Abstract
Chronic kidney disease (CKD) is estimated to affect 10–14% of global population. Kidney fibrosis, characterized by excessive extracellular matrix deposition leading to scarring, is a hallmark manifestation in different progressive CKD; However, at present no antifibrotic therapies against CKD exist. Kidney fibrosis is identified by tubule atrophy, interstitial chronic inflammation and fibrogenesis, glomerulosclerosis, and vascular rarefaction. Fibrotic niche, where organ fibrosis initiates, is a complex interplay between injured parenchyma (like tubular cells) and multiple non-parenchymal cell lineages (immune and mesenchymal cells) located spatially within scarring areas. Although the mechanisms of kidney fibrosis are complicated due to the kinds of cells involved, with the help of single-cell technology, many key questions have been explored, such as what kind of renal tubules are profibrotic, where myofibroblasts originate, which immune cells are involved, and how cells communicate with each other. In addition, genetics and epigenetics are deeper mechanisms that regulate kidney fibrosis. And the reversible nature of epigenetic changes including DNA methylation, RNA interference, and chromatin remodeling, gives an opportunity to stop or reverse kidney fibrosis by therapeutic strategies. More marketed (e.g., RAS blockage, SGLT2 inhibitors) have been developed to delay CKD progression in recent years. Furthermore, a better understanding of renal fibrosis is also favored to discover biomarkers of fibrotic injury. In the review, we update recent advances in the mechanism of renal fibrosis and summarize novel biomarkers and antifibrotic treatment for CKD.
Subject terms: Kidney diseases, Kidney diseases
Introduction
Chronic kidney disease (CKD) is defined as abnormalities of renal structure or function, present for >3 months, with implications for health.1 The most commonly used diagnostic criteria in clinics are estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or urinary albumin-to-creatinine ratio (ACR) ≥ 30 mg/g. Ascertaining the actual prevalence of CKD is a difficult task owing to its early-stage asymptomatic nature, but the disease is predicted to affect 10–14% of global population.2–4 Regardless of diverse causes, CKD is featured by progressive and irreversible nephron loss, microvascular damage, decreased regenerative capacity, inflammation, oxidative stress, and metabolic changes, which ultimately led to renal failure and end-stage kidney disease.5 The impact of CKD on worldwide morbidity and mortality is rapidly increasing,6,7 indicating the shortcoming of therapeutic drugs at present.
Kidney fibrosis is the common pathological feature and final manifestation of CKD, whose morphological characteristics include glomerulosclerosis, tubule atrophy, interstitial chronic inflammation, and fibrogenesis, as well as vascular rarefaction.8 Fibrosis occurs when wound healing is deregulated, leading to excessive extracellular matrix (ECM) protein accumulation such as fibronectin and collagens.9 When kidneys are injured, local fibroblasts and pericytes are activated, increasing their contractility, secreting inflammatory mediators, and synthesizing ECM components, which trigger wound healing. However, if the damage is repetitious or severe, the ECM proteins persistently accumulate in the kidneys, resulting in tissue disruption, renal dysfunction, and ultimately organ failure.10 Despite substantial progress in understanding kidney fibrotic mechanism, there remains a translational barrier from the identification of the promising antifibrotic therapeutic drug target to the transformation of this knowledge into clinical application for human health.
The kidney organ consists of some anatomically and functionally discrete segments, where diverse cell types with sophisticated mechanisms participate in the occurrence and progression of renal fibrosis.11 In particular, the rapidly developing technologies of spatial and single-cell transcriptomics could facilitate dissecting genetic programming, signal pathway, and the mechanism of cell crosstalk that underlie kidney function in physiological conditions, as well as the dysfunctions in fibrotic condition.12
Epigenetics controls gene expression without altering DNA sequence and affect the gene and environment crosstalk.13 Epigenetic regulation exerted fundamental and crucial functions in cell biology of kidneys through the action of DNA methylation, chromatin modifications via epigenetic factors and interaction with transcription factors, and non-coding RNAs.14 New findings in epigenetics can drive the development of not only the mechanism of kidney fibrosis, but also biomarkers and targeted drugs for the CKD’s diagnosis, prognosis, and therapy.
In the review, we updated recent advances in the mechanism of kidney fibrosis from new perspectives and summarize the new and promising biomarker and antifibrotic treatment for patients with fibrotic kidney diseases.
Fibrotic niche in kidney fibrosis
Increasing evidence has supported the idea that organ fibrosis starts from ‘fibrotic niche’—a complex interplay between the injured parenchyma and multiple non-parenchymal cell lineages spatially located within areas of scarring.15–17 Kidney spatial transcriptomic analysis demonstrated that mesenchymal cells, immune cells, and specific types of tubular epithelial cells were the cellular components of fibrotic niche within the human kidney.18 Although the main cell types of fibrotic niche have been identified, the functional heterogeneity and the interaction of cell lineages need further clarifications (Fig. 1).
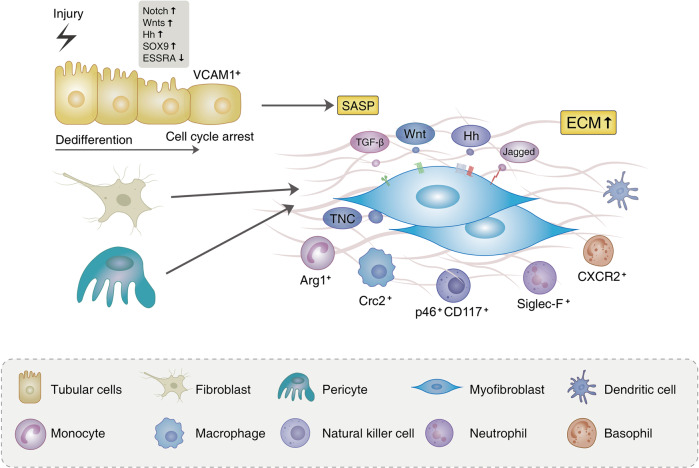
Fig. 1.
Origination and activation of myofibroblasts. In tubulointerstitium, injury results in epithelial dedifferentiation, which is characterized by the upregulation of Notch, Wnt, Hedgehog (Hh), and SOX9 pathways. Persistent damage leads to cycle arrest and senescence of tubular epithelial cells, accompanying the secretion of profibrotic factors and senescence-associated secretory phenotype (SASP). Injured VCAM-1+ tubules secrete paracrine mediators such as TGF-β, Hh, and Wnt ligands, which impact interstitial pericytes and fibroblasts to activate myofibroblast differentiation, proliferation, and ECM accumulation. Of note, the different population of immune cell including macrophage, lymphocyte, neutrophil, and basophil also have been found in the interstitial space. And these cells expressing specific markers play an important part in kidney fibrosis
Myofibroblast
Myofibroblasts activation and subsequent ECM accumulation are major events in kidney fibrosis. The activated myofibroblast works as the prominent contributor to renal fibrosis due to its ability to produce the most matrix.19 The alpha-smooth-muscle actin (α-SMA) is a specific marker that activates fibroblasts into myofibroblasts. Likewise, other interstitial cells such as pericyte and vascular smooth-muscle cells also express α-SMA. To distinguish myofibroblasts, vimentin, collagen-1α1 (Col1a1), CD73, platelet-derived growth factor receptor beta (PDGFRβ), and fibroblast-specific protein-1 (FSP1)/S100A4 could be used.10,12 The number of myofibroblast is rare in normal condition, but increases sharply in fibrotic kidneys. However, the origin of myofibroblast remains controversial. Resident fibroblasts,20,21 pericytes,21,22 mesenchymal stem cell (MSC)-like cells,23 epithelial cells,24,25 endothelium,26,27 and circulating bone marrow-derived cells are all candidates for possible precursors.28–30 Recently, a published single-cell atlas of CKD in human could go a long way toward answering this question. Christoph et al. totally profiled 87362 kidney cortex cells from 13 patients of CKD owing to hypertensive nephrosclerosis.18 After defining myofibroblasts that express a large proportion of ECM genes, they reported three main myofibroblast sources in kidneys: PDGFRα+PDGFRβ+MEG3+ fibroblast; PDGFRβ+COLEC11+CXCL12+ fibroblast; and PDGFRα–PDGFRβ+RGS5+NOTCH3+ pericytes. And the identified chemokine CXCL12-expressing cells are exactly similar to the Gli1+ MSC-like cells.23 Cessation of cell cycle characterized the pericyte- or fibroblast-to-myofibroblast differentiation. The early activating protein-1 (AP-1) signaling and late TGF-β signaling played regulatory roles in the differentiation of fibroblasts and pericytes into myofibroblasts. Another study that conducted a combination of ATAC-seq (a method detecting chromatin accessibility) with single-cell sequencing identified transcription factor Runx1 was a direct driver of human kidney fibroblasts transdifferentiating into myofibroblast, and abnormal Runx1 triggered the expression of several myofibroblast genes (Fn1, Col13A1, Tgfbr1, Twist2, and Postn).31 In addition, the increase of ECM genes was inapparent in tubular epithelial cells and suggested that the long-debated epithelial-to-mesenchymal transition (EMT) exerted a minor contribution to kidney fibrosis.18
The mechanism of myofibroblast activation is the central issue in kidney fibrosis. As above-mentioned, a fibrotic niche forms after injury, where the injured tubular cells and the infiltrated immune inflammatory cells could secrete various profibrotic mediators, which target myofibroblast precursors via autocrine or paracrine pathway, leading to the activation of myofibroblast.16 The sources of profibrotic factors (Table 1) and key signals that mediate myofibroblast activation are introduced in the subsequent part.
Table 1.
Tubule-derived factors regulating myofibroblast activation
| Profibrotic factor | Main effects | Ref. |
|---|---|---|
| TGF-β | TGF-β (1) directly triggers ECM synthesis via Smad3-dependent or independent manners, (2) suppresses the ECM degradation by inhibiting MMPs and inducing natural inhibitors of MMPs (like TIMPs), (3) induces trans-differentiation of myofibroblasts, and (4) mesangial cells proliferation and elimination of TECs, podocytes, and endothelial cells | 22–31,273–276 |
| HER2 | Tubule-derived HER2 induces the profibrotic CTGF expression via activating STAT3 | 277 |
| FGF2 | Autophagy-mediated expression and secretion of FGF2 activates fibroblasts | 278,279 |
| Wnt | Epithelial-derived Wnt ligands induce fibroblasts proliferation and differentiation into myofibroblasts | 68,69 |
| Hh | Hh promotes the transcription factor Glis nuclear translocation, and Shh upregulates the expression of Snail, α-SMA, fibronectin, and collagen I in fibroblasts | 75,280 |
| PDGFs | PDGFs mediate the proliferation, migration, and activation of mesangial cells, fibroblasts, and vascular smooth-muscle cells | 22,281 |
| CTGF | CTGF (1) is a co-factor for TGF-β, (2) activates the Wnt signaling pathway via interacting with LRP6, and (3) induces the trans-differentiation of pericytes and fibroblasts into myofibroblasts | 282–284 |
| TIMP | Timp1 promotes fibroblast proliferation | 285 |
| Lcn2 | Lcn2 increases collagen production in tubular cells | 286 |
| INHBB | INHBB promotes interstitial fibroblast activation through the release of profibrotic activin B from the injured TECs | 83 |
| Connexin 43 | Cx43 mediates the ATP release from TECs, inducing the release of CXCL10 from peritubular macrophage and activation of intrarenal fibroblasts | 287 |
| DACH1 | DACH1 controls the expression of cell cycle and myeloid chemotactic factors (e.g., CSF1, CCL2), contributing to macrophage infiltration and fibrosis development. | 288 |
α-SMA alpha-smooth-muscle actin, ATP adenosine triphosphate, CCL2 chemokine (C-C motif) ligand 2, CSF1 colony-stimulating factor 1, CTGF connective tissue growth factor, CXCL10 C-X-C motif chemokine ligand 10, Cx43 Connexin 43, DACH1 Dachshund homolog 1, ECM extracellular matrix, FGF2 fibroblast growth factor 2, HER2 human epidermal growth factor receptor 2, Hh hedgehog, INHBB inhibin subunit beta B, Lcn2 lipocalin, LRP6 low-density lipoprotein receptor-related protein 6, MMP matrix metallopeptidase, PDGF platelet-derived growth factor, Shh Sonic hedgehog, STAT3 signal transducer and activator of transcription 3, TEC tubular epithelial cell, TGF-β transforming growth factor-b, TIMP tissue inhibitor of matrix metalloproteinase 1
There is a growing recognition that certain markers of myofibroblasts could be employed as therapeutic targets. Christoph et al. found that naked cuticle homolog 2 (NKD2) was especially expressed in terminally differentiated human and mouse myofibroblasts.18 Activity of TGF-β, Wnt, and tumor necrosis factor (TNF) signal pathway increased in NKD2+ myofibroblasts rather than NKD2− cells. And NKD2 gene knockout cells showed the loss of ECM modulators, collagens, and glycoproteins. Therefore, the NKD2-marked myofibroblasts in fibrotic kidneys are essential for the expression of collagen and represent a promising drug target. Although Gli1+ MSC-like cells is the main source of myofibroblast and could be therapeutically targeted, the absence of Gli1+ MSCs alone is also able to trigger capillary rarefaction,32 which means the direct inhibition of Gli1+ cells may cause hypoxia-induced kidney fibrosis.33 Liang et al. have shown that conditional knockout of Yap/Taz in Gli1+ cells of mice retarded unilateral ureteral occlusion (UUO)-induced myofibroblast accumulation, ECM deposition, and tubulointerstitial fibrosis.34 Thus, the precise regulation of Gli1+ cells is required to treat or delay fibrosis. Myofibroblasts have other specific markers, such as integrin αvβ3,35 fibronectin,36 and CD248,37 which have been used to design targeted drug delivery systems to treat kidney fibrosis. However, the development of effective antifibrotic drugs remains challenging.
Tubules and tubulointerstitial crosstalk
The tubules make up the majority of kidney organ and are sensitive to various injuries such as ischemia, hypoxia, toxins, proteinuria, and metabolic disturbance. In CKD, the injured epithelium is now recognized as both victims and contributors to the progression of fibrotic kidney diseases.38 Although tubular epithelial-to-mesenchymal transition provided a minor contribution to the myofibroblast pool, tubulointerstitial crosstalk initiated by injured tubules is a core driver of CKD progression. As acknowledged, tubular epitheliums possess the formidable capability of self-repair.39 However, when the insult is repetitive and ongoing, injured tubules fail to re-differentiate after dedifferentiation,40 which produce and release bioactive molecules to recruit inflammatory cells and activate myofibroblast differentiation, proliferation, and matrix secretion.41 The cytokines, growth factors, and key mediators in kidneys produced by epitheliums are listed in Table 1.
Profibrotic features of injured tubules
In addition to the above-mentioned characteristics, single-cell RNA transcriptomics (scRNA-seq) is beneficial to the precise identification of tubular cells with profibrotic characteristics. A population of dedifferentiated VCAM-1+ proximal tubule cells has been shown to exert broad relevance in fibrotic kidneys.42,43 A recent human kidney cell atlas revealed that adaptive and/or maladaptive tubule epithelial cells in proximal tubule (PT) and thick ascending limb (TAL) shared the common expression of prominin 1 (PROM1), hepatitis A virus cellular receptor 1 (HAVCR1), doublecortin domain containing protein-2 (DCDC2), and occupied a core location in fibrotic niche via multiple cell-cell interactions, thus inducing ECM deposition, myofibroblast differentiation, and inflammation.44 Another mouse single-cell transcriptomic analysis found that transient inflammatory proximal tubule cell state appeared after slight kidney damage, with the significant downregulation of glutathione metabolism-related genes which triggering these cells sensitive to ferroptotic stress.45 This study indicates that ferroptotic stress recruits proinflammatory cells as key participant in maladaptive repair.
Signal pathways in tubular differentiation
Notch, Wnt, and Hedgehog have been recognized as key developmental signaling pathways closely related to cell differentiation. The transient activation of the three signal pathways is required for injured tissue/organ repair, but sustained activation is believed to aggravate fibrotic progression. Evidence from single-cell transcriptomic data verify their functions and roles in renal fibrosis.
Notch
Activation of Notch signaling scarcely appears in adult kidney, and its re-expression in injured and fibrotic kidney is related to regeneration and repair.46 Although the proliferation of undifferentiated cells seems to be crucial for the replacement of lost tubular cells, on the other hand, the impeding effect of Notch signaling on differentiation impairs kidney function with a high probability.47 In the kidneys of CKD patients and mice, the Notch signaling is reinduced during fibrosis. In mouse experimental models, inducing the cleaved Notch1 expression caused tubular dilatation and atrophy, accompanied by matrix accumulation, myofibroblast activation, and immune cell infiltration in interstitium.46 In contrast, genetic or pharmacological inhibition of Notch signaling significantly reduced renal tubulointerstitial fibrosis.25,48,49 Moreover, as a key crosstalk signal pathway, Notch signaling is a major component of tubule-interstitial communication by paracrine effects. Increased tubular epithelial Notch expression was related with an elevated TGF-β level, which directly activates myofibroblasts.50,51 Snai1, one of the downstream genes of Notch, was once regarded as a main regulator of the EMT program.52 However, the linage-tracing study did not advocate the effects of epithelial-to-mesenchymal transition in renal fibrosis.18,53 Later, partial EMT has been proposed, which indicated that dedifferentiated tubules do not fully translate into myofibroblasts, but exacerbated kidney fibrosis via the secretion of mediators to trigger the differentiation of myofibroblast.52,54
Wnt
Wnt is comparatively invisible in normal condition of adult kidneys, but is initiated in human kidney diseases.55 Activation of Wnt after acute kidney injury (AKI) facilitates repair and regeneration of damaged tubules.56 However, the prolonged activation of Wnt exacerbates fibrotic kidney diseases.57 Tubular expression of most of nineteen Wnts and ten frizzled receptors (Fzd) was elevated in fibrotic kidneys of UUO experimental model, except for Wnt5b, Wnt8b, Wnt9b, Fzd4 and Fzd5.58 Although conditional deletion of tubular β-catenin exerted no protective effects against tubulointerstitial fibrosis of UUO model,59 several antifibrotic treatments interfering with Wnt signaling by diverse strategies were promising. These acknowledged Wnt inhibitors included kallistatin,60 dapper3,61 dickkopf1 (Dkk1),58,62 Dkk2,63 ICG-001,64 paricalcitol,65 secreted frizzled-related protein (Sfrp) 166 and Sfrp4.67 As key targets of Wnt ligands, the interstitial fibroblast and pericyte are also recognized in abundant studies. By paracrine pathway, tubule-derived Wnts contribute to the activation of myofibroblasts.68–70 What’s more, fibroblast and pericyte canonical Wnt4/β-catenin constitutive activation displayed spontaneous interstitial myofibroblast differentiation even without stimulant or injury.71
Hedgehog
Hedgehog (Hh) is also a vital mammalian developmental signal pathway that controls tissue/organ patterning, cell growth, and differentiation.72 Desert hedgehog (Dhh), Indian hedgehog (Ihh), and Sonic hedgehog (Shh) are three well-known Hh ligands. In human kidney tissues, the ligands of Hh are expressed and secreted in tubule cells, and these interstitial cells with respondency to Hh ligands.73,74 And Gli1 is one of Hh target genes.75 The lineage-tracing investigation indicated that the expression of Ihh and Shh were increased in UUO surgery-injured tubules.73,75,76 As mentioned before, Gli1+ MSC-like cells play a key contribution to the myofibroblast pool.23 Therefore, Hh signaling participates in the occurrence and progression of kidney fibrosis, mainly via the secretion/release of these ligands from tubular cells and the increase of Gli1 in myofibroblast.
SRY-related high-mobility-group box 9 (SOX9)
SOX9 as a transcription factor is responsible for cell growth and differentiation in multiple organs including kidneys.77 Kang et al. have found that SOX9-positive cells of kidneys showed progenitor-like functionalities, and contributed to the regeneration of epitheliums after injury.78 Some studies have consistently explored that the loss of SOX9 function underlined a failure of survived PT cells to repair after AKI.79–81 However, SOX9 is also crucial for kidney fibrosis. Neuron navigator 3 (NAV3)82 and homodimer of inhibin subunit beta B (INHBB),83 the acting downstream of SOX9, were thought to be potential antifibrotic targets for pharmacological intervention.
Estrogen-related receptor alpha (ERRα)
In addition to classical pathways that influence cell differentiation, nuclear receptors such as ERRα were also investigated to maintain the metabolism and differentiation of PT cells via directly control of these cell-specific genes.84 Dhillon et al. proved that fatty acid oxidation (FAO) and oxidative phosphorylation in proximal tubules exhibited a positive and reproducible relationship with cell differentiation and disease progression. Furthermore, it was ERRα and peroxisomal proliferation-activated receptor (PPAR) alpha that coupled with metabolism and proximal tubule cell-specific gene expression in mouse and human specimens, while defending against kidney disease of an experimental mouse model.84
Tubular cell cycle arrest and senescence
Cell cycle arrest is usually beneficial to DNA repair before initiating their proliferation. Both rapid progression through cell cycle phases with incorrect checkpoints, and the prolonged cell cycle arrest, are detrimental for tubules.85 Cellular senescence is a fate where cell cycle arrest is permanent and irreversible.86 The p16Ink4a and p53-p21Cip1/Waf1 are both critical cyclin-dependent kinase (CDK) inhibitors, leading to cellular senescence.87,88 Senescent cell is resistant to apoptosis and continually produces a complex secretome as senescence-associated secretory phenotype (SASP) such as proinflammatory and profibrotic mediators, etc.86 Numerous studies have cleared that the G1/S and G2/M arrest of tubular cells is crucial drivers in maladaptive repair and kidney fibrosis.89–91 Importantly, these senescent cells could be pharmacologically targeted to reduce fibrosis. Recently, several creative studies validated the efficacy and safety of senescent cell clearances, applying transgenic mice with selectively sensitive pharmacological agents,92,93 or the mice access to antiapoptotic drugs.92,94,95 Moreover, various strategies are being tested to prevent the formation of senescent cells (e.g., exercise, weight loss),96–98 trigger the apoptosis of senescent cell (e.g., ABT-263, FOXO4-DRI),92,99 reduce the secretion of SASP (e.g., metformin, sirolimus),100,101 and take advantage of senescent cell metabolic activity to activate compounds (e.g., galactooligosaccharide conjugated drugs).102 In addition, the treatment of CDK4/6 inhibitor PD-0332991, could result in a transient cell cycle arrest after injury, suppressed DNA damage, tubular epithelial cell apoptosis, interstitial inflammatory response, and restored kidney function.103,104 The induction of transient cycle arrest or pharmacological quiescence by CDK4/6 inhibition in proximal tubule cells could allow correcting DNA damage with decreasing early apoptosis, senescence as well as AKI-to-CKD transition.
Immune cells
Immune cells also are the core participants in the fibrotic niche. In tissue specimens from CKD patients, fibrotic niches were associated with infiltrating CD68+, myeloperoxidase+ (MPO+), and CD3+ cells. And predominantly CD3+ immune cell activity was observed in neighborhoods of adaptive/maladaptive PT and TAL. The positive relationship between specific renal structures and leukocytes was also identified, including MPO+ cells with glomeruli and CD68+ cells with proximal tubule epithelium.44 In mice, normal kidney was governed by tissue-resident macrophages, followed by T and B cells, as well as a relatively small percentage of monocytes, neutrophils, natural killer (NK) cells and dendritic cells (DCs). The myeloid compartment consistently predominated after injury. Whereas, early after ischemia reperfusion injury (IRI) (day 7), the complete knockout of B cells and partial knockout of T cells were observed. If fibrosis occurs after injury (day 28), T cells re-expand.105
Macrophages
Similar to the tubular epithelium, although monocyte exerts a limited contribution to myofibroblasts,105 monocytes/macrophages play critical roles in kidney fibrosis. As acknowledged, the proinflammatory M1 and antiinflammatory M2 macrophage are closely related to organ/tissue injury, repair and fibrosis. Recently, relevant knowledge have been summarized and is not covered in this review.106 A new atlas of single-cell RNA transcriptomics has reported that myeloid heterogeneity was involved in the progression and regression of kidney disease. Monocytes recruited to the injured kidney in early stage and adopt rapidly a proinflammatory and profibrotic phenotype that expressed Arginase 1, before transforming to C-C chemokine receptor 21+ (Ccr21+) macrophages which accumulated in the late stage of kidney damage. However, mannose receptor 1+ (Mrc1+) and matrix metallopeptidase 12+ (Mmp12+) macrophages could be characterized by scavenger receptor expression and might contribute to the degradation of matrix.107
Lymphocytes
A single-cell RNA sequencing of CD4+ T cells in fibrotic kidneys uncovered the expansion of T helper 17 (Th17) cells and regulatory T cells. However, the expansion of tissue-resident IL-33R and IL-2Rα positive Tregs before injury could protect kidney damage and scaring.108 Tertiary lymphoid tissue (TLT) is inducible ectopic lymphoid tissue in the process of chronic inflammation, which functioned as the priming site of local immune responses.109 In the aging kidney, T and B cells, together with resident fibroblasts, form TLTs that cause uncontrollable inflammation and delay tissue repair.110 Several studies have documented that TLT is related with the progression of kidney damage.110–112 Targeting IL-17A113 or CD153/CD30114 might slow the progression of kidney diseases by attenuating TLT formation. The natural killer cell is a population of lymphoid cells that exerts crucial functions in innate immunity. Law et al. found an increased number of interstitial CD56bright NK cells (NKp46+CD117+) in fibrotic kidneys, contributing to tissue scaring by producing interferon (IFN)-γ.115
Neutrophils
Neutrophils are an important component of innate immune cells. Recently, the most prevalent population of immune cells in the advanced fibrotic kidney is neutrophils by flow cytometric analysis of UUO mice.116 TGF-β1 and GM-CSF could trigger neutrophils converting into Siglec-F+ (usually seen as an eosinophil marker) neutrophils, which produced profibrotic mediators and secreted collagen-1. Depletion of Siglec-F+ neutrophils reduced collagen deposition and disease progression, indicating its promising therapeutic strategy for fibrotic kidney disease.116
Other immune cells
Dendritic cells and mast cells also participated in kidney fibrosis, and the clearance of DCs or mast cells could alleviate fibrosis.117,118 The latest study identified that CXCR2+ basophils were recruited by CXCL1 (secreted from profibrotic tubules) as key contributors to kidney fibrosis and suggested that targeting these cells may be a promising guideline for the treatment of CKD.119
ECM network
The ECM network, also termed the decellularized kidney tissue scaffolds, plays an indispensable role in fibrotic niche formation. Using proteomics by unbiased mass spectrometry, researchers decoded almost one thousand ECM proteins, which were divided into four main categories: structural ECM protein (collagens, fibronectin, and elastin), matricellular protein (fibrillin 1 (FBN1), tenascin-C (TNC), connective tissue growth factor (CTGF), and periostin (POSTN)), matrix-modifying proteins and proteoglycans.16 Among them, the most increased proteins in fibrotic kidney tissue scaffolds were matricellular proteins, highlighting that they are pivotal contributors in fibrotic niche. TNC, an extracellular matrix glycoprotein, has been confirmed to be a major component of fibrotic niche in renal fibrosis.120,121 In adult kidney and other organs, no or little TNC is detected, while the de novo expression of TNC is prominent in fibrotic kidneys. TNC, produced primarily by fibroblasts, promotes the activation and proliferation of fibroblasts, as well as ECM production and deposition via activating integrin/adhesive plaque kinase (FAK)/mitogen-activated protein kinase (MAPK) signaling. The knockdown of TNC in vivo inhibited fibroblast activation and proliferation, which in turn reduced renal fibrosis. Further studies revealed that TNC not only activated signaling pathways in fibroblasts, but its unique hexametric structure also acted as a sponge to trap and concentrated other profibrotic factors such as Wnts, thus forming a local microenvironment enriched with the high concentrations of profibrotic mediators, and initiating and accelerating the progression of fibrosis.120
Mechanisms of glomerulosclerosis
Both progressive primary glomerular diseases and secondary glomerular diseases culminate in glomerulosclerosis. In a single-cell transcriptome atlas of glomerulus in mice, five known cell types including podocytes, endothelium, tubules, mesangium, and immune cells are present in glomeruli.122 The atlas of human kidneys identifies glomerular endothelial cells, podocytes, mesangial cells, and parietal epithelial cells in glomerulus.11 However, some methodological challenges of single-cell technology still exist in glomerular diseases, especially in humans.123 For example, the biopsy by core needle provided a small-sized sample, and some specimens are defective to identify glomerular cells.123 Moreover, there is a lack of animal experimental models that could well simulate glomerular diseases. Thus, our understanding of the trajectory of glomerulosclerosis is not as clear as that of tubulointerstitial fibrosis.
Diabetic kidney disease
Chung et al. compared glomerular cells from the leptin-deficient ob/ob obese and lean mice by principal component analysis. The alterations of gene expression were found in mesangial cells and podocytes, but not endothelial cells. The pathway enrichment analysis exhibited the pathway of glucose and lipid metabolism-related gene alterations in both mesangial cells and podocytes. The pathway of apoptotic genes was enriched in podocytes, and the pathway of cell proliferation were triggered in mesangial cells. Furthermore, the number of podocytes decreased whereas the number of mesangial cells increased at 21th week. And it was not surprising to find that the expression of matrix/matrix-modifying proteins was increased in both mesangial cells and podocytes.124 However, in another single-cell transcriptomics profiling of DKD experimental model, fewer mesangial cells and podocytes were found in the kidneys of diabetic mice compared to those of control mice, with a greater number of immune cells and glomerular endothelial cells in kidneys of diabetic mice. The data of pathway enrichment analysis from diabetic kidney tissues indicated that the regulation of migration and angiogenesis pathway-related genes was changed in endothelial cells, and the genes involved in the pathway of translation, gene expression, and protein stabilization were highly enriched in mesangial cells. However, a small number of podocytes was captured in the kidneys of diabetic mice. Ctgf, Vegf, Tnfa, and leucine-rich α-2-glycoprotein 1 (LRG1) in diabetic kidneys were highly expressed in glomerular mesangial cells, podocytes, endothelial cells, and immune cells, respectively. The study also found that M1 phenotype macrophages were predominant in glomerular immune cells. Some well-established pairs of ligand-receptor in the crosstalk of glomerular cells were identified such as endothelial Flt1-podocyte Vegfa.122 The recent study of early-stage diabetic nephropathy patients by single-nucleus RNA transcriptomics showed no significant difference (P = 0.66) in the number of podocytes because of the limited sample number.125 The expression podocyte PLA2R1 and THSD7A in early-stage diabetic patients were increased in comparison to a previous study of later-stage diabetes whose PLA2R1 was greatly reduced. For mesangial cells, the angiogenesis-related genes by gene ontology (GO) analysis were enriched which were driven by the increased expression of regulatory genes (MYH9, NR4A1, SLIT3, ADAMTS12) and ECM components (COL4A1, COL4A2). The cluster of cells characterized by VCAM-1, CFH, AKAP12, CLDN1 gene are most likely parietal epithelial cells, whose CFH gene expression being decreased. In endothelial cells, the related genes of ECM components (COL4A1), angiogenesis regulators (VCAM, VEGFC, ITGB1, HDAC9, MYH9, TMEM204, PRCP, MEF2C, NR4A1), and glucose transporters (SLC2A14, SLC2A3) were differentially expressed.125 The pairings of ligand-receptor were also investigated among various cell types in glomeruli. In diabetic mesangial cells, CCN1 and SLIT3 expression was elevated. The growth factor-inducible CCN1 controls tissue and organ repair through the interactions with endothelial ITGB3 and podocyte ITGAV, ITGB3, ITGB5.126 SLIT3 interacting with podocyte/endothelial cell-expressed ROBO2 regulates cellular migration.127 Diabetic podocytes displayed decreased INSR expression, and the NAMPT expression from mesangial cells regulated pancreatic-β cell insulin secretion.128 The increased LTBP1 level of diabetic endothelial cells could control the targeting of latent complexes of TGF-β.129
Lupus nephritis
As a secondary glomerular disease, lupus nephritis is a severe manifestation of systemic lupus erythematosus (SLE) associated with a complicated immune mechanism. Single-cell techniques offer new perspectives for understanding this disease. For example, the results from scRNA transcriptomics of human skin and renal biopsies indicated that the analysis of skin as biomarkers could predict kidney disease, because IFN-inducible genes, correlated to chronicity index, immunoglobulin (Ig) G deposition, quantity of proteinuria, and therapeutic response upregulated parallelly in keratinocytes and tubular cells in lupus nephritis.130 Furthermore, the high expression of type I interferon-induced genes of tubules could separate proliferative lupus nephritis from membranous symptom.131 Another study focused on immune cells revealed that various leukocytes were activated, and explicit interferon responses were detected in most of the cells broadly expressing CX3CR1 and CXCR4. The study also indicated that urine specimens may be a replacement for renal biopsy, in which immune cell gene expression was highly correlated between kidney and urine. Among immune cells, an intrarenal subset of “M2-like” CD16+ macrophages with their high CD163 and CXCL12 level was speculated to decoy other immune cells.132 Importantly, soluble urine CD163 could be used to distinguish patients with proliferative or membranous lupus nephritis, and also discriminate active lupus nephritis from other forms of SLE patients.133 In a model of TLR7-induced mouse lupus, IFN-λ was found to promote systemic immune dysregulation via local consequences in kidney and skin.134 In the meanwhile, IFN-λ aggravated lupus-related renal pathological damage and also activated mouse mesangial cells to highly express IFN-stimulated and chemokine genes.134
IgA nephropathy
Multiple ligand-receptor connections were also detected in glomerular cells of IgA nephropathy (IgAN) mice. Glomerular endothelial cells occupied the dominant position by activating and recruiting leukocytes at the initial phase of IgAN.135 Among primary glomerular cell types, mesangial cell-expressed Slit3 could activate Robo2 and 4 in podocytes and endothelial cells, respectively. Mesangial cell-expressed secreted phosphoprotein 1 (Spp1) could bind to endothelial sphingosine-1-phosphate receptor 1 (S1pr1). Podocyte-expressed Col1a1 could be binding to endothelial and mesangial cell CD36. At the early stage of IgAN, proximal tubules were surprisingly influenced by the identification of glomerulo-tubular crosstalk.135 However, another human study of IgAN emphasized a central role for mesangial cells, which may recognize and transport IgA via the upregulation of joining chain of multimeric IgM and IgA expression.136 In addition, mesangial-tubule and mesangial-immune crosstalk also triggered tubulointerstitial inflammation and fibrosis.
Podocytopathy
Podocyte injury is responsible for proteinuria. Chung et al. identified and validated that Hippo pathway was indispensable to repair podocyte injury. Knockout of the Hippo downstream effector YAP or TAZ led to more prolonged and severe proteinuria and worse glomerulosclerosis.124 Focal segmental glomerulosclerosis (FSGS) is initiated from primary, secondary, or genetic podocyte injury. Besides podocytes, parietal epithelial cells were paid more attention in the progression of FSGS. A recent study also found that the transcriptional level of glomerular alpha-2-macroglobulin (A2M) was related to lower proteinuria remission rates, connecting long-term outcomes and endothelial function in FSGS.137 Single-cell RNA transcriptomics from urine samples of FSGS patients was also used to investigate disease-related molecular signatures. Renal epithelial and immune cells (predominantly monocytes) were identified in the urine. And shedding of podocytes showed a high level of EMT genes in the urine. Consistent with these results, the genes related to immune signature and EMT signature in urine cells were more highly expressed in kidney biopsies of FSGS patients in comparison to those of minimal change nephropathy (MCD).138
Pathogenesis of vascular rarefaction
Vascular rarefaction, which refers to a decrease in capillary density leading to ischemic and hypoxic conditions, is a pathological feature, a progressive factor, and a consequence of kidney fibrosis. Vascular rarefaction is related to the apoptosis, detachment, and dysfunction of endothelial cells.139 Previous studies mainly concentrated on the dysfunction of angiogenic mediators such as VEGF, which is an important factor of vascular repair and angiogenesis. The level of VEGF was decreased in chronic ischemic kidney disease, resulting in kidney function deterioration and microvascular rarefaction.140 However, VEGF is also a key anti-tumor drug target and the restoration of VEGF might pose a risk.141 Therefore, other mechanisms regulating vascular rarefaction need to be discovered.
As the other family member of angiogenic growth factor, angiopoietins (Angpt) control vascular stability and maturation.142 Inflammation and hypoxia triggered endothelial cell angpt2 expression,143 which could destabilized the integrity of endothelium.144 A latest published study has proven that angpt2 inhibition by angpt1 or a Fc-fusion peptide L1-10 could attenuate endothelial inflammation, apoptosis, and renal fibrosis in progressive kidney diseases.145 The LRG1 as an angiogenic factor could promotes angiogenesis of myocardial infarction and ocular disease mouse model.146,147 Liu et al. reported that plasma and kidney LRG1 expression was increased in CKD patients and proved that LRG1 regulated capillary-like formation. Gene knockout of LRG1 exacerbated capillary rarefaction, inflammatory cells infiltration, and kidney fibrosis following UUO surgery.148
Caspase-3 is a well-known effector of programmed cell death or cell apoptosis.149 Active caspase-3 has capacity to regulate apoptosis during kidney injury.150 It is interesting to find that, in caspase-3 knockout mice, tubular damage and kidney dysfunction are worsening in early-stage IRI, while microvascular integrity is alleviated during overall process of disease. The caspase-3 gene knockout mice exhibit the remission of tubular damage, microvascular dropout, and tubulointerstitial fibrosis in the long term. These findings confirm the significance of caspase-3 in controlling IRI-induced kidney fibrosis and microvascular endothelial cell apoptosis.151 Activin A receptor like type 1 (ALK1) as a type I receptor for TGF-β family proteins regulated the ECM accumulation and organ fibrosis in the liver, heart, skin, and kidneys.152,153 In a recent study of ALK1 heterozygous mice, the recovery of fibrotic kidney benefited by myofibroblast clearance and low degree of vascular rarefaction, indicating that ALK1 deficiency ameliorated UUO-injured peritubular microvasculature.152
Klotho is a well-known renoprotective gene/protein. Recently, a study reveals a promising mechanism by which klotho improved peritubular capillary rarefaction and delayed renal fibrosis. The study concluded that VEGFR2 was indispensable for vascular integrity, where Klotho exerted antifibrotic and vascular protective effects partially through the modulation of VEGFR2 function in kidneys.154
As above-mentioned, FBN1 as a component of ECM network, is increased in the kidneys of CKD animals and patients. Li et al. recently found that FBN1 inhibited endothelial cell proliferation and induced their apoptosis in vitro. Furthermore, FBN1-triggered endothelial injury could be abolished by the suppression of integrin αvβ6/TGF-β pathway and FBN1 blockage mitigated vascular rarefaction and ameliorated fibrotic scaring in kidneys.155 The results identify FBN1 as a mediator to orchestrate a detrimental microenvironment for endothelial cells in vascular rarefaction. Lysyl oxidase (LOX) catalyzes the crosslinking of ECM proteins and plays a key function in stabilizing resistant matrix degradation.156 Not unexpectedly, LOX takes part in the process of renal fibrosis. Recently, a study observed that LOX inhibition could partially restore microvascular rarefaction, inhibit the loss of pericyte and maintain endothelial cell-pericyte interaction in UUO-induced fibrotic kidneys.157
Pericytes embedded in capillary basement membrane could directly communicate with endothelial cells. Pericytes contribute to microvascular stability and modulate cortical/medullary flow by dilating/contracting in response to stimuli, which were produced by neighboring tubular and endothelial cells.158 A study showed that complement activation in IRI caused the occurrence of pericyte-to-myofibroblast and the decreased area of peritubular capillaries lumen. Of note, complement 5a exerted profibrotic capacity to drive pericytes toward maladaptive phenotype via the activation of ERK.159 Another study explored that bone marrow-derived mesenchymal stem cells (BMSCs) transplantation could improve pericyte vitality and suppress pericyte detachment and trans-differentiation. The direct BMSCs differentiation into pericytes by the transplantation of BMSCs also takes part in AKI-induced peritubular capillary repair, archiving the effect of preserving renal function and reducing kidney fibrosis.160
It is exciting to find that a sodium-glucose cotransporter 2 (SGLT2) inhibitor Luseogliflozin ameliorate renal interstitial fibrosis and peritubular capillary rarefaction in animal models.161 And a bioengineered fusion of elastin-like polypeptide with VEGF was also designed to restore kidney function of renovascular disease and increase renal microvascular density.162,163 However, there are data from single-cell technology about vascular rarefaction, so advances in technology will be a tremendous help in understanding the mechanisms that vascular rarefaction occurs.
Epigenetics in kidney fibrosis
Conrad Waddington for the first time reported ‘epigenetics’ to illuminate genes interacting with the environment. Epigenetics is increasingly explored as one of the major determinants in the kinds of miscellaneous diseases.13 Epigenetics is a heritable alteration in the expression pattern of genes, which is caused by a mechanism other than the changes in DNA sequence. The RNA interference, chromatin remodeling, and DNA methylation are all well-known epigenetic mechanisms.14 As for the patients of CKD, the alteration of many factors including oxidative stress, inflammation, uremic toxins, and metabolic state such as hyperglycemia, could control genome epigenetic reprogramming, further contributing to the progression of kidney disease.164
Metabolic and hypoxic memory
DKD is a typical CKD affected by environmental and genetic factors. Considerable studies indicate that the DKD-featured gene expressions are modulated by classical signaling, but also by epigenomics being responded to environmental changes. Notably, the epigenetic mechanism, which is termed as “metabolic memory”, could regulate perpetually a long-term statement of DKD-associated genes and phenotypes initiated priorly by hyperglycemia, regardless of consequent blood glucose control.164 Therefore, controlling the occurrence of epigenetic events in early-stage DKD might be worthy of early diagnosis and timely treatment, which is believed to delay or stop disease progression. With the technology of epigenome-wide association studies (EWASs), the epigenetic signatures of DKD patients could be valuable for personalized precision medicines. In addition to epigenetic mechanisms shared with tubulointerstitial fibrosis, unique epigenetics, and epigenomics also play important roles in DKD.165
Like metabolic memory, “hypoxic memory” has been proposed to understand the AKI-to-CKD transition.166 During and after AKI episodes, hypoxia sustainedly exists owing to capillary rarefaction. Hypoxia triggers sterile inflammatory response and fibrotic scaring, and in turn kidney fibrosis exacerbates the degree of tissue hypoxia. As known, renal fibrosis caused capillary loss and intensified the distance between tubular cells and capillaries, resulting in the decreased efficiency of oxygen diffusion.166–169 Moreover, hypoxia-induced epigenetic alterations including chromosome conformational change, histone modification (e.g., demethylation of H3K9me2 and methylation of H3K27me3), DNA methylation, and these changes of non-coding RNAs (ncRNAs).166 In kidneys, hypoxia is recorded as epigenetic alterations which are termed as “hypoxic memory”, and exerted long-term effects after the recovery from the initial AKI episode. Therefore, targeting hypoxic memory are a potential strategy to delay or stop the AKI-to-CKD transition.
DNA methylation
DNA methylation is a reaction by adding a methyl group to carbon 5 position of the cytosine residue (CpG site) which is catalyzed by DNA methyltransferases (DNMTs), usually contributing to the loss of gene expression.170 Hypomethylation or DNA methylation on specific CpG site exert key functions in kidney development, whereas abnormal methylation or hypermethylation also could happen in various kidney diseases. Recently, Susztak et al. reported the genotype and DNA methylation profiles of more than 500 kidney samples and identified approximately 140,000 CpG with methylation QTLs (meQTLs).171 In this study, methylation variation explained a larger percentage of heritability than gene expression. DNA methylation-mediated heritability was significantly tissue-specific and enriched in kidney-specific enhancer regions.171 A study of cytosine methylation in CKD patients discovered these differentially methylated loci in tubules and significant difference of methylation was found in 1061 genes, many of which are known in kidney fibrosis such as TGFβ receptor 3 (TGFBR3), SMAD3, and SMAD6.172 Some of the genes, like hepatocyte nuclear factor (HNF)173 and SIX2,174 are involved in renal transcription factors, and other genes, like collagens and laminins, are indicators of cell adhesion pathways. Moreover, 19 differential CpG sites of whole-blood DNA methylation in an EWAS of two population-based cohorts including 4,859 participants were significantly and reproducibly related to eGFR or CKD, as well as kidney fibrosis clinical endpoint.175 In the corresponding kidney biopsies, the gene DNA methylations of protein tyrosine phosphatase non-receptor type 6 (PTPN6)/prohibitin 2 (PHB2), ankyrin repeat domain containing 11 (ANKRD11), trinucleotide repeat containing 18 (TNRC18), solute carrier family 66 member 1 (SLC66A1), and pre-mRNA processing factor 8 (PRPF8) were significantly correlated with the degree of fibrosis.175 Another genome-wide study reported that DNA hypomethylation and hypermethylation were present at different loci in whole-blood of CKD patients.176 Previously, the differentially methylated genes containing engulfment and cell motility 1 (ELMO1), cut like homeobox 1 (CUX1), FKBP5, protein tyrosine phosphatase receptor type N2 (PTPRN2), and inhibin subunit beta A antisense RNA 1 (INHBA-AS1) have been concluded in transcription regulation, signaling, and apoptosis pathway.176 Notably, the consistent trend of the correlation between kidney function and DNA methylation was shown in both whole-blood samples and kidneys, suggesting that the profiles of whole-blood DNA methylation could reflect the DNA methylation in kidneys.
Additionally, the methylation of many specific genes also is closely related with the progression of CKD. At first, ECM-related genes could be modulated by their corresponding promoters’ methylation. For example, SMAD3 and SMAD6 methylated changes were correlated with their transcriptional levels in samples of CKD pateints.172 The RAS protein activator-like 1 (RASAL1) hypermethylation was related to kidney fibrosis with the perpetuation of myofibroblast activation, where TGF-β mediated the hypermethylation of RASAL1 with DNMT1 effect in fibrogenesis.177 In whole-kidney biopsy and fibroblasts, hypermethylation and transcriptional silencing of RASAL1 was also related to severe fibrosis. DNA methylation could be inherited by progeny, which could explain the perpetuation of myofibroblast activation.177 Moreover, hypermethylation of antifibrotic genes like secreted frizzled-related proteins 5 (sFRP5),178 KLOTHO,179,180 and Krüppel-like factor 4 (KLF4) also contribute to renal fibrosis.181 Collectively, targeting DNA methylation might be a therapeutic strategy, as RASAL1 hypermethylation could be revoked by bone morphogenic protein 7 (BMP-7), which is a well-known endogenous antagonist of TGF-β.182
Histone acetylation
The acetylation and methylation of lysine residues are the most important two kinds of histone modifications.164 In most cases, histone acetylation mediated by histone acetyltransferases (HATs) could promote transcriptional activation, while histone deacetylases (HDACs)-mediated histone deacetylation could repress gene expression.183 Histone methylation with the different modified lysine residues could function as gene transcription activation or repression, respectively.184 Beyond those, histone phosphorylation, ubiquitylation, and glycosylation are extensively investigated, and their modified location also spreads to serine, threonine, and arginine residues.185
Histone acetylation could facilitate chromatin opening, which DNA is more accessible to the transcription machinery and promote gene transcription.186 Histone acetylation could be reversibly modulated by HATs, HDACs, and bromodomain and extraterminal (BET) proteins (the “readers” of lysine acetylation),187 which participate in various pathophysiological events of kidney fibrosis such as partial EMT,188 myofibroblast activation,189,190 inflammation,191,192 and profibrotic factor secration.193,194 The HATs mainly included the P300/CREB binding protein (CBP) and P300/CBP-associated factor (PCAF). P300 inhibitors, L002,195 C646,196,197 and C66198 reduced renal fibrosis in the hypertensive and diabetic kidneys. Garcinol, a PACF inhibitor, alleviated kidney fibrosis of UUO model by inhibiting the activation of NF-κB and NRF2.193
Histone deacetylases
There are four categories of HDACs containing 18 members. The HDACs of class I includes four subtypes of 1, 2, 3, and 8; The class II HDACs contain two subclasses IIa (4, 5, 7, and 9) and class IIb (6, 10). Sirtuin (SIRT) 1–7 are the members of III HDACs, and HDAC11 is only one of IV HDAC.187 The pan HDAC I and II inhibitor Trichostatin A could delay fibrotic kidney via suppressing TGF-β1 and JNK/Notch2 pathways,190,199 as well as preserving E-cadherin expression.200 The selective HDAC I inhibitor MS-275 retarded fibrosis by suppressing Smad3 and STAT3 phosphorylation, and reduced TGF-β1 expression in UUO-injured kidneys.201 However, some natural and synthetic compounds activating SIRTs also present renoprotective effects, where resveratrol and honokiol activate SIRT1 and SIRT3, respectively. Activating SIRT1 by resveratrol reduces interstitial fibrosis via suppressing acetylated Smad3 and MMP-7 in UUO mice.202,203 Honokiol alleviated the acetylation level and kidney fibrosis, whose mechanism was related to the SIRT3-mediated metabolic reprogramming.204,205 In addition, honokiol attenuated angiotensin II (Ang II)-triggered kidney dysfunction and fibrotic scaring by inhibiting KLF15-mediated ECM protein expression.206 SRT1720, SRT2183, and SRT3025 are all synthetic SIRT1 activators, showing antifibrotic effects in CKD models.207–209 SIRT6 was also found to improve renal fibrosis by epigenetically suppressing β-catenin signaling.210 These results are consistent with those obtained in SIRT1, SIRT3, or SIRT6 genetically engineered mice.210–212 However, SIRT2 seems to play the other way around. He et al. reported that chemical inhibition and genetic knockdown of SIRT2 alleviated TGF-β1-triggered activation of fibroblast and tubulointerstitial fibrosis via suppressing murine double minute 2 (MDM2) expression.213
Up to now, several isoform-selective HDAC inhibitors have been developed, which are beneficial to individual HDAC member functional studies in fibrotic kidneys. RGFP966 as an HDAC3-selective inhibitor could improve kidney fibrotic damage in both aristolochic acid nephropathy and UUO mice.214 PCI34051, a highly selective inhibitor of HDAC8, decreased the number of tubular epithelial cell G2/M-phase arrest as well as suppressed UUO surgery-induced Smad3, STAT3, β-catenin phosphorylation and Snail expression.188,200 Furthermore, inhibition of HDAC8 restored the reduction of Klotho and BMP-7 in UUO experimental model. HDAC4 inhibition by MC1568 also decreased the level of profibrotic factors and preserved Klotho, BMP-7, and Smad7 expression.188 Tubastatin A and ACY-1215 as HDAC6 inhibitors presented antifibrotic effects in different CKD models.215,216 Quisinostat alleviated renal fibrosis in UUO and Ang II-induced hypertension by inhibiting HDAC11 and subsequently repressing KLF15.217
Bromodomain and extra terminal proteins
BET proteins as epigenetic readers contain Brd2-4, and Brdt.218 Increasing studies report that BET proteins could modulate kinds of cell functions such as pericyte/fibroblast activation, inflammation, cell growth, and differentiation.218,219 Using JQ1, I-BET151, and ZLD2218 to inhibit BET protein could prevent the inflammatory response, inhibit G2/M-phase cell arrest, and suppress profibrotic signaling activation, contributing to the delay of renal fibrosis.220–223
Acetylation of non-histone proteins
Unlike demethylated agents against progressive CKD, the HATs, HDACs, and BET inhibitors have all demonstrated therapeutic effects on renal fibrosis, which indicates other mechanisms beyond histone acetylation exist. For example, STAT3 and NF-κB could be acetylated in mammalian cells.224 Non-histone protein acetylation could mediate various functions including enzymatic activity, protein stability, and the interactions of protein-protein or protein-DNA.225
Histone methylation
As known, histone lysine (K) or arginine (R) methylation are also essential epigenetic modifications to control gene expression in CKD, which are mainly regulated by methyltransferases. The methyltransferases could mediate substrate-specific methylation at one or two lysine residue(s) in a single histone.226 The methylation effects do not alter the charge of histone and not interfere with DNA association, which controls gene transcription via histone readers.227 In Fig. 2 and Table 2, the functions of histone methylation and methyltransferase in kidney fibrosis are summarized.
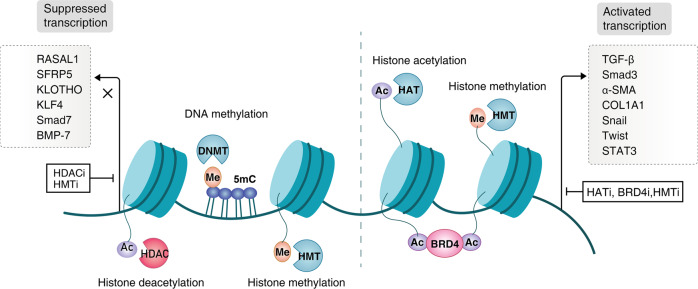
Fig. 2.
Histone modification and DNA methylation in kidney fibrosis. Suppression of antifibrotic genes (e.g., RASAL1, KLOTHO, KLF4, Smad7) could be accomplished by DNA methyltransferase (DNMT)-governed DNA methylation, HDACs-induced deacetylation, and histone methyltransferase (HMT)-driven methylation (e.g., H3K9me, H3K27me). On the contrary, HATs-mediated histone acetylation, HMT-ruled H3K4me, and BET proteins are involved in profibrotic gene transcriptional activation (TGF-β, Smad3, α-SMA, Snail, Twist, STAT3, et al.) α-SMA alpha-smooth-muscle actin, BMP-7 bone morphogenic protein 7, BRD4 bromodomain-containing protein 4, Col1a collagen-1α, KLF4 Krüppel-like factor 4, RASAL1 RAS protein activator like 1, SFRP5 secreted frizzled-related proteins 5, STAT3 signal transducer and activator of transcription 3, TGF-β transforming growth factor beta
Table 2.
Histone methylation and methyltransferases in kidney fibrosis
| Methyltransferase | Histone site | Interventions | Experimental model | Results | Ref. |
|---|---|---|---|---|---|
| DOT1L | H3K79me2 | EPZ5676 | UUO | TGF-β↓, G2/M arrest↓, Snail↓, Twist↓, Notch↓, SMAD3↓, EGFR↓, PDGFR↓, STAT3↓, AKT↓, NF-κB↓, KLOTHO↑, SMAD7↑ | 289 |
| H3K79 | EPZ004777 | IRI | ROS↓, PI3K/AKT↓ | 290 | |
| G9a | H3K9me1 | BIX01294 | UUO | α-SMA↓, fibronectin↓, KLOTHO↑ | 291 |
| SMYD2 | H3K36me3 | AZ505 | UUO | SMAD3↓, ERK1/2↓, AKT↓, STAT3↓, NF-kB↓, Snail↓, Twist↓, SMAD7↑ | 292 |
| SETD7 | H3K4 | PFI-2 | folic acid, UUO | M2 macrophages-myofibroblasts transition↓, myeloid fibroblasts activation↓, inflammation↓ | 293 |
| H3K4me1 | sinefungin | UUO | mesenchymal markers↓, ECM↓ | 294 | |
| H3K4me2 | - | type 1 diabetes (STZ) | Col1a↑ | 295 | |
| SUV39H1 | H3K9me2 | - | type 1 diabetes (STZ) | Col1a↑ | 295 |
| EZH2 | H3k27me3 | 3-DZNeP, GSK126 | UUO | SMAD7↑, PTEN↑ | 296 |
| H3k27me3 | 3-DZNeP | hyperuricemia | serum uric acid↓, XOD↓, TGF-β/Smad3↓, EGFR↓, ERK1/2↓, EMT↓, apoptosis↓, fibroblast activation↓, ECM↓, NF-κB↓ | 297 | |
| H3k27me3 | GNA | UUO, folic acid | Smad7↑, TGF-β/Smad3↓, α-SMA↓, fibronectin↓, collagens↓ | 298 | |
| PRMT1 | H4R3Me2a | AMI-1 | UUO | TGF-β/Smad3↓ | 299 |
| MLL1/WDR5 | H3K4me3 | MM-102, OICR-9429 | IRI | p16↓ | 300 |
AKT protein kinase B, α-SMA alpha-smooth-muscle actin, Col1a collagen-1α, DOT1L disruptor of telomeric silencing 1-like, ECM extracellular matrix, EGFR epidermal growth factor receptor, EMT epithelial-to-mesenchymal transition, ERK extracellular signal-regulated kinase, EZH2 enhancer of zester homolog 2, GNA gambogenic acid, IRI ischemia-reperfusion injury, MLL1 mixed-lineage leukemia 1, NF-κB nuclear factor kappa B, PI3K phosphatidylinositol 3-kinase, PDGFR platelet-derived growth factor receptor, PRMT-1 protein arginine methyltransferase 1, PTEN phosphatase and tensin homolog, ROS reactive oxygen species, STED7 SET domain containing 7 histone lysine methyltransferase, SMYD2 SET and MYND domain containing 2, STAT3 signal transducer and activator of transcription 3, STZ streptozotocin, TGF-β transforming growth factor beta, UUO unilateral ureteral occlusion, WDR5 WD repeat domain 5, XOD xanthine oxidase, 3-DZNeP 3-deazaneplanocin A
Other histone modifications
Histone H2AX serine 139 phosphorylation (γH2AX) is a common histone modification, which is induced by ATM, ATR, and DNA-PKs.228 The γH2AX is an important DNA damage marker and identified in DKD and AKI.229,230 Importantly, DNA damage is closely associated with proximal tubular maladaptive repair and resultant kidney fibrogenesis. Additionally, the crotonylation and lactylation of histone also take part in kidney diseases, which are gradually gaining attention.231
Non-coding RNAs
The ncRNAs are a kind of functional RNA molecule, and mainly contained miRNAs, lncRNAs, circRNAs, small nucleolar RNAs (snoRNAs), and transfer RNAs (tRNAs).232 Accumulating evidence suggests that miRNA and lncRNA in kidneys play important roles in damage, repair, and fibrosis.233
miRNA
The miRNAs are a class of evolutionarily conserved and small ncRNAs, contributing to the control of translation and mRNA degradation.234 The miRNA could theoretically target masses of mRNAs to exert diverse functions such as regulating development, differentiation, apoptosis, and stress.235 The role of microRNAs in kidney fibrosis is different: some miRNAs are fibrotic promotors and some are repressors.236 Here, we summarize miRNAs in both humans and mice (Table 3).
Table 3.
Role of microRNAs in kidney fibrosis
| microRNA | Targets | Ref. |
|---|---|---|
| Pro-fibrosis | ||
| miR-21 | TGF-β1/Smad3, Smad7, MMP9/TIMP1, PTEN/Akt, PPARα, Mpv17l, Cdc25a/Cdk6, Spry1 | 301–307 |
| miR-33 | CPT1α, CROT, HADHB | 308 |
| miR-34a | KLOTHO, Bcl-2 | 309,310 |
| miR-132 | TGF-β signaling, STAT3/ERK pathways | 311 |
| miR-150 | SOCS1 | 312 |
| miR-192 | MDM2/p53, Zeb1, ZEB2/TGF-β/CTGF, GLP-1R | 313–317 |
| miR-214 | ULK1, PTEN, E-cadherin, mt-Nd6, mt-Nd4l | 318–321 |
| miR-324 | Prep | 322 |
| miR-382 | HSPD1, PTEN/Akt, SIRP-α/STAT3, kallikrein 5, SOD-2 | 323–327 |
| miR-433 | Azin1, TGF-β/Smad3 | 328 |
| Anti-fibrosis | ||
| miR-29 | Col1-4, TPM1, HDAC4, YY1, TGF-β3, PI3K/AKT | 329–333 |
| miR-30 | SOX9, CTGF, Snail1, Snail2, Zeb2 | 334–336 |
| miR-181 | Egr1 | 337 |
| miR-200 | ZEB1, ZEB2, Snail2, KEAP1, TGF-β1 | 338–340 |
| miR-204 | PTEN11/p-STAT3, SP1 | 341,342 |
| Let-7 | Col1a2, Col4a1, TNFAIP3 | 343,344 |
AKT protein kinase B, Azin1 antizyme inhibitor 1, Cdc25a cell division cycle 25a, Cdk6 cyclin-dependent kinase 6, Col collagen, CPT1α carnitine palmitoyltransferase 1 A, CROT carnitine O-octanoyltransferase, CTGF connective tissue growth factor, Egr1 early growth response 1, ERK extracellular signal-regulated kinase, GLP-1R glucagon like peptide 1 receptor, HADHB hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta, HDAC4 histone deacetylase 4, HSPD1 heat shock protein family D (Hsp60) member 1, Keap1 kelch like ECH associated protein-1, MDM2 murine double minute 2, MMP matrix metallopeptidase, Mpv17l MPV17 mitochondrial inner membrane protein like, mt-Nd mitochondrial genes-NADH-dehydrogenase, PI3K phosphatidylinositol 3-kinase, PPARα peroxisome proliferator activated receptor alpha, Prep prolyl endopeptidase, PTEN phosphatase and tensin homolog, SIRP-α signal regulatory protein alpha, SOCS1 suppressor of cytokine signaling-1, SOD-2 superoxide dismutase 2, SOX9 SRY-box transcription factor 9, Spry1 sprout RTK signaling antagonist 1, STAT3 signal transducer and activator of transcription 3, STZ streptozotocin, TGF-β transforming growth factor beta, TIMP tissue inhibitor of matrix metalloproteinase, TNFAIP3 tumor necrosis factor alpha-induced protein 3, TPM1 tropomyosin 1, ULK1 unc-51 like autophagy activating kinase 1, YY1 Yin Yang 1, ZEB zinc finger E-box binding homeobox
lncRNA
The lncRNAs are a kind of conserved non-coding transcripts with >200 nucleotides, which are not translated into protein with little or no open reading frame.237 In fact, lncRNAs exert multiple biological functions by binding to RNA, DNA, or protein. Cytoplasm lncRNA could function as competing endogenous RNA (ceRNA) and modulate the stability, degradation, and translation of mRNAs to control gene expression. The lncRNAs localized in the nucleus could modulate chromosome conformation, and the rate of gene transcription activation.237 Recently, Xia et al. have summarized the effects of lncRNA on renal fibrosis and not covered in detail herein.238
Biomarkers and therapeutic medicines for kidney fibrosis
Novel biomarkers
As diagnostic criteria for CKD, the GFR, and albuminuria are not good at assessing the degree of renal fibrosis. Although tubulointerstitial fibrosis is well established to correlate with renal dysfunction, fibrotic initiation in kidneys usually happens before the GFR decrease.239 And albuminuria mainly reflects glomerular injury. Up to now, the biopsy still is a gold standard for investigating kidney fibrosis with the limitation of invasive detection. Therefore, quantified biomarkers of plasma or urine specimens are very valuable for evaluating the degree of fibrosis. Although many biomarkers have been found to reflect renal function or predict GFR decline, their direct relationship to kidney fibrosis has not been demonstrated. Here, we summarized blood or urinary biomarkers that are associated with biopsy-proved kidney fibrosis (Fig. 3, Table 4).
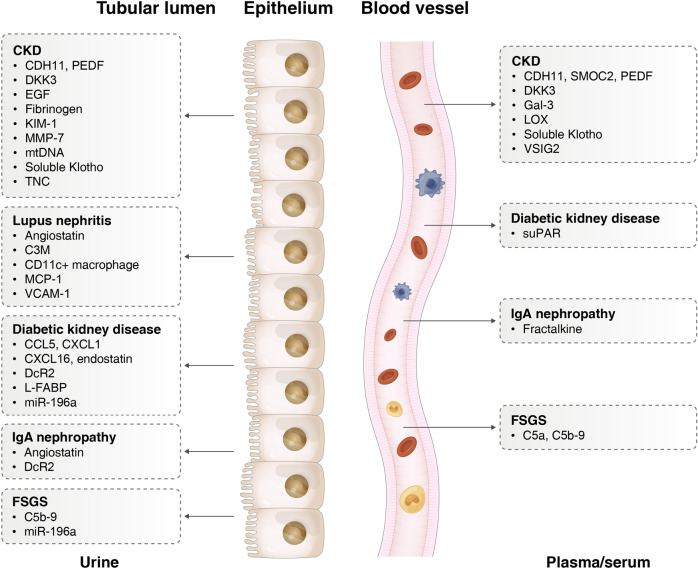
Fig. 3.
Non-invasive biomarkers of kidney fibrosis in urine and blood. Blood or urinary biomarkers that are associated with biopsy-proven kidney fibrosis. CCL5 C-C motif chemokine ligand 5, CDH11 Cadherin-11, CXCL C-X-C motif chemokine ligand, DcR2 decoy receptor 2, DKK3 Dickkopf-3, EGF epidermal growth factor, Gal-3 Galectin-3, KIM-1 kidney injury molecule 1, L-FABP liver fatty acid binding protein, LOX lysyl oxidase, MCP-1 monocyte chemoattractant protein-1, MMP-7 matrix metalloproteinase 7, mtDNA mitochondrial DNA, PEDF pigment epithelium-derived factor, PTC-EMP peritubular capillary-endothelial microparticles, SMOC2 Sparc-related modular calcium binding protein-2, suPAR soluble urokinase plasminogen activator receptor, TNC Tenascin-C, VCAM-1 vascular cell adhesion molecule 1, VSIG2 V-set and immunoglobulin domain containing 2
Table 4.
The blood or urinary molecules as non-invasive biomarkers of kidney fibrosis
| Biomarker | Relationship in kidney fibrosis | Ref. |
|---|---|---|
| Angiostatin | The levels of urinary angiostatin/creatinine in patients are associated with tubular atrophy/interstitial fibrosis | 345 |
| C3M | C3M in urine inversely correlates with tubular atrophy/interstitial fibrosis | 346 |
| C5a, C5b-9 | The plasma C5a level is correlated with the proportion of segmental sclerotic glomeruli. The plasma and urinary levels of C5b-9 are correlated with interstitial fibrosis | 347 |
| CCL5, CXCL1 | Urinary sediment CCL5 and CXCL1 mRNAs are associated with interstitial fibrosis degree | 348 |
| CD11c+ macrophages | The numbers and proportions of urinary CD11c+ macrophages are associated with the values of chronicity indices such as tubular atrophy/interstitial fibrosis | 349 |
| CDH11, SMOC2, PEDF | Higher levels of plasma CDH11, SMOC2, and PEDF and urinary CDH11 and PEDF are significantly associated with increasing severity of interstitial fibrosis and tubular atrophy | 350 |
| CXCL16, Endostatin | Urinary CXCL16 and endostatin are correlated with tubular atrophy/interstitial fibrosis score | 351 |
| DcR2 | Urinary DcR2 level is correlated with scores of tubulointerstitial injury | 352,353 |
| DKK3 | Urinary DKK3 is related to the extent of tubulointerstitial fibrosis. DKK3 in serum is associated with higher chronicity index at biopsy and flares rate | 354–356 |
| EGF | Urinary EGF shows significant correlation with intrarenal EGF mRNA, and tubular atrophy/interstitial fibrosis | 357 |
| L-FABP | Urinary L-FABP is positively associated with interstitial fibrosis | 358 |
| Fibrinogen | Urinary fibrinogen correlates with tubular atrophy/interstitial fibrosis | 359 |
| Fractalkine | Plasma fractalkine is associated with mesangial hypercellularity pathological damage, CD68+ macrophage, and CD20+ B cell infiltration in renal tissue and renal outcome in IgAN patients | 360 |
| Gal-3 | Higher plasma Gal-3 levels are associated with interstitial fibrosis, tubular atrophy, and vascular intimal fibrosis | 361 |
| KIM-1 | Urinary KIM-1 is associated with inflammation, renal function, and also reflects KIM-1 in tissue. Plasma KIM-1 is also associated with tubular atrophy/interstitial fibrosis | 362,363 |
| LOX | Serum LOX levels correlates with the area of kidney fibrosis | 364 |
| MMP-7 | Urinary MMP-7 correlates with renal fibrosis scores in CKD patients | 365 |
| miR-196a | Urinary miR-196a is associated with glomerular sclerosis and tubular atrophy/interstitial fibrosis | 366,367 |
| MCP-1 | Urinary MCP-1 correlates with chronic damage, especially fibrous crescents, and tubular atrophy/interstitial fibrosis | 368 |
| mtDNA | Urinary mtDNA has positive correlations with interstitial fibrosis | 369,370 |
| PTC-EMPs | Urinary PTC-EMPs levels correlates directly with interstitial fibrosis, and inversely with the number of peritubular capillary | 371 |
| Soluble klotho | High serum klotho is associated with decreased odds ratios of interstitial fibrosis and segmental sclerosis. Patients with a lower urinary klotho-to-creatinine ratio are more likely to have diffuse foot process effacement | 372,373 |
| suPAR | Serum suPAR is correlated with tubular atrophy/interstitial fibrosis score and interstitial inflammation score | 374 |
| TNC | There is a robust correlation between urinary TNC and kidney fibrotic score | 121 |
| VCAM-1 | Urinary VCAM-1 is associated with fibrous crescents | 375,376 |
| VSIG2 | Plasma VSIG2 is positively associated with interstitial fibrosis/tubular atrophy | 362 |
CCL5 C-C motif chemokine ligand 5, CDH11 Cadherin-11, CKD chronic kidney disease, CXCL C-X-C motif chemokine ligand, DcR2 decoy receptor 2, DKK3 Dickkopf-3, EGF epidermal growth factor, Gal-3 Galectin-3, IgAN IgA nephropathy, KIM-1 kidney injury molecule 1, L-FABP liver fatty acid binding protein, LOX lysyl oxidase, MCP-1 monocyte chemoattractant protein-1, MMP-7 matrix metalloproteinase 7, mtDNA mitochondrial DNA, PEDF pigment epithelium-derived factor, PTC-EMP peritubular capillary-endothelial microparticles, SMOC2 Sparc-related modular calcium binding protein-2, SUPER soluble urokinase plasminogen activator receptor, TNC Tenascin-C, VCAM-1 vascular cell adhesion molecule 1, VSIG2 V-set and immunoglobulin domain containing 2
Treatment of kidney fibrosis or CKD
With the help of gene editing and high-throughput sequencing technology, more kidney fibrotic mechanisms are continually explored. Generally, the potential of the therapeutic drugs will come from the target of these crucial mechanisms. Unfortunately, no effective drugs at present exist against kidney fibrosis. The renin-angiotensin system (RAS) blockade,8,240,241 SGLT2 inhibitor,242,243 GLP-1 receptor agonist,244,245 Atrasentan (endothelin-1 blocker),246 Tolvaptan (vasopressin receptor 2 antagonist)247 and Finerenone (non-steroidal anti-mineralocorticoid)248 can delay the progression of CKD (especially in DKD) to varying degrees. In fact, they are not designed to treat CKD. Some studies have revealed their antiinflammatory, and antifibrotic effects in cultured cells and animal models.249–251 Herein, we summarized therapeutic strategies tested in clinical trials targeting fibrotic drivers and signal pathways of tubular damage, regeneration, and inflammatory response (Table 5). It is also important to note that targeting single pathogenetic phenotype might lead to unpredictable events in the co-occurring process.
Table 5.
Clinical trials of antifibrotic drug in CKD
| Drug | Target | Disease | Phase | Outcome | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| FG-3019 | CTGF | FSGS | 1 | Terminated | NCT00782561 |
| Microalbuminuric DKD | 1 |
Treatment was well tolerated; Decrease in albuminuria |
NCT00102297 | ||
| DKD with urine ACR > 200 mg/g | 1 | Unknown | NCT00754143 | ||
| Type 2 diabetes and DKD with urine ACR > 200–3000 mg/g | 2 | Terminated early for business purposes | NCT00913393 | ||
| RG-012 | miR-21 | Alport syndrome | 1 | Unknown | NCT03373786 |
| Bardoxolone methyl | Nrf2 | CKD at risk of rapid progression | 2 | Increase in the eGFR | NCT04702997 |
| Patients with advanced CKD and type 2 diabetes | 2 | Increase in the eGFR | NCT00811889 (BEAM study) | ||
| CKD patients with type 2 diabetes | 2 | Increase in measured GFR | NCT02316821 (TSUBAKI study) | ||
| IgAN, CKD associated with type 1 diabetes, FSGS, ADPKD | 2 | Increase in the eGFR | NCT03366337 | ||
| Patients with type 2 diabetes and stage 4 CKD | 3 |
No reduction in the risk of ESKD or death from cardiovascular causes; Increased rate of cardiovascular events |
NCT01351675 (BEACON study) | ||
| Alport syndrome | 2/3 | Preservation in eGFR | NCT03019185 (CARDINAL study) | ||
| Pirfenidone | TGF-β | FSGS | 2 |
Decreased GFR loss; No change in proteinuria |
NCT00001959 |
| DKD | 1/2 | GFR increased in the pirfenidone 1200-mg/d group | NCT00063583 | ||
| DKD | 3 | Unknown | NCT02689778 | ||
| GCS-100 | Galectin-3 | CKD | 1 | Unknown | NCT01717248 |
| CKD | 2 | Unknown | NCT01843790, NCT02155673 | ||
| DKD | 2 | Unknown | NCT02312050 | ||
| Apabetalone | BET | Fabry Disease | 1/2 | Unknown | NCT03228940 |
| CKD subjects with a history of CVD | 2 |
Apabetalone was well tolerated; Favorable effects of apabetalone on ALP and eGFR |
Post-hoc analysis of NCT01423188 (SUSTAIN study) and NCT01067820 (ASSURE study) | ||
| Patients with CKD and type 2 diabetes | 3 | Apabetalone was associated with a reduced hazard for MACE and heart failure hospitalization | CKD subgroup analysis of NCT02586155 (BETonMACE study) |
ACR albumin-creatinine ratio, ADPKD autosomal dominant polycystic kidney disease, ALP alkaline phosphatase, BET bromodomain and extraterminal, CKD chronic kidney disease, CTGF connective tissue growth factor CVD cardiovascular disease, DKD diabetic kidney disease, eGFR estimated glomerular filtration rate, ESKD end-stage kidney disease, FSGS focal segmental glomerulosclerosis, IgAN IgA nephropathy, MACE major adverse cardiovascular event, TGF-β transforming growth factor beta
Pirfenidone
TGF-β is a core regulator in fibrosis, and several TGF-β-neutralizing antibodies and small-molecule inhibitors have been developed for clinical trials. Fresolimumab and LY2382770, both anti-TGF-β antibodies, failed to show efficacy in steroid-resistant FSGS and DKD patients, respectively (NCT01665391, NCT01113801).252 As a small synthetic molecule, pirfenidone is protected against pulmonary fibrosis by the inhibition of TGF-β1 expression.253,254 What’s more, it is encouraging to find Pirfenidone could restore the eGFR in patients with DKD or FSGS.255 And more phase 2 and 3 trials are ongoing to study the effect of pirfenidone on CKD.
FG-3019
As a TGF-β downstream effector, CTGF could trigger fibroblast activation and ECM accumulation. In phase 1 clinical study, anti-CTGF antibody FG-3019 was well tolerated and led to a reduction of microalbuminuria in DKD patients.256 However, another phase 1 trial in primary FSGS was prematurely terminated. A phase 2 trial recruiting subjects with type 2 diabetes and persistent proteinuria was also terminated due to business purpose.
Bardoxolone methyl
Bardoxolone methyl, an NRF2 activator, was found to restore eGFR of different kidney diseases (e.g., DKD, IgAN, FSGS, Alport syndrome, and polycystic kidney disease) in a phase 2 trial. Several phase 2 trials reproduced the result that Bardoxolone methyl could increase the eGFR in CKD patients.257,258 However, in a larger phase 3 trial including more than 2000 patients with stage 4 CKD and diabetes (BEACON study), bardoxolone methyl failed to reduce the risk of ESRD or death from cardiovascular causes, along with an increased rate of cardiovascular events.259 Researchers conducted post-hoc analysis of BEACON study, and found that patients randomized to bardoxolone methyl were significantly less likely to experience the composite renal endpoint.260 Therefore, studies to further assess the potential risk-benefit profile of bardoxolone methyl are ongoing in patients without identified risk factors for fluid overload. These studies aim to ascertain whether increases in eGFR with bardoxolone methyl offer the potential to prevent or delay kidney function decline and progression to ESKD.
Apabetalone
Apabetalone is an oral inhibitor of BET proteins. Although in a large multicenter randomized controlled trial (BETonMACE study), apabetalone added to standard therapy did not significantly reduce the risk of major adverse cardiovascular events (MACE) among patients with recent acute coronary syndrome, type 2 diabetes, and low high-density lipoprotein cholesterol levels,261 a post-hoc analysis showed apabetalone demonstrated a potential cardioprotective effect in CKD patients with favorable effects on eGFR.262 Another post-hoc analysis of CKD subjects from the SUSTAIN and ASSURE randomized controlled trials also exhibit favorable effects of apabetalone on eGFR.263
Other drugs
RG-012, an inhibitor of miR-21, is currently evaluated in a Phase I trial for treating Alport syndrome. Galectin-3 regulates basic cellular functions such as cell-cell and cell-matrix interactions, growth, proliferation, differentiation, and inflammation. So, this protein is involved in fibrosis, chronic inflammation and scarring affecting many different tissues.264 Galectin-3 has been proposed as a novel biomarker of heart failure and cardiac fibrosis. And higher plasma galectin-3 levels were associated with an elevated risk of developing incident CKD.265 GCS-100 is a modified citrus pectin and galectin-3 antagonist. Clinical trials of different stages that study effect of GCS-100 on CKD are ongoing and promising results are expected.
Conclusion and perspectives
The global prevalence of CKD has been a tremendous burden. Regardless of etiology, kidney fibrosis is a hallmark of most progressive CKD. After decades of work, the key steps in the understanding of kidney fibrosis have been unremittingly revealed: firstly, kidney damage-triggered inflammation activation and immune cell infiltration; secondly, profibrotic mediator release such as growth factors, chemokines, and cytokines; thirdly, myofibroblast activation and excessive ECM accumulation in tubulointerstitium, owing to ECM synthesis/degradation imbalance; fourthly, phenotype alteration and irreversible loss of parenchymal cells; fifthly, kidney microvasculature reduction. Detailed molecular mechanisms have been uncovered gradually. A recent report of human kidney atlas identified these primary cell types in kidneys, recapitulated several known subtypes, and clarified their key functions, guiding kidney disease classification when intricate pathophysiologic mechanisms underlie convergently clinical symptoms. Important findings also included the fact that PT S3 segment and medullary thick ascending limb (mTAL) were the two most vulnerable sites to kidney injury.11 Now, targeting the main collagen-producing myofibroblast activation is a promising strategy to prevent tubule damage and exert antifibrotic activity. However, no effective drugs exist at present and most of therapies only retard the progression of fibrotic kidney disease, underscoring the urgent need to explore innovative approaches to reverse or stop disease progression.
For a long time, we studied the mechanism of fibrotic kidney using whole-kidney tissue. However, this in reality may only represent changes in the renal tubular cells, as the tubules make up the majority of the kidney. Following the advancement of single-cell technology, many key questions have been answered, such as what kind of renal tubules are profibrotic, where myofibroblasts originate, which immune cells get involved, and how cells communicate with each other. Genetics and epigenetics are deeper mechanisms that regulate renal fibrosis. Although the reversible feature of epigenetics provides a fighting chance to treat the disease, epigenetic drugs are approximately restricted in the oncology field and few pioneering studies explore epigenetic clinical significance in kidney disease. However, genomic and epigenomic analyses offer ponderable diagnostic or prognostic biomarkers in the progression of kidney disease.
While exploring approaches against kidney fibrosis, investigators have sought to use drug delivery systems to target specific cells (PT cells, mesangial cells, myofibroblasts, et al.) for inhibiting myofibroblast activity and ECM production, simultaneously avoiding drug toxicity. Extracellular vesicles (EVs) are kinds of lipid bilayer-delimited particles secreted by almost all types of cells in physiopathologic conditions.266 EVs-contained mRNAs, miRNAs, proteins, and lipids from parent cells could be functionally transferred to recipient cells, and these messages mediate cell-to-cell communication. EVs are not only mediators of paracrine secretion, they have been applied as a drug delivery system for kidney diseases.267,268 Both traditional and biological drug delivery systems have demonstrated good safety and therapeutic efficacy in preclinical studies, but investigators appear to be very cautious about conducting clinical trials with them.
Overall, it is exciting to find more and more existing drugs, e.g., RAS blockage, SGLT2 inhibitors, vasopressin receptor 2 antagonist, and non-steroidal anti-mineralocorticoid can delay the progression of CKD. Many Chinese herbal formulas, single herbs, and Chinese herbal compounds have been shown to reduce kidney fibrosis.269,270 Solid evidence from well-designed experimental studies has revealed their antifibrotic mechanism and activity.271,272 However, challenges and barriers of traditional Chinese medicines still limited their participation in clinical trials for the treatment of kidney disease. The side-effect from traditional Chinese medicine is drastically under-reported. There is still lack of long-term follow-up data for some patients taking traditional Chinese medicine. Most of clinical investigations referred to traditional Chinses medicines against kidney diseases have issues such as flawed methodologic approach, suboptimal reporting, and small-sample size, etc.269
Kidney fibrosis is the common outcome across all kinds of progressive CKD, which means that kidney injury is in an advanced stage. This may be the reason why antifibrotic treatment is difficult. The prerequisite for early treatment is early detection. However, there is a lack of good biomarkers to predict and assess kidney fibrosis in clinic. There is no doubt that technological advances drive a deeper understanding of diseases. With a fuller understanding of fibrotic kidney mechanism, access to drugs stopping or even reversing renal fibrosis is challenging and promising.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (82200973), the Science/Technology Project of Sichuan province (2021YFQ0027, 2022YFS0589, 2022YFS0589), and 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (ZYGD18027).
Author contributions
R.S.H. and L.M. completed the concept and critical design of the study. R.S.H., and L.M. collected the literature and analyzed the data. R.S.H. and L.M. wrote the draft and revised the manuscript. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Contributor Information
Ping Fu, Email: Fupinghx@scu.edu.cn.
Liang Ma, Email: Liang_m@scu.edu.cn.
References
- 1.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat. Rev. Nephrol. 2017;13:104–114. doi: 10.1038/nrneph.2016.163. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020;16:269–288. doi: 10.1038/s41581-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R, et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin. Kidney J. 2021;14:1719–1730. doi: 10.1093/ckj/sfab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Q, Tang B, Zhang C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target Ther. 2022;7:182. doi: 10.1038/s41392-022-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N. Engl. J. Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 10.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat. Rev. Nephrol. 2015;11:233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J, et al. A reference tissue atlas for the human kidney. Sci. Adv. 2022;8:eabn4965. doi: 10.1126/sciadv.abn4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreibing F, Kramann R. Mapping the human kidney using single-cell genomics. Nat. Rev. Nephrol. 2022;18:347–360. doi: 10.1038/s41581-022-00553-4. [DOI] [PubMed] [Google Scholar]
- 13.Waddington CH. The epigenotype. 1942. Int. J. Epidemiol. 2012;41:10–13. doi: 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 2018;378:1323–1334. doi: 10.1056/NEJMra1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran P, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Fu H, Liu Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat. Rev. Nephrol. 2022;18:545–557. doi: 10.1038/s41581-022-00590-z. [DOI] [PubMed] [Google Scholar]
- 17.Joshi N, et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020;55:1900646. doi: 10.1183/13993003.00646-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuppe C, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589:281–286. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Q, Tan RJ, Liu Y. Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv. Exp. Med. Biol. 2019;1165:253–283. doi: 10.1007/978-981-13-8871-2_12. [DOI] [PubMed] [Google Scholar]
- 20.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura J, et al. Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 2019;95:526–539. doi: 10.1016/j.kint.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Chen YT, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 23.Kramann R, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011;80:41–50. doi: 10.1038/ki.2011.77. [DOI] [PubMed] [Google Scholar]
- 25.Su J, et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577:566–571. doi: 10.1038/s41586-019-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X, Jin J, Lan HY. Driving role of macrophages in transition from acute kidney injury to chronic kidney disease. Chin. Med J. (Engl.) 2022;135:757–766. doi: 10.1097/CM9.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang H, et al. The IL-4 receptor α has a critical role in bone marrow-derived fibroblast activation and renal fibrosis. Kidney Int. 2017;92:1433–1443. doi: 10.1016/j.kint.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Zhang Z, Yang J, Mitch WE, Wang Y. JAK3/STAT6 stimulates bone marrow-derived fibroblast activation in renal fibrosis. J. Am. Soc. Nephrol. 2015;26:3060–3071. doi: 10.1681/ASN.2014070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, et al. Chromatin-accessibility estimation from single-cell ATAC-seq data with scOpen. Nat. Commun. 2021;12:6386. doi: 10.1038/s41467-021-26530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1(+) pericyte loss induces capillary rarefaction and proximal tubular injury. J. Am. Soc. Nephrol. 2017;28:776–784. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami T, Mimura I, Shoji K, Tanaka T, Nangaku M. Hypoxia and fibrosis in chronic kidney disease: crossing at pericytes. Kidney Int. Suppl. 2014;4:107–112. doi: 10.1038/kisup.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang M, et al. Yap/Taz deletion in Gli(+) cell-derived myofibroblasts attenuates fibrosis. J. Am. Soc. Nephrol. 2017;28:3278–3290. doi: 10.1681/ASN.2015121354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, et al. Targeting interstitial myofibroblast-expressed integrin αvβ3 alleviates renal fibrosis. Mol. Pharm. 2021;18:1373–1385. doi: 10.1021/acs.molpharmaceut.0c01182. [DOI] [PubMed] [Google Scholar]
- 36.Li R, et al. Targeted delivery of celastrol to renal interstitial myofibroblasts using fibronectin-binding liposomes attenuates renal fibrosis and reduces systemic toxicity. J. Control Release. 2020;320:32–44. doi: 10.1016/j.jconrel.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, et al. Antibody-drug conjugates targeting CD248(+) myofibroblasts effectively alleviate renal fibrosis in mice. FASEB J. 2022;36:e22102. doi: 10.1096/fj.202101441R. [DOI] [PubMed] [Google Scholar]
- 38.Grgic I, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl Acad. Sci. USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang HM, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transpl. 2015;30:575–583. doi: 10.1093/ndt/gfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA. 2020;117:15874–15883. doi: 10.1073/pnas.2005477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muto Y, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat. Commun. 2021;12:2190. doi: 10.1038/s41467-021-22368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. bioRxiv, https://www.biorxiv.org/content/10.1101/2021.07.28.454201v1 (2021). [DOI] [PMC free article] [PubMed]
- 45.Ide S, et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. Elife. 2021;10:e68603. doi: 10.7554/eLife.68603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bielesz B, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirin Y, Susztak K. Notch in the kidney: development and disease. J. Pathol. 2012;226:394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sörensen-Zender I, et al. Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury. Am. J. Physiol. Ren. Physiol. 2014;306:F907–F915. doi: 10.1152/ajprenal.00030.2014. [DOI] [PubMed] [Google Scholar]
- 49.Xiao Z, et al. The Notch γ-secretase inhibitor ameliorates kidney fibrosis via inhibition of TGF-β/Smad2/3 signaling pathway activation. Int. J. Biochem. Cell Biol. 2014;55:65–71. doi: 10.1016/j.biocel.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Dees C, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann. Rheum. Dis. 2011;70:1304–1310. doi: 10.1136/ard.2010.134742. [DOI] [PubMed] [Google Scholar]
- 51.Liu T, et al. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am. J. Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grande MT, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 53.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng L, Zhuang S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front Physiol. 2020;11:569322. doi: 10.3389/fphys.2020.569322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halt K, Vainio S. Coordination of kidney organogenesis by Wnt signaling. Pediatr. Nephrol. 2014;29:737–744. doi: 10.1007/s00467-013-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou D, et al. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao L, et al. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He W, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou D, Tan RJ, Zhou L, Li Y, Liu Y. Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci. Rep. 2013;3:1878. doi: 10.1038/srep01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, et al. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–4238. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue H, et al. Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/β-catenin signaling. J. Biol. Chem. 2013;288:15006–15014. doi: 10.1074/jbc.M113.458448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren S, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl Acad. Sci. USA. 2013;110:1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J. Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 64.Hao S, et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuyama M, Nomori A, Nakakuni K, Shimono A, Fukushima M. Secreted Frizzled-related protein 1 (Sfrp1) regulates the progression of renal fibrosis in a mouse model of obstructive nephropathy. J. Biol. Chem. 2014;289:31526–31533. doi: 10.1074/jbc.M114.584565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 68.Luo C, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018;29:1238–1256. doi: 10.1681/ASN.2017050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou D, et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 2017;28:2322–2336. doi: 10.1681/ASN.2016080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maarouf OH, et al. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J. Am. Soc. Nephrol. 2016;27:781–790. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 73.Ding H, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J. Am. Soc. Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rauhauser AA, et al. Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am. J. Physiol. Ren. Physiol. 2015;309:F770–F778. doi: 10.1152/ajprenal.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fabian SL, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am. J. Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou D, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J. Am. Soc. Nephrol. 2014;25:2187–2200. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reginensi A, et al. SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum. Mol. Genet. 2011;20:1143–1153. doi: 10.1093/hmg/ddq558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang HM, et al. Sox9-positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Rep. 2016;14:861–871. doi: 10.1016/j.celrep.2015.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu L, et al. Embryonic stem cell-derived extracellular vesicles promote the recovery of kidney injury. Stem Cell Res. Ther. 2021;12:379. doi: 10.1186/s13287-021-02460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soofi A, Kutschat AP, Azam M, Laszczyk AM, Dressler GR. Regeneration after acute kidney injury requires PTIP-mediated epigenetic modifications. JCI Insight. 2020;5:e130204. doi: 10.1172/jci.insight.130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar S, et al. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12:1325–1338. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 82.Raza S, et al. SOX9 is required for kidney fibrosis and activates NAV3 to drive renal myofibroblast function. Sci. Signal. 2021;14:eabb4282. doi: 10.1126/scisignal.abb4282. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, et al. Tubule-derived INHBB promotes interstitial fibroblast activation and renal fibrosis. J. Pathol. 2022;256:25–37. doi: 10.1002/path.5798. [DOI] [PubMed] [Google Scholar]
- 84.Dhillon P, et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 2021;33:379–394.e378. doi: 10.1016/j.cmet.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee K, Gusella GL, He JC. Epithelial proliferation and cell cycle dysregulation in kidney injury and disease. Kidney Int. 2021;100:67–78. doi: 10.1016/j.kint.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Childs BG, et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melk A, et al. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65:510–520. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 88.Megyesi J, et al. Increased expression of p21WAF1/CIP1 in kidney proximal tubules mediates fibrosis. Am. J. Physiol. Ren. Physiol. 2015;308:F122–F130. doi: 10.1152/ajprenal.00489.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canaud G, et al. Cyclin G1 and TASCC regulate kidney epithelial cell G(2)-M arrest and fibrotic maladaptive repair. Sci. Transl. Med. 2019;11:eaav4754. doi: 10.1126/scitranslmed.aav4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hannken T, Schroeder R, Zahner G, Stahl RAK, Wolf G. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27(Kip1): role in angiotensin II-mediated hypertrophy of proximal tubular cells. J. Am. Soc. Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- 92.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baker DJ, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu M, et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat. Commun. 2020;11:889. doi: 10.1038/s41467-020-14734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernier M, et al. Disulfiram treatment normalizes body weight in obese mice. Cell Metab. 2020;32:203–214.e204. doi: 10.1016/j.cmet.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parry-Williams G, Gati S, Sharma S. The heart of the ageing endurance athlete: the role of chronic coronary stress. Eur. Heart J. 2021;42:2737–2744. doi: 10.1093/eurheartj/ehab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han X, et al. FOXO4 peptide targets myofibroblast ameliorates bleomycin-induced pulmonary fibrosis in mice through ECM-receptor interaction pathway. J. Cell Mol. Med. 2022;26:3269–3280. doi: 10.1111/jcmm.17333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang R, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16:564–574. doi: 10.1111/acel.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim H, et al. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell. 2021;20:e13317. doi: 10.1111/acel.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang W, et al. Lactobacillus plantarum 69-2 combined with galacto-oligosaccharides alleviates d-galactose-induced aging by regulating the AMPK/SIRT1 signaling pathway and gut microbiota in mice. J. Agric. Food Chem. 2021;69:2745–2757. doi: 10.1021/acs.jafc.0c06730. [DOI] [PubMed] [Google Scholar]
- 103.DiRocco DP, et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Ren. Physiol. 2014;306:F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pabla N, et al. Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc. Natl Acad. Sci. USA. 2015;112:5231–5236. doi: 10.1073/pnas.1424313112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kramann R, et al. Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight. 2018;3:e99561. doi: 10.1172/jci.insight.99561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019;15:144–158. doi: 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- 107.Conway BR, et al. Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J. Am. Soc. Nephrol. 2020;31:2833–2854. doi: 10.1681/ASN.2020060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.do Valle Duraes F, et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight. 2020;5:e130651. doi: 10.1172/jci.insight.130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375:eabf9419. doi: 10.1126/science.abf9419. [DOI] [PubMed] [Google Scholar]
- 110.Sato Y, et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight. 2016;1:e87680. doi: 10.1172/jci.insight.87680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato Y, et al. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney Int. 2020;98:448–463. doi: 10.1016/j.kint.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 112.Pei G, et al. Lymphangiogenesis in kidney and lymph node mediates renal inflammation and fibrosis. Sci. Adv. 2019;5:eaaw5075. doi: 10.1126/sciadv.aaw5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo R, et al. Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A. Theranostics. 2021;11:117–131. doi: 10.7150/thno.48624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sato Y, et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest. 2022;132:e146071. doi: 10.1172/JCI146071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Law BMP, et al. Interferon-γ production by tubulointerstitial human CD56(bright) natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int. 2017;92:79–88. doi: 10.1016/j.kint.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Ryu S, et al. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J. Clin. Invest. 2022;132:e156876. doi: 10.1172/JCI156876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitamoto K, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J. Pharm. Sci. 2009;111:285–292. doi: 10.1254/jphs.09227FP. [DOI] [PubMed] [Google Scholar]
- 118.Summers SA, et al. Mast cell activation and degranulation promotes renal fibrosis in experimental unilateral ureteric obstruction. Kidney Int. 2012;82:676–685. doi: 10.1038/ki.2012.211. [DOI] [PubMed] [Google Scholar]
- 119.Doke T, et al. Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis. Nat. Immunol. 2022;23:947–959. doi: 10.1038/s41590-022-01200-7. [DOI] [PubMed] [Google Scholar]
- 120.Fu H, et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J. Am. Soc. Nephrol. 2017;28:785–801. doi: 10.1681/ASN.2016020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu H, et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int. 2020;97:1017–1031. doi: 10.1016/j.kint.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fu J, et al. Single-Cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J. Am. Soc. Nephrol. 2019;30:533–545. doi: 10.1681/ASN.2018090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deleersnijder D, et al. Current methodological challenges of single-cell and single-nucleus RNA-sequencing in glomerular diseases. J. Am. Soc. Nephrol. 2021;32:1838–1852. doi: 10.1681/ASN.2021020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung JJ, et al. Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury. J. Am. Soc. Nephrol. 2020;31:2341–2354. doi: 10.1681/ASN.2020020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilson PC, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl Acad. Sci. USA. 2019;116:19619–19625. doi: 10.1073/pnas.1908706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park MH, et al. CCN1 interlinks integrin and hippo pathway to autoregulate tip cell activity. Elife. 2019;8:e46012. doi: 10.7554/eLife.46012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang YH, Manning Fox JE, Zhang KL, MacDonald PE, Johnson JD. Intraislet SLIT-ROBO signaling is required for beta-cell survival and potentiates insulin secretion. Proc. Natl. Acad. Sci. USA. 2013;110:16480–16485. doi: 10.1073/pnas.1214312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Revollo JR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Robertson IB, et al. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Der E, et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight. 2017;2:e93009. doi: 10.1172/jci.insight.93009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Der E, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 2019;20:915–927. doi: 10.1038/s41590-019-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arazi A, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019;20:902–914. doi: 10.1038/s41590-019-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang T, et al. Association of urine sCD163 with proliferative lupus nephritis, fibrinoid necrosis, cellular crescents and intrarenal M2 macrophages. Front. Immunol. 2020;11:671. doi: 10.3389/fimmu.2020.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goel RR, et al. Interferon lambda promotes immune dysregulation and tissue inflammation in TLR7-induced lupus. Proc. Natl Acad. Sci. USA. 2020;117:5409–5419. doi: 10.1073/pnas.1916897117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zambrano S, et al. Molecular insights into the early stage of glomerular injury in IgA nephropathy using single-cell RNA sequencing. Kidney Int. 2022;101:752–765. doi: 10.1016/j.kint.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 136.Zheng Y, et al. Single-cell transcriptomics reveal immune mechanisms of the onset and progression of IgA nephropathy. Cell Rep. 2020;33:108525. doi: 10.1016/j.celrep.2020.108525. [DOI] [PubMed] [Google Scholar]
- 137.Menon R, et al. Single cell transcriptomics identifies focal segmental glomerulosclerosis remission endothelial biomarker. JCI Insight. 2020;5:e133267. doi: 10.1172/jci.insight.133267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Latt KZ, et al. Urine single-cell RNA sequencing in focal segmental glomerulosclerosis reveals inflammatory signatures. Kidney Int. Rep. 2022;7:289–304. doi: 10.1016/j.ekir.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Afsar B, et al. Capillary rarefaction from the kidney point of view. Clin. Kidney J. 2018;11:295–301. doi: 10.1093/ckj/sfx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Doi K, Noiri E, Fujita T. Role of vascular endothelial growth factor in kidney disease. Curr. Vasc. Pharm. 2010;8:122–128. doi: 10.2174/157016110790226606. [DOI] [PubMed] [Google Scholar]
- 141.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 143.Fiedler U, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 144.Hakanpaa L, et al. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat. Commun. 2015;6:5962. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang FC, et al. Angiopoietin-2 inhibition attenuates kidney fibrosis by hindering chemokine C-C motif ligand 2 expression and apoptosis of endothelial cells. Kidney Int. 2022;102:780–797. doi: 10.1016/j.kint.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 146.Wang X, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013;499:306–311. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumagai S, et al. Myeloid cell-derived LRG attenuates adverse cardiac remodelling after myocardial infarction. Cardiovasc. Res. 2016;109:272–282. doi: 10.1093/cvr/cvv273. [DOI] [PubMed] [Google Scholar]
- 148.Liu TT, et al. LRG1 mitigates renal interstitial fibrosis through alleviating capillary rarefaction and inhibiting inflammatory and pro-fibrotic cytokines. Am. J. Nephrol. 2021;52:228–238. doi: 10.1159/000514167. [DOI] [PubMed] [Google Scholar]
- 149.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 150.Wang B, et al. Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis. Cell Death Dis. 2022;13:333. doi: 10.1038/s41419-022-04794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang B, et al. Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2018;29:1900–1916. doi: 10.1681/ASN.2017050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martínez-Salgado C, Sánchez-Juanes F, López-Hernández FJ, Muñoz-Félix JM. Endothelial activin receptor-like kinase 1 (ALK1) regulates myofibroblast emergence and peritubular capillary stability in the early stages of kidney fibrosis. Front. Pharm. 2022;13:843732. doi: 10.3389/fphar.2022.843732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roman BL, Hinck AP. ALK1 signaling in development and disease: new paradigms. Cell Mol. Life Sci. 2017;74:4539–4560. doi: 10.1007/s00018-017-2636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi M, Maique J, Cleaver O, Moe OW, Hu MC. VEGFR2 insufficiency enhances phosphotoxicity and undermines Klotho’s protection against peritubular capillary rarefaction and kidney fibrosis. Am. J. Physiol. Ren. Physiol. 2022;324:F106–F123. doi: 10.1152/ajprenal.00149.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li L, et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci. Adv. 2021;7:eabc7170. doi: 10.1126/sciadv.abc7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu SB, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30:1599–1609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 157.Saifi MA, Godugu C. Inhibition of lysyl oxidase ameliorates renal injury by inhibiting CD44-mediated pericyte detachment and loss of peritubular capillaries. Life Sci. 2020;243:117294. doi: 10.1016/j.lfs.2020.117294. [DOI] [PubMed] [Google Scholar]
- 158.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM. Renal pericytes: regulators of medullary blood flow. Acta Physiol. (Oxf.) 2013;207:212–225. doi: 10.1111/apha.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Castellano G, et al. Complement activation during ischemia/reperfusion injury induces pericyte-to-myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front. Immunol. 2018;9:1002. doi: 10.3389/fimmu.2018.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu W, et al. Bone marrow-derived mesenchymal stem cells transplantation attenuates renal fibrosis following acute kidney injury by repairing the peritubular capillaries. Exp. Cell Res. 2022;411:112983. doi: 10.1016/j.yexcr.2021.112983. [DOI] [PubMed] [Google Scholar]
- 161.Zhang Y, et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 2018;94:524–535. doi: 10.1016/j.kint.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 162.Guise E, et al. Biopolymer-delivered vascular endothelial growth factor improves renal outcomes following revascularization. Am. J. Physiol. Ren. Physiol. 2019;316:F1016–F1025. doi: 10.1152/ajprenal.00607.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Engel JE, Williams E, Williams ML, Bidwell GL, 3rd, Chade AR. Targeted VEGF (Vascular Endothelial Growth Factor) therapy induces long-term renal recovery in chronic kidney disease via macrophage polarization. Hypertension. 2019;74:1113–1123. doi: 10.1161/HYPERTENSIONAHA.119.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019;15:327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng W, Guo J, Liu ZS. Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective. Clin. Epigenetics. 2021;13:87. doi: 10.1186/s13148-021-01079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nangaku M, Hirakawa Y, Mimura I, Inagi R, Tanaka T. Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition. Nephron. 2017;137:256–259. doi: 10.1159/000476078. [DOI] [PubMed] [Google Scholar]
- 167.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 168.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 169.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 170.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum. Mol. Genet. 2007;16:R50–R59. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- 171.Liu H, et al. Epigenomic and transcriptomic analyses define core cell types, genes and targetable mechanisms for kidney disease. Nat. Genet. 2022;54:950–962. doi: 10.1038/s41588-022-01097-w. [DOI] [PubMed] [Google Scholar]
- 172.Ko YA, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013;14:R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boutet A, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kobayashi A, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chu AY, et al. Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat. Commun. 2017;8:1286. doi: 10.1038/s41467-017-01297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smyth LJ, McKay GJ, Maxwell AP, McKnight AJ. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics. 2014;9:366–376. doi: 10.4161/epi.27161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bechtel W, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu Y, et al. DNA hypermethylation of sFRP5 contributes to indoxyl sulfate-induced renal fibrosis. J. Mol. Med. 2017;95:601–613. doi: 10.1007/s00109-017-1538-0. [DOI] [PubMed] [Google Scholar]
- 179.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81:640–650. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gu Y, et al. Hydrogen sulfide attenuates renal fibrosis by inducing TET-dependent DNA demethylation on Klotho promoter. FASEB J. 2020;34:11474–11487. doi: 10.1096/fj.201902957RR. [DOI] [PubMed] [Google Scholar]
- 181.Xiao X, Tang W, Yuan Q, Peng L, Yu P. Epigenetic repression of Krüppel-like factor 4 through Dnmt1 contributes to EMT in renal fibrosis. Int. J. Mol. Med. 2015;35:1596–1602. doi: 10.3892/ijmm.2015.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zeisberg M, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 183.Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022;23:329–349. doi: 10.1038/s41580-021-00441-y. [DOI] [PubMed] [Google Scholar]
- 184.Michalak EM, Burr ML, Bannister AJ, Dawson MA. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019;20:573–589. doi: 10.1038/s41580-019-0143-1. [DOI] [PubMed] [Google Scholar]
- 185.Li X, Egervari G, Wang Y, Berger SL, Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018;19:563–578. doi: 10.1038/s41580-018-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y, et al. Identification of histone deacetylase 8 as a novel therapeutic target for renal fibrosis. FASEB J. 2020;34:7295–7310. doi: 10.1096/fj.201903254R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Smith ER, Wigg B, Holt S, Hewitson TD. TGF-β1 modifies histone acetylation and acetyl-coenzyme A metabolism in renal myofibroblasts. Am. J. Physiol. Ren. Physiol. 2019;316:F517–F529. doi: 10.1152/ajprenal.00513.2018. [DOI] [PubMed] [Google Scholar]
- 190.Pang M, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nguyễn-Thanh T, et al. Inhibition of histone deacetylase 1 ameliorates renal tubulointerstitial fibrosis via modulation of inflammation and extracellular matrix gene transcription in mice. Int. J. Mol. Med. 2018;41:95–106. doi: 10.3892/ijmm.2017.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar P, et al. Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am. J. Physiol. Ren. Physiol. 2017;313:F781–F795. doi: 10.1152/ajprenal.00166.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chung S, et al. Inhibition of p300/CBP-associated factor attenuates renal tubulointerstitial fibrosis through modulation of NF-kB and Nrf2. Int. J. Mol. Sci. 2019;20:1554. doi: 10.3390/ijms20071554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hewitson TD, et al. Epigenetic modifications to H3K9 in renal tubulointerstitial cells after unilateral ureteric obstruction and TGF-β1 stimulation. Front Pharm. 2017;8:307. doi: 10.3389/fphar.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rai R, et al. A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis. Epigenetics. 2017;12:1004–1013. doi: 10.1080/15592294.2017.1370173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lazar AG, Vlad ML, Manea A, Simionescu M, Manea SA. Activated histone acetyltransferase p300/CBP-related signalling pathways mediate up-regulation of NADPH oxidase, inflammation, and fibrosis in diabetic kidney. Antioxid. (Basel) 2021;10:1356. doi: 10.3390/antiox10091356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ni J, et al. P300-dependent STAT3 acetylation is necessary for angiotensin II-induced pro-fibrotic responses in renal tubular epithelial cells. Acta Pharm. Sin. 2014;35:1157–1166. doi: 10.1038/aps.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Y, et al. Novel curcumin analog C66 prevents diabetic nephropathy via JNK pathway with the involvement of p300/CBP-mediated histone acetylation. Biochim. Biophys. Acta. 2015;1852:34–46. doi: 10.1016/j.bbadis.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tung CW, et al. Trichostatin A ameliorates renal tubulointerstitial fibrosis through modulation of the JNK-dependent Notch-2 signaling pathway. Sci. Rep. 2017;7:14495. doi: 10.1038/s41598-017-15162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Choi SY, et al. Class I HDACs specifically regulate E-cadherin expression in human renal epithelial cells. J. Cell Mol. Med. 2016;20:2289–2298. doi: 10.1111/jcmm.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu N, et al. Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One. 2013;8:e54001. doi: 10.1371/journal.pone.0054001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am. J. Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xiao Z, Chen C, Meng T, Zhang W, Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Exp. Biol. Med. (Maywood) 2016;241:140–146. doi: 10.1177/1535370215598401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Quan Y, et al. Sirtuin 3 activation by honokiol decreases unilateral ureteral obstruction-induced renal inflammation and fibrosis via regulation of mitochondrial dynamics and the renal NF-κBTGF-β1/smad signaling pathway. Int. J. Mol. Sci. 2020;21:402. doi: 10.3390/ijms21020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang Y, et al. Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis. Cell Death Dis. 2021;12:847. doi: 10.1038/s41419-021-04134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li N, et al. SIRT3-KLF15 signaling ameliorates kidney injury induced by hypertension. Oncotarget. 2017;8:39592–39604. doi: 10.18632/oncotarget.17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chang JW, et al. Up-regulation of SIRT1 reduces endoplasmic reticulum stress and renal fibrosis. Nephron. 2016;133:116–128. doi: 10.1159/000447067. [DOI] [PubMed] [Google Scholar]
- 208.He W, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang Y, et al. Sirtuin 1 activation reduces transforming growth factor-β1-induced fibrogenesis and affords organ protection in a model of progressive, experimental kidney and associated cardiac disease. Am. J. Pathol. 2017;187:80–90. doi: 10.1016/j.ajpath.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 210.Cai J, et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2020;97:106–118. doi: 10.1016/j.kint.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 211.Lin JR, et al. Suppression of endothelial-to-mesenchymal transition by SIRT (Sirtuin) 3 alleviated the development of hypertensive renal injury. Hypertension. 2018;72:350–360. doi: 10.1161/HYPERTENSIONAHA.118.10482. [DOI] [PubMed] [Google Scholar]
- 212.Li P, et al. SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discov. 2021;7:59. doi: 10.1038/s41420-021-00443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He FF, et al. Inhibition of SIRT2 alleviates fibroblast activation and renal tubulointerstitial fibrosis via MDM2. Cell Physiol. Biochem. 2018;46:451–460. doi: 10.1159/000488613. [DOI] [PubMed] [Google Scholar]
- 214.Chen F, et al. Histone deacetylase 3 aberration inhibits Klotho transcription and promotes renal fibrosis. Cell Death Differ. 2021;28:1001–1012. doi: 10.1038/s41418-020-00631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Choi SY, et al. Tubastatin A suppresses renal fibrosis via regulation of epigenetic histone modification and Smad3-dependent fibrotic genes. Vasc. Pharm. 2015;72:130–140. doi: 10.1016/j.vph.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 216.Chen X, et al. Histone deacetylase 6 inhibition mitigates renal fibrosis by suppressing TGF-β and EGFR signaling pathways in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2020;319:F1003–F1014. doi: 10.1152/ajprenal.00261.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mao L, et al. Histone deacetylase 11 contributes to renal fibrosis by repressing KLF15 transcription. Front. Cell Dev. Biol. 2020;8:235. doi: 10.3389/fcell.2020.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discov. 2018;8:24–36. doi: 10.1158/2159-8290.CD-17-0605. [DOI] [PubMed] [Google Scholar]
- 219.Morgado-Pascual JL, Rayego-Mateos S, Tejedor L, Suarez-Alvarez B, Ruiz-Ortega M. Bromodomain and extraterminal proteins as novel epigenetic targets for renal diseases. Front Pharm. 2019;10:1315. doi: 10.3389/fphar.2019.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang X, et al. Bromodomain-containing protein 4 contributes to renal fibrosis through the induction of epithelial-mesenchymal transition. Exp. Cell Res. 2019;383:111507. doi: 10.1016/j.yexcr.2019.111507. [DOI] [PubMed] [Google Scholar]
- 221.Zhou B, et al. Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-β-mediated Nox4 expression. Redox Biol. 2017;11:390–402. doi: 10.1016/j.redox.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tao S, et al. Discovery of indol-6-yl-pyrrolo[2,3-c]pyridin-7-one derivatives as bromodomain-containing protein 4 (BRD4) inhibitors for the treatment of kidney fibrosis. Eur. J. Med Chem. 2022;231:114153. doi: 10.1016/j.ejmech.2022.114153. [DOI] [PubMed] [Google Scholar]
- 223.Xiong C, et al. Pharmacologic targeting of BET proteins attenuates hyperuricemic nephropathy in rats. Front Pharm. 2021;12:636154. doi: 10.3389/fphar.2021.636154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sun HJ, et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 2021;38:101813. doi: 10.1016/j.redox.2020.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Narita T, Weinert BT, Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019;20:156–174. doi: 10.1038/s41580-018-0081-3. [DOI] [PubMed] [Google Scholar]
- 226.Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- 227.Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol. Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhu K, et al. NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Radic. Biol. Med. 2015;83:21–30. doi: 10.1016/j.freeradbiomed.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 230.Digby JLM, Vanichapol T, Przepiorski A, Davidson AJ, Sander V. Evaluation of cisplatin-induced injury in human kidney organoids. Am. J. Physiol. Ren. Physiol. 2020;318:F971–F978. doi: 10.1152/ajprenal.00597.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Martinez-Moreno JM, et al. The contribution of histone crotonylation to tissue health and disease: focus on kidney health. Front. Pharm. 2020;11:393. doi: 10.3389/fphar.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang L, Wang B, Ma L, Fu P. An update of long-noncoding RNAs in acute kidney injury. Front. Physiol. 2022;13:849403. doi: 10.3389/fphys.2022.849403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu Z, et al. Non-coding RNAs in kidney injury and repair. Am. J. Physiol. Cell Physiol. 2019;317:C177–C188. doi: 10.1152/ajpcell.00048.2019. [DOI] [PubMed] [Google Scholar]
- 234.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 235.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev. Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.van Zonneveld AJ, Kölling M, Bijkerk R, Lorenzen JM. Circular RNAs in kidney disease and cancer. Nat. Rev. Nephrol. 2021;17:814–826. doi: 10.1038/s41581-021-00465-9. [DOI] [PubMed] [Google Scholar]
- 237.Tsagakis I, Douka K, Birds I, Aspden JL. Long non-coding RNAs in development and disease: conservation to mechanisms. J. Pathol. 2020;250:480–495. doi: 10.1002/path.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xia W, et al. Long non-coding RNA: an emerging contributor and potential therapeutic target in renal fibrosis. Front. Genet. 2021;12:682904. doi: 10.3389/fgene.2021.682904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol. 2017;28:2838–2844. doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hou FF, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 241.Hsu TW, et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174:347–354. doi: 10.1001/jamainternmed.2013.12700. [DOI] [PubMed] [Google Scholar]
- 242.Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 243.Wheeler DC, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 244.Gerstein HC, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 245.Mann JFE, et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 246.Heerspink HJL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 247.Torres VE, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 248.Bakris GL, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 249.Miao J, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18:e13004. doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li J, et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight. 2020;5:e129034. doi: 10.1172/jci.insight.129034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Spires D, et al. Prevention of the progression of renal injury in diabetic rodent models with preexisting renal disease with chronic endothelin A receptor blockade. Am. J. Physiol. Ren. Physiol. 2018;315:F977–F985. doi: 10.1152/ajprenal.00182.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Vincenti F, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int. Rep. 2017;2:800–810. doi: 10.1016/j.ekir.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.King TE, Jr, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 254.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 255.Sharma K, et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Adler SG, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pergola PE, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 258.Warady BA, et al. Effects of bardoxolone methyl in alport syndrome. Clin. J. Am. Soc. Nephrol. 2022;17:1763–1774. doi: 10.2215/CJN.02400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.de Zeeuw D, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chin MP, et al. Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am. J. Nephrol. 2018;47:40–47. doi: 10.1159/000486398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ray KK, et al. Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: a randomized clinical Trial. JAMA. 2020;323:1565–1573. doi: 10.1001/jama.2020.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kalantar-Zadeh K, et al. Effect of apabetalone on cardiovascular events in diabetes, CKD, and recent acute coronary syndrome: results from the BETonMACE randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2021;16:705–716. doi: 10.2215/CJN.16751020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kulikowski E, et al. Apabetalone mediated epigenetic modulation is associated with favorable kidney function and alkaline phosphatase profile in patients with chronic kidney disease. Kidney Blood Press Res. 2018;43:449–457. doi: 10.1159/000488257. [DOI] [PubMed] [Google Scholar]
- 264.Nangia-Makker P, Hogan V, Balan V, Raz A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J. Biol. Chem. 2022;298:102622. doi: 10.1016/j.jbc.2022.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rebholz CM, et al. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. 2018;93:252–259. doi: 10.1016/j.kint.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van Niel G, et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022;23:369–382. doi: 10.1038/s41580-022-00460-3. [DOI] [PubMed] [Google Scholar]
- 267.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 268.Wang J, et al. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy. Biomaterials. 2021;276:121056. doi: 10.1016/j.biomaterials.2021.121056. [DOI] [PubMed] [Google Scholar]
- 269.Zhong Y, Menon MC, Deng Y, Chen Y, He JC. Recent advances in traditional chinese medicine for kidney disease. Am. J. Kidney Dis. 2015;66:513–522. doi: 10.1053/j.ajkd.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 270.Yang L, Wang B, Ma L, Fu P. Traditional Chinese herbs and natural products in hyperuricemia-induced chronic kidney disease. Front. Pharm. 2022;13:971032. doi: 10.3389/fphar.2022.971032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Guo L, et al. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017;8:878. doi: 10.1038/s41467-017-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhong Y, et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 2019;10:4523. doi: 10.1038/s41467-019-12433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hong Q, et al. Modulation of transforming growth factor-β-induced kidney fibrosis by leucine-rich α-2 glycoprotein-1. Kidney Int. 2022;101:299–314. doi: 10.1016/j.kint.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Meng XM, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J. Am. Soc. Nephrol. 2010;21:1477–1487. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jung SW, et al. Midbody plays an active role in fibroblast-myofibroblast transition by mediating TGF-β signaling. FASEB J. 2022;36:e22272. doi: 10.1096/fj.202101613R. [DOI] [PubMed] [Google Scholar]
- 276.Loeffler I, Hopfer U, Koczan D, Wolf G. Type VIII collagen modulates TGF-β1-induced proliferation of mesangial cells. J. Am. Soc. Nephrol. 2011;22:649–663. doi: 10.1681/ASN.2010010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Li H, et al. Upregulation of HER2 in tubular epithelial cell drives fibroblast activation and renal fibrosis. Kidney Int. 2019;96:674–688. doi: 10.1016/j.kint.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 278.Livingston MJ, et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy. 2022;19:256–277. doi: 10.1080/15548627.2022.2072054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Livingston MJ, et al. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy. 2016;12:976–998. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu X, et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 2020;97:1181–1195. doi: 10.1016/j.kint.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 281.Wang M, et al. Exogenous bone marrow derived-putative endothelial progenitor cells attenuate ischemia reperfusion-induced vascular injury and renal fibrosis in mice dependent on pericytes. Theranostics. 2020;10:12144–12157. doi: 10.7150/thno.48562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geng H, et al. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am. J. Pathol. 2012;181:1236–1249. doi: 10.1016/j.ajpath.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Johnson BG, et al. Connective tissue growth factor domain 4 amplifies fibrotic kidney disease through activation of LDL receptor-related protein 6. J. Am. Soc. Nephrol. 2017;28:1769–1782. doi: 10.1681/ASN.2016080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sakai N, et al. Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor. Kidney Int. 2017;91:628–641. doi: 10.1016/j.kint.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lu Y, et al. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol. Cells. 2011;31:225–230. doi: 10.1007/s10059-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bienaime F, et al. Stat3 controls tubulointerstitial communication during CKD. J. Am. Soc. Nephrol. 2016;27:3690–3705. doi: 10.1681/ASN.2015091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Xu H, et al. Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis. 2022;13:511. doi: 10.1038/s41419-022-04910-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Doke T, et al. Transcriptome-wide association analysis identifies DACH1 as a kidney disease risk gene that contributes to fibrosis. J. Clin. Invest. 2021;131:e141801. doi: 10.1172/JCI141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Liu L, et al. Blocking the histone lysine 79 methyltransferase DOT1L alleviates renal fibrosis through inhibition of renal fibroblast activation and epithelial-mesenchymal transition. FASEB J. 2019;33:11941–11958. doi: 10.1096/fj.201801861R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yang C, Chen Z, Yu H, Liu X. Inhibition of disruptor of telomeric silencing 1-like alleviated renal ischemia and reperfusion injury-induced fibrosis by blocking PI3K/AKT-mediated oxidative stress. Drug Des. Devel Ther. 2019;13:4375–4387. doi: 10.2147/DDDT.S224909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Irifuku T, et al. Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney Int. 2016;89:147–157. doi: 10.1038/ki.2015.291. [DOI] [PubMed] [Google Scholar]
- 292.Liu L, et al. Critical roles of SMYD2 lysine methyltransferase in mediating renal fibroblast activation and kidney fibrosis. FASEB J. 2021;35:e21715. doi: 10.1096/fj.202000554RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu B, et al. Pharmacological inhibition of SETD7 by PFI-2 attenuates renal fibrosis following folic acid and obstruction injury. Eur. J. Pharm. 2021;901:174097. doi: 10.1016/j.ejphar.2021.174097. [DOI] [PubMed] [Google Scholar]
- 294.Sasaki K, et al. Inhibition of SET domain-containing lysine methyltransferase 7/9 ameliorates renal fibrosis. J. Am. Soc. Nephrol. 2016;27:203–215. doi: 10.1681/ASN.2014090850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Goru SK, et al. Histone H2AK119 and H2BK120 mono-ubiquitination modulate SET7/9 and SUV39H1 in type 1 diabetes-induced renal fibrosis. Biochem. J. 2016;473:3937–3949. doi: 10.1042/BCJ20160595. [DOI] [PubMed] [Google Scholar]
- 296.Zhou X, et al. Enhancer of Zeste homolog 2 inhibition attenuates renal fibrosis by maintaining smad7 and phosphatase and tensin homolog expression. J. Am. Soc. Nephrol. 2016;27:2092–2108. doi: 10.1681/ASN.2015040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shi Y, et al. Blockade of enhancer of zeste homolog 2 alleviates renal injury associated with hyperuricemia. Am. J. Physiol. Ren. Physiol. 2019;316:F488–F505. doi: 10.1152/ajprenal.00234.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tao S, et al. Gambogenic acid alleviates kidney fibrosis via epigenetic inhibition of EZH2 to regulate Smad7-dependent mechanism. Phytomedicine. 2022;106:154390. doi: 10.1016/j.phymed.2022.154390. [DOI] [PubMed] [Google Scholar]
- 299.Zhu Y, Yu C, Zhuang S. Protein arginine methyltransferase 1 mediates renal fibroblast activation and fibrogenesis through activation of Smad3 signaling. Am. J. Physiol. Ren. Physiol. 2020;318:F375–F387. doi: 10.1152/ajprenal.00487.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shimoda H, et al. Inhibition of the H3K4 methyltransferase MLL1/WDR5 complex attenuates renal senescence in ischemia reperfusion mice by reduction of p16(INK4a) Kidney Int. 2019;96:1162–1175. doi: 10.1016/j.kint.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 301.Zhao S, et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics. 2021;11:8660–8673. doi: 10.7150/thno.62820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kölling M, et al. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol. Ther. 2017;25:165–180. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chuppa S, et al. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4. Kidney Int. 2018;93:375–389. doi: 10.1016/j.kint.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wang J, et al. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem. Biophys. 2013;67:537–546. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 305.McClelland AD, et al. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. (Lond.) 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 306.Liu E, et al. METTL3/N6-methyladenosine/ miR-21-5p promotes obstructive renal fibrosis by regulating inflammation through SPRY1/ERK/NF-κB pathway activation. J. Cell Mol. Med. 2021;25:7660–7674. doi: 10.1111/jcmm.16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chung KW, et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 2018;29:1223–1237. doi: 10.1681/ASN.2017070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Price NL, et al. Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight. 2019;4:e131102. doi: 10.1172/jci.insight.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liu Y, et al. MicroRNA-34a promotes renal fibrosis by downregulation of klotho in tubular epithelial cells. Mol. Ther. 2019;27:1051–1065. doi: 10.1016/j.ymthe.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhou Y, et al. Secreted fibroblast-derived miR-34a induces tubular cell apoptosis in fibrotic kidney. J. Cell Sci. 2014;127:4494–4506. doi: 10.1242/jcs.155523. [DOI] [PubMed] [Google Scholar]
- 311.Bijkerk R, et al. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int. 2016;89:1268–1280. doi: 10.1016/j.kint.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 312.Zhou H, et al. miR-150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J. Am. Soc. Nephrol. 2013;24:1073–1087. doi: 10.1681/ASN.2012080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Overstreet JM, Gifford CC, Tang J, Higgins PJ, Samarakoon R. Emerging role of tumor suppressor p53 in acute and chronic kidney diseases. Cell Mol. Life Sci. 2022;79:474. doi: 10.1007/s00018-022-04505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Putta S, et al. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wang B, et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes. 2010;59:1794–1802. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jia Y, et al. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp. Mol. Med. 2018;50:1–13. doi: 10.1038/s12276-018-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kato M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ma Z, et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J. Clin. Invest. 2020;130:5011–5026. doi: 10.1172/JCI135536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bera A, et al. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am. J. Physiol. Cell Physiol. 2017;313:C430–C447. doi: 10.1152/ajpcell.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liu M, et al. Hypoxia-induced activation of Twist/miR-214/E-cadherin axis promotes renal tubular epithelial cell mesenchymal transition and renal fibrosis. Biochem. Biophys. Res. Commun. 2018;495:2324–2330. doi: 10.1016/j.bbrc.2017.12.130. [DOI] [PubMed] [Google Scholar]
- 321.Bai M, et al. MicroRNA-214 promotes chronic kidney disease by disrupting mitochondrial oxidative phosphorylation. Kidney Int. 2019;95:1389–1404. doi: 10.1016/j.kint.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 322.Macconi D, et al. MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J. Am. Soc. Nephrol. 2012;23:1496–1505. doi: 10.1681/ASN.2011121144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fang Y, et al. miR-382 contributes to renal tubulointerstitial fibrosis by downregulating HSPD1. Oxid. Med. Cell Longev. 2017;2017:4708516. doi: 10.1155/2017/4708516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wang X, et al. Upregulation of miR-382 contributes to renal fibrosis secondary to aristolochic acid-induced kidney injury via PTEN signaling pathway. Cell Death Dis. 2020;11:620. doi: 10.1038/s41419-020-02876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wang X, et al. MicroRNA-382 promotes M2-like macrophage via the SIRP-α/STAT3 signaling pathway in aristolochic acid-induced renal fibrosis. Front. Immunol. 2022;13:864984. doi: 10.3389/fimmu.2022.864984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kriegel AJ, et al. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol. Genomics. 2012;44:259–267. doi: 10.1152/physiolgenomics.00173.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kriegel AJ, et al. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res. 2010;38:8338–8347. doi: 10.1093/nar/gkq718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Li R, et al. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int. 2013;84:1129–1144. doi: 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 329.Wang B, et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012;23:252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Fang Y, et al. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-α activation. Am. J. Physiol. Ren. Physiol. 2013;304:F1274–F1282. doi: 10.1152/ajprenal.00287.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lin CL, et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014;25:1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wang H, et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol. Ther. 2019;27:571–583. doi: 10.1016/j.ymthe.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hu H, et al. miR-29b regulates Ang II-induced EMT of rat renal tubular epithelial cells via targeting PI3K/AKT signaling pathway. Int. J. Mol. Med. 2018;42:453–460. doi: 10.3892/ijmm.2018.3579. [DOI] [PubMed] [Google Scholar]
- 334.Li H, et al. TGF-β-mediated upregulation of Sox9 in fibroblast promotes renal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:520–532. doi: 10.1016/j.bbadis.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 335.Wang J, et al. Downregulation of miR-30c promotes renal fibrosis by target CTGF in diabetic nephropathy. J. Diabetes Complicat. 2016;30:406–414. doi: 10.1016/j.jdiacomp.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 336.Cao G, et al. Schisandrin B attenuates renal fibrosis via miR-30e-mediated inhibition of EMT. Toxicol. Appl. Pharm. 2019;385:114769. doi: 10.1016/j.taap.2019.114769. [DOI] [PubMed] [Google Scholar]
- 337.Zhang X, Yang Z, Heng Y, Miao C. MicroRNA‑181 exerts an inhibitory role during renal fibrosis by targeting early growth response factor‑1 and attenuating the expression of profibrotic markers. Mol. Med. Rep. 2019;19:3305–3313. doi: 10.3892/mmr.2019.9964. [DOI] [PubMed] [Google Scholar]
- 338.Oba S, et al. miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One. 2010;5:e13614. doi: 10.1371/journal.pone.0013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hajarnis S, et al. Suppression of microRNA activity in kidney collecting ducts induces partial loss of epithelial phenotype and renal fibrosis. J. Am. Soc. Nephrol. 2018;29:518–531. doi: 10.1681/ASN.2017030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wu H, et al. C66 ameliorates diabetic nephropathy in mice by both upregulating NRF2 function via increase in miR-200a and inhibiting miR-21. Diabetologia. 2016;59:1558–1568. doi: 10.1007/s00125-016-3958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cheng Y, et al. Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J. Am. Soc. Nephrol. 2020;31:1539–1554. doi: 10.1681/ASN.2019101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chen SJ, et al. miR-204 regulates epithelial-mesenchymal transition by targeting SP1 in the tubular epithelial cells after acute kidney injury induced by ischemia-reperfusion. Oncol. Rep. 2017;37:1148–1158. doi: 10.3892/or.2016.5294. [DOI] [PubMed] [Google Scholar]
- 343.Park JT, et al. Repression of let-7 by transforming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am. J. Physiol. Ren. Physiol. 2014;307:F1390–F1403. doi: 10.1152/ajprenal.00458.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liu J, Zhu L, Xie GL, Bao JF, Yu Q. Let-7 miRNAs modulate the activation of NF-κB by targeting TNFAIP3 and are involved in the pathogenesis of lupus nephritis. PLoS One. 2015;10:e0121256. doi: 10.1371/journal.pone.0121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wu T, et al. Urinary angiostatin–a novel putative marker of renal pathology chronicity in lupus nephritis. Mol. Cell Proteom. 2013;12:1170–1179. doi: 10.1074/mcp.M112.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Genovese F, et al. Collagen type III and VI remodeling biomarkers are associated with kidney fibrosis in lupus nephritis. Kidney. 2021;2:1473–1481. doi: 10.34067/KID.0001132021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Huang J, et al. Complement activation profile of patients with primary focal segmental glomerulosclerosis. PLoS One. 2020;15:e0234934. doi: 10.1371/journal.pone.0234934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Feng ST, et al. Urinary sediment CCL5 messenger RNA as a potential prognostic biomarker of diabetic nephropathy. Clin. Kidney J. 2022;15:534–544. doi: 10.1093/ckj/sfab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kim J, et al. Clinical and histological significance of urinary CD11c(+) macrophages in lupus nephritis. Arthritis Res. Ther. 2020;22:173. doi: 10.1186/s13075-020-02265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Schmidt IM, et al. Cadherin-11, Sparc-related modular calcium binding protein-2, and pigment epithelium-derived factor are promising non-invasive biomarkers of kidney fibrosis. Kidney Int. 2021;100:672–683. doi: 10.1016/j.kint.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lee YH, et al. Urinary chemokine C-X-C motif ligand 16 and endostatin as predictors of tubulointerstitial fibrosis in patients with advanced diabetic kidney disease. Nephrol. Dial. Transplant. 2021;36:295–305. doi: 10.1093/ndt/gfz168. [DOI] [PubMed] [Google Scholar]
- 352.Chen J, et al. Urinary DcR2 is a novel biomarker for tubulointerstitial injury in patients with diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2017;313:F273–F281. doi: 10.1152/ajprenal.00689.2016. [DOI] [PubMed] [Google Scholar]
- 353.Chen J, et al. DCR2, a cellular senescent molecule, is a novel marker for assessing tubulointerstitial fibrosis in patients with immunoglobulin A nephropathy. Kidney Blood Press Res. 2019;44:1063–1074. doi: 10.1159/000502233. [DOI] [PubMed] [Google Scholar]
- 354.Zewinger S, et al. Dickkopf-3 (DKK3) in urine identifies patients with short-term risk of eGFR loss. J. Am. Soc. Nephrol. 2018;29:2722–2733. doi: 10.1681/ASN.2018040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Schunk SJ, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394:488–496. doi: 10.1016/S0140-6736(19)30769-X. [DOI] [PubMed] [Google Scholar]
- 356.Sciascia S, et al. Dickkopf homolog 3 (DKK3) as a prognostic marker in lupus nephritis: a prospective monocentric experience. J. Clin. Med. 2022;11:2977. doi: 10.3390/jcm11112977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ju W, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci. Transl. Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Xu Y, Xie Y, Shao X, Ni Z, Mou S. L-FABP: a novel biomarker of kidney disease. Clin. Chim. Acta. 2015;445:85–90. doi: 10.1016/j.cca.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 359.Wang H, et al. Urinary fibrinogen as a predictor of progression of CKD. Clin. J. Am. Soc. Nephrol. 2017;12:1922–1929. doi: 10.2215/CJN.01360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Luo R, et al. Plasma fractalkine levels are associated with renal inflammation and outcomes in immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2019;34:1549–1558. doi: 10.1093/ndt/gfy169. [DOI] [PubMed] [Google Scholar]
- 361.Ou SM, et al. Identification of galectin-3 as potential biomarkers for renal fibrosis by RNA-sequencing and clinicopathologic findings of kidney biopsy. Front. Med. 2021;8:748225. doi: 10.3389/fmed.2021.748225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 363.Schmidt IM, et al. Circulating plasma biomarkers in biopsy-confirmed kidney disease. Clin. J. Am. Soc. Nephrol. 2022;17:27–37. doi: 10.2215/CJN.09380721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhang XQ, et al. Serum lysyl oxidase is a potential diagnostic biomarker for kidney fibrosis. Am. J. Nephrol. 2020;51:907–918. doi: 10.1159/000509381. [DOI] [PubMed] [Google Scholar]
- 365.Zhou D, et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J. Am. Soc. Nephrol. 2017;28:598–611. doi: 10.1681/ASN.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.An Y, et al. Increased urinary miR-196a level predicts the progression of renal injury in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2020;35:1009–1016. doi: 10.1093/ndt/gfy326. [DOI] [PubMed] [Google Scholar]
- 367.Zhang C, et al. Urinary miR-196a predicts disease progression in patients with chronic kidney disease. J. Transl. Med. 2018;16:91. doi: 10.1186/s12967-018-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Puthumana J, et al. Biomarkers of inflammation and repair in kidney disease progression. J. Clin. Invest. 2021;131:e139927. doi: 10.1172/JCI139927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wei Z, et al. Urinary mitochondrial DNA level as a biomarker of tissue injury in non-diabetic chronic kidney diseases. BMC Nephrol. 2018;19:367. doi: 10.1186/s12882-018-1178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wei PZ, et al. Urinary mitochondrial DNA level is an indicator of intra-renal mitochondrial depletion and renal scarring in diabetic nephropathy. Nephrol. Dial. Transplant. 2018;33:784–788. doi: 10.1093/ndt/gfx339. [DOI] [PubMed] [Google Scholar]
- 371.Sun IO, et al. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension. 2018;72:1180–1188. doi: 10.1161/HYPERTENSIONAHA.118.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Cho NJ, et al. Soluble klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS One. 2018;13:e0194617. doi: 10.1371/journal.pone.0194617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Drew DA, et al. Association between soluble klotho and change in kidney function: the health aging and body composition study. J. Am. Soc. Nephrol. 2017;28:1859–1866. doi: 10.1681/ASN.2016080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Lupușoru G, et al. Serum soluble urokinase plasminogen activator receptor as a potential biomarker of renal impairment severity in diabetic nephropathy. Diabetes Res. Clin. Pract. 2021;182:109116. doi: 10.1016/j.diabres.2021.109116. [DOI] [PubMed] [Google Scholar]
- 375.Soliman S, et al. Urine angiostatin and VCAM-1 surpass conventional metrics in predicting elevated renal pathology activity indices in lupus nephritis. Int. J. Rheum. Dis. 2017;20:1714–1727. doi: 10.1111/1756-185X.13197. [DOI] [PubMed] [Google Scholar]
- 376.Jia Y, Xu H, Yu Q, Tan L, Xiong Z. Identification and verification of vascular cell adhesion protein 1 as an immune-related hub gene associated with the tubulointerstitial injury in diabetic kidney disease. Bioengineered. 2021;12:6655–6673. doi: 10.1080/21655979.2021.1976540. [DOI] [PMC free article] [PubMed] [Google Scholar]