Highlights
-
•
Biology-guided radiation therapy (BgRT) uses PET imaging to guide radiotherapy.
-
•
Metastatic renal cell carcinoma (RCC) patients may benefit from BgRT using PSMA PET.
-
•
The largest published cohort of RCC patients that underwent PSMA PET was considered.
-
•
More than 60% of targets were suitable for BgRT in RCC patients.
-
•
Bone, lung, pancreas, and liver metastases were optimal candidates for BgRT.
Abbreviations: Abdo, abdomen; BgRT, biology-guided radiotherapy; BTZ, biological tracking zone; GTV, gross tumor volume; LN&ST, lymph node and soft tissue; MIP, maximum intensity projection; nSUV, normalized SUV; PET, positron emission tomography; PSMA, prostate specific membrane antigen; RCC, renal cell carcinoma; SUV, standardized uptake value; TPS, treatment planning system
Keywords: BgRT, PSMA, RCC, BTZ
Abstract
Background
Biology-guided radiotherapy (BgRT) is a novel treatment where the detection of positron emission originating from a volume called the biological tracking zone (BTZ) initiates dose delivery. Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) is a novel imaging technique that may improve patient selection for metastasis-directed therapy in renal cell carcinoma (RCC). This study aims to determine the feasibility of BgRT treatment for RCC.
Material and methods
All consecutive patients that underwent PSMA PET/CT scan for RCC staging at our institution between 2014 and 2020 were retrospectively considered for inclusion. GTVs were contoured on the CT component of the PET/CT scan. The tumor-to-background ratio was quantified from the normalized standardized uptake value (nSUV), defined as the ratio between SUVmax inside the GTV and SUVmean inside the margin expansion. Tumors were classified suitable for BgRT if (1) nSUV was greater or equal to an nSUV threshold and (2) if the BTZ was free of any PET-avid region other than the tumor.
Results
Out of this cohort of 83 patients, 47 had metastatic RCC and were included in this study. In total, 136 tumors were delineated, 1 to 22 tumors per patient, mostly in lung (40%). Using a margin expansion of 5 mm/10 mm/20 mm and nSUV threshold = 3, 66%/63%/41% of tumors were suitable for BgRT treatment. Uptake originating from another tumor, the kidney, or the liver was typically inside the BTZ in tumors judged unsuitable for BgRT.
Conclusions
More than 60% of tumors were found to be suitable for BgRT in this cohort of patients with RCC. However, the proximity of PET-avid organs such as the liver or the kidney may affect BgRT delivery.
Introduction
Biology-guided radiotherapy (BgRT) is a novel treatment modality that utilizes positron emission tomography (PET) to guide radiation delivery. To enable this, radiotherapy is delivered by a linear accelerator equipped with dual 90° PET detectors (PET-linac) (RefleXion Medical, Hayward, California) [1], [2]. The PET-linac is able to generate a 6 MV photon beam with a nominal dose rate of 8.5 Gy/min and to deliver radiation beamlets with sub-second latency. Only photons emanating from a volume called the biological tracking zone (BTZ), defined as the union of the gross tumor volume (GTV) and an isotropic margin expansion of the GTV, can trigger radiotherapy delivery. By relying on PET emissions originating from target lesions, BgRT has the potential to deliver tracked radiation to multiple lesions more accurately and efficiently.
Prostate-specific membrane antigen (PSMA) PET is a highly sensitive imaging modality for prostate cancer [3], [4], [5], [6]. Despite the name, PSMA is also highly expressed by endothelial cells of the neovasculature of solid tumors other than prostate cancer, including renal cell carcinoma (RCC) [7], [8], [9]. Recent studies demonstrated that PSMA PET with 68Ga-PSMA [10], [11], [12], [13], [14], [15], [16] or 18F-DCFPyL [17], [18], [19] tracer has the potential to detect metastatic RCC and improves the accuracy of staging compared to conventional imaging. Considering the PSMA avidity, BgRT incorporating 68Ga-PSMA or 18F-DCFPyL tracers may be a novel and effective approach to delivering radiotherapy to metastatic RCCs.
The feasibility of BgRT treatment for metastatic RCC is addressed in this study by using the largest published cohort that underwent PSMA PET/CT scan. We first aim to describe the pattern of disease distribution and quantify the PSMA PET uptake in a patient cohort that underwent PSMA PET/CT scan for staging purposes at our institution. We further aim to determine the proportion of tumors that may be suitable for BgRT treatment based on different parameters that may be achieved by the PET-linac system.
Materials and methods
We retrospectively evaluated a cohort of 83 patients that underwent PSMA PET/CT for RCC staging purposes at our institution between 2014 and 2020. Patients were included in this study based on the existence of a corresponding contrast-enhanced CT and the suspicion of the presence of metastatic RCC on conventional imaging.
Either 68Ga-PSMA or 18F-DCFPyL PET tracer was administrated depending on their availability. Images were acquired with the Discovery 690 or 710 PET/CT scanner (General Electric Medical System, Milwaukee, USA). The PET/CT pixel spacing was 2.86 mm or 3.65 mm/1.07 mm or 1.37 mm, both with 3.27 mm slice thickness.
GTVs were delineated on the CT component of the PET/CT by using the PET component as a guide by a genitourinary radiation oncologist after importation of the datasets to the treatment planning system (TPS) (Eclipse v16.1, Varian Medical System, Palo Alto, USA). The resulting structure sets and the PET/CT images were then imported to the MIM software (v6.9.4, MIM inc., Chicago, USA) for registration and metrics extraction. The PET distribution was evaluated from the standardized uptake value (SUV) normalized by body weight to allow comparison between patients.
Patient movement or physiological motion may result in misregistration between the CT and the PET component of the PET/CT scan. Misregistration was evaluated on a per tumor basis and corrected manually by moving the PET distribution to the fixed contours on CT.
Tumors were categorized as lung, bone, lymph node and soft tissue (LN&ST), and abdomen (Abdo) depending on their anatomical location. The details of each anatomical location are shown in Table 1. Tumors were also analyzed based on the type of PET tracers administered.
Table 1.
Anatomical location of tumors.
| Site | Anatomical location | Number of tumors | |
|---|---|---|---|
| 1. | Lung | Lung, pleura | 55 |
| 2. | Bone | Ribs, humerus, scapula, iliac crest, sacrum, femur, sternum, spine, acetabulum, ischium, clavicle, skull | 41 |
| 3. | Lymph node and soft tissue | Para-aortic, hilar, subcarinal, oesophagus, pelvic, retrocrural, paratracheal, mediastinal, thyroid, thigh | 21 |
| 4. | Abdomen | Subdiaphragmatic, pancreas, omental, diaphragmatic, liver, abdominal, kidney, renal bed, adrenal | 19 |
BgRT requires a strong PET signal-to-background ratio and the absence of any PET-avid region other than the tumor inside the BTZ, assuming that only one tumor is being treated within the specific BTZ [20], [21]. BTZs were generated from an isotropic three-dimensional outer margin expansion of the GTV of size 5 mm/10 mm/20 mm to study the impact of BTZ size on the proportion of tumors suitable for BgRT.
The normalized SUV (nSUV), defined as the ratio of SUVmax inside the GTV and SUVmean inside the outer margin expansion, was calculated to quantify the tumor to background signal within the BTZ. The signal-to-background ratio was deemed sufficiently strong for BgRT if nSUV was greater or equal to an nSUV threshold. The value of the nSUV threshold had not yet been finalized on the PET-linac system. We, therefore, evaluated a range of nSUV thresholds between 2 and 6 and explicitly reported results for nSUV = 3 as the most likely value to be recommended on the PET-linac system.
In addition to meeting the nSUV threshold, there should be no PET avid regions within the outer shell GTV margin for BgRT to be effective. The detection of a PET avid region within the outer shell GTV margin was automated [20]. To do so, 3D isotropic outer margin expansions of the GTV of 1 mm thickness were successively applied at a distance = [3,30] mm from the GTV. SUVmax inside these shells was subsequently extracted. An increase in SUVmax in two adjacent shells indicated the presence of an avid region. The proximity of PET uptake within the outer shell GTV margin was confirmed manually and the PET-avid organ was recorded. In summary, a tumor was judged suitable for BgRT if (1) nSUV ≥ nSUV threshold and (2) there was no PET avidity within the outer margin expansion of the GTV.
The difference in the medians of distribution was evaluated by using the Mann-Whitney U test. The null hypothesis was rejected at the 95% confidence level. Results are reported in terms of mean ± standard deviation of the distribution unless specified otherwise. Correlations were evaluated with the Spearman correlation coefficient (r) and its associated p-value. The statistical analysis was done using the SciPy module (v1.5.2) with Python.
Results
Of this cohort of 83 patients, 47 had metastatic disease and were included in this study resulting in 136 GTVs. Patient characteristics are detailed in Table 2. 68Ga-PSMA was administered to 19 patients (79 tumors, 58% of all tumors) and 18F-DCFPyL to 28 patients (57 tumors, 42% of all tumors). The uptake time was greater with 18F-DCFPyL than 68Ga-PSMA (mean uptake time = 108 ± 24/61 ± 24 min for 18F-DCFPyL/68Ga-PSMA, p-value < 0.001). The registration between the CT and the PET component was of the order of the PET resolution (mean 3D shift = 2.86 ± 3.90 mm; range = 0–25 mm). Large shift (>2 standard deviations = 7.8 mm) occurred in lung (9 tumors), abdomen (2 tumors, omental and pancreas), lymph nodes (2 tumors, subcarinal), and bone (1 tumor, scapula).
Table 2.
Patient characteristics.
| Characteristic | Total (n = 47) | |||
| Sex | n | (%) | ||
| Male | 26 | (55%) | ||
| Female | 21 | (45%) | ||
| Age | Years | |||
| Mean (standard deviation) | 66.5 (9.7) | |||
| Median (range) | 66 (45-91) | |||
| Interquartile range | 60.5–73 | |||
| Primary tumor size | (mm) | |||
| Mean (standard deviation) | 69.9 (37.5) | |||
| Median (range) | 60 (19-165) | |||
| Interquartile range | 43.5 – 83 | |||
| Histology | n | (%) | ||
| Clear cell | 44 | (94%) | ||
| Chromophobe | 2 | (4%) | ||
| Papillary | 0 | (0%) | ||
| Unclassified variant | 1 | (2%) | ||
| Primary tumor grade (G) | n | (%) | ||
| Clear cell | ||||
| G1 | 5 | (11%) | ||
| G2 | 10 | (21%) | ||
| G3 | 15 | (32%) | ||
| G4 | 9 | (19%) | ||
| Unknown | 5 | (11%) | ||
| Chromophobe | ||||
| G2 | 2 | (4%) | ||
| Unclassified variant | ||||
| Unknown | 1 | (2%) | ||
| Number of tumors per patient | n | (%) | ||
| 1 | 18 | (38%) | ||
| 2 | 11 | (23%) | ||
| 3 | 7 | (15%) | ||
| 4 | 4 | (9%) | ||
| 5 | 4 | (9%) | ||
| 7 | 1 | (2%) | ||
| 10 | 1 | (2%) | ||
| 22 | 1 | (2%) | ||
SUV distribution
The maximum intensity projection of the anatomical locations of the GTVs and their corresponding PET distribution is shown in Fig. 1 for six different patients. The distribution of tumors per patient is further shown in Fig. 2. In this cohort, 36 (77%) patients had three tumors or less and 45 (96%) patients had seven tumors or less. Two patients (4%) had ten tumors or more (10 tumors and 22 tumors). The lung was the most common site of metastases (40% of all tumors), followed by bone (30%), lymph node and soft tissue (16%), and abdomen (14%).
Fig. 1.
(a) Maximum intensity projection (MIP) of all GTV contours projected on the middle slice along the anteroposterior direction of six different patients (top row). (b) MIP of the PET image normalized by the maximum SUV (%) for the same patients (bottom row).
Fig. 2.
Boxplots of the number of tumors per patient for all patients and for each anatomical site. The medians of the distributions are shown (solid red rectangle). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
GTV volume and SUVmax per anatomical site and per PET tracer are shown in the supplementary material. GTV volume ranged from 0.1 cm3 to 152 cm3 (mean = 20.8 ± 33.2 cm3). Lung GTV volumes were smaller than all other sites (volume = 7.4 ± 22.0 cm3/29.8 ± 36.3 cm3 in lung/all other sites, n = 55/81, p-value < 0.001). GTV volumes other than lung sites were statistically similar to each other (p-value = [0.2–0.4]). No significant differences in GTV volume were observed when guided by 68Ga-PSMA or 18F-DCFPyL PET tracer (p-value = 0.3).
SUVmax inside GTV ranged from 1 to 69 (mean = 14 ± 13). SUVmax was lower for lung metastases compared with metastases from other sites (SUVmax = 8 ± 7/18 ± 14 in lung/all other sites, n = 55/81, p-value < 0.001), which can be partly explained form respiratory movement [22]. SUVmax in sites other than lung were all statistically similar to each other (p-value = [0.2–0.4]). Tumor SUVmax was higher for 68Ga-PSMA than 18F-DCFPyL PET (SUVmax = 16 ± 12/11 ± 13 with 68Ga-PSMA/18F-DCFPyL, p-value < 0.001).
All tumors considered, GTV volume was moderately correlated with SUVmax (r = 0.6, p-value < 0.001). The correlation between GTV volume and SUVmax was strong in lung metastases (r = 0.8, p-value < 0.001), weak in bone metastases (r = 0.3, p-value = 0.03), but not significant in other sites (p-value = [0.1–0.7]).
Suitability for BgRT
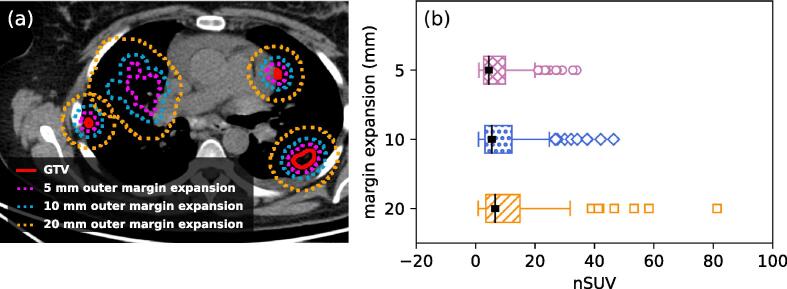
An illustration of the margin expansion used to calculate nSUV is shown in Fig. 3 (a) and the determined nSUV distributions are shown in Fig. 3 (b). Complete nSUV data are shown in the supplementary material. For all margin expansions considered, nSUV was higher for bone metastases as compared with all the other sites (nSUV = 14.3 ± 8.8/4.7 ± 3.7, p-value < 0.001; 17.5 ± 11.8/5.9 ± 4.8, p-value < 0.001; 20.9 ± 17.3/7.0 ± 6.0, p-value < 0.001; by using 5 mm/10 mm/20 mm margin expansion, respectively). nSUV was higher for the 68Ga-PSMA PET tracer than the 18F-DCFPyL tracer for all margin expansions (nSUV = 8.2 ± 7.4/6.8 ± 6.9, p-value = 0.01; 10.6 ± 9.9/7.7 ± 8.0, p-value < 0.01; 13.2 ± 14.2/8.3 ± 9.1, p-value < 0.001; by using 5 mm/10 mm/20 mm margin expansion, respectively).
Fig 3.
(a) Example of the 5 mm/10 mm/20 mm outer margin expansion of GTVs. (b) nSUV distribution for all patients resulting from margin expansion of 5 mm/10 mm/20 mm. The medians of the distributions are shown (solid black rectangle).
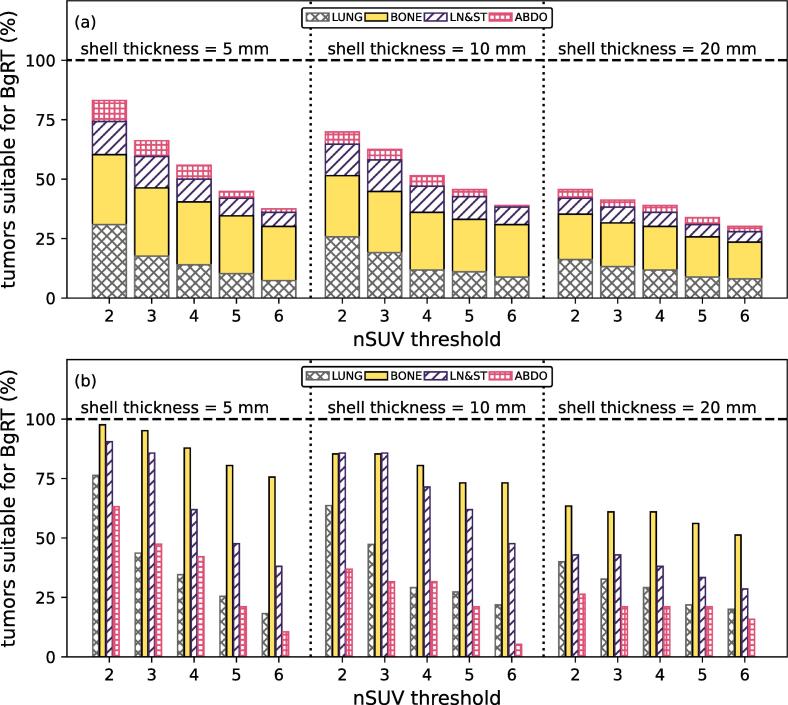
Fig. 4(a) shows the proportion of tumors suitable for BgRT, classified per site while Fig. 4(b) demonstrates the proportion of tumors suitable for BgRT in each site. By using the nSUV threshold = 3, 66%/63%/41% of all tumors were suitable for BgRT with margin expansions of 5 mm/10 mm/20 mm. Of all sites, bone metastases generally demonstrated the highest rates of BgRT suitability (50% to 98% of tumors located in bone site were suitable for BgRT depending on the margin expansion and nSUV threshold considered).
Fig. 4.
(a) Proportion of the total number of tumors suitable for BgRT treatment (%) for different values of the nSUV threshold by using an outer margin expansion of 5 mm/10 mm/20 mm. Results are classified per site. (b) The proportion of tumors suitable for BgRT treatment in each site (%) for different values of the nSUV threshold by using an outer margin expansion of 5 mm/10 mm/20 mm.
By using a margin expansion of 5 mm, five tumors (4%) were not suitable for BgRT because of the presence of physiological PET avidity within the outer shell GTV margin expansion. PET avidity was originating from liver (2), kidney (2), and bowel (1). With a margin expansion of 10 mm, 22 tumors (16%) had physiological uptake inside the margin expansion, originating from kidney (7), another tumor (6), liver (4), bowel (2), spleen (2), and bladder (1).
Discussion
In this study, the feasibility of PSMA PET BgRT for metastatic RCC was evaluated for the first time. This was an analysis of the largest published cohort (to our knowledge) of consecutive patients that underwent PSMA PET/CT scan in the staging of RCC with suspicion of metastases based on conventional imaging. Target tumor's suitability for BgRT depends on a sufficient nSUV and the absence of any PET avidity within the outer shell GTV margin, both of which can be influenced by the size of the outer shell GTV margin. For example, nSUV was generally larger for BTZs generated with a margin expansion of 20 mm, compared to BTZs generated with a margin expansion of 5 or 10 mm, because more low SUV values were included in the calculation of SUVmean. However, the margin expansion of 20 mm resulted in the lowest number of tumors suitable for BgRT because the probability that a PET-avid organ of being present inside the BTZ increases with the size of the margin expansion. Overall, the findings from the study suggest that 5 mm or 10 mm margin expansions may be optimal in the setting of RCC metastases despite a lower nSUV. Further strategies could be considered to treat tumors that are deemed unsuitable for BgRT treatment. For example, the margin expansion might be anisotropic to mask out adjacent PET-avid regions from non-tumor structures.
Of the 47 patients considered in this study, 25 (53%)/23 (49%) patients had all of their tumors suitable for BgRT with a margin expansion of 5 mm/10 mm with the nSUV threshold = 3. Twenty-nine (62%) patients had multiple metastases. These patients may be particularly well suited to BgRT treatment due to the PET-linac’s potential to treat multiple targets rapidly in a single session.
The anatomical site with the largest proportion of RCC metastases suitable for BgRT was RCC bone metastases (30% of all tumors) due to the large tumor to background PET ratio. These metastases could benefit from the potential of the PET-linac system to treat multiple lesions in a single session. RCC metastases located in lung, pancreas, liver, and around the diaphragm were also associated with a large number of tumors well suited for BgRT. These RCC metastases tend to be mobile and may benefit from the real-time tracking ability of the PET-linac system.
The epidemiology of metastatic RCC has been recently described in detail by Dudani et al. (n = 10 105) by utilizing the International mRCC Database Consortium database [23]. The findings from our study were consistent with the findings of Dudani et al.with clear cell RCC (ccRCC) making up the majority of the histologic subtypes (94% of ccRCC in this current study versus 91%) and lung being the most common site of RCC metastases (43% had lung metastasis in this current study versus 70%). In both the current study and the published study by Dudani et al., approximately a third of metastases were in the bone.
The SUVmax distribution was comparable to other studies that incorporated 68Ga-PSMA [mean SUVmax = 20; range = 2–48, n = 86 [14], mean = 10 ± 8, range = 3-26, n = 8 [16], mean = 16 ± 12, range = 1-69, n = 79 (this study)] or 18F-DCFPyL [median SUVmax = 10, IQR = 5–16, n = 24 [19], range = 2–20, n = 5 [11], median = 8, IQR = 3–13, range = 1–58 (this study)] tracers for patients with metastatic RCC. SUVmax was statistically higher in tumors imaged with 68Ga-PSMA than with 18F-DCFPyL. Consequently, the proportion of tumors that satisfied the condition nSUV ≥ nSUV threshold was higher with the 68Ga-PSMA PET tracer and therefore more tumors were suitable for BgRT with this tracer (the proportion of tumors suitable for BgRT for each tracer is shown in the supplementary material). This observation differs from PSMA PET studies in prostate cancer where either no difference between the two tracers was observed (n = 46, p-value = 0.66) [24] or where SUVmax was higher with 18F-DCFPyL than with 68Ga-PSMA (n = 14, p-value = 0.03) [25]. Whether indeed 68Ga-PSMA is associated with greater uptake than 18F-DCFPyL for metastatic RCC would need to be addressed from a patient intra-variability study.
This study included some limitations. First, the optimal BTZ size and the value of nSUV threshold are not well established in the PET-linac system. A range of values and their effect on the suitability for BgRT were therefore investigated. Furthermore, GTV delineation was performed on the CT component of a PET/CT scan. This step differs from the BgRT workflow where a planning CT would first be acquired and then registered to the CT component of a PET/CT acquisition for delineation. Moreover, it was assumed that nSUV stayed constant from the BgRT planning session to the end of treatment. A decline in SUVmax or an increase in the SUVmean in the margin expansion from the timing of the planning scan to completion of BgRT may result in a decrease in nSUV which can potentially affect the feasibility of BgRT. This is relevant when considering more than a single fraction of radiotherapy or if considering concurrent systemic treatment that can impact the SUV of RCC metastases and surrounding tissue [18].
PSMA PET SUVmax of WHO/ISUP grade 3–4 ccRCC tumors are generally higher than that of grade 1–2 ccRCC tumors [10]. Thus, grade 3–4 ccRCC tumors may be more suitable for BgRT than grade 1–2 ccRCC tumors. In the current study, WHO/ISUP grades of primary tumors were available but not of metastatic tumors. Therefore, we were unable to evaluate the difference in rates of BgRT feasibility for grade 1–2 ccRCC versus grade 3–4 ccRCC metastases. Guhne et al. previously demonstrated that the grade of ccRCC metastases can be different from the primary and therefore, we did not assume that the ccRCC grades of metastases were equivalent to that of the primary [26]. Future studies may attempt to incorporate such data to determine whether PSMA BgRT is suitable for both high and low grade ccRCC targets, though biopsy of all individual tumors may be clinically impractical.
Conclusions
The feasibility of PSMA PET BgRT for metastatic RCC was evaluated for the first time. Suitable RCC targets for PSMA PET BgRT often included moving targets located in lung or upper abdomen and were characterized by PSMA PET avid metastases surrounded by an area of non-avid normal tissue.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This work is funded in part by the Peter MacCallum Cancer Centre Foundation. This research is partially funded by RefleXion Medical. The funders had no role in study design, analysis or manuscript writing.
Acknowledgments
This work is funded in part by the Peter MacCallum Cancer Centre Foundation for research software licences. Shankar Siva is supported by the Victorian Cancer Council Colebatch Fellowship. Michael Hofman is supported by the Prostate Cancer Foundation (PCF) funded by CANICA Oslo Norway, Peter MacCallum Foundation, Medical Research Future Fund (MRFF) and a NHMRC Investigator Grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100608.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Oderinde O.M., Shirvani S.M., Olcott P.D., Kuduvalli G., Mazin S., Larkin D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin Transl Radiat Oncol. 2021;29:106–112. doi: 10.1016/j.ctro.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirvani S.M., Huntzinger C.J., Melcher T., Olcott P.D., Voronenko Y., Bartlett-Roberto J., et al. Biology-guided radiotherapy: redefining the role of radiotherapy in metastatic cancer. Br J Radiol. 2021;94(1117):20200873. doi: 10.1259/bjr.20200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofman M.S., Iravani A., Nzenza T., Murphy D.G. Advances in urologic imaging. Urol Clin N Am. 2018;45:503–524. doi: 10.1016/j.ucl.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Eiber M., Fendler W.P., Rowe S.P., Calais J., Hofman M.S., Maurer T., et al. Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med. 2017;58(Supplement 2):67S–76S. doi: 10.2967/jnumed.116.186767. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian J.J., Gorin M.A., Ross A.E., Pienta K.J., Tran P.T., Schaeffer E.M. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15–25. doi: 10.1038/nrurol.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho S.Y., Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014;44:93–109. doi: 10.1053/j.semnuclmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siva S., Udovicich C., Tran B., Zargar H., Murphy D.G., Hofman M.S. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol. 2020;17:107–118. doi: 10.1038/s41585-019-0272-5. [DOI] [PubMed] [Google Scholar]
- 8.Urso L., Castello A., Rocca G.C., Lancia F., Panareo S., Cittanti C., et al. Role of PSMA-ligands imaging in renal cell carcinoma management: current status and future perspectives. J Cancer Res Clin Oncol. 2022;148(6):1299–1311. doi: 10.1007/s00432-022-03958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siva S., Callahan J., Pryor D., Martin J., Lawrentschuk N., Hofman M.S. Utility of 68Ga prostate specific membrane antigen – positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J Med Imaging Radiat Oncol. 2017;61:372–378. doi: 10.1111/1754-9485.12590. [DOI] [PubMed] [Google Scholar]
- 10.Gao J., Xu Q., Fu Y., He K., Zhang C., Zhang Q., et al. Comprehensive evaluation of 68Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2021;48(2):561–569. doi: 10.1007/s00259-020-04916-6. [DOI] [PubMed] [Google Scholar]
- 11.Golan S., Aviv T., Groshar D., Yakimov M., Zohar Y., Prokocimer Y., et al. Dynamic 68Ga-PSMA-11 PET/CT for the primary evaluation of localized renal mass: a prospective study. J Nucl Med. 2021;62(6):773–778. doi: 10.2967/jnumed.120.251272. [DOI] [PubMed] [Google Scholar]
- 12.Gühne F., Seifert P., Theis B., Steinert M., Freesmeyer M., Drescher R. PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: Histopathological correlations of imaging findings. Diagnostics. 2021;11:1–8. doi: 10.3390/diagnostics11071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raveenthiran S., Esler R., Yaxley J., Kyle S. The use of 68Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46:2280–2288. doi: 10.1007/s00259-019-04432-2. [DOI] [PubMed] [Google Scholar]
- 14.Rhee H., Blazak J., Tham C.M., Ng K.L., Shepherd B., Lawson M., et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6(1) doi: 10.1186/s13550-016-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tariq A., Kwok M., Pearce A., Rhee H., Kyle S., Marsh P., et al. The role of dual tracer PSMA and FDG PET/CT in renal cell carcinoma (RCC) compared to conventional imaging: a multi-institutional case series with intra-individual comparison. Urologic Oncology: Seminars and Original Investigations. 2022;40(2):66.e1–66.e9. doi: 10.1016/j.urolonc.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Sawicki L.M., Buchbender C., Boos J., Giessing M., Ermert J., Antke C., et al. Diagnostic potential of PET/CT using a 68Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: initial experience. Eur J Nucl Med Mol Imaging. 2017;44(1):102–107. doi: 10.1007/s00259-016-3360-2. [DOI] [PubMed] [Google Scholar]
- 17.Meyer A.R., Carducci M.A., Denmeade S.R., Markowski M.C., Pomper M.G., Pierorazio P.M., et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2019;33:617–623. doi: 10.1007/s12149-019-01371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittlmeier L.M., Unterrainer M., Rodler S., Todica A., Albert N.L., Burgard C., et al. 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: preliminary results. Eur J Nucl Med Mol Imaging. 2021;48(6):2031–2037. doi: 10.1007/s00259-020-05165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe S.P., Gorin M.A., Hammers H.J., Som Javadi M., Hawasli H., Szabo Z., et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2015;29(10):877–882. doi: 10.1007/s12149-015-1017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudreault M., Chang D., Hardcastle N., Jackson P., Kron T., Hanna G.G., et al. Utility of biology-guided radiotherapy to de novo metastases diagnosed during staging of high-risk biopsy-proven prostate cancer. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.854589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudreault M., Chang D., Hardcastle N., Jackson P., Kron T., Hofman M.S., et al. Feasibility of biology-guided radiotherapy using PSMA-PET to boost to dominant intraprostatic tumour. Clin Transl Radiat Oncol. 2022;35:84–89. doi: 10.1016/j.ctro.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang T-C, Wang Y-C. Deformation Effect on SUVmax Changes in Thoracic Tumors Using 4-D PET/CT Scan. PLoS One 2013;8:e58886. https://doi.org/10.1371/journal.pone.0058886. [DOI] [PMC free article] [PubMed]
- 23.Dudani S, de Velasco G, Wells JC, Gan CL, Donskov F, Porta C, et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw Open 2021;4:e2021869. https://doi.org/10.1001/jamanetworkopen.2020.21869. [DOI] [PMC free article] [PubMed]
- 24.Hoberück S., Löck S., Borkowetz A., Sommer U., Winzer R., Zöphel K., et al. Intraindividual comparison of [68 Ga]-Ga-PSMA-11 and [18F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res. 2021:11. doi: 10.1186/s13550-021-00845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietlein M., Kobe C., Kuhnert G., Stockter S., Fischer T., Schomäcker K., et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17(4):575–584. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gühne F., Seifert P., Theis B., Steinert M., Freesmeyer M., Drescher R. PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: histopathological correlations of imaging findings. Diagnostics. 2021;11:1142. doi: 10.3390/diagnostics11071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.