Abstract
Objectives
The oral microbiological environment may be implicated in the corrosion of orthodontic metals. This study aimed to examine the prevalence of sulfate-reducing bacteria (SRB) in orthodontic patients undergoing fixed appliance treatment.
Methods
Sixty-nine orthodontic and 69 healthy non-orthodontic participants were enrolled in the study. Supragingival and subgingivaloral biofilm were collected and tested for the presence of SRB. The DNA extraction, polymerase chain reaction (PCR), and 16sRNA Sanger sequencing method was performed from the SRB-positive samples. The sequenced PCR products were analysed and compared with databases to identify the bacterial genus.
Results
Amongst 69 orthodontic patients, characteristic black precipitates developed in 14, indicating the presence of iron sulfides which demonstrates the likelihood of SRB. Alternatively, 2 out of 69 showed the presence of SRB in healthy non-orthodontic participants (controls). Desulfovibrio spp was confirmed by analyses of 16sRNA sequencing, which revealed that the SRB prevalence was 20% in the examined participants with orthodontic appliances.
Conclusions
The prevalence of SRB was found to be significantly higher amongst orthodontic patients compared to non-orthodontic participants. Presence of stainless steel in the oral environment may have facilitated the colonisation of SRB.
Key words: Desulfovibrio, Microbial corrosion, Nickel-titanium, Orthodontics, Periodontal disease, Sulfate-reducing bacteria
Introduction
Orthodontics uses wires and brackets to exert physiologic forces to correct malocclusions.1 It is estimated that approximately 25% to 53% of people worldwide need orthodontic treatment at some point in their life.2,3 In spite of the popularity of clear aligners, fixed orthodontic metal appliances remain a mainstay of most orthodontic practices.4,5 Efficient orthodontic treatment is predicated on unimpaired force application through wires and brackets. Alternatively, treatment efficiency can be affected by oral ecological conditions. Microbiological corrosion of dental alloys in the oral cavity is a fairly well-known phenomenon.6 Corrosion can weaken the parts of a fixed orthodontic appliance, damaging them and causing deterioration in its mechanical properties, and resulting in breakages and a longer treatment time.7,8
A wide microbial profile is evident on fixed orthodontic appliances during treatment.9 The mouth is host to more than 700 different bacterial species forming a complex microflora, with each individual's oral microbiome being unique at the species level. Sulfate-reducing bacteria (SRB) are a group of microorganisms responsible for the oxidation of sulfur compounds from the surrounding environment.10 They are commonly found in the gastrointestinal tracts of animals and human.11 They are believed to be a part of the oral microflora, yet their exact composition in the oral ecology remains unclear. There are more than 2 dozen genera of SRB including Desulfovibrio, Desulfomonas, Desulfobacter, and Desulfomena. Desulfovibrio desulfuricans have been detected commonly in the oral cavity.12 SRB are believed to be involved in destructive periodontal diseases.13 The evidence tying SRB to microbiological corrosion is incontrovertible.14,15 Biofilms formed by the SRB can accelerate corrosion by its metabolites or its by-products within a gelatinous matrix that adheres to the metal interface.16 Previous studies have shown the microbiological corrosive activity of D desulfuricans in endodontic files17 and stainless steel.18
Orthodontic literature has little definitive research examining oral microbiological corrosion of metal brackets and wires used in patients. Several classes of microorganisms including bacteria, fungi, and algae showed potential to corrode metals. Amongst these, SRB play a significant role in the corrosion process.19 SRB access their energy for growth through reduction of sulfate to sulfide (hydrogen sulfide) using electrons from hydrogen present in the environment. Two mechanisms of corrosion are suggested: the undissociated protons in Hydrogen Sulfide accept protons from iron causing corrosion by chemical means.20 Second, SRB utilise electrons from iron by direct uptake through electrical means as the source of reducing equivalents for sulfate reduction.21
Iron can easily corrode in a cast form or stainless steel form, both under aerobic and anaerobic conditions, due to its ability to give off electrons very easily.22 With its potential in causing corrosion of iron under favourable conditions, research has generally focussed on biocorrosion due to SRB in the context of pipeline industries.23 To date, microbiological corrosion in orthodontic patients has received scant attention. There have been no attempts to examine the prevalence of biocorrosion-causing bacteria in the oral cavity. Hence, this study sought to assess the prevalence of oral SRB in a group of patients wearing fixed orthodontic appliances and a control group not undergoing such treatment.
Materials and methods
Participant selection
The study was conducted following the Declaration of Helsinki and was approved by the Institutional Ethics Review Board of Sri Venkateswara Dental College and Hospital, Chennai, India (IEC/SVDCH/1906). Informed consent was obtained from all participants involved in the study. Sample size was calculated based on Willis et al.24 A total of 138 participants (69; male = 36, female = 33) undergoing orthodontic treatment and 69 controls (male = 34, female = 35) were recruited for this study based on simple random sampling method by following inclusion and exclusion criteria. Inclusion criteria were as follows: (1) Adolescent or young adult age (10–35 years) and (2) good oral hygiene assessed based on oral hygiene index score (ranging between 0 and 1.2). (3) For the orthodontic treatment group, the participants were treated with stainless steel metal braces (Victory series, 3M) and wires (G&H Wires), and levelling wires were to be stainless steel. (4) Finally, participants should be under orthodontic treatment for at least 6 months, as the establishment of any bacterial population takes a minimum of 6 months.25 Exclusion criteria include the following: (1) patients with gingivitis and/or periodontal diseases; (2) patients with systemic disease or under treatment including antibiotics, antiseptic mouth rinses, and periodontal issues and patients under systemic medications which might affect salivary flow or composition. (3) Those with orthodontic treatment other than stainless steel brackets were excluded. All screening examination was performed by the dental practitioners. Each participant was examined by 3 practitioners to avoid subjective decisions on the participant oral/dental status. Practitioners were blinded to the decisions of other practitioners, and if there was a difference in opinion it would be resolved by further assessment by a fourth examiner (this did not happen in our case). All 138 samples were collected within a period of 1 to 2 weeks. Average age of the control group was 22 ± 1.7 years; for the treatment group, who received treatment for 19 ± 9 months, average age was 18.7 ± 4.8 years. Informed consent was obtained from all participants. The participants were given proper oral hygiene instructions before introduction into the study. Hygiene instructions and their significance were reinforced at every visit to avoid the influence of periodontal disease on the prevalence of SRB.
Sample collection
Samples were collected from each individual using sterilised cotton buds and a curette from numerous locations, including anterior and posterior palate, buccal and vestibular mucosa just above the parotid duct opening, dorsum of the tongue, supra, and subgingival sites. Further, the samples of each person at numerous sites were pooled to conduct a microbiological examination.
Medium preparation
A collection medium was prepared by suspending 25.4 g of sulfate in American petroleum institute (API) agar following the manufacturer recommendation (Himedia) containing 1000 mL of purified distilled water. Then, 4 mL of sodium lactate was added and the mixture was brought to a boiling temperature to ensure complete dissolution of the material. Following that, 1.5 mL of the mixture was distributed equally to 138 (69 treated and 69 control) screw cap tubes and autoclaved at 12 °C at 15 pounds’ pressure for 10 minutes. All samples were processed at the Department of Microbiology, Madras Veterinary College, Chennai, India, for bacterial identification. The collected sample was immediately placed in the screw cap tubes with a reducing medium. Subsequently, samples were transferred to the culture medium containing sulfate API agar with 14 g/L of agar for the cultivation of pure colonies. Samples inoculated in separate tubes with the selective medium are incubated at 30 °C for 28 days and observed for the appearance of characteristic black colonies. The colonies were further confirmed through genus-specific polymerase chain reaction (PCR).
DNA extraction and purification from cultures
From the colonies that turned black, we randomly picked 3 regions for further molecular analysis. DNA was extracted using the method described by Queipo-Ortuno et al with certain modifications (the centrifuge time was increased to 15 minutes and bacterial pellet was washed twice with phosphate-buffered saline [PBS] to obtain pure culture).26 Overnight bacterial cultures were centrifuged at 6000 rpm for 10 minutes at room temperature. The bacterial pellet was washed twice by resuspending in 5 mL of PBS at 6000 rpm for 10 minutes at room temperature. Then the bacterial pellet was resuspended in 100 µL of nuclease-free water and boiled at 100 °C in a water bath for 10 minutes and snap chilled on ice for 5 minutes. The lysate was centrifuged at 12,000 rpm for 10 minutes, and the supernatant was collected and stored at −20 °C until further use.
Polymerase chain reaction
Genus-specific PCR was performed using a modified method described by Fite et al27 with the primer sequences (forward (DSV691-F):5’-CCGTAGATATCTGGAGGAACATCAG-3’ and reverse (DSV826-R):5’-ACATCTAGCATCCATCGTTTACAGC-3’) by following the PCR condition to confirm presence of Desulfovibrio genus in the samples.27 Additionally, the PCR for bacterial 16S rRNA using universal primers (16S-For: 5′-AGAGTTTGATYMTGGCTCAG-3’ and 16S-Rev: 5’-GYTACCTTGTTACGACTT-3’) was performed following the usual PCR cyclic conditions. The reaction mixture was prepared containing master mix (25 µL), forward (1 µL), and reverses primers (1 µL) and sample DNA (5 µL) with the nuclease free water to make the total volume 50 µL. Then it was placed in a PCR thermal cycle machine for initial denaturation at 95 °C for 5 minutes followed by cyclical denaturation, annealing (55 °C), and extension (72 °C) lasting 10 minutes. Simultaneously, the PCR products were sequenced using the Sanger sequencing method for the 7 randomly selected samples of amplified product of bacterial 16S rRNA. The obtained 16S rRNA sequences were compared to those already in GenBank (www.ncbi.nlm.nih.gov).
Statistical analysis
The statistical analysis for the prevalence data obtained from this experiment was carried out using SPSS software version 21. Further, we use chi-square test to determine the differences in SRB prevalence based on age, sex, and duration (months) within the orthodontic treatment group.
Results
Biochemical assessment of SRBs
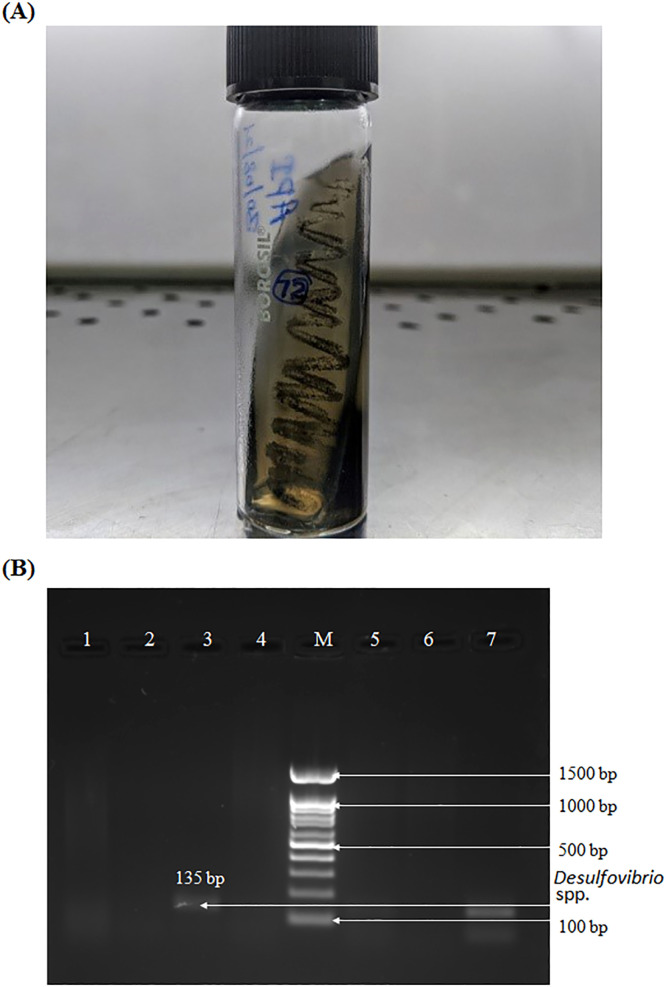
The media was visually analysed for the colour change with black precipitates dispersed throughout the medium. The blackening indicated the presence of iron sulfide. The iron in the API medium binds with sulfides formed by SRBs as a result of their reducing process leading to the formation of iron sulfide. Samples were taken from the grow pure cultures for DNA extraction. We were able to see pure characteristic black colonies during the process (Figure 1A). Amongst 69 participants in the orthodontic treatment group, the characteristic black precipitates developed in 14. However, from 69 control participants in the non-orthodontic treatment group, 2 were observed to have black precipitates.
Fig. 1.
A, Characteristic black colonies presenting the likelihood of sulfate-reducing bacteria (SRB). B, polymerase chain reaction (PCR) products from the pure culture showing the presence of Desulfovibrio genus by gel electrophoresis. The PCR product (Lane 3 and 5 showing Desulfovibrio spp specific product size of 135 bp. Lane 7: Positive control and M: 100 bp DNA Ladder.
Molecular identification
The genomic DNA was extracted from the grown pure cultures. Qualitative and quantitative analysis of the DNA was performed in Thermo Scientific NanoDrop™ 2000 and revealed that the absorbance ratio of 260/280 was in the range of 1.75 to 1.85; thus, extracted genomic DNA was free from any other contamination. The concentration of genomic DNA was in the range of 560 to 730 µg/mL. We performed conventional PCR to establish the presence of SRB in the oral environment of the orthodontic treatment group (Figure 1B). All PCR products from the pure culture showed the presence of Desulfovibrio genus by gel electrophoresis. Further, DNA amplification based on universal 16S rRNA and subsequent sequencing confirmed the presence of SRB specific to D desulfuricans, D piger, and D fairfieldensis based on the identify in the existing database at GenBank. The obtained sequences were submitted to the NCBI GenBank repository, which can be accessed using the accession numbers ON183261, ON183262, and ON183263. Overall, using genus-level primers, the samples were detected with Desulfovibrio, whilst sequencing and matching with the database Desulfovibrio spp have been determined in the samples.
Subgroup analysis
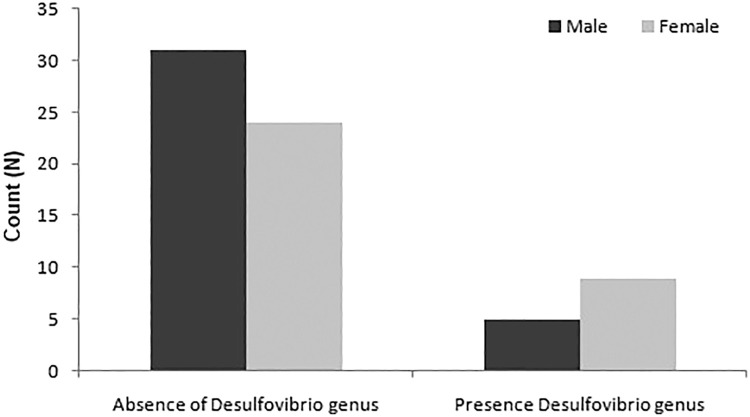
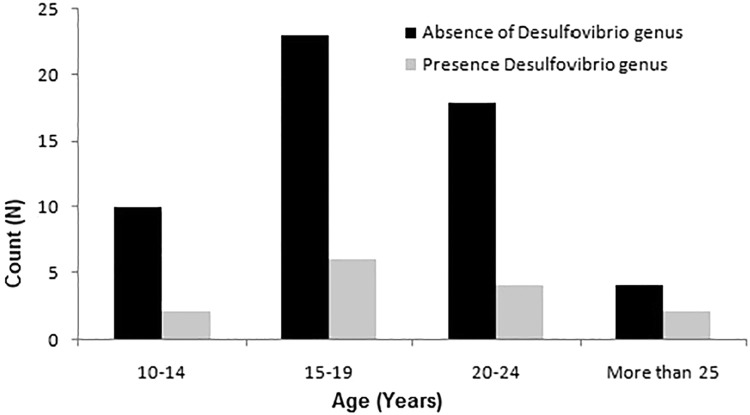
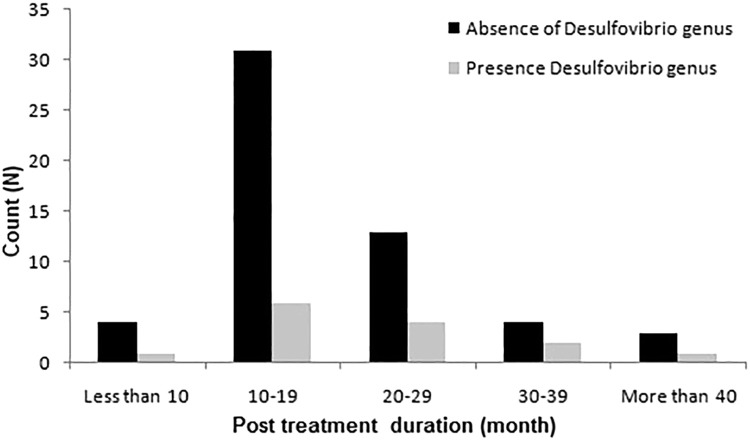
Significant changes in SRB prevalence based on sex, age, and treatment duration were assessed using the chi-square test. The analysis revealed no significant difference in the prevalence percentage amongst sex, age, or months of treatment (Table, Fig. 2, Fig. 3, Fig. 4).
Table.
Subgroup analysis based on sex, age, and orthodontic treatment duration.
| Parameter | Subgroup | Absence of Desulfovibrio genus (N) | Presence Desulfovibrio genus (N) | χ2 value | P value |
|---|---|---|---|---|---|
| Sex | Male | 31 | 5 | 1.90 | .167 |
| Female | 24 | 9 | |||
| Age group (years) | 10–14 | 10 | 2 | 0.79 | .85 |
| 15–19 | 23 | 6 | |||
| 20–24 | 18 | 4 | |||
| >25 | 4 | 2 | |||
| Months into orthodontic treatment | <10 | 4 | 1 | 1.17 | .88 |
| 10–19 | 31 | 6 | |||
| 20–29 | 13 | 4 | |||
| 30–39 | 4 | 2 | |||
| >40 | 3 | 1 |
Fig. 2.
Bar chart for sex-based association of SRB prevalence.
Fig. 3.
Bar chart for age-based association of SRB prevalence
Fig. 4.
Bar chart for treatment duration and the association of sulfate-reducing bacteria prevalence.
Discussion
Corrosion is the gradual destruction of any metal due to environmental conditions leading to deterioration in material properties and appearance.28 In the wider world, microbial corrosion has huge economic consequences.29 Several microorganisms including SRB in the oral cavity have been implicated in the corrosion of dental materials.6 This study examined the prevalence of SRB in the oral cavity of orthodontic patients, which is one of the causative factors for corrosion of orthodontic wires and brackets made with stainless steel. Our results found evidence for the presence of SRB, with a prevalence of 20% in the oral cavity of patients who underwent orthodontic treatment. However, only a 2.89% prevalence was seen in the control group. Results were based on sequential analysis from (1) the biochemical analysis to test the presence of SRB evident due to the dark-coloured iron sulfide precipitates as a result of bacterial metabolism. Further, (2) molecular analysis based on PCR and DNA sequencing revealed prevalence of Desulfovibrio spp in the samples.
-Desulfovibrio is a Gram-negative SRB implicated in metal corrosion.30 The stainless steel metal surface of orthodontic brackets can provide iron atoms that can combine with the sulfide ions created by the reduction of sulfates to sulfides by SRBs, resulting in the formation of iron sulfide.31 Produced sulfide acts on the layer of stainless steel, dissolving it into metallic ions taken up by the SRB.32 Geesey et al examined the action of SRB on stainless steel using electron microscopy and found that the bacterial action can penetrate up to a depth of 2 to 5 nm.33 Deng et al recently demonstrated that iron sulfide functions as an excellent electron conductor, playing a role in microbial energy production.34 Particularly, Desulfovibrio is said to weaken the passive metallic layer on the surface of stainless steel through the dissolution of the ions.34 These ions appear to have a effect on the metabolism of the SRB, enhancing microbiologic corrosion.32 Additionally, the prevalence of SRB in the oral cavity of orthodontic patients is significant and has wider implications on oral health. The higher prevalence of SRB is concerning because their metabolic by-products such as hydrogen sulfide can cause cellular damage, contributing to the initialisation and propagation of periodontal disease.13 Particularly, SRB are implicated in oral malodor due to their release of volatile sulfur compounds (VSCs).35 Besides resulting in halitosis, these VSCs may contribute to the aetiology of gingivitis and periodontitis.36
Given its proven potential in causing corrosion and oral pathologic effect,25 the confirmed prevalence of SRB in orthodontic patients raises concern. Our study found a prevalence of 20% SRB in orthodontic and 2.89% in non-orthodontic samples examined. Most existing research on the prevalence of SRB is confined to spheres of periodontal disease and gastrointestinal diseases. Although we have not tested corrosion, we try to establish that our results are partly in agreement with a previous study by Heggendorn et al, who found a prevalence of 29.7% of SRB in the oral microbiome of a random sampling of individuals.37 Similarly, our findings are consistent with previous work by Langendijk et al, who found that 64% of patients presented with SRB in cases of gingivitis and pocket formation.13 Langendijk-Genevaux et al found that elimination of the SRB through mechanical periodontal treatment led to appreciable clinical improvement.38 Mechanical debridement through scaling and root planning effectively eliminated up to 89% of the SRB at 95% of the sites.38 This is in accord with evidence that oral health promotion through effective prophylaxis and plaque oral biofilm control can lead to better orthodontic and periodontal treatment outcomes.39,40
Overall, our study provides benefit in elucidating the prevalence of SRB in patients that contributes to corrosion of orthodontic metals. It is important to acknowledge the advantages of this study and that this is the first study to undertake an analysis of the prevalence of SRB in orthodontic patients. Also, it provides evidence based on 16S rRNA sequences (submitted to the GenBank repository) that indicate the presence of Desulfovibrio spp. Alternatively, there are few limitations1: We have not analysed corrosion by SRB or its toxicity. However, published literature on the ions associated with metal corrosion and its toxicity have been discussed. Finally, this study can be extended in the future by addressing the prevalence of SRB in orthodontic metals made from other materials. The material properties of an orthodontic bracket and wire are fundamental to their effectiveness. Formation of SRB biofilms on these might lead to degradation of the material over time, altering treatment duration and efficiency. Hence, a deeper understanding of the effects of microbiological corrosion of orthodontic appliances is warranted through further studies.
Conclusions
The present study set out to establish the prevalence of SRB in the oral cavity of orthodontic patients. We identified that SRB have a prevalence of 20% in orthodontic patients. The samples developed black precipitates that are characteristic of iron sulfides formed from bacterial metabolism. Further research on the biochemical functions and complex interplay amongst SRB, iron sulfides, and dental materials is needed to prove the corrosion of orthodontic materials and also to develop strategies to prevent microbiological corrosion in the oral cavity.
Conflicts of Interest
None disclosed.
REFERENCES
- 1.Yamaguchi K, Nanda RS, Morimoto N, Oda Y. A study of force application, amount of retarding force, and bracket width in sliding mechanics. Am J Orthod Dentofac Orthop. 1996;109(1):50–56. doi: 10.1016/s0889-5406(96)70162-2. [DOI] [PubMed] [Google Scholar]
- 2.de Almeida AB, Leite ICG. Orthodontic treatment need for Brazilian schoolchildren: a study using the Dental Aesthetic Index. Dental Press J Orthod. 2013;18(1):103–109. doi: 10.1590/s2176-94512013000100021. [DOI] [PubMed] [Google Scholar]
- 3.Utomi I, Onyeaso C. Malocclusion and orthodontic treatment need of patients attending the Lagos University Teaching Hospital, Lagos, Nigeria. Odontostomatol Trop. 2015;38(150):23–30. [PubMed] [Google Scholar]
- 4.Antonio-Zancajo L, Montero J, Albaladejo A, Oteo-Calatayud MD, Alvarado-Lorenzo A. Pain and oral-health-related quality of life in orthodontic patients during initial therapy with conventional, low-friction, and lingual brackets and aligners (Invisalign): a prospective clinical study. J Clin Med. 2020;9(7):2088. doi: 10.3390/jcm9072088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, Tang JS, Skulski B, et al. Evaluation of Invisalign treatment effectiveness and efficiency compared with conventional fixed appliances using the Peer Assessment Rating index. Am J Orthod Dentofac Orthop. 2017;151(2):259–266. doi: 10.1016/j.ajodo.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Kameda T, Oda H, Ohkuma K, et al. Microbiologically influenced corrosion of orthodontic metallic appliances. Dent Mater J. 2014;33:187–195. doi: 10.4012/dmj.2013-297. [DOI] [PubMed] [Google Scholar]
- 7.Zapata-Noreña Ó, Barbosa-Liz DM, Carvajal-Flórez Á, SP P. Factors related to orthodontic treatment duration for a graduate orthodontics program. Rev Fac Odontol Univ Antioquia. 2017;13:17–25. [Google Scholar]
- 8.Bichara LM, Aragón MLC de, Brandão GAM, Normando D. Factors influencing orthodontic treatment time for non-surgical class III malocclusion. J Appl Oral Sci. 2016;24(5):431–436. doi: 10.1590/1678-775720150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gujar AN, Al-Hazmi A, Raj AT, Patil S. Microbial profile in different orthodontic appliances by checkerboard DNA-DNA hybridization: an in-vivo study. Am J Orthod Dentofacial Orthop. 2020;157(1):49–58. doi: 10.1016/j.ajodo.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Kushkevych I, Coufalová M, Vítězová M, Rittmann SKMR. Sulfate-reducing bacteria of the oral cavity and their relation with periodontitis—recent advances. J Clin Med. 2020;9(8):1–20. doi: 10.3390/jcm9082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushkevych I, Dordević D, Vítězová M. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Open Med. 2019;14(1):66–74. doi: 10.1515/med-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzierzewicz Z, Szczerba J, Lodowska J, et al. The role of Desulfovibrio desulfuricans lipopolysaccharides in modulation of periodontal inflammation through stimulation of human gingival fibroblasts. Arch Oral Biol. 2010;55(7):515–522. doi: 10.1016/j.archoralbio.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Langendijk PS, Hanssen JTJ, Van Der Hoeven JS. Sulfate-reducing bacteria in association with human periodontitis. J Clin Periodontol. 2000;27(12):943–950. doi: 10.1034/j.1600-051x.2000.027012943.x. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Whitfield M, Van Vliet KJ. Beating the bugs: roles of microbial biofilms in corrosion. Corros Rev. 2013;31(3–6):73–84. [Google Scholar]
- 15.Mallard E, Kervadec D, Gil O, Lefevre Y, Malard S. Interactions between steels and sulphide-producing bacteria—corrosion of carbon steels and low-alloy steels in natural seawater. Electrochim Acta. 2008;54:8–13. [Google Scholar]
- 16.Videla HA, Characklis WG. Biofouling and microbially influenced corrosion. Int Biodeterior Biodegradation. 1992;29(3–4):195–212. [Google Scholar]
- 17.Heggendorn FL, Gonçalves LS, Dias EP, de Oliveira FreitasLione V, Lutterbach MTS. Biocorrosion of endodontic files through the action of two species of sulfate-reducing bacteria: Desulfovibrio desulfuricans and Desulfovibrio fairfieldensis. J Contemp Dent Pract. 2015;16(8):665–673. doi: 10.5005/jp-journals-10024-1738. [DOI] [PubMed] [Google Scholar]
- 18.Beech IB, Gaylarde CC. Microbial polysaccharides and corrosion. IntBiodeterior. 1991;27(2):95–107. [Google Scholar]
- 19.Muyzer G, Stams AJM. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6(6):441–454. doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton WA. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling. 2003;19(1):65–76. doi: 10.1080/0892701021000041078. [DOI] [PubMed] [Google Scholar]
- 21.Iverson WP. Academic Press; 1987. Microbial corrosion of metals; pp. 1–36. Laskin AIBT-A in AM, editor. [Google Scholar]
- 22.Enning D, Garrelfs J. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol. 2014;80(4):1226–1236. doi: 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qing Y, Bai Y, Xu J, Wu T, Yan M, Sun C. Effect of alternating current and sulfate-reducing bacteria on corrosion of X80 pipeline steel in soil-extract solution. Materials (Basel) 2019;12(1):144. doi: 10.3390/ma12010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis CL, Gibson G, Allison C, Macfarlane S, Holt JS. Growth, incidence and activities of dissimilatory sulfate-reducing bacteria in the human oral cavity. FEMS Microbiol Lett. 1995;129(2–3):267–271. doi: 10.1111/j.1574-6968.1995.tb07591.x. [DOI] [PubMed] [Google Scholar]
- 25.Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89(1):8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 26.Queipo-Ortuño MI, De Dios Colmenero J, Macias M, Bravo MJ, Morata P. Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin Vaccine Immunol. 2008;15(2):293–296. doi: 10.1128/CVI.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fite A. Identification and quantitation of mucosal and faecaldesulfovibrios using real time polymerase chain reaction. Gut. 2004;53(4):523–529. doi: 10.1136/gut.2003.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibars JR, Moreno DA, Ranninger C. Microbial corrosion of stainless steel. Microbiologia. 1992;8(2):63–75. [PubMed] [Google Scholar]
- 29.Tang H-Y, Yang C, Ueki T, et al. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021;15(10):3084–3093. doi: 10.1038/s41396-021-00990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TMP, Sheng X, Ting Y-P, Pehkonen SO. Biocorrosion of AISI 304 stainless steel by Desulfovibrio desulfuricans in seawater. Ind Eng Chem Res. 2008;47(14):4703–4711. [Google Scholar]
- 31.Kumar AVR, Singh R, Nigam RK. Mössbauer spectroscopy of corrosion products of mild steel due to microbiologically influenced corrosion. J Radioanal Nucl Chem. 1999;242(1):131–137. [Google Scholar]
- 32.Lopes FA, Morin P, Oliveira R, Melo LF. Interaction of Desulfovibrio desulfuricans biofilms with stainless steel surface and its impact on bacterial metabolism. J Appl Microbiol. 2006;101(5):1087–1095. doi: 10.1111/j.1365-2672.2006.03001.x. [DOI] [PubMed] [Google Scholar]
- 33.Geesey GG, Gillis RJ, Avci R, et al. The influence of surface features on bacterial colonization and subsequent substratum chemical changes of 316L stainless steel. Corros Sci. 1996;38(1):73–95. [Google Scholar]
- 34.Chen G, Clayton CR. The influence of sulfate-reducing bacteria on the passivity of type 317L austenitic stainless steel. J Electrochem Soc. 1998;145(6):1914–1922. [Google Scholar]
- 35.Tanabe S, Desjardins J, Bergeron C, Gafner S, Villinski JR, Grenier D. Reduction of bacterial volatile sulfur compound production by licoricidinand licorisoflavan A from licorice. J Breath Res. 2012;6(1):16006. doi: 10.1088/1752-7155/6/1/016006. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, McCombs GB, Darby ML, Marinak K. Sulphur by-product: the relationship between volatile sulphur compounds and dental plaque-induced gingivitis. J Contemp Dent Pract. 2004;5(2):27–39. [PubMed] [Google Scholar]
- 37.Heggendorn FL, Gonçalves LS, Dias EP, Silva Junior A, Galvão MM, Lutterbach MTS. Detection of sulphate-reducing bacteria in human saliva. Acta Odontol Scand. 2013;71(6):1458–1463. doi: 10.3109/00016357.2013.770163. [DOI] [PubMed] [Google Scholar]
- 38.Langendijk-Genevaux PS, Hanssen JTJ. Van der Hoeven JS. Decrease of sulfate-reducing bacteria after initial periodontal treatment. J Dent Res. 2001;80(7):1637–1642. doi: 10.1177/00220345010800070801. [DOI] [PubMed] [Google Scholar]
- 39.Gray D, McIntyre G. Does oral health promotion influence the oral hygiene and gingival health of patients undergoing fixed appliance orthodontic treatment? A systematic literature review. J Orthod. 2008;35(4):262–269. doi: 10.1179/14653120722770. [DOI] [PubMed] [Google Scholar]
- 40.Migliorati M, Isaia L, Cassaro A, et al. Efficacy of professional hygiene and prophylaxis on preventing plaque increase in orthodontic patients with multibracket appliances: a systematic review. Eur J Orthod. 2015;37(3):297–307. doi: 10.1093/ejo/cju044. [DOI] [PubMed] [Google Scholar]