Abstract
Purpose
CDKN1B mutations were established as a cause of multiple endocrine neoplasia 4 (MEN4) syndrome in patients with MEN1 phenotype without a mutation in the MEN1 gene. In addition, variants in other cyclin-dependent kinase inhibitors (CDKIs) were found in some MEN1-like cases without the MEN1 mutation. We aimed to describe novel germline mutations of these genes in patients with primary hyperparathyroidism (PHPT).
Methods
During genetic screening for familial hyperparathyroidism, three novel CDKIs germline mutations in three unrelated cases between January 2019 and November 2021 were identified. In this report, we describe clinical features, DNA sequence analysis, and familial segregation studies based on these patients and their relatives. Genome-wide DNA study of loss of heterozygosity (LOH), copy number variation (CNV), and p27/kip immunohistochemistry was performed on tumour samples.
Results
DNA screening was performed for atypical parathyroid adenomas in cases 1 and 2 and for cystic parathyroid adenoma and young age at diagnosis of PHPT in case 3. Genetic analysis identified likely pathogenic variants of CDKN1B in cases 1 and 2 and a variant of the uncertain significance of CDKN2C, with uniparental disomy in the tumour sample, in case 3. Neoplasm screening of probands showed other non-endocrine tumours in case 1 (colon adenoma with dysplasia and atypical lipomas) and case 2 (aberrant T-cell population) and a non-functional pituitary adenoma in case 3.
Conclusion
Germline mutations in CDKIs should be included in gene panels for genetic testing of primary hyperparathyroidism. New germline variants here described can be added to the current knowledge.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-022-01948-7.
Keywords: Parathyroid, Primary hyperparathyroidism, Multiple endocrine neoplasia type 1, Multiple endocrine neoplasia type 4, Cyclin dependent kinase inhibitors
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is a syndrome characterized by the pituitary, parathyroid and entero-pancreatic neuroendocrine tumours (NETs). Primary hyperparathyroidism (PHPT) affects 95% of MEN1 patients and is its most common clinical feature [1, 2]. This syndrome is caused by loss-of-function variants of the MEN1 tumour-suppressor gene (11q13), which encodes menin, a protein responsible for genome stability, transcription, and cell division [3–6]. Germline mutations of MEN1 gene have been found in around 85% to 90% of clinically suspected MEN1 patients [7]. Thus, in a meaningful number of patients with MEN1-like phenotype, around 10%, germinal MEN1 mutations are not detected [4, 8–10]. This lack of detection could possibly be explained by genetic abnormalities in non-coding regions or whole-gene deletion of MEN1, however, large deletions of MEN1 appear to be uncommon [5, 11–14]. Therefore, other predisposing genes, such as cyclin-dependent kinase inhibitors (CDKIs), have been suggested by different authors to play a role in patients with MEN1-like phenotype without the MEN1 mutation [12, 15, 16] since it is known that menin affects gene transcription of some cell cycle regulators, such as CDKN1B/p27 and CDKN2C/p18 [17]. In particular, CDKN1B germline pathogenic variants have been reported in some patients with MEN1-like phenotype without a MEN1 gene mutation, a condition termed MEN4 syndrome (OMIM 610755) [12, 14, 18–20].
PHPT is the most common clinical feature of both MEN1 and MEN4 syndromes. It is known that 10% of cases of PHPT are hereditary due to germline mutations of different genes, a condition called familial PHPT (FHPT) [1]. FHPT involves syndromic forms, such as multiple endocrine neoplasia types 1 to 4 and hyperparathyroidism-jaw tumour syndrome. Besides, FHPT may present as non-syndromic PHPT, which includes familial hypercalcemia hypocalciuric forms, neonatal severe hyperparathyroidism, and others, that are associated with several genes and are all classified as familial isolated hyperparathyroidism [21]. Until now, a consensual systematized genetic diagnosis for FHPT has not been implemented due to a lack of firm evidence about which individuals have to be tested and which mutations should be determined. Even so, most authors agree on the recommendation of testing for germline mutations in individuals with PHPT plus some specific situations: (1) PHPT occurring before the age of 45 years, (2) recurrent or persistent PHPT, (3) multi-gland disease (MGD), (4) atypical parathyroid adenoma (APA), (5) parathyroid carcinoma, (6) existence of other tumours related to syndromic PHPT, and/or (7) being a first-degree relative of a known mutation carrier or having a first-degree relative with PHPT [22, 23]. Germline mutation testing for possible FHPT may include MEN1, CASR, CDC73, CDKN1A, CDKN1B, CDKN2B, CDKN2C, RET, PTH, GNA11, AP2S1, GCM2, and AIP genes according to recent data [1, 23, 24].
Minimal information concerning MEN1-like patients due to CDKIs germline mutations have been reported to date, and this lack of reporting suggests that this type of FHPT probably remains underdiagnosed. This report describes three individuals with a history of PHPT who were evaluated based on a suspicion of FHPT and carried novel germline variants of CDKIs genes.
Material and methods
Phenotype
We re-evaluated data from three patients with a history of PHPT in whom we performed genetic screening for suspicion of FHPT. Clinical information about probands and their first-degree relatives (including clinical features, biochemical results, and radiological findings) was obtained from medical records and routine visits in our hospital (Endocrinology and Nutrition Department). Table 1 presents the clinical findings.
Table 1.
Clinical characteristics of patients
| Mutation | LOH/UPD | PHPT history | Screening of related tumors after genetic diagnosis | Additional Medical History of Endocrine or Cancer Disorders (or remarkable) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ca max (mmol/L) | PTH max (pmol/L) | Histology diagnosis | Pituitary MRI | CT thorax and abdomen | Abnormal lab tests | ||||
| Case 1 | CDKN1B c.280_281delinsG, p.(Pro94Alafs*25) | No | 3.39 | 4,950 | APA | Lack of neurohypophyseal bright signal |
Enlarged right adrenal gland Bilateral renal cysts |
CgA: 493.9 μg/L |
Colon tubule-villous adenoma with low-grade dysplasia Atypical lipomas Obesity T2DM Primary hypothyroidism |
| Case 2 | CDKN1B c.169C>T, p.(Gln57*) | No | 3.37 | 33,963 | MGD: 3 glands parathyroid hyperplasia and 1 APA | Normal | No tumours |
CgA: 1,947.1 μg/L Gastrin: 132.6 pmol/L Prl: 6,127.2 pmol/L FSH: 106 UI/L LH: 79.4 UI/L T(t): 4.23 nmol/L T(f): 0.01 nmol/L IGF1: 5.85 nmol/L |
Subclinical hypothyroidism Obesity Aberrant T-cell population Chronic idiopathic axonal polyneuropathy |
| Case 3 | CDKN2C c.319T>G, p.(Leu107Val) | Yes | 2.84 | 846 | MGD: 1 cystic adenoma and 1 parathyroid adenoma | Nonfunctioning cystic microadenoma | No tumours |
CgA 110.3 μg/L Prl 1,358.6 pmol/L UFC 225.5 pg/24 h |
Uterine leiomyomas Bilateral ovarian cysts |
LOH: loss of heterozygosity; UPD: uniparental disomy; RV: reference value; Ca max: higher blood calcium levels before parathyroid surgery (RV: 2.1–2.55); PTH max: serum higher parathyroid hormone before parathyroid surgery (RV: 95–618); APA: atypical parathyroid adenoma; MGD: multiglandular disease; CgA: chromogranin A (RV < 100); T2DM: type 2 diabetes mellitus; Prl: Prolactin (RV 212.6–1,034.5); Gastrin RV 6.2–54.8; UFC: Urinary free cortisol (RV 36–137), FSH: follicle-stimulating hormone (RV: 1.5–12.4); LH: luteinizing hormone (RV: 1.7–8.6); T(t): total testosterone (RV: 6.69–25.6); T(f): free testosterone (RV: 0.02–0.08); IGF1: insulin-like growth factor 1 (RV: 7.54–26.52); MRI: magnetic resonance imaging; CT: computed tomography
Gene panel sequencing and variant interpretation
DNA was extracted from blood and other tissues samples using the Gentra Puregene DNA reagent (Qiagen, Valencia, CA). The customized gene panel of FHPT screening in our centre includes sequence analysis and copy number variation (CNV) analysis of the following genes: AIP, AIRE, AP2S1, CASR, CDC73, CDKN1A, CDKN1B, CDKN2B, CDKN2C, GCM2, GNA11, MEN1, PTH, RET, and TRPV6.
In the analysis, the mean sequencing depth was > 150 times, and > 99% of target nucleotides were covered with > 20 × sequencing depth for all assays. The target nucleotides included all protein-coding exons of the genes on the panels in addition to 20 base pairs (bp) inside each intron/exon boundary. The panel was also customized by adding oligonucleotides that targeted deep intronic variants (≥ 20 bp from the intron/exon boundary) and non-coding variants (promoter region, 5′ or 3′ untranslated regions [UTR]), which have been reported as disease causing in association with hyperparathyroidism. Sequence reads of each sample were mapped to the human reference genome (GRCh37/hg19). Burrows–Wheeler Aligner (BWA–MEM) software was used for reading alignment. Duplicate read marking, local realignment around indels, base quality score recalibration, and variant calling were performed using genomic analysis toolkit (GATK) algorithms (Sentieon) for nDNA. Variant data were annotated using a collection of tools (VcfAnno and VEP) with a variety of public variant databases, including but not limited to Genome Aggregation Database control population cohorts (gnomAD), ClinVar, and Human Gene Mutation Database (HGMD). Duplicate read marking, local realignment around indels, base quality score recalibration and variant calling were performed using GATK algorithms [25].
The sequence variant analysis pipeline was validated in a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) accredited Blueprint Genetics diagnostic laboratory. The series of selected quality criteria included a variant call quality score, variant genomic location, sequence content, and integrative genomics viewer visual analysis. This algorithm was established based on the outcome of an internal validation performed in the CLIA and CAP-accredited Blueprint Genetics diagnostic laboratory.
CNV analysis was performed bioinformatically from next-generation sequencing (NGS) data using a bioinformatic pipeline; one component is a CNV kit and another one involves in-house developed proprietary technology. CNVs were confirmed using digital polymerase chain reaction (PCR). The CNV analysis pipeline was validated in the CLIA and CAP-accredited Blueprint Genetics diagnostic laboratory.
Sanger sequencing of the candidate variants was performed on the patient and the family member samples to confirm the presence of the variant and the pattern of inheritance. Variants were classified following the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guidelines [26].
Study of loss of heterozygosity (LOH) and CNV in tumour
Tumour tissue samples from formalin-fixed, paraffin-embedded (FFPE) were used for this analysis. DNA of tumour samples from patients who had undergone parathyroidectomy was extracted using Qiagen DNA extraction kit (QIAGEN, Valencia CA). Genome-wide DNA study of LOH and CNV was performed using OncoScan CNV Assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA). This assay is designed to cover the entire genome with higher resolution in well-known cancer drivers (900 cancer genes). Data analysis was done using the software Chromosome Analysis Suite 4.3 (ChAS 4.3) for CNV and LOH detection.
Immunohistochemistry
Immunohistochemical study with antip27/kip1 antibody (clone SX53G8, Ventana) was performed on slides obtained from formalin-fixed paraffin-embedded parathyroidectomy tissues using the Ventana Benchmark immunostaining system (Ventana Medical System, Tucson, USA). Endothelial cells were used as positive controls. Immunohistochemistry for p18 was not performed due to the lack of a commercially available monoclonal antip18 antibody.
Results
Case 1
Medical history and clinical presentation
A 47-year-old man was referred to our department for a cervical node. In his personal history, he presented obesity, primary hypothyroidism, and obstructive sleep apnoea syndrome. In addition, he had undergone surgery for two atypical lipomas and had an endoscopic polypectomy of a tubule-villous adenoma with low-grade dysplasia. He had no family history of parathyroid disorders.
On examination, he had no obvious neck masses or goiter. A neck ultrasound scan (US) showed a homogeneously hypoechoic nodule of 3 cm posterior to the thyroid gland, suggesting a parathyroid lesion. Blood tests revealed severe hypercalcemia (3.39 mmol/L; reference value [RV] 2.1–2.55), hypophosphatemia (0.58 mmol/L, RV 0.87–1.45), and elevated parathyroid hormone (PTH) levels (4950 pmol/L, RV 95–618), consistent with PHPT (Table 1). No clinical history of fractures or nephrolithiasis was present. Treatment was initiated with cinacalcet and oral hydration, and he underwent surgical removal of a solitary abnormal parathyroid gland at the age of 48. Intra-operative PTH (ioPTH) monitoring confirmed the excision of the adenoma as the PTH dropped from 4950 pmol/L (pre-excision) to 618 pmol/L (30′ after excision). Histological study evidenced a nodule (size: 4.5 × 3.5 × 2.5 cm; weigh: 15.9 g) with histopathological characteristics consistent with an atypical parathyroid adenoma (APA), such as fibrous bands associated with haemosiderin deposition and mitotic activity (2 mitotic events/10 high-power fields). Proliferation index (Ki67) was 3%.
After surgery, serum calcium nadir was 2.2 mmol/L, and during the next 10 years, it oscillated between 2.07 and 2.56 mmol/L. Ionic serum calcium remained within normal limits until now.
Given the atypical features of adenoma, genetic testing was performed. Sequence analysis using the panel described above identified a heterozygous frameshift likely pathogenic variant in CDKN1B (NM 004064.4) c.280_281delinsG, p.(Pro94Alafs*25).
Germline genetic analysis and LOH/CNV study in tumour
This variant deletes two base pairs, inserts one base pair in exon 1 (of three total exons) and generates a frameshift, leading to a premature stop codon at position 25 in a new reading frame. This setup is predicted to lead to loss of normal protein function, either through protein truncation or nonsense-mediated mRNA decay. This variant is absent in the gnomAD. To our knowledge, this variant has not been described in the medical literature or reported in disease-related variation databases, such as ClinVar or HGMD. Loss of CDKN1B function is an established disease mechanism, and other truncating variants in the gene have been described in patients with phenotypes consistent with CDKN1B-related disease, which is inherited in an autosomal dominant (AD) manner. This CDKN1B variant is classified as likely pathogenic based on the established association between the gene and the patient’s phenotype, the variant’s absence in control populations, and the variant type (frameshift). The genetic analysis of tumour DNA did not show LOH or CNV.
p27/kip1 immunohistochemistry
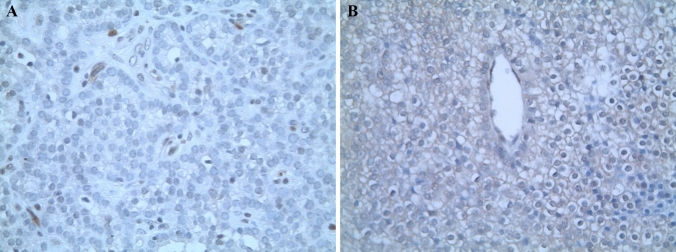
Immunohistochemical analysis of the excised parathyroid adenoma demonstrated loss of nuclear expression of p27/kip1 in neoplastic cells. This result is consistent with a lower expression of the tumour suppressor protein due to the described pathogenic variant (Fig. 1a).
Fig. 1.
Immunostaining with p27/kip1 of parathyroid adenomas from cases 1 (a) and 2 (b). Absence of nuclear expression in adenoma cells. × 400
Clinical evolution after the genetic result
Specific guidelines for MEN4 management are lacking as few cases have been reported until now, however, most authors recommend following MEN1 guidelines in these cases [7, 27]. Thus, we performed a complete radiological and serological study (Table 1). Lab tests did not show alterations in pituitary hormones, and pituitary magnetic resonance imaging (MRI) manifested a lack of a neurohypohyseal bright signal, which was attributed to a normal physiological occurrence in the absence of any clinical suspicion [28]. A computed tomography (CT) of the chest and abdomen indicated enlarged right adrenal gland and bilateral renal cysts without clinical significance. Urinary free cortisol was normal. Screening for asymptomatic NETs showed a non-specific mild elevated chromogranin A ([CgA] 493.9 μg/L, RV < 100) that has almost normalized over time.
Family screening after genetic results
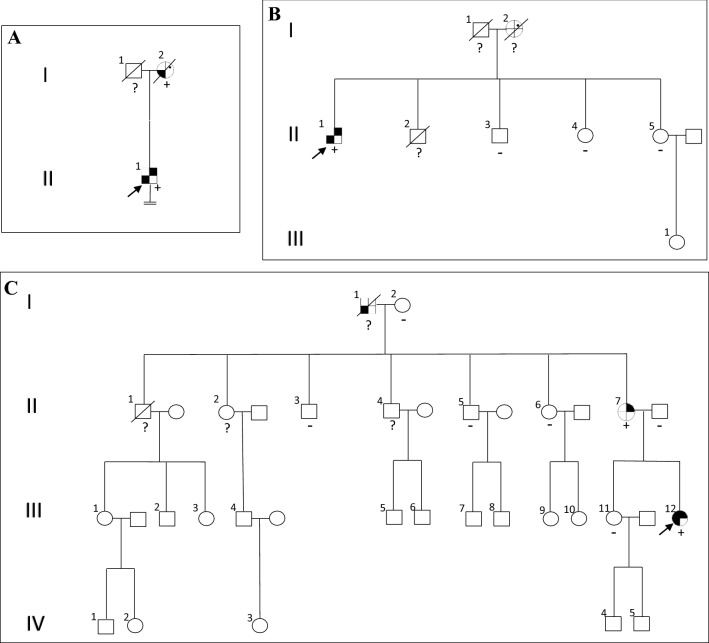
A complete family history was taken after the genetic diagnosis was obtained. No first-degree relatives were still alive. Even so, we were able to analyse a DNA sample from a surgical specimen from the mother (I.2, Fig. 2a), who had died years before from a colon neoplasia. In addition, she presented hypercalcemia on some occasions, which had not been evaluated. She carried the same variant in the CDKN1B gene as her son.
Fig. 2.
Pedigreeandtumors of probands (withconfirmatedmutation) andtheirrelatives. a Case 1, variant c.280_281delinsG, p.(Pro94Alafs*25) CDKN1B; b case 2, variant c.169C > T, p.(Gln57*) CDKN1B; c case 3, variant c.319 T > G, p.(Leu107Val) CDKN2C. Generation number is indicated with roman numerals. Arrows indicate index case; circle, female; square, male; double line below descent line, no offspring; empty symbol, unaffected family member; slashed symbol, deceased; + , mutation positive; −, mutation negative; ?, no mutation test performed; filled upper right quadrant, PHPT; filled upper rightspot, history of nephrolithiasis, renal colics or hypercalcemia; filled upper left quadrant, pituitary adenoma; filled lower right quadrant, neuroendocrine tumor; filled lower left quadrant, tumor unspecified
Case 2
Medical history and clinical presentation
We report a case of a 57-year-old man with a history of chronic kidney disease (CKD) due to interstitial nephropathy diagnosed at the age of 28, which progressed to end-stage renal disease (ESRD) and was treated with haemodialysis since he was 43. As additional significant medical history, he was diagnosed with chronic idiopathic axonal polyneuropathy, aberrant T-cell population at the age of 46, obesity, and subclinical hypothyroidism. As the only family history of interest, his mother had had renal colic. He was diagnosed with tertiary hyperparathyroidism six years after presenting with CKD. Lab tests showed corrected calcium of 3.37 mmol/L and PTH of 33,963 pmol/L. A bone mineral density (BMD) scan was not obtained. He had a previous history of symptomatic kidney stones and complicated urinary infections. Thus, the possibility of having a PHPT prior to CKD could not be excluded. Neck US revealed a hypoechoic nodule (1.3 cm in size) posterior to the thyroid gland. Conversely, scintigraphy study with technetium 99 m sestamibi (TcMIBI) did not show high radiotracer uptake. Treatment with cinacalcet was initiated but yielded a poor response, so he finally underwent bilateral neck exploration and total parathyroidectomy without autotransplantation. Histology diagnosis showed MGD due to three hyperplastic parathyroid glands plus one APA (1.7 g weight).
Post-operatively, lab tests showed a nadir PTH of 561 pmol/L and nadir serum-corrected calcium level of 1.75 mmol/L, suggesting post-surgical hypoparathyroidism. Treatment with calcium supplements and calcitriol was initiated, and normal serum levels were recovered.
Considering his young age at diagnosis and the identification of APA and MGD with three hyperplastic parathyroid glands, a screening test of the most likely genetic causes of FHPT was performed. Sequence analysis of DNA obtained from blood identified a heterozygous nonsense variant of CDKN1B c.169C>T, p.(Gln57*) (NM 004064.4).
Germline genetic analysis and LOH/CNV study in tumour
This CDKN1B variant is classified as likely pathogenic based on the established association between the gene, patient’s phenotype, variant’s absence in control populations and type of variant (nonsense). It generates a premature stop codon in exon 1 (of 3 exons) and is predicted to produce a loss of normal protein function, either through protein truncation or nonsense-mediated mRNA decay. This variant was detected in 48% (132 out of 276) NGS reads. As the patient had an aberrant T-cell population, and some pathogenic CDKN1B variants have been associated with hematologic malignancy, the identified variant could represent either a germline or somatic variant. Therefore, we performed genetic analyses on other tissues (colon, thyroid, and saliva) [29, 30], and found that all of them showed the presence of this variant; thus, we concluded that c.169C>T was a germline variant. This variant has been reported several times in the Catalogue Of Somatic Mutations In Cancer (COSMIC) in more tissues (skin, breast, prostate, haematopoietic, and lymphoid tissues). These data strongly support the pathogenicity of this variant. This variant is absent in gnomAD and, according to our knowledge, it has not been described as a germline variant in the literature or reported in disease-related variation databases. It should be mentioned that although it is a variant that when expressed somatically is associated with a significant risk phenotype, in this case the patient developed a rather mild tumoral phenotype (PHPT due to APA and three hyperplastic parathyroid glands and aberrant T-cell population without clinical affectation for the moment). The genetic analysis of tumour DNA did not show LOH or CNV.
p27/kip1 immunohistochemistry
Immunohistochemical analysis of the excised parathyroid glands (three hyperplastic parathyroid glands and one APA) demonstrated loss of staining for p27 in neoplastic cells. This result is consistent with a lower expression of the tumour suppressor protein due to the described pathogenic variant (Fig. 1b).
Clinical evolution after the genetic result
We completed the screening for MEN4-related comorbidities (Table 1). CT scan of the chest and abdomen did not show any abnormalities. Measurement of pancreatic polypeptide (PP) and plasma vasoactive intestinal peptide (VIP) was normal as was a dexamethasone suppression test. Serum CgA (1947.1 μg/L) and serum gastrin (132.6 pmol/L, RV 6.2–54.8) were elevated. Since the proband did not indicate clinical or radiological signs of NETs, these results were attributed to proton pump inhibitor therapy and ESRD [31]. Concerning pituitary hormones, he showed moderated prolactin elevation, hypergonadotropic hypogonadism, and low insulin-like growth factor 1 (IGF1) levels, which could be explained by CKD [32]. Pituitary MRI did not show abnormalities.
Family screening after the genetic result
Family members were referred for genetic counselling. In terms of first-degree alive relatives, he had two living sisters and one brother. All of them had undergone predictive testing, which did not detect the CDKN1B c.169C>T p.(Gln57*) variant in tested samples (Fig. 2b). Parental samples were not available.
Case 3
Medical history and clinical presentation
We present a 44-year-old woman that noticed a neck lump when she was 28. She had no significant medical history. No family history of parathyroid known alterations was reported, but it should be noted that her mother had a history of renal colic episodes. Neck US showed an extrathyroidal hypoechoic nodule of 1.1 cm posterior to the right thyroid lobe and a large cystic nodule (3 cm) on the left side. A neck CT scan revealed the same cystic lesion in a left para-traqueal location, measuring a maximum diameter of 3 cm. It remained unclear whether it was of parathyroid or thyroid origin. Scintigraphy using TcMIBI showed avid tracer uptake near the right thyroid lobe, suggesting a single parathyroid lesion. Fine-needle aspiration biopsy of the cystic nodule showed non-specific cystic content. Lab tests revealed a PHPT pattern with corrected calcium levels of 2.84 mmol/L and PTH of 846 pmol/L. BMD showed normal bone mineralization, and abdominal US excluded renal lithiasis at that moment. Bilateral neck exploration with left cyst excision and right extrathyroidal nodule extraction was performed. The final pathologic diagnosis was MGD due to one parathyroid adenoma (0.1 g weight) and one mainly cystic parathyroid adenoma (3.5 cm diameter, unknown weight due to splitting of the cyst).
After surgery, PTH levels dropped to 409 pmol/L after which she remained asymptomatic and normocalcemic during the post-surgery years. Genetic screening of FHPT forms was performed given the detection of MGD, the existence of a large cystic parathyroid adenoma, and her young age at diagnosis of PHPT. The patient was heterozygous for c.319T>G, p.(Leu107Val) in CDKN2C (NM 001262.2).
Germline genetic analysis and LOH/CNV study in tumour
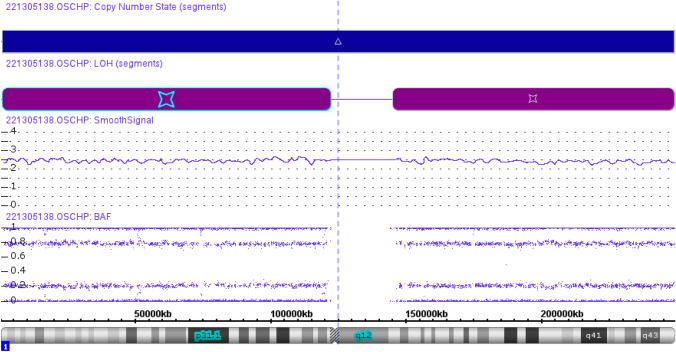
This CDKN2C variant was genetically classified as a variant of uncertain significance (VUS) according to the ACMG criteria. This variant affects a conserved amino acid, and it is predicted to be damaging by most in silico tools. Only two missense variants in CDKN2C are currently reported in the HGMD: (1) one in association with MEN1 and (2) the other in association with parathyroid adenoma [16, 33]. Agarwal et al. reported a missense germline variant in a family with parathyroid tumours in which a decrease in protein expression as a probable cause of sporadic endocrine tumours was found [16]. The c.319T>G variant in CDKN2C has been reported in gnomAD in 9/251,454 alleles with a low allele frequency (3.58 × 10−5). It should be noted that the gnomAD database excludes individuals known to be affected with the severe paediatric disease in addition to their first-degree relatives [34]. The clinical age-related penetrance in patients with CDKN2C variants could be low or limited due to the small number of cases described in the literature. Functional studies would help to a better classification of this variant. Sporadic adenomas have not been extensively investigated for candidate variants in CDKI genes and specifically for CDKN2C. This gene has been examined in a small sporadic adenomas series and to date, has no known identified variant [34, 35]. In this case, the analysis of LOH and CNV in tumour sample showed a gain of whole chromosome 1 and the genotype for three copies were identical leading to somatic uniparental disomy of chromosome 1 (Fig. 3). The detection of this event may reinforce the pathogenicity of this variant although this result should be viewed with caution.
Fig. 3.
Genome-wide DNA study of loss of heterozygosity (LOH) and copy number variation (CNV) in case 3. CNV track shows a gain of chromosome 1 and B-allele frequency (BAF) track shows uniparental disomy of whole chromosome 1
Clinical evolution after the genetic result
After finding the CDKN2C variant, physical, imaging, and lab exams were performed following MEN1 guidelines (Table 1). Concerning dermatological features, physical examination revealed a preauricular right tag and seborrheic keratosis. A CT scan of the abdomen and chest showed uterine leiomyomas and some ovarian cysts. No adrenal alteration was seen. MRI indicated a nodule (0.6 × 0.7 cm), suggestive of cystic non-functional pituitary microadenoma, and the only alteration of pituitary lab test was mild elevated prolactin (1358.64 pmol/L, RV 212.6–1034.5) without clinical significance. Urinary free cortisol level was elevated twice (225.5 pg/24 h and 156.9 pg/24 h; RV 36–137), but no clinical features of Cushing syndrome (CS) were detected. In addition, a salivary cortisol and dexamethasone suppression test showed no alterations, so CS diagnosis was not confirmed. Screening for asymptomatic NETs showed only a non-specific mild elevated CgA (110.3 μg/L).
Family screening after the genetic result
First-degree relatives underwent genetic counselling. Proband’s mother (II.7, Fig. 2c) carried the variant. She was offered clinical assessment, including biochemical and imaging tests of the pancreas, the pituitary, and the adrenal glands. Lab tests showed hypercalcemia (2.97 mmol/L) and elevated PTH (2423 pmol/L). US of the neck revealed a hypoechoic nodule (0.5 × 0.3 cm) posterior to the left thyroid lobe and scintigraphy using TcMIBI showed an increased tracer uptake in the same location, so she was diagnosed with PHPT. Abdominal US confirmed the presence of renal lithiasis. Due to densitometric osteoporosis (lumbar spine 0.637 g/cm2 and T score − 4.69 SD; femoral neck 0.593 g/cm2 and T score − 3.23 SD), alendronate was prescribed. We performed unilateral neck exploration with the removal of a single parathyroid adenoma. PTH levels declined to normal after excision. CT scan of the chest and abdomen did not show abnormalities. Regarding pituitary hormones, lab test showed a low morning serum cortisol level (5.04 µg/dL; RV 6.02–18.4) with low adrenocorticotropin hormone level (4.2 pg/mL; RV 7.2–63.3), pattern suggesting secondary adrenal insufficiency. We performed a 250 µg cosyntropin stimulation test, which showed normal post-stimulation cortisol levels: (1) 30 min − 26.9 µg/dL and (2) 60 min 31.9 µg/dL (RV > 14 µg/dL). No other lab pituitary hormone alterations or radiological abnormalities of the gland were detected.
Other family members (proband’s father, sister, grandmother, and great uncles) did not present the variant and showed normal phospho-calcium metabolism lab tests (Fig. 2c).
Discussion
We report three novel CDKIs germline variants in three different patients with PHPT identified during the course of screening for FHPT causes: (1) two likely pathogenic variants of CDKN1B gene (cases 1 and 2) and (2) one VUS of CDKN2C gene (case 3).
As stated above, CDKIs germline mutations have been proposed as a cause of MEN1-like syndromes without MEN1 mutation. This situation is called MEN4 when a CDKN1B germline mutation is found. However, the clinical course of patients affected by mutations in CKDIs is still poorly understood since few cases have been reported until now. We reviewed medical literature and genetic databases (ClinVar, HGMD) and found that a few more than 100 cases with MEN4, including 52 different variants of CDKN1B, have been reported to date [12, 16, 20, 29, 36–54]. As supplementary material, we added a table with the total number of CDKIs pathogenic/likely pathogenic/VUS germline variants thus far reported along with the type of parathyroid lesion or other tumours found (if reported) and pathogenicity level of the variants, which was assessed following the ACMG guidelines [26] (see supplementary material Appendix 1). It should be noted that 34 of these CDKN1B variants have been reported from a single submitter as criteria provided and 10/55 are classified as VUS. All these facts support that so very little knowledge about the clinical implications of CDKIs pathogenic variants is available to date. In addition, four other variants of CDKN1B have been reported in patients with medulloblastoma, paraganglioma, acute lymphoblastic leukaemia and familial colorectal cancer [30, 55–57].
Germline mutations in three other CDKI genes (CDKN2B, CDKN2C, and CDKN1A) were found in MEN1-like cases without detectable MEN1 mutations [16]. Afterall, a reliable prevalence of CDKIs mutations in MEN1-like patients or patients with PHPT is difficult to estimate as genetic screening of these genes has not been widely established. We report three novel CDKIs germline mutations in three different patients with PHPT. The possibility of including these cases within the term MEN4 or MEN1-like states should be assessed.
MEN4–PHPT affects approximately 80–90% of the reported MEN4 cases to date [7, 53]. This finding is in accordance with MEN1 classical presentation [58]. On the contrary, in most MEN4 cases, the clinical course of PHPT seems to be less severe than in patients with MEN1 syndrome and exhibits a female predominance. Only one case of MEN4 presented recurrent PHPT after subtotal parathyroidectomy [39, 53]. All cases that we reported agree with this as all of them showed remission of PHPT after surgery to date (at least nine years follow-up). However, moderate to severe hypercalcemia was seen in our three cases in contrast to other cases of PHPT associated with CDKIs mutations [53] (Table 1). Considering that most of these patients presented remission after surgery, differing from many MEN1 cases, a minimally invasive parathyroid surgery could be contemplated. Regarding endocrine histology, most of the cases of PHPT-related to CDKIs mutations described to date were caused by a single parathyroid adenoma [53], except for a few cases of MGD [16, 39]. To our knowledge, we report for the first time two cases of APA and one case of cystic parathyroid adenoma related to CDKIs germline mutations (Table 1).
Regarding pituitary neoplasms, up to now, some cases of these tumours were described in patients with MEN4 (43%) [12, 38, 45, 47, 49, 50, 52, 53]. These findings agree with the estimated prevalence of pituitary tumours in patients with MEN1 (40%). Recently, in a large cohort of 211 patients (mostly paediatric) with Cushing disease, five germline CDKN1B variants were identified (2.6%) [50]. In addition, three germline CDKN1B variants were detected in three paediatric patients with pituitary adenomas, with no other manifestations related to MEN4 [47]. In addition, one case of macroprolactinoma was described in a case with germline CDKN1A mutation [16]. Somatotroph, corticotroph, and non-functioning pituitary adenomas have been found to be the most frequent pituitary tumours in MEN4 cases. On the contrary, prolactinomas seem less frequent in MEN4 patients [45, 52]. Non-functioning microadenomas are usual in the general population, and it remains challenging to distinguish between incidentalomas and MEN-related pituitary tumours [53]. In this regard, a non-functioning microadenoma was detected in case 3. The age of onset of pituitary tumours in MEN4 is still unclear due to the few number of cases reported until now.
Concerning NETs, we did not find any radiological image or analytical alteration suggestive of tumour in our cases. That finding fits with the last data about MEN4 cases, which indicates a lower prevalence of NETs than in MEN1 [53].
Other tumours, such as meningiomas, liver haemangiomas, skin tumours, adrenal nodes, colon cancer, breast cancer, and genitourinary neoplasms, have been described in patients with germline mutations of CDKIs [12, 16, 20, 36, 38, 53]. We describe a colon adenoma with low-grade dysplasia and atypical lipomas in case 1 and a colon neoplasia in her mother who carried the same variant in CDKN1B. In addition, we report a malignant hemopathy (aberrant T-cell population) in a CDKN1B carrier (case 2).
In the firsts two cases (cases 1 and 2), genetic analysis of tumour samples showed nor LOH neither CNV for CDKN1B, findings that agree with those in the literature. We reviewed recent data of MEN4 published cases describing CDKN1B pathogenic variants that have analysed tumour tissue to assess the presence of a possible LOH. In most of the cases, tumour testing failed to demonstrate a second somatic event in the wildtype allele. Therefore, in contrast to MEN1 syndrome, haploinsufficiency for CDKN1B appears to be enough for tumours to occur in MEN4 syndrome [41, 53, 59]. Conversely, in case 3, genetic tumour testing showed uniparental disomy of chromosome 1, including CDKN2C gene, which could support a pathogenic role of this variant. Another germline variant of CDKN2C with LOH was described in a patient with parathyroid adenoma [33]. In addition, some reports propose the contribution of CDKN2C as tumour suppressor gene in different neoplasms [60–63]. However, further studies are required to establish whether CDKN2C germline pathogenic variants cause genetic predisposition to FHPT or multiple endocrine neoplasms. In addition, immunohistochemistry testing for p18 (not available in our laboratory) could be of interest in future studies of similar cases to determine the protein expression in tumour tissue.
To summarize, we report two novel likely pathogenic variants of CDKN1B gene in patients with suspected familial PHPT. The first one was found in a patient with atypical parathyroid adenoma, atypical lipomas, and a colon tubule-villous adenoma with low-grade dysplasia. His mother, who carried the same variant, died from colon neoplasia and once presented with non-studied hypercalcemia. The second one found in a patient with PHPT is associated with aberrant T-cell population and other comorbidities, such as obesity, subclinical hypothyroidism, and chronic idiopathic axonal polyneuropathy. In addition, we describe a novel variant in CDKN2C gene with uniparental disomy in tumour sample that was classified as uncertain significance in a patient with PHPT and a non-functional pituitary adenoma. Although the case 3 variant was classified as VUS, we would like to highlight its clinical relevance since both the proband and her mother, who carried the same variant, presented PHPT.
As a reflection, we would like to point out that genetic screening of FHPT could be considered in patients with features such as APA or in the presence of other tumours apart from PHPT. Considering all reported cases of MEN1-like patients with CDKIs germline mutations with PHPT as the predominant feature, we theorize that patients with mutations in different CDKIs apart from CDKN1B (such as CDKN2C) may be encompassed in the nomenclature of MEN4. However, further studies should be done for a better understanding of this entity, and development of specific guidelines for these patients should be established. New germline variants described in this study can be added to the current knowledge. In conclusion, we can say that germline mutations in CDKIs may represent an etiology of FHPT. Therefore, these genes should be included in gene panels for screening this disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the staff of the Endocrinology and Nutrition Department, Pathology Department, Genetic Department, and Surgery Departmen of our hospital for guidance and suggestions, the Blueprint Genetic laboratory for the clinical support and Elena Aller (Thermo Fisher Scientific) for her help in LOH interpretation.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. This article did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest that could prejudice the impartiality of the article.
Ethics approval
The protocol for the study was approved by the institutional Ethics Committee of Institut d’Investigació i Innovació Parc Taulí I3PT.
Research involving human participants and/or animals
All procedures performed were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thakker RV. Genetics of parathyroid tumours. J Intern Med. 2016;280:574–583. doi: 10.1111/joim.12523. [DOI] [PubMed] [Google Scholar]
- 2.Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) Q J Med. 1996;89:653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 3.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) Best Pract Res Clin Endocrinol Metab. 2010;24:355–370. doi: 10.1016/j.beem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Brandi ML, Gagel RF, Angeli A, et al. CONSENSUS guidelines for diagnosis and therapy of MEN type 1 and type 2. JCEM. 2001;86(12):5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 5.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 6.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 7.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 8.Namihira H, Sato M, Matsubara S, et al. Mutation MEND gene in a case of familial neoplasia type of germline or somatic multiple. Endocr J. 1999;46:811–816. doi: 10.1507/endocrj.46.811. [DOI] [PubMed] [Google Scholar]
- 9.Pellegata NS. MENX and MEN4. Clinics. 2012;67:13–18. doi: 10.6061/clinics/2012(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandeva S, Daly AF, Petrossians P, et al. Somatic and germline mutations in the pathogenesis of pituitary adenomas. Eur J Endocrinol. 2019;181:R235–R254. doi: 10.1530/EJE-19-0602. [DOI] [PubMed] [Google Scholar]
- 11.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Mol Cell Endocrinol. 2014;386:2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natalia P, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Jochen Graw MJA. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavaco BM, Domingues R, Bacelar MC, et al. Mutational analysis of Portuguese families with multiple endocrine neoplasia type 1 reveals large germline deletions. Clin Endocrinol (Oxf) 2002;56:465–473. doi: 10.1046/j.1365-2265.2002.01505.x. [DOI] [PubMed] [Google Scholar]
- 14.Owens M, Ellard S, Vaidya B. Analysis of gross deletions in the MEN1 gene in patients with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2008;68:350–354. doi: 10.1111/j.1365-2265.2007.03045.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Pellegata NS. Multiple endocrine neoplasia type 4. Front Horm Res. 2013;41:63–78. doi: 10.1159/000345670. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–1834. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schernthaner-Reiter MH, Trivellin G, Stratakis CA. MEN1, MEN4, and carney complex: pathology and molecular genetics. Neuroendocrinology. 2016;103:18–31. doi: 10.1159/000371819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozawa A, Agarwal SK, Mateo CM, et al. The parathyroid/pituitary variant of multiple endocrine neoplasia type 1 usually has causes other than p27Kip1 mutations. J Clin Endocrinol Metab. 2007;92:1948–1951. doi: 10.1210/jc.2006-2563. [DOI] [PubMed] [Google Scholar]
- 19.Igreja S, Chahal HS, Akker SA, et al. Blackwell Publishing Ltd assessment of p27 (cyclin-dependent kinase inhibitor 1B) and aryl hydrocarbon receptor-interacting protein (AIP) genes in multiple endocrine neoplasia (MEN1) syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol. 2009;70:259–264. doi: 10.1111/j.1365-2265.2008.03379.x. [DOI] [PubMed] [Google Scholar]
- 20.Occhi G, Regazzo D, Trivellin G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet. 2013 doi: 10.1371/journal.pgen.1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberto F. Genetics of parathyroids disorders: overview. Best Pract Res Clin Endocrinol Metab. 2018;32:781–790. doi: 10.1016/j.beem.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Starker LF, Åkerström T, Long WD, et al. Frequent germ-line mutations of the MEN1, CASR, and HRPT2/CDC73 genes in young patients with clinically non-familial primary hyperparathyroidism. Horm Cancer. 2012;3:44–51. doi: 10.1007/s12672-011-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science (80-) 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 24.Mariathasan S, Andrews K, Thompson E, et al. Genetic testing for hereditary hyperparathyroidism in a large UK cohort. Endocr Abstr. 2019;63(OC1):1. doi: 10.1530/endoabs.63.OC1.1. [DOI] [PubMed] [Google Scholar]
- 25.Van der Auwera GA, Carneiro MO, Hartl C, et al. From fastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinform. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonnell JE, Gild ML, Clifton-Bligh RJ, Robinson BG. Multiple endocrine neoplasia: an update. Intern Med J. 2019;49:954–961. doi: 10.1111/imj.14394. [DOI] [PubMed] [Google Scholar]
- 28.Klyn V, Dekeyzer S, Van Eetvelde R, et al. Presence of the posterior pituitary bright spot sign on MRI in the general population: a comparison between 1.5 and 3T MRI and between 2D–T1 spin-echo- and 3D–T1 gradient-echo sequences. Pituitary. 2018;21:379–383. doi: 10.1007/s11102-018-0885-3. [DOI] [PubMed] [Google Scholar]
- 29.Pappa V, Papageorgiou S, Papageorgiou E, et al. A novel p27 gene mutation in a case of unclassified myeloproliferative disorder. Leuk Res. 2005;29:229–231. doi: 10.1016/j.leukres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi C, Takeuchi S, Ikezoe T, et al. Parathyroid tumor suppressor on 1p: analysis of the p18 cyclin-dependent kinase inhibitor gene as a candidate. Leukemia. 2002;16:956–958. doi: 10.1038/sj/leu/2402408. [DOI] [PubMed] [Google Scholar]
- 31.Lundell L, Vieth M, Gibson F, et al. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 32.Meuwese CL, Carrero JJ. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res. 2013;44:591–600. doi: 10.1016/j.arcmed.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Costa-Guda J, Soong CP, Parekh VI, et al. Germline and somatic mutations in cyclin-dependent kinase inhibitor genes CDKN1A, CDKN2B, and CDKN2C in sporadic parathyroid adenomas. Horm Cancer. 2013;4:301–307. doi: 10.1007/s12672-013-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahara H, Smith AP, Gaz RD, et al. Parathyroid tumor suppressor on 1p: analysis of the p18 cyclin-dependent kinase inhibitor gene as a candidate. J Bone Miner Res. 1997;12:1330–1334. doi: 10.1359/jbmr.1997.12.9.1330. [DOI] [PubMed] [Google Scholar]
- 35.Tahara H. Loss of chromosome arm 9p DNA and analysis of the ~16 and ~15 Cyclin-dependent kinase inhibitor genes in human parathyroid adenomas*. JCEM. 1996;81:3663–3667. doi: 10.32388/zhve0o. [DOI] [PubMed] [Google Scholar]
- 36.Georgitsi M, Raitila A, Karhu A, et al. Brief report: germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92:3321–3325. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 37.Belar O, De La Hoz C, Pérez-Nanclares G, et al. Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clin Endocrinol (Oxf) 2012;76:719–724. doi: 10.1111/j.1365-2265.2011.04269.x. [DOI] [PubMed] [Google Scholar]
- 38.Molatore S, Marinoni I, Lee M, et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010;31:1825–1835. doi: 10.1002/humu.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonelli F, Giudici F, Giusti F, et al. A heterozygous frameshift mutation in exon 1 of cdkn1B gene in a patient affected by MEN4 syndrome. Eur J Endocrinol. 2014 doi: 10.1530/EJE-14-0080. [DOI] [PubMed] [Google Scholar]
- 40.Pardi E, Mariotti S, Pellegata NS, et al. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4. Endocr Connect. 2015;4:1–8. doi: 10.1530/EC-14-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seabrook A, Wijewardene A, de Sousa S, et al. MEN4, the MEN1 mimicker; a case series of 3 phenotypically heterogenous patients with unique CDKN1B mutations. J Clin Endocrinol Metab. 2022;107:2339–2349. doi: 10.1210/clinem/dgac162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa-Guda J, Marinoni I, Molatore S, et al. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2011;96:E701–E706. doi: 10.1210/jc.2010-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bugalho MJ, Domingues R. Uncommon association of cerebral meningioma, parathyroid adenoma and papillary thyroid carcinoma in a patient harbouring a rare germline variant in the CDKN1B gene. BMJ Case Rep. 2016 doi: 10.1136/bcr-2015-213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borsari S, Pardi E, Pellegata NS, et al. Loss of p27 expression is associated with MEN1 gene mutations in sporadic parathyroid adenomas. Endocrine. 2017;55:386–397. doi: 10.1007/s12020-016-0941-6. [DOI] [PubMed] [Google Scholar]
- 45.Sambugaro S, Di Ruvo M, Ambrosio MR, et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5′UTR region. Endocrine. 2015;49:58–64. doi: 10.1007/s12020-015-0540-y. [DOI] [PubMed] [Google Scholar]
- 46.Malanga D, De Gisi S, Riccardi M, et al. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol. 2012;166:551–560. doi: 10.1530/EJE-11-0929. [DOI] [PubMed] [Google Scholar]
- 47.Chasseloup F, Pankratz N, Lane J, et al. Germline CDKN1B loss-of-function variants cause pediatric Cushing’s disease with or without an MEN4 phenotype. J Clin Endocrinol Metab. 2020;105:1–23. doi: 10.1210/clinem/dgaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elston MS, Meyer-rochow GY, Dray M, et al. Early onset primary hyperparathyroidism associated with a novel germline mutation in CDKN1B. Case Rep Endocrinol. 2015;2015:1–4. doi: 10.1155/2015/510985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tichomirowa MA, Lee M, Barlier A, et al. Cyclin-dependent kinase inhibitor 1B(CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr Relat Cancer. 2012;19:233–241. doi: 10.1530/ERC-11-0362. [DOI] [PubMed] [Google Scholar]
- 50.de LaPiscina IM, Najera NP, Rica I, et al. Clinical and genetic characteristics in patients under 30 years with sporadic pituitary adenomas. Eur J Endocrinol. 2021;185:485–496. doi: 10.1530/EJE-21-0075. [DOI] [PubMed] [Google Scholar]
- 51.Lavezzi E, Brunetti A, Smiroldo V, et al. Case report: new CDKN1B mutation in multiple endocrine neoplasia type 4 and brief literature review on clinical management. Front Endocrinol. 2022;13:1–7. doi: 10.3389/fendo.2022.773143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chevalier B, Odou MF, Demonchy J, et al. Multiple endocrine neoplasia type 4: novel CDNK1B variant and immune anomalies. Ann Endocrinol (Paris) 2020;81:124–125. doi: 10.1016/j.ando.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Frederiksen A, Rossing M, Hermann P, et al. Clinical features of multiple endocrine neoplasia type 4: novel pathogenic variant and review of published cases. J Clin Endocrinol Metab. 2019;104:3637–3646. doi: 10.1210/jc.2019-00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brock P, Bustamante Alvarez J, Mortazavi A, et al. Co-occurrence of multiple endocrine neoplasia type 4 and spinal neurofibromatosis: a case report. Fam Cancer. 2020;19:189–192. doi: 10.1007/s10689-019-00152-6. [DOI] [PubMed] [Google Scholar]
- 55.Esteban-Jurado C, Vila-Casadesús M, Garre P, et al. Whole-exome sequencing identifies rare pathogenic variants in new predisposition genes for familial colorectal cancer. Genet Med. 2015;17:131–142. doi: 10.1038/gim.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitworth J, Smith PS, Martin JE, et al. Comprehensive cancer-predisposition gene testing in an adult multiple primary tumor series shows a broad range of deleterious variants and atypical tumor phenotypes. Am J Hum Genet. 2018;103:3–18. doi: 10.1016/j.ajhg.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waszak SM, Northcott PA, Buchhalter I, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018;19:785–798. doi: 10.1016/S1470-2045(18)30242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal SK, Kester MB, Debelenko LV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2018;24:1–4. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 59.Inoue K, Fry EA. Haploinsufficient tumor suppressor genes. Adv Med Biol. 2017;118:83–122. [PMC free article] [PubMed] [Google Scholar]
- 60.Costa-Guda J, Arnold A. Genetic and epigenetic changes in sporadic endocrine tumors: Parathyroid tumors. Mol Cell Endocrinol. 2014;386:46–54. doi: 10.1016/j.mce.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Naofal M, Kim A, Yon HY, et al. Role of CDKN2C fluorescence in situ hybridization in the management of medullary thyroid carcinoma. Ann Clin Lab Sci. 2017;47:523–528. [PMC free article] [PubMed] [Google Scholar]
- 62.Georgitsi M. MEN-4 and other multiple endocrine neoplasias due to cyclin-dependent kinase inhibitors (p27Kip1 and p18INK4C) mutations. Best Pract Res Clin Endocrinol Metab. 2010;24:425–437. doi: 10.1016/j.beem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Kirsch M, Mörz M, Pinzer T, et al. Frequent loss of the CDKN2C (p18INK4c) gene product in pituitary adenomas. Genes Chromosomes Cancer. 2009;48:143–154. doi: 10.1002/gcc.20621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.