Visual Abstract
Abstract
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a potentially curative treatment for patients with high-risk acute leukemias, but unfortunately disease recurrence remains the major cause of death in these patients. Infusion of donor lymphocytes (DLI) has the potential to restore graft-versus-leukemia immunologic surveillance; however, efficacy varies across different hematologic entities. Although relapsed chronic myeloid leukemia, transplanted in chronic phase, has proven remarkably susceptible to DLI, response rates are more modest for relapsed acute myeloid leukemia and acute lymphoblastic leukemia. To prevent impending relapse, a number of groups have explored administering DLI preemptively on detection of measurable residual disease (MRD) or mixed chimerism. Evidence for the effectiveness of this strategy, although encouraging, comes from only a few, mostly single-center retrospective, nonrandomized studies. This article seeks to (1) discuss the available evidence supporting this approach while highlighting some of the inherent challenges of MRD-triggered treatment decisions post-transplant, (2) portray other forms of postremission cellular therapies, including the role of next-generation target-specific immunotherapies, and (3) provide a practical framework to support clinicians in their decision-making process when considering preemptive cellular therapy for this difficult-to-treat patient population.
Edited by Associate Editor Robert Zeiser, this How I Treat series focuses on post–allogeneic transplant management of high-risk patients. These patients are at risk for relapse of malignancy and severe graft-versus-host disease (GVHD). Two articles in the series discuss adoptive cellular immunotherapy and maintenance therapy to preempt relapse of acute myeloid leukemia. The third article addresses the state of the art for preventing GVHD in this subset of stem cell transplant recipients.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a potentially curative treatment for acute leukemias. However, relapse is the major cause of death in this patient population. Second alloHSCT and infusion of donor lymphocytes (DLI) represent the only established treatment options with the potential for cure. Unfortunately, both measures convey equally disappointing results with long-term survival of 5% to 20%1, 2, 3, 4, 5.
The concept of DLI was introduced in the early 1990s6,7 to enhance the immunological graft-versus-leukemia (GvL) effect, driven in part by restoration of T-cell immunity and reversal of T-cell exhaustion in resident CD8+ T cells.8 Response to DLI, however, varies dramatically depending on the underlying disease. Relapsed chronic myeloid leukemia (CML), when transplanted in chronic phase, proved particularly susceptible to DLI, with response rates of 70% to 80%.7,9 For patients transplanted in the accelerated phase, response rates dropped to ∼40%, and those transplanted in blast crisis were resistant to DLI in an early seminal study.7 Relapsed acute myeloid leukemia (AML), similarly, is disproportionally more resistant than chronic-phase CML, with response rates of 30% to 40%.7,10 DLI performs even worse in the context of relapsed acute lymphoblastic leukemia (ALL), with remissions of less than 15%.7,11
In this article, we will discuss the available clinical evidence on the different cellular strategies pursued to treat and prevent relapse of high-risk AML after alloHSCT. Although others have comprehensively reviewed the evolution of cell therapy,12,13 this article will place an emphasis on how these strategies have been applied in the posttransplant setting. We will conclude with our institutional perspective on how to manage this hard-to-treat patient population. Although the primary focus of this article will be on AML, we will introduce seminal work conducted in the context of CD19+ lymphoid malignancies when it has been instrumental in driving forward innovations for the treatment of AML.
What is the role of preemptive cellular therapy after allogeneic transplant?
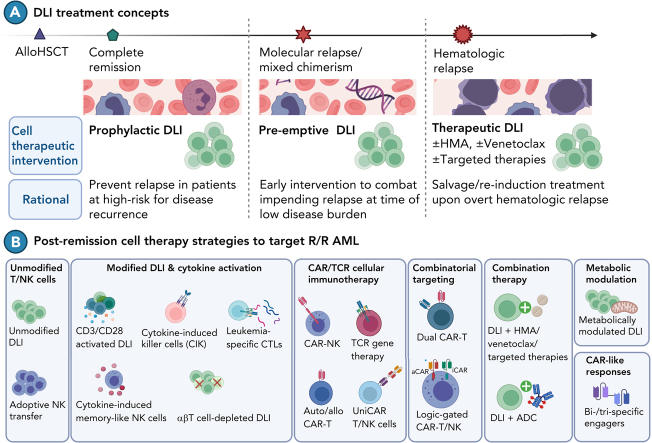
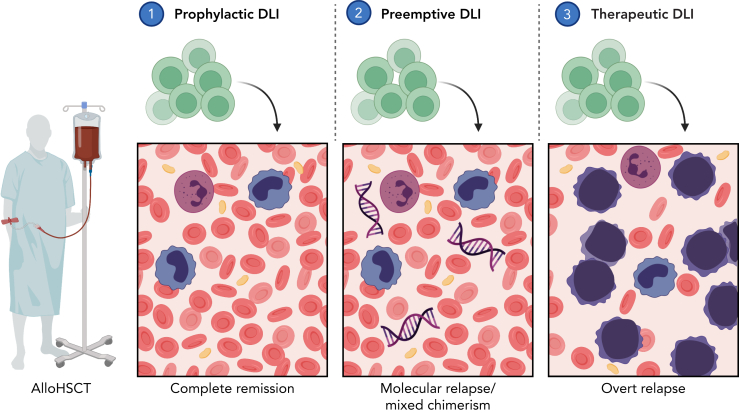
Given the limited efficacy of DLI at overt relapse, several groups have explored administering DLI earlier when disease burden is lower. Prophylactic and preemptive DLI both aim to augment the GvL effect before overt hematologic relapse. Figure 1 provides an overview of the different strategies pursued to refine DLI treatment.
Figure 1.
Overview of different strategies to administer DLI. (1) Prophylactic DLI is administered after a defined interval posttransplant to reduce the risk of relapse. (2) Preemptive DLI serves to restore GvL on impending relapse as detected by persisting MRD or LOC. (3) Therapeutic DLI is administered to treat overt hematologic relapse. Abbreviations: DLI, donor lymphocyte infusion; GvL, graft-versus-leukemia effect; LOC, loss of complete donor chimerism; MRD, measurable residual disease. Image created in BioRender.
Prophylactic DLI is administered to patients at high risk for relapse at a defined interval posttransplant to prevent disease recurrence. Identification of high-risk patients is based on pretransplant clinical features (unfavorable cytogenetics, chemo-refractory disease, remission status including measurable residual disease [MRD] at time of transplant), transplant-associated factors (use of anti-T-cell antibodies, T cell-depleted grafts, reduced-intensity conditioning [RIC] regimens, recipients of bone marrow grafts) and posttransplant considerations (graft-versus-host disease [GvHD], uncontrolled infections).14, 15, 16, 17, 18, 19
Preemptive DLI aims to combat impending relapse triggered either by detection of MRD or loss of complete donor chimerism (LOC).20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31,34,35
Both strategies need to be carefully weighed against potential toxicity, especially acute and chronic GvHD, which may occur in up to 12% and 31%, respectively.32, 33, 34
What is the clinical evidence to support preemptive DLI?
The evidence for preemptive DLI comes from a handful of mostly single-center, nonrandomized studies20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31,34,35 (Table 1) that encompass a broad range of different disease entities including AML but also spanning aggressive lymphatic malignancies, making interpretation of their results challenging. In 2007, Dominietto et al investigated preemptive DLI on detection of MRD after alloHSCT in patients with AML and ALL. The risk of relapse was reduced (6% vs 63%) and 3-year overall survival (OS) improved (80% vs 26%) compared with no intervention.20 Of note, this was a retrospective study and the non-DLI arm was composed only of patients ineligible for DLI because of uncontrolled GvHD, absence of a potential donor, or evidence of overt relapse, thus limiting any definitive conclusions. In a follow-up study, improved response rates (45% vs 33%) and prolonged 2-year survival (43% vs 19%) were reported when DLI was administered at the time of molecular relapse instead of overt relapse.26 Moreover, patients with relapsed/refractory (R/R) AML positive for Wilms’ tumor protein (WT1-MRD+) who received DLI at a lower cutoff threshold (100 WT1 copies/104 ABL) fared significantly better than those with a higher threshold of 180 WT1 copies/104 ABL (cumulative incidence of relapse [CIR]: 29% vs 76%; leukemia-free survival at 4 years: 74% vs 23%), underscoring the notion that DLI may be more effective at a time of lower disease burden.35
Table 1.
Overview of studies evaluating preemptive DLI
| Reference | Study design | Disease | Graft type, conditioning regimen, donor source, GvHD prophylaxis | Trigger | Cell dose | Study arms compared | Efficacy outcomes | Grade III/IV GvHD |
|---|---|---|---|---|---|---|---|---|
| 20 | Prospective, nonrandomized | AML (n = 36), ALL (n = 44) | NR | MRD | Starting CD3 dose Matched related: 1 × 106/kg MUD/MMUD: 1 × 105/kg |
Preemptive DLI vs no DLI |
CIR: 6% 3-y OS: 80% —— CIR: 63% 3-y OS: 26% |
NR |
| 21 | Case report | AML (n = 1) | NR | MRD, MC | Not reported | Single case report | Conversion to MRD negativity | NR |
| 22 | Prospective, nonrandomized | AML (n = 61) ALL (n = 38) MDS (n = 6) Total n = 105 |
T cell-replete MAC-BM/PBSC-alloHSCT Conditioning regimens/donor source: matched related (n = 40): Bu/Cy or Cy/TBI Haplo (n = 53) and MUD (n = 12): Bu/Cy + ATG or Cy/TBI + ATG GvHD prophylaxis: CSA, MMF, MTX (± ATG) |
MRD | Median CD3 dose: 3.8 × 107/kg | Preemptive DLI (n = 56) vs IL-2 (n = 49) |
CIR: 28% 3-y OS: 58% 3-y DFS: 56% —— CIR: 64% 3-y OS: 28% 3-y DFS: 24% |
8% (P = .471) —— 4% |
| 30 | Retrospective | AML (n = 14) ALL (n = 11) CML (n = 2) |
T cell-replete MAC-PBSC-alloHSCT Conditioning regimens/donor source: matched related (n = 14): Bu/Cy Haplo (n = 2) and MUD (n = 11): Bu/Cy + ATG + cytarabine GvHD prophylaxis: CSA, MMF, MTX (± ATG) |
MRD | Median CD3 dose: 5.51 × 107/kg | Preemptive DLI vs Therapeutic DLI |
1-y OS: 94% —— 1-y OS: 27% |
6% —— 9% |
| 24 | Prospective, nonrandomized phase 2 trial | AML (n = 14) MDS (n = 4) MPN (n = 2) Others (n = 16) |
T cell-replete low-dose alemtuzumab RIC-PBSC-alloHSCT Conditioning regimens: Myeloid: Flu/Bu Lymphoid: Flu/Cy (± rituximab) Donor source: MUD: 33 MMUD: 3 GvHD prophylaxis: Tacrolimus, MTX, alemtuzumab |
MC (<50% at d+60) | Starting CD3 dose: 0.5–1.0 × 107/kg | Preemptive DLI | 2-y OS: 57%; 2-y DFS: 38%; 1-y NRM: 14% |
0% |
| 25 | Retrospective | AML (n = 16) NHL (n = 6) HL (n = 5) MM (n = 4) ALL (n = 3) Others (n = 6) |
T cell-replete HLA-haplo-BM-alloHSCT Conditioning regimens: MAC (n = 6): Bu/Cy RIC (n = 34): Flu/Cy/TBI Donor source: Haplo (n = 40) GvHD prophylaxis: Tacrolimus, MMF, PTCy |
MRD, LOC | Starting CD3 dose 1 × 105/kg-1 × 106/kg | Preemptive DLI (n = 5) vs Therapeutic DLI (n = 35) |
ORR: 60% —— ORR: 26% |
NR |
| 23 | Prospective, nonrandomized | AML (n = 17) | T cell-replete BM/PBSC-MAC-alloHSCT Conditioning regimens/donor source: matched related (n = 43): Bu/Cy or Cy/TBI Haplo (n = 44) and MUD (n = 5): Bu/Cy + ATG or Cy/TBI + ATG GvHD prophylaxis: CSA, MMF, MTX (± ATG) |
MRD | NR | Preemptive DLI vs No DLI |
2-y CIR: 24% 2-y OS: 64% —— 2-y CIR: 84% 2-y OS: 0% |
NR |
| 26 | Retrospective | AML (n = 22) ALL (n = 9) MM (n = 1) |
T cell-replete HLA-haplo-MAC-BM-alloHSCT Conditioning regimens: Flu/Bu/TT Flu/TBI Donor source: Haplo (n = 32) GvHD prophylaxis: CSA, MMF, PTCy |
MRD | Starting CD3 dose 1 × 104/kg-1 × 105/kg | Preemptive DLI vs Therapeutic DLI |
ORR: 45% 2-y OS: 43% —— ORR: 33% 2-y OS: 19% |
5% —— 8% |
| 35 | Retrospective | AML (n = 207) | T cell-replete MAC-BM/PBSC/CB-alloHSCT Conditioning regimens: Bu/Flu (+TT), Bu/Cy, Cy/Mel/TT Cy/TT, Cy/TBI Donor source: Matched related (n = 82) MUD (n = 66) Haplo (n = 59) GvHD prophylaxis: CSA, MTX |
MRD | Starting CD3 dose: Matched-sibling: 1 × 106/kg MUD: 1 × 105/kg; HaploDLI: 1 × 104/kg |
WT1 100 copies/104 ABL vs WT1 180 copies/104 ABL |
CIR: 29% 4-y LFS 74% —— CIR: 76% 4-y LFS: 23% |
0% 0% |
| 31 | Retrospective | AML (n = 31) NHL (n = 19) MDS (n = 8) Others (n = 28) |
T cell-replete BM/PBSC-MAC/RIC-alloHSCT Conditioning regimens: RIC (n = 60): Flu, Mel, Alemtuzumab MAC (n = 26): Bu/Cy, Bu/Flu, TBI/Cy, TBI/Cy/VP Donor sources: Matched related (n = 53) MUD (n = 25) MMUD (n = 8) GvHD prophylaxis: Not reported for MAC; alemtuzumab for RIC |
MC | Starting CD3 dose: Matched-sibling: 3 × 106-1 × 107/kg MUD: 1-3 × 106/kg |
Preemptive DLI vs Therapeutic DLI |
5-y OS: 69% 5-y PFS: 71% —— 5-y OS: 28% 5-y PFS: 18% |
26% —— 31% |
| 28 | Retrospective | AML (n = 14) MDS (n = 20 ALL/NHL (n = 5) CML (n = 2) |
T-cell replete PB-haploHCT Conditioning regimens: MAC (n = 8) RIC (n = 13) Donor source: Haplo (n = 21) GvHD prophylaxis: tacrolimus, MMF/ sirolimus, MMF, PTCy |
MC | Most common starting CD3 dose: 1 × 106/kg |
Preemptive DLI (n = 2) vs Therapeutic DLI (n = 12) |
120-d OS: 100% —— 120-d OS: 29% |
NR |
| 27 | Retrospective, matched-pair analysis | AML, MDS, ALL (n = 20) |
T cell-replete BM/PBSC-MAC-alloHSCT Conditioning regimens/donor source: matched related (n = 8): Bu/Cy Haplo (n = 12): Bu/Cy + cytarabine + ATG GvHD prophylaxis: CSA, MMF, MTX |
MRD | Median CD3 dose: 2.5 × 107/kg | No control | MRD conversion: 75%; 2-y CIR 31%; 2-y OS 69% |
Grade II-IV aGvHD: 4 (20%) |
| 29 | Retrospective single-center landmark analysis | AML (n = 42) | T cell-replete PBSC-MAC/RIC-alloHSCT Conditioning regimens: MAC (n = 12): Bu/Cy, TT/Bu/Flu RIC (n = 30): Flu/BCNU/Mel, Flu/TT Donor source: matched related (n = 11) MUD (n = 23) MMUD (n = 7) Haplo (n = 1) GvHD prophylaxis: CSA/Alemtuzumab, CSA/MMF, MTX ± ATG |
MRD (n = 18) MC (n = 24) |
Median starting CD3 dose: 0.69 × 106/kg |
Preemptive DLI vs Therapeutic DLI |
5-y OS: 43% —— 5-y OS: 10% |
7% —— 6% |
| 34 | Retrospective registry study | AML (n = 153) ALL (n = 39) |
BM/PBSC-MAC /RIC-alloHSCT with (n = 149) and without (n = 42) T-cell depletion Conditioning regimens: MAC (n = 74) RIC (n = 107) Donor source: Matched related (n = 129) MUD (n = 63) GvHD prophylaxis: No IST regimens reported |
MRD (n = 23) MC (n = 169) |
Median starting CD3 dose: 1 × 106/kg | Preemptive DLI for MRD (n = 23) Preemptive DLI for MC (n = 169) |
Decreasing MRD/improving peripheral blood counts: 71% (16/21); 5-y NRM: 9% 5-y CIR: 44% 5-y LFS: 47% 5-y OS: 51% —— Improving MC: 70% (110/158); 5-y NRM: 15% 5-y CIR: 28% 5-y LFS: 57% 5-y OS: 63% |
7% (18/248 patients from entire cohort including preemptive DLI for MRD/MC and prophylactic DLI) |
aGvHD, acute graft-versus-host disease; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BCNU, carmustine; Bu, busulfan; CIR, cumulative incidence of relapse; CSA, cyclosporine A; Cy, cyclophosphamide; DFS, disease-free survival; DLI, donor lymphocyte infusion; Flu, fludarabine; Haplo, HLA-haploidentical donor; LFS, leukemia-free survival; MAC, myeloablative conditioning; MC, mixed chimerism; MDS, myelodysplastic syndrome; Mel, melphalan; MMUD, mismatched unrelated donor; MMF, mycophenolate mofetil; MRD, measurable residual disease; MTX, methotrexate; MUD, matched unrelated donor; NR, not reported; NRM, nonrelapse mortality; ORR, overall response rate; OS, overall survival; PTCy, posttransplant cyclophosphamide; RIC, reduced-intensity conditioning; TBI, total body irradiation; TT, thiotepa; VP, etoposide.
A large prospective trial evaluated preemptive DLI for MRD after alloHSCT across a spectrum of hematologic entities including AML. Preemptive DLI significantly reduced CIR at 3 years (28% vs 64%) without increasing the incidence of ≥grade 3 GvHD compared with interleukin-2 (IL-2).22 Two follow-up studies found similar results in AML and myelodysplastic syndrome (MDS).23,27 Interestingly, the addition of chemotherapy before DLI did not add any benefit.27 Another group tested preemptive DLI triggered by the detection of <50% donor T-cell chimerism at day d+60 in 25 patients receiving alemtuzumab-containing RIC-unrelated transplant for hematologic malignancies including AML. This approach was safe with no cases of high-grade GvHD and led to a promising 2-year OS of 57%.24
In 2021, Rettig et al studied preemptive DLI as triggered by either MRD or mixed chimerism (MC) vs therapeutic DLI in patients with AML.29 Preemptive DLI improved 5-year OS (43% vs 10%) with minimal incidence of high-grade GvHD. Patients with MC fared significantly better than those treated for MRD, possibly signifying a more favorable biological trajectory. Surprisingly, there was no significant difference regarding the time to first DLI between the 2 cohorts calling into question the traditional “early-warning” concept and potentially pointing to inherent differences in the underlying disease characteristics.29 On the contrary, timing has been suggested as a crucial determinant for both DLI efficacy and safety with unacceptable toxicity when administered before d+100 and potentially lost efficacy for prophylactic DLI administered beyond 6 months posttransplant.36
Although the available data support a role for preemptive DLI to treat impending relapse posttransplant, reliably establishing an MRD marker in the context of the heterogeneous genomic landscape of AML is challenging.29,37 Using MC to trigger preemptive DLI is even more troublesome because recrudescent recipient cells do not necessarily point toward relapse.1 Hence, it remains elusive when, and if at all, preemptively treated patients would have relapsed, had they not been given DLI infusions, leading to both lead time and selection biases.
What are the consensus guidelines regarding administration of preemptive DLI?
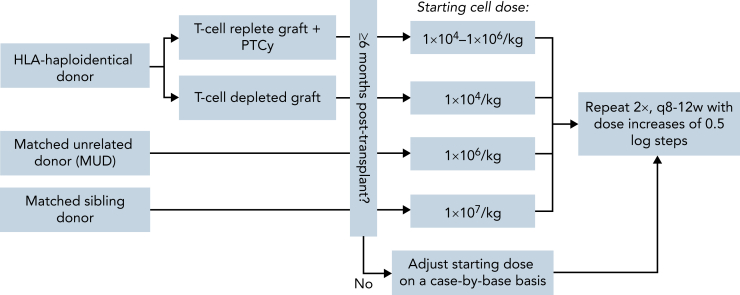
Based on the available evidence, the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) outlined in their 2016 Guidelines an individualized and risk-adapted approach to identify patients with AML at high risk for relapse for which preemptive DLI should be considered. Although acknowledging that their recommendations lie outside the current standard of care, they advocate for aggressive weaning and discontinuation of all immunosuppression upon detection of MRD and/or increasing MC and starting DLI as early as d+100 in suitable high-risk patients while closely monitoring for GvHD.1 The EBMT Handbook similarly describes the use of preemptive DLI for persisting or reemergent MRD/MC. According to the authors, DLI should only be initiated in the absence of GvHD and uncontrolled infections with a recommended starting cell dose of 1 × 105/kg to 1 × 106/kg for both related and unrelated matched donors, and 1 × 104/kg for HLA-haploidentical donors because of toxicity concerns arising from higher HLA disparity and risk of GvHD. DLI may be repeated 3 to 4 times at 4- to 12-week intervals with incremental dose increases of 0.5 to 1 log steps until achieving MRD negativity or complete donor chimerism.38 In their 2020 Consensus Recommendations on the Clinical Use of Haploidentical DLI, the Acute Leukemia Working Party notes the potential benefits of preemptive DLI and proposes repeated administration of DLI for persistent MRD or LOC starting at a dose of 1 × 105 CD3 cells/kg. The authors recommend tapering all immunosuppression before initiating DLI and excluding patients whose donor chimerism has fallen below 50% for concerns of marrow aplasia.39
What other strategies have been pursued to restore GvL using DLI?
Manipulated DLI
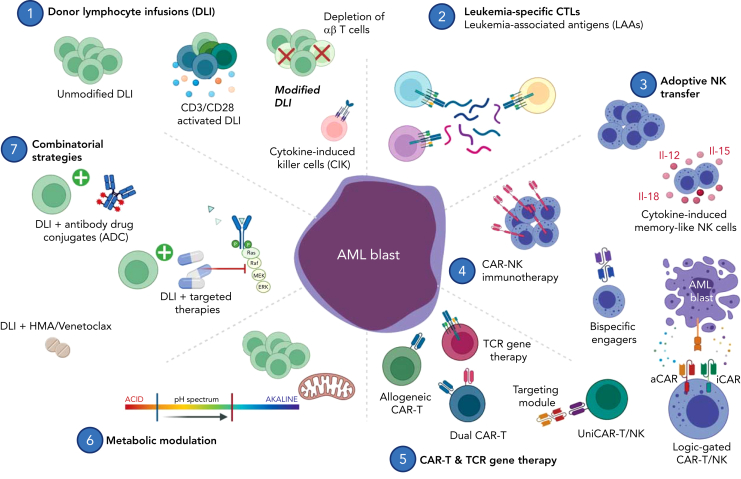
To discriminate GvL from GvHD, several groups have manipulated donor T cells ex vivo with the goal of favoring T-cell subsets that can kill leukemic cells while sparing healthy tissue. Figure 2 provides an overview of the various postremission strategies being pursued.
Figure 2.
Postremission adoptive cellular therapy for high-risk acute leukemia. (1) Donor lymphocyte infusions aim to restore GvL and have been applied clinically to treat overt relapse posttransplant, prevent relapse in high-risk patients (prophylactic intent), and to restore GvL on impending relapse as defined by detection of MRD or LOC (preemptive intent). Several groups have refined this strategy with depletion and enrichment of specific T-cell subsets. Cytokine-induced killer cells (CIK) mimic an NK-like phenotype and are capable of HLA-unrestricted killing mediated through NKG2D and DAP10. (2) CTLs are primed ex vivo with leukemia-associated antigen (LAA) peptide mixes to redirect specificity. (3) Adoptive transfer of NK cells supports immune reconstitution early posttransplant. Ex vivo cytokine induction using interleukins-12, -15, -18 primes memory-like NK cells for enhanced killing. (4) CAR-modified NK cells are redirected for specific target engagement using a genetically engineered synthetic receptor. Logic-gated CAR-NK cells can discriminate between leukemic blasts and healthy progenitor cells by recognition of both activating and inhibitory stimuli. (5) CAR-T cells are genetically engineered to specifically recognize and kill targets in an HLA-unrestricted manner. Universal allogeneic CAR-T candidates are genetically edited for safe clinical use despite being derived from third-party donors. Dual-CAR-T constructs target multiple antigens simultaneously to circumvent immune escape arising from antigen-negative disease. TCR-engineered T cells recognize leukemia-associated antigens such as WT1 in an HLA-dependent manner. (6, 7) DLI combination therapy promises both synergistic antileukemic activity and immune modulation to augment GvL. Abbreviations: aCAR, activating chimeric antigen receptor; ADC, antibody-drug conjugate; Bcl-2, B-cell lymphoma-2; BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; CIK, cytokine-induced killer cells; CTL, cytotoxic T lymphocytes; DLI, donor lymphocyte infusion; HDACi, histone deacetylase inhibitor; HMA, hypomethylating agent; iCAR, inhibitory chimeric antigen receptor; LAA, leukemia-associated antigen; LOC, loss of complete donor chimerism; TCR, T-cell receptor; WT1, Wilms’ tumor protein. Image created in BioRender.
Donor lymphocyte subsets
Early attempts to differentiate GvL from GvHD originated in the mid-1990s when groups sought to selectively deplete DLI of T-cell subsets that had been implicated in mediating GvHD.40, 41, 42, 43 Seeking to reduce relapse rates for recipients of T cell-depleted allografts, groups in the mid-2000s tested the prophylactic administration of CD8-depleted DLI to accelerate immune reconstitution early posttransplant. One study investigated infusion of CD8-depleted DLI on days +60 and +120 after T cell-depleted HLA-identical RIC alloHSCT and demonstrated it to be safe and capable of restoring full donor chimerism in 4 trial subjects with declining chimerism.44 For T cell-depleted HLA-haploidentical grafts, this approach was shown to promote T-cell recovery without increasing the risk of nonrelapse mortality (NRM; 1-year NRM: 15%).45
With time, research focus shifted to removing regulatory T-cell subsets (Treg) with the aim of unleashing alloreactivity and enhancing GvL. As hypothesized, Treg depletion resulted in enhanced response rates and improved event-free survival in patients with relapsed hematologic malignancies, without excess GvHD.46, 47, 48 Another approach, shown in preclinical studies to enhance GvL without increasing GvHD, is to enrich for memory T cells. This subset was believed to have a lower ability to induce GvHD because of a skewed T-cell receptor (TCR) repertoire secondary to prior antigen exposure.49,50 However, results from a phase 1 trial in patients with relapsed hematologic malignancies, including AML, yielded disappointing long-term disease control with only 3 of 15 patients (20%) in complete remission (CR) and a median disease-free survival of 4.9 months.51
Adopted from the field of pediatric haploidentical allo-transplant, DLI depleted of alloreactive αβ T cells52, 53, 54, 55 aim to skew the immune cell composition toward gamma-delta (γδ) T cells and natural killer (NK) cells that kill leukemic blasts in a non-major histocompatibility complex (MHC)-restricted manner without inducing GvHD. Evidence for this strategy remains scarce, with only 1 case report documenting the course of a patient with AML relapse after transplant. After sorafenib reinduction, the patient received a total of eight αβ T cell-depleted DLI infusions that helped induce and transiently maintain an MRD-negative remission before a second fulminant relapse, unresponsive to DLI.56 A phase 1 trial is under way to further explore this concept in the posttransplant setting (NCT03939585).57 A related effort aims to eradicate alloreactive T cells using photodepletion. In recipients of TCD-haploHSCT, this approach was shown to reduce NRM and prolong survival.58
Activated DLI and cytokine-induced killer cells
Carl June’s group explored in a phase 1 trial ex vivo activated DLI using CD3/CD28 stimulation and reported complete remissions in a subset of patients with relapsed leukemia after HSCT without causing excessive GvHD.59 In a follow-up study, they tested prophylactic administration of activated DLI on days +120 and +180 in patients with high risk for relapse following alloHSCT. This approach, although safe, had a limited effect in preventing relapse60 potentially because of delayed administration or intrinsically inferior potency compared with unstimulated DLI. In support of their expansion protocol, the authors report no phenotypic signs of T-cell exhaustion and point to previous studies of genetically modified T cells expanded up to >1000-fold using the same approach with evidence of in vivo persistence for up to a decade.
CIK cells are CD8+ T lymphocytes cultured ex vivo with interferon-γ, IL-2, and anti-CD3 to induce an NK-like phenotype with non-HLA-restricted cytotoxicity mediated through NKG2D and DAP10.61,62 Infusion of CIK cells has been explored in patients with relapsed hematologic malignancies posttransplant including AML and has been shown to induce clinically meaningful responses in 30% to 50% of patients without inducing high-grade GvHD.63,64
G-CSF mobilized DLI
Granulocyte colony-stimulating factor (G-CSF)-mobilized donor lymphocytes, derived from the initial stem cell product, have been explored in a small number of retrospective studies.65, 66, 67, 68, 69, 70, 71, 72 The cellular composition of the G-CSF DLI product has been reported to be different from that of steady-state DLI, with increased frequencies of immunoregulatory myeloid-derived suppressor cells, which might constrain severe forms of GvHD and favorably affect clinical outcomes. Furthermore, higher numbers of CD34+ progenitor cells might accelerate engraftment after myeloablative conditioning.72 Although one retrospective study reported improved conversion to full donor chimerism and lower CIR for G-CSF-primed DLI,67 most studies found both approaches to be equally effective and safe.
Leukemia-specific CTLs
In contrast to the previously mentioned forms of cellular immunotherapy, antigen-specific cytotoxic T cells (CTLs) aim to selectively target leukemia-associated antigens (LAAs) by priming donor-derived T cells with peptide mixes ex vivo in combination with cytokines.73, 74, 75 In AML, the adoptive transfer of donor-derived WT1-specific T cells was pioneered by Greenberg’s group and found to exert antileukemic activity, including induction of a prolonged MRD-negative remission in 1 patient.76 Extending the scope of CTLs beyond a single antigen, infusion of multispecific CTLs against PRAME, WT1, Survivin, and NY-ESO-1, is currently under investigation both with therapeutic and prophylactic intent in patients with AML after alloHSCT in a trial run by the Baylor group, with very promising early reports.77
How to combine DLI with other agents
DLI combination strategies build on the molecular rationale to modulate the T cell-driven antileukemic response and favorably impact GvL while mitigating GvHD. Epigenetic modulators, for instance, have been shown to augment GvL by upregulating previously silenced LAAs,78 minor histocompatibility antigens,79 and MHC class I/II molecules80, 81, 82 and may prevent GvHD by activating Tregs.83, 84, 85 The available clinical evidence both to combat overt relapse86, 87, 88 and with prophylactic intent,89, 90, 91, 92 however, is disappointing, with limited long-term success for most patients. More promising, a retrospective study found a survival benefit for patients with Flt3-ITD-mutant AML who received DLI and sorafenib as salvage treatment for relapse after alloHSCT.93 Venetoclax with DLI has also been explored but larger studies are needed to assess potential synergies.94 DLI has similarly been tested alongside novel leukemia-directed bispecific T cell engagers95, 96, 97, 98 and antibody-drug conjugates,99 such as gemtuzumab ozogamicin in the case of R/R AML (NCT03374332).
Other groups have examined ways to restore GvL, circumventing the direct transfer of immune cells by leveraging the power of immune checkpoint blockade. For example, CTLA-4 blockade after alloHSCT has been shown to induce remissions without causing significant GvHD,100 emulating some of the immunological effects observed with DLI.8 In a recent study, metabolic reprograming of infused lymphocytes restored GvL in patients with relapsed AML and augmented T-cell metabolic fitness as assessed by elevated levels of oxidative phosphorylation.101
How can NK cells prevent relapse posttransplant?
DLIs exert their antileukemic potency through HLA-restricted killing which is hampered by genomic HLA class I loss or downregulation of HLA II molecules, in effect rendering leukemic blasts invisible to donor T cells.102
In AML, especially in the HLA-haploidentical and mismatched setting, the selective pressure arising from HLA disparity has been shown to result in relapse associated with genomic loss of the mismatched HLA class I/II.103, 104, 105, 106 Seminal studies have shown epigenetic silencing of HLA class II molecules in up to 50% of patients with R/R AML after alloHSCT, including those transplanted from matched donors.107,108 Adoptive NK immunotherapy promises to overcome this mechanism of immune escape by relying on a set of germline-encoded endogenous killer-cell immunoglobulin-like receptors that prime NK cells for HLA-unrestricted killing.
Early evidence for NK-driven alloreactivity was observed in patients undergoing T cell-depleted haploHSCT, in which NK cells provided the dominant force of antileukemic immunity early after transplant.109, 110, 111 Evidence supporting the safe clinical use of NK adoptive cell therapy emerged in the early 2000s from the group in Minnesota and later by multiple other investigators that tested unmodified NK cells and ex vivo expanded NK cells to treat and prevent relapse after alloHSCT.112, 113, 114, 115, 116, 117, 118, 119, 120, 121 More recently, adoptive transfer of cytokine-induced memory-like NK cells, which are stimulated ex vivo with IL-12/15/18, has been shown to reduce the frequency of pathogenic variant alleles including WT1, TP53, TET2, ASXL1, in the first 3 patients with relapsed AML after alloHSCT in an ongoing phase 1 trial.122, 123, 124
Prophylactic NK immunotherapy has been explored to provide recipients of HLA-haploidentical grafts with an early line of immunologic defense to mitigate some of the morbidity and mortality secondary to delayed T-cell reconstitution.125,126 In a trial reported in 2021, infusion of up to 1 × 108 NK cells peritransplant led to early NK-dominant immune reconstitution and significantly reduced CIR compared with historical controls (4% vs 38%) with minimal incidence of high-grade GvHD.126 With the increasing adoption of posttransplant cyclophosphamide as GvHD prophylaxis both in the HLA-mismatched and matched transplantation setting, the benefits of early immune reconstitution afforded by adoptive transfer of ex vivo expanded NK cells are likely to become more relevant even beyond haploHSCT.
Cellular immunotherapy using engineered lymphocytes
The advent of genetically engineered lymphocytes has opened novel avenues to treat and prevent AML relapse posttransplant. The first clinical application to leverage these tools was to engineer T cells to express suicide genes including inducible Caspase9127,128 or thymidine kinase129 as safety switches, which allow their elimination in vivo in the event of GvHD.
CAR-redirected cellular therapy
The next leap in genetic engineering came with CD19-chimeric antigen receptor (CAR)-modified T lymphocytes, and some early studies focused on how these could be exploited to treat relapsed ALL after alloHSCT.130,131 In AML, there is a relative paucity of suitable antigens, and most efforts have focused on targeting CD33132, 133, 134, 135, 136 and CD123.135,137,138 Aside from the quest to identify viable targets, research efforts have also focused on engineering more sophisticated CAR constructs. Today’s CAR molecules build on optimized intracellular costimulatory domains and may include molecular payloads for enhanced functionality including transgenic expression of cytokines or immunomodulatory agents (ie, fourth-generation CAR-T cells, sometimes referred to as TRUCKs).139 Efforts are also ongoing to increase immune cell trafficking, extend in vivo persistence, and overcome functional suppression from the tumor microenvironment leveraging multiplexed genetic editing approaches.140, 141, 142, 143 Dual targeting has been proposed to overcome tumor immune evasion from antigen loss144, 145, 146, 147 and logic-gated CAR circuits now allow the selective targeting of cancerous cells while sparing healthy tissues with shared antigen expression.148, 149, 150 In AML, logic-gated CARs, which are activated by specific leukemic antigens but inhibited by concurrent recognition of surface molecules expressed on healthy HSCs, have been reported.151, 152, 153, 154 Another approach to target shared antigens while avoiding excessive toxicity builds on short-lived adaptor molecules that cross-link universal CAR molecules expressed on either T or NK cells with their respective targets.155, 156, 157, 158, 159 Recent first-in-human data demonstrated that this strategy can yield responses including CRs in relapsed AML160 (NCT04230265).
Despite these advancements, the cumbersome manufacturing process of autologous CAR-T products continues to restrict the scope of eligible patients, and several groups are exploring universal allogeneic CAR-T cells to overcome these logistical hurdles as well as immunologic anergy of autologous lymphocytes.161, 162, 163 In AML, allogeneic CAR-T cells targeting CD123 (NCT03190278, NCT02159495, NCT03114670, NCT03556982) and CD33 (NCT02799680) are being investigated in the clinic and could present an option for patients relapsing after alloHSCT.
CAR-NK immunotherapy offers a highly scalable, allogeneic cell product.164 Current NK cell therapy programs rely on different sources of NK cells including cord blood,164, 165, 166 peripheral blood,123,124,167 induced pluripotent stem cells (iPSCs)168, 169, 170 and cell lines,171,172 with each platform presenting its unique advantages and limitations, as has been reviewed previously.173,174 Clinically, CAR-NK therapy has demonstrated highly encouraging responses in patients with CD19+ malignancies.175,176 Current trial protocols have excluded patients who had previously undergone alloHSCT. Given its favorable safety record, CAR-NK immunotherapy may, however, be an attractive treatment strategy after transplant for future studies to explore. Next-generation CAR-NK therapies are already on the horizon, with many efforts ongoing to enhance NK cell fitness including through metabolic modulation.170,177
Another approach that circumvents the complexities of genetically engineered lymphocytes relies on bi-178, 179, 180, 181 or tri-specific engagers,182 which cross-link tumor and immune cells to emulate CAR-like responses, an approach starting to emerge to target AML.183, 184, 185
TCR gene therapy
Targeting AML is difficult because shared surface antigen expression across leukemic blasts and healthy stem cells. TCR gene therapy allows the reprogramming of T cells to recognize LAAs, presented through HLA molecules and promises to selectively target transformed cells.186 Greenberg and his group pioneered this approach by prophylactically infusing 12 patients after alloHSCT with genetically modified T cells targeting WT1.187, 188, 189 Compared with matched controls, they found an encouraging relapse-free survival rate of 100% vs 54% at a median follow-up of 44 months. Very recently, HLA-independent T cells have emerged and leverage a modified TCR-CD3 complex to target extracellular antigens. By design, HLA-independent T cells have dramatically increased antigen sensitivity and allow the targeting of antigens expressed at very low densities, another strategy to overcome antigen-low immune escape.190
How do we manage imminent relapse after alloHSCT using preemptive cell therapy?
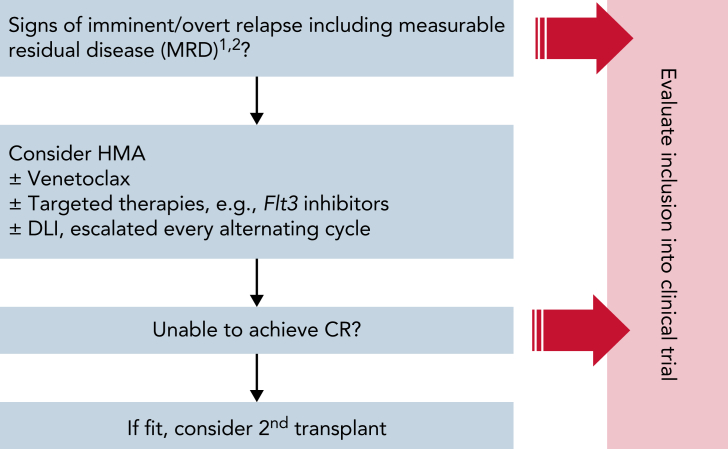
As evidence for the preemptive use of DLI remains scarce and available studies may be confounded by nonrandom selection bias, the decision for preemptive administration of DLI must be meticulously weighed against the risk of inducing GvHD. Although we cannot recommend a definitive guideline because of the lack of unequivocal data, we provide our institutional algorithm (Figure 3) to guide clinicians in their decision-making process to identify patients who might benefit from preemptive cellular therapy.
Figure 3.
MD Anderson Cancer Center institutional algorithm to manage patients with high-risk AML after alloHSCT outside of a clinical trial. The presented algorithm is adopted from MDACC’s institutional guidelines to help identify patients who may be candidates for cell therapy post-alloHSCT and to structure the clinical decision-making process that feeds into the management of this hard-to-treat patient population. As a general rule, we propose an individualized risk-stratified approach that takes into account both pretransplant clinical features (eg, remission status, molecular and cytogenetic risk profile, comorbidities, chemorefractory disease), transplant-associated aspects (eg, graft type, donor source, conditioning regimen, degree of HLA disparity, type of GvHD prophylaxis), and posttransplant parameters including the emergence and severity of aGvHD, MRD markers, and chimerism studies. Whenever possible, patients should be evaluated for inclusion into a clinical trial to benefit from novel innovative treatment strategies. Outside of clinical trials, patients should be treated on protocols whenever possible. Candidates for DLI should be discussed at a patient management conference. (1) MRD studies should be performed in accordance with Food and Drug Administration guidance and based on either multiparameter flow cytometry, reverse transcriptase-quantitative polymerase chain reaction (PCR), allele-specific oligonucleotide PCR (ASO-PCR), or targeted NGS. Assessment of MRD levels should be performed longitudinally (ie, both pre- and posttransplant) to provide the highest degree of sensitivity and specificity in detecting clinically relevant leukemia-driving clones taking into account the manufacturer’s guidance for the specific diagnostic test used. Results should be carefully interpreted in light of the test’s inherent sensitivity and specificity as well as diagnostic limitations arising from clonal evolution and clonal heterogeneity. (2) Chimerism assessment is done on peripheral blood or bone marrow. Due to the lack of unequivocal data and the concern of inducing GvHD, our institutional guidelines preclude any conclusions regarding the use of preemptive cell therapy based on mixed T-cell chimerism studies only.
Our institutional guidelines recommend performing T and myeloid chimerism studies on peripheral blood and if available also on bone marrow samples. For MRD studies, bone marrow samples would be preferred when available. Within our institution, MRD is routinely assessed by multiparameter flow cytometry but may be complemented with polymerase chain reaction or next-generation sequencing-based detection methods, bearing in mind the inherent limitations of MRD assessment in the posttransplant setting.37 Our institution regards mixed myeloid chimerism, as defined by the CD33+ fraction, a particular concern reflecting imminent relapse. Mixed T-cell chimerism, as defined by the CD3+ fraction, on the other hand, is frequently observed with busulfan/fludarabine conditioning regimens and should, by itself, not entail DLI infusions.
For patients with molecular relapse and/or mixed chimerism, we suggest evaluation for inclusion into a clinical trial. If no suitable trials are available, preemptive DLI may be considered in an individualized risk-stratified approach. Cell doses should be adjusted according to the donor source with starting doses of 1 × 106-7/kg in the matched-sibling/matched unrelated donor setting and 1 × 104-6/kg for haplo-DLI depending on patient and transplant-related factors including graft type and GvHD prophylaxis (Figure 4). Epigenetic modulators, venetoclax, and targeted therapies may also be considered. If DLI fails to convert MRD, HLA loss should be ruled out, preferably using polymerase chain reaction-based assays.191 Second alloHSCT should be reserved for overt hematologic relapse, in which chemotherapy-based reinduction should similarly be evaluated. For documented HLA loss, a different donor may be considered for the patient to benefit from renewed GvL.192,193 To illustrate our institutional management, we have included a case vignette of a patient with relapsed t-MDS after alloHSCT who received DLI with azacitidine and venetoclax to restore complete donor chimerism (Figure 5).
Figure 4.
MD Anderson Cancer Center institutional guidelines for DLI starting dose. DLI starting dose should be selected based on donor and graft type. If DLI is administered earlier than 6 months after transplant, the starting cell dose should be adjusted on a case-by-case basis to minimize the risk of GvHD. DLI is repeated every alternating cycle of HMA therapy, escalating cell doses incrementally in 0.5 log steps. q, every; w, weeks.
Figure 5.
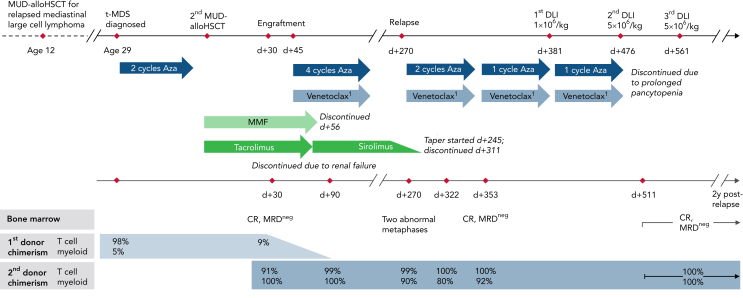
Case vignette. A 32-year-old man with a history of MUD-alloHSCT for relapsed mediastinal large cell lymphoma underwent second MUD-alloHSCT for therapy-related MDS (t-MDS) with complex karyotype and del7q. He relapsed at day (d) +270 with chimerism studies revealing 99% donor T cells and 90% donor myeloid cells. Chimerism studies on day +322 indicated further decrease of donor myeloid chimerism to 80%. Immunosuppression with sirolimus was tapered and ended by day +311. Administration of azacitidine in combination with dose-adjusted venetoclax due to anti-fungal prophylaxis led to MRDneg complete remission (CR) by day +353 with concomitant increase in donor myeloid chimerism to 92%. Three courses of DLI were administered from d+381 through d+561 at escalating dose levels of 1 × 106 to 5 × 106/kg. Donor myeloid chimerism was fully restored 30 days after the second course of DLI. The patient continues to be in MRDneg CR with complete donor chimerism at 2 years after relapse. 1Dose adjusted to 100 mg daily because of concomitant antifungal prophylaxis.
Conclusions and future directions
The quest to provide patients with high-risk AML with effective and tolerable treatment options has come a long way. However, despite the many strategies tested to improve outcomes for this patient group, the rate of relapse remains high, and long-term survival is unacceptably low.
On a more optimistic note, the available studies have linked preemptive DLI to reduced CIR and improved survival without excessive toxicity. Nevertheless, future prospective studies are needed to better delineate the role of preemptive DLI in preventing relapse. Although randomized, controlled trials are desirable, the complexity of studying patients in the posttransplant setting makes these studies challenging. Future research initiatives should embrace collaborative international efforts while fostering harmonization of standards to detect MRD to arrive at a meaningfully large sample size of real-world evidence.
The advent of genetically engineered lymphocytes has opened novel treatment avenues and laid the groundwork for further innovations.12,143,173 Going forward, we project that DLI will continue to play an important role in the management of patients with high-risk AML after alloHSCT. Aside from unmodified DLI, we anticipate multi-LAA-specific T cells and CAR/TCR-engineered cell therapies will gain considerable momentum and expand the current treatment armamentarium. Whether antigen-specific cell therapies will evolve to serve as preemptive measures to eradicate MRD/MC after alloHSCT remains to be seen. Notwithstanding, the powerful tools of genetic engineering promise an exciting new era of next-generation cell therapies, with novel concepts to modulate the immune response being explored to overcome some of the most notorious hurdles of AML therapy.
Conflict-of-interest disclosure: K.R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical and Affimed GmbH. K.R. participates on the Scientific Advisory Board for GemoAb, AvengeBio, Virogin Biotech, GSK, Caribou Biosciences, Navan Technologies, and Bayer. A.B. declares no competing financial interests.
Acknowledgments
The authors thank Judy Moyes for critical review and edit of the manuscript.
This work was supported by the German Research Foundation (A.B., as Walter Benjamin Postdoctoral Fellow) (464778766); by grants from CPRIT (RP160693), by a Stand Up To Cancer Dream Team Research Grant (grant number: SU2C-AACR-DT-29-19), by grants from the National Institutes of Health (NIH) National Cancer Institute (1 R01 CA211044-01, 5 P01CA148600-03, and P50CA100632-16), the Specialized Program of Research Excellence (SPORE) in Leukemia grant (P50CA100632), by a grant (CA016672) to the MD Anderson Cancer Center from the NIH, and the generous support of The University of Texas Anderson Cancer Center Moon Shots Program. The SU2C research grant is administered by the American Association for Cancer Research, the scientific partner of SU2C.
Authorship
Contribution: A.B. and K.R. wrote and approved the article.
References
- 1.Tsirigotis P, Byrne M, Schmid C, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016;51(11):1431–1438. doi: 10.1038/bmt.2016.167. [DOI] [PubMed] [Google Scholar]
- 2.Stein AS, Kantarjian H, Gökbuget N, et al. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1498–1504. doi: 10.1016/j.bbmt.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Spyridonidis A, Labopin M, Schmid C, et al. Immunotherapy Subcommittee of Acute Leukemia Working Party. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26(6):1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, et al. EBMT Acute Leukemia Working Party. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31):4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 5.Kharfan-Dabaja MA, Labopin M, Polge E, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018;4(9):1245–1253. doi: 10.1001/jamaoncol.2018.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. [PubMed] [Google Scholar]
- 7.Kolb H-J, Schattenberg A, Goldman JM, et al. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 8.Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123(9):1412–1421. doi: 10.1182/blood-2013-08-523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalandon Y, Passweg JR, Schmid C, et al. Chronic Leukemia Working Party of European Group for Blood and Marrow Transplantation. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2010;45(3):558–564. doi: 10.1038/bmt.2009.177. [DOI] [PubMed] [Google Scholar]
- 10.Takami A, Yano S, Yokoyama H, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2014;20(11):1785–1790. doi: 10.1016/j.bbmt.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Collins RH, Jr., Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26(5):511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 12.Wendel P, Reindl LM, Bexte T, et al. Arming immune cells for battle: a brief journey through the advancements of T and NK cell immunotherapy. Cancers (Basel) 2021;13(6):1481. doi: 10.3390/cancers13061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cieri N, Maurer K, Wu CJ. 60 years young: the evolving role of allogeneic hematopoietic stem cell transplantation in cancer immunotherapy. Cancer Res. 2021;81(17):4373–4384. doi: 10.1158/0008-5472.CAN-21-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedlickova Z, Schmid C, Koenecke C, et al. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(5):663–667. doi: 10.1038/bmt.2015.234. [DOI] [PubMed] [Google Scholar]
- 15.Yan CH, Liu QF, Wu DP, et al. Prophylactic donor lymphocyte infusion (DLI) followed by minimal residual disease and graft-versus-host disease-guided multiple DLIs could improve outcomes after allogeneic hematopoietic stem cell transplantation in patients with refractory/relapsed acute leukemia [published correction appears in Biol Blood Marrow Transplant. 2020;26(1):214] Biol Blood Marrow Transplant. 2017;23(8):1311–1319. doi: 10.1016/j.bbmt.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Cauchois R, Castagna L, Pagliardini T, et al. Prophylactic donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation for high risk haematological malignancies: a retrospective bicentric analysis of serial infusions of increasing doses of CD3+ cells. Br J Haematol. 2019;185(3):570–573. doi: 10.1111/bjh.15544. [DOI] [PubMed] [Google Scholar]
- 17.Greiner J, Götz M, Bunjes D, Hofmann S, Wais V. Immunological and clinical impact of manipulated and unmanipulated DLI after allogeneic stem cell transplantation of AML patients. J Clin Med. 2019;9(1):E39. doi: 10.3390/jcm9010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X-N, Lin J, Wang S-H, et al. Donor lymphocyte infusion for prevention of relapse after unmanipulated haploidentical PBSCT for very high-risk hematologic malignancies. Ann Hematol. 2019;98(1):185–193. doi: 10.1007/s00277-018-3482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid C, Labopin M, Schaap N, et al. EBMT Acute Leukaemia Working Party. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia - a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol. 2019;184(5):782–787. doi: 10.1111/bjh.15691. [DOI] [PubMed] [Google Scholar]
- 20.Dominietto A, Pozzi S, Miglino M, et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood. 2007;109(11):5063–5064. doi: 10.1182/blood-2007-02-072470. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann S, Götz M, Herbst C, et al. Preemptive donor lymphocyte infusion induces polyspecific T-cell responses in a patient with AML with NPM1 Mutation. Blood. 2011;118(21):4311. [Google Scholar]
- 22.Yan C-H, Liu D-H, Liu K-Y, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(14):3256–3262. doi: 10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wu D-P, Liu Q-F, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124(12):1880–1886. doi: 10.1182/blood-2014-03-563403. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SR, Sizemore CA, Zhang X, et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: a prospective phase II trial. Bone Marrow Transplant. 2014;49(5):616–621. doi: 10.1038/bmt.2014.2. [DOI] [PubMed] [Google Scholar]
- 25.Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(3):314–318. doi: 10.1016/j.bbmt.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghiso A, Raiola AM, Gualandi F, et al. DLI after haploidentical BMT with post-transplant CY. Bone Marrow Transplant. 2015;50(1):56–61. doi: 10.1038/bmt.2014.217. [DOI] [PubMed] [Google Scholar]
- 27.Mo X-D, Zhang X-H, Xu L-P, et al. Comparison of outcomes after donor lymphocyte infusion with or without prior chemotherapy for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2017;96(5):829–838. doi: 10.1007/s00277-017-2960-7. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith SR, Slade M, DiPersio JF, et al. Donor-lymphocyte infusion following haploidentical hematopoietic cell transplantation with peripheral blood stem cell grafts and PTCy. Bone Marrow Transplant. 2017;52(12):1623–1628. doi: 10.1038/bmt.2017.193. [DOI] [PubMed] [Google Scholar]
- 29.Rettig AR, Ihorst G, Bertz H, et al. Donor lymphocyte infusions after first allogeneic hematopoietic stem-cell transplantation in adults with acute myeloid leukemia: a single-center landmark analysis. Ann Hematol. 2021;100(9):2339–2350. doi: 10.1007/s00277-021-04494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan Y, Du K, Luo Y, et al. Superiority of preemptive donor lymphocyte infusion based on minimal residual disease in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Transfusion. 2014;54(6):1493–1500. doi: 10.1111/trf.12524. [DOI] [PubMed] [Google Scholar]
- 31.Caldemeyer LE, Akard LP, Edwards JR, Tandra A, Wagenknecht DR, Dugan MJ. Donor lymphocyte infusions used to treat mixed-chimeric and high-risk patient populations in the relapsed and nonrelapsed settings after allogeneic transplantation for hematologic malignancies are associated with high five-year survival if persistent full donor chimerism is obtained or maintained. Biol Blood Marrow Transplant. 2017;23(11):1989–1997. doi: 10.1016/j.bbmt.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;41(5):483–493. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 33.Castagna L, Sarina B, Bramanti S, Perseghin P, Mariotti J, Morabito L. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfus Apheresis Sci. 2016;54(3):345–355. doi: 10.1016/j.transci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Schmid C, Labopin M, Schaap N, et al. Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia. Bone Marrow Transplant. 2022;57(2):215–223. doi: 10.1038/s41409-021-01515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Grazia C, Pozzi S, Geroldi S, et al. Wilms tumor 1 expression and pre-emptive immunotherapy in patients with acute myeloid leukemia undergoing an allogeneic hemopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(7):1242–1246. doi: 10.1016/j.bbmt.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Tsirigotis P, Gkirkas K, Kitsiou V, et al. Repetitively administered low-dose donor lymphocyte infusion for prevention of relapse after allogeneic stem cell transplantation in patients with high-risk acute leukemia. Cancers (Basel) 2021;13(11):2699. doi: 10.3390/cancers13112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spyridonidis A. How I treat measurable (minimal) residual disease in acute leukemia after allogeneic hematopoietic cell transplantation. Blood. 2020;135(19):1639–1649. doi: 10.1182/blood.2019003566. [DOI] [PubMed] [Google Scholar]
- 38.Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer International Publishing; Cham: 2019. [PubMed] [Google Scholar]
- 39.Dholaria B, Savani BN, Labopin M, et al. Clinical applications of donor lymphocyte infusion from an HLA-haploidentical donor: consensus recommendations from the Acute Leukemia Working Party of the EBMT. Haematologica. 2020;105(1):47–58. doi: 10.3324/haematol.2019.219790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giralt S, Hester J, Huh Y, et al. CD8-depleted donor lymphocyte infusion as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation. Blood. 1995;86(11):4337–4343. [PubMed] [Google Scholar]
- 41.Alyea EP, Soiffer RJ, Canning C, et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91(10):3671–3680. [PubMed] [Google Scholar]
- 42.Soiffer RJ, Alyea EP, Hochberg E, et al. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood Marrow Transplant. 2002;8(11):625–632. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 43.Orti G, Lowdell M, Fielding A, et al. Phase I study of high-stringency CD8 depletion of donor leukocyte infusions after allogeneic hematopoietic stem cell transplantation. Transplantation. 2009;88(11):1312–1318. doi: 10.1097/TP.0b013e3181bbf382. [DOI] [PubMed] [Google Scholar]
- 44.Meyer RG, Britten CM, Wehler D, et al. Prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantation. Blood. 2007;109(1):374–382. doi: 10.1182/blood-2006-03-005769. [DOI] [PubMed] [Google Scholar]
- 45.Dodero A, Carniti C, Raganato A, et al. Haploidentical stem cell transplantation after a reduced-intensity conditioning regimen for the treatment of advanced hematologic malignancies: posttransplantation CD8-depleted donor lymphocyte infusions contribute to improve T-cell recovery. Blood. 2009;113(19):4771–4779. doi: 10.1182/blood-2008-10-183723. [DOI] [PubMed] [Google Scholar]
- 46.Hicheri Y, Bouchekioua A, Hamel Y, et al. Donor regulatory T cells identified by FoxP3 expression but also by the membranous CD4+CD127low/neg phenotype influence graft-versus-tumor effect after donor lymphocyte infusion. J Immunother. 2008;31(9):806–811. doi: 10.1097/CJI.0b013e318184908d. [DOI] [PubMed] [Google Scholar]
- 47.Maury S, Lemoine FM, Hicheri Y, et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med. 2010;2(41):41ra52. doi: 10.1126/scitranslmed.3001302. [DOI] [PubMed] [Google Scholar]
- 48.Nikiforow S, Kim HT, Daley H, et al. A phase I study of CD25/regulatory T-cell-depleted donor lymphocyte infusion for relapse after allogeneic stem cell transplantation. Haematologica. 2016;101(10):1251–1259. doi: 10.3324/haematol.2015.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117(6):1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muffly L, Sheehan K, Armstrong R, et al. Infusion of donor-derived CD8+ memory T cells for relapse following allogeneic hematopoietic cell transplantation. Blood Adv. 2018;2(6):681–690. doi: 10.1182/bloodadvances.2017012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang P, Feuchtinger T, Teltschik HM, et al. Improved immune recovery after transplantation of TCRαβ/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015;50(2 suppl 2):S6–S10. doi: 10.1038/bmt.2015.87. [DOI] [PubMed] [Google Scholar]
- 53.Locatelli F, Merli P, Pagliara D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood. 2017;130(5):677–685. doi: 10.1182/blood-2017-04-779769. [DOI] [PubMed] [Google Scholar]
- 54.Lang PJ, Schlegel PG, Meisel R, et al. Safety and efficacy of Tcralpha/beta and CD19 depleted haploidentical stem cell transplantation following reduced intensity conditioning in children: results of a prospective multicenter phase I/II clinical trial. Blood. 2017;130:214. [Google Scholar]
- 55.Bertaina A, Zecca M, Buldini B, et al. Unrelated donor vs HLA-haploidentical α/β T-cell- and B-cell-depleted HSCT in children with acute leukemia. Blood. 2018;132(24):2594–2607. doi: 10.1182/blood-2018-07-861575. [DOI] [PubMed] [Google Scholar]
- 56.Kordelas L, Buttkereit U, Lindemann M, et al. αβ-T-cell depleted donor lymphocyte infusion for leukemia relapse after allogeneic stem cell transplantation. Bone Marrow Transplant. 2017;52(12):1668–1670. doi: 10.1038/bmt.2017.185. [DOI] [PubMed] [Google Scholar]
- 57.Otegbeye F, Ramsey C, Fox R, et al. Manufacturing a donor lymphocyte product depleted of TCR-αβ T cells and CD19+ B cells for prophylactic infusion following allogeneic stem cell transplantation. Cytotherapy. 2020;22(5 suppl):S134. [Google Scholar]
- 58.Roy DC, Walker I, Maertens J, et al. ATIR101 administered after T-cell-depleted haploidentical HSCT reduces NRM and improves overall survival in acute leukemia. Leukemia. 2020;34(7):1907–1923. doi: 10.1038/s41375-020-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107(4):1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 60.Kumar AJ, Hexner EO, Frey NV, et al. Pilot study of prophylactic ex vivo costimulated donor leukocyte infusion after reduced-intensity conditioned allogeneic stem cell transplantation [published correction appears in Biol Blood Marrow Transplant. 2015;125(16):2583] Biol Blood Marrow Transplant. 2013;19(7):1094–1101. doi: 10.1016/j.bbmt.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 61.Verneris MR, Karimi M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells [published correction appears in Blood. 2015;125(16):2583] Blood. 2004;103(8):3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 62.Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112(6):2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1679–1687. doi: 10.1016/j.bbmt.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]
- 65.Abbi KK, Zhu J, Ehmann WC, et al. G-CSF mobilized vs conventional donor lymphocytes for therapy of relapse or incomplete engraftment after allogeneic hematopoietic transplantation. Bone Marrow Transplant. 2013;48(3):357–362. doi: 10.1038/bmt.2012.144. [DOI] [PubMed] [Google Scholar]
- 66.Lee Y, Yoon JS, Lee IH, Moon JH, Sohn SK. Efficacy of G-CSF primed DLI using cells reserved at the time of harvest in patients with hematologic malignancies who lately relapsed after allogeneic PBSCT. Blood. 2016;128(22):5809. [Google Scholar]
- 67.Schneidawind C, Jahnke S, Schober-Melms I, et al. G-CSF administration prior to donor lymphocyte apheresis promotes anti-leukaemic effects in allogeneic HCT patients. Br J Haematol. 2019;186(1):60–71. doi: 10.1111/bjh.15881. [DOI] [PubMed] [Google Scholar]
- 68.Lamure S, Paul F, Gagez AL, et al. A retrospective comparison of DLI and gDLI for post-transplant treatment. J Clin Med. 2020;9(7):E2204. doi: 10.3390/jcm9072204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De León AG, Colunga-Pedraza PR, Garcia-Camarillo DE, et al. Donor lymphocyte infusion from G-CSF-primed, unmanipulated whole blood is safe and improves chimerism in HLA-matched and haploidentical transplantation. Biol Blood Marrow Transplant. 2019;25(3 suppl):S171–S172. [Google Scholar]
- 70.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007;92(3):414–417. doi: 10.3324/haematol.10570. [DOI] [PubMed] [Google Scholar]
- 71.Antonenas V, Garvin F, Yehson K, Hansra G, Micklethwaite K, Gottlieb D. G-CSF primed donor haematopoietic stem cell collections are associated with reduced viable T cell yield for donor lymphocyte infusion. Cytotherapy. 2015;17(6):S57. [Google Scholar]
- 72.Jaiswal SR, Zaman S, Chakrabarti A, et al. Improved outcome of refractory/relapsed acute myeloid leukemia after post-transplantation cyclophosphamide-based haploidentical transplantation with myeloablative conditioning and early prophylactic granulocyte colony-stimulating factor-mobilized donor lymphocyte infusions. Biol Blood Marrow Transplant. 2016;22(10):1867–1873. doi: 10.1016/j.bbmt.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Gerdemann U, Vera JF, Christin AS, et al. 29. Multi-tumor-antigen-specific cytotoxic T lymphocytes for cancer immunotherapy. Mol Ther. 2010;18:S12–S13. [Google Scholar]
- 74.Weber G, Gerdemann U, Caruana I, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013;27(7):1538–1547. doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber G, Gerdemann U, Hensel NF, Leen AM, Bollard CM, Barrett AJ. Generation of multi-antigen specific T cells for adoptive immunotherapy of myeloid leukemia and identification of MHC class I and II-restricted peptides for WT1, proteinase 3 and human neutrophil elastase. Blood. 2011;118(21):2985. [Google Scholar]
- 76.Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lulla PD, Naik S, Vasileiou S, et al. Clinical effects of administering leukemia-specific donor T cells to patients with AML/MDS after allogeneic transplant [published correction appears in Blood. 2022;139(8):1257] Blood. 2021;137(19):2585–2597. doi: 10.1182/blood.2020009471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 79.Hambach L, Ling K-W, Pool J, et al. Hypomethylating drugs convert HA-1-negative solid tumors into targets for stem cell-based immunotherapy. Blood. 2009;113(12):2715–2722. doi: 10.1182/blood-2008-05-158956. [DOI] [PubMed] [Google Scholar]
- 80.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96(12):3847–3856. [PubMed] [Google Scholar]
- 81.Magner WJ, Kazim AL, Stewart C, et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165(12):7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 82.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Ødum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65(23):11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder T, Fröbel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. 2013;27(9):1910–1913. doi: 10.1038/leu.2013.64. [DOI] [PubMed] [Google Scholar]
- 84.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116(1):129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119(14):3361–3369. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 86.Steinmann J, Bertz H, Wäsch R, et al. 5-Azacytidine and DLI can induce long-term remissions in AML patients relapsed after allograft. Bone Marrow Transplant. 2015;50(5):690–695. doi: 10.1038/bmt.2015.10. [DOI] [PubMed] [Google Scholar]
- 87.Tessoulin B, Delaunay J, Chevallier P, et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(4):567–571. doi: 10.1038/bmt.2013.233. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27(6):1229–1235. doi: 10.1038/leu.2013.7. [DOI] [PubMed] [Google Scholar]
- 89.Guillaume T, Malard F, Magro L, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019;54(11):1815–1826. doi: 10.1038/s41409-019-0536-y. [DOI] [PubMed] [Google Scholar]
- 90.Bug G, Burchert A, Wagner E-M, et al. Phase I/II study of the deacetylase inhibitor panobinostat as maintenance therapy after an allogeneic stem cell transplantation in patients with high-risk MDS or AML: the Panobest-Trial. Blood. 2015;126(23):4344. [Google Scholar]
- 91.Kalin B, van Norden Y, van Gelder M, et al. Panobinostat and decitabine prior to donor lymphocyte infusion in allogeneic stem cell transplantation. Blood Adv. 2020;4(18):4430–4437. doi: 10.1182/bloodadvances.2020002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oran B, de Lima M, Garcia-Manero G, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients [published correction appears in Blood Adv. 2021;5(6):1755–1756] Blood Adv. 2020;4(21):5580–5588. doi: 10.1182/bloodadvances.2020002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathew NR, Baumgartner F, Braun L, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells [published correction appears in Nat Med. 2018;24(4):526] Nat Med. 2018;24(3):282–291. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amit O, On YB, Perez G, Shargian-Alon L, Yeshurun M, Ram R. Venetoclax and donor lymphocyte infusion for early relapsed acute myeloid leukemia after allogeneic hematopoietic cell transplantation. A retrospective multicenter trial. Ann Hematol. 2021;100(3):817–824. doi: 10.1007/s00277-021-04398-y. [DOI] [PubMed] [Google Scholar]
- 95.Ueda M, de Lima M, Caimi P, et al. Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2016;51(9):1253–1255. doi: 10.1038/bmt.2016.104. [DOI] [PubMed] [Google Scholar]
- 96.Bondarenko SN, Maschan AA, Parovichnikova EN, et al. The efficacy and toxicity of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia: Russian Multicenter Experience. Blood. 2017;130(suppl 1):2604. [Google Scholar]
- 97.Durer C, Durer S, Shafqat M, et al. Concomitant use of blinatumomab and donor lymphocyte infusion for post-transplant relapsed CD19 positive acute lymphoblastic leukemia: systematic review. Blood. 2018;132(suppl 1):5742. [Google Scholar]
- 98.Paul S, Rausch CR, Nasnas PE, Kantarjian H, Jabbour EJ. Treatment of relapsed/refractory acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2019;17(3):166–175. [PubMed] [Google Scholar]
- 99.Papayannidis C, Sartor C, Dominietto A, et al. ALL-073: inotuzumab ozogamicin (IO) and donor lymphocyte infusion (DLI) are a safe and promising combination in relapsed acute lymphoblastic leukemia (ALL) after allogeneic hematopoietic stem cell transplant (HSCT) Clin Lymphoma Myeloma Leuk. 2020;20:S161. [Google Scholar]
- 100.Davids MS, Kim HT, Bachireddy P, et al. Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uhl FM, Chen S, O’Sullivan D, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci Transl Med. 2020;12(567) doi: 10.1126/scitranslmed.abb8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeiser R, Vago L. Mechanisms of immune escape after allogeneic hematopoietic cell transplantation. Blood. 2019;133(12):1290–1297. doi: 10.1182/blood-2018-10-846824. [DOI] [PubMed] [Google Scholar]
- 103.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 104.Villalobos IB, Takahashi Y, Akatsuka Y, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115(15):3158–3161. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 105.McCurdy SR, Iglehart BS, Batista DA, et al. Loss of the mismatched human leukocyte antigen haplotype in two acute myelogenous leukemia relapses after haploidentical bone marrow transplantation with post-transplantation cyclophosphamide. Leukemia. 2016;30(10):2102–2106. doi: 10.1038/leu.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grosso D, Johnson E, Colombe B, et al. Acquired uniparental disomy in chromosome 6p as a feature of relapse after T-cell replete haploidentical hematopoietic stem cell transplantation using cyclophosphamide tolerization. Bone Marrow Transplant. 2017;52(4):615–619. doi: 10.1038/bmt.2016.324. [DOI] [PubMed] [Google Scholar]
- 107.Christopher MJ, Petti AA, Rettig MP, et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379(24):2330–2341. doi: 10.1056/NEJMoa1808777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toffalori C, Zito L, Gambacorta V, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603–611. doi: 10.1038/s41591-019-0400-z. [DOI] [PubMed] [Google Scholar]
- 109.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 110.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Velardi A. Natural killer cell alloreactivity 10 years later. Curr Opin Hematol. 2012;19(6):421–426. doi: 10.1097/MOH.0b013e3283590395. [DOI] [PubMed] [Google Scholar]
- 112.Koehl U, Sörensen J, Esser R, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33(3):261–266. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 113.Passweg JR, Tichelli A, Meyer-Monard S, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18(11):1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 114.Slavin S, Morecki S, Shapira MY, et al. Use of matched or mismatched rIL-2 activated donor lymphocytes positively selected for CD56+ for immunotherapy of resistant leukemia after allogeneic stem cell transplantation. J Clin Oncol. 2004;22(14 suppl):6516. [Google Scholar]
- 115.Koehl U, Esser R, Zimmermann S, et al. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr. 2005;217(6):345–350. doi: 10.1055/s-2005-872520. [DOI] [PubMed] [Google Scholar]
- 116.Passweg JR, Koehl U, Uharek L, Meyer-Monard S, Tichelli A. Natural-killer-cell-based treatment in haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2006;19(4):811–824. doi: 10.1016/j.beha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Barkholt L, Alici E, Conrad R, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1(5):753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 118.Rizzieri DA, Storms R, Chen DF, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(8):1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uharek L, Friedrichs B, Nogai A, et al. Successful treatment of high-risk and refractory acute myeloid leukemia with haploidentical stem cell transplantation plus NK cell therapy. Blood. 2010;116(21):2370. [Google Scholar]
- 120.Farhan S, Lee DA, Champlin RE, Ciurea SO. NK cell therapy: targeting disease relapse after hematopoietic stem cell transplantation. Immunotherapy. 2012;4(3):305–313. doi: 10.2217/imt.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant. 2016;22(7):1290–1298. doi: 10.1016/j.bbmt.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shapiro RM, Nikiforow S, Rambaldi B, et al. Cytokine-induced memory-like NK cells exhibit massive expansion and long-term persistence after infusion post-haploidentical stem cell transplantation: a report of the first three cases in a phase I trial. Blood. 2020;136(suppl 1):8–9. [Google Scholar]
- 123.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357) doi: 10.1126/scitranslmed.aaf2341. 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation [published correction appears in Blood. 2018;132(26):2782] Blood. 2017;130(16):1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ciurea SO, Kongtim P, Soebbing D, et al. Decrease post-transplant relapse using donor-derived expanded NK-cells. Leukemia. 2022;36(1):155–164. doi: 10.1038/s41375-021-01349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou X, Di Stasi A, Tey SK, et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood. 2014;123(25):3895–3905. doi: 10.1182/blood-2014-01-551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125(26):4103–4113. doi: 10.1182/blood-2015-02-628354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ciceri F, Bonini C, Stanghellini MTL, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 130.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marin V, Pizzitola I, Agostoni V, et al. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010;95(12):2144–2152. doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dutour A, Marin V, Pizzitola I, et al. In vitro and in vivo antitumor effect of anti-cd33 chimeric receptor-expressing EBV-CTL against acute myeloid leukemia. Adv Hematol. 2012;2012 doi: 10.1155/2012/683065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang QS, Wang Y, Lv HY, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28(8):1596–1605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 136.Tambaro FP, Singh H, Jones E, et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021;35(11):3282–3286. doi: 10.1038/s41375-021-01232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mardiros A, Dos Santos C, McDonald T, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.El Khawanky N, Hughes A, Yu W, et al. Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leukemia. Nat Commun. 2021;12(1):6436. doi: 10.1038/s41467-021-26683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holzinger A, Abken H. CAR T cells: a snapshot on the growing options to design a CAR. HemaSphere. 2019;3(1):e172. doi: 10.1097/HS9.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katti A, Diaz BJ, Caragine CM, Sanjana NE, Dow LE. CRISPR in cancer biology and therapy. Nat Rev Cancer. 2022;22(5):259–279. doi: 10.1038/s41568-022-00441-w. [DOI] [PubMed] [Google Scholar]
- 142.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Biederstädt A, Rezvani K. Engineering the next generation of CAR-NK immunotherapies. Int J Hematol. 2021;114(5):554–571. doi: 10.1007/s12185-021-03209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cordoba S, Onuoha S, Thomas S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27(10):1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Petrov JC, Wada M, Pinz KG, et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia. 2018;32(6):1317–1326. doi: 10.1038/s41375-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-cell-based immunotherapy of acute myeloid leukemia: current concepts and future developments. Leukemia. 2021;35(7):1843–1863. doi: 10.1038/s41375-021-01253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Srivastava S, Salter AI, Liggitt D, et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell. 2019;35(3):489–503. doi: 10.1016/j.ccell.2019.02.003. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wallstabe L, Göttlich C, Nelke LC, et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4(18) doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho JH, Okuma A, Sofjan K, Lee S, Collins JJ, Wong WW. Engineering advanced logic and distributed computing in human CAR immune cells. Nat Commun. 2021;12(1):792. doi: 10.1038/s41467-021-21078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzalez A, Roguev A, Frankel NW, et al. Abstract LB028: Development of logic-gated CAR-NK cells to reduce target-mediated healthy tissue toxicities. Cancer Res. 2021;81(13 suppl):LB028. [Google Scholar]
- 152.Garrison BS, Deng H, Yucel G, et al. Precise targeting of AML with first-in-class OR/NOT logic-gated gene circuits in CAR-NK Cells. Presented at the 24th ASGCT Annual Meeting Abstract 77. 2021;29:4S1. [Google Scholar]
- 153.Richards RM, Zhao F, Freitas KA, et al. NOT-gated CD93 CAR T cells effectively target AML with minimized endothelial cross-reactivity. Blood Cancer Discov. 2021;2(6):648–665. doi: 10.1158/2643-3230.BCD-20-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haubner S, Mansilla-Soto J, Nataraj S, et al. “IF-better” gating: combinatorial targeting and synergistic signaling for enhanced CAR T cell Efficacy. Blood. 2021;138(suppl 1):2774. [Google Scholar]
- 155.Loff S, Dietrich J, Meyer JE, et al. Rapidly switchable universal CAR-T cells for treatment of CD123-positive leukemia. Mol Ther Oncolytics. 2020;17:408–420. doi: 10.1016/j.omto.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bachmann D, Aliperta R, Bergmann R, et al. Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19 positive tumor cells. Oncotarget. 2017;9(7):7487–7500. doi: 10.18632/oncotarget.23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6(8):e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meyer JE, Loff S, Dietrich J, et al. Evaluation of switch-mediated costimulation in trans on universal CAR-T cells (UniCAR) targeting CD123-positive AML. OncoImmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1945804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mitwasi N, Feldmann A, Arndt C, et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci Rep. 2020;10(1):2141. doi: 10.1038/s41598-020-59082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wermke M, Kraus S, Ehninger A, et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood. 2021;137(22):3145–3148. doi: 10.1182/blood.2020009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 162.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 163.Galetto R, Lebuhotel C, Gouble A, et al. 397. Allogenic CAR T-cells targeting CD123 effectively eliminate myeloid leukemia cells. Mol Ther. 2016;24(suppl 1):S157–S158. [Google Scholar]
- 164.Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dolstra H, Roeven MWH, Spanholtz J, et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23(15):4107–4118. doi: 10.1158/1078-0432.CCR-16-2981. [DOI] [PubMed] [Google Scholar]
- 166.Hoogstad-van Evert JS, Bekkers R, Ottevanger N, Jansen JH, Massuger L, Dolstra H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol Oncol. 2020;157(3):810–816. doi: 10.1016/j.ygyno.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 167.Gang M, Marin ND, Wong P, et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020;136(20):2308–2318. doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhu H, Blum RH, Bernareggi D, et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell. 2020;27(2):224–237.e6. doi: 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burger MC, Mildenberger IC, Cieplik HC, et al. P07.01 The CAR2BRAIN study: a monocentric phase I trial with ErbB2-specific NK-92/5.28.z cells in recurrent glioblastoma. Neuro-oncol. 2017;19(suppl_3):iii51–iii52. [Google Scholar]
- 172.Romanski A, Uherek C, Bug G, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20(7):1287–1294. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Daher M, Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov. 2021;11(1):45–58. doi: 10.1158/2159-8290.CD-20-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol. 2021;193(2):216–230. doi: 10.1111/bjh.17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bachanova V, Ghobadi A, Patel K, et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood. 2021;138(suppl 1):823. [Google Scholar]
- 177.Daher M, Basar R, Gokdemir E, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood. 2021;137(5):624–636. doi: 10.1182/blood.2020007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kerbauy LN, Marin ND, Kaplan M, et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30+ malignancies. Clin Cancer Res. 2021;27(13):3744–3756. doi: 10.1158/1078-0432.CCR-21-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–2103. [PubMed] [Google Scholar]
- 180.Topp MS, Duell J, Zugmaier G, et al. Anti-B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J Clin Oncol. 2020;38(8):775–783. doi: 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 181.Hipp S, Tai YT, Blanset D, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo [published correction appears in Leukemia. 2017;31(10):2278] Leukemia. 2017;31(8):1743–1751. doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- 182.Gauthier L, Morel A, Anceriz N, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177(7):1701–1713. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 183.Reusing SB, Vallera DA, Manser AR, et al. CD16xCD33 bispecific killer cell engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL [published correction appears in Cancer Immunol Immunother. 2021;70(12):3709] Cancer Immunol Immunother. 2021;70(12):3701–3708. doi: 10.1007/s00262-021-03008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ravandi F, Stein AS, Kantarjian HM, et al. A phase 1 first-in-human study of AMG 330, an anti-CD33 bispecific T-cell engager (BiTE®) antibody construct, in relapsed/refractory acute myeloid leukemia (R/R AML) Blood. 2018;132(suppl 1):25. [Google Scholar]
- 185.Jitschin R, Saul D, Braun M, et al. CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells. J Immunother Cancer. 2018;6(1):116. doi: 10.1186/s40425-018-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schmitt TM, Aggen DH, Stromnes IM, et al. Enhanced-affinity murine T-cell receptors for tumor/self-antigens can be safe in gene therapy despite surpassing the threshold for thymic selection. Blood. 2013;122(3):348–356. doi: 10.1182/blood-2013-01-478164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chapuis A, Egan DN, Bar M, et al. EBV-specific donor cells transduced to express a high-affinity WT1 TCR can prevent recurrence in post-HCT patients with high-risk AML. Blood. 2016;128(22):1001. [Google Scholar]
- 189.Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25(7):1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mansilla-Soto J, Eyquem J, Haubner S, et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat Med. 2022;28(2):345–352. doi: 10.1038/s41591-021-01621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ahci M, Toffalori C, Bouwmans E, et al. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood. 2017;130(10):1270–1273. doi: 10.1182/blood-2017-05-784306. [DOI] [PubMed] [Google Scholar]
- 192.Rovatti PE, Gambacorta V, Lorentino F, Ciceri F, Vago L. Mechanisms of leukemia immune evasion and their role in relapse after haploidentical hematopoietic cell transplantation. Front Immunol. 2020;11(147):147. doi: 10.3389/fimmu.2020.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vago L, Ciceri F. Choosing the alternative. Biol Blood Marrow Transplant. 2017;23(11):1813–1814. doi: 10.1016/j.bbmt.2017.09.009. [DOI] [PubMed] [Google Scholar]