Summary
Background
Physical sequelae related to multi-drug resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are emerging and under-recognised global challenges. This systematic review and meta-analysis aimed to quantify the prevalence and the types of long-term physical sequelae associated with patients treated for MDR- and XDR-TB.
Methods
We systematically searched CINAHL (EBSCO), MEDLINE (via Ovid), Embase, Scopus, and Web of Science from inception through to July 1, 2022, and the last search was updated to January 23, 2023. We included studies reporting physical sequelae associated with all forms of drug-resistant TB, including rifampicin-resistant TB (RR-TB), MDR-TB, Pre-XDR-TB, and XDR-TB. The primary outcome of interest was long-term physical sequelae. Meta-analysis was conducted using a random-effect model to estimate the pooled proportion of physical sequelae. The sources of heterogeneity were explored through meta-regression using study characteristics as covariates. The research protocol was registered in PROSPERO (CRD42021250909).
Findings
From 3047 unique publications identified, 66 studies consisting of 37,380 patients conducted in 30 different countries were included in the meta-analysis. The overall pooled estimate was 44.4% (95% Confidence Interval (CI): 36.7–52.1) for respiratory sequelae, 26.7% (95% CI: 23.85–29.7) for hearing sequelae, 10.1% (95% CI: 7.0–13.2) for musculoskeletal sequelae, 8.4% (95% CI: 6.5–10.3) for neurological sequelae, 8.1% (95% CI: 6.3–10.0) for renal sequelae, 7.3% (95% CI: 5.1–9.4) for hepatic sequelae, and 4.5% (95% CI: 2.7–6.3) for visual sequelae. There was substantial heterogeneity in the estimates. The stratified analysis showed that the pooled prevalence of hearing sequelae was 26.6% (95% CI: 12.3–40.9), neurological sequelae was 31.5% (95% CI: 5.5–57.5), and musculoskeletal sequelae were 21.5% (95% CI: 9.9–33.1) for patients with XDR-TB, which were higher than the pooled prevalence of sequelae among patients with MDR-TB. Respiratory sequelae were the highest in low-income countries (59.3%) and after completion of MDR-TB treatment (57.7%).
Interpretation
This systematic review found that long-term physical sequelae such as respiratory, hearing, musculoskeletal, neurological, renal, hepatic, and visual sequelae were common among survivors of MDR- and XDR-TB. There was a significant difference in the prevalence of sequelae between patients with MDR- and XDR-TB. Post-MDR- and XDR-TB treatment surveillance for adverse outcomes needs to be incorporated into the current programmatic management of MDR-TB to enable early detection and prevention of post-treatment sequelae.
Funding
Australian National Health and Medical Research Council, through an Emerging Leadership Investigator grant, and the Curtin University Higher Degree Research scholarship.
Keywords: Multidrug-resistant tuberculosis, Extensively drug-resistant tuberculosis, Physical sequelae, Systematic review, Meta-analysis
Research in context.
Evidence before this study
The burden of physical sequelae among Multidrug-Resistant Tuberculosis (MDR-TB) and Extensively Drug-Resistant Tuberculosis (XDR-TB) is expected to be higher than the burden from drug-susceptible tuberculosis (DS-TB) due to the high toxicity and longer duration of MDR- and XDR-TB treatment. We performed systematic searches in CINHAL (EBSCO), MEDLINE (via OVID), Embase, Scopus, and Web of Science databases from inception until July 01, 2022, without language restrictions using keywords related to “MDR-TB”, “XDR-TB” and “sequelae”. One meta-analysis combined with 131 studies showed that the global pooled prevalence of long-term disabilities among patients with DS-TB was 15–60%. Another meta-analysis reported the prevalence of mental health disorders, social stressors, and health-related quality of life in patients with MDR-TB. However, we did not identify any systematic review and meta-analysis that quantified the burden of long-term physical sequelae among patients with MDR-TB and XDR-TB only.
Added value of this study
The findings of our study showed that long-term physical sequelae (i.e., respiratory, hearing, musculoskeletal, neurological, renal, hepatic, and visual sequelae) were frequent among survivors of MDR- and XDR-TB. We found that the risk of developing physical sequelae was much higher among patients with XDR-TB than among patients with MDR-TB.
Implications of all the available evidence
Our study findings highlight the prevalence of physical sequelae among survivors of MDR- and XDR-TB. The strong relationship between the burden of long-term physical sequelae and the timing after completion of treatment indicates the need for incorporating post-MDR- and XDR-TB treatment surveillance into the current programmatic management of MDR-TB.
Introduction
Globally, Multidrug-resistant tuberculosis (MDR-TB) and Extensively Drug-Resistant Tuberculosis (XDR-TB) pose a major threat to human health. Patients treated with MDR/XDR-TB are at higher risk of long-term sequelae, which arise because of the disease process or side effects related to the use of second-line drugs.1 For instance, respiratory sequelae are mainly caused by the progression of the disease process.2,3 On the other hand, hearing sequelae is mainly drug-related sequelae, particularly to aminoglycoside agents such as Kanamycin, Amikacin, and Capreomycin.4 One challenge to tackling MDR/XDR-TB sequelae is a lack of understanding of the burden of the problem, as there is no routine post-treatment follow-up done as part of the surveillance system, and data are sparse.5 The current World Health Organization (WHO) guidelines for MDR-TB management define ‘treatment success’ using microbiological outcomes and survival only. Moreover, MDR/XDR-TB-associated morbidity remains solely focused on the period before and during treatment. The global End-TB strategies neglect post-treatment care and a lack of follow-up to TB or MDR-TB survivors after treatment completion.
The treatment of MDR/XDR-TB requires the use of second-line TB medicines, which provided for longer than drug-susceptible (DS)-TB (nine months to two years for MDR-TB, compared to six to nine months) and include medicines that cause serious adverse events. Therefore, the burden of physical sequelae is thought to be higher in patients with MDR- and XDR-TB than in patients with DS-TB,6 although this has not been empirically quantified. Physical sequelae related to MDR/XDR-TB also vary according to the affected body site. For example, people with a history of pulmonary MDR-TB would suffer from a range of long-lasting respiratory sequelae: Chronic Obstructive Pulmonary Disease (COPD), bronchiectasis, chronic pulmonary aspergillosis, pulmonary hypertension, and pulmonary fibrosis.7, 8, 9, 10, 11 Spinal MDR-TB can result in paraparesis and quadriparesis that occurs due to spinal deformity and damage to the neural structures, resulting in permanent physical sequelae.12,13
Long-term sequelae increase the risk of permanent disability, premature mortality, ongoing stigma, and poor quality of life.14 Previous studies demonstrated that the prevalence of long-term physical sequelae: respiratory, visual, neurological, and hearing sequelae, and mental health disorders were frequently reported among patients with DS-TB.15,16 Studies have also estimated end-of-treatment outcomes for MDR/XDR-TB in specific locations.17, 18, 19 There are also systematic reviews and modeling studies investigating the burden of long-term sequelae among DS-TB. A recently published report by Alene et al. on the global burden of TB-related disability showed that the prevalence of respiratory function sequelae among survivors of TB ranged from 15 to 60%.20 However, previous studies were mainly focused on DS-TB and presented single estimates only for MDR-TB, without stratified the analysis by study level characteristics. Moreover, the previous systematic review by Alene et al. did not cover physical sequelae among patients with XDR-TB, and included studies only published in English and reported data from 2000 to 2019. To our knowledge, there has not been a comprehensive study estimating the global prevalence and identifying the common types of physical sequelae following MDR- and XDR-TB treatment.
Quantifying the burden and identifying the type of sequelae arising as a result of MDR/XDR-TB or their medications are essential to inform service providers and policymakers, particularly in countries where MDR- and XDR-TB are common, in the design of effective preventive strategies.21 Therefore, we conducted this systematic review and meta-analysis to estimate the pooled proportion of long-term sequelae and identify the most common types of sequelae among patients with MDR- and XDR-TB.
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.22 The research protocol was registered in PROSPERO (CRD42021250909). We searched CINAHL(EBSCO), MEDLINE (via Ovid), PsycINFO, Embase, and Web of science for articles without restrictions on language, geography, or year of publication from the inception of each database to 01 July 2022. The last search was updated to 23 January 2023 to identify studies published since our initial search. We used relevant Medical Subject Heading (MeSH) headings and keywords for DR-TB and sequelae. Reference lists of included studies were checked for additional studies. Additionally, we searched the grey literature using the Google search engine. Corresponding authors were contacted by email when additional information was required. Our search strategy was developed by consulting a medical librarian. A detailed summary of all search terms can be found in Supplementary Information (Supplementary file: Table S1).
Study selection and eligibility criteria
Studies were eligible for inclusion if they reported any types of physical sequelae among patients with MDR- or XDR-TB. Studies that reported at least one of the primary outcomes (i.e., physical sequelae with a permanent residual effect, which could include respiratory sequelae, radiological abnormalities, hearing sequelae, renal sequelae, hepatic sequelae, blindness, neurological or other abnormalities). Mental health sequelae were not included as they were outside the scope of this study and will be considered in further research. All relevant studies reporting permanent or long-term physical sequelae were included in the study. Studies reporting temporary side effects of medications only were excluded. We also excluded case reports, case series, correspondence, reviews, abstracts, and animal studies. Studies conducted only on drug-susceptible TB or latent TB were also excluded. We used Google Translate for non-English language articles.
Outcomes of the study
The primary outcome of interest was the prevalence of any form of physical sequelae that occurred due to MDR/XDR-TB or its medication, which included but was not limited to hearing sequelae, respiratory sequelae, renal sequelae, neurological sequelae, visual sequelae, hepatic sequelae, and musculoskeletal sequelae. The primary outcome was measured as the proportion of patients with MDR and/or XDR-TB who developed any form of physical sequelae. The definition of each physical sequelae is summarised in the supplementary document (Supplementary file: Table S2).
Data extraction
All articles identified through our search strategies were imported into an EndNote Library (Thomson Reuters, London) and all duplicates were removed. The articles were then exported to Rayyan software for screening. Two reviewers (TYA and HFW) independently screened the titles, abstracts, and full texts to identify eligible studies. Differences were resolved by discussion with a third reviewer (KAA). Data from the included studies were independently extracted by the same two investigators, and information was collected in a Microsoft Excel (version 2014) spreadsheet. We collected information about the characteristics of the studies (the name of the first author, year of publication, country of the study, study setting, and study design), characteristics of the participants (study population such as MDR-TB, XDR-TB, or both, mean or median age, the proportion of participants who were male, sample size, type of MDR/XDR-TB medications, duration of treatments, and comorbidities, including HIV and diabetes mellitus (DM)), and outcomes of interest (type of physical sequelae, and the proportion of people with the sequela).
Quality assessment
The quality of the included studies was assessed by the same two researchers (TYA and HFW). The Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality and risk of bias for cohort and case–control studies and a modified version was used for cross-sectional studies.23 The tools contained criteria for the selection of study groups (4 points), comparability of groups (2 points), and ascertainment of the outcome of interest (3 points). The tools allocated scores ranging from zero to nine, with low (1–4), medium (5–7), and high (8–9) quality groupings.
Data synthesis
Owing to the heterogeneity between studies, meta-analysis was performed using random-effects models for each of the long-term sequelae when two or more studies were available for the outcome of interest. The pooled prevalence and 95% confidence intervals (CI) were presented as summary effect estimates for each type of sequelae. The sources of heterogeneity were explored through meta-regression using study characteristics as covariates. An adjusted odds ratio with a 95% CI was used to interpret the findings. Sub-group analysis was conducted by drug-resistant type, region, the timing of sequelae, study design, countries' income level, and HIV prevalence. We assessed publication bias by visual inspection of funnel plots and Egger's regression test. The analyses were performed by STATA version 17 software. Finally, sensitivity analysis was performed by trimming low-quality studies.
Ethics
Ethical review was not applicable since it was a systematic review and meta-analysis of the data from published literature.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Search results
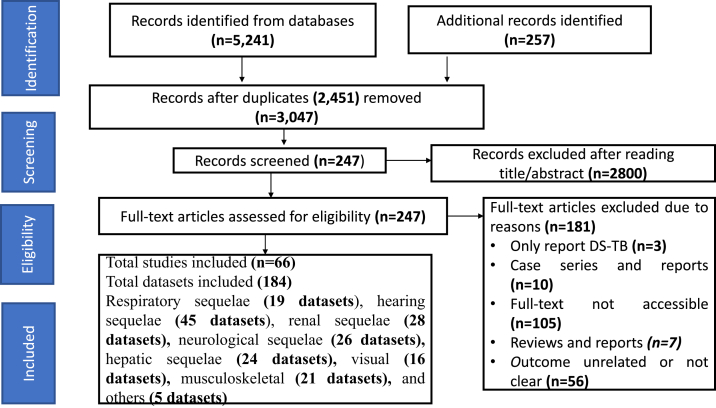
A total of 5498 records were identified from the database searches. After removing duplicates, 3047 unique publications were screened by title and abstract and 247 publications were eligible for full-text review. After a full-text review, 66 publications (comprising 184 datasets)3,7,11,24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86 including data on 37,380 patients were included (Fig. 1).
Fig. 1.
Study profile. DS-TB = drug susceptible tuberculosis.
Characteristics of included studies
The characteristics of the included studies are summarised in Table 1. The studies were conducted in 30 different countries and the data were collected from 1995 to 2022. Among the included studies, 30 (16.3%) studies were from India, 29 (15.8%) were from South Africa, and 15 (8.2%) were from China. The mean/median age of study participants was 35.66 years, with a standard deviation of 9.8 years. Nearly two-thirds (63.3%) of cases were males. Nearly two-thirds (64.1%, n = 118) of studies included MDR-TB only, 14.7% (n = 27) of studies included both MDR- and XDR-TB, 11.4% (n = 21) of studies were unclassified, and 9.8% (n = 18) of studies included XDR-TB only.
Table 1.
Characteristics of included studies.
| First Author | Publication year | Country | Country income | Type of DR-TB | Year of data collection | Study design | Male proportion (%) | Mean age (years) | Sample size (n) |
|---|---|---|---|---|---|---|---|---|---|
| Respiratory sequelae | |||||||||
| Anthony et al. | 2017 | Peru | UMICs | MDR-TB | 2014 | Prospective cohort | 57.6 | 29 | 33 |
| Godoy et al. | 2012 | Brazil | UMICs | MDR-TB | 2008–2012 | Cross-sectional | 67 | 43.7 | 18 |
| Muñoz et al. | 2020 | Multicounty | UMICs | MDR- & XDR-TB | 2014–2019 | Cross-sectional | 59.3 | 43.7 | 27 |
| Murniati et al. | 2020 | Indonesia | LMICs | MDR- & XDR-TB | 2017 | Cross-sectional | 51.7 | 42.3 | 60 |
| Edwin et al. | 2020 | Uganda | LICs | MDR-TB | 2018 | Cross-sectional | 60 | 39 | 95 |
| Sana et al. | 2017 | Pakistan | LMICs | MDR-TB | 2014–2015 | Cohort | 78.5 | 45.5 | 100 |
| Neeta et al. | 2009 | India | LMICs | MDR-TB | 2009 | Cross-sectional | 55.6 | 33.5 | 51 |
| Rupak et al. | 2018 | India | LMICs | MDR-TB | Cross-sectional | 54.3 | 27.6 | 46 | |
| Syed et al. | 2021 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2017 | Retrospective cohort | NA | NA | 19 |
| Sergo et al. | 2019 | Georgia | UMICs | MDR- & XDR-TB | Cross-sectional | 57 | 31 | 58 | |
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1999 | Retrospective cohort | 70.5 | 33 | 44 |
| Piubella et al. | 2014 | Niger | LICs | MDR-TB | 2008–2010 | Prospective cohort | 81.5 | 31 | 65 |
| Nianlan et al. | 2022 | China | UMICs | Unclassified | 2008–2017 | Retrospective cohort | 61.2 | 45.8 | 516 |
| Farouq et al. | 2021 | Nigeria | LMICs | Unclassified | 2018–2020 | Prospective cohort | 87.2 | 32 | 39 |
| Atif et al. | 2017 | India | LMICs | MDR-TB | 2010–2014 | Retrospective cohort | 71.2 | 35.7 | 147 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR | 2008–2016 | Retrospective cohort | 58.1 | 32 | 74 |
| Aziz et al. | 2018 | Indonesia | LMICs | MDR-TB | 2013–2015 | Cross-sectional | 52 | 39.9 | 183 |
| Duo et al. | 2017 | China | UMICs | MDR-TB | 2012–2016 | Retrospective cohort | 65.2 | 39 | 89 |
| Oladimeji et al. | 2022 | Nigeria | LMICs | MDR- & XDR | 2010–2016 | Retrospective cohort | 66.93 | NA | 2555 |
| Hearing impairment | |||||||||
| Sana et al. | 2017 | Pakistan | LMICs | MDR-TB | 2014–2015 | Cohort | 78.5 | 45.5 | 100 |
| Sagwa et al. | 2016 | Nambia | UMICs | MDR-TB | 2015 | Cross-sectional | 56 | 45.5 | 36 |
| Delicia et al. | 2016 | South Africa | UMICs | MDR-TB | NA | Cohort | 52 | 34 | 52 |
| Jager et al. | 2001 | Netherlands | HICs | MDR-TB | 1995–2000 | Retrospective cohort | 73.6 | 35.7 | 110 |
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1998 | Retrospective cohort | 70.5 | 33 | 44 |
| Chawangwa et al. | 2014 | Botswana | UMICs | MDR-TB | 2006–2012 | Retrospective cohort | 55 | 38 | 437 |
| Kiran et al. | 2018 | India | LMICs | MDR-TB | 2014–2015 | Retrospective cohort | 66.7 | 32 | 108 |
| Piubella et al. | 2014 | Niger | LICs | MDR-TB | 2008–2010 | Prospective cohort | 81.5 | 31 | 65 |
| Rajendra et al. | 2015 | India | LMICs | MDR-TB | 2009–2010 | Prospective cohort | 69.4 | 29.3 | 98 |
| Lebogang et al. | 2012 | South Africa | UMICs | MDR & XDR | Not reported | Cross-sectional | 49.1 | 33 | 53 |
| Sagwa et al. | 2015 | Namibia | UMICs | MDR-TB | 2004–2014 | Retrospective cohort | 56.1 | 36.5 | 353 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | Retrospective cohort | 53 | 8 | 19 |
| Kathryn et al. | 2016 | South Africa | UMICs | MDR-TB | 2012–2014 | Retrospective cohort | 49 | 35 | 94 |
| Usha et al. | 2020 | India | LMICs | MDR-TB | 2017–2018 | Retrospective cohort | 73.6 | 44.97 | 110 |
| Karen et al. | 2013 | South Africa | UMICs | XDR-TB | 2014–2015 | Retrospective cohort | NA | NA | 115 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Sen et al. | 2020 | China | UMICs | MDR-TB | 1999–2015 | Retrospective cohort | 68.6 | 31.2 | 272 |
| Pelden et al. | 2021 | Bhutan | LMICs | MDR-TB | 2018–2019 | Prospective cohort | 47.6 | 28.3 | 42 |
| Alexander et al. | 2021 | Uzbekistan | LMICs | MDR-TB | 2018–2020 | Retrospective cohort | 70.5 | 45.6 | 45 |
| Olusola et al. | 2017 | Nigeria | LMICs | Unclassified | 2014–2015 | Prospective cohort | 65.9 | 34.5 | 132 |
| Olusola et al. | 2017 | Nigeria | LMICs | Unclassified | 2014–2015 | Prospective cohort | 62.9 | 34.6 | 70 |
| Jonathan et al. | 2020 | South Africa | UMICs | MDR-TB | 2011–2015 | Prospective cohort | 36 | 33 | 174 |
| Amber et al. | 2017 | UK | HICs | MDR & XDR-TB | Retrospective cohort | 68 | 28 | 93 | |
| Muhammad et al. | 2017 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2015 | Retrospective cohort | 53.8 | 37.2 | 80 |
| Parvaneh et al. | 2011 | Iran | LMICs | MDR-TB | 2006–2009 | Retrospective cohort | 55 | 40.6 | 80 |
| Buziashvili et al. | 2019 | Georgia | UMICs | MDR- & XDR-TB | 2010–2012 | Retrospective cohort | 65 | 35.3 | 147 |
| Atif et al. | 2017 | India | LMICs | MDR-TB | 2010–2015 | Retrospective cohort | 71.2 | 35.7 | 147 |
| Safurah et al. | 2021 | India | LMICs | MDR-TB | 2015–2018 | Prospective cohort | 76 | 40.3 | 400 |
| Jing et al. | 2021 | China | UMICs | MDR- & XDR-TB | 2018–2020 | Prospective cohort | 70 | 36 | 1162 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR-TB | 2008–2016 | Retrospective cohort | 58.1 | 32 | 74 |
| Harouna et al. | 2019 | Niger | LICs | MDR-TB | 2008–2013 | Retrospective cohort | 82.5 | 31 | 120 |
| Hong et al. | 2020 | South Africa | UMICs | MDR-TB | 2014–2017 | Prospective cohort | 54 | 35 | 1315 |
| Huerga et al. | 2017 | Kenya | LMICs | MDR-TB | 2006–2012 | Retrospective cohort | 55 | 29 | 169 |
| Petros et al. | 2011 | India | LMICs | MDR-TB | 2007–2011 | Prospective cohort | 56 | 35 | 71 |
| Kalpesh et al. | 2013 | India | LMICs | MDR-TB | 2009–2011 | Prospective cohort | 62 | 31.4 | 130 |
| Innocent et al. | 2020 | DR. Congo | LICs | XDR-TB | 2015–2017 | Retrospective cohort | 56.3 | 32.4 | 32 |
| Kuban et al. | 2015 | Cameroon | LMICs | MDR-TB | 2008–2012 | Prospective cohort | 51.3 | 33.7 | 106 |
| Ronnie et al. | 2020 | Zimbabwe | LMICs | MDR-TB | 2010–2015 | Retrospective cohort | 48.6 | 34 | 473 |
| Nair et al. | 2017 | India | LMICs | MDR-TB | 2009–2011 | Retrospective cohort | 68 | NA | 788 |
| Seda et al. | 2021 | Turkey | UMICs | MDR-TB | 2011–2018 | Retrospective cohort | 73.2 | 75.9 | 13 |
| Nesri et al. | 2020 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Natasha et al. | 2021 | Canada | HICs | MDR- & XDR-TB | 2010–2016 | Retrospective cohort | 55 | 31 | 49 |
| Sagwa et al. | 2012 | Namibia | UMICs | XDR-TB | 2008–2010 | Cross-sectional | 64 | 34.7 | 59 |
| Koirala et al. | 2021 | Nepal | LMICs | MDR-TB | 2018–2020 | Prospective cohort | 71.8 | 34 | 301 |
| Vishal et al. | 2016 | India | LMICs | MDR-TB | 2012–2014 | Prospective cohort | 66.7 | 37.5 | 100 |
| Renal impairment | |||||||||
| Sana et al. | 2017 | Pakistan | LMICs | MDR-TB | 2014–2015 | Cohort | 78.5 | 45.5 | 100 |
| Jager et al. | 2001 | Netherlands | HICs | MDR-TB | 1995–2001 | retrospective cohort | 73.6 | 37.7 | 110 |
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1998 | retrospective cohort | 70.5 | 33 | 44 |
| Rajendra et al. | 2016 | India | LMICs | MDR-TB | 2009–2010 | Prospective cohort | 69.4 | 29.3 | 98 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | retrospective cohort | 53 | 8 | 19 |
| Karen et al. | 2013 | South Africa | UMICs | XDR-TB | 2002–2008 | retrospective cohort | NA | NA | 115 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Aleksande et al. | 2021 | Uzbekistan | LMICs | MDR-TB | 2018–2020 | Retrospective cohort | 70.5 | 45.6 | 45 |
| Ilse et al. | 2020 | South Africa | UMICs | MDR-TB | 2018–2019 | Retrospective cohort | 59.8 | 35 | 62 |
| Jonathan et al. | 2021 | South Africa | UMICs | MDR-TB | 2011–2016 | Prospective cohort | 36 | 33 | 206 |
| Muhammad et al. | 2017 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2015 | Retrospective cohort | 53.8 | 37.2 | 80 |
| Mehari et al. | 2019 | Ethiopia | LICs | MDR- & XDR-TB | 2010–2017 | Retrospective cohort | 56.8 | 28 | 570 |
| Parvaneh et al. | 2011 | Iran | LMICs | MDR-TB | 2006–2009 | Retrospective cohort | 55 | 40.6 | 80 |
| Mariana et al. | 2021 | Georgia | UMICs | MDR-& XDR-TB | 2016–2018 | Retrospective cohort | 78.3 | 39.6 | 73 |
| Buziashvili et al. | 2019 | Georgia | UMICs | MDR- & XDR-TB | 2010–2012 | Retrospective cohort | 65 | 35.3 | 147 |
| Safurah et al. | 2021 | India | LMICs | MDR-TB | 2015–2020 | Prospective cohort | 76 | 40.3 | 400 |
| Jing et al. | 2021 | China | UMICs | MDR- & XDR-TB | 2018–2020 | Prospective cohort | 70 | 36 | 1162 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR-TB | 2008–2016 | Retrospective cohort | 58.1 | 32 | 74 |
| Harouna et al. | 2019 | Niger | LICs | MDR-TB | 2008–2013 | Retrospective cohort | 82.5 | 31 | 120 |
| Hong et al. | 2020 | South Africa | UMICs | MDR-TB | 2014–2017 | Prospective cohort | 54 | 35 | 1313 |
| Huerga et al. | 2017 | Kenya | LMICs | MDR-TB | 2006–2012 | Retrospective cohort | 55 | 29 | 169 |
| Petros et al. | 2011 | India | LMICs | MDR-TB | 2007–2011 | Prospective cohort | 56 | 35 | 71 |
| Jackie et al. | 2019 | South Africa | UMICs | Unclassified | 2015–2016 | Retrospective cohort | 38 | 30 | 32 |
| Nair et al. | 2017 | India | LMICs | MDR-TB | 2009–2011 | Retrospective cohort | 68 | NA | 788 |
| Seda et al. | 2021 | Turkey | UMICs | MDR-TB | 2011–2018 | Retrospective cohort | 73.2 | 75.9 | 13 |
| Nesri et al. | 2020 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Natasha et al. | 2021 | Canada | HICs | MDR-& XDR-TB | 2010–2016 | Retrospective cohort | 55 | 31 | 49 |
| Yimer et al. | 2019 | Ethiopia | LICs | MDR-TB | 2012–2014 | Cross-sectional | 53.6 | 32.7 | 250 |
| Neurological impairment | |||||||||
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1998 | retrospective cohort | 70.5 | 33 | 44 |
| Rajendra et al. | 2016 | India | LMICs | MDR-TB | 2009–2010 | Prospective cohort | 69.4 | 29.3 | 98 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | retrospective cohort | 53 | 8 | 19 |
| Karen et al. | 2013 | South Africa | UMICs | XDR-TB | 2002–2008 | retrospective cohort | NA | NA | 115 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Sen et al. | 2019 | China | UMICs | MDR-TB | 199–2015 | retrospective cohort | 68.6 | 31.2 | 272 |
| Ilse et al. | 2020 | South Africa | UMICs | MDR-TB | 2018–2019 | retrospective cohort | 59.8 | 35 | 62 |
| Muhammad et al. | 2017 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2015 | retrospective cohort | 53.8 | 37.2 | 80 |
| Mehari et al. | 2019 | Ethiopia | LICs | MDR- XDR-TB | 2010–2017 | retrospective cohort | 56.8 | 28 | 570 |
| Parvaneh et al. | 2011 | Iran | LMICs | MDR-TB | 2006–2009 | retrospective cohort | 55 | 40.6 | 80 |
| Mariana et al. | 2021 | Georgia | UMICs | MDR- & XDR-TB | 2016–2020 | retrospective cohort | 78.3 | 39.6 | 73 |
| Farouq et al. | 2021 | Nigeria | LMICs | Unclassified | 2018–2020 | Prospective cohort | 87.2 | 32 | 39 |
| Safurah et al. | 2021 | India | LMICs | MDR-TB | 2015–2019 | Prospective cohort | 76 | 40.3 | 400 |
| Jing et al. | 2021 | China | UMICs | MDR- & XDR-TB | 2018–2020 | Prospective cohort | 70 | 36 | 1162 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR-TB | retrospective cohort | 58.1 | 32 | 74 | |
| Harouna et al. | 2019 | Niger | LICs | MDR-TB | 2008–2013 | retrospective cohort | 82.5 | 31 | 120 |
| Huerga et al. | 2017 | Kenya | LMICs | MDR-TB | 2006–2012 | retrospective cohort | 55 | 29 | 169 |
| Petros et al. | 2011 | India | LMICs | MDR-TB | 2007–2011 | Prospective cohort | 56 | 35 | 71 |
| Kalpesh et al. | 2013 | India | LMICs | MDR-TB | 2009–2011 | Prospective cohort | 62 | 31.4 | 130 |
| Innocent et al. | 2020 | DR. Congo | LICs | XDR-TB | 2015–2017 | retrospective cohort | 56.3 | 32.4 | 32 |
| Ronnie et al. | 2020 | Zimbabwe | LMICs | MDR-TB | 2010–2015 | retrospective cohort | 48.6 | 34 | 473 |
| Olatunde et al. | 2019 | South Africa | UMICs | XDR-TB | 2014–2018 | Prospective cohort | 61.9 | 37 | 63 |
| Seda et al. | 2021 | Turkey | UMICs | MDR-TB | 2011–2018 | retrospective cohort | 73.2 | 75.9 | 13 |
| Nesri et al. | 2020 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Sagwa et al. | 2012 | Namibia | UMICs | MDR- & XDR-TB | 2008–2010 | Cross-sectional | 64 | 34.7 | 59 |
| Yimer et al. | 2019 | Ethiopia | LICs | MDR-TB | 2012–2014 | Cross-sectional | 53.6 | 32.7 | 250 |
| Hepatic impairment | |||||||||
| Sana et al. | 2017 | Pakistan | LMICs | MDR-TB | 2014–2015 | Cohort | 78.5 | 45.5 | 100 |
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1998 | Retrospective cohort | 70.5 | 33 | 44 |
| Kiran et al. | 2018 | India | LMICs | MDR-TB | 2014–2015 | Retrospective cohort | 66.7 | 32 | 108 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | Retrospective cohort | 53 | 8 | 19 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Sen et al. | 2019 | China | UMICs | MDR-TB | 1999–2015 | Retrospective cohort | 68.6 | 31.2 | 272 |
| Aleksandr et al. | 2021 | Uzbekistan | LMICs | MDR-TB | 2018–2020 | Retrospective cohort | 70.5 | 45.6 | 45 |
| Ilse et al. | 2020 | South Africa | UMICs | MDR-TB | 2018–2019 | Retrospective cohort | 59.8 | 35 | 62 |
| Muhammad et al. | 2017 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2015 | Retrospective cohort | 53.8 | 37.2 | 80 |
| Parvaneh et al. | 2011 | Iran | LMICs | MDR-TB | 2006–2009 | Retrospective cohort | 55 | 40.6 | 80 |
| Mariana et al. | 2021 | Georgia | UMICs | MDR- & XDR-TB | 2016–2018 | Retrospective cohort | 78.3 | 39.6 | 73 |
| Atif et al. | 2017 | India | LMICs | MDR-TB | 2010–2016 | Retrospective cohort | 71.2 | 35.7 | 147 |
| Jing et al. | 2021 | China | UMICs | MDR- & XDR-TB | 2018–2020 | Prospective cohort | 70 | 36 | 1162 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR-TB | 2008–2016 | Retrospective cohort | 58.1 | 32 | 74 |
| Kalpesh et al. | 2013 | India | LMICs | MDR-TB | 2009–2011 | Prospective cohort | 62 | 31.4 | 130 |
| Innocent et al. | 2020 | DR. Congo | LICs | XDR-TB | 2015–2017 | Retrospective cohort | 56.3 | 32.4 | 32 |
| Keshavjee et al. | 2013 | India | LMICs | MDR-TB | 2000–2004 | Retrospective cohort | 82 | 34 | 568 |
| Ronnie et al. | 2020 | Zimbabwe | LMICs | MDR-TB | 2010–2015 | Retrospective cohort | 48.6 | 34 | 473 |
| Nair et al. | 2017 | India | LMICs | MDR-TB | 2009–2011 | Retrospective cohort | 68 | NA | 788 |
| Seda et al. | 2021 | Turkey | UMICs | MDR-TB | 2011–2018 | Retrospective cohort | 73.2 | 75.9 | 13 |
| Nesri et al. | 2021 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Sagwa et al. | 2012 | Namibia | UMICs | MDR- & XDR | 2008–2010 | Cross-sectional | 64 | 34.7 | 59 |
| Yimer et al. | 2019 | Ethiopia | LICs | MDR-TB | 2012–2014 | Cross-sectional | 53.6 | 32.7 | 250 |
| Koirala et al. | 2021 | Nepal | LMICs | MDR-TB | 2018–2019 | Prospective cohort | 71.8 | 34 | 301 |
| Visual impairment | |||||||||
| Sana et al. | 2017 | Pakistan | LMICs | MDR-TB | 2014–2015 | Cohort | 78.5 | 45.5 | 100 |
| Geerligs et al. | 2000 | Netherlands | HICs | MDR-TB | 1985–1998 | Retrospective cohort | 70.5 | 33 | 44 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | Retrospective cohort | 53 | 8 | 19 |
| Karen et al. | 2013 | South Africa | UMICs | Unclassified | 2002–2008 | Retrospective cohort | NA | NA | 115 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Aleksandr et al. | 2021 | Uzbekistan | LMICs | MDR-TB | 2018–2020 | Retrospective cohort | 70.5 | 45.6 | 45 |
| Ilse et al. | 2020 | South Africa | UMICs | MDR-TB | 2018–2019 | Retrospective cohort | 59.8 | 35 | 62 |
| Jonathan et al. | 2021 | South Africa | UMICs | MDR-TB | 2011–2018 | Prospective cohort | 36 | 33 | 187 |
| Farouq et al. | 2021 | Nigeria | LMICs | Unclassified | 2018–2020 | Prospective cohort | 87.2 | 32 | 39 |
| Atif et al. | 2017 | India | LMICs | Unclassified | 2010–2013 | Retrospective cohort | 71.2 | 35.7 | 147 |
| Harouna et al. | 2019 | Niger | LICs | MDR-TB | 2008–2013 | Retrospective cohort | 82.5 | 31 | 120 |
| Innocent et al. | 2020 | DR. Congo | LICs | XDR-TB | 2015–2017 | Retrospective cohort | 56.3 | 32.4 | 32 |
| Ronnie et al. | 2020 | Zimbabwe | LMICs | MDR-TB | 2010–2015 | Retrospective cohort | 48.6 | 34 | 473 |
| Seda et al. | 2021 | Turkey | UMICs | MDR-TB | 2011–2018 | Retrospective cohort | 73.2 | 75.9 | 13 |
| Nesri et al. | 2020 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Sagwa et al. | 2012 | Namibia | UMICs | MDR- & XDR-TB | 2008–2010 | Cross-sectional | 64 | 34.7 | 59 |
| Musculoskeletal impairment | |||||||||
| Safurah et al. | 2021 | India | LMICs | MDR-TB | 2015–2021 | Prospective cohort | 76 | 40.3 | 400 |
| Gina et al. | 2019 | Italy | HICs | MDR- & XDR-TB | 2008–2016 | Retrospective cohort | 58.1 | 32 | 74 |
| Kalpesh et al. | 2013 | India | LMICs | MDR-TB | 2009–2011 | Prospective cohort | 62 | 31.4 | 130 |
| Jackie et al. | 2019 | South Africa | UMICs | Unclassified | 2015–2016 | Prospective cohort | 38 | 30 | 32 |
| Innocent et al. | 2020 | DR. Congo | LICs | XDR-TB | 2015–2017 | Retrospective cohort | 56.3 | 32.4 | 32 |
| Olatunde et al. | 2019 | South Africa | UMICs | XDR-TB | 2014–2018 | Prospective cohort | 61.9 | 37 | 63 |
| Nesri et al. | 2020 | South Africa | UMICs | XDR-TB | 2014–2015 | Prospective cohort | 47.7 | 33 | 151 |
| Sagwa et al. | 2012 | Namibia | UMICs | MDR- & XDR-TB | 2008–2010 | Cross-sectional | 64 | 34.7 | 59 |
| Rajendra et al. | 2016 | India | LMICs | MDR-TB | 2009–2010 | Prospective cohort | 69.4 | 29.3 | 98 |
| Piubella et al. | 2014 | Niger | LICs | MDR-TB | 2008–2010 | Prospective cohort | 81.5 | 31 | 65 |
| Hind et al. | 2012 | Lesotho | LMICs | MDR-TB | 2007–2011 | Retrospective cohort | 53 | 8 | 19 |
| Karen et al. | 2013 | South Africa | UMICs | XDR-TB | 2002–2008 | Retrospective cohort | NA | NA | 115 |
| Ilse et al. | 2020 | South Africa | UMICs | MDR-TB | 2018–2019 | Retrospective cohort | 59.8 | 35 | 62 |
| Aleksandr et al. | 2021 | Uzbekistan | LMICs | MDR-TB | 2018–2020 | Retrospective cohort | 70.5 | 45.6 | 45 |
| Yang et al. | 2017 | China | UMICs | MDR-TB | 2009–2016 | Retrospective cohort | 73.2 | 44 | 751 |
| Muhammad et al. | 2017 | Pakistan | LMICs | MDR- & XDR-TB | 2014–2015 | Retrospective cohort | NA | NA | 80 |
| Parvaneh et al. | 2011 | Iran | LMICs | MDR-TB | 2006–2009 | Retrospective cohort | 55 | 40.6 | 80 |
| Farouq et al. | 2021 | Nigeria | LMICs | Unclassified | 2018–2020 | Prospective cohort | 87.2 | 32 | 39 |
| Atif et al. | 2017 | India | LMICs | MDR-TB | 2010–2013 | Retrospective cohort | 71.2 | 35.7 | 147 |
| Huerga et al. | 2017 | Kenya | LMICs | MDR-TB | 2006–2012 | Retrospective cohort | 55 | 29 | 169 |
| Petros et al. | 2011 | India | LMICs | MDR-TB | 2007–2011 | Prospective cohort | 56 | 35 | 71 |
DR.Congo: Democratic Republic of Congo, DR-TB: Drug Resistant Tuberculosis, HICs: High-Income Countries, LICs: Low-Income Countries, LMICs: Lower-Middle-Income Countries, MDR-TB: Multidrug Resistant Tuberculosis, UK: United Kingdom, UMICs: Upper-Middle-Income Countries, and XDR-TB: Extensively Drug-Resistant Tuberculosis.
Approximately half of the studies (46.7%, n = 86) included patients with pulmonary MDR/XDR-TB only (25.0%, n = 46) studies included both pulmonary and extrapulmonary, and 3 (1.6%) studies included extrapulmonary MDR/XDR-TB only. However, 49 (26.6%) studies did not mention the affected body sites. The HIV status of study participants was reported in 72.3% (n = 133) of studies, and the prevalence of HIV infection in these studies varied from 0% to 100%. The review showed that 9.8% (n = 18) of studies were reported from Low-Income Countries (LICs). The majority, 44.0% (n = 81) and 37.0% (n = 68) studies were reported from lower-middle-income countries (LMICs) and upper-middle-income countries (UMICs), respectively. All the studies reported the timing of sequelae, and most studies (92.4%, n = 170) reported that sequelae occurred during treatment. Only 58 (31.5%) of included articles had data on the duration of treatment (Table 1).
The global prevalence of sequelae and source of heterogeneity
The most common type of sequelae was respiratory sequelae (44.40%, 95% CI: 36.70–52.10), followed by hearing sequelae (26.7%, 95% CI: 23.9–29.7), renal sequelae (8.1%, 95% CI: 6.3–10.0), neurological sequelae (8.4%, 95% CI: 6.5–10.3), visual sequelae (4.5%, 95% CI: 2.7–6.3), hepatic sequelae (7.3% with a 95% CI: 5.1–9.4), and musculoskeletal sequelae (10.1% 7.0–13.2) (Supplementary file: Figs. S2, S4–S9). Other physical sequelae reported in a few studies were cardiac failure and multiple organ failure (Table 2).
Table 2.
Pooled prevalence of respiratory impairment, hearing impairment, renal impairment, neurological impairment, visual impairment, hepatic Impairment, and musculoskeletal impairment, stratified by study characteristics.
| Categories | Respiratory impairment |
Hearing impairment |
Renal impairment |
Neurologic impairment |
Visual impairment |
Hepatic impairment |
Musculoskeletal impairment |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | Number of studies | Prevalence of sequelae | |
| Overall | 19 | 44.4 | 45 | 26.8 | 28 | 8.1 | 26 | 8.4 | 16 | 4.5 | 24 | 7.3 | 21 | 10.1 |
| Countries income | ||||||||||||||
| HICs | 2 | 10.2 | 5 | 23.6 | 4 | 13.6 | 2 | 14.6 | 1 | 2.3 | 2 | 22.9 | 1 | 37.8 |
| UMICs | 6 | 45.8 | 16 | 35.2 | 11 | 15.9 | 10 | 9.9 | 7 | 6.6 | 9 | 11.1 | 7 | 12.8 |
| LMICs | 9 | 48.1 | 21 | 22.4 | 10 | 1.3 | 10 | 8.4 | 6 | 3.5 | 11 | 2.9 | 11 | 5.3 |
| LICs | 2 | 59.3 | 3 | 17.7 | 3 | 5.2 | 4 | 7.1 | 2 | 3.7 | 2 | 1.9 | 2 | 24.1 |
| Type of TB | ||||||||||||||
| MDR-TB | 11 | 52.3 | 31 | 25.0 | 19 | 4.9 | 17 | 5.6 | 10 | 3.7 | 16 | 6.1 | 11 | 7.3 |
| XDR-TB | NA | NA | 4 | 26.6 | 2 | 17.6 | 3 | 31.5 | 3 | 11.3 | 3 | 3.1 | 2 | 21.5 |
| MDR &XDR | 5 | 45.1 | 6 | 32.6 | 4 | 24.2 | 3 | 10.0 | NA | NA | 4 | 16.7 | 4 | 20.4 |
| Unclassified | 3 | 15.1 | 4 | 31.6 | 3 | 7.8 | 3 | 12.1 | 3 | 2.1 | 1 | 2.0 | 4 | 5.6 |
| Timing of sequelae | ||||||||||||||
| During treatment | 9 | 29.8 | 43 | 27.0 | 27 | 8.4 | 26 | 8.4 | 15 | 4.2 | 23 | 7.2 | 21 | 10.1 |
| After treatment | 10 | 57.7 | 2 | 22.0 | 1 | 2.0 | NA | NA | 1 | 10.0 | 1 | 10.0 | NA | -NA |
| Region | ||||||||||||||
| Southeast Asia | 6 | 62.8 | 13 | 18.1 | 6 | 0.68 | 5 | 6.4 | 2 | 6.5 | 7 | 2.6 | 7 | 2.8 |
| Sub-Saharan | 4 | 43.0 | 20 | 36.2 | 11 | 10.2 | 13 | 13.6 | 10 | 5.2 | 7 | 3 | 7 | 13.1 |
| European& CA | 4 | 28.3 | 7 | 29.9 | 7 | 23.0 | 4 | 12.7 | 3 | 3.0 | 6 | 19.7 | 6 | 26.6 |
| East Asia & Pacific | 3 | 29.9 | 3 | 4.7 | 2 | 3.2 | 3 | 2.5 | 2.5 | 3 | 3 | 10.0 | 2.6 | 17.4 |
| MENA | NA | NA | 1 | 10.0 | 1 | 3.8 | 1 | 8.8 | NA | NA | 1 | 5.0 | 1 | 7.5 |
| Region of America | 2 | 48.1 | 1 | 12.2 | 1 | 10.2 | NA | NA | NA | NA | NA | NA | NA | NA |
| Study design | ||||||||||||||
| Prospective cohort | 3 | 46.7 | 16 | 31.0 | 7 | 10.1 | 8 | 16.2 | 3 | 11.1 | 4 | 7.2 | 9 | 7.2 |
| Retrospective cohort | 8 | 35.5 | 26 | 22.6 | 20 | 7.9 | 16 | 6.0 | 12 | 2.7 | 18 | 8.1 | 11 | 12.9 |
| Cross-sectional | 8 | 52.3 | 3 | 37.5 | 1 | 4.8 | 2 | 3.7 | 1 | 4.5 | 2 | 0.9 | 1 | 11.9 |
| HIV status | ||||||||||||||
| <1% | 5 | 62.8 | 2 | 27.3 | 1 | 1.0 | 1 | 21.4 | NA | NA | 1 | 16.0 | 1 | 2.0 |
| 1–50% | 5 | 33.0 | 19 | 29.2 | 13 | 10.0 | 10 | 11.2 | 5 | 3.4 | 10 | 4.9 | 10 | 12.8 |
| 51–100% | 2 | 18.4 | 10 | 31.4 | 7 | 14.1 | 8 | 14.2 | 7 | 6.9 | 6 | 4.9 | 6 | 11.1 |
| Not recorded | 7 | 45.6 | 14 | 13.1 | 7 | 3 | 7 | 3.0 | 4 | 3.4 | 7 | 8.2 | 4 | 7.8 |
| Median age | ||||||||||||||
| Not reported | 2 | 64.1 | 2 | 6.5 | 2 | 3.3 | 1 | 11.3 | 1 | 0.9 | 1 | 0.8 | 1 | 2.6 |
| < median age | 8 | 49.3 | 28 | 29.4 | 21 | 7.5 | 17 | 10.2 | 10 | 5.8 | 14 | 5.8 | 13 | 10.9 |
| Median age & above | 9 | 35.3 | 15 | 24.6 | 5 | 3.5 | 8 | 6.5 | 5 | 4.2 | 9 | 10.8 | 6 | 11.7 |
| Site of DR-TB | ||||||||||||||
| Not reported | 1 | 8.8 | 17 | 36.6 | 8 | 8.6 | 5 | 3.2 | 5 | 2.6 | 6 | 8.1 | 4 | 12.3 |
| Pulmonary | 17 | 48.8 | 17 | 21.4 | 11 | 9.9 | 12 | 18.2 | 8 | 7.3 | 10 | 5.3 | 10 | 7.7 |
| Extrapulmonary | NA | NA | 1 | 0.4 | NA | NA | 1 | 2.2 | NA | NA | 1 | 2.2 | NA | NA |
| Both | 1 | 6.8 | 10 | 24.7 | 9 | 6.8 | 8 | 5.5 | 3 | 3.7 | 7 | 10.8 | 6 | 17.0 |
| DM status | ||||||||||||||
| Not reported | 10 | 46.9 | 26 | 29.9 | 18 | 7.5 | 19 | 8.1 | 10 | 5.3 | 10 | 6.7 | 11 | 8.4 |
| <1% | 2 | 84.1 | 1 | 20.0 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | 3.1 |
| 1–50% | 6 | 26.3 | 18 | 22.6 | 10 | 11.0 | 7 | 18.3 | 6 | 3.5 | 14 | 8.7 | 8 | 10.5 |
| ≥50% | 1 | 51.9 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
CA: Central Asia, DM: Diabetes Mellitus, DR-TB: Drug-Resistant Tuberculosis, HICs: High-Income Countries, HIV: Human Immune Deficiency Virus, LICs: Low-Income Countries, LMICs: Lower-Middle-Income Countries, MENA: Middle East and North Africa, MDR-TB: Multi-drug Resistant Tuberculosis, NA: No data reported, TB: Tuberculosis, UMICs: Upper-Middle-Income Countries, and XDR-TB: Extensively Drug-Resistant Tuberculosis.
Large heterogeneity in the prevalence of sequelae was identified in the included studies. Nine variables namely: the type of MDR-TB, the country's income status, HIV prevalence, the timing of sequelae, WHO region, study design, median age, Diabetes Mellitus (DM) status, and site of MDR-TB, were identified as sources of heterogeneity and used as a primary stratification. The pooled prevalence of sequelae did not differ significantly by any of the following study characteristics: median/mean age of study participants, study design, Human Immune Deficiency Virus (HIV) status, and DM status. The results of each physical sequelae were summarised in Table 3, Table 4.
Table 3.
Sub-group analysis on the pooled prevalence of physical sequelae.
| Stratification variable | Respiratory sequelae | Hearing sequelae | Renal sequelae | Neurologic sequelae | Hepatic sequelae | Visual sequelae | Musculoskeletal sequelae |
|---|---|---|---|---|---|---|---|
| Type of TB | |||||||
| MDR-TB | 52.3 (40.0, 64.6) | 25.0 (21.6, 28.4) | 4.9 (3.2, 6.7) | 5.6 (3.8, 7.5) | 6.1 (4.0, 8.3) | 3.7 (1.7.5.8) | 7.3 (3.3, 11.2) |
| XDR-TB | – | 26.6 (12.3, 40.9) | 17.6 (3.6, 31.6) | 31.5 (5.5, 57.5) | 3.1 (0.9, 5.3) | 11.3 (−0.1, 22.8) | 21.5 (9.9, 33.1) |
| MDR &XDR | 45.1 (28.3, 61.9) | 32.6 (14.9, 50.3) | 24.2 (3.8, 44.6) | 10.0 (1.7, 18.3) | 16.7 (6.6, 26.9) | – | 20.4 (13.0, 53.8) |
| Unclassified | 15.1 (9.4, 20.7) | 31.6 (11.3, 52.0) | 7.8 (5.81, 9.7) | 12.12 (3.0, 21.3) | 2.0 (0.2, 4.3) | 2.1 (−0.1, 4.2) | 5.6 (0.3, 10.9) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| By country income | |||||||
| HI | 10.2 (3.5, 16.9) | 13.6 (3.5, 23.6) | 13.6 (3.5, 23.6) | 14.6 (1.2, 30.4) | 22.9 (9.4, 36.4) | 2.3 (−2.1, 6.7) | 37.8 (28.8, 48.9) |
| UMI | 45.8 (26.5, 65.1) | 15.9 (11.2, 20.5) | 15.9 (11.2, 20.5) | 9.9 (6.4, 13.4) | 11.1 (6.1, 16.1) | 6.6 (3.0, 10.2) | 12.8 (5.9, 19.7) |
| LMI | 48.1 (35.0, 61.2) | 1.3 (0.5, 2.0) | 1.3 (0.5, 2.0) | 8.4 (4.7, 12.0) | 2.9 (1.5, 4.3) | 3.5 (0.5, 6.5) | 5.3 (2.7, 7.8) |
| LI | 59.3 (11.5, 130) | 5.2 (2.0, 8.5) | 5.2 (2.0, 8.5) | 7.1 (2.1, 12.1) | 1.9 (0.2.4, 6.1) | 3.7 (0.7, 6.7) | 24.1 (18.7, 67.0) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| Timing of sequelae | |||||||
| During treatment | 29.8 (18.7, 41.0) | 27.0 (24.0–30.0) | 8.44 (6.5, 10.4) | 8.4 (6.5, 10.3) | 7.2 (5.0, 9.4) | 4.2 (2.4, 6.0) | 10.1 (7.0, 13.2) |
| After treatment | 57.7 (47.3, 68.2) | 22.0 (16.1–27.8) | 2.00 (−0.7, 4.7) | – | 10.0 (4.1, 15.9) | 10.0 (4.1, 15.9) | – |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| By study design | |||||||
| Prospective cohort | 46.7 (15.5, 77.8) | 3.01 (17.6.44.5) | 10.1 (6.0, 14.2) | 16.2 (10.3, 22.0) | 7.2 (0.1, 14.4) | 11.1 (1.2, 21.0) | 10.9 (6.8, 15.0) |
| Retrospective cohort | 35.5 (25.6, 45.3) | 22.7 (16.7, 28.6) | 7.9 (5.5, 10.4) | 6.0 (4.0, 7.9) | 8.1 (5.6, 10.6) | 2.7 (1.2, 4.2) | 11.7 (4.3, 19.1) |
| Cross-sectional | 52.3 (39.0, 65.6) | 37.5 (12.3, 62.7) | 4.8 (2.2, 7.5) | 3.7 (1.3, 6.0) | 0.9 (0.2, 1.9) | 5.1 (0.5, 10.7) | 2.6 (−0.3, 5.5) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| By HIV status | |||||||
| Not recorded | 45.6 (39.1, 52.2) | 16.3 (13.3.19.2) | 3.0 (1.2, 4.8) | 3.5 (1.7, 5.3) | 8.2 (2.7, 13.6) | 3.4 (0.5, 6.3) | 7.8 (0.4, 15.2) |
| <1% | 62.8 (44.1, 81.4) | 26.6 (22.6.30.5) | 1.0 (0.9, 3.0) | 21.4 (13.3, 29.6) | 16.0 (13.0, 19.0) | – | 2.0 (0.8, 4.8) |
| 1%–50% | 33.0 (17.2, 48.8) | 32.3 (25.9.38.8) | 10.0 (6.1, 13.9) | 11.2 (7.2, 15.2) | 4.85 (2.64, 7.07) | 3.4 (1.6, 5.2) | 12.8 (7.5, 18.0) |
| 51%–100% | 18.4 (9.1, 27.7) | 30.9 (20.7.41.1) | 14.1 (4.8, 23.4) | 14.2 (6.6, 21.7) | 4.9 (1.7, 8.1) | 6.88 (1.9, 11.9) | 11.1 (4.5, 17.7) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| By region | |||||||
| South Asia | 62.8 (40.5, 85.0) | 18.1 (14.6, 21.6) | 0.7 (0.3, 1.1) | 6.4 (2.6, 10.2) | 2.6 (1.0, 4.3) | 6.5 (0.8, 12.2) | 2.8 (0.6, 5.0) |
| SSA | 43.0 (33.0, 53.0) | 36.2 (28.4, 44.0) | 10.19 (6.1, 14.3) | 13.6 (9.4, 17.8) | 2.6 (0.9, 4.4) | 5.2 (2.4, 7.9) | 13.1 (8.0, 18.2) |
| ECA | 28.3 (16.6, 39.9) | 29.9 (14.2, 45.6) | 21.99 (8.0, 36.0) | 12.7 (4.2, 21.2) | 19.7 (14.9, 24.5) | 3.0 (1.4, 7.3) | 26.7 (4.8, 48.5) |
| EAP | 29.9 (20.3, 39.4) | 4.7 (0.4, 9.7) | 3.19 (0.6, 5.8) | 2.45 (0.4, 4.5) | 10.0 (1.3, 18.66) | 2.5 (1.4, 3.7) | 17.4 (14.3, 20.2) |
| MENA | – | 10.0 (9.3, 10.7) | 3.8 (0.4, 7.9) | 8.75 (2.6, 14.9) | 5.00 (0.2, 10.0) | – | 7.5 (1.7, 13.3) |
| North America | 48.1 (1.1, 95.1) | 12.2 (11.3, 13.2) | 10.2 (1.7, 18.7) | – | – | – | – |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.00 | I2 = 94.7%, p = 0.000 |
| By DM status | |||||||
| Not reported | 46.9 (36.0, 57.7) | 29.8 (21.5, 38.1) | 7.3 (5.1, 9.5) | 8.1 (5.7, 10.5) | 6.7 (3.0, 10.4) | 5.3 (2.5, 8.2) | 8.4 (4.9, 11.9) |
| <1% | 84.1 (61.4, 106.8) | 20.0 (10.3, 29.7) | – | – | – | – | 3.1 (1.1, 7.3) |
| 1%–50% | 26.3 (20.0, 32.6) | 22.4 (14.8, 30.1) | 11.0 (6.3, 15.8) | 18.3 (9.6, 27.0) | 8.7 (5.3, 12.1) | 3.5 (1.8, 5.3) | 10.5 (4.3, 16.7) |
| 51%–100% | – | – | – | – | – | – | – |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| Mean/median age | |||||||
| < median age | 49.3 (36.7, 61.9) | 29.4 (19.9, 38.9) | 9.8 (6.7, 12.8) | 10.2 (7.2, 13.1) | 5.8 (3.5, 8.2) | 5.8 (2.3, 9.2) | 10.9 (6.8, 15.0) |
| ≥median age | 35.3 (30.1, 40.5) | 24.5 (15.3, 33.8) | 3.6 (1.2, 6.0) | 6.5 (3.4, 9.5) | 10.8 (5.8, 15.7) | 4.2 (1.5, 6.9) | 11.7 (4.3, 19.1) |
| Not reported | 64.1 (4.4, 123.8) | 5.8 (1.7, 9.8) | 3.3 (0.3, 9.9.6) | 11.3 (5.5, 17.1) | 0.8 (3.5, 8.2) | 0.9 (0.2, 2.6) | 2.6 (0.3, 5.5) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.3%, p = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
| Site of DR-TB | |||||||
| Pulmonary | 48.8 (40.5, 57.1) | 21.2 (13.0, 29.4) | 9.9 (5.7, 14.0) | 18.2 (11.4, 25.0) | 5.3 (2.7, 7.9) | 7.3 (2.8, 11.8) | 7.7 (4.0, 11.3) |
| Extra pulmonary | – | 0.6 (0.2, 1.1) | – | 2.2 (0.5, 4.0) | 2.2 (0.5, 4.0) | – | – |
| Both | 6.8 (6.6, 6.9) | 23.7 (14.7, 32.8) | 6.8 (4.2, 9.5) | 5.5 (3.4, 7.7) | 10.8 (3.5, 18.1) | 3.7 (0.4, 7.0) | 17.0 (6.7, 27.3) |
| Not reported | 8.8 (8.7, 9.0) | 35.0 (21.1, 48.9) | 8.6 (5.0, 12.2) | 3.2 (1.3, 5.2) | 8.1 (3.2, 13.0) | 2.6 (0.7, 4.5) | 12.3 (2.8, 21.9) |
| Overall, DL | I2 = 100%, P = 0.000 | I2 = 99.6%, P = 0.000 | I2 = 95.1%, p = 0.000 | I2 = 92.1%, p = 0.000 | I2 = 94.7%, p = 0.000 | I2 = 85.1%, p = 0.000 | I2 = 94.7%, p = 0.000 |
EAP: East Asia and Pacific, ECA: European and Central Asia, DM: Diabetes Mellitus, DR-TB: Drug-Resistant Tuberculosis, HI: High Income, HIV: Human Immune Deficiency Virus, LMI: Lower Middle Income, LI: Low Income, MDR-TB: Multidrug-resistant Tuberculosis, MENA: Middle East and North Africa, UMI: Upper Middle Income, SSA: Sub-Saharan Africa, XDR-TB: Extensively Drug-Resistant Tuberculosis.
–Indicates no study reported in that specific category.
Table 4.
Meta-regression output for respiratory sequelae, hearing sequelae, renal sequelae, neurological sequelae, visual sequelae, hepatic sequelae, and musculoskeletal sequelae.
| Variable | Respiratory sequelae |
Hearing sequelae |
Renal sequelae |
Neurological sequelae |
||||
|---|---|---|---|---|---|---|---|---|
| AOR with 95% CI | P-value | AOR with 95% CI | P-value | AOR with 95% CI | P-value | AOR with 95% CI | P-value | |
| Type of TB | ||||||||
| MDR | 1 | 1 | 1 | 1 | ||||
| XDR-TB | NA | NA | 1.7 (1.1, 2.7) | 1.6 (0.2, 13.8) | 0.638 | 7.8 (1.4, 42.7) | 0.021 | |
| MDR- & XDR-TB | 3.30 (0.36, 30.07) | 0.069 | 1.5 (0.8, 3.0) | 1.0 (0.1, 22.5) | 0.996 | 1.3 (0.2, 8.4) | 0.761 | |
| Unclassified | 2.79 (0.45, 17.18) | 0.018 | 1.1 (0.6, 2.0) | 1.2 (0.1, 12.8) | 0.875 | 2.5 (0.3, 19.1) | 0.347 | |
| DM Status | ||||||||
| <1% | 1 | 1 | ||||||
| 1–50% | 0.05 (0.01, 0.21) | 0.015 | NA | NA | NA | NA | 13.8 (3.12, 60.7) | 0.002 |
| 51–100% | 0.06 (0.002, 0.40) | 0.001 | – | – | ||||
| Not reported | 0.14 (0.05, 0.41) | 0.011 | 2.5 (0.99, 6.2) | 0.052 | ||||
| Timing of sequelae | ||||||||
| During treatment | 1 | 1 | 1 | |||||
| After treatment | 5.42 (1.38, 21.28) | 0.004 | 2.1 (1.3, 3.3) | 0.6 (0.2, 1.9) | 0.335 | NA | NA | |
| Site of DR-TB | ||||||||
| Pulmonary | 1 | 1 | ||||||
| Extrapulmonary | NA | NA | 0.4 (0.2, 0.6) | NA | NA | 0.14 (0.05, 0.4) | 0.001 | |
| Both | 1.1 (0.6, 2.1) | 0.2 (0.1, 0.8) | 0.031 | |||||
| Not reported | 1.2 (0.7, 1.9) | 0.2 (0.1, 0.5) | 0.003 | |||||
| Country Income | ||||||||
| HI | 1 | 1 | ||||||
| UMI | NA | NA | 0.6 (0.3, 0.9) | 0.39 (0.35, 4.5) | 0.437 | NA | NA | |
| LMI | 0.7 (0.5, 1.2) | 0.2 (0.01, 1.6) | 0.109 | |||||
| LI | 1.2 (0.6, 2.2) | 0.07 (0.1, 0.5) | 0.007 | |||||
| Median age | ||||||||
| <Median | NA | NA | NA | NA | NA | NA | NA | |
| Median & above | ||||||||
| Unclassified | ||||||||
| Study design | ||||||||
| Prospective cohort | NA | NA | NA | NA | NA | NA | NA | |
| Retrospective cohort | ||||||||
| Cross-sectional | ||||||||
| HIV status | ||||||||
| 0–50% | NA | NA | NA | NA | NA | NA | NA | |
| 51–100% | ||||||||
| Unclassified | ||||||||
| Meta-regression output continued | ||||||
|---|---|---|---|---|---|---|
| Variable | Hepatic sequelae |
Visual sequelae |
Musculoskeletal sequelae |
|||
| AOR with 95% CI | P-value | AOR with 95% CI | P-value | AOR with 95% CI | P-value | |
| Type of TB | ||||||
| MDR | 1 | 1 | 1 | |||
| XDR-TB | 1.02 (0.3, 31.2) | 0.993 | 6.4 (1.3, 31.8) | 0.028 | 7.7 (1.1, 51.9) | 0.038 |
| MDR- & XDR-TB | 0.4 (0.2, 9.0) | 0.525 | 1.1 (0.3, 4.7) | 0.868 | 0.8 (0.04, 17.9) | 0.884 |
| Unclassified | 0.4 (0.2, 6.3) | 0.451 | – | – | – | – |
| DM Status | ||||||
| <1% | ||||||
| 1–50% | NA | NA | NA | NA | NA | NA |
| 51–100% | ||||||
| Not reported | ||||||
| Timing of sequelae | ||||||
| During treatment | NA | NA | NA | NA | NA | NA |
| After treatment | ||||||
| Site of DR-TB | ||||||
| Pulmonary | 1 | |||||
| Extrapulmonary | 0.2 (0.01, 5.3) | 0.309 | NA | NA | NA | NA |
| Both | 0.7 (0.5, 8.3) | 0.718 | ||||
| Not reported | 0.3 (0.27, 4.0) | 0.352 | ||||
| Country Income | ||||||
| HICs | 1 | |||||
| UMICs | 0.03 (0.008, 0.79) | 0.038 | NA | NA | NA | NA |
| LMICs | 0.2 (0.008, 0.34) | 0.012 | ||||
| LICs | 0.02 (0.004, 0.86 | 0.043 | ||||
| Median age | ||||||
| <Median | 1 | 1 | ||||
| Median & above | 3.58 (0.24.53.01) | 0.323 | 1.1 (0.9, 11.9) | 0.957 | NA | NA |
| Unclassified | 0.18 (0.03, 1.23) | 0.075 | 0.4 (0.1, 3.0) | 0.320 | ||
| Study design | ||||||
| Prospective cohort | 1 | NA | NA | |||
| Retrospective cohort | NA | NA | 0.7 (0.2, 3.4) | 0.623 | ||
| Cross-sectional | 0.3 (0.1, 1.4) | 0.124 | ||||
| HIV status | ||||||
| 0–50% | 1 | |||||
| 51–100% | NA | NA | NA | NA | 0.5 (0.1, 4.7) | 0.485 |
| Unclassified | 0.1 (0.03, 0.6) | 0.009 | ||||
NA: Not significant in the bivariable analysis at a p-value of <0.2.
MDR-TB: DM: Diabetes Mellitus, HICs: XDR-TB: Extensively Drug-Resistant Tuberculosis, High-Income Countries, HIV: Human Immune Deficiency Virus, LICs: Low-Income Countries, LMI: Lower Middle-Income Countries, Multidrug-Resistant Tuberculosis, UMICs: Upper Middle-Income Countries.
Respiratory sequelae
A total of 18 papers reported respiratory sequelae, and the pooled prevalence was 44.40% (95% CI: 36.70–52.10) Table 2 and Supplementary file, Fig. S2. The prevalence of respiratory sequelae was 52.3% (95% CI: 40.0, 64.6) (I2 = 100%, p = 0.000) for patients with MDR-TB, and there was no report among patients with XDR-TB only. The sub-group analysis finding showed that the highest prevalence of respiratory sequelae 57.7% (95% CI: 47.3, 68.2) (overall, DL I2 = 100%, p = 0.000) was reported after completion of DR-TB treatment compared to sequelae during treatment 29.8% (95% CI: 18.7, 41.0) (Overall, DL I2 = 100%, p = 0.000) (Table 3). The meta-regression analysis showed that the prevalence of respiratory sequelae after DR-TB treatment completion was nearly five times higher than during treatment (AOR = 5.42, 95% CI: 1.38–21.28 (Table 4).
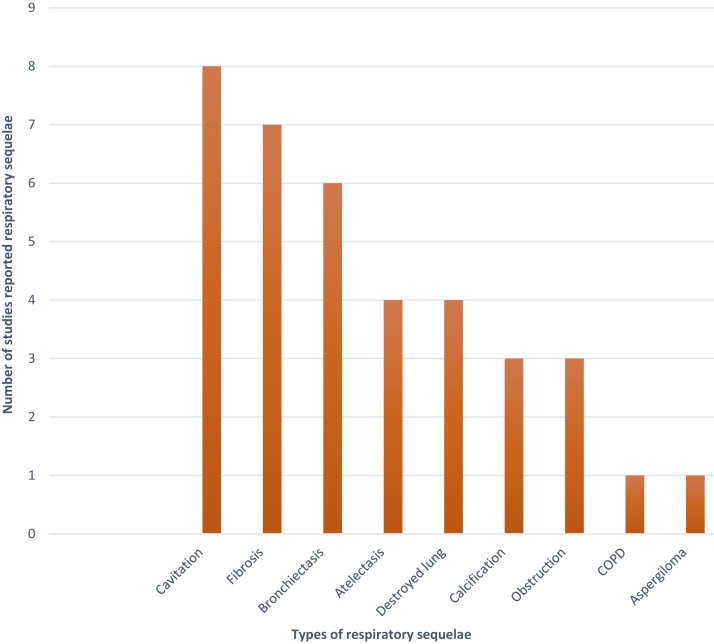
Types of respiratory sequelae
Cavitations, bronchiectasis, fibrosis, atelectasis, and destroyed lung disease were the commonest types of respiratory sequelae reported among patients with MDR- and XDR-TB (Fig. 2).
Fig. 2.
Respiratory sequelae reported among patients with MDR-TB and XDR-TB. MDR-TB = multi-drug resistant tuberculosis. XDR-TB = extensively drug-resistant tuberculosis.
Hearing sequelae
A total of 45 papers reported hearing sequelae, and the pooled prevalence was 26.65% (95% CI: 20.81–32.50) (Table 2 and Supplementary file: Fig. S4). The pooled prevalence of hearing sequelae was 25.0% (95% CI: 21.6, 28.4) (overall, DL I2 = 99.3%, p = 0.000) for patients with MDR-TB and 26.6% (95% CI: 12.3, 40.9) (overall, DL I2 = 99.3%, p = 0.000) for patients with XDR-TB (Table 3). The meta-regression analysis showed that patients with XDR-TB had a 70% higher risk of developing hearing sequelae than patients with MDR-TB (AOR = 1.70, 95% CI: 1.1–2.7). The prevalence of developing hearing sequelae after completion of DR-TB treatment was nearly two times compared with during DR-TB treatment (AOR = 2.1, 95% CI: 1.3–3.3) (Table 4).
Renal sequelae
A total of 28 papers reported renal sequelae, and the pooled prevalence was 8.13% (95% CI: 6.25–10.00) (Table 2 and Supplementary file: Fig. S5). The highest prevalence of renal sequelae reported in UMICs was 15.9% (95% CI: 11.2, 20.5) (overall, DL I2 = 95.1%, p = 0.000) and High-Income Countries (HICs) 13.6% (95% CI: 3.5, 23.6) (overall, DL I2 = 95.1%, p = 0.000) countries. The lowest prevalence of renal sequelae reported in LMICs was 1.3% (95% CI: 0.5, 2.0) (Overall, DL I2 = 95.1%, p = 0.000) and Low-Income Countries (LICs) (5.2%) countries (Overall, DL I2 = 95.1%, p = 0.000) (Table 3). The prevalence of renal sequelae among LICs was nearly 90% lower than HICs (AOR = 0.07, 95% CI (0.1, 0.5)) (Table 4).
Neurological sequelae
A total of 26 papers reported neurological sequelae, and the pooled prevalence was 8.41% (95% CI: 6.52–10.29) (Table 2 and Supplementary file: Fig. S6). The highest prevalence of neurological sequelae was reported in patients with XDR-TB; 31.5% (95% CI: 5.5, 57.5) (overall, DL I2 = 92.1%, p = 0.000) and was nearly seven times higher than in patients with MDR-TB 5.6% (95% CI: 3.8, 7.5) (overall, DL I2 = 92.1%, p = 0.000). The highest prevalence of neurological sequelae was reported among patients with pulmonary DR-TB; 18.2% (95% CI: 11.4, 25.0) (Overall, DL I2 = 92.1%, p = 0.000) (Table 3). The prevalence of neurological sequelae among patients with XDR-TB was nearly eight times higher risk than patients with MDR-TB (AOR = 7.8, 95% CI: 1.4–42.7). Similarly, the prevalence of neurological sequelae among patients with DM was nearly 14 times higher than among patients without DM (AOR = 13.8, 95% CI: 3.12, 60.7) (Table 4).
Visual sequelae
A total of 16 studies reported visual sequelae, and the pooled prevalence was 4.51% (95% CI: 2.71–6.30) (Table 2 and Supplementary file: Fig. S7). Patients with XDR-TB were at nearly six times higher risk than patients with MDR-TB (AOR = 6.4, 95% CI: 1.3, 31.8) (Table 4).
Hepatic sequelae
A total of 24 papers reported hepatic sequelae, and the pooled prevalence was 7.27% (95% CI: 5.11–9.43) (Table 2 and Supplementary file: Fig. S8). HICs had the highest prevalence of hepatic sequelae at 22.9% (95% CI: 9.4, 36.4) (Overall, DL I2 = 94.7%, p = 0.000), followed by UMICs countries at 11.1% (95% CI: 6.1, 16.1) (Overall, DL I2 = 94.7%, p = 0.000). The prevalence of hepatic sequelae was 10.0% (95% CI: 4.1, 15.9) (Overall, DL I2 = 94.7%, p = 0.000) two years after completion of MDR-TB treatment. The prevalence of hepatic sequelae was 6.1% (95% CI: 4.0, 8.3) for MDR-TB (Overall, Dl I2 = 94.7%, p = 0.000) (Table 3). The prevalence of hepatic sequelae among LMICs (AOR = 0.2, 95% CI (0.008, 0.34)) and LICs (AOR = 0.02, 95% CI: 0.004, 0.86) was lower than HICs (Table 4).
Musculoskeletal sequelae
A total of 21 articles reported musculoskeletal sequelae, and the pooled prevalence was 10.1% (95% CI: 6.97–13.7) (Table 2 and Supplementary file: Fig. S9). The prevalence of musculoskeletal sequelae among patients with MDR-TB was 7.3% (95% CI: 3.3, 11.2) (overall, DL I2 = 94.7%, p = 0.000), for patients with XDR-TB; 21.5% (95% CI: 9.9, 33.1) (overall, DL I2 = 94.7%, p = 0.000). The highest prevalence of musculoskeletal sequelae was reported among people from communities or studies with HIV prevalence of 1–50%; 12.8% (95% CI: 7.5, 18.0) (Overall, DL I2 = 94.7%, p = 0.000) and HIV prevalence >50%; 11.1% (95% CI: 4.5, 17.7) (Overall, DL I2 = 94.7%, p = 0.000) compared with studies with HIV prevalence of <1% (Overall, DL I2 = 94.7%, p = 0.000) (Table 3). Meta-regression output showed that the prevalence of musculoskeletal sequelae among patients with XDR-TB was nearly eight times higher than among patients with MDR-TB (AOR = 7.7, 95% CI: 1.1, 51.9) (Table 4).
Publication bias
The asymmetrical funnel plots and Egger's test indicate potential publication bias among the included studies for almost all types of physical sequelae. However, the Egger test showed no evidence of substantial publication bias for respiratory sequelae (p = 0.544) according to our significance level (Supplementary file: Figs. S10–16). We have applied trim and fill methods to adjust for the publication bias in the analysis. As a result, the pooled prevalence after adjusting the publication bias was reduced significantly for each physical sequelae. However, the funnel plot asymmetry was corrected after adjusting the therorethical missing studies by the trim and fill methods. The finding is summarized in the Supplementary file: Figs. S17–22.
Quality assessment
The overall quality assessment for the included studies was low to high, with a median score of 6 points and an Inter Quartile Range (IQR) of 3 points. Of 66 included studies, seven had eight or nine points, regarded as high-quality studies. The majority, 42 (63.63%), studies had 5 to 7 points, classified as medium-quality studies. The other studies having a score of 4 points or lower were low-quality studies.
Sensitivity analysis
Sensitivity analysis was conducted by removing low-quality studies (Supplementary file: Figs. S23–29). The pooled prevalence of respiratory sequelae after removing low-quality studies was 45.13% (95% CI: 39.97–50.29) (Overall, DL (I2 = 100%, p = 0.000)) (Fig. S23). The finding after adjustment was the same as before adjustment (43.77% (95% CI: 40.09–47.48)). The prevalence of hearing sequelae after trimming poor-quality studies was 23.32% (95% CI: 18.49–28.15) (overall, DL (I2 = 98.7%, p = 0.000)) (Fig. S24). However, there was no significant difference in estimates between the original (26.65% (95% CI: 20.81–32.50)) and trimmed prevalence.
The prevalence of renal sequelae after adjustment of low-quality studies was 8.63% (95% CI: 5.81–10.93) (overall, DL (I2 = 95.7%, p = 0.000)) (Fig. S25). However, the finding showed that there was no difference between the original (8.13% (95% CI: 6.25–10.00)) and trimmed prevalence (9.37% (95% CI: 7.03–11.7)). The prevalence of neurological sequelae after trimming of low-quality studies was 9.37% (95% CI: 7.03–11.7) (overall, DL (I2 = 92.3%, p = 0.000)) (Fig. S26). However, there was no difference between the prevalence of neurological sequelae between the original (8.41% (95% CI: 6.52–10.29)) and after trimming of low-quality studies. Moreover, the prevalence of hepatic sequelae after trimming of low-quality studies was 8.37% (95% CI: 5.81–10.93) (overall, DL (I2 = 95.7%, p = 0.000)) (Fig. S27). However, there was no difference in the prevalence of hepatic sequelae between the original (7.27% (95% CI: 5.11–9.43)) and the trimmed prevalence.
The prevalence of visual sequelae after trimming of low-quality studies was 4.47 (95% CI: 2.40–6.53) (overall, DL (I2 = 92.3%, p = 0.000)) (Fig. S28). However, there was no difference in the prevalence of visual sequelae between the original (4.51% (95% CI: 2.71–6.30)) and after trimming of low-quality studies. The overall prevalence of musculoskeletal sequelae after removing low-quality studies was 9.33 (95% CI (5.58–13.09) (overall, DL (I2 = 92.3%, p = 0.000))) (Fig. S29). However, there was no difference between the original (10.1% (95% CI: 6.97–13.7)) and trimmed prevalence.
Discussion
To the best of our knowledge, this is the first reported comprehensive systematic review and meta-analysis to quantify the prevalence of physical sequelae among patients with MDR/XDR-TB and to identify the most common types of sequelae. The prevalence of long-term physical sequelae was high among patients with MDR/XDR-TB. The most common types of sequelae that have been reported in the existing literature were respiratory, hearing, renal, neurological, hepatic, visual, and musculoskeletal sequelae.
We found that respiratory and hearing sequelae were the most common type of long-term sequelae among patients with MDR/XDR-TB, with an overall pooled prevalence of 43.8% and nearly 30%, respectively. Our finding is significantly higher than a systematic review conducted among patients with DS-TB amongst whom the pooled prevalence of respiratory sequelae was 33.1%87 and 14.5% of patients developed hearing sequelae.87 The higher hearing sequelae could be due to the inclusion of aminoglycoside agents in MDR-TB treatment regimens, which are the major cause of ototoxicity.88
Previously the WHO used to recommend the treatment of MDR-TB with an intensive phase of injectable aminoglycosides (amikacin, kanamycin, and capreomycin), which can lead to hearing sequelae.89 However, the WHO currently recommends MDR-TB treatment without aminoglycosides90 to minimise drug-related hearing sequelae. Therefore, the high prevalence of hearing sequelae in the current study could be due to the inclusion of aminoglycosides in the past treatment regimens of MDR- and XDR-TB.
In our review, nearly one in twenty patients with MDR-TB developed visual sequelae. The finding was lower than for a systematic review conducted among patients with DS-TB (11.9%). A possible reason could be due to the prolonged use of the drug ethambutol in patients with DS-TB,91 which is not used in patients with MDR-TB. Ethambutol is a potent anti-TB drug that commonly affects the optic nerve and can cause irreversible visual sequelae.92
The prevalence of physical sequelae was heterogeneous when stratified by type of TB, the timing of treatment, country income, and DM status. For instance: respiratory sequelae were more prevalent among patients with MDR-TB after completing their treatment compared with patients with MDR-TB during treatment. This could be explained by the endobronchial involvement that causes extended parenchymal destruction11 further resulting in poor compliance, bronchiectasis, aspergilloma, and other parenchymal airway diseases.93 It is also expected that chemotherapy-cured patients with MDR-TB, who usually end-up with significant lung destruction and chronic complications like fibrosis, bronchiectasis, and cavitations.94 Although patients become negative bacteriologically, a significant number of patients with MDR-TB are left with persistent symptoms and lung function sequelae.95 A likely explanation is the occurrence of common residual conditions like fibrosis, scarring, cavitation, and distortion of lung architecture that led to volume loss and bronchiectasis.95 Pulmonary TB also involves airways and results in hyperplasia and hypertrophy of mucous glands, thus affecting the functioning of airways, and causing a reduction of total lung capacity. Hence, there will be a significant increase in functional limitations among patients with MDR-TB.96
The highest prevalence of respiratory sequela was reported among studies conducted in LICs countries (59.3%). The possible reason could be due to weak healthcare systems, inadequate financing, poverty, and poor access (in terms of quality, geographic access, financial access, and acceptability of services) in LICs and LMICs countries as compared with HICs and UMICs countries.97,98 The other possible reason could be due to the presence of possible diagnosis delay, treatment delay, and the long and less effective medication regimens of patients with MDR-TB in LICs and LMICs countries.95 Moreover, low-income countries have poor access to education and poor health insurance,99 which improves the patient's health outcome.100 Non-invasive respiratory support and adjunct surgical resection, which is less affordable in LICs and LMICs countries, were associated with an increase in maintaining a residual capacity to prevent lung complications101 and improved symptoms of respiratory sequelae by enhancing pulmonary function and elastic recoil.81 The high burden of respiratory sequelae may also relate to the high burden of TB in LICs and LMIC countries. Moreover, patients with MDR-TB might be exposed to multiple exposures like smoking, occupational exposures, and indoor biomass fuels that could further result in respiratory failure.102
On the other hand, we found that the prevalence of hearing, renal, and hepatic sequelae was higher in UMICs and HICs countries than in LMICs and LICs countries. The lower prevalence of hearing sequelae in LICs and LMICs could be due to a lack of audiometric tools to measure hearing sequelae in LICs and LMICs countries.103 The lower prevalence of renal and hepatic sequelae in LICs and LMICs countries could be due to the high burden of DM104 and hypertension105 in HICs and UMICs countries. DM and hypertension commonly increase the risk of developing renal sequelae, including end-stage renal failure.106 Moreover, comorbidities including DM and hypertension negatively impact on the patient's immune response, precipitate treatment failure.107 It could be also due to the high cost and complexity of diagnosing renal sequelae in LICs and LMICs countries.
A high prevalence of neurological sequelae was reported among patients with a high prevalence of DM. A possible reason could be that DM causes endothelial dysfunction, inflammation, platelet aggregation, and vascular smooth muscle cell dysfunction, which leads to atherosclerosis, which in turn can impact neurological outcomes.108 The prevalence of musculoskeletal sequelae significantly differed by HIV status. This could be due to the high prevalence of HIV among patients with XDR-TB (>50% prevalence in 75% of studies) than patients with MDR-TB (>50% in none of the studies).
Patients with MDR and XDR-TB developed sequelae during or post-TB treatment due to either the nature of the second-line drugs or the disease itself. MDR-TB by itself causes active lesions of the parenchymal (infiltration, cavitations, consolidations, and glass opacity) that further cause extensive tissue damage by the long duration of the disease compared to DS-TB.3 The dominant cavities and consolidations in MDR- and XDR-TB occurred following a failure of post-TB treatment.2 Unhealed cavities, in turn, cause new cavities and consolidations in patients with MDR-TB.2 Bronchiectasis, a non-lung parenchymal morphology, is a dilation of multiple bronchi resulting from the course of active lesions of post-pulmonary TB and affects the surrounding structures such as bronchus and bronchioles.109 Non-active parenchymal lesions (fibrosis, calcification, and atelectasis) are common post-MDR-TB sequelae in patients with DR-TB. It causes the reactivation of active TB in the long run and leads to recovery.2,3 Therefore, we can conclude that respiratory sequelae are mainly caused by the disease progression and not a drug-related problems.
On the other hand, aminoglycosides such as Amikacin, Capreomycin, and Kanamycin are the common causes of hearing sequelae among patients with MDR- and XDR-TB.110 For instance: a previous study showed that Kanamycin-based regimens were associated with a higher risk of hearing loss.4,111 WHO 2018 report recommended the replacement of aminoglycoside agents with less toxic agents such as Bedaquiline-based regimens to minimise permanent hearing loss.89 This evidence supports our hypothesis aminoglycoside agents cause hearing-related sequelae, and it is possible to conclude that hearing sequelae is mainly drug-related sequelae.
In the current study, patients with XDR-TB were at a higher risk of developing visual sequelae compared with patients with MDR-TB. The higher prevalence of visual sequelae among patients with XDR-TB could be because of higher doses and longer durations of linezolid, it is a well-known cause of optic neuropathy among patients with MDR-TB.112 Similarly, patients with MDR-TB were at a higher risk of developing neurological sequelae due to the drug linezolid. Its effect increases with the dose and duration of treatment. As a result, patients with DR-TB were at a higher risk of neurological and visual sequelae. For instance, reducing linezolid dose from 600 to 300 g reduced the risk of neurological abnormalities and improved treatment outcomes.113 This finding strengthens our hypothesis of both neurological and visual sequelae are more drug-related sequelae. However, the effect of linezolid on neurological sequelae among patients with MDR- and XDR-TB needs further study. In general, neurological sequelae are irreversible and patient's quality of life through functional, mental, and social impairment.91,114
Adequate and standardised monitoring of patients with MDR- and XDR-TB during and after the completion of treatment will be necessary to minimise the burden of physical sequelae. DR-patients with TB are declared cured or completed based on clinical, smear, and radiological examinations.1 However, there is a lack of follow-up and support for patients with MDR-TB and XDR-TB after the completion of their treatment. Therefore, policymakers, planners, researchers, and clinicians need to work collaboratively to decrease the burden of long-term physical sequelae after the completion of their treatment. Preparing standard monitoring guidelines, expanding the availability of objective tools, and discussions with stakeholders on the management of post-MDR-TB sequelae will reduce the burden of physical sequelae.
Secondly, creating awareness of physical sequelae among clinicians and patients will be necessary to ensure the provision of integrated care during and after the completion of treatment. Thirdly, the ministries of health of each country should implement a strategy to build skills and systems for long-term care for patients. Finally, the scaling-up of a rehabilitation centre and tools (hearing aids, vision aids, and wheelchairs) will play a vital role. LICs and LMICs countries need international support to manage the high costs associated with patient care with long-term sequelae.
This study has some important limitations. The first limitation was the presence of high heterogeneity between included studies. We investigated the sources of heterogeneity by conducting a sub-group analysis for each of the outcomes. Secondly, each study used different methodologies or tools for outcome ascertainment. This will lead to errors in estimates of the prevalence of physical sequelae. Thirdly, since more than 50% of studies did not report the dose and type of DR-TB regimen, it wasn't possible to determine drug-specific sequelae among patients with DR-TB. The study lacks information on whether the sequelae developed because of the DR-TB drugs or the disease itself. Fourthly, it is impossible to assess the severity of sequelae as there isn't reported data on the severity of sequelae. The existing publication bias will under or overestimate the overall finding. As a result, it could affect the representativeness of the study. The study also lacks information on what fraction of patients had been previously treated for TB as included studies reported previously treated cases of TB, MDR-TB, and XDR-TB combined.
This systematic review found that long-term physical sequelae such as respiratory, hearing, musculoskeletal, neurological, renal, hepatic, and visual sequelae were common among survivors of MDR- and XDR-TB. There were significant differences in the prevalence of sequelae between patients with MDR- and XDR-TB. There is a substantial need for incorporating post-MDR-TB and XDR-TB treatment surveillance into the current programmatic management of MDR-TB to early detect and prevent post-treatment sequelae, and for health systems to be supported and strengthened to be able to provide appropriate ongoing care to patients after cessation of TB treatment.
Contributors
TYA, ACAC, and KAA conceptualised the study. TYA, ACAC, HFW, and KAA performed data curation. TYA and KAA carried out the statistical analysis and accessed and verified the data. TYA wrote the first draft of the manuscript with input from ACAC and KAA. All authors reviewed the final draft of the manuscript for intellectual content. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Data sharing statement
Data will be available upon request from the corresponding author.
Declaration of interests
All authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (NHMRC) through an Emerging Leadership Investigator Grant APP1196549. KAA is a senior researcher at Curtin University who received the fund. TYA is also supported by Curtin University Higher Degree Research (HDR) Scholarship and acknowledges Curtin University for providing support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101900.
Appendix A. Supplementary data
References
- 1.World Health Organization . World Health Organization; 2019. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva, License: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 2.Zahirifard S., Bakhshayeshkaram M. The radiological spectrum of pulmonary multidrug-resistant tuberculosis in HIV-negative patients. Iran J Radiol. 2003;1(3&4):161–166. [Google Scholar]
- 3.Icksan A.G., Napitupulu M.R.S., Nawas M.A., Nurwidya F. Chest X-ray findings comparison between multi-drug-resistant tuberculosis and drug-sensitive tuberculosis. J Nat Sci Biol Med. 2018;9(1):42–46. doi: 10.4103/jnsbm.JNSBM_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nizamuddin S., Khan F.A., Khan A.R., Kamaal C.M. Assessment of hearing loss in multi-drug resistant tuberculosis (MDR-TB) patients undergoing aminoglycoside treatment. Int J Res Med Sci. 2015;3(7):1734–1740. [Google Scholar]
- 5.Alene K.A., Clements A.C.A., McBryde E.S., et al. Sequelae of multidrug-resistant tuberculosis: protocol for a systematic review and meta-analysis. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-019593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alene K.A., Clements A.C., McBryde E.S., et al. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Infect. 2018;77(5):357–367. doi: 10.1016/j.jinf.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Byrne A.L., Marais B.J., Mitnick C.D., et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res. 2017;3(3) doi: 10.1183/23120541.00026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kampen S.C., Wanner A., Edwards M., et al. International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Glob Health. 2018;3(4) doi: 10.1136/bmjgh-2018-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral A.F., Coton S., Kato B., et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46(4):1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akkara S., Shah A., Adalja M., Akkara A., Rathi A., Shah D. Pulmonary tuberculosis: the day after. Int J Tuberc Lung Dis. 2013;17(6):810–813. doi: 10.5588/ijtld.12.0317. [DOI] [PubMed] [Google Scholar]
- 11.Godoy M.D., Mello F.C., Lopes A.J., et al. The functional assessment of patients with pulmonary multidrug-resistant tuberculosis. Respir Care. 2012;57(11):1949–1954. doi: 10.4187/respcare.01532. [DOI] [PubMed] [Google Scholar]
- 12.Garg R.K., Somvanshi D.S. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain A. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 14.Tahaoğlu K., Törün T., Sevim T., et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345(3):170–174. doi: 10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Herdman T., Saunders M., et al. Rising burden of visual and auditory disability in patients after tuberculosis therapy in Peruvian slums. Int J Infect Dis. 2018;73(Suppl):348–349. [Google Scholar]
- 16.Gunasekeran D.V., Gupta B., Cardoso J., Pavesio C.E., Agrawal R. Visual morbidity and ocular complications in presumed intraocular tuberculosis: an analysis of 354 cases from a non-endemic population. Ocul Immunol Inflamm. 2018;26(6):865–869. doi: 10.1080/09273948.2017.1296580. [DOI] [PubMed] [Google Scholar]
- 17.Orenstein E.W., Basu S., Shah N.S., et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9(3):153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 18.Ettehad D., Schaaf H.S., Seddon J.A., Cooke G.S., Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):449–456. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson K.R., Tierney D.B., Jeon C.Y., Mitnick C.D., Murray M.B. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51(1):6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alene K.A., Wangdi K., Colquhoun S., et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med. 2021;19(1):1–19. doi: 10.1186/s12916-021-02063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uplekar M., Weil D., Lonnroth K., et al. WHO's new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G., Shea B., O'Connell D., et al. 2014. Newcastle-Ottawa quality assessment scale cohort studies. [Google Scholar]
- 24.Appana D., Joseph L., Paken J. An audiological profile of patients infected with multi-drug resistant tuberculosis at a district hospital in KwaZulu-Natal. S Afr J Commun Disord. 2016;63(1):e1–e12. doi: 10.4102/sajcd.v63i1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold A., Cooke G.S., Kon O.M., et al. Adverse effects and choice between the injectable agents amikacin and capreomycin in multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61(9) doi: 10.1128/AAC.02586-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atif M., Bashir A., Ahmad N., Fatima R.K., Saba S., Scahill S. Predictors of unsuccessful interim treatment outcomes of multidrug resistant tuberculosis patients. BMC Infect Dis. 2017;17(1):655. doi: 10.1186/s12879-017-2746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baghaei P., Tabarsi P., Dorriz D., et al. Adverse effects of multidrug-resistant tuberculosis treatment with a standardized regimen: a report from Iran. Am J Ther. 2011;18(2):e29–e34. doi: 10.1097/MJT.0b013e3181c0806d. [DOI] [PubMed] [Google Scholar]
- 28.Buziashvili M., Mirtskhulava V., Kipiani M., et al. Rates and risk factors for nephrotoxicity and ototoxicity among tuberculosis patients in Tbilisi, Georgia. Int J Tuberc Lung Dis. 2019;23(9):1005–1011. doi: 10.5588/ijtld.18.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng N., Wu S., Luo X., et al. A comparative study of chest computed tomography findings: 1030 cases of drug-sensitive tuberculosis versus 516 cases of drug-resistant tuberculosis. Infect Drug Resist. 2021;14:1115–1128. doi: 10.2147/IDR.S300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayyab F., Iliyasu G., Ahmad B., Habib A. Early safety and efficacy of linezolid-based combination therapy among patients with drug-resistant tuberculosis in North-western Nigeria. Int J Mycobacteriol. 2021;10(2):129–135. doi: 10.4103/ijmy.ijmy_57_21. [DOI] [PubMed] [Google Scholar]
- 31.de Jager P., van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6(7):622–627. [PubMed] [Google Scholar]
- 32.Dela A.I., Tank N.K.D., Singh A.P., Piparva K.G. Adverse drug reactions and treatment outcome analysis of DOTS-plus therapy of MDR-TB patients at district tuberculosis centre: a four year retrospective study. Lung India. 2017;34(6):522–526. doi: 10.4103/0970-2113.217569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatima S., Syeda M.F., Adla N., Devi R. Ambispective study of adverse drug reactions in multi-drug resistant tuberculosis patients in Warangal, Telangana. Lung India: official organ of Indian Chest Society. 2021;38(4):330–337. doi: 10.4103/lungindia.lungindia_118_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J.T., Du J., Wu G.H., et al. Bedaquiline-containing regimens in patients with pulmonary multidrug-resistant tuberculosis in China: focus on the safety. Infect Dis Poverty. 2021;10(1):32. doi: 10.1186/s40249-021-00819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geerligs W.A., Van Altena R., De Lange W.C.M., Van Soolingen D., Van Der Werf T.S. Multidrug-resistant tuberculosis: long-term treatment outcome in The Netherlands. Int J Tuberc Lung Dis. 2000;4(8):758–764. [PubMed] [Google Scholar]
- 36.Gualano G., Mencarini P., Musso M., et al. Putting in harm to cure: drug related adverse events do not affect outcome of patients receiving treatment for multidrug-resistant Tuberculosis. Experience from a tertiary hospital in Italy. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harouna S.H., Ortuno-Gutierrez N., Souleymane M.B., et al. Short-course treatment outcomes and adverse events in adults and children-adolescents with MDR-TB in Niger. Int J Tuberc Lung Dis. 2019;23(5):625–630. doi: 10.5588/ijtld.17.0871. [DOI] [PubMed] [Google Scholar]
- 38.Hong H., Dowdy D.W., Dooley K.E., et al. Risk of hearing loss among multidrug-resistant tuberculosis patients according to cumulative aminoglycoside dose. Int J Tuberc Lung Dis. 2020;24(1):65–72. doi: 10.5588/ijtld.19.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huerga H., Bastard M., Kamene M., et al. Outcomes from the first multidrug-resistant tuberculosis programme in Kenya. Int J Tuberc Lung Dis. 2017;21(3):314–319. doi: 10.5588/ijtld.16.0661. [DOI] [PubMed] [Google Scholar]
- 40.Isaakidis P., Cox H.S., Varghese B., et al. Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain K., Desai M., Solanki R., Dikshit R.K. Treatment outcome of standardized regimen in patients with multidrug resistant tuberculosis. J Pharmacol Pharmacother. 2014;5(2):145–149. doi: 10.4103/0976-500X.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones J., Mudaly V., Voget J., Naledi T., Maartens G., Cohen K. Adverse drug reactions in South African patients receiving bedaquiline-containing tuberculosis treatment: an evaluation of spontaneously reported cases. BMC Infect Dis. 2019;19(1):544. doi: 10.1186/s12879-019-4197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashongwe I.M., Mawete F., Mbulula L., et al. Outcomes and adverse events of pre- and extensively drug-resistant tuberculosis patients in Kinshasa, Democratique Republic of the Congo: a retrospective cohort study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keshavjee S., Gelmanova I.Y., Shin S.S., et al. Hepatotoxicity during treatment for multidrug-resistant tuberculosis: occurrence, management and outcome. Int J Tuberc Lung Dis. 2012;16(5):596–603. doi: 10.5588/ijtld.11.0591. [DOI] [PubMed] [Google Scholar]
- 45.Kuaban C., Noeske J., Rieder H.L., Ait-Khaled N., Abena Foe J.L., Trebucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis. 2015;19(5):517–524. doi: 10.5588/ijtld.14.0535. [DOI] [PubMed] [Google Scholar]
- 46.Li D., He W., Chen B., Lv P. Primary multidrug-resistant tuberculosis versus drug-sensitive tuberculosis in non-HIV-infected patients: comparisons of CT findings. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matambo R., Takarinda K.C., Thekkur P., et al. Treatment outcomes of multi drug resistant and rifampicin resistant Tuberculosis in Zimbabwe: a cohort analysis of patients initiated on treatment during 2010 to 2015. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0230848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merid M.W., Gezie L.D., Kassa G.M., Muluneh A.G., Akalu T.Y., Yenit M.K. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;19(1):286. doi: 10.1186/s12879-019-3919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modongo C., Sobota R.S., Kesenogile B., et al. Successful MDR-TB treatment regimens including amikacin are associated with high rates of hearing loss. BMC Infect Dis. 2014;14:542. doi: 10.1186/1471-2334-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muñoz-Torrico M., Cid-Juárez S., Gochicoa-Rangel L., et al. Functional impact of sequelae in drug-susceptible and multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2020;24(7):700–705. doi: 10.5588/ijtld.19.0809. [DOI] [PubMed] [Google Scholar]
- 51.Murniati M., Handayani D., Isbaniah F. Two-years biannual evaluation of drug-resistant tuberculosis patients completing their treatment at persahabatan general hospital jakarta. Respir Sci. 2020;1(1):15–32. [Google Scholar]
- 52.Nair D., Velayutham B., Kannan T., et al. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. Public Health Action. 2017;7(1):32–38. doi: 10.5588/pha.16.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nuwagira E., Stadelman A., Baluku J.B., et al. Obstructive lung disease and quality of life after cure of multi-drug-resistant tuberculosis in Uganda: a cross-sectional study. Trop Med Health. 2020;48:34. doi: 10.1186/s41182-020-00221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olayanju O., Esmail A., Limberis J., Gina P., Dheda K. Linezolid interruption in patients with fluoroquinolone-resistant tuberculosis receiving a bedaquiline-based treatment regimen. Int J Infect Dis. 2019;85:74–79. doi: 10.1016/j.ijid.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Onur S.T., Ortakoylu M.G., Iliaz S., Alkan F. Treatment outcome and mortality among geriatric patients diagnosed with multiple-drug resistant tuberculosis: a comparative analysis from a tertiary referral center. Haseki Tip Bulteni. 2021;59(5):377–380. [Google Scholar]
- 56.Padayatchi N., Bionghi N., Osman F., et al. Treatment outcomes in patients with drug-resistant TB-HIV coinfection treated with bedaquiline and linezolid. Int J Tuberc Lung Dis. 2020;24(10):1024–1031. doi: 10.5588/ijtld.20.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piparva K.G., Jansari G., Singh A.P. Evaluation of treatment outcome and adverse drug reaction of directly observed treatment (DOT) plus regimen in multidrug-resistant tuberculosis (MDR-TB) patients at district tuberculosis centre Rajkot. Perspect Clin Res. 2018;9(4):165–169. doi: 10.4103/picr.PICR_165_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piubello A., Harouna S.H., Souleymane M.B., et al. High cure rate with standardised short-course multidrugresistant tuberculosis treatment in Niger: No relapses. Int J Tuberc Lung Dis. 2014;18(10):1188–1194. doi: 10.5588/ijtld.13.0075. [DOI] [PubMed] [Google Scholar]
- 59.Prasad R., Singh A., Srivastava R., Hosmane G.B., Kushwaha R.A.S., Jain A. Frequency of adverse events observed with second-line drugs among patients treated for multidrug-resistant tuberculosis. Indian J Tuberc. 2016;63(2):106–114. doi: 10.1016/j.ijtb.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 60.Ramma L., Ibekwe T.S. Cochleo-vestibular clinical findings among drug resistant Tuberculosis Patients on therapy-a pilot study. Int Arch Med. 2012;5:3. doi: 10.1186/1755-7682-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rehman S., Munir M.K., Iqbal R., Saeed M.S., Aasim M. Long term follow up of multi drug resistance tuberculosis patients after 3-4 Years of starting second line anti tubercular drugs. Ann King Edw Med Univ Lahore Pakistan. 2017;23(3):328–334. [Google Scholar]
- 62.Sabur N.F., Brar M.S., Wu L., Brode S.K. Low-dose amikacin in the treatment of multidrug-resistant tuberculosis (MDR-TB) BMC Infect Dis. 2021;21(1):254. doi: 10.1186/s12879-021-05947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagwa E., Mantel-Teeuwisse A.K., Ruswa N., et al. The burden of adverse events during treatment of drug-resistant tuberculosis in Namibia. South Med Rev. 2012;5(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- 64.Sagwa E.L., Ruswa N., Mavhunga F., Rennie T., Leufkens H.G.M., Mantel-Teeuwisse A.K. Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacol Toxicol. 2015;16:36. doi: 10.1186/s40360-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sagwa E.L., Ruswa N., Mavhunga F., et al. Renal function of MDR-TB patients treated with kanamycin regimens or concomitantly with antiretroviral agents. Int J Tuberc Lung Dis. 2017;21(12):1245–1250. doi: 10.5588/ijtld.16.0953. [DOI] [PubMed] [Google Scholar]
- 66.Satti H., McLaughlin M.M., Omotayo D.B., et al. Outcomes of comprehensive care for children empirically treated for multidrug-resistant tuberculosis in a setting of high HIV prevalence. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnippel K., Berhanu R.H., Black A., et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study. BMC Infect Dis. 2016;16(1):593. doi: 10.1186/s12879-016-1933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seddon J.A., Thee S., Jacobs K., Ebrahim A., Hesseling A.C., Schaaf H.S. Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect. 2013;66(4):320–329. doi: 10.1016/j.jinf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Seid Y., Ketema B., Yilma M., Desalegn Z. Severe adverse effects associated with multidrug resistant tuberculosis medications among patients attending alert hospital, Ethiopia. Ethiop Med J. 2019;57(1):49–58. [Google Scholar]
- 70.Shah N.P., Pyakurel P., Khanal M., et al. High success and low recurrence with shorter treatment regimen for multidrug-resistant tb in Nepal. Public Health Action. 2021;11:38–45. doi: 10.5588/pha.21.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma V., Bhagat S., Verma B., Singh R., Singh S. Audiological evaluation of patients taking kanamycin for multidrug resistant tuberculosis. Iran J Otorhinolaryngol. 2016;28(86):203–208. [PMC free article] [PubMed] [Google Scholar]
- 72.Shastri U., Khateeb Z.F., Khan M.A., Kanagokar V. Hearing in individuals enrolled for drug-resistant tuberculosis treatment: a retrospective study from a South Indian city. Hear Bal Commun. 2021;19(2):118–125. [Google Scholar]
- 73.Shean K., Streicher E., Pieterson E., et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singla N., Singla R., Fernandes S., Behera D. Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tuberc. 2009;56(4):206–212. [PubMed] [Google Scholar]
- 75.Singla R., Mallick M., Mrigpuri P., Singla N., Gupta A. Sequelae of pulmonary multidrug-resistant tuberculosis at the completion of treatment. Lung India. 2018;35(1):4–8. doi: 10.4103/lungindia.lungindia_269_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith J.P., hi N.R., Shah N.S., et al. The impact of concurrent antiretroviral therapy and MDR-TB treatment on adverse events. J Acquir Immune Defic Syndr. 2020;83(1):47–55. doi: 10.1097/QAI.0000000000002190. (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sogebi O.A., Adefuye B.O., Adebola S.O., Oladeji S.M., Adedeji T.O. Clinical predictors of aminoglycoside-induced ototoxicity in drug-resistant Tuberculosis patients on intensive therapy. Auris Nasus Larynx. 2017;44(4):404–410. doi: 10.1016/j.anl.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Sogebi O.A., Fadeyi M.O., Adefuye B.O., Soyinka F.O. Hearing thresholds in patients with drug-resistant tuberculosis: baseline audiogram configurations and associations. J Bras Pneumol. 2017;43(3):195–201. doi: 10.1590/S1806-37562016000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tack I., Dumicho A., Ohler L., et al. Safety and effectiveness of an all-oral, bedaquiline-based, shorter treatment regimen for rifampicin-resistant tuberculosis in high human immunodeficiency Virus (HIV) burden rural South Africa: a retrospective cohort analysis. Clin Infect Dis. 2021;73(9):E3563–E3571. doi: 10.1093/cid/ciaa1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trubnikov A., Hovhannesyan A., Akopyan K., et al. Effectiveness and safety of a shorter treatment regimen in a setting with a high burden of multidrug-resistant tuberculosis. Int J Environ Res Public Health. 2021;18(8):4121. doi: 10.3390/ijerph18084121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vashakidze S.A., Kempker J.A., Jakobia N.A., et al. Pulmonary function and respiratory health after successful treatment of drug-resistant tuberculosis. Int J Infect Dis. 2019;82:66–72. doi: 10.1016/j.ijid.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wangchuk P., Ram Adhikari T., Nima G., Dendup P. Audiological monitoring of patients undergoing multidrug resistant tuberculosis treatment at jigme dorji wangchuk national referral hospital and gidakom hospital, Bhutan. J Clin Tuberc Other Mycobact Dis. 2021;23 doi: 10.1016/j.jctube.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S., Yu Y., Ji Y., et al. Multi-drug resistant spinal tuberculosis-epidemiological characteristics of in-patients: a multicentre retrospective study. Epidemiol Infect. 2020;148:e11. doi: 10.1017/S0950268820000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Wu S., Xia Y., et al. Adverse events associated with treatment of multidrug-resistant tuberculosis in China: an ambispective cohort study. Med Sci Monit. 2017;23:2348–2356. doi: 10.12659/MSM.904682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zubair S.M., Ali M.G., Irfan M. Post tuberculosis radiological sequelae in patients treated for pulmonary and pleural tuberculosis at a tertiary center in Pakistan. Monaldi Arch Chest Dis. 2021;92(1) doi: 10.4081/monaldi.2021.1814. [DOI] [PubMed] [Google Scholar]
- 86.Oladimeji O., Adeniji-Sofoluwe A.T., Othman Y., et al. Chest X-ray features in drug-resistant tuberculosis patients in Nigeria; a retrospective record review. Medicines (Basel, Switzerland) 2022;9(9):46. doi: 10.3390/medicines9090046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alene K.A., Wangdi K., Colquhoun S., et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med. 2021;19(1):203. doi: 10.1186/s12916-021-02063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13(1):119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization . World Health Organization; Geneva: 2018. Rapid Communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) [Google Scholar]
- 90.WHO consolidated guidelines on tuberculosis: module 4: treatment - Drug-resistant tuberculosis treatment . World Health Organization; Geneva: 2020. WHO guidelines approved by the guidelines review committee. [PubMed] [Google Scholar]
- 91.Maguire G., Anstey N.M., Ardian M., et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis. 2009;13(12):1500–1506. [PubMed] [Google Scholar]
- 92.Lim S.A. Ethambutol-associated optic neuropathy. Ann Acad Med Singap. 2006;35(4):274–278. [PubMed] [Google Scholar]
- 93.Lopez-Pastorini A., Plönes T., Ludwig C., Stoelben E. Intrapulmonary aspergilloma in an old tuberculous cavity with access to the bronchial system. Eur J Cardiothorac Surg. 2014;46(1):144. doi: 10.1093/ejcts/ezt533. [DOI] [PubMed] [Google Scholar]
- 94.Dheda K., Booth H., Huggett J.F., Johnson M.A., Zumla A., Rook G.A. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192(7):1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 95.Dheda K., Chang K.C., Guglielmetti L., et al. Clinical management of adults and children with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Microbiol Infect. 2017;23(3):131–140. doi: 10.1016/j.cmi.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27(147) doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters D.H., Garg A., Bloom G., Walker D.G., Brieger W.R., Rahman M.H. Poverty and access to health care in developing countries. Ann N Y Acad Sci. 2008;1136:161–171. doi: 10.1196/annals.1425.011. [DOI] [PubMed] [Google Scholar]
- 98.Cox V., Cox H., Pai M., Stillo J., Citro B., Brigden G. Health care gaps in the global burden of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23(2):125–135. doi: 10.5588/ijtld.18.0866. [DOI] [PubMed] [Google Scholar]
- 99.Lazar M., Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35(1):28–37. doi: 10.1080/07370016.2018.1404832. [DOI] [PubMed] [Google Scholar]
- 100.Fan H., Yan Q., Coyte P.C., Yu W. Does public health insurance coverage lead to better health outcomes? Evidence from Chinese adults. Inquiry. 2019;56 doi: 10.1177/0046958019842000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gulla K.M., Kabra S.K., Lodha R. Feasibility of pediatric non-invasive respiratory support in low- and middle-income countries. Indian Pediatr. 2021;58(11):1077–1084. doi: 10.1007/s13312-021-2377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allwood B.W., Byrne A., Meghji J., Rachow A., Van Der Zalm M.M., Schoch O.D. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration. 2021;100(8):751–763. doi: 10.1159/000512531. [DOI] [PubMed] [Google Scholar]
- 103.Dillard L.K., Martinez R.X., Perez L.L., Fullerton A.M., Chadha S., McMahon C.M. Prevalence of aminoglycoside-induced hearing loss in drug-resistant tuberculosis patients: a systematic review. J Infect. 2021;83(1):27–36. doi: 10.1016/j.jinf.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 104.Shahwan M., Hassan N., Shaheen R.A., et al. Diabetes mellitus and renal function: current medical research and opinion. Curr Diabetes Rev. 2021;17(9) doi: 10.2174/1573399817999210111205532. [DOI] [PubMed] [Google Scholar]
- 105.Monhart V. [Hypertension and chronic renal insufficiency--chronic kidney failure] Vnitr Lek. 2003;49(5):388–394. [PubMed] [Google Scholar]
- 106.Thomas P.P. Changing profile of causes of chronic renal failure. Saudi J Kidney Dis Transpl. 2003;14(4):456–461. [PubMed] [Google Scholar]
- 107.Krishna S., Jacob J.J. In: Endotext. Feingold K.R., Anawalt B., Boyce A., et al., editors. 2000. Diabetes mellitus and tuberculosis.www.Endotext.org South Dartmouth (MA) [Google Scholar]
- 108.Soyoye D.O., Abiodun O.O., Ikem R.T., Kolawole B.A., Akintomide A.O. Diabetes and peripheral artery disease: a review. World J Diabetes. 2021;12(6):827–838. doi: 10.4239/wjd.v12.i6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prasad R. Multidrug and extensively drug-resistant tuberculosis management: evidences and controversies. Lung India. 2012;29:154–159. doi: 10.4103/0970-2113.95321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hong H., Dowdy D.W., Dooley K.E., et al. Aminoglycoside-induced hearing loss among patients being treated for drug-resistant tuberculosis in South Africa: a prediction model. Clin Infect Dis. 2020;70(5):917–924. doi: 10.1093/cid/ciz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghafari N., Court R., Chirehwa M.T., et al. Pharmacokinetics and other risk factors for kanamycin-induced hearing loss in patients with multi-drug resistant tuberculosis. Int J Audiol. 2020;59(3):219–223. doi: 10.1080/14992027.2019.1690170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park D.H., Park T.K., Ohn Y.H., Park J.S., Chang J.H. Linezolid induced retinopathy. Doc Ophthalmol. 2015;131(3):237–244. doi: 10.1007/s10633-015-9518-6. [DOI] [PubMed] [Google Scholar]
- 113.Singh B., Cocker D., Ryan H., Sloan D.J. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3(3):CD012836. doi: 10.1002/14651858.CD012836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mafukidze A.T., Calnan M., Furin J. Peripheral neuropathy in persons with tuberculosis. J Clin Tuberc Other Mycobact Dis. 2016;2:5–11. doi: 10.1016/j.jctube.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.