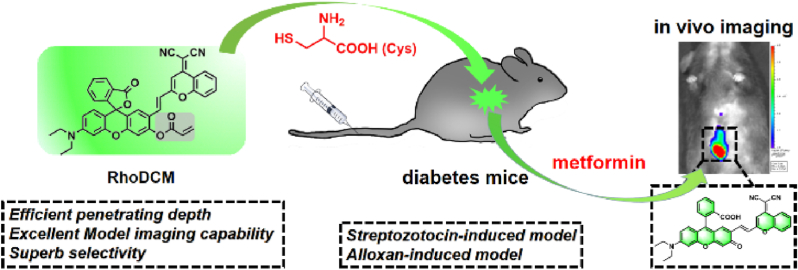
Abstract
Herein, a novel fluorescent probe RhoDCM was developed for monitoring the cysteine (Cys) dynamics. For the first time, the Cys-triggered implement was applied in relatively complete diabetic mice models. The response of RhoDCM towards Cys suggested advantages including practical sensitivity, high selectivity, rapid reaction, and steadiness in various pH and temperature conditions. RhoDCM could basically monitor the intracellular Cys level, both exogenous and endogenous. It could further monitor the glucose level via detecting consumed Cys. Furthermore, the diabetic mice models including the no diabetic control group, the induced model groups by streptozocin (STZ) or alloxan, and the treatment groups induced by STZ and treated with vildagliptin (Vil), dapagliflozin (DA), or metformin (Metf) were constructed. The models were checked by oral glucose tolerance test and significant liver-related serum indexes. Based on the models, the in vivo imaging and penetrating depth fluorescence imaging both indicated that RhoDCM could characterize the status of the development and treatment in the diabetic process via monitoring the Cys dynamics. Consequently, RhoDCM seemed beneficial for inferring the order of severity in the diabetic process and evaluating the potency of therapeutic schedules, which might be informatic for correlated investigations.
Keywords: Diabetic process, Cysteine dynamics, Fluorescent imaging, Redox status indicator
Graphical abstract

Highlights
-
•
First trial for Cys-triggered probe applied in diabetic mice models of both the development and treatment.
-
•
A novel fluorescent probe developed based on the robustness and applicability.
-
•
Achieving characterizing the status of diabetic process via monitoring the Cys dynamics.
-
•
Accompanied by the checking with oral glucose tolerance test, serum indexes, and histopathological staining.
Diabetes mellitus (DM), which usually presents abnormal insulin synthesis and release, is a tremendous risk for healthcare [1,2]. As the World Health Organization demonstrated, the population over 18 with diabetes in 2014 (422 million) was four times higher than that in 1980 (108 million) [3]. The common symptoms of DM include polyuria, fatigue, and emotion disorders, while the internal lesion is mainly on the liver and kidneys [[4], [5], [6]]. Without diagnosis in time, DM may cause serious complications [7]. Therefore, it is of great significance to accurately diagnose and treat DM as soon as possible after the occurrence [8].
The diagnosis indexes of DM are basically universal, but different according to the categories. Diabetic disorders can be generally classified into Type I, Type II, gestational, and other specific syndromes [9]. As the most common two groups, Type I is an auto-immune disorder caused by immune-mediated destruction of insulin-producing β-cells [10]; while Type II is chronic metabolic disorder of the insulin receptors on various cells, which presents insulin desensitization or resistance [11]. As the most convenient index, blood glucose elevation and glycated albumin have been commonly used but were considered post hoc events [12]. Another universal index might be the functional loss of β-cell mass (BCM), which could be induced by hyperglycemia via apoptosis, dedifferentiation, and so on [13]. However, BCM seems not a convenient index to bear the advantages such as real-time, in situ, and accuracy. Recent investigations indicated that the increase in blood glucose might be associated with cellular status, especially oxidative-reductive stress [14]. The inspired research broadened the potential indexes to enzymatic ones including alkaline phosphatase [15], sodium-glucose cotransporter-2 (SGLT2) [16], dipeptide peptidase IV (DPP-IV) [17] as well as environmental ones such as viscosity [18], homocysteine (Hcy) [19], and Cys [[20], [21], [22], [23], [24]]. In clinical research, the close relationship between Hcy and diabetic disease has been confirmed by previous research [19,25,26]. Meanwhile, an increase in blood glucose in organisms might be accompanied by the production of oxidative stress [27]. To keep the balance of the redox system, some reducing substances are present in the cell to clear the excess reactive oxygen species, and Cys is an essential one among these reducing substances. Accordingly, the level of Cys is closely related to diabetes and oxidative stress [28,29]. In combination with convenience, accuracy, and predictability, the cysteine dynamics seemed a potential study node to intensify the connection between the oxidative-reductive status and the diabetic process.
Although the detection of Cys with fluorescent probes have been a hotspot in recent year [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]], the practical monitoring in the development and treatment of diabetic process was few due to the applicability of the implementation and, what might be more notable, the completeness of the models. In 2017, Yin et al. developed one typical fluorescent probe for visualization of Cys metabolism based on the rational design of dual recognition sites for Cys and its metabolite SO2, respectively. They provided a deeper insight into the physiological processes of Cys [53]. To study the oxidative stress process in cells more conveniently, Meng’s group exploited a ratiometric fluorescent probe that could achieve real-time tracking and visualization of the early stage of LPS-induced apoptosis by monitoring oxidative stress levels in mitochondria [51]. To further develop real-time imaging fluorescent probes for Cys in model mice, in 2019, Chen’s group successfully applied a fluorescence probe to evaluate the level of Cys in an orthotopic lung cancer model. The probe could discriminate tumor lesions from normal tissues [52]. In 2022, Lin and co-workers designed a novel mitochondria-targeted fluorescent probe to assay Cys [20]. Importantly, by using the new probe, they discovered that mitochondrial Cys levels were significantly decreased in diabetic cells and mice. Accordingly, the detection in DM models was rarely reported and not elaborate. Therefore, there was an urgent need to develop Cys fluorescent probes in the diagnosis and treatment of diabetes to provide important information for clinical diabetes research.
In this work, a novel fluorescent probe RhoDCM (named from the fluorophores) was developed for monitoring the Cys dynamics and, for the first time, the Cys-triggered implement was applied in the mice models containing both the development and treatment of diabetic process (Fig. 1). Compared with the previously reported Cys-triggered implements in Table S1, RhoDCM focused on robustness and applicability, with the strong fluorescent signal and typical response mechanism. RhoDCM had high selectivity, and steadiness in various pH and temperature conditions. It could discriminate Cys from glutathione (GSH)/Hcy. Compared with other Cys probes [37,38,50], the linear range of 0–100 μM and the calculated limit of detection (LOD) as 0.23 μM also inferred that RhoDCM was applicable for monitoring the physiological and pathological range of Cys level. Consequently, RhoDCM not only had a low detection limit, but also had a wide linear range, which could match the physiological detection range of Cys. RhoDCM could respond to the fluorescence of Cys in a short period (less than 5 min), and has good stability (more than 60 min). It could conduct real-time imaging of Cys in different mice models. Although the recognition group was a reported applicative one, the combination of the fluorophore and the recognition group was structurally and functionally new. Meanwhile, the diabetic mouse model was also further developed to involve both the development and treatment of diabetic process for the first time. Thus, the application of the probe in this new model was also application innovation. The strong fluorescent signal after the activation by Cys might guarantee the biological imaging of RhoDCM in diabetic procedures in living cells as well as in mice. Along with the unfolding of the investigations here, an informatic presentation of the Cys dynamic in a relatively complete diabetic process was provided, which might be helpful in correlational research.
Fig. 1.
The general illustration of the fluorescent probe for monitoring the Cysteine dynamics in diabetic models.
1. Experimental section
General. The details of the experimental information and protocols were present in the Supporting Information.
Synthesis of RhoDCM. The general synthesis route of the probe RhoDCM (Fig. S1) and the processes for preparing compound 5 was detailed in the Supporting Information. Then, acryloyl chloride (45 mg, 0.5 mmol) and compound 5 (182 mg, 0.3 mmol) were added into CH2Cl2 at 0 °C. Triethylamine (TEA) was used as a catalyzer, and the reaction solution was stirred overnight. After that, the solvent was removed, and then the mixture was purified by silica gel column chromatography to afford the probe RhoDCM (119 mg, 60%). 1H NMR (400 MHz, DMSO-d6) δ 8.77 (d, J = 8.1 Hz, 1H), 8.27 (d, J = 16.2 Hz, 1H), 8.14 (d, J = 8.7 Hz, 1H), 8.07-7.95 (m, 2H), 7.88 (d, J = 7.6 Hz, 1H), 7.77-7.63 (m, 3H), 7.58 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 7.7 Hz, 1H), 7.15 (s, 1H), 7.06 (s, 1H), 6.74 (d, J = 2.5 Hz, 1H), 6.50 (dd, J = 9.0, 2.6 Hz, 1H), 6.40 (d, J = 9.0 Hz, 1H), 6.04 (d, J = 17.8 Hz, 1H), 5.95 (d, J = 10.6 Hz, 1H), 5.70 (dd, J = 17.2, 10.5 Hz, 1H), 3.41 (q, J = 6.8 Hz, 4H), 1.13 (t, J = 7.0 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 169.77, 162.64, 158.30, 153.71, 152.59, 150.98, 150.64, 150.12, 149.77, 136.00, 135.81, 134.38, 131.06, 129.96, 128.59, 126.78, 126.46, 126.21, 125.23, 125.12, 123.85, 122.06, 121.48, 119.98, 119.30, 117.61, 111.96, 109.97, 108.03, 97.46, 80.79, 61.25, 44.13, 12.89. ESI-MS m/z = [M+H]+ Calcd. for 660.2090, found 660.2105.
Imaging in mice diabetic models. For imaging the cysteine dynamics in mice diabetic models, male C57BL/6J mice were used. After 10 days of adaptive feeding, six specific groups were set according to the corresponding conditions. I) Control group: Saline was used instead in both the induction period (Day 11–14) and the treating period (Day 15–43), then RhoDCM (100 μL, 0.1 mM in 1% DMSO) was intravenously injected for imaging (Day 45); II) Model group induced by streptozocin (STZ): STZ in citrate buffer (10 mM, pH 4.5) at a dose of 150 mg/kg was intraperitoneally injected each day in the induction period (Day 11–14), while saline was used once per 7 days in the treating period (Day 15–43) before imaging (Day 45); III) Model group induced by alloxan: Alloxan (out of light and freshly prepared before administration) at a dose of 100 mg/kg was intraperitoneally injected instead of STZ, and other conditions were the same as that in II); IV) to VI): Treatment groups: STZ was used in the induction period (Day 11–14) with the same conditions as in II), whereas in the treating period (Day 15–43) before imaging (Day 45), the oral gavage of vildagliptin (Vil), dapagliflozin (DA), and metformin (Metf) in solution (100 μL, 100 mg/kg of body weight) was conducted once per 7 days. Additionally, one day before the imaging (Day 44), a typical oral glucose tolerance test (OGTT) was conducted. During the imaging procedure, all the animals were anesthetized by isoflurane and used to record images at various time points with the small animal optical imaging system. After the above experiments were completed, mice were sacrificed after anesthetization. The organs were separated by opening the abdominal cavity. The tissues were then pictured by fluorescence imaging. All the animal experimental protocols complied with the Ethical Committee of Nanjing Medical University, and approval for the study was also granted by Nanjing Medical University.
2. Results and discussion
Synthesis of the probe RhoDCM. To reveal the oxidative-reductive status in the development and treatment of the diabetic process, monitoring the Cys dynamics might be a reliable approach. Here a practical fluorescent probe was developed with the design concept containing robustness and applicability. The dicyanomethylene-benzopyran (DCM)-modified rhodamine derivative was selected to offer stable fluorescence signals [54,55], while a typical acryloyl-deprotection mechanism was chosen to ensure the selective response to Cys [21,23]. The sulfhydryl of Cys could add onto the α, β-unsaturated carbonyl group, and cause the intramolecular cyclization. Afterwards, the seven-membered ring departed and the fluorophore was released. Other thiols could not generate a similar ring (GSH, H2S), or the generated ring was not steady (Hcy). Accordingly, the probe RhoDCM was synthesized via the route in Fig. S1. The chemical structures were analyzed by 1H NMR, 13C NMR, and HRMS (Supporting Information). The mechanism was also illustrated and checked (Fig. S2). To further confirm the response mechanism, we have presented the LC-MS results of the reaction between RhoDCM and Cys in Fig. S3. Before the reaction, only the peak of RhoDCM appeared (m/z = 660.2117). After the reaction between RhoDCM and Cys, the system presented the main peaks of Cys, compound A-C, and compound 5 (m/z = 122.0270, 781.2327, 783.2483, 176.0737, and 606.2017, respectively).
Optical response of RhoDCM towards cysteine. Initially, the optical properties of the probe RhoDCM towards Cys were investigated. A stock solution (1.0 mM) of RhoDCM was prepared in DMSO. To acquire other concentrations of RhoDCM, the stock solution was diluted by DMSO. The fluorescence spectral experiments were carried out in PBS buffer (10 mM, 1% DMSO, pH 7.4). The absorption spectrum of RhoDCM itself exhibited a peak at 480 nm, while the addition of Cys (200 μM) led to an obvious enhancement of the peak (Fig. 2a). Correspondingly, the fluorescence spectrum of RhoDCM itself under the excitation of 480 nm showed a weak peak at 570 nm, while the addition of Cys (200 μM) could cause a remarkable increase of almost 20 times at the same wavelength (Fig. 2b). The fluorescence quantum yields of Compound 5 and RhoDCM were calculated as 0.395 and 0.049, respectively, via a reference method with Rhodamine B in ethanol solution as the standard. The above results guaranteed that RhoDCM could realize the turn-on response towards Cys. Moreover, the conditional parameters including response time, pH, and temperature were studied. For the response time, the response of RhoDCM towards Cys could reach the saturation value within 5 min (Fig. 2c), which was relatively rapid among the reported probes. Considering that the structure of RhoDCM contains two ester groups, hydrolysis may occur in aqueous solution for a long time. Consequently, we measure the time-resolved fluorescence spectra of RhoDCM within 60 min to check the stability at pH 7.4. In Fig. S4, the fluorescence intensity of RhoDCM and its reaction product remained unchanged within 60 min, which proved that RhoDCM had good stability. For the pH, RhoDCM indicated a stable fluorescence intensity towards Cys within the range of 6.0-8.0 (Fig. 2d). For the temperature, the response signal could stay steady within the range of 30–40 °C (Fig. S5). All the results of conditional parameters checking suggested that RhoDCM was practical for physiological applications. Considering the C C conjugate structure of RhoDCM, we check whether RhoDCM and its reaction products have absorption and fluorescence spectra for sensing viscosity (Fig. S6). However, RhoDCM and the reaction product were not affected by the viscosity of the solution.
Fig. 2.
a) The absorption spectra of RhoDCM without and with Cys. b) The fluorescence spectra of RhoDCM without and with Cys. c) The time-dependent fluorescence response of RhoDCM towards Cys in PBS buffer. d) The fluorescence intensity changes of RhoDCM in different pH conditions in the absence or presence of Cys in PBS buffer. e) The fluorescence changes of RhoDCM with the addition of Cys (0-1.0 mM). f) The relationship between the fluorescence signal and the Cys level (0-1.0 mM). Insert: the linear relationship of the fluorescence signal changes of RhoDCM with Cys (0–100 μM). g) The fluorescence intensity changes of RhoDCM with the addition of different species. h) The fluorescence intensity of RhoDCM in the co-existence system containing competitive substances together with Cys in PBS buffer. RhoDCM: 10 μM, Cys: 200 μM, others: 1 mM. λex = 480 nm, λem = 570 nm; Slit widths 5.0 nm*5.0 nm; Photomultiplier voltage 700 V.
Subsequently, the correlation between the fluorescence intensity of the response system and the concentration of Cys was investigated. As shown in Fig. 2e & f, the fluorescence intensity of the peak at 570 nm enhanced along with the increase of the Cys concentration and reached the plateau when the Cys concentration was 200 μM (20 equivalent (Eq)). The range in which there reflected the linear correlation was 0–100 μM (0–10 Eq). Accordingly, the LOD was determined as 0.23 μM from the formula 3σ/slope (σ: background noise of 30 tests; slope: from the linear equation). In cells, the normal content of Cys is 30–200 μM, and aberrant percentage of Cys can give rise to terrible diseases such as edema, liver damage and lethargy [22,23]. Compared with the physiological and pathological range of Cys level and the LOD values of the recently reported probes in Table S1, the results above inferred that RhoDCM was applicable.
In consideration of the complex intracellular micro-environment, the developed probe RhoDCM should have high selectivity towards Cys, and the response signal should workable anti-interference. As shown in Fig. 2g & S8a, RhoDCM could distinguish Cys from other biologically relevant species including H2S, reactive oxygen species (ROS), reactive nitrogen species (RNS), S2O32−, S2O42−, S2O52−, SCN−, SO42−, HSO42−, GSH, Hcy, and glucose, because no other analyte except for Cys could cause obvious fluorescence signal at 570 nm. When the competing species were broadened to amino acids, metal ions, and anions, RhoDCM still indicated high selectivity towards Cys (Fig. S7). In the co-existence system containing both Cys and the competing species (such as GSH and Hcy), the response signal of RhoDCM toward Cys was almost not interfered (Fig. 2h & S8b). Therefore, both the selectivity and anti-interference of the detecting system were guaranteed.
Fluorescence imaging in living cells. Before the fluorescence imaging in living cells, the cell cytotoxicity was conducted on human embryonic kidney cell line HEK 293T and human ovarian cancer cell line HeLa with a typical MTT assay. As shown in Fig. S9, even though the concentration of RhoDCM reached 50 μM, the cell viability of both cell lines was still over 90%, which suggested that RhoDCM was low-toxic.
For the fluorescence imaging of Cys level, the living HeLa cells were divided into five groups. Initially, all the groups were pre-treated with 2 mM N-ethylmaleimide (NEM) to clean up the biological thiols. Except for the addition of the probe RhoDCM, the five groups were incubated with buffer, 100 μM Hcy, 100 μM GSH, 50 μM Cys, and 100 μM Cys, respectively for 30 min (Fig. 3A & D). No obvious fluorescence enhancement could be observed in the Hcy- or GSH-incubated groups, while in the Cys-incubated groups, there seemed a basically dose-dependent fluorescence enhancement. Thus, RhoDCM could basically visualize the intracellular Cys level. Since the cytoplasmic environment is usually different from the solution system in vitro, the ratio coefficient of fluorescence intensity signal towards the cysteine concentration is also different [56,57]. Furthermore, to verify that RhoDCM could monitor the Cys generation in living cells, HeLa cells were divided into three groups and treated with RhoDCM for different time conditions (0, 15, and 30 min). As shown in Fig. 3B & E, along with the extension of the incubation time, the fluorescence signal in the green channel enhanced gradually. Since the response of RhoDCM towards Cys was rapid, the time-dependent fluorescence enhancement inferred that the probe could detect the endogenous generation of Cys in living cells.
Fig. 3.
A) The confocal images of the HeLa cells were incubated with NEM (2 mM) first and then under various conditions for 30 min (a1-a4) The cells were incubated with RhoDCM (20 μM) directly. (b1-e4) The cells were pre-treated with RhoDCM (20 μM) and then treated with (b1-b4) 100 μM Hcy, (c1-c4) 100 μM GSH, (d1-d4) 50 μM Cys, and (e1-e4) 100 μM Cys, respectively. B) The confocal images of the HeLa cells under different incubation time conditions. (f1-h4) The cells were incubated with RhoDCM (20 μM) directly for 0, 15, and 30 min, respectively. C) The confocal images of the HeLa cells with RhoDCM (20 μM) after the incubation of different concentrations of high glucose stimulation for 30 min (i1-k4) The cells were pre-treated with 0, 50, and 100 mM of glucose, respectively, and then incubated with RhoDCM (20 μM). D) Relative pixel ratio of the consistent groups in A). E) Relative pixel ratio of the consistent groups in B). F) Relative pixel ratio of the consistent groups in C). Scale bar: 20 μm. n = 3, error bars meant standard derivation (SD). Statistical analysis was performed with a two-tailed Student’s t-test with unequal variance, *p value < 0.05, **p value < 0.01, ***p value < 0.001.
At present, it is generally believed that oxidative stress damage caused by continuous high glucose stimulation is an important part of the pathogenesis of diabetes [20]. In addition, the correlation between glucose level and Cys concentration has been reported [58,59]. Cys and glucose did not react directly as shown in the selectivity experiment. Based on the fact that Cys in living cells could accumulate to a certain concentration, the glucose-induced groups initiated with the accumulated Cys level. Therefore, we studied the fluctuation of Cys concentration in cells incubated with high glucose. The living HeLa cells were incubated with various concentrations of glucose (0, 50, and 100 mM) for 30 min, then washed with PBS three times, and incubated with RhoDCM (20 μM) for 30 min. As shown in Fig. 3C & F, along with the increase of the glucose concentration, the fluorescence signal in the green channel decreased gradually, which indicated the decrease of Cys level. This result agreed with the recent report that glucose was accompanied by oxidative stress, and could consume the intracellular Cys.
Imaging Cys dynamics in mice models. To study the optical performance of the probe RhoDCM in vivo, fluorescence imaging was initially conducted in 4T1-xenograft mice as a preliminary trial before the diabetic model. As detailed in the Supporting Information, BALB/C nude mice (5–6 weeks old, male) were divided into six groups. The radiance intensity of the mice and the separate tumor was all measured. As shown in Fig. S10Aa1-f1 & S10B, compared with the blank group with saline only, the control group with RhoDCM injected directly exhibited an obvious radiance signal; when H2O2 (Cys consumer) or NEM (biothiols cleaner) was injected before the injection of RhoDCM, the tested radiance signal was slightly weaker; while the injection of N-acetylcysteine (NAC, precursor of cysteine) or Cys could lead to the enhancement of the radiance signal. In the separate tumors, as shown in Fig. S10Aa2-f2 & S10B, the tendency was almost the same and the differences were even more obvious. Furthermore, in each group, the radiance changes at different time points (0, 10, 20, and 30 min) after the injection of RhoDCM were analyzed. As shown in Figs. S11A and S12, all the tested groups indicated a slight increase in radiance intensity along with the extension of time; on the other hand, the differences between the groups basically agreed with the findings in Figs. S10A and S10B. Additionally, despite the tumor tissues, the organs including the heart, liver, spleen, lung, kidney, and intestine of the mice in Fig. S10A were also separated to test the radiance values. The corresponding pictures as well as the analysis were displayed in Figs. S11B and S13. In each group, except for the strongest signal in the tumor, the other relatively observable signal was mainly in the liver, intestine, and kidney. The above results suggested that RhoDCM could achieve imaging in living mice, and the metabolism sites, especially the liver, were the same as the location of the diabetic process.
Subsequently, as one of the highlighted designs, the mice models containing both the development and treatment of the diabetic process were constructed according to the schedule in Fig. 4a. The control group was normal mice with no diabetic symptoms; the model groups were C57BL/6J mice with Type II DM then induced by STZ and alloxan (both were Type I DM inducers), respectively; while the treatment groups were model mice induced by STZ first and then treated with Vil, DA, and Metf (marketed drugs with different mechanisms), respectively. The induction and treatment of the diabetic process were mainly checked by the oral glucose tolerance test (OGTT) as shown in Fig. 4b. The diabetic status was evaluated to the blood glucose level as well as the ability to metabolize the blood glucose. In the control group, the blood glucose level elevated from ∼5 mmol/L to ∼25 mmol/L in 1 h and then recovered to ∼5 mmol/L in the next hour. In the STZ-induced group, the initial blood glucose level was ∼25 mmol. It could rise to ∼40 mmol/L in 45 min, and took 75 min to recover to ∼25 mmol/L. The alloxan-induced group indicated a similar tendency to the STZ-induced group, but the affection was slightly weaker. Accordingly, the STZ- or alloxan-induced groups exhibited a typical feature of the diabetic process. On the other hand, in the treatment groups with different drugs, the curve was closer to that of the control group. Metf suggested the strongest potency among the selected drugs. Moreover, the significant liver-related serum indexes including alanine aminotransferase (ALT), aspartate aminotransferase (AST), Uric acid, and Creatinine (Fig. 4c–4f) were also measured. Generally, the levels of these indexes showed an increase in the model groups and the recovery after being treated with the selected drugs. In recovering these indexes, Metf seemed also the most potency one. In brief, the mice models in this work could represent the development and treatment of the diabetic process.
Fig. 4.
(a) The schedule for the control group (no diabetic mice), the model group, and the treatment group with different stages. (b) The measurements of blood glucose for the control group, the model groups (STZ and alloxan), and the treatments group (STZ + Vil, STZ + DA, STZ + Metf) at Day 44 of the model establishment and treatments course during OGTT. (c) Blood ALT levels of the studied groups. (d) Blood AST levels of the studied groups. (e) Blood uric acid levels of the studied groups. (f) Blood creatinine levels of the studied groups.
One step further, for the first time, the Cys-triggered implement was applied in the relatively complete models built above. One day after the OGTT, the mice, as well as the separated organs, were imaged with RhoDCM to monitor the Cys dynamics (Fig. 5 & S14-16). As shown in Fig. 5Aa1-f1, it could be observed at the first glance that the body habitus of the model groups were larger than the control/treatment groups. Meanwhile, there was an observable radiance signal in the control group, whereas the signal almost disappeared in the model groups. The radiance signal recovered in the treatment groups. In the separated liver tissues, the tendency of radiance changes was the same as that in the mice (Fig. 5Aa2-f2 & 5B). For the time condition, all the studied groups inferred a slight enhancement in radiance intensity as time prolonged, while the differences between the groups agreed with that in Fig. 5A & B. Moreover, the main organs including the heart, liver, spleen, lung, kidney, and intestine were separated and measured. As shown in Fig. 5C & S16, the differences between the groups also agreed with that in Fig. 5A & B, while in each group, the radiance signal was strongest in liver, followed by kidney and lung. In brief, RhoDCM could monitor the Cys dynamics in diabetic mice models, thus imaging the diabetic status in both the development and treatment processes.
Fig. 5.
A) The fluorescence imaging of mice and livers from (a) the control group, (b–c) diabetic groups, and (d–f) treatment groups after the intravenous injection with RhoDCM (100 μM). B) Relative fluorescence intensity in A). C) The fluorescence imaging and photos of the main organs from the studied groups as well as the average fluorescence intensity of the separate organs. n = 3, error bars meant standard derivation (SD). Statistical analysis was performed with a two-tailed Student’s t-test with unequal variance, *p value < 0.05, **p value < 0.01, ***p value < 0.001.
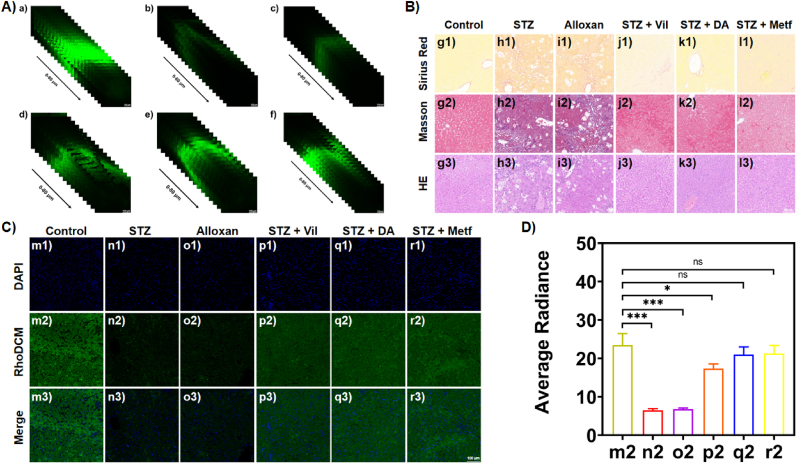
Finally, for further confirming the fluorescence imaging performance of RhoDCM, as well as the diabetic status, the penetrating depth fluorescence imaging together with the histopathological staining of the liver tissues was conducted (Fig. 6). Through a total depth of 80 μm (∼20 pieces in each sample), the fluorescence imaging of the liver tissues from each group, was conducted in the green channel (Fig. 6A). The slices of the liver tissue from the control group exhibited strong fluorescence signal in the green channel (Fig. 6Aa). On the contrary, the fluorescence signals in the liver slices from the diabatic model groups became much weaker (Fig. 6Ab-c). There was almost no fluorescence in the STZ-induced group (Fig. 6Ab).
Fig. 6.
A) The penetrating depth fluorescence imaging of liver tissues with RhoDCM (100 μM): (a) control; (b) STZ-induced; (c) alloxan-induced; (d) STZ-induced then Vil-treated; (e) STZ induced then DA-treated; (f) STZ-induced then Metf-treated. B) The histopathological staining of the liver slices from the studied groups with Sirius Red, Masson, and HE protocols. C) The fluorescence imaging of the liver slices with RhoDCM (100 μM) and DAPI (10 μM) together. D) The relative quantitative analysis of the fluorescence intensity in C). λex = 488 nm, Scale bar = 100 μm. n = 3, error bars meant standard derivation (SD). Statistical analysis was performed with a two-tailed Student’s t-test with unequal variance, *p value < 0.05, **p value < 0.01, ***p value < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
However, in the liver slices from the treatment groups, the green fluorescence signals recovered (Fig. 6Ad-f). Among them, the fluorescence signal intensity in the Metf-treated group seemed the closest to that in the control group. The above results showed that the probe could achieve imaging in deep tissue with practicability. Besides, the reference on the analogue of the selected fluorophore reported the in vivo imaging of liver injury and inflammation models in mice, which also supported good tissue penetration ability [54]. The single liver slices of all the studied groups were also incubated with DAPI to show the position of the nucleus in the blue channel together with the signals caused by RhoDCM in the green channel (Fig. 6C). In the single liver slices from the studied mice groups, the changes of the fluorescence intensity in the green channel (Fig. 6C & D) agreed with that of the radiance signal in the in vivo imaging section. In brief, the fluorescence signals decreased during the development of the diabetic process and recovered during the treatment. Independent from the optical signals, the histopathological staining results also showed consistency with the above tendency. In Sirius Red staining pictures (Fig. 6Bg1-l1), the control group was light yellow and dense, whereas the model groups were orange and loose, and the treatment groups exhibited a recovery to the status of the control. In Masson staining pictures (Fig. 6Bg2-l2), the development of the diabetic process could transfer the pink and dense status to the purple and loose status, which could also be recovered by the treatment. In Hematoxylin-eosin (HE) staining pictures (Fig. 6Bg3-l3), the above-mentioned changes could also be observed according to the features of HE staining.
Therefore, we found that the optical signal-based results (in vivo imaging and fluorescence imaging) and the non-optical-based results (OGTT, serum indexes, and histopathological staining) were consistent with each other. Accordingly, by using the approaches based on RhoDCM, the status of both the development and the treatment in the diabetic process could be characterized via monitoring the Cys dynamics. This kind of characterization could cover different induction mechanisms (STZ or alloxan) and various treating strategies (Vil, DA, or Mtef). It could also infer the order of severity in the diabetic process, and evaluate the potency of different therapeutic schedules.
3. Conclusion
To conclude, a fluorescent probe RhoDCM for monitoring the Cys dynamics was developed, and, as a novel trial, it was applied in relatively complete mice models containing both the development and treatment of the diabetic process. High selectively towards Cys, RhoDCM could report a remarkable fluorescence peak at 570 nm under the excitation of 480 nm. The response could rapidly complete within 5 min, and was stable within the pH range of 6.0-8.0 as well as the temperature range of 30–40 °C. With low cytotoxicity, RhoDCM could monitor the intracellular Cys level, both exogenous and endogenous. Since the increase of glucose concentration could consume the intracellular Cys, RhoDCM could also monitor the glucose level via the decrease of fluorescence signal. Moreover, RhoDCM could achieve the imaging in living 4T1-xenograft mice, and the metabolism sites, especially the liver, was the same as the location of the diabetic process. Accordingly, the diabetic mice models were constructed to involve the no diabetic control group, the induced model groups (by STZ or alloxan), and the treatment groups (induced by STZ and then treated with marketed drugs including Vil, DA, and Metf). The diabetic process was mainly checked by the OGTT and the significant liver-related serum indexes. The in vivo imaging and the penetrating depth fluorescence imaging both indicated that RhoDCM could characterize the status of both the development and the treatment in diabetic process via monitoring the Cys dynamics. Thus, RhoDCM could also infer the order of severity in diabetic process and evaluate the potency of different therapeutic schedules, which might be informatic and applicable in future investigations.
Funding sources
This work was supported by the National Natural Science Foundation of China (No. 81803349), Jiangsu Provincial Health Commission Project (Z2022066), the Science and Technology Development Fund Project of Nanjing Medical University, China (NMUB20210311). The authors would like to thank Wen-Dong Li from Shiyanjia Lab (www.shiyanjia.com) for the LC-MS analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102660.
Contributor Information
Zhi-Gang Hu, Email: jswxhzg@163.com.
Yu-Shun Yang, Email: ys_yang@nju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Targher G., Lonardo A., Byrne C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 2018;14:99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 2.Donath M.Y., Dinarello C.A., Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019;19:734–746. doi: 10.1038/s41577-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; 2016. Global Report on Diabetes. [Google Scholar]
- 4.Fisher L., Gonzalez J.S., Polonsky W.H. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet. Med. 2014;31:764–772. doi: 10.1111/dme.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Felice F.G., Ferreira S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 6.Rines A.K., Sharabi K., Tavares C.D.J., Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016;15:786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H., Moschen A.R., Roden M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2018;14:32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 8.Kim W., Bang A., Kim S., Lee G.-J., Kim Y.-H., Choi S. Adiponectin-targeted SERS immunoassay biosensing platform for early detection of gestational diabetes mellitus. Biosens. Bioelectron. 2022;213 doi: 10.1016/j.bios.2022.114488. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 10.Ilonen J., Lempainen J., Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:635–650. doi: 10.1038/s41574-019-0254-y. [DOI] [PubMed] [Google Scholar]
- 11.Milardi D., Gazit E., Radford S.E., Xu Y., Gallardo R.U., Caflisch A., Westermark G.T., Westermark P., Rosa C.L., Ramamoorthy A. Proteostasis of islet amyloid polypeptide: a molecular perspective of risk factors and protective strategies for type II diabetes. Chem. Rev. 2021;121:1845–1893. doi: 10.1021/acs.chemrev.0c00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang M., Daya N., Coresh J., Christenson R.H., Selvin E. Glycated albumin for the diagnosis of diabetes in US adults. Clin. Chem. 2022;68:413–421. doi: 10.1093/clinchem/hvab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami T., Fujimoto H., Inagaki N. Non-invasive beta-cell imaging: visualization, quantification, and beyond. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.714348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P.J., Li T., Wu X.Y., Nice E.C., Huang C.H., Zhang Y.Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang W.X., Jiang W.L., Guo H., Li Y.F., Li C.Y. Real-time imaging of alkaline phosphatase activity of diabetes in mice via a Near-Infrared fluorescent probe. Chem. Commun. 2021;57:480–483. doi: 10.1039/d0cc07292c. [DOI] [PubMed] [Google Scholar]
- 16.Yu W.L., Huang J., Lin M.G., Wei G.M., Yang F., Tang Z.X., Zeng F., Wu S.Z. Fluorophore-dapagliflozin dyad for detecting diabetic liver/kidney damages via fluorescent imaging and treating diabetes via inhibiting SGLT2. Anal. Chem. 2021;93:4647–4656. doi: 10.1021/acs.analchem.1c00223. [DOI] [PubMed] [Google Scholar]
- 17.Wang J.M., Zhang L., Qu Y., Yang Y.X., Cao T., Cao Y.P., Iqbal A., Qin W.W., Liu Y. Long-wavelength ratiometric fluorescent probe for the early diagnosis of diabetes. Anal. Chem. 2021;93:11461–11469. doi: 10.1021/acs.analchem.1c01491. [DOI] [PubMed] [Google Scholar]
- 18.Chen B.C., Mao S.M., Sun Y.Y., Sun L.Y., Ding N., Li C.D., Zhou J. A mitochondria-targeted Near-Infrared fluorescent probe for imaging viscosity in living cells and a diabetic mice model. Chem. Commun. 2021;57:4376–4379. doi: 10.1039/d1cc01104a. [DOI] [PubMed] [Google Scholar]
- 19.Avci E., Uncu G., Avci G.A. An independent risk factor for cardiovascular diseases in diabetic nephropathy; Homocysteine. Free Radical Biol. Med. 2018;120:153–154. [Google Scholar]
- 20.Yue L.Z., Huang H.W., Song W.H., Lin W.Y. Research on mitochondrial oxidative stress accompanying the diabetic process under airborne particulate matter pollution by NIR fluorescence imaging of cysteine. Chem. Eng. J. 2022;441 [Google Scholar]
- 21.Dong J., Lu G., Tu Y., Fan C. Recent research progress of red-emitting/Near-Infrared fluorescent probes for biothiols. New J. Chem. 2022;46:10995–11020. [Google Scholar]
- 22.Dong J., Wang Y., Fan C., Tu Y., Pu S. Chalcone dye-based fluorescent probe for selective and specific detection of cysteine in lysosomes of living cells. Dyes Pigments. 2023;210 [Google Scholar]
- 23.Lu G., Dong J., Fan C., Tu Y., Pu S. A coumarin-based fluorescent probe for specific detection of cysteine in the lysosome of living cells. Bioorg. Chem. 2022;119 doi: 10.1016/j.bioorg.2021.105558. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Lu G., Tu Y., Pu S. A turn-on fluorescent probe for the discrimination of Cys/Hcy and GSH with dual emission signals. J. Fluoresc. 2021;31:599–607. doi: 10.1007/s10895-021-02684-6. [DOI] [PubMed] [Google Scholar]
- 25.Lu J.T., Chen K.G., Chen W., Liu C., Jiang X.P., Ma Z.L., Li D., Shen Y.J., Tian H. Association of serum homocysteine with cardiovascular and all-cause mortality in adults with diabetes: a prospective cohort study. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/2156483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shargorodsky M., Boaz M., Pasternak S., Hanah R., Matas Z., Fux A., Beigel Y., Mashavi M. Serum homocysteine, folate, vitamin B12 levels and arterial stiffness in diabetic patients: which of them is really important in atherogenesis? Diabetes-Metab. Res. 2009;25:70–75. doi: 10.1002/dmrr.902. [DOI] [PubMed] [Google Scholar]
- 27.Bhansali S., Bhansali A., Walia R., Saikia U.N., Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front. Endocrinol. 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes C.E., Coody T.K., Jeong M.-Y., Berg J.A., Winge D.R., Hughes A.L. Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell. 2020;180:296–310. doi: 10.1016/j.cell.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badgley M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.-J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M., Decker A.R., Sastra S.A., Palermo C.F., Andrade L.R., Sajjakulnukit P., Zhang L., Tolstyka Z.P., Hirschhorn T., Lamb C., Liu T., Gu W., Seeley E.S., Stone E., Georgiou G., Manor U., Iuga A., Wahl G.M., Stockwell B.R., Lyssiotis C.A., Olive K.P. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Wang Z.Q., Mao G.J., Jiang W.L., Tan M., Xu F., Li C.Y. A Near-Infrared fluorescent probe with large Stokes shift for imaging Cys in tumor mice. Anal. Chim. Acta. 2021;1171 doi: 10.1016/j.aca.2021.338655. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z.Y., Si S.F., Zhang Z.J., Tan H.Y., Qin T.Y., Wang Z.L., Wang D., Wang L., Liu B. A fluorescent probe with dual acrylate sites for discrimination of different concentration ranges of cysteine in living cells. Anal. Chim. Acta. 2021;1176 doi: 10.1016/j.aca.2021.338763. [DOI] [PubMed] [Google Scholar]
- 32.Wang S., Zhang Q., Chen S.J., Wang K.P., Hu Z.Q. A diazabenzoperylene derivative as ratiometric fluorescent probe for cysteine with super large Stokes shift. Anal. Bioanal. Chem. 2020;412:2687–2696. doi: 10.1007/s00216-020-02500-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L.H., He X., Huang Y.B., Zhang S.Q., Han H., Xu L.L., Wang X.H., Song D.Q., Ma P.Y., Sun Y. A novel Near-Infrared fluorescent probe for intracellular detection of cysteine. Anal. Bioanal. Chem. 2020;412:7211–7217. doi: 10.1007/s00216-020-02853-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y.D., Wang X., Bai X.Y., Li P., Su D., Zhang W., Zhang W., Tang B. Highly specific Cys fluorescence probe for living mouse brain imaging via evading reaction with other biothiols. Anal. Chem. 2019;91:8591–8594. doi: 10.1021/acs.analchem.9b01878. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H.C., Liu C.Y., Rong X.D., Zhang Y., Su M.J., Wang X., Liu M.Y., Zhang X.H., Sheng W.L., Zhu B.C. A new isothiocyanate-based Golgi-targeting fluorescent probe for Cys and its bioimaging applications during the Golgi stress response. Bioorg. Chem. 2022;122 doi: 10.1016/j.bioorg.2022.105741. [DOI] [PubMed] [Google Scholar]
- 36.Shen B.X., Qian Y. Red emission cysteine probe with high selectivity based on fluorescent protein chromophores and turn-on fluorescence in cell cultures. Dyes Pigments. 2019;166:350–356. [Google Scholar]
- 37.Chen X.X., Huang X.Q., Liu G., Tu Y.Y., Fan C.B., Pu S.Z. A highly selective colorimetric and fluorescent probe for cysteine sensing: application in live cell imaging and test strips. Dyes Pigments. 2021;196 [Google Scholar]
- 38.Zhang X., Zhang L., Ma W.W., Zhou Y., Lu Z.N., Xu S.Y. Near-Infrared ratiometric fluorescent probe for highly selective recognition and bioimaging of cysteine. Front. Chem. 2019;7:32. doi: 10.3389/fchem.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai S.T., Liu C., Jiao X.J., Zhao L.C., Zeng X.S. A lysosome-targeted Near-Infrared fluorescent probe for imaging endogenous cysteine (Cys) in living cells. J. Mater. Chem. B. 2020;8:2269–2274. doi: 10.1039/c9tb02609f. [DOI] [PubMed] [Google Scholar]
- 40.Liu D.J., Lv Y., Chen M., Cheng D., Song Z.L., Yuan L., Zhang X.B. A long wavelength emission two-photon fluorescent probe for highly selective detection of cysteine in living cells and an inflamed mouse model. J. Mater. Chem. B. 2019;7:3970–3975. [Google Scholar]
- 41.Zhu M.Q., Fan F.G., Zhao Z.Y., Wu X.Q., Wang L.J., Na R.S., Wang Y. An ICT-based ratiometric fluorescent probe for cysteine and its application in biological issues. J. Mol. Liq. 2019;296 [Google Scholar]
- 42.Ren H.X., Huo F.J., Zhang Y., Zhao S.H., Yin C.X. An NIR ESIPT-based fluorescent probe with large Stokes shift for specific detection of Cys and its bioimaging in cells and mice. Sensor Actuat. B-Chem. 2020;319 [Google Scholar]
- 43.Huang Y.L., Ren Q.F., Li S.Q., Feng Y., Zhang W., Fang G.S., Li L.K., Sun C., Wang X., Meng X.M. A dual-emission two-photon fluorescent probe for specific-cysteine imaging in lysosomes and in vivo. Sensor Actuat. B-Chem. 2019;293:247–255. [Google Scholar]
- 44.Qin J.C., Li Z.W., Fu Z.H., Zhang Z.H. Development of a NIR fluorescent probe for detection of cysteine and its application in bioimaging. Sensor Actuat. B-Chem. 2022;357 [Google Scholar]
- 45.Tu Y.X., Vijay N., Ko H.X., Lo Y.P., Velmathi S., Wu S.P. Specific two-photon fluorescent probe for cysteine detection in vivo. Spectrochim. Acta. 2022;267 doi: 10.1016/j.saa.2021.120521. [DOI] [PubMed] [Google Scholar]
- 46.Hou X.F., Li Z.S., Li Y.Q., Zhou Q.H., Liu C.H., Fan D., Wang J.J., Xu R.J., Xu Z.H. ICT-modulated NIR water-soluble fluorescent probe with large Stokes shift for selective detection of cysteine in living cells and zebrafish. Spectrochim. Acta A. 2021;246 doi: 10.1016/j.saa.2020.119030. [DOI] [PubMed] [Google Scholar]
- 47.Li Y.L., He X., Huang Y.B., Xu L.B., Zhao L.H., Li X.L., Sun Y., Wang X.H., Ma P.Y., Song D.Q. Development of a water-soluble Near-Infrared fluorescent probe for endogenous cysteine imaging. Spectrochim. Acta. 2020;226 doi: 10.1016/j.saa.2019.117544. [DOI] [PubMed] [Google Scholar]
- 48.Qiao L.Q., Yang Y.X., Cai J.H., Lv X., Hao J.S., Li Y.P. Long wavelength emission fluorescent probe for highly selective detection of cysteine in living cells. Spectrochim. Acta A. 2022;264 doi: 10.1016/j.saa.2021.120247. [DOI] [PubMed] [Google Scholar]
- 49.Ji Y., Dai F., Zhou B. Developing a julolidine-fluorescein-based hybrid as a highly sensitive fluorescent probe for sensing and bioimaging cysteine in living cells. Talanta. 2019;197:631–637. doi: 10.1016/j.talanta.2019.01.084. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X.Y., Liu H., Ma Y.Y., Qu W.B., He H.P., Zhang X.H., Wang S.F., Sun Q., Yu F.B. Development of a novel Near-Infrared fluorescence light-up probe with a large Stokes shift for sensing of cysteine in aqueous solution, living cells and zebrafish. Dyes Pigments. 2019;171 [Google Scholar]
- 51.Huang Y.L., Zhou Q., Feng Y., Zhang W., Fang G.S., Fang M., Chen M., Xu C.Z., Meng X.M. Rational design of a ratiometric two-photon fluorescent probe for real-time visualization of apoptosis. Chem. Commun. 2018;54:10495–10498. doi: 10.1039/c8cc05594g. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.Y., He N., Huang Y., Yu F.B., Li B.W., Lv C.J., Chen L.X. Mitochondria-targeting Near-Infrared ratiometric fluorescent probe for selective imaging of cysteine in orthotopic lung cancer mice. Sensor Actuat. B-Chem. 2019;282:69–77. [Google Scholar]
- 53.Yue Y.K., Huo F.J., Ning P., Zhang Y.B., Chao J.B., Meng X.M., Yin C.X. Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells. J. Am. Chem. Soc. 2019;139:3181–3185. doi: 10.1021/jacs.6b12845. [DOI] [PubMed] [Google Scholar]
- 54.Wen L., Ma X.Y., Yang J., Jiang M.M., Peng C., Ma Z.Y., Yu H., Li Y.H. A new ratiometric design strategy based on modulation of π-conjugation unit for developing fluorescent probe and imaging of cellular peroxynitrite. Anal. Chem. 2022;94:4763–4769. doi: 10.1021/acs.analchem.1c05447. [DOI] [PubMed] [Google Scholar]
- 55.Sedgwick A.C., Wu L.L., Han H.H., Bull S.D., He X.P., James T.D., Sessler J.L., Tang B.Z., Tian H., Yoon J.Y. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018;47:8842–8880. doi: 10.1039/c8cs00185e. [DOI] [PubMed] [Google Scholar]
- 56.Liu C., Liu J., Zhang W., Wang Y.L., Liu Q., Song B., Yuan J., Zhang R. Two birds with one stone" ruthenium(II) complex probe for biothiols discrimination and detection In vitro and in vivo. Adv. Sci. 2020;7 doi: 10.1002/advs.202000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K., Wang W., Guo M.Y., Chen S.Y., Yang Y.S., Wang B.Z., Xu C., Zhu H.L. Design and synthesis of a novel "turn-on" long range measuring fluorescent probe for monitoring endogenous cysteine in living cells and Caenorhabditis elegans. Anal. Chim. Acta. 2021;1152 doi: 10.1016/j.aca.2021.338243. [DOI] [PubMed] [Google Scholar]
- 58.Simpson R.C., Freedland R.A. Relative importance of the two major pathways for the conversion of cysteine to glucose in the perfused rat liver. J. Nutr. 1975;105:1440–1446. doi: 10.1093/jn/105.11.1440. [DOI] [PubMed] [Google Scholar]
- 59.Gazit V., Ben-Abraham R., Coleman R., Weizman A., Katz Y. Cysteine-induced hypoglycemic brain damage: an alternative mechanism to excitotoxicity. Amino Acids. 2004;26:163–168. doi: 10.1007/s00726-003-0045-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.