Abstract
Periconceptional maternal obesity is linked to adverse maternal and neonatal outcomes. Identifying periconceptional biomarkers of pathways affected by maternal obesity can unravel pathophysiologic mechanisms and identify individuals at risk of adverse clinical outcomes. The literature was systematically reviewed to identify periconceptional biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways influenced by maternal obesity. A search was conducted in Embase, Ovid Medline All, Web of Science Core Collection and Cochrane Central Register of Controlled Trials databases, complemented by manual search in PubMed until December 31st, 2020. Eligible studies were those that measured biomarker(s) in relation to maternal obesity, overweight/obesity or body mass index (BMI) during the periconceptional period (14 weeks preconception until 14 weeks post conception). The ErasmusAGE score was used to assess the quality of included studies. Fifty-one articles were included that evaluated over 40 biomarkers. Endocrine biomarkers associated with maternal obesity included leptin, insulin, thyroid stimulating hormone, adiponectin, progesterone, free T4 and human chorionic gonadotropin. C-reactive protein was associated with obesity as part of the inflammatory pathway, while the associated one-carbon metabolism biomarkers were folate and vitamin B12. BMI was positively associated with leptin, C-reactive protein and insulin resistance, and negatively associated with Free T4, progesterone and human chorionic gonadotropin. Concerning the remaining studied biomarkers, strong conclusions could not be established due to limited or contradictory data. Future research should focus on determining the predictive value of the optimal set of biomarkers for their use in clinical settings. The most promising biomarkers include leptin, adiponectin, human chorionic gonadotropin, insulin, progesterone and CRP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11154-022-09762-5.
Keywords: Obesity, Body mass index, Periconceptional period, Biomarker
Introduction
The global prevalence of obesity almost tripled since 1975, affecting 15% of adult women worldwide [World Health Organization (WHO) 2016] [1]. The rise in this epidemic is alarming for its association with increased reproductive and pregnancy complications [2, 3]. These complications can originate during the periconceptional period (defined as the period from 14 weeks prior to, until 10 weeks following, conception) during which gametogenesis, fertilization, implantation, embryogenesis and placentation take place [4, 5]. From a life-course perspective, maternal obesity impacts the health of the woman and her offspring commencing from the periconceptional period, with the effects persisting into adulthood [4, 6].
Maternal obesity leads to impaired oogenesis, infertility and anovulation [7–10]. In utero, maternal obesity is linked to production of blastocysts with fewer cells, accelerated preimplantation embryonic development, decreased post-implantation embryonic and fetal growth trajectories, and impaired fetal cardiac function [11–16]. Furthermore, obesity continues to pose risks throughout pregnancy such as that for miscarriage, gestational diabetes mellitus (GDM), preeclampsia and delivery complications [17–19]. This, in turn, increases the risk of adverse birth outcomes, neural tube and congenital heart defects in offspring of obese women [20–26].
Unraveling the pathophysiologic mechanisms can aid in understanding the link between maternal obesity and adverse clinical outcomes. Obesity disrupts the endocrine and inflammatory pathways at both systemic and local levels which leads to, or is a consequence of, perturbations in metabolic processes such as one-carbon metabolism [27, 28]. The disruptions can be identified clinically using biomarkers for early diagnosis, for detection and prevention of adverse clinical outcomes [29]. These biomarkers could potentially be used as screening tools to identify population at risk, and to predict outcomes for the mother and offspring. However, the applicability of these biomarkers in a clinical setting is limited and requires more information, particularly among the obese population [30]. For example, it is generally known that low folate levels increase the risk of neural tube defects in offspring, while first trimester inflammatory cytokines are associated with increased risk of preterm birth among obese women [31–33]. Furthermore, endocrine and inflammatory pathways are involved in the manifestation of obesity-related pregnancy complications such as GDM and preeclampsia [34–37].
Identification of biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways affected by maternal obesity during the periconceptional period can aid in our understanding of the pathophysiologic basis of adverse clinical outcomes to be used as an early detection marker of patients at risk [4]. Therefore, the aim of this review is to identify how maternal obesity impacts the different types of biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways during the periconceptional period.
Methods
Sources
A literature search was performed by a biomedical information specialist (W.B.) specialized in systematic reviews using the databases of Embase, Ovid Medline All, Web of Science Core Collection and Cochrane Central Register of Controlled Trials until December 31st, 2020. The keywords used for the search strategy included but were not limited to: obesity, maternal obesity, pregnancy, preconception, periconception, first trimester, biomarker, endocrinology, leptin, inflammation, carbon metabolism (Table S1). The Boolean operators used for the search outcome were “AND”, “OR” and “NEAR”. In addition, the database of PubMed was manually searched to identify relevant articles. The review was structured in accordance to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. A protocol of this systematic review was designed and registered under the PROSPERO international prospective registry of systematic reviews (2020: CRD42021240883).
Eligibility
All types of observational human studies that associated maternal obesity with a certain biomarker measured during the periconceptional period were eligible for inclusion. Biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways were all considered, with no limitation on the source of samples. In addition, eligible studies had to include biomarkers studied in population with obesity or overweight/obesity, or in relation to BMI. Inclusion and exclusion criteria for this systematic review are shown in Table 1.
Table 1.
Inclusion and exclusion criteria
| Criteria | Inclusion | Exclusion | Reason for exclusion |
|---|---|---|---|
| Study design | - Observational | - Intervention | The systematic review is not focused on the impact of intervention on biomarker level |
| Publication type | - Research papers published in full text |
- Non-English papers - No full text available - Reviews - Case reports - Abstracts |
Not relevant |
| Population | - Women |
- Men - Animal studies |
The systematic review comprises the female population |
| Period |
- Fourteen weeks preconception period - First trimester of pregnancy |
- Pregnant women in second and third trimester of pregnancy - Pregnant women with unreported gestational age - Non-pregnant women at any age but not in the 14 weeks preconception period |
The time frame of the systematic review encompasses the periconceptional period and additional part of the first trimester of pregnancy |
| Indication | - Population includes obese women |
- Population does not include obese women - Women with identified pregnancy related complications - Women with polycystic ovary syndrome - Women with identified acute and chronic diseases |
Population does not include a group of obese or healthy obese women |
| Outcome | - Endocrine, inflammatory, and one-carbon metabolism biomarkers |
- No biomarker measured - No obesity related biomarker outcome - Biomarkers not related to the Endocrine, inflammatory, and one-carbon metabolic pathways |
The systematic review is focused on the biomarkers of endocrine, inflammatory, and one-carbon metabolic pathways |
Since the aim of this systematic review was to encompass the periconceptional period and the first trimester of pregnancy, articles with a time frame spanning 14 weeks preconception up and until 14 weeks of gestation were considered eligible (we extended the post-conception period from 10 to 14 weeks to include the first trimester of pregnancy).
Selection strategy
An abstract – title evaluation was performed by two independent reviewers (B.H. and S.S.) on all publications from the search. When both reviewers did not agree on the inclusion of certain articles, a third reviewer (M.R.) repeated the evaluation of the articles for a final decision.
Thereafter, a full text review and data extraction was performed for the selected publications. Data extraction included year of publication, country, study design, detailed sample size, age, BMI, biomarker, biomarker class, gestational age when biomarker and BMI were assessed, biomarker source, statistical analysis and adjustments, results and conclusion.
The systematic review comprised the population of obese women with different fertility status. Obesity was classified based on criteria indicated by the authors of each study, or according to the WHO classification of BMI ≥ 30 kg/m2 when no criteria were mentioned. Studies including outcomes related to BMI or combined overweight/obesity were included if the population included obese women identified from the population BMI of ≥ 30 kg/m2 or if the authors indicated N number of obese women, though not studied separately.
Quality score assessment and risk of bias
The ErasmusAGE score was used to assess the quality of studies included in the systematic review (Table S2) [38]. This tool is based on previously published scoring systems and is applicable for intervention and observational studies, as well as for systematic reviews and meta-analysis [38]. The quality score is based on 5 items covering the study design and size, exposure and outcome, and adjustments. The parameters for these items were adapted, based on the literature and discussion with other researchers, as relevant for the review. The allocated scores for each item were: study design (0 = cross-sectional study, 1 = longitudinal study, 2 = intervention study), study size N (small: 0 = < 100, intermediate: 1 = 100 to 500, large: 2 = > 500 participants), exposure measurement method (0 = not reported/inadequate, 1 = moderate quality, 2 = adequate), outcome measurement method (0 = not reported/inadequate, 1 = moderate quality, 2 = adequate) and adjustments in analysis (0 = no adjustments, 1 = controlled for age, 2 = controlled for additional confounders) (Table S2). The score ranges from 0 to 10 and was considered as low (ErasmusAGE score ≤ 5) or high (ErasmusAGE score ≥ 6) for each study.
Results
Study selection
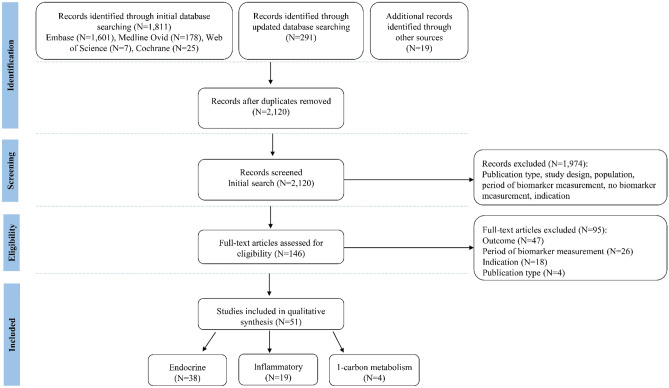
The flowchart depicted in Fig. 1 summarizes the process of literature screening and study selection. The initial and updated electronic search of the databases resulted in 2,102 records (including 1 identified as duplicate) and the manual search resulted in 19 additional records. A total of 1,974 articles were excluded after title-abstract screening for the eligibility criteria, leaving 146 articles for full text assessment. Eventually, 51 articles were left for analysis in this systematic review.
Fig. 1.
PRISMA flow chart of the systematic review
Study characteristics
Table S3 summarizes the general characteristics and ErasmusAGE quality score of the selected studies. The included studies consisted of prospective (N = 28) and retrospective (N = 3) cohort, cross-sectional (N = 14) and case–control (N = 6) studies. Most studies based their obesity classification according to WHO criteria of BMI ≥ 30 kg/m2 (N = 27), or BMI ≥ 25 kg/m2 based on BMI references for the Asian population (N = 2). Sources for biomarker sampling included serum, plasma, follicular fluid, placental tissue, red blood cells, urine and oocytes.
Quality of studies
Details on the quality score and risk of bias assessment for each study are provided in Fig. 2. The quality score of the studies ranged between 2 to 9 (mean ErasusAGE score 4.4 out of 10.0) (Fig. S1). Seventy-six percent of studies were of low quality (N = 39) and 24% were of high quality (N = 12). Fifty-five percent of studies had small sample size (N = 28), 49% did not specify the tool for anthropometrics screening (N = 25), 4% did not specify the analysis tool for biomarker measurement (N = 2), and 78% did not adjust for confounding factors (N = 40). In studies with adjustments for confounding factors (N = 11), the important confounders considered were maternal age (N = 11), gestational age (N = 7), smoking (N = 7) and gravidity/parity (N = 6). Other confounders were alcohol use, race/ethnicity, socio-economic status, marital status, miscarriage, biomarkers, sex of newborn, day of embryo transfer, stress, nausea, weight gain and conception mode; where each was adjusted in one or two studies.
Fig. 2.
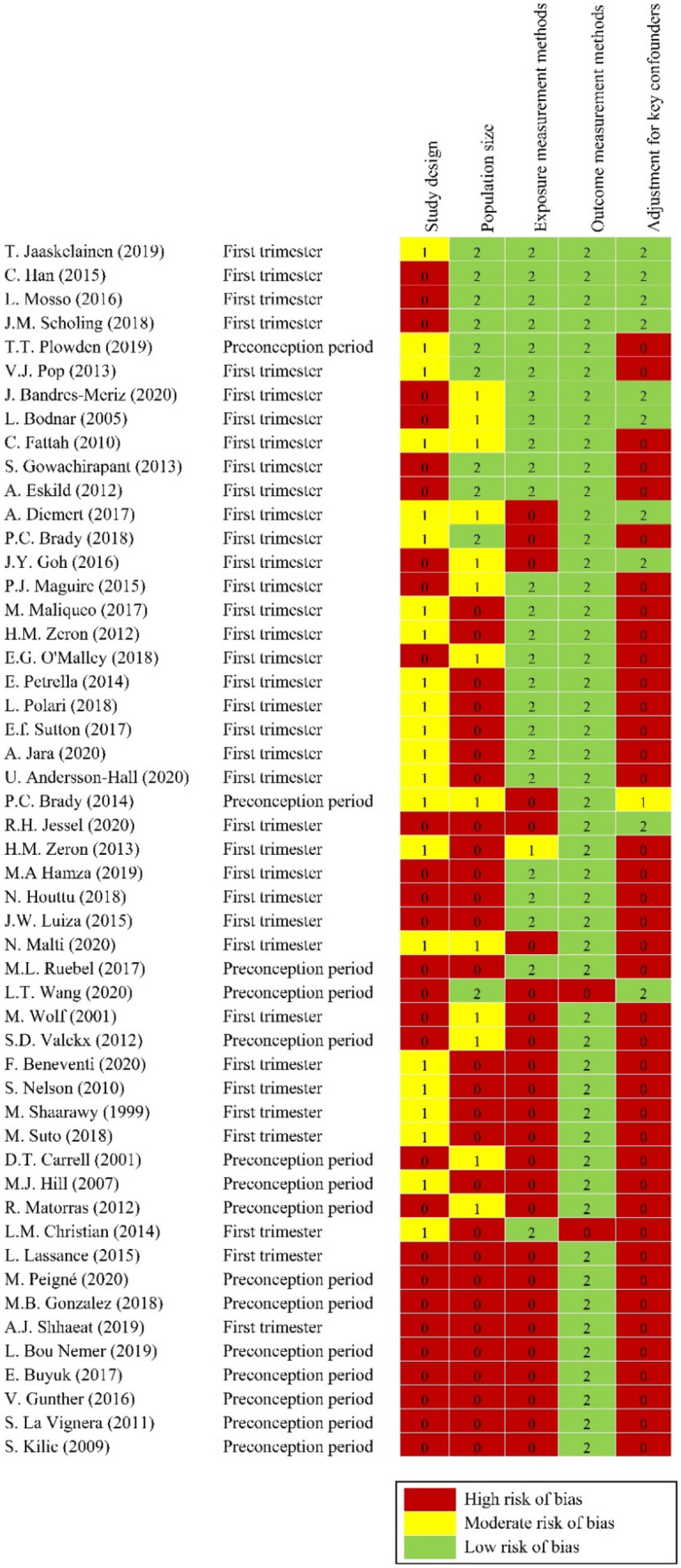
Quality score assessment and risk of bias for all studies included in this systematic review
Endocrine biomarkers
Endocrine biomarkers are considered secretions released into circulation from different glands in the body, interacting with each other in feedback loops. These biomarkers are involved in regulating various body functions including metabolism, growth, appetite and inflammation [39]. Tables 2 and 3 summarize associations between maternal obesity and the endocrine biomarkers for the preconception period and first trimester, respectively.
Table 2.
Summary of studies examining the association between maternal obesity and biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways during the preconception period sorted according to ErasmusAGE quality score (QS) and biomarker pathway
| Author, year | Country | NTotal, NObesity | Maternal age (years) | BMI criteria (kg/m2) | Biomarker | Source | Main findings | QS | Pathw |
|---|---|---|---|---|---|---|---|---|---|
|
Plowden et al (2019) [40] |
USA | 1,053 | 28.9 ± 4.7 | n/a | Leptin | S |
Incr. BMI ↑ Leptin (r = 0.8) Incr. waist circumference ↑ Leptin (r = 0.8) |
7 | EC |
|
Brady et al (2014) [41] |
USA | 229 (13) | 27.1 ± 4.0 |
Obesity ≥ 30.0 Overweight 25–29.9 Normal < 25.0 |
Progesterone | S |
Obesity vs normal weight ↓ Progesterone (Median (IQR): 16.0 (10.0–50.0) vs 25.0 (6.0–16.0) ng/ml) BMI Predictor for progesterone <20 ng/ml |
5 | EC |
|
Ruebel et al (2017) [42] |
USA | 24 (11a) |
Overweight /obesity 32.1 ± 1.5 Normal 31.6 ± 0.9 |
Overweight /obesity > 25.0 Normal 18.5–24.9 |
Estradiol, insulin, FSH leptin, LH, CCL2, CRP, IL-6, TNF-α, genes | S, FF, oocyte |
Overweight/obesity vs normal weight ↑ S HOMA-IR, leptin, CCL2 (Mean ± SD: 1.8 ± 0.3 vs 0.7 ± 0.1, p = 0.002; 27.2 ± 6.6 vs 5.3 ± 1.0 ng/ml, p < 0.003; 403.6 ± 51.0 vs 238.6 ± 22.4 pg/ml, p = 0.005, respectively) ↑ FF leptin, CRP (Mean ± SEM: 28.0 ± 2.0 vs 12.6 ± 6.0 ng/ml, p < 0.05; 5.0 ± 2.0 vs 2.0 ± 0.5 μg/ml, p < 0.05) Ten genes involved in chemokine and cytokine pathways differentially regulated (p = 0.007 and p = 0.011, respectively) Incr. BMI ↑ CXCL3, IL-34 gene expression (R = 0.6, p = 0.04) Incr. fat mass percent ↑ FF TNF-α (R = 0.7, p = 0.001) No effect Obesity on S estradiol, FSH, insulin, LH, CRP, IL-6, TNF-α, and FF insulin, CCL2, TNF-α |
4 | EC /Inf |
|
Wang et al (2020) [43] |
China | 2,319 (848) |
Group I < 35.0 Group II > 35.0 |
Obesity 25.0–35.1 Overweight 23.0–25.0 Normal 8.5–23.0 |
Estradiol, LH, progesterone | S |
Obesity vs normal weight Natural cycle (group I): ↓ estradiol, LH (Median (IQR): 927.3 (696.1–1201.0) vs 1105.5 (808.8–1426.8), p < 0.05; 25.3 (14.4–43.1) vs 34.3 (19.3–54.5), p < 0.05, respectively) Ovulation induction cycle (group I): ↓ estradiol, LH, progesterone (Median (IQR): 933.5 (525.7–1607.8) vs 1538.0 (827.9–2828.5); 10.70 (7.0–19.0) vs 12.8 (8.1–23.3); 2.1 (1.5–2.9) vs 2.4 (1.7–3.2), ps < 0.05, respectively) Incr. BMI ↓ Estradiol, LH, progesterone (r = -0.2, p < 0.001; r = -0.1, p = 0.001; r = -0.1, p < 0.001, respectively) No effect Group I (natural cycle): obesity on progesterone Group II (natural and ovulation cycles): obesity on estradiol, LH, progesterone |
4 | EC |
|
Valckx et al (2012) [44] |
Belgium | 106 (20) |
Obesity 35.0 ± 6.1 Overweight 32.0 ± 4.6 Normal 34.0 ± 4.7 |
Obesity ≥ 30.0 Overweight 25–29.9 Normal 18.5–24.9 |
Insulin, CRP, IGF-1 | S, FF |
Obesity vs normal weight ↑ S insulin, CRP, IGF-1 (Mean ± SD: 16.0 ± 9.1 vs 9.0 ± 3.8 mUI/l, p < 0.01; 4.0 ± 3.6 vs 2.0 ± 3.3 mg/l, p < 0.01; 150.0 ± 48.3 vs 185.0 ± 59.7 ng/ml, p = 0.03, respectively) ↑ FF insulin, CRP, IGF-1 (Mean ± SD: 12.0 ± 6.7 vs 8.0 ± 5.3 mUI/l, p < 0.01; 2.0 ± 1.8 vs 2 ± 2.6 mg/l, p < 0.01; 101.0 ± 39.1 vs 128.0 ± 42.4 ng/ml, p = 0.04, respectively) Incr. BMI ↓ QUICKI, rQUICKI (ps < 0.01) |
3 | EC / Inf |
|
Hill et al (2007) [45] |
Hawaii | 10 | 33.7 ± 2.8 | n/a | Leptin | S, FF |
Incr. BMI ↑ S and FF leptin (r = 0.8, p < 0.01) |
3 | EC |
|
Carrell et al (2001) [46] |
USA | 247 (34) | n/a |
High > 30.0 Medium 20.0 < BMI < 30.0 Low < 20.0 |
Estradiol, hCG | FF |
Obesity vs medium BMI ↓ hCG (Mean ± SD: 73.6 ± 8.8 vs 121.4 ± 6.0 mIU/ml, p < 0.001) Obesity vs low BMI ↓ hCG (Mean ± SD: 73.6 ± 8.8 vs 153.2 ± 11.2 mIU/ml, p < 0.001) Incr. BMI ↓ hCG (r = -0.4, p < 0.001) No effect Obesity on S estradiol |
3 | EC |
|
Matorras et al (2012) [47] |
Spain | 473 | 34.7 ± 2.9 |
BMI < 20.0 BMI 20.0–25.0 BMI 25.0–30.0 BMI > 30.0 |
hCG | P |
Incr. BMI ↓ hCG (r = -0.4, p < 0.05) |
3 | EC |
|
Peigné et al (2020) [48] |
France | 37 (16) |
Obesity 31.5 Normal 32.0 |
Obesity ≥ 30.0 Normal ≤ 25.0 |
AMH, ASD, estradiol, insulin, LH, FSH, SHBG, testosterone | S |
Obesity vs normal weight ↓ API, SHBG (34.6 vs 39.0%, p = 0.015; median (5th-90th percentile): 36.3 (17.2–65.8) vs 70.4 (40.7–103.8) pmol/l, p < 0.001, respectively) No effect Obesity on total AMH, ASD, estradiol, LH, FSH, insulin, proAMH, testosterone |
2 | EC |
|
Buyuk et al (2017) [49] |
USA |
S 39 (10) FF 40 (13) |
27–43 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal < 25.0 |
eotaxin, estradiol FSH, CRP, EGF, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, MCP-1, TNF-α | S, FF |
Obesity vs normal weight ↓ S FSH (Mean ± SD: 6.8 ± 1.7 vs 11.4 ± 4.5 IU/L, p = 0.005) ↑ S CRP, MCP-1 (Mean ± SD: 1586.2 ± 816.5 vs 585.2 ± 321.8 ng/mL, p = 0.0003; 921.1 ± 308.5 vs 558.0 ± 252.7 pg/mL, p = 0.0002, respectively) ↑ FF MCP-1(Mean ± SD: 821.0 ± 403.0 vs 444.0 ± 210.0 pg/ml, p = 0.02) Incr. BMI ↑ S CRP, MCP-1, G-CSF and (β = 0.4, p = 0.008; β = 0.4, p = 0.03; β = 0.4, p = 0.03, respectively) ↑ FF MCP-1 (β = 0.5, p = 0.002) No effect Obesity on S eotaxin, estradiol, EGF, G-CSF, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and FF estradiol, FSH |
2 | EC /Inf |
|
Bou Nemer et al (2019) [50] |
USA | 25 (5) | 35.0–45.0 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal 18.5–24.9 Non-obese < 30.0 |
adiponectin, C-peptide, ghrelin, glucagon, GLP-1, Insulin, leptin, resistin, visfatin, methionine |
FF |
Obesity vs non-obese ↑ C-peptide, glucagon, GLP-1, insulin, leptin (Mean ± SD: 86.0 ± 12.6 vs 56.5 ± 4.9 ng/dl, p = 0.0001; 32.9 ± 6.3 vs 25.3 ± 2.6 pg/ml, p = 0.0008; 43.3 ± 4.8 vs 31.4 ± 1.2 pg/ml, p = 0.0018; 398.0 ± 150.2 vs 96.4 ± 17.5 pg/ml, p = 0.0008; 12.8 ± 1.4 vs 3.6 ± 0.8 ng/ml, p = 0.0001, respectively) Obesity vs normal weight ↑ Methionine (p < 0.05) Incr. BMI ↑ Methionine (p < 0.05) No effect Obesity on adiponectin, ghrelin, resistin No assoc BMI with visfatin |
2 | EC / Inf / 1-CM |
|
Gonzalez et al (2018) [51] |
Australia | 45–48 (15–16) | n/a |
Obesity > 30.0 Overweight 25.0–29.9 Normal n/a |
Adiponectin, leptin, CRP, IL-6, IL-10, MCP-1, sICAM-1, TNF-α | FF |
Incr. BMI ↑ Leptin and CRP (β = 0.1, p < 0.001) ↑ 1 kg/m2 assoc with ↑ 14% CRP No assoc BMI with adiponectin, IL-6, IL-10, MCP-1, sICAM-1, TNF-α |
2 | EC /Inf |
|
Kilic et al (2009) [52] |
Turkey | 82 (24) | n/a |
Obesity ≥ 30.0 Overweight 25.0- 30.0 Normal < 25.0 |
LH, FSH, IL-18 | S, FF |
No effect Obesity on S and FF IL-18, LH, FSH |
2 | EC /Inf |
|
Gunther et al (2016) [53] |
Germany | 90 (5) | 25.0–43.0 |
BMI < 20.0 BMI 20.0–25.0 BMI 25.0–30.0 BMI > 30.0 |
IL-18 | S, FF |
Incr. BMI ↑ S IL-18 with BMI class (p = 0.027) No assoc BMI with FF IL-18 |
2 | Inf |
|
La Vignera et al (2011) [54] |
Italy | 40 (16) | 34.0 |
Obesity (II) 35.0–39.9 Obesity 30.0–34.9 Overweight 25.0–29.9 Normal 18.5–24.9 |
IL-6, IL-8, TNF-α | FF |
Obesity II vs normal weight ↑ IL-6, TNF-α (Mean ± SD: 23.1 ± 0.8 vs 14.2 ± 0.6 pg/ml, p < 0.05; 29.8 ± 2.5 vs 15.2 ± 0.6 pg/ml, p < 0.05, respectively) Obesity vs normal weight ↑ IL-6 (Mean ± SD: 21.1 ± 1.9 vs 14.2 ± 0.6 pg/ml, p < 0.05) No effect Obesity on IL-8 |
2 | Inf |
1-CM 1-carbon metabolism, AMH anti mullerian hormone, API anti mullerian hormone prohormone index, ASD androstenedione, assoc association, b/w between, CCL2 chemokine (C–C motif) ligand 2, CXCL3 chemokine (C-X-C motif) ligand 3, EC endocrine, EGF epidermal growth factor, FF follicular fluid, FSH follicle stimulating hormone, GIP gastric inhibitory polypepetide, GM-CSF granulocyte macrophage-colony stimulating factor, hCG human ghorionic gonadotropin, IGF-1 insulin-like growth factor 1, IL interleukin, Incr. increased, Inf inflammatory, LH luteinizing hormone, MCP-1 monocyte chemotactic protein-1, n/a not available, pathw pathway, IQR interquartile range, QS quality score, S serum, SEM standard error mean, SD standard deviation, SHBG sex hormone-binding globulin, sOB-R soluble leptin receptor, TNF tumor necrosis factor, P plasma
(↑) Higher
(↓) Lower
Table 3.
Summary of studies examining the association between maternal obesity and biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways during the first trimester of pregnancy sorted according to ErasmusAGE quality score (QS) and biomarker pathway
| Author, year | Country | NTotal, Nobesity | Maternal age (years) | BMI criteria (kg/m2) | Biomarker | Source | Main findings | QS | Pathw |
|---|---|---|---|---|---|---|---|---|---|
|
Han et al (2015) [55] |
China | 6,303 (90) | 19.0–40.0 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal 18.5–24.9 |
FT4 and TSH | S |
Obesity vs overweight ↑ TSH (Median (2.5th–97.5th percentile): 2.5 (0.6–13.8) vs 2.1 (0.4–6.8) mIU/l, p < 0.001) ↑ TSH > 5.2 mIU/l (OR 2.4, 95% CI 1.2, 5.0) ↓ FT4 (Median (2.5th–97.5th percentile): 14.7 (10.3–18.8) vs 15.3 (11.9–19.7) pmol/l, p < 0.001) Incr. BMI 1 kg/m2 assoc with ↓ 0.12 pmol/l FT4 (p < 0.05) |
8 | EC |
|
Mosso et al (2016) [56] |
Chile | 720 (145) | 25.4 ± 6.6 |
Obesity ≥ 30.0 Normal 20.0–24.9 |
FT4, TSH, TT4 | S |
Obesity vs normal weight ↑ TSH (Median: 2.3 vs 2.0 mIU/l, p = 0.036) ↓ FT4 (Median: 13.9 vs 14.8 pmol/l, p < 0.01) Incr. BMI ↑ TSH (R2 = 0.009, p = 0.04) ↓ FT4 (R2 = -0.09, p < 0.001) ↓ TT4 with BMI class (p = 0.002) |
8 | EC |
|
Pop et al (2013) [57] |
NL | 1,035 (160) | 30.5 ± 3.6 |
Obesity (III) > 40.0 Obesity (II) 35.0–40.0 Obesity (I) 30.0–35.0 Pre-obesity 25.0–30.0 Normal 18.5–25.0 Underweight > 18.5 |
FT4 and TSH | n/a |
Incr. BMI ↓FT4 (r = -0.14, p < 0.001) Diff. in FT4 and TSH b/w BMI classes (p < 0.001 and p = 0.02, respectively) No assoc BMI with TSH |
7 | EC |
|
Bandres-Meriz et al (2020) [58] |
Austria | 323 (24) | 29.5 ± 7.0 | n/a | C-peptide, insulin, leptin | S |
Obesity vs normal/ underweight ↑ C-peptide, leptin (Median (IQR): 545.9 (431.7–680.8) vs 329.1 (258.0–422.1) pmol/l, p < 0.001; 20.6 (16.4–29.1) vs 8.0 (4.0–11.7) ng/ml, p < 0.001, respectively) ↓ ISOHOMA (Median (IQR): 0.5 (0.4–0.7) vs 0.8 (0.6–1.1), p < 0.001) Incr. BMI ↑ Leptin (r = 0.5, p < 0.001) |
7 | EC |
|
Diemert et al (2017) [59] |
Germany | 220 (18) | 32.0 ± 3.7 |
Obesity > 30.0 |
Progesterone | S |
Incr. BMI ↓ Progesterone (β = -1.1, p < 0.0001) |
6 | EC |
|
Eskild et al (2012) [60] |
Norway | 2,626 (231) | 32.7 |
BMI < 20.0 BMI 20.0–25.0 BMI 25.0–30.0 BMI 30.0–35.0 BMI ≥ 35.0 |
hCG | S |
BMI ≥ 35.0 vs BMI < 20.0 Singleton prg.: ↓ hCG (Mean ± SD: 97.0 ± 51.0 vs 136.0 ± 76.0 IU/l, p < 0.05) Multiple prg.: ↓ hCG (Mean ± SD:152.0 ± 68.0 vs 252.0 ± 123.0 IU/l, p < 0.05) Incr. BMI Total pop.: ↓ hCG (R2 = -0.013, p < 0.0001) Singleton prg.: ↓ hCG (R2 = -0.012, p < 0.0001) Multiple prg.: ↓ hCG (R2 = -0.03, p < 0.0001) |
6 | EC |
|
Gowachirapant et al (2014) [61] |
Thailand | 514 (79) | 30.0 ± 5.0 |
Obesity ≥ 25.0 Normal 18.5–22.9 |
FT4, Tg, TSH | S |
Incr. BMI ↑ Tg (β = 0.1, p < 0.023) ↓FT4 (β = -0.2, p < 0.001) No assoc BMI not predictor for TSH |
6 | EC |
|
Fattah et al (2011) [62] |
Ireland | 100 (17) | 28.1 ± 5.1 |
Obesity > 29.9 |
Leptin | S |
Incr. BMI ↑ Leptin (R = 0.5) Incr. fat mass ↑ Leptin (R = 0.5, p < 0.0001) |
6 | EC |
|
Brady et al (2018) [63] |
USA | 541 |
Obesitya 34.4 ± 3.9 Non-obesitya 33.3 ± 3.3 |
Obesity III > 40.0 Obesity II 35.0–39.9 Obesity I 30.0–34.9 Non-obese < 30.0 |
hCG | S |
Obesity vs non-obese ↓ hCG (β = -170.38, 95% CI -199.6, -141.2) ↑ hCG < 100 mIU/ml (OR 3.8, 95% CI 1.8, 7.9) Incr. BMI ↓ hCG with BMI class (p < 0.0001) |
5 | EC |
|
Goh et al (2016) [64] |
Singapore | 194 (14) | 29.9 ± 4.0 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal 18.5–24.9 |
Progesterone | S |
Obesity vs normal weight ↓ Progetseone (Mean ± SD: 46.9 ± 18.1 vs 65.5 ± 21.6 nmol/l, p = 0.004); ↑ Progesterone < 35 nmol/l (OR 9.1, p = 0.003) Incr. BMI ↓ Progesterone with BMI class (p = 0.015) |
5 | EC |
|
Andersson-Hall et al (2020) [65] |
Sweden | 49 (19) |
Obesity 31.8 ± 3.5 Healthy 31.7 ± 3.4 |
Obesity ≥ 30.0 Healthy 18.5–24.9 |
Adiponectin, insulin, leptin, sOB-R | S |
Obesity vs healthy weight ↑ Free leptin index, HOMA-IR, leptin, leptin/adiponectin (ps ≤ 0.001) ↓ Adiponectin and sOB-R (ps ≤ 0.001) Incr. fat mass percent ↑ HOMA-IR (β = 0.38, p = 0.037) |
5 | EC |
|
Jara et al (2020) [66] |
USA | 80 (19) | 25.6 ± 4.3 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal 18.5–24.9 |
Adiponectin, leptin | S |
Obesity vs normal weight ↑ Leptin (p < 0.005) ↓ Adiponectin (p < 0.02) Incr. BMI ↑ Leptin (r > 0.55, p < 0.001) ↓ Adiponectin (r < -0.28, p < 0.01) |
5 | EC |
|
Maliqueo et al (2017) [67] |
Chile | 71 (36) | 24.0 |
Obesity ≥ 30.0 Normal 20.0–24.9 |
Adiponectin, ASD, estradiol, estrone, insulin, progesterone, SHBG, testosterone | S |
Obesity vs normal weight Total pop.: ↑ HOMA-IR, fasting insulin, FAI, testosterone (Median (IQR): 2.4 (2.3–3.2) vs 1.7 (1.2–2.2), p < 0.001; 14.0 (12.6–17.5) vs 10.0 (7.4–12.6) μUI/ml, p < 0.001; 0.5 (0.4–0.7 vs 0.25 (0.24–0.6), p = 0.003; 2.1 (3.1–1.5) vs 1.25 (1.0–3.0) nmol/l, p = 0.01, respectively) ↓ Adiponectin and progesterone (Median (IQR): 8.6 ( 6.3–11.5) vs 11.7 (10.1–13.2) μg/ml, p = 0.001; 7.0 (5.0–9.0) vs 8.0 (7.5–10.0) p = 0.014, respectively) Pregnancies with male fetus: ↓ Progesterone (Median (IQR): 7.0 (5.0–8.5) vs 10.0 (8.5–12.5), p = 0.034) ↑ FAI, testosterone (Median (IQR): 0.7 (0.4–1.4) vs 0.3 (0.2–0.4), p = 0.011; 3.0 (1.9–4.0) vs 1.8 (1.5–2.0) nmol/l, p = 0.023, respectively) No effect Obesity on ASD, estradiol, estrone, SHBG |
5 | EC |
|
Zeron et al (2012) [68] |
Mexico | 40b (21) |
Overweight /obesity 26.1 ± 4.6 Normal 24.8 ± 5.7 |
Obesity ≥ 30.0 Overweight /obesity ≥ 25.0 Overweight 25.0- 30.0 Normal < 25.0 |
Leptin | S |
Overweight/obesity vs normal weight ↑ Leptin (Mean ± SD: 29.6 ± 11.0 vs 45.8 ± 13.1 ng/ml, p < 0.001) |
5 | EC |
|
Petrella et al (2014) [69] |
Italy | 22 (12) |
Overweight /obesity 32.1 ± 5.4 Normal 25.6 ± 4.5 |
Overweight /obesity ≥ 25.0 Normal 18.0–25.0 |
Insulin | S |
Overweight/obesity vs normal weight ↑ Fasting insulin (Mean ± SD: 17.6 ± 16.5 vs 8.0 ± 5.1 mUI/l, p < 0.01) |
5 | EC |
|
Houttu et al (2018) [70] |
Finland | 99 (47) | 30.0 ± 5.0 |
Obesity ≥ 30.0 Overweight 25.0–29.9 |
Insulin, CRP, GlycA | S |
Obesity vs overweight ↑ HOMA2-IR, insulin, CRP, GlycA (6.1 vs 4.0 mg/l, p = 0.002; 14.0 vs 9.0 mU/I, p < 0.001; 1.7 vs 1.2, p < 0.001; 1.6 ± 0.2 vs 1.5 ± 0.1 mmol/l, p < 0.001, respectively) Incr. BMI ↑ CRP, GlycA (rho = 0.4, p < 0.001; rho = 0.5, p < 0.001, respectively) Explanatory factor for HOMA2-IR (β = 0.072, p < 0.001) |
4 | EC /Inf |
|
Malti et al (2020) [71] |
Algeria | 120 (60) |
Obesity 31.0 ± 3.0 Normal 28.0 ± 3.0 |
Obesity ≥ 30.0 Normal 19.0–25.0 |
leptin and insulin | P |
Obesity vs normal weight ↑ Insulin, leptin (Mean ± SD: 2.7 ± 0.2 vs 1.2 ± 0.1 ng/ml, p = 0.006; 30.3 ± 2.2 vs 11.5 ± 1.2 ng/ml, p < 0.05, respectively) |
4 | EC |
|
Luiza et al (2015) [72] |
USA | 50 |
Depressed 22.3 ± 3.1 Non-depressed 22.2 ± 3.0 |
Obesity ≥ 30.0 |
Cortisol | P, U |
Obesity vs non-obese Non-depressed pop.: ↓ P cortisol (Median (IQR: 22.0 (19.0–38.0) vs 40.0 (25.0–51.0) ng/ml, p < 0.05) Incr. BMI Non-depressed pop.: ↓ P cortisol (r2 = -0.29, p < 0.01) Incr. fat mass and percent Total pop.: ↓ P cortisol (r2 = -0.10, p < 0.05) Non-depressed pop.: ↓ P cortisol (r2 = -0.29, p < 0.01; r2 = -0.26, p < 0.01, respectively) No effect Depressed pop.: obesity on P cortisol No assoc Total pop.: BMI with U and P cortisol Depressed pop.: BMI and fat with P cortisol |
4 | EC |
|
Hamza et al (2019) [73] |
Iraq |
Prg.c 20 (8d) |
LP 24.0 ± 3.8 OP 27.6 ± 3.9 |
Overweight /obesity > 25.0 Normal ≤ 24.9 |
Betatrophin | n/a |
Overweight/obesity vs normal weight ↓ Betatrophin (Mean: 2.5 vs 6.1 ng/ml, p < 0.001) |
4 | EC |
|
Zeron et al (2013) [74] |
Mexico | 42 (21) |
Overweight/obesity 28.0 Normal 21.0 |
Overweight /obesity ≥ 25.0 Normal < 25.0 |
Adiponectin and leptin | S |
Overweight/obesity vs normal weight ↑ Leptin (Median (IQR): 18.3 (11.0–24.9) vs 9.6 (2.3–13.3) ng/ml, p < 0.001) ↓ Adiponectin/leptin (Median (IQR): 4.7 (3.1–7.1) vs 12.0 (4.1–46.2), p < 0.05) No effect Obesity on adiponectin |
4 | EC |
|
Beneventi et al (2019) [35] |
Italy | 82 (35d) |
Overweight/obesity 31.0–36.0 Controls 29.7–38.0 |
Overweight /obesity ≥ 24.9 Normal < 24.9 |
Leptin and IL-33 | S |
Incr. BMI ↑ Leptin (rho = 0.4, p < 0.001) ↓ IL-33 (rho = -0.4, p < 0.001) |
3 | EC /Inf |
|
Nelson et al (2010) [75] |
UK | 60 | n/a | n/a | AMH | P |
Incr. BMI ↓ AMH (r = -0.33, p = 0.01) Incr. waist circumference ↓ AMH (r = -0.31, p = 0.018) |
3 | EC |
|
Shaarawy (1999) [76] |
Egypt | 11e | 31 ± 5.1 | n/a | Leptin | S |
Incr. BMI ↑ Leptin (r = 0.89, p < 0.01) |
3 | EC |
|
Suto et al. (2019) [77] |
Japan | 89 (18d) |
Overweight/obesity 29.2 ± 4.3 Normal 31.5 ± 5.2 Lean 29.1 ± 6.9 |
Oveweight /obesity ≥ 25.0 Normal 18.0–25.0 Lean < 18.5 |
Adiponectin, leptin, resistin, visfatin | S |
Overweight/obesity vs normal weight ↑ Leptin, visfatin (Mean: 25.0 vs 12.0 ng/ml, p < 0.001; 1.1 vs 0.8 ng/ml, p < 0.05, respectively) ↓ Adiponectin (Mean: 8.0 vs 12.5 μg/ml, p = 0.054) No effect Obesity on resistin |
3 | EC |
|
Lassance et al (2015) [78] |
USA | 33 (16) |
Obesity 24.3 ± 4.9 Normal 25.5 ± 7.0 |
Obesity > 30.0 Normal < 25.0 |
Insulin, leptin, placental insulin regulated genes | P, Plac. T |
Obesity vs normal weight ↑ Insulin, leptin (Mean ± SD: 13.2 ± 4.9 vs 7.0 ± 5.2 μU/mL, p = 0.002; 40.8 ± 20.8 vs 16.5 ± 12.8 ng/ml, p < 0.001, respectively) Insulin treated cells: ↓ genes regulated by insulin (-30x, 87 vs 2875) Untreated cells: 1342 genes differentially expressed (90% down expressed) |
2 | EC |
|
Jaaskelainen et al (2019) [79] |
Finland | 2510 | n/a |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal < 25.0 |
CRP | S |
Obesity vs normal weight ↑ CRP (Mean ± SD: 9.2 ± 7.3 mg/l vs 4.2 ± 7.8 mg/l, p < 0.001) BMI Assoc with CRP in PE and non-PE (R2 = 0.2, p < 0.001) |
9 | Inf |
|
Scholing et al (2018) [80] |
NL | 4243 (211) | 30.9 ± 4.9 |
Obesity ≥ 30.0 Normal 18.5–24.9 |
CRP, folate, vitamin B12 | S |
Obesity vs normal weight ↑ CRP (Median (IQR): 8.2 (5.0–14.4) vs 2.8 (1.2–5.2) mg/l, p < 0.001) ↓ Folate, vitamin B12 (β = -2.3, p < 0.01; -15%, p < 0.001, respectively) ↑ Folate def., vitamin B12 def. (OR = 2.0, p < 0.01; OR = 2.1, p < 0.001, respectively) |
8 | Inf /1-CM |
|
Bodnar et al (2005) [81] |
USA | 220 (36) |
PE 25.3 ± 6.1 Controls 25.4 ± 6.1 |
Obesity ≥ 30.0 Normal 18.5–24.9 Underweight < 18.5 |
CRP | S |
Obesity vs normal weight ↑ CRP (Mean: 0.7 vs 0.3 mg/dl, p < 0.05) BMI ↑ 5 units assoc with ↑ 46% CRP (95% CI 33, 61) |
7 | Inf |
|
Maguire et al (2015) [82] |
Ireland | 146 (22) | 18.6–43.7 | n/a | CRP | S |
Incr. BMI ↑ CRP (R2 = 0.1, p < 0.001) |
5 | Inf |
|
Polari et al (2018) [83] |
Finland | 78 (9) | n/a |
Obesity > 30.0 Overweight 25.0–30.0 Normal 18.5–24.9 |
MCP-1, IL-1β, IL-10 | S |
Obesity vs normal weight ↑ MCP-1 (+ 42%, p < 0.001) No effect Obesity on IL-10, IL-1β |
5 | Inf |
|
Sutton et al (2018) [84] |
USA | 43 (29) | 29.0 ± 5.0 |
Obesity ≥ 30.0 Overweight 25.0–29.9 |
FGF21 | S |
Incr. BMI ↑ FGF21 (rho = 0.5, p = 0.008) Incr. fat mass ↑ FGF21 (rho = 0.5, p = 0.01) |
5 | Inf |
|
Wolf et al (2001) [85] |
USA | 120 | 29.5 | n/a | CRP | S |
Incr. BMI ↑ CRP (r = 0.4, p < 0.01) |
3 | Inf |
|
Christian et al (2014) [86] |
USA | 57 (24) |
Obesity 24.3 ± 3.7 Overweight 24.1 ± 3.5 Normal 24.3 ± 3.7 |
Obesity ≥ 30.0 Overweight 25.0–29.9 Normal 18.5–24.9 |
CRP, IL-1β, IL-6, IL-8, TNF-α | S |
Obesity vs normal weight ↑ IL-6 (Mean: 0.3 vs 0.1 log pg/ml, p < 0.04) No effect Obesity on CRP (p ≥ 0.05), IL-1β, IL-8, TNF-α |
3 | Inf |
|
Shhaeat et al (2019) [87] |
Iraq | 60 | 25–35 |
Obesity > 30.0 Healthy n/a |
CRP | S |
Obesity vs healthy weight ↑ CRP (Mean ± SD: 8.7 ± 0.2 vs 5.2 ± 0.1 mg/l, p < 0.05) |
2 | Inf |
|
O'Malley et al (2018) [88] |
Ireland | 496 (97) | 30.7 ± 5.5 |
Obesity ≥ 30.0 Normal 18.5–24.9 |
Folate and vitamin B12 | S, P, RBC |
Obesity vs normal weight ↓ S folate, P vitamin B12 (Median (IQR): 32.0 (20.2) vs 36.2 (16.3) nmol/l, p = 0.02; 203.0 vs 208.0 pmol/l, p = 0.03, respectively) ↑ Vitamin B12 < 148 pmol/l (24.7% vs 15.3%, p = 0.037) Incr. BMI ↓ S folate, P vitamin B12 (rho = -0.1, p = 0.03; rho = -0.1, p = 0.026) No effect Obesity on RBC folate No assoc BMI with RBC folate |
5 | 1-CM |
|
Jessel et al (2020) [89] |
USA | 24 (11) |
Obesity 29.5 ± 1.5 Normal 23.3 ± 1.5 |
Obesity ≥ 30.0 Normal 18.5–24.9 |
Folate, MTHF, MVM FR-α, MVM PCFT, MVM RFC | S, Plac. T |
Obesity vs normal weight ↓ Expression of MVM FR-α, MVM RFC (-17% p = 0.037; -19%, p = 0.026, respectively) ↓ MTHF uptake (-52%, p = 0.016) No effect Obesity on S folate and MVM PCFT expression |
4 | 1-CM |
1-CM 1-carbon metabolism, AMH anti mullerian hormone, assoc association, b/w between, CRP C-reactive protein, def. deficiency, diff. difference, EC endocrine, FGF21 fibroblast growth factor 21, FR-α folate receptor α, GlycA glycoprotein acetylation, GA gestation weeks, Incr. increased, IL interleukin, Inf inflammatory, LP lean pregnant, MCP-1 monocyte chemotactic protein-1, MTHF methyl tetrahydrofolate, MVM microvillous plasma membrane, NL Netherlands, n/a not available, OP obese pregnant, P plasma, pathw pathway, PCFT proton coupled folate transporter, PE preeclemptic, Plac. T placental tissue, pop. population, Prg. pregnant, IQR interquartile range, QS quality score, RBC red blood cells, RFC reduced folate carrier, S serum, SD standard deviation, SHBG sex hormone-binding globulin, sOB-R soluble leptin receptor, Tg thyroglobulin, TNF tumor necrosis factor, TSH thyroid stimulating hormone, U urine
a oocyte age
b N total population: 40 (healthy) and 16 (GDM)
c N total population: 59 (all three trimesters) and 22 (non-pregnant)
d Overweight/obesity
e N total population: 52 (all three trimesters) and 30 (non-pregnant women)
(↑) Higher
(↓) Lower
Adipokines
The studied adipokines included leptin, adiponectin, visfatin and resistin and they were analyzed in seventeen different studies (cohort N = 11, cross-sectional N = 4, case–control N = 2) [58, 62, 65–68, 71, 74, 76–78].
Leptin
Leptin was analyzed in sixteen different studies (mean ErasmusAGE score 4.1 out of 10.0), three of high quality and thirteen of low quality.
Preconception: Higher levels of follicular fluid and serum leptin were observed in obese and overweight/obese women compared to women of normal weight in two studies [42, 50]. Additionally, BMI was positively correlated with follicular fluid leptin levels in three studies (i.e., higher levels of leptin with increasing BMI) [40, 45, 51].
First trimester: The levels of leptin were higher in obese (five studies) and overweight/obese (three studies) women compared to women of normal weight [58, 65, 66, 68, 71, 74, 77, 78]. Also, BMI positively correlated with maternal leptin levels in five studies [35, 58, 62, 66, 76]. Furthermore, Fattah et al. demonstrated a positive correlation between fat mass and leptin levels [62].
Adiponectin
Adiponectin was analyzed in seven different studies of low quality (mean ErasmusAGE score 3.7 out of 10.0).
Preconception: No difference in follicular fluid adiponectin levels was observed between obese and non-obese women in one study; another study reported no correlation with BMI [50, 51].
First trimester: Obese women exhibited lower levels of adiponectin compared to women of normal weight in three studies [65–67]. BMI was also negatively correlated with adiponectin levels in one study (i.e., lower levels of adiponectin with increasing BMI) [66]. One study showed that adiponectin levels in overweight/obese women were lower compared to women of normal weight, while no difference was observed in another study [74, 77].
Visfatin and resistin
Visfatin and resistin were analyzed in two different studies of low quality (mean ErasmusAGE score 2.5 out of 10.0).
Preconception: In one study the effect of obesity and BMI on resistin and visfatin levels was investigated, but no difference or correlation was found [50].
First trimester: Suto et al. reported higher levels of visfatin in overweight/obese women compared to women of normal weight, whereas no difference was observed in resistin levels [77].
Thyroid biomarkers
Biomarkers of the thyroid function included free T4 (FT4), thyroid stimulating hormone (TSH), total T4 (TT4) and thyroglobulin (Tg). These were analyzed in four different studies (cohort N = 2, cross-sectional N = 2) [55–57, 61].
FT4
FT4 was analyzed in four different studies of high quality (mean ErasmusAGE score 7.3 out of 10.0).
First trimester: In three different studies, FT4 levels were lower in obese compared to non-obese women [55–57]. In three further studies, BMI was negatively associated with FT4 levels (i.e., lower levels of FT4 with increasing BMI) [56, 57, 61]. Han et al. illustrated this effect by showing that each 1 kg/m2 increase in BMI was associated with a 0.12 pmol/l lower FT4 levels [55].
TSH
TSH was analyzed in four different studies of high quality (mean ErasmusAGE score 7.3 out of 10.0).
First trimester: TSH levels were elevated in obese compared to non-obese women in three studies [56, 57]. Also, Han et al. showed an association between obesity and increased odds for higher TSH levels [55]. A positive association between BMI and TSH levels was shown in one study, whereas no association was established in two other studies [56, 57, 61].
Tg and TT4
First trimester: BMI was positively associated with Tg concentrations in one study (ErasmusAGE score 6.0 out of 10.0), whereas lower TT4 levels were observed with increasing BMI class in another study (ErasmusAGE score 8.0 out of 10.0) [56, 61].
Steroids
Steroid analyses were reported in ten studies and included estrogen, progesterone, androestardione, testosterone, and cortisol (cohort N = 6, cross-sectional N = 2, case–control N = 2) [41–43, 46, 48, 49, 59, 64, 67, 72].
Estrogen
Estrogen was analyzed in six different studies of low quality (mean ErasmusAGE score 3.3 out of 10.0).
Preconception: No difference was observed in either serum or follicular fluid levels of estradiol between obese women compared to women of normal weight in five studies, with an exception of a subgroup of women aged < 35 years that showed that lower levels were associated with obesity in one study [42, 43, 46, 48, 49]. Additionally, one study showed a negative correlation between BMI and serum estradiol levels [43].
First trimester: In one study, no difference was observed in serum estradiol and estrone levels between obese women compared to women of normal weight [67].
Progesterone
Progesterone was analyzed in five different studies (mean ErasmusAGE score 5.0 out of 10.0), one of high quality and four of low quality.
Preconception: In two different studies, obese women undergoing assisted reproductive technology (ART) treatment had lower progesterone levels compared to women of normal weight, whereas in one study, no difference was observed in obese women compared to women of normal weight in natural cycle pregnancies, nor in women aged > 35 years [41, 43].
First trimester: Obese women had lower progesterone levels compared to women of normal weight in two studies [64, 67]. This difference was evident in the total studied population and in pregnancies with male fetuses; as well as by association between obesity and higher odds for lower progesterone levels [64, 67]. Also, a negative association was observed between BMI and progesterone levels in two studies [59, 64].
Androstenedione, testosterone, cortisol
Testosterone and androstenedione were analyzed in two different studies of low quality (mean ErasmusAGE score 3.5 out of 10.0), whereas cortisol was analyzed once (ErasmusAGE score 4.0 out of 10.0).
Preconception: No difference in testosterone and androstenedione levels was observed between obese women and women of normal weight in one study [48].
First trimester: One study showed that the levels of testosterone and the free androgen index were higher in obese women compared to women of normal weight in the total study population, and in pregnancies with male fetuses; whereas no difference was observed in androstenedione levels [67]. In a group of depressed and non-depressed women, plasma cortisol levels were negatively associated with fat mass and fat percentage, while no association was evident with BMI [72]. Further subgroup analysis showed that plasma cortisol levels were lower in obese compared to non-obese women (but only for those that were non-depressed), and levels of cortisol associated negatively with BMI, fat mass and fat percentage [72].
Gonadotropins
Gonadotropins were analyzed in nine different studies and included follicular stimulating hormone (FSH), luteinizing hormone (LH) and human chorionic gonadotropin (hCG) (cohort N = 3, cross-sectional N = 5, case–control N = 1) [42, 43, 46–49, 52, 60, 63].
FSH and LH
FSH and LH were analyzed in four different studies of low quality (mean ErasmusAGE score 2.5 and 3.0 out of 10.0, respectively).
Preconception: Apart from one study that revealed lower levels of serum FSH in obese compared to non-obese women, no differences were observed in four studies in either serum or follicular fluid FSH levels [42, 48, 49, 52]. Similarly, only one study with a subgroup of women aged < 35 years, reported lower levels of serum LH in obese women compared to women of normal weight, whereas no difference was observed in women aged > 35 years. This was similar to the findings of three other studies [42, 43, 48, 52]. Finally, in one study, BMI correlated negatively with serum LH levels [43].
HCG
HCG was analyzed in four different studies (mean ErasmusAGE score 4.3 out of 10.0), one of high and three of low quality.
Preconception: Lower follicular fluid hCG levels in obese compared to non-obese women were established in one study, and plasma and follicular fluid levels correlated negatively with BMI in two further studies [46, 47].
First trimester: Serum hCG levels were lower in obese compared to non-obese women, and correlated negatively with BMI in two studies [60, 63].
Insulin
Insulin was analyzed in eleven different studies (cohort N = 9, case–control N = 1, cross-sectional N = 1; mean ErasmusAGE score 3.9 out of 10.0), predominantly of low quality (N = 10 out of 11).
Preconception: Two studies showed that serum or follicular fluid levels of insulin were higher in obese women compared to women of normal weight, whereas no difference was observed in either sources in two other studies [42, 44, 48, 50]. The increase in insulin levels was supported by a negative correlation between BMI and insulin sensitivity in one study, and increased insulin resistance in overweight/obese women compared to women of normal weight in another study [42, 44].
First trimester: Higher levels of insulin were observed in obese (four studies) and overweight/obese (one study) women compared to normal weight or overweight women [67, 69–71, 78]. HOMA-IR and HOMA2-IR scores (used to assess insulin resistance) were higher in obese women compared to normal weight or overweight women in three studies; whereas the ISHOMA score, which assesses insulin sensitivity, was lower [58, 65, 67, 70]. Also, insulin sensitivity decreased with increasing BMI in one study; while BMI was a significant explanatory factor for HOMA2-IR in another study [58, 70]. What is more, fat mass correlated positively with HOMA-IR score in one study [65]. Another study on insulin treated placental trophoblasts showed that, among obese women, genes responding to insulin (87 identified) were 30 times less abundant compared to women of normal weight (2,875 genes) [78]. Moreover, in untreated placental cells, 1,342 genes were differentially expressed between obese women compared to women of normal weight; with 90% showing down regulated expression [78].
Other endocrine biomarkers
Anti-Müllerian hormone (AMH), C-peptide and sex hormone binding globulin (SHBG) were each analyzed in two different studies of low quality (mean ErasmusAGE score 2.5, 4.5 and 3.5 out of 10.0, respectively), whereas betatrophin, glucagon, glucagon-like peptide-1 (GLP-1), ghrelin, insulin like growth factor-1 (IGF-1), and soluble leptin receptor (sOB-R) were analyzed only once (ErasmusAGE scores ≤ 5.0 out of 10).
Preconception: Serum IGF-1 and follicular fluid IGF-1, glucagon, GLP-1 and C-peptide were analyzed once and showed higher levels in obese women compared to women of normal weight [44, 50]. One other study reported lower serum SHBG levels in obese women compared to women of normal weight [48]. AMH, proAMH and ghrelin were each analyzed once and showed no difference between obese and non-obese women [48, 50].
First trimester: One study analyzed AMH and reported a negative correlation with BMI and waist circumference [75]. Also, levels of sOB-R and betatrophin were each lower in obese and overweight/obese women compared to women of normal weight, respectively [65, 73]. In contrast, obese women had higher levels of C-peptide compared to women of normal weight in one study [58]. No difference was observed in SHBG levels between obese women and women of normal weight in one study [67].
Highlight
Obesity alters several endocrine biomarkers throughout the periconceptional period. In particular, leptin and insulin levels are increased, whereas levels of adiponectin, FT4, hCG and progesterone are decreased.
Inflammatory biomarkers
It is widely accepted that obesity represents a state of chronic inflammation. The inflammatory process is a body defense mechanism against injury or infection triggered by cellular and tissue damage during which various substances are released into circulation. These represent inflammatory biomarkers. These biomarkers can be used as a measure of health status and disease progression. Tables 2 and 3 summarize associations between maternal obesity and inflammatory biomarkers for the preconception period and first trimester, respectively.
CRP
CRP was analyzed in twelve studies (cohort N = 7, case–control N = 3, cross-sectional N = 2; mean ErasmusAGE score 4.3 out of 10.0), three of high quality and nine of low quality.
Preconception: Higher levels of serum or follicular fluid CRP in obese and overweight/obese women, compared to women of normal weight, were established in three studies, whereas one study of overweight/obese women showed no difference in serum levels [42, 44, 49]. Also, a positive correlation was observed between BMI and CRP levels in two studies, in which each 1kg/m2 increase in BMI was associated with 14% increase in follicular fluid CRP [49, 51].
First trimester: CRP was shown to be higher among obese women compared to lower BMI groups in five studies [70, 79–81, 87]. However, one study reported non-significantly higher levels of CRP among obese women [86]. Also, BMI was positively correlated with serum CRP levels in five studies [70, 79, 81, 82, 85]. In the Bodnar et al. study, a 5 unit increase in BMI was associated with 46% increase in serum CRP [81].
Interleukins (ILs)
The ILs were analyzed in nine studies of low quality and included IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, IL-33, IL-1α and IL-1β (cohort N = 4, cross-sectional N = 3, case–control N = 2; mean ErasmusAGE 2.8 out of 10.0).
Preconception: Higher levels of follicular fluid IL-6 were observed in obese women compared to lower BMI groups in one study; whereas no difference was reported in the serum levels in two other studies [42, 49, 54]. BMI positively correlated with serum IL-18 levels in one study [53]. However, no effect of obesity was found on the levels of serum IL-1α, IL-1β, IL-2, IL-4, IL-8, IL-10 and IL-18, nor follicular fluid levels of IL-8 and IL-18 [49, 52, 54]. Also, BMI did not correlate with follicular fluid levels of IL-6, IL-10 or IL-18 in two studies [51, 53].
First trimester: Levels of IL-6 were higher among obese women compared to women of normal weight in one study [86]. In another study, BMI correlated negatively with IL-33 levels [35]. No significant difference was observed in serum levels of IL-1β, IL-8 and IL-10 between obese women compared to women of normal weight in any study [83, 86].
TNF-α
TNF-α was analyzed in five different studies of low quality (mean ErasmusAGE score 2.6 out of 10.0).
Preconception: No difference was observed in serum or follicular fluid TNF-α levels in obese and overweight/obese women compared to women of normal weight; and there was no correlation with BMI in three studies [42, 49, 51]. However, in one study, obesity class II women had higher TNF-α levels compared to lower BMI groups [54]. In another study, TNF-α levels correlated positively with fat percentage [42].
First trimester: No difference in TNF-α levels was observed between obese women and women of normal weight [86].
Other inflammatory biomarkers
Monocyte chemotactic factor-1 (MCP-1) was analyzed in three different studies of low quality (mean ErasmusAGE score 3.0 out of 10.0), whereas chemokine (C–C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 3 (CXCL3) and IL-34 genes, eotaxin, epidermal growth factor (EGF), fibroblast growth factor 21 (FGF21), glycoprotein acetylation A (GlycA), granulocyte macrophage-colony stimulating factor (GM-CSF), and soluble intercellular adhesion molecule-1 (sICAM-1) were analyzed only once (ErasmusAGE scores ≤ 5.0 out of 10.0).
Preconception: Ten genes involved in chemokine and cytokine pathways were differentially regulated between overweight/obese women and women of normal weight, with BMI correlating positively with CXCL3 and IL-34 gene expression [42]. One study showed higher serum and follicular fluid MCP-1 levels in obese women compared to women of normal weight and a positive correlation with BMI, whereas no correlation between follicular fluid MCP-1 and BMI was observed in another study [49, 51]. Serum levels of CCL2 were higher in overweight/obese women compared to women of normal weight, whereas no difference was observed in follicular fluid levels [42]. However, BMI correlated positively with GM-CSF levels [49]; although no difference was observed in the serum levels of GM-CSF, EGF or eotaxin between obese women compared to women of normal weight [49]. Also, BMI did not correlate with sICAM-1 levels in follicular fluid [51].
First trimester: Serum MCP-1 levels were higher by 42% in obese women compared to women of normal weight in one study [83]. Levels of GlycA were higher in obese women compared to overweight women and showed a positive association with BMI [70]. Also, FGF21 levels were positively correlated with BMI and fat mass [84].
Highlight
Obesity increases CRP levels thus exacerbating the inflammatory process across the periconceptional period.
One-carbon metabolism biomarkers
One-carbon metabolism is a sequence of interlinked metabolic cycles providing one-carbon units for biosynthetic processes fundamental for cellular function [90]. Tables 2 and 3 summarize associations between maternal obesity and one-carbon metabolism biomarkers for the preconception period and first trimester, respectively.
Folate
Folate was analyzed in three studies (cohort N = 2, case–control N = 1; mean ErasmusAGE score 5.7 out of 10.0), one of high quality and two of low quality.
First trimester: Obese women had lower serum folate levels compared to women of normal weight in two studies, and higher odds for folate deficiency in one study, whereas no difference was observed in either serum or red blood cell (RBC) in two studies [80, 88, 89]. In one study, BMI negatively associated with serum folate levels whereas no association was observed with RBC folate [88].
Vitamin B12
Vitamin B12 was analyzed in two cohorts (mean ErasmusAGE score 6.5 out of 10.0), one of high and one of low quality.
First trimester: Obese women had lower levels of vitamin B12 compared to women of normal weight in two studies, and they revealed higher odds for vitamin B12 deficiency in one study [80, 88]. Also, BMI negatively associated with vitamin B12 levels in one study [88].
Methionine
Preconception: Methionine was analyzed in one study (ErasmusAGE score 2.0 out of 10.0) and showed higher levels in obese women compared to women of normal weight; and there was a positive correlation with BMI [50].
Folate transporters
First trimester: One study analyzed placental tissue (ErasmusAGE score 4.0 out of 10.0) and reported that obese women had lower mean expression of microvillus plasma membrane (MVM) reduced folate carrier (by 19%), MVM folate receptor alpha (by 17%) and methyl tetrahydrofolate uptake (by 52%) compared to women of normal weight [89]. However, no difference in expression levels were observed for MVM proton coupled folate transporter between obese women and women of normal weight [89].
Highlight
Obesity reduces folate and vitamin B12 levels in the first trimester of pregnancy which interferes with one-carbon metabolic pathways.
Discussion
This systematic review addressed the impact of maternal obesity on several biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways during the periconceptional period, extended to cover the first trimester of pregnancy. Outcomes were presented as a function of obesity, combined overweight/obesity or BMI. Throughout the periconceptional period, obesity was associated with a variety of biomarkers of the endocrine, inflammatory, as well as one-carbon metabolic pathways. Dysregulation in these three pathways, as a consequence of obesity, can lead to adverse maternal, fetal and offspring health outcomes. Here we focus on biomarkers relevant to obesity and pregnancy that were analyzed in multiple studies, as well as biomarkers from single studies of high quality based on ErasmusAGE scores.
Endocrine biomarkers
Adipokines
Leptin
The present review demonstrated that levels of leptin are elevated in obese women and correlate positively with BMI at both systemic and local levels throughout the periconceptional period [35, 40, 42, 45, 50, 51, 62, 65, 66, 68, 71, 74, 76–78]. The results are consistent with other studies, confirming the effect of obesity on increasing leptin levels [91, 92]. Moreover, levels of leptin in follicular fluid are also positively correlated with serum levels in pregnant and non-pregnant women, suggesting a connection between serum and follicular fluid levels [45]. Leptin is directly secreted from the white adipose tissue and its levels are proportional to the level of adiposity [93, 94]. Circulating leptin acts on the brain to regulate energy homeostasis via its actions on satiety [95]. Leptin transport across the blood brain barrier is decreased and endoplasmic reticulum stress is increased in obese individuals, which may contribute to the development of leptin resistance, a pathological condition that induces hyperleptinemia [95]. Weight loss is associated with reduced adiposity, therefore decreasing leptin levels. This was demonstrated by a recent meta-analysis that showed that Orlistat use (a weight loss drug) was effective in decreasing leptin levels [96].
Adiponectin
Obesity increases CRP levels and is linked to mitochondrial dysfunction in adipose tissue leading to decreased adiponectin synthesis [97–99]. Moreover, obesity increases the expression of caveolin-1, a major component of the caveolae (small membrane invaginations), which attenuates leptin-dependent adiponectin secretion [100, 101]. This is consistent with results from the first trimester when obesity was related to reduced adiponectin levels; which would hinder its anti-inflammatory effect [65–67, 102]. Consistent with our findings, two previous studies demonstrated that obese women have low circulating adiponectin levels compared to non-obese women [103, 104]. Moreover, a decrease in body weight during a weight loss program was associated with increased serum adiponectin levels in overweight/obese women [105]. On the other hand, adiponectin concentrations in follicular fluid were not altered by obesity preconceptionally and were not related to serum levels. This suggests that regulatory mechanisms controlling adiponectin concentrations are different at systemic and local levels [106, 107].
Thyroid biomarkers
Thyroid hormones are involved in regulating body metabolism, yet it is unclear whether altered thyroid function is a cause or consequence of obesity [108]. In this review, obese women exhibited reduced FT4 levels; whereas levels of TSH were increased during first trimester [55–57, 61]. Similarly, TSH and FT4 levels in pregnant women were influenced by maternal weight [109]. Placental secretion of hCG contributes to increased FT4 levels in normal pregnancies whereas, in obese women, levels of hCG are decreased which diminishes the thyrotropic effect of hCG [110]. Subsequently, low FT4 levels reduce the negative feedback of FT4 on TSH secretion [111]. Moreover, higher Tg antibody positivity and iodine deficiency, associated with obesity, might contribute to the lower TT4 levels reported [55, 56]. However, no association between BMI and TSH levels at a mean of 16.6 weeks gestational age was observed [113]. This suggests that other factors, such as gestational age and iodine status, can influence the association between adiposity and thyroid hormone levels.
Steroids and gonadotropins
Estrogen, LH, FSH
Obesity did not alter estradiol and LH levels except in an Asian population of younger women [42, 43, 46, 48, 49, 52]. Results are consistent with other studies demonstrating the absence of effect [114, 115]. However, the effect of ethnicity requires further exploration [116]. Because estrogen was not influenced by obesity, its effect on FSH may be similar to that observed in women of normal weight, which was reported in the majority of studies analyzing FSH [42, 48, 52, 117]. Nevertheless, Buyuk et al. reported lower FSH levels with no alteration in estradiol [49]. However, the small sample size beholds the possibility of bias. Also, the purpose of ART treatment is to provide optimal conditions for successful ovulation, which might explain the absence of difference in sex hormones measured after a short time of hormonal treatment.
Progesterone
A negative relationship between obesity and progesterone levels was demonstrated during the first trimester which can in part be explained by the effect of leptin on reducing progesterone secretion [59, 64, 67, 118]. Also, lower hCG levels associated with obesity might contribute to low progesterone levels, as hCG supports the production of progesterone by the corpus luteum [119]. Lower progesterone levels were present in obese pregnant women with a male but not a female fetus [67]. Likewise, the association between progesterone levels and fetal steroid profile, timing of delivery and birth weight were also dependent on fetal sex [59, 120, 121]. During the preconception period, the effect of obesity on progesterone levels was only established in sub-fertile women undergoing ART treatment and in younger women, which suggests an additional effect of age and mode of conception on progesterone levels [41, 43].
HCG
HCG is secreted from trophoblasts, used for pregnancy testing and required for the maintenance of pregnancy [122]. During the preconception period, hCG is detectable in women undergoing ART or can originate from tumors [123, 124]. The levels of hCG in the periconceptional period, as a result of exogenous hCG treatment or pregnancy, were decreased in obese women and associated negatively with BMI [46, 47, 60, 63]. The pathophysiology of this relationship is unclear, but results are consistent with previous studies, supporting this negative relationship [125, 126]. By way of illustration, when obese women are injected with hCG, they have lower Cmax and hCG compared to women of normal weight [127].
Insulin
Metabolic alterations during pregnancy can lead to decreased insulin sensitivity which might be exacerbated in obese women [128, 129]. The associated increase in insulin levels and in insulin resistance with obesity was reported during the first trimester of pregnancy; observations comparable to other studies, emphasizing the risk of hyperinsulinemia among obese women [58, 65, 67, 69–71, 78, 130, 131]. During the preconception period, two out of four studies did not show an effect of obesity on insulin levels, however, they were of small sample size and did not adjust for any confounders [42, 48]. The mechanisms behind the impact of obesity on insulin levels can be related to a decrease in total and high affinity insulin receptors, thus requiring increased insulin secretion [132]. Moreover, CRP impairs insulin receptor substrate 1 (IRS-1) which might explain the state of insulin resistance in obesity due to elevated CRP levels [133].
Other endocrine biomarkers
Visfatin and resistin were analyzed only once at each time period and from different sources, with inconsistent outcomes, thus a relationship cannot be established, especially since the involved outcomes were from low quality score studies [50, 77]. Increased testosterone levels in the first trimester might be due to the increased expression of 17beta-hydroxysteroid dehydrogenase type 5 (involved in the conversion of androstenedione to testosterone) in obese individuals [134, 135]. Yet, no change in testosterone levels were reported for the preconception period, which suggests a possible role of pregnancy in modifying biomarker levels in obese women [134, 135]. However, outcomes were from small sample sizes, thus lacking power to establish clear relationships. The relationship between cortisol and obesity was dependent on emotional state demonstrating a potential role of stress on cortisol levels, which is consistent with other findings [72, 136–138]. Outcomes on the effect of obesity on AMH levels were from low quality score studies, thus they lacked power to establish a clear relationship with obesity. However, lower levels were previously reported in obese women [139, 140].
Inflammatory biomarkers
CRP
Obesity is a chronic inflammatory state associated with elevated CRP levels, a recognized marker of systemic inflammation. This was evident in the present systematic review throughout the periconceptional period [70, 79–82, 85–87]. Outcomes are in line with a previous meta-analysis that showed an association between BMI and CRP levels in women [141]. Moreover, increased CRP levels were also reported in follicular fluid suggesting the activation of an inflammatory cascade in follicles of obese women [42, 44, 51]. This effect can be related to the role of adipose tissue inducing CRP gene expression in obese individuals [142]. For example, genes involved in chemokine and cytokine pathways were differentially expressed in the obese state [42]. Moreover, adipose tissue secretes IL-6, which can be elevated in obese individuals, promoting the release of CRP [54, 86, 143, 144].
Interleukins
There was no effects of obesity on the majority of interleukins studied during the periconceptional period [42, 49–54, 83, 86]. Outcomes for IL-6 were inconsistent, where two studies showed no effect preconceptionally, while one study reported elevated levels in obese individuals during the preconception period and first trimester [42, 49, 54, 86]. Increased serum IL-6 during the first trimester can be explained by the increased expression of IL-6 and IL-6 receptor in adipose tissue of obese women, and the effect of leptin in inducing IL-6 secretion by trophoblasts [118, 143]. Considering ILs, all studies were of low quality, thus limiting confidence in the effects of obesity, especially as the results contradict other studies in humans and rodents reporting a relationship with obesity [145–147].
TNF-α
TNF-α is implicated in the state of inflammation and metabolic complications associated with obesity [148]. From this review, the association between obesity and TNF-α levels during the periconceptional period remains controversial, with the majority of studies not showing a relationship [42, 49, 51, 86, 149]. Despite an absence of differences in TNF-α levels between overweight/obese and normal weight women, TNF-α levels correlated positively with fat mass which is a better indicator for adiposity [42]. Moreover, these studies were of low quality and those showing no relationship contradict others that showed increased levels, and adipose tissue expression, of TNF-α in obese individuals [42, 49, 51, 86, 150, 151].
Other inflammatory biomarkers
MCP-1 levels were elevated in obese women during the periconceptional period [49, 83]. Outcomes were from low quality score studies but comparable to others showing induced overexpression and levels of MCP-1 in obese individuals, suggesting an effect of obesity during the periconceptional period [152, 153]. In a mouse model, obesity was proposed to be a FGF21- resistant condition which might explain why its positive association with BMI was reported in the first trimester [84, 154]. Other inflammatory biomarkers such as CCL2, GM-CSF, EGF, sICAM-1, eotaxin and GlycA were analyzed once in low quality score studies, thus making it difficult to establish clear relationships.
One-carbon metabolism biomarkers
Obesity was associated with lower vitamin B12 levels during the first trimester, whereas associations between obesity and folate levels were inconsistent; two studies showed decreased levels whereas one study, with a small sample size, demonstrated no association [80, 88, 89]. Low levels of folate and vitamin B12 perturb the functioning of one-carbon metabolism impairing DNA synthesis and chromatin methylation [4]. Previous studies reported a relationship between obesity and low folate and vitamin B12 levels; however, the cause-consequence relationship remains controversial [155, 156]. Inadequate dietary intake, altered distribution and absorption of micronutrients may contribute to the effect of obesity on lowering serum folate and vitamin B12 levels [157, 158]. On the other hand, folate deficiency and low vitamin B12 levels induce adipogenesis, which is attenuated with increased vitamin B12 and folate levels [159–161].
Obesity was associated with increased methionine levels in one low quality score study which had a small sample size [50]. This is not consistent with what would be expected due to the effect of obesity on reducing folate and vitamin B12 levels, both acting as cofactors in the remethylation of homocysteine to methionine, thus reducing methionine levels [4, 162].
The endocrine, inflammatory and one-carbon metabolic axes
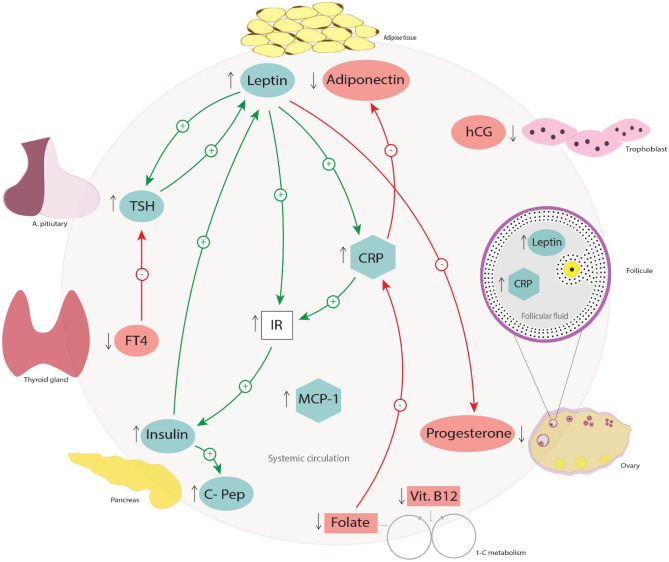
Figure 3 illustrates the possible connections between the major biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways affected by obesity during the periconceptional period based on human and animal studies. As leptin is a potential biomarker of obesity, an interplay between leptin and other biomarkers can be considered. In trophoblast cells, the secretion of hCG and IL-6 was stimulated with the treatment and secretion of leptin [118, 163]. In turn, hCG has a stimulatory effect on leptin secretion and expression via the mitogen-activated protein kinase (MAPK) pathway [164, 165]. Yet, reduced levels were observed in obese individuals. In mice, obesity activates the MAPK pathway, suggesting that other biomarkers might also be involved through this pathway; stimulating leptin expression independent of hCG levels [165, 166]. In the obese state, leptin is elevated and this reduces insulin responsiveness and progesterone secretion [118, 167]. At the same time, insulin and cortisol promote the production of leptin in human adipocytes, indicating the possibility of crosstalk among biomarkers within the endocrine pathway of obese individuals [168]. Subsequently, elevated levels of C-peptide are related to elevated insulin levels as demonstrated in the present systematic review [50, 58]. The cause-consequence link between obesity and thyroid dysfunction is controversial. Low FT4 levels reduce negative feedback inhibition on TSH secretion. TSH stimulates leptin secretion, and hypothyroidism is linked to increased weight gain [169, 170]. Simultaneously, leptin induces TSH by stimulating expression of thyroid-releasing hormone [171]. In addition, leptin influences the inflammatory pathway through its actions on CRP [172]. On the other hand, CRP impairs IRS-1 and a positive association was found between insulin and CRP [133, 173]. Moreover, co-incubation of adipocytes with CRP reduced adiponectin gene expression [99]. On the other hand, a reduction in CRP levels is observed with folic acid supplement use, as well as improvement in adiponectin levels and insulin resistance with vitamin B12 supplement use [174–176].
Fig. 3.
Major biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways affected by maternal obesity, and possible connections throughout the periconceptional period based on human and animal studies. Levels of leptin, TSH, insulin, C-pep, MCP-1 and CRP are increased (↑) in obese women, whereas the levels of adiponectin, hCG, progesterone, vitamin B12, folate and FT4 are decreased (↓). FT4 inhibits (-) TSH secretion. TSH promotes (+) leptin secretion. Leptin promotes (+) TSH and CRP and inhibits (-) progesterone secretion. CRP and leptin increase (+) IR. IR increases (+) insulin levels. Insulin promotes (+) leptin secretion and increases (+) C-pep production. CRP inhibits (-) adiponectin production. Folate decreases (-) CRP production. Abbreviations: CRP, C-reactive protein; C-pep, C-peptide; FT4, free T4; IR; insulin resistance; MCP-1, monocyte chemoattractant protein-1; TSH, thyroid stimulating hormone; vit.; vitamin; 1-C, one-carbon
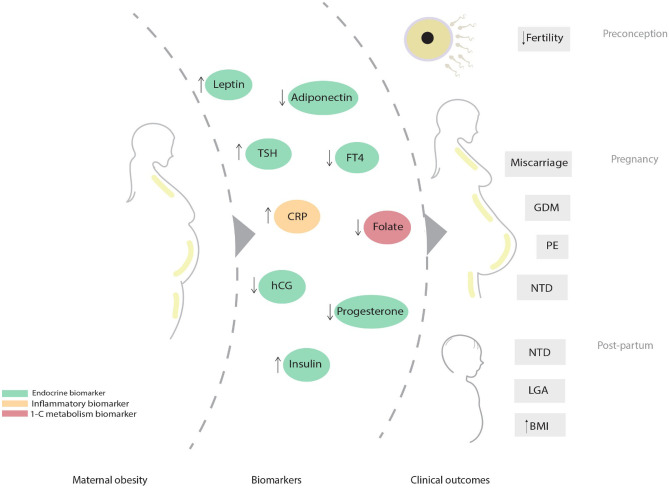
Dysregulation in endocrine, inflammatory and one-carbon metabolism biomarkers and clinical outcomes
Dysregulation in biomarker levels of the endocrine, inflammatory, and one-carbon metabolic pathways, as a result of maternal obesity, may impose adverse clinical outcomes related to fertility, pregnancy, and offspring health. Figure 4 illustrates the relationship between biomarkers of these three pathways and clinical outcomes. Obese women are at increased risk of subfertility which can be related to lower progesterone and increased leptin levels [40, 177]. Leptin can modulate reproductive function by affecting ovarian folliculogenesis and ovulation, and by perturbing the hypothalamic-pituitary-gonadal axis, such as by lowering progesterone and gonadotropin-releasing hormone levels [178, 179]. Subsequently, decreased progesterone levels can lead to changes in the endometrium physiology adversely affecting fertility, implantation and the maintenance of pregnancy [180]. Besides low progesterone levels, maternal obesity was also associated with lower HCG and higher TSH levels which can partly explain the increased risk of pregnancy loss among this population [181–187]. Low levels of HCG affect the uterine vasculature and placentation, whereas TSH is involved in endometrial physiology [188, 189]. During pregnancy, obese women showed increased insulin resistance, increased leptin and CRP levels, and decreased FT4 and adiponectin levels, which are associated with increased risk of cardio-metabolic pregnancy complications such as GDM and preeclampsia [34–37, 54, 81, 85, 173, 190, 191]. Endothelial dysfunction can be promoted by leptin, FT4, insulin and CRP while adiponectin improves endothelial dysfunction by inducing the production of nitric oxide [192–196]. Furthermore, leptin and CRP increase insulin resistance whereas adiponectin improves insulin sensitivity; primary features of GDM.
Fig. 4.
Hypothesized connection between maternal obesity, biomarkers of the endocrine, inflammatory and one-carbon metabolic pathways, and important clinical outcomes. Abbreviations: 1-C, one-carbon; BMI, body mass index; CRP, C-reactive protein; FT4, free T4; GDM, gestational diabetes mellitus; hCG, human chorionic gonadotropin; LGA, large for gestational age; NTD, neural tube defect; PE, preeclampsia; TSH, thyroid stimulating hormone. (↑) Increased levels. (↓) Decreased levels
In terms of offspring health, high leptin levels are associated with increased infant BMI [197]. Similarly, increased insulin levels (mainly due to insulin resistance) and decreased adiponectin levels are correlated with increased risk of macrosomia and LGA newborns [191, 198–200]. Alterations in these biomarkers can influence placental nutrient transport, leading to a larger baby [201, 202]. Furthermore, placental and embryonic cerebellar growth can be influenced by the perturbations in one-carbon metabolism during the periconceptional period [203, 204]. Both folate and vitamin B12 deficiency can increase the risk of neural tube defects. Despite folic acid supplement use, obese women have an elevated risk of giving rise to children with neural tube defects, which can partly be explained by the lower expression of certain MVM placental folate transporters [89, 205].
Strengths and limitations
This systematic review included a study population of obese women from different regions of the world supporting the general applicability of outcomes. Results are presented separately for associations between obesity, BMI or overweight/obesity and biomarkers; and between pre- and post-conception periods, permitting longitudinal interpretations and providing a clearer distinction of the origin of outcomes observed.
Limitations to be considered are, firstly, half of the studies (N = 25, 49%) did not report the tool for anthropometrics screening. Also, eight studies (14%) reported obesity based on self-reported anthropometrics. Though this tool is widely used in studies, the risk of self-reporting bias cannot be excluded [206, 207]. A second limitation relates to heterogeneity in time of reporting and measurement of body weight. Thus, outcomes were based on different BMI time-point measurements, although all were within the periconceptional period. Moreover, there was heterogeneity in the obese and control strata. For example, some studies included groups of combined overweight/obese women, which may interfere with interpreting the results solely attributable to obesity. With respect to the control group, some studies considered controls as non-obese or low BMI with BMI ranges transcending the known classifications, or they only had an overweight control group. Thirdly, several biomarkers analyzed for their relation to obesity or BMI were determined only on one occasion, thus making it difficult to interpret any dynamic effect. Also, some biomarkers were analyzed in low quality score studies, and/or in a small population, increasing the risk of a type II error. However, most observations were interpreted with support from high quality score studies for the same biomarker. Fourthly, the majority of included studies were not adjusted for covariates which are known to influence biomarker levels; particularly age and lifestyle factors [208, 209]. Finally, publication bias was not formally assessed, for example by using a funnel plot test, due to the different type of outcomes and few number of studies that analyzed each biomarker (N = 20, 46% of biomarkers were analyzed in one study; N = 15, 35% of biomarkers were analyzed in two to four studies; N = 5, 12% of biomarkers were analyzed in five to nine studies; N = 3, 7% of biomarkers were analyzed in at least ten studies).
Implications for clinical practice and future research
This review suggests that maternal obesity is associated with altered periconceptional biomarker levels of the endocrine, inflammatory and one-carbon metabolic pathways which can affect fertilization, pregnancy, maternal and offspring health. The majority of these biomarkers were analyzed in clinical settings (i.e., by means of blood or follicular fluid sampling). Therefore, implementation into clinical practice could be considered appropriate for routine laboratory testing, as an early screening tool to identify and monitor the obese population at risk preconceptionally and shortly after conception, so within the periconceptional period. For example, analyzing and monitoring levels of leptin, adiponectin, FT4, insulin and CRP could assist in early identification of women at risk for preeclampsia or development of GDM. Identification of a high-risk population will allow for anticipatory obstetrical management including counseling for healthier lifestyle behaviour and more antenatal appointments. Besides their predictive value, these biomarkers can be also used for more personalized and effective management of adverse associated health risks later in life [210–212]. Of foremost importance, findings of this review support the known importance of weight loss to counteract the detrimental effects caused by obesity. This can be complemented by monitoring the reported biomarker levels. Furthermore, patient-tailored interventions (particularly those targeting lifestyle) to optimize health and biomarker levels among obese women, in general and more specifically the ones wanting to conceive, are recommended. For example, a change in dietary patterns and physical training were effective in reducing leptin, insulin and CRP levels, and increasing adiponectin levels in obese adults [213–215].
In light of these findings, additional research to identify the predictive value of the most promising biomarkers, particularly leptin, adiponectin, hCG, insulin, progesterone and CRP is recommended. Also, we encourage separate studies with obese and overweight individuals, and to have a normal BMI control group identified based on previously established criteria.
Conclusion
Findings from this systematic review reveal that maternal obesity can alter levels of several biomarkers throughout the periconceptional period associated with disruption of the endocrine, inflammatory and one-carbon metabolic pathways. Importantly, maternal obesity was associated with higher leptin, insulin, TSH and CRP levels, and lower adiponectin, progesterone, FT4, hCG, folate and vitamin B12 levels. These biomarkers help to identify possible underlying pathophysiological mechanisms leading to adverse clinical outcomes. While the measurement of biomarkers is an applicable tool to potentially predict the risk of future adverse health outcomes, their clinical usefulness is still limited. Additional research on the predictive value of the optimal set of biomarkers is warranted for their use in clinical settings. Based on the current analysis, biomarkers of most interest include leptin, adiponectin, hCG, insulin, progesterone and CRP.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Wichor M. Bramer from the Medical Library Department of the Erasmus MC, for his assistance in the literature search and assessment strategy.
Abbreviations
- AMH
Anti-Müllerian hormone
- ART
Assisted reproductive technology
- BMI
Body mass index
- CCL2
Chemokine (C–C motif) ligand 2
- CRP
C-reactive protein
- CXCL3
Chemokine (C-X-C motif) ligand 3
- EGF
Epidermal growth factor
- FGF21
Fibroblast growth factor 21
- FSH
Follicle-stimulating hormone
- FT4
Free T4
- GDM
Gestational diabetes mellitus
- GLP-1
Glucagon-like peptide 1
- GlycA
Glycoprotein acetylation A
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- HCG
Human chorionic gonadotropin
- HOMA
Homeostatic model assessment
- HOMA-IR
HOMA for insulin resistance
- HOMA-IS
HOMA for insulin sensitivity
- IGF-1
Insulin-like growth factor 1
- IL
Interleukin
- IRS-1
Insulin receptor substrate 1
- LGA
Large for gestational age
- LH
Luteinizing hormone
- MCP-1
Monocyte chemoattractant protein-1
- MVM
Microvillus plasma membrane
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- SHBG
Sex hormone-binding globulin
- SICAM-1
Soluble intercellular adhesion molecule-1
- SOB-R
Soluble leptin receptor
- Tg
Thyroglobulin
- TNFα
Tumor necrosis factor alpha
- TSH
Thyroid stimulating hormone
- TT4
Total T4
- WHO
World Health Organization
Author contribution
Conceptualization S.S., M.R., R.S.T and B.H.; Literature search B.H.; Article selection B.H., S.S., M.R.; Data extraction B.H.; Quality assessment B.H., S.S; Validation B.H., S.S., M.R., R.S.T; Writing—Original draft B.H.; Writing—Review & Editing B.H., S.S., M.R., K.D.S, R.S.T., A.D.; Visualization B.H.; Supervision S.S., M.R. All authors approved the final version of the review.
Funding
This research leading to these results received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 812660 (DohART-NET). K.D.S. was in receipt of funding from the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/K017810/1).
Data availability
Not applicable.
Declarations
Financial interests
A.D. is founder and employee of BioTalentum Ltd. B.H., M.R., K.D.S., R.S.T and S.S. have nothing to disclose.
Conflict of interest
No conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Hleath Organization: Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020). Accessed 12 2020.
- 2.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161. 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed]
- 3.Dag ZO, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111–117. doi: 10.5152/jtgga.2015.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19(6):640–655. doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- 5.Rubini E, Baijens IMM, Horanszky A, Schoenmakers S, Sinclair KD, Zana M, et al. Maternal One-Carbon Metabolism during the Periconceptional Period and Human Foetal Brain Growth: A Systematic Review. Genes (Basel). 2021;12(10):1634. 10.3390/genes12101634. [DOI] [PMC free article] [PubMed]
- 6.Hemond J, Robbins RB, Young PC. The Effects of Maternal Obesity on Neonates, Infants, Children, Adolescents, and Adults. Clin Obstet Gynecol. 2016;59(1):216–227. doi: 10.1097/GRF.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez MA. Impacto de la sobrenutricion materna sobre el periodo de periconcepcion. Endocrinologia y Nutricion. 2015;62(5):246–253. doi: 10.1016/j.endonu.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2149;95(6):2146–9. 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed]
- 9.Christensen MW, Ingerslev HJ, Degn B, Kesmodel US. Effect of Female Body Mass Index on Oocyte Quantity in Fertility Treatments (IVF): Treatment Cycle Number Is a Possible Effect Modifier. A Register-Based Cohort Study. PLoS One [Electronic Resource]. 2016;11(9):e0163393. 10.1371/journal.pone.0163393. [DOI] [PMC free article] [PubMed]
- 10.Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol Reprod. 2005;73(5):918–926. doi: 10.1095/biolreprod.105.041483. [DOI] [PubMed] [Google Scholar]
- 11.Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122–132. doi: 10.1093/humrep/deu276. [DOI] [PubMed] [Google Scholar]
- 12.Koning IV, Dudink J, Groenenberg IAL, Willemsen SP, Reiss IKM, Steegers-Theunissen RPM. Prenatal cerebellar growth trajectories and the impact of periconceptional maternal and fetal factors. Hum Reprod. 2017;32(6):1230–1237. doi: 10.1093/humrep/dex079. [DOI] [PubMed] [Google Scholar]
- 13.Thagaard IN, Krebs L, Holm JC, Christiansen M, Moller H, Lange T, et al. The effect of obesity on early fetal growth and pregnancy duration: a cohort study. J Matern-Fetal Neo M. 2018;31(22):2941–2946. doi: 10.1080/14767058.2017.1359825. [DOI] [PubMed] [Google Scholar]
- 14.Patey O. Re: Maternal obesity affects fetal myocardial function as early as in the first trimester. C. B. Ingul, L. Loras, E. Tegnander, S. H. Eik-Nes and A. Brantberg. Ultrasound Obstet Gynecol 2016; 47: 433–442. Ultrasound Obst Gyn. 2016;47(4):415-. 10.1002/uog.15883. [DOI] [PubMed]
- 15.van Duijn L, Rousian M, Hoek J, Willemsen SP, van Marion ES, Laven JSE, et al. Higher preconceptional maternal body mass index is associated with faster early preimplantation embryonic development: the Rotterdam periconception cohort. Reprod Biol Endocrin. 2021;19(1):145. 10.1186/s12958-021-00822-0. [DOI] [PMC free article] [PubMed]
- 16.van Duijn L, Rousian M, Laven JSE, Steegers-Theunissen RPM. Periconceptional maternal body mass index and the impact on post-implantation (sex-specific) embryonic growth and morphological development. Int J Obesity. 2021;45(11):2369–2376. doi: 10.1038/s41366-021-00901-7. [DOI] [PubMed] [Google Scholar]
- 17.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9(2):140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 18.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19(7):1644–1646. doi: 10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- 19.Saadia Z. Association between maternal obesity and cesarean delivery complications. Cureus. 2020;12(3):e7163. 10.7759/cureus.7163. [DOI] [PMC free article] [PubMed]
- 20.Han YS, Ha EH, Park HS, Kim YJ, Lee SS. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. Int J Obes. 2011;35(4):570–577. doi: 10.1038/ijo.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Haroush A, Hadar E, Chen R, Hod M, Yogev Y. Maternal obesity is a major risk factor for large-for-gestational-infants in pregnancies complicated by gestational diabetes. Arch Gynecol Obstet. 2009;279(4):539–543. doi: 10.1007/s00404-008-0767-4. [DOI] [PubMed] [Google Scholar]
- 22.Hildén K, Hanson U, Persson M, Magnuson A, Simmons D, Fadl H. Gestational diabetes and adiposity are independent risk factors for perinatal outcomes: a population based cohort study in Sweden. Diabet Med. 2019;36(2):151–157. doi: 10.1111/dme.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison MS, Thorsten VR, Dudley DJ, Parker CB, Koch MA, Hogue CJR, et al. Stillbirth, Inflammatory Markers, and Obesity: Results from the Stillbirth Collaborative Research Network. Am J Perinatol. 2018;35(11):1071–1078. doi: 10.1055/s-0038-1639340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brite J, Laughon SK, Troendle J, Mills J. Maternal overweight and obesity and risk of congenital heart defects in offspring. Int J Obes. 2014;38(6):878–882. doi: 10.1038/ijo.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon DM, Liu J, Zhang H, Torres ME, Best RG. Maternal obesity, folate intake, and neural tube defects in offspring. Birth Defects Res Part A Clin Mol Teratol. 2013;97(2):115–122. doi: 10.1002/bdra.23113. [DOI] [PubMed] [Google Scholar]
- 26.Ou X, Thakali KM, Shankar K, Andres A, Badger TM. Maternal adiposity negatively influences infant brain white matter development. Obesity. 2015;23(5):1047–1054. doi: 10.1002/oby.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbenhardt C, Miller JW, Song X, Brown EC, Cheng TY, Wener MH, et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr. 2014;144(5):714–721. doi: 10.3945/jn.113.183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adaikalakoteswari A, Wood C, Mina TH, Webster C, Goljan I, Weldeselassie Y, et al. Vitamin B12 deficiency and altered one-carbon metabolites in early pregnancy is associated with maternal obesity and dyslipidaemia. Sci Rep. 2020;10(1):11066. doi: 10.1038/s41598-020-68344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahab ZJ, Semaan SM, Sang QX. Methodology and applications of disease biomarker identification in human serum. Biomark Insights. 2007;2:21–43. doi: 10.1177/117727190700200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrera TT, Garcia JL, Britton GB. Blood-based biomarkers of adverse perinatal outcomes in maternal obesity. J Matern Fetal Neonatal Med. 2017;30(24):2991–2997. doi: 10.1080/14767058.2016.1271406. [DOI] [PubMed] [Google Scholar]
- 31.Wald NJ, Law MR, Morris JK, Wald DS. Quantifying the effect of folic acid. Lancet. 2001;358(9298):2069–2073. doi: 10.1016/s0140-6736(01)07104-5. [DOI] [PubMed] [Google Scholar]
- 32.Sun B, Bertolet M, Simhan HN, Parks WT, Catov JM. Early-pregnancy inflammatory markers and preterm birth classified according to placental features. Reprod Sci (Thousand Oaks, Calif) 2018;25(1):86A–A87. [Google Scholar]
- 33.Sun B, Parks WT, Simhan HN, Bertolet M, Catov JM. Early pregnancy immune profile and preterm birth classified according to uteroplacental lesions. Placenta. 2020;89:99–106. doi: 10.1016/j.placenta.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Bozkurt L, Gobl CS, Baumgartner-Parzer S, Luger A, Pacini G, Kautzky-Willer A. Adiponectin and Leptin at Early Pregnancy: Association to Actual Glucose Disposal and Risk for GDM-A Prospective Cohort Study. Int J Endocrinol. 2018;2018:5463762. doi: 10.1155/2018/5463762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beneventi F, Locatelli E, De Amici M, Cavagnoli C, Bellingeri C, De Maggio I, et al. Maternal and fetal Leptin and interleukin 33 concentrations in pregnancy complicated by obesity and preeclampsia. J Matern -Fetal Neonatal Med. 2019 doi: 10.1080/14767058.2019.1593359. [DOI] [PubMed] [Google Scholar]
- 36.Thagaard IN, Hedley PL, Holm JC, Lange T, Larsen T, Krebs L, et al. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019;15:78–83. doi: 10.1016/j.preghy.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Wolf M, Sandler L, Hsu K, Vossen-Smirnakis K, Ecker JL, Thadhani R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26(3):819–824. doi: 10.2337/diacare.26.3.819. [DOI] [PubMed] [Google Scholar]
- 38.Project EPHP: Quality assessment tool for quantitative studies. https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/. Accessed 15 Mar 2021.
- 39.Chrousos GP. Organization and Integration of the Endocrine System. Sleep Med Clin. 2007;2(2):125–145. doi: 10.1016/j.jsmc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plowden TC, Zarek SM, Devilbiss EA, Radoc JG, Kim K, Sjaarda LA, et al. Preconception Leptin and Fecundability, Pregnancy, and Live Birth among Women with a History of Pregnancy Loss. J Endocr Soc. 2019;3(11):1958–1968. doi: 10.1210/js.2019-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady PC, Kaser DJ, Ginsburg ES, Ashby RK, Missmer SA, Correia KF, et al. Serum progesterone concentration on day of embryo transfer in donor oocyte cycles. J Assist Reprod Genet. 2014;31(5):569–575. doi: 10.1007/s10815-014-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, et al. Obesity modulates inflammation and lipid metabolism oocyte gene expression: A single-cell transcriptome perspective. J Clin Endocrinol Metab. 2017;102(6):2029–2038. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang LT, Wang CX, Sun HL, Wang X, Li XF, Wang YL, et al. Effect of BMI on blood value of patients on HCG day with IUI treatment. BMC Womens Health. 2020;20(1):105. doi: 10.1186/s12905-020-00963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, et al. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. 2012;27(12):3531–3539. doi: 10.1093/humrep/des350. [DOI] [PubMed] [Google Scholar]
- 45.Hill MJ, Uyehara CF, Hashiro GM, Frattarelli JL. The utility of serum leptin and follicular fluid leptin, estradiol, and progesterone levels during an in vitro fertilization cycle. J Assist Reprod Genet. 2007;24(5):183–188. doi: 10.1007/s10815-007-9106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrell DT, Jones KP, Peterson CM, Aoki V, Emery BR, Campbell BR. Body mass index is inversely related to intrafollicular HCG concentrations, embryo quality and IVF outcome. Reprod Biomed Online. 2001;3(2):109–111. doi: 10.1016/s1472-6483(10)61977-3. [DOI] [PubMed] [Google Scholar]
- 47.Matorras R, Meabe A, Mendoza R, Prieto B, Ramon O, Mugica J, et al. Human chorionic gonadotropin (hCG) plasma levels at oocyte retrieval and IVF outcomes. J Assist Reprod Genet. 2012;29(10):1067–1071. doi: 10.1007/s10815-012-9826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peigne M, Pigny P, Pankhurst MW, Drumez E, Loyens A, Dewailly D, et al. The proportion of cleaved anti-Mullerian hormone is higher in serum but not follicular fluid of obese women independently of polycystic ovary syndrome. Reprod Biomed Online. 2020;41(6):1112–1121. doi: 10.1016/j.rbmo.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Buyuk E, Asemota OA, Merhi Z, Charron MJ, Berger DS, Zapantis A, et al. Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: a pilot study. Fertility & Sterility. 2017;107(3):632–40 e3. 10.1016/j.fertnstert.2016.12.023. [DOI] [PubMed]
- 50.Bou Nemer L, Shi H, Carr BR, Word RA, Bukulmez O. Effect of Body Weight on Metabolic Hormones and Fatty Acid Metabolism in Follicular Fluid of Women Undergoing In Vitro Fertilization: A Pilot Study. Reprod Sci. 2019;26(3):404–411. doi: 10.1177/1933719118776787. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez MB, Lane M, Knight EJ, Robker RL. Inflammatory markers in human follicular fluid correlate with lipid levels and Body Mass Index. J Reprod Immunol. 2018;130:25–29. doi: 10.1016/j.jri.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Kilic S, Moraloglu O, Yuksel B, Ozaksit G, Tasdemir N, Dogan M. IL-18 and IL-18 binding protein concentrations in serum and ovarian follicular fluid do not affect in vitro fertilization outcomes in obese, overweight, and non-obese patients. J Reprod Immunol. 2009;79(2):211–214. doi: 10.1016/j.jri.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Gunther V, Alkatout I, Fuhs C, Salmassi A, Mettler L, Hedderich J, et al. The Role of Interleukin-18 in Serum and Follicular Fluid during In Vitro Fertilization and Intracytoplasmic Sperm Injection. Biomed Res Int. 2016;2016:6379850. doi: 10.1155/2016/6379850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Vignera S, Condorelli R, Bellanca S, La Rosa B, Mousavi A, Busa B, et al. Obesity is associated with a higher level of pro-inflammatory cytokines in follicular fluid of women undergoing medically assisted procreation (PMA) programs. Eur Rev Med Pharmacol Sci. 2011;15(3):267–273. [PubMed] [Google Scholar]
- 55.Han C, Li C, Mao J, Wang W, Xie X, Zhou W, et al. High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. BioMed Res Int. 2015;2015. 10.1155/2015/351831. [DOI] [PMC free article] [PubMed]
- 56.Mosso L, Martínez A, Rojas MP, Latorre G, Margozzini P, Lyng T, et al. Early pregnancy thyroid hormone reference ranges in Chilean women: the influence of body mass index. Clin Endocrinol. 2016;85(6):942–948. doi: 10.1111/cen.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pop VJ, Biondi B, Wijnen HA, Kuppens SM, Lvader H. Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin Endocrinol (Oxf) 2013;79(4):577–583. doi: 10.1111/cen.12177. [DOI] [PubMed] [Google Scholar]
- 58.Bandres-Meriz J, Dieberger AM, Hoch D, Pöchlauer C, Bachbauer M, Glasner A, et al. Maternal Obesity Affects the Glucose-Insulin Axis During the First Trimester of Human Pregnancy. Front Endocrinol. 2020;11. 10.3389/fendo.2020.566673. [DOI] [PMC free article] [PubMed]
- 59.Diemert A, Goletzke J, Hecher K, Arck P, Barkmann C. Maternal progesterone levels are modulated by maternal BMI and predict birth weight sex-specifically in human pregnancies. J Reprod Immunol. 2017;121:49–55. doi: 10.1016/j.jri.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Eskild A, Fedorcsak P, Morkrid L, Tanbo TG. Maternal body mass index and serum concentrations of human chorionic gonadotropin in very early pregnancy. Fertil Steril. 2012;98(4):905–910. doi: 10.1016/j.fertnstert.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Gowachirapant S, Melse-Boonstra A, Winichagoon P, Zimmermann MB. Overweight increases risk of first trimester hypothyroxinaemia in iodine-deficient pregnant women. Matern Child Nutr. 2014;10(1):61–71. doi: 10.1111/mcn.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fattah C, Barry S, O'Connor N, Farah N, Stuart B, Turner MJ. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol Endocrinol. 2011;27(4):263–266. doi: 10.3109/09513590.2010.491167. [DOI] [PubMed] [Google Scholar]
- 63.Brady PC, Farland LV, Ginsburg ES. Serum human chorionic gonadotropin among women with and without obesity after single embryo transfers. J Clin Endocrinol Metab. 2018;103(11):4209–4215. doi: 10.1210/jc.2018-01057. [DOI] [PubMed] [Google Scholar]
- 64.Goh JY, He S, Allen JC, Malhotra R, Tan TC. Maternal obesity is associated with a low serum progesterone level in early pregnancy. Horm Mol Biol Clin Invest. 2016;27(3):97–100. doi: 10.1515/hmbci-2015-0030. [DOI] [PubMed] [Google Scholar]
- 65.Andersson-Hall U, Svedin P, Svensson H, Lönn M, Mallard C, Holmäng A. Longitudinal changes in adipokines and free leptin index during and after pregnancy in women with obesity. Int J Obes. 2020;44(3):675–683. doi: 10.1038/s41366-019-0452-7. [DOI] [PubMed] [Google Scholar]
- 66.Jara A, Dreher M, Porter K, Christian LM. The association of maternal obesity and race with serum adipokines in pregnancy and postpartum: Implications for gestational weight gain and infant birth weight. Brain Behav Immun Health. 2020;3. 10.1016/j.bbih.2020.100053. [DOI] [PMC free article] [PubMed]
- 67.Maliqueo M, Cruz G, Espina C, Contreras I, García M, Echiburú B, et al. Obesity during pregnancy affects sex steroid concentrations depending on fetal gender. Int J Obes. 2017;41(11):1636–1645. doi: 10.1038/ijo.2017.159. [DOI] [PubMed] [Google Scholar]
- 68.Mendieta Zerón H, García Solorio VJ, Nava Díaz PM, Garduño Alanís A, Santillán Benítez JG, Domínguez García V, et al. Hyperleptinemia as a prognostic factor for preeclampsia: a cohort study. Acta Medica (Hradec Kralove) 2012;55(4):165–71. doi: 10.14712/18059694.2015.41. [DOI] [PubMed] [Google Scholar]
- 69.Petrella E, Pignatti L, Neri I, Facchinetti F. The l-arginine/nitric oxide pathway is impaired in overweight/obese pregnant women. Pregnancy Hypertens. 2014;4(2):150–155. doi: 10.1016/j.preghy.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Houttu N, Mokkala K, Laitinen K. Overweight and obesity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clin Nutr. 2018;37(6):1955–1966. doi: 10.1016/j.clnu.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 71.Malti N, Merzouk H, Bouhmama L, Saker M, Elhabiri M, Cherrak S. Time course of changes in leptin levels and their relationships with oxidant status biomarkers in pregnant women with obesity. J Clin Diagn Res. 2020;14(4):CC01-CC5. 10.7860/jcdr/2020/43475.13640.
- 72.Luiza JW, Gallaher MJ, Powers RW. Urinary cortisol and depression in early pregnancy: Role of adiposity and race. BMC Pregnancy Childbirth. 2015;15(1). 10.1186/s12884-015-0466-7. [DOI] [PMC free article] [PubMed]
- 73.Hamza MA, Tamer YYAL, Al-Habib OAM. Variation in circulating betatrophin/ ANGPTL8 levels in normal and overweight/obese pregnant women. J Clin Diagn Res. 2019;13(8):BC04-BC8. 10.7860/jcdr/2019/41808.13073.
- 74.Mendieta Zerón H, Garduño Alanís A, Montserrat Nava Díaz P, Guadalupe Santillán Benítez J, De La Rosa Parra JA, Conzuelo Rodríguez G. Low activity in pregnancy does not modify neither adiponectin nor leptin serum levels. Gazz Med Ital Arch Sci Med. 2013;172(10):773–9.
- 75.Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of antimüllerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2010;93(4):1356–1358. doi: 10.1016/j.fertnstert.2009.07.1676. [DOI] [PubMed] [Google Scholar]
- 76.Shaarawy M, El-Mallah SY. Leptin and gestational weight gain: Relation of maternal and cord blood leptin to birth weight. J Soc Gynecol Invest. 1999;6(2):70–73. doi: 10.1016/s1071-5576(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 77.Suto M, Maeda K, Sato M, Kaji T, Irahara M. Plasma adipokine concentrations in overweight/obese pregnant women: a longitudinal study. Gynecol Endocrinol. 2019;35(3):242–246. doi: 10.1080/09513590.2018.1512572. [DOI] [PubMed] [Google Scholar]
- 78.Lassance L, Haghiac M, Leahy P, Basu S, Minium J, Zhou J, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am J Obstet Gynecol. 2015;212(5):647.e1-.e11. 10.1016/j.ajog.2015.02.026. [DOI] [PMC free article] [PubMed]
- 79.Jääskeläinen T, Heinonen S, Hämäläinen E, Pulkki K, Romppanen J, Laivuori H, et al. Impact of obesity on angiogenic and inflammatory markers in the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort. Int J Obes. 2019;43(5):1070–1081. doi: 10.1038/s41366-018-0217-8. [DOI] [PubMed] [Google Scholar]
- 80.Scholing JM, Olthof MR, Jonker FA, Vrijkotte TG. Association between pre-pregnancy weight status and maternal micronutrient status in early pregnancy. Public Health Nutr. 2018;21(11):2046–2055. doi: 10.1017/S1368980018000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 82.Maguire PJ, Power KA, O'Higgins AC, Jackson S, Harley R, Le Roux CW, et al. Maternal C-reactive protein in early pregnancy. 2015;193:79-82. 10.1016/j.ejogrb.2015.07.005 [DOI] [PubMed]
- 83.Polari L, Kumar H, Rautava S, Salminen S, Isolauri E. Increase in serum Interleukin-10 does not alleviate pro-inflammatory MCP-1 production in obese pregnancies. Cytokine. 2018;108:67–70. doi: 10.1016/j.cyto.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 84.Sutton EF, Morrison CD, Stephens JM, Redman LM. Fibroblast growth factor 21, adiposity, and macronutrient balance in a healthy, pregnant population with overweight and obesity. Endocr Res. 2018;43(4):275–283. doi: 10.1080/07435800.2018.1473421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstet Gynecol. 2001;98(5):757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 86.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014;70(2):134–140. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shhaeat AJ, Khalifa AA. The study of malondialdehyde, uric acid, and C-reactive protein in obese and healthy pregnant women in different trimesters. Ann Trop Med Public Health. 2019;22(8). 10.36295/asro.2019.220817.
- 88.O'Malley EG, Reynolds CME, Cawley S, Woodside JV, Molloy AM, Turner MJ. Folate and vitamin B12 levels in early pregnancy and maternal obesity. 2018;231:80–4. 10.1016/j.ejogrb.2018.10.001. [DOI] [PubMed]
- 89.Jessel RH, Rosario FJ, Chen YY, Erickson K, Teal SB, Kramer A, et al. Decreased placental folate transporter expression and activity in first and second trimester in obese mothers. J nutr biochem. 2019;77:108305. doi: 10.1016/j.jnutbio.2019.108305. [DOI] [PubMed] [Google Scholar]
- 90.Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25(1):27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farooq R, Lutfullah S, Ahmed M. Serum leptin levels in obese infertile men and women. Pak J Pharm Sci. 2014;27(1):67–71. [PubMed] [Google Scholar]
- 92.Sitticharoon C, Klinjampa R, Souvannavong-Vilivong X, Chatree S, Boonpuan P, Sripong C, Nway NC, Lertbunnaphong T. Serum neuropeptide Y and leptin levels compared between non-pregnant and pregnant women in overall, non-obese, and obese subjects. Siriraj Med J. 2018;70(3):204–12. 10.14456/smj.2018.34.
- 93.Lonnqvist F, Nordfors L, Jansson M, Thorne A, Schalling M, Arner P. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J Clin Investig. 1997;99(10):2398–404. 10.1172/JCI119422. [DOI] [PMC free article] [PubMed]
- 94.Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R226–R234. doi: 10.1152/ajpregu.00392.2001. [DOI] [PubMed] [Google Scholar]
- 95.Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–51. 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed]
- 96.Derosa G, Maffioli P, Sahebkar A. Improvement of plasma adiponectin, leptin and C-reactive protein concentrations by orlistat: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81(5):819–834. doi: 10.1111/bcp.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ejarque M, Ceperuelo-Mallafre V, Serena C, Maymo-Masip E, Duran X, Diaz-Ramos A, et al. Adipose tissue mitochondrial dysfunction in human obesity is linked to a specific DNA methylation signature in adipose-derived stem cells. Int J Obes. 2019;43(6):1256–1268. doi: 10.1038/s41366-018-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56(12):2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- 99.Yuan G, Chen X, Ma Q, Qiao J, Li R, Li X, et al. C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol. 2007;194(2):275–281. doi: 10.1677/JOE-07-0133. [DOI] [PubMed] [Google Scholar]
- 100.Singh P, Sharma P, Sahakyan KR, Davison DE, Sert-Kuniyoshi FH, Romero-Corral A, et al. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int J Obes. 2016;40(2):266–274. doi: 10.1038/ijo.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parton RG. Caveolae: Structure, Function, and Relationship to Disease. Annu Rev Cell Dev Biol. 2018;34:111–136. doi: 10.1146/annurev-cellbio-100617-062737. [DOI] [PubMed] [Google Scholar]
- 102.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380(1–2):24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sitticharoon C, Souvannavong-Vilivong X, Klinjampa R, Churintaraphan M, Nway NC, Keadkraichaiwat I, Lertbunnaphong T. Serum Adiponectin, Visfatin, and Omentin Compared between Non-pregnant and Pregnant Women in Overall, Non-obese, and Obese subjects. Siriraj Med J. 2018;70(3):219-226. 10.14456/smj.2018.36.
- 104.Rolland YM, Haren MT, Patrick P, Banks WA, Malmstrom TK, Miller DK, et al. Adiponectin levels in obese and non-obese middle-aged African-American women. Obes Res Clin Pract. 2007;1(1):1–78. doi: 10.1016/j.orcp.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foula WH, Emara RH, Eldeeb MK, Mokhtar SA, El-Sahn FA. Effect of a weight loss program on serum adiponectin and insulin resistance among overweight and obese premenopausal females. J Egypt Public Heal. 2020;95(1). 10.1186/s42506-020-00060-z. [DOI] [PMC free article] [PubMed]
- 106.Bersinger NA, Wunder DM. Adiponectin isoform distribution in serum and in follicular fluid of women undergoing treatment by ICSI. Acta Obstet Gyn Scan. 2010;89(6):782–788. doi: 10.3109/00016341003657918. [DOI] [PubMed] [Google Scholar]
- 107.Mastrolia R, Lee C, Anasti JN. Adiponectin levels in follicular fluid from gonadotropin-stimulated follicles. Obstetrics and Gynecology. 2006;107(4):54s-s. 10.1097/00006250-200604001-00127.
- 108.Cordido M, Juiz-Valina P, Urones P, Sangiao-Alvarellos S, Cordido F. Thyroid function alteration in obesity and the effect of bariatric surgery. J Clin Med. 2022;11(5):1340. 10.3390/jcm11051340. [DOI] [PMC free article] [PubMed]
- 109.Bestwick JP, John R, Maina A, Guaraldo V, Joomun M, Wald NJ, et al. Thyroid stimulating hormone and free thyroxine in pregnancy: Expressing concentrations as multiples of the median (MoMs) Clin Chim Acta. 2014;430:33–37. doi: 10.1016/j.cca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 110.Hershman JM. The role of human chorionic gonadotropin as a thyroid stimulator in normal pregnancy. J Clin Endocr Metab. 2008;93(9):3305–3306. doi: 10.1210/jc.2008-1461. [DOI] [PubMed] [Google Scholar]
- 111.Pirahanchi Y, Toro F, Jialal I. Physiology, thyroid stimulating hormone. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed]
- 113.Haddow JE, Craig WY, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, et al. Impact of Adjusting for the Reciprocal Relationship Between Maternal Weight and Free Thyroxine During Early Pregnancy. Thyroid. 2013;23(2):225–230. doi: 10.1089/thy.2012.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bernasconi D, Del Monte P, Meozzi M, Randazzo M, Marugo A, Badaracco B, et al. The impact of obesity on hormonal parameters in hirsute and nonhirsute women. Metab Clin Exp. 1996;45(1):72–75. doi: 10.1016/s0026-0495(96)90202-4. [DOI] [PubMed] [Google Scholar]
- 115.Roth LW, Allshouse AA, Bradshaw-Pierce EL, Lesh J, Chosich J, Kohrt W, et al. Luteal phase dynamics of follicle-stimulating and luteinizing hormones in obese and normal weight women. Clin Endocrinol (Oxf) 2014;81(3):418–425. doi: 10.1111/cen.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paxton RJ, King DW, Garcia-Prieto C, Connors SK, Hernandez M, Gor BJ, et al. Associations between body size and serum estradiol and sex hormone-binding globulin levels in premenopausal African American women. J Clin Endocrinol Metab. 2013;98(3):E485–E490. doi: 10.1210/jc.2012-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Charlesworth MC, Schwartz NB. Estrogen inhibition of LH and FSH secretion: effects of a GnRH antagonist. Am J Physiol. 1986;250(4 Pt 1):E341–E345. doi: 10.1152/ajpendo.1986.250.4.E341. [DOI] [PubMed] [Google Scholar]
- 118.Cameo P, Bischof P, Calvo JC. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol Reprod. 2003;68(2):472–477. doi: 10.1095/biolreprod.102.006122. [DOI] [PubMed] [Google Scholar]
- 119.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cathey AL, Watkins DJ, Rosario ZY, Vega CMV, Mukherjee B, O'Neill MS, et al. Gestational Hormone Concentrations Are Associated With Timing of Delivery in a Fetal Sex-Dependent Manner. Front Endocrinol (Lausanne) 2021;12:742145. doi: 10.3389/fendo.2021.742145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siemienowicz KJ, Wang Y, Mareckova M, Nio-Kobayashi J, Fowler PA, Rae MT, et al. Early pregnancy maternal progesterone administration alters pituitary and testis function and steroid profile in male fetuses. Sci Rep. 2020;10(1):21920. doi: 10.1038/s41598-020-78976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nwabuobi C, Arlier S, Schatz F, Gu007Aeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: Biological functions and clinical applications. Int J Mol Sci. 2017;18(10):2037. 10.3390/ijms18102037. [DOI] [PMC free article] [PubMed]
- 123.Syrigos KN, Fyssas I, Konstandoulakis MM, Harrington KJ, Papadopoulos S, Milingos N, et al. Beta human chorionic gonadotropin concentrations in serum of patients with pancreatic adenocarcinoma. Gut. 1998;42(1):88–91. doi: 10.1136/gut.42.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Du YB, Yan L, Sun M, Sheng Y, Li XF, Feng ZH, et al. Effect of human chorionic gonadotropin injection before frozen embryo transfer on pregnancy outcomes in endometriosis-associated infertility. Front Med-Lausanne. 2020;14(7):592921. 10.3389/fmed.2020.592921. [DOI] [PMC free article] [PubMed]
- 125.Korevaar TI, Steegers EA, de Rijke YB, Schalekamp-Timmermans S, Visser WE, Hofman A, et al. Reference ranges and determinants of total hCG levels during pregnancy: the Generation R Study. Eur J Epidemiol. 2015;30(9):1057–1066. doi: 10.1007/s10654-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elkind-Hirsch KE, Bello S, Esparcia L, Phillips K, Sheiko A, McNichol M. Serum human chorionic gonadotropin levels are correlated with body mass index rather than route of administration in women undergoing in vitro fertilization–embryo transfer using human menopausal gonadotropin and intracytoplasmic sperm injection. Fertil Steril. 2001;75(4):700–704. doi: 10.1016/s0015-0282(00)01790-8. [DOI] [PubMed] [Google Scholar]
- 127.Shah DK, Missmer SA, Correia KF, Ginsburg ES. Pharmacokinetics of human chorionic gonadotropin injection in obese and normal-weight women. J Clin Endocrinol Metab. 2014;99(4):1314–1321. doi: 10.1210/jc.2013-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140(3):365–71. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sonagra AD, Biradar SM, K D, Murthy DSJ. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res. 2014;8(11):CC01–3. 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed]
- 130.Kim MK, Reaven GM, Kim SH. Dissecting the Relationship Between Obesity and Hyperinsulinemia: Role of Insulin Secretion and Insulin Clearance. Obesity. 2017;25(2):378–383. doi: 10.1002/oby.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in Individuals with Obesity: Role of Insulin Clearance. Obesity. 2015;23(12):2430–2434. doi: 10.1002/oby.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borissova AM, Tankova TI, Koev DJ. Insulin secretion, peripheral insulin sensitivity and insulin-receptor binding in subjects with different degrees of obesity. Diabetes Metab. 2004;30(5):425–431. doi: 10.1016/S1262-3636(07)70138-6. [DOI] [PubMed] [Google Scholar]
- 133.D'Alessandris C, Lauro R, Presta I, Sesti G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia. 2007;50(4):840–849. doi: 10.1007/s00125-006-0522-y. [DOI] [PubMed] [Google Scholar]
- 134.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity–a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183(2):331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 135.Rice S, Patel B, Bano G, Ugwumadu A, Whitehead SA. Aromatase expression in abdominal omental/visceral and subcutaneous fat depots: a comparison of pregnant and obese women. Fertil Steril. 2012;97(6):1460–6.e1. doi: 10.1016/j.fertnstert.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 136.Moyer AE, Rodin J, Grilo CM, Cummings N, Larson LM, Rebuffe-Scrive M. Stress-induced cortisol response and fat distribution in women. Obes Res. 1994;2(3):255–262. doi: 10.1002/j.1550-8528.1994.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 137.Vicennati V, Pasqui F, Cavazza C, Pagotto U, Pasquali R. Stress-related development of obesity and cortisol in women. Obesity (Silver Spring) 2009;17(9):1678–1683. doi: 10.1038/oby.2009.76. [DOI] [PubMed] [Google Scholar]
- 138.Al-Safi ZA, Polotsky A, Chosich J, Roth L, Allshouse AA, Bradford AP, et al. Evidence for disruption of normal circadian cortisol rhythm in women with obesity. Gynecol Endocrinol. 2018;34(4):336–340. doi: 10.1080/09513590.2017.1393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bernardi LA, Carnethon MR, de Chavez PJ, Ikhena DE, Neff LM, Baird DD, et al. Relationship between obesity and anti-Mullerian hormone in reproductive-aged African American women. Obesity (Silver Spring) 2017;25(1):229–235. doi: 10.1002/oby.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LCL, Strauss JF. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101–106. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 141.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 142.Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101(8):1824–33. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 143.Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: Significance for metabolic inflammation. PLoS One. 2015;10(7):e0133494. 10.1371/journal.pone.0133494. [DOI] [PMC free article] [PubMed]
- 144.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. 10.1038/nature05487. [DOI] [PubMed]
- 145.Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol. 2007;157(4):465–471. doi: 10.1530/EJE-07-0206. [DOI] [PubMed] [Google Scholar]
- 146.Kondo H, Abe I, Gotoh K, Fukui A, Takanari H, Ishii Y, et al. Interleukin 10 treatment ameliorates high-fat diet-induced inflammatory atrial remodeling and fibrillation. Circ-Arrhythmia Elec. 2018;11(5):e006040. 10.1161/CIRCEP.117.006040. [DOI] [PubMed]
- 147.Straczkowski M, Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87(10):4602–4606. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- 148.Kern L, Mittenbuhler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity-induced TNFalpha and IL-6 signaling: The missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers (Basel). 2018;11(1):24. 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed]
- 149.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 150.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Investig. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 152.Malavazos AE, Cereda E, Morricone L, Coman C, Corsi MM, Ambrosi B. Monocyte chemoattractant protein 1: a possible link between visceral adipose tissue-associated inflammation and subclinical echocardiographic abnormalities in uncomplicated obesity. Eur J Endocrinol. 2005;153(6):871–877. doi: 10.1530/eje.1.02033. [DOI] [PubMed] [Google Scholar]
- 153.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100(12):7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ortega RM, Lopez-Sobaler AM, Andres P, Rodriguez-Rodriguez E, Aparicio A, Perea JM. Folate status in young overweight and obese women: changes associated with weight reduction and increased folate intake. J Nutr Sci Vitaminol (Tokyo). 2009;55(2):149–55. 10.3177/jnsv.55.149. [DOI] [PubMed]
- 156.Knight BA, Shields BM, Brook A, Hill A, Bhat DS, Hattersley AT, et al. Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic white British population. PLoS One [Electronic Resource]. 2015;10(8):e0135268. 10.1371/journal.pone.0135268. [DOI] [PMC free article] [PubMed]
- 157.da Silva VR, Hausman DB, Kauwell GP, Sokolow A, Tackett RL, Rathbun SL, et al. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int J Obes. 2013;37(12):1608–10. doi: 10.1038/ijo.2013.41. [DOI] [PubMed] [Google Scholar]
- 158.Kobylinska M, Antosik K, Decyk A, Kurowska K. Malnutrition in obesity: Is it possible? Obes Facts. 2022;15(1):19–25. doi: 10.1159/000519503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bellner L, Nichols A, Pandey V, Vanella L, Gilliam C, Gupte R, et al. Effect of vitamin B12 and nutrients on adipogenesis-adipogenic markers in 3T3 Cells. Faseb J. 2015;29:996.2. 10.1096/fasebj.29.1_supplement.996.2
- 160.Adaikalakoteswari A, Vatish M, Alam MT, Ott S, Kumar S, Saravanan P. Low Vitamin B12 in Pregnancy Is Associated With Adipose-Derived Circulating miRs Targeting PPARgamma and Insulin Resistance. J Clin Endocrinol Metab. 2017;102(11):4200–4209. doi: 10.1210/jc.2017-01155. [DOI] [PubMed] [Google Scholar]
- 161.Chan CW, Chan PH, Lin BF. Folate Deficiency Increased Lipid Accumulation and Leptin Production of Adipocytes. Front Nutr. 2022;9:852451. doi: 10.3389/fnut.2022.852451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Windt M, Schoenmakers S, van Rijn B, Galjaard S, Steegers-Theunissen R, van Rossem L. Epidemiology and (Patho)Physiology of Folic Acid Supplement Use in Obese Women before and during Pregnancy. Nutrients. 2021;13(2):331. 10.3390/nu13020331. [DOI] [PMC free article] [PubMed]
- 163.Chardonnens D, Cameo P, Aubert ML, Pralong FP, Islami D, Campana A, et al. Modulation of human cytotrophoblastic leptin secretion by interleukin-1alpha and 17beta-oestradiol and its effect on HCG secretion. Mol Hum Reprod. 1999;5(11):1077–1082. doi: 10.1093/molehr/5.11.1077. [DOI] [PubMed] [Google Scholar]
- 164.Dos Santos E, Dieudonne MN, Leneveu MC, Pecquery R, Serazin V, Giudicelli Y. In vitro effects of chorionic gonadotropin hormone on human adipose development. J Endocrinol. 2007;194(2):313–325. doi: 10.1677/JOE-06-0101. [DOI] [PubMed] [Google Scholar]
- 165.Maymo JL, Perez Perez A, Sanchez-Margalet V, Duenas JL, Calvo JC, Varone CL. Up-regulation of placental leptin by human chorionic gonadotropin. Endocrinology. 2009;150(1):304–313. doi: 10.1210/en.2008-0522. [DOI] [PubMed] [Google Scholar]
- 166.Zhang X, Chen J, Huang B, Wang J, Shan Z, Liu J, et al. Obesity Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances via MAPK Pathway Activation in Intervertebral Disk Degeneration. Front Physiol. 2019;10:1284. doi: 10.3389/fphys.2019.01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem. 1997;272(16):10585–10593. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- 168.Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45(10):1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 169.Jonklaas J, Nsouli-Maktabi H. Weight changes in euthyroid patients undergoing thyroidectomy. Thyroid. 2011;21(12):1343–1351. doi: 10.1089/thy.2011.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Menendez C, Baldelli R, Camina JP, Escudero B, Peino R, Dieguez C, et al. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7–12. doi: 10.1677/joe.0.1760007. [DOI] [PubMed] [Google Scholar]
- 171.Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004;145(5):2221–2227. doi: 10.1210/en.2003-1312. [DOI] [PubMed] [Google Scholar]
- 172.Viikari LA, Huupponen RK, Viikari JSA, Marniemi J, Eklund C, Hurme M, et al. Relationship between leptin and C-reactive protein in young Finnish adults. J Clin Endocr Metab. 2007;92(12):4753–4758. doi: 10.1210/jc.2007-0103. [DOI] [PubMed] [Google Scholar]
- 173.Missel AL, Saslow LR, Griauzde DH, Marvicsin D, Sen A, Richardson CR, et al. Association between fasting insulin and C-reactive protein among adults without diabetes using a two-part model: NHANES 2005–2010. Diabetol Metab Syndr. 2021;13(1):29. doi: 10.1186/s13098-021-00645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fatahi S, Pezeshki M, Mousavi SM, Teymouri A, Rahmani J, Kord Varkaneh H, et al. Effects of folic acid supplementation on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2019;29(5):432–439. doi: 10.1016/j.numecd.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 175.Satapathy S, Bandyopadhyay D, Patro BK, Khan S, Naik S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial. Complement Ther Med. 2020;53:102526. 10.1016/j.ctim.2020.102526. [DOI] [PubMed]
- 176.Twigt JM, Bezstarosti K, Demmers J, Lindemans J, Laven JSE, Steegers-Theunissen RP. Preconception folic acid use influences the follicle fluid proteome. Eur J Clin Invest. 2015;45(8):833–841. doi: 10.1111/eci.12478. [DOI] [PubMed] [Google Scholar]
- 177.Sanchez EG, Giviziez CR, Sanchez HM, Agostinho PL, Barros PS, Approbato MS. Low progesterone levels and ovulation by ultrasound assessment in infertile patients. JBRA Assist Reprod. 2016;20(1):13–16. doi: 10.5935/1518-0557.20160004. [DOI] [PubMed] [Google Scholar]
- 178.Brannian JD, Hansen KA. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Seminars in Reproductive Medicine. 2002;20(2):103–112. doi: 10.1055/s-2002-32501. [DOI] [PubMed] [Google Scholar]
- 179.Ahn SY, Yang SW, Lee HJ, Byun JS, Om JY, Shin CH. Excess of leptin inhibits hypothalamic KiSS-1 expression in pubertal mice. Korean J Pediatr. 2012;55(9):337–343. doi: 10.3345/kjp.2012.55.9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cable JK, Grider MH. Physiology, Progesterone. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed]
- 181.Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17(1):101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 182.Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29(6):507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- 183.Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res. 2002;10(6):551–554. doi: 10.1038/oby.2002.74. [DOI] [PubMed] [Google Scholar]
- 184.Radwanska E, Frankenberg J, Allen EI. Plasma progesterone levels in normal and abnormal early human pregnancy. Fertil Steril. 1978;30(4):398–402. doi: 10.1016/S0015-0282(16)43571-5. [DOI] [PubMed] [Google Scholar]
- 185.Hernandez M, Lopez C, Soldevila B, Cecenarro L, Martinez-Barahona M, Palomera E, et al. Impact of TSH during the first trimester of pregnancy on obstetric and foetal complications: Usefulness of 2.5 mIU/L cut-off value. Clin Endocrinol (Oxf). 2018;88(5):728–34. 10.1111/cen.13575. [DOI] [PubMed]
- 186.Li JS, Liu AH, Liu HX, Li CY, Wang WW, Han C, et al. Maternal TSH levels at first trimester and subsequent spontaneous miscarriage: a nested case-control study. Endocr Connect. 2019;8(9):1288–1293. doi: 10.1530/Ec-19-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Homan G, Brown S, Moran J, Homan S, Kerin J. Human chorionic gonadotropin as a predictor of outcome in assisted reproductive technology pregnancies. Fertil Steril. 2000;73(2):270–4. doi: 10.1016/S0015-0282(99)00512-9. [DOI] [PubMed] [Google Scholar]
- 188.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrin. 2010;8:102. 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed]
- 189.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95(1):230–7, 7.e1–2. 10.1016/j.fertnstert.2010.06.079. [DOI] [PubMed]
- 190.Zhang Y, Dai X, Yang S, Zhang C, Han M, Huang HF, et al. Maternal low thyroxin levels are associated with adverse pregnancy outcomes in a Chinese population. PLoS One [Electronic Resource]. 2017;12(5):e0178100. 10.1371/journal.pone.0178100. [DOI] [PMC free article] [PubMed]
- 191.Lekva T, Roland MCP, Michelsen AE, Friis CM, Aukrust P, Bollerslev J, et al. Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J Clin Endocrinol Metab. 2017;102(7):2552–2559. doi: 10.1210/jc.2017-00289. [DOI] [PubMed] [Google Scholar]
- 192.Saif A, Mousa S, Assem M, Tharwat N, Abdelhamid A. Endothelial dysfunction and the risk of atherosclerosis in overt and subclinical hypothyroidism. Endocr Connect. 2018;7(10):1075–1080. doi: 10.1530/EC-18-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huby AC, Otvos L Jr, Belin de Chantemele EJ. Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice. Hypertension. 2016;67(5):1020–8. 10.1161/HYPERTENSIONAHA.115.06642. [DOI] [PMC free article] [PubMed]
- 194.Devaraj S, Kumaresan PR, Jialal I. C-Reactive Protein Induces Release of Both Endothelial Microparticles and Circulating Endothelial Cells In Vitro and In Vivo: Further Evidence of Endothelial Dysfunction. Clin Chem. 2011;57(12):1757–1761. doi: 10.1373/clinchem.2011.169839. [DOI] [PubMed] [Google Scholar]
- 195.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes. 2010;34(1):165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 196.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Telschow A, Ferrari N, Deibert C, Flock A, Merz WM, Gembruch U, et al. High Maternal and Low Cord Blood Leptin Are Associated with BMI-SDS Gain in the First Year of Life. Obes Facts. 2019;12(5):575–585. doi: 10.1159/000502421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kayemba-Kay's S, Peters C, Geary MP, Hill NR, Mathews DR, Hindmarsh PC. Maternal hyperinsulinism and glycaemic status in the first trimester of pregnancy are associated with the development of pregnancy-induced hypertension and gestational diabetes. Eur J Endocrinol. 2013;168(3):413–418. doi: 10.1530/EJE-12-0609. [DOI] [PubMed] [Google Scholar]
- 199.Yang Y, Wang Z, Mo M, Muyiduli X, Wang S, Li M, et al. The association of gestational diabetes mellitus with fetal birth weight. J Diabetes Complications. 2018;32(7):635–642. doi: 10.1016/j.jdiacomp.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 200.Nanda S, Akolekar R, Sarquis R, Mosconi AP, Nicolaides KH. Maternal serum adiponectin at 11 to 13 weeks of gestation in the prediction of macrosomia. Prenatal Diag. 2011;31(5):479–483. doi: 10.1002/pd.2723. [DOI] [PubMed] [Google Scholar]
- 201.Aye IL, Powell TL, Jansson T. Review: Adiponectin–the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34(Suppl):S40–S45. doi: 10.1016/j.placenta.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ruiz-Palacios M, Ruiz-Alcaraz AJ, Sanchez-Campillo M, Larque E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Ann Nutr Metab. 2017;70(1):16–25. doi: 10.1159/000455904. [DOI] [PubMed] [Google Scholar]
- 203.Hoek J, Schoenmakers S, Ringelberg B, Reijnders IF, Willemsen SP, De Rijke YB, et al. Periconceptional maternal and paternal homocysteine levels and early utero-placental (vascular) growth trajectories: The Rotterdam periconception cohort. Placenta. 2021;115:45–52. doi: 10.1016/j.placenta.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 204.Koning IV, Groenenberg IAL, Gotink AW, Willemsen SP, Gijtenbeek M, Dudink J, et al. Periconception maternal folate status and human embryonic cerebellum growth trajectories: The Rotterdam predict study. PLoS One. 2015;10(10):e0141089. 10.1371/journal.pone.0141089. [DOI] [PMC free article] [PubMed]
- 205.Zhang L, Zhang Y, Li Z, Ren A, Liu J, Ye R. Maternal periconceptional body mass index and risk for neural tube defects: results from a large cohort study in China. J Matern Fetal Neonatal Med. 2021;34(2):274–280. doi: 10.1080/14767058.2019.1606192. [DOI] [PubMed] [Google Scholar]
- 206.Carrilho TRB, Rasmussen KM, Farias DR, Costa NCF, Batalha MA, Reichenheim ME, et al. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. Bmc Pregnancy Childb. 2020;20(1):734. doi: 10.1186/s12884-020-03354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self-reported height and weight in a large, nationwide cohort of US adults. PLoS One [Electronic Resource] 2020;15(4):e0231229. doi: 10.1371/journal.pone.0231229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Artese A, Stamford BA, Moffatt RJ. Cigarette Smoking: An Accessory to the Development of Insulin Resistance. Am J Lifestyle Med. 2019;13(6):602–605. doi: 10.1177/1559827617726516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schautz B, Later W, Heller M, Peters A, Muller MJ, Bosy-Westphal A. Impact of age on leptin and adiponectin independent of adiposity. Br J Nutr. 2012;108(2):363–370. doi: 10.1017/S0007114511005605. [DOI] [PubMed] [Google Scholar]
- 210.Ng KYB, Steegers-Theunissen R, Willemsen S, Wellstead S, Cheong Y, Macklon N. Smartphone-based lifestyle coaching modifies behaviours in women with subfertility or recurrent miscarriage: a randomized controlled trial. Reprod Biomed Online. 2021;43(1):111–119. doi: 10.1016/j.rbmo.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 211.Brouwer IA, van Dusseldorp M, West CE, Meyboom S, Thomas CM, Duran M, et al. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J Nutr. 1999;129(6):1135–1139. doi: 10.1093/jn/129.6.1135. [DOI] [PubMed] [Google Scholar]
- 212.Kanakkaparambil R, Singh R, Li D, Webb R, Sinclair KD. B-vitamin and homocysteine status determines ovarian response to gonadotropin treatment in sheep. Biol Reprod. 2009;80(4):743–752. doi: 10.1095/biolreprod.108.072074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rostas I, Poto L, Matrai P, Hegyi P, Tenk J, Garami A, et al. In middle-aged and old obese patients, training intervention reduces leptin level: A meta-analysis. PLoS One [Electronic Resource] 2017;12(8):e0182801. doi: 10.1371/journal.pone.0182801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rahimi GRM, Yousefabadi HA, Niyazi A, Rahimi NM, Alikhajeh Y. Effects of Lifestyle Intervention on Inflammatory Markers and Waist Circumference in Overweight/Obese Adults With Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol Res Nurs. 2022;24(1):94–105. doi: 10.1177/10998004211044754. [DOI] [PubMed] [Google Scholar]
- 215.Marquis-Gravel G, Hayami D, Juneau M, Nigam A, Guilbeault V, Latour E, et al. Intensive lifestyle intervention including high-intensity interval training program improves insulin resistance and fasting plasma glucose in obese patients. Prev Med Rep. 2015;2:314–318. doi: 10.1016/j.pmedr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.