Abstract
This study aimed to evaluate the individual and combined effects of chemically protected sodium butyrate (CSB) and xylo-oligosaccharide (XOS) on performance, anti-inflammatory and antioxidant capacity, intestinal morphology and microbiota of broilers. A total of 280 one-day-old Arbor Acres broilers were randomly distributed into 5 treatments: basal diet (CON), basal diet supplemented with 100 mg/kg aureomycin and 8 mg/kg enramycin (ABX), 1000 mg/kg CSB (CSB), 100 mg/kg XOS (XOS), and mixture of 1000 mg/kg CSB and 100 mg/kg XOS (MIX), respectively. On d 21, ABX, CSB, and MIX decreased feed conversion ratio compared with CON (CON: ABX: CSB: MIX = 1.29: 1.22: 1.22: 1.22), whereas body weight of CSB and MIX was increased by 6.00% and 7.93%, and average daily gain was increased by 6.62% and 8.67% at 1-21 d, respectively (P < 0.05). The main effect analysis showed that both CSB and XOS treatments increased ileal villus height and villus height to crypt depth ratio (VCR) (P < 0.05). Moreover, broilers in ABX showed lower 21.39% ileal crypt depth and higher 31.43% VCR than those in CON (P < 0.05). Dietary CSB and XOS were added individually or collectively increased total antioxidant capacity and superoxide dismutase, and anti-inflammatory cytokines interleukin-10 and transforming growth factor-β, whereas decreased malondialdehyde, and proinflammatory cytokines IL-6 and tumor necrosis factor-α content in serum (P < 0.05). Meanwhile, MIX showed the best effect of antioxidant and anti-inflammatory capacity among the 5 groups (P < 0.05). There was an interaction between CSB and XOS treatments on increasing cecal acetic acid, propionic acid, butyric acid and total short-chain fatty acid (SCFA) (P < 0.05), and the one-way ANOVA showed that propionic acid in CSB was 1.54 times that of CON, whereas butyric acid and total SCFAs in XOS were 1.22 times and 1.28 times that of CON, respectively (P < 0.05). Furthermore, dietary combination of CSB and XOS changed phyla Firmicutes and Bacteroidota, and increased genera Romboutsia and Bacteroides (P < 0.05). In conclusion, dietary CSB and XOS improved growth performance of broilers, and the combined addition of them had the best effect on anti-inflammatory and antioxidant capacity, and intestinal homeostasis of broilers in current study, indicating that it may be a potential natural alternative to antibiotics.
Key words: sodium butyrate, xylo-oligosaccharide, antioxidant capacity, immune function, gut microbiota
INTRODUCTION
Broiler is vulnerable to stimulation by external factors such as diseases, nutritional and environmental challenges, especially when the intestinal function and immune function are not fully developed, resulting in poor health and growth performance (Wang et al., 2021). For a long time, antibiotics have maintained the dominant position in feed additives due to the bacteriostatic or bactericidal characteristics, and the promotion of animal growth. However, as people pay more attention to the problem of antibiotic resistance and residues in animals (Frieri et al., 2017), the prohibition of antibiotic use in animal feed has been promulgated and implemented in the European Union and China (Salaheen et al., 2017; Wang et al., 2020; Wen et al., 2022). This means that antibiotic-free feed has become an inevitable trend worldwide. Nevertheless, we cannot ignore the problems such as low growth performance and high intestinal diseases in livestock and poultry without the protection of antibiotics. In recent years, researchers have been devoting themselves to seek antibiotics alternatives, such as acidifiers, prebiotics, probiotics and plant extracts, etc., and alternative proposals (Abiala et al., 2016; Chen et al., 2018b; Liu et al., 2018b; Sanders et al., 2019).
Xylo-oligosaccharides (XOS) are emerging prebiotics that have recently been gained a great interest in antibiotic substitution. XOS are functional polymerized sugars with straight or branched chains formed by 2-7 xylose molecules bonded by β-1, 4-glycosidic bonds (Chen et al., 2021a). XOS are not be degraded by endogenous enzymes, but serve as substrates for microbial fermentation to produce short-chain fatty acids (SCFAs) in animals, thus affecting the growth and development of animals (Rahimifard and Naseri, 2016; Zhuang et al., 2019). It can selectively stimulate the growth and/or activities of beneficial bacteria such as Bifidobacterium and Lactobacillus, etc., and/or restrict the colonization of pathogenic bacteria (de Figueiredo et al., 2020). Previous studies in our laboratory have found that XOS supplementation stimulated the increase of Lactobacillus to enhance intestinal health (Chen et al., 2021c). Six strains of Lactobacillus, such as Lactobacillus gallinarum, Lactobacillus johnsonii, Lactobacillus salivarius, etc., have been reported to be beneficial to the intestinal health of broilers (Neveling et al., 2020). However, it is still unclear whether XOS has the same positive regulatory effect on Lactobacillus levels in broiler at early stage. Besides, XOS can improve the growth performance of weaned piglets by improving serum antioxidant defense system, serum immunoglobulin G (IgG), small intestinal structure and intestinal barrier function (Chen et al., 2021b).
Sodium butyrate is widely used as a sodium salt of organic acid (butyrate) in feed additives. In addition to providing energy and mediating immune response, butyrate, 1 of the 3 main SCFAs (acetate, propionate and butyrate), also serves to balance intestinal flora in animals (Bortoluzzi et al., 2017; Chen et al., 2018a; Liu et al., 2018a). However, butyrate is easily volatilized and its peculiar odor leads to the poor palatability of the feed. In general, sodium butyrate or coated sodium butyrate could enhance the stability of butyrate, reduce the odor of butyrate, and even regulate the location or rate of release of butyrate in an animal. The effects of different coated sodium butyrate have been widely studied (Wu et al., 2018; Wambacq et al., 2020; Makowski et al., 2022; Zhang et al., 2022), but there are still many differences and controversies in related research and techniques. In this study, experimented butyrate, named chemically protected sodium butyrate (CSB), is protected by a physical and chemical matrix of buffer salts, which avoided dissociation at low pH in stomach or gizzard and is able to release enough butyrate in small intestine (Zhao et al., 2022). Furthermore, Lan et al. (2020b) reported that similar CSB had positive effects on growth performance and gastrointestinal development of broilers, and its effect and mechanism on poultry need more study to verify and investigate.
As dietary supplements designed to improve animal health, CSB and XOS have garnered considerable attention due to their unique properties. At present, it is unclear whether the combination of CSB and XOS could get better effects, and the research on the relevant mechanism is relatively lacking. Therefore, the purpose of this study was conducted to evaluate the effects of XOS, CSB and their combination on the growth performance, intestinal morphology, digestive enzyme activities, antioxidant capacity, immune function and cecal microflora in broilers at early stage, finally providing reference and theoretical basis for the application of their mixed preparations in poultry.
MATERIALS AND METHODS
Animals, Diets, and Experimental Design
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Chinese Academy of Agriculture Sciences and experiments were approved by the Animal Ethics Committee of Experimental Animal Welfare and Ethical of Institute of Animal Science, Chinese Academy of Agriculture Sciences (IAS2021-105).
A total of 280 one-day-old male Arbor Acres broilers (Beijing Huadu Broiler Company, Beijing, China) were randomly divided into 5 treatments with 8 replicates and 7 birds/replicate. The broilers fed a basal diet (CON, a corn-soybean meal), CON diet supplemented with 100 mg/kg aureomycin and 8 mg/kg enramycin (ABX) of diet, CON diet supplemented with 1000 mg/kg CSB (CSB) of diet, CON diet supplemented with 100 mg/kg XOS (XOS) of diet, and CON diet supplemented with 1000 mg/kg CSB and 100 mg/kg XOS (MIX) of diet, respectively. The supplementation CSB (Beijing Shengtaiyuan Bio-Technology Co., Ltd., Beijing, China) contains 54% sodium butyrate protected by a physical and chemical matrix of buffer salts (Lan et al., 2020a; Lan et al., 2020b), and the supplementation XOS (Longlive Biotechnology Co., Ltd., Shandong, China) contains 95% XOS (Chen et al., 2021b; Chen et al., 2021c).
The light schedule was 23 h of light and 1 h of dark throughout the experimental period, for 21 d. Broilers were given free access to water and feed. The CON diet was formulated according to the Feeding Standard of Broiler Chicken (China, 2004; Table 1).
Table 1.
Composition and nutrient levels of the basal corn–soybean meal diets (as-fed basis).
| Items (% unless noted) | Contents |
|---|---|
| Ingredients | |
| Corn (7.9%, crude protein) | 55.00 |
| Soybean meal (43.6%, crude protein) | 36.30 |
| Soybean oil | 4.15 |
| Dicalcium phosphate | 1.80 |
| Sodium chloride | 0.30 |
| Limestone | 0.90 |
| Choline chloride (50%) | 0.10 |
| L - Lysine • HCl (99%) | 0.21 |
| DL - Methionine (98%) | 0.24 |
| Premix1 (1%) | 1.00 |
| Total | 100.00 |
| Calculated nutrient levels | |
| Metabolic energy (Mcal/kg) | 2.97 |
| Crude protein | 21.10 |
| Available phosphorus | 0.46 |
| Calcium | 1.05 |
| Lysine | 1.32 |
| Methionine | 0.58 |
The premix provided per kilogram of diets: vitamin A 12,000 IU, vitamin B1 3.5 mg, vitamin B2 8.6 mg, vitamin B12 0.02 mg, vitamin D3 25,000 IU, vitamin E 20 IU, vitamin K3 32.5 mg, biotin 0.20 mg, folic acid 1.00 mg, D-pantothenic acid 15 mg, nicotinic acid 50 mg, Cu (as copper sulfate) 8 mg, Fe (as ferrous sulfate) 80 mg, Mn (as manganese sulfate) 120 mg, Zn (as zinc sulfate) 110 mg, Se (as sodium selenite) 0.30 mg.
Sample Collection
At 21 d of age, 1 bird per replicate close to the average body weight (BW) was selected and weighed. Fresh blood was collected from the jugular vein in nonheparinized centrifuge tubes, and then these tubes were centrifuged at 1,700 × g for 15 min at 4°C to separate serum. The isolated serum was stored at −20°C until analysis. Later, the birds were killed by cervical dislocation. Open the abdominal cavity and separate the duodenum, jejunum, ileum and cecum. Then, about 1-cm middle portion of the duodenum, jejunum and ileum were individually fixed in 4% paraformaldehyde, and stored at room temperature until analysis. Finally, the digesta of jejunum, ileum and cecum were removed and collected in sterile freezer tubes, snap-frozen in liquid nitrogen, and then stored at −80°C until analysis.
Growth Performance
The BW of 1- and 21-day-old broilers was recorded as the initial BW and the final BW (FBW). BW and feed consumption of broilers were recorded for each replicate throughout the experimental period. In this way, at 21 d of age, average daily weight gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated for every bird.
Small Intestinal Histomorphology Analysis
Samples of duodenum, jejunum and ileum fixed with 4% paraformaldehyde were dehydrated with 70, 80, and 90% alcohol, and cleaned by treating with xylene solution. Then, the tissues were embedded with paraffin and sectioned at a thickness of 3 to 5 μm. After staining with Periodic Acid Schiff, images were captured using a DM300 microscope (Leica Microsystems, Wetzlar, Germany). The villus height (VH) and crypt depth (CD) (Luo et al., 2021) were observed under an optical microscope, and VH to CD ratio (VCR) were calculated. Ten points of each section were randomly selected for measurement and the average value was taken.
Small Intestinal Digestive Enzyme Activities Determination
Approximately 0.5 g jejunal or ileal digesta were homogenized by adding 9 times the volume of cold normal saline, and then centrifuged at 1,200 × g for 10 min at 4°C to take the supernatant for analysis. The total protein of homogenized supernatant was determined using Pierce BCA protein assay kit (Thermo Scientific Inc., Waltham, MA). Trypsin, amylase and lipase activities were measured by using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and all results were normalized by total protein of each sample for inter-sample comparison.
Serum Biochemical Analysis
Total antioxidant capacity (T-AOC), superoxide dismutase (SOD), malondialdehyde (MDA), immunoglobulin A (IgA), IgG, and immunoglobulin M (IgM) in serum were measured using relevant reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. Interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) were determined according to the manufacturer's instructions (Beijing Jinhaikeyu Biological Technology Development Co. Ltd, Beijing, China), and measured with ST-360 microplate reader (Shanghai Kehua Bio-Engineering Co. Ltd, Shanghai, China).
Microbial 16S rRNA Analysis of Cecal Digesta
DNA was extracted according to the FastDNA SPIN for soil kit (MP Biomedicals, Solon, OH) instructions. DNA purity and concentration were detected by NanoDrop2000, and DNA integrity was detected by 1% agarose gel electrophoresis. PCR was used to amplify V3-V4 region of 16S rRNA gene with universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The reaction parameters of PCR amplification were performed as follows: initial denaturation at 95°C for 3 min, followed by 27 cycles of denaturing at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s, and single extension at 72°C for 10 min, and end at 10°C. Purified amplicons were pooled in equimolar and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Cecal SCFAs Analysis
Acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid were quantitatively determined in cecum digesta by gas chromatography (Tang et al., 2021). The standard curve was prepared by mixing mother liquor, 25% metaphosphoric acid and ultrapure water in different proportions. Cecal chyme was homogenized with ultra-pure water, centrifuged, supernatant mixed with 25% metaphosphoric acid 9:1, filtered by 0.45 μm Milled-LG (Millipore, Billerica, MA), and SCFAs analysis was performed by Agilent 7890 N gas chromatograph (Agilent, Santa Clara, CA).
Statistical Analysis
The SAS 9.4 statistical software (SAS Inst. Inc., Cary, NC) was used to analyze data, except the venn diagram, principal co-ordinate analysis (PCoA) and community bar plot analysis, by using 1-way ANOVA following by Duncan's multiple range test among 5 groups and a 2 × 2 factorial arrangement (CSB and XOS), excluding ABX, to consider the main effects of CSB and XOS supplementation as well as CSB × XOS interaction. Data were expressed as mean and SEM, mean ± SE or mean and value from minimum to maximum only in table, bar graph or box plot, respectively. P < 0.05 indicated statistically significant, and 0.05 ≤ P < 0.10 was defined as a tendency towards significance. Operational taxonomic units (OTUs) clustering based on ribosomal database project classifier Bayesian algorithm at 97% similarity level, venn diagram on OTU level, community bar plot on phylum and genus level, and PCoA analysis based on the unweighted-unifrac distance metrics and ANOSIM test of cecal flora, were performed by Majorbio I-Sanger Cloud Platform (www.i-sanger.com).
RESULTS
Growth Performance
As shown in Table 2, broilers fed CSB supplementation diets showed the higher FBW and ADG, and the lower FCR than those fed diets without CSB (P < 0.05). There was no interaction between CSB and XOS treatments (P > 0.05), however, the 1-way ANOVA analysis results showed that broilers in MIX group exhibited higher FBW and ADG as compared with those in CON and XOS groups (P < 0.05). FCR of broilers in ABX group was lower than CON group (P < 0.05), to a level equal to that of CSB and MIX groups (P > 0.05). No significant difference was observed in ADFI among the 5 groups (P > 0.05).
Table 2.
Effects of CSB or/and XOS on growth performance in broilers.
| Treatments | IBW (g) | FBW (g) | ADG (g/d) | ADFI (g/d) | FCR (g/g) |
|---|---|---|---|---|---|
| CON | 46.81 | 599.21c | 26.30c | 33.97 | 1.29a |
| ABX | 46.33 | 619.24abc | 27.28abc | 33.31 | 1.22b |
| CSB | 46.44 | 635.18ab | 28.04ab | 34.22 | 1.22b |
| XOS | 46.49 | 609.15bc | 26.79bc | 33.45 | 1.25ab |
| MIX | 46.55 | 646.73a | 28.58a | 34.87 | 1.22b |
| SEM | 0.08 | 5.60 | 0.27 | 0.26 | 0.01 |
| P-value | 0.38 | 0.04 | 0.04 | 0.32 | 0.01 |
| Main effects | |||||
| CSB | |||||
| - | 46.65 | 604.18y | 26.55y | 33.71 | 1.27x |
| + | 46.49 | 640.95x | 28.31x | 34.55 | 1.22y |
| XOS | |||||
| - | 46.63 | 617.19 | 27.17 | 34.10 | 1.26 |
| + | 46.52 | 627.94 | 27.69 | 34.16 | 1.24 |
| P-values | |||||
| CSB | 0.39 | 0.01 | 0.01 | 0.19 | 0.01 |
| XOS | 0.56 | 0.40 | 0.40 | 0.92 | 0.19 |
| CSB × XOS | 0.25 | 0.95 | 0.96 | 0.35 | 0.25 |
Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; IBW, initial body weight; FBW, final body weight; FCR, feed conversion ratio (feed: gain, g/g); MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; XOS, 100 mg/kg XOS.
Superscripts for means belong to 1-way ANOVA following by Duncan's multiple range test among 5 groups (n = 8).
Superscripts for means belong to 2 × 2 factorial arrangement (CSB and XOS), excluding ABX.
Means in a column with different superscripts are significantly different (P < 0.05).
Small Intestinal Histomorphology
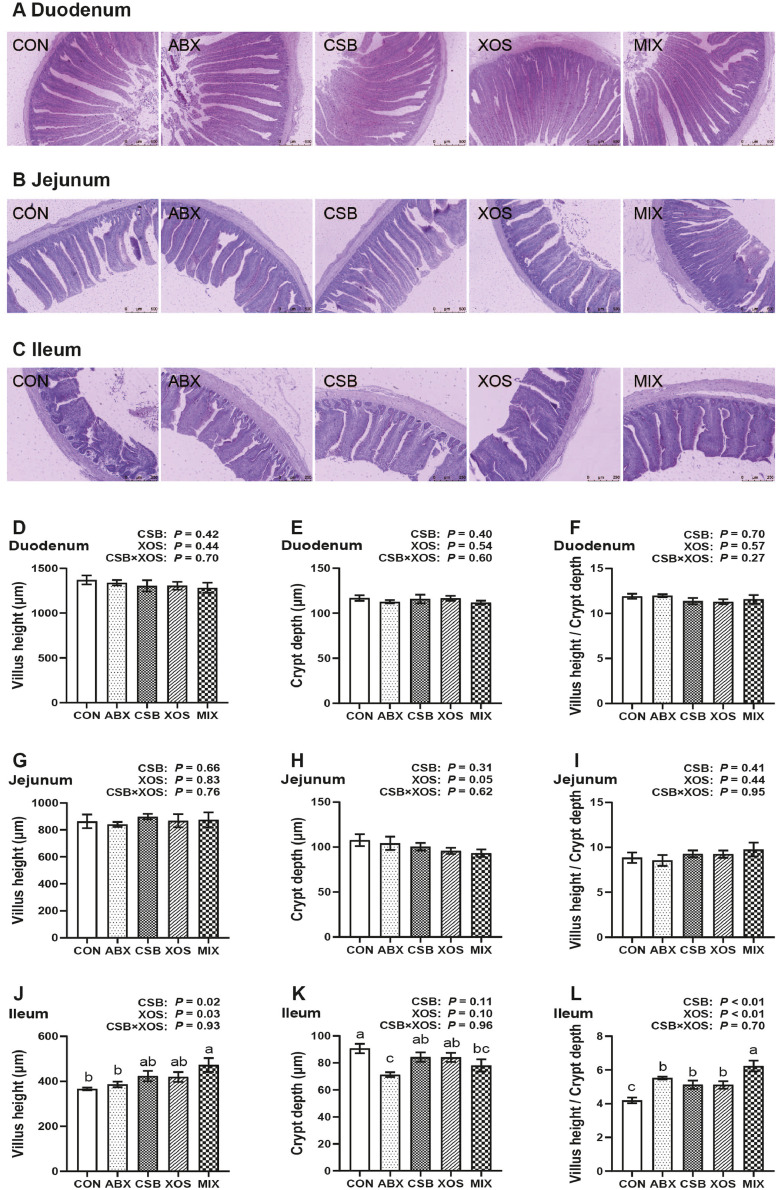
The morphology structure of duodenum, jejunum and ileum was observed on d 21 (Figures 1A–1C). The main effect analysis results showed that both CSB and XOS treatments increased ileal VH and VCR (P < 0.05; Figures 1J and 1L), and dietary XOS had a tendency to decrease jejunal CD (P = 0.05; Figure 1H). The 1-way ANOVA results showed that broilers in MIX group showed the highest ileal VH and VCR among 5 groups, as well as lower ileal CD than those in CON group (P < 0.05; Figures 1J–1L). Meanwhile, the ileal CD in ABX group broilers were significantly lower than that in CON, CSB and XOS groups (P < 0.05; Figure 1K). Besides, no obvious changes were found in duodenum and jejunum among the 5 groups (P > 0.05, Figures 1D–1I). On the whole, VH, CD and VCR were the greatest in duodenum, followed by jejunum and ileum.
Figure 1.
Effects of CSB or/and XOS on small intestinal histomorphology in broilers. The pictures of periodic acid-schiff-stained sections of duodenum (A, ×10), jejunum (B, ×10) and ileum (C, ×5), and villus height, crypt depth and villus height/crypt depth of duodenum (D–F), jejunum (G–I), and ileum (J–L). Data represent mean ± SE (n = 8), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; XOS, 100 mg/kg XOS.
Small Intestinal Digestive Enzyme Activities
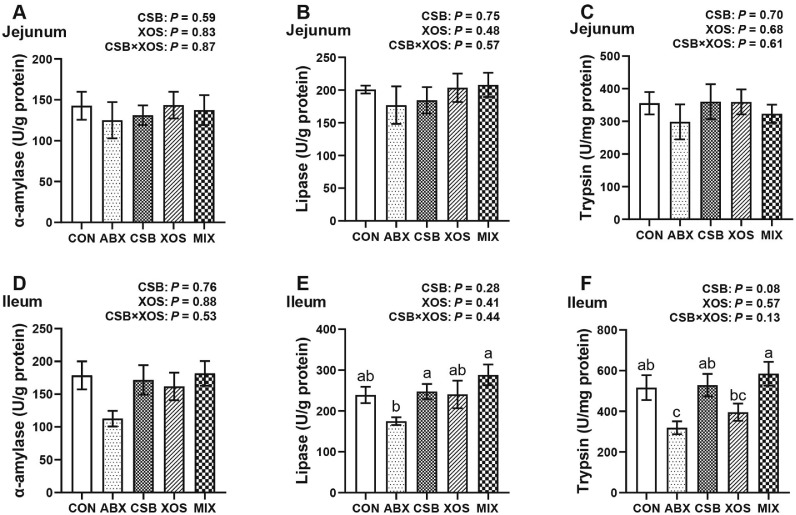
CSB had no interaction with XOS (P > 0.05; Figures 2A–2F), and the main effect analysis results showed dietary CSB had a tendency to increase ileal trypsin (P = 0.08; Figure 2F). Moreover, the results of 1-way ANOVA showed that both CSB and MIX increased ileal lipase of broilers compared with ABX (P < 0.05; Figure 2E), and ileal trypsin in MIX group was significantly higher than that in ABX and XOS groups (P < 0.05; Figure 2F).
Figure 2.
Effects of CSB or/and XOS on small intestinal digestive enzyme activities in broilers. The α-amylase, lipase and trypsin activity of jejunum (A–C) and ileum (D–F). Data represent mean ± SE (n = 6–7), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; XOS, 100 mg/kg XOS.
Serum Antioxidant Status
As exhibited in Table 3, results of the main effect analysis showed that both CSB and XOS increased T-AOC concentration and SOD activity and decreased MDA concentration in serum (P < 0.05). CSB and XOS had no interaction effect on serum antioxidant capacity of broilers (P > 0.05), however, the 1-way ANOVA results showed that the combination of CSB and XOS resulted in the optimal antioxidant effect of broilers compared with the other 4 groups (P < 0.05). T-AOC concentration of broilers in ABX group was lower than that in CSB, XOS and MIX groups (P > 0.05), but there was no difference in SOD activity and MDA concentration (P < 0.05).
Table 3.
Effects of CSB or/and XOS on serum antioxidant capacity in broilers.
| Treatments | T-AOC (U/mL) | SOD (U/mL) | MDA (nmol/mL) |
|---|---|---|---|
| CON | 7.68bc | 126.07b | 5.49a |
| ABX | 7.42c | 124.06b | 5.54a |
| CSB | 7.90b | 128.81b | 5.29a |
| XOS | 7.84b | 128.56b | 5.32a |
| MIX | 8.38a | 134.82a | 4.91b |
| SEM | 0.08 | 0.93 | 0.06 |
| P-value | <0.01 | <0.01 | <0.01 |
| Main effects | |||
| CSB | |||
| - | 7.76y | 127.31y | 5.40x |
| + | 8.14x | 131.82x | 5.10y |
| XOS | |||
| - | 7.79y | 127.44y | 5.39x |
| + | 8.11x | 131.69x | 5.12y |
| P-values | |||
| CSB | 0.02 | 0.02 | 0.02 |
| XOS | 0.05 | 0.03 | 0.03 |
| CSB × XOS | 0.32 | 0.35 | 0.40 |
Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MDA, malondialdehyde; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; XOS, 100 mg/kg XOS.
Superscripts for means belong to 1-way ANOVA following by Duncan's multiple range test among 5 groups (n = 8).
Superscripts for means belong to 2 × 2 factorial arrangement (CSB and XOS), excluding ABX.
Means in a column with different superscripts are significantly different (P < 0.05).
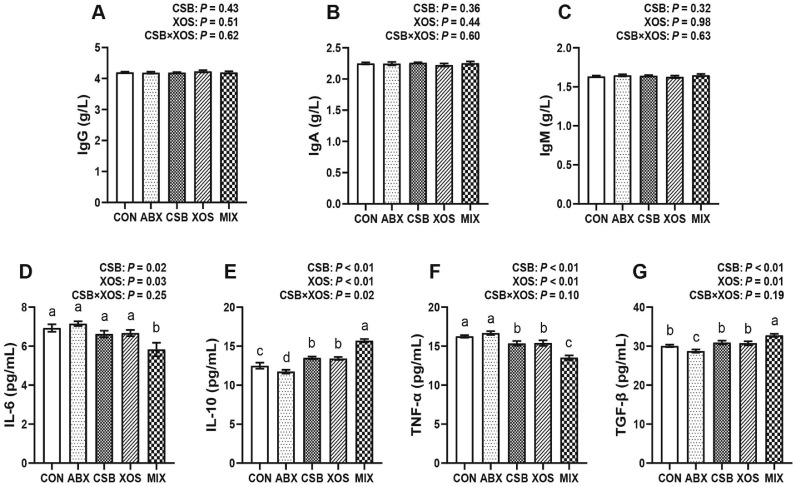
Serum Immunoglobulins and Inflammatory Factors
This study did not find a significant difference in serum IgG, IgA and IgM of broilers at 21 d among 5 groups (P > 0.05, Figures 3A–3C). The main effect analysis showed that both CSB and XOS treatments down-regulated the level of proinflammatory cytokines IL-6 and TNF-α, and up-regulated the level of anti-inflammatory cytokines IL-10 and TGF-β in serum (P < 0.05, Figures 3D–3G). Furthermore, there was a significant interaction effect between CSB and XOS on IL-10 (P = 0.02; Figure 3E). The 1-way ANOVA results showed that broilers in MIX group had the lowest concentrations of IL-6 and TNF-α and the highest concentrations of IL-10 and TGF-β in serum among 5 groups (P < 0.05, Figures 3D–3G). Whereas, diet supplementation with ABX decreased the concentrations of IL-10 and TGF-β compared with the other 4 groups (P < 0.05, Figures 3E and 3G).
Figure 3.
Effects of CSB or/and XOS on serum immunoglobulins (A–C) and cytokines (D–G) in broilers. Data represent mean ± SE (n = 8), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-6, interleukin-6; IL-10, interleukin-10; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; XOS, 100 mg/kg XOS.
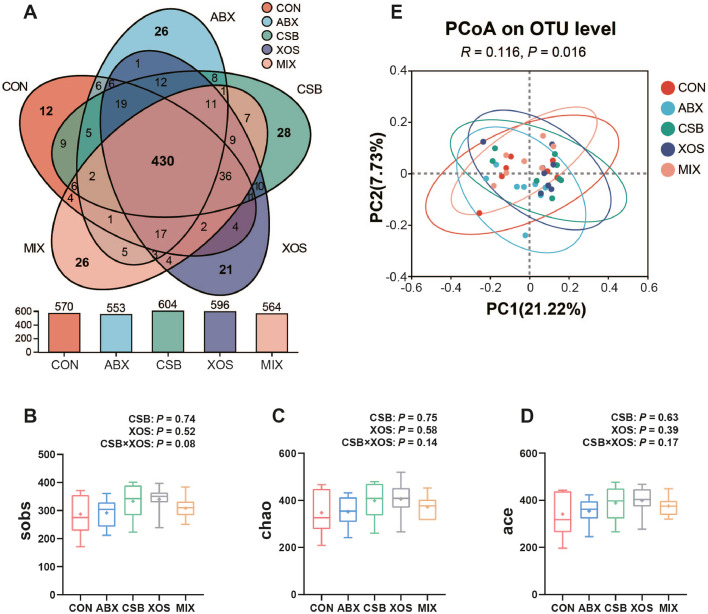
Cecal Microbial Diversity
The venn diagram showed 430 co-owned OTUs (clustering at 97% similarity level) among 5 groups and unique OTUs of CON, ABX, CSB, XOS and MIX group broilers was 12, 26, 28, 21, and 26, respectively (Figure 4A). Sobs (Figure 4B), chao (Figure 4C) and ace (Figure 4D) indices used to characterize the α diversity of cecal microorganisms increased numerically in the other 4 groups compared with CON, which did not reach the level of statistical significance (P > 0.05), and a tendency of interaction between CSB and XOS in the sobs index (P = 0.08; Figure 4A) was observed. Additionally, PCoA analysis based on unweighted-unifrac distance metrics and ANOSIM test revealed that a clear microbiota community shift occurred at the OTU level among 5 groups (R = 0.116, P = 0.016; Figure 4E).
Figure 4.
Effects of CSB or/and XOS on cecal microbial diversity in broilers. The venn diagram (A), box plots of α-diversity as measured by sobs index (B), chao index (C) and ace index (D) at OTU level, and PCoA plot (E) of the cecal microbiome based on unweighted-unifrac distance metrics at OTU level. Data (B–D) represent mean and from minimum to maximum (n = 7–8), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; OTU, operational taxonomic units; PCoA, principal co-ordinate analysis; XOS, 100 mg/kg XOS.
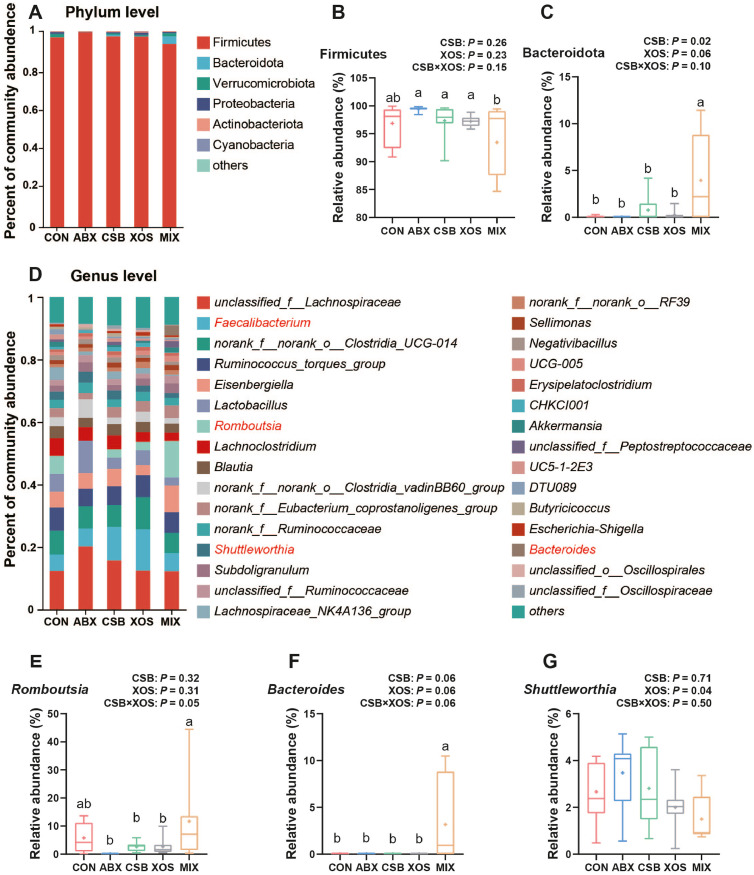
Cecal Microbial Community Compositions
Microbial community composition was analyzed at the phylum (Figure 5A) and genus levels (Figure 5D). At the phylum level, Firmicutes were the first dominant phyla among all 5 groups, and the 1-way ANOVA analysis showed that its relative abundance in MIX group broilers was significantly lower than that in the ABX, CSB and XOS groups (P < 0.05; Figure 5B). However, broilers in MIX group had higher relative abundance of Bacteroidota than those in the other 4 groups (P < 0.05; Figure 5C). Moreover, the main effect analysis showed that CSB significantly increased relative abundance of Bacteroidota (P < 0.05), and XOS had a tendency to increased Bacteroidota abundance (P = 0.06; Figure 5C).
Figure 5.
Effects of CSB or/and XOS on cecal microbial composition in broilers. Community bar plot analysis at phylum level (A) or genus level (D). Comparative analysis of relative abundance of major bacterial at phylum level (B, C) or genus level (E–G). Data represent mean and from minimum to maximum (n = 7–8), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; XOS, 100 mg/kg XOS.
At the genus level, the relative abundance of Faecalibacterium in XOS (13.29%) and CSB groups broilers (10.67%) was nearly twice that of MIX (5.86%), ABX (5.79%), and CON groups (5.33%), although it did not reach statistical significance (P > 0.05; Figure 5D). A tended interaction of Romboutsia was observed between CSB and XOS group broilers (P = 0.05), and the 1-way ANOVA analysis showed that its relative abundance increased significantly in MIX group broilers than that in XOS, CSB and CON groups (P < 0.05; Figure 5E). Furthermore, broilers in MIX group had higher relative abundance of Bacteroides than those in the other 4 groups (P < 0.05; Figure 5F). The main effect analysis results showed that XOS treatment significantly decreased relative abundance of Shuttleworthia (P < 0.05; Figure 5G).
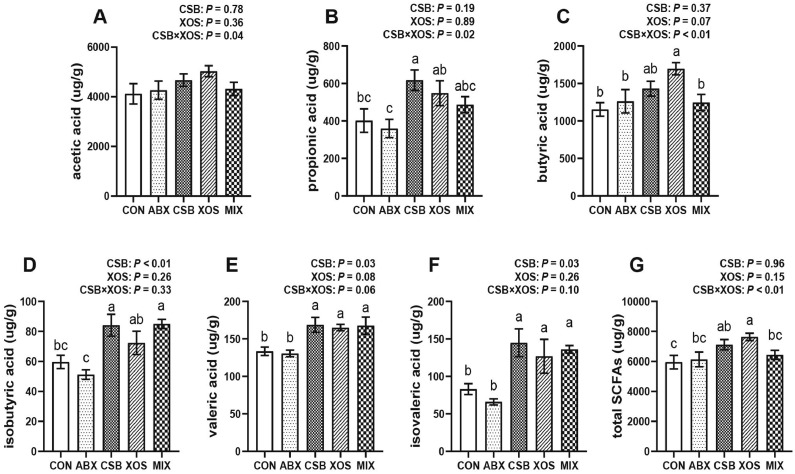
Cecal SCFAs
There was an interaction between CSB and XOS treatments on increasing contents of acetic acid, propionic acid, butyric acid and total SCFAs (P < 0.05; Figures 6A–C and 6G) in cecal digesta of broilers. Moreover, the 1-way ANOVA results showed that CSB increased contents of propionic acid (P < 0.05; Figure 6B) and XOS increased contents of butyric acid and total SCFAs (P < 0.05; Figures 6C and 6G) as comparing with CON and ABX group broilers. Besides, compared with the other 4 groups, XOS treatment also increased content of acetic acid from the value of view, which did not reach the level of statistical significance (P > 0.05, Figure 6A). The main effect analysis results showed that dietary CSB increased contents of isobutyric acid, valeric acid and isovaleric acid (P < 0.05; Figures 6D–6F), whereas dietary XOS had a tendency to increase contents of valeric acid (P = 0.08; Figure 6E). In addition, the 1-way ANOVA results showed that contents of isobutyric acid, valeric acid and isovaleric acid of broilers in MIX group were higher than those in CON and ABX group (P < 0.05; Figures 6D–6F), and similar to those in CSB and XOS group (P > 0.05; Figures 6D–6F).
Figure 6.
Effects of CSB or/and XOS on cecal SCFAs in broilers. The concentrations of acetic acid (A), propionic acid (B), butyric acid (C), isobutyric acid (D), valeric acid (E), isovaleric acid (F), and total SCFAs (G) in cecal digesta. Data represent mean ± SE (n = 8), and bars with different letters (a, b, c) analyzed by 1-way ANOVA following by Duncan's multiple range test among 5 groups differ significantly (P < 0.05); P values means the significant from 2 × 2 factorial analysis (CSB and XOS), excluding ABX. Abbreviations: ABX, 100 mg/kg aureomycin and 8 mg/kg enramycin; CON, a corn-soybean meal; CSB, 1,000 mg/kg CSB; MIX, 1,000 mg/kg CSB and 100 mg/kg XOS; SCFAs, short-chain fatty acids; XOS, 100 mg/kg XOS.
DISCUSSION
As growth-promoting antibiotics are banned in animal feed, finding effective alternatives to enhance animal health and maintain high-quality products is urgent. A major focus of this study was the potential effectiveness of CSB and XOS together in replacing growth-promoting antibiotics. We found the CSB treatment increased the FBW and ADG and decreased the FCR of the broilers at early stage (1–21 d), which was consistent with the previous studies (Bortoluzzi et al., 2017; Lan et al., 2020a). However, the main effect analysis of results demonstrated that the XOS did not affected FBW, ADG, and FCR of the broilers at early stage, which is contrary to previous studied suggesting that supplementation with XOS could improve the growth performance of broilers (De Maesschalck et al., 2015; Suo et al., 2015; Yuan et al., 2018). One most possible explanation is that XOS has prebiotic properties which cannot be digested by endogenous digestive enzymes of single-stomach animals such as broilers (Holscher, 2017), and 1-21d broilers have poor microbiota and digestive system. A more significant effect was observed when extending the age to 35 d or 42 d in our research (unpublished data). Even so, in this study, broilers in the mixed group (CSB + XOS) obtained the largest BW and ADG among the 5 groups, and significantly reduced FCR. According to this finding, the combination of CSB and XOS was shown to have good potential as antibiotic alternatives for improving broiler growth. A broiler's growth performance was strongly related to its digestive and absorption capacity, especially the capacity of its small intestine (Gao et al., 2018; Li et al., 2019). Therefore, we further analyzed the morphologic alterations of the small intestinal and digestive enzyme activities of broilers.
The morphologic structure of the small intestine plays a key role in the digestion of nutrient substrates and is closely related to the performance of broilers. Small intestine absorption capacity can be estimated from the VH and VCR, and a higher ratio indicates a more efficient absorption rate (Olukosi and Dono, 2014; Walton et al., 2016). Increasing pieces of evidence have demonstrated beneficial effects of CSB (Chamba et al., 2014; González-Ortiz et al., 2019) or XOS (Luo et al., 2021; Wang et al., 2021; Yang et al., 2022) on the morphology and structure of small intestine at different phases of broiler growth, although these results had some variations, which is accordance with the present results that the CSB or XOS treatment elevated the VH and VCR of ileum at broiler early stage. Additionally, we found that the combination of CSB and XOS had a better effect on improving mucosal morphology of the ileum as compared with individual supplementation of CSB or XOS without obvious synergistic effect. Traditionally, the small intestinal is responsible for the majority of digested products absorption (from fat, starch, and protein) (Oso et al., 2019). Accordingly, we hypothesized that XOS or CSB may improve broiler performance by affecting intestinal mucosa morphology and enhancing intestinal health more efficiently. On the other part, the level of digestive enzyme activities is one of the important reference indexes affecting the ability of livestock and poultry to digest and absorb nutrients. The main analysis results in this study exhibited that administration of CSB and XOS did not affect the digestive enzyme (lipase, trypsin, and amylase) activities in jejunum or ileum at broiler early stage, suggesting the enhanced feed conversion efficiency and growth performance may be independent of the alterations of digestive enzyme activities.
Normally, animal body cells metabolize and produce reactive oxygen species (ROS) which are maintained at normal levels. However, external stimuli such as shock and heat stress caused oxidative stress with excessive ROS production which destroyed the cellular structures of carbohydrates, nucleic acids, lipids and proteins, thus changing their functions in the body (Song et al., 2018). Antioxidant enzymes whose activity indirectly reflects the ability to scavenge ROS (Liu et al., 2020) play a key role in animal defense against oxidative stress caused by xenobiotic (Wu et al., 2016). Lan et al. (2020a) reported that sodium butyrate increased the activities of SOD and glutathione peroxidase, the key antioxidant enzymes, and decreased the content of MDA, the product of lipid peroxided degradation (Mousavi et al., 2018; Liu et al., 2020), in the serum of broilers. Wang et al. (2022) reported that dietary XOS had a tendency to increase T-AOC, and XOS +IAPS significantly increased T-AOC activity and decreased MDA content in the serum of broilers. Similar results of the CSB or XOS administration in present study were observed, exhibiting the increased antioxidant capacity of CSB or XOS in the early stage of broiler. Besides, broilers treated with CSB and XOS together had the highest activities of T-AOC and SOD and the lowest content of MDA among the 5 treatments, indicating that CSB combined with XOS played a better antioxidant effect to avoid the excessive energy waste in broilers.
We further evaluated the effects of CSB and XOS on the immune function in broiler early stage. The results in this study demonstrated that no significant alterations of the immunoglobulins (IgG, IgA, and IgM), several globulins with antibody activity or similar chemical structure to antibody (Balan et al., 2019; Megha and Mohanan, 2021), were observed in serum of broilers treated with CSB or XOS, meaning the CSB or XOS might not regulate the secretion of IgG, IgA, and IgM. However, the main analysis results in present study demonstrated that administration of CSB and XOS increased the anti-inflammatory cytokines IL-10 and TGF-β, and decreased the proinflammatory cytokines IL-6 and TNF-α. This finding was consistent with the results that supplementary XOS modulated the level of cytokine (increasing anti-inflammatory cytokines and decreasing proinflammatory cytokines) in the serum or intestine of piglets, enhancing animal immune function in our laboratory's previous studies (Chen et al., 2021b; Tang et al., 2022). Additionally, we found that there was a synergy between CSB and XOS on increasing serum IL-10, and the 1-way ANOVA results showed that the combination of CSB and XOS also had the best effect for regulating the serum cytokines level to improve the immune function of broilers.
The gut microbiota harboring the animal gastrointestinal tract was involved in regulating key host metabolic and immunologic health, including nutrient digestion and absorption, maintaining energy balance and immune system development (Martin-Gallausiaux et al., 2021), and the balance of microflora is the key to the healthy growth of animal. Therefore, we profiled the response of cecal microbiota composition to dietary XOS or CSB supplementation. The diversity of cecum microbial community was not affected by dietary changes, but there were differences in the relative abundance of individual phylum and genus in this study. Similar to previous studies (Lourenco et al., 2019), Firmicutes was the first dominant microflora among the cecal contents of broilers in the 5 treatment groups. In addition, the altered relative abundance of Bacteroidota that involved in the degradation of complex carbohydrates (Louis et al., 2014) was more sensitively responded to mix of CSB and XOS treatment without obvious synergistic effect, suggesting that the microbiota utilizing XOS or CSB were mainly Bacteroidota phyla.
Specific bacteria (e.g., Bacteroides spp., Faecalibacterium spp., Shuttleworthia, etc.) were identified to be influenced by a dietary mix of CSB and XOS in the cecum. For instance, Bacteroides spp. (belonging to the Bacteroidota) that could provide nutrition for other microbial residents and protection for the host from pathogens in the gut (Zafar and Saier, 2021) was the highest in MIX group broilers. The Romboutsia observed to adapt well to mixing CSB and XOS in this study was a new genus isolated from the gut in recent years (Gerritsen et al., 2014). It can utilize different and partially redundant pathways of carbohydrates, including diet-derived FOS and host-derived sugars released by other microorganisms, and adapt to a nutrient-rich environment rich in carbohydrates, amino acids, and vitamins (Gerritsen et al., 2017), finally playing a key role in maintaining the health of the host (Mangifesta et al., 2018). Besides, we found that XOS administration had significant lower cecal Shuttleworthia abundance, the potential pathogenic genus (Zhao et al., 2019), compared with broilers not treated with XOS, which was accord with previously similar results that the intake of isomaltose reduced the level of pathogens such as Shuttleworthia (Yang et al., 2021). In addition, whether CSB and XOS affect the changes of Lactobacillus strains in the gut of broilers at early stage is also our concern. In this study, Lactobacillus strains, such as L. johnsonii and L. salivarius, have been recognized as having positive effects on the intestinal health of broilers (Neveling et al., 2020), showed no significant difference among 5 groups, indicating that Lactobacillus may be not the main bacteria regulated by CSB or/and XOS in broilers at early stage.
Microbial metabolites, closely related to the composition of gut microbiota, are incredibly crucial for the systemic metabolism and immunologic health (Wu et al., 2021), and the SCFA, a kind of very vital microbial metabolites, was further analyzed in this research. Our results demonstrated that dietary addition of CSB, XOS, or a mixture of CSB and XOS, could increase the content of various SCFAs in cecal contents to a certain extent, which was similar to previous studies (Wu et al., 2018; Chen et al., 2021c). A large amount of evidence had shown that SCFAs provided energy for intestinal epithelial cells and played an important role in regulating immune function and enhancing intestinal barrier function in animals (Parada Venegas et al., 2019). Intriguing, the addition of CSB eliminated the increment of butyric acid content in the cecal chyme after the supplementary of XOS, indicating that there might be also a negative feedback inhibition pathway for butyric acid production of gut microbiota. Besides, the alteration of the butyric acid content might be related to the abundance of cecal Faecalibacterium spp., one of the main components of intestinal microbiota (Ferreira-Halder et al., 2017) related to Faecalibacterium Prausnitzii (Oikonomou et al., 2013), that could metabolize intestinal sugars unabsorbed by host to produce large amounts of butyric acid (Flint et al., 2012). Moreover, we observed the interaction between CSB and XOS on the change of acetic acid, propionic acid and butyric acid content, which may be related to microbial symbiosis, but the specific mechanism needs to be further studied.
CONCLUSIONS
Taken together, dietary supplementary of CSB, XOS, or their combination could improve the morphology of ileum tissue, therefore enhancing the feed utilization of broilers at the early stage (1–21 d), which was independent of the alterations of digestive enzyme activities. Besides, the CSB, XOS, or their combination treatment enhanced immunity and provided a better oxidation-deoxidation environment and intestinal homeostasis (microbiota composition and SCFA content), guaranteeing the broilers in a healthier state. Moreover, broilers receiving the mix addition of CSB and XOS demonstrated the best effects on the improvement of small gut morphology and intestinal homeostasis, and enhancement of anti-inflammatory and antioxidant capacity, eventually elevating growth performance. These results suggested that combination of CSB and XOS may be a potential natural alternative to antibiotics.
ACKNOWLEDGMENTS
This research was funded by National Key R&D Program of China (2022YFD1300605), China Agriculture Research System of MOF and MARA (CARS-41), and Agricultural Science and Technology Innovation Program (ASTIP-IAS07).
DISCLOSURES
The authors have declared that no competing interests exist.
REFERENCES
- Abiala M., Olayiwola J., Babatunde O., Aiyelaagbe O., Akinyemi S. Evaluation of therapeutic potentials of plant extracts against poultry bacteria threatening public health. BMC Complement. Altern. Med. 2016;16:417. doi: 10.1186/s12906-016-1399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan P., Sik-Han K., Moughan P.J. Impact of oral immunoglobulins on animal health—a review. Anim. Sci. J. 2019;90:1099–1110. doi: 10.1111/asj.13258. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Chamba F., Puyalto M., Ortiz A., Torrealba H., Mallo J.J., Riboty R. Effect of partially protected sodium butyrate on performance, digestive organs, intestinal villi and E. coli development in broilers chickens. Int. J. Poult. Sci. 2014;13:390–396. [Google Scholar]
- Chen G., Ran X., Li B., Li Y., He D., Huang B., Fu S., Liu J., Wang W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wen C., Zhou Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J. Sci. Food Agric. 2018;98:3343–3350. doi: 10.1002/jsfa.8838. [DOI] [PubMed] [Google Scholar]
- Chen Y., Xie Y., Ajuwon K.M., Zhong R., Li T., Chen L., Zhang H., Beckers Y., Everaert N. Xylo-oligosaccharides, preparation and application to human and animal health: a review. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.731930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xie Y., Zhong R., Han H., Liu L., Chen L., Zhang H., Beckers Y., Everaert N. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J. Anim. Sci. 2021;99:1–8. doi: 10.1093/jas/skab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xie Y., Zhong R., Liu L., Lin C., Xiao L., Chen L., Zhang H., Beckers Y., Everaert N. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.641172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo F.C., Ranke F.F.D., de Oliva-Neto P. Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and Salmonella typhimurium. LWT. 2020;118 [Google Scholar]
- Ferreira-Halder C.V., Faria A.V.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Flint H.J., Scott K.P., Duncan S.H., Louis P., Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieri M., Kumar K., Boutin A. Antibiotic resistance. J. Infect. Public Health. 2017;10:369–378. doi: 10.1016/j.jiph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:e166–e175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Gerritsen J., Fuentes S., Grievink W., van Niftrik L., Tindall B.J., Timmerman H.M., Rijkers G.T., Smidt H. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int. J. Syst. Evol. Microbiol. 2014;64:1600–1616. doi: 10.1099/ijs.0.059543-0. [DOI] [PubMed] [Google Scholar]
- Gerritsen J., Hornung B., Renckens B., van Hijum S., Martins Dos Santos V.A.P., Rijkers G.T., Schaap P.J., de Vos W.M., Smidt H. Genomic and functional analysis of Romboutsia ilealis CRIB(T) reveals adaptation to the small intestine. PeerJ. 2017;5:e3698. doi: 10.7717/peerj.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ortiz G., Dos Santos T.T., Vienola K., Vartiainen S., Apajalahti J., Bedford M.R. Response of broiler chickens to xylanase and butyrate supplementation. Poult. Sci. 2019;98:3914–3925. doi: 10.3382/ps/pez113. [DOI] [PubMed] [Google Scholar]
- Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R., Zhao Z., Li S., An L. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions. Poult. Sci. 2020;99:5491–5500. doi: 10.1016/j.psj.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R.X., Li S.Q., Zhao Z., An L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020;6:491–499. doi: 10.1002/vms3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang X.F., Ren L.N., Li J.L., Zhu X.D., Xing T., Zhang L., Gao F., Zhou G.H. Protective effects of γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: a double-edged sword for health? Adv. Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.B., Yan H.L., Zhang Y., Hu Y.D., Zhang H.F. Effects of stale maize on growth performance, immunity, intestinal morphology and antioxidant capacity in broilers. Asian-Australas J. Anim. Sci. 2020;33:605–614. doi: 10.5713/ajas.19.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., Kwon W.B., Mathai J.K., Navarro D., Jaworski N.W., Stein H.H. Non-antibiotic feed additives in diets for pigs: a review. Anim. Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Lourenco J.M., Rothrock M.J., Fluharty F.L., Callaway T.R. The successional changes in the gut microbiome of pasture-raised chickens fed soy-containing and soy-free diets. Front. Sustain. Food Syst. 2019;3:35. [Google Scholar]
- Luo D., Li J., Xing T., Zhang L., Gao F. Combined effects of xylo-oligosaccharides and coated sodium butyrate on growth performance, immune function, and intestinal physical barrier function of broilers. Anim. Sci. J. 2021;92:e13545. doi: 10.1111/asj.13545. [DOI] [PubMed] [Google Scholar]
- Makowski Z., Lipiński K., Mazur-Kuśnirek M. The effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals (Basel) 2022;12:1836. doi: 10.3390/ani12141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangifesta M., Mancabelli L., Milani C., Gaiani F., de'Angelis N., de'Angelis G.L., van Sinderen D., Ventura M., Turroni F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018;8:13974. doi: 10.1038/s41598-018-32413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gallausiaux C., Marinelli L., Blottière H.M., Larraufie P., Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- Megha K.B., Mohanan P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021;169:28–38. doi: 10.1016/j.ijbiomac.2020.12.073. [DOI] [PubMed] [Google Scholar]
- Mousavi S.N., Faghihi A., Motaghinejad M., Shiasi M., Imanparast F., Amiri H.L., Shidfar F. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-fed high fat diet. Biol. Trace Elem. Res. 2018;181:288–295. doi: 10.1007/s12011-017-1059-2. [DOI] [PubMed] [Google Scholar]
- Neveling D.P., Ahire J.J., Laubscher W., Rautenbach M., Dicks L.M.T. Genetic and phenotypic characteristics of a multi-strain probiotic for broilers. Curr. Microbiol. 2020;77:369–387. doi: 10.1007/s00284-019-01797-3. [DOI] [PubMed] [Google Scholar]
- Oikonomou G., Teixeira A.G., Foditsch C., Bicalho M.L., Machado V.S., Bicalho R.C. Fecal microbial diversity in pre-weaned dairy calves as described by pyrosequencing of metagenomic 16S rDNA. Associations of Faecalibacterium species with health and growth. PLoS One. 2013;8:e63157. doi: 10.1371/journal.pone.0063157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O.A., Dono N.D. Modification of digesta pH and intestinal morphology with the use of benzoic acid or phytobiotics and the effects on broiler chicken growth performance and energy and nutrient utilization. J. Anim. Sci. 2014;92:3945–3953. doi: 10.2527/jas.2013-6368. [DOI] [PubMed] [Google Scholar]
- Oso A.O., Suganthi R.U., Reddy G.B.M., Malik P.K., Thirumalaisamy G., Awachat V.B., Selvaraju S., Arangasamy A., Bhatta R. Effect of dietary supplementation with phytogenic blend on growth performance, apparent ileal digestibility of nutrients, intestinal morphology, and cecal microflora of broiler chickens. Poult. Sci. 2019;98:4755–4766. doi: 10.3382/ps/pez191. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimifard N., Naseri M. Bifidobacteria bifidum and Bifidobacteria infantis Effects on Salmonella Enteritidis. J. Pure Appl. Microbiol. 2016;10:1885–1889. [Google Scholar]
- Salaheen S., Kim S.W., Haley B.J., Van Kessel J.A.S., Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Suo H.-q., Lu L., Xu G.-h., Xiao L., Chen X.-g., Xia R.-r., Zhang L.-y., Luo X.-g. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J. Integr. Agric. 2015;14:2050–2057. [Google Scholar]
- Tang S., Chen Y., Deng F., Yan X., Zhong R., Meng Q., Liu L., Zhao Y., Zhang S., Chen L., Zhang H. Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model. Carbohydr. Polym. 2022;294 doi: 10.1016/j.carbpol.2022.119776. [DOI] [PubMed] [Google Scholar]
- Tang S., Zhong R., Yin C., Su D., Xie J., Chen L., Liu L., Zhang H. Exposure to high aerial ammonia causes hindgut dysbiotic microbiota and alterations of microbiota-derived metabolites in growing pigs. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.689818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K.D., Freddo A.M., Wang S., Gumucio D.L. Generation of intestinal surface: an absorbing tale. Development. 2016;143:2261–2272. doi: 10.1242/dev.135400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambacq W.A., van Doorn D.A., Rovers-Paap P.M., Ducatelle R., Vlaminck L., Lourenço M., Hesta M. Dietary supplementation of micro-encapsulated sodium butyrate in healthy horses: effect on gut histology and immunohistochemistry parameters. BMC Vet. Res. 2020;16:121. doi: 10.1186/s12917-020-02332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated astragalus polysaccharides on the immune response, antioxidant capacity, and intestinal microbiota composition of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu C., Zhang R., Chen Y., Shen Y., Hu F., Liu D., Lu J., Guo Y., Xia X., Jiang J., Wang X., Fu Y., Yang L., Wang J., Li J., Cai C., Yin D., Che J., Fan R., Wang Y., Qing Y., Li Y., Liao K., Chen H., Zou M., Liang L., Tang J., Shen Z., Wang S., Yang X., Wu C., Xu S., Walsh T.R., Shen J. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: an epidemiological comparative study. Lancet Infect. Dis. 2020;20:1161–1171. doi: 10.1016/S1473-3099(20)30149-3. [DOI] [PubMed] [Google Scholar]
- Wen R., Li C., Zhao M., Wang H., Tang Y. Withdrawal of antibiotic growth promoters in China and its impact on the foodborne pathogen Campylobacter coli of swine origin. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang K., Wang X., Pang Y., Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12:360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xiao Z., An W., Dong Y., Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou Y., Lu C., Ahmad H., Zhang H., He J., Zhang L., Wang T. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tang X.W., Liu X., Yang H., Bin D.M., Liu H.J., Tang Q.H., Tang J.Y. Effects of dietary oligosaccharides on serum biochemical index, intestinal morphology, and antioxidant status in broilers. Anim. Sci. J. 2022;93:e13679. doi: 10.1111/asj.13679. [DOI] [PubMed] [Google Scholar]
- Yang Z.D., Guo Y.S., Huang J.S., Gao Y.F., Peng F., Xu R.Y., Su H.H., Zhang P.J. Isomaltulose exhibits prebiotic activity, and modulates gut microbiota, the production of short chain fatty acids, and secondary bile acids in rats. Molecules. 2021;26:2464. doi: 10.3390/molecules26092464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Li W., Huo Q., Du C., Wang Z., Yi B., Wang M. Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ. 2018;6:e4435. doi: 10.7717/peerj.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H., Saier M.H., Jr Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang K., Wang J., Bai S., Zeng Q., Peng H., Zhang B., Xuan Y., Ding X. Effects of coated sodium butyrate on performance, egg quality, nutrient digestibility, and intestinal health of laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Bai H., Deng F., Zhong R., Liu L., Chen L., Zhang H. Chemically protected sodium butyrate improves growth performance and early development and function of small intestine in broilers as one effective substitute for antibiotics. Antibiotics (Basel) 2022;11:132. doi: 10.3390/antibiotics11020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li K., Luo H., Duan L., Wei C., Wang M., Jin J., Liu S., Mehmood K., Shahzad M. Comparison of the intestinal microbial community in ducks reared differently through high-throughput sequencing. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/9015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M., Shang W.T., Ma Q.C., Strappe P., Zhou Z.K. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201801187. [DOI] [PubMed] [Google Scholar]