Abstract
Gel-forming mucins secreted by conjunctival goblet cells have been implicated in the clearance of allergens, pathogens, and debris. However, their roles remain incompletely understood. Here we show that human and mouse conjunctival goblet cell mucins have Alcian blue-detectable sialic acids, but not sulfates in the steady state. Interestingly, Balb/c mouse strain lacks this sialylation due to a point mutation in a sialyltransferase gene, St6galnac1, which is responsible for sialyl-Tn synthesis. Introduction of intact St6galnac1 to Balb/c restores the sialylation of conjunctival goblet cell mucus. Sialylated mucus efficiently captures and encapsulates the allergen particles in an impenetrable layer, leading to the protection of mice from the development of allergic conjunctivitis. Expression of ST6GALNAC1 and sialyl-Tn is upregulated in humans under conditions with chronic stimuli. These results indicate that the sialylated glycans on the ocular mucins play an essential role in maintaining the conjunctival mucosa by protecting from the incoming foreign bodies such as allergen particles.
Subject terms: Mucosal immunology, Molecular medicine, Conjunctival diseases
Conjunctival goblet cells secrete mucins that are heavily glycosylated. Here, the authors show that sialylation of mucins is essential for capturing and encapsulates allergen particles and thus reduces the risk for developing allergic conjunctivitis.
Introduction
The goblet cell is a specialized epithelial cell type that plays a role in the first-line defense on the wet surfaces of the body including the ocular surface1–3. Goblet cells provide hydration and lubrication of the mucosal surface by secreting high molecular weight mucins that are heavily glycosylated2–4. The mucus granules containing gel-forming mucins are constitutively exocytosed at the steady state. Upon detection of incoming threats, a burst of release occurs, which entraps the pathogens and debris to facilitate their removal5–7. The importance of gel-forming mucins is best exemplified by the fact that deficiency of an intestinal gel-forming mucin, Muc2, leads to spontaneous colitis and enhanced colon cancer development8,9. However, in contrast to the intestinal mucosa, which is continuously challenged by luminal bacteria, the roles of single gel-forming mucins in the ocular surface remain elusive especially in specific pathogen-free environments10,11. The major gel-forming mucins expressed in the ocular surface include Muc5ac and Muc5b2. Although MUC5AC is believed to play a pivotal role in maintaining the rheological properties of the tear film2, mice lacking Muc5ac or Muc5b showed only mild, or no dry eye phenotypes10,11. Even with the complete loss of conjunctival goblet cells, the dry eye phenotype was mild to moderate12. Interestingly, two important phenotypes were observed; an increase of debris in the fornix, which can be attributed to the loss of gel-forming mucins13, and a loss of tolerogenic response, which is supported by goblet cell-derived retinoic acid14. However, a Pseudomonas aeruginosa infection experiment did not prove any protective roles of goblet cells against bacterial infection in the eye13 despite the loss of anticipated regulatory effects of gel-forming mucins15. Thus, the roles of goblet cell-derived gel-forming mucins in the protection of ocular surface remain incompletely understood.
The mucin proteins harbor repetitive core sequences that are rich in serine and threonine residues, which are known to be extensively O-glycosylated. The major gel-forming mucin in the conjunctiva, MUC5AC, is one of the largest glycoproteins known to date. Its molecular mass reaches more than 20 mDa after glycosylation and multimerization16. The O-glycosylated tandem repeat domain is flanked by cysteine-rich domains, and intermolecular disulfide bonding of these domains allows multimerization of the molecules, giving them a gel-like property4.
Mucin oligosaccharides account for 50–90% of the molecular mass of the mature glycoprotein. The average glycan mass of the human ocular surface mucin is reported to account for 55%17. Most of the glycans are O-glycans, although N-glycans are also found18–20. Negative charges of the terminal sialic acid or sulfate group of the glycan chains confer anti-adhesive properties to the mucins21. Alterations of mucin glycosylation, including sialylated glycans have been reported in pathological states of ocular surfaces, such as dry eye22, contact lens wear pathology23, pterygium24–26, and ocular rosacea27, as well as in the airway28, gastrointestinal tract29, and cancer30. However, whether these alterations are detrimental or protective to the host remains largely unknown, except for cancer O-glycan chains, which have been shown to contribute to biological and pathological consequences30,31.
ST6GALNAC is a subfamily of sialyltransferases, which transfers sialic acid with an α2-6 linkage to N-acetylgalactosamine (GalNAc) in O-glycans and glycolipids. Among the six members identified to date in mice and humans, ST6GALNAC1, 2, 4, and to a lesser extent, 3, have an activity on the GalNAc residues in O-linked glycans32,33. While ST6GALNAC3 and 4 require sialylation of the acceptor substrate, ST6GALNAC1 and 2 can target non-sialylated structures such as Tn and T antigens34–36. In addition, ST6GALNAC1 and 2 have differential preference for their acceptor substrates; ST6GALNAC1 prefers Tn antigen, while ST6GALNAC2 prefers T and sialyl-T antigens in vivo34,37. A recent report has shown that the ST6GALNAC1 expressed in the intestine affects sialylation of N-glycans, which plays a role in stabilizing MUC2 mucins against bacterial proteolytic degradation38. Although ST6GALNAC1 is reportedly one of the most expressed ST6GALNACs in the human conjunctiva39, how ST6GALNAC1 affects MUC5AC and intrinsic properties of the mucus is not known.
The prevalence of ocular allergy has increased in the past decades40–42 and is estimated to have reached up to 40–50%, depending on the country41,42. The manifestation ranges from less severe but recurrent seasonal and perennial allergic conjunctivitis to severe forms including vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), which potentially lead to corneal involvement and ultimately to vision loss42,43. Alterations of both transmembrane and gel-forming mucin expression have been observed in VKC and AKC44,45. More recently, it has been reported that there are increased amounts of sialylated species in the N-glycans present in the tears of VKC and AKC patients46. However, the roles of O-glycans and their sialylation in ocular allergy remain unexplored19,46.
In this study, we found that goblet cells in the conjunctiva of humans and C57BL/6 J (B6J) mice are Alcian Blue (AB)-positive due to sialylation, while those of Balb/c mice lack it. The major responsible gene was identified as a sialyltransferase, St6galnac1. In humans, ST6GALNAC1 and its enzymatic oligosaccharide product, sialyl-Tn, was found to be increased in pathologic conditions under chronic mechanical/inflammatory stimuli. By transferring the intact St6galnac1 gene to the St6galnac1-deficient Balb/c strain, and by generating St6galnac1-knockout B6J strain, we investigated the roles of sialylated mucins in vivo.
Results
Conjunctival goblet cells of humans and B6J mice, but not those of Balb/c mice, are sialylated in the steady state
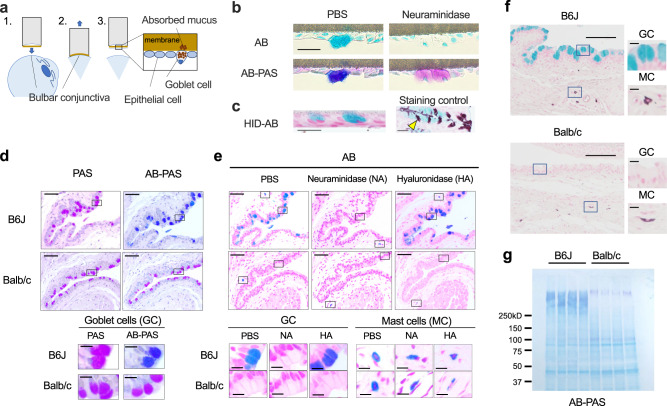
Previous studies have demonstrated that Alcian Blue (AB) stains human, rat, and mouse conjunctival goblet cells differentially or simultaneously with Periodic acid-Schiff (PAS) staining depending on the disease state and age47–50. Because AB stains acidic residues such as sialic acid, hyaluronic acid, and sulfate groups on the glycoproteins, we used AB staining in attempt to elucidate the responsible acidic residues in the goblet cell mucus granules. Steady state human conjunctival goblet cells were positive for AB, but negative for high iron diamine (HID), which stains sulfate groups (Fig. 1a–c). In addition, desialylated conjunctival goblet cells became negative for AB, suggesting that the AB positivity of human goblet cells are derived from their sialic acid (Fig. 1b). However, goblet cells of B6J and Balb/c mice were stained differently; B6J goblet cells were positive for both AB and PAS, while Balb/c goblet cells were positive for PAS staining only (Fig. 1d). Neuraminidase, but not hyaluronidase treatment, abolished the AB staining of the B6J goblet cells, indicating that sialylated glycans are the major substances stained by AB, and these are present only in the B6J goblet cells (Fig. 1e). Goblet cells of both strains were negative for HID (Fig. 1f). Of note, mast cell AB staining was retained after neuraminidase and hyaluronidase treatments, consistently with the positive HID staining (Fig. 1e, f). AB-PAS staining of the swab extract of the conjunctiva revealed that the major AB-positive (and negative in Balb/c) PAS-positive protein has a high molecular weight, indicating that the mucins are the major substances that harbor sialylated glycan stained in conjunctival goblet cells (Fig. 1g).
Fig. 1. Conjunctival goblet cell mucus of humans and C57BL/6 J mice, but not that of Balb/c mice, is sialylated in the steady state.
a A schematic diagram of impression cytology. b Periodic acid–Schiff (PAS) and Alcian Blue (AB) (pH2.5) staining of the bulbar conjunctival goblet cells from healthy subjects with or without neuraminidase treatment. Bar, 20 μm. c High-iron diamine (HID) and AB staining of sulfomucin of the human conjunctival goblet cells. Staining control: mouse colon. The arrowhead indicates an example of HID-positive (brown) goblet cell. Bar, 20 μm. PAS and AB-PAS (d), or AB staining (e) of mouse conjunctiva. Bar 50 μm. Inset: Bar, 10 μm. Tissue sections were treated with neuraminidase (NA) or hyaluronidase (HA) before staining in (e). GC, goblet cells; MC, mast cells. f HID-AB staining of the mouse conjunctivas. Bar, 100 μm. Inset: Bar, 10 μm. g AB-PAS staining of membrane-transferred conjunctival swab proteins. Data are representative of at least two independent experiments (b–g). Source data are provided as a Source Data file.
Aberrant splicing disrupts the sialyl motif of St6galnac1 in Balb/c mice
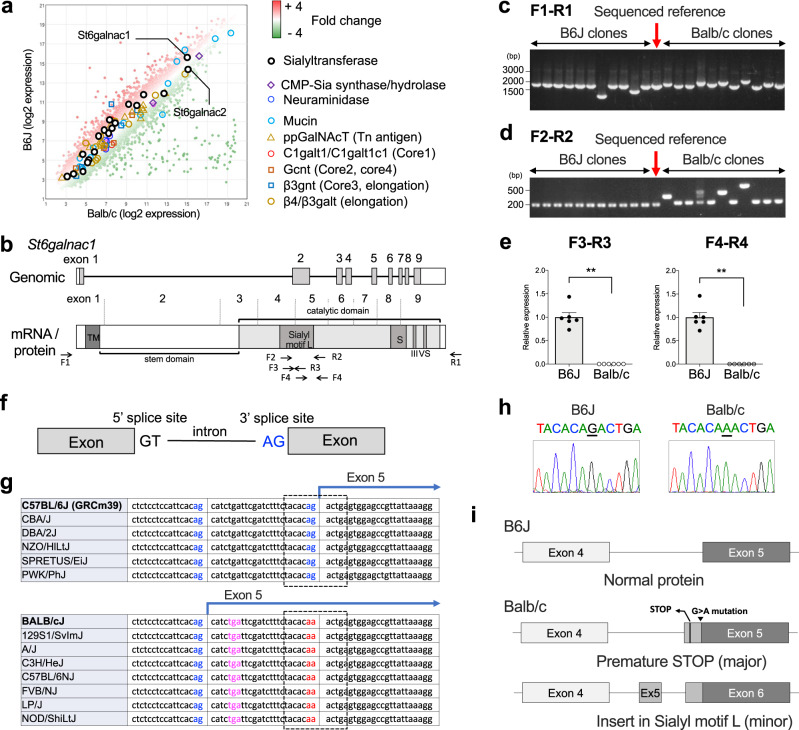
In search of the genes responsible for differential sialylation between B6J and Balb/c strains, we compared the expression profiles of the conjunctival tissues using gene microarray. To our surprise, there were no genes related to sialylation showing all-or-none expressions between the two strains (Fig. 2a). Instead, high expression of a few sialyltransferases, including St6galnac1, was observed in both the strains (Fig. 2a). This indicated that qualitative, rather than quantitative, differences led to the all-or-none phenotype of sialylation.
Fig. 2. Aberrant splicing disrupts sialyl motif of St6galnac1 in Balb/c mice.
a Pooled total RNA from three eyes for each strain was subjected to microarray analysis. CMT-Sia, cytidine-5ʹ -monophospho-N-acetylneuraminic acid; ppGalNAcT, peptidyl-N-acetylgalactosaminyl-transferases; Gcnt, glucosaminyl (N-acetyl) transferase; β3gnt, β3-GlcNAc-transferase; galt, galactosyltransferase. b Schematic representation of St6galnac1 gene and its product. The catalytic domain has four conserved sialyl motifs (L, S, III, VS). Priming sites for PCR used in (c–e) are shown. TM, transmembrane domain. Colony PCR (c) and nested PCR (d) of cloned St6galnac1. Data are representative of two independent experiments. e Quantitative PCR for intact sialyl motif L. n = 6 for each strain. **p < 0.01 (p = 0.0022 for F3-R3 and p = 0.0022 for F4-R4) by two-tailed Mann–Whitney test. Data represents mean ± S.E.M. Data are representative of two independent experiments. f Schematic representation of splicing sites. g Strain comparison of 3ʹ splice site sequences available at Ensembl database52. Blue, potential 3ʹ splice sites; Red, disabled 3ʹ splice site; Magenta, premature stop codon. The range of Sanger sequencing shown in (h) is circled with a dotted line. h Sanger sequencing of 3ʹ splice site of intron before exon 5. i Schematic representation of exon usages of cloned St6galnac1. Source data are provided as a Source Data file (c, d, e).
To examine whether there are any functional differences in St6galnac1 which is expressed highest in the conjunctiva of the two strains, we cloned St6galnac1 mRNA expressed in the conjunctiva. St6galnac1 is a type 2 transmembrane protein residing in the Golgi apparatus, that is comprised of an N-terminus transmembrane domain followed by a long stem and a catalytic domain (Fig. 2b). The catalytic domain has four regions called “sialyl motifs”, which are conserved among all classes of sialyltransferases51. The amplicon sizes of the cloned St6galnac1 were highly variable in Balb/c mice, compared to those of the relatively uniform B6J clones (Fig. 2c). Furthermore, the lengths of the Balb/c amplicons corresponding to the site of Sialyl motif L were different from the known RefSeq sequence (Fig. 2d), suggesting a difference in the coding sequences. In fact, two pairs of quantitative-PCR primers detected no mRNA coding intact Sialyl motif L in the Balb/c conjunctiva (Fig. 2e). Examination of genomic and cloned cDNA sequences revealed that a point mutation at the 3ʹ splice site (G > A) before exon 5 led to aberrant splicing in Balb/c mice (Fig. 2f–i). Comparison of publicly available mouse genomic sequences52 revealed that the G > A mutation was found across several mouse strains (Fig. 2g). Most of the Balb/c clones resulted in the premature stop codon, with loss of most of the catalytic domain (Fig. 2g,i). We searched for potentially functional Balb/c clones that are derived from in-frame alternative splicing and found a rare clone with an extra exon that results in an insertion in the Sialyl motif L amino acid sequence (Fig. 2i).
Defective St6galnac1 activity in Balb/c mice
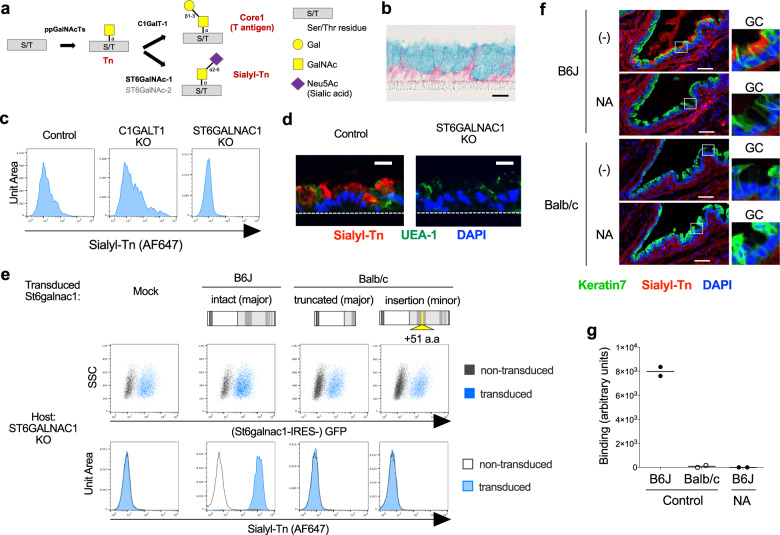
To test the enzymatic activity of cloned St6galnac1 isoforms to synthesize sialyl-Tn (Fig. 3a), we employed a human goblet cell line HT29-MTX-E12 (E12)53. E12 cells differentiated into mature goblet cells, by approximately two weeks of culture after becoming confluent (Fig. 3b). E12 was found to expresses a small amount of sialyl-Tn, which was enhanced upon CRISPR/Cas9-mediated knockout of core-1 synthase (C1GALT1), and abrogated by knockout of ST6GALNAC1 (Fig. 3c and Supplementary Fig. 1). The goblet cell mucus granules were positive for sialyl-Tn, suggesting that the sialyl-Tn is present on the mucins (Fig. 3d). Retroviral transduction of intact full-length St6galnac1, but not the Balb/c-derived clones, dramatically increased sialyl-Tn expression (Fig. 3e). Although lectins known to bind some sialylated glycan structures did not bind B6J conjunctival goblet cells (Supplementary Fig. 2a,b), sialyl-Tn was detected in the B6J conjunctival goblet cells and released mucus, but not in the Balb/c goblet cells (Fig. 3f). Consistently with this, the conjunctival swab from Balb/c mice did not have any detectable sialyl-Tn (Fig. 3g). These results indicate that only the St6galnac1 protein derived from the intact B6J transcript is functional, while those from Balb/c transcripts are not.
Fig. 3. Defective St6galnac1 activity in Balb/c goblet cells.
a Biosynthetic pathways of Tn, Core1, and sialyl-Tn. S/T, serine or threonine residue. b AB and Kernechtrot staining of differentiated HT29-MTX-E12 (E12) cell line. Bar, 20 μm. Polyclonal knockout (KO) of C1GALT1 or ST6GALNAC1 in E12 cells by genome editing affects sialyl-Tn expression assessed by FACS (c) and immunostaining (d). UEA-1, Ulex Europaeus Agglutinin I. Bar, 20 μm. e ST6GALNAC1-KO E12 cells were transduced with cloned St6galnac1 sequences. Expression levels of sialyl-Tn and GFP in transduced (blue histogram) and un-transduced (gray empty histogram) cells were evaluated by FACS. a.a, amino acids. f B6J and Balb/c conjunctivas were stained with a monoclonal antibody against sialyl-Tn (clone MLS132) with or without neuraminidase treatment. GC, goblet cells. Bar, 50 μm. g Swab extract of conjunctival sac (n = 2 for each condition) was subjected to neuraminidase or BSA (control) treatment before coating onto the plate. Sialyl-Tn was detected by anti-sialyl-Tn antibody. Source data are provided as a Source Data file. Data are representative of two independent experiments.
Expression of intact St6galnac1 restores sialylation of goblet cell mucins in Balb/c mice
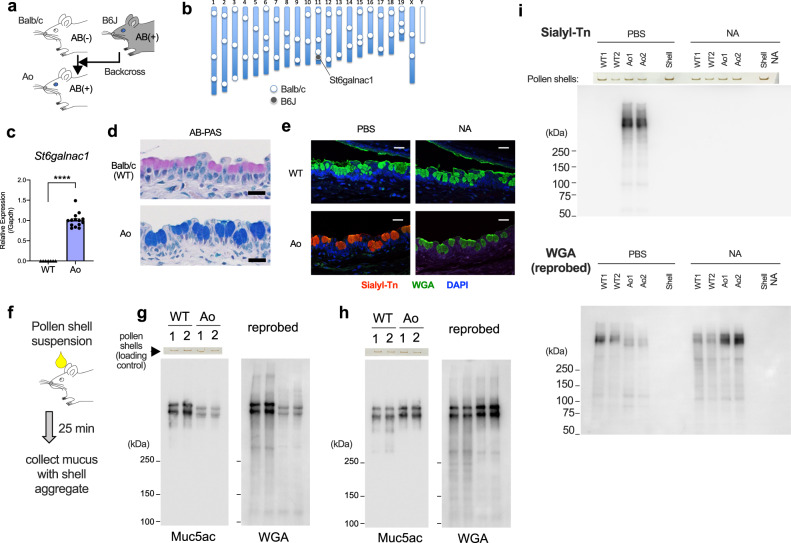
To assess the physiological role of St6galnac1, we introduced B6J-type intact St6galnac1 allele to the Balb/c strain by backcrossing (Fig. 4a, b and Supplementary Fig. 3). Forty eight simple sequence length polymorphism markers were genotyped54 to confirm the replacement of chromosomal arms other than the St6galnac1 locus, after nine generations of backcrossing (Fig. 4b). The conjunctival tissue of fully backcrossed mice, termed Ao mice, expressed sialyl-motif-intact St6galnac1 (Fig. 4c) and had AB-positive goblet cells (Fig. 4d). The presence of sialyl-Tn was confirmed by immunostaining (Fig. 4e). However, there was no difference in the amount of tear volume between WT and Ao mice (Supplementary Fig. 4). To assess the properties of mature mucus proteins, we stimulated mucus secretion by applying ragweed pollen shells essentially devoid of proteins and collected them as the shell aggregates (Fig. 4f). Most of the WGA-reactive glycoprotein was found to be Muc5ac, with different mobilities between WT and Ao mice (Fig. 4g). The mobilities were the same after neuraminidase treatment, indicating that the difference may be attributable to negative charges of the sialic acids (Fig. 4h). The sialylation was represented by the existence of sialyl-Tn (Fig. 4i). These results demonstrate that St6galnac1 is sufficient for the synthesis of sialic acid-capped glycans, including sialyl-Tn, in murine conjunctival goblet cells.
Fig. 4. Expression of intact St6galnac1 restores Sialyl-Tn expression in conjunctival goblet cell mucins in vivo.
a Schematic representation of backcrossing of B6J-derived intact St6galnac1 gene to Balb/c mice. b Chromosomal distribution of PCR-confirmed strain-specific markers in Ao mice. c Intact St6galnac1 expression in the conjunctiva. n = 7 for WT and n = 14 for Ao mice. ****p < 0.0001 by two-tailed Mann–Whitney test. Error bar represents S.E.M. d AB-PAS staining of the conjunctiva. Bar, 20 μm. e Immunostaining of sialyl-Tn in conjunctiva with or without neuraminidase treatment. WGA, wheat germ agglutinin Bar, 20 μm. f A schematic diagram of secreted mucus collection. Western blotting of the secreted mucus with (h, i) or without (g, i) neuraminidase treatment. The photographs of pollen shell amount left in the wells of polyacrylamide gels after the electrophoresis are also shown as loading controls (g–i). Data are representative of at least two independent experiments (d, e, g–i). Source data are provided as a Source Data file (c, g, h, i).
Sialylated mucins encapsulate pollens with an impenetrable layer
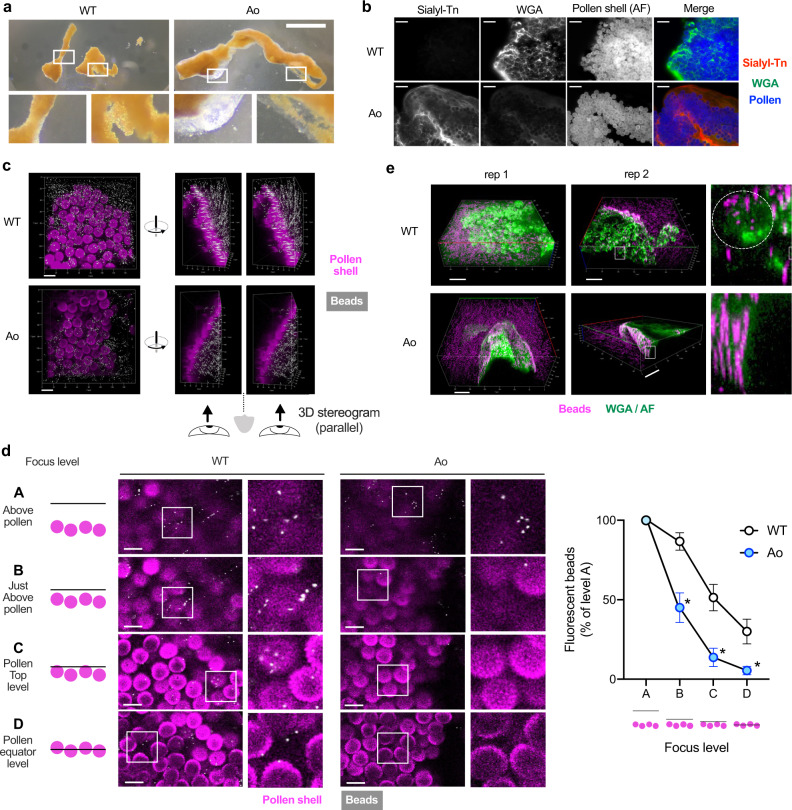
To investigate the functions of sialylated mucins, we examined the pollen aggregates under a microscope. Intriguingly, mucus from Ao mice, but not that from WT Balb/c mice, encapsulated the pollen aggregate with a layer of gel-like structure (Fig. 5a and Supplementary Fig 5). The layer was sialyl-Tn-positive, but the immunostaining intensity was lower compared to that of the mucus inside the aggregate, implying that the water content is higher in the gel-like layer (Fig. 5b). To assess the penetrability of the mucus layer, we took an inverse penetration approach; the mucus-encapsulated pollen aggregates were soaked into the fluorescent particle suspension, and the space accessible to the fluorescent particles was assessed. In WT Balb/c, the pollen shell surface was readily accessible to the particles with a diameter of 0.5 μm (Fig. 5c, d and Supplementary Fig. 6). However, in Ao mice, most of the particles did not reach the shell surface (Fig. 5c, d and Supplementary Fig. 6). Furthermore, additional staining with WGA revealed the difference of surface texture of the mucus; Ao mucus completely covered the pollen aggregate, while the WT mucus did not hide the pollen balls (Fig. 5e). Given that the sizes of most of the bacteria are in the similar order (0.5–2 μm) to the fluorescent particles used, and so do the subpollen particles that carry allergenic proteins (0.5–4.5 μm)55, the sialylated mucins may have a role in quarantining potentially harmful particles, as a first-line of defense on the ocular surface.
Fig. 5. Sialylated mucins encapsulate pollens with an impenetrable layer.
a Representative pictures of pollen shell-mucus aggregates 25 min after challenge. Bar, 1 mm. See also Supplementary Fig. 5 for more examples. b Immunostaining of pollen shell aggregates retrieved from the conjunctival sac. AF, autofluorescence. Bar, 50 μm. c The pollen shell aggregates from indicated strain were soaked in the fluorescent beads-suspended PBS. Parallel-view 3D stereogram of the side-view is also shown. Bar, 20 μm along the closest edge. d The bead distribution was enumerated at various focus levels. Bar, 20 μm. n = 6 for WT and n = 7 for Ao mice. *p < 0.05 (adjusted p = 0.0101, 0.0101, and 0.0233 for levels B, C, and D) by two-tailed Two-way ANOVA with Holm-Sidak’s multiple comparisons. Focus level A is excluded from the statistical analysis. n = 6 for WT and n = 7 for Ao mice. Error bar represents S.E.M. Source data are provided as a Source Data file. See also Supplementary Fig. 6. e The pollen shell aggregates were soaked in fluorescent beads-suspended PBS containing WGA. The detail of the white-squared area is also shown. The white dotted circle indicates a pollen shell. Bar, 50 μm along the closest edge; rep, representative pollen shell aggregate. Data are representative of at least two independent experiments (a–e).
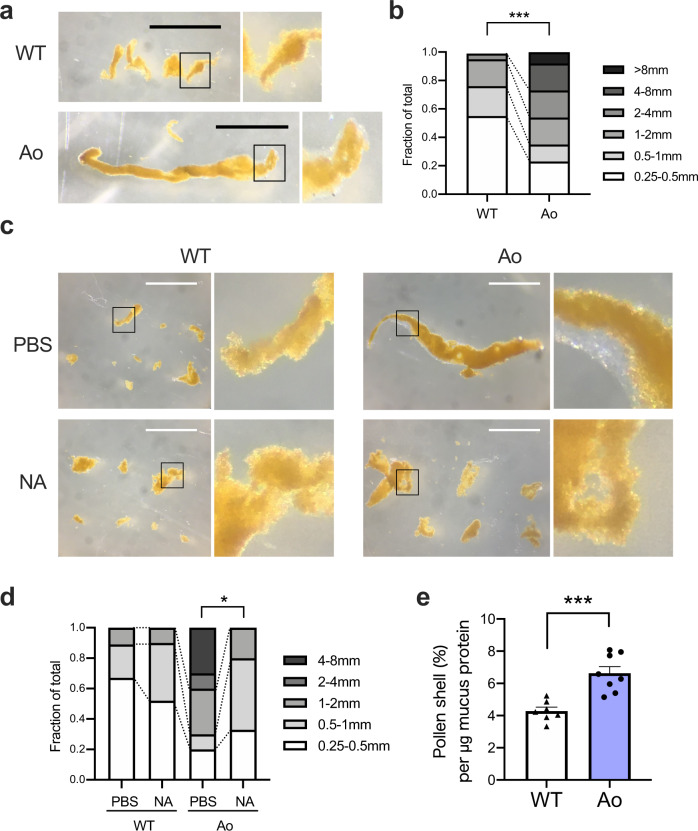
Sialylated mucins efficiently forms large aggregates of pollen shells
In addition to the gel-like layer, we noticed that secreted mucus from Ao mice formed significantly larger aggregates compared to that from WT Balb/c mice (Figs. 5a, 6a, b and Supplementary Fig. 5). The aggregate sizes were decreased when the pollen shells were administered together with neuraminidase (Fig. 6c, d). Gel-like layer also disappeared by sialic acid removal (Fig. 6c). Furthermore, significantly larger amount of pollen shells were captured by the same amount of mucus protein in Ao mice, as compared to those captured in WT Balb/c mice (Fig. 6e).
Fig. 6. Sialylation is required for the efficient entrapment of pollen shells.
a Representative picture of pollen shell-mucus aggregates from one eye. Bar, 1 mm. b Frequencies of aggregates with indicated lengths. Pooled data from 8 eyes each. ***p < 0.001 (p = 0.0009) by two-tailed Fisher’s exact test for the ratio of fractions <1 mm and ≧1 mm. c The pollen shells were instilled into eyes with or without 5 U/mL neuraminidase. Bar, 1 mm. d Frequencies of aggregates with indicated lengths. Pooled data from 4 eyes each. *p < 0.05 (p = 0.0344) by two-tailed Fisher’s exact test for the ratio of fractions <1 mm and ≧1 mm. e The pollen shell amount in the aggregate was divided by the amount of mucus protein in the same aggregate. n = 7 for WT and n = 8 for Ao mice. ***p < 0.001 (p = 0.0004) by two-tailed Welch’s t test. Error bar represents S.E.M. Data are representative of at least three independent experiments (a–e). Source data are provided as a Source Data file (b, d, e).
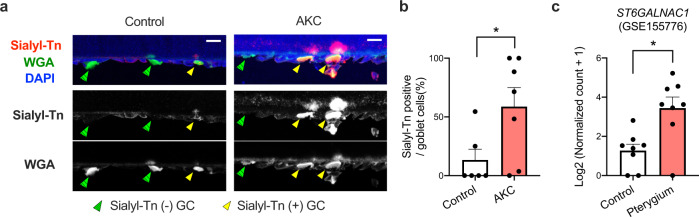
Chronic stimulatory conditions upregulate sialyl-Tn and ST6GALNAC1 in humans
Since sialylated mucins could play a protective role by capturing and quarantining incoming foreign bodies to the eyes, it may be increased in conditions with chronic mechanical and/or inflammatory stimuli. Indeed, we found increase of sialyl-Tn-positive goblet cells in the conjunctiva of AKC patients (Fig. 7a, b). In addition, analysis of a published dataset56 revealed that ST6GALNAC1 expression levels were significantly increased in the biopsies of pterygium, a relatively common proliferative ocular surface disorder associated with UV exposure and inflammation57, compared to those in healthy conjunctiva (Fig. 7c).
Fig. 7. Sialyl-Tn or ST6GALNAC1 is upregulated in chronic stimulatory conditions in humans.
a Representative immunostaining of impression cytology specimens. GC, goblet cells. Bar, 20 μm. b Frequencies of sialyl-Tn-positive goblet cells in impression cytology specimens from control (n = 6) and AKC (n = 7) subjects. *p < 0.05 (p = 0.0443) by two-tailed Mann–Whitney test. Error bar represents S.E.M. c ST6GALNAC1 expression in an RNA-seq dataset (GSE155776) of pterygium (n = 8) and control (n = 8) biopsies. *p < 0.05 (p = 0.0103) by two-tailed Mann–Whitney test. Error bar represents S.E.M. Source data are provided as a Source Data file (a, b).
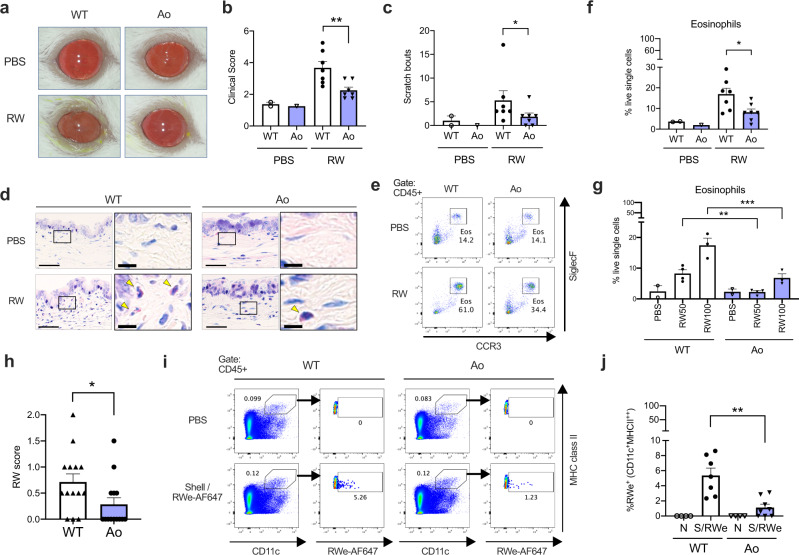
St6galnac1 protects mice from pollen-induced allergic conjunctivitis
Finally, we investigated whether sialylated mucins are functionally protective in vivo. We applied a well-established murine model of ragweed (RW) pollen-induced conjunctivitis to WT Balb/c and Ao mice (Supplementary Fig. 7a). Ao mice showed significantly ameliorated clinical manifestations (Fig. 8a–c). In addition, the eosinophil accumulations were attenuated in Ao mice (Fig. 8d–g and Supplementary Fig. 8). Dose-response analysis showed that the presence of intact St6galnac1 increases the antigen dose threshold for inducing eosinophil accumulation (Fig. 8g). Although the humoral responses were similar after immunization (Supplementary Fig. 7b–d), RW-specific IgE was elevated after pollen challenges in WT mice, but not in Ao mice, suggesting that the sialylated mucins can reduce the antigen responses (Supplementary Fig. 7b–d). Indeed, the amounts of pollens remaining in the conjunctival sac of Ao mice after challenges were significantly smaller than those of WT Balb/c mice, suggesting that the sialylated mucus can efficiently remove the pollen particles compared to the non-sialylated one (Fig. 8h and Supplementary Fig. 9). Furthermore, the fraction of the migrating dendritic cells ingesting the instilled allergen was found to be smaller in Ao mice than in WT Balb/c mice in the draining lymph node (Fig. 8i, j). These results suggest that the sialylated mucins play an essential role in protecting the ocular surface from incoming foreign bodies including allergens (Supplementary Fig. 10).
Fig. 8. St6galnac1 protects mice against allergic conjunctivitis.
a Clinical appearance of PBS- or ragweed pollen (RW)-challenged eyes. b Clinical scores. n = 2, 1, 7, and 7 for PBS-WT, PBS-Ao, RW-WT, and RW-Ao conditions, respectively (b, c, f). **p < 0.01 (p = 0.0093) by two-tailed Mann–Whitney test. c Scratch bouts. *p < 0.05 (p = 0.0449) by two-tailed Mann–Whitney test. d Giemsa staining of the conjunctiva. Arrowheads indicate eosinophils. Bar, 50 μm. Inset: Bar, 10 μm. e Eosinophil frequency among CD45 + cells in the conjunctiva. Eosinophil frequencies in live single cells after challenges of 100 mg/mL (f) or indicated amount (mg/mL) (g) of RW pollen or PBS. *p < 0.05 (p = 0.0379) by two-tailed Mann–Whitney test (f). **p < 0.01 (adjusted p = 0.0091, WT vs Ao in RW 50 mg/mL condition), ***p < 0.001 (adjusted p = 0.0006, WT vs Ao in RW 100 mg/mL condition) by two-tailed ANOVA with Holm-Sidak’s multiple comparisons test (g). h RW scores represent the amount of remaining pollen in the conjunctival sac 30 min after pollen challenges. n = 14 for each strain. *p < 0.05 (p = 0.0325) by two-tailed Mann–Whitney test. i, j Accumulation of migratory dendritic cells (CD11c+MHC class II high) to the draining lymph nodes 12 h after instillation of pollen shells and RW extract labeled with AF647. FACS gating for pooled data (i) and enumeration (j) are shown. N, PBS-instilled; S/RWe, pollen shell and AF647-labeled RW extract. PBS-instilled data were used for setting AF647 gate and therefore excluded from statistical analysis. n = 4, 7, 4, and 8 for WT-N, WT-S/RWe, Ao-N, and Ao-S/RWe conditions, respectively. **p < 0.01 (p = 0.0037) by two-tailed Mann–Whitney test. Data are representative of four (a–h) or three (i, j) independent experiments. Error bar represents S.E.M. (b, c, f, g, h, j) Source data are provided as a Source Data file (b, c, f, g, h, j).
To further confirm the role of sialylated mucins in vivo, we generated St6galnac1 knockout (KO) mice on the B6J background using CRISPR-Cas9 technique (Supplementary Fig. 11a). As expected, conjunctival goblet cells of St6galnac1-KO mice were completely negative for sialyl-Tn, indicating the successful disruption of St6galnac1 gene (Supplementary Fig. 11b). However, there were some sialyl-Tn-negative AB-positive goblet cells left in the St6galnac1 KO conjunctiva (Supplementary Fig. 11b). The AB-stained substance was sialic acid, as there were no AB-positive cells after neuraminidase treatment (Supplementary Fig. 11b). This indicated that another sialyltransferase activity was constitutively operative, or expressed as a compensatory mechanism, in a minor subset of B6J goblet cells, and it was inactive in Balb/c mice. As we found some missense mutations in Balb/c sialyltransferases other than St6galnac1 (Supplementary Table 2), these might affect their functionality. Interestingly, partial formation of gel-like layer was observed around the pollen aggregates from St6galnac1 KO mice, suggesting that partially expressed sialylated glycan other than sialyl-Tn may contribute to the gel-like layer formation (Supplementary Fig. 11c). However, the lengths of the pollen aggregates were smaller (Supplementary Fig. 11d), and the eosinophilic inflammation upon induction of allergic conjunctivitis was exacerbated in St6galnac1 KO mice compared to those in WT mice (Supplementary Fig 11e,f). These results suggest that the decrease in sialylation of mucins in St6galnac1 KO mice results in less-protective phenotypes, illustrating the protective function of sialylated mucins.
Discussion
In the present study, we report the protective role of sialylated mucins present in conjunctival goblet cells. The humans and B6J mice have sialylated mucins in conjunctival goblet cells, while Balb/c mice lacked this sialylation. The difference was largely due to a point mutation at the 3ʹ splice site of a sialyltransferase, St6galnac1, which we found is highly expressed in the mouse conjunctiva, similarly to the human conjunctiva39. In Balb/c strain, loss of St6galnac1 activity, in addition to another sialyltransferase activity found in a minor population of conjunctival goblet cells, led to loss of sialylation on goblet cell mucins. Expressing intact St6galnac1 in Balb/c mice restored this sialylation, which was required for the efficient entrapment and encapsulation of the allergen into a gel-like layer, which was impenetrable to sub-micrometer particles. Human conjunctiva was found to express increased amount of ST6GALNAC1 and sialyl-Tn under chronic stimuli. Finally, the pollen-induced allergic conjunctivitis was ameliorated by expressing sialylated mucins, with enhanced removal of the pollen particles.
The apical surface barriers of mucosal tissue may consist of several layers, depending on the location. For instance, the intestinal surface of the ileum is protected by a single layer of penetrable mucus layer, under which is a rigid glycocalyx on the apical side of the epithelial cells7,58. In the distal colon, where the bacterial load is highest in the intestine, there are two mucus layers; the outer layer serves as a habitat for microbiota, while the inner layer is a separating border that is free of bacteria7,59. The tear film of the ocular surface is composed of three layers; the lipid layer that prevents water evaporation, the aqueous/mucoaqueous layer that contains soluble and gel-forming mucins, and the glycocalyx that serves as a physical barrier2,60. Recently, a new model of the distal colon mucus layers has been proposed, in which the two mucus layers are encapsulating the feces, rather than lining the epithelial surface, to limit the bacterial contact with the epithelial surface61,62. For this to be functional, the proximal colon-derived O-glycosylated mucus was required61. Importantly, allowing bacterial contact to the epithelium would expose the host to the risk of inflammation, even in the case of commensals63. Our observation that the released mucus encapsulates the particulate allergens suggests that the goblet cells enforce two strategies to protect the ocular surface by gel-forming mucins: maintaining the stability of the tear film at steady state, and capturing and quarantining the large foreign bodies to enhance their removal in emergency. Our results indicate that sialylation of mucins is necessary for the latter to be effective.
The molecular sieve of the bacteria-impenetrable inner layer of colon mucus is reported to be denser compared to that of the outer layer7,64. The penetrability is associated not only with the thickness, but also with the glycan component63. At the protein or small compound level, the mucins can inhibit drug diffusion65, but the retention of IgA is enhanced by the presence of the mucus layer66, which is beneficial for the protection of mucosal surfaces67. Interestingly, both the outer and inner layers of the colon mucus, and the penetrable layer of the ileum are composed of the same mucin, Muc2, suggesting that the same gene can produce mucus layers with different physical properties7. In line with this, loss of Core-1 synthase C1galt1 in the intestinal epithelial cells has been reported to impair the integrity of the mucus layer63,68. Interestingly, C1galt1-deficient colonic goblet cells have increased Tn antigen, which implies a reduction of the total negative charges of sialylation or sulfation on the mucin glycans68. In the conjunctiva, our results indicated that St6galnac1-dependent sialylation can provide negative charges to the mucin glycans as observed by the increased mobility on SDS-PAGE. In addition, sialylation increased pollen-capturing efficiency, and the impenetrable capsule layer was missing without mucus sialylation. Thus, we propose that the sialylation is one of the most important factors that regulate Muc5ac gel-forming mucin functions in the conjunctiva.
We previously reported that the pollen shells play an essential role in conjunctival local sensitization as well as in the elicitation phase of the allergic conjunctivitis69. In addition, raw ragweed pollens have been shown to release sub-pollen particles upon being soaked in water, which in turn enhance the allergic inflammation55. Our observation that the pollen particles can be encapsulated with a sialylated impenetrable layer suggest that the layer may have a role in protecting epithelial cells from mechanical damage and in preventing the microparticles to be released. As such, the enhanced removal of pollen particles and reduced migration of allergen-bearing DCs to the lymph nodes was observed in mice capable of producing sialylated mucins. Further investigation is required to elucidate the consequences of sialylation on other mucus properties such as stability against various proteases and permeability to the proteins and small molecules.
In summary, our study elucidated the role of sialylated goblet cell mucins in providing protection against foreign bodies such as allergen particles.
Methods
Mice
B6J and Balb/c mice were bred in the animal facility of Juntendo University or purchased from Sankyo Labo Service Corporation (Tokyo, Japan). Ao mice and St6galnac1 knockout mice were generated as described below. All mice used in this study were 5-9 weeks old. Male and female mice were equally used. Mice were fed ad libitum with a standard diet (Charles River formula-1 (CRF-1), Catalog number (Cat.#) 2108200, Oriental Yeast, Japan) and maintained in plastic cages containing wood chip in an air-conditioned and light-controlled animal room (23 ± 1 C, 55 ± 5% humidity, 12 h light/ 12 h dark).
Generation of Ao mouse
Ao mice were generated by backcrossing B6J mice to Balb/c mice. See Supplemental Fig. 3 and Supplemental Table 1 for the detail of genotyping information. Simple sequence length polymorphism regions (D1Mit231, D1Mit218, D1MIt150, D2Mit149, D2Mit328, D2Mit265, D3Mit164, D3Mit189, D3Mit19, D4Mit193, D4Mit203, D5Mit233, D5Mit161, D6Mit138, D6Mit8, D6Mit36, D7Mit267, D7Mit101, D8Mit178, D8Mit211, D9Mit8, D9Mit18, D10Mit213, D10Mit230, D11Mit226, D11Mit39, D11Mit333, D12Mit12, D12Mit114, D12nDs2, D13Mit16, D13Mit213, D14Mit60, D14Mit194, D15Mit265, D15Mit80, D15Mit34, D16Mit131, D16Mit140, D17Mit133, D17Mit123, D18Mit222, D18Mit184, D19Mit68, D19Mit19, D19Mit1, DXMit48, DXMit153) were genotyped to confirm the replacement of chromosomes54.
Generation of St6galnac1-knockout mice
St6galnac1-knockout mice were generated by CRISPR/Cas9 system70 in our facility. Ribonucleoprotein was assembled using Alt-R S.p. HiFi Cas9 Nuclease V3 (Integrated DNA technologies, USA), Alt-R CRISPR-Cas9 tracrRNA (Integrated DNA technologies), and Alt-R CRISPR-Cas9 crRNA (Mm.Cas9.ST6GALNAC1.1.AB,/AlTR1/rArUrArCrArCrUrUrGrGrArGrUrArCrCrCrArGrUrGrUrUrUrUrArGrArGrCrUrArUrGrCrU/AlTR2/, Integrated DNA technologies) and was microinjected into the cytoplasm of fertilized 1-cell eggs at the pronuclei stage from C57BL/6 J female mice (Charles River Laboratories Japan, Inc., Kanagawa Japan) superovulated by intraperitoneal injection of PMSG followed by hCG at an interval of 48 h and mated overnight with C57BL/6 J male mice (Charles River Laboratories Japan, Inc., Kanagawa Japan). Microinjections were performed by micromanipulators (Leica, Wetzlar, Germany) with a PMM-150 FU piezo-impact drive unit (Prime Tech Inc., Ibaragi, Japan) using a blunt-ended mercury-containing injection pipette with approximately 6 μm of inner diameter71. After microinjection, 2-cell stage embryos, cultured in modified Whitten’s medium72 (Mitsubishi Kagaku Iatron Inc., Tokyo, Japan) for approximately 24 h and developed from fertilized 1-cell eggs, were transferred into the oviducts of pseudopregnant ICR females (Charles River Laboratories Japan, Inc., Kanagawa, Japan) at 0.5 dpc.
Mutations were evaluated by Sanger sequencing of PCR product from genomic DNA. Targeted region was PCR amplified using Q5 High-Fidelity DNA Polymerase (New England Biolabs, USA) and following primers: Forward: 5ʹ-GCTGCTTCAGAGCTGAGG-3ʹ and reverse: 5ʹ-AGCCCATATCTACAAGCAACG-3ʹ.
The product was cleaned using NucleoSpin Gel and PCR Clean-up (MACHEREY-NAGEL, Germany) kit following manufacturer’s instructions. Sanger sequence was performed using the reverse primer as the sequencing primer. Obtained knockout lines were crossed with wild-type C57BL/6 J mice to segregate the knockout alleles. The heterozygous pups were genotyped by Sanger sequencing, and the pups harboring the same mutation was crossed to generate homozygous knockout mice.
Allergic conjunctivitis models
The experimental allergic conjunctivitis models were induced as previously described69. For Balb/c background strains, each foot pad of Balb/c or Ao mice received 50 μg of RW pollens emulsified in 50 μL of Imject Alum Adjuvant (ThermoFisher Scientific) on day 0. On day 14, 0.1 mg of RW pollens suspended in 200 μL of PBS was injected intraperitoneally. From day 28, each conjunctival sac received 2.5 μL of 100, 50, or 0 mg/mL RW pollen suspension once a day for 4 consecutive days (Supplementary Fig. 7a). For B6J background strains, the local sensitization model was used69. Each conjunctival sac received 2.5 μL of 200 mg/mL RW pollen suspension once a day for 5 days per week for 3 weeks. 3 days later, the last challenge was performed at the same dose (Supplementary Fig. 11e).
Evaluation of allergic conjunctivitis model
Mouse eyes were scored for the signs of immediate hypersensitivity responses 30 min after the topical challenges of RW pollen suspension, according to previously published criteria69. Conjunctival redness, chemosis, tearing, and eyelid edema were graded as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe) by a person blind to the mouse identity. Signs that appeared to be in the middle of the grades were scored in increments of 0.5. Scores of the left and right eyes were averaged to calculate the final clinical score assigned to each mouse. The remaining ragweed pollen amount was graded from 0 to 2, as shown in Supplementary Fig. 9 and assigned to each eye. Scratching behavior with hind legs were also counted for 30 minutes. Mice were sacrificed 24 h after the last challenge and subjected to analysis.
Cell culture
HT29-MTX-E12 (E12) cell line was obtained from European Collection of Authenticated Cell Cultures (ECACC, UK). Cells were cultured in DMEM supplemented with 10% fetal calf serum, 1% nonessential amino acids and 1% L-glutamine. For differentiation, cells were seeded on Millicell culture inserts (Merck Millipore, Germany) and cultivated for 2 weeks. The medium was refreshed every other day.
Genome editing
LentiGuide-Puro and lentiCas9-Blast were gifts from Feng Zhang (Addgene plasmids # 52963, http://n2t.net/addgene:52963, RRID:Addgene_52963 and #52962, http://n2t.net/addgene:52962, RRID:Addgene_52962, respectively)73. Streptococcus pyogenes Cas9 sequence was subcloned into pMXs-Blasti, following which E12 cells were retrovirally transduced. After selection, single guide sequences for C1GALT1 and ST6GALNAC1 were cloned into lentiGuide-Puro, or the non-targetting control. The lentivirus was produced in 293FT cells using the ViraPower Lentiviral Expression System (Thermo Fisher Scientific). The knockout efficiencies were determined by cloning of the genomic sequences corresponding the guide-targeted regions. The targeted regions were PCR amplified from the extracted DNA using following primers: C1GALT1 Forward: 5ʹ-GGGAGAAAAGGTTGACACCCA-3ʹ; C1GALT1 Reverse: 5ʹ-GCGGGTTAATGATGTCACACAG-3ʹ; ST6GALNAC1 Forward-1: 5ʹ-GACAGTGGGTACCTGAACCAG-3ʹ; ST6GALNAC1 Reverse-1: 5ʹ- GACAGTGGGTACCTGAACCAG-3ʹ; ST6GALNAC1 Forward-2: 5ʹ-TCAAGAACGTGCCTCTTGGG-3ʹ; ST6GALNAC1 Reverse-2: 5ʹ-GGGTCTGTGCCTGTGGTTAG-3ʹ; ST6GALNAC1 Forward-3: 5ʹ-TCTGCACAGATTGAGCGGAG-3ʹ; ST6GALNAC1 Reverse-3: 5ʹ-TGTCCAACCCTTTAGAGCCAC-3ʹ. The amplicons were cloned into pCR-Blunt vector (Thermo Fisher Scientific), which was then transformed into DH5α competent cells. A total of 24 colonies (24 colonies for C1GALT1, and 8 colonies per primer pair for ST6GALNAC1) for each knockout cell culture were tested for colony PCR using GoTaq Green Master Mix (Promega, USA). Plasmids from PCR-positive colonies were extracted and subjected to Sanger sequencing.
Histology and immunofluorescence microscopy
Mouse conjunctiva biopsies were obtained after euthanasia. AB, PAS, or Giemsa staining was performed for PFA-fixed paraffin-embedded sections before and after neuraminidase treatment.
For immunofluorescence microscopy, the biopsy was fixed in 4% PFA at 4 °C for 1 h, washed in PBS, and embedded in O.C.T. compound (Sakura Finetek, Japan). Cryosections were treated with and without neuraminidase (1 U/ml, Nacalai Tesque, Japan) at 37 °C overnight. The specimen was blocked with streptavidin and biotin solutions and Carbo-Free blocking solution (both from Vector Laboratories, USA). Lectin staining was performed with biotinylated Peanut Agglutinin (PNA, Cat.# B-1075-5, Vector Laboratories, 1:1000 dilution), biotinylated Maackia Amurensis Lectin II (MAL-II, Cat.# B-1265-1, Vector Laboratories, 1:200 dilution), or biotinylated Sambucus Nigra Lectin (SNA, Cat.# B-1305-2, Vector Laboratories, 1:1000 dilution) followed by incubation with streptavidin conjugated with AlexaFluor 568 (Cat.# S11226, Thermo Fisher Scientific, 1:1000 dilution), or WGA conjugated with CF488A (Cat.# 29022-1, Biotium, USA). For competition experiments, lectins were incubated with 500 mM of sialic acid (Wako, Japan) prior to application to tissue specimen. Immunostaining was performed with anti-cytokeratin7 (clone EPR17078, Cat.# ab181598, Abcam, UK, 1:1300 dilution), and anti-Sialyl-Tn (clone MLS132, Cat.# 010-25881, FujiFilm, 1:275 dilution) as primary antibodies, and anti-mouse AlexaFluor 568 (Cat.# A11031, ThermoFisher Scientific, 1:200 dilution), anti-mouse AlexaFluor 647 (Cat.# A21236, Thermo Fisher Scientific, 1:200 dilution), anti-rabbit AlexaFluor 488 (Cat.# A11034, Thermo Fisher Scientific, 1:200 dilution), and anti-rabbit IgG Superclonal recombinant antibody AlexaFluor 488 (A27034, Thermo Fisher Scientific, 1:200 dilution) as secondary antibodies. After staining with DAPI (Dojindo, Japan, 1:1000 dilution), the coverslips were mounted with Slowfade Diamond solution (Thermo Fisher Scientific) and fluorescence was observed under the LSM 700 confocal microscope using ZEN 2011 SP3 software (Zeiss, Germany). For separating pollen shell-derived autofluorescence, fluorescence was observed under Nuance Multispectral Imaging System (PerkinElmer).
For the cultured goblet cells, the membranes of culture inserts were rinsed with PBS, fixed in Fixation buffer (Biolegend, USA), and embedded in O.C.T. compound. Sections were stained with either Alcian Blue (pH2.5) and Kernechtrot solution (#4087-1, Muto Pure Chemicals, Japan) or with anti-sialyl-Tn antibody (clone MLS132, FujiFilm, 1:275 dilution) and biotinylated Ulex Europaeus Agglutinin I (UEA-1, Cat.# B-1065-2, Vector Laboratories, 1:500 dilution), followed by anti-mouse AlexaFluor 647 (#A21236, Themo Fisher Scientific, 1:200 dilution) and avidin AlexaFluor 488 (#A21370, Themo Fisher Scientific, 1:200 dilution).
Enzyme-linked immunosorbent assay (ELISA)
For the detection of sialyl-Tn in the secreted mucin molecule, the conjunctival swab extract was diluted to the concentration of 2 µg/ml and immobilized onto a black microplate at 37 °C for 1 h. The plate was blocked with Carbo-Free blocking solution (SP-5040, Vector Laboratories, USA). After washes, the plates were incubated with 1 µg/ml of sialyl-Tn antibody diluted in PBS (clone MLS132, Cat.# 010-25881, FujiFilm, Japan, 1:1100 dilution) at RT for 30 min. After washes, bound antibody was detected by HRP-conjugated anti-mouse IgG antibody (Cat.# 7076, Cell Signaling Technology, USA, 1:3000 dilution) in PBS containing 1% BSA. After extensive washings, Western Lightning ECL Pro (PerkinElmer) was added as a substrate, and luminescence was measured by GloMax luminescence plate reader (Promega, USA).
For the quantitation of ragweed-specific immunoglobulins in sera, black microplates (Greiner Bio-one, Germany) were coated with 5 μg/mL of RW extract. After blocking with 30% ImmunoBlock reagent (KAC, Japan), diluted mouse serum was applied to the wells and incubated for 2 h at room temperature. After washing, the plates were incubated with biotin-conjugated detection antibodies (rat anti-mouse IgE, clone R35-118, Cat. #553419, BD Biosciences, USA, 1:500 dilution; goat anti-IgG1, #1070-08, SouthernBiotech, USA, 1:10,000 dilution; goat anti-IgG2a, #1080-08, SouthernBiotech, 1:5000 dilution) together with avidin-HRP (Cat.# 405103, BioLegend, CA, USA, 1:1000 dilution) for 1 h. After washings, the plate was subjected to chemiluminescence measurement as described above.
Flow cytometry
For the measurement of glycan expression, E12 cells were dissociated and fixed with Fixation Buffer (Biolegend, USA) for 20 min at room temperature (RT) before permeabilization with Intracellular Staining Permeabilization Wash Buffer (Biolegend). The permeabilized cells were incubated with anti-sialyl-Tn monoclonal antibody (mAb) (clone MLS132, FujiFilm, Japan, 1:250 dilution) followed by Alexa Fluor 647-conjugated anti-mouse IgG antibody (A21236, Thermo Fisher Scientific, USA, 1:200 diluition).
Conjunctival leukocyte infiltration was evaluated as previously described69. Conjunctival tissue was dissected, minced, and digested in RPMI1640 supplemented with 10% FCS, 1 mg/mL collagenase (FujiFilm, Japan) and 0.5 mg/mL DNase I with continuous stirring for 1 h at 37 °C. The dispersed cell suspension was filtered through a nylon mesh. Lymph node cells were directly dispersed using a nylon mesh. The washed cells were blocked with anti-CD16/CD32 antibody (clone 2.4G2, Cat.# 553141, BD Biosciences) for 10 min at 4 °C. The cells were further incubated with surface-staining antibodies at 4 °C for 20 min. For the conjunctival cells, following antibodies were used: anti-CD11b, FITC (clone M1/70, Cat.# 101206, BioLegend, 1:200 dilution), anti-CCR3, PE (clone 83101, Cat.# FAB729P, R&D SYSTEMS, 1:100 dilution), anti-CD45, PerCP/Cyanine5.5 (clone 30-F11, Cat.# 103132, BioLegend, 1:500 dilution), anti-CD11c, PE/Cyanine7 (clone N418, Cat.# 117318, BioLegend, 1:200 dilution), and anti-Siglec-F, Alexa Fluor647 (clone E50-2440, Cat.# 562680, BD Biosciences, 1:500 dilution) for the conjunctival cells. For the lymph node cells, following antibodies were used: Anti-CD11b, FITC (clone M1/70, Cat.# 101206, BioLegend, 1:200 dilution), anti-mouse I-A/I-E, PE (clone M5/114.15.2, Cat.# 107608, BioLegend, 1:1000 dilution), anti-CD45, biotin (clone 30-F11, Cat.# 103104, BioLegend, 1:250 dilution), anti-CD11c, PE/Cyanine7 (clone N418, Cat.# 117318, BioLegend, 1:200 dilution), and streptavidin, BV711 (Cat.# 563262, BD Biosciences, 1:250 dilution). 4ʹ,6-diamidino-2-phenylindole (DAPI, Cat.# 340-07971, Dojindo, 1:1000 dilution) was added to the cell suspension prior to acquisition.
Fluorescence intensity was measured using FACSuite software (BD Biosciences, USA) and FACSVerse flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (BD Biosciences). The gating is shown in Supplementary Fig. 8.
SDS-PAGE and western blotting
The proteins in the swab extract or the proteins in the secreted mucus were denatured in reducing condition, separated by SDS-PAGE, and electroblotted to PVDF membranes (Millipore). Membranes were stained with AB solution pH 2.5 (Wako, Japan). After differentiation with 3% acetic acid followed by 100% methanol, the membrane was further stained using PAS staining kit (MUTO Pure Chemicals, Japan) according to the manufacturer’s instructions.
For the sialyl-Tn detection by Western blotting, the membranes were blocked with Carbo-Free blocking solution (SP-5040, Vector Laboratories), and incubated with the anti-Sialyl-Tn (clone MLS132, Cat.# 010-25881, FujiFilm, 1:1100 dilution) or anti-Muc5ac (clone 45M1, Cat.# ab3649, Abcam, 1:500 dilution), followed by the HRP-conjugated anti-mouse IgG antibody (Cat.# 7076, Cell Signaling Technology, 1:3000 dilution). After stripping, the same membrane was incubated with WGA-CF488A (Cat.# 29022-1, Biotium, 1:200 diliution). The ECL luminescence and the fluorescence were visualized with LAS-4000 (FujiFilm).
Micorarray analysis
Total RNA was extracted from mouse conjunctival tissues using RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The same amount of total RNA from 3 mice were mixed for each cohort and a microarray analysis was performed using Clariom S assay for mouse (Thermo Fisher Scientific, USA) following the manufacturer’s instruction. Data were analyzed by Transcriptome Analysis Console Software (Thermo Fisher Scientific).
Cloning and retroviral transduction of St6galnac1
St6galnac1 transcripts were amplified from the mouse conjunctival cDNA by PCR using Q5 DNA polymerase (New England Biolabs, USA). The amplicons were cloned into pCR-Blunt vector (Thermo Fisher Scientific), which was then transformed into DH5α competent cells. Colony PCR was performed using GoTaq Green Master Mix (Promega, USA). The obtained clones were sequenced and subcloned into pMXs-IG vectors. Retrovirus was produced by transfection into Plat-A cells, and then applied to E12 cells.
Sanger sequencing
The genomic DNA was extracted from mouse tails and the St6galnac1 genomic sequence containing exon 5 (PCR region 2 in Supplementary Fig. 3b) was amplified with B6J primers (Supplementary Table 1). The amplicon was purified and Sanger-sequenced using Common-R primer for region 2 in the laboratory of FASMAC (FASMAC, Japan).
Collection of secreted mucus
The shells of RW pollen were produced as previously described69. The RW pollens were defatted with acetone at 60 °C overnight, followed by an incubation in phosphoric acid at 60 °C for 7 d. In this step, most of the pollen content was removed from the navel pore. After washings, the pollens were further incubated in 6% w/v KOH solution at 80 °C for 6 h. The process was repeated to remove heat- and base-labile components such as proteins and endotoxins. The washed pollen shells were dried overnight. The shells were weighed and suspended in PBS at the same particle concentrations as 200 mg/mL of RW pollen suspension.
Each mouse conjunctival sac received 2.5 μL of shell suspension to induce mucus secretion. Twenty-five min later, the mice were deeply anesthetized or sacrificed, and the pollen shell-mucus aggregates were carefully collected from the conjunctival sac. The morphology of the aggregates was observed under a stereo microscope. The aggregates were either fixed in 4% PFA overnight at 4 °C before immunostaining, treated with or without neuraminidase at 37 °C for two days before Western Blotting, directly subjected to reverse penetrability assessment, or subjected to the measurement of protein concentration.
Measurement of protein concentration and pollen shell amount
The protein amount was measured using Micro BCA Protein Assay Kit (ThermoFisher) following manufacturer’s instructions. After measurement, the released pollen shells were washed in water, and their amount was measured using their autofluorescence at an excitation wavelength 405 nm and an emission filter 500–550 nm using GloMax luminescence plate reader (Promega).
Reverse penetration assay
The pollen aggregates collected in PBS was soaked in PBS containing FluoSphere Carboxylate-Modified Microspheres, 0.5 µm, red fluorescent (580/605) (#F8812, ThermoFisher) and/or WGA-CF488A (Biotium) in a well of µ-Plate 96 Well Black plate (#89626, ibidi, Germany). The fluorescence was observed under the LSM 700 confocal microscope (Zeiss) using ZEN2011 SP3 software (Zeiss).
Human samples
Six control subjects and seven atopic keratoconjunctivitis patients were recruited. Clinical parameters other than diagnosis, such as age and sex information were not collected. The recruitment was announced for consecutive patients who were visiting the Department of Ophthalmology Juntendo University, Juntendo and Urayasu Hospital, Tokyo and Chiba, Japan. When conjunctival samples were collected from AKC patients, there was no treatment of eye drops to these patients. Healthy volunteers who did not show signs of allergic conjunctivitis or corneal diseases were recruited. Written informed consent was received prior to participation. Impression cytology specimens were obtained using EYEPRIM (OPIA Technologies SAS, France) as shown in Fig. 1a. The cells were fixed with 4% paraformaldehyde in PBS, rinsed with PBS, and embedded in O.C.T. compound. The sections were subjected to AB and PAS staining, HID staining, or immunostaining. In some cases, sections were treated with neuraminidase from Arthrobacter ureafaciens (#24229-74, Nacalai Tesque, Japan) before staining.
Statistics & reproducibility
Statistical analysis was performed using Prism 8 software (GraphPad, CA). Data are presented as mean ± S.E.M. in all figure parts in which error bars are shown. No statistical method was used to predetermine sample size. We determined sample sizes by referring to previously published papers in the field56,69. The investigators were blinded to allocation during experiments and outcome assessment. Groups were analyzed by two-tailed tests. P values of <0.05 were considered statistically significant. All experiments except for the one using human samples were replicated at least twice. All attempts to replication were successful. Human experiment (Fig. 7b) was not replicated because of the limitation of the sample availability. Analysis based on the published database (Fig. 7c) was not replicated because of the data availability.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We wish to acknowledge the support from the members of the Laboratory of Morphology and Image Analysis, Research Support Center, Juntendo University Graduate School of Medicine for their technical assistance. This study was partly funded by the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04217(J.K.), 20H03721(J.K.), 17K10253(T.A.), 20K08808(T.A.)), by the Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder (T.A.) and by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan (J.K. and T.A.).
Source data
Author contributions
T.A. and J.K. conceived the project; M.M. and T.A. designed the study; M.M., T.A., S.F., M.K., Y.K., T.I., K.I, A.Kaitani, M.H., and A.Kamei performed experiments; E.N. and N.T. generated St6galnac1 knockout mice; A.M. and N.E. collected human samples; M.M. and T.A. analyzed and interpreted the results, and prepared figures; N.N., K.M., H.O., K.O., A.M, N.E., J.K. contributed to the interpretation of data; T.A. and M.M. wrote the manuscript; J.K. and K.O. supervised the entire project; All authors provided critical feedback and helped shape the research, analysis and manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Source data are provided with this paper. The microarray data for mouse conjunctival gene expression profiles is deposited in Gene Expression Omnibus (GEO; accession no. GSE220182). The genomic DNA sequences for various mouse strains were retrieved through Ensembl database (https://www.ensembl.org). Publicly available dataset GSE155776 was retrieved from Gene Expression Omnibus. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments were approved by the Institutional Animal Care and Use Committee at Juntendo University (Approval numbers: 2022107 and 2020136), and performed in accordance with guidelines of the Laboratory Animal Experimentation at Juntendo University and with the Association for Research in Vision and Ophthalmology (ARVO) statement for the animal use in Ophthalmic and Vision Research. All human procedures were approved by the Ethical Review Board, Juntendo University Faculty of Medicine (approval no. 2019244). Written informed consent was received prior to participation. Participants did not receive any compensation. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomoaki Ando, Email: tm-ando@juntendo.ac.jp.
Jiro Kitaura, Email: j-kitaura@juntendo.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-37101-y.
References
- 1.Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018;11:1551–1557. doi: 10.1038/s41385-018-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portal C, Gouyer V, Gottrand F, Desseyn JL. Ocular mucins in dry eye disease. Exp. Eye Res. 2019;186:107724. doi: 10.1016/j.exer.2019.107724. [DOI] [PubMed] [Google Scholar]
- 3.Hodges RR, Dartt DA. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 2013;117:62–78. doi: 10.1016/j.exer.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demouveaux B, Gouyer V, Gottrand F, Narita T, Desseyn JL. Gel-forming mucin interactome drives mucus viscoelasticity. Adv. Colloid Interface Sci. 2018;252:69–82. doi: 10.1016/j.cis.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Birchenough GM, Nystrom EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352:1535–1542. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wlodarska M, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest Liver Physiol. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 10.Floyd AM, et al. Mucin deficiency causes functional and structural changes of the ocular surface. PLoS One. 2012;7:e50704. doi: 10.1371/journal.pone.0050704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marko CK, Tisdale AS, Spurr-Michaud S, Evans C, Gipson IK. The ocular surface phenotype of Muc5ac and Muc5b null mice. Invest Ophthalmol Vis. Sci. 2014;55:291–300. doi: 10.1167/iovs.13-13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marko CK, et al. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am. J. Pathol. 2013;183:35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gipson IK. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin Eye Res. 2016;54:49–63. doi: 10.1016/j.preteyeres.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko BY, Xiao Y, Barbosa FL, de Paiva CS, Pflugfelder SC. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight. 2018;3:e98222. doi: 10.1172/jci.insight.98222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler KM, et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 2019;4:2146–2154. doi: 10.1038/s41564-019-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheehan JK, et al. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem. 2004;279:15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- 17.Chao CC, Butala SM, Herp A. Studies on the isolation and composition of human ocular mucin. Exp Eye Res. 1988;47:185–196. doi: 10.1016/0014-4835(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi T, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J. Biol. Chem. 2017;292:11079–11090. doi: 10.1074/jbc.M116.770123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez Benavente MC, Argueso P. Glycosylation pathways at the ocular surface. Biochem. Soc. Trans. 2018;46:343–350. doi: 10.1042/BST20170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockhausen I, Elimova E, Woodward AM, Argueso P. Glycosylation pathways of human corneal and conjunctival epithelial cell mucins. Carbohydr Res. 2018;470:50–56. doi: 10.1016/j.carres.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marczynski M, et al. Charged glycan residues critically contribute to the adsorption and lubricity of mucins. Colloids Surf. B Biointerfaces. 2020;187:110614. doi: 10.1016/j.colsurfb.2019.110614. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, et al. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Vis. Sci. 2004;45:2212–2217. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]
- 23.Fukui M, Yamada M, Akune Y, Shigeyasu C, Tsubota K. Fluorophotometric analysis of the ocular surface glycocalyx in soft contact lens wearers. Curr. Eye Res. 2016;41:9–14. doi: 10.3109/02713683.2014.999948. [DOI] [PubMed] [Google Scholar]
- 24.Kawano K, Uehara F, Ohba N. Lectin-cytochemical study on epithelial mucus glycoprotein of conjunctiva and pterygium. Exp Eye Res. 1988;47:43–51. doi: 10.1016/0014-4835(88)90022-X. [DOI] [PubMed] [Google Scholar]
- 25.Creuzot-Garcher C, et al. Alteration of sialyl Lewis epitope expression in pterygium. Invest Ophthalmol Vis. Sci. 1999;40:1631–1636. [PubMed] [Google Scholar]
- 26.Kase S, Kitaichi N, Furudate N, Yoshida K, Ohno S. Increased expression of mucinous glycoprotein KL-6 in human pterygium. Br. J. Ophthalmol. 2006;90:1208–1209. doi: 10.1136/bjo.2006.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An HJ, et al. Glycomics analyses of tear fluid for the diagnostic detection of ocular rosacea. J. Proteome Res. 2005;4:1981–1987. doi: 10.1021/pr0501620. [DOI] [PubMed] [Google Scholar]
- 28.Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15:747–775. doi: 10.1093/glycob/cwi061. [DOI] [PubMed] [Google Scholar]
- 29.Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/Corfield. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia R, et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223–236. doi: 10.1007/s10555-018-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munkley J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016;17:275. doi: 10.3390/ijms17030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takashima S. Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci. Biotechnol. Biochem. 2008;72:1155–1167. doi: 10.1271/bbb.80025. [DOI] [PubMed] [Google Scholar]
- 33.Dimitroff CJ. Galectin-binding O-glycosylations as regulators of malignancy. Cancer Res. 2015;75:3195–3202. doi: 10.1158/0008-5472.CAN-15-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcos NT, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 35.Kurosawa N, Inoue M, Yoshida Y, Tsuji S. Molecular cloning and genomic analysis of mouse Galbeta1, 3GalNAc-specific GalNAc alpha2,6-sialyltransferase. J. Biol. Chem. 1996;271:15109–15116. doi: 10.1074/jbc.271.25.15109. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa N, Kojima N, Inoue M, Hamamoto T, Tsuji S. Cloning and expression of Gal beta 1,3GalNAc-specific GalNAc alpha 2,6-sialyltransferase. J. Biol. Chem. 1994;269:19048–19053. doi: 10.1016/S0021-9258(17)32272-X. [DOI] [PubMed] [Google Scholar]
- 37.Sewell R, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 38.Yao Y, et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185:1172–1188 e1128. doi: 10.1016/j.cell.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzman-Aranguez A, Mantelli F, Argueso P. Mucin-type O-glycans in tears of normal subjects and patients with non-Sjogren’s dry eye. Invest Ophthalmol Vis. Sci. 2009;50:4581–4587. doi: 10.1167/iovs.09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: Four repeated surveys from 1993-2014. Respir Med. 2015;109:982–990. doi: 10.1016/j.rmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J. Allergy Clin. Immunol. 2010;126:778–783 e776. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki D, et al. Air pollution significantly associated with severe ocular allergic inflammatory diseases. Sci. Rep. 2019;9:18205. doi: 10.1038/s41598-019-54841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dogru M, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63:1324–1334. doi: 10.1111/j.1398-9995.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 45.Hu Y, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62:917–925. doi: 10.1111/j.1398-9995.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 46.Messina A, et al. Tear N-glycomics in vernal and atopic keratoconjunctivitis. Allergy. 2021;76:2500–2509. doi: 10.1111/all.14775. [DOI] [PubMed] [Google Scholar]
- 47.Henriksson JT, et al. Morphologic alterations of the palpebral conjunctival epithelium in a dry eye model. Cornea. 2013;32:483–490. doi: 10.1097/ICO.0b013e318265682c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams GG, Dilly PN. Differential staining of ocular goblet cells. Eye (Lond) 1989;3:840–844. doi: 10.1038/eye.1989.128. [DOI] [PubMed] [Google Scholar]
- 49.Huang AJ, Tseng SC, Kenyon KR. Morphogenesis of rat conjunctival goblet cells. Invest Ophthalmol Vis. Sci. 1988;29:969–975. [PubMed] [Google Scholar]
- 50.Aragona P, Micali A, Paladino G, Ferreri F, Puzzolo D. Effects of tear substitutes on conjunctival epithelium of mice. Ophthalmic Res. 2007;39:265–275. doi: 10.1159/000108120. [DOI] [PubMed] [Google Scholar]
- 51.Moremen KW, et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 2018;14:156–162. doi: 10.1038/nchembio.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keane TM, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens I, Stenberg P, Artursson P, Kissel T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm Res. 2001;18:1138–1145. doi: 10.1023/A:1010974909998. [DOI] [PubMed] [Google Scholar]
- 54.Gurumurthy CB, et al. Validation of simple sequence length polymorphism regions of commonly used mouse strains for marker assisted speed congenics screening. Int. J. Genomics. 2015;2015:735845. doi: 10.1155/2015/735845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J. Allergy Clin. Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf J, et al. Characterization of the cellular microenvironment and novel specific biomarkers in pterygia using RNA sequencing. Front Med. (Lausanne) 2021;8:714458. doi: 10.3389/fmed.2021.714458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther. Adv. Ophthalmol. 2021;13:25158414211020152. doi: 10.1177/25158414211020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Layunta E, Javerfelt S, Dolan B, Arike L, Pelaseyed T. IL-22 promotes the formation of a MUC17 glycocalyx barrier in the postnatal small intestine during weaning. Cell Reports. 2021;34:108757. doi: 10.1016/j.celrep.2021.108757. [DOI] [PubMed] [Google Scholar]
- 59.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 60.Swamynathan SK, Wells A. Conjunctival goblet cells: Ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul. Surf. 2020;18:19–26. doi: 10.1016/j.jtos.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergstrom K, et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science. 2020;370:467–472. doi: 10.1126/science.aay7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamphuis JBJ, Mercier-Bonin M, Eutamene H, Theodorou V. Mucus organisation is shaped by colonic content; a new view. Sci. Rep. 2017;7:8527. doi: 10.1038/s41598-017-08938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson ME, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falavigna M, Stein PC, Flaten GE, di Cagno MP. Impact of mucin on drug diffusion: development of a straightforward in vitro method for the determination of drug diffusivity in the presence of mucin. Pharmaceutics. 2020;12:168. doi: 10.3390/pharmaceutics12020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogier EW, Frantz AL, Bruno ME, Kaetzel CS. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 2014;3:390–403. doi: 10.3390/pathogens3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boullier S, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 68.Fu J, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukase S, et al. Pollen shells and soluble factors play non-redundant roles in the development of allergic conjunctivitis in mice. Ocul. Surf. 2021;22:152–162. doi: 10.1016/j.jtos.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 72.Whitten WK. Nutrient requirements for the culture of preimplantation embryos in vitro. Adv. Biosci. 1971;6:129–141. [Google Scholar]
- 73.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided with this paper. The microarray data for mouse conjunctival gene expression profiles is deposited in Gene Expression Omnibus (GEO; accession no. GSE220182). The genomic DNA sequences for various mouse strains were retrieved through Ensembl database (https://www.ensembl.org). Publicly available dataset GSE155776 was retrieved from Gene Expression Omnibus. Source data are provided with this paper.