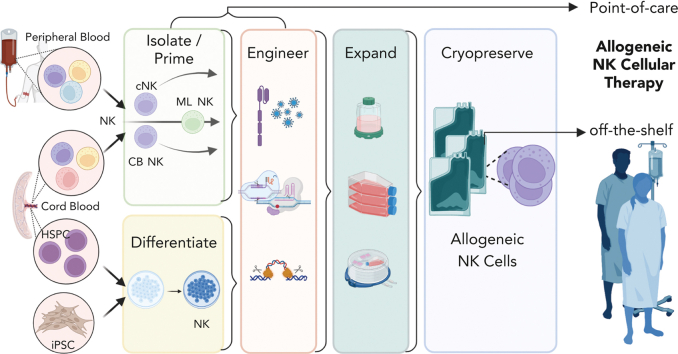
Visual Abstract
Abstract
Interest in adoptive cell therapy for treating cancer is exploding owing to early clinical successes of autologous chimeric antigen receptor (CAR) T lymphocyte therapy. However, limitations using T cells and autologous cell products are apparent as they (1) take weeks to generate, (2) utilize a 1:1 donor-to-patient model, (3) are expensive, and (4) are prone to heterogeneity and manufacturing failures. CAR T cells are also associated with significant toxicities, including cytokine release syndrome, immune effector cell–associated neurotoxicity syndrome, and prolonged cytopenias. To overcome these issues, natural killer (NK) cells are being explored as an alternative cell source for allogeneic cell therapies. NK cells have an inherent ability to recognize cancers, mediate immune functions of killing and communication, and do not induce graft-versus-host disease, cytokine release syndrome, or immune effector cell–associated neurotoxicity syndrome. NK cells can be obtained from blood or cord blood or be derived from hematopoietic stem and progenitor cells or induced pluripotent stem cells, and can be expanded and cryopreserved for off-the-shelf availability. The first wave of point-of-care NK cell therapies led to the current allogeneic NK cell products being investigated in clinical trials with promising preliminary results. Basic advances in NK cell biology and cellular engineering have led to new translational strategies to block inhibition, enhance and broaden target cell recognition, optimize functional persistence, and provide stealth from patients’ immunity. This review details NK cell biology, as well as NK cell product manufacturing, engineering, and combination therapies explored in the clinic leading to the next generation of potent, off-the-shelf cellular therapies for blood cancers.
Complex patient-specific manufacturing and variable potency due to poor T-cell fitness limit advances in autologous chimeric antigen receptor T therapies and support the potential attractiveness of having potent immune effector cell therapy that is readily available “off the shelf.” Edited by Associate Editor Helen Heslop and authored by leading experts, this review series focuses on several types of banked allogeneic immune cell therapies under development, highlighting their attributes and speculating on their anticipated future place in the therapeutic armamentarium.
Introduction
Natural killer (NK) cells are innate lymphocytes critical for controlling viral infections and eliminating malignant cells, originally identified by their ability to kill target cells without prior sensitization.1,2 NK cells are the founding member of innate lymphoid cells, which are defined by their effector functions, localization, and transcription factor requirements.3 Human NK cells recognize malignant cells, especially hematologic cancers, and respond by killing and producing cytokines and chemokines.4 Landmark hematopoietic cell transplantation (HCT) and adoptive NK cell clinical trials revealed remarkable safety and preliminary clinical responses. Advances in our basic understanding of NK cell biology, along with methods for NK cell isolation, expansion, engineering, differentiation from stem cells, and discovery of memory programs have led to an explosion of preclinical and clinical research testing NK cell therapeutics. Through these advances, NK cell–based therapies provide a complementary clinical strategy to, and overcome limitations of, US Food and Drug Administration–approved chimeric antigen receptor (CAR) T-cell therapies. Moreover, there are many strategies that leverage immunotherapy combinations with NK cell therapy. This review focuses on foundational NK cell biology and allogeneic NK cell therapies derived from primary cells (blood, cord blood [CB], induced pluripotent stem cells [iPSCs]) in clinical testing. These span point-of-care rapid NK cell manufacture to “off-the-shelf” products with ex vivo expansion, engineering, and cryopreservation.
NK cell biology and anticancer responses
NK cell development and subsets
NK cells develop in the bone marrow from the common lymphoid progenitor into immature CD56bright NKG2A+CD16−KIR− NK cells and then differentiate into mature CD56dimNKG2A+/−CD16+KIR+ NK cells.5 Conditioning of NK cells to acquire functional competence (licensing) requires recognition of at least 1 self-inhibitory receptor that binds to major histocompatibility complex 1 (MHC I) or MHC I-like molecules.6 CD56bright NK cells predominate in secondary lymphoid organs, whereas CD56dim NK cells are most abundant in the blood.7,8 CD56bright NK cells produce abundant cytokines and initially were considered primarily immunoregulatory.9,10 However, CD56bright NK cells have increased proliferative potential, robust killing, and other functional responses after priming with interleukin-15 (IL-15),11 the homeostatic cytokine required for proper NK cell development and function.12,13 As NK cells mature from CD56bright to CD56dim, increased CD16a and cytotoxic molecules are expressed.6,8,14 Expression of CD16a (FcγRIIIa) allows for antibody-dependent cellular cytotoxicity (ADCC).15 These 2 NK cell subsets were more recently confirmed in unbiased approaches using mass cytometry and cellular indexing of transcriptomes and epitopes by sequencing, and have differential cell surface and transcription factor expression, as well as unique functional attributes.16 Importantly, CD56 expression can be upregulated by activation when generating therapeutic NK cell products, but high CD56 expression in this context does not necessarily equate these cells with the traditional CD56bright NK cell subset.
Recognition of target cells: activating and inhibitory receptors
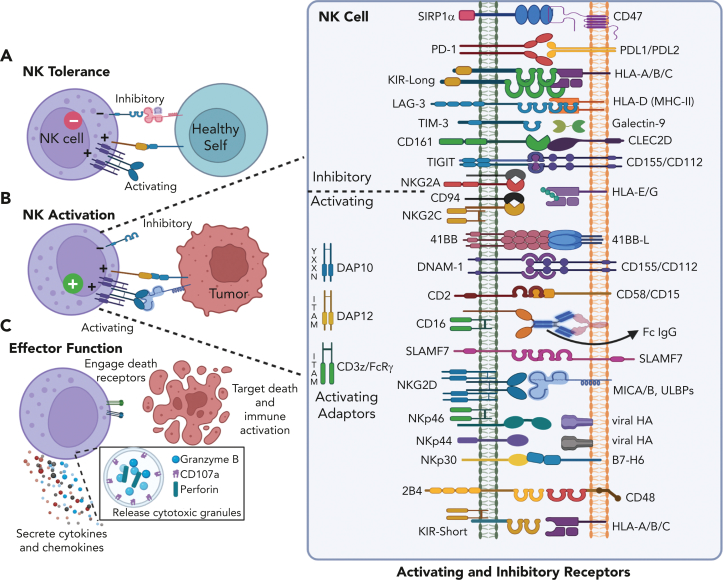
NK cells are important for tumor surveillance17 and antitumor responses depend upon the balance between signals through stochastically expressed activating and inhibitory receptors (Figure 1).18 Activating NK cell receptors recognize a variety of ligands expressed selectively on transformed or infected cells, and these signals are transmitted through multiple distinct intracellular signaling pathways. Most activating receptors require coactivation with cytokines or engagement of a second activating receptor to generate robust functional responses, whereas CD16 initiates responses independently.19,20 Inhibitory receptors include inhibitory killer immunoglobulin-like receptors (iKIRs) that recognize polymorphic regions of MHC class I (primarily HLA-C/B), and NKG2A, which recognizes nonclassical HLA-E/G. The downregulation or loss of inhibitory ligands (“missing self”) promotes NK cell activation,21 and KIR immunogenetics have been studied extensively in early NK-cell therapy studies.22 Expression of NK cell receptors can be altered with activation or affected by the cancer microenvironment.23,24 NK cells can be induced to express inhibitory checkpoint molecules such as TIGIT, LAG-3, Tim-3, CD161, PD-1, and SIRP1-α.24,25
Figure 1.
NK cell responses are determined by the balance of signals received through activating and inhibitory receptors. (A) NK cells sense healthy self-tissues through interactions between KIR and MHC-I molecules, which inhibit NK cell killing. (B) Malignant or virally infected cells upregulate stress ligands recognized through NK activating receptors, which can trigger killing. In the event that infected or transformed cells downregulate MHC-I, NK cells kill via missing-self response. (C) NK cells can induce apoptosis by releasing perforin and granzyme-containing granules or engaging death receptors on target cells; in addition, NK cells produce immune modulating cytokines and chemokines upon activation. (Inset) main activating and inhibitory receptors expressed by NK cells and their ligands, present on target cells. KIR-Long contain intracellular immunoreceptor tyrosine-based inhibitory motifs whereas KIR-Short lack intracellular immunoreceptor tyrosine-based inhibitory motifs and instead associate with membrane adapters with activating motifs.
Cytokine receptor signals
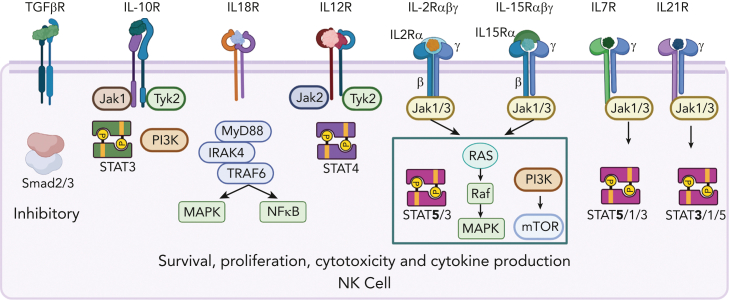
NK cells constitutively express a variety of cytokine receptors that tune their responses (Figure 2).9,26,27 Cytokine receptor stimulation of NK cells results in cytokine and chemokine production, proliferation, improved cytotoxicity, survival, and enhanced metabolism.9,26 Cytokine receptor signals can enhance expression of activating and inhibitory receptors, thereby altering their sensitivity to a specific target. Common gamma-chain cytokines (γc; IL-2, IL-15, IL-7, IL-21) promote NK cell proliferation, survival, cytotoxicity, and cytokine production.26,27 Signals via the IL-2/IL-15 receptor are critical for NK cell homeostasis and in vivo persistence.28 Furthermore, multiple negative regulators of IL-2/15 signaling have been identified, including SOCS1, SOCS3, and CISH.29,30 In addition, IL-12 and IL-18, produced by activated dendritic cells and macrophages, potently promote NK cell effector cytokine production and cytotoxic potential.31 IL-10, although conventionally considered immunosuppressive, is a pleiotropic cytokine that improves NK cell cytotoxicity and proliferation.32 In contrast, transforming growth factor β (TGFβ) profoundly inhibits NK cell responses in the tumor environment,24,33 promoting NK cell differentiation to be innate lymphoid cell-1-like with abrogated effector function.34
Figure 2.
Constitutively expressed cytokine receptors on NK cells. Common gamma chain (γc) receptors constitutively expressed by NK cells include intermediate affinity IL-2/IL-15 (βγc) receptor. High-affinity IL-15Rα is presented in trans by other cell types. CD56bright NK cell subsets constitutively express high-affinity IL-2Rα and IL-7R, CD56dim NK cells upregulate these receptors upon activation. IL-12R is constitutively expressed and required for adaptive memory responses. TGFβR inhibits IL-15R signal transduction and significantly alters NK cell phenotype and effector functions.
Innate memory: adaptive and memory-like NK cell biology
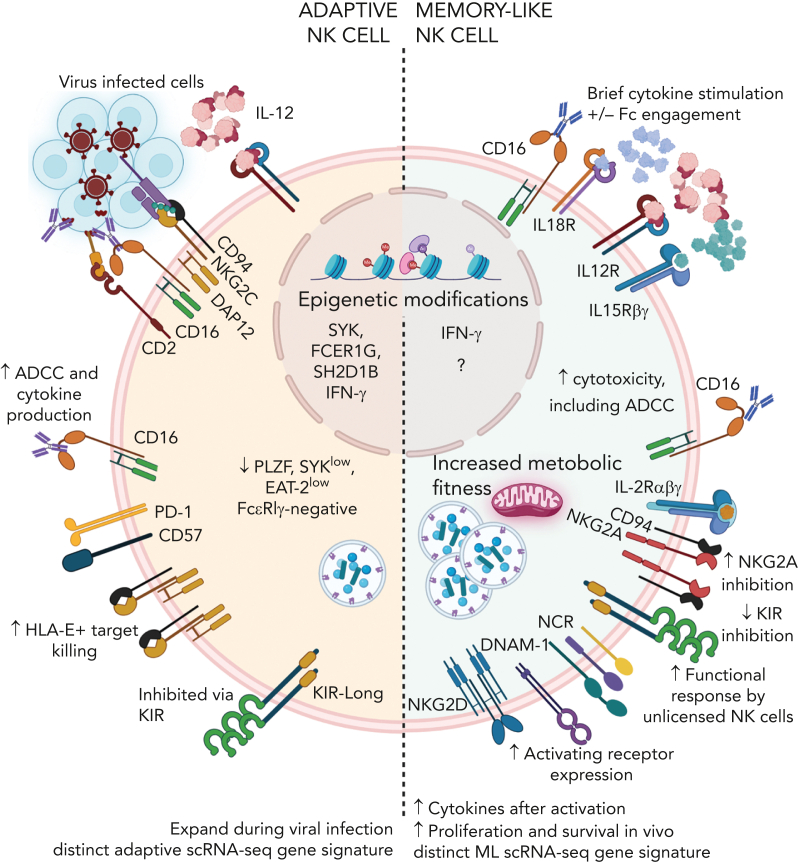
Human NK cells exhibit several types of innate memory.35, 36, 37 Most relevant for clinical translation, to date, are adaptive NK cells induced via cytomegalovirus infection and memory-like NK cells that differentiate after brief activation with cytokines IL-12, IL-15, and IL-18 (Figure 3).38,39 These 2 NK cell types are functionally distinct and have different molecular programs. Human adaptive NK cells were first identified after cytomegalovirus infections with skewing and expansion of NKG2C+ NK cells,40 with biased functionality toward responses via CD16 and reduced capacity for cytokine production.41 These cells undergo epigenetic changes resulting in PLZF, EAT-2, and FCεR1γ downregulation41, 42, 43 that are not evident in memory-like NK cells.44 Memory-like NK cells demonstrate enhanced effector functions in response to multiple second stimuli, including CD16, tumor targets, and cytokine stimulation, as well as enhanced metabolic features and penetration into lymphomas.45, 46, 47 Memory-like NK cells pass on their enhanced function after cell division, exhibit a unique epigenetic and single-cell transcriptomic signature, and functionally persist for months in the immune compatible setting.44,48 Because of their enhanced functional properties, adaptive and memory-like NK cells are being explored clinically.38,39
Figure 3.
Adaptive and cytokine-induced memory-like NK cells. Adaptive and cytokine-induced memory-like NK cells are distinct memory NK cell types. Adaptive NK cells are induced after viral infection and demonstrate enhanced responses to antibody-mediated activation, and they proliferate robustly after subsequent virus exposure. Memory-like NK cells are induced through IL-12, IL-15, IL-18 and with the addition of CD16 engagement. Memory-like NK cells demonstrate enhanced cytokine production and killing in response to multiple stimuli, including Fc-receptor ligation, activating receptor ligation, and cytokine receptor activation.
NK cellular therapy
Allogeneic NK cell therapies have multiple advantages over existing CAR T-cell therapies. Allogeneic T-cell–based therapies cause graft-versus-host disease (GVHD), whereas NK cells do not. Compared with T cells, NK cells have remarkably reduced cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome toxicity, which may be explained by expansion kinetics or differences in their receptor and cytokine biology. Off-the-shelf and point-of-care NK cell therapies are immediately available, obviating weeks for autologous CAR T-cell production, and have economy of scale with many patients treated by a single NK donor, which markedly reduces cost. Furthermore, both engineered and nonengineered NK cells have the capacity to recognize tumors in multiple ways, potentially reducing antigen-escape failures.
Rapid NK cellular therapy was originally achieved via point-of-care NK cell isolation from donor apheresis, activation, and fresh infusion. Newer methods can differentiate and/or expand NK cells ex vivo, which then are cryopreserved for storage and administered to a patient after being thawed at the bedside. NK cells are readily engineered to have enhanced efficacy using multiple approaches, including viral and nonviral gene transfer and genetic deletion using CRISPR/Cas9 and base-editing technologies. These approaches allow for improved NK cell products via broadened specificity, enhanced quality of the NK cell response/persistence, or reduced immunogenicity. Thus, there are several approaches to generate allogenic NK cells for rapid point-of-care or off-the-shelf use that are being explored in the clinic (Figure 4).
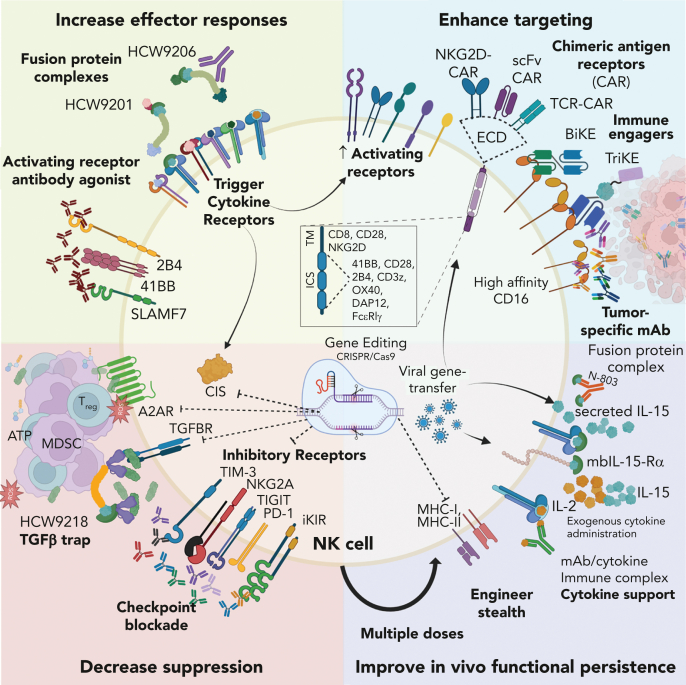
Figure 4.
Strategies for improving NK cell–based therapy. NK cells used in therapy can be improved by increasing effector responses, enhancing targeting, decreasing suppression, and improving in vivo functional persistence. ECD, extracellular domain; HCW9201, IL-12/IL-15/IL-18 receptor agonist; HCW9206, IL-7/IL-15/IL-21 receptor agonist; N-803, IL-15 receptor super agonist; mbIL-15-Rα, membrane-bound IL-15 receptor associated with high-affinity IL-15 receptor alpha subunit; HCW9218, IL-15 receptor agonist fused with TGF-β trap; BiKE/TriKE, bi- or tri-specific killer engagers, respectively; TM/ICS, transmembrane/intracellular signaling domain.
Early investigations of NK cells in HCT and adoptive transfer
Although allogeneic HCT is curative for many patients with acute leukemia via graft-versus-leukemia immune-cell responses, these patients also have substantial morbidity and mortality from infections, organ toxicity, and GVHD mediated by T cells. The concept of NK cells as a major contributor to graft-versus-leukemia during allogeneic HCT was demonstrated in patients with high-risk leukemia undergoing MHC-haploidentical HCT,49 catalyzing the field and leading to studies of NK cell receptor immunogenetics across many types of allogeneic HCT.50, 51, 52, 53 These studies set the stage for testing allogeneic adoptive NK cell therapy.
In the first rapid point-of-care NK cell therapy, Miller et al utilized a donor apheresis followed by NK cell enrichment (40%-50%), overnight IL-2 activation, and the product was administrated to patients with cancer within 24 to 48 hours.54 This important study established the need for lymphodepletion that induced endogenous IL-15 to facilitate in vivo donor NK cell expansion. Of the patients with relapsed/refractory acute myeloid leukemia (r/r AML) receiving this enriched allogeneic NK cell product, 25% had complete remission with no major toxicities observed. This approach has since been tested in an expanded number of patients, which confirmed initial results.55, 56, 57 This early approach demonstrated the feasibility and safety of NK cell therapy and thus paved the way for modern approaches. However, many open questions remain, including optimal NK cell source, dose, administration schedule, cytokine support, and mechanisms of response and resistance.
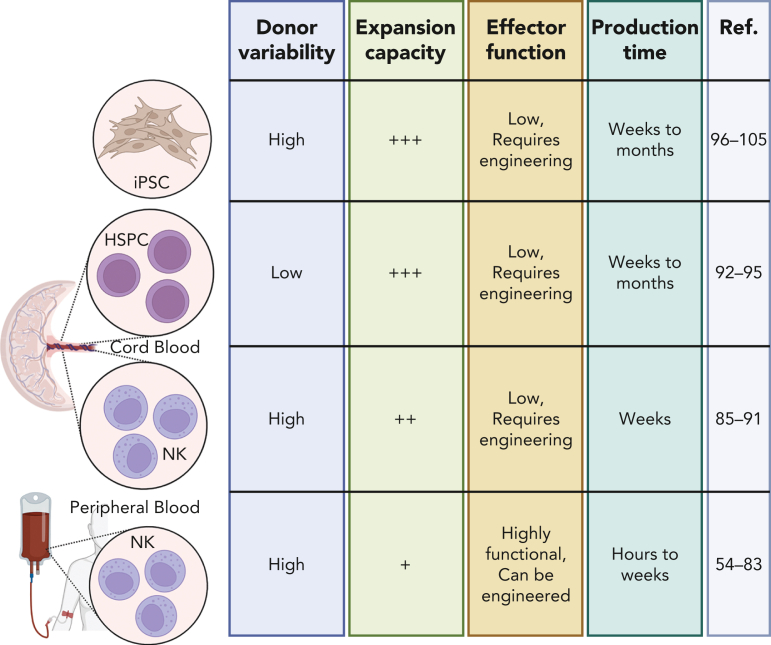
In modern NK cell therapy, there are 3 main tissue sources for allogeneic NK cells: donor peripheral blood (PB), CB, or differentiation from CB hematopoietic stem and progenitor cells (HSPCs) or iPSCs (Table 1; Figure 5). Although immortalized NK cell lines have been investigated extensively, they have many challenges that limit their current therapeutic potential.58 Currently, there are advantages and challenges to each tissue source for NK cells, and all are being investigated in the clinical setting.
Table 1.
Selected off-the-shelf and point-of-care NK products in clinical development derived from PB, CB, or iPSCs from 490 NK cell clinical trials
| Sponsor | Therapy name | CAR | Target | Cell source | Disease | Clinical trial number | Clinical phase |
|---|---|---|---|---|---|---|---|
| Wuhan Union Hospital, China | — | + | CD19 | CB | B-cell malignancies | NCT04796675 | Phase 1 |
| Hangzhou Cheetah Cell Therapeutics Co, Ltd | — | + | NKG2D | CB | AML | NCT05247957 | Phase 1 |
| Xinqiao Hospital of Chongqing | — | + | BCMA | CB | MM | NCT05008536 | Phase 1 |
| Washington University | CIML NK cells | − | PBMC memory-like |
r/r AML after allo-HCT |
NCT03068819 | Phase 1/2∗ | |
| Washington University | CIML NK cells | − | PBMC memory-like |
AML with haplo-HCT | NCT02782546 | Phase 2∗ | |
| Wugen Inc | WU-NK-101 | − | PBMC memory-like, expanded | r/r AML | NCT05470140 | Phase 1 | |
| MDACC | CAR.70/IL5 | CD70 | CB | r/r hematologic malignancies | NCT05092451 | Phase 1/2 | |
| MDACC | AFM13-NK | − | CD30 | CB | HL, NHL | NCT04074746 | Phase 1/2 |
| MDACC | iC9/CAR.19/IL15 | + | CD19 | CB | r/r B-lymphoid malignancies | NCT03056339 | Phase 1/2 |
| Takeda | TAK-007 | + | CD19 | CB | r/r NHL | NCT05020015 | Phase 2 |
| CHLA | Haplo–IL-21 NK | − | — | PBMC IL-21 expanded |
AML with HCT |
NCT04836390 | Phase 2∗ |
| Ohio State | KDS-1001 | − | PBMC mIL-21 expanded | AML | NCT04220684 | Phase 1 | |
| Ohio State/NWC | UD-NK mIL-21 | PBMC mIL-21 UD expanded | r/r AML | NCT05503134 | Phase 1 | ||
| Ohio State | UD-NK mIL-21 | − | PBMC mIL-21 UD expanded |
CTLC | NCT04848064 | Phase 1 | |
| Kiadis/Sanofi | KDS-1001 | − | PBMC mIL-21 expanded | CML | NCT04808115 | Phase 1 | |
| Nkarta Inc | NKX101 | + | NKG2D | PBMC | AML, MDS | NCT04623944 | Phase 1 |
| Nkarta Inc | NKX019 | + | CD19 | PBMC | r/r NHL, CLL B-ALL |
NCT05020678 | Phase 1 |
| Nkarta Inc | NKX019 | + | CD19 | PBMC | r/r NHL, CLL B-ALL |
NCT05020678 | Phase 1 |
| Fate Therapeutics | FT576 | + | CD38 and BCMA | iPSC | r/r MM | NCT05182073 | Phase 1 |
| Fate Therapeutics | FT596 | + | CD19 | iPSC | NHL | NCT04245722 | Phase 1 |
| Fate Therapeutics | FT536 | − | CD38 mAb | iPSC | MM, solid tumors | NCT05395052 | Phase 1 |
| Fate Therapeutics | FT516 | − | CD20, B7-53, PD-L1 | iPSC | NHL, ovarian, RCC, Merkel cell | NCT04023071NCT04630769NCT04551885 | Phase 1 |
| Fate Therapeutics | FT500 | − | PD-1 | iPSC | NHL, solid tumors | NCT03841110 | Phase 1 |
Only clinical trials with available information on www.clinicaltrials.gov were considered.
ALL, acute lymphoblastic leukemia; allo, allogenic; BCMA, B-cell maturation antigen; CHLA, Children's Hospital Los Angeles; CIML, cytokine-induced memory-like; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CTLC, cutaneous T-cell lymphoma; HL, Hodgkin lymphoma; MDACC, MD Anderson Cancer Center; MDS, myelodysplastic syndrome; mIL-21, membrane-bound IL-21; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; PBMC, peripheral blood mononuclear cells; RCC, renal cancer cell; UD, universal donor.
Point-of-care allogeneic product.
Figure 5.
Multiple sources for NK cells used in allogeneic NK cell therapy. Source of NK cells for therapy include iPSCs, HSPCs, and CB-derived and PB- or blood-derived NK cells.
Off-the-shelf PB NK cells
PB NK cells may be enriched by CD3/CD19-depletion (∼50% CD56+CD3−)54,55 or CD3-depletion and CD56-selection (>90% CD56+CD3−).44,48,55,59, 60, 61 An additional advantage of off-the-shelf products is the ability to return to the same donor for future product generation, which allows for donor selection and optimization. Challenges with PB NK cells include donor-to-donor variability and a finite NK cell number that can be obtained from a single apheresis. PB NK cell heterogeneity can arise from subsets,8 the relative proportion of self-KIR–licensed NK cells, the expression of activating and inhibitory NK cell receptors, the alloreactive NK cell fraction, the activating KIR component encoded in the NK gene complex, and memory programs. Multiple strategies were developed to expand PB NK cells, thereby providing the ability to test different dosing schedules. This also eases the barrier to cellular engineering and gene editing, overcoming the challenge of limiting NK cell yield from a single donor.
Memory-like NK cell therapy
Point-of-care memory-like NK cells (Figure 3) were developed at Washington University and tested in a phase 1 clinical trial.59,60 Purified PB NK cells were stimulated with IL-12, IL-15, and IL-18 for 12 to 16 hours to initiate the memory-like program, washed, and infused as a single dose into patients with r/r AML after fludarabine/cyclophosphamide lymphodepletion. NK cells expanded and differentiated into memory-like NK cells in vivo. Importantly, the overall safety profile was excellent and composite complete remissions (CRs) occurred in 47% of patients.60,62 Memory-like NK cells have also been studied as a way to augment reduced intensity–conditioned allogeneic HCT, which resulted in an 87% composite CR rate, >1000-fold NK cell expansion in vivo, and persistence of memory-like NK cells for >3 months.44 Additional studies have explored memory-like NK cells for treating post-HCT relapse.48,63 Data spanning 4 different clinical trials indicate that memory-like programs enhance NK cell responses against leukemia and provide a platform for ongoing innovation in NK cellular therapy. Wugen has developed a commercial version of memory-like NK cells that preserves memory-like biology but supports expansion and viable cryopreservation.64 These allogeneic off-the-shelf memory-like NK cells (WU-NK-101) are clinically being tested for r/r AML (NCT05470140).
Expanded blood NK cells
To provide both cytokine and activating receptor signals to PB NK cells, the Campana group engineered K562 feeder cells with membrane-bound (mb) IL-15 and 4-1BB (CD137) ligand and exogenous IL-2.65,66 This yielded expansion of 25 fold after 7 days, resulting in NK cells with increased activity against AML targets. Commercial off-the-shelf CAR-NK cells using mbIL-15-K562 accessory cells have been developed by Nkarta, engineered with anti-NKG2D-CAR (NKX101) or anti-CD19-CAR (NKX019) utilizing OX40/CD3ζ signaling domains. Preclinically, these expanded CAR NK cells exhibited killing against tumors and persistence in vivo, improved over conventional NK cells and CAR19-T cells.67 Phase 1 clinical trials using PB expanded CAR-NK cells are ongoing for patients with r/r AML and myelodysplastic syndrome using NKX101 and for patients with B-cell malignancies with NKX019.68,69
Pioneered at MDACC, K562/41BBL/mbIL-21 is a feeder line that expands NK cells without causing senescence, resulting in a higher fold NK cell expansion compared with mbIL-15 feeder cells. mbIL-21–expanded NK cells showed enhanced function compared with mbIL-15–expanded NK cells despite similar expression of NCRs, CD16, and NKG2D.70 mbIL-21–expanded NK cells retain inhibition by iKIR ligands and mediate ADCC. These NK cells were translated into phase 1 trials in AML that demonstrated safety when administered pre-HCT71 or post-HCT.72 This approach was commercialized by CytoSen/Kiadis/Sanofi and is being developed as SAR445419 with phase 1 studies ongoing.73,74
Additional approaches include utilizing irradiated PB mononuclear cells or B-lymphoblastoid lines to expand human NK cells.75, 76, 77, 78, 79, 80 Commercial approaches developed by Miltenyi using IL-2 in dedicated medium result in expansion of CAR-NK cells (15%-50% range), which killed CD19+ lymphomas and primary acute lymphocytic leukemia blasts in vitro, and controlled CD19+ Daudi cells in vivo.81 Despite remarkable cell expansion, feeder cells used during NK expansion create a regulatory issue and complicate consistency of scale manufacturing. Thus, several strategies have been developed for feeder-free, cytokine-based expansion of allogeneic NK cells for cancer therapy that mimic the scaffold-function of the accessory cell.82,83
CB NK cells
CB contains ∼30% NK cells that exhibit similar NK cell subsets and capacity for cytokine secretion, albeit with reduced cytotoxicity, compared with PB NK cells.84 In addition, CD34+ HSPCs and NK progenitors are also present in higher frequencies, facilitating differentiation. CB-derived NK cells can be expanded ex vivo (∼1000-2000-fold) using cytokines and feeder cells, and likely result from both progenitor differentiation and ex vivo NK expansion.85 Thus, CB-derived NK cells have the advantages of a ready source of NK cells and progenitors that could be expanded, easily engineered, and differentiated into NK cells. Challenges include the finite starting cell source, ex vivo differentiation, and inability to pursue repeat donors.
Clinical-grade expanded CB-derived NK cells have been developed.85,86 Initial approaches utilized feeder cells and cytokines,87 with later innovations of artificial feeder cells expressing mbIL-15 or mbIL-21 and 4-1BBL. Rezvani’s group developed expanded CB NK cells using K562/mbIL-21/41BBL engineered to express a CD19-CAR and IL-15.88 This strategy was translated into a landmark first-in-human phase 1 clinical study, demonstrating that CB CD19-CAR NK cells induced objective responses in 73% of patients with r/r CD19+ B-cell cancers. Responses were not formally assessed for durability but occurred after multiple prior lines of therapy. In line with prior experiences with allogeneic NK cells, CD19-CAR CB-NK cells were safe and well tolerated despite the HLA mismatch between the patients and their CAR-NK products.89 These initial clinical data supported an improved safety profile over traditional CAR T cells, while exhibiting antilymphoma responses, sparking intense investigation in CAR NK cells. Innovation of the feeder cells has included CD48 with 4-1BBL and mbIL-21 that expand CB NK cells >900-fold in 2 weeks with favorable metabolic fitness and effector function.90 The field of CB-derived CAR NK cells is an active area of investigation with many companies advancing this version of NK cell therapy, and many clinical trials initiated as recently cataloged.58
NK cells can also differentiate and expand from isolated CD34+ HSPCs, utilizing cytokines and stroma-based feeder cells91 or cytokines alone.92 This has been adapted to clinical scale with refinements in cellular manufacturing,93 resulting in very large expansions of functional NK cells. However, some persistent phenotypic and functional differences remain, including inconsistent KIR expression and an unclear effect on receptor-based licensing, and reduced CD16 expression. Dolstra et al94 reported the first clinical study of CD34+ HSPC-derived NK cells as a consolidation therapy in older patients with high-risk AML. This approach was advanced as a commercial off-the-shelf product (GTA002) by Glycostem, with early clinical data supporting safety. Larger patient cohorts will be needed to understand efficacy, and future work will address their capacity for engineering.
iPSC-derived NK cells
iPSC technology uses stepwise differentiation of somatic cells into HSPCs and then NK cells, which are then expanded (iNK).95,96 This sequential differentiation using cytokines and stromal cells result in the differentiation of NK cells that express CD56, low levels of KIR, minimal CD16, and intact expression of other activating NK cell receptors.95 This approach was clinically scaled, providing adequate iPSC-derived NK cells for clinical testing,96 with preclinical data demonstrating responses against cancer.97 The iNK methods continue to evolve, with improvements in efficiency,98 and potential for feeder-free chemically defined methods.99 Advantages of iPSC-derived NK cells are their consistency, with a nearly unlimited source of same-donor cells, and uniformity of the resulting NK cell product. Challenges overlap with CB HSPC-derived NK cells, including a lack of complete recapitulation of the physiologic NK cell differentiation process and conditioning, and the potential for a constrained NK cell receptor repertoire.100, 101, 102 As an example, FT596 are iNK edited to express CD16a, mbIL-15/15R, and a CD19-CAR,103 with a preliminary report of 20 patients demonstrating safety, with no GVHD or immune effector cell–associated neurotoxicity syndrome, and low-grade cytokine release syndrome (10%). In the 17 evaluable patients, 11 achieved an objective response, including 4 patients previously receiving CD19-CAR T cells.104 Although these early results are encouraging, comparing the durability of the clinical responses (especially CRs) with that of autologous CAR T cells will be an important clinical outcome. Furthermore, published reports of in vivo persistence are lacking. iPSC-derived off-the-shelf cellular therapies, including NK cells, are discussed in detail by Miller et al in this review series.105
Enhancing allogeneic NK cell responses
Although current NK cell therapies are being evaluated in a variety of clinical trials, the field remains in its infancy. Multiple strategies are advancing to develop the next generation of NK cell therapies and combinations including blocking inhibitory signals, enhancing target recognition, increasing effector functionality and persistence, and masking from host immunity.
Blocking inhibitory signals
Inhibitory receptors can be constitutive or induced on NK cells (Figure 1). Blocking monoclonal antibodies (mAbs) have been developed against families of inhibitory KIR2D (lirilumab) with the premise that blocking iKIR binding would unleash NK cells through “missing self.” Two phase 1 clinical trials demonstrated safety, mAb occupancy, and preliminary evidence of response in AML and myeloma,106,107 whereas subsequent phase 1,108 and randomized phase 2 studies showed no clinical improvement.109 This might be owing to loss of licensing with prolonged KIR blockade.
Monalizumab blocks the inhibitory NKG2A receptor from its HLA-E/G ligands. This approach has been tested in solid tumors and found to be safe,110 however, combinations with tumor-targeting mAb in solid tumors failed to demonstrate clinical improvements in a randomized study (INTERLINK-1, phase 3; NCT04590963). Notably, clinical data in hematologic malignancies are lacking, and monalizumab might have improved effects when used in diseases with dominant NKG2A impairment of NK cell responses, or in conjunction with adoptive allogeneic NK cell therapy in which NKG2A is a dominant checkpoint.60 Gene editing approaches have been developed to delete NKG2A that eliminate NKG2A inhibitory signals.60,111 Additional mAbs evaluated preclinically to block induced NK cell inhibitory receptors include TIM-3, TIGIT, LAG3, PD-1, CD161, and CD96.112, 113, 114 Rational development of these inhibitory checkpoints should be guided by basic and functional biology in specific hematologic malignancies.
Cytokines also induce regulatory checkpoints, with IL-15R signaling resulting in upregulation of several suppressors that quench IL-15R–based JAK/STAT signals. The cytokine-inducible SH2-containing protein (CIS) has been identified as a negative regulator of NK cell survival and function in the presence of IL-15.29 Multiple groups have demonstrated that deletion of CIS results in enhanced NK cell survival and functionality,115,116 whereas memory-like NK cells have reduced CIS expression.117 TGFβ is a potent negative regulator of NK cell functionality and expressed in several hematologic malignancies.118 Gene editing to remove TGFβR2 from NK cells is being explored preclinically.119 Strategies to provide cytokines to restore or enhance NK cell responses or to inhibit the negative effects of TGFβ are also in the clinic.26
Suppressive cell types also limit NK cell activity. Regulatory T cells (Treg) produce suppressive TGFβ and myeloid-derived suppressor cells can inhibit NK cell functions by secreting reactive oxygen species, by directly inducing Tregs, and by direct cell contact via inhibitory ligand expression.120 In the hypoxic tumor environment, high levels of adenosine triphosphate are released from the cancer cells and myeloid-derived suppressor cells that are then consecutively degraded through ectoenzymes CD39 and CD73 to become adenosine (ADO). ADO suppresses NK cell function, and several groups are investigating approaches to minimize ADO effects on NK cells, including engineering of CD73-NK CAR as well as inhibition of the ADO A2A receptor.121, 122, 123
Enhancing target cell recognition and effector responses
Multiple mechanisms to enhance NK cell tumor targeting are being explored clinically. Tumor targeting with mAbs has widely been employed for hematologic cancers as a therapeutic strategy to recruit NK cells expressing CD16a to tumor cells leading to ADCC.15 Bispecific and trispecific engagers build upon mAb and are small molecules that can enhance targeting and function (reviewed in Demaria et al124). The ligation of coactivating receptors by anti-CD137 and anti-SLAMF7 agonist mAbs are being explored as an alternative strategy to enhancing expansion and persistence.125
CAR engineering is readily applicable to NK cells to provide synthetic activating receptors and thereby broaden target specificity.125 First-generation NK CARs employed intracellular signaling domains, which were used in CAR T cells (Figure 4),89 but intracellular signaling domains specific to NK cells have been tested in vitro,126 and it is likely that each NK cell product will have individual optimal signaling preferences. Alternative targeting approaches are being explored with NKG2D and TCR-like CAR.127,128 Complex CAR targeting systems incorporating an activating CAR against a tumor antigen and NK self-recognizing inhibitory CAR are currently also being explored.129
Improving functional persistence: cytokines and stealth
Early NK cell trials correlated expansion with improved clinical activity against AML,55 which is consistent with CAR T cells correlation of expansion/persistence with clinical responses.130 As a living drug, NK cells can expand after transfer, circulate, traffic to tissues and sites of disease, and persist depending on the homeostatic signals and programs available. Because NK cell exhaustion has been reported, emphasis is placed on functional expansion and persistence, which are likely governed by different signals and programs from T cells.24 In general, approaches to enhance functional persistence, or the cellular area-under-the-curve, include using cytokine support in vivo, harnessing memory-like or adaptive programs, or engineering autonomous support systems into NK cell products, employing repeat dosing schedules, and shielding allogeneic NK cells from host rejection.
NK cells require homeostatic cytokine support after transfer. Providing intermediate or low dose rhIL-2 for 2 weeks after transfer has been tested in multiple clinical trials and is generally considered safe with evidence of in vivo functional persistence.54,55,60 Synthetic IL-15R agonists have been developed,131 and based on favorable in vivo pharmacokinetics are being explored as rhIL-2 alternatives.13 However, caution should be used selecting an IL-15R agonist because systemic administration at moderate to high doses can result in unwanted recipient CD8 T-cell activation and rapid allo-mediated rejection.44,62 Furthermore, prolonged exposure to IL-15R stimulation can result in a cytokine-exhausted state,132 suggesting that intermittent or low-level stimulation might be optimal. Alternative engineering approaches have been employed to provide autocrine cytokine support through soluble cytokines or through the engineering of mbIL-15. Many groups are testing IL-15 “armored” CAR NK cells clinically89,133 with persistence being reported by quantitative polymerase chain reaction as long as 1 year with CD19-CAR with hIL-15.
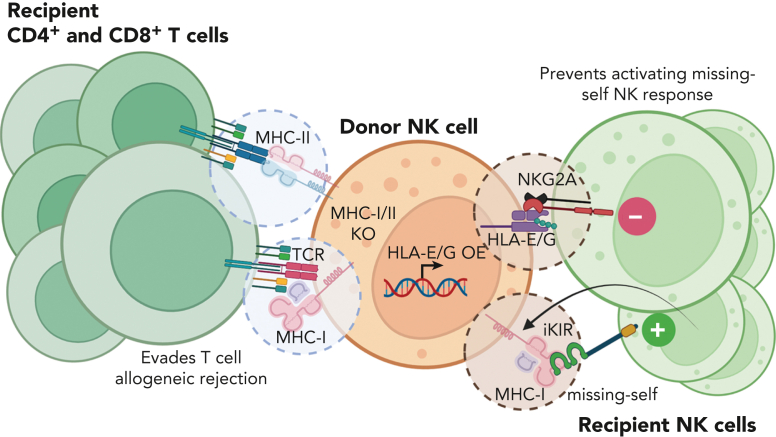
Extrinsic factors, such as the host immune system, also contribute to limited allogenic NK cell persistence. A major barrier for allogenic NK cells, and all allogenic cell products, in the clinical setting is immune rejection resulting in decreased persistence of adoptively transferred cells, which likely limits clinical responses. Thus, strategies are needed to cloak allogenic NK cells from the host immune system, including endogenous T cells and NK cells. Approaches that hide donor NK cells from recipient T cells, commonly MHC-I-deletion,21 make the donor NK cells susceptible to recipient NK killing. Strategies currently being explored to cloak donor cells from endogenous NK and T cells include engineering to express HLA-E, HLA-G, or CD47, and knock out of MHC class I and II (Figure 6).134,135
Figure 6.
Strategies for stealth NK cell products. Approaches that limit T-cell responses to donor allo-antigens, specifically MHC-I, make NK cell products susceptible to recipient NK cell killing via missing-self responses. Current strategies include knocking out MHC-I and overexpressing nonclassical MHC-I molecules to protect donor NK cells from recipient lymphocyte-mediated rejection.
Conclusions
The future of off-the-shelf cellular NK cell products for treating blood cancers is promising because they overcome many of the limitations of autologous CAR T cells. Advances in the fields of gene editing have allowed for rapid development of next-generation allogenic NK cellular therapies, although rejection of allogenic NK cells by host immune system remains a major barrier in the field. The ultimate success of off-the-shelf NK cell products will likely require a combinatorial approach to enhance targeting, inhibit negative regulators, and enhance survival and donor NK cell persistence.
Conflict-of-interest disclosure: M.M.B.-E. and T.A.F. have equity, consulting, and/or potential royalty interest in Wugen Inc. T.A.F. serves on the scientific advisory board of Wugen, Indapta Therapeutics, Orca Bio, and Affimed, and received research funding from HCW Biologics and Affimed. M.T.J. declares no competing financial interests.
Acknowledgments
The authors apologize to investigators for not being able to cite them owing to space constraints. The authors thank Pamela Wong, David Russler-Germain, Jennifer Foltz, and other members of the Fehniger laboratory for critical review of the manuscript. All figures were created with BioRender.com.
The research reported in this publication was supported by grants from the National Institutes of Health, National Cancer Institute P50CA171963 (T.A.F. and M.M.B.-E.), R01CA205239 (T.A.F.), P30CA91842 (T.A.F.), K12CA167540 (M.T.J.). Additional support was provided by the Siteman Cancer Center (T.A.F.), the ASCO Young Investigator Award via Conquer Cancer Foundation (M.T.J.), the Paula C. and Rodger O. Riney Blood Cancer Initiative (T.A.F.), and the Lymphoma Research Foundation (T.A.F.).
Authorship
Contribution: M.M.B.-E., M.T.J., and T.A.F. wrote the manuscript.
References
- 1.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. 1. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 3.Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cichocki F, Grzywacz B, Miller JS. Human NK cell development: one road or many? Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson HA, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101(08):27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 7.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47(5):820–833. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 10.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143(10):3183–3191. [PubMed] [Google Scholar]
- 11.Wagner JA, Rosario M, Romee R, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127(11):4042–4058. doi: 10.1172/JCI90387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 13.Ma S, Caligiuri MA, Yu J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 2022;43(10):833–847. doi: 10.1016/j.it.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau JE, Hsu KC. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends Immunol. 2018;39(3):222–239. doi: 10.1016/j.it.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capuano C, Pighi C, Battella S, et al. Harnessing CD16-mediated NK cell functions to enhance therapeutic efficacy of tumor-targeting mAbs. Cancers. 2021;13(10):2500. doi: 10.3390/cancers13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crinier A, Milpied P, Escalière B, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity. 2018;49(5):971–986.e5. doi: 10.1016/j.immuni.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannello A, Thompson TW, Ardolino M, Marcus A, Raulet DH. Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr Opin Immunol. 2016;38:52–58. doi: 10.1016/j.coi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmberg K-J, Carlsten M, Björklund A, et al. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Kärre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9(5):477–480. doi: 10.1038/ni0508-477. [DOI] [PubMed] [Google Scholar]
- 22.Stringaris K, Barrett AJ. The importance of natural killer cell killer immunoglobulin-like receptor-mismatch in transplant outcomes. Curr Opin Hematol. 2017;24(6):489–495. doi: 10.1097/MOH.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 23.Cózar B, Greppi M, Carpentier S, et al. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11(1):34–44. doi: 10.1158/2159-8290.CD-20-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino AM, Kim H, Miller JS, Cichocki F. Unraveling exhaustion in adaptive and conventional NK cells. J Leukoc Biol. 2020;108(4):1361–1368. doi: 10.1002/JLB.4MR0620-091R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckle I., Guillerey C. Inhibitory receptors and immune checkpoints regulating natural killer cell responses to cancer. Cancers. 2021;13(17):4263. doi: 10.3390/cancers13174263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014 doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J-X, Leonard WJ. Fine-tuning cytokine signals. Annu Rev Immunol. 2019;37:295–324. doi: 10.1146/annurev-immunol-042718-041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 29.Delconte RB, Kolesnik TB, Dagley LF, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol. 2016;17(7):816–824. doi: 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- 30.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19(4):414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “L’union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 32.Carson WE, Lindemann MJ, Baiocchi R, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85(12):3577–3585. [PubMed] [Google Scholar]
- 33.Konjević GM, Vuletić AM, Mirjačić Martinović KM, Larsen AK, Jurišić VB. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine. 2019;117:30–40. doi: 10.1016/j.cyto.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Nieves U.Y., Tay J.K., Saumyaa S., et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc Natl Acad Sci U S A. 2021;118(28) doi: 10.1073/pnas.2101169118. e2101169118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34(6):251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rölle A, Pollmann J, Cerwenka A. Memory of infections: an emerging role for natural killer cells. PLoS Pathog. 2013;9(9):1–3. doi: 10.1371/journal.ppat.1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43(4):634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol. 2016;37(12):877–888. doi: 10.1016/j.it.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gang M, Wong P, Berrien-Elliott MM, Fehniger TA. Memory-like natural killer cells for cancer immunotherapy. Semin Hematol. 2020;57(4):185–193. doi: 10.1053/j.seminhematol.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gumá M, Angulo A, Vilches C, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 41.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013;190(4):1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berrien-Elliott MM, Foltz JA, Russler-Germain DA, et al. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci Transl Med. 2022;14(633) doi: 10.1126/scitranslmed.abm1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bednarski JJ, Zimmerman C, Berrien-Elliott MM, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood. 2022;139(11):1670–1683. doi: 10.1182/blood.2021013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 50.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 51.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Blood. 2002;100(10):3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 52.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C –dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson JK, Miller JS. Current strategies exploiting NK-cell therapy to treat haematologic malignancies. Int J Immunogenet. 2018;45(5) doi: 10.1111/iji.12387. [DOI] [PubMed] [Google Scholar]
- 54.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 55.Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high-risk acute myeloid leukemia patients. Blood. 2011;118(12):3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 58.Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22(10):557–575. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357) doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berrien-Elliott MM, Cashen AF, Cubitt CC, et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020;10(12):1854–1871. doi: 10.1158/2159-8290.CD-20-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee DA, Verneris MR, Campana D. Acquisition, preparation, and functional assessment of human NK cells for adoptive immunotherapy. Methods Mol Biol. 2010;651:61–77. doi: 10.1007/978-1-60761-786-0_4. [DOI] [PubMed] [Google Scholar]
- 62.Berrien-Elliott MM, Becker-Hapak M, Cashen AF, et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood. 2021;139(8):1177–1183. doi: 10.1182/blood.2021011532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shapiro RM, Birch GC, Hu G, et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J Clin Invest. 2022;132(11) doi: 10.1172/JCI154334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan R, Mathyer M, Govero J, et al. Development of WU-NK-101, a feeder cell-free expanded allogeneic memory NK cell product with potent anti-tumor activity. J Immunother Cancer. 2021;9(suppl 2) Abstract 1188. [Google Scholar]
- 65.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morisot N, Wadsworth S, Davis T, et al. 127 Preclinical evaluation of NKX019, a CD19-targeting CAR NK Cell. J Immunother Cancer. 2020;8(suppl 3):A78. [Google Scholar]
- 68.Dickinson M, Hamad N, Bryant CE, et al. A phase 1 study of NKX019, a CD19 chimeric antigen receptor natural killer (CAR NK) cell therapy, in subjects with B-cell malignancies [abstract] Blood. 2021;138(suppl 1) Abstract 3868. [Google Scholar]
- 69.Bachier C, Borthakur G, Hosing C, et al. A phase 1 study of NKX101, an allogeneic CAR natural killer (NK) cell therapy, in subjects with relapsed/refractory (R/R) acute myeloid leukemia (AML) or higher-risk myelodysplastic syndrome (MDS) [abstract] Blood. 2020;136(suppl 1) Abstract 1040. [Google Scholar]
- 70.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant. 2016;22(7):1290–1298. doi: 10.1016/j.bbmt.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciurea S, Schafer J, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasu S, Bejanyan N, Devine SM, et al. BMT CTN 1803: haploidentical natural killer cells (K-NK002) to prevent post-transplant relapse in AML and MDS (NK-REALM) Transplant Cell Ther. 2021;27(3):S430–S431. [Google Scholar]
- 74.Rein L., Rizzieri D.A. A phase I trial of incorporating natural killer (K-NK) cells for patients with chronic myeloid leukemia (CML) and molecular residual disease after tyrosine kinase inhibitor (TKI) therapy. Transplant Cell Ther. 2021;27(3):S161. [Google Scholar]
- 75.Perussia B, Ramoni C, Anegon I, et al. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6(4):171–188. [PubMed] [Google Scholar]
- 76.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991;135(2):454–470. doi: 10.1016/0008-8749(91)90290-r. [DOI] [PubMed] [Google Scholar]
- 77.Igarashi T, Wynberg J, Srinivasan R, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104(1):170–177. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 78.Berg M, Lundqvist A, McCoy P, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11(3):341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carlens S, Gilljam M, Chambers BJ, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62(10):1092–1098. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 80.Passweg JR, Koehl U, Uharek L, Meyer-Monard S, Tichelli A. Natural-killer-cell-based treatment in haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2006;19(4):811–824. doi: 10.1016/j.beha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Quintarelli C, Sivori S, Caruso S, et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B-cell precursor acute lymphoblastic leukemia. Leukemia. 2019;34(4):1102–1115. doi: 10.1038/s41375-019-0613-7. [DOI] [PubMed] [Google Scholar]
- 82.Oyer JL, Pandey V, Igarashi RY, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 2016;18(5):653–663. doi: 10.1016/j.jcyt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Shrestha N, Dee M, Chaturvedi P, et al. A novel, non-feeder-cell approach to generate large numbers of cytokine-induced memory-like NK cells for adoptive cells therapies. J Immumolunol. 2020;204(suppl 1):A88.7. [Google Scholar]
- 84.Dalle JH, Menezes J, Wagner É, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res. 2005;57(5 pt 1):649–655. doi: 10.1203/01.PDR.0000156501.55431.20. [DOI] [PubMed] [Google Scholar]
- 85.Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med. 2016;2:1–10. doi: 10.3389/fmed.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah N., Martin-Antonio B., Yang H., et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076781. e76781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu E, Ang SOT, Kerbauy L, et al. GMP-compliant universal antigen presenting cells (uAPC) promote the metabolic fitness and antitumor activity of armored cord blood CAR-NK cells. Front Immunol. 2021;12:626098. doi: 10.3389/fimmu.2021.626098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller JS, Verfaillie C, McGlave P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood. 1992;80(9):2182–2187. [PubMed] [Google Scholar]
- 92.Yu H, Fehniger TA, Fuchshuber P, et al. Flt3 ligand promotes the generation of a distinct CD34+ human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92(10):3647–3657. [PubMed] [Google Scholar]
- 93.Spanholtz J, Tordoir M, Eissens D, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One. 2010;5(2):e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dolstra H, Roeven MWH, Spanholtz J, et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23(15):4107–4118. doi: 10.1158/1078-0432.CCR-16-2981. [DOI] [PubMed] [Google Scholar]
- 95.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113(24):6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2(4):274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hermanson DL, Bendzick L, Pribyl L, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells. 2016;34(1):93–101. doi: 10.1002/stem.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu H, Kaufman DS. An improved method to produce clinical-scale natural killer cells from human pluripotent stem cells. Methods Mol Biol. 2019;2048:107–119. doi: 10.1007/978-1-4939-9728-2_12. [DOI] [PubMed] [Google Scholar]
- 99.Lupo KB, Moon J Il, Chambers AM, Matosevic S. Differentiation of natural killer cells from induced pluripotent stem cells under defined, serum- and feeder-free conditions. Cytotherapy. 2021;23(10):939–952. doi: 10.1016/j.jcyt.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodridge JP, Mahmood S, Zhu H, et al. FT596: translation of first-of-kind multi-antigen targeted off-the-shelf CAR-NK cell with engineered persistence for the treatment of B cell malignancies [abstract] Blood. 2019;134(suppl 1) Abstract 301. [Google Scholar]
- 102.Patel K, Bachanova V, Goodman AM, et al. Phase I study of FT516, an off-the-shelf iPSC-derived NK cell therapy, in combination with rituximab in patients with relapsed/refractory B-cell lymphoma [abstract] Blood. 2021;138(suppl 1) Abstract 3873. [Google Scholar]
- 103.Cichocki F., Goodridge J., Bjordahl R., et al. Dual-antigen targeted off-the-shelf NK cells show durable response and prevent antigen escape in lymphoma and leukemia. Blood. 2022;140(23):2451–2462. doi: 10.1182/blood.2021015184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bachanova V., Ghobadi A., Patel K., et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma [abstract] Blood. 2021;138(suppl 1) Abstract 823. [Google Scholar]
- 105.Cichocki F., van der Stegen S.J.C., Miller J.S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood. 2023;141(8):846–855. doi: 10.1182/blood.2022016205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vey N, Bourhis J-H, Boissel N, et al. A phase I trial of the anti-inhibitory KIR monoclonal antibody IPH2101 for acute myeloid leukemia (AML) in complete remission. Blood. 2012;120(22):4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 107.Benson DM, Cohen AD, Jagannath S, et al. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin Cancer Res. 2015;21(18):4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vey N, Karlin L, Sadot-lebouvier S, et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin- like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget. 2018;9(25):17675–17688. doi: 10.18632/oncotarget.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vey N, Dumas P-Y, Recher C, et al. Randomized phase 2 trial of lirilumab (anti-KIR monoclonal antibody, mAb) as maintenance treatment in elderly patients (pts) with acute myeloid leukemia (AML): results of the Effikir Trial [abstract] Blood. 2017;130(suppl 1) Abstract 889. [Google Scholar]
- 110.André P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bexte T, Alzubi J, Reindl LM, et al. CRISPR-Cas9 based gene editing of the immune checkpoint NKG2A enhances NK cell mediated cytotoxicity against multiple myeloma. Oncoimmunology. 2022;11(1):2081415. doi: 10.1080/2162402X.2022.2081415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 113.Hsu J, Hodgins JJ, Marathe M, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blake SJ, Stannard K, Liu J, et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6(4):446–459. doi: 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 115.Zhu H, Blum RH, Bernareggi D, et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell. 2020;27(2):224–237.e6. doi: 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daher M, Basar R, Gokdemir E, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood. 2021;137(5):624–636. doi: 10.1182/blood.2020007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Becker-Hapak MK, Shrestha N, McClain E, et al. A fusion protein complex that combines IL-12, IL-15, and IL-18 signaling to induce memory-like NK cells for cancer immunotherapy. Cancer Immunol Res. 2021;9(9):1071–1087. doi: 10.1158/2326-6066.CIR-20-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Batlle E, Massagué J. Transforming grown factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaim H, Shanley M, Basar R, et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest. 2021;131(14) doi: 10.1172/JCI142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma T, Renz BW, Ilmer M, et al. Myeloid-derived suppressor cells in solid tumors. Cells. 2022;11(2):310. doi: 10.3390/cells11020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chambers AM, Lupo KB, Wang J, et al. Engineered natural killer cells impede the immunometabolic CD73-adenosine axis in solid tumors. Elife. 2022;11 doi: 10.7554/eLife.73699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (review) Int J Oncol. 2008;32(3):527–535. [PubMed] [Google Scholar]
- 123.Mittal D, Young A, Stannard K, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74(14):3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 124.Demaria O, Gauthier L, Debroas G, Vivier E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021;51(8):1934–1942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 125.Storti P, Costa F, Marchica V, et al. Novel approaches to improve myeloma cell killing by monoclonal antibodies. J Clin Med. 2020;9(9):2864. doi: 10.3390/jcm9092864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leivas A, Córdoba L, Valeri A, et al. NKG2D CAR-expressing lymphocytes target acute myeloid leukemia cells [abstract] Blood. 2019;134(suppl 1) Abstract 2667. [Google Scholar]
- 128.Dong H, Ham JD, Hu G, et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc Natl Acad Sci U S A. 2022;119(25) doi: 10.1073/pnas.2122379119. e2122379119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y., Basar R., Wang G., et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med. 2022;28(10):2133–2144. doi: 10.1038/s41591-022-02003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romee R, Cooley S, Berrien-Elliott M, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Merino A, Zhang B, Dougherty P, et al. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J Clin Invest. 2019;129(9):3770–3785. doi: 10.1172/JCI125916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woan KV, Kim H, Bjordahl R, et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell. 2021;28(12):2062–2075.e5. doi: 10.1016/j.stem.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gornalusse GG, Hirata RK, Funk SE, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35(8):765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37(3):252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]