Abstract
Introduction
Finding a non-invasive and repeatable tool has been recommended to make an accurate diagnosis of Alzheimer’s disease (AD) and Parkinson’s disease (PD).
Methods
70 volunteers participated in three groups: 24 with mild dementia of AD, 24 in the first and second stages of PD, and 22 healthy controls. After valuing the scores of cognitive tests, the salivary levels of phosphorylated tau (p-tau), total alpha-synuclein (α-syn), and beta-amyloid 1–42 (Aβ) proteins have been evaluated. Finally, the cutoff points, receiver operating characteristic (ROC), sensitivity, and specificity have been calculated to find accurate and detectable biomarkers.
Results
Findings showed that the salivary level of Aβ was higher in both PD (p < 0.01) and AD (p < 0.001) patients than in controls. Moreover, the level of α-syn in both PD and AD patients was similarly lower than in controls (p < 0.05). However, the level of p-tau was only higher in the AD group than in the control (p < 0.01). Salivary Aβ 1–42 level at a 60.3 pg/ml cutoff point revealed an excellent performance for diagnosing AD (AUC: 0.81).
Conclusion
Evaluation of p-tau, α-syn, and Aβ 1–42 levels in the saliva of AD and PD patients could help the early diagnosis. The p-tau level might be valuable for differentiation between AD and PD. Therefore, these hopeful investigations could be done to reduce the usage of invasive diagnostic methods, which alone is a success in alleviating the suffering of AD and PD patients. Moreover, introducing accurate salivary biomarkers according to the pathophysiology of AD and PD should be encouraged.
Abbreviations: AD, Alzheimer's disease; Aβ, Beta-amyloid 1–42; BDRS, Blessed Dementia Rating Scale; CSF, Cerebrospinal fluid; CT scan, Computed tomography scan; ELISA, Enzyme-linked immunosorbent assay; MRI, Magnetic resonance imaging; MMSE, MCI (mild cognitive impairment mini-mental state examination; MDS-UPDRS, MDS-Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; NIA-AA, National Institute on Aging-Alzheimer’s Association; NFTs, Neurofibrillary Tangles; PD, Parkinson's disease; p-tau, Phosphorylated tau; ROC, Receiver operating characteristic; α-syn, Total alpha-synuclein
Keywords: Phosphorylated tau, Total alpha-synuclein, Beta-amyloid, Alzheimer's disease, Parkinson's disease
1. Introduction
The most common age-related neurodegenerative disorders are Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Lesage and Brice, 2009, Hilton and Shivane, 2015), characterized by progressive trends of impairment in neuronal functions (Kovacs, 2016). The pathophysiology of these diseases involves the synthesis of particular proteins, alpha-synuclein in PD and beta-amyloid, and hyper-phosphorylated tau in AD, leading to neuronal death (Mayeux, 2010, Bidinosti et al., 2012, Herzig et al., 2012).
Clinically, AD has three stages: preclinical, MCI (mild cognitive impairment), and AD-associated with dementia (mild, moderate, and severe) (Mayeux, 2010, Lannfelt, 1998, Mckhann et al., 2011). So, the range of symptoms in AD differs from minor memory impairment in the initial stages to extensive cognitive impairment, which can result in severe mental disorders in advanced stages (Mayeux, 2010).
Besides motor impairments, PD has some non-motor symptoms, including depression, cognitive dysfunction, and complex behavioral disorders. (Poewe, 2008). Since the neuropathological process commences some decades before the appearance of clinical signs and symptoms, finding early detectable biomarkers in body fluids like cerebrospinal fluid (CSF) and performing related imaging methods is recommended (Mayeux, 2010, Rosén et al., 2013, Dubois et al., 2009, Ismail et al., 2020).
Since CSF evaluation requires a lumbar puncture, which may occasionally be painful or cause other complications, finding biomarkers in plasma or other body fluids has been suggested. However, the results of plasma Aβ levels were conflicting, and no study has yet confirmed the adequate sensitivity of this biomarker in AD (Bermejo-Pareja et al., 2010, Shi et al., 2011). On the other hand, few studies have examined saliva in AD or PD patients around the world, and the results of these studies could not have replaced those about CSF. Their findings revealed a significant increase in levels of Aβ1–42 and higher p-tau/t-tau proportions in the saliva of AD patients, compared to healthy controls. In addition, the levels of total α-syn were decreased in PD patients compared to the healthy participants, while oligomeric α-syn and the proportion of oligomeric α-syn/total α-syn noticeably were increased. Although the aim of those studies is to compare the levels of the salivary biomarkers to healthy controls, determining a cutoff point for the levels and ratios is still complicated, and requires performing more uniform studies (Bermejo-Pareja et al., 2010, Devic et al., 2011, Stewart et al., 2014, Wolgin et al., 2022). Moreover, the salivary levels of p-tau protein in PD patients and total α-syn protein in AD patients have not been reported (Pawlik and Błochowiak, 2021, Wolgin et al., 2022).
Therefore, our aim was to evaluate the alteration of salivary phosphorylated tau (p-tau), total alpha-synuclein (α-syn), and beta-amyloid 1–42 (Aβ) proteins levels in patients with AD and PD and compare them with healthy participants. In addition, we aimed to achieve the accurate, safe, painless, and repeatable diagnostic tool in the early stages of these neurodegenerative diseases.
2. Methods & Materials
2.1. Study sample
The present cross-sectional study was performed on 70 volunteers over 50 years old in three groups, including 24 patients with AD and 24 patients with PD referred to the Neurology Department of Rasool Akram and Firouzgar hospitals in Tehran, and 22 healthy controls. The Iran University of Medical Sciences Ethics Committee approved all study stages and procedures (ethical code: IR.IUMS.REC.1394.9211314202). The study process and examinations were clearly explained to the volunteers, who were requested to read and sign an informed consent before any procedures. In addition, the patients or their caregivers were asked to complete a questionnaire about their demographic information, educational levels, duration of the symptoms, and oral health. Also, neurological assessments, reduced functional status, cognitive weakening stated by caregivers, mental status evaluation, and physical examinations were done as part of regular patients’ evaluations. There was no history of consuming drugs other than medications for treating the underlying neurological disease. All of the cases had a measurement of B12, folate, thyroid hormone levels, and other routine factors from blood samples. Brain Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) scan was performed on the patients for further investigations. Also, they have received the standard therapy for their AD or PD conditions. The control cases were formed from caregivers of patients or their family members, whom a neurologist clinically interviewed. They had normal functional and cognitive scores based on the mini-mental state examination (MMSE score equal to or more than 26) and no history of neurological diseases. Excluding criteria for all participants were a history of vascular dementia, stroke, head injury, and liver or kidney failure.
2.2. AD diagnosis
A neurologist using Core Clinical Criteria recommended by the National Institute on Aging-Alzheimer’s Association (NIA-AA) (Mckhann et al. (2011)) established the clinical diagnosis of probable AD dementia. This criterion includes considerable cognitive or behavioral impairment and symptoms that interfere with daily activities, gradual onset of manifestations, amnestic (learning and recall of new information), or non-amnestic (language, visuospatial, executive) presentations. Based on the criteria, participants with a history of cerebrovascular dementia, stroke, and head injury, core features of other dementias such as Lewy bodies, frontotemporal dementia, or primary progressive aphasia, evidence of another concurrent neurological or non-neurological disorders or medical drugs that affect cognition such as liver or kidney failure, were excluded from the study. In addition, Patients with vascular dementia based on neuroimaging and a modified ischemic score of more than four were excluded. Findings on behalf of AD pathophysiological degenerations, such as Neurofibrillary Tangles NFTs or amyloid plaques, increase the accuracy of the diagnosis (Mckhann et al., 2011, Pawlik and Błochowiak, 2021). Dementia severity classification was performed based on the MMSE, and patients with mild dementia degree of AD (MMSE score: 20–25) were included.
2.3. PD diagnosis
An expert neurologist in movement disorders, using Movement Disorder Society criteria, accomplished the diagnosis of clinically probable PD (Postuma et al., 2015). According to the Hoehn and Yahr rating scale (Perlmutter, 2009), PD patients in the first or second stages of the disease, based on the PD motor symptoms, have entered the study. Also, their motor symptoms had been controlled well based on the MDS-Unified Parkinson’s Disease Rating Scale (mean MDS-UPDRS:20.4 ± 3.2) (Goetz et al., 2008). There were no clinical signs of cognitive impairment based on the MMSE score ≥ 26. A complete neurological examination, routine blood tests like B12 vitamin level measurements, and imaging examinations like MRI were done for further evaluation.
2.4. Cognitive tests
The Montreal Cognitive Assessment (MoCA) and Blessed Dementia Rating Scale (BDRS) were used for the cognitive assessment of all participants in this study. The scores of MoCA range from 0 to 30, and scores equal to or above 26 indicate normal cognition. On the other hand, BDRS scores range from 0 to 17 and are usually classified into three modes: 0–5 (mild dementia), 6–11 (moderate dementia), and 12–17 (advanced dementia).
2.5. Collection of salivary samples
All participants were asked to take a low-protein diet for five days before saliva sampling and consume fluids frequently. In addition, they had to follow a special tooth brushing and mouth washing protocol and be fast for four hours before sampling. The patients’ families controlled these conditions through the coordination of the research team. For collecting the salivary fluid, a dental cotton roll was laid on the oral side of the participant's cheek to be completely moist. Moist rolls were located inside the salivary collector tubes and were centrifuged at 1500 rpm for five minutes. Then, the remaining fluids at the bottom of the tubes were divided into microtubes and maintained at −80 °C before starting the enzyme-linked immunosorbent assay (ELISA) tests. All procedures were performed hygienically (by using disposable dental cotton rolls, microtubes, and also sterilized salivary collector tubes).
2.6. Detection of protein levels by ELISA assay
Before doing ELISA tests, the salivary sample tubes were placed at room temperature. Next, the level of p-tau, total α-syn, and Aβ 1–42 proteins was measured by ELISA kits under their manufacturer’s guidance. The ELISA kits contained human Aβ peptide 1–42 (DLdevelop; Wuxi Donglin Sci and Tech Development Company, China), human pMAPT/pTAU (Elabscience; Biotechnology Company, US), and human α-syn (Cloud-Clone-Corp Company; US).
The detection ranges of Aβ 1–42, pMAPT/pTAU, and SNCa kits were 15.62–1000, 31.25–2000, and 15.6–1000 pg/ml, respectively. The minimum detectable dose or sensitivity for Aβ 1–42 and SNCa were less than 5.48 and 6.8 pg/ml, respectively, and for pMAPT/pTAU was 18.75 pg/ml. For detecting Aβ 1–42 protein, 50 μl of saliva sample was added to each well of the kit, while for detecting p-tau and α-syn proteins, 100 μl were added.
2.7. Data analysis
We used SPSS version 26.0 for data analysis. Kolmogorov-Smirnov was utilized to examine the normality of data scattering. Mann-Whitney U test was applied to compare the data with asymmetrical distribution. The student’s t-test evaluated differences among variables with normal distribution. Chi-square was conducted for the examination of stratified parameters. In addition, spearman’s correlation test was applied to approximate the relationship between variable factors. Distributed symmetric variables are presented as mean±SD (standard deviation), and skewed data are presented as Median±IQR (interquartile range). We calculated the cutoff points by Youden’s index. Also, receiver operating characteristic (ROC) analysis was performed for diagnostic accuracy using the R statistical software program (version 4.3.4). Significance differences are shown by considering a p-value less than 0.05.
3. Results
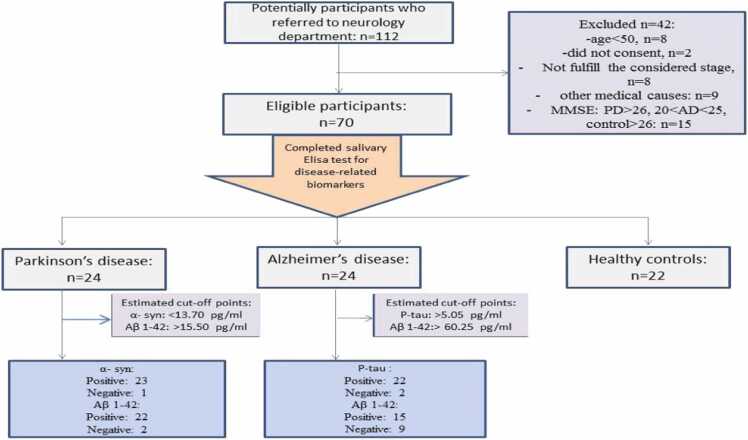
We have presented the comparisons between each of the patient groups (AD & PD) and the control group (Fig. 1 demonstrates the patients’ flow diagram). Therefore, the two groups of patients were not compared with each other.
Fig. 1.
Patients’ flow diagram.
3.1. Demographic Information
Table 1 shows the data on participants' age, sex, and educational levels. Although the independent samples t-test showed that the control and PD groups were homogeneous in age distribution, the control, and AD groups were not homogeneous (p < 0.01), and the control group had a younger mean age than the AD patients. The results of correlation analysis, based on spearman's two-tailed test, revealed that age did not correlate with the other variables in each group. The Chi-square test revealed that the control group is homogeneous in sex distribution with both PD and AD patient groups. Participants' educational levels were defined in three levels, i: literacy or education under five years, ii: diploma, and iii: academic education. The Chi-square test revealed that the control group is homogeneous regarding educational levels distribution with both AD and PD groups compared to the control group (Table 1).
Table 1.
Demographic data of participants.
| Groups |
Age (years) |
Sex |
Educational level |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean ±SD | Minimum | Maximum | Female | Male | Literacy | Diploma | Academic | |
| Control | 64.1 (±9.2) | 53 | 85 | 13 | 9 | 9 | 5 | 8 |
| AD | 73.5 (±9.8) | 60 | 89 | 10 | 14 | 10 | 9 | 5 |
| PD | 61.2 (±8.7) | 51 | 82 | 10 | 14 | 8 | 6 | 10 |
AD: Alzheimer’s disease, PD: Parkinson’s disease.
3.2. Cognitive tests
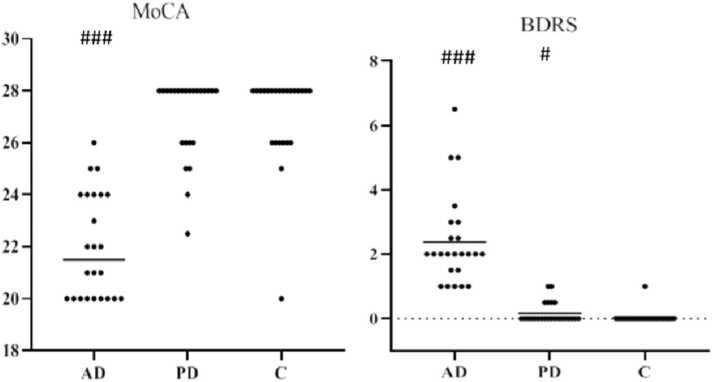
Results of cognitive tests showed that the median±IQR of the MoCA in control, AD, and PD groups were 28 (±2), 21.5 (±4), and 28 (±2), respectively. In addition, the median ± IQR of the BDRS test in the control, AD, and PD groups were 0 ( ± 0), 2 ( ± 1.4), and 0 ( ± 0.4), respectively. The findings of both the Mann-Whitney test (without considering the age confounding variable) and analysis of covariance (by adjusting the age variable) showed that the differences in both MoCA and BDRS scores between AD patients and healthy controls were significant (p < 0.001, with and without adjusting for age). AD patients had lower cognition scores in both MoCA and BDRS tests. At the same time, PD patients had only an upper score on the BDRS test than the control group (p < 0.05), as is shown in Fig. 2.
Fig. 2.
Scatter plot of the groups' MoCA & BDRS scores (median ± IQR). Median values are indicated with a horizontal line. AD: Alzheimer’s disease, PD: Parkinson’s disease, C: control. Data in the AD group are presented without considering the age-confounding variable). #p < 0.05, ### p < 0.001 compared to the control group.
3.3. Proteins levels in saliva
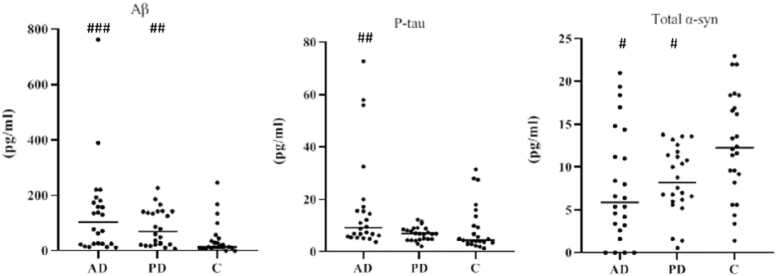
The median ± IQR of the salivary levels of Aβ 1–42 protein (pg/ml) in control, AD, and PD groups were 13.5 ( ± 21.5), 104.3 ( ± 155.2), and 69.5 ( ± 120.4), respectively. As shown in Fig. 3, the results of the Mann-Whitney test, regardless of the age-confounding variable, showed that the median of Aβ 1–42 level was higher in AD patients than in the normal controls (Z = −3.6, p < 0.001). Analysis of covariance by adjusting the age variable showed a lower difference between the AD and the control (p = 0.045). Moreover, the Mann-Whitney test revealed that the median salivary level of Aβ 1–42 protein was higher in the PD than in the healthy controls (Z = −3.2, p = 0.001).
Fig. 3.
Scatter plot of the median ± IQR of salivary Aβ (1-42), and P-tau and mean ± SD of salivary total α-syn protein levels in the groups. Median values are indicated with a horizontal line. AD: Alzheimer’s disease, PD: Parkinson’s disease, C: control. Data in the AD group are presented without considering the age confounding variable. #p < 0.05, ## p < 0.01, ### p < 0.001 compared to the control group. .
The median ± IQR for salivary p-tau levels (pg/ml) in control, AD, and PD groups were 4.2 ( ± 6.1), 9.2 ( ± 10.9), and 6.9 ( ± 4.2), respectively. The results of the Mann-Whitney test, regardless of the age-confounding variable, showed that the median of the p-tau level was higher in AD than in control (Z = −3.2, p = 0.001) (Fig. 3). Moreover, analysis of covariance by adjusting the age variable revealed a difference between AD patients and healthy controls (p = 0.030). On the other hand, the Mann-Whitney test showed that the median p-tau protein salivary level was not significantly different in PD compared to the control group (Z = −1.6, p = 0.104).
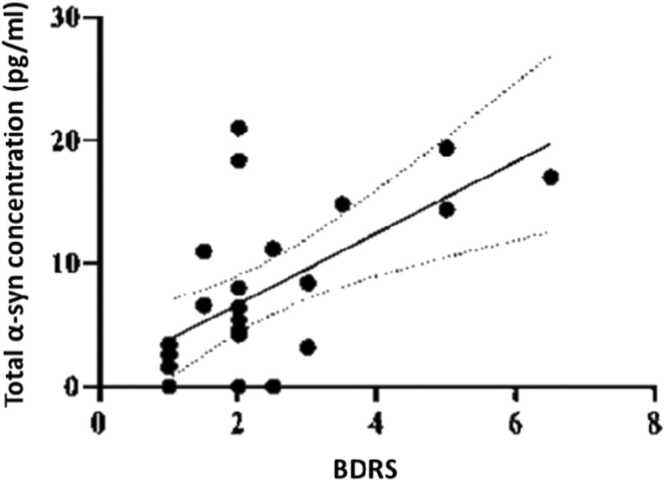
The mean ± SD of salivary levels of total α-syn protein (pg/ml) in control, AD, and PD groups were 12.5 ( ± 6.3), 7.8 ( ± 6.6), and 8.4 ( ± 4), respectively. The results of the two-sample independent t-test, regardless of the age-confounding variable, showed that the mean total α-syn protein level in AD was lower than in the control group (t = −2.4, df=44, p = 0.018) (Fig. 3). Applying the analysis of covariance by adjusting age showed a difference between AD patients and healthy controls, too (p = 0.021). On the other hand, a two-sample independent t-test revealed that the mean total α-syn protein level in PD was lower (p = 0.015) than in the control group (t = −2.6, df=34.7). The results of spearman’s correlation test between the protein concentrations and cognitive tests in each group of the study revealed that there was just a significant correlation (r: 0.6, p < 0.01) between salivary total α-syn protein concentration and BDRS in the AD group (Fig. 4).
Fig. 4.
Spearman’s correlation test between total α-syn protein concentration in the saliva and BDRS score in the AD (Alzheimer’s disease) group. The P-value was < 0.01 between the two variables.
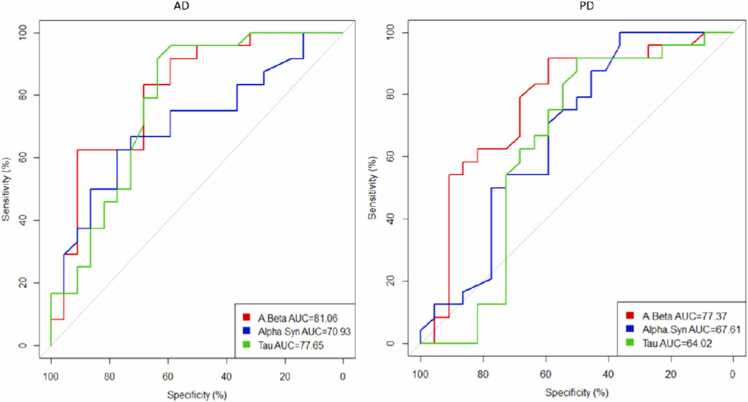
3.4. Sensitivity and specificity of the proteins’ levels
We used Youden’s index (to calculate the sensitivity and specificity of protein concentrations) and receiver operating characteristic (ROC) analysis for diagnostic accuracy of protein concentrations in the saliva in AD and PD patients (Table 2, Table 3, Fig. 5). The salivary Aβ 1–42 concentrations for diagnosing AD in the mild dementia stage revealed an excellent performance with a cutoff point equal to 60.3 pg/ml (AUC: 0.81, specificity: 91%, and sensitivity: 62.5%). Also, p-tau provided a more accurate diagnosis with a cutoff point equal to 5.1 pg/ml (AUC: 0.78, specificity: 63.6%, and sensitivity: 91.7%). Although the total α-syn level might be an acceptable indicator for AD diagnosis with a cutoff point equal to 9.4 pg/ml (AUC: 0.71), it did not reach adequate specificity or sensitivity (Table 2 & Fig. 5).
Table 2.
Diagnostic accuracy of proteins in AD (Alzheimer’s disease) group.
| Cutoff point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | P-value | |
|---|---|---|---|---|---|---|---|
| Aβ | 60.3 | 62.5 | 91 | 88.2 | 68.9 | 0.81 | < .001 |
| P-tau | 5.1 | 91.7 | 63.6 | 73.3 | 87.5 | 0.78 | .001 |
| Total α-syn | 9.4 | 66.7 | 68.2 | 69.5 | 65.2 | 0.71 | .015 |
PPV & NPV: Positive predictive value and negative predictive value. AUC: Area under the curve.
Table 3.
Diagnostic accuracy of proteins in the PD (Parkinson’s disease) group.
| Cutoff point | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) | AUC | P-value | |
|---|---|---|---|---|---|---|---|
| Aβ | 15.5 | 59.1 | 91.7 | 70.9 | 86.6 | 0.77 | .001 |
| P-tau | 4.1 | 50 | 91.7 | 66.6 | 84.6 | 64.0 | .104 |
| Total α-syn | 13.7 | 36.4 | 95.8 | 62.1 | 88.8 | 0.68 | .041 |
PPV & NPV: Positive and negative predictive values. AUC: Area under the curve.
Fig. 5.
ROC (receiver operating characteristic) curves for AD: Alzheimer’s disease, PD: Parkinson’s disease. AUC: Area under the curve.
In the case of diagnosing PD, the results of salivary biomarker concentrations in the early stages showed that total α-syn displayed an acceptable performance at the cutoff point equal to 13.7 pg/ml (AUC: 0.68, specificity: 36.4%, and sensitivity: 95.8%). Also, an acceptable result was obtained with Aβ 1–42 at the cutoff point equal to 15.5 pg/ml (AUC: 0.77, specificity: 59.1%, and sensitivity: 91.7%) (Table 3 & Fig. 5).
4. Discussion
Biomarkers can be helpful for rapid and precise diagnosis, disease prognosis identification, and drug target assessment (O'bryant et al. (2017)). Therefore, here, we evaluated the salivary levels of Aβ 1–42, p-tau, and total α-syn proteins in early stages of PD (first and second stages) and AD (with mild dementia based on the MMSE score) patients and compared the findings in each group with healthy controls to investigate the changes of these biomarkers.
In the present study, the AD patients' mean age was higher than the control. However, the age of participants did not correlate with the other variables in each group. Another study reported similar findings to our study, and the age of participants did not affect the other variables. They had only 15 CE patients in mild to moderate stages and seven healthy controls (Sabbagh et al., 2018).
The assessment results of MoCA and BDRS cognitive tests showed that AD patients had lower cognitive functions in the initial stages than healthy control. Although the PD patients were without signs of dementia, the mean score on the BDRS score was lower than the control group. Previous studies showed that the MoCA could be a valid test for evaluating cognition in AD patients in the initial stages and monitoring its impairment (Costa et al., 2014). It was shown similar results for the BDRS score in a previous study (Zhu et al., 2006). In contrast, a negative correlation was demonstrated between the MoCA score and total α -syn levels (Vivacqua et al., 2016). There are some results suggest that more research required to confirm the psychometric properties of MoCA concepts for PD patients in different populations (Benge et al., 2017).
We showed that the changes in salivary levels of Aβ 1–42 and α-syn proteins in both AD and PD patient groups were almost similar. In comparison, the salivary level of p-tau protein was high only in AD patients. Therefore, the level of p-tau protein in the saliva may be more important for differential diagnosis of AD in the initial stages.
In a pilot study, in which AD patients were arranged in all three stages of the disease, salivary Aβ 1–42 level was high in AD patients. However, this elevation was only significant in mild AD compared to the healthy control groups, similar to our results. Despite this significant finding in mild AD patients, the salivary Aβ 1–42 levels were higher in our measurements; 104.3 ( ± 155.2)* pg/ml vs. 7.7 ( ± 16.3) pg/ml, which could be due to the different assay method somewhat. Also, they revealed that this biomarker is independent of AD risk factors such as age and Apo E genotype (Bermejo-Pareja et al., 2010). Recently, some scientists recommended that beta-amyloid deposition could not be the main reason for dementia in PD patients (Melzer et al., 2019). In contrast to our investigations which PD patients had a significant increase in the salivary Aβ 1–42; 69.5 ( ± 120.35) pg/ml, compared to the control group, in their study, the mean level of this protein was not different in PD patients; 3.7 ( ± 4.2) pg/ml (Bermejo-Pareja et al., 2010). In another study, AD patients were in the mild and moderate stages. The results showed a high level of salivary Aβ 1–42 in AD patients by a 2.45-fold increase compared with healthy control groups. This proportion was higher in our study by a 7.73-fold increase in AD patients, which is because their AD patients' inclusion criteria involve moderate dementia participants with lower MMSE scores in addition to mild dementia (Sabbagh et al., 2018). Also, it is indicated that the Aβ 1–42 level in AD patients was 40 pg/ml, a twofold increase in saliva compared to control and PD participants. The authors did not establish any significant difference between AD stages (Lee et al., 2017).
In contrast to other published results, a study demonstrated that Aβ 1–42 correlates with the intensity of dementia in AD. This heterogeneity can be related to using a different method, antibody-based magnet nanoparticles immunoassay, for the detection of Aβ 1–42 concentration (Kim and Song, 2014). Besides the investigations of these small pilot studies, a few papers indicated that Aβ 1–42 was not detectable by Luminex assay in the saliva of AD patients (Shi et al., 2011). Moreover, no study compared the salivary Ab42/Ab40 ratio (Lee et al., 2019). Based on the sensitivity, specificity, and AUC of our Aβ 1–42 concentration in saliva results (62.5%, 90.9%, and 0.81, respectively) and the investigations of previous studies, it can be used as a diagnostic method in the early stages of the disease, and evaluation of the AD severity and progression in accompany of other biomarkers such as p-tau.
Although a major number of studies in this field have investigated the salivary Aβ 1–42 detection, there are a few research projects and efforts to measure p-tau and t-tau levels in saliva. Some researchers assessed the salivary level of p-tau and t-tau proteins in AD patients by Luminex assay. In summary, their results could not reach the required significant differences besides the t-tau levels decreasing and the p-tau levels increasing in the AD patients compared to the healthy controls. Additionally, they found that the p-tau/t-tau ratio was significantly higher in AD than in normal controls (Shi et al., 2011). Similarly, another study was shown that using western blot analysis, demonstrated a significant increase in the salivary t-tau/t-tau proportion in AD participants compared to both MCI and control groups (Pekeles et al., 2019).
In contrast to the mentioned study (Shi et al., 2011), our findings revealed a trend toward increasing salivary p-tau levels in AD patients. This difference was statistically significant whether adjusting the age variable or not. This conflict may be justified by their different AD patients' illness stages or their different methods (Luminex assay) for measuring tau proteins in the saliva. The sensitivity, specificity, and AUC of salivary p-tau in our study have reached higher levels (91.7%, 63.4%, and 0.77, respectively), which may be due to the investigation of only one stage of AD (mild dementia). In addition, we measured the p-tau levels in the saliva of PD patients for the first time, as we knew. However, there was a trend towards an increase in salivary secretions, but this difference was not statistically significant compared to healthy control groups. Other researchers suggested that the ratio of tau and amyloid plaques in the brain tissue of PD patients does not relate to their cognitive status (Winer et al., 2018).
In our study, the salivary level of total α-syn in both AD and PD was lower than in the healthy controls. There is a conflict in the findings of prior studies, which have measured salivary α-syn, indicating either an increase or no change in the PD patients' α-syn amount compared to healthy control participants. Similar to our results, some others demonstrated a statistically significant decrease in total salivary α-syn of PD patients compared to healthy participants. Their measured total α-syn level in PD patients was higher than our results; 65 ( ± 52.2) pg/ml vs. 8.44 ( ± 3.96) pg/ml, possibly due to the different types of Elisa kits usage (Al-Nimer et al., 2014). Also, the results of another study confirmed that the salivary level of total α-syn in PD patients was lower, while the oligomeric form and the oligomeric α-syn/total α-syn ratio were higher than in normal controls. They suggested that since α-syn can be aggregated in the salivary nuclei or salivary ganglion neurons in the initial stages of PD, a significant reduction in the level of this protein might occur in the saliva. Gradually, α-syn spreads through the axons, reaches the epithelial cells around the salivary glands, and finally accumulates in the saliva (Vivacqua et al., 2016). Although similar results were obtained by one other study, there was no significant correlation between the total α-syn levels and the severity of PD (Shaheen et al., 2020). Contrary, a cohort study conducted on 201 PD patients and 67 healthy controls revealed no significant difference in α-syn salivary levels between PD participants and controls (Kang et al., 2014). Our study's sensitivity, specificity, and AUC of total salivary α-syn were 95.8%, 36.4%, and 0.68, respectively. Based on our investigations and the findings of previous studies assessing total α-syn concentration in PD patients' saliva may be a beneficial diagnostic tool, especially in the early stages. In addition, the Oligomeric α-syn/total α-syn proportion increased with PD progression. Also, as we knew, we measured the total alpha-synuclein levels in the saliva of AD patients for the first time. The results showed a significant decrease in salivary secretions compared to healthy groups. The total α-syn cutoff point for diagnosing AD patients was lower than PD patients (9.4 pg/ml vs. 13.7 pg/ml), which approves the lower specificity rate of total salivary alpha-synuclein (36.4%) as a diagnostic tool in PD patients.
Although there is a considerable progression in the analysis and detection of salivary biomarkers in AD and PD diagnosing, studies’ findings on biomarkers in saliva are still inadequate (Pawlik and Błochowiak, 2021). Our study had noteworthy strengths. We evaluated p-tau, Aβ 1–42, and total α-syn in the homogenous groups of AD and PD participants at their primary stages of the disease. Based on the recent articles, for the first time, we assessed the salivary p-tau in PD participants and total α-syn in AD patients (Pawlik and Błochowiak, 2021, Wolgin et al., 2022). Also, we determined the sensitivity, specificity, PPV, NPV, and AUC of these three biomarkers for standardizing our results and making the primary steps for introducing them as a diagnostic tool. We had several limitations during our study period. Due to the lack of uniform and standardized Elisa kits for detecting and measuring salivary biomarkers, it was difficult to make a specific cutoff point for diagnosing these neurodegenerative diseases. Another limitation in our study was the smaller number of patients in the early stages than in advanced one. Indeed, further research projects with larger groups in different disease stages and following up them for several years are required to make a standardized diagnostic protocol for salivary biomarkers in AD and PD. Also, there are some reports about the contribution between the oral cavity microbiome, salivary exosomes, and cytokines to AD and PD progression (Rani et al., 2021, Orr et al., 2020), which could be a consideration for future studies to find out if there any correlation between the mentioned biomarkers and these new factors.
5. Conclusion
Considering the accessibility of the salivary samples, tracing the biomarkers in the saliva may be a secure and non-invasive approach to making a more accurate and reasonable diagnosis. Evaluation of the salivary levels of p-tau, total α-syn, and Aβ 1–42 proteins in AD and PD patients revealed that the changes in these biomarkers could be a helpful diagnostic tool in the early stages. In addition, p-tau biomarker levels in saliva may be a more accurate and valuable tool for differentiation between these two neurodegenerative diseases. Based on our and previous investigations further studies with more homogenous AD and PD subjects need to be conducted to obtain a standardized diagnostic protocol for salivary biomarkers. Thus, these hopeful efforts could be done to decrease the usage of invasive diagnostic methods, which alone is an achievement in alleviating the suffering of AD and PD patients. Also, detecting new salivary biomarkers and confirming the findings of previously assessed biomarkers based on the pathophysiology of AD and PD should be studied and encouraged.
Funding
This study was supported financially by the Iran University of Medical Sciences (No: 26176).
Ethical statement
All procedures performed in this study that involved human participants were permitted by the Ethics Committee of Iran University of Medical Sciences (ethical code: IR.IUMS.REC.1394.9211314202), under the latest variety of ethical standards of the 1964 Helsinki declaration.
Authors' contributions
Study conception and design: Saba Rahimian, Homa Rasoolijazi & Masoumeh Sabaei, Data collection: Masoumeh Sabaei & Mahmoud Faramarzi, Data analysis and interpretation: Homa Rasoolijazi, Saba Rahimian & Arsh Haj Mohamad Ebrahim Ketabforoush, Drafting the article: Masoumeh Sabaei, Homa Rasoolijazi, Saba Rahimian & Mansoureh Soleimani, Critical revision of the article: Arsh Haj Mohamad Ebrahim Ketabforoush & Homa Rasoolijazi, Supervision of study: Homa Rasoolijazi, Clinical approaches for forming the study groups: Fahimeh Hajiakhoundi, Babak Zamani, & Gholamali Shahidi, All authors reviewed the results and approved the final version of the manuscript.
CRediT authorship contribution statement
Saba Rahimian: Conceptualization, Project administration, Methodology, Investigation. Masoumeh Sabaei: Formal analysis, Project administration. Arsh Haj Mohamad Ebrahim Ketabforoush: Formal analysis, Writing – original draft, Investigation, Writing – review & editing. Homa Rasoolijazi: Supervision, Writing – original draft, Writing – review & editing. Mansoureh Soleimani: Visualization, Investigation. Fahimeh Hajiakhoundi: Writing – review & editing. Babak Zamani: Writing – review & editing. Gholamali Shahidi: Formal analysis. Mahmood Faramarzi: Formal analysis.
Conflict of Interest
No author has a conflict of interest related to the publication of this manuscript.
Acknowledgment
The authors want to thank all patients, their family, and participating volunteers for their humanity and kindness. We also thank Mr. Saeed Mohammadi (MSc of biostatistics, Iran University of Medical Sciences) for his guidance in analyzing the data.
References
- Al-Nimer M.S., Mshatat S.F., Abdulla H.I. Saliva α-synuclein and a high extinction coefficient protein: a novel approach in assessment biomarkers of Parkinson's disease. North Am. J. Med. Sci. 2014;6:633. doi: 10.4103/1947-2714.147980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benge J.F., Balsis S., Madeka T., Uhlman C., Lantrip C., Soileau M.J. Factor structure of the montreal cognitive assessment items in a sample with early Parkinson's disease. Park. Relat. Disord. 2017;41:104–108. doi: 10.1016/j.parkreldis.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F., Antequera D., Vargas T., Molina J.A., Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer's disease: a pilot study. BMC Neurol. 2010;10:1–7. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidinosti M., Shimshek D.R., Mollenhauer B., Marcellin D., Schweizer T., Lotz G.P., Schlossmacher M.G., Weiss A. Novel one-step immunoassays to quantify α-synuclein: applications for biomarker development and high-throughput screening. J. Biol. Chem. 2012;287:33691–33705. doi: 10.1074/jbc.M112.379792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.S., Reich A., Fimm B., Ketteler S.T., Schulz J.B., Reetz K. Evidence of the sensitivity of the MoCA alternate forms in monitoring cognitive change in early Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2014;37:95–103. doi: 10.1159/000351864. [DOI] [PubMed] [Google Scholar]
- Devic I., Hwang H., Edgar J.S., Izutsu K., Presland R., Pan C., Goodlett D.R., Wang Y., Armaly J., Tumas V. Salivary α-synuclein and DJ-1: potential biomarkers for Parkinson's disease. Brain. 2011;134 doi: 10.1093/brain/awr015. e178-e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Picard G., Sarazin M. Early detection of Alzheimer's disease: new diagnostic criteria. Dialog-. Clin. Neurosci. 2009;11:135. doi: 10.31887/DCNS.2009.11.2/bdubois. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez‐Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov. Disord.: Off. J. Mov. Disord. Soc. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Herzig M.C., Bidinosti M., Schweizer T., Hafner T., Stemmelen C., Weiss A., Danner S., Vidotto N., Stauffer D., Barske C. High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PloS One. 2012;7 doi: 10.1371/journal.pone.0036581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D.A., Shivane A.G. Springer; 2015. Neuropathology simplified: a guide for clinicians and neuroscientists. [Google Scholar]
- Ismail R., Parbo P., Madsen L.S., Hansen A.K., Hansen K.V., Schaldemose J.L., Kjeldsen P.L., Stokholm M.G., Gottrup H., Eskildsen S.F. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J. Neuroinflamm. 2020;17:1–11. doi: 10.1186/s12974-020-01820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W.-Y., Yang Q., Jiang X.-F., Chen W., Zhang L.-Y., Wang X.-Y., Zhang L.-N., Quinn T.J., Liu J., Chen S.-D. Salivary DJ-1 could be an indicator of Parkinson's disease progression. Front. Aging Neurosci. 2014;6:102. doi: 10.3389/fnagi.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-B., Song K.-B. Antibody-based magnetic nanoparticle immunoassay for quantification of salivary beta-amyloid peptides. Biophys. J. 2014;106:616a. doi: 10.1117/1.JBO.19.5.051205. [DOI] [PubMed] [Google Scholar]
- Kovacs G.G. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int. J. Mol. Sci. 2016;17:189. doi: 10.3390/ijms17020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L. Biochemical diagnostic markers to detect early Alzheimer’s disease. Neurobiol. Aging. 1998;19:165–167. doi: 10.1016/s0197-4580(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Kim S.J., Hong S., Kim Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019;51:1–10. doi: 10.1038/s12276-019-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Guo J.-P., Kennedy K., Mcgeer E.G., Mcgeer P.L. A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels. J. Alzheimer'S. Dis. 2017;55:1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- Lesage S., Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Early Alzheimer's disease. N. Engl. J. Med. 2010;362:2194–2201. doi: 10.1056/NEJMcp0910236. [DOI] [PubMed] [Google Scholar]
- Mckhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr, Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer'S. Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer T.R., Stark M.R., Keenan R.J., Myall D.J., Macaskill M.R., Pitcher T.L., Livingston L., Grenfell S., Horne K.-L., Young B.N. Beta amyloid deposition is not associated with cognitive impairment in Parkinson's disease. Front. Neurol. 2019;10:391. doi: 10.3389/fneur.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'bryant S.E., Mielke M.M., Rissman R.A., Lista S., Vanderstichele H., Zetterberg H., Lewczuk P., Posner H., Hall J., Johnson L. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimer'S. Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M.E., Reveles K.R., Yeh C.K., Young E.H., Han X. Can oral health and oral‐derived biospecimens predict progression of dementia? Oral. Dis. 2020;26:249–258. doi: 10.1111/odi.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik P., Błochowiak K. The role of salivary biomarkers in the early diagnosis of Alzheimer’s disease and Parkinson’s disease. Diagnostics. 2021;11:371. doi: 10.3390/diagnostics11020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekeles H., Qureshi H.Y., Paudel H.K., Schipper H.M., Gornistky M., Chertkow H. Development and validation of a salivary tau biomarker in Alzheimer's disease. Alzheimer's & Dementia: Diagnosis. Assess. Dis. Monit. 2019;11:53–60. doi: 10.1016/j.dadm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter J.S. Assessment of Parkinson disease manifestations. Curr. Protoc. Neurosci. 2009;49:10.1. 1–10.1. 14. doi: 10.1002/0471142301.ns1001s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W. Non‐motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E. MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- Rani K., Rastogi S., Vishwakarma P., Bharti P.S., Sharma V., Renu K., Modi G.P., Vishnu V.Y., Chatterjee P., Dey A.B. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods. 2021;347 doi: 10.1016/j.jneumeth.2020.108980. [DOI] [PubMed] [Google Scholar]
- Rosén C., Hansson O., Blennow K., Zetterberg H. Fluid biomarkers in Alzheimer’s disease–current concepts. Mol. Neurodegener. 2013;8:1–11. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M.N., Shi J., Lee M., Arnold L., Al-Hasan Y., Heim J., Mcgeer P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC Neurol. 2018;18:1–4. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen H., Sobhy S., EL Mously S., Abuomira M., Mansour M. Salivary alpha-synuclein (total and oligomeric form): potential biomarkers in Parkinson’s disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2020;56:1–6. [Google Scholar]
- Shi M., Sui Y.-T., Peskind E.R., Li G., Hwang H., Devic I., Ginghina C., Edgar J.S., Pan C., Goodlett D.R. Salivary tau species are potential biomarkers of Alzheimer's disease. J. Alzheimer'S. Dis. 2011;27:299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T., Sui Y.-T., Gonzalez-Cuyar L.F., Wong D.T., Akin D.M., Tumas V., Aasly J., Ashmore E., Aro P., Ginghina C. Cheek cell–derived α-synuclein and DJ-1 do not differentiate Parkinson's disease from control. Neurobiol. Aging. 2014;35:418–420. doi: 10.1016/j.neurobiolaging.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua G., Latorre A., Suppa A., Nardi M., Pietracupa S., Mancinelli R., Fabbrini G., Colosimo C., Gaudio E., Berardelli A. Abnormal salivary total and oligomeric alpha-synuclein in Parkinson’s disease. PloS One. 2016;11 doi: 10.1371/journal.pone.0151156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J.R., Maass A., Pressman P., Stiver J., Schonhaut D.R., Baker S.L., Kramer J., Rabinovici G.D., Jagust W.J. Associations between tau, β-amyloid, and cognition in Parkinson disease. JAMA Neurol. 2018;75:227–235. doi: 10.1001/jamaneurol.2017.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgin M., Zobering M., Dvornyk V., Braun R.J., Kielbassa A.M. Systematic review on saliva biomarkers in patients diagnosed with morbus Alzheimer and Morbus Parkinson. Biomedicines. 2022;2022(10):1702. doi: 10.3390/biomedicines10071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.W., Scarmeas N., Torgan R., Albert M., Brandt J., Blacker D., Sano M., Stern Y. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67:998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]