STRUCTURED ABSTRACT:
Objective:
Our recent studies using a porcine model of metabolic syndrome (MS) and chronic myocardial ischemia demonstrate that extracellular vesicle (EV) therapy improves blood flow and arteriogenesis in ischemic myocardium, though mechanisms of these changes are unclear. We hypothesized that in the setting of MS, EV therapy would decrease anti-angiogenic signaling to mediate increased blood flow to chronically ischemic myocardium.
Methods:
Yorkshire swine were fed a high fat diet for four weeks to induce MS, then underwent placement of an ameroid constrictor to the left circumflex artery to induce chronic myocardial ischemia. Two weeks later, pigs underwent intramyocardial injection of vehicle (control, n=6) or human bone marrow-derived EVs (n=8). Five weeks later, left ventricular myocardium in ischemic territory was harvested. Protein expression was measured by immunoblotting, and data was analyzed via Wilcoxon rank sum test. Myocardial perfusion was measured with isotope-labeled microspheres, and correlation data was analyzed using Spearman’s rank correlation coefficient.
Results:
EV treatment was associated with decreased expression of anti-angiogenic proteins angiostatin (p<0.001) and endostatin (p=0.043) in ischemic myocardium compared to control. In EV-treated pigs, there was a negative correlation between blood flow to ischemic myocardium and angiostatin (rs=−0.76, p=0.037), but not endostatin expression (rs=0.02, p=0.98). EV treatment was also associated with decreased cathepsin D, which cleaves precursors to produce angiostatin and endostatin, in ischemic myocardium (p=0.020).
Conclusions:
In the setting of MS and chronic myocardial ischemia, EV therapy is associated with decreased expression of anti-angiogenic proteins, which may contribute to increased blood flow to chronically ischemic myocardium.
Keywords: Extracellular Vesicles, Ischemia, Angiogenesis, Metabolic Syndrome, Coronary
INTRODUCTION:
Therapeutic options are limited in patients with chronic coronary artery disease (CAD) with poor surgical and percutaneous revascularization options.1 Further, coronary disease is often co-morbid with and exacerbated by metabolic syndrome, characterized by insulin resistance, obesity, hyperlipidemia, and hypertension.2 With suboptimal CAD treatment in this setting, chronic myocardial ischemic injury contributes to myocardial necrosis, adverse remodeling, and heart failure.3 Therefore, there is an ongoing need to develop and better understand therapeutic options for patients with chronic coronary disease using experimental models that reflect common co-morbidities in patients with CAD.
Stem-cell based therapies are an attractive option in treatment of chronic CAD, though clinical trials have typically shown modest or transient benefit.4 Among stem-cell based therapies, a promising therapeutic option may be extracellular vesicles (EVs), which can be derived from many sources including human bone marrow derived stem cells, and mediate the paracrine effects of these cells.4 EVs are small lipid-bound bioactive molecules that contain growth factors, kinase receptors, and non-coding RNAs which when released can produce regenerative and angiogenic effects on tissues and organs. Some advantages of EVs compared to the mesenchymal stem cells from which they are derived include decreased immunogenicity, higher safety profile, and ability to cross biological barriers.4 Several animal models have shown that EVs have cardioprotective effects, including improved function and revascularization in small and large animal models of acute myocardial ischemia, which have prompted an ongoing clinical trial of EV therapy for acute myocardial ischemia (NCT04327635).4 We have previously shown that in a swine model of chronic myocardial ischemia and metabolic syndrome, intramyocardial injection of EVs was associated with improved myocardial perfusion during pacing and arteriolar vessel density, among other benefits including improved myocardial function, reduced fibrosis, and reduced oxidative stress.5–7 However, the molecular mechanisms by which EV therapy mediates these benefits are still unclear.
Collateralization in the setting of myocardial ischemia is regulated by a balance of pro- and anti-angiogenic factors.8 Two important anti-angiogenic signaling markers are angiostatin and endostatin, which are produced by cathepsin- and matrix metalloproteinase (MMP)-mediated cleavage of plasminogen and collagen 18 respectively.9,10 These anti-angiogenic factors are increased in the setting of hyperlipidemia and diabetes in animal and human studies, and may impair myocardial perfusion.11–14 Therefore downregulation of these factors may provide benefit to ischemic myocardium in the setting of metabolic syndrome.
Given the beneficial effects of EVs on perfusion to chronically ischemic myocardium, we sought to further investigate the effects of intramyocardial EV therapy on pro- and antiangiogenic signaling markers in the setting of chronic myocardial ischemia and metabolic syndrome, and how these effects may contribute to myocardial perfusion.
METHODS:
Animal Model:
Fourteen male Yorkshire swine at age 6 weeks were started on a high-fat diet (HFD), consisting of a 500g/d high cholesterol diet comprised of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO) to induce metabolic syndrome.5 These diets continued for the duration of the study. The swine involved in this study have been previously shown to have impaired glucose tolerance and hyperlipemia including markedly elevated LDL and total cholesterol when compared to swine receiving a normal diet.5,6
Four weeks after initiation of a high fat diet, pigs underwent a thoracotomy for placement of an ameroid constrictor (Research Instruments SW, Escondido, CA) around the proximal left circumflex artery (LCx) to induce chronic myocardial ischemia. Animals underwent a second thoracotomy procedure two weeks later for intramyocardial injection of either saline vehicle (control group, n=6) or EVs (high fat diet with myocardial EV injection group “HVM”, n=8). Five weeks after injection with either EVs or vehicle, animals underwent a terminal harvest procedure for analysis. (Figure 1) In order to investigate the effects of EVs in non-metabolic syndrome swine, an additional fifteen Yorkshire swine receiving a normal diet underwent ameroid placement at 11 weeks of age, and were divided into two groups: one received intramyocardial EV injection two weeks later (normal diet with myocardial EV injection group “MVM”, n=8) and the other received no treatment (normal diet control “NDC”, n=7), with terminal harvest occurring an additional five weeks later. All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital (#017316, 10/18/16), and animals were cared for in coordination with veterinary technicians at Rhode Island Hospital in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals.
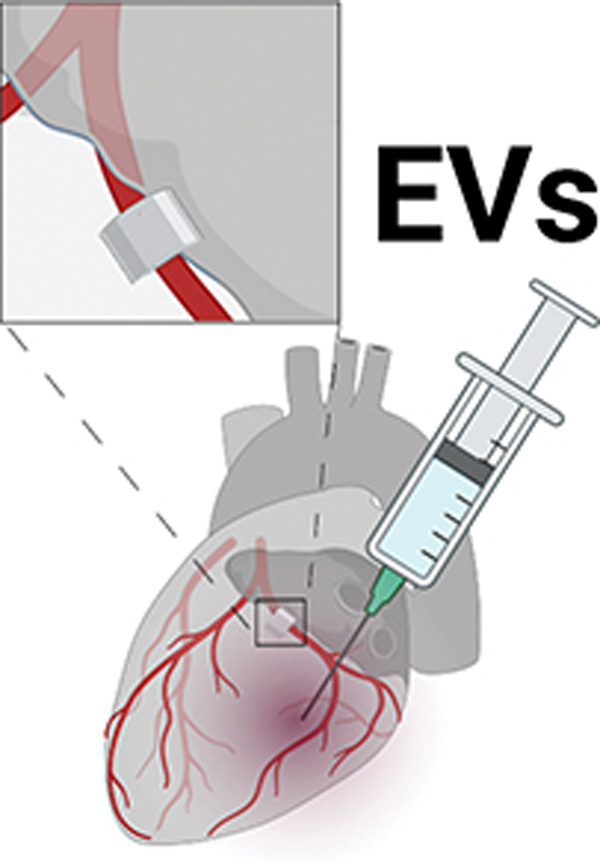
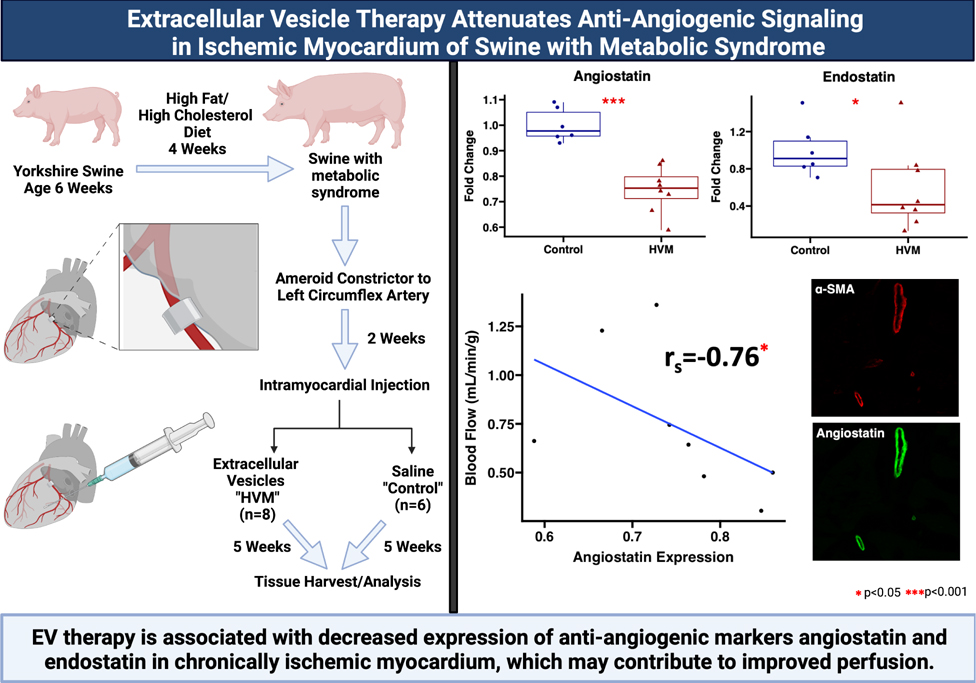
Figure 1: Graphical abstract.
Swine at age 6 weeks were given four weeks of high fat and high cholesterol diet to induce metabolic syndrome. An ameroid constrictor was placed on the proximal left circumflex artery to induce chronic myocardial ischemia. Two weeks later, animals received intramyocardial injection of either saline vehicle (“control,” n=6) or extracellular vesicles (high fat diet with myocardial EV injection group “HVM,” n=8). Five weeks later myocardial tissue was harvested for analysis. Ischemic myocardium in the HVM group had decreased expression of anti-angiogenic signaling markers angiostatin and endostatin. Angiostatin expression localized the coronary vasculature as determined by α-smooth muscle actin (α-SMA) staining. Among EV-treated pigs, angiostatin expression was inversely related to blood flow to ischemic myocardium during pacing. EV = extracellular vesicle. In boxplots, upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. Created with BioRender.com.
EV Isolation
Human bone marrow-derived mesenchymal stem cells (Lonza, Allendale, NJ) were cultured according to Lonza recommendations in growth media (MSCGM Bulletkit PT-3001; Lonza, Allendale, NJ). According to certificates of analysis supplied by Lonza, donors were age 18 (female) and 22 (male), with negative viral testing. Cells were grown to 80% confluence in normoxia conditions and passaged up to six times for cell-line expansion. Twenty-four hours prior to EV isolation, growth media was removed and replaced with serum-free Roswell Park Memorial Institute medium. After 24 hours of incubation, the serum-free media was collected and centrifuged at 2,000 rpm at 4 degrees Celsius to remove cellular debris. Exosomes and microsomes were then isolated by ultracentrifugation at 100,000g for 70 minutes, washed with PBS at 100,000g for 70 minutes, resuspended in 1% dimethyl sulfoxide in PBS, snap frozen with liquid nitrogen, and stored at −80 degrees Celsius. Characterization was determined by immunoblotting for CD81, CD9, and Alix to establish presence of EV transmembrane and cytosolic proteins, and absence of albumin to confirm EV purity.15 EV size and concentrations were quantified via nanoparticle tracking analysis (Supplemental Figure 1). Protein quantification was performed using a radioimmunoprecipitation assay (Thermo Fisher Scientific) to quantify a standard dose for intramyocardial injection. 50 μg of EVs, a dose previously effective in chronic myocardial ischemia with intramyocardial delivery,5 were thawed and resuspended in 2mL of 0.9% sterile saline on the day of administration. EVs were only subjected to a single freeze/thaw cycle.
Placement of Ameroid Constrictor
Procedure anesthesia, perioperative analgesia, and prophylaxis were carried out as previously described.5 A left mini-thoracotomy was made, the pericardium was opened to expose the heart, and the LCx was identified and isolated with a vessel loop near the takeoff from the left main coronary artery. The pig was systemically heparinized, and the vessel loop was lifted to occlude the LCx for two minutes as confirmed by ST and/or T wave changes on ECG. During occlusion, 5mL of gold microspheres (BioPal, Worcester, MA) were injected into the left atrium via syringe and 22 gauge needle. After two minutes, the vessel loop was relaxed and ECG changes were allowed to return to baseline. An ameroid constrictor, consisting of hygroscopic casein material cased in titanium (Research Instruments SW, Escondido, CA) was sized according to the LCx diameter and placed around the artery, which gradually occludes the artery over the course of two to three weeks to induce chronic myocardial ischemia. Nitroglycerin was topically applied over the vessel as needed to reverse vasospasm. The incision was closed in layers, and the pigs were recovered from anesthesia in a monitored setting.
Intramyocardial Injection
After previously described pre-operative preparation, a left mini-thoracotomy was made one rib space below the prior incision, and the pericardium was opened to expose the heart. Animals then underwent intramyocardial injection of either 50 μg EVs suspended in 2mL normal saline (HVM group) or 2mL normal saline (control group) using a 27-gauge needle inserted 0.5cm into the myocardium. Injections were made at eight locations of the left ventricular myocardium at risk of ischemia as determined by tracking branches off the LCx and spacing injections 2–3cm apart. The wound was closed in layers, and pigs were recovered from anesthesia in a monitored setting. Swine were monitored for vital sign abnormalities and other signs of toxicity from immunogenic reaction to treatment, of which there were none.
Harvest Procedure
Five weeks after intramyocardial injection, pigs underwent a terminal harvest procedure. Femoral artery access was obtained, and a pressure catheter was inserted to monitor blood pressure. A median sternotomy was performed to expose the heart. For blood flow analysis, isotope-labeled microspheres were injected into the left atrium with a syringe and 22 gauge needle while simultaneously withdrawing 10mL of blood from the femoral artery catheter. At the end of the procedure, the heart was excised, and the myocardial tissue was quickly divided into 16 segments based on location with respect to the LAD and LCx. Myocardial tissue segments were snap frozen in liquid nitrogen for immunoblotting and frozen sectioning, or air dried for microsphere analysis.
Myocardial Perfusion
Myocardial perfusion was determined using isotope-labeled microspheres (BioPal, Worcester, MA) injected at the time of the ameroid procedure and the harvest procedure. During the first surgical procedure for ameroid placement, 5mL of gold microspheres were injected into the left atrium to determine the at-risk territory of the LV perfused by the LCx. During the harvest procedure, 5mL of Lutetium microspheres were injected into the left atrium while simultaneously withdrawing 10mL of blood from the femoral artery in order to calculate blood flow to the ischemic territory. This process was repeated during pacing to 150bpm using Samarium microspheres. Blood samples and LV myocardial samples from 10 sections based on proximity of location to the LAD and LCx were weighed, dried, and sent to BioPal for analysis of blood flow to ischemic tissue segments. Complete perfusion data in these swine has been previously reported.5
Immunoblotting
Total protein (40μg) was fractionated on a 4–12% Bis-Tris gel (ThermoFisher) and transferred onto a nitrocellulose membrane (ThermoFisher Scientific, Waltham, MA). Membranes were incubated overnight at 4 degrees Celsius with 1:1000 dilutions of individual rabbit polyclonal primary antibodies to angiostatin, plasminogen, collagen 18 (Abcam, Cambridge, UK), Tie2, tissue inhibitor of metalloproteinases 2 (TIMP2), TIMP3, angiopoietin 1, cathepsin L, pro-cathepsin L, cathepsin D, pro-Cathepsin D, cathepsin B, matrix metalloproteinase-2 (MMP2), ß-Catenin, delta like ligand 4 (DLL4), VE-cadherin, vascular endothelial growth factor A (VEGF-A), fibroblast growth factor receptor 1 (FGFR1), monocyte chemoattractant protein-1 (MCP-1) (Cell Signaling, Danvers, MA), and endostatin (MilliporeSigma, Burlington, MA). Membranes were then washed and incubated with goat polyclonal anti-rabbit or anti-mouse secondary antibodies at 1:3000 dilutions for one hour at room temperature. Membranes were washed and processed for chemiluminescent detection and captured with a digital camera system (Bio-Rad ChemiDoc MP, Life Science, Hercules, CA). Membranes were probed with GAPDH (Cell Signaling, Danvers, MA) to correct for loading error. Densitometric analysis of band intensity was performed with NIH Image J Software.
Immunohistochemistry
Immunohistochemistry was performed as previously described16. Briefly, frozen section slides were thawed, fixed with 10% paraformaldehyde, blocked, and incubated with antibody to angiostatin (Abcam, Cambridge, UK). Images were analyzed at 20X magnification with an Olympus VS200 Slide Scanner (Olympus, Tokyo, Japan).
Data Analysis:
By analyzing data from prior protocols, using a 2-tailed α-level of 0.01, a ß-error level of 0.20, and the known standard deviation (0.150 mL/mg/min), we obtained a minimum sample size of 6 animals per group. Immunoblot results are reported as median fold change values compared to average value of the control group with interquartile ranges, and statistically analyzed via Wilcoxon rank-sum test. Correlation data were statistically analyzed using Spearman’s rank correlation coefficient. Probability values <0.05 were considered significant.
RESULTS:
Pro- and Anti-Angiogenic Protein Expression
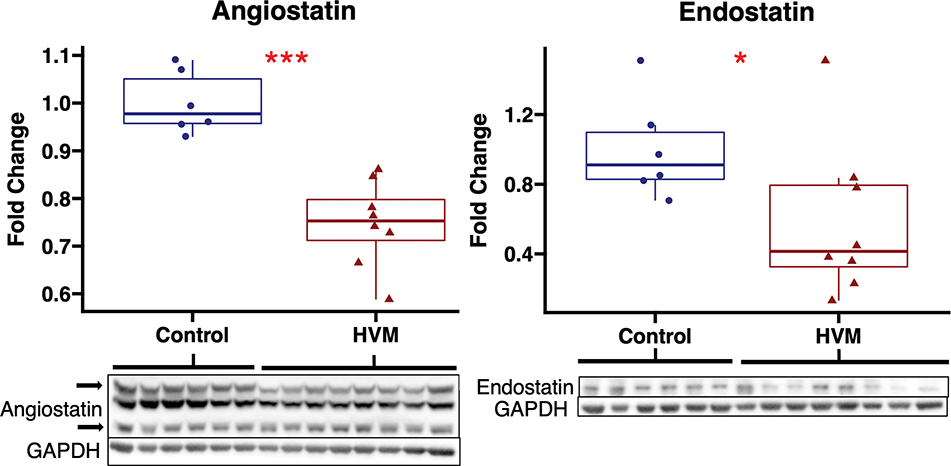
In chronically ischemic myocardium of HFD pigs, EV treatment was associated with decreased expression of anti-angiogenic markers angiostatin and endostatin compared to control. There were no differences in expression of anti-angiogenic marker DLL4 between groups. EV treatment was not associated significant changes in proangiogenic markers angiopoietin 1, ß-catenin, VE-cadherin, Tie2, VEGF-A, FGFR1, or MCP-1 compared to control. (Figure 2, Table 1) There were no significant differences in expression of angiostatin or endostatin with EV treatment in pigs receiving a normal diet (Supplemental Figure 2).
Figure 2: Angiostatin and endostatin expression are decreased with extracellular vesicle therapy in chronically ischemic myocardium.
The high-fat diet with EV injection (HVM, n=8) group had significant decreases in expression of anti-angiogenic markers angiostatin and endostatin compared to the control group (n=6). Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. ***p<0.001, *p<0.05 as determined by Wilcoxon rank-sum test.
Table 1:
Angiogenic protein expression in ischemic myocardium of EV-treated swine.
| Protein | Median (IQR) | P Value |
|---|---|---|
|
| ||
| Anti-Angiogenic | ||
| Angiostatin | 0.75 (0.71–0.79) | <0.001 |
| Endostatin | 0.41 (0.33–0.79) | 0.043 |
| DLL4 | 0.76 (0.66–1.02) | 0.95 |
| Pro-Angiogenic | ||
| Angiopoietin 1 | 1.31 (1.11–1.45) | 0.14 |
| β-Catenin | 1.09 (0.56–1.44) | 0.66 |
| VE-Cadherin | 0.67 (0.44–0.76) | 0.28 |
| Tie2 | 0.82 (0.59–0.99) | 0.29 |
| VEGF-A | 0.62 (0.37–0.95) | 0.14 |
| FGFR1 | 0.91 (0.83–1.00) | 1 |
| MCP-1 | 1.07 (0.92–1.10) | 0.85 |
Statistics presented as median fold change (interquartile range) of protein level in metabolic syndrome pigs treated with extracellular vesicles (HVM, n=8) compared to control (n=6). P values calculated using Wilcoxon rank sum test. DLL4, delta like ligand 4; VEGF-A, vascular endothelial growth factor A; FGFR1, fibroblast growth factor receptor 1; MCP-1, monocyte chemoattractant protein 1.
Blood Flow Correlation
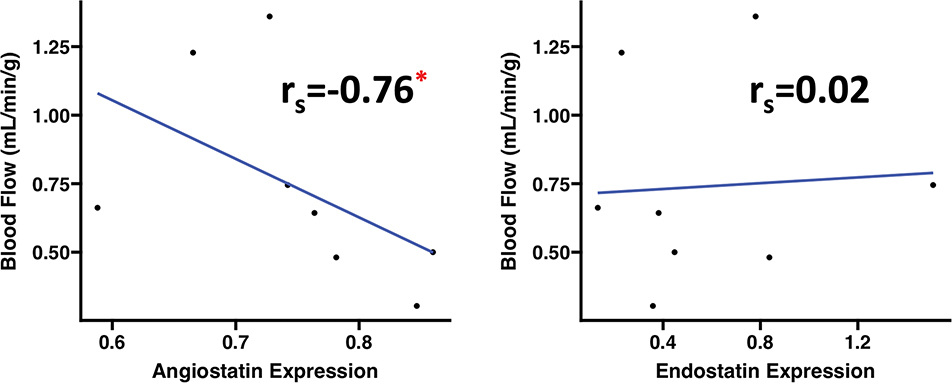
Among HFD EV-treated pigs, angiostatin expression was inversely correlated to absolute blood flow in ischemic myocardial tissue during pacing. Endostatin expression had no significant correlation to absolute blood flow in ischemic myocardium. (Figure 3)
Figure 3: Angiostatin expression is correlated with perfusion to ischemic territory among EV-treated swine.
There was significant negative correlation between angiostatin expression and absolute blood flow to the ischemic myocardial territory during pacing to 150bpm among EV-treated swine (n=8). Correlation data analyzed using Spearman’s rank correlation coefficient (rs). *p<0.05
Angiostatin Localization in Myocardial Tissue
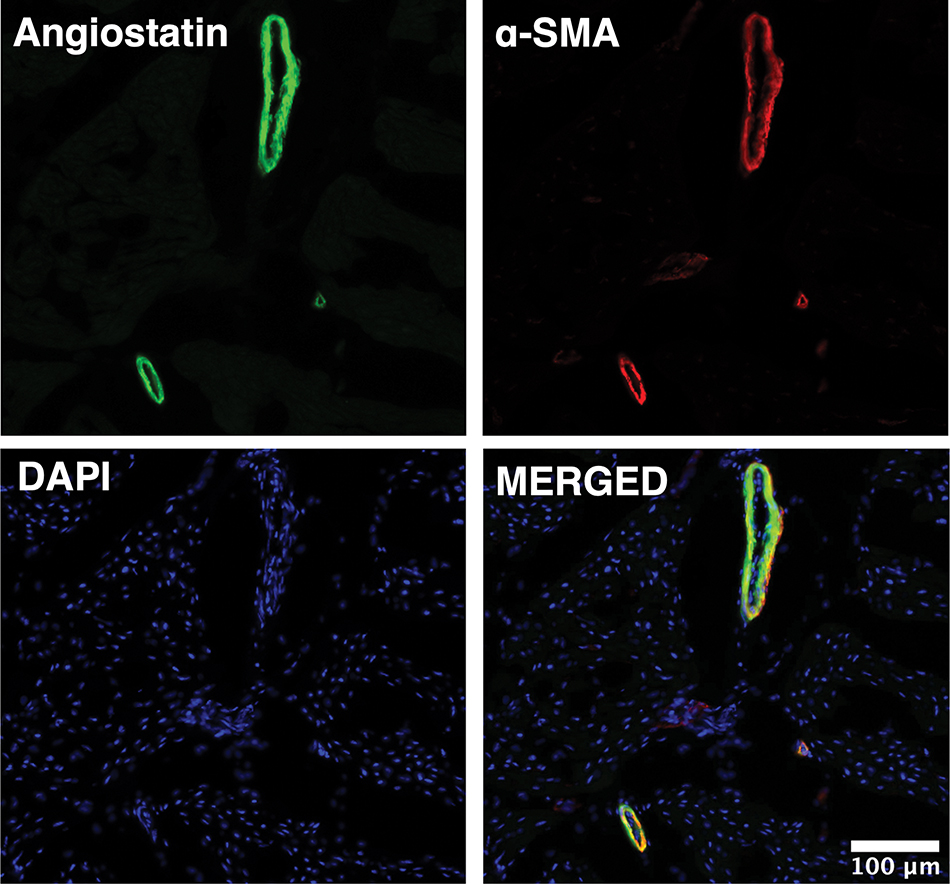
Immunofluorescence studies in ischemic myocardial tissue from HFD pigs demonstrated that angiostatin localized predominately to the coronary microvasculature as determined by co-staining with antibodies against angiostatin and α-SMA. (Figure 4)
Figure 4: Angiostatin localizes to coronary microvasculature in chronically ischemic myocardial tissue.
Representative image of left ventricular chronically ischemic myocardium obtained at 20X magnification demonstrates that angiostatin (green) localizes to coronary smooth muscle cells as stained by α-smooth muscle actin (α-SMA, red). Nuclei stained with DAPI (blue).
Expression of Proteins Involved in Angiostatin and Endostatin Production
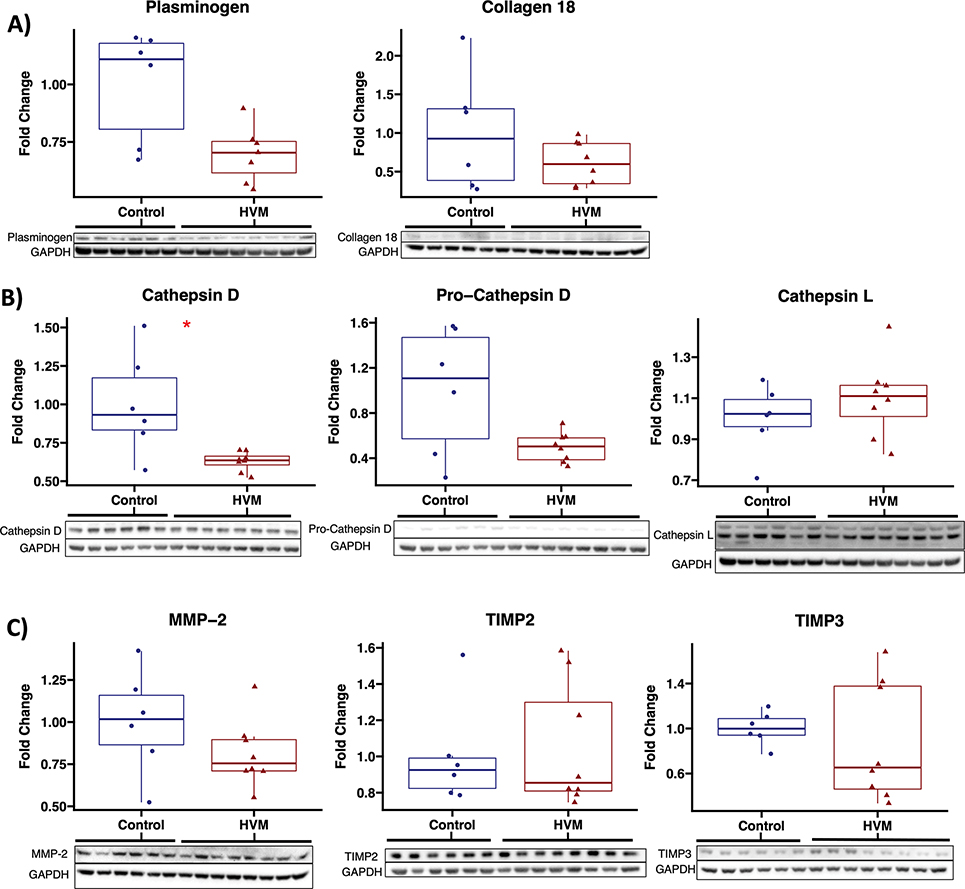
In chronically ischemic myocardium of HFD pigs, there was a non-significant trend toward decreased plasminogen expression in EV-treated pigs (n=7, one outlier removed which was greater than 2.5 standard deviations above mean) compared to control (n=6). There were no differences in expression of collagen 18 between groups. EV treatment was associated with a significant reduction in expression of Cathepsin D, and a trend towards reduced expression of pro-cathepsin D. There were no differences in expression of cathepsin L, pro-cathepsin L, Cathepsin B, MMP-2, TIMP2, or TIMP3. (Figure 5, Table 2)
Figure 5: Among proteins involved in production of angiostatin and endostatin, cathepsin D is significantly decreased with EV therapy.
Immunoblot results are shown for A) precursors to angiostatin and endostatin, B) cathepsins D and L which cleave precursors to generate angiostatin and endostatin, and C) matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of metalloproteinases (TIMP) 2 and 3 which regulate generation of angiostatin and endostatin. HVM, high fat diet swine that received intramyocardial extracellular vesicles (n=8), control, high fat diet swine that received saline vehicle (n=6). Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. *p<0.05 as determined by Wilcoxon rank-sum test.
Table 2:
Protein expression related to production of angiostatin and endostatin.
| Protein | Median (IQR) | P Value |
|---|---|---|
|
| ||
| Plasminogen | 0.70 (0.62–0.75) | 0.051 |
| Collagen 18 | 0.60 (0.34–0.86) | 0.49 |
| Cathepsin D | 0.64 (0.50–0.86) | 0.020 |
| Pro-cathepsin D | 0.50 (0.39–0.58) | 0.18 |
| Pro-cathepsin D | 0.50 (0.39–0.58) | 0.18 |
| Cathepsin L | 1.11 (1.01–1.16) | 0.41 |
| Pro-cathepsin L | 0.96 (0.73–1.71) | 0.85 |
| Cathepsin B | 1.10 (0.95–1.13) | 0.41 |
| MMP-2 | 0.75 (0.71–0.90) | 0.23 |
| TIMP2 | 0.85 (0.81–1.30) | 0.95 |
| TIMP3 | 0.65 (0.46–1.38) | 0.49 |
Statistics presented as median fold change (interquartile range) of protein level in metabolic syndrome pigs treated with extracellular vesicles (HVM, n=8) compared to control (n=6). P values calculated using Wilcoxon rank sum test. MMP-2, matrix metalloproteinase 2; TIMP2, tissue inhibitor of metalloproteinases 2, TIMP3, tissue inhibitor of metalloproteinases 3.
DISCUSSION:
In the present study, we found that in the setting of chronic myocardial ischemia and metabolic syndrome, intramyocardial EV injection was associated with decreased expression of anti-angiogenic markers angiostatin and endostatin (Video Abstract). Angiostatin localized to coronary microvessels and its expression was inversely correlated with myocardial perfusion to ischemic territory. EV therapy was also associated with decreased expression of cathepsin D, which could contribute to decreased anti-angiogenic signaling in this setting.
Video: Clinical Implications of Reduced Anti-Angiogenic Signaling with EV Therapy in Swine with Metabolic Syndrome.
Our group has previously shown that intramyocardial extracellular vesicle (EV) therapy improves myocardial perfusion in our swine model of chronic myocardial ischemia and metabolic syndrome, a large animal model that is relevant to patients with coronary disease and common co-morbidities. This study shows that EV therapy decreases anti-angiogenic signaling, correlating with improved perfusion, in ischemic myocardial tissue in swine with metabolic syndrome. These findings are significant as patients with coronary disease and co-morbid diabetes have increased expression of antiangiogenic signaling markers which impairs coronary collateralization and flow, and EV-mediated reduction in these markers could provide benefit.
In the setting of myocardial ischemia, microvascular collateralization is regulated by a balance of pro- and anti-angiogenic factors.8 Two potent anti-angiogenic proteins are angiostatin and endostatin, which are cleaved from plasminogen and collagen 18 respectively.9,10 Cleavage to generate the active form of both of these factors is mediated by cathepsins and MMPs. MMP activity is regulated by tissue inhibitors of matrix metalloproteinases (TIMP). In addition to anti-angiogenic activity, these factors may negatively regulate coronary vasodilation.
More specifically, angiostatin is proteolytically cleaved from plasminogen by several factors including MMPs and cathepsin D, and is known to counteract angiogenic growth factors.9,17,18 Angiostatin is often investigated in the context of tumor biology, but also plays an important role in the coronary vasculature, particularly in the setting of myocardial ischemia.11,19,20 We found that EV treatment significantly decreased angiostatin expression in chronically ischemic myocardium. Further, immunofluorescence studies indicated that angiostatin was localized to the coronary vascular walls as stained by α-SMA, which is consistent with prior findings that angiostatin binds to coronary smooth muscle cells.21 Importantly, angiostatin does not only inhibit endothelial cell proliferation, but also inhibits vascular smooth muscle cell proliferation and endothelium-dependent vasodilation in the coronary microvasculature.20,21 These additional functions of angiostatin may provide a mechanistic explanation for our finding that angiostatin expression was inversely correlated with perfusion to ischemic myocardium. Reduced angiostatin activity in EV-treated myocardium may promote smooth muscle cell proliferation thereby improving arteriogenesis and augmenting blood flow, or perhaps reductions in angiostatin expression, independent of alterations in collateralization, may attenuate impaired microvascular vasodilation to improve blood flow.
Given our findings of reduced angiostatin expression and its relationship to perfusion, we further investigated key proteins involved in the generation of angiostatin. EV treatment significantly reduced expression of the protease cathepsin D, which is known to cleave plasminogen to form angiostatin. There was also a nonsignificant trend towards decreased expression of the precursor to cathepsin D, procathepsin D, with EV treatment. 18,22 Additionally, there was a strong trend toward decreased plasminogen expression with EV treatment, and reduced bioavailability of this angiostatin precursor could also contribute to reduced angiostatin expression. An important consideration is the mechanisms by which EV therapy may regulate cathepsin D expression. Similar to many proteins, cathepsins may be regulated by changes at transcriptional, translational, post-translational, and epigenetic levels.23 EVs can mediate effects at each of these levels with the cargo that they contain, and non-coding RNAs, specifically microRNAs (miRNA), are particularly effective as they have a longer half-life than EVs themselves and mediate transcriptional and epigenetic changes that have prolonged downstream effects.4,24 Further characterization of the transcriptome of our EVs to determine microRNA content may better elucidate the mechanisms by which transcriptional or epigenetic regulation of cathepsin D may occur.
In addition to reducing angiostatin expression, EV therapy was associated with reduced endostatin expression. Endostatin is proteolytically cleaved from collagen 18, a process that is mediated by MMP2, and several cathepsins, including cathepsins L, B, and D. 10,25 In addition to its potent anti-angiogenic activity on endothelial cells, endostatin also impairs recruitment of smooth muscle actin-expressing perivascular cells to reduce collateralization,26 and impairs coronary endothelium-dependent vasodilation.27 In contrast to angiostatin, endostatin expression was not correlated with myocardial perfusion to ischemic territory, a finding that could be due to several reasons. It is possible that due to a small sample size, the correlation analysis was underpowered, however there was no correlative trend and that may be a less likely explanation. More likely, endostatin downregulation may be contributing to the balance of pro- and anti-angiogenic and vasodilatory signaling factors in the dysfunctional microenvironment induced by metabolic syndrome, but without a strong enough influence to correlate directly with perfusion.
In order to better understand angiogenic signaling pathways regulated by EV-therapy, we investigated several proangiogenic proteins, including angiopoietin 1, ß-catenin, VE-cadherin, Tie2, VEGF-A, FGFR1, and MCP-1, the latter two which are more involved in arteriogenesis. Though there was a trend toward increased angiopoietin and decreased VEGF-A, all of these pro-angiogenic proteins were not significantly changed. Our group has shown in previous studies that in the setting of chronic myocardial ischemia without metabolic syndrome, EVs improve proangiogenic signaling via mitogen activated protein kinases, endothelial nitric oxide synthase, and Akt, though these effects were not seen in the setting of metabolic syndrome. Therefore, the lack of upregulation of other pro-angiogenic markers is consistent with what we consider to be a blunted effect of EVs in the setting of metabolic syndrome, which may be secondary to altered signaling cascades, redox state, and endothelial dysfunction that occur in the setting of metabolic syndrome co-morbidities.28 These previous findings also underscore the significance of reduced anti-angiogenic signaling markers with EV treatment in this study, which may provide novel, though indirect, mechanistic evidence for improvements in perfusion.
There are several limitations in this study to consider. First, the study involved only intact male swine, and although the swine were young and we would not expect sex hormones to play a major physiological role, we have since modified our pig protocols to include equal numbers of male and female pigs to account for any possible sex-related differences. Additionally, given the large animal model, our study has a relatively low sample size, which may have masked significant differences in our protein expression analysis. Further, EVs have a short half-life, and the sustained effect of EV therapy five weeks after injection are likely mediated by early molecular changes. It would be informative to harvest tissue sooner after EV injection to determine the early signaling pathways affected, which would help provide more direct evidence of the effect of EVs on anti-angiogenic signaling markers and their relationship with myocardial perfusion, though this can be logistically challenging in a large animal model. Additionally, intramyocardial injection is a less practical delivery method than intravenous or intracoronary delivery. We have previously shown that intravenous injection of EVs is not very effective likely due to poor myocardial uptake.29 Intracoronary delivery is another approach that may warrant further investigation, though limitation of this approach in our model is that at the time of delivery the ameroid would likely be closed and myocardial uptake of EVs would be dependent of collateral perfusion. Finally, we have not yet fully characterized our EVs, particularly with transcriptome analysis to assess for non-coding RNA contents, and ongoing studies by our lab in this area will help elucidate the molecular mechanisms by which EV therapy provides benefit to chronically ischemic myocardium in swine with metabolic syndrome.
CONCLUSION:
In the setting of metabolic syndrome and chronic myocardial ischemia, EV therapy is associated with decreased expression of the anti-angiogenic proteins angiostatin and endostatin, which may contribute to increased blood flow to ischemic myocardium (Video).
Supplementary Material
Supplemental Figure 2: Angiostatin and endostatin expression are unchanged in chronically ischemic myocardium of normal diet swine receiving EV therapy. The normal diet with EV injection (MVM, n=8) group had no significant difference in expression of anti-angiogenic markers angiostatin and endostatin compared to the normal diet control (NDC, n=7) group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. Statistical significance defined by p>0.05 as determined by Wilcoxon rank-sum test.
Supplemental Figure 1: EV Characterization. A) CD81, CD9 and Alix were identified by western blot to establish the presence of EV transmembrane proteins and cytosolic proteins. The absence of albumin confirmed EV purity. B) The EV size and concentrations were quantified via nanoparticle tracking analysis (mean EV size 233.4 nm ± 120.4 nm).
CENTRAL MESSAGE:
Extracellular vesicle therapy reduced expression of anti-angiogenic signaling markers in chronically ischemic myocardium.
PERSPECTIVE STATEMENT:
Chronic coronary artery disease, often comorbid with metabolic syndrome, contributes to significant morbidity and mortality. Metabolic syndrome is known to increase anti-angiogenic signaling markers angiostatin and endostatin. In a large animal model of chronic myocardial ischemia and metabolic syndrome, extracellular vesicle therapy reduced anti-angiogenic signaling markers to improve perfusion.
Funding:
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) 1F32HL160063-01 (S.A.S.); 1R01HL133624 (M.R.A.); R01HL46716 and R01HL128831 (F.W.S.).
GLOSSARY OF ABBREVIATIONS
- CAD
coronary artery disease
- DLL4
delta like ligand 4
- EV
extracellular vesicle
- FGFR1
fibroblast growth factor receptor 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HVM
high fat diet with myocardial extracellular vesicle injection group
- LAD
left anterior descending artery
- LCx
left circumflex artery
- MCP-1
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinases
- VEGF-A
vascular endothelial growth factor A
Biography

Footnotes
Disclosures: None
Presentation: To be presented as an oral presentation at the Western Thoracic Surgical Association 48th Annual Meeting, June 22–25, 2022, Koloa, HI.
All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital (#017316, 10/18/16).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106(6):897–909. doi: 10.1007/s00395-011-0200-1 [DOI] [PubMed] [Google Scholar]
- 2.Hirode G, Wong RJ. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–2528. doi: 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 4.Karbasiafshar C, Sellke FW, Abid MR. Mesenchymal stem cell-derived extracellular vesicles in the failing heart: past, present, and future. Am J Physiol Heart Circ Physiol. 2021;320(5):H1999–H2010. doi: 10.1152/ajpheart.00951.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scrimgeour LA, Potz BA, Aboul Gheit A, et al. Extracellular Vesicles Promote Arteriogenesis in Chronically Ischemic Myocardium in the Setting of Metabolic Syndrome. J Am Heart Assoc. 2019;6;8(15):e012617. doi: 10.1161/JAHA.119.012617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aboulgheit A, Potz BA, Scrimgeour LA, et al. Effects of High Fat Versus Normal Diet on Extracellular Vesicle–Induced Angiogenesis in a Swine Model of Chronic Myocardial Ischemia. J Am Heart Assoc. 2021;10(4):e017437. doi: 10.1161/JAHA.120.017437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboulgheit A, Karbasiafshar C, Sabra M, et al. Extracellular vesicles improve diastolic function and substructure in normal and high-fat diet models of chronic myocardial ischemia. J Thorac Cardiovasc Surg. Published online October 13, 2021:S0022–5223(21)01420–3. doi: 10.1016/j.jtcvs.2021.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemanthakumar KA, Kivelä R. Angiogenesis and angiocrines regulating heart growth. Vasc Biol. 2020;2(1):R93–R104. doi: 10.1530/VB-20-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soff GA. Angiostatin and angiostatin-related proteins. Cancer Metastasis Rev. 2000;19(1–2):97–107. doi: 10.1023/a:1026525121027 [DOI] [PubMed] [Google Scholar]
- 10.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaissé J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486(3):247–251. doi: 10.1016/s0014-5793(00)02249-3 [DOI] [PubMed] [Google Scholar]
- 11.Sodha NR, Clements RT, Boodhwani M, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296(2):H428–434. doi: 10.1152/ajpheart.00283.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodha NR, Boodhwani M, Clements RT, Xu SH, Khabbaz KR, Sellke FW. Increased Antiangiogenic Protein Expression in the Skeletal Muscle of Diabetic Swine and Patients. Arch Surg. 2008;143(5):463–470. doi: 10.1001/archsurg.143.5.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boodhwani M, Nakai Y, Mieno S, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. [DOI] [PubMed] [Google Scholar]
- 14.Robich MP, Chu LM, Chaudray M, et al. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 2010;148(2):453–462. doi: 10.1016/j.surg.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmadhun NY, Lassaletta AD, Chu LM, Liu Y, J F, Sellke FW. Atorvastatin increases oxidative stress and modulates angiogenesis in Ossabaw swine with the metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2012;144:1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274(41):29568–29571. doi: 10.1074/jbc.274.41.29568 [DOI] [PubMed] [Google Scholar]
- 18.Morikawa W, Yamamoto K, Ishikawa S, et al. Angiostatin generation by cathepsin D secreted by human prostate carcinoma cells. J Biol Chem. 2000;275(49):38912–38920. doi: 10.1074/jbc.M005402200 [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105(18):2185–2191. doi: 10.1161/01.cir.0000015856.84385.e9 [DOI] [PubMed] [Google Scholar]
- 20.Koshida R, Ou J, Matsunaga T, et al. Angiostatin: a negative regulator of endothelial-dependent vasodilation. Circulation. 2003;107(6):803–806. doi: 10.1161/01.cir.0000057551.88851.09 [DOI] [PubMed] [Google Scholar]
- 21.Walter JJ, Sane DC. Angiostatin Binds to Smooth Muscle Cells in the Coronary Artery and Inhibits Smooth Muscle Cell Proliferation and Migration In Vitro. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(9):2041–2048. doi: 10.1161/01.ATV.19.9.2041 [DOI] [PubMed] [Google Scholar]
- 22.Perchick GB, Jabbour HN. Cyclooxygenase-2 overexpression inhibits cathepsin D-mediated cleavage of plasminogen to the potent antiangiogenic factor angiostatin. Endocrinology. 2003;144(12):5322–5328. doi: 10.1210/en.2003-0986 [DOI] [PubMed] [Google Scholar]
- 23.Yadati T, Houben T, Bitorina A, Shiri-Sverdlov R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells. 2020;9(7):1679. doi: 10.3390/cells9071679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402. doi: 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walia A, Yang JF, hui Huang Y, Rosenblatt MI, Chang JH, Azar DT. Endostatin’s Emerging Roles in Angiogenesis, Lymphangiogenesis, Disease, and Clinical Applications. Biochim Biophys Acta. 2015;1850(12):2422–2438. doi: 10.1016/j.bbagen.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skovseth DK, Veuger MJT, Sorensen DR, De Angelis PM, Haraldsen G. Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo. Blood. 2005;105(3):1044–1051. doi: 10.1182/blood-2004-03-1164 [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Zhang Y, Li PL. Dependence of cathepsin L-induced coronary endothelial dysfunction upon activation of NAD(P)H oxidase. Microvasc Res. 2009;78(1):45–50. doi: 10.1016/j.mvr.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal. 2009;11(8):1945–1959. doi: 10.1089/ars.2009.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scrimgeour LA, Potz BA, Aboul Gheit A, et al. Intravenous injection of extracellular vesicles to treat chronic myocardial ischemia. PLoS One. 2020;15(9):e0238879. doi: 10.1371/journal.pone.0238879 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2: Angiostatin and endostatin expression are unchanged in chronically ischemic myocardium of normal diet swine receiving EV therapy. The normal diet with EV injection (MVM, n=8) group had no significant difference in expression of anti-angiogenic markers angiostatin and endostatin compared to the normal diet control (NDC, n=7) group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. Statistical significance defined by p>0.05 as determined by Wilcoxon rank-sum test.
Supplemental Figure 1: EV Characterization. A) CD81, CD9 and Alix were identified by western blot to establish the presence of EV transmembrane proteins and cytosolic proteins. The absence of albumin confirmed EV purity. B) The EV size and concentrations were quantified via nanoparticle tracking analysis (mean EV size 233.4 nm ± 120.4 nm).