Abstract
Objective
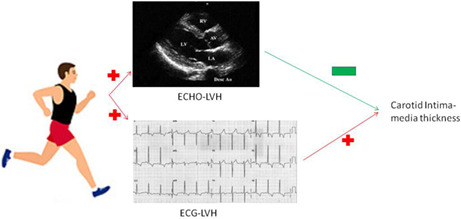
Both electrocardiographic and echocardiographic left ventricular hypertrophy (LVH) have been reported with an association with greater carotid intima‐media thickness (cIMT), a marker of subclinical atherosclerosis in patients with hypertension, while the associations are unclear in physically fit young adults.
Methods
A total of 1822 Taiwanese military personnel, aged 18–40 years, received an annual health examination including electrocardiography (ECG) and echocardiography in 2018–2020. Left carotid bulb cIMT was measured by high‐resolution ultrasonography. Multiple logistic regression analysis with adjustments for age, sex, smoking, alcohol consumption, body mass index, mean blood pressure, and physical fitness was used to determine the associations between echocardiographic and ECG parameters and the highest quintile of cIMT (≥0.8 mm).
Results
Cornell‐based LVH, Myers et al.‐based RVH and heart rate ≥75/min were associated with cIMT ≥0.8 mm [odds ratios (ORs) and 95% confidence intervals: 1.54 (1.01, 2.35), 1.66 (1.18, 2.33), and 1.39 (1.06, 1.83), respectively], while echocardiographic LVH defined as ≥46.0 g/m2.7 for men and ≥38.0 g/m2.7 for women was inversely associated with cIMT ≥0.8 mm [OR: 0.45 (0.24, 0.86)].
Conclusion
In tactical athletes of military, the associations of ECG and echocardiographic LVH with cIMT were in opposite directions. Higher physical fitness may cause cardiac muscle hypertrophy and reduce the atherosclerosis severity, possibly leading to the paradoxical echocardiographic finding. This study suggests that ECG‐based LVH remains a good marker of subclinical atherosclerosis in our military population.
Keywords: carotid intima‐media thickness, echocardiography, electrocardiography, military personnel, tactical athletes
In tactical athletes of military, the associations of ECG and echocardiographic LVH with cIMT were in opposite directions. Higher physical fitness may cause cardiac muscle hypertrophy and reduce the atherosclerosis severity, possibly leading to the paradoxical echocardiographic finding. This study suggests that ECG‐based LVH remains a good marker of subclinical atherosclerosis in our military population.
1. INTRODUCTION
Atherosclerosis is an inflammatory process in the arterial bed related to many vascular risk factors, for example, high blood pressure (BP) and low‐density lipoprotein cholesterol (LDL‐C) (Lin, Li, et al., 2016; Lin, Liu, et al., 2016). An unhealthy lifestyle, such as cigarette smoking, alcohol consumption and sedentary behavior, may increase systemic inflammation and contribute to the progress in atherosclerosis (Chevli et al., 2020; Lavie et al., 2019; Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022). The subclinical atherosclerosis burden can be evaluated, using ultrasonography, to measure the carotid artery intima‐media thickness (cIMT) and assessed by coronary imaging in those with symptomatic angina. Presence of end‐organ damage may have a synergic effect with the pre‐existed vascular risk factors on the progression of atherosclerosis, resulting in a greater incidence of cardiovascular diseases (CVDs). Prior studies have shown that both electrocardiography (ECG) and echocardiography‐based left ventricular hypertrophy (LVH) were associated with a higher risk of great cIMT level, coronary atherosclerosis, myocardial infarction, and a composite of CVD events in patients with hypertension or diabetes (Chahal et al., 2012; Kishi et al., 2015; Liao et al., 2021; Meijs et al., 2009). However, it is unclear whether the association of ECG and echocardiography‐based LVH with atherosclerosis is consistently present in those maintaining good health and without long‐term hypertension.
As is known, regular exercise can modulate heart function and structure, which increases left ventricular mass (LVM) and augments electrical conduction, for example, ECG‐LVH (Lin et al., 2021; Liu et al., 2021). In addition, exercises can reduce the prevalence and severity of vascular risk factors, for example, obesity, dyslipidemia, and hypertension, which are related to the atherosclerosis progression (Lavie et al., 2019; Lin, Li, et al., 2016; Lin, Liu, et al., 2016). Several prior studies have shown that both aerobic and resistant exercises were associated with reduced burden of atherosclerosis assessed by cIMT in children and young adults (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022; Melo et al., 2015). However, the impacts of echocardiography and ECG‐based‐LVH on atherosclerosis were rarely investigated in physically active individuals, such as military personnel. Therefore, the present study aimed to clarify the associations between ECG and echocardiographic parameters and subclinical atherosclerosis, that is, cIMT in tactical athletes of military in Taiwan.
2. METHODS
2.1. Study population
The study population is obtained from the cardiorespiratory fitness and health in eastern armed forces (CHIEF)—atherosclerosis study, which included 1822 military men and women, ages of 18–43 years, free of taking any medication for hypertension or dyslipidemia in Hualien, Taiwan during the period between 2018 and 2021 (Lin, Li, et al., 2016; Lin, Liu, et al., 2016; Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022). All participants received exercise training regularly in their military bases and completed the annual health examination for laboratory tests and a self‐report regarding the habit of toxic substances use, for example, alcohol consumption and cigarette smoking (former or never vs. active) in the Hualien Armed Forces General Hospital. In the same year after the health examination performed, participants completed a 3000‐m run field test in the Huadong Military Physical Training and Testing Center to evaluate endurance capacity (Chen et al., 2017). A 12‐lead surface ECG and a transthoracic echocardiography were performed for evaluating the cardiac structure and an ultrasonography was carried out for measuring cIMT, after the run test and before the end of index year (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022).
2.2. Measurements of anthropometric and hemodynamic parameters
Waist circumference, body weight, and body height of each study participant were measured in a standing position. Body mass index (BMI) was defined as body weight divided by body height squared (kg/m2). The BP of each participant was measured at rest over the right arm in a sitting position by the automatic detection device (FT201 Parama‐Tech Co., Ltd, Fukuoka, Japan), using the oscillometric method. Both the anthropometric and hemodynamic measurements were supervised by an experienced medical staff. If the systolic BP level was ≥140 mmHg or the diastolic BP level was ≥90 mmHg, another two times of BP measurement were carried out and averaged as the final BP level (Lin, Fan, et al., 2020; Lin, Liu, et al., 2020). Mean BP was defined as (1 × systolic BP + 2 × diastolic BP)/3. Blood biochemical tests, that is, lipid profiles, fasting glucose, and serum uric acid were measured, using an auto analyzer (AU640, Olympus, Kobe, Japan) (Lin, Fan, et al., 2020; Lin, Liu, et al., 2020).
2.3. The 3000‐M run field test
The 3000‐m run field test was performed outdoor on a flat playground in the Huadong Military Physical Training and Testing Center at 16:00 pm, if there was no heavy raining and the heat stroke risk coefficient, which is defined as the product of outdoor temperature (Celsius scale) and relative humidity (%) × 0.1 <40 (Chen et al., 2017). All participants completed the run test without bearing a load.
2.4. cIMT measurements
Measurement of the cIMT dimension over the left far‐wall carotid artery bulb was performed, using an ultrasound which was equipped with a linear transducer of 4–8 MHz, by the iE33 device (Philips Medical Systems, Andover, MA, USA). The distance between the leading edges of the media–adventitia interface and the lumen‐intima interface was calculated as the cIMT. For verifying the cIMT, the measurement procedure was performed repeatedly, and the coefficient of variation was up to 96.8% (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022). In whole of the carotid sonographic examinations, no carotid plagues were observed.
2.5. Twelve‐lead surface ECG
The 12‐lead ECG was operated via the CARDIOVIT MS‐2015 machine (Schiller AG, Baar, Switzerland). The ECG report was interpreted by a board‐certified cardiologist. The Cornell voltage criterion for LVH is defined, when (R‐aVL + S‐V3) ≥18 mm and ≥6 mm respectively for Asian young men and women were met (Su et al., 2017, 2020). ECG‐based RVH is defined, when (1) the R/S ratio in V5 or the R/S ratio in V1 > 1, or (2) R‐V1 > 6 mm, on the basis of the Myers et al. (1948) criteria, or when (R‐V1 + S‐V5 or S‐V6) >10.5 mm according to the Sokolow‐Lyon voltage criterion (Sokolow & Lyon, 1949) is fulfilled. ECG‐based left atrial enlargement is defined if a notched P wave ≥0.12 s in lead II or a notch of ≥0.04 s is fulfilled (Alpert & Munuswamy, 1989). Left axis deviation means the QRS axis between −30° and −90°, and right axis deviation means the QRS axis between +90。and +180° (Lévy, 1991).
2.6. Echocardiographic measurements
All participants underwent a transthoracic echocardiography equipped with a 1–5 MHz transducer (iE33; Philips Medical Systems, Andover, MA, USA) at the Hualien‐Armed Forces General Hospital. The cardiac dimensions and diastolic functions were measured, according to the recommendations of the American Society of Echocardiography (Lang et al., 2015). LVM was calculated at end diastole, using the Devereux et al. formula (Devereux et al., 1984), which was described as 0.8 × {1.04 × [(left ventricular internal diameter (LVIDd) + posterior wall thickness + interventricular septal thickness3 − LVIDd3)]} + 0.6. The highest 5% of the LVM controlled for the body height (m2.7), was 46.0 g/m2.7 in men (Lin, Fan, et al., 2020; Lin & Liu, 2020; Lin & Lu, 2020) and 38.0 g/m2.7 in women (Su et al., 2020), which were defined as echocardiographic LVH in this study. The highest 5% of the RV wall thickness was 5.5 mm in men and 5.2 mm in women (Lin, Fan, et al., 2020; Lin& Lu, 2020; Meng et al., 2017; Su et al., 2020) which were defined as echocardiographic RVH in this study.
2.7. Statistical analysis
The highest quintile of the cIMT (≥0.8 mm) was defined as the significant group (N = 402) and the other quintiles were defined as the control group (N = 1420). Characteristics of participants were expressed as numbers (%) for categorical variables and mean ± standard deviation for continuous variables, respectively. Analysis of covariance (ANCOVA) was used to examine the differences in the mean values of echocardiographic and ECG continuous variables between the significant and the control groups with adjustment for age, sex, BMI, mean BP and time for a 3000‐m field run test. Multiple linear regression analysis was used to determine the association between aerobic fitness assessed by time for a 3000‐m run and LVM scaled to body height (m2.7). Multiple logistic regression analysis was used to determine the independent ECG and echocardiographic predictors for cIMT ≥0.80 mm. Two models were used for multiple regression analyses. In model 1, age, sex, BMI, alcohol intake, smoking, and mean BP were adjusted. In model 2, time for a 3000‐m run field test was further adjusted. A value of p < .05 was considered significant. All analyses were performed by SPSS version 25.0 software for Windows (IBM Corp., Armonk, NY, USA). The Institutional Review Board of the Mennonite Christian Hospital (No. 16–05‐008) in Taiwan approved the study, and written informed consent was obtained from all participants.
3. RESULTS
3.1. The demographic and laboratory findings
The clinical characteristics of participants in the significant cIMT group and the control group are shown in Table 1. The mean age of participants was 27 years, the mean BMI level was 24.6 kg/m2, and the mean BP level was 101.6 mmHg. No differences in demographic variables, anthropometric variables, BP levels, and plasma metabolic biomarkers between the two groups were found.
TABLE 1.
Clinical characteristics of participants with ultrasonography for carotid intima‐media thickness
| cIMT <0.8 mm (N = 1420) | cIMT ≥0.8 mm (N = 402) | p value | |
|---|---|---|---|
| cIMT (mm) | 0.64 ± 0.08 | 0.86 ± 0.09 | <.001 |
| Range: min–max | (0.41–0.79) | (0.80–1.59) | |
| Age (years) | 27.14 ± 6.02 | 26.97 ± 6.15 | .62 |
| Male sex (%) | 1262 (88.9) | 250 (87.1) | .31 |
| Alcohol drinking (%) | 558 (39.3) | 170 (42.3) | .27 |
| Cigarette smoking (%) | 573 (40.4) | 177 (44.0) | .18 |
| Body mass index (kg/m2) | 24.57 ± 3.75 | 24.71 ± 3.67 | .49 |
| Waist circumference (cm) | 82.38 ± 9.91 | 82.58 ± 9.78 | .72 |
| Systolic BP (mmHg) | 117.73 ± 13.38 | 117.66 ± 13.24 | .92 |
| Diastolic BP (mmHg) | 69.74 ± 10.23 | 69.12 ± 10.48 | .29 |
| Mean BP (mmHg) | 101.73 ± 11.45 | 101.48 ± 11.47 | .69 |
| Blood test | |||
| Total cholesterol (mg/dl) | 173.15 ± 32.80 | 174.88 ± 34.73 | .35 |
| LDL‐C (mg/dl) | 106.88 ± 29.83 | 107.43 ± 31.49 | .74 |
| HDL‐C (mg/dl) | 50.40 ± 10.88 | 50.80 ± 11.57 | .52 |
| Triglycerides (mg/dl) | 102.64 ± 81.58 | 108.94 ± 86.14 | .17 |
| Fasting glucose (mg/dl) | 93.28 ± 10.40 | 93.32 ± 16.38 | .95 |
| Serum uric acid (mg/dl) | 6.52 ± 1.45 | 6.53 ± 1.49 | .94 |
| Time for a 3000‐m run (s) | 877.10 ± 105.25 | 906.47 ± 126.71 | .004 |
Note: Continuous variables are expressed as mean ± SD, and categorical variables as numbers (%).
Abbreviations: BP, blood pressure; cIMT, carotid intima‐media thickness; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
3.2. The echocardiographic findings
The results of a comparison in echocardiographic parameters between groups are shown in Table 2. In the univariate comparison, participants with cIMT ≥0.8 mm had a greater aortic valve opening diameter (20.08 mm vs. 19.83 mm) and mitral peak A velocity (51.49 cm/s vs. 49.56 cm/s) than those with cIMT ≥0.8 mm. In addition, participants with cIMT ≥0.8 mm had a greater prevalence in mild aortic, mitral and tricuspid regurgitation than those with cIMT <0.8 mm. However, participants with cIMT ≥0.8 mm had a lower LVM index than those with cIMT <0.8 mm (76.34 g/m2.7 vs. 78.60 g/m2.7), which was consistent with the results of ANCOVA. In addition, there was a lower prevalence in LVH in participants with cIMT ≥0.8 mm than those with cIMT <0.8 mm (3.5% vs. 7.1%). No differences in LV diastolic function indices, RV outflow tract diameter and RV wall thickness were found.
TABLE 2.
Echocardiographic findings of participants with ultrasonography for carotid intima‐media thickness
| cIMT <0.8 mm (N = 1420) | cIMT ≥0.8 mm (N = 402) | p‐value | p‐value a | |
|---|---|---|---|---|
| Aortic valve open (mm), PLAX | 19.83 ± 2.30 | 20.08 ± 1.98 | .04 | .12 |
| Aortic root dimension (mm), PLAX | 29.64 ± 3.31 | 29.80 ± 3.07 | .37 | .11 |
| LV mass (g), PLAX | 144.53 ± 34.28 | 140.97 ± 31.85 | .06 | .06 |
| LV mass index (g/m2.7), PLAX | 78.60 ± 15.30 | 76.34 ± 13.26 | .007 | .02 |
| LV posterior wall (mm), PLAX | 8.47 ± 1.08 | 8.37 ± 1.02 | .09 | .21 |
| LV internal dimension in diastole (mm), PLAX | 48.74 ± 3.76 | 48.52 ± 3.62 | .29 | .22 |
| LV internal dimension in systole (mm), PLAX | 30.51 ± 3.41 | 30.51 ± 3.54 | .99 | .37 |
| Interventricular septum, (mm), PLAX | 8.69 ± 1.19 | 8.58 ± 1.12 | .10 | .18 |
| RV wall thickness (mm), PLAX | 4.63 ± 0.60 | 4.62 ± 0.57 | .93 | .98 |
| RV outflow tract dimension in diastole (mm), PLAX | 26.35 ± 4.13 | 26.38 ± 4.06 | .88 | .56 |
| Left atrial dimension (mm), PLAX | 32.71 ± 4.08 | 32.56 ± 3.97 | .49 | .23 |
| LV hypertrophy | 101 (7.1) | 14 (3.5) | .008 | |
| RV hypertrophy | 102 (7.2) | 24 (6.0) | .39 | |
| LV ejection fraction (%), PLAX | 62.02 ± 4.97 | 61.89 ± 4.76 | .63 | .72 |
| Aortic regurgitation ≥ mild grade | 23 (1.6) | 13 (3.2) | .04 | |
| Mitral regurgitation ≥ mild grade | 1103 (77.7) | 336 (83.6) | .01 | |
| Pulmonary regurgitation ≥ mild grade | 973 (68.5) | 269 (66.9) | .54 | |
| Tricuspid regurgitation ≥ mild grade | 1170 (82.4) | 350 (87.1) | .02 | |
| RV systolic pressure (mmHg) | 27.85 ± 4.30 | 27.97 ± 4.05 | .61 | .76 |
| Peak E velocity (cm/s) | 87.36 ± 27.54 | 87.59 ± 15.45 | .87 | .61 |
| Peak A velocity (cm/s) | 49.56 ± 10.68 | 51.49 ± 27.33 | .03 | .03 |
| E/A ratio | 1.85 ± 0.91 | 1.86 ± 1.06 | .86 | .87 |
| Peak E′ velocity (cm/s) | 17.35 ± 6.69 | 17.75 ± 5.64 | .27 | .54 |
| Peal A′ velocity (cm/s) | 8.63 ± 4.12 | 8.51 ± 2.21 | .58 | .50 |
| E′/A′ ratio | 2.18 ± 1.00 | 2.23 ± 0.88 | .38 | .57 |
| LV diastolic dysfunction | 45 (3.2) | 16 (4.0) | .43 | |
| E/A < 0.8 | 11 (0.8) | 5 (1.2) | .37 | |
| E′ < 10 cm/s | 38 (2.7) | 13 (3.2) | .56 | |
| E/E′ > 14 | 1 (0.1) | 1 (0.2) | .34 |
Note: Continuous variables are expressed as mean ± standard deviation and categorical variables as numbers (%).
Abbreviations: LV, left ventricle; PLAX, parasternal long axis window; RV, right ventricle.
ANCOVA for continuous variables with adjustment for age, sex, body mass index, mean blood pressure, and time for a 3000‐m run.
3.3. The ECG findings
The results of a comparison in ECG parameters between groups are revealed in Table 3. In the univariate comparison, compared with those with cIMT ≥0.8 mm, participants with cIMT ≥0.8 mm had a greater P wave duration (106.28 ms vs. 104.04 ms) and QTc interval (397.83 ms vs. 394.29 ms), which were relevant to the results of ANCOVA. With adjustments for covariates in ANCOVA, heart rate (68.04 beats/min vs. 66.98 beats/min) and QRS duration (96.68 ms vs. 95.81 ms) were found greater in those with cIMT ≥0.8 mm than those with cIMT <0.8 mm. The prevalence of ECG‐based LVH (10.4% vs. 7.6%, p = .07), ECG‐based RVH (15.9% vs. 11.5%) and right axis deviation (12.4% vs. 8.3%) were higher in those with cIMT ≥0.8 mm than those with cIMT <0.8 mm.
TABLE 3.
Electrocardiographic findings of participants with ultrasonography for carotid intima‐media thickness
| cIMT <0.8 mm (N = 1420) | cIMT ≥0.8 mm (N = 402) | p‐value | p‐value a | |
|---|---|---|---|---|
| Heart rate (beats per minute) | 66.98 ± 11.19 | 68.04 ± 11.25 | .09 | .04 |
| P duration (ms) | 104.04 ± 16.14 | 106.28 ± 15.18 | .01 | .01 |
| PR interval (ms) | 154.90 ± 20.40 | 155.73 ± 17.58 | .45 | .30 |
| QRS duration (ms) | 95.81 ± 10.48 | 96.68 ± 9.96 | .13 | .04 |
| QTc interval (ms) | 394.29 ± 25.26 | 397.83 ± 25.53 | .01 | .01 |
| QRS axis (degree) | 62.88 ± 28.81 | 63.40 ± 28.69 | .74 | .63 |
| ECG‐based LVH | 108 (7.6) | 42 (10.4) | .07 | |
| ECG‐based RVH | 164 (11.5) | 64 (15.9) | .01 | |
| ECG‐based LAE | 280 (19.7) | 66 (16.4) | .13 | |
| Sinus bradycardia | 371 (26.1) | 89 (22.1) | .10 | |
| Ectopic P rhythm | 62 (4.4) | 14 (3.5) | .43 | |
| First‐degree atrioventricular block | 38 (2.7) | 7 (1.7) | .28 | |
| Left axis deviation | 16 (1.1) | 4 (1.0) | .82 | |
| Right axis deviation | 118 (8.3) | 50 (12.4) | .01 | |
| Complete RBBB | 32 (2.3) | 10.(2.5) | .78 | |
| Incomplete RBBB | 71 (5.0) | 23 (5.7) | .56 | |
| QTc prolongation | 12 (0.8) | 3 (0.7) | .84 | |
| Inferior T wave inversion | 56 (3.9) | 18 (4.5) | .63 |
Note: Continuous variables are expressed as mean ± SD, and categorical variables as numbers (%).
Abbreviations: ECG, electrocardiography; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; RVH, right ventricular hypertrophy.
ANCOVA for continuous variables with adjustment for age, sex, body mass index, mean blood pressure, and time for a 3000‐m run.
3.4. The aerobic fitness and left ventricular mass index association
The multiple linear regression analysis results for the association between time for a 3000‐m run (s) and LVM scaled to body height (m2.7) are revealed in Table 4. Time for a 3000‐m run (s) was negatively associated with LVM index (g/m2.7) in the univariate analysis and Model 1 (β and 95% confidence intervals (CIs): −0.026 (−0.033, −0.020) and −0.014 (−0.021, −0.007), respectively).
TABLE 4.
Association between aerobic fitness assessed by time for A 3000‐M run and left ventricular mass
| Crude model | Model 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| R | β | 95% CI | p‐value | R | β | 95% CI | p‐value | |
| Time for a 3000‐m run (s) | 0.20 | −0.026 | −0.033, −0.020 | <.001 | 0.46 | −0.014 | −0.021, −0.007 | <.001 |
Note: Data are presented as β and 95% confidence interval (CI). Multiple linear regression analysis model is used to determine the association between time for a 3000‐m run (s) and left ventricular mass scaled to body height (m2.7). Model 1: age, sex, body mass index, alcohol intake, tobacco smoking, mean blood pressure adjustments.
3.5. The ECG and echocardiographic markers for significant carotid intima‐media thickness
The multiple logistic regression analysis results for the associations of ECG and echocardiographic markers with cIMT ≥0.8 mm, are revealed in Table 5. In the univariate analysis, those with ECG‐based RVH and heart rate ≥75 beats/min were more likely to have cIMT ≥0.8 mm (odds ratios (ORs): 1.57 (1.13, 2.19) and 1.39 (1.06, 1.82), respectively). There were borderline significant associations for ECG‐based LVH and right axis deviation (ORs: 1.47, p = .07 and 1.38, p = .09, respectively). In Model 1 and further adjustment for time for a 3000‐m run in Model 2, the associations remained significant for ECG‐based RVH and heart rate ≥ 75 beats/min (ORs: 1.63 (1.16, 2.29) and 1.35 (1.02, 1.78), respectively in Model 2). It is notable that the associations for ECG‐based LVH was significant in both Models 1 and 2 (OR: 1.54 (1.01, 2.35) and 1.52 (1.00, 2.32), respectively). On the contrary, participants with echocardiographic LVH were less likely to have cIMT ≥0.8 mm in the crude Model, Model 1 and Model 2 (ORs: 0.51 (0.28, 0.95), 0.45 (0.24, 0.86) and 0.44 (0.23, 0.84), respectively). No associations of LV diastolic dysfunction with cIMT ≥0.8 mm were found in all models.
TABLE 5.
Associations of electrocardiographic and echocardiographic markers with carotid intima‐media thickness ≥0.8 mm
| Crude model | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Echo‐based LVH | 0.51 | 0.28, 0.95 | .03 | 0.45 | 0.24, 0.86 | .01 | 0.44 | 0.23, 0.84 | .01 |
| ECG‐based LVH | 1.47 | 0.97, 2.23 | .07 | 1.54 | 1.01, 2.35 | .04 | 1.52 | 1.00, 2.32 | .05 |
| ECG‐based RVH | 1.57 | 1.13, 2.19 | .008 | 1.66 | 1.18, 2.33 | .003 | 1.63 | 1.16, 2.29 | .005 |
| Right axis deviation | 1.38 | 0.95, 2.01 | .09 | 1.44 | 0.98, 2.11 | .06 | 1.44 | 0.98, 2.12 | .06 |
| HR ≥75 beats/min | 1.39 | 1.06, 1.82 | .01 | 1.39 | 1.06, 1.83 | .01 | 1.35 | 1.02, 1.78 | .03 |
| LVDD | 1.54 | 0.81, 2.93 | .19 | 1.55 | 0.80, 3.01 | .19 | 1.50 | 0.77, 2.92 | .23 |
Note: Data are presented as odds ratio (OR) and 95% confidence interval (CI). Multiple logistic regression analysis models are used to determine the association of electrocardiographic and echocardiographic markers with carotid intima‐media thickness ≥0.8 mm. Model 1: age, sex, body mass index, alcohol intake, tobacco smoking, mean blood pressure adjustments. Model 2: age, sex, body mass index, alcohol intake, tobacco smoking, mean blood pressure, and time for 3000‐m run adjustments.
Abbreviations: ECG, electrocardiography; HR, heart rate; LAE, left atrial enlargement; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; RVH, right ventricular hypertrophy.
4. DISCUSSION
The main findings in this study were that in tactical athletes, a greater LV mass index, possibly related to greater aerobic fitness, was associated with a lower cIMT level. No other echocardiographic LV or RV indices, that is, RV chamber size and LV diastolic function were associated with cIMT levels. On the contrary, ECG‐based LVH and ECG‐based RVH and right axis deviation were associated with greater cIMT levels. All these associations were established independently of age, sex, body weight, mean BP, and aerobic fitness.
This study shows a paradoxical finding in the association of echocardiographic LVH with significant cIMT compared with the previous study findings. Among patients with hypertension, higher BP level is a crucial shared risk factor and plays a major role in the positive relationship between LVM and cIMT (Meijs et al., 2009). In tactical athletes, such as our military personnel, prior studies have revealed that physical fitness could increase LV muscle hypertrophy and diastolic function (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022; Liu et al., 2022). Greater cardiorespiratory fitness and muscular strength levels have revealed an inverse association with lower cIMT levels (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022). It is reasonable that greater LVM related to more intensive physical training was inversely associated with lower cIMT, which highlights that echocardiographic LVH may be a protective marker for atherosclerosis in physically fit individuals in this study. Moreover, most of the increased LV diastolic functions related to exercise training directed from reduced heart rate (Lin, Liu, Tsai, et al., 2022; Lin, Lloyd‐Jones, Colangelo, et al., 2022; Lin, Tsai, Chang, et al., 2022; Lin, Tsai, Han, et al., 2022), which revealed an association with significant cIMT in this study, reconfirming the physical effects on both LVM and cIMT.
In another respect, this study reveals a consistent finding for the association of ECG‐based LVH with cIMT compared with the previous report (Liao et al., 2021). Our prior study has shown that prehypertension was a potent risk factor of ECG‐based LVH in both physically fit young men and women (Tsai et al., 2022). Similarly, hypertension was also found as a risk factor of ECG‐based LVH in the general population (Cao et al., 2019; Lehtonen et al., 2021). Right axis deviation implying a greater RV electrical force and ECG‐based RVH were found as highly prevalent as ECG‐based LVH in patients with hypertension (Cuspidi et al., 2009, 2013). By contrast, in our prior study for physically fit young men, aerobic fitness and muscular strength levels were not significantly associated with ECG‐based LVH and RVH (Chao et al., 2019). Therefore, the associations of ECG‐based LVH and RVH with cIMT might be linked to elevated BP, which outweighed the impact of physical fitness in this study.
4.1. Study strengths and limitations
The major strength of this study was that military personnel received regular training in Taiwan, and the military environments were unified, which could reduce unmeasured confounders. In contrast, there had some restrictions in this study. First, since this study was a cross‐sectional design, the temporal associations of ECG and echocardiographic parameters with cIMT could not be assessed. Second, the cIMT was merely measured over the left carotid bulb, and there might have some variations if the other sites of the carotid artery were assessed. Third, this study's findings may not be applied appropriately to non‐athletes. An increase in LVM in the general population, where hypertension is much more prevalent and fitness lower, has much worse implications and likely would be directly associated with cIMT. Finally, we could not exclude the presence of residual confounding completely although many potential covariates were adjusted in the model.
5. CONCLUSION
In tactical athletes of military, the associations of ECG and echocardiographic LVH with cIMT were in opposite directions. Greater aerobic fitness may cause cardiac muscle hypertrophy and reduce the atherosclerosis severity, possibly leading to the paradoxical echocardiographic finding. By contrast, this study suggests that ECG‐based LVH and RVH which were more related to higher BP than physical fitness remain good markers of subclinical atherosclerosis in the military population.
AUTHOR CONTRIBUTIONS
Yen‐Po Lin and Yi‐Chiung Hsu contributed equally to writing the article; Kun‐Zhe Tsai collected, analyzed, and interpreted the data; Wei‐Chun Huang and Chih‐Lu Han made critical comments and revised this study; Gen‐Min Lin was the principal investigator and responsible for this study.
FUNDING INFORMATION
This study was supported by the Medical Affairs Bureau Ministry of National Defense (MND‐MAB‐D‐112182) and Hualien Armed Forces General Hospital (HAFGH‐D‐112004).
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
The Institutional Review Board of the Mennonite Christian Hospital (No. 16‐05‐008) in Taiwan approved the study, and written informed consent was obtained from all participants.
ACKNOWLEDGMENTS
None.
Lin, Y.‐P. , Hsu, Y.‐C. , Tsai, K.‐Z. , Huang, W.‐C. , Han, C.‐L. , & Lin, G.‐M. (2023). Electrocardiographic and echocardiographic predictors of greater carotid intima‐media thickness in tactical athletes: The CHIEF atherosclerosis study. Annals of Noninvasive Electrocardiology, 28, e13045. 10.1111/anec.13045
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alpert, M. A. , & Munuswamy, K. (1989). Electrocardiographic diagnosis of left atrial enlargement. Archives of Internal Medicine, 149(5), 1161–1165. [PubMed] [Google Scholar]
- Cao, X. , Broughton, S. T. , Waits, G. S. , Nguyen, T. , Li, Y. , & Soliman, E. Z. (2019). Interrelations between hypertension and electrocardiographic left ventricular hypertrophy and their associations with cardiovascular mortality. The American Journal of Cardiology, 123(2), 274–283. [DOI] [PubMed] [Google Scholar]
- Chahal, H. , Backlund, J. Y. , Cleary, P. A. , Lachin, J. M. , Polak, J. F. , Lima, J. A. , Bluemke, D. A. , & DCCT/EDIC Research Group . (2012). Relation between carotid intima‐media thickness and left ventricular mass in type 1 diabetes mellitus from the epidemiology of diabetes interventions and complications [EDIC] study. The American Journal of Cardiology, 110(10), 1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, W. H. , Su, F. Y. , Lin, F. , Yu, Y. S. , & Lin, G. M. (2019). Association of electrocardiographic left and right ventricular hypertrophy with physical fitness of military males: The CHIEF study. European Journal of Sport Science, 19(9), 1214–1220. [DOI] [PubMed] [Google Scholar]
- Chen, Y. J. , Chen, K. W. , Shih, Y. L. , Su, F. Y. , Lin, Y. P. , Meng, F. C. , Lin, F. , Yu, Y. S. , Han, C. L. , Wang, C. H. , Lin, J. W. , Hsieh, T. Y. , Li, Y. H. , & Lin, G. M. (2017). Chronic hepatitis B, nonalcoholic steatohepatitis and physical fitness of military males: CHIEF study. World Journal of Gastroenterology, 23(25), 4587–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevli, P. A. , Hari, K. J. , Kanaya, A. M. , Talegawkar, S. A. , Needham, B. L. , & Herrington, D. (2020). Association of alcohol consumption and ideal cardiovascular health among South Asians: The mediators of atherosclerosis in south Asians living in America (MASALA) study. Alcoholism, Clinical and Experimental Research, 44(9), 1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi, C. , Negri, F. , Giudici, V. , Valerio, C. , Meani, S. , Sala, C. , Esposito, A. , Masaidi, M. , Zanchetti, A. , & Mancia, G. (2009). Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. Journal of Hypertension, 27(4), 854–860. [DOI] [PubMed] [Google Scholar]
- Cuspidi, C. , Sala, C. , Muiesan, M. L. , De Luca, N. , Schillaci, G. , & Working Group on Heart . (2013). Hypertension of the Italian Society of Hypertension. Right ventricular hypertrophy in systemic hypertension: An updated review of clinical studies. Journal of Hypertension, 31(5), 858–865. [DOI] [PubMed] [Google Scholar]
- Devereux, R. B. , Casale, P. N. , Eisenberg, R. R. , Miller, D. H. , & Kligfield, P. (1984). Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. Journal of the American College of Cardiology, 3, 82–87. [DOI] [PubMed] [Google Scholar]
- Kishi, S. , Magalhaes, T. A. , George, R. T. , Dewey, M. , Laham, R. J. , Niinuma, H. , Friedman, L. A. , Cox, C. , Tanami, Y. , Schuijf, J. D. , Vavere, A. L. , Kitagawa, K. , Chen, M. Y. , Nomura, C. H. , Brinker, J. A. , Rybicki, F. J. , Di Carli, M. F. , Arbab‐Zadeh, A. , & Lima, J. A. (2015). Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: The CORE320 multicenter study. European Heart Journal Cardiovascular Imaging, 16(2), 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , Flachskampf, F. A. , Foster, E. , Goldstein, S. A. , Kuznetsova, T. , Lancellotti, P. , Muraru, D. , Picard, M. H. , Rietzschel, E. R. , Rudski, L. , Spencer, K. T. , Tsang, W. , & Voigt, J. U. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28(1), 1–39.e14. [DOI] [PubMed] [Google Scholar]
- Lavie, C. J. , Ozemek, C. , Carbone, S. , Katzmarzyk, P. T. , & Blair, S. N. (2019). Sedentary behavior, exercise, and cardiovascular health. Circulation Research, 124(5), 799–815. [DOI] [PubMed] [Google Scholar]
- Lehtonen, A. O. , Suvila, K. , Jula, A. M. , & Niiranen, T. J. (2021). Association between self‐reported hypertension onset age and electrocardiographic left ventricular hypertrophy. Journal of Human Hypertension, 35(5), 479–482. [DOI] [PubMed] [Google Scholar]
- Lévy, S. (1991). Diagnostic approach to cardiac arrhythmias. Journal of Cardiovascular Pharmacology, 17(Suppl 6), S24–S31. [PubMed] [Google Scholar]
- Liao, Y. Y. , Gao, K. , Fu, B. W. , Yang, L. , Zhu, W. J. , Ma, Q. , Chu, C. , Yan, Y. , Wang, Y. , Zheng, W. L. , Hu, J. W. , Wang, K. K. , Sun, Y. , Chen, C. , & Mu, J. J. (2021). Risk factors for electrocardiographic left ventricular hypertrophy in a young Chinese general population: The Hanzhong adolescent cohort study. BMC Cardiovascular Disorders, 21(1), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , Li, Y. H. , Lee, C. J. , Shiang, J. C. , Lin, K. H. , Chen, K. W. , Chen, Y. J. , Wu, C. F. , Lin, B. S. , Yu, Y. S. , Lin, F. , Su, F. Y. , & Wang, C. H. (2016). Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in eastern Taiwan. World Journal of Cardiology, 8(8), 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , & Liu, K. (2020). An electrocardiographic system with anthropometrics via machine learning to screen left ventricular hypertrophy among young adults. IEEE Journal of Translational Engineering in Health and Medicine, 8, 1800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , Liu, K. , Colangelo, L. A. , Lakoski, S. G. , Tracy, R. P. , & Greenland, P. (2016). Low‐density lipoprotein cholesterol concentrations and Association of High‐Sensitivity C‐reactive protein concentrations with incident coronary heart disease in the multi‐ethnic study of atherosclerosis. American Journal of Epidemiology, 183(1), 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , Liu, P. Y. , Tsai, K. Z. , Lin, Y. K. , Huang, W. C. , & Lavie, C. J. (2022). Cardiorespiratory fitness and carotid intima‐media thickness in physically active young adults: CHIEF atherosclerosis study. Journal of Clinical Medicine, 11(13), 3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , Lloyd‐Jones, D. M. , Colangelo, L. A. , Szklo, M. , Heckbert, S. R. , Chen, L. Y. , Lima, J. A. C. , & Liu, K. (2022). Secondhand tobacco smoke exposure, urine cotinine, and risk of incident atrial fibrillation: The multi‐ethnic study of atherosclerosis. Progress in Cardiovascular Diseases, 74, 38–44. [DOI] [PubMed] [Google Scholar]
- Lin, G. M. , & Lu, H. H. (2020). A 12‐Lead ECG‐based system with physiological parameters and machine learning to identify right ventricular hypertrophy in young adults. IEEE Journal of Translational Engineering in Health and Medicine, 8, 1900510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. M. , Tsai, K. Z. , Chang, Y. C. , Huang, W. C. , Sui, X. , & Lavie, C. J. (2022). Muscular strength and carotid intima‐media thickness in physically fit young adults: The CHIEF atherosclerosis study. Journal of Clinical Medicine, 11(18), 5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. K. , Liu, P. Y. , Fan, C. H. , Tsai, K. Z. , Lin, Y. P. , Lee, J. M. , Lee, J. T. , & Lin, G. M. (2020). Metabolic biomarkers and long‐term blood pressure variability in military young male adults. World Journal of Clinical Cases, 8(11), 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. K. , Tsai, K. Z. , Han, C. L. , Lee, J. T. , & Lin, G. M. (2022). Athlete's heart assessed by sit‐up strength exercises in military men and women: The CHIEF heart study. Frontiers in Cardiovascular Medicine, 8, 737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. K. , Tsai, K. Z. , Han, C. L. , Lin, Y. P. , Lee, J. T. , & Lin, G. M. (2021). Obesity phenotypes and electrocardiographic characteristics in physically active males: CHIEF study. Frontiers in Cardiovascular Medicine, 8, 738575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. P. , Fan, C. H. , Tsai, K. Z. , Lin, K. H. , Han, C. L. , & Lin, G. M. (2020). Psychological stress and long‐term blood pressure variability of military young males: The cardiorespiratory fitness and hospitalization events in armed forces study. World Journal of Cardiology, 12(12), 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. Y. , Tsai, K. Z. , Huang, W. C. , Lavie, C. J. , & Lin, G. M. (2022). Electrocardiographic and cardiometabolic risk markers of left ventricular diastolic dysfunction in physically active adults: CHIEF heart study. Frontiers in Cardiovascular Medicine, 9, 941912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. Y. , Tsai, K. Z. , Lima, J. A. C. , Lavie, C. J. , & Lin, G. M. (2021). Athlete's heart in Asian military males: The CHIEF heart study. Frontiers in Cardiovascular Medicine, 8, 725852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijs, M. F. , Doevendans, P. A. , Cramer, M. J. , Vonken, E. J. , Velthuis, B. K. , van der Graaf, Y. , Visseren, F. L. , Mali, W. P. , Bots, M. L. , & SMART Study Group . (2009). Relation of common carotid intima‐media thickness with left ventricular mass caused by shared risk factors for hypertrophy. Journal of the American Society of Echocardiography, 22(5), 499–504. [DOI] [PubMed] [Google Scholar]
- Melo, X. , Santa‐Clara, H. , Santos, D. A. , Pimenta, N. M. , Minderico, C. S. , Fernhall, B. , & Sardinha, L. B. (2015). Independent association of muscular strength and carotid intima‐media thickness in children. International Journal of Sports Medicine, 36(8), 624–630. [DOI] [PubMed] [Google Scholar]
- Meng, F. C. , Lin, Y. P. , Su, F. Y. , Yu, Y. S. , & Lin, G. M. (2017). Association between electrocardiographic and echocardiographic right ventricular hypertrophy in a military cohort in Taiwan: The CHIEF study: ECG criteria for RVH. Indian Heart Journal, 69, 331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, G. B. , Klein, H. A. , & Stofer, B. E. (1948). The electrocardiographic diagnosis of right ventricular hypertrophy. American Heart Journal, 35(1), 1–40. [DOI] [PubMed] [Google Scholar]
- Sokolow, M. , & Lyon, T. P. (1949). The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. American Heart Journal, 38(2), 273–294. [DOI] [PubMed] [Google Scholar]
- Su, F. Y. , Li, Y. H. , Lin, Y. P. , Lee, C. J. , Wang, C. H. , Meng, F. C. , Yu, Y. S. , Lin, F. , Wu, H. T. , & Lin, G. M. (2017). A comparison of Cornell and Sokolow‐Lyon electrocardiographic criteria for left ventricular hypertrophy in a military male population in Taiwan: The cardiorespiratory fitness and hospItalization events in armed forces study. Cardiovascular Diagnosis and Therapy, 7(3), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, F. Y. , Lin, Y. P. , Lin, F. , Yu, Y. S. , Kwon, Y. , Lu, H. H. , & Lin, G. M. (2020). Comparisons of traditional electrocardiographic criteria for left and right ventricular hypertrophy in young Asian women: The CHIEF heart study. Medicine (Baltimore), 99(42), e22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, K. Z. , Liu, P. Y. , Huang, W. C. , Lima, J. A. C. , Lavie, C. J. , & Lin, G. M. (2022). Sex‐specific cardiometabolic risk markers of left ventricular mass in physically active young adults: The CHIEF heart study. Scientific Reports, 12(1), 11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


