the platform zone. The ANOVA revealed considerable intergroup differences for number of entries [F (5,42) = 12.17, P < 0.0001] and time spent in target quadrant [F (5,42) = 11.51, P < 0.0001]. During the probe session, the scopolamine-treated rats showed impaired cognitive abilities as evident by their reduced frequency of visits to the rescue quadrant and lesser swimming duration with P < 0.01 and P < 0.001, respectively. These amnesic impacts of scopolamine were markedly prevented by piracetam as animals entered more often with an increased duration of stay in the platform quadrant with P < 0.0001 (Fig. 6b). Likewise, continuing treatment with AA resulted in preserved memory in a dose-dependent manner as rats in AA 100 and 200 showed an increased number of entries in the platform quadrant with P < 0.01 and P < 0.0001 as compared to scopolamine-amnesic rats, revealing their remembrance for the rescue quadrant (Fig. 6c).
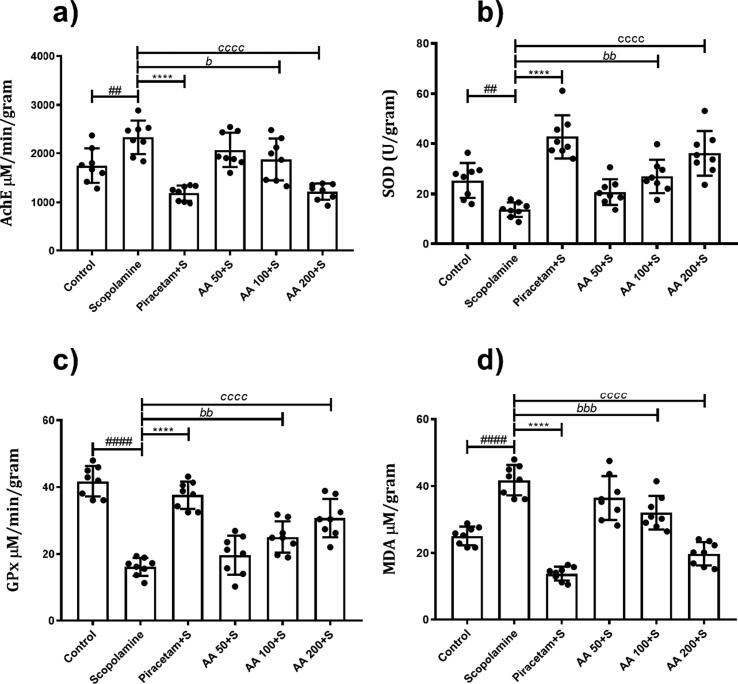
3.2.7. Effects of AA on brain biochemical analysis
The one-way ANOVA showed that significant variation in AChE levels in groups (F (5, 42) = 16.85, P < 0.0001. In comparison to control animals, the levels of AChE were increased in animals treated with scopolamine with P < 0.005. But, these elevated levels of AChE levels were reduced in animals treated with AA 100 and 200 mg/kg with P < 0.05 and P < 0.0001, respectively, as compared to scopolamine-treated animals (Fig. 7a).
Fig. 7.
Effects of AA on brain biochemical analysis: The brain homogenates of rats (n = 8) were analyzed for (a) Acetylcholinesterase, (b) Glutathione peroxidase, (c) Superoxide dismutase and (d) Malondialdehyde levels to examine the effects of AA on biochemical parameters. All data were expressed as mean ± SD and evaluated by one-way ANOVA followed by Dunnet’s test. #P < 0.05, *P < 0.05, bP < 0.05 and cP < 0.05 were considered as significant for control, piracetam, AA 100 and AA 200 compared to scopolamine-treated amnesic rats, respectively.
The levels of SOD varied significantly among different groups (F (5, 42) = 18.82, P < 0.0001). The administration of scopolamine to the animals decreased the levels of SOD in the brain with P < 0.05, as compared to the control. But, treatment with AA 100 and 200 mg/kg led to increase these levels with P < 0.05 and P < 0.0001, respectively (Fig. 7b).
A significant variation in the levels of GPx enzyme was observed among different groups (F (5, 42) = 36.25, P < 0.0001). It was observed that the brains of scopolamine-treated animals had noticeably reduced levels of GPx enzyme as compared to control (P < 0.0001). But the administration of AA resulted in the dose-dependent elevation of GPx as animals treated with 100 and 200 mg/kg showed an increase in enzyme levels as compared to scopolamine-amnesic rats with P < 0.05 and P < 0.0001, respectively (Fig. 7c).
Similarly, the levels of MDA in the brains were variable in differently treated animals (F (5, 42) = 46.50, P < 0.0001). There were increased MDA levels in scopolamine-treated animals (P < 0.0001) indicating the scopolamine-induced increased oxidative stress. However, this increased MDA levels was reduced by AA at doses 100 and 200 mg/kg with P < 0.001 and P < 0.0001 in comparison to scopolamine-treated animals (Fig. 7d).
3.3. In-silico studies
3.3.1. ADME studies
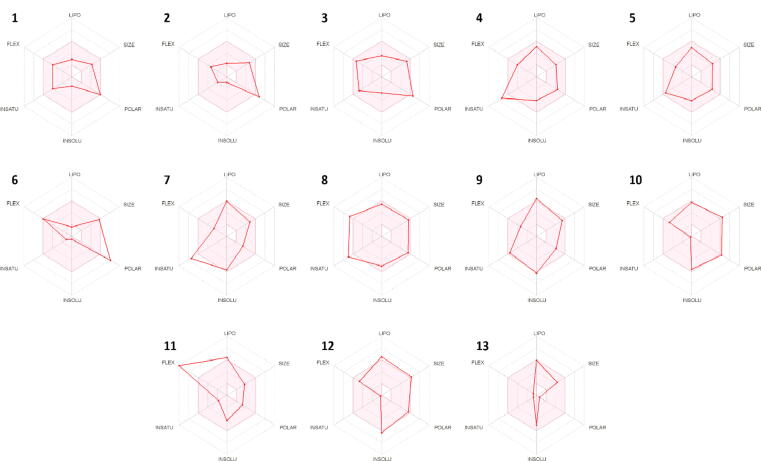
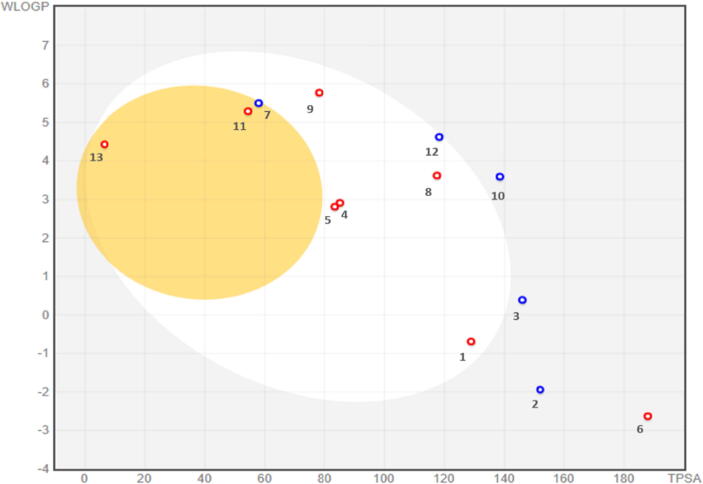
Many compounds with potential biological activity cannot reach clinical trials because of their poor dynamics of pharmacokinetic properties. Thus, predicting the pharmacokinetic characteristics of compounds through in silico studies is an alternative to experimental techniques in the earlier process of drug discovery. Herein, SwissADME was utilized to examine the ADME profile of the detected phytocompounds and obtained values are summarized in Table 3. The fast appraisal of drug-likeness of the compounds was achieved by plotting bioavailability radar which determines the six physicochemical features; size, lipophilicity, polarity, solubility, flexibility and saturation (Fig. 8). The properties of a molecule should fall into the pick area of the radar to be considered as drug-like. It was observed that the physiochemical range of all the compounds falls into a considerable area except compound 11 which displayed high flexibility due to the presence of hydrocarbon chain. Similarly, the boiled-egg plot depicted the absorption in human intestinal tract, permeation through blood–brain and p-glycoprotein (Fig. 9). P-glycoprotein permeability is a key to determining effusion through biological membranes. The plot demonstrated the high gastrointestinal absorption for most of the compounds while only compounds 7, 11 and 13 were permeable to the blood–brain barrier (BBB). However, compounds 2, 3, 7, 10 and 12 were found as a substrate of p-glycoprotein. Taken together the isolated compounds appreciably met the criteria of considering them as drug-like.
Table 3.
ADME profile of 13 secondary metabolites of methanolic extract of Ailanthus altissima.
| Ligands |
Mol. Wt. |
HBA | HBD | TPSA | LOG P | GI Absorption | BBB | P-gp Substrate | Lipinski Violations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 342.34 | 8 | 5 | 128.84 | 1.55 | High | No | No | 0 |
| 2 | 388.37 | 10 | 4 | 151.98 | 2.19 | Low | No | Yes | 0 |
| 3 | 434.44 | 9 | 5 | 145.91 | 1.72 | Low | No | Yes | 0 |
| 4 | 330.33 | 6 | 2 | 85.22 | 2.59 | High | No | No | 0 |
| 5 | 360.36 | 7 | 1 | 83.45 | 3.65 | High | No | No | 0 |
| 6 | 476.47 | 12 | 6 | 187.9 | 2.08 | Low | No | No | 2 |
| 7 | 400.42 | 5 | 0 | 57.9 | 4.06 | High | Yes | Yes | 0 |
| 8 | 465.5 | 7 | 4 | 117.48 | 3.46 | High | No | No | 0 |
| 9 | 448.51 | 6 | 1 | 78.13 | 4.7 | High | No | No | 0 |
| 10 | 536.74 | 7 | 6 | 138.45 | 2.82 | Low | No | Yes | 2 |
| 11 | 296.44 | 3 | 1 | 54.37 | 3.72 | High | Yes | No | 0 |
| 12 | 520.74 | 6 | 5 | 118.22 | 3.34 | High | No | Yes | 1 |
| 13 | 356.59 | 2 | 0 | 6.48 | 4.32 | High | Yes | No | 1 |
Fig. 8.
Bioavailability radar of secondary metabolites of Ailanthus altissima. Pink region represents the acceptable range of six physiochemical properties including size, lipophilicity, polarity, solubility, flexibility and saturation.
Fig. 9.
Boiled-egg model for secondary metabolites of Ailanthus altissima. The yellowish egg yolk-like section represents the optimal area for BBB permeability while white section represents the optimal area for GI absorption. Blue and red dots predicted the effluated and non-effluated compounds from the CNS by the p-glycoprotein, respectively.
3.3.2. Molecular docking studies
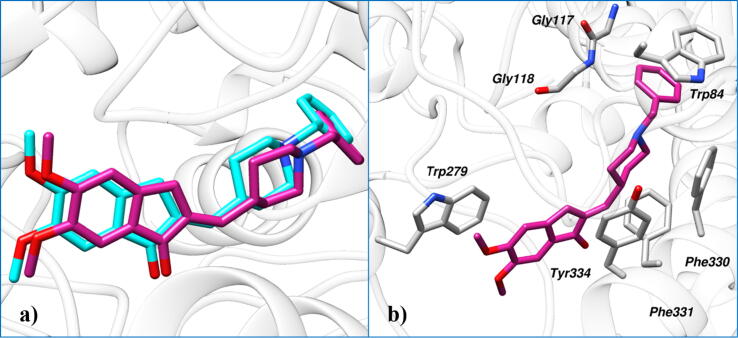
Initially, the docking protocol was evaluated by the redocking experiment. The deviation between the coordinates of the crystal pose and the redock pose was found to be 0.34 Å which indicates that the docking protocol is able to correctly emulate experimental binding and could be used to study the binding modes of secondary metabolites of Ailanthus altissima in the active site of AChE (Fig. 10a). Insight into the binding mode of cognate ligand in the binding site of AChE, it was observed that terminal phenyl rings stacked with Trp84 and Trp279 by mediating parallel π–π stacking (Fig. 10b). The nitrogen of the piperidine ring interacts with Phe330 via a cation–π interaction. Moreover, a network of hydrophobic interaction was also observed with Trp84, Gly117, Gly118, Phe330, Phe331 and Tyr334.
Fig. 10.
A) Redocking results show the cognate ligand (violet red) and the redock pose (cyan) produced by MOE. B) Molecular interaction between the active site residues of AChE and cocrystallized ligand.
Molecular docking studies of metabolites of Ailanthus altissima suggested that all the compounds have gained access to the active site of AChE with the binding affinity ranging from −9.89 to −7.48 kcal/mol. The compounds 6, 9, 10 and 12 displayed a high binding affinity than that of cognate ligand (Table 4).
Table 4.
Binding affinity of secondary metabolites of Ailanthus altissima with AChE.
| Ligand No. | Ligands Chemical Name | Binding Affinity (kcal/mol) |
Type of Interactions |
|
|---|---|---|---|---|
| Hydrophobic Interactions | Hydrogen Bonding | |||
| 1. | Sphalleroside A | −8.20 | Phe290, Phe331, Tyr334 | Ser122, Glu199, His440 |
| 2. | Verbenalin | −8.60 | Asp72, Trp84, Tyr121, Phe330 | Gly118 |
| 3. | 4′-O-Methyldavidioside | −8.35 | Phe330, Phe331, Tyr334 | Asp72, Tyr121, Ser286 |
| 4. | 2′,4′-Dihydroxy-2,3′,6′-trimethoxychalcone | −7.48 | Phe331, Tyr334 | Gly117, Tyr130, Glu199 |
| 5. | Heteroflavanone A | −8.34 | Tyr121, Phe330, Phe331, Tyr334 | Phe288 |
| 6. | Kanokoside A | −9.89 | Tyr121, Leu282, Phe290, Tyr334 | Trp84, Gly118, Glu199, Phe288, His440 |
| 7. | Lophirone J | −8.25 | Asp72, Trp84, Tyr121, Phe330, Tyr334 | --- |
| 8. | (E)-Squamosamide | −8.79 | Tyr121, Trp279, Phe330, Phe331, Tyr334 | Glu199, Phe288, Phe330 |
| 9. | Lonchocarpenin | −9.04 | Asp72, Trp84, Phe330, Phe331, Tyr334, Ile444 | Tyr121, Phe288 |
| 10. | Cyclotricuspidogenin C | −9.08 | Tyr70, Asp72, Trp84, Trp279, Phe290, Phe330, Phe331, Tyr334, Ile444 | Gly118, Glu199, Phe330, His440 |
| 11. | 12-oxo-10Z-octadecenoic acid | −8.00 | Trp84, Phe330, Phe331, Tyr334 | --- |
| 12. | Cyclopassifloic acid B | −9.45 | Trp84, Tyr121, Phe290, Phe330, Phe331, Tyr334 | Tyr70, Gly117, Gly118, Glu199 |
| 13. | Conessine | −8.31 | Trp84, Tyr121, Phe330, Phe331, Tyr334, Ile444 | Gly117 |
| 14. | Cognate Ligand | −9.04 | Trp84, Gly117, Gly118, Trp279 Phe330, Phe331, Tyr334. | --- |
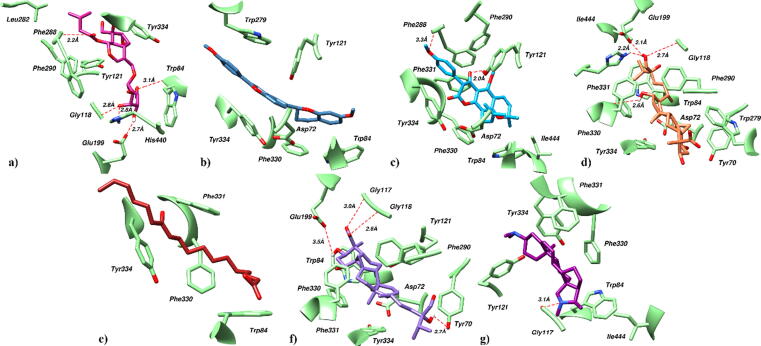
Compound 6 in the active aromatic gorge of AChE establishes five hydrogen bonds contacts (Fig. 11a). Terminal hydroxyl group mediates hydrogen bond with Trp84. Similarly, hydroxyl group on pyran ring mediates three hydrogen bonds with Gly118, Glu199 and His440 while fifth hydrogen bond was observed between carbonyl group of the ligand and Phe288. Moreover, compound 6 form π-alkyl interactions with Tyr121, Phe190 and Tyr334. Furthermore, anchorage was provided by the alkyl-alkyl interactions between the terminal hydrocarbon chain of ligand and Leu282.
Fig. 11.
Docked pose of compound a) 6, b) 7, c) 9, d) 10, e) 11, f) 12 and g) 13 in the active site of AChE. Protein residues are shown in lime green color as sticks while ligands are shown in different colors. Red dotted lines represented the hydrogen bond contacts.
In case of compound 7 which was predicted to be blood brain barrier permeable, a network of extensive hydrophobic interactions was observed with the binding site residues (Fig. 11b). Six-membered aromatic rings in the ligand mediates π-alkyl interactions with Asp72, Trp84, Tyr121, Trp279, Phe330 and Tyr334. Similarly, furan ring stacked between Tyr121 and Tyr334 by mediating π-π stacking.
Similarly, the terminal methoxy group and hydroxyl group on pyran ring of compound 9 establish hydrogen bond links with the active site residues of AChE including Tyr121 and Phe288 (Fig. 11c). The phenyl ring of ligand stacked with Phe290 by mediating π-stacking. Further ligand stabilized itself by extensive hydrophobic contacts with the surrounding residues including Asp72, Trp84, Phe330, Phe331, Tyr334 and Ile444.
Compound 10 in the active site of AChE demonstrates four hydrogen bonds contacts and significant hydrophobic interactions (Fig. 11d). The oxygen of polar hydroxyl groups of ligand establishes hydrogen bonds with Gly118, Glu199, Phe330 and His440. Similarly, non-polar methyl groups demonstrate hydrophobic contact with surrounding residues including Tyr70, Asp72, Trp84, Trp279, Phe290, Phe331, Tyr334 and Ile444.
Compound 11 in the binding site of AChE is stabilized by a network of hydrophobic interactions (Fig. 11e). Hydrocarbons in the ligand establish hydrophobic interactions with Trp84, Phe330, Phe331 and Tyr334.
Likewise, a docked pose of compound 12 in the active site of AChE demonstrates a network of significant hydrogen bonding and hydrophobic interactions (Fig. 11f). Oxygen of carboxyl and hydroxyl group establishes four hydrogen bond contacts with Tyr70, Gly117, Gly118 and Glu119. Further, hydrophobic interactions with Trp84, Tyr121, Phe290, Phe330, Phe331 and Tyr334 contribute to the stability of the protein–ligand complex.
Similarly, in the binding site of AChE, N-dimethyl amino group of compound 13 demonstrated a hydrogen bond with Gly117 while rest of the interactions were hydrophobic (Fig. 11g). Rings and terminal methyl groups establish π-alkyl and alkyl-alkyl interactions with Trp84, Tyr121, Phe330, Phe331, Tyr334 and Ile444.
Docking results reveal that the binding conformation of metabolites of Ailanthus altissima in the active site of AChE closely resembles to that of cognate ligand, extending from the bottom of the anionic subsite to the peripheral anionic site. The phytocompounds mediate interactions with the similar residues that displayed by the cognate ligand. However, in case of phytocompounds additional interactions with other crucial residues were also observed which pronounces the observed memory-improving activity.
4. Discussion
In the current study, the Ailanthus altissima was found enriched with flavonoids, phenols and free radical scavenging potential. The existence of a plethora of chemically diverse phytocompounds was revealed by UHPLC-MS analysis. The chronically treated rats with AA showed reduced anxiety and improved memory in a set of behavioral experiments. The biochemical analysis of isolated brains revealed reduced malondialdehyde and AChE levels while increased antioxidant enzymes in animals treated with AA as compared to scopolamine-treated rats.
The plant-derived medicinal products are gaining increased acceptance as people around the world find them affordable with safe therapeutic profiles as compared to synthetic drugs. Plants have been enriched with pharmacologically active agents and few of these have been advanced into drugs. The fascinating pharmacological characteristics and structural properties have made the plants a source of lead molecules for drug discovery (Zhao et al., 2008). Phenols and flavonoids are the vital classes of bioactive phytocompounds owned by therapeutically well-known plants. Sufficient scientific evidence reports that these phytoconstituents have the capacity to produce multiple biological effects. Thus, inspecting the flavonoids and phenols content in any plant sample is an important step to estimate its therapeutic potential (Sun et al., 2008). In the current study, the in-vitro experimentation revealed that the extract of bark of Ailanthus altissima possessed a substantial amount of phenols and flavonoids and findings are in-line with the previous study by Mohammed et al (Mohamed et al., 2021).
Despite multifaced epigenetic and genetic factors contributing to the development of neurodegenerative ailments, the deteriorative role of oxidative stress has always been central (Pedre et al., 2018). After ingestion, phenolic compounds are quickly transformed into glucuronide derivatives and distributed to different organs including the brain (Cosme et al., 2020). When tested, the AA showed noticeable radical scavenging capacity in DPPH and ABTS assays which might be attributed to its phenolic and flavonoid content. These outcomes are supported by the findings of Luis et al. who tested the crude extract of Ailanthus altissima and reported its antioxidant potential (Luís et al., 2012). A study by Albouchi et al., also provided scientific validation for radical scavenging activity and correlated it with the plant’s higher phenolic content (Alboucshi et al. 2013). Furthermore, the AA profiling through UHPLC-MS analysis revealed the existence of 13 phytocompounds belonging to diverse classes i.e. phenols, flavonoids, glucosides, fatty acids, coumarin, chalcones and triterpenoids. The existence of these phytocompounds is supported by earlier studies conducted on aerial parts of Ailanthus altissima (Luís et al., 2012, Tsao et al., 2002).
Many scientists have reported that phenolic phytocompounds not only halt neurodegeneration by free radical scavenging but might also regulate certain signaling pathways crucial for neuroinflammation (Matuszewska et al., 2018). Flavonoids play a neuroprotective role by improving neuronal degeneration by inhibiting lipoxygenases and cyclooxygenases (Panche et al., 2016). Thus, the neuroprotective benefits noted in the current study might be attributed to the anti-inflammatory properties of AA phytocompounds. An earlier study detected the presence of kaempferol, ethyl gallate, quercetin, and luteolin in leaves extract of Ailanthus altissima and revealed its ameliorative role neuroinflammation as oral administration of its leaves extract inhibited iNOS and COX-2 expression in LPS-stimulated astrocytes (Kim et al., 2022)In previous study by Jin et al., pretreatment with ethanol extract of Ailanthus altissima reduced eosinophil infiltration into the airway and downregulated inflammatory mediators in ovalbumin sensitized mice model of lung inflammation (Jin et al., 2006). Scopolamine-induced increased oxidative stress and neuroinflammation leading to neuronal death in hippocampus, a structure of brain regulating learning capacity and memory retention (Balaban et al., 2017, Muhammad et al., 2019). Scopolamine administration results in increased caspase-3 and Bax levels and apoptotic neurons in rodent’s brains (Xu et al., 2016). Demirci et al. also reported scopolamine-induced enhanced expression of caspase-3 leading to neuronal cell death in brains of dementia animal models (Demirci et al., 2017). Lophirone A and B, phytocompounds owned by AA, have been previously reported to reverse the caspase-3 activation by regulating the generation of oxidative free radicals in rats hepatocytes (Ajiboye et al., 2016). Thus, Lophirone J belonging to flavonoids might be interrupting the caspase-3 activity to exert these observed pharmacological effects. Ajiboye et al. isolated lophirones from stem bark of Lophira alata and correlated it's antimutagenic potential to the free radical scavenging potential (Ajiboye et al., 2014).
Anxiety is becoming an important research zone of neuropharmacology as its 16.6 % prevalence makes it one of the most common mental disorders worldwide (Martin et al., 2009). Anxiety is associated with dysregulated neurotransmitters. Plant-based several natural and derived compounds specifically flavonoids are known for anxiolytic potential without any additional CNS effects. In behavioral experiments, AA showed anxiolytic-like effects in OFT, L/D and EPM tests as rats chronically treated with AA were fear-free for open, illuminated and exposed areas of maze. Diazepam, incorporated as a standard antianxiety drug, modulates the action of the neuro-inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Flavonoids are known to interact with GABAA receptors resulting in increased GABA potentiation and anxiolytic effects (Hanrahan et al., 2011). The radical scavenging properties of flavonoids have also been linked with reduced anxiety (Ko et al., 2020).
Similarly, the AA caused protection from scopolamine-induced amnesia in a dose-dependent manner as animals had a memory of previously visited arms, familiar object and platform zone in y-maze, NOR and MWM tests, respectively. These memory-improving potentials of the plant might be attributed to its BBB secondary metabolites detected in UHPLC-MS. The AA was found to possess conessine (Compound 13), an alkaloid, that has anti-inflammatory and antioxidant activities. It freely crosses the BBB with slow clearance from the central nervous system (Zhao et al., 2008). Previously, Bandaru et al. investigated the impact of chronic administration of conessine on neurobehavioral activities in rat models of AD. The animals treated with conessine showed better memory retrieval and consolidation in passive avoidance and novel object recognition tests with improved antioxidant enzyme levels in the hippocampal area (Bandaru et al., 2020). A derivative of conessine, DMNG-3, can cross the BBB and is a novel acetylcholinesterase inhibitor. The study by Zhang et al. reported that DMNG-3 could be progressed to develop a new remedy for AD as it dose-dependently improved the memory in scopolamine amnesic mice (Zhang et al., 2016). Another study explored the effect of crude extract of Holarrhena antidysenterica rich in conessine in learning and memory of amnesic mice and reported its benefits in the management of dementia (Kaur et al., 2020). Various studies report that diets lacking fatty acids are correlated with impairment in cognitive function (Albouchi et al., 2013). Chalcones, detected in AA, are the phytochemicals that might protect the neurons against Aβ-induced death in ischemic brain injury (Kumar and Khanum, 2012). Triterpenoids are also active phytochemicals known to revitalize and strengthen neuronal function (Cho et al., 2020).
5. Conclusion
The outcomes of this study suggest that prolonged pretreatment with AA alleviates anxiety and scopolamine-induced memory impairment in rats. The biochemical analysis of brain homogenates provided a clue that these outcomes might be the result of anticholinesterase and the antioxidant potential of its secondary metabolites. The docking studies also predicted that AA has compounds with good BBB permeability and lower binding energies with acetylcholinesterase. In the future, this plant might be a valuable source of neuroprotective phytocompounds. Their isolation and detailed investigation of the exact mechanism of action might prove fruitful contributions to the development of newer neuroprotective therapeutic options.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors are thankful to Mr. Muhammad Imran, an animal house attendant for taking care of animals. Furthermore, the authors extended their appreciation to Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia for funding this work through Research Supporting Project Number (RSP2023R131).
Ethics Statement
All animal studies were performed after availing authorization from the departmental ethical committee of BZU, Multan (09/PEC/2015) and were accomplished by following instructions of the “Institute of Laboratory Animal Resources” (ILAR), Commission on Life Sciences, National Research Council (NRC, 1996).
Authors’ contributions
II, WA, FA and HMAR designed this study and performed the experiments. II, SJ, MFR, SMMA and TA contributed to manuscript writing and statistical assessment of data. SAK, HS, ZUH and AR performed the chemical characterization and molecular docking studies. All authors read and approved the final manuscript.
Funding
This work was funded by Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia through research supporting project Number (RSP2023R131).
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Faleh Alqahtani, Email: afaleh@ksu.edu.sa.
Imran Imran, Email: imran.ch@bzu.edu.pk.
References
- Adebiyi O.E., Olayemi F.O., Ning-Hua T., Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci. 2017;6:10–14. doi: 10.1016/j.bjbas.2016.12.003. [DOI] [Google Scholar]
- Ajiboye T.O., Yakubu M.T., Oladiji A.T. Cytotoxic, antimutagenic, and antioxidant activities of methanolic extract and chalcone dimers (Lophirones B and C) derived from Lophira alata (Van Tiegh. Ex Keay) stem bark. J. Evidence-Based Complement. Altern. Med. 2014;19:20–30. doi: 10.1177/2156587213505112. [DOI] [PubMed] [Google Scholar]
- Ajiboye T.O., Yakubu M.T., Oladiji A.T. Lophirones B and C prevent aflatoxin B1-induced oxidative stress and DNA fragmentation in rat hepatocytes. Pharm. Biol. 2016;54:1962–1970. doi: 10.3109/13880209.2015.1137603. [DOI] [PubMed] [Google Scholar]
- Albouchi F., Hassen I., Casabianca H., Hosni K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. South African J. Bot. 2013;87:164–174. doi: 10.1016/J.SAJB.2013.04.003. [DOI] [Google Scholar]
- Albright T.P., Chen H., Chen L., Guo Q. The ecological niche and reciprocal prediction of the disjunct distribution of an invasive species: The example of Ailanthus altissima. Biol. Invasions. 2010;12:2413–2427. doi: 10.1007/S10530-009-9652-8. [DOI] [Google Scholar]
- Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/METABO9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z., Shah R.C., Bennett D.A. Diagnosis and management of dementia: review. JAMA. 2019;322:1589–1599. doi: 10.1001/JAMA.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban H., Nazıroğlu M., Demirci K., Övey İ.S. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol. Neurobiol. 2017;54:2852–2868. doi: 10.1007/S12035-016-9835-0. [DOI] [PubMed] [Google Scholar]
- Bandaru N., Komavari C., Gorla U.S., Koteswarao G.S.N., Kulandaivelu U., Ankarao A. Neuroprotective effect of Conessinin on Elevated oxidative stress induced Alzheimers’disease in rats. Res. J. Pharm. Technol. 2020;13:2703–2707. doi: 10.5958/0974-360X.2020.00481.3. [DOI] [Google Scholar]
- Caliandro R., Pesaresi A., Cariati L., Procopio A., Oliverio M., Lamba D. Kinetic and structural studies on the interactions of Torpedo californica acetylcholinesterase with two donepezil-like rigid analogues. J. Enzyme Inhib. Med. Chem. 2018;33:794–803. doi: 10.1080/14756366.2018.1458030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Khan S., Avula B., Lata H., Yang M.H., ElSohly M.A., Khan I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evidence-based Complement. Altern. Med. 2014;2014:1–9. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.M., Ha T.K.Q., Doan T.P., Dhodary B., An J.P., Lee B.W., Yang J.L., Oh W.K. Neuroprotective effects of triterpenoids from Camellia japonica against amyloid β-Induced neuronal damage. J. Nat. Prod. 2020;83:2076–2086. doi: 10.1021/acs.jnatprod.9b00964. [DOI] [PubMed] [Google Scholar]
- Chow C.K., Tappel A.L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972;7:518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Cosme P., Rodríguez A.B., Espino J., Garrido M. Plant phenolics: bioavailability as a key determinant of their potential health-promoting applications. Antioxidants. 2020;9:1–20. doi: 10.3390/ANTIOX9121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S.L., Silva V.D.A., dos Santos Souza C., Santos C.C., Paris I., Muñoz P., Segura-Aguilar J. Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox. Res. 2016;30:41–52. doi: 10.1007/S12640-016-9600-1. [DOI] [PubMed] [Google Scholar]
- Demirci K., Nazıroğlu M., Övey İ.S., Balaban H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017;32:321–329. doi: 10.1007/S11011-016-9903-1. [DOI] [PubMed] [Google Scholar]
- Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Griffin S.L., Van Reekum R., Masanic C. A review of cholinergic agents in the treatment of neurobehavioral deficits following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2003;15:17–26. doi: 10.1176/JNP.15.1.17. [DOI] [PubMed] [Google Scholar]
- Hanrahan J.R., Chebib M., Johnston G.A.R. Flavonoid modulation of GABAA receptors. Br. J. Pharmacol. 2011;163(2):234–245. doi: 10.1111/j.1476-5381.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illouz T., Madar R., Louzon Y., Griffioen K.J., Okun E. Unraveling cognitive traits using the Morris water maze unbiased strategy classification (MUST-C) algorithm. Brain. Behav. Immun. 2016;52:132–144. doi: 10.1016/J.BBI.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Imran I., Javaid S., Waheed A., Rasool M.F., Majeed A., Samad N., Saeed H., Alqahtani F., Ahmed M.M., Alaqil F.A. Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 2021;7 doi: 10.3389/fnut.2020.587367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid U., Javaid S., Ashraf W., Rasool M.F., Noman O.M., Alqahtani A.S., Majeed A., Shakeel W., Albekairi T.H., Alqahtani F., Imran I. Chemical profiling and dose-dependent assessment of fear reducing and memory-enhancing effects of Solanum virginianum in Rats. Dose. Response. 2021;19 doi: 10.1177/1559325821998486. 1559325821998486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M.H., Yook J., Lee E., Lin C.X., Quan Z., Son K.H., Bae K.H., Kim H.P., Kang S.S., Chang H.W. Anti-inflammatory activity of Ailanthus altissima in ovalbumin-induced lung inflammation. Biol. Pharm. Bull. 2006;29:884–888. doi: 10.1248/BPB.29.884. [DOI] [PubMed] [Google Scholar]
- Jin M.H., Yook J., Lee E., Lin C.X., Quan Z., Son K.H., Bae K.H., Kim H.P., Kang S.S., Chang H.W. Anti-inflammatory activity of Ailanthus altissima in ovalbumin-induced lung inflammation. Biol. Pharm. Bull. 2006;29:884–888. doi: 10.1248/BPB.29.884. [DOI] [PubMed] [Google Scholar]
- John O.O., Amarachi I.S., Chinazom A.P., Adaeze E., Kale M.B., Umare M.D., Upaganlawar A.B. Phytotherapy: A promising approach for the treatment of Alzheimer’s disease. Pharmacol. Res. - Mod. Chinese Med. 2022;2 doi: 10.1016/J.PRMCM.2021.100030. [DOI] [Google Scholar]
- Kang T.H., Choi I.Y., Kim S.J., Moon P.D., Seo J.U., Kim J.J., An N.H., Kim S.H., Kim M.H., Um J.Y., Hong S.H., Kim H.M., Jeong H.J. Ailanthus altissima swingle has anti-anaphylactic effect and inhibits inflammatory cytokine expression via suppression of nuclear factor-kappaB activation. Vitr. Cell. Dev. Biol. - Anim. 2010;46:72–81. doi: 10.1007/S11626-009-9237-Y/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- Kaur J., Kumar M., Bansal N. Amelioration of dementia and antioxidant activity of Holarrhena antidysenterica bark in mice. Curr. Psychopharmacol. 2020;9:43–57. doi: 10.2174/2211556009666200129123134. [DOI] [Google Scholar]
- Kim H.M., Lee J.S., Sezirahiga J., Kwon J., Jeong M., Lee D., Choi J.H., Jang D.S. A new canthinone-type alkaloid isolated from Ailanthus altissima Swingle. Molecules. 2016;21:642. doi: 10.3390/MOLECULES21050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Park Y., Li M.o., Kim Y.K., Lee S., Son S.Y., Lee S., Lee J.S., Lee C.H., Park H.H., Lee J.-Y., Hong S., Cho Y.-C., Kim J.-W., Yoo H.M., Cho N., Lee H.-S., Lee S.H. Anti-inflammatory effect of Ailanthus altissima (Mill.) Swingle leaves in lipopolysaccharide-stimulated astrocytes. J. Ethnopharmacol. 2022;286:114258. doi: 10.1016/j.jep.2021.114258. [DOI] [PubMed] [Google Scholar]
- Ko Y.H., Kim S.K., Lee S.Y., Jang C.G. Flavonoids as therapeutic candidates for emotional disorders such as anxiety and depression. Arch. Pharm. Res. 2020;43:1128–1143. doi: 10.1007/S12272-020-01292-5. [DOI] [PubMed] [Google Scholar]
- Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6:81. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li Y., Ma S., Zhao Q., Wu J., Duan L., Xie Y., Wang S. Traditional uses, phytochemistry, and pharmacology of Ailanthus altissima (Mill.) Swingle bark: a comprehensive review. J. Ethnopharmacol. 2021;275 doi: 10.1016/J.JEP.2021.114121. [DOI] [PubMed] [Google Scholar]
- Liu L., Xuan C., Shen P., He T., Chang Y., Shi L., Tao S., Yu Z., Brown R.E., Wang J. Hippocampal mechanisms underlying impairment in spatial learning long after establishment of noise-induced hearing loss in CBA mice. Front. Syst. Neurosci. 2018;12:35. doi: 10.3389/FNSYS.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A. Current therapeutic options for Alzheimer’s disease. Curr. Genomics. 2007;8:550–558. doi: 10.2174/138920207783769549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luís Â., Gil N., Amaral M.E., Domingues F., Duarte A.P. Ailanthus altissima (Miller) Swingle: A source of bioactive compounds with antioxidant activity. Bioresources.com. 2012;7:2105–2120. doi: 10.15376/BIORES.7.2.2105-2120. [DOI] [Google Scholar]
- Malik H., Javaid S., Fawad Rasool M., Samad N., Rizwan Ahamad S., Alqahtani F., Imran I. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of Ficus benghalensis in behavioral experimental models. Medicina (B. Aires) 2020;56:144. doi: 10.3390/medicina56030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.I., Ressler K.J., Binder E., Nemeroff C.B. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr. Clin. North Am. 2009;32(3):549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewska A., Jaszek M., Stefaniuk D., Ciszewski T., Matuszewski Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS One. 2018;13:e0197044. doi: 10.1371/JOURNAL.PONE.0197044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedel C.J., Patton J.M., Miedel A.N., Miedel E.S., Levenson J.M. Assessment of spontaneous alternation, novel object recognition and limb clasping in transgenic mouse models of amyloid and tau neuropathology. J. Vis. Exp. 2017;123:55523. doi: 10.3791/55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed H.R., El-Wakil E.A., Abdel-Hameed E.S.S., El-Hashash M.M., Shemis M. Evaluation of total phenolics, flavonoids, and antioxidant and cytotoxic potential of Ailanthus altissima (Mill.) swingle leaves. J. Reports Pharm. Sci. 2021;10:130. doi: 10.4103/JRPTPS.JRPTPS_7_21. [DOI] [Google Scholar]
- Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev. Neurother. 2008;8(11):1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad T., Ali T., Ikram M., Khan A., Alam S.I., Kim M.O. Melatonin rescue oxidative stress-mediated neuroinflammation/ neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J. Neuroimmune Pharmacol. 2019;14:278–294. doi: 10.1007/S11481-018-9824-3. [DOI] [PubMed] [Google Scholar]
- Naskar S., Islam A., Mazumder U.K., Saha P., Haldar P.K., Gupta M. In vitro and in vivo antioxidant potential of hydromethanolic extract of Phoenix dactylifera Fruits. J. Sci. Res. 2009;2:144–157. doi: 10.3329/JSR.V2I1.2643. [DOI] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:1–15. doi: 10.1017/JNS.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedre L.L., Gallardo J.M., Chacón L.M.M., García A.V., Flores-Mendoza M., Neri-Gómez T., Díaz B.E., Cruz-Xenes R.M., Fuentes N.P., Orozco-Suárez S. Oxidative stress in patients with drug resistant partial complex seizure. Behav. Sci. (Basel) 2018;8:59. doi: 10.3390/bs8060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/JCC.20084. [DOI] [PubMed] [Google Scholar]
- Rabelo A.C.S., de Pádua Lúcio K., Araújo C.M., de Araújo G.R., de Amorim Miranda P.H., Carneiro A.C.A., de Castro Ribeiro É.M., de Melo Silva B., de Lima W.G., Costa D.C. Baccharis trimera protects against ethanol induced hepatotoxicity in vitro and in vivo. J. Ethnopharmacol. 2018;215:1–13. doi: 10.1016/j.jep.2017.12.043. [DOI] [PubMed] [Google Scholar]
- Rahman H.M.A., Javaid S., Ashraf W., Rasool M.F., Anjum S.M.M., Saleem H., Siddique F., Chtita S., Sivandzade F., Alqahtani F., Alotaibi M.R., Imran I. Neuropharmacological investigation, ultra-high performance liquid chromatography analysis, and in silico studies of Phyla nodiflora. J. Physiol. Pharmacol. 2021;72:637–654. doi: 10.26402/JPP.2021.4.15. [DOI] [PubMed] [Google Scholar]
- Rashed K., Slowing K., Said A., Cueto M. Analgesic, antipyretic and antiulcer activities of Ailanthus altissima (Mill.) Swingle. Phytopharmacol. 2012;3:341–350. [Google Scholar]
- Rashid S., Ahmad M., Zafar M., Sultana S., Ayub M., Khan M.A., Yaseen G. Ethnobotanical survey of medicinally important shrubs and trees of Himalayan region of Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol. 2015;166:340–351. doi: 10.1016/J.JEP.2015.03.042. [DOI] [PubMed] [Google Scholar]
- Rehman Z., Farooq T., Javaid S., Ashraf W., Fawad Rasool M., Samad N., Tariq M., Muhammad Muneeb Anjum S., Sivandzade F., Alotaibi F., Alqahtani F., Imran I. Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi Pharm. J. 2022;30(5):494–507. doi: 10.1016/j.jsps.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjad Haider M., Ashraf W., Javaid S., Fawad Rasool M., Muhammad Abdur Rahman H., Saleem H., Muhammad Muneeb Anjum S., Siddique F., Morales-Bayuelo A., Kaya S., Alqahtani F., Alasmari F., Imran I. Chemical characterization and evaluation of the neuroprotective potential of Indigofera sessiliflora through in-silico studies and behavioral tests in scopolamine-induced memory compromised rats. Saudi J. Biol. Sci. 2021;28(8):4384–4398. doi: 10.1016/j.sjbs.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel, W., Javaid, S., Rasool, M.F., Samad, N., Alasmari, F., Alasmari, A.F., Alaqil, F.A., Alqarni, S.A., Alotaibi, F.M., Alqahtani, F., Imran, I., Sciences, A., Arabia, S., 2020. time course evaluation of lacosamide alone and in polypharmacy on behavioral manifestations and oxidative stress in lithium-pilocarpine-induced model. 71, 547–564. https://doi.org/10.26402/jpp.2020.4.10 [DOI] [PubMed]
- Sharifi-Rad M., Lankatillake C., Dias D.A., Docea A.O., Mahomoodally M.F., Lobine D., Chazot P.L., Kurt B., Tumer T.B., Moreira A.C., Sharopov F., Martorell M., Martins N., Cho W.C., Calina D., Sharifi-Rad J. Impact of natural compounds on neurodegenerative disorders: from preclinical to pharmacotherapeutics. J. Clin. Med. 2020;9:1061. doi: 10.3390/JCM9041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Botanical phenolics and brain health. Neuromolecular Med. 2008;10(4):259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarawneh R., Holtzman D.M. The clinical problem of symptomatic alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012;2(5) doi: 10.1101/cshperspect.a006148. a006148 a6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R., Romanchuk F.E., Peterson C.J., Coats J.R. Plant growth regulatory effect and insecticidal activity of the extracts of the Tree of Heaven (Ailanthus altissima L.) BMC Ecol. 2002;2:1–6. doi: 10.1186/1472-6785-2-1/tables/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.Q., Xu Y.J., Yang C., Tang Y., Li L., Cai H.B., Hou B.N., Chen H.F., Wang Q., Shi X.G., Zhang S.J. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. Biomed Res. Int. 2016;2016:9852536. doi: 10.1155/2016/9852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.L., Yuan Y.L., Zhang D.M., Li F., Ye W.C., Shinjulactone O. A new quassinoid from the root bark of Ailanthus altissima. Nat. Prod. Res. 2014;28:1432–1437. doi: 10.1080/14786419.2014.909418/SUPPL_FILE/GNPL_A_909418_SM4086.DOC. [DOI] [PubMed] [Google Scholar]
- Zang C.X., Bao X.Q., Li L., Yang H.Y., Wang L., Yu Y., Wang X.L., Yao X.S., Zhang D. The protective effects of Gardenia jasminoides (Fructus Gardenia) on Amyloid-β-Induced mouse cognitive impairment and neurotoxicity. Am. J. Chin. Med. 2018;46:389–405. doi: 10.1142/S0192415X18500192. [DOI] [PubMed] [Google Scholar]
- Zhang X.G., Kou F., Ma G.D., Tang P., Yang Z.D. Pharmacokinetics and pharmacodynamics of a novel Acetylcholinesterase Inhibitor, DMNG-3. Acta Neurobiol. Exp. (Wars) 2016;76:117–124. doi: 10.21307/ANE-2017-011. [DOI] [PubMed] [Google Scholar]
- Zhao C., Sun M., Bennani Y.L., Gopalakrishnan S.M., Witte D.G., Miller T.R., Krueger K.M., Browman K.E., Thiffault C., Wetter J., Marsh K.C., Hancock A.A., Esbenshade T.A., Cowart M.D. The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. J. Med. Chem. 2008;51:5423–5430. doi: 10.1021/JM8003625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.