Abstract
Shaniodendron subeaqualis is a tertiary relict plant unique to China. This species has high greening, ecological, and scientific research value, and has been listed as a national I-level key protected plant. Clarifying the main climatic factors restricting the geographical distribution of S. subeaqualis and predicting the potential geographical distribution pattern of this species can provide a scientific basis for the protection of the germplasm resources of this rare species. Based on 104 S. subeaqualis natural distribution records and 9 climate factors, the MaxEnt software was used to predict the potential suitable areas of S. subeaqualis in different periods (LGM, MH, Current, SSP245-2050s, SSP245-2090s, SSP585-2050s, SSP585-2090s). The results showed that the contemporary AUC predicted by MaxEnt is 0.996, with high simulation accuracy; Precipitation in the driest season (Bio17), the average temperature in the coldest season (Bio11) are main factors affecting the distribution of S. subeaqualis. At present, the suitable area of S. subeaqualis is mainly distributed in Jiangsu, Anhui, and Zhejiang province, with a total area of 11.575 × 104 km2, of which the high suitable area is 1.424 × 104 km2 and the medium suitable area is 3.826 × 104 km2. In the LGM, the area of S. subeaqualis was roughly similar to that of the contemporary period, but there was a southward migration phenomenon in some areas, such as the suitable area in the south of Zhejiang. In order to avoid the influence of ice age, S. subeaqualis moved to nearby refuge places, such as Dabie Mountain area of Anhui province, the west of Tianmu Mountain area of Zhejiang province and mountain area of Jiangsu province. In the MH, the suitable area for S. subeaqualis was reduced and moved northward to a small extent. In the future period, the suitable range of S. subeaqualis will not change greatly, but the overall degree of fragmentation will intensify. If effective measures are not taken, it is bound to bring severe challenges to the survival of S. subeaqualis. In order to protect S. subeaqualis germplasm resources more effectively, it is suggested to dynamically monitor the existing S. subeaqualis population and take various measures actively to reduce the negative effects of climate change on S. subeaqualis.
Keywords: Climate change, Shaniodendron subeaqualis, MaxEnt, Prediction of suitable areas
1. Introduction
The influence of climate on vegetation has become a hotspot in the fields of ecology and botany in recent years. According to the IPCC (United Nations Intergovernmental Panel on Climate Change) report, anthropogenic greenhouse gas emissions have already caused global warming, and the global average surface temperature by the end of this century will be 0.3–4.8 °C higher than that in 1986–2005 [1]. Continued global warming is likely to get a major impact on the world's ecosystems [2,3]. Vegetation play an important role in the interaction among the atmosphere, soil, and water, as well as in terrestrial ecosystems [4,5]. However, vegetation is extremely sensitive to climate change. Studies have shown that climate warming will cause changes in vegetation community and genetic structure, and even reduce the suitable range of vegetation, further leading to species extinction [[6], [7], [8], [9]]. Under the current background of global warming, the risk of species extinction has increased, which has seriously threatened the global biodiversity security [10]. Therefore, it is of profound significance to reveal the changes in species distribution areas under climate change and formulate reasonable conservation strategies for maintaining global biodiversity.
In recent years, Species Distribution Models (SDMs) have become an effective tool for predicting the potential distribution and suitable habitat of target species [11]. The species distribution model predicts the potentially suitable areas of species in different periods by using the species distribution information and the environmental variable factors of the region and combining the two based on a certain algorithm [12]. So far, various SDMs have been developed, such as BIOCLIM (Bioclimate modeling), Domain (Domain environment envelope), CLIMEX (Climate change experiment), GARP (Genetic algorithm for rule-set production) and MaxEnt (Maximum entropy), etc. Studies have shown that MaxEnt outperforms other models due to its simplicity of operation and high accuracy of prediction results [13]. After years of research and application, the maximum entropy model (MaxEnt) has gradually been recognized by a wide range of researchers [[14], [15], [16]]. The MaxEnt model is a computer learning model that predicts the distribution of a target species based on the maximum entropy algorithm [17]. Previous studies have shown that the MaxEnt model is not sensitive to species distribution samples, and only a few species distribution data need to be obtained more accurate prediction results [[18], [19], [20]]. Therefore, the model is extremely suitable for the prediction of the geographical distribution pattern of extremely small endangered species. Currently, the MaxEnt model has been applied to Cunninghamia Konishi [21], Pomatosace filicula [22], Glyptostrobus pensilis [23], Gymnocladus assamicus [24] and other endangered plant potential suitable areas prediction research. In addition, the MaxEnt model can also be applied to the prehistoric geological period (the Last interglacial, the Last glacial maximum and the mid Holocene, etc.), which provides some auxiliary information for the study of relict plant phylogeography [23].
Shaniodron subeaqualis was originally named Parrotia subeaqualis, which has been renamed according to the latest phylogenetic status [25]. This species is a deciduous small tree of the genus Shaniodron in Hamamelidae, with a straight trunk, beautiful shape and seasonal leaf color, so it has become an excellent urban greening tree species [26]. Its wood texture is dense, very hard, for first-class furniture and technology materials. Its stem bark contains secondary metabolites such as rhodiola rosea, which has certain potential value [27]. Its well-developed root system can grow in stony barren environment and has strong resistance to stress, so it is a good material for studying plant physiology and stress resistance [28]. S. subeaqualis has an ancient origin and is an endemic tertiary relict plant in China. It is an important research plant for studying the origin and differentiation of Hamamelidaceae, flora, paleontology and other directions. However, the distribution of S. subeaqualis is extremely narrow and the population size is mostly small. The reasons for this unfavorable situation may be as follows: First, the unique reproductive physiological habits of S. subeaqualis. For example, S. subeaqualis blooms once every few years. Due to the influence of early cold and spring injury, the flower bud of S. subeaqualis. could not complete its development cycle smoothly, thus reducing the possibility of producing sexual offspring [29]. Second, unique growing environment. S. subeaqualis, mostly grows in isolated mountains and valleys, which can not effectively use wind pollination, its pollination mode is greatly limited, the ratio of pollen flow and seed flow is significantly reduced, and it is easy to produce inbred offspring, resulting in reduced genetic variation and adverse consequences [30]. Thirdly, the competition ability is weak. Compared with other tree species, S. subeaqualis is at a disadvantage in the competition for environmental factors such as sunlight and moisture, and has a higher mortality rate than other tree species. From the perspective of community structure, S. subeaqualis only played a secondary and subordinate role [31]. Finally, human activity. For example, social infrastructure construction and tourism development will have a certain impact on it. Based on its special functions and unfavorable community status, S. subeaqualis is currently listed as a national Class I priority plant, a 120 species for conservation of very small populations, and an IUCN (International Union for Convervation of Nature) critically endangered (CR) species [25,[32], [33], [34]]. At present, the research on S. subaqualis mainly focuses on community investigation [35,36], conservation and reproduction [37], genetic diversity [30,38,39] and other fields. However, the current MaxEnt research on S. subaqualis mainly focuses on the changes of its distribution pattern at present and in the future, and does not involve the prehistoric geological period and in-depth discussion on how the main climate variable factors affect its distribution [40]. In addition, The Shared Socioeconomic Pathway (SSPs) climate data from the Sixth International Coupled Model Comparison Program (CMIP6), published in 2020, have been shown to outperform the CMIP5 Common Climate Model (CCSM4) simulations [41,42]. Therefore, it is of great significance to use CMIP6 data through MaxEnt model to predict the suitability of S. subeaqualis.
Therefore, the MaxEnt software was used to simulate the potential suitable areas of the Last glacial maximum (LGM) and the mid Holocene (MH) in the two ancient periods of the Quaternary, the contemporary period and the different climate change scenarios (SSP245 and SSP585) in the future periods (2050s and 2090s). The main factors restricting their distribution were comprehensively evaluated by the percentage contribution, the significance of displacement and the Jackknife method; Determine the optimal growth environment range of S. subeaqualis according to the response curve; Based on ArcGIS10.4, the temporal and spatial change map of potential suitable habitat and future suitable habitat of Hamamelis under different climate scenarios was drawn. The objectives of our study were to (1) explore how the main climatic variables affect influence the distribution of S. subeaqualis; (2) reveal how the distribution pattern of S. subeaqualis has changed from the past to the future; (3) study and develop conservation strategies for S. subeaqualis.
2. Materials and methods
2.1. The study area
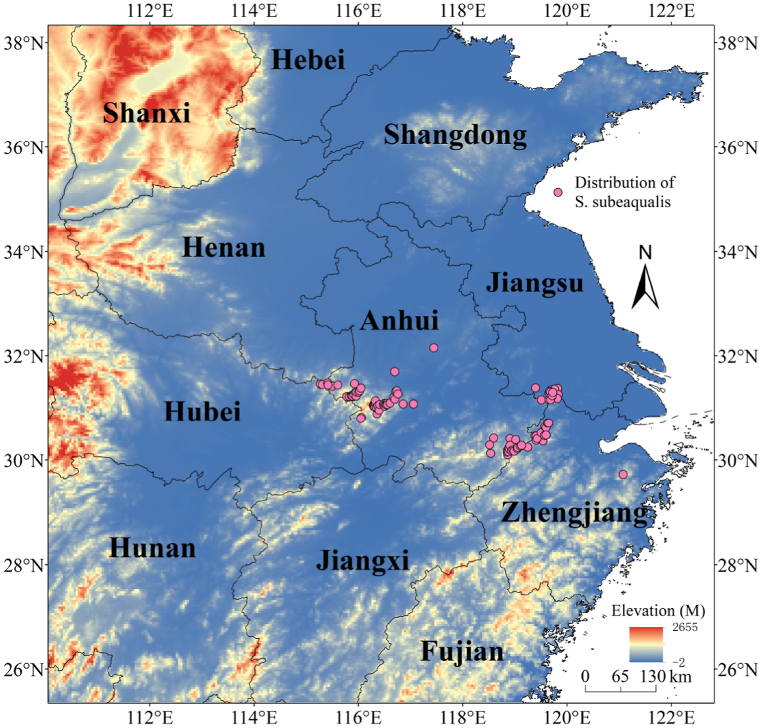
According to the literature, S. subeaqualis has a narrow distribution, and its main distribution is in the eastern part of China. The climate in this region is warm and humid, with an annual average rainfall of 1100–1400 mm and an average annual temperature in the range of 8.3–16.0 °C. S. subeaqualis is mainly distributed in Jiangsu, Zhejiang, and Anhui province [43]. Since the purpose of the study was the prediction of the potential distribution area of S. subeaqualis, to avoid missing some areas where S. subeaqualis may exist, 28°–34°N and 110°–122°E were set as the study area in this paper (Fig. 1).
Fig. 1.
The distribution of S. subeaqualis.
2.2. Distribution point collection and processing
The distribution data of S. subaqualis were obtained from the specimen database and literature records. Database including Global Biodiversity Information Facility (GBIF, www.gbif.org), National Specimen Information Infrastructure (NSII, http://www.nsii.org.cn/), Plant Photo Dank of China (PPBC, http://ppbc.iplant.cn/), Chinese Virtual Herbarium (CVH, http://www.cvh.ac.cn/), etc. The literature records include documents collected in various literature databases such as China National Knowledge Infrastructure (CNKI) and Web of Science (SCI), as well as native flora. A total of 163 species records of S. subaqualis were obtained. Artificial planting distribution points, fuzzy recording distribution points and repeated distribution points were removed from the obtained S. subaqualis distribution data. At the same time, in order to minimize the model error caused by clustering effect, one distribution point was reserved in each 1 km × 1 km grid [44]. The geographical coordinate transformation and correction were carried out on the screened record points, and 104 S. subaqualis natural distribution points were finally determined (Fig. 1), and CSV files were exported for use.
2.3. Climatic variable factor data acquisition and screening
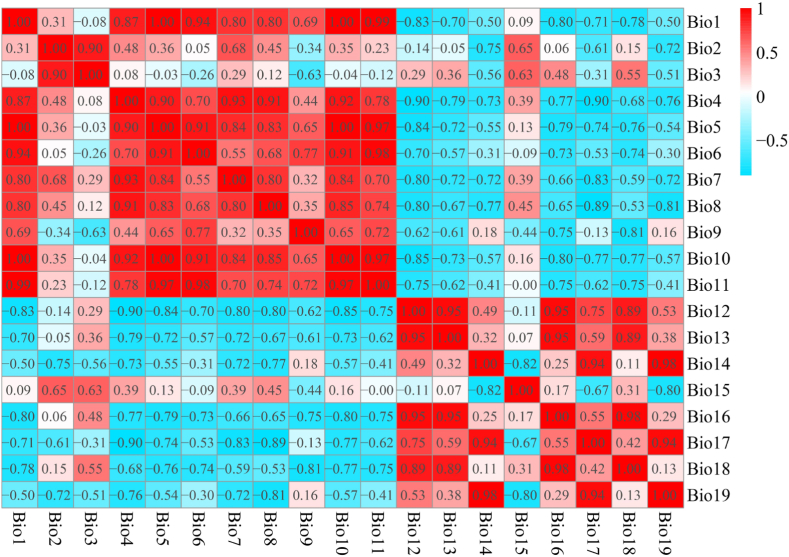
The maps of China's provincial administrative districts used in this study were derived from the National Geomatics Center of China (http://www.ngcc.cn/ngcc/). Climatic data of the Last glacial maximum (LGM, 22ka bp) and the mid Holocene (MH, 10ka bp) were obtained using the CCSM4 model in Worldclim 1.4 (http://worldclim.org), while the current and future climatic data were obtained using BCC-CMN2-MR in Worldclim 2.1. Future climate data include SSP2-RCP 4.5 (SSP245) and SSP5-RCP 8.5 (SSP585) climate models, in which “SSP” represents Shared Socioeconomic Pathway, “RCP” represents Representative Concentration Pathway [45], and SSP245 and SSP585 represent medium and high gas emission patterns, respectively. The emission models for both future concentrations were downloaded for the periods 2050s (2041–2060) and 2090s (2081–2100). Each period contains 19 climate variable factors, and the spatial resolution accuracy of climate variable factors is 2.5′. In order to avoid the problem of over fitting the model due to the multiple collinearity between climate variables, this study used Pearson correlation analysis on the R platform to screen climate factors (Fig. 2), and retained the climate factors with correlation coefficient |r| < 0.9. Among the climate factors with correlation coefficient |r| > 0.9 [21], the climate factors with the closest relationship between retention and growth of S. subeaqualis were selected for prediction, and finally nine climate factors were screened, as shown in Table 1.
Fig. 2.
Correlation analysis of 19 climate factors.
Table 1.
Filtered climate data.
| Data | Variable | Specific name | Unit |
|---|---|---|---|
| Climate factor | Bio1 | Annual mean air temperature | °C |
| Bio4 | Air temperature seasonality | °C | |
| Bio6 | Min air temperature of the coldest month | °C | |
| Bio8 | Mean air temperature of the wettest quarter | °C | |
| Bio9 | Mean air temperature of the driest quarter | °C | |
| Bio11 | Mean air temperature of the coldest quarter | mm | |
| Bio14 | Precipitation of the driest month | mm | |
| Bio17 | Precipitation of the driest quarter | mm | |
| Bio19 | Precipitation of the coldest quarter | mm |
2.4. Model parameter setting and accuracy evaluation
In this study, the MaxEnt model was used to predict the potential suitable areas in seven different climatic backgrounds of S. subaqualis. After importing the distribution points and climate factors of S. subaqualis samples collected into MaxEnt software, 75% sample data were randomly selected for training data and 25% for test data, and the maximum number of iterations was set as 1000. Bootstrap10 is repeated, and other parameters are set to default values. The accuracy of simulation results was evaluated by Receiver Operating Characteristic curve (ROC). The Area enclosed by the ROC and the abscissa is the AUC (Area Under Curve) value. The AUC value ranges from 0 to 1, and the closer the AUC value is to 1, the better the prediction effect is. It is generally believed that AUC less than 0.7 indicates very poor prediction effect, 0.7–0.8 indicates relatively accurate prediction effect, 0.8–0.9 prediction effect is very accurate, and 0.9–1 prediction effect is extremely accurate [44,46,47].
2.5. Evaluation of model prediction results
2.5.1. Climatic factor importance assessment
The output results of MaxEnt software included Percent Contribution, Permutation Importance and Jackknife test. The above three results were used to comprehensively evaluate the importance of climate factors on the contemporary geographical distribution pattern of S. subaqualis. The Percentage Contribution is based on the specific algorithm to obtain the optimal solution, and the gain value is improved by modifying the corresponding factor coefficient step by step. Finally, the gain value is allocated to the climate variable that determines the factor and presented as a percentage. In this process, if there are high correlation climate variables, the results will not be accurate. The Permutation Importance only depends on the final result, which has nothing to do with the exact algorithm. The result is based on the value of climate variables in the training point set by random replacement, and the decline amplitude of the training AUC value caused by climate variables is measured. After normalization, the AUC value is output in the form of percentage. Jackknife test is a demonstration method that selects one climate variable in turn or excludes one climate variable in turn to build a species prediction model. The importance of climate variables to species was evaluated by comparing the differences of Regularized training gain, Test gain and AUC values in the test results.
2.5.2. Response curve analysis
Response curve according to the curve of the species suitable as climate factor changes, the curve for a single climate factor, the abscissa denotes the numerical range, in a logical output value as the ordinate, the logic of the output value is expressed as the climate factor numerical probability species exist, namely suitable degree, of which 10% of MaxEnt simulation training existence threshold is defined as a species threshold [48]. Therefore, the logical output values in the response curves were used to determine the appropriate range of climate conditions for S. subaqualis.
2.6. Classification of fitness degree and change of geographical distribution pattern
The simulation result files of seven different climate scenarios of S. subaqualis obtained by MaxEnt were imported into ArcGIS for grading zoning of suitable areas. In this study, the Jenks' Natural breaks was used to divide the suitable areas into 4 grades: not suitable (0 < P < 0.15), low suitabile (0.15 = P < 0.3), moderately suitable (0.3 = P < 0.5), and highly suitable (0.5 = P < 1) [49].
There are three survival modes of species in response to climate and environmental changes: disappearing, maintaining the status quo, and migrating. Therefore, in this study, the “superposition analysis” tool in ArcGIS 10.4 is used to take the contemporary suitable areas of S. subaqualis as reference, and the suitable range of the remaining periods is compared with it. According to the changes in the range of S. subaqualis, it is defined as the following three types: (1) Preserving suitable areas (the intersection of suitable areas in different periods and suitable areas in contemporary times); (2) Increase suitable areas. This kind of situation can be divided into the areas suitable for contemporary but unsuitable for the last interglacial, the areas unsuitable for contemporary but suitable for the future. (3) Loss of suitable areas. This situation can be divided into areas suitable for the last interglacial but not suitable for the present and areas suitable for the present but not suitable for the future. Finally, ArcGIS 10.4 was used for area statistics and visual representation [50].
3. Results
3.1. Analysis of model results
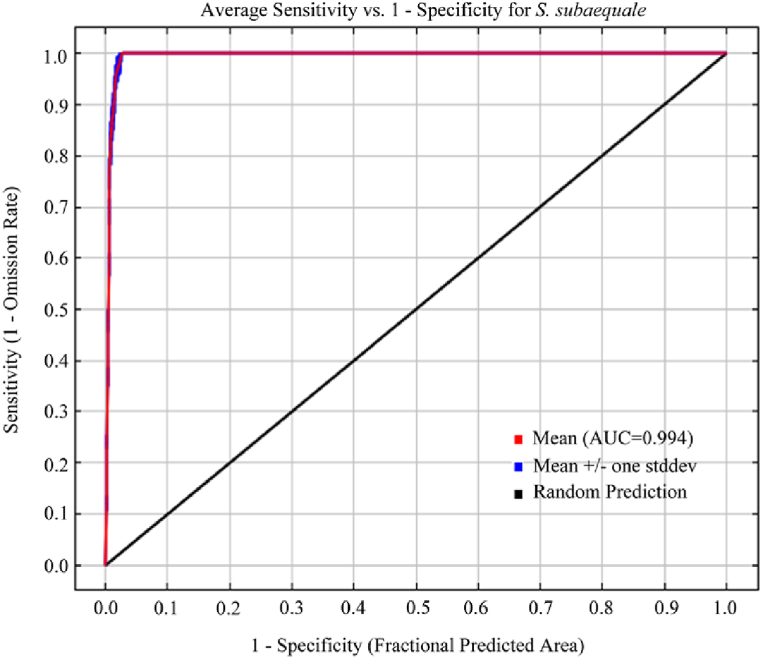
In this study, the MaxEnt model was used to predict the modern distribution area of S. subaqualis based on 104 species distribution points. The output results of the model showed that the AUC value of the ROC curve of S. subaqualis was 0.994 (Fig. 3), which was greater than 0.9, indicating that the accuracy of simulation results was extremely accurate.
Fig. 3.
ROC curve of MaxEnt results.
3.2. Climatic factors affecting the spatial distribution of S. subeaqualis
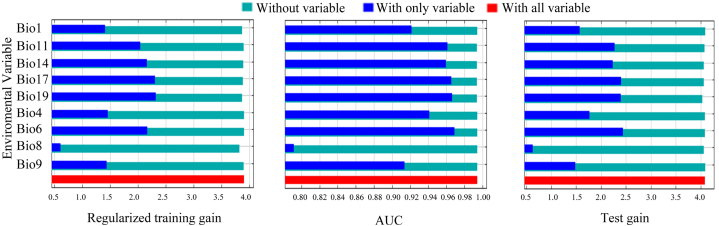
In this study, nine climatic factors were selected for model building. Table 2 shows that the Precipitation of the driest quarter (Bio17, 60.1%), the Mean air temperature of the coldest quarter (Bio11, 25.4%), and the Annual mean air temperature (Bio1, 8.2%) ranked the top three, with a total contribution rate of 87.8%. The top three Permutation Importance values are Mean air temperature of the coldest quarter (Bio11, 32.3%), Precipitation of the driest quarter (Bio17, 31.5%), and Precipitation of the coldest quarter (bio19, 16.2%), with a cumulative value of 80%. When a single environmental variable is excluded successively, Regularized training gain of the model decreases most in Mean air temperature of the wettest quarter (Bio8), Annual mean air temperature (Bio1), and the Precipitation of the coldest quarter (Bio19). The top three factors with the largest decrease in test gain are Precipitation of the coldest quarter (bio19), Precipitation of the driest month (Bio14), and Mean air temperature of the wettest quarter (Bio8). The most decreasing AUC values are Precipitation of the coldest quarter (Bio19), Precipitation of the driest month (bio14), Precipitation of the driest quarter (Bio17), Mean air temperature of the coldest quarter (Bio11). The above results indicate that these climatic factors had no effect on the growth of S. subaqualis. The above results show that these climatic factors play an important role in the growth of S. subaqualis compared with other factors. When only a single environmental variable is used, the top three Regularized training gain of the model are Precipitation of the coldest quarter (Bio19), Precipitation of the driest quarter (Bio17), Min air temperature of the coldest month (Bio6). Min air temperature of the coldest month (Bio6), Precipitation of the driest quarter (Bio17), Precipitation of the coldest quarter (Bio19) rank the top three in the Test gain. The three highest AUC factors are Min air temperature of the coldest month (bio6), Precipitation of the coldest quarter (Bio19), Precipitation of the driest quarter (Bio17). These results indicate that these factors play an important role in the growth of S. subaqualis (Fig. 4). Based on the above analysis, the precipitation factor is the first and the temperature factor is the second most closely related to the distribution pattern of S. subaqualis. According to all the evaluation indicators, it can be concluded that the main climatic factors affecting the distribution of contemporary S. subaqualis are Bio17, Bio19, Bio11, Bio6 and Bio8.
Table 2.
Various parameters of the main environmental variables of S. subeaqualis.
| Environmental variables | PC (%) | PI (%) | RTGw | RTGo | TGw | TGo | AUCw | AUCo |
|---|---|---|---|---|---|---|---|---|
| Precipitation of the driest quarter (Bio17) | 60.1 | 31.5 | 3.8861 | 2.3083 | 4.0622 | 2.4032 | 0.9938 | 0.9656 |
| Mean air temperature of the coldest quarter (Bio11) | 19.5 | 32.3 | 3.8968 | 2.0426 | 4.0717 | 2.2693 | 0.9939 | 0.9611 |
| Annual mean air temperature (Bio1) | 8.1 | 9 | 3.8678 | 1.4126 | 4.0869 | 1.5731 | 0.994 | 0.9216 |
| Min air temperature of the coldest month (Bio6) | 5.7 | 0 | 3.9043 | 2.1665 | 4.0819 | 2.44 | 0.994 | 0.9688 |
| Mean air temperature of the wettest quarter (Bio8) | 2.6 | 8 | 3.8195 | 0.6134 | 4.0619 | 0.633 | 0.9941 | 0.7915 |
| Mean air temperature of the driest quarter (Bio9) | 1.4 | 1 | 3.8996 | 1.4406 | 4.0856 | 1.4849 | 0.994 | 0.9138 |
| Air temperature seasonality (Bio4) | 1.2 | 0.8 | 3.9031 | 1.4615 | 4.0819 | 1.7673 | 0.994 | 0.9413 |
| Precipitation of the coldest quarter (Bio19) | 1.1 | 16.2 | 3.8687 | 2.3228 | 4.0275 | 2.3941 | 0.9937 | 0.9662 |
| Precipitation of the driest month (Bio14) | 0.2 | 1.3 | 3.8926 | 2.1583 | 4.0562 | 2.2392 | 0.9938 | 0.9594 |
Note: PC is Percent contribution; PI is Permutation importance; RTGw is the regulariaed training gain using without the variable; RTGo is the regulariaed training gain using the only variable; TGw is the test gain using without the variable; TGO is the test gain using the only variable; AUCw is the area under the receiver operating characteristic curve using without the variable; AUCo is the area under the working characteristic curve of the subjects using the only variable.
Fig. 4.
Main evaluation parameters for the main environmental variables.
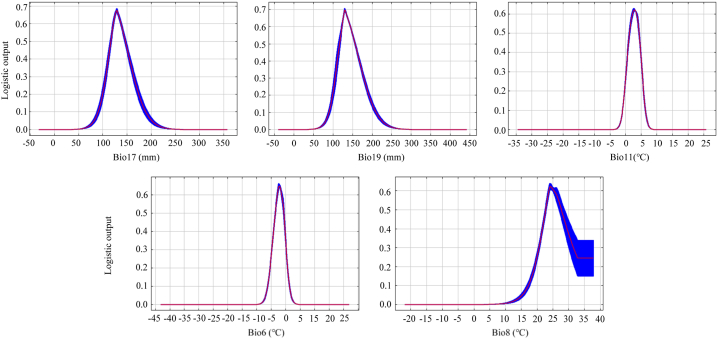
MaxEnt software running results show that the 10% training existence threshold is 0.3. According to the Response curve analysis (Fig. 5), the suitable range of the main climatic factors affecting the distribution of S. subaqualis can be seen as follows: The Precipitation of the driest quarter (Bio17) is 100–175 mm, and the Precipitation of the coldest quarter (Bio19) is 110–180 mm, the Mean air temperature of the coldest quarter (Bio11) is 1–4 °C, the Min air temperature of the coldest month (Bio6) is 5–0 °C, and the Mean air temperature of the wettest quarter (Bio8) is 22 °C–30 °C (the existence probability of S. subaqualis is unchanged after 30 °C).
Fig. 5.
Response curve analysis of main climatic variables of S. subeaqualis.
3.3. Geographical distribution of S. subeaqualis under different climate scenarios
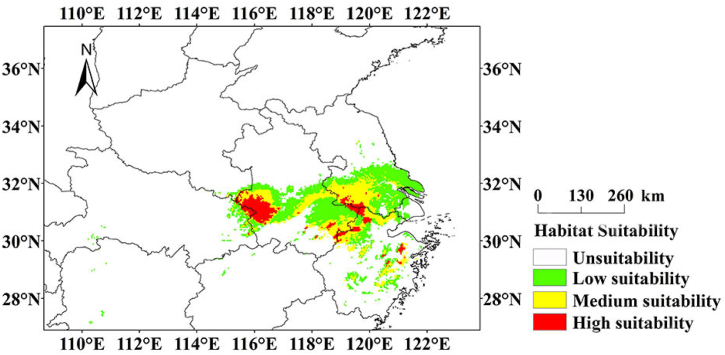
According to the collected information of S. subaqualis, the database of domestic and foreign papers and the data platform of plant specimens, S. subaqualis is mainly distributed in Jiangsu, Anhui, Zhejiang, Henan and Hubei province in China. The prediction results of this model (Fig. 6) shows that the potentially suitable area of S. subaqualis in contemporary times is much larger than the actual collection and distribution range of this species (Figs. 1 and 6). In this period, the total suitable area of S. subaqualis is 11.575 × 104 km2, accounting for 1.21% of the total area of the country (Table 3). Among them, the highly suitable areas are mainly distributed in the Dabie Mountain area of Anhui, the southwestern area of Jiangsu, the junction of Jiangsu and Anhui, the junction of Anhui and Zhejiang, and the scattered areas of Zhejiang, with an area of 1.424 × 104 km2, accounting for 12.30% of the total suitable area. Medium suitability areas cover Dabie Mountain area in the southwest of Anhui, southeast of Anhui, central and southern Jiangsu, and a few areas in Zhejiang. The overall area of moderately suitable areas is about 3.826 × 104 km2, accounting for 33.06% of the total suitable area. Compared with medium and high suitability areas, low suitability areas are wider in southern Henan, northeastern Hubei, central Anhui, central and southeast Jiangsu, and northwest Zhejiang. The total area of low suitability areas is 6.325 × 104 km2, accounting for 54.64% of the total suitability areas. On the whole, the high suitability and moderate suitability areas of S. subaqualis have shown an obvious “island” discontinuous distribution, but the low suitability area connects the moderate suitability area and high suitability area, so the whole suitability area of S. subaqualis has a certain connectivity.
Fig. 6.
Prediction of modern geographical distribution pattern of S. subeaqualis by MaxEnt model.
Table 3.
The change of suitable area of S. subeaqualis in different periods (unit: × 104 km2).
| Period | LGM | MH | Current | SSP245 |
SSP585 |
||
|---|---|---|---|---|---|---|---|
| 2050s | 2090s | 2050s | 2090s | ||||
| Lowly suitable area | 6.885 | 5.847 | 6.325 | 7.278 | 6.262 | 5.660 | 7.469 |
| Modium suitable area | 3.726 | 4.594 | 3.826 | 3.618 | 3.469 | 2.488 | 4.038 |
| Highly suitable area | 2.653 | 1.424 | 1.424 | 1.174 | 1.622 | 1.406 | 1.418 |
| Total suitable area | 13.264 | 11.865 | 11.575 | 12.069 | 11.352 | 9.554 | 12.925 |
| Percentage of total suitable area | 1.382% | 1.236% | 1.206% | 1.257% | 1.183% | 0.995% | 1.346% |
Note: The percentage is the ratio of suitable areas to national land surface area (960 × 104 km2) under different climatic scenarios.
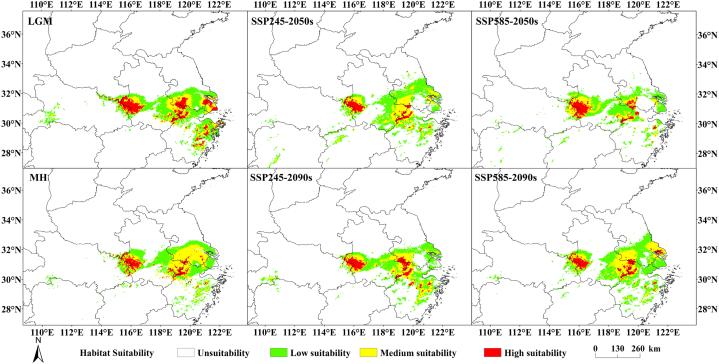
Based on the climate data of four climate emission scenarios, LGM, MH, SSP245 and SSP585, the potential geographical distribution pattern of S. subaqualis under different climate scenarios was simulated using MaxEnt, and the geographical distribution map (Fig. 6) and area (Table 3) of S. subaqualis under different climate scenarios were obtained. The results showed that the geographical design of the distribution area of S. subaqualis for all climatic scenarios was generally consistent and contained the areas of Henan, Jiangsu, Anhui and Zhejiang province. In the LGM, the total suitable area of S. subaqualis increased by 1.689 × 104 km2 compared with the present, among which, the high suitable area and low suitable area increased by 1.229 × 104 km2 and 0.561 × 104 km2, respectively, while the medium suitable area decreased by 0.101 × 104 km2. In the MH, the total suitable area of S. subaqualis was only 0.290 × 104 km2 more than that of the present. The highly suitable area was 1.424 × 104 km2, and the medium suitable area was 0.767 × 104 km2 more than that of the present. The low suitable area was 0.477 × 104 km2 less than the contemporary area. Compared with the current period, under the two future emission scenarios (SSP245 and SSP585), the change of the total adaptation area of S. subaqualis presented two different trends. From contemporary to 2090s, the suitable area of S. subaqualis in SSP545 situation showed an increase and then a decrease, while that in SSP585 situation showed a decrease and then an increase. In the SSP245 climate scenario, the medium area of S. subaqualis broke in 2050 and resumed connectivity in 2090. In the 2050s, the areas of low and high suitable areas of S. subaqualis were both larger than that of the present, while the areas of medium suitable areas were smaller than that of the present. The overall suitable area of 2090s is reduced by 0.222 × 104 km2, but the highly suitable area was increased by 0.198 × 104 km2. In the scenario of SSP585, the suitable area of S. subaqualis in 2050 was greatly reduced, and the reduced area was 2.021 × 104 km2, which was smaller than the contemporary area. In 2090, there was a discontinuity in the suitable area of S. subaqualis, but the total area rose up and was larger than the contemporary area, and the increased part was low and medium suitable area.
3.4. Distribution pattern change of S. subaqualis in future climate scenario
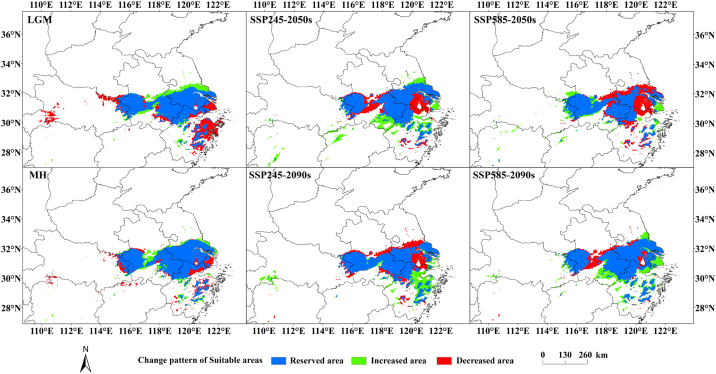
Based on the distribution of potential suitable areas of S. subaqualis under different climate scenarios predicted by MaxEnt (Figs. 6 and 7), the spatial and temporal changes of potential suitable areas of S. subaqualis under different climate scenarios in different periods were obtained by visual processing using ArcGIS14.0 software (Fig. 8). The results (Table 4) show that the change degree of the LGM period is greater, as high as 13.08%, and the loss area is 3.660 × 104 km2. The loss area is mainly in eastern Zhejiang, the junction of Hubei and Henan, and a few areas in northern Anhui and Jiangsu, and the increase area is mainly in Anhui and Jiangsu province. In the MH, the suitable area of S. subaqualis changes little, only by 1.80%. The area of increase and decrease is small, and sporadically appear around the suitable area. Under the SSP245 scenario, the habitat of S. subaqualis increases by 3.359 × 104 km2 in 2050s, with an increase rate of 29.24%, while the area of retains habitat is 8.595 × 104 km2, with a retention rate of 65.14%, and 2.896 × 104 km2 is lost, with a loss rate of 21.95%. And by 2090, the increase area of S. subaqualis decreases and the loss area is the same as in 2050, making the overall still 1.71% less than contemporary. Under the SSP585 scenario, in 2050, the suitable area of S. subaqualis increases by 1.805 × 104 km2, and 3.928 × 104 km2 is lost, making the overall area 16.09% smaller than the contemporary area. But in 2090, S. subaqualis increases by 3.049 × 104 km2, and only lost 1.725 × × 104 km2, making the overall area 10.03% more than the contemporary area.
Fig. 7.
Geographical distribution of S. subeaqualis under different climatic scenarios.
Fig. 8.
Spatial change of geographical distribution pattern of S. subeaqualis under different climate scenarios.
Table 4.
Spatial variation in suitable distribution area of S. subeaqualis in different periods.
| Period |
Area ( × 104 km2) |
Change Rate (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Increase | Reserved | Lost | Change | Increase rate | Reserved rate | Lost rate | Change rate | |
| LGM | 1.934 | 9.534 | 3.660 | −1.726 | 14.66% | 72.26% | 27.74% | −13.08% |
| MH | 1.684 | 9.774 | 1.921 | −0.237 | 14.40% | 74.08% | 14.56% | −1.80% |
| SSP245-2050s | 3.359 | 8.595 | 2.896 | 0.463 | 29.24% | 65.14% | 21.95% | 3.51% |
| SSP245-2090s | 2.615 | 8.656 | 2.841 | −0.226 | 22.74% | 65.61% | 21.53% | −1.71% |
| SSP585-2050s | 1.805 | 7.566 | 3.928 | −2.123 | 15.70% | 57.34% | 29.77% | −16.09% |
| SSP585-2090s | 3.049 | 9.768 | 1.725 | 1.323 | 26.53% | 74.04% | 13.08% | 10.03% |
4. Discussion
4.1. Reliability analysis of model prediction
In this study, the MaxEnt model and ArcGIS software were used to predict and analyze the potential habitat range of the endemic and endangered plant S. subaqualis in China. The present study predicts that S. subaqualis is mainly distributed in southern Henan , central and southern Anhui and Jiangsu province , scattered areas in northwestern and central Zhejiang province, and a few areas in eastern Hubei Province, in which the range of medium and high suitable areas predicted by the results is basically consistent with the known distribution of S. subaqualis [51] and previous predictions [40], which greatly indicates that the results of this study have a high degree of confidence. However, comparing with previous studies, it was found that Ge and Zhang [40] in their study predicted the suitable area of contemporary S. subaqualis to be only 13.305 × 104 km2, which is somewhat different from the 11.575 × 104 km2 predicted in this paper. The reasons for this may be as follows: (1) the difference in the number of species samples retained and involved in modeling can cause different prediction results; (2) the choice of climate models and the difference in accuracy can cause different results; (3) the difference in the choice of climate factors involved in modeling can also affect the prediction results (4) different methods are used to classify the suitable habitat. In addition, the above-mentioned reasons are equally influencing the accuracy of the prediction results of the species distribution models, such as the reliability of the species distribution samples or whether the climate variables involved in the modeling are closely related to the habits of the organisms after screening by correlation analysis. Therefore, in the future research process, the collection of sample sites should be prioritized with reference to existing records and combined with fieldwork, and the modeling should be based on the habitat of species to screen out relevant climatic factors to improve the accuracy of prediction results as much as possible.
In comparison with the available literature, the results of this study showed that the range of suitability of contemporary S. subaqualis was larger, probably due to the fact that only climatic factors were used to discuss the effect of hydrothermal conditions on S. subaqualis in this study. The main reason for using only climatic factors in this study is to investigate the dynamics of the distribution patterns of S. subaqualis caused by different climatic scenarios. Although climatic factors are the main determinants of species distribution at global, continental or regional scales [[52], [53], [54]], the distribution of species can be influenced not only by climate but may be limited by habitat conditions and biological factors [55]. For example, human activity is the most important environmental parameter affecting the distribution of the three Ephedra species [56]. The weak interspecific competition and the strong influence of human activities [[57], [58], [59], [60]] have been shown to greatly constrain the survival space and population size of S. subaqualis. It is expected that subsequent studies will include human activities and more environmental variables to explore the key factors governing the geographical distribution of S. subaqualis more comprehensively and systematically, so as to further improve the accuracy of prediction.
4.2. Analysis of factors restricting the distribution of S. subaqualis
The results showed that the cumulative contribution rate of precipitation factor is 59.6%, and the cumulative contribution rate of temperature factor is 40.4%. The reason for this result may be related to the environmental requirements of S. subaqualis, which is mainly distributed in mountain slopes and gullies and has a great demand for water. For example, the photosynthesis of S. subaqualis is mainly restricted by water conditions. When the soil moisture content was more than 60%, S. subaqualis had the maximum photosynthetic rate [36,61]. In addition, atmospheric relative humidity (RH) was the main factor affecting the net photosynthetic rate of sapling hamamelis in May [62]. Good water conditions are conducive to the accumulation of more organic matter in S. subaqualis, so as to better promote the growth and development of S. subaqualis. At the same time, water also affects the pollination process of S. subaqualis. Its pollen is easily lost by rain, which hinders cross pollination and makes pollination difficult. This also becomes one of the reasons why S. subaqualis is in an endangered state [29]. Among the water factors, the Precipitation of the driest quarter (Bio 17) is the key water factor that restricts the geographical distribution of S. subaqualis. With the decrease of precipitation, the suitability of S. subaqualis is decreasing. S. subaquali is mainly distributed in East China, where the driest season is in winter and spring, and the precipitation in winter and spring is one of the important conditions for the survival of endemic plants in East China [63]. In addition, the phenology showed that the fruit stage of S. subaqualis was about the middle of October, and the seeds popped in the second half of that month. When the winter is too dry, the seeds of most plants that mature in the fall are more susceptible to dehydration and death [64,65]. Therefore, the Precipitation of the driest quarter (Bio 17) was closely related to the successful completion of life history of S. subaqualis. Besides water factor, temperature factor also restricts the distribution of S. subaqualis. The strong cold current attack is not conducive to the growth of the flower buds in the germinating stage, and the appropriate temperature can help to break the dormancy of seeds and to improve the germination rate and germination potential of seeds [66,67]. Previous studies have shown that temperature is an important factor affecting the germination of S. subaqualis seeds, and the optimal germination temperature range is 20–25 °C [68]. Therefore, temperature is also important for the growth of S. subaqualis. It is worth noting that the Mean air temperature of the coldest quarter (Bio11) among the temperature factors has the greatest impact on the distribution of S. subaqualis. This again shows that S. subaqualis has a high demand for water and a certain demand for temperature. Based on the above analysis, it can be concluded that the key factor affecting the distribution of S. subaqualis is the Precipitation of the driest quarter (Bio17), and the temperature factor is the Mean air temperature of the coldest quarter (Bio11), which is consistent with the biological characteristics of S. subaqualis that likes humidity and temperature.
The geographical distribution pattern of plants is often restricted by hydrothermal conditions. Studies have shown that heat affects the distribution of plants in the northern and southern latitudes, and water affects the distribution of vegetation from the coast to the interior [69]. Based on the response curves, we boldly speculate that the Precipitation of the driest quarter (Bio17) limits the growth of S. subaqualis to central Hubei, and the Mean air temperature of the coldest quarter (Bio11) limits the growth of S. subaqualis to northern Jiangsu and southern Zhejiang. These factors may be one of the important reasons for limiting the growth of S. subaqualis in the subtropical area of East China.
4.3. Distribution pattern and change of S. subaqualis in different periods
Since Quaternary, species distribution patterns have been strongly influenced by climate [70]. During the LGM, the global cooling caused the decrease of forest area in different regions, and the forests in the warm temperate zone in eastern China gradually moved south [71]. The results of this study showed that S. subaqualis involved more extensive areas in the southern suitable area of LGM, such as the eastern part of Zhejiang province, than in the contemporary period, indicating that the cold climate affected the suitable range of S. subaqualis to a certain extent. It is worth noting that the suitable area of S. subaqualis in the LGM period was larger than that in the contemporary period, especially the area of high suitability increased by 1.229 × 104 km2, which may be due to the influence of terrain factors. Even if the temperature decreased, the humidity in the subtropical mountain area in the LGM period did not change much, which was conducive to the growth of hygro-loving plants. Studies on plant phylogeography have revealed the existence of climatic refuges for plants to cope with climate change, for example, some areas of Sierra Madre in eastern Mexico are the climatic refuges of Fagus mexicana in LGM, a gene relicent plant that is about to die out [72]. MaxEnt results showed that the highly suitable areas of S. subaqualis were mainly located in the mountainous areas of Jiangsu province, Dabie Mountain area of Anhui province, and West Tianmu Mountain area of Zhejiang province during the LGM period. As one of the highly suitable areas for S. subaqualis, the western Tianmu Mountain in Zhejiang province has an ancient geology, and only the foothills were slightly invaded during the Quaternary glacial period, so the native vegetation was intact. By using AFLP markers and chloroplast DNA (cpDNA) sequences, some scholars have speculated that the western Tianmu mountain area is one of the climate shelters for residual Ginkgo biloba [73]. Compared with the contemporary distribution area of S. subaqualis, the suitability of these mountain areas did not change significantly, indicating that these areas could provide a suitable environment for S. subaqualis population during the glacial period. Therefore, it is speculated that the mountain areas of Jiangsu , Dabie Mountain area of Anhui and western Tianmu Mountain area of Zhejiang province were the sanctuaries of S. subaqualis during LGM.
From LGM to MH, some suitable areas of S. subaqualis had higher latitude. The reason for this phenomenon may be that the climate of the MH was more humid and the precipitation intensity was greater than that of the LGM. In this climate, the vegetation that liked temperature and humidity would migrate northward in a small extent. However, in MH, the suitable area of S. subaqualis was reduced to 11.865 × 104 km2, and the overall suitable area fragmentation was intensified. The reason may be that the increase of precipitation in MH led to the change of the suitable area of S. subaqualis. According to the response curve in the results (Fig. 5), when the precipitation exceeds a certain limit, the distribution of S. subaqualis will be greatly restricted.
As the global climate continues to warm [74,75], plants and animals will gradually migrate to higher altitudes or higher latitudes [76,77] and the potential suitable areas of species will change accordingly. The results of this study showed that the future change trend of S. subaqualis suitable areas were complex and varied, and there was no obvious trend of migration to high latitude and altitude. However, from the perspective of distribution range, the suitable area of SSP245 and SSP585 did not decrease significantly at the end of this century under different scenarios, especially the SSP585-2090s increased by 1.351 × 104 km2 compared with the contemporary period. This situation is different from the results of the study that the distribution range of the large group will be reduced [78]. So why has this happened to S. subaqualis? Studies have shown that certain plants with narrow geographic ranges but relatively wide altitude ranges may be more tolerant to climate change and have the potential to expand [79]. This also suggests that S. subaqualis may migrate to higher altitudes in response to climate change in the future. In addition, small-scale species living in a warm climate environment will continue to benefit even from climate change [80]. S. Subqualis likes warm and humid climate, so it may benefit from climate change.
Taken together the above analysis, from the LGM to the future, the potential distribution pattern of S. subaqualis has a tendency of repeated contraction and expansion.
4.4. Conservation strategies of S. subaqualis
In response to global climate change, countries have actively implemented a series of strategies, measures and actions. There is reason to believe that the future direction of climate change is more likely to be towards SSP245 or even lower emissions. Such a development direction seems to be more conducive to the survival of S. subaqualis. However, according to the study in this paper, under the SSP245 scenario, S. subaqualis still has the status quo of habitat fragmentation. The discontinuity of habitats will lead to the isolation of S. subaqualis populations, limit their species diffusion, hinder gene exchange among populations, and lead to the reduction of genetic diversity, then affect the quantity and quality of individuals in the population, which is not conducive to the evolution of S. subaqualis. The overall degree of fragmentation of contemporary suitable areas is not serious, but the distribution of medium and highly suitable areas has shown an “island”. Therefore, under the current situation, we should take active and effective measures to gradually slow down the adverse effects of climate change on S. subaqualis. In addition, these stable areas should be divided into S. subaqualis germplasm resource conservation area and artificial planting area. This not only strengthens the protection of S. subaqualis germplasm resources, but also helps to improve the quality of cultivation, so that it can give full play to its ecological benefits and scientific research value. At the same time, the corresponding protection policies should be formulated, the protection publicity should be intensified, and the man-made damage should be reduced as far as possible. For S. subaqualis increasing area, it is suggested to make a reasonable and comprehensive land planning, set up S. subaqualis introduction demonstration area, observe the introduction effect in advance, and prepare for the protection of S. subaqualis. For its loss areas such as Anhui and Jiangsu province, S. subaqualis germplasm bank should be established in advance, and the changes of S. subaqualis germplasm resources should be fully investigated, collected and dynamically monitored to avoid the loss of genetic information of S. subaqualis in different provenance areas. In addition to the macro planning, special attention should be paid to the biological characteristics and habitat protection of S. subaqualis. At present, S. subaqualis has problems such as pollination difficulty, low seed setting rate, weak interspecific competitiveness, and vulnerability to pests and diseases. Solving the above problems is also a key link to protecting the germplasm resources of S. subaqualis.
5. Conclusion
-
(1)
In this study, MaxEnt model was used to simulate the potential habitat of S. subaqualis under different climatic backgrounds. The results showed that the main environmental factors affecting the geographical distribution pattern of S. subaqualis were Precipitation of the driest quarter (Bio17), Precipitation of the coldest quarter (Bio19), Mean air temperature of the coldest quarter (Bio11), Min air temperature of the coldest month (Bio6) and Mean air temperature of the wettest quarter (Bio8). Water factor is the main climatic factor restricting the growth and distribution of S. subaqualis.
-
(2)
The potential suitable areas for S. subaqualis are mainly distributed in the junction of Jiangsu, Anhui, Zhejiang and Henan, Hubei and Anhui province under contemporary climatic conditions. From the past to the future, S. subaqualis population decreased to different degrees, but the distribution range remained the same in all periods. During the LGM, The S. subaqualis population includes the great mountains of Anhui and the west of Tianmu mountains of Zhejiang province as glacial refugia. After entering the MH, the suitable area of S. subaqualis undergoes a small northward shift. In the future climate scenario, S. subaqualis may migrate to higher elevations. With the gradual warming of the climate, S. subaqualis population may benefit from climate change, but the problem of habitat fragmentation is still serious and the survival crisis of the population still exists, which may not be conducive to the evolution of S. subaqualis population.
-
(3)
The distribution pattern of S. subaqualis in the future will be retained, increased, and lost. Therefore, when formulating a protection strategy for S. subaqualis against climate change, it is necessary to combine the actual situation and implement regional policies. In addition, the development of germplasm resources protection strategy for S. subaqualis should not only macro planning, but also specific to its biological characteristics.
Author contribution statement
Wenfeng Lai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chenyang Shi: Conceived and designed the experiments; Wrote the paper.
Guowei Wen: Analyzed and interpreted the data.
Zengwei Lü: Performed the experiments; Wrote the paper.
Liqi Ye; Qiuliang Huang; Guofang Zhang: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research was supported by Fujian Science and Technology Plan Project [2022I0008]; East-West Collaborative Project [KH180062A, 11891008004, KH190315A]; Science and Technology Innovation Fund of Fujian Agriculture and Forestry University [CXZX2019046].
Data availability statement
The authors do not have permission to share data.
Additional information
No additional information is available for this paper.
Declaration of interest's statement
The authors declare no conflict of interest.
References
- 1.IPCC . Cambridge University Press; New York: 2014. Climate Change 2014: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 2.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 3.Balazy K., Boehnke R., Trudnowska E., Søreide J.E., Błachowiak-Samołyk K. Phenology of Oithona similis demonstrates that ecological flexibility may be a winning trait in the warming Arctic. Sci. Rep. 2021;11:1–13. doi: 10.1038/S41598-021-98068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arneth A. Climate science: uncertain future for vegetation cover. Nature. 2015;524:44–45. doi: 10.1038/524044a. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Yang Q., Huang W. Analysis of the spatial and temporal changes of NDVI and its driving factors in the Wei and Jing river basins. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph182211863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidel D.J., Qiang F., Randel W.J., Reichler T.J. Widening of the tropical belt in a changing climate. Nat. Geosci. 2008:21–24. doi: 10.1038/ngeo.2007.38. [DOI] [Google Scholar]
- 7.Lesica P. Arctic-alpine plants decline over two decades in glacier national park, Montana, U.S.A. Arctic, Antarctic, Alpine Res. 2018;46:327–332. doi: 10.1657/1938-4246-46.2.327. [DOI] [Google Scholar]
- 8.Román-Palacios C., Wiens J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. U. S. A. 2020;117:4211–4217. doi: 10.1073/pnas.1913007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Zhang Y., Yang X., Chen N., Li S., Wang P., Jiang L. Warming alters plant phylogenetic and functional community structure. J. Ecol. 2020;108:2406–2415. doi: 10.1111/1365-2745.13448. [DOI] [Google Scholar]
- 10.Trew B.T., Maclean I.M. Vulnerability of global biodiversity hotspots to climate change. Global Ecol. Biogeogr. 2021;30:768–783. doi: 10.1111/geb.13272. [DOI] [Google Scholar]
- 11.Akhter S., McDonald M.A., van Breugel P., Sohel S., Kjaer E.D., Mariott R. Habitat distribution modelling to identify areas of high conservation value under climate change for Mangifera sylvatica Roxb. of Bangladesh. Land Use Pol. 2017;60:223–232. doi: 10.1016/j.landusepol.2016.10.027. [DOI] [Google Scholar]
- 12.Guisan A., Zimmermann N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000;135:147–186. doi: 10.1016/S0304-3800(00)00354-9. [DOI] [Google Scholar]
- 13.Padalia H., Srivastava V., Kushwaha S. Modeling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. in India: comparison of MaxEnt and GARP. Ecol. Inf. 2014;22:36–43. doi: 10.1016/j.ecoinf.2014.04.002. [DOI] [Google Scholar]
- 14.Booth T.H., Nix H.A., Busby J.R., Hutchinson M.F., Franklin J. Bioclim: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2013;20:1–9. doi: 10.1111/ddi.12144. [DOI] [Google Scholar]
- 15.Radosavljevic A., Anderson R.P. Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 2014;41:629–643. doi: 10.1111/jbi.12227. [DOI] [Google Scholar]
- 16.Guisan A., Thuiller W., Zimmermann N.E. Cambridge University Press; 2017. Habitat Suitability and Distribution Models: with Applications in R. [Google Scholar]
- 17.Phillips S.J., Anderson R.P., Schapire R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- 18.Pearson R.G., Raxworthy C.J., Nakamura M., Townsend Peterson A. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34:102–117. doi: 10.1111/j.1365-2699.2006.01594.x. [DOI] [Google Scholar]
- 19.Abdelaal M., Fois M., Fenu G., Bacchetta G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inf. 2019;50:68–75. doi: 10.1016/j.ecoinf.2019.01.003. [DOI] [Google Scholar]
- 20.Wang H.H., Wonkka C.L., Treglia M.L., Grant W.E., Smeins F.E., Rogers W.E. Incorporating local-scale variables into distribution models enhances predictability for rare plant species with biological dependencies. Biodivers. Conserv. 2019;28:171–182. doi: 10.1007/s10531-018-1645-4. [DOI] [Google Scholar]
- 21.Nguyen T.T., Gliottone I., Pham M.P. Current and future predicting habitat suitability map of Cunninghamia konishii Hayata using MaxEnt model under climate change in Northern Vietnam. Eur. J. Ecol. 2021;7:1–17. doi: 10.17161/eurojecol.v7i2.15079. [DOI] [Google Scholar]
- 22.Chen K., Wang B., Chen C., Zhou G. MaxEnt modeling to predict the current and future distribution of Pomatosace filicula under climate change scenarios on the Qinghai–Tibet plateau. Plants. 2022;11:670. doi: 10.3390/plants11050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye X., Zhang M., Yang Q., Ye L., Liu Y., Zhang G., Chen S., Lai W., Wen G., Zheng S. Prediction of suitable distribution of a critically endangered plant Glyptostrobus pensilis. Forests. 2022;13:257. doi: 10.3390/f13020257. [DOI] [Google Scholar]
- 24.Sarma K., Roy S.J., Kalita B., Baruah P.S., Bawri A., Nath M.J., Baruah U.D., Sahariah D., Saikia A., Tanti B. Habitat suitability of Gymnocladus assamicus-A critically endangered plant of Arunachal Pradesh, India using machine learning and statistical modeling. Acta Ecol. Sin. 2022;42:398–406. doi: 10.1016/j.chnaes.2022.05.009. [DOI] [Google Scholar]
- 25.Flore of China Parrotia subaequalis. 2022. http://www.iplant.cn/info/Parrotia%20subaequalis?t=r (accessed 20 August 2022)
- 26.Zhang C.Q., Xu B.C. First report of Canker on Chinese Hickory Carya cathayensis) caused by botryosphaeria dothidea in China. Plant Dis. 2011;95 doi: 10.1094/PDIS-05-11-0457. 1319-1319. [DOI] [PubMed] [Google Scholar]
- 27.Wu X.L., Zhou R.H., Duan J.A. Preliminary studies on the chemical constituents in the stem bark of Shaniodendron subaequale (H. T. Chang) M. B. Deng, H. T. Wei et X. Q. Wang. J. Plant Resour. Environ. 1998;4:59–60. (in Chinese) [Google Scholar]
- 28.Sefidi K., Mohadjer M., Etemad V., Copenheaver C.A. Stand characteristics and distribution of a relict population of Persian ironwood (Parrotia persica C.A. Meyer) in northern Iran. Flora. 2011;206:418–422. doi: 10.1016/j.flora.2010.11.005. [DOI] [Google Scholar]
- 29.Deng M., Jin Y., Sheng G., Yang Q. Observation on the growth of flower buds and flowering habit of Shaniodondron subaequale. Chin. J. Appl. Environ. Biol. 1997;3:226–229+300. (in Chinese) [Google Scholar]
- 30.Zhang Y., Shi E., Yang Z., Geng Q., Qiu Y., Wang Z. Development and application of genomic resources in an endangered palaeoendemic tree, Parrotia subaequalis (Hamamelidaceae) from eastern China. Front. Plant Sci. 2018;9:246. doi: 10.3389/fpls.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Li Y., Fang Y. Prediction of potential suitable distribution areas of Quercus fabri in China based on an optimized maxent model. Sci. Silvae Sin. 2018;54(8):153–164. doi: 10.11707/j.1001-7488.20180817. (in Chinese) [DOI] [Google Scholar]
- 32.Qin H., Yang Y., Dong S., He Q., Jia Y., Zhao L., Yu S., Liu H., Liu B., Yan Y., Xiang J., Xia N., Peng H., Li Z., Zhang Z., He X., Yin L., Lin Y., Liu Q., Hou Y., Liu Y., Liu Q., Cao W., Li J., Chen S., Jin X., Gao T., Chen W., Ma H., Geng Y., Jin X., Chang C., Jiang H., Cai L., Zang C., Wu J., Ye J., Lai Y., Liu B., Lin Q., Xue N. Threatened species list of China's higher plants. Biodivers. Sci. 2017;25(7):696–744. doi: 10.17520/biods.2017144. (in Chinese) [DOI] [Google Scholar]
- 33.National Forestry and Grassland Administration of China List of National Key Protected Wild Plants. 2021. https://www.forestry.gov.cn/main/3954/20210908/163949170374051.html (accessed 20 August 2022)
- 34.Zhang G., Xiong T., Sun T., Li K., Shao L. Diversity, distribution, and conservation of rare and endangered plant species in Jiangsu Province. Biodivers. Sci. 2022;30(2) doi: 10.17520/biods.2021335. (in Chinese) [DOI] [Google Scholar]
- 35.Gong B., Xia Y.J., Zhang G.F., Lu Y., Sun G. Population structure and spatial pattern of Parrotia subaequalis, a rare and endangered species endemic to China. J. Ecol. Rural Environ. 2012;28:638–646. [Google Scholar]
- 36.Li W., Zhang G. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora-Morphology, Distribution. Function. Ecol. Plants. 2015;212:10–18. doi: 10.1016/j.flora.2015.02.002. [DOI] [Google Scholar]
- 37.Geng Q., Yao Z., Yang J., He J., Wang D., Wang Z., Liu H. Effect of Yangtze River on population genetic structure of the relict plant Parrotia subaequalis in eastern China. Ecol. Evol. 2015;5:4617–4627. doi: 10.1002/ece3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Zhang M., Hu Y., Zhuang X., Xu W., Li P., Wang Z. Mining and characterization of novel EST-SSR markers of Parrotia subaequalis (Hamamelidaceae) from the first Illumina-based transcriptome datasets. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Jiang J., Nan C., Xue X. ISSR analysis on genetic diversity of endangered plant Parrotia subaequalis in dalonggou of Yixing, Jiangsu. E3S Web Conf. 2020;145 doi: 10.1051/e3sconf/202014501026. [DOI] [Google Scholar]
- 40.Ge Y., Zhang G. Predicting the potential distribution of endangered Parrotia subaequalis in China. Forests. 2022;13:1595. doi: 10.3390/f13101595. [DOI] [Google Scholar]
- 41.Wu T., Lu Y., Fang Y., Xin X., Li L., Li W., Jie W., Zhang J., Liu Y., Zhang L. The Beijing Climate Center climate system model (BCC-CSM): the main progress from CMIP5 to CMIP6. Geosci. Model Dev. (GMD) 2019;12:1573–1600. doi: 10.5194/gmd-12-1573-2019. [DOI] [Google Scholar]
- 42.Chen G., Ling J., Zhang R., Xiao Z., Li C. The MJO from CMIP5 to CMIP6: perspectives from Tracking MJO precipitation. Geophys. Res. Lett. 2022;49 doi: 10.1029/2021GL095241. [DOI] [Google Scholar]
- 43.Lai F. Anqing Normal University; 2022. Preliminary Study on Population Restoration of Endangered Plant Parrotia subaequalis. (in Chinese) [Google Scholar]
- 44.Mahmoodi S., Heydari M., Ahmadi K., Khwarahm N.R., Karami O., Almasieh K., Naderi B., Bernard P., Mosavi A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: implications for ecological conservation and restoration. Ecol. Indicat. 2022;137 doi: 10.1016/j.ecolind.2022.108752. [DOI] [Google Scholar]
- 45.Popp A., Calvin K., Fujimori S., Havlik P., Humpenöder F., Stehfest E., Bodirsky B.L., Dietrich J.P., Doelmann J.C., Gusti M. Land-use futures in the shared socio-economic pathways. Global Environ. Change. 2017;42:331–345. doi: 10.1016/j.gloenvcha.2016.10.002. [DOI] [Google Scholar]
- 46.Zhang Y., Tang J., Ren G., Zhao K., Wang X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-96041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai X., Wu W., Ji L., Tian S., Yang B., Guan B., Wu D. MaxEnt model-based prediction of potential distributions of Parnassiawightiana (Celastraceae) in China. Biodivers. Data J. 2022;10(2022) doi: 10.3897/BDJ.10.e81073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H., Zhao H., Xu C. The potential geographical distribution of Alsophila spinulosain under climate change in China. Chin. J. Ecol. 2021;40(4):968–979. doi: 10.13292/j.1000-4890.202104.022. (in Chinese) [DOI] [Google Scholar]
- 49.Elias W., Sintayehu C.D.W., Arbo B.F., Hadera A.K. Modelling the distribution of Oxytenanthera abyssinica (A. Richard) under changing climate: implications for future dryland ecosystem restoration. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khwarahm N.R. Mapping current and potential future distributions of the oak tree (Quercus aegilops) in the Kurdistan Region, Iraq. Ecol. Process. 2022;9:1–16. doi: 10.1186/s13717-020-00259-0. [DOI] [Google Scholar]
- 51.Hu Y., Fang G., Luo X. Status of Parrotia subaequalis in Taxonomy, reasons for its endangerment and protective measures. Anhui Forest. Sci. Technol. 2011;37(2):46–48. (in Chiense) [Google Scholar]
- 52.Pearson R.G., Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- 53.Hortal J., Rourapascual N., Sanders N.J., Rahbek C., Hortal J., Rourapascual N., Sanders N.J., Rahbek C. Understanding (insect) species distribution across spatial scales. Ecography. 2010;33:51–53. doi: 10.1111/j.1600-0587.2009.06428.x. [DOI] [Google Scholar]
- 54.Soberón J.M. Niche and area of distribution modeling: a population ecology perspective. Ecography. 2010;33:159–167. doi: 10.1111/j.1600-0587.2009.06074.x. [DOI] [Google Scholar]
- 55.Bradie J., Leung B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2007;44:1344–1361. doi: 10.1111/jbi.12894. [DOI] [Google Scholar]
- 56.He P., Li J., Li Y., Xu N., Gao Y., Guo L., Huo T., Peng C., Meng F. Habitat protection and planning for three Ephedra using the MaxEnt and Marxan models. Ecol. Indicat. 2021;133 doi: 10.1016/j.ecolind.2021.108399. [DOI] [Google Scholar]
- 57.Huang S., Fang Y., Peng Z., Yan J., Fang S. The Niche study of Shaniodendron subaequale population of longchi mountain. J. Cent. South Forest. Univ. 2005;25(6):80–83. (in Chinese) [Google Scholar]
- 58.Zhu T., Yue C., Jin S. Ecophysiological trait comparison fo Shaniodendron subaequale and accompanying species. J. Zhejiang For. Coll. 2008;25(2):176–180. (in Chinese) [Google Scholar]
- 59.Zhang G., Yao R., Jiang Y., Chen F., Zhang W. Intraspecific and interspecific competition intensity of Parrotia subaequalis in different habitats from Wanfoshan nature reserve, Anhui province. Chin. J. Ecol. 2016;35(7):1744–1750. doi: 10.13292/j.l000-4890.201607.029. (in Chinese) [DOI] [Google Scholar]
- 60.Liu M., Zhu B., Yuan S., Zhou Z. Pollen morphology of an endangered emdemic species, Parrotia subaequalis. Acta Micropalaeontol. Sin. 2020;37(1):99–104. doi: 10.16087/j.cnki.1000-0674.2020.01.008. (in Chinese) [DOI] [Google Scholar]
- 61.Yue C., Jin S., Chang J., Jiang H. Response of photosynthesis in Shaniodendron subaequale to soil water status. Ann. Bot. Fenn. 2006;43:389–393. [Google Scholar]
- 62.Yan C., Wang Z., An S., Chen S., Wei N., Lu X. Differences in photosynthetic capacity a mong different diameter -classes of Parrotia subaequalis populations and their implications to regeneration limitation. Acta Ecol. Sin. 2008;28(9):4153–4161. doi: 10.1016/j.flora.2015.02.002. (in Chiense) [DOI] [Google Scholar]
- 63.Huang Y., Jacques F., Su T., Ferguson D.K., Tang H., Chen W., Zhou Z. Distribution of Cenozoic plant relicts in China explained by drought in dry season. Sci. Rep. 2015;5:1–7. doi: 10.1038/srep14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts E.H. Predicting the storage life of seeds. Proceedings. 1973;1:499–514. [Google Scholar]
- 65.Zhou Z., Huang J., Ding W. The impact of major geological events on Chinese flora. Biodivers. Sci. 2017;25:123–135. doi: 10.17520/biods.2016120. (in Chinese) [DOI] [Google Scholar]
- 66.Maraghni M., Gorai M., Neffati M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. South Afr. J. Bot. 2010;76:453–459. doi: 10.1016/j.sajb.2010.02.092. [DOI] [Google Scholar]
- 67.Kumar B., Verma S.K., Singh H.P. Effect of temperature on seed germination parameters in Kalmegh (Andrographis paniculata Wall. ex Nees.) Ind. Crop. Prod. 2011;34:1241–1244. doi: 10.1016/j.indcrop.2011.04.008. [DOI] [Google Scholar]
- 68.Hu G., Lu Y., Li H., Yu L., Jin S. Study on seed germination Characters of Parrotia subaequalis. J. Zhejiang Forest. Sci. Technol. 2012;32:48–51. (in Chinese) [Google Scholar]
- 69.Li L., Zhang G., Wang M., Wang J., Zhu J. Structure, spatial pattern and regeneration of Parrotia subaequalis population in Liyang mountainous area of Jiangsu Province. J. Jiangsu Forest. Sci. Technol. 2018;45:16–28. (in Chinese) [Google Scholar]
- 70.Hewitt G.M. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. Biol. Sci. 2004;359(1442):183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison S., Yu G., Takahara H., Prentice I. Diversity of temperate plants in east Asia. Nature. 2001;413:129–130. doi: 10.1038/35093166. [DOI] [PubMed] [Google Scholar]
- 72.Ames-Martínez F.N., Luna-Vega I., Dieringer G., Rodríguez-Ramírez Ernesto C. The effect of climate change on Arcto-Tertiary Mexican beech forests: exploring their past, present, and future distribution. Ecol. Evol. 2022;12:e9228. doi: 10.1002/ece3.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong W., Chen C., Dobeš C., Fu C.X., Koch M.A. Phylogeography of a living fossil: pleistocene glaciations forced Ginkgo biloba L.(Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol. Phylogenet. Evol. 2008;48:1094–1105. doi: 10.1016/j.ympev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Cai W., Borlace S., Lengaigne M., Van Rensch P., Collins M., Vecchi G., Timmermann A., Santoso A., McPhaden M.J., Wu L. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change. 2004;4:111–116. doi: 10.1038/nclimate2100. [DOI] [Google Scholar]
- 75.Patricola C.M., Chang P., Saravanan R. Impact of Atlantic SST and high frequency atmospheric variability on the 1993 and 2008 Midwest floods: regional climate model simulations of extreme climate events. Clim. Change. 2015;129:397–411. doi: 10.1007/s10584-013-0886-1. [DOI] [Google Scholar]
- 76.Parmesan C., Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 77.Lenoir J., Gégout J.C., Marquet P.A., de Ruffray P., Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- 78.Yu F., Wang T., Groen T.A., Skidmore A.K., Yang X., Ma K., Wu Z. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019;659:515–528. doi: 10.1016/j.scitotenv.2018.12.223. [DOI] [PubMed] [Google Scholar]
- 79.Valladares F., Matesanz S., Guilhaumon F., Araújo M.B., Balaguer L., Benito-Garzón M., Cornwell W., Gianoli E., van Kleunen M., Naya D.E. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014;17:1351–1364. doi: 10.1111/ele.12348. [DOI] [PubMed] [Google Scholar]
- 80.Frishkoff L.O., Karp D.S., Flanders J.R., Zook J., Hadly E.A., Daily G.C., M'Gonigle L.K. Climate change and habitat conversion favour the same species. Ecol. Lett. 2016;19:1081–1090. doi: 10.1111/ele.12645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.