Abstract
Objective
Various modifications in formulation of glass ionomer cements (GICs) have been made in order to improve the clinical performance of these restorations. The aim of this work was to evaluate the microleakage and microshear bond strength (μSBS) of bacterial cellulose nanocrystal (BCNC)–modified glass ionomer cement (GIC) restorations in primary dentition.
Methods
A total number of 60 freshly extracted primary molar teeth were selected. Half of the samples were used for μSBS testing (in 2 groups, n = 15). In group 1, conventional GIC (CGIC) of Fuji IX (GC) was placed in cylindrical molds on dentinal surfaces. In group 2, CGIC of Fuji IX containing 1% wt of BCNCs was used. μSBS was evaluated using a universal testing machine. In another part of the study, microleakage of class V restorations was assessed according to the mentioned groups (n = 15). The sectioned samples were observed under stereomicroscope, and microleakage scores were recorded. SPSS version 16.0 (SPSS), independent samples t test, and Mann–Whitney U test were used for statistical analysis at a significance level of P < .05.
Results
Results showed statistically significant differences between the μSBS of CGIC and modified GIC groups (P < .0001). The BCNC-modified GIC group recorded significantly higher bond strength values (3.51 ± 0.033 vs 1.38 ± 0.034 MPa). Also, microleakage scores of CGIC and BCNC-modified GIC restorations were not significantly different (P = .57).
Conclusions
Based on our findings, it was concluded that incorporating BCNCs (1% wt) into the CGIC of Fuji IX significantly increased the μSBS to the dentin structure of the primary teeth.
Key words: Cellulose, Glass ionomer cement, Microleakage, Nanocrystals, Shear bond strength
Introduction
Considering the high rate of tooth decay in children and its consequences, treatment methods and materials used are very important.1 Recently, conservative restorative dentistry has been focused on preserving the dental hard tissue during cavity preparation.2 Hence, restoring a carious primary tooth in the preliminary stages is a rational treatment option that leads to preservation of the arch length as well as the functions of mastication and speech. In paediatric dentistry, simplicity of the clinical application of the restorative material along with other properties should be considered.3
Glass ionomer cements (GICs) are water-based materials that set by acid-based setting reaction between polyalkenoic acid and fluro-alumino silicate glass.4 They are used as full restorative materials in occlusal and proximal cavities of primary teeth, liners and bases, fissure sealants, bonding agents for orthodontic brackets, and atraumatic restorative treatment materials, especially in paediatric dentistry.5,6,7
The main advantages of GICs include short time needed to fill the cavity, chemical bond to the tooth structure, biocompatibility, and fluoride-releasing properties.5,8 However, the clinical performance of conventional GICs is not desirable due to the poor mechanical properties including low flexural strength, low fracture resistance, and desiccation and elastic deformation in masticatory movements.9 Several research works were concentrated on overcoming these disadvantages. For example, modifications with filler particles such as bioactive glass particles, hydroxyapatite powders, metallic powders, nanoclay, and discontinuous glass fibers were made.10 Recently, incorporation of cellulose nanocrystals (CNCs) improved the physicochemical properties needed in clinical situations.11
Cellulose is extracted from abundant sources like plant-based materials and some bacteria. Bacterial cellulose nanocrystals (BCNCs) are natural hydrogels, produced by Gluconacetobacter xylinum in culture, and constitute a 3-dimensional network of ribbon-shaped bundles of cellulose microfibrils.12 The supramolecular structure of the bacterial cellulose is known to be different from plant cellulose, although the chemical structure is comparable.13 They have unique characteristics such as high crystallinity, water retention capacity, and mechanical strength in the wet state. Other favourable characteristics of these nanocrystals include biocompatibility, low density, and biodegradability.13, 14, 15, 16, 17, 18 In addition to excellent dispersion in the matrix and forming a solid scaffold in different directions, these nanocrystals maintain the integrity and purity of the material.10,19 It is noteworthy to mention that many researchers prefer BCNCs for medical applications due to their excellent mechanical and thermal properties.13
Modification of GICs with CNCs showed great improvements in resistance to abrasion, compressive strength, diametral tensile strength, modulus of elasticity, and bond strength to the dental structure.19 CNC-modified GICs seem to be promising restorative materials that might be indicated as alternatives to dental amalgams.10,11,19
In a recent study, the effect of BCNC incorporation on the mechanical properties of a resin-modified glass ionomer cement (RMGIC) including compressive strength, diametral tensile strength, and modulus of elasticity was evaluated. The addition of BCNCs to the RMGIC led to an increase in all of the tested mechanical properties compared with the control group, with a significant increase observed for 1% wt BCNCs. The authors also concluded that the newly developed RMGIC with BCNCs might represent an ideal and promising novel dental material in restorative dentistry.19
Considering the clinical success of such novel materials, good adhesion between CNC-modified GIC restorative material and the dentinal surface is a determinant aspect.20 Furthermore, the complete and perfect seal of the restoration margins is another important property responsible for ideal performance of such restorative materials in the oral cavity. This properties are particularly important for primary teeth because the floor of the prepared cavity is closer to the pulp.21
Although several research works have investigated various mechanical properties of CNC-modified GIC materials, no research has been conducted to evaluate microleakage and microshear bond strength (μSBS) of BCNC-modified restorations. Hence, the aim of this study was to assess the microleakage and μSBS of BCNC-modified GIC restorations in primary dentition.
Materials and methods
BCNCs (Nano Novin Polymer Co.) and GIC of Fuji IX (GC) were used in this study.
Teeth selection
The study protocol was approved by the local ethics committee of Shiraz Dental School (IR.SUMS.DENTAL.REC.1399.099). A total number of 60 freshly extracted maxillary and mandibular primary molar teeth were selected from the pool of recently extracted teeth (from June 2020 to August 2020). Parents or guardians provided written informed consent at the time of tooth extraction. The purpose of the study, privacy preservation, and data anonymity were explained to them.
In order to rule out samples with fractures, fissures, carious lesions, abrasive or erosion lesions, and restorations, all teeth were examined macroscopically and microscopically (20× magnification) by a stereomicroscope (12× SZ51/61, Olympus). A hand scaler (Zeffiro) was used to remove soft tissue remnants. The teeth were disinfected in 0.1% chloramine T for 48 hours and subsequently shifted to distilled water at 4 ° C until use.
μSBS test
Thirty extracted primary molar teeth with intact occlusal surfaces were used for μSBS testing. The teeth were mounted in self-polymerising acrylic resin (Acropars), using rectangular molds (30 mm × 20 mm × 15 mm) as their occlusal portions were accessible for bonding. A diamond disk (D&Z) under water cooling was used to expose a flat superficial dentinal surface just beneath the dentino-enamel junction.
The exposed dentinal surfaces of all teeth were wet grounded with 300-, 600-, and 1000-grit silicon carbide abrasive papers to ensure a flat dentinal surface and uniform smear layer.
The specimens were randomly divided into 2 groups (n = 15):
Group 1 (CGICs): Conventional glass ionomer cements powder of Fuji IX (GC) was mixed with its liquid according to the manufacturer's instructions (powder to liquid ratio; 3.2:1 g) for 25 seconds. After applying 10% polyacrylic acid conditioner for 20 seconds, the prepared paste was placed in cylindrical molds (1.5 mm diameter and 1 mm height), prepared by putty (Speedex, Coltene), on the centre of the superficial exposed dentinal surfaces.
Group 2 (BCNC-modified GICs): BCNC powder (Nano Novin Polymer Co.) at a concentration of 1% in weight was added to the powder of Fuji IX and mixed with liquid (powder to liquid ratio 3. 2:1g), then the restoration was done with the prepared paste similar to the group 1.
After setting of the materials within 24 hours and removing of the molds, the specimens were placed in a jig attached to the universal testing machine (Zwick/Roll Z020, Zwick GmbH & Co). Shear force was applied at a crosshead speed of 0.5 mm/min, using a thin wire (0.3 mm thick) looped around the tooth/glass ionomer interface, until debonding. The μSBS was calculated by dividing the maximum load at failure by the cross-sectional surface area of the bonded surface. In situations of spontaneous debonding, a new sample was replaced.22
Failure mode of each fractured specimen was determined using a stereomicroscope (12× SZ51/61, Olympus) at 30× magnification. The modes of failures were classified as follows:
I: Adhesive failure: the failure between the GIC and the dentin
II: Cohesive failure: the failure in the GIC
III: Mixed failure: the combination of adhesive and cohesive failures
Microleakage test
Thirty extracted primary molar teeth with intact buccal or lingual surface were used for microleakage test. A 0.08 mm diamond fissure bur (Tizkavan) on high-speed handpiece (NSK) with water coolant was used to prepare classical rectangular class V cavity preparations with 90-degree cavosurface angles (3-mm width, 2-mm height, and 2-mm depth). All preparations and dimensions were measured with a periodontal probe to ensure a calibrated size and depth cut.
The teeth were randomly divided into 2 groups (n = 15), and cavities were restored according to the groups and materials used in the μSBS testing section. Sof-Lex discs (3M ESPE AG) in a slow-speed handpiece were used for finishing and polishing of the restorations. Then, the teeth were stored in distilled water at 37 °C for 24 hours. Following thermocycling (1000 times between 5 ± 2 °C and 55 ± 2 °C, with a dwell time of 30 seconds and transfer time of 3 seconds), the specimens were prepared for immersion in dye solution.
Two coats of nail varnish were applied on all the tooth surfaces, except the restoration and a 1-mm zone adjacent to its margins. The root apices were sealed with sticky wax. Then coated teeth were immersed in 2% basic fuchsin dye solution (Ranbaxy Fine Chemicals Ltd) for a period of 24 hours at 37 °C and washed thoroughly with pumice slurry to remove residual dye.23 The specimens were embedded in clear auto-polymerising resin (Castin' Craft Clear Plastic Casting Resin, ETI) and longitudinally sectioned in the occlusogingival direction at the centre of each restoration by using a diamond disk (Tizkavan, Tehran, Iran) mounted on a straight handpiece with water coolant (NSK). Each section was then observed under a stereomicroscope (12 × SZ51/61, Olympus) with a magnification of 30×. The degree of microleakage of both halves was assessed. The section showing the maximum degree of dye penetration was chosen for grading the microleakage.
The extent of the microleakage was noted according to the following scoring criteria:
0. No marginal leakage
1. Up to 1/3 cavity depth
2. 1/3-2/3 cavity depth
3. >2/3 cavity depth but not involving the axial wall
4. Involving the axial wall
Preparation for visualisation using scanning electron microscopy (SEM)
Two samples of each group were selected to observe the sheared dentinal surfaces using SEM with magnifications of up to 10,000×, with emphasis on areas of adhesive or cohesive failures. The specimens were mounted on aluminum stubs with conductive silver liquid, gold sputter-coated, and examined under a field-emission SEM (TE-SCAN, VEGA3) for verification of the type of failure.
Statistical analysis
SPSS version 16.0 (SPSS) was used to analyse the mean values and standard deviations. Kolmogorov–Smirnov test was used for data normality analysis. Independent t test was used for statistical analysis of μSBS test and the Mann–Whitney U test for microleakage evaluation at a significance level of P < .05.
Results
The mean μSBS values and standard deviations for the 2 groups of the study are summarised in Table 1. Independent samples t test results revealed statistically significant differences between μSBS values of 2 groups of the study (P < .0001). The BCNC-modified GIC group recorded significantly higher μSBS values in comparison to the CGIC group (3.51 ± 0.033 vs 1.38 ± 0.034 MPa).
Table 1.
The mean μSBS values (MPa) and standard deviation for all groups of the study.
| Groups | N | Mean (MPa) | SD | P value |
|---|---|---|---|---|
| CGIC | 15 | 1.38 | ±0.34 | <.001 |
| CNCs-modified GIC | 15 | 3.51 | ±0.33 |
CGIC, conventional glass ionomer cement; CNC, cellulose nanocrystals; GIC, glass ionomer cement.
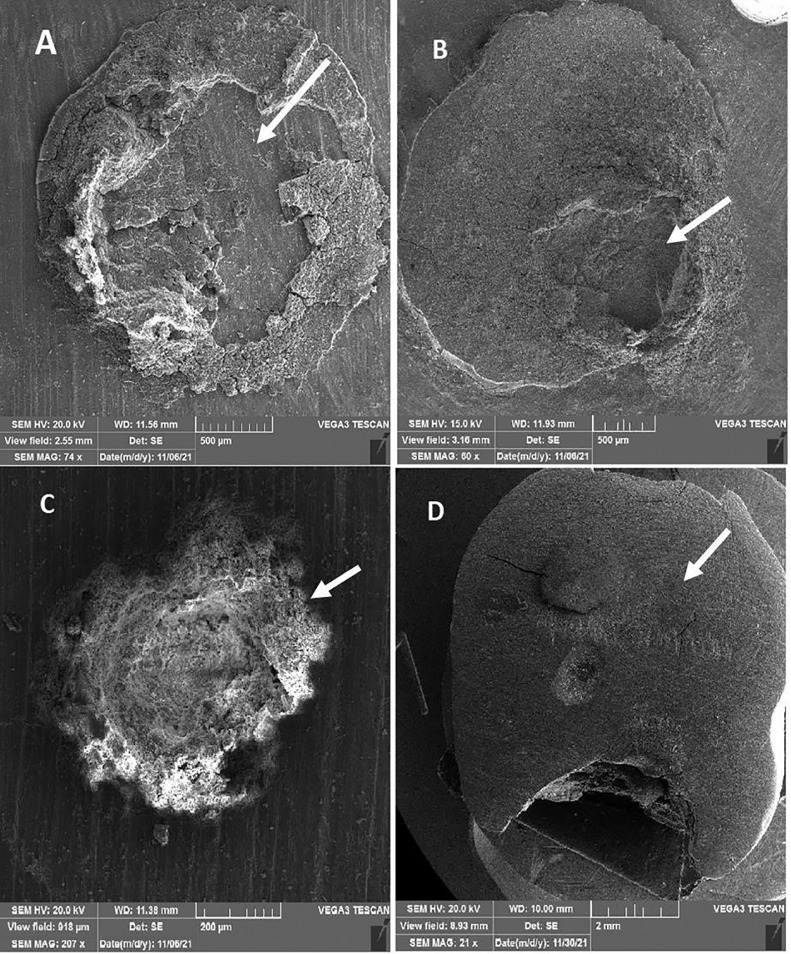
According to the failure mode analysis, mixed failure was the most prevalent mode of failure in both groups (Table 2). Although more cohesive failures were recorded within the BCNC-modified GIC group, Fisher exact test showed no statistically significant differences (P = 1.00). SEM observation of sheared surfaces also confirmed the results of stereomicroscopic evaluation (Figure 1).
Table 2.
Different modes of failure according to stereomicroscopic analysis.
| Mode of failure/group | CGIC, No. (%) | CNC-modified GIC, No. (%) |
|---|---|---|
| Adhesive | 4 (26.7%) | 2 (13.3%) |
| Cohesive | 2 (13.3%) | 5 (33%) |
| Mixed | 9 (60%) | 8 (53.3%) |
| P value* | 1.00 | |
CGIC, conventional glass ionomer cement; CNC, cellulose nanocrystals; GIC, glass ionomer cement.
Fig. 1.
Scanning electron microscopy observation of sheared surfaces: A, mixed failure of the conventional glass ionomer cements (CGIC) group; B, mixed failure of the bacterial cellulose nanocrystal (BCNC)–modified glass ionomer cement (GIC) group; C, cohesive failure of the BCNC-modified GIC group; D, adhesive failure of the CGIC group). The area of failure is indicated with arrows.
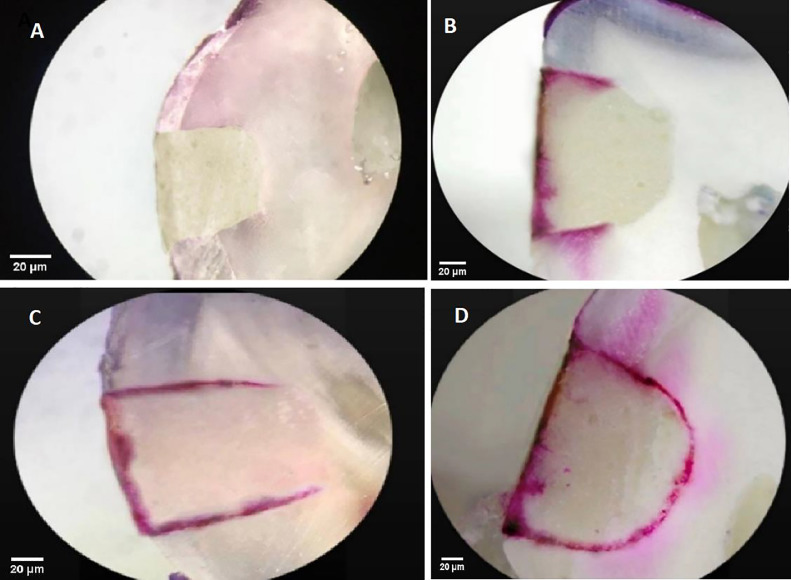
Table 3 shows the mean microleakage scores and standard deviations of all groups. Sixty percent of the samples within the CGIC group and 73% of the samples within the BCNC-modified GIC group showed no penetration (score 0). However, no statistically significant differences were observed in either group (P = .57). Figure 2 shows different microleakage scores observed under stereomicroscope.
Table 3.
Distribution of samples with corresponding microleakage scores.
| Group | Number of sample | Microleakage scores, No. (%) |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| CGIC | 15 | 8 (53%) | 0 | 1 (6.7%) | 2 (13.3%) | 4 (26.7%) |
| CNC_CGIC | 15 | 11 (73%) | 0 | 2 (12.5%) | 1 (6.3%) | 2 (13.3%) |
| P value | 0.572 | |||||
CGIC, conventional glass ionomer cement; CNC, cellulose nanocrystals.
Fig. 2.
Representative samples of microleakage scores under stereomicroscope: A, score 0 of the bacterial cellulose nanocrystal (BCNC)–modified glass ionomer cement (GIC) group; B, score 2 of the BCNC-modified GIC group; C, score 3 of the conventional glass ionomer cement (CGIC) group; D, score 4 of the CGIC group.
Discussion
Despite special properties of GICs, such as being self-adhesive to the tooth structure and fluoride-releasing potential,24,25 poor mechanical properties have been shown in regular use of these restorative materials in dentistry.26 Various modifications have been made in the cement powder and liquid of these materials.27, 28, 29, 30, 31, 32, 33 Previous studies have shown that incorporating CNCs into the GIC improved the mechanical properties of the material.34, 35, 36 Furthermore, it has been demonstrated that a scaffold based on cellulose microfibers and nanocrystals retains the biocompatibility of the original GIC.36
Incorporation of 1% wt of discontinuous cellulose microfibers into the GIC of Fuji IX displayed the highest compressive strength values amongst all tested groups in the study by Garoushi et al.38 Nishimura et al also showed that powdery cellulose nanofiber addition improved the flexural, compressive, and diametral tensile strengths of the GIC of Fuji VII (GC).39
The present study is the first study evaluating the μSBS and microleakage of BCNC-modified GIC restorations in primary dentition. The obtained results showed that adding BCNCs (1% wt) significantly increased the μSBS of the Fuji IX GIC to the dentin structure compared to the control group (P < .001).
Moradian et al investigated the effect of BCNCs on shear bond strength (SBS) of an RMGIC to the dentin of permanent teeth in 3 samples of 0.3%, 0.5%, and 1% wt of BCNCs.40 Similar to our results, they concluded that the RMGICs containing 1% wt of BCNCs represented significantly higher shear bond strength values to the dentin of permanent teeth compared to the control group.40
It is noteworthy that, due to the specific area of the nanomaterials, very small concentrations are required for reinforcement. According to the study by Silva et al, when the concentrations of CNCs were greater than 1%, the nanoparticles aggregated, causing the mechanical properties to fail.37 Based on this scientific point and the results of the study by Moradian et al,40 BCNC powder at concentration of 1% in weight was added to the powder of Fuji IX in the current study.
Silva et al also evaluated the physicochemical properties of the GICs, modified with different concentrations of fibers. They found that adding CNCs to the GICs did not interfere with its working and setting times, solubility, disintegration in water, and diametral tensile strength. Moreover, improvements in the compressive strength, abrasion resistance, and bond strength to the dental structure were observed.11
Another related study showed improvement in the release of flouride ions, compressive, and tensile strength of the modified GIC of Fuji IV with CNCs (0.2% by weight). Fibrillar aggregation of the nanoparticles interspersed in the matrix of GICs was observed by transmission electron microscopy and SEM. This dispersion of the fibers and obtained reinforcement of the cement could be attributed to the anionic nature of the CNCs that leads to a strong electrostatic interaction between the positive charges of the GICs and the negative charges of the CNCs.34
Several factors including the type of dental tissue could influence the bond strength of the GICs. So, due to the heterogeneous structure, low surface energy, and complex nature of the inorganic/organic materials, bonding to the dentin tissue is more compromised.41 Considering the extensive use of GICs in paediatric dentistry, improvement of bond strength to the dentin, observed in our study, is a substantial finding that may help the GIC to resist the masticatory occlusal forces produced in clinical situations.
The ability of BCNCs to bind to the hydroxyl groups of the glass particles and to the carboxylic groups of the polyacrylic acid by means of hydrogen bonding can be considered as the probable reason for improvement of the mechanical properties.19 This bonding, in addition to the uniform distribution of the interrelated nanocrystal in the cement matrix may participate in significant increase in the bond strength detected with the addition of BCNCs.
Incorporation of CNCs into the GICs may also result in a wider range of particle size distribution. Therefore, these small nanocrystal and fiber networks can occupy the empty spaces between the larger glass particles and may provide additional bonding sites for the polyacrylic polymer and thereby reinforce the GIC.34 Another probable reason for increasing μSBS by incorporating BCNCs is that cellulose swelling with the polyacrylic acid solution increases the crystals’ reactivity with the cementations’ matrix and tooth structure. There are many hydroxyl groups in the chemical structure of the crystals. This probably increases the chemical bonds between the hydroxyl groups and calcium and phosphate ions in the dentin structure.11
According to the failure mode analysis, mixed failures were the most prevalent failure modes observed under stereomicroscope in both groups. Although more cohesive failures were recorded within the BCNC-modified GIC group, no statistically significant differences were observed between different failure modes (P = 1.00). SEM observation of the sheared surfaces also confirmed the results of the stereomicroscopic evaluation.
Although a more cohesive fracture in the substrate has been proposed as the reflection of higher bond strength values,42 no significant relationship between the bond strength and the fracture type was reported by other studies, which was similar to our results.43, 44, 45, 46
Additionally, we compared the microleakage of CGIC and BCNC-modified GIC restorations in the current study. According to the results, no statistically significant difference in microleakage scores was observed in comparison to the control group (P = .572). Thus, it can be declared that incorporating BCNCs improves the bond strength properties of the GICs, whilst it does not interfere with the adhesive property or the risk of microleakage of these restorations.
One reason for this result may be that Fuji IX GICs have a coefficient of thermal expansion close to that of the tooth structure and exhibit a good marginal seal.47, 48, 49 Only 4 samples in the control group showed dye penetration involving the axial wall in the current study.
In agreement with our results, Abraham et al also reported no statistically significant differences in microleakage scores of the Chitosan-modified GIC of Fuji IX restorations.48 However, further studies with larger sample sizes are suggested to show the probable positive effect of adding BCNCs on the microleakage of modified GIC restorations.
The results obtained from in vitro studies may not be directly attributed to the clinical situations. However, they create some information regarding the performance of the restorative materials evaluated. Based on our results, BCNC-modified GIC is a favourable restorative dental material to be used for general clinical application in paediatric dentistry as well as restorations in permanent dentition not in stress-bearing areas. To confirm the observed superior properties and characteristics of BCNC-modified GIC restorations, studies with larger sample sizes, evaluating other properties such as biocompatibility and colour stability, and also clinical studies are suggested.
Conclusions
Based on the obtained results, the authors concluded that the addition of BCNCs (1% wt) significantly increased the μSBS of the Fuji IX GIC to the dentin structure of the primary teeth. Considering the extensive use of GICs in paediatric dentistry, although no statistically significant difference was observed between microleakage scores of the 2 groups, improved bond strength to the dentin tissue is a substantial finding. BCNC modification may help the GICs to resist the masticatory occlusal forces produced in clinical situations.
Conflict of interest
None disclosed.
Acknowledgements
The authors thank the Vice-Chancellery of Shiraz University of Medical Sciences for supporting this research (Grant #24118). The manuscript is derived from the dissertations performed by Dr Lida Vaziri Borazjani.
REFERENCES
- 1.Gisovar EF, Hedayati N, Shadman N, Shafiee L. Casein phosphopeptide-amorphous calcium phosphate and shear bond strength of adhesives to primary teeth enamel. Iran Red Crescent Med. 2015;17(2):e11167. doi: 10.5812/ircmj.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jowkar Z, Jowkar M, Shafiei F. Mechanical and dentin bond strength properties of the nanosilver enriched glass ionomer cement. J Clin Exp Dent. 2019;11(3):e275. doi: 10.4317/jced.55522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varughese RE, Andrews P, Sigal MJ, Azarpazhooh A. An assessment of direct restorative material use in posterior teeth by American and Canadian pediatric dentists: I. Material choice. Pediatr Dent. 2016;38(7):489–496. [PubMed] [Google Scholar]
- 4.Barandehfard F, Rad MK, Hosseinnia A, et al. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram Int. 2016;42(15):17866–17875. [Google Scholar]
- 5.Pargaonkar S, Padubidri M, Gunjal S, Patil P, Musmade D, Sankhe C. Glass ionomer cement: brief review in pediatric dentistry. Int J Healthc Biomed Res. 2018;6(03):13–17. [Google Scholar]
- 6.Ching HS, Luddin N, Kannan TP, Ab Rahman I, Abdul Ghani NR. Modification of glass ionomer cements on their physical-mechanical and antimicrobial properties. J Esthet Restor Dent. 2018;30(6):557–571. doi: 10.1111/jerd.12413. [DOI] [PubMed] [Google Scholar]
- 7.Borges AB, Torres C, Cassiano KV, Toyama RV, Pucci CR. Influence of matrix and insertion technique on the microleakage and microhardness of posterior composite restorations. Gen Dent. 2009;57(2):163–170. [PubMed] [Google Scholar]
- 8.Croll TP, Nicholson JW. Glass ionomer cements in pediatric dentistry: review of the literature. Pediatr Dent. 2002;24(5):423–429. [PubMed] [Google Scholar]
- 9.Oznurhan F, Ozturk C. Evaluation of polypropylene fiber reinforced glass ionomer cement: a comparative in-vitro study. J Adv Oral Res. 2020;11(2):196–201. [Google Scholar]
- 10.Menezes-Silva R, de Oliveira BMB, Fernandes PHM, et al. Effects of the reinforced cellulose nanocrystals on glass-ionomer cements. Dent Mater. 2019;35(4):564–573. doi: 10.1016/j.dental.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Silva R, Santos P, Souza L, Dumont V, Soares J, Santos M. Effects of cellulose fibers on the physical and chemical properties of glass ionomer dental restorative materials. Mater Res Bull. 2013;48(1):118–126. [Google Scholar]
- 12.Gea S, Reynolds CT, Roohpour N,, et al. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a twostep purification process. Bioresour Technol. 2011;102(19):9105–9110. doi: 10.1016/j.biortech.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 13.Gelin K, Bodin A, Gatenholm P, Mihranyan A, Edwards K, Strømme M. Characterization of water in bacterial cellulose using dielectric spectroscopy and electron microscopy. Polymer. 2007;48(26) [Google Scholar]
- 14.Hu Y, Catchmark JM, Zhu Y, et al. Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering. J Mater Res. 2014;29(22):2682–2693. [Google Scholar]
- 15.Petersen N, Gatenholm P. Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol. 2011;91(5):1277–1286. doi: 10.1007/s00253-011-3432-y. [DOI] [PubMed] [Google Scholar]
- 16.Bäckdahl H, Helenius G, Bodin A, et al. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials. 2006;27(9):2141–2149. doi: 10.1016/j.biomaterials.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Iguchi M, Yamanaka S, Budhiono A. Bacterial cellulose—a masterpiece of nature's arts. J Mater Sci. 2000;35(2):261–270. [Google Scholar]
- 18.George J, Sajeevkumar V, Kumar R, Ramana K, Sabapathy S, Bawa A. Enhancement of thermal stability associated with the chemical treatment of bacterial (Gluconacetobacter xylinus) cellulose. J Appl Polym Sci. 2008;108(3):1845–1851. [Google Scholar]
- 19.Moradian M, Nosrat Abadi M, Jafarpour D, Saadat M. Effects of bacterial cellulose nanocrystals on the mechanical properties of resin-modified glass ionomer cements. Eur J Dent. 2021;15(02):197–201. doi: 10.1055/s-0040-1717051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fattah Z, Jowkar Z, Rezaeian S. Microshear bond strength of nanoparticle-incorporated conventional and resin-modified glass ionomer to caries-affected dentin. Int J Dent. 2021;2021 doi: 10.1155/2021/5565556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahluwalia P, Chopra S, Thomas A. Strength characteristics and marginal sealing ability of chlorhexidine-modified glass ionomer cement: an in vitro study. J Indian Soc Pedod Prev Dent. 2012;30(1):41. doi: 10.4103/0970-4388.95580. [DOI] [PubMed] [Google Scholar]
- 22.Fattah Z, Shafiei F, Rajabi F. Effect of tannic acid and quercetin antioxidants on bond strength of resin cement to dentin after internal bleaching. Eur J Prosthodont Restor Dent. 2022;30(2):126–133. doi: 10.1922/EJPRD_2324Shafiei08. [DOI] [PubMed] [Google Scholar]
- 23.Ahluwalia P, Chopra S, Thomas AM. Strength characteristics and marginal sealing ability of chlorhexidine-modified glass ionomer cement: an in vitro study. J Indian Soc Pedod Prev Dent. 2012;30(1):41–46. doi: 10.4103/0970-4388.95580. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19(6):485–494. doi: 10.1016/s0142-9612(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 25.Glasspoole EA, Erickson RL, Davidson CL. Effect of surface treatments on the bond strength of glass ionomers to enamel. Dent Mater. 2002;18(6):454–462. doi: 10.1016/s0109-5641(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 26.Shintome LK, Nagayassu MP, Di Nicoló R, Myaki SI. Microhardness of glass ionomer cements indicated for the ART technique according to surface protection treatment and storage time. Braz Oral Res. 2009;23:439–445. doi: 10.1590/s1806-83242009000400014. [DOI] [PubMed] [Google Scholar]
- 27.Boyd D, Towler M. The processing, mechanical properties and bioactivity of zinc based glass ionomer cements. J Mater Sci Mater Med. 2005;16(9):843–850. doi: 10.1007/s10856-005-3578-1. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Yap A, Cheang P, Khor K. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC) Biomaterials. 2005;26(7):713–720. doi: 10.1016/j.biomaterials.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Yap A, Cheang P, Khor K. Zirconia–glass ionomer cement––a potential substitute for Miracle Mix. Scr Mater. 2005;52(2):113–116. [Google Scholar]
- 30.Zoergiebel J, Ilie N. Evaluation of a conventional glass ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Investig. 2013;17(2):619–626. doi: 10.1007/s00784-012-0733-1. [DOI] [PubMed] [Google Scholar]
- 31.Cibim DD, Saito MT, Giovani PA, et al. Novel nanotechnology of TiO2 improves physical-chemical and biological properties of glass ionomer cement. Int J Biomater. 2017;2017 doi: 10.1155/2017/7123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kheur M, Kantharia N, Iakha T, Kheur S, Husain NA-H, Özcan M. Evaluation of mechanical and adhesion properties of glass ionomer cement incorporating nano-sized hydroxyapatite particles. Odontology. 2020;108(1):66–73. doi: 10.1007/s10266-019-00427-5. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 2015;23:321–328. doi: 10.1590/1678-775720140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menezes-Silva R, de Oliveira BMB, Fernandes PHM, et al. Effects of the reinforced cellulose nanocrystals on glass-ionomer cements. Dent Mater. 2019;35(4):564–573. doi: 10.1016/j.dental.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Dwifulqi H, Tjandrawinata R, Kusnoto J. The effects of reinforced cellulose nanocrystals from sugarcane bagasse fiber on the hardness of glass ionomer cements. Sci Dent J. 2021;5(1):33. [Google Scholar]
- 36.Silva RM, Pereira FV, Mota FA, Watanabe E, Soares SM, Santos MH. Dental glass ionomer cement reinforced by cellulose microfibers and cellulose nanocrystals. Mater Sci Eng C Mater Biol Appl. 2016;58:389–395. doi: 10.1016/j.msec.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 37.Menezes-Silva R, Pereira FV, Santos MH, Soares JA, Soares SMCS, Miranda JLd. Biocompatibility of a new dental glass ionomer cement with cellulose microfibers and cellulose nanocrystals. Braz Dent J. 2017;28:172–178. doi: 10.1590/0103-6440201701059. [DOI] [PubMed] [Google Scholar]
- 38.Garoushi S, He J, Obradovic J, Fardim P, Vallittu PK, Lassila L. Incorporation of cellulose fiber in glass ionomer cement. Eur J Oral Sci. 2020;128(1):81–88. doi: 10.1111/eos.12668. [DOI] [PubMed] [Google Scholar]
- 39.Fabianelli A, Pollington S, Davidson CL, Cagidiaco MC, Goracci C. The relevance of microleakage studies. Int Dent SA. 2007;9(3):64–74. [Google Scholar]
- 40.Moradian M, Jafarpour D, Saadat M, Tahmasebi F. The effect of bacterial cellulose nanocrystals on the shear bond strength of resin modified glass ionomer cement to dentin. J Clin Exp Dent. 2021;13(8):e784. doi: 10.4317/jced.58153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohbauer U. Dental glass ionomer cements as permanent filling materials?–properties, limitations and future trends. Materials. 2010;3(1):76–96. [Google Scholar]
- 42.Tanumiharja M, Burrow M, Tyas M. Microtensile bond strengths of glass ionomer (polyalkenoate) cements to dentine using four conditioners. J Dent. 2000;28(5):361–366. doi: 10.1016/s0300-5712(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 43.Feilzer A, De Gee A, Davidson C. Relaxation of polymerization contraction shear stress by hygroscopic expansion. J Dent Res. 1990;69(1):36–39. doi: 10.1177/00220345900690010501. [DOI] [PubMed] [Google Scholar]
- 44.Davidson CL. Resisting the curing contraction with adhesive composites. J Prosthet Dent. 1986;55(4):446–447. doi: 10.1016/0022-3913(86)90173-3. [DOI] [PubMed] [Google Scholar]
- 45.Asafarlal S. Comparative evaluation of microleakage, surface roughness and hardness of three glass ionomer cements–Zirconomer, Fujii IX Extra GC and Ketac Molar: an in vitro study. Dentistry. 2017;7(5) 2161-1122.1000427. [Google Scholar]
- 46.Joseph A, Santhosh L, Hegde J, Panchajanya S, George R. Microleakage evaluation of Silorane-based composite and methacrylate-based composite in class II box preparations using two different layering techniques: an in vitro study. Indian J Dent Res. 2013;24(1):148. doi: 10.4103/0970-9290.114943. [DOI] [PubMed] [Google Scholar]
- 47.Sumitha D, Rao A. Nanoionomer: evaluation of microleakage. J Indian Soc Pedod Prev Dent. 2011;29(1):20–24. doi: 10.4103/0970-4388.79919. [DOI] [PubMed] [Google Scholar]
- 48.Abraham D, Abi Mathew Thomas SC, Koshy S. A comparative evaluation of microleakage of glass ionomer cement and chitosan-modified glass ionomer cement: an in vitro study. Int J Clin Pediatr. 2014;7(1):6. doi: 10.5005/jp-journals-10005-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frankenberger R, Sindel J, Krämer N. Viscous glass-ionomer cements: a new alternative to amalgam in the primary dentition? Quintessence Int. 1997;28(10) [PubMed] [Google Scholar]