Graphical abstract
Keywords: Nitrosamines, Nitrosation, Control limits, Sartans, Ranitidine
Abbreviations: AI, acceptable intake; APIs, active pharmaceutical ingredients; ARBs, angiotensin II receptor blockers; AZBC, 4′-(azidomethyl)-[1.1′-biphenyl]-2-carbonitile; AZBT, 5-(4′-(azidomethyl)-[1,1′-biphenyl]-2-yl)-1H-tetrazole; AZTT, 5-(4′-((5-(azidomethyl)-2-butyl-4-chloro-1H-imidazol-1-yl) methyl)-[1,1′-biphenyl]-2-yl)-1H-tetrazole; CDER, center for drug evaluation and research; CPNP, 1-cyclopentyl-4-nitrosopiperazine; DBA, N,N-dibutylamine; DEA, N,N-diethylamine; DIPEA, N,N-diisopropylethylamine; DMA, dimethylamine; DMF, N,N-dimethyl formamide; DPA, N,N-dipropylamine; EMA, European Medicines Agency; EPA, Environmental Protection Agency; FDA, Food and Drug Administration; HSA, Health Sciences Authority; IARC, International Agency for Research on Cancer; ICH, International Council for Harmonisation; LD50, median lethal dose; MBA, N-methylamino-N-butyric acid; MDD, maximum daily dose; MNP, 1-methyl-4-nitrosopiperazine; NAP, nitrosation assay procedure; NDBA, N-nitrosodibutylamine; NDEA, N-nitrosodiethylamine; NDIPA, N-nitrosodiisopropylamine; NDMA, N-nitrosodimethylamine; NDSRIs, Nitrosamine drug substance-related impurities; NEIPA, N-nitroso ethylisopropylamine; NMBA, N-nitroso-N-methyl-4-aminobutyric acid; NMP, N-methyl pyrrolidinone; NOCs, N-nitroso compounds; PPRs, proportionate reporting ratios; SARs, structure–activity relationships; TD50, median toxic dose; TEA, triethylamine; TMA, trimethylamine; TTC, threshold of toxicological concern; USFDA, United States Food Drug and Administration; USP, United States Pharmacopoeia; WHO, World Health Organization
Abstract
Over the last two years, global regulatory authorities have raised safety concerns on nitrosamine contamination in several drug classes, including angiotensin II receptor antagonists, histamine-2 receptor antagonists, antimicrobial agents, and antidiabetic drugs. To avoid carcinogenic and mutagenic effects in patients relying on these medications, authorities have established specific guidelines in risk assessment scenarios and proposed control limits for nitrosamine impurities in pharmaceuticals. In this review, nitrosation pathways and possible root causes of nitrosamine formation in pharmaceuticals are discussed. The control limits of nitrosamine impurities in pharmaceuticals proposed by national regulatory authorities are presented. Additionally, a practical and science-based strategy for implementing the well-established control limits is notably reviewed in terms of an alternative approach for drug product N-nitrosamines without published AI information from animal carcinogenicity testing. Finally, a novel risk evaluation strategy for predicting and investigating the possible nitrosation of amine precursors and amine pharmaceuticals as powerful prevention of nitrosamine contamination is addressed.
1. Introduction
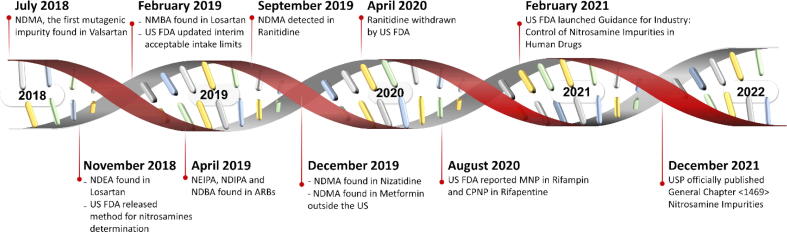
Nitrosamines have been classified based on their carcinogenic potential into four groups by the International Agency for Research on Cancer (IARC), World Health Organization (WHO) (1987). Group 1 compounds, e.g., N-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), have sufficient incidences of carcinogenic effects on humans while Group 2A compounds such as N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitroso-N-methyl-4-aminobutyric acid (NMBA) are probably carcinogenic to humans with limited evidence in humans but sufficient evidence in animals. Examples of Group 2B such as 1-methyl-4-nitrosopiperazine (NMP) and 1-cyclopentyl-4-nitrosopiperazine (CPNP) are considered to be possibly carcinogenic to humans with limited evidence in humans as well as in animals. The nitrosamines, with inadequate data on their carcinogenicity in human and experimental animals, are classified in group 3 such as N-nitrosodiphenylamine and N-nitrosoguvacoline. In the ICH guideline for industry M7 (R1), they are listed as a representative “cohort of concern” of mutagenic carcinogens and are categorized as Class 1 when known rodent carcinogenicity and mutagenicity data were characterized such as NDMA and NDEA. Nitrosamine impurities in pharmaceuticals have concerned several national regulatory agencies since 2018, leading to critical drug supply shortages (Brian Byrd et al., 2019). The timeline of emerging nitrosamine contamination is depicted in Fig. 1 and described below.
Fig. 1.
Evolution timeline of nitrosamine-contaminated pharmaceuticals. NDMA = N-nitrosodimethylamine, NDEA = N-nitrosodiethylamine, NMBA = N-nitroso-N-methyl-4-aminobutyric acid, NEIPA = N-nitroso ethylisopropylamine, NDIPA = N-nitrosodiisopropylamine, NDBA = N-nitrosodibutylamine.
When NDMA was raised as the first mutagenic impurity in valsartan-containing medicines in mid-2018, voluntary recalls were announced by the United States Food and Drug Administration (US FDA) (https://www.fda.gov/news-events/press-announcements/fda-announces-voluntary-recall-several-medicines-containing-valsartan-following-detection-impurity, accessed 13 Jul 2018) and the European Medicines Agency (EMA) (https://www.ema.europa.eu/en/documents/referral/angiotensin-ii-receptor-antagonists-sartan-article-31-referral-chmp-list-questions-be-addressed-api_en.pdf, accessed 16 Jul 2018). Since the report of NDMA contamination in valsartan, nitrosamine species have been detected in other sartan-containing medicines such as losartan, irbesartan, and candesartan (https://www.ema.europa.eu/en/documents/other/tempory-interim-limit-nmba-dipna-eipna-impurities-sartan-blood-pressure-medicines_en.pdf, accessed 20 Aug 2019). These species include N-nitroso diethylamine (NDEA), N-nitroso-N-methyl-4-aminobutyric acid (NMBA), N-nitroso ethylisopropylamine (NEIPA), N-nitrosodiisopropylamine (NDIPA), and N-nitrosodibutylamine (NDBA). In 2019, ranitidine and nizatidine were found to be associated with NDMA contamination because they are inherently unstable; consequently, the US FDA (https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market, accessed 01 Apr 2020) and EMA (https://www.ema.europa.eu/en/news/suspension-ranitidine-medicines-eu, accessed 30 Apr 2020) terminated ranitidine use in 2020 after 30 years of clinical therapeutic applications. Furthermore, the Singapore Health Sciences Authority (HSA) announced the metformin recall in 2019 due to NMDA contamination (https://www.hsa.gov.sg/announcements/news/hsa-recalls-three-out-of-46-metformin-medicines, accessed 04 Dec 2019). The FDA’s Center for Drug Evaluation and Research (CDER) has also raised awareness of NMDA contamination in metformin outside the US (https://www.fda.gov/news-events/press-announcements/statement-janet-woodcock-md-director-fdas-center-drug-evaluation-and-research-impurities-found, accessed 05 Dec 2019). Nitrosamines have been reported not only in wholly synthetic drugs, but also in semisynthetic antimicrobial agents such as rifampin and rifapentine. More specifically, 1-methyl-4-nitrosopiperazine (MNP) and 1-cyclopentyl-4-nitrosopiperazine (CPNP), which are structurally related to the medicines (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-nitrosamines-rifampin-and-rifapentine, accessed 26 Aug 2020), have been detected in rifampin and rifapentine, respectively.
To date, various nitrosamines have been found in several therapeutic classes, including angiotensin II receptor antagonists or angiotensin II receptor blockers (ARBs), histamine-2 receptor antagonists, antimicrobial agents, and antidiabetic drugs. National regulatory authorities in many countries have responded to nitrosamine contamination with numerous press releases, specific guidance, and risk assessment strategies to determine the root cause of nitrosamine contamination. The acceptable limits of nitrosamines in pharmaceuticals have been established based on their toxic levels (https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf, accessed 23 Jun 2019; https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan, accessed 28 Feb 2019). In addition, several pieces of literature have reviewed the formation and regulatory control of nitrosamine impurities in APIs and drug products (Aldawsari et al., 2021, Beard and Swager, 2021, Borths et al., 2021, Dattatraya et al., 2022, Shaik et al., 2020, Shaikh et al., 2020, Snodin and Elder, 2019, Tuesuwan and Vongsutilers, 2021). These review articles include nitrosamine chemistry and reactivity, pathways of N-nitroso compound formation, regulatory agencies’ actions, the source of the NDMA contamination, a range of possible risk assessments mainly based on ICH M7 (R1) criteria and analytical aspects as summarized in Table 1. In 2019, the short commentary by Snodin and Elder (2019) focused on NDMA contamination of valsartan products. Later, Shaikh et al. (2020) reported the possibility of nitrosamine contamination in pharmaceuticals. Limit criteria and published analytical methods for NDMA and NDEA in sartans were specified. At the same time, basic principles and recently updated information on methodologies and sample preparation techniques for trace analysis of nitrosamines in pharmaceuticals were discussed by Shaik et al. (2020). Moreover, López-Rodriguez et al. (2020) mainly focused on the chemical principles of possible formation pathways of N-nitroso compounds in terms of reaction conditions and reactants. In 2021, the review of nitrosamine contamination in sartan, ranitidine and nizatidine was presented by Tuesuwan and Vongsutilers (2021). In such a review, the basic methodologies for nitrosamine determination were reported, and the risk assessments-based approach was outlined. Aldawsari et al. (2021) reported the specific observation highlighted in the mechanisms for NDMA from ranitidine degradation. Chronological events of the EU regulatory network responses and the Croatian market situation specific to NDMA and NDEA contamination in marketed pharmaceuticals have been summarized by Sedlo et al. (2021). The case reports and risk analysis of drug product recalls by global pharmaceutical industries from the FDA database were reported by Bharate (2021). The perspectives on chemistry, reactivity and synthetic applications of N-nitrosamines were discussed by Beard and Swager, 2021 and Borths et al. (2021). Many nitrosamine decomposition and deactivation processes were summarized in the reviews (Beard and Swager, 2021, Borths et al., 2021). Recently, a short review of the contamination events in pharmaceutical and control measures from the global regulatory agencies was presented by Dattatraya et al. (2022).
Table 1.
Summary of the main focus of previous review articles on nitrosamine contamination.
| Year of Publication | Title | Main focus | Ref. |
|---|---|---|---|
| 2019 | Short commentary on NDMA (N-nitrosodimethylamine) contamination of valsartan products | Specific review of NDMA contamination in valsartan products | Snodin and Elder (2019) |
| 2020 | Nitrosamine impurities in drug substances and drug products | Review of nitrosamine cross-contamination possibilities to pharmaceuticals, limits and acceptable intake of NDMA and NDEA in sartans and published analytical techniques. | Shaikh et al. (2020) |
| 2020 | Regulatory updates and analytical methodologies for nitrosamine impurities detection in sartans, ranitidine, nizatidine, and metformin along with sample preparation techniques | Basic principles and recently updated information on methods and sample preparation for nitrosamine analysis. | Shaik et al. (2020) |
| 2020 | Pathways for N-nitroso compound formation: Secondary amines and beyond | The reaction conditions and reactant leading to N-nitroso compound formations. | López-Rodriguez et al. (2020) |
| 2021 | Nitrosamine contamination in pharmaceuticals: Threat, Impact, and Control | The source of nitrosamine contamination in pharmaceuticals such as sartans, ranitidine and nizatidine. Discuss the methodologies for trace nitrosamine analyses, risk assessment-based approach and control measures. | Tuesuwan and Vongsutilers (2021) |
| 2021 | N-nitrosodimethylamine (NDMA) contamination of Ranitidine Products: A review of recent findings | Specific observation of ranitidine degradation to NDMA | Aldawsari et al. (2021) |
| 2021 | Presence of nitrosamine impurities in medicinal products | Short communication of the EU regulatory response and the Croatian market situation on NDMA and NDEA contamination events in pharmaceutical products | Sedlo et al. (2021) |
| 2021 | Critical analysis of drug product recalls due to nitrosamine impurities. | Case reports and risk analysis of drug product recalls due to the nitrosamine impurities | Bharate (2021) |
| 2021 | An organic chemist’s guide to N-nitrosamines: Their structure, reactivity, and role as contaminants | The perspective on the chemical structure, reactivity, and synthetic applications of alkyl N-nitrosamines | Beard and Swager (2021) |
| 2021 | Nitrosamine reactivity: A survey of reactions and purge processes. | The development of effective chemical processes for the control of nitrosamine contamination. | Borths et al. (2021) |
| 2022 | A review of nitrosamine impurities present in drugs | Short report the contamination events in pharmaceuticals and control measures from the global regulatory agencies such as EMA, CHMP, USFDA | Dattatraya et al. (2022) |
Currently, nitrosamine formation pathways and approaches for establishing specific control limits have been reported due to an increase in concerns about nitrosamine contamination in pharmaceuticals. In this review, we update the current status of nitrosation pathways including basic and advanced chemistry of nitrosamine formation and chemical reactivity of nitrosamines to complement other recent publications. All known possible root causes of nitrosamine formation in pharmaceuticals are systematically summarized. The control limits of nitrosamine impurities in pharmaceuticals proposed by national regulatory authorities are re-visited. Novel approaches to AI estimation for unique and complex N-nitroso pharmaceutical analogues without sufficient animal carcinogenicity data are addressed in this review article. In addition, a novel risk evaluation strategy for scrutinizing possible nitrosation of amine precursors and amine pharmaceuticals during drug development and registration is discussed.
2. Nitrosation pathways of nitrosamine formation
Nitrosamines in pharmaceutical products arise from APIs, manufacturing processes, direct and indirect cross-contamination from solvents and equipment, and chemical degradation during storage. During the API manufacturing process, nitrosamine contaminants can be sourced from the starting materials, intermediates, solvents, and reagents during the synthesis. Determining the root cause of nitrosamine formation and contamination in specific pharmaceuticals is essential for developing pharmaceutical products, in which the nitrosamines remain within acceptable limits. For example, nitrosamines are commonly formed by nitrosation of an amine precursor co-existing with a nitrosating agent. These contaminants can be minimized under strong acidic conditions that reduce the amine reactivity. Although the nitrosation process is enhanced at acidic pH, it is less reactive at very low pH due to the protonation of amines. The preferred condition of nitrosation balances the pH against the basicity of the amine precursors. Most nitrosating agents used in API synthesis are the oxidized forms of nitrogen-containing compounds such as nitrous acid (HNO2) and nitrite (NO2–). Other nitrosating reagents are dinitrogen trioxide/nitrous anhydride (N2O3), dinitrogen tetroxide (N2O4), nitrosyl chloride (NOCl), nitrosonium tetrafluoroborate (NOBF4), and nitrothiocyanate (CN2OS).
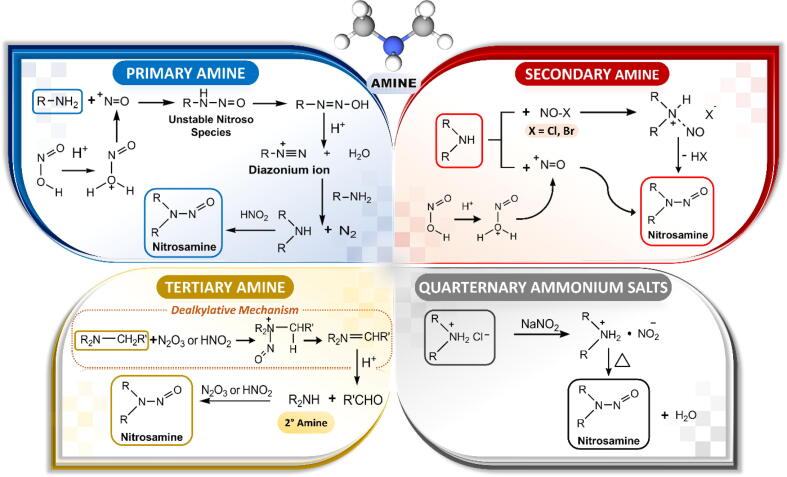
The possible mechanisms of nitrosation of primary, secondary, and tertiary amines and ammonium salts are illustrated in Fig. 2. Primary amines are readily nitrosated by nitrosating agents, generating unstable N-nitroso products that rapidly decompose into diazonium salts. However, the nitrosation of secondary amines to diazonium species is rare because the process is hindered by alkyl groups. Therefore, secondary amine precursors are promptly converted to their corresponding nitrosamines and are considered as the most reactive species. Tertiary amines can directly convert to secondary amines via a dealkylative mechanism (https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf, accessed 23 Jun 2019) and thereafter react with nitrites to form the corresponding N-nitrosamine (Smith and Loeppky, 1976). Various amine and amide precursors of associated nitrosamine impurities are summarized in Table 2.
Fig. 2.
Pathways of nitrosamine formation between nitrosating agents and primary, secondary, and tertiary amines and ammonium salts.
Table 2.
Amine and amide precursors of nitrosamine impurities in pharmaceuticals.
| Nitrosamines | Amine and Amide Precursors |
|---|---|
| N-nitrosodimethylamine (NDMA) |
N,N-dimethyl formamide (DMF) dimethylamine (DMA) trimethylamine (TMA) |
| N-nitrosodiethylamine (NDEA) |
N,N-diethylamine (DEA) triethylamine (TEA) |
| N-nitroso-N-methyl-4-aminobutyric acid (NMBA) |
N-methylamino-N-butyric acid (MBA) N-methyl pyrrolidinone (NMP) |
| N-nitrosodiisopropylamine (NDIPA) | N,N-diisopropylethylamine (DIPEA) |
| N-nitrosoethylisopropylamine (NEIPA) | N,N-diisopropylethylamine (DIPEA) |
| N-nitrosodibutylamine (NDBA) | N,N-dibutylamine (DBA) |
| 1-methyl-4-nitrosopiperazine (MNP) | 1-methyl piperazine |
| 1-cyclopentyl-4-nitrosopiperazine (CPNP) | 1-cyclopentyl piperazine |
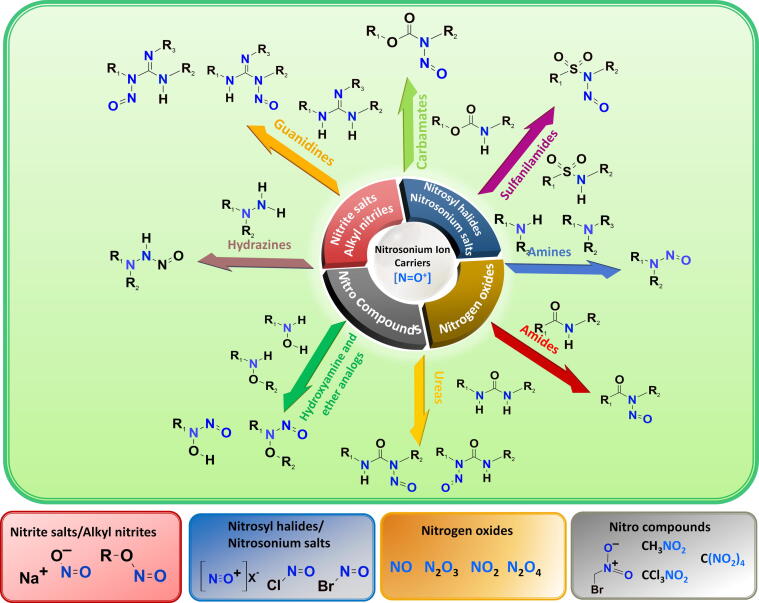
For the preventive control strategy, understanding the reaction conditions (i.e. reactants and specific conditions) of nitrosamine formation in drug synthesis steps is essential for risk evaluation of an API manufacturing process. López-Rodriguez et al. (2020) have revealed nitrosamine formation pathways between NH-containing substrates and various nitrosating agents, i.e., nitrosonium ion carriers ([NO+]). In addition to secondary and tertiary amines, N-nitrosation can be formed via other secondary NH-containing compounds such as amides, carbamates, sulfonamides, hydroxylamines and their ethers, hydrazines, hydrazones, hydrazides, ureas, and guanidines as illustrated in Fig. 3 (López-Rodriguez et al., 2020). Although those functionalities are not as reactive as typical amines because of less nucleophilic lone pair electrons, enhanced electrophilicity of nitrosating agents with some acidic reagents, e.g., acetic acid, acetic anhydride, formic acid, or inorganic acid (such as hydrochloric acid) is required to accelerate the chemical reactivity (López-Rodriguez et al., 2020). The nucleophilicity characteristics of nitrogen functionalities, the reactivity of nitrosonium ion carriers and reaction conditions (i.e., solvents, temperature, pH, etc.) contribute to the rate of nitrosation reaction. Secondary amines usually require weaker nitrosating agents and milder conditions (lower temperature and shorter reaction time) compared to tertiary amines, hetero amides and other related NH-compounds (López-Rodriguez et al., 2020). In contrast, tertiary amines with an electron-rich aromatic substituent such as gramine and bromhexine are prone to undergo nitrosative dealkylation and rapid nitrosamine formation (López-Rodriguez et al., 2020). Likewise, secondary amides, carbamates and ureas have been proven to be good substrates for nitrosation, leading to N-nitroso amides, N-nitroso carbamates and N-nitroso ureas, respectively (Shiri et al., 2010). Hydroxylamines and their ethers contain more reactive nucleophilic nitrogens than amide-type nitrogen and readily react with sodium nitrite in aqueous acid resulting in N-nitrosohydroxylamines and N-nitrosohydroxylamine ethers, respectively (Gordon and Maskill, 2001; Khlestkin et al., 2000). Typically, hydrazide nitrosation occurs at a β-position nitrogen to carbonyl, whereas hydrazines undergo nitrosation on both sides of functionalized nitrogens (Rehse and König, 1995) or via an N—N cleavage/nitrosation process for N,N-disubstituted hydrazines (Ohwada et al., 2001). Guanidines can be N-nitrosated with sodium nitrite under acidic conditions, although very few cases are reported (Abou-Gharbia et al., 1981; Rice et al., 1985). Regarding nitrosating agents, it is well-established that nitrite cannot promptly react with secondary amine substrates without [NO+] conversion, which is not generally feasible under neutral and basic conditions. Interestingly, some carbonyl compounds (i.e., formaldehyde, pyridoxal and benzaldehydes, etc.) induce N-nitrosation of nitrites in neutral and alkaline solutions via an iminium ion intermediate, but all are less reactive than those in typical acidic solutions (Keeper and Roller, 1973). Apart from aqueous media, in the presence of sodium nitrite, appropriate Brϕnsted organic acids such as oxalic acid (Zolfigol, 1998), para-toluenesulfonic acid (PTSA) (Borikar and Paul, 2010) or Lewis acids such as tin (IV) chloride (Célariès and Párkányi, 2006) and bismuth (III) chloride (Chaskar et al., 2009) in dichloromethane (DCM) can rapidly trigger the formation of nitrosamines from secondary amines. Likewise, in the presence of ZnCl2 as a Lewis acid catalyst, NDMA and NDEA impurities in valsartan have arisen from NaNO2, which is used to quench the remaining NaN3 in the step of tetrazole ring formation (Xiaoren et al., 2014).
Fig. 3.
Possible pathways of N-Nitroso compound formation (López-Rodriguez et al., 2020).
Apart from nitrite salts, alkyl nitrites, nitrosyl halides, nitrogen oxides and organic nitro compounds are powerful nitrosonium carriers as shown in Fig. 3. Alkyl nitrites are very powerful nitrosating agents under mild reaction conditions in both aqueous and organic media (Iglesias and Casado, 2002). In aqueous acidic media, they readily undergo acid-catalyzed hydrolysis to yield nitrous acid-like nitrosating agents (López-Rodriguez et al., 2020). In basic aqueous media, alkyl nitrites can convert to innocuous nitrite, but the reaction rate is much slower than in acidic aqueous hydrolysis (López-Rodriguez et al., 2020). In organic media, tert-butyl nitrite (TBN) is most frequently used for N-nitrosation of secondary and tertiary amines (López-Rodriguez et al., 2020). Preferably, the polar protic and aprotic solvents such as DCM, tetrahydrofuran (THF) and acetonitrile are selected as reaction solvents (López-Rodriguez et al., 2020). The N-nitrosation of less reactive secondary amides has also been achieved with alkyl nitrites at room temperature (Yedage and Bhanage, 2017). Furthermore, some organic nitro compounds such as bromonitromethane (Challis and Yousaf, 1990), trichloronitromethane (Demir et al., 1992), and tetranitromethane (Fan et al., 1978) can act as effective nitrosating agents. The reaction was driven by an iminium intermediate and nitrite formation after the nitro-substitution on a secondary amine substrate (Challis and Yousaf, 1990). In addition, nitrogen oxides are one of the most classical [NO+] carriers. Four members of this class are nitric oxide (NO), nitrogen dioxide (NO2), dinitrogen trioxide/nitrous anhydride (N2O3), and dinitrogen tetroxide (N2O4) (López-Rodriguez et al., 2020). NO itself is a non-effective nitrosating agent. In the presence of catalytic oxygen, NO is oxidized to NO2, which can subsequently dimerize to N2O4 and/or combine with NO2 to yield N2O3. Under an anaerobic environment, NO can be activated by iodine to yield nitrosyl iodide (López-Rodriguez et al., 2020). In addition to in situ generations of nitrosyl iodide, nitrosyl halides and nitrosonium salts are the most common commercially available reagents as nitrosating agents (López-Rodriguez et al., 2020). Due to their instability, the aqueous solution is inappropriate for the reaction with nitrosyl halides. DCM, acetonitrile, toluene and dimethylformamide are most frequently used for the reaction solvents (López-Rodriguez et al., 2020). Consequently, nitrosyl halides and nitrosonium salts are considered efficient nitrosating agents in organic solvents. N-nitrosation of sulfonamides (Dewynter et al., 1996), ureas (Miyahara, 1986), and carbamates (Martinez et al., 1982) has been achieved by utilizing nitrosyl halides (i.e., NOCl) and nitrosonium salts (i.e., NOBF4) (Thakkalapally and Benin, 2005) in organic solvents under mild condition. Both N2O3 and N2O4 are active nitrosating forms of [NO+] which can nitrosate at NH of secondary and tertiary amines in organic and aqueous solutions (López-Rodriguez et al., 2020). In aqueous solutions, both proceed smoothly and more rapidly under a basic condition while slowly undergoing hydrolysis at pH > 5 to innocuous NO2– and NO3– (López-Rodriguez et al., 2020). However, the hydrolysis rate is much less rapid than the N-nitrosation process. In organic solvents, the reaction of secondary amines and N2O3 ends up with the corresponding nitrosamines and nitrous acid by-products at room temperature or lower because the reaction equilibrium can be remarkably promoted by the consumption of nitrous acid by-products with starting unprotonated amine (López-Rodriguez et al., 2020).
On the other hand, understanding the chemistry principle and reactivity of nitrosamines can further contribute to the development of effective remediation via the chemical process as the alternative scenario to deactivate nitrosamines in pharmaceuticals. Beard and Swager (2021) and Borths et al. (2021) have recently reported and discussed aspects of the basic chemical attributes of nitrosamines, reaction chemistry involving denitrosation of nitrosamines and reductive nitrosamine chemistry, and their chemical reactivity to develop the effective decomposition and deactivation processes. Beard and Swager (2021) described chemical reactions such as protolytic denitrosation and reactions with electrophiles to deactivate N-nitroso functionality. Lithium diisopropylamide (LDA) used in the nitrosamine eradication process can yield α-lithiated nitrosamines, leading to subsequent denitrosation with electrophilic displacement (Beard and Swager, 2021; Borths et al., 2021). Alternatively, organolithium and Grignard reagents can be used to decompose nitrosamines. Catalytic hydrogenation using Pd/C, Pt/C and Rh/C is of interest as an in-process control in industrial routes to convert nitrosamines to the corresponding hydrazines (Beard and Swager, 2021; Borths et al., 2021). Metal catalysts such as Pd/alumina, Ni/alumina, and Ru/alumina can reduce NDMA to DMA (Borths et al., 2021). Raney nickel is another reductive catalyst that cleaves the resulting N—N bond (Borths et al., 2021). Metal amides such as lithium, sodium, potassium, and calcium amides are capable of reducing simple nitrosamines (Borths et al., 2021). The photolytic method can also be used for nitrosamine decomposition (Beard and Swager, 2021). The above processes can be integrated into manufacturing to remediate, mitigate, and control nitrosamine adulteration in pharmaceuticals.
3. Possible root causes of nitrosamine contamination in pharmaceuticals
3.1. ARBs
With the exception of eprosartan, the core structure of ARBs or sartans is a biphenyl functional group. Structurally, ARBs are divided into two categories: tetrazole analogs and nontetrazole analogs. Nitrosamines presenting in ARBs are generally formed during API manufacturing and are identified as in-process impurities. Under the preferred condition of the nitrosation reaction, co-existing primary, secondary, and tertiary amines or quaternary ammonium salts react with nitrosating agents to generate nitrosamine impurities during the synthesis of ARBs. The chemical structures of ARBs are unrelated to those of the contaminating nitrosamines because the amine precursor arises from residual amines or amides in the organic solvents used in API synthesis. Nitrosating agents, including sodium nitrite, nitrous acid, nitrous anhydride, and nitrosyl halides, can arise from recycled solvents or reused catalysts from different processes or across manufacturing lines with inadequate control and inappropriate monitoring. Amines and nitrosating agents during the synthesis of ARBs can also arise from 1) impure starting materials or intermediates in the upstream step, 2) carry-over of other manufacturing processes along the same production line, and 3) cross-reaction between nitrocellulose in the packaging materials and some printing inks.
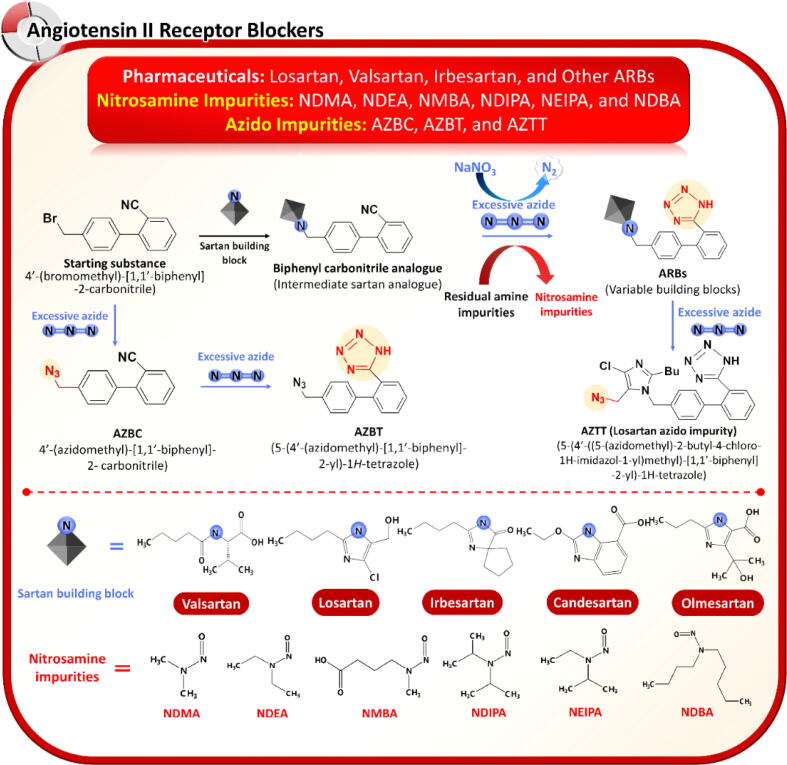
Nitrosamines found in ARBs depend on the synthetic route. They are mainly attributed to a tetrazole formation step. ARBs containing a tetrazole ring such as valsartan, losartan, irbesartan, candesartan, and olmesartan are prone to nitrosamine contamination whereas non-tetrazole ARB analogs such as eprosartan, telmisartan, and azilsartan are unlikely to contain nitrosamine impurities. The tetrazole ring is usually formed by the addition of hydrazoic acid to alkyl carbonitrile (Mihina and Herbst, 1950). To avoid the use of toxic and explosive hydrazoic acid, a tetrazole ring can be constructed by the formal [1 + 3] cycloaddition of nitriles and azide (Himo et al., 2002). Tetrazole formation using azide reagents is implemented primarily during the final step of sartan synthesis (Madasu et al., 2012). Sodium azide (NaN3) and organometallic azide derivatives such as tributyltin azide (Bu3SnN3) and trimethyltin azide (Me3SnN3) are preferable azide reagents despite their human hazardous potential, owing to their convenient handling and easy disposal. The unreacted azides from the tetrazole formation step are commonly eliminated by adding sodium nitrite in an acidic environment. This step generates nitrous acid with the subsequent release of nitrogen gas and nitrous oxide by-products (Stedman, 1959). During the disposition process of unreacted azides, any alkyl amine residues from the solvents, reagents or upstream contamination will likely generate associated nitrosamines. This contamination risk is unintended and unforeseen.
Valsartan was the first ARB recalled due to NDMA contamination. NDMA in valsartan derives from dimethylamine (DMA) as a residual contaminant in N,N-dimethylformamide (DMF), a common solvent used in API synthesis (Brian Byrd et al., 2019; USFNF, 2022). In addition, DMF degradation to DMA can coincide with ARB synthesis. Meanwhile, NDEA has been detected in losartan, indicating a potential implication of triethylamine (TEA), an auxiliary base used in the synthesis of losartan. Although TEA is not a direct precursor of NDEA and is plausibly contaminated with diethylamine (DEA), a precursor of NDEA, previously reported by Tuesuwan and Vongsutilers (2021), it can be directly dealkylated to DEA by nitrite or nitrous acid illustrated in Fig. 2. Other nitrosamine species, e.g., N-nitroso N-methyl butyric acid (NMBA), N-nitrosodiisopropylamine (NDIPA), and N-nitrosoethylisopropylamine (NEIPA), have also been observed in ARBs. NMBA contamination was first noted in losartan and irbesartan. The secondary amine precursor of NMBA is N-methyl amino N-butyric acid (MBA), which is potentially formed through degradation of N-methyl pyrrolidinone (NMP), an organic solvent used during tetrazole synthesis. Similar to NDEA formation, N,N-diisopropylethylamine (DIPEA), a common base in API synthesis, can possibly produce dealkylative products, such as N,N-diisopropylamine (DIPA) and N-ethyl N-isopropylamine (EIPA), as respective precursors of NDIPA and NEIPA, respectively. Referring to risk-based evaluation and manufacturers’ evidence-based recalls, tetrazole-containing ARBs can be contaminated with an enormous variety of nitrosamine species when diversified amine reagents are involved in the tetrazole-building step.
While the nitrosamine issue remains incompletely resolved, new organic azide by-products called azido impurities have been recently found in tetrazole-containing ARBs (Jireš et al., 2021). These azido impurities, namely, 4′-(azidomethyl)-[1,1′-biphenyl]-2-carbonitile and 5-(4′-(azidomethyl)-[1,1′-biphenyl]-2-yl)-1H-tetrazole, arise from the side reaction between sodium azide and residual intermediates left from the upstream step (Jireš et al., 2021). The Canadian FDA has announced the voluntary recalls of multiple lots of ARB drug products contaminated with azido impurities (https://recalls-rappels.canada.ca/en/alert-recall/multiple-lots-irbesartan-losartan-and-valsartan-drugs-recalled, accessed 30 May 2021). Losartan products contaminated with the losartan azido impurity 5-(4′-((5-(azidomethyl)-2-butyl-4-chloro-1H-imidazol-1-yl) methyl)-[1,1′-biphenyl]-2-yl)-1H-tetrazole (AZTT) have also been recalled. AZTT is formed by direct reaction between a residual azide and losartan (https://recalls-rappels.canada.ca/en/alert-recall/multiple-lots-irbesartan-losartan-and-valsartan-drugs-recalled, accessed 30 May 2021; https://www.edqm.eu/en/news/risk-presence-mutagenic-azido-impurities-losartan-active-substance, accessed 29 Sep 2021). The possible root causes of nitrosamine and azido impurities found in ARBs are presented in Fig. 4.
Fig. 4.
Possible root causes of nitrosamines and azido impurities in angiotensin II receptor blockers.
To mitigate the risks of nitrosamine contamination, nitrosating agents such as sodium nitrite should be avoided during the crucial azide-removal step in the synthetic route of ARBs. Sodium nitrite can be omitted by additional clean-up of inorganic azides through the quenching process. For example, a quenching method that removes residual azides through an aqueous layer of hydrogen peroxide added during the quenching process in losartan synthesis has been patented in China (2019) (CN Patent no. 109748905A). However, the quenching procedure is difficult to control and is considered relatively violent. The reaction generates magnificent bubbles and risks punching explosions during scale-up production. Moreover, side reactions of over-reactive peroxide might form by-products of excessive oxidation. Alternatively, triarylphosphine can eradicate residual azides via the Staudinger reaction (Leffler and Temple, 1967). Azide quenching with triphenylphosphine has been disclosed in a European patent as in-process remediation (Zuwei et al., 2021). Triphenylphosphine can react with an azide, forming a phosphazide intermediate that immediately liberates N2 to generate iminophorane, followed by an aqueous workup providing amine or amine derivatives and phosphine oxide. Replacing sodium nitrite with trace amounts of triphenylphosphine can therefore remove residual inorganic azide ions without risk of nitrosamine contamination. For this reason, triphenylphosphine is beneficial not only for inorganic decomposition but also for eradicating some organic azide by-products.
3.2. Histamine-2 (H2) receptor antagonists
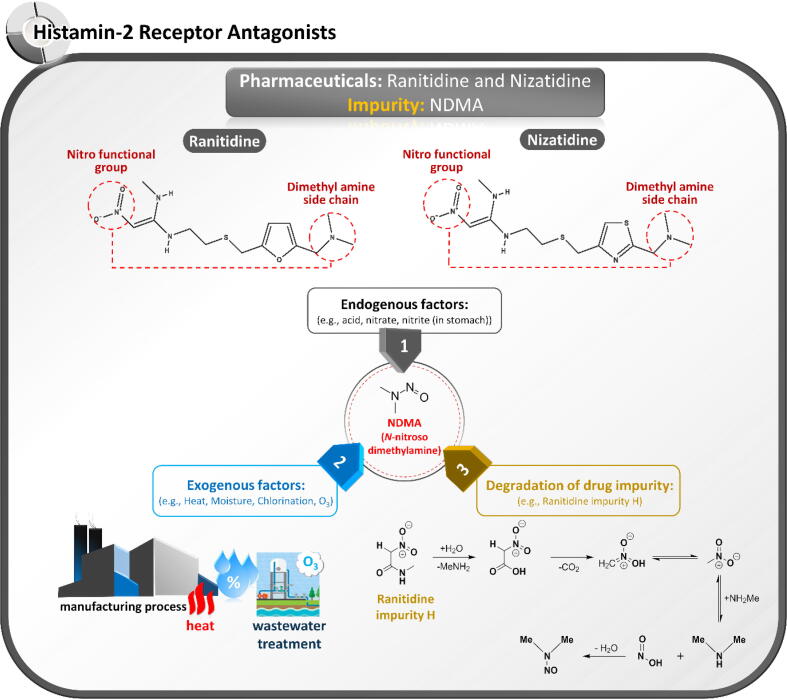
NDMA is a disinfection by-product of ozonation and chloramination that typically causes environmental problems (Alaba et al., 2018). Therefore, its elimination has attracted the attention of chemists and environmentalists in many countries (Lv et al., 2017; Roux et al., 2012). Recently, NDMA was found in the wastewater treatment process of a ranitidine manufacturer, implying a relationship between NDMA and ranitidine, an H2-receptor antagonist that can potentially be an NDMA precursor (Lv et al., 2017; Roux et al., 2012). The risk of NDMA formation is related to the chemical structures of compounds containing functional groups for N-nitrosation. The nitro functional group and dimethylamine side chain found in ranitidine and nizatidine are probably implicated in NDMA generation. In contrast, cimetidine and famotidine are free from NDMA contamination. The inherent instability of H2-receptor antagonists has been inevitably investigated in ranitidine and nizatidine (El-Shaheny et al., 2019; Yokoo et al., 2021). The possible causes of NDMA contamination in these compounds are contamination during the manufacturing process, the inherent instability of APIs, and degradation of drug impurities as summarized in Fig. 5.
Fig. 5.
Possible root causes of N-nitroso dimethylamine (NDMA) contamination in ranitidine and nizatidine and their pharmaceutical products.
Unlike NDMA contamination in ARBs, NDMA levels in ranitidine and nizatidine products significantly depend on storage time and temperature. Yokoo et al. (2021) reported that NDMA forms directly via ranitidine and is released much faster from ranitidine impurities. DMA is believed to be generated from ranitidine and its impurities containing dimethylamino and/or nitro functional groups. Ranitidine impurity H in the solid state promptly degrades to NDMA despite lacking a dimethylamino moiety in its structure. The proposed mechanism of NDMA generation from ranitidine impurity H is demonstrated in Fig. 5. Besides the exogenous degradation of ranitidine, endogenous decomposition via metabolism to NDMA has been noted. In an in vitro study, the endogenously formed N-nitroso degradation product of nizatidine substantially increased under the acidic conditions of the gastric environment (El-Shaheny et al., 2019). Whereas in vitro experiments have indicated a strong connection between ranitidine and NDMA formation, in vivo clinical studies in animals and humans have highlighted the potential carcinogenicity of ranitidine. The genotoxic effects of ranitidine have been studied in rodents since 1983 (Brambilla et al., 1983). Experiments have demonstrated that ranitidine induces DNA fragmentation in the liver or mucosa in the presence of sodium nitrite. In a clinical trial, NDMA was found in urinary excretions after oral intake of ranitidine, suggesting that long-term administration of ranitidine or its related structures increases the cancer risk (Zeng and Mitch, 2016). Furthermore, several cohort studies demonstrated the relationship between cumulative individual cancer incidence and long-term ranitidine use (McGwin, 2020; Wang et al., 2022). For instance, McGwin (2020) reported that proportionate reporting ratios (PPRs) of the significant and elevated malignancy observation on pharyngeal, esophageal, stomach, colorectal, hepatic, and pancreatic cancers were 9.24, 3.56, 1.48, 16.31, 2.64, and 2.18, respectively. The study results support the hypothesis that NDMA-contaminated ranitidine can increase the epidemiologic cancer risk. Likewise, the clinically meaningful results from Taiwan National Health Insurance Research Database demonstrated that long-term ranitidine use is associated with a higher likelihood of liver cancer development in comparison with the control groups of famotidine or proton-pump inhibitors (Wang et al., 2022). However, Kim et al. (2021) reported that increased gastric cancer was unrelatable to ranitidine use in South Korean patients. After a long usage history, the US FDA and EMA eventually decided to withdraw and close this over-the-counter medicine. However, more in-depth investigations of nizatidine are required to assess the risk of NDMA contamination.
To the best of our knowledge, there is no report on a solution to the abovementioned problem. At this point, we suggest a potential solution by controlling/monitoring stability-related factors such as light, humidity and temperature during the manufacturing process and drug storage. Due to the temperature-dependent characteristic of NDMA formation in ranitidine (Abe et al., 2020), the storage condition of both API and drug product should be lower than 25 °C for the solid dosage form and between 2 and 8 °C for the parenteral dosage form. The amount of NDMA during the storage period should be closely monitored in a vigilance process.
3.3. Antidiabetic agents
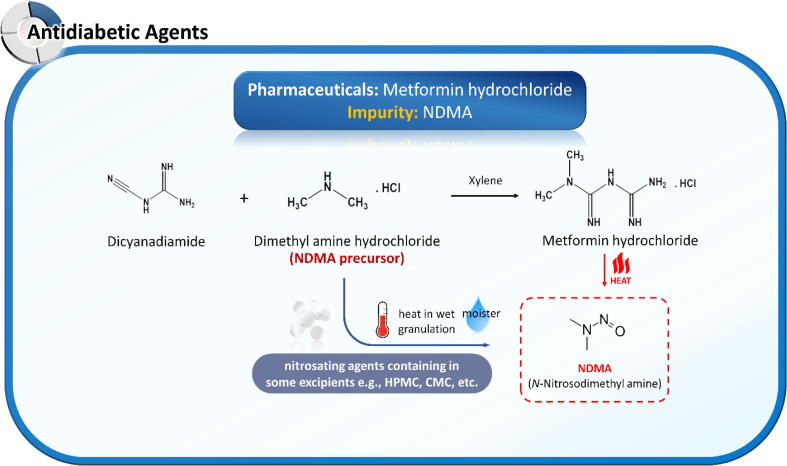
Metformin, a dimethyl guanidine analog, is structurally classified as a guanidine derivative. Unlike ranitidine, metformin lacks a nitro functional group in its chemical structure and requires an exogenous nitrosating agent for nitrosamine formation. Badran et al. (2019) reported that incomplete oxy-cracked metformin could carry several amine by-products, including NDMA, DMF, N,N-dimethylurea, dimethylguanidine, and hydroxyacetonitrile (Badran et al., 2019). In the same year, the Singapore HSA (https://www.hsa.gov.sg/announcements/news/hsa-recalls-three-out-of-46-metformin-medicines, accessed 04 Dec 2019) and US FDA (https://www.fda.gov/news-events/press-announcements/statement-janet-woodcock-md-director-fdas-center-drug-evaluation-and-research-impurities-found, accessed 05 Dec 2019) cautioned the risk of NMDA contamination in metformin preparations sold in several countries outside the US.
In 2020, the US FDA revealed that NDMA contamination in several metformin extended-release products exceeded the recommended acceptable threshold (https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin, accessed 28 May 2020). The root cause of NDMA contamination in metformin differs from that in ARBs and histamine-2 receptor antagonists. Metformin is relatively stable and requires critical co-existing external factors such as nitrosating agents, moisture, and heat for conversion to NDMA. According to Nasr et al. (2021), NDMA is generated during the manufacturing process of metformin drug products. Nitrites and nitrates available in pharmaceutical excipients, such as CMC sodium, HPMC E5, HPMC K15M, and PolyoxTM, are essential factors of nitrosamine contamination. Additional moisture in wet granulation and excessive heat during the drying process initiate nitrosation reactions in the manufacturing process of metformin tablets (Nasr et al., 2021). The NDMA contamination risk can be reduced by avoiding nitrite- or nitrate-containing excipients or the wet granulation process. NDMA contamination in metformin can be sourced from the reaction between dimethylamine and the nitrosating agent during the API manufacturing process, the reaction between APIs and nitrosating agents from excipients under the wet granulation process, and degradation of APIs under very high temperatures. These possible source mechanisms are presented in Fig. 6.
Fig. 6.
Possible root causes of N-nitroso dimethylamine (NDMA) contamination in metformin and its pharmaceutical products.
Another blood-sugar-lowering substance, pioglitazone, has also been cautioned by EMA (https://www.ema.europa.eu/en/documents/press-release/update-nitrosamine-impurities-ema-continues-work-prevent-impurities-medicines_en.pdf, accessed 26 Apr 2019) for NDMA contamination. As preliminarily reported by the manufacturer, NDMA in pioglitazone is sourced from sodium nitrite and hydrobromic acid found in an early step (before the use of DMF) and hydrochloride in a later step (https://veeprho.com/blog/nitrosamine-impurities-traces-in-pioglitazone/, accessed 16 Jul 2020). Sodium nitrite or other nitrosating agents can be carried over multiple unitary operations during the API synthesis.
3.4. Antimicrobial agents
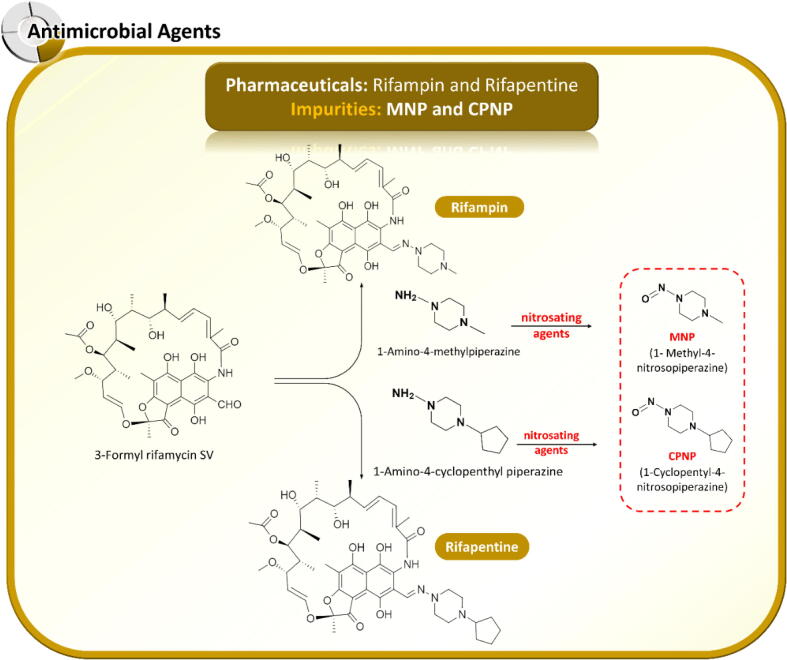
In August 2020, the FDA noted two nitrosamine impurities in the antituberculosis agents rifampin and rifapentine (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-nitrosamines-rifampin-and-rifapentine, accessed 26 Aug 2020). The contaminated nitrosamines are structurally related to the APIs, implying that two nitrosamine analogs (MNP and CPNP) originate in the drug synthesis process. CPNP is thought to arise from an intermediate step in rifapentine production. The root cause of MNP formation remains unidentified by manufacturers but probably originates similarly to rifapentine contamination. The nitrosamine levels in many rifampin and rifapentine products in the US exceed the AI interim limits (0.16 ppm for MNP in rifampin and 0.1 ppm for CPNP in rifapentine) (https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-rifampinrifapentine-products, accessed 28 Jan 2021). To maintain the supply of these lifesaving drugs in clinical treatments, the US FDA has set the maximum levels of MNP in rifampin and CPNP in rifapentine at 5 and 20 ppm, respectively (https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-rifampinrifapentine-products, accessed 28 Jan 2021). Singapore HSA (https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/nitrosamine-impurity-1-methyl-4-nitrosopiperazine-(mnp)-found-in-rifampicin-products, accessed 23 Apr 2021) has also permitted the supply of rifampin products with trace amounts of MNP, as these medicines are essential to patients. The toxicophores (structural and toxicological relationships) of MNP and CPNP (Helguera et al., 2008) suggest that these nitrosamines are less carcinogenic than NDMA. Although the FDA has applied the long-term interim AI of NDMA instead of the tight control limits (Frick and Cloëz, 2021), preventive action from drug-substance manufacturers and intensive follow-up programs from authorities are necessary. As summarized in Fig. 7, MNP and CPNP contamination in rifampin and rifapentine can occur when nitrosating agents introduced during the manufacturing step react with 1-methyl piperazine and 1-cyclopentyl piperazine, respectively.
Fig. 7.
Possible root causes of 1-methyl-4-nitrosopiperazine (MNP) contamination in rifampin and 1-cyclopentyl-4-nitrosopiperazine (CPNP) contamination in rifapentine during the synthesis of active pharmaceutical ingredients.
3.5. Other medicines
Over two years of nitrosamine detection in ARBs, global health authorities and pharmaceutical industries have learned important lessons. Nitrosamines can contaminate not only tetrazole-containing sartans, but also other tetrazole drugs such as cefamandole, ceftezole, tedizolid, letrozole, and tomelukast with similar risk. This issue has dramatically impacted the quality and reliability of drug products, leading to a drug supply shortage. Moreover, to address patient safety concerns, some physicians and healthcare professionals have switched their therapeutic regimens. Although nitrosamine does not contaminate all medicines, a number of medications have been identified as nitrosamine precursors in wastewater treatment (Shen and Andrews, 2010; Wang et al., 2015). Concurrent formation of nitrosamines is favored by the chloramine disinfection process. Shen and Andrews (2010) conducted a test of nitrosamine formation potential. They identified several APIs—ranitidine, nizatidine, carbinoxamine, chlorpheniramine, diphenhydramine, doxylamine, azithromycin, clarithromycin, erythromycin, roxithromycin, amitriptyline, diltiazem, metformin, escitalopram, sumatriptan, tramadol, tetracycline, and venlafaxine—with potential NDMA-conversion ability after treatment with chloramine disinfectants. In contrast, lidocaine is prone to NDEA contamination. Pretreatment of wastewater with oxidizing agents can effectively minimize NDMA formation by adding lone-pair electrons to nitrogen via an ozonation pathway leading to N-oxide (Wang et al., 2015). Such findings imply that amine-based pharmaceuticals contribute significantly to nitrosamine conversion and, consequently, to pharmaceutical contamination. Risk evaluation and routine quality-control testing of pharmaceutical products are expected to place enormous burdens on both reference and generic drug manufacturers, who must deliver qualified, efficient, and safe medicines to patients.
4. Acceptable limits of nitrosamines in pharmaceuticals
Since 2017, the ICH Guideline M7 (R1) has been established for the assessment and control of mutagenic impurities in pharmaceuticals. Nitrosamines are classified as Class-1 impurities as they are known mutagenic carcinogens. The ICH Guideline M7 (R1) is based on the “Threshold of Toxicological Concern” (TTC) concept, which defines the tolerable amounts of lifetime exposure with negligible human cancer risk. The TTC is theoretically associated with the potential of significant carcinogenic risk, stating that the cancer risk increases to one case in 100,000 (a theoretical excess lifetime cancer risk of 10−5) when the acceptable limits of mutagenic impurities in drug substances and drug products are set at 1.5 μg/day. The TTC value can be justified and generally applied in the AI estimation for mutagenic agents (Class-2 and Class-3) but is only a conservative approach (Ruepp et al., 2021). For class-1 nitrosamines, the ICH Guideline M7 (R1) also states that when sufficient carcinogenicity data from the animal are available, AI should be derived from compound-specific risk assessments (the TD50 or Benchmark Dose Limit (BMDL10) of animal carcinogenicity studies performed with the specific nitrosamine) rather than by the TTC concept. The calculations can be extrapolated from animal to human toxicity levels. Thus, the interim limit criteria for nitrosamines set by the US FDA (https://www.fda.gov/news-events/press-announcements/fda-announces-voluntary-recall-several-medicines-containing-valsartan-following-detection-impurity, accessed 13 Jul 2018) and EMA (https://www.ema.europa.eu/en/documents/other/temporary-interim-limits-nmba-dipna-eipna-impurities-sartan-blood-pressure-medicines_en.pdf, accessed 20 Aug 2019) are based on the median tumorigenic dose (TD50) data from the US Environmental Protection Agency (EPA). The maximum consumption of nitrosamines is considered reasonably safe throughout a person’s lifetime of 70 years, resulting in less than one additional case of cancer per 100,000 people. For example, the TD50 values of NDMA are 0.096 mg/kg/day for the most sensitive rat species (Peto et al., 1991) and 0.189 mg/kg/day for mice (https://www.nlm.nih.gov/databases/download/cpdb.html). Typically, the lower TD50 is chosen for AI calculations. Dividing the TD50 by 50,000 and multiplying the result by the average human body weight (50 kg), the AI of NDMA is determined as 96 ng/day. More importantly, the ICH Guideline M7 (R1) also suggested that the flexible AI value for mutagenic impurities could be adjustable based on the drug exposure duration (less than a lifetime (LTL)). For example, the control of NDEA based on the LTL AI concept was proven the potential carcinogenic risk protection under the typical exposure durations of clinical trials and therapeutic medication (Bercu et al., 2021). However, this paradigm was questioned by experts consulted as part of the Article 5(3) scientific review on nitrosamine of the EU regulatory network in case of potent mutagenic and carcinogenic nitrosamine (https://www.ema.europa.eu/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf, accessed 22 Sep 2021). However, CHMP recommended the calculation of nitrosamine AI limits should be based on lifetime exposure but may be exceptional in case of the high-risk event of medicine shortage (Ruepp et al., 2021). In such cases, the manufacturer must report and coordinate with the relevant authorities to evaluate clinical benefits and mutagenic risks. Additionally, General chapter < 1469 > nitrosamine impurities suggested converting AI to a maximum allowance of nitrosamine concentration limits, in ppm, specific for individual drug products by dividing the maximum daily dose (MDD) (mg/day) of the drug substance (USPNF, 2022). To facilitate the development of an analytical method for nitrosamine determination with sufficient sensitivity based on these values, we have summarized the interim limit criteria for nitrosamines in various pharmaceuticals in Table 3 (https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf, accessed 23 Jun 2019).
Table 3.
Interim limit criteria of nitrosamines in pharmaceuticals.
|
Drug |
Maximum daily dose (mg/day) | Acceptable intake NDMA and NMBA (ng/day)Π |
Acceptable intake NDMA and NMBA (ppm)≠ |
Acceptable intake NDEA, DIPNA, and EIPNA (ng/day)Π | Acceptable intake NDEA (ppm) ≠ | Acceptable intake MNP (ng/day) | Acceptable intake MNP (ppm) |
|---|---|---|---|---|---|---|---|
| Valsartan | 320 | 96 | 0.3 | 26.5 | 0.083 | – | – |
| Losartan | 100(US) 150(EMA) |
96 96 |
0.96* 0.64** |
26.5 26.5 |
0.27* 0.177** |
– - |
– - |
| Irbesartan | 300 | 96 | 0.32 | 26.5 | 0.088 | – | – |
| Azilsartan | 80 | 96 | 1.2 | 26.5 | 0.33 | – | – |
| Olmesartan | 40 | 96 | 2.4 | 26.5 | 0.66 | – | – |
| Eprosartan | 800 | 96 | 0.12 | 26.5 | 0.033 | – | – |
| Candesartan | 32 | 96 | 3.0 | 26.5 | 0.83 | – | – |
| Telmisartan | 80 | 96 | 1.2 | 26.5 | 0.33 | – | – |
| Metformin | 3000 | 96 | 0.032 | – | – | – | – |
| Rifampin | 600 | – | – | – | – | 96 | 0.16 |
If multiple nitrosamines are present in pharmaceuticals, the sum of nitrosamines must be lower than 26.5 ng/day (based on the AI of the most toxic nitrosamine (US-FDA).
The acceptable intake defines the daily exposure to a compound such as NDMA, NDEA, NMBA, DIPNA, and EIPNA that raises the cancer risk to approximately 1:100,000 after 70 years of exposure; ≠ These values are based on the maximum daily dose given on the drug label; * US FDA limit criteria; **EMA limit criteria.
The current interim limits are applicable only when a drug product contains a single nitrosamine. According to the US FDA guidance for industry and USP general chapter <1469> nitrosamine impurities, if multiple nitrosamine impurities are detected, their total quantity should not exceed 26.5 ng/day (the AI of the most toxic nitrosamine). For drug products with a maximum daily dose (MDD) below 880 mg/day, the recommended limit of total nitrosamines is 26.5 ng/day (>30 ppb). For drug products with MDDs above 880 mg/day, the limit of total nitrosamines should be adjusted to < 30 ppb to meet the recommended 26.5 ng/day (US FDA Guidance for Industry, 2021; USPNF, 2022). The low AI limit recommended for nitrosamine assays demands a sensitive analytical method with a limit of quantification (LOQ) at or below an acceptable threshold. For example, if the MDD is 1,200 mg, the LOQ of the method should be lower than 22 ppb. Therefore, before developing and validating an analytical method of nitrosamine determination, researchers must establish the AI limits of each nitrosamine impurity for specified APIs and pharmaceutical products.
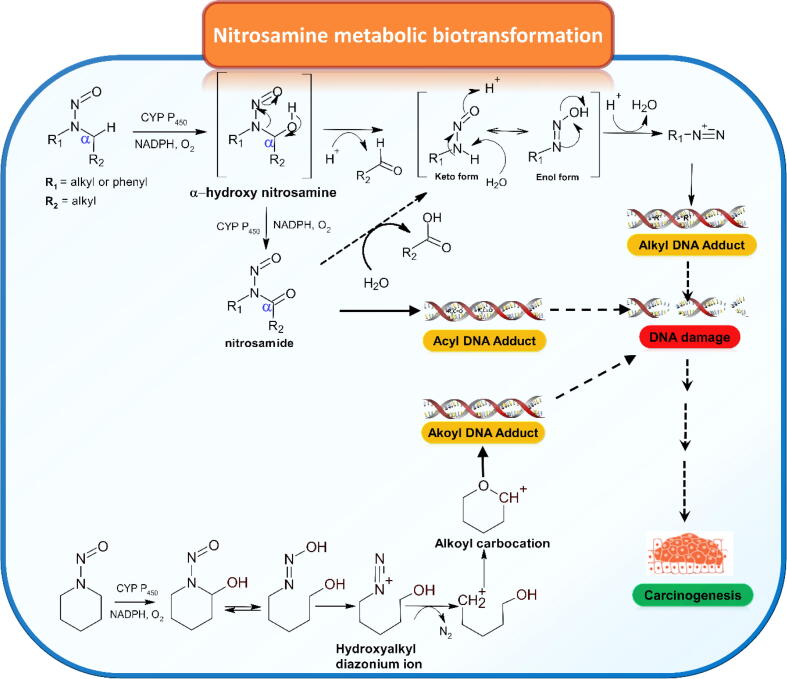
Apart from small alkyl nitrosamines found in medicines such as NDMA, NDEA, and NMBA, the discovery of unique and complex N-nitroso pharmaceutical analogues, the so-called nitrosamine drug substance-related impurities (NDSRIs), have been detected in amine-based pharmaceuticals. (https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/lupin-pharmaceuticals-Inc-issues-voluntarily-nationwide-recall-all-irbesartan-tablets-and-irbesartan, accessed 14 Oct 2021; https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-nitrosamine-varenicline-chantix, accessed 02 Sep 2021; https://www.pfizer.com/news/press-release/press-release-detail/pfizer-voluntary-nationwide-recall-lots-accuretictm, accessed 21 Mar 2022). The specific AIs of these mutagenic impurities have not yet been assigned due to the insufficient availability of animal carcinogenicity data. In such cases, two approaches for AI estimation are proposed under Article 5(3) recommended by EMA (https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf, accessed 22 Sep 2021). The first approach is the application of a conservative class limit TTC of 18 ng/day based on the most potent N-nitrosamine. In addition, EMA has recently announced that a twelve-month interim AI of 178 ng/day for total nitrosamines can be adopted by the relevant authorities for marketed medicines identified to contain one or more nitrosamines. The other approach is to estimate a suitable AI, which is derived by a TD50 of structurally related N-nitrosamine derivatives with appropriate scientific-based justification. The latter approach is consistent with ICH M7 (R1) recommendation. When the latter approach is adopted, it seems not to define all N-nitrosamines as exceptionally potent carcinogens. More interestingly, based on animal carcinogenicity data derived from 228 low-molecular-weight N-nitrosamines, 18 % of those were found to have noncarcinogenic effects (Thresher et al., 2020). Theoretically, N-nitrosamines (e.g., N,N-dialkyl, cycloalkyl, and N-alkyl N-aryl analogues) undergo oxidative metabolic activation via cytochrome P450 (CYP P450) enzymes to exhibit carcinogenic effects (Fig. 8). CYP P450 enzymes associated with hydroxylation at the α-position of the carbon atom to the N-nitroso moiety results in the formation of an N-nitrosocarbinolamine species (α-hydroxylation of nitrosamines), which subsequently decomposes to the corresponding aldehyde and diazohydroxide intermediates (enol form of nitroso primary amine). The unstable enol tautomer species further spontaneously decomposes to yield noxiously reactive N-alkyl diazonium species and generate covalent DNA adducts, especially O6-methylguanine leading to Increased carcinogenic Incidences (Li and Hecht, 2022). Although extensive data have strongly suggested this pathway, it was questionable whether those unstable intermediates can be survived until DNA interaction due to their short half-life. Carlson et al. (2017) have proven that the metabolic pathway can undergo though another key intermediate, which is more stable nitrosamides after cytochrome P450 oxidation. Most interestingly, the nitrosamide intermediates can either directly bind to DNA to form novel acyl DNA adducts or subsequently convert to alkyl diazohydroxide to generate typical alkyl DNA adducts illustrated in Fig. 8. Labile hydrogen at α-carbon position to N-nitroso functionality is one key criterion of the triggered bioactivation associated with mutagenic and carcinogenic properties of those poisonous nitrosamines. More importantly, the structural morphology of N-nitroso analogues specific to metabolic transformation, stability of the diazonium ion and DNA adducts in tissues, and metabolic repair efficiency and capacity may play other crucial roles in carcinogenic potential. For several instances, the metabolic transformation of N-nitroso-hydrochlorothiazide and N-nitroso derivatives of niridiazole and tolbutamide end up with diazophenyl (Ph-N+≡N) and diazo amide (–NH-CO-N+≡N) species, respectively, which are less potent than diazoalkane metabolite (Bharate, 2021). This observation supported that all N-nitrosamines were not the “cohort of concern” of mutagenic carcinogens.
Fig. 8.
Nitrosamine metabolic biotransformation pathways.
To avoid AI overestimation, Cross and Ponting (2021) developed mechanistically based structure–activity relationships. The driving force of high carcinogenic prevalence results from metabolic hydroxylation on α-position carbon of nitrosamines; however, many factors affect nitrosamine carcinogenic potency. The carcinogenic potency and mutagenic prevalence of the suspected analogues were considered according to the chemical features to determine the nature of their metabolic activation via α-carbon hydroxylation and any chemical attributes impacting on increasing or reducing the opportunity of DNA adducts (Cross and Ponting, 2021). They reported that the steric hindrance via the degree of α-carbon substitution and electronic effect of β-carbon via the presence or absence of electron-withdrawing groups play a crucial role in nitrosamine carcinogenic potency and mutagenic prevalence. Based on Ames study data and rodent carcinogenicity data, increasing the number of α-carbon substitution trends decreases the degree of potency and positivity of mutagenicity. The complete removal of all α-carbon hydrogens is not a critical criterion to neutralize the carcinogenicity of nitrosamines. More interestingly, by increasing the chain length of the substituent, ring size, and molecular weight, steric hindrance can reduce nitrosamines' relative potency compared to small substituents, e.g., methyl and ethyl functional groups (Cross and Ponting, 2021). Strong electron-withdrawing substituents at β-position carbon result in decreased carcinogenicity and increased prevalence of negative malignancy (Cross and Ponting, 2021). Substitutions with the stronger electron-withdrawing groups at β-position carbon on both sides produce an even more pronounced effect, whereas weaker β electron-withdrawing groups exhibit lesser effects on potency (Cross and Ponting, 2021). The additional categories of nitrosamine SAR are still being investigated and further elucidated on their carcinogenic potency.
As an alternative, Dobo et al. (2022) proposed the systematic, practical, scientific-based and conservative AI calculation approach for a large number of the structural diversity of complex N-nitroso analogues independent of carcinogenicity data from the compound-specific based testing. Firstly, the impurities were divided into 13 commonly distinct structural features containing the substituents based on the different electronic and steric environments in their chemical structures. The thirteen groups of AIs were assigned from LD50 of the most potent N-nitrosamine obtained from the Lhasa Carcinogenicity Database (LCDB)) (Dobo et al., 2022). The N-nitrosamine impurities (approximately 76 %) were assigned to the groups with higher estimated AIs than the precautionary default limit of 18 ng/day. For example, Group 1 assigned as N,N-dialkylnitrosoamines was considered to have structures equivalent to simple dialkyl nitrosamines such as NDMA and NDEA (Dobo et al., 2022). Consequently, the estimated AI for Group 1 is recommended to be assigned to 26.5 ng/day, which is the most potent published AI of NDEA from animal studies. The AIs of Groups 2, 6, and 8 were estimated by the EMA default limit of 18 ng/day due to the inadequate carcinogenicity database of their derived simple nitrosamine structures to support these groups (Dobo et al., 2022). In contrast, the established AIs of the remaining 9 groups (Groups 3, 4, 5, 7, and 9–13) were estimated according to published carcinogenicity data of corresponding N-nitrosamine analogues (Dobo et al., 2022). The lowest published or calculated TD50 was chosen to establish the specific AIs for each group. Seven assigned groups had at least one N-nitrosamine with TD50 equivalent to or higher than the default lifetime TTC of 1.5 μg/day defined in ICH M7 (R1), and five assigned groups had at least one noncarcinogenic N-nitrosamine assignment (Dobo et al., 2022).
5. Case reports of nitrosamine and N-nitroso analogue contamination
Since 2018, the nitrosamine crisis has caused>1800 recalled batches of drug products in the United States (Schmidtsdorff et al., 2022). Drug products of valsartan, irbesartan, metformin, ranitidine, nizatidine, rifampin, and rifapentine were recalled or even withdrawn due to the detection of small alkyl nitrosamine contaminants exceeding the interim threshold limits. Most drug product recalls (approximately 34 %) are related to NDMA contamination (Schmidtsdorff et al., 2022). According to the FDA database, 81 % of the recalls resulted from sartan-containing pharmaceutical products. Comparatively, the highest number of sartan recalls (approximately 24 %) were associated with losartan (Bharate, 2021). The recalls of fixed-dose combination drug products containing those APIs have also been related to this situation (Bharate, 2021). Among these sartan recalls, 42 % of the total were counted as fixed-dose formulations (Bharate, 2021).
The number of drug product recalls are varied depending on the manufacturers. Initially, Zhejiang Huahi Pharmaceutical in China found that valsartan was contaminated with NDMA, NDEA, and NMBA after replacing tributyltin azide with sodium azide during the manufacturing process (Xiaohui et al., 2018). After the recalls of Zhejiang Huahi, several companies in the United States have extensively inspected the nitrosamine contamination in their valsartan-containing drugs. Twenty-five percent of valsartan-containing products have been recalled by Mylan Pharma. In addition, Teva Pharma and Torrent Pharma have the most recalls of valsartan-HCTZ (26.9 %) and Amlodipine-Valsartan (30.7 %), respectively (Bharate, 2021).
Irbesartan is another sartan manufactured by Sanofi, Aurobindo, and Alembic (Bharate, 2021). The formation of tetrazole in the last synthesis step results in nitrosamine contamination. Alternatively, Srini pharmaceuticals implemented a different synthesis approach with the earlier built-up tetrazole formation, leading to a lower risk of nitrosamine adulteration (Bharate, 2021). According to an FDA press release, NDEA was found in some batches of the irbesartan manufactured by ScieGen Pharmaceuticals, in which tetrazole was formed using a metal azide in the final synthesis step (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan, accessed 12 Sep 2022). The recall affects approximately 1 percent of the irbesartan drug products in the US market. The voluntary recalls of one lot of irbesartan and seven lots of irbesartan and HCTZ fixed-dose combination tablets using the irbesartan APIs distributed by Solo Healthcare LLC, a Prinston Pharmaceutical Inc. subsidiary, were announced by USFDA because of NDEA detection above the interim limit of 0.088 ppm (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan, accessed 12 Sep 2022).
Several batches of losartan products were recalled from the market because of NDEA and NMBA contaminations (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan, accessed 12 Sep 2022). According to the risk assessment of the manufacturing process, there are two different synthetic routes proposed by DuPont (Carini et al., 1991) and Merck. (Larsen et al., 1994). Merck’s manufacturing process avoided using azide-relating reagents in the late step of the synthesis route using 5-phenyltetrazole as the starting material. In contrast, the DuPont method generated tetrazole functionality by the cyclization of nitrile under sodium azide. The use of sodium nitrite for the azide quenching process in combination with DMF/NMP as solvents were identified as the cause of NDEA and NMBA formation. According to the FDA database, losartan and losartan/HCTZ drug products have been voluntarily recalled from many pharmaceutical companies such as Camber Pharma Inc., Sandoz Inc., Teva Pharma, Torrent Pharma ltd. and Macleods Pharma ltd.
While small alkyl nitrosamines in pharmaceuticals haven’t yet been readily resolved, several N-nitroso drug analogues have been alerted by global pharmaceutical companies. Initially, Lupin Pharmaceuticals announced a nationwide voluntary recall of irbesartan and HCTZ tablets due to N-nitroso irbesartan (https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/lupin-pharmaceuticals-Inc-issues-voluntarily-nationwide-recall-all-irbesartan-tablets-and-irbesartan, accessed 14 Oct 2021). However, Lin et al. (2021) argued that N-nitroso-irbesartan was unlikely to exist in ten oxidative stress reactions of irbesartan with nitrous acid. They postulated that the N-nitrosation process was not feasible due to the poor nucleophilicity of tetrazole nitrogen. More interestingly, E- and Z-isomers of irbesartan oxime impurities were instead detected and structurally characterized (Lin et al., 2021). According to the FDA press releases and company announcements, several high-molecular-weight nitrosamines such as N-nitroso varenicline, N-nitroso-quinapril, and N-nitroso orphenadrine were unexpectedly found in varenicline (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-nitrosamine-varenicline-chantix, accessed 02 Sep 2021), quinapril (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-voluntary-nationwide-recall-lots-accuretictm, accessed 21 Mar 2022), and orphenadrine (https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/sandoz-Inc-issues-nationwide-recall-13-lots-orphenadrine-citrate-100-mg-extended-release-tablets-due, accessed 22 Mar 2022), respectively.
6. Conclusions
Nitrosamine impurities have roused safety concerns across the pharmaceutical landscape, which encompasses tetrazole-based medicines and has extended to amine-related analogs. The risks of chemical reagents or possible by-products that generate reactive genotoxic impurities such as azido and nitroso compounds, epoxides, hydrazines, alkyl halide derivatives, etc. must be evaluated by pharmaceutical industries in drug product registration dossier according to ICH M7 (R1) guidance or other quality legislation. Recently, updated guidelines have been announced by national authority agencies and General Chapter <1469> in the US pharmacopoeia. A risk assessment-based approach has been recommended for evaluating the potential root cause of nitrosamine contamination, investigating the possibility of contamination, and establishing a suitable control strategy and an acceptable contamination threshold. However, the risk assessment is based on the risk likelihood, which requires prior knowledge of previous incident reports. Without any previous threats, the risks will be ignored or lowly valued and the risk evaluation will be ineffective. Therefore, the control strategies in terms of the preventive and corrective actions not only rely on the in-depth chemistry understanding of the nitrosamine formation and decomposition, but also depend on the suitable built-in concept of control limit criteria.
7. Future perspectives
Several preventive measures have been proposed to predict and manage suspected contamination with nitrosamines or potential mutagenic impurities in pharmaceuticals. Common risk assessments are not possible to investigate and evaluate all potential risks due to several unusual chemical reactions that happen unintentionally under drug substance and product manufacturing processes leading to potential impurities. Since 1980, IARC has proposed a nitrosation assay procedure (NAP) to evaluate the reactivity of nitrogen-containing drugs to nitrosating agents, but it has not been implemented in the drug discovery process (World Health Organization (WHO) IARC monographs, 1980). Recently, the NAP test has become the first prospective approach in drug development to identify and screen the nitrosation possibility on a target drug substance with all relevant reagents in synthetic reactions and thirty-three of the investigated pharmaceuticals presented suspected signals at the exact masses of their corresponding N-nitroso compounds (NOCs) associated with drug-nitrite interaction after nitrosation (Schmidtsdorff et al., 2022). Therefore, it is recommended to implement the NAP test in the drug development step and include it in the registration dossier of drug substances and products. The estimated AIs based on the structure–activity relationships (SARs) of existing LD50 in the LCDB database are the second direction to establish a more reliable control limit to be an essential part of the risk assessment scenario (Cross and Ponting, 2021, Dobo et al., 2022). Another direction is to tighten the sum acceptable threshold of < 26.5 ng/day when multiple nitrosamine impurities are presented in an individual drug product as suggested by the US FDA Guidance for Industry (2021) and USPNF (2022). Nonetheless, the allowance level of contamination should be appropriately implemented with a much stricter in sum acceptable limit in case of multiple classes of mutagenic impurities contamination such as different organic azido impurities in sartans and various hydrazines in COX-2 inhibitors in addition to several nitrosamine impurities. Last but not least, high-throughput chromatographic-based methodology and fluorescence probe sensor technology have to promptly support the routine monitoring of mutagenic contamination in the pharmaceutical industry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by the Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University for the Center of Excellence in Natural Products for Ageing and Chronic Diseases under Grant No. GCE 6503433003-1 (Pornchai Rojsitthisak).
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any authors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abe Y., Yamamoto E., Yoshida H., Usui A., Tomita N., Kanno H., Masada S., Yokoo H., Tsuji G., Uchiyama N., Hakamatsuka T., Demizu Y., Izutsu K.-I., Goda Y., Okuda H. Temperature-Dependent Formation of N-Nitrosodimethylamine during the Storage of Ranitidine Reagent Powders and Tablets. Chem. Pharm. Bull. 2020;68:1008–1012. doi: 10.1248/cpb.c20-00431. [DOI] [PubMed] [Google Scholar]
- Abou-Gharbia M.A., Pylypiw H., Harrington G.W., Swern D. Synthesis of N-nitrosocimetidine hydrate and nitrate and tritium-labeling studies. J. Org. Chem. 1981;46:2193–2194. doi: 10.1021/jo00323a049. [DOI] [Google Scholar]
- Alaba P.A., Sani Y.M., Olupinla S.F., Wan Daud W.M., Mohammed I.Y., Enweremadu C.C., Ayodele O.O. Toward N-nitrosamines free water: Formation, prevention, and removal. Crit. Rev. Environ. Sci. Technol. 2018:1–42. doi: 10.1080/10643389.2018.1430438. [DOI] [Google Scholar]
- Aldawsari F.S., Alshehry Y.M., Alghamdi T.S. N-nitrosodimethylamine (NDMA) contamination of Ranitidine Products: A review of recent findings. J. Food Drug Anal. 2021;29(1):39–45. doi: 10.38212/2224-6614.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran I., Manasrah A.D., Nassar N.N. A combined experimental and density functional theory study of metformin oxy-cracking for pharmaceutical wastewater treatment. RSC Adv. 2019;9:13403–13413. doi: 10.1039/C9RA01641D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J.C., Swager T.M. An Organic Chemist’s Guide to N-Nitrosamines: Their Structure, Reactivity, and Role as Contaminants. J. Org. Chem. 2021;86:2037–2057. doi: 10.1021/acs.joc.0c02774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercu J.P., Masuda-Herrera M., Johnson G., Czich A., Glowienke S., Kenyon M., Thomas R., Ponting D.J., White A., Cross K., Waechter F., Rodrigues M.A.C. Use of less-than-lifetime (LTL) durational limits for nitrosamines: Case study of N-Nitrosodiethylamine (NDEA) Regul. Toxicol. Pharmacol. 2021;123 doi: 10.1016/j.yrtph.2021.104926. [DOI] [PubMed] [Google Scholar]
- Bharate S.S. Critical Analysis of Drug Product Recalls due to Nitrosamine Impurities. J. Med. Chem. 2021;64:2923–3936. doi: 10.1021/acs.jmedchem.0c02120. [DOI] [PubMed] [Google Scholar]
- Borikar S.P., Paul V. N-nitrosation of secondary amines using p-TSA-NaNO2 as a novel nitrosating agent under mild conditions. Synth. Commun. 2010;40:654–660. doi: 10.1080/00397910903009448. [DOI] [Google Scholar]
- Borths C.J., Burns M., Curran T., Ide N.D. Nitrosamine Reactivity: A Survey of Reactions and Purge Processes. Org. Process Res. Dev. 2021;25:1788–1801. doi: 10.1021/acs.oprd.1c00162. [DOI] [Google Scholar]
- Brambilla G., Cavanna M., Faggin P., Maura A., Pino A., Ricci R., Robbiano L. Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite. Carcinogenesis. 1983;4(10):1281–1285. doi: 10.1093/carcin/4.10.1281. [DOI] [PubMed] [Google Scholar]
- Brian Byrd J., Chertow G.M., Bhalla V. Hypertension hot potato-Anatomy of the angiotensin-receptor blocker recalls. N. Engl. J. Med. 2019;380:1589–1591. doi: 10.1056/NEJMp1901657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini D.J., Duncia J.V., Aldrich P.E., Chiu A.T., Johnson A.L., Pierce M.E., Price W.A., Santella J.B., III, Wells G.J., Wexler R.R., Wong P.C., Yoo S.-E., Timmermans P.B.M.W.M. Nonpeptide angiotensin II receptor antagonists: The discovery of a seriers of N-(bipheylylmethyl)imidazoles as potent, orally active antihypertensives. J. Med. Chem. 1991;34:2525–2547. doi: 10.1021/jm00112a031. [DOI] [PubMed] [Google Scholar]
- Carlson E.S., Upadhyaya P., Hecht S.S. A general method for detecting nitrosamide formation in the in vitro metabolism of nitrosamines by cytochrome P450s. J. Vis. Exp. 2017;127:e56312. doi: 10.3791/56312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Célariès B., Párkányi C. Tin (IV) chloride-sodium nitrite as a new nitrosating agent for N-nitrosation of amines, amides and ureas under mild and heterogeneous conditions. Synthesis. 2006;2006:2371–2375. doi: 10.1055/S-2006-942397. [DOI] [Google Scholar]
- Challis B.C., Yousaf T.I. Facile formation of N-nitrosamines from bromonitromethane and secondary amines. J. Chem. Soc., Chem. Commun. 1990:1598–1599. doi: 10.1039/C39900001598. [DOI] [Google Scholar]
- Chaskar A.C., Langi B.P., Deorukhkar A., Deokar H. Bismuth chloride-sodium nitrite: A novel reagent for chemoselective N-nitrosation. Synth. Commun. 2009;39:604–612. doi: 10.1080/00397910802413881. [DOI] [Google Scholar]
- Cross K.P., Ponting D.J. Developing structure-activity relationships for N-nitrosamine activity. Comput. Toxicol. 2021;20:100186–100197. doi: 10.1016/j.comtox.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattatraya J.P., Arun S.N., Deshmukh V.K., Ghawate4 V.G., Pund A.R. A review on nitrosamine impurities present in drugs. Pharm. Reson. 2022;4(2):44–48. https://pharmacy.dypvp.edu.in/pharmaceutical-resonance/downloads/original-research-articles/Volume-4-Issue-2/7.pdf. [Google Scholar]
- Demir A.S., Mahasneh A.S., Aksoy H., Gercek Z. The mild N-nitrosation of secondary amines with trichloro nitromethane. Synth. Commun. 1992;22:2607–2611. doi: 10.1080/00397919208021659. [DOI] [Google Scholar]
- Dewynter G., Abdaoui M., Regainia Z., Montero J.-L. Synthesis of N-Sulfamoyl oxazolidinones and -Perhydrooxazinones reactivity and use as donors in the transsulfamoylation reaction; Application to the preparation of 2-chloroethylnitrososulfamides IV. Tetrahedron. 1996;52:14217–14224. doi: 10.1016/0040-4020(96)00860-5. [DOI] [Google Scholar]
- Dobo K.L., Kenyon M.O., Dirat O., Engel M., Fleetwood A., Martin M., Mattano S., Musso A., Christopher Mcwilliams J., Papanikolaou A., Parris P., Whritenour J., Yu S., Kalgutkar A.S. Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N-Nitrosamine Impurities. Chem. Res. Toxicol. 2022;35:475–489. doi: 10.1021/acs.chemrestox.1cc00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shaheny R., Radwan M., Yamada K., El-Maghrabey M. Estimation of nizatidine gastric nitrosatability and product toxicity via an integrated approach combining HILIC, in silico toxicology, and molecular docking. J. Food Drug Anal. 2019;27:915–925. doi: 10.1016/j.jfda.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T.Y., Vita R., Fine D.H. C-Nitro compounds: a new class of nitrosating agents. Toxicol. Lett. 1978;2:5–10. doi: 10.1016/0378-4274(78)90057-7. [DOI] [Google Scholar]
- Frick, M., Cloëz, S., 2021. TAG Information Note: N-nitrosamines and tuberculosis medicines rifampicin and rifapentine, 1-14.
- Gordon I.M., Maskill H. Solvolysis of substituted benzyl azoxyarenesulfonates: characterisation of the transition state and the selectivity of benzylic intermediates in 50% aqueous 2,2,2-trifluoroethanol. J. Chem. Soc. Perkin Trans. 2001;2:2059–2062. doi: 10.1039/B106887N. [DOI] [Google Scholar]
- Helguera A.M., Cordeíro M.N.D., Pérez M.Á.C., Combes R.D., González M.P. Quantitative structure carcinogenicity relationship for detecting structural alerts in nitroso-compounds; Sex: Male; Route of administration: Water. Toxicol. Appl. Pharmacol. 2008;231(2):197–207. doi: 10.1016/j.taap.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Himo F., Demko Z.P., Noodleman L., Shampless K.B. Mechanisms of tetrazole formation by addition of azide to nitriles. J. Am. Chem. Soc. 2002;124:12210–12216. doi: 10.1021/ja0206644. [DOI] [PubMed] [Google Scholar]
- Iglesias E., Casado J. Mechanisms of hydrolysis and nitrosation reactions of alkyl nitrites in various media. Int. Rev. Phys. Chem. 2002;21:37–74. doi: 10.1080/01442350110092693. [DOI] [Google Scholar]
- ICH Harmonised Guideline: Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7 (R1). Current Step 4 version. https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf (accessed 31 May 2017).
- Jireš, J., Gibala, P., Kalášek, S., Douša, M., Doubsky¢, J., 2021. The determination of two analogues of 4-(azidomethyl)-1,1¢-biphenyl as potential genotoxic impurities in the active pharmaceutical ingredient of several sartans containing a tetrazole group. J. Pham. Biomed. Anal., 205: 114300-6. https://doi.org/10.1016/j.jpba.2021.114300. [DOI] [PubMed]
- Keeper L.K., Roller P.P. N-nitrosation by nitrite ion in neutral and basic medium. Science. 1973;181:1245–1247. doi: 10.1126/science.181.4106.1245. [DOI] [PubMed] [Google Scholar]
- Khlestkin V.K., Mazhukin D.G., Tikhonov A.Y., Rybalova T.V., Bagryanskaya I.Y., Gatilov Y.V. Intramolecular cyclization of 1,2-Bis (N-alkoxy-N-nitrosoamino) alkanes: A unique route to 4,5-dihydro-1,2,3-triazole 2-oxides. Synthesis. 2000:681–690. doi: 10.1055/s-2000-6399. [DOI] [Google Scholar]
- Kim S., Lee S., Hong J., Ko I., Kim J.-Y., Kim D.-K. Effect of ranitidine intake on the risk of gastric cancer development. Healthcare. 2021;9:1071–1079. doi: 10.3390/healthcare9081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R.D., King A.O., Chen C.Y., Corley E.G., Foster B.S., Roberts F.E., Yang C., Lieberman D.R., Reamer R.A., Tschaen D.M., Verhoeven T.R., Reider P.J., Lo Y.S., Rossano L.T., Brookes A.S., Meloni D., Moore J.R., Arnett J.F. Efficient synthesis of losartan, a nonpeptide angiotensin II receptor antagonist. J. Org. Chem. 1994;59:6391–6394. doi: 10.1021/jo00100a048. [DOI] [Google Scholar]
- Leffler J.E., Temple R.D. Staudinger reaction between triarylphosphines and azides mechanism. J. Am. Chem. Soc. 1967;89(20):5235–5246. doi: 10.1021/ja00996a027. [DOI] [Google Scholar]
- Li Y., Hecht S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022;23:4559. doi: 10.3390/ijms23094559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Zhou Q., Jia R., Liu W., Hou H., Ma J., Li D., Chen N., Chen W., Ye J., Hu W. Reaction of irbesartan with nitrous acid produces irebesartan oxime derivatives, rather than N-nitrosoirbesartan. Org. Process Res. Dev. 2021;26(4):1236–1246. doi: 10.1021/acs.oprd.1c00494. [DOI] [Google Scholar]
- López-Rodriguez R., McManus J.A., Murphy N.S., Ott M.A., Burns M.J. Pathways for N-Nitroso Compound Formation: Secondary Amines and Beyond. Org. Process Res. Dev. 2020;24:1558–1585. doi: 10.1021/acs.oprd.0c00323. [DOI] [Google Scholar]
- Lv J., Wang L., Li Y. Characterization of N-nitrosodimethylamine formation from the ozonation of ranitidine. J. Environ. Sci. 2017;58:116–126. doi: 10.1016/j.jes.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Madasu S.B., Vekariya N.A., Koteswaramma C., Islam A., Sanasi P.D., Korupolu R.B. An efficient, commercially viable, and safe process for preparation of losartan potassium, an angiotensin II receptor antagonist. Org. Process Res. Dev. 2012;16(12):2025–2030. doi: 10.1021/op300179u. [DOI] [Google Scholar]
- Martinez J., Oiry J., Imbach J.L., Winternitz F. Activated N-nitrosocarbamates for regioselective synthesis of N-nitrosoureas. J. Med. Chem. 1982;25(2):178–182. doi: 10.1021/jm00344a017. [DOI] [PubMed] [Google Scholar]
- McGwin G. The association between ranitidine use and gastrointestinal cancers. Cancers. 2020;13:24–32. doi: 10.3390/cancers13010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihina J.S., Herbst R.M. The reaction of nitriles with hydrazoic acid: synthesis of monosubstituted tetrazoles. J. Org. Chem. 1950;15(5):1082–1892. doi: 10.1021/jo01151a027. [DOI] [Google Scholar]
- Miyahara M. Substituent effects on nitrosation of 1,3-diarylureas with nitrosyl chloride, dinitrogen trioxide, and dinitrogen tetroxide. Chem. Pharm. Bull. 1986;34:1950–1960. https://pharmacy.dypvp.edu.in/pharmaceutical-resonance/downloads/original-research-articles/Volume-4-Issue-2/7.pdf [Google Scholar]
- Nasr N.E.H., Metwaly G.M., Ahmed E.O., Fares A.R., ElMeshad A.N. Investigating the root cause of N-nitrosodimethylamine formation in metformin pharmaceutical products. Exp. Opin. Drug Saf. 2021;20(7):1–8. doi: 10.1080/14740338.2021.1917547. [DOI] [PubMed] [Google Scholar]
- Ohwada T., Miura M., Tanaka H., Sakamoto S., Yamaguchi K., Ikeda H., Inagaki S. Structural Features of Aliphatic NNitrosamines of 7-Azabicyclo[2.2.1]Heptanes That Facilitate N-NO Bond Cleavage. J. Am. Chem. Soc. 2001;123:10164–10172. doi: 10.1021/ja010917d. [DOI] [PubMed] [Google Scholar]
- Peto R., Gray R., Brantom P., Grasso P. Dose and time relationships for tumor induction in the liver and esophagus of 4080 inbred rats by chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine. Cancer Res. 1991;51:6452–6469. https://aacrjournals.org/cancerres/article/51/23_Part_2/6452/497278/Dose-and-Time-Relationships-for-Tumor-Induction-in. [PubMed] [Google Scholar]
- Rehse K., König P. New NO-donors with antithrombotic and vasodilating Activities, XII: mesoionic oxatriazoles and related noncyclic nitrosohydrazine derivatives. Arch. Pharm. (Weinheim, Ger.) 1995;328:137–142. doi: 10.1002/ardp.19953280209. [DOI] [PubMed] [Google Scholar]
- Rice S., Ichinotsubo D., Mower H., Mandel M. Structure elucidation of nitrosocimetidine, a mutagenic charge-transfer system. J. Am. Chem. Soc. 1985;107(26):8023–9029. doi: 10.1021/ja00312a037. [DOI] [Google Scholar]
- Roux J.L., Gallard H., Croué J., Papot S., Deborde M. NDMA formation by chloramination of ranitidine: kinetics and mechanism. Environ. Sci. Technol. 2012;46:11095–11103. doi: 10.1021/es3023094. [DOI] [PubMed] [Google Scholar]
- Ruepp R., Frötschl R., Bream R., Filancia M., Girard T., Spinei A., Weise M., Whomsley R. The EU Response to the Presence of Nitrosamine Impurities in Medicines. Front. Med. 2021;8 doi: 10.3389/fmed.2021.782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtsdorff S., Neumann J., Schmidt A.H., Parr M.K. Risk assessment for nitrosated pharmaceuticals: A future perspective in drug development. Arch. Pharm. 2022;355(4):2100435–2100445. doi: 10.1002/ardp.202100435. [DOI] [PubMed] [Google Scholar]
- Sedlo I., Kolonić T., Tomić S. Presence of nitrosamine impurities in medicinal products. Arh. Hig. Rada. Toksikol. 2021;72:1–5. doi: 10.2478/aiht-2021-72-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik K.M., Sarmah B., Wadekar G.S., Kumar P. Regulatory updates and analytical methodologies for nitrosamine impurities detection in sartans, ranitidine, nizatidine, and metformin along with sample preparation techniques. Crit. Rev. Anal. Chem. 2020;52(1):53–71. doi: 10.1080/10408347.2020.1788375. [DOI] [PubMed] [Google Scholar]
- Shaikh T., Gosar A., Sayyed H. Nitrosamine impurities in drug substances and drug products. J. Adv. Pharm. Pract. 2020;2(1):48–57. doi: 10.5281/zenodo.3629095. [DOI] [Google Scholar]
- Shen R., Andrews S.A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res. 2010;45:944–952. doi: 10.1016/j.watres.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Shiri M., Zolfigol M.A., Kruger H.G., Tanbakouchian Z. Advances in the application of N2O4/NO2 in organic reactions. Tetrahedron. 2010;66:9077–9106. doi: 10.1016/j.tet.2010.09.057. [DOI] [Google Scholar]
- Smith P.A.S., Loeppky R.N. Nitrosative cleavage of tertiary amines. J. Am. Chem. Soc. 1976;89(5):1147–1157. doi: 10.1021/ja00981a021. [DOI] [Google Scholar]
- Snodin D.J., Elder D.P. Short commentary on NDMA (N-nitrosodimethylamine contamination of valsartan products. Regul. Toxicol. Pharmacol. 2019;103:325–329. doi: 10.1016/j.yrtph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Stedman, G., 1959. 591. Mechanism of the azide-nitrite reaction. Part II. J. Chem. Soc. (Resumed), 2949-2954.
- Thakkalapally A., Benin V. Synthesis, structural studies and desilylation reaction of some N-2-(Trimethylsilyl) ethyl-N-nitrosocarbamates. Tetrahedron. 2005;61:4939–4948. doi: 10.1016/j.tet.2005.03.044. [DOI] [Google Scholar]
- The processing method of a kind of azides ion and without the husky smooth class bulk pharmaceutical chemicals of genotoxicity impurity and its intermediate. CN Pat. 109748905A., Zhuhai Rundu Pharmaceutical.
- Thresher A., Foster R., Ponting D.J., Stalford S.A., Tennant R.E., Thomas R. Are all nitrosamines concerning? A. review of mutagenicity and carcinogenicity data. Regul. Toxicol. Pharmacol. 2020;116 doi: 10.1016/j.yrtph.2020.104749. [DOI] [PubMed] [Google Scholar]
- Tuesuwan B., Vongsutilers V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021;110:3118–3128. doi: 10.1016/j.xphs.2021.04.021. [DOI] [PubMed] [Google Scholar]
- United State Pharmacopeia. The National Formulary. General Chapter <1469> NITROSAMINE IMPURITIES. USPNF 2022 Issue 2; United States Pharmacopeia: Rockville, MD, USA, 2008; p. 1-15. https://doi.org/10.31003/USPNF_M15715_02_01.
- US FDA. Guidance for Industry: Control of nitrosamine impurities in human drugs. US Department of Health and Human Services, Food and Drug Administration: Washington, DC, 2021, Rev. 1.
- Wang C.-H., Chen I., Chen C.-H., Tseng Y.-T. Pharmacoepidemiological research on N-nitrosodimethylamine contaminated ranitidine use and long-term cancer risk: A population-based longitudinal cohort study. Int. J. Environ. Res. Public Health. 2022;19:12469–12484. doi: 10.3390/ijerph191912469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yang H., Zhou B., Wang X., Xie Y. Effect of oxidation on amine-based pharmaceutical degradation and N-Nitrosodimethylamine formation. Water Res. 2015;87:403–411. doi: 10.1016/j.watres.2015.07.045. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 1980. IARC monographs on the evaluation of carcinogenic risks to humans, Some pharmaceutical drugs. Lyon (SW), Vol. 24.
- World Health Organization (WHO), 1987. IARC monographs on the evaluation of carcinogenic risks to humans, Some N-nitroso Compounds. Lyon (FR), Vol. 17.
- Xiaohui, Z., Xiaoren, Z., Yuanxun, Z., Peng, D., Peng, W., Jinsheng, L., Wenquan, Z., Min, L., 2018. A kind of synthetic method of valsartan. CN Pat. 108752285 A, Zhejiang Huahai Pharmaceutical.
- Xiaoren, Z., Nianping, S., Wenling, Z., Peng, W., 2014. Improved method for preparing tetrazole for valsartan. CN Pat. 104045602A.
- Yedage S.L., Bhanage B.M. Tert-butyl nitrite-mediated synthesis of N-nitrosoamides, carboxylic acids, benzocoumarins, and isocoumarins from amides. J. Org. Chem. 2017;82:5769–5781. doi: 10.1021/acs.joc.7b00570. [DOI] [PubMed] [Google Scholar]
- Yokoo H., Yamamoto E., Masada S., Uchiyama N., Tsuji G., Hakamatsuka T., Demizu Y., Izutsu K., Goda Y. N-nitrosodimethylamine (NDMA) formation from ranitidine impurities: possible root causes of the presence of NDMA in ranitidine hydrochloride. Chem Pharm Bull. 2021;69:872–876. doi: 10.1248/cpb.c21-00289. [DOI] [PubMed] [Google Scholar]
- Zeng T., Mitch W.A. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethyl amine. Carcinogenesis. 2016;37(6):625–634. doi: 10.1093/carcin/bgw034. [DOI] [PubMed] [Google Scholar]
- Zolfigol M.A. Efficient and chemoselective N-nitrosation of secondary amines under mild and heterogeneous conditions with sodium nitrite and oxalic acid two hydrate. Synth. Commun. 1998;29:905–910. doi: 10.1080/00397919908086051. [DOI] [Google Scholar]
- Zuwei, H., Gang, L., Dequan, S., Ruiyun, L.V., Wei, X., Zhen, W., Guorong, Z., Yongjun, T., 2021. Preparation method for losartan. EU Pat. WO2021036193A1., Zhejiang Tianyu Pharmaceutical.