Summary
The expression splanchnic vein thrombosis encompasses Budd-Chiari syndrome and portal vein thrombosis. These disorders have common characteristics: they are both rare diseases which can cause portal hypertension and its complications. Budd-Chiari syndrome and portal vein thrombosis in the absence of underlying liver disease share many risk factors, among which myeloproliferative neoplasms represent the most common; a rapid comprehensive work-up for risk factors of thrombosis is needed in these patients. Long-term anticoagulation is indicated in most patients. Portal vein thrombosis can also develop in patients with cirrhosis and in those with porto-sinusoidal vascular liver disease. The presence and nature of underlying liver disease impacts the management of portal vein thrombosis. Indications for anticoagulation in patients with cirrhosis are growing, while transjugular intrahepatic portosystemic shunt is now a second-line option. Due to the rarity of these diseases, studies yielding high-grade evidence are scarce. However, collaborative studies have provided new insight into the management of these patients. This article focuses on the causes, diagnosis, and management of patients with Budd-Chiari syndrome, portal vein thrombosis without underlying liver disease, or cirrhosis with non-malignant portal vein thrombosis.
Keywords: Cavernoma, Portal biliopathy, Direct oral anticoagulants, Portal vein recanalisation, Vascular liver diseases
Abbreviations: BCS, Budd-Chiari syndrome; CALR, calreticulin; DOACs, direct-acting oral anticoagulants; EHPVO, extrahepatic portal vein obstruction; GFR, glomerular filtration rate; JAK2, Janus kinase 2; LMWH, low-molecular-weight heparin; MPN, myeloproliferative neoplasm; MTHFR, methylene-tetrahydrofolate reductase; PNH, paroxysmal nocturnal hemoglobinuria; PVT, portal vein thrombosis; SVT, splanchnic vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt; VKAs, vitamin K antagonists
Key points.
-
•
Patients with Budd-Chiari syndrome or portal vein thrombosis in the absence of underlying liver disease should be systematically screened for myeloproliferative neoplasms.
-
•
The diagnosis of Budd-Chiari syndrome or portal vein thrombosis is suspected using abdominal Doppler ultrasonography and confirmed using contrast-enhanced CT or MRI; liver biopsy is generally not necessary.
-
•
In patients with Budd-Chiari syndrome, long-term anticoagulation is recommended; prompt identification and treatment of the causal factor have a beneficial impact on patient outcomes.
-
•
Spontaneous recanalization is rare in patients with portal vein thrombosis in the absence of underlying liver disease but occurs in ∼40% of patients with cirrhosis.
-
•
In patients with portal vein thrombosis in the absence of underlying liver disease, long-term anticoagulation is generally recommended.
-
•
In patients with portal vein thrombosis in the absence of underlying liver disease, preliminary data suggest that portal vein recanalization with or without TIPS is a safe option for the treatment of refractory complications of portal hypertension or portal cavernoma cholangiopathy when performed in expert centres.
-
•
In patients with cirrhosis and portal vein thrombosis, anticoagulation is recommended for all liver transplant candidates and, among those who are not candidates, for those with recent (<6 months) thrombosis occluding >50% of the lumen of the main portal vein.
-
•
In patients with cirrhosis, TIPS can be considered as the second-line option for the treatment of portal vein thrombosis, especially in case of significant concomitant complications of portal hypertension.
-
•
There is growing evidence demonstrating that DOACs are a safe and effective option, though high-grade evidence is still needed before making strong recommendations on the use of DOACs in patients with splanchnic vein thrombosis.
Introduction
The expression splanchnic vein thrombosis (SVT) encompasses Budd-Chiari syndrome (BCS) and portal vein thrombosis (PVT). PVT can develop in patients without underlying liver disease or affect patients with cirrhosis. BCS and PVT in the absence of underlying liver disease share several similarities. First, they belong to rare disorders since they affect fewer than 1 in 2,000 people in the general population.1 Second, they are commonly associated with risk factors for thrombosis. Third, portal hypertension is a common consequence. PVT can also occur in patients with cirrhosis, where it is usually non-occlusive and found in the absence of symptoms. Risk factors for thrombosis in patients with PVT and cirrhosis are uncommon. However, PVT in cirrhosis carries specific challenges, especially in liver transplant candidates.
The present review focuses on recent findings on the management of BCS and PVT without underlying liver disease or with underlying cirrhosis. PVT occurring in patients with porto-sinusoidal vascular liver disorder will not be discussed here, since it has recently been reviewed elsewhere.2 Management of BCS and PVT is multidisciplinary, with a growing place for interventional radiology. Importantly, besides treating portal hypertension-related complications, managing patients with SVT largely depends on associated risk factors for thrombosis or extrahepatic conditions. Indeed, the prognosis of patients with vascular liver diseases differs according to the causal factors.
Causes of SVT in the absence of underlying liver diseases
Causes for SVT include general risk factors for thrombosis, namely systemic acquired prothrombotic diseases and inherited thrombophilia, and local factors. General risk factors for thrombosis are found in approximately 70% of patients with SVT, while local factors are identified in 20% and 5% of patients with PVT and BCS, respectively.
A combination of two or more genetic or acquired risk factors is found in 26%–46% and 10–23% of patients with BCS or PVT, respectively.[3], [4], [5], [6] Furthermore, 36% of patients with PVT and a local factor also have a general risk factor for thrombosis.5,7 These results justify comprehensive investigations, even when predisposing or precipitating factors have already been identified (Table 1). This work-up should be performed at the diagnosis of SVT since control of some conditions (e.g., myeloproliferative neoplasms [MPNs], Behcet’s disease, paroxysmal nocturnal hemoglobinuria [PNH]) influences patient outcomes.[8], [9], [10], [11]
Table 1.
Prevalence of risk factors for BCS and PVT in the absence of underlying liver disease and proposed diagnostic work-up and specific management (see[4], [5], [6], [7],13,14).
| Condition | Prevalence |
Recommended work-up | |
|---|---|---|---|
| BCS | PVT | ||
| Myeloproliferative neoplasm | 30–57% | 21–25% | Systematic genetic testing of the V617F mutation of the JAK2 gene in all patients. If negative:
|
| JAK2 V617F | 28–45% | 15–21% | |
| CALR mutation | 1–3% | 1–2% | |
| Inherited thrombophilic disorders | Genetic testing for prothrombin G202101A and Factor V Leiden mutationsProtein S activity Protein C activity Antithrombin activity Protein S, C and antithrombin activities should be assessed in the absence of VKAs. Cautious interpretation of impaired liver function |
||
| G20210A prothrombin gene mutation | 12% | 5% | |
| Factor V Leiden mutation | 4% | 8% | |
| Antithrombin deficiency | 3% | 5% | |
| Protein C deficiency | 2% | 1% | |
| Protein S deficiency | 2% | 2% | |
| Acquired thrombophilic disorders | |||
| Antiphospholipid antibody syndrome | 5% | 5% | Lupus anticoagulant, anti-cardiolipin, and anti-beta2 glycoprotein 1 antibody testing Repeat testing after 12 weeks in case of positive testing |
| Paroxysmal nocturnal hemoglobinuria | 10% | 0-0.5% | Flow cytometry analysis |
| Behcet’s disease | 1-2% | Uncommon | No specific testing, clinical diagnosis Suspect Behcet's disease if: male sex, Mediterranean origin IVC stenosis, genital/oral ulcers, deep vein thrombosis in other sites, arterial thrombosis |
| Coeliac disease | 1.4% | 0.7% | Anti-transglutaminase antibody +/- duodenal biopsies |
Other systemic factors
|
Search clinical and/or laboratory features CMV IgM and CMV PCR (blood) |
||
Hormonal factors
|
∼30% | ∼20% | Clinical context Search for introduction/modification of oral contraception within 6 months before diagnosis |
Local factors
|
0-3% | 20 % | CT scan Colonoscopy |
| No identified factor | 10-29% | 15-40% | |
BCS, Budd-Chiari syndrome; CMV, cytomegalovirus; IVC, inferior vena cava; PVT, portal vein thrombosis; VKAs, vitamin K antagonists.
Myeloproliferative neoplasms
MPNs represent the most common risk factor for BCS and PVT in Europe and Asia. In Europe, the reported prevalence of MPNs in patients with BCS and PVT is 30-57% and 21-25%, respectively.[4], [5], [6], [7],[12], [13], [14] In Asia, the prevalence of MPNs among patients with BCS or PVT ranges from 5–28%.15,16 The Janus kinase 2 (JAK2) V617F mutation can be detected in over 90% of patients with SVT and a MPN. Thus, routine screening for the JAK2 V617F mutation should be performed in all patients with SVT. Somatic mutations of the gene encoding calreticulin (CALR) are identified in about 2% of patients with SVT without the JAK2 V617F mutation. CALR mutations should be searched for in patients with spleen height ≥16 cm and a platelet count ≥200x 109/L since 33-56% of patients who meet both these criteria have CALR mutations.6,13,14 MPL exon 10 and JAK2-exon 12 mutations are even less common in patients with SVT.6,10,12 Next-generation sequencing (NGS) could play a role in diagnosing an underlying MPN in patients without JAK2 V617F or CALR mutations.10,17,18 Moreover, in patients with SVT, molecular profiling by NGS carries prognostic information since some molecular risk factors predict the risk of thrombosis recurrence in patients without MPN10 and the risk of haematological transformation and poorer survival in patients with MPN.17 Portal hypertension, by causing hypersplenism and haemodilution, often masks increased blood cell counts and makes the diagnosis of MPN challenging.19 Therefore, given the frequency of MPN in patients with SVT and the consequences of its diagnosis, referral to a haematologist should be systematically considered to discuss NGS and/or bone marrow biopsy (Table 1).
Other acquired prothrombotic disorders
Antiphospholipid antibodies are reportedly present in between 5% and 30% of patients with SVT,[4], [5], [6],13,14 while these antibodies can be found in the absence of antiphospholipid syndrome in up to 5% of healthy individuals.20 A meta-analysis did not show an association between antiphospholipid antibodies and BCS or PVT, except for IgG anticardiolipin antibodies.21 Therefore, if antiphospholipid antibodies are detected at the diagnosis of SVT, repeat testing 12 weeks after the diagnosis is recommended.
The association between Behcet’s disease and SVT mainly concerns BCS and is especially relevant in the Mediterranean population.9,22 Behcet’s disease should be suspected in case of inferior vena cava obstruction, oral and/or genital ulcers, male sex, deep venous thrombosis in other territories, and systemic inflammatory syndrome.9 Early medical therapy, including anticoagulation and immunosuppressive agents, may improve symptoms of BCS (including requirement of invasive treatments) in patients with Behcet’s disease.9
PNH has been observed in up to 10% of patients with BCS.4,23 Conversely, PNH appears extremely rare (less than 2%) among patients with PVT.5,6 Still, systematic screening for PNH is recommended in all patients with SVT since specific therapies, especially eculizumab, have been shown to decrease the recurrence of thrombosis and mortality in this setting.11
In a cohort of 115 Algerian patients with BCS, coeliac disease was found in 11% of patients.24 This association is less common in European countries.4,13 Cytomegalovirus (CMV) disease is another recently highlighted rare (<5%) risk factor for PVT. This association should be suspected in patients with recent PVT displaying features of mononucleosis syndrome. CMV disease should not deter a complete work-up since a general risk factor for thrombosis (especially the G20210A prothrombin gene mutation) was found in 50% of patients with CMV.25 CMV disease does not influence thrombosis extension or recanalization. In addition, acute SVT has been reported in patients with SARS-CoV-2 infection. Although rare, SVT may be severe in this setting, suggesting that patients with SARS-CoV-2 infection and severe gastrointestinal symptoms should be screened for SVT.26 Although rare cases of SVT occurring after COVID-19 vaccination were reported, the risk of thrombosis is higher after SARS-CoV-2 infection than after COVID-19 vaccination.27 Furthermore, since SARS-CoV-2 infection seems to be more frequent and severe in patients with vascular liver diseases than in the general population,28 a history of SVT should not contraindicate COVID-19 vaccination.
Inherited thrombophilia
Factor V Leiden and G20210A prothrombin gene mutations have been reported in 12% and 4% of patients with BCS, respectively. In patients with PVT, the prevalence of Factor V Leiden and G20210A prothrombin gene mutations are 5% and 8%, respectively.6,14 Compared to healthy individuals, the Factor V Leiden mutation is associated with an increased risk of both BCS and PVT, whereas the G20210A prothrombin gene mutationis associated with PVT but not with BCS.29
The prevalence of deficiency in antithrombin, protein C or protein S is 3%, 2% and 2% in BCS, respectively, and 5%, 1% and 2% in those with PVT, respectively.6,14 Diagnosis of inherited deficiencies in antithrombin, protein C, and protein S may be difficult to establish because liver dysfunction can induce a non-specific decrease in these natural anticoagulants, as recently highlighted with antithrombin deficiency.30,31 Although not widely available, genetic testing might be considered in cases of re-thrombosis, family history of deep vein thrombosis or doubtful interpretation of antithrombin, protein C, and protein S concentrations.
Hyperhomocysteinemia and/or homozygous C677T methylene-tetrahydrofolate reductase (MTHFR) gene polymorphisms have been observed in 22% of patients with BCS4 and 11% of those with PVT.5 However, the role of hyperhomocysteinemia as a risk factor for SVT is difficult to assess because homocysteine levels are highly influenced by diet and by vitamin B6, B12, or B9 deficiencies. The role of homozygous C677T MTHFR mutations as a risk factor for BCS seems more relevant in Asia than in Europe.15,32 The prevalence of the C677T MTHFR mutation does not differ between patients with PVT and healthy individuals32
Hormonal factors
Pregnancy and oral contraceptives have been associated with BCS. Up to 74% of western women with BCS have been using oral contraceptive agents and a temporal link between pregnancy and BCS has been described.3,4,33 Local or other general prothrombotic factors are commonly associated with pregnancy or oral contraceptives in women with BCS. Regarding PVT, exposure to female hormones does not appear to cause PVT, as illustrated by the absence of a female predominance among patients with PVT (contrary to BCS).5,34,35
Local factors
Local risk factors for SVT include abdominal surgery and infectious or inflammatory conditions involving splanchnic organs, such as cancer or inflammatory bowel disease5 (Table 1). Certain local factors can be identified on the CT scan performed at SVT diagnosis. In PVT, colonoscopy is recommended to screen for colon cancer or inflammatory bowel disease, although the level of evidence is low.36 PVT associated with alcoholic pancreatitis has some specificity since it does not seem to be favoured by general risk factors for thrombosis, so whether a comprehensive work-up for risk factors of thrombosis is warranted in this setting remains questionable.37 Visceral adipose tissue might also promote PVT. Indeed, in a retrospective case-control study of 79 patients with PVT and 79 healthy individuals, features of the metabolic syndrome were more frequent in those with “idiopathic PVT” (i.e. no risk factor for PVT identified) than in those with either secondary PVT (i.e. risk factor for PVT identified) or healthy individuals. Specifically, increased waist circumference was observed in ∼75% of patients with “idiopathic PVT” vs. ∼30% of patients with either secondary PVT or healthy individuals, pointing to visceral adipose tissue as a potential risk factor for PVT.7 Confirmatory studies addressing the link between visceral obesity and PVT are needed.
In patients with BCS, local factors appear to be rare. However, in countries with a high prevalence of Echinococcus granulosus, liver hydatid cysts have been associated with BCS in up to 4% of patients.24
Budd-Chiari syndrome
BCS is caused by hepatic venous outflow tract obstruction, originating anywhere from the small hepatic veins to the entry of the inferior vena cava into the right atrium. BCS is usually caused by thrombosis. Heart failure, constrictive pericarditis, and sinusoidal obstruction syndrome/veno-occlusive disease are differential diagnoses.
Diagnosis
BCS should be considered in patients with any acute or chronic liver disease. The clinical presentation of BCS varies from asymptomatic (3% of cases) to severe portal hypertension or liver insufficiency. In most patients, clinical manifestations include abdominal pain and ascites.4
The diagnosis of BCS is based on radiological findings on Doppler ultrasonography and contrast-enhanced CT or MRI. Because the diagnosis of BCS can be difficult, radiologists should be experienced and aware of the clinical suspicion of BCS. Radiological features of BCS include (i) direct evidence of obstruction, including solid non-enhancing endoluminal material or transformation of the veins into a cord devoid of flow signal, and (ii) indirect evidence of outflow obstruction, including dilatation of the vein upstream of the obstruction, inter-hepatic venous collateral or inverted venous flow, atrophy/hypertrophy of affected/unaffected segments.38 “Classical” forms of BCS do not require liver biopsy for diagnosis, whereas it can be helpful in the rare cases where the obstruction is located in small hepatic veins and the large hepatic veins remain patent. Variations in the anatomical location of obstruction have been observed between regions: 62% of BCS in Western countries are pure hepatic vein obstruction, whereas membranous obstruction of the inferior vena cava is more common in Asia.39 The difficulty of diagnosing BCS is illustrated by a French epidemiological study where the duration between first clinical manifestations and diagnosis of primary BCS exceeded 6 months in 15% of patients and 1 year in 6% of patients.40
Management
BCS requires referral to centres with expertise in managing patients with vascular liver diseases; access to interventional radiology and liver transplantation is crucial. The approach should be multidisciplinary, including specialists in haemostasis, haematology, diagnostic and interventional radiology, and liver transplantation. For more than 15 years, a stepwise treatment strategy, according to response to previous therapy (from less to more invasive), has been proposed and is now used worldwide.[41], [42], [43] With this strategy, patient outcomes have dramatically improved, with 5-year overall survival ranging from 77% to 87%.[44], [45], [46] Among patients alive at 5 years, BCS is controlled with medical therapy alone in ∼30%, interventional radiology in 35% and liver transplantation in 10%.45 The exact timings for treatment escalation have not been defined. Improvement (with no need for further intervention) is characterised by the presence of a combination of several of the following features: decreasing rate of ascites formation, decreasing serum bilirubin, decreasing serum creatinine and decreasing international normalised ratio (INR) (or increasing factor V in patients receiving vitamin K antagonists [VKAs]).43 Several prognostic indices have been proposed for all patients with BCS (Child-Pugh score, model for end-stage liver disease (MELD), Clichy prognostic index, Rotterdam BCS index, New Clichy prognostic index) and one for patients with BCS in whom transjugular intrahepatic portosystemic shunt (TIPS) is being considered as a therapeutic option (BCS-TIPS). These prognostic indices are accurate to assess transplant-free survival and invasive therapy-free survival. However, because they were derived from retrospective cohorts collected over several decades (with drastically different therapeutic options and outcomes) and because they exhibited low prognostic accuracy in most recent studies, they are considered insufficiently accurate to guide the management of individual patients with BCS.45,[47], [48], [49]
Medical therapy
Prompt identification of MPN, PNH, or Behcet’s disease associated with BCS is essential since targeting the underlying condition positively influences patient outcomes.[8], [9], [10], [11] Anticoagulation should be started at BCS diagnosis, even in the absence of an identified prothrombotic disorder. Despite the absence of a randomised study comparing anticoagulation vs. no treatment, long-term anticoagulation is currently recommended for all patients with BCS based on survival vs. historical comparators.50 Only a few cases of anticoagulation interruption have been described in patients with BCS in whom the prothrombotic factor was treated.3,51 However, there is no clear or sufficient argument currently to stop anticoagulation once BCS is stabilised and the causal factor adequately treated. Because of a high rate of heparin-induced thrombocytopenia, mainly observed with unfractionated heparin (15%), low-molecular-weight heparin (LMWH) is currently recommended.41,43,52,53 LMWH is usually substituted with VKA in patients with stable disease, with a target INR between 2 and 3. Although experience is limited, direct oral anticoagulants (DOACs) seem safe and effective in patients with BCS, but larger prospective studies are needed54,55 (Table 2). A recent retrospective case-control study found that dabigatran was associated with similar stent patency and complication rates after endovascular intervention as VKAs.56 DOACs are currently not recommended in patients with antiphospholipid syndrome as they have been associated with an increased risk of recurrent arterial thrombosis.57 Ascites, gastrointestinal bleeding, infections, renal failure, and encephalopathy should be treated as recommended for patients with cirrhosis, due to an absence of specific data in the BCS population. Severe bleeding related to paracentesis has been reported in patients with BCS receiving anticoagulation. Thus, a brief interruption of anticoagulation could be considered before paracentesis.58
Table 2.
Considerations for the choice of anticoagulant to treat splanchnic vein thrombosis and suggestion of doses (adapted from149).
| Considerations | Low-molecular-weight heparin | Vitamin K antagonists | Direct oral anticoagulants |
|||
|---|---|---|---|---|---|---|
| Apixaban | Rivaroxaban | Edoxaban | Dabigatran | |||
| Liver function | ||||||
| Child-Pugh class A | No action needed | No action needed Target INR 2-3 |
No action needed 5 mg twice a day |
No action needed 20 mg once a day |
No action needed 60 mg once a day |
No action needed 150 mg twice a day |
| Child-Pugh class B | No action needed | Possible Target INR 2-3 |
Use with caution 2.5 mg twice a day |
Use with caution 15 mg once a day |
Use with caution 30 mg once daily |
Use with caution 110 mg twice a day |
| Child-Pugh class C |
No action needed |
Possible Target INR 2-3 |
Contraindicated |
Contraindicated |
Contraindicated |
Contraindicated |
| Renal function | ||||||
| eGFR 30-50 ml/min | No action needed | Possible Target INR 2-3 |
No action needed 5 mg twice a day |
Use with caution 15 mg once a day |
Use with caution 30 mg once daily |
Use with caution 110 mg twice a day |
| eGFR <30 ml/min | Use with caution | Possible Target INR 2-3 |
Use with caution 2.5 mg twice a day |
Use with caution 15 mg once a day |
Use with caution 30 mg once daily |
Contraindicated |
| eGFR <15 ml/min |
Contraindicated |
Possible Target INR 2-3 |
Contraindicated |
Contraindicated |
Contraindicated |
Contraindicated |
| Other considerations | ||||||
| Drug-drug interaction Other medication with P-gp protein or Cytochrome 3A4 metabolism |
No action needed | No action needed Target INR 2-3 |
No action needed | Use with caution 15 mg once a day |
No action needed | Use with caution 110 mg twice a day |
| History of peptic ulcer disease with or without gastrointestinal bleeding | No action needed | Use with caution Consider 15 mg once a day |
No action needed | Use with caution Consider 110 mg twice a day |
||
| Specific consideration | Monitoring may be difficult in patients with liver insufficiency Factor II concentration may be useful in this setting |
Contraindicated in patients with antiphospholipid syndrome No data on pharmacokinetics after TIPS placement | ||||
eGFR, estimated glomerular filtration rate; INR, international normalised ratio; P-gp, Permeability glycoprotein; TIPS, transjugular intrahepatic portosystemic shunt.
Interventional radiology
Short-length hepatic vein stenosis should be systematically searched for to re-establish the physiological drainage of portal and sinusoidal blood. When identified, i.e. in ∼15% of patients, percutaneous transluminal angioplasty of accessible stenosis should be performed as this procedure is associated with good efficacy and low morbidity.39,41,44,45,59 In a recent Chinese randomised-controlled trial including 88 patients with BCS and short-length stenosis, stent placement improved hepatic vein patency and reduced symptom recurrence over percutaneous transluminal angioplasty alone.60
TIPS has become the standard of care for patients with BCS and incomplete response to medical therapy and/or angioplasty.45,46,48 TIPS is currently needed in 40% of patients with BCS, persistent ascites being the most common indication.45 The technical success rate exceeds 90% in expert centres, with 10-year liver transplantation-free survival rates reaching 76%. Long-term anticoagulation should be maintained after TIPS placement.44 TIPS dysfunction occurs in 42% and was mostly due to re-thrombosis of the stent which can be managed with TIPS revision, leading to a secondary patency rate close to 100%. Late hepatic encephalopathy, which is mostly transient and easily medically controlled with no reappearance, occurs at an incidence of 25% in these patients.61
Surgery
Surgical portosystemic shunt placement for BCS has now been almost completely abandoned because of high perioperative mortality, averaging 25%,62 and a high rate of shunt dysfunction due to early or late thrombosis or late stenosis, reaching 30% in series with long-term follow-up.63
Liver transplantation
Despite medical therapy and interventional radiology, liver transplantation is necessary for ∼10% of patients with BCS. Previous TIPS does not compromise the results of liver transplantation.64
Specific issues
Pregnancy
Pregnancy is not contraindicated in women with controlled BCS. Three retrospectives studies, including 55 pregnancies completed between 1985 and 2015, reported no maternal death.[65], [66], [67] Liver-related complications were rare in women with BCS that had been diagnosed and treated before pregnancy. Bleeding events occurred in women receiving anticoagulation and were unrelated to portal hypertension. The reported rate of miscarriages or ectopic pregnancies before the 20th week of pregnancy was about 30%, higher than in healthy women of similar age. On the other hand, after 20 weeks of pregnancy, 93% of children were healthy, but the prematurity rate was high. Practical management of pregnancy in women with vascular liver diseases is described in Table 3. All VKAs must be switched to LMWH before the 6th week of gestation as VKAs cross the placenta and can cause foetal haemorrhage and foetal VKA syndrome, especially between 6 to 12 weeks of gestation.68
Table 3.
Practical management of pregnancy in patients with vascular liver diseases.
| Advice | |
|---|---|
| Before conception | Early counselling should always be proposed before conception. Pregnancy should be planned when the liver disease and the prothrombotic condition are well-controlled. Cytoreductive treatments for myeloproliferative neoplasms should be stopped before conception as they are teratogenic. |
| Anticoagulation | VKAs must be switched to LMWH before the 6th week of amenorrhea, as they cross placenta and can cause foetal warfarin syndrome or warfarin embryopathy. LMWH are then continued during the whole pregnancy. DOACs are contraindicated during the whole pregnancy. |
| Portal hypertension | Gastroesophageal varices should ideally be investigated in the year before conception or during the second trimester of pregnancy. Variceal haemorrhage occurring during pregnancy should be prevented and managed as in non-pregnant patients. |
| Delivery | Vaginal delivery should be preferred even in case of portal hypertension. Caesarean section reserved only for obstetrical indications. Platelet count >20 × 109/L and >50 × 109/L considered as safe for vaginal delivery and caesarean section respectively. Platelet counts >75 × 109/L are considered safe for epidural anaesthesia and over 50 × 109/L for spinal anaesthesia. Stop anticoagulation therapy 24 h before epidural analgesia |
| Post-partum | Oestrogen-derived oral contraceptives are contraindicated. Breastfeeding is possible with beta-blocker therapy and warfarin but not with other VKA molecules or DOACs. |
DOACs, direct oral anticoagulants, LMWH, low-molecular-weight heparin; VKAs, vitamin K antagonists.
Liver nodules
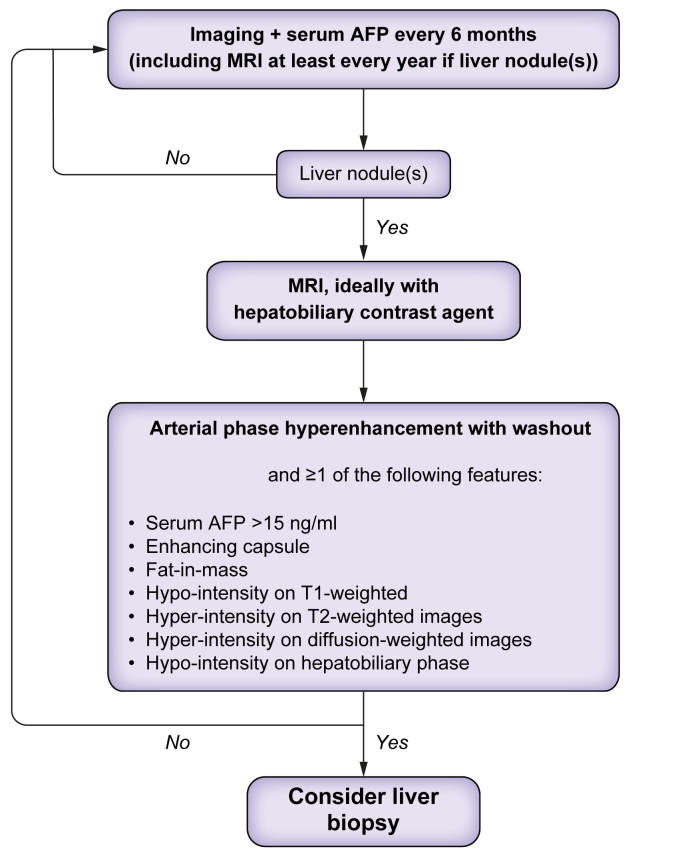
An early decrease in portal perfusion associated with a compensatory increase in hepatic arterial perfusion is thought to contribute to the development of liver nodules in chronic BCS. Most liver nodules are benign regenerative nodules, also called focal nodular hyperplasia-like nodules, but hepatocellular adenomas or hepatocellular carcinoma (HCC) can also arise.[69], [70], [71], [72], [73] The cumulative incidence of HCC is about 4%,69,74 which is similar to that reported in other chronic liver diseases; so, like for cirrhosis, screening for liver nodules every 6 months can be proposed.43 When liver nodules are detected, MRI is the imaging procedure of choice, ideally with the injection of hepatobiliary contrast agent, to distinguish malignant from benign nodules. The vast majority of nodules show arterial phase hyper-enhancement.73,75 Washout is observed in 75% of HCC vs. 29% of benign lesions and is thus not specific for HCC. Additional features are helpful to differentiate benign nodules from HCC, including serum alpha-fetoprotein >15 ng/ml, fat content, capsule, hypo-intensity on T1-weighted sequence, hyper-intensity on T2-weighted sequence, hyper-intensity on high b value diffusion-weighted imaging, or hypo-intensity on hepatobiliary phase.69,73,75 The combination of a hyper-enhanced nodule with washout and one of these features reaches a specificity of between 88% and 100% for HCC.73 A liver biopsy is required in case of suspicion of HCC (Fig. 1). Management of hepatocellular adenoma and HCC should be discussed on a case-by-case basis in specialised centres. Ablation, transarterial chemoembolisation, and liver transplantation can be considered.69,70
Fig. 1.
Management of liver nodules in patients with Budd-Chiari syndrome. AFP, alpha-fetoprotein.
PVT in patients without underlying cirrhosis
Diagnosis
Recent PVT refers to the recent formation (<6 months) of a thrombus within the portal vein and/or its branches and/or radicles. Abdominal pain is the most frequent clinical feature (91%); high leukocyte count and C-reactive protein levels are common.5 Conversely, signs of peritoneal irritation, organ failure, clinical ascites, and/or high lactate levels are rare and should raise suspicion of PVT complicated with intestinal necrosis, i.e. a complication requiring emergency surgery (see below).76
The diagnosis of recent PVT is based on imaging. Ultrasound coupled with Doppler is usually the first-line approach, allowing for the direct detection of the thrombus in the portal vein and the absence of flow in case of complete PVT. Contrast-enhanced CT or MRI is recommended to (i) confirm the diagnosis of PVT, showing a hyperattenuating (hyperintense) thrombus on unenhanced CT (MRI) and a lack of enhancement of the lumen in the contrast-enhanced portal venous phase; enlargement of the portal vein can be observed when PVT is complete; (ii) determine the extension of the thrombus to splenic and mesenteric veins; (iii) identify potential local factors; and (iv) search for complications including signs of acute mesenteric ischaemia and intestinal necrosis.77 Bowel wall thickening, mesenteric fat stranding, and ascites are common both in patients without acute mesenteric ischaemia and those with acute mesenteric ischaemia and intestinal necrosis.76,78 By contrast, decreased bowel wall enhancement (especially of the mucosa) is more suggestive of intestinal necrosis.76
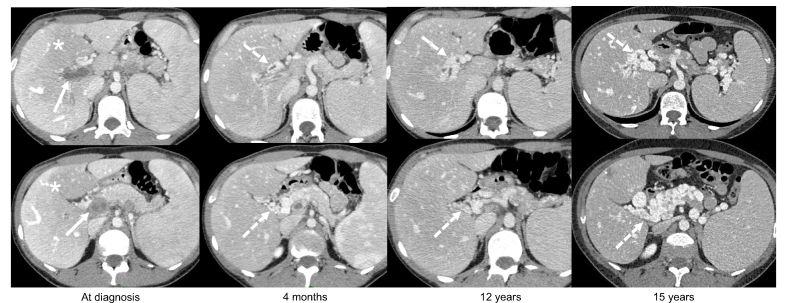
Chronic extrahepatic portal vein obstruction (EHPVO) in the absence of underlying liver disease refers to incomplete resolution of the portal vein obstruction 6 months after recent PVT or to portal cavernoma (Fig. 2). Portal cavernoma is a network of porto-portal collaterals that develop following portal vein obstruction. Obstruction leading to cavernoma is mainly related to thrombosis in adults and is less likely in children and young adults.43 The main signs of EHPVO include gastroesophageal varices (80%), splenomegaly (70%), and thrombocytopenia (30%).79 The diagnosis of EHPVO is based on an inability to visualise the portal vein on contrast-enhanced CT or MRI, which is usually associated with cavernoma enhancement after contrast injection.80 A liver biopsy is needed when an underlying chronic liver disease (cirrhosis or porto-sinusoidal vascular liver disorder) is suspected based on abnormal liver morphology and/or increased liver stiffness measurement. Liver stiffness measurement <10 kPa can rule-out underlying cirrhosis in this setting.81
Fig. 2.
Example of acute portal vein thrombosis with progressive development of a cavernous transformation. A female patient presented with abdominal pain. Contrast-enhanced CT (all portal venous phase, axial view) showed a complete occlusion of an enlarged portal trunk and intrahepatic portal branches by non-enhancing and hypoattenuating material (arrow) consistent with an acute portal vein thrombosis. Note the heterogeneous enhancement of the hepatic parenchyma with central hypoenhancement relative to the liver periphery (zonal perfusion, ∗). Over time, CT shows the progressive development of numerous tortuous veins in the hepatic pedicle and the hepatic hilum corresponding to a cavernous transformation of the portal vein (dashed arrows). Note the absence of recanalization of the portal veins and the progressive enlargement of the cavernoma, with progressive extension to the pancreas. No bile duct dilatation was noted.
Management
Medical therapy
In patients with recent PVT, immediate initiation of anticoagulation is recommended because it has been associated with the prevention of thrombus extension and a reduction in the incidence of intestinal infarction to only 2%, compared with 30% in patients not receiving anticoagulants.5,82 In a study on 67 patients with acute mesenteric ischaemia (arterial and venous), administration of oral antibiotics (gentamicin 80 mg/day + metronidazole 1.5 g/day) was associated with a decreased incidence of intestinal necrosis.83
Recanalization of the thrombosed veins is achieved in ∼30% of patients treated with anticoagulation and takes place within the first 6 months of therapy.5,84,85 Spontaneous recanalization is uncommon.84 Factors associated with recanalization include the site of thrombosis (splenic or superior mesenteric veins having a higher rate of recanalization than the main portal vein) and early anticoagulation (<15 days after first symptoms).5,84,86 By contrast, in patients with recent PVT, ascites, an occluded splenic vein, and underlying prothrombotic disorders have been associated with failure to recanalize the portal vein.5 Accordingly, anticoagulants should be given for at least 6 months in patients with recent PVT.43 In most studies, unfractionated heparin or LMWH were used initially, before being substituted for VKAs, with a target INR between 2 and 3.5,35,86 Unfractionated heparin should be avoided because of the high risk (up to 20%) of heparin-induced thrombocytopenia, especially in patients with MPNs.43,52 Although mostly derived from small retrospective unselected cohort studies, DOACs are now part of the therapeutic arsenal in patients with recent PVT. Despite the absence of direct comparison between LMWH or VKA and DOACs, the rates of PVT recanalization seem similar, without a higher risk of bleeding in patients receiving DOACs. Dedicated studies are still needed to assess the efficacy and safety of each DOAC in patients with PVT in the absence of underlying liver disease. The choice of anticoagulant therapy in PVT should be individualised, considering comorbidities and risk factors for PVT (Table 2).
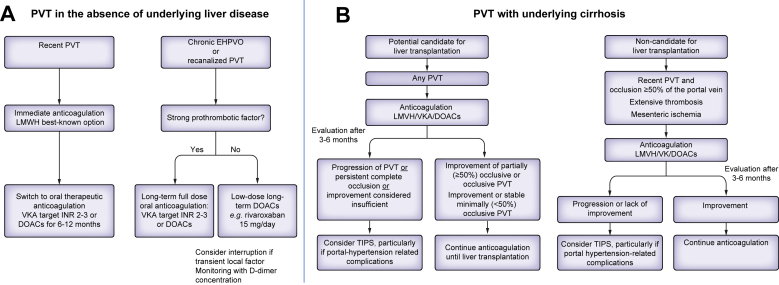
In patients with past PVT (i.e. who achieved recanalization after 6 months of anticoagulation) or in patients with chronic EHPVO, long-term anticoagulation is indicated in most cases since it decreases the incidence of recurrent thrombosis.87,88 Recently, the Baveno VII consensus conference recommended adapting the dosage of anticoagulants according to the aetiologic work-up (grade B recommendation). In patients with a permanent and strong risk factor for thrombosis (MPN, PNH, Behcet’s diseases, antiphospholipid syndrome, personal or first degree familial history of spontaneous venous thrombosis or a history of intestinal necrosis due to mesenteric ischaemia), full-dose long-term oral anticoagulation should be maintained.42,43 VKAs targeting an INR between 2 and 3 have long been used in this situation.43 Although solid data are still lacking, DOACs at therapeutic dosage (e.g., rivaroxaban 20 mg once a day or apixaban 5 mg twice a day) appear an attractive alternative in patients without antiphospholipid syndrome.57,87 In patients without underlying permanent and strong risk factors for thrombosis, the recent RIPORT randomised-controlled trial found that rivaroxaban at the dose of 15 mg per day decreased the incidence of recurrent thrombosis from 19/100 patient-years to 0/100 patient-years. Of note, in this trial, plasma D-dimer concentrations <500 ng/ml 1 month after interruption of anticoagulation were predictive of a low risk of recurrence.88 Furthermore, recurrence of thrombosis was uncommon in patients with an isolated transient local factor for PVT. Therefore, in patients with no general risk factor for thrombosis and a transient local factor, anticoagulation might be discontinued, with D-dimer monitoring 1 month later to determine whether or not to resume anticoagulation43 (Fig. 3A).
Fig. 3.
Proposed algorithm for anticoagulant therapy in patients with portal vein thrombosis with and without cirrhosis. DOACs, direct-acting oral anticoagulants; EHPVO, extrahepatic portal vein obstruction; LMWH, low-molecular-weight heparin; PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt; VKAs, vitamin K antagonists.
In patients with recent PVT or chronic EHPVO, endoscopy should be performed within the first months after diagnosis to screen for gastroesophageal varices. In patients with recent PVT, varices mainly develop during the first year of follow-up,85 so endoscopy should be repeated 1 year after the diagnosis of PVT.43 Non-invasive methods, including transient elastography, are not accurate to rule-out large varices.81 In patients with chronic EHPVO, a study on 178 patients showed that the course of gastroesophageal varices is similar to that in patients with cirrhosis. Therefore, primary prophylaxis is usually based on non-selective beta-blockers or endoscopic band ligation, and secondary prophylaxis on the combination of both.79 A study of 471 endoscopies showed that endoscopic band ligation is safe in patients with EHPVO treated with VKAs.43,89 This suggests that oral anticoagulation can be maintained in patients undergoing scheduled endoscopic variceal band ligation.
Radiology
In patients with recent PVT, invasive strategies including transjugular or transhepatic thrombus aspiration, local fibrinolysis, and/or TIPS have been proposed in the first weeks after the PVT diagnosis in highly selected patients, namely those with PVT and extension to the superior mesenteric vein, particularly when features predictive of intestinal necrosis are present.90,91 However, further studies are needed to clarify indications and patient outcomes.
In patients with chronic EHPVO, portal vein recanalization with or without TIPS can be considered in patients with portal hypertension-related bleeding not controlled with endoscopic treatment or in patients with symptomatic portal cavernoma cholangiopathy. Various approaches to shunt placement have been reported, including transjugular, transhepatic and trans-splenic. This procedure is technically successful in more than 80% of cases when performed in expert centres if intrahepatic portal branches are patent.92 The usefulness of long-term anticoagulation after portal vein recanalization is unknown. Maintaining anticoagulation seems reasonable, especially in patients with risk factors for thrombosis. The clinical outcome seems favourable. Recent data suggest successful portal vein recanalization is associated with increased muscle mass and decreased spleen volume.[92], [93], [94], [95] However, these results are mainly based on small, retrospective, mostly single-centre, unselected cohort studies. Dedicated comparative studies are needed to evaluate the impact of portal vein recanalization on long-term outcome and to determine the best technical approach.
Surgery
In patients with acute mesenteric ischaemia and signs suggestive of intestinal necrosis, emergency surgery is indicated to assess bowel viability. Patients should be transferred to a referral centre to enable multidisciplinary management.43 Shunt surgery to treat portal hypertension-related complications is increasingly being replaced by radiological portal vein recanalization.
Specific issues
Pregnancy
Pregnancy is not contraindicated in women with stable chronic EHPVO. The best information stems from three series of patients, two Indian and one European, including 104 pregnancies.33,[96], [97], [98] Anticoagulation was administered on a case-by-case basis. Rates of miscarriage and preterm birth were 14% and 14%, respectively. Foetal and maternal outcomes were favourable for most pregnancies. Only five episodes of variceal haemorrhage were reported, including three among patients without adequate prophylaxis for portal hypertension-related bleeding. This highlights the importance of upper gastrointestinal endoscopy before conception or during the second trimester of pregnancy in women not receiving beta-blockers (Table 2). Thrombotic events occurred in two patients.
Portal cavernoma cholangiopathy
‘‘Portal cavernoma cholangiopathy’’ refers to abnormalities of the biliary tract in patients with chronic EHPVO.99 These abnormalities are due to the pressure of dilated collaterals on the bile ducts or their lumen and ischaemic damage to the biliary tree.100,101 Magnetic resonance cholangiography coupled with MR angiography is the reference technique for diagnosing portal cavernoma cholangiopathy.100,102 MR cholangiography shows bile duct changes, including stenoses, upstream dilatation, and irregularities in the calibre of bile ducts; MR angiography shows cavernomatous veins in the vicinity of stenoses.102,103 At MRI, bile duct changes are common (found in 80% of patients).[102], [103], [104] However, the clinical impact is much more limited than morphologic changes: moderate liver enzyme abnormalities are observed in 50% of patients;103 severe biliary complications, including cholangitis, pancreatitis, jaundice, and pruritus, occur in ∼10% of patients.[102], [103], [104] Such complications were described only in patients with grade III cholangiopathy (namely strictures with dilations), in whom the risk was 41%.104
Treatment of portal cavernoma cholangiopathy should be discussed on an individual basis at referral centres. Specific treatments of portal cavernoma cholangiopathy include endoscopic retrograde cholangiopancreatography and portal vein recanalization. These treatments should be considered only in patients with cholangitis, pancreatitis, jaundice, or pruritus. Endoscopic retrograde cholangiopancreatography enables bile stone extraction and temporary stenting of biliary strictures.100 The risk of bleeding from bile duct varices should be kept in mind. Treatment with ursodeoxycholic acid following endoscopic therapy was associated with a reduction of symptoms in ∼50% of patients.102,104 Portal vein recanalization may also be helpful in this setting.95 Bilio-enteric by-pass in the absence of portal decompression is not recommended because it is associated with high morbidity and mortality.100
Non-malignant portal vein thrombosis in patients with cirrhosis
Cirrhosis accounts for ∼40% of unselected cases of PVT.35 The prevalence of PVT increases in parallel with the severity of cirrhosis: 10% in patients with compensated cirrhosis, 17% in patients with decompensated cirrhosis, and up to 26% in liver transplant candidates.105 Longitudinal studies reported an incidence of PVT ranging from 11% at 5 years in patients with compensated cirrhosis106 to 24% per year in patients awaiting liver transplantation.105,107
Causes
In patients with cirrhosis and PVT, systematic screening for factor V Leiden and G20210A prothrombin gene mutations is not systematically recommended because their prevalence is low in this setting.29,108,109 Features of metabolic syndrome, including higher body mass index,106 obesity110 and diabetes110,111 have been associated with the occurrence of PVT, although the impact of these features on PVT development might be limited. Local risk factors, such as abdominal surgery, have also been reported.112
The main risk factors for PVT are features reflecting the severity of portal hypertension, including large oesophageal varices and low platelet count.106,108,111,113 Low portal vein flow velocity (<15 cm/s) has also been reported in several large studies, although not systematically.106,108,113 The link between non-selective beta-blockers and PVT occurrence remains debated.108,114,115 The only study to include a time-dependent analysis of the association between beta-blockers and PVT occurrence did not find any association.108
Diagnosis
In patients with cirrhosis, PVT is usually found either in the absence of symptoms during HCC screening or following a decompensating event.43,106 Although PVT is generally recognised on ultrasound, CT or MR angiography are recommended to assess the degree and extent of obstruction. In contrast to findings in patients without cirrhosis, non-occlusive PVT is the predominant form in patients with cirrhosis, accounting for around 70% of cases.105 Recent recommendations concur regarding the need to provide information on the following characteristics as part of the description of PVT: time course (recent, i.e. <6 months, or chronic, i.e. ≥6 months), degree of occlusion (minimally occlusive, i.e. <50% of the lumen obstructed, partially occlusive, i.e. ≥50% of the lumen obstructed, or completely occlusive, i.e. no persistent lumen), and response to treatment or interval change (progression, stability or regression).42,43 Patients with HCC are at risk of tumoral invasion of the portal vein but also at higher risk of non-tumoral PVT since HCC may tilt the haemostatic balance towards hypercoagulability.116
The presence of three or more features among serum alpha-fetoprotein concentrations >1,000 μg/L, venous expansion, thrombus enhancement at arterial phase, neovascularity, and PVT adjacent to HCC have a 100% sensitivity and 94% specificity for the diagnosis of tumoral invasion of the portal vein.117 In the absence of these specific features, fine needle ultrasound-guided biopsy of the thrombi may be safe and useful to obtain a definitive diagnosis.118
Natural history of PVT and impact on patient outcomes
Spontaneous regression or even recanalization of PVT may occur in ∼40% of patients, mainly in those with non-occlusive PVT and compensated cirrhosis.106,119,120 By contrast, the rate of spontaneous recanalization is very low in patients with occlusive PVT.121 In patients with cirrhosis, with recent PVT and extension to the superior mesenteric vein, acute mesenteric ischaemia or intestinal necrosis can occur, though they are uncommon in this context.122
The link between PVT and decompensation of cirrhosis has been analysed in several studies,[123], [124], [125] including a large prospective study in over 1,200 patients with compensated cirrhosis: occurrence of PVT was not associated with subsequent decompensation of cirrhosis,106 suggesting that PVT is a marker but not a direct cause of the progression of liver disease. By contrast, the impact of PVT in the context of liver transplantation is significant. Pre-transplantation PVT has been associated with increased short-term mortality and graft failure after transplantation in large databases.126 However, in the latter studies, the link between the size and extent of PVT and post-transplant outcomes was not evaluated. The impact of PVT appears to depend on the possibility of restoring physiologic portal perfusion to the graft using anatomical (physiological) procedures. Good results are obtained when the superior mesenteric vein is patent. By contrast, an irremovable thrombus occluding the superior mesenteric vein requires left renal to portal vein anastomosis, or portocaval hemi-transposition. These interventions that do not relieve portal hypertension and do not restore graft perfusion with portal blood are associated with a high rate of failure or complications.127 Thus, persistent complete extensive thrombosis may preclude liver transplantation.
Management
Medical therapy
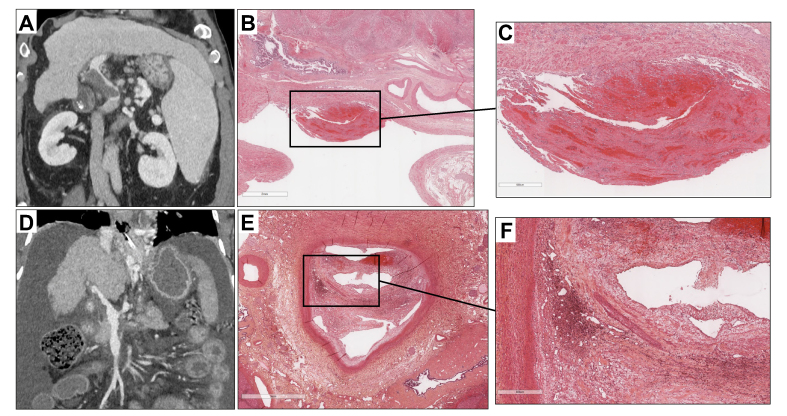
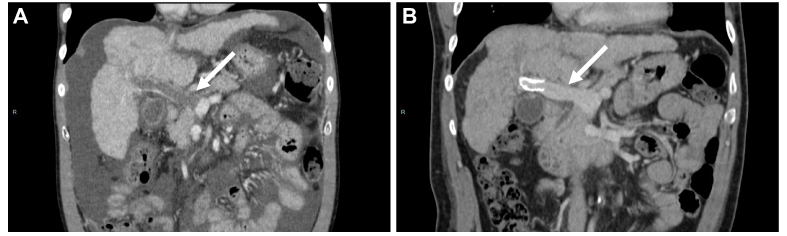
In patients with cirrhosis and PVT who are potential candidates for liver transplantation – meaning those without a definitive contraindication to liver transplantation – the main objective of anticoagulation is to maintain or obtain a patent portal vein trunk to enable liver transplantation. The efficacy of anticoagulants in this setting has been evaluated in several cohort studies, mostly retrospective.105 In a meta-analysis of eight studies involving 353 patients, PVT progression was observed in 9% of treated patients compared to 33% of untreated patients. Portal vein recanalization was achieved in 71% of treated patients compared to 42% of untreated patients.119 A detailed analysis of the structure and composition of portal vein thrombi showed that portal vein obstruction consists of intimal fibrosis with an additional fibrin-rich thrombus in only one-third of cases, which may account for the unsystematic recanalization observed after anticoagulation128 (Fig. 4). Likewise, factors associated with recanalization in patients receiving anticoagulants include recent PVT and a duration between PVT diagnosis and treatment initiation of <6 months.129 Less severe liver disease and a non-occlusive PVT are also associated with a higher rate of recanalization.129
Fig. 4.
Correlation between radiological findings and pathology. (A) Recent portal vein thrombosis in a 59-year-old man with viral cirrhosis. Contrast-enhanced CT (coronal view, portal venous phase) shows partially occlusive non-enhancing material corresponding to the clot in the lumen of the portal trunk (arrows). The downstream venous branches are patent. (B) HES staining. Low magnification. Recent portal vein partial thrombosis. (C) HES staining. Higher magnification. Recent portal vein thrombi composed of fibrinous deposits associated with red blood cells. (D) Chronic portal vein thrombosis in a 52-year-old man with alcohol-related cirrhosis. Contrast-enhanced CT (coronal view, portal venous phase) shows minimally occlusive non-enhancing material corresponding to the incorporation of the clot in the wall of the portal trunk and the superior mesenteric vein (arrows). (E) HES staining. Low magnification. Non-malignant chronic portal vein thrombi. (F) HES staining. Higher magnification. Chronic portal vein thrombi with fibro-oedematous intimal thickening with numerous siderophagous and neovessels partially repermeabilizing the lumen.
In patients with cirrhosis and PVT who are not candidates for liver transplantation, the beneficial effect of anticoagulation might go beyond its impact on PVT. A landmark prospective randomised-controlled trial in 70 patients with cirrhosis without PVT showed that treatment with 4,000 IU/day enoxaparin for 48 weeks effectively prevented PVT occurrence and, more importantly, decreased decompensation of cirrhosis and death.130 In a large individual patient data meta-analysis of five studies, including 500 patients with cirrhosis and PVT, anticoagulation increased overall survival, and the beneficial effect was independent of portal vein recanalization.131 Based on these data, the recent Baveno VII consensus conference recommended initiating anticoagulation (i) in all patients with cirrhosis and PVT who are potentially candidates for liver transplantation, independently of the degree of occlusion and extension and (ii) in patients with cirrhosis and recent (<6 months) completely or partially occlusive (>50%) thrombosis of the portal vein trunk even if not a candidate for liver transplantation.43 Recanalization is usually achieved within 6 months.132 Therefore, anticoagulants should be maintained until portal vein recanalization or for a minimum of 6 months.43 After that, the decision to maintain anticoagulation depends on the response to therapy and the prospect of liver transplantation. Anticoagulation is generally maintained until liver transplantation, except if a TIPS is performed in potential liver transplant candidates. In other patients, the continuation of anticoagulation should be considered in patients without complete PVT based on regular evaluation and consideration of the benefits and risks of anticoagulation (Fig. 3B).
Bleeding events not related to portal hypertension occur in ∼10% of patients with cirrhosis receiving anticoagulation.119 This rate does not seem to be higher than that observed in patients with cirrhosis and PVT not receiving anticoagulation nor in patients without cirrhosis receiving anticoagulation.119,133 In patients with cirrhosis treated with VKAs, platelet count <50x 109/L was predictive of bleeding.132 Data obtained in the setting of atrial fibrillation suggest that VKAs are associated with a higher risk of major bleeding events than DOACs in patients with cirrhosis.133 Bleeding events related to portal hypertension are actually less frequent in patients receiving anticoagulants, possibly due to the beneficial effect of anticoagulation on intrahepatic vascular resistance.119,133,134 Therefore, administering anticoagulation does not imply a more frequent screening for gastroesophageal varices nor more intense prophylaxis for portal hypertension-related complications.43
The best type of anticoagulation in patients with cirrhosis and PVT remains to be defined. LMWH is the best-known option in this setting. LMWH should be used cautiously in patients with moderate kidney dysfunction (glomerular filtration rate [GFR] <30 ml/min) and is contraindicated in patients with a GFR <15 ml/min. Although data are scarce, especially on safety, fondaparinux might be an option in patients with PVT and cirrhosis.135 An alternative option consists of oral VKAs targeting an INR between 2 and 3. However, the accuracy of INR for VKA monitoring is uncertain in patients with liver insufficiency. Experience with DOACs in patients with cirrhosis is growing. The main advantages of DOACs over VKAs are that they are given at fixed doses without laboratory monitoring. The liver disease could influence several aspects of DOAC pharmacokinetics, including systemic elimination, plasma protein binding, cytochrome P450-mediated metabolism, and biliary excretion.136 The Child-Pugh score is used to guide dosing and the use of DOACs. In the context of PVT treatment, all DOACs can be used in patients with Child-Pugh A cirrhosis; DOACs can be used with caution and/or adjusted doses in patients with Child-Pugh class B cirrhosis; DOACs are usually not recommended in patients with Child-Pugh class C cirrhosis or coagulopathy.43,137,138 Renal insufficiency (GFR <30 ml/min), potential drug-drug interactions, and previous gastrointestinal bleeding should be evaluated before introducing DOACs139 (Table 2). Although limited, available data suggest that the efficacy of DOACs in patients with cirrhosis and PVT is at least equivalent to that of LMWH or VKAs in achieving portal vein recanalization.54,[140], [141], [142]
Transjugular intrahepatic portosystemic shunt
PVT is no longer considered a contraindication to TIPS, which has been reported to be feasible in 73-100% of patients with PVT.124,[143], [144], [145] In patients with either completely occlusive PVT or cavernoma, TIPS might still be feasible through a transjugular or trans-splenic approach, although the technical success rate is lower.143,146 Indeed, factors associated with technical failure of TIPS insertion are cavernomatous transformation, inability to identify intrahepatic portal vein branches, and extension to the superior mesenteric vein.105 In a recent meta-analysis collating data on 367 patients with cirrhosis and PVT treated with TIPS, the 12-month recanalization rate was 84%145 (Fig. 5). Importantly, these procedures were performed in expert centres, which may explain these promising results. After successful TIPS insertion, a randomised-controlled trial did not find any advantage of maintaining anticoagulation; recanalization was achieved in most patients even without anticoagulation.147 Most patients included in the latter study had non-occlusive PVT, and only 3 out 64 had cavernoma. This suggests that long-term anticoagulation is not necessary after successful TIPS placement in patients with non-occlusive PVT. Further studies are needed to make broad recommendations against long-term anticoagulation after TIPS insertion, irrespective of the type of PVT. Severe procedural complications occur in 10% of patients with cirrhosis and PVT following TIPS placement.145 Catheter-directed thrombolysis with or without thrombus aspiration have been associated with higher rates of bleeding complications. The rate of hepatic encephalopathy is similar to that observed in patients without PVT at around 25%.145 Based on these data, beyond the usual indications for TIPS (refractory ascites and recurrent variceal bleeding), TIPS can be considered as a second-line option for the treatment of PVT, especially in case of significant concomitant complications of portal hypertension or after lack of improvement (i.e. persistent complete occlusion or progression) after anticoagulation43 (Fig. 3B). In liver transplant candidates with complete or extensive PVT, TIPS (transjugular or trans-splenic) may improve transplantation feasibility if it leads to portal vein recanalization.146 In patients with complete PVT, reno-portal anastomosis during liver transplantation is another option, since it has been reported to be feasible in 89% of patients, and is associated with a 1-year survival rate of 87%.148
Fig. 5.
CT scan showing a patient with decompensated cirrhosis and occlusive portal vein thrombosis in whom portal vein recanalization was achieved after TIPS insertion. Patient with (A) occlusive portal vein thrombosis (arrow) despite anticoagulation for 3 months, in whom (B) portal vein recanalization was achieved after TIPS insertion. TIPS, transjugular intrahepatic portosystemic shunt.
Conclusion
In patients without underlying cirrhosis, medical management of SVT is based on a complete aetiological work-up, specific aetiological treatment, and generally long-term anticoagulation. Interventional radiology, performed in expert centres, is the second-line therapy for both BCS and chronic EHPVO. In patients with cirrhosis and PVT, recent guidelines have extended the indications for anticoagulation, even to some patients who are not candidates for liver transplantation. Beyond its usual indications in cirrhosis (refractory ascites or recurrent variceal bleeding), TIPS is a second-line therapy for PVT, especially in patients with concomitant complications of portal hypertension. Although the use of DOACs is growing, further studies are needed to clarify the best option, considering both patient and disease heterogeneity.
Financial support
The research laboratory is supported by the “Institut National de la Santé et de la Recherche Médicale” (ATIP AVENIR), the “Agence Nationale de la Recherche” (ANR-18-CE14-0006-01, RHU QUID-NASH, ANR-18-IDEX-0001, ANR-22-CE14-0002) by « Émergence, Ville de Paris », by Fondation ARC and by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 847949.
Authors’ contributions
Concept and writing of the original draft: LE, AP, PER. Critical revision: MR, DV, AP, VP, LDA.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgement
We thank Dr Aurélie Beaufrère for her help with preparation of the figures.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100667.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Jones D.E.J., Sturm E., Lohse A.W. Access to care in rare liver diseases: new challenges and new opportunities. J Hepatol. 2018;68:577–585. doi: 10.1016/j.jhep.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 2.De Gottardi A., Sempoux C., Berzigotti A. Porto-sinusoidal vascular disorder. J Hepatol. 2022;77:1124–1135. doi: 10.1016/j.jhep.2022.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Denninger M.H., Chaït Y., Casadevall N., Hillaire S., Guillin M.C., Bezeaud A., et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587–591. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 4.Darwish Murad S., Plessier A., Hernandez-Guerra M., Fabris F., Eapen C.E., Bahr M.J., et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167–175. doi: 10.7326/0003-4819-151-3-200908040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Plessier A., Darwish-Murad S., Hernandez-Guerra M., Consigny Y., Fabris F., Trebicka J., et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210–218. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 6.Poisson J., Plessier A., Kiladjian J.-J., Turon F., Cassinat B., Andreoli A., et al. Selective testing for calreticulin gene mutations in patients with splanchnic vein thrombosis: a prospective cohort study. J Hepatol. 2017;67:501–507. doi: 10.1016/j.jhep.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Bureau C., Laurent J., Robic M.A., Christol C., Guillaume M., Ruidavets J.B., et al. Central obesity is associated with non-cirrhotic portal vein thrombosis. J Hepatol. 2016;64:427–432. doi: 10.1016/j.jhep.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Chagneau-Derrode C, Roy L, Guilhot J, Goria O, Ollivier-Hourmand I, Bureau C, et al. Impact of cytoreductive therapy on the outcome of patients with myeloproliferative neoplasms and hepato-splanchnic vein thrombosis. Hepatology. 2013;58:847A–898A. doi: 10.1002/hep.26866. [DOI] [Google Scholar]
- 9.Desbois A.C., Rautou P.E., Biard L., Belmatoug N., Wechsler B., Resche-Rigon M., et al. Behcet’s disease in Budd-Chiari syndrome. Orphanet J Rare Dis. 2014;9:104. doi: 10.1186/s13023-014-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magaz M., Alvarez-Larrán A., Colomer D., López-Guerra M., García-Criado M.Á., Mezzano G., et al. Next-generation sequencing in the diagnosis of non-cirrhotic splanchnic vein thrombosis. J Hepatol. 2021;74:89–95. doi: 10.1016/j.jhep.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Plessier A., Esposito-Farèse M., Baiges A., Shukla A., Pagan J.C.G., De Raucourt E., et al. Paroxysmal nocturnal hemoglobinuria and vascular liver disease: eculizumab therapy decreases mortality and thrombotic complications. Am J Hematol. 2022 doi: 10.1002/ajh.26474. [DOI] [PubMed] [Google Scholar]
- 12.Smalberg J.H., Arends L.R., Valla D.C., Kiladjian J.-J., Janssen H.L.A., Leebeek F.W.G. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921–4928. doi: 10.1182/blood-2011-09-376517. [DOI] [PubMed] [Google Scholar]
- 13.Turon F., Cervantes F., Colomer D., Baiges A., Hernández-Gea V., Garcia-Pagán J.C. Role of calreticulin mutations in the aetiological diagnosis of splanchnic vein thrombosis. J Hepatol. 2015;62:72–74. doi: 10.1016/j.jhep.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Plompen E.P.C., Valk P.J.M., Chu I., Darwish Murad S., Plessier A., Turon F., et al. Somatic calreticulin mutations in patients with Budd-Chiari syndrome and portal vein thrombosis. Haematologica. 2015 doi: 10.3324/haematol.2014.120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi X., Han G., Guo X., De Stefano V., Xu K., Lu Z., et al. Review article: the aetiology of primary Budd-Chiari syndrome - differences between the West and China. Aliment Pharmacol Ther. 2016;44:1152–1167. doi: 10.1111/apt.13815. [DOI] [PubMed] [Google Scholar]
- 16.Fan J., Wang Q., Luo B., Chen H., Wang Z., Niu J., et al. Prevalence of prothrombotic factors in patients with Budd-Chiari syndrome or non-cirrhotic nonmalignant portal vein thrombosis: a hospital-based observational study. J Gastroenterol Hepatol. 2020;35:1215–1222. doi: 10.1111/jgh.14925. [DOI] [PubMed] [Google Scholar]
- 17.Debureaux P.-E., Cassinat B., Soret-Dulphy J., Mora B., Verger E., Maslah N., et al. Molecular profiling and risk classification of patients with myeloproliferative neoplasms and splanchnic vein thromboses. Blood Adv. 2020;4:3708–3715. doi: 10.1182/bloodadvances.2020002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiladjian J.-J., Debureaux P.-E., Plessier A., Soret-Dulphy J., Valla D., Cassinat B., et al. Benefits of molecular profiling with next-generation sequencing for the diagnosis and prognosis of myeloproliferative neoplasms in splanchnic vein thrombosis. J Hepatol. 2021;74:251–252. doi: 10.1016/j.jhep.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Chait Y., Condat B., Cazals-Hatem D., Rufat P., Atmani S., Chaoui D., et al. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005;129:553–560. doi: 10.1111/j.1365-2141.2005.05490.x. [DOI] [PubMed] [Google Scholar]
- 20.Levine J.S., Branch D.W., Rauch J. The antiphospholipid syndrome. New Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 21.Qi X., De Stefano V., Su C., Bai M., Guo X., Fan D. Associations of antiphospholipid antibodies with splanchnic vein thrombosis: a systematic review with meta-analysis. Medicine (Baltimore) 2015;94:e496. doi: 10.1097/MD.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakr M.A., Reda M.A., Ebada H.E., Abdelmoaty A.S., Hefny Z.M., Ibrahim Z.H., et al. Characteristics and outcome of primary Budd-Chiari syndrome due to Behçet’s syndrome. Clin Res Hepatol Gastroenterol. 2020;44:503–512. doi: 10.1016/j.clinre.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra J., Leebeek F.W.G., Plessier A., Raffa S., Darwish Murad S., Heller J., et al. Paroxysmal nocturnal hemoglobinuria in Budd-Chiari syndrome: findings from a cohort study. J Hepatol. 2009;51:696–706. doi: 10.1016/j.jhep.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Afredj N., Guessab N., Nani A., Faraoun S.A., Ouled Cheikh I., Kerbouche R., et al. Aetiological factors of Budd-Chiari syndrome in Algeria. World J Hepatol. 2015;7:903–909. doi: 10.4254/wjh.v7.i6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Broucker C., Plessier A., Ollivier-Hourmand I., Dharancy S., Bureau C., Cervoni J.-P., et al. Multicenter study on recent portal venous system thrombosis associated with cytomegalovirus disease. J Hepatol. 2022;76:115–122. doi: 10.1016/j.jhep.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Buso G., Becchetti C., Berzigotti A. Acute splanchnic vein thrombosis in patients with COVID-19: a systematic review. Dig Liver Dis. 2021;53:937–949. doi: 10.1016/j.dld.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baiges A., Cerda E., Amicone C., Téllez L., Alvarado-Tapias E., Puente A., et al. Impact of SARS-CoV-2 pandemic on vascular liver diseases. Clin Gastroenterol Hepatol. 2022;20:1525–1533.e5. doi: 10.1016/j.cgh.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi X., Ren W., De Stefano V., Fan D. Associations of coagulation factor V Leiden and prothrombin G20210A mutations with Budd-Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1801–1812.e7. doi: 10.1016/j.cgh.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Fisher N.C., Wilde J.T., Roper J., Elias E. Deficiency of natural anticoagulant proteins C, S, and antithrombin in portal vein thrombosis: a secondary phenomenon? Gut. 2000;46:534–539. doi: 10.1136/gut.46.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baiges A., de la Morena-Barrio M.E., Turon F., Miñano A., Alberto Ferrusquía J., Magaz M., et al. Congenital antithrombin deficiency in patients with splanchnic vein thrombosis. Liver Int. 2020;40:1168–1177. doi: 10.1111/liv.14342. [DOI] [PubMed] [Google Scholar]
- 32.Qi X., Yang Z., De Stefano V., Fan D. Methylenetetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia in Budd-Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis of observational studies. Hepatol Res. 2014;44:E480–E498. doi: 10.1111/hepr.12348. [DOI] [PubMed] [Google Scholar]
- 33.Bissonnette J., Durand F., de Raucourt E., Ceccaldi P.-F., Plessier A., Valla D., et al. Pregnancy and vascular liver disease. J Clin Exp Hepatol. 2015;5:41–50. doi: 10.1016/j.jceh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen H.L., Meinardi J.R., Vleggaar F.P., van Uum S.H., Haagsma E.B., van Der Meer F.J., et al. Factor V Leiden mutation, prothrombin gene mutation, and deficiencies in coagulation inhibitors associated with Budd-Chiari syndrome and portal vein thrombosis: results of a case-control study. Blood. 2000;96:2364–2368. [PubMed] [Google Scholar]
- 35.Rajani R., Björnsson E., Bergquist A., Danielsson A., Gustavsson A., Grip O., et al. The epidemiology and clinical features of portal vein thrombosis: a multicentre study. Aliment Pharmacol Ther. 2010;32:1154–1162. doi: 10.1111/j.1365-2036.2010.04454.x. [DOI] [PubMed] [Google Scholar]
- 36.Soret J., Debray D., de Fontbrune F.S., Kiladjian J.-J., Saadoun D., de Latour R.P., et al. Risk factors for vascular liver diseases: vascular liver diseases: position papers from the francophone network for vascular liver diseases, the French Association for the Study of the Liver (AFEF), and ERN-rare liver. Clin Res Hepatol Gastroenterol. 2020;44:410–419. doi: 10.1016/j.clinre.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Rebours V., Boudaoud L., Vullierme M.-P., Vidaud D., Condat B., Hentic O., et al. Extrahepatic portal venous system thrombosis in recurrent acute and chronic alcoholic pancreatitis is caused by local inflammation and not thrombophilia. Am J Gastroenterol. 2012;107:1579–1585. doi: 10.1038/ajg.2012.231. [DOI] [PubMed] [Google Scholar]
- 38.Van Wettere M., Bruno O., Rautou P.-E., Vilgrain V., Ronot M. Diagnosis of Budd-Chiari syndrome. Abdom Radiol (NY) 2018;43:1896–1907. doi: 10.1007/s00261-017-1447-2. [DOI] [PubMed] [Google Scholar]
- 39.Valla D.-C. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018;12:168–180. doi: 10.1007/s12072-017-9810-5. [DOI] [PubMed] [Google Scholar]
- 40.Ollivier-Hourmand I., Allaire M., Goutte N., Morello R., Chagneau-Derrode C., Goria O., et al. The epidemiology of Budd-Chiari syndrome in France. Dig Liver Dis. 2018;50:931–937. doi: 10.1016/j.dld.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Plessier A., Sibert A., Consigny Y., Hakime A., Zappa M., Denninger M.-H., et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology. 2006;44:1308–1316. doi: 10.1002/hep.21354. [DOI] [PubMed] [Google Scholar]
- 42.Northup P.G., Garcia-Pagan J.C., Garcia-Tsao G., Intagliata N.M., Superina R.A., Roberts L.N., et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;73:366–413. doi: 10.1002/hep.31646. [DOI] [PubMed] [Google Scholar]
- 43.de Franchis R., Bosch J., Garcia-Tsao G., Reiberger T., Ripoll C., Abraldes J.G., et al. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eapen C.E., Velissaris D., Heydtmann M., Gunson B., Olliff S., Elias E. Favourable medium term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut. 2006;55:878–884. doi: 10.1136/gut.2005.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seijo S., Plessier A., Hoekstra J., Dell’era A., Mandair D., Rifai K., et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962–1968. doi: 10.1002/hep.26306. [DOI] [PubMed] [Google Scholar]
- 46.Tripathi D., Macnicholas R., Kothari C., Sunderraj L., Al-Hilou H., Rangarajan B., et al. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome. Aliment Pharmacol Ther. 2014;39:864–872. doi: 10.1111/apt.12668. [DOI] [PubMed] [Google Scholar]
- 47.Rautou P.-E., Moucari R., Escolano S., Cazals-Hatem D., Denié C., Chagneau-Derrode C., et al. Prognostic indices for Budd-Chiari syndrome: valid for clinical studies but insufficient for individual management. Am J Gastroenterol. 2009;104:1140–1146. doi: 10.1038/ajg.2009.63. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Pagán J.C., Heydtmann M., Raffa S., Plessier A., Murad S., Fabris F., et al. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808–815. doi: 10.1053/j.gastro.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 49.EASL Clinical Practice Guidelines Vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 50.Zeitoun G., Escolano S., Hadengue A., Azar N., El Younsi M., Mallet A., et al. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30:84–89. doi: 10.1002/hep.510300125. [DOI] [PubMed] [Google Scholar]
- 51.Al-Jafar H.A., AlDallal S.M., Askar H.A., Aljeraiwi A.M., Al-Alansari A. Long standing eculizumab treatment without anticoagulant therapy in high-risk thrombogenic paroxysmal nocturnal hemoglobinuria. Hematol Rep. 2015;7:5927. doi: 10.4081/hr.2015.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randi M.L., Tezza F., Scapin M., Duner E., Scarparo P., Scandellari R., et al. Heparin-induced thrombocytopenia in patients with Philadelphia-negative myeloproliferative disorders and unusual splanchnic or cerebral vein thrombosis. Acta Haematol. 2010;123:140–145. doi: 10.1159/000280466. [DOI] [PubMed] [Google Scholar]
- 53.Zaman S., Wiebe S., Bernal W., Wendon J., Czuprynska J., Auzinger G. Increased prevalence of heparin-induced thrombocytopenia in patients with Budd-Chiari syndrome: a retrospective analysis. Eur J Gastroenterol Hepatol. 2016;28:967–971. doi: 10.1097/MEG.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 54.De Gottardi A., Trebicka J., Klinger C., Plessier A., Seijo S., Terziroli B., et al. Antithrombotic treatment with direct-acting oral anticoagulants (DOACs) in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2016 doi: 10.1111/liv.13285. [DOI] [PubMed] [Google Scholar]
- 55.Semmler G., Lindorfer A., Schaefer B., Bartl S., Hametner-Schreil S., Gensluckner S., et al. Outcome of Budd-Chiari syndrome (BCS) patients treated with direct oral anticoagulants (DOACs) - an Austrian multicenter study. Clin Gastroenterol Hepatol. 2022;S1542–3565(22):449. doi: 10.1016/j.cgh.2022.04.024. -9. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S., Kumar R., Rout G., Gamanagatti S.R., Shalimar Null. Dabigatran as an oral anticoagulant in patients with Budd-Chiari syndrome post-percutaneous endovascular intervention. J Gastroenterol Hepatol. 2020;35:654–662. doi: 10.1111/jgh.14843. [DOI] [PubMed] [Google Scholar]
- 57.Dufrost V., Wahl D., Zuily S. Direct oral anticoagulants in antiphospholipid syndrome: meta-analysis of randomized controlled trials. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2020.102711. [DOI] [PubMed] [Google Scholar]
- 58.Rautou P.-E., Douarin L., Denninger M.-H., Escolano S., Lebrec D., Moreau R., et al. Bleeding in patients with Budd–Chiari syndrome. J Hepatol. 2011;54:56–63. doi: 10.1016/j.jhep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Mukund A., Sarin S.K. Budd–Chiari syndrome: a focussed and collaborative approach. Hepatol Int. 2018;12:483–486. doi: 10.1007/s12072-018-9900-z. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q., Li K., He C., Yuan X., Luo B., Qi X., et al. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:686–697. doi: 10.1016/S2468-1253(19)30177-3. [DOI] [PubMed] [Google Scholar]
- 61.Hayek G., Ronot M., Plessier A., Sibert A., Abdel-Rehim M., Zappa M., et al. Long-term outcome and analysis of dysfunction of transjugular intrahepatic portosystemic shunt placement in chronic primary Budd-Chiari syndrome. Radiology. 2017;283:280–292. doi: 10.1148/radiol.2016152641. [DOI] [PubMed] [Google Scholar]
- 62.Langlet P., Valla D. Is surgical portosystemic shunt the treatment of choice in Budd-Chiari syndrome? Acta Gastroenterol Belg. 2002;65:155–160. [PubMed] [Google Scholar]
- 63.Bachet J.-B., Condat B., Hagège H., Plessier A., Consigny Y., Belghiti J., et al. Long-term portosystemic shunt patency as a determinant of outcome in Budd-Chiari syndrome. J Hepatol. 2007;46:60–68. doi: 10.1016/j.jhep.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Segev D.L., Nguyen G.C., Locke J.E., Simpkins C.E., Montgomery R.A., Maley W.R., et al. Twenty years of liver transplantation for Budd-Chiari syndrome: a national registry analysis. Liver Transplant. 2007;13:1285–1294. doi: 10.1002/lt.21220. [DOI] [PubMed] [Google Scholar]
- 65.Rautou P.-E., Angermayr B., Garcia-Pagan J.-C., Moucari R., Peck-Radosavljevic M., Raffa S., et al. Pregnancy in women with known and treated Budd-Chiari syndrome: maternal and fetal outcomes. J Hepatol. 2009;51:47–54. doi: 10.1016/j.jhep.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 66.Khan F., Rowe I., Martin B., Knox E., Johnston T., Elliot C., et al. Outcomes of pregnancy in patients with known Budd-Chiari syndrome. World J Hepatol. 2017;9:945–952. doi: 10.4254/wjh.v9.i21.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shukla A., Sadalage A., Gupta D., Gupte A., Mahapatra A., Mazumder D., et al. Pregnancy outcomes in women with Budd Chiari Syndrome before onset of symptoms and after treatment. Liver Int. 2018;38:754–759. doi: 10.1111/liv.13552. [DOI] [PubMed] [Google Scholar]
- 68.Bates S.M., Greer I.A., Middeldorp S., Veenstra D.L., Prabulos A.-M., Vandvik P.O., et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e691S–e736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moucari R., Rautou P.-E., Cazals-Hatem D., Geara A., Bureau C., Consigny Y., et al. Hepatocellular carcinoma in Budd-Chiari syndrome: characteristics and risk factors. Gut. 2008;57:828–835. doi: 10.1136/gut.2007.139477. [DOI] [PubMed] [Google Scholar]
- 70.Gwon D., Ko G.-Y., Yoon H.-K., Sung K.-B., Kim J.H., Lee S.S., et al. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617–626. doi: 10.1148/radiol.09090738. [DOI] [PubMed] [Google Scholar]
- 71.Sempoux C., Balabaud C., Paradis V., Bioulac-Sage P. Hepatocellular nodules in vascular liver diseases. Virchows Arch. 2018;473:33–44. doi: 10.1007/s00428-018-2373-6. [DOI] [PubMed] [Google Scholar]
- 72.Vilgrain V., Paradis V., Van Wettere M., Valla D., Ronot M., Rautou P.-E. Benign and malignant hepatocellular lesions in patients with vascular liver diseases. Abdom Radiol (NY) 2018;43:1968–1977. doi: 10.1007/s00261-018-1502-7. [DOI] [PubMed] [Google Scholar]
- 73.Van Wettere M., Purcell Y., Bruno O., Payancé A., Plessier A., Rautou P.-E., et al. Low specificity of washout to diagnose hepatocellular carcinoma in nodules showing arterial hyperenhancement in patients with Budd-Chiari syndrome. J Hepatol. 2019;70:1123–1132. doi: 10.1016/j.jhep.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Paul S.B., null Shalimar, Sreenivas V., Gamanagatti S.R., Sharma H., Dhamija E., et al. Incidence and risk factors of hepatocellular carcinoma in patients with hepatic venous outflow tract obstruction. Aliment Pharmacol Ther. 2015;41:961–971. doi: 10.1111/apt.13173. [DOI] [PubMed] [Google Scholar]
- 75.Van Wettere M., Paulatto L., Raynaud L., Bruno O., Payancé A., Plessier A., et al. Hepatobiliary MR contrast agents are useful to diagnose hepatocellular carcinoma in patients with Budd-Chiari syndrome. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nuzzo A., Maggiori L., Ronot M., Becq A., Plessier A., Gault N., et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol. 2017;112:597–605. doi: 10.1038/ajg.2017.38. [DOI] [PubMed] [Google Scholar]
- 77.Berzigotti A., García-Criado Á., Darnell A., García-Pagán J.-C. Imaging in clinical decision-making for portal vein thrombosis. Nat Rev Gastroenterol Hepatol. 2014;11:308–316. doi: 10.1038/nrgastro.2013.258. [DOI] [PubMed] [Google Scholar]
- 78.Elkrief L., Corcos O., Bruno O., Larroque B., Rautou P.-E., Zekrini K., et al. Type 2 diabetes mellitus as a risk factor for intestinal resection in patients with superior mesenteric vein thrombosis. Liver Int Off J Int Assoc Study Liver. 2013 doi: 10.1111/liv.12386. [DOI] [PubMed] [Google Scholar]
- 79.Noronha Ferreira C., Seijo S., Plessier A., Silva-Junior G., Turon F., Rautou P.-E., et al. Natural history and management of esophagogastric varices in chronic noncirrhotic, nontumoral portal vein thrombosis. Hepatology. 2016;63:1640–1650. doi: 10.1002/hep.28466. [DOI] [PubMed] [Google Scholar]
- 80.De Gaetano A.M., Lafortune M., Patriquin H., De Franco A., Aubin B., Paradis K. Cavernous transformation of the portal vein: patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol. 1995;165:1151–1155. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 81.Sharma P., Mishra S.R., Kumar M., Sharma B.C., Sarin S.K. Liver and spleen stiffness in patients with extrahepatic portal vein obstruction. Radiology. 2012;263:893–899. doi: 10.1148/radiol.12111046. [DOI] [PubMed] [Google Scholar]
- 82.Acosta S., Alhadad A., Svensson P., Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245–1251. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 83.Nuzzo A., Maggiori L., Paugam-Burtz C., Cazals-Hatem D., Ronot M., Huguet A., et al. Oral antibiotics reduce intestinal necrosis in acute mesenteric ischemia: a prospective cohort study. Am J Gastroenterol. 2019;114:348–351. doi: 10.1038/s41395-018-0389-9. [DOI] [PubMed] [Google Scholar]
- 84.Condat B., Pessione F., Helene Denninger M., Hillaire S., Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32:466–470. doi: 10.1053/jhep.2000.16597. [DOI] [PubMed] [Google Scholar]
- 85.Turnes J., García-Pagán J.C., González M., Aracil C., Calleja J.L., Ripoll C., et al. Portal hypertension-related complications after acute portal vein thrombosis: impact of early anticoagulation. Clin Gastroenterol Hepatol. 2008;6:1412–1417. doi: 10.1016/j.cgh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 86.Amitrano L., Guardascione M.A., Scaglione M., Pezzullo L., Sangiuliano N., Armellino M.F., et al. Prognostic factors in noncirrhotic patients with splanchnic vein thromboses. Am J Gastroenterol. 2007;102:2464–2470. doi: 10.1111/j.1572-0241.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 87.Valeriani E., Di Nisio M., Riva N., Cohen O., Garcia-Pagan J.-C., Magaz M., et al. Anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis. Blood. 2020 doi: 10.1182/blood.2020006827. [DOI] [PubMed] [Google Scholar]
- 88.Plessier A., Goria O., Cervoni J.P., Ollivier-Hourmand I., Bureau C., Poujol-Robert A., et al. Rivaroxaban prophylaxis in noncirrhotic portal vein thrombosis. NEJM Evid. 2022;1(12) doi: 10.1056/EVIDoa2200104. [DOI] [PubMed] [Google Scholar]
- 89.Guillaume M., Christol C., Plessier A., Corbic M., Péron J.-M., Sommet A., et al. Bleeding risk of variceal band ligation in extrahepatic portal vein obstruction is not increased by oral anticoagulation. Eur J Gastroenterol Hepatol. 2018;30:563–568. doi: 10.1097/MEG.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 90.Hernández-Gea V., De Gottardi A., Leebeek F.W.G., Rautou P.-E., Salem R., Garcia-Pagan J.C. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175–199. doi: 10.1016/j.jhep.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Benmassaoud A., AlRubaiy L., Yu D., Chowdary P., Sekhar M., Parikh P., et al. A stepwise thrombolysis regimen in the management of acute portal vein thrombosis in patients with evidence of intestinal ischaemia. Aliment Pharmacol Ther. 2019;50:1049–1058. doi: 10.1111/apt.15479. [DOI] [PubMed] [Google Scholar]
- 92.Marot A., Barbosa J.V., Duran R., Deltenre P., Denys A. Percutaneous portal vein recanalization using self-expandable nitinol stents in patients with non-cirrhotic non-tumoral portal vein occlusion. Diagn Interv Imaging. 2018 doi: 10.1016/j.diii.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 93.Fanelli F., Angeloni S., Salvatori F.M., Marzano C., Boatta E., Merli M., et al. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig Liver Dis. 2011;43:78–84. doi: 10.1016/j.dld.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Knight G.M., Clark J., Boike J.R., Maddur H., Ganger D.R., Talwar A., et al. TIPS for adults without cirrhosis with chronic mesenteric venous thrombosis and EHPVO refractory to standard-of-care therapy. Hepatology. 2021;74:2735–2744. doi: 10.1002/hep.31915. [DOI] [PubMed] [Google Scholar]
- 95.Artru F., Vietti-Violi N., Sempoux C., Vieira Barbosa J., Becce F., Sah N., et al. Portal vein recanalisation alone to treat severe portal hypertension in non-cirrhotic patients with chronic extrahepatic portal vein obstruction. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal N., Chopra S., Raveendran A., Suri V., Dhiman R.K., Chawla Y.K. Extra hepatic portal vein obstruction and pregnancy outcome: largest reported experience. J Obstet Gynaecol Res. 2011;37:575–580. doi: 10.1111/j.1447-0756.2010.01407.x. [DOI] [PubMed] [Google Scholar]
- 97.Mandal D., Dattaray C., Sarkar R., Mandal S., Choudhary A., Maity T.K. Is pregnancy safe with extrahepatic portal vein obstruction? An analysis. Singapore Med J. 2012;53:676–680. [PubMed] [Google Scholar]
- 98.Hoekstra J., Seijo S., Rautou P.E., Ducarme G., Boudaoud L., Luton D., et al. Pregnancy in women with portal vein thrombosis: results of a multicentric European study on maternal and fetal management and outcome. J Hepatol. 2012;57:1214–1219. doi: 10.1016/j.jhep.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 99.Dhiman R.K., Saraswat V.A., Valla D.C., Chawla Y., Behera A., Varma V., et al. Portal cavernoma cholangiopathy: consensus statement of a working party of the Indian national association for study of the liver. J Clin Exp Hepatol. 2014;4:S2–S14. doi: 10.1016/j.jceh.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dhiman R.K., Behera A., Chawla Y.K., Dilawari J.B., Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puri P. Pathogenesis of portal cavernoma cholangiopathy: is it compression by collaterals or ischemic injury to bile ducts during portal vein thrombosis? J Clin Exp Hepatol. 2014;4:S27–S33. doi: 10.1016/j.jceh.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Condat B., Vilgrain V., Asselah T., O’Toole D., Rufat P., Zappa M., et al. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–1308. doi: 10.1053/jhep.2003.50232. [DOI] [PubMed] [Google Scholar]
- 103.Malkan G.H., Bhatia S.J., Bashir K., Khemani R., Abraham P., Gandhi M.S., et al. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–348. doi: 10.1016/s0016-5107(99)70011-8. [DOI] [PubMed] [Google Scholar]
- 104.Llop E., de Juan C., Seijo S., García-Criado Á., Abraldes J.G., Bosch J., et al. Portal cholangiopathy: radiological classification and natural history. Gut. 2011;60:853–860. doi: 10.1136/gut.2010.230201. [DOI] [PubMed] [Google Scholar]
- 105.Senzolo M., Garcia-Tsao G., García-Pagán J.C. Current knowledge and management of portal vein thrombosis in cirrhosis. J Hepatol. 2021;75:442–453. doi: 10.1016/j.jhep.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 106.Nery F., Chevret S., Condat B., de Raucourt E., Boudaoud L., Rautou P.-E., et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660–667. doi: 10.1002/hep.27546. [DOI] [PubMed] [Google Scholar]
- 107.Francoz C., Valla D., Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57:203–212. doi: 10.1016/j.jhep.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 108.Turon F., Driever E.G., Baiges A., Cerda E., García-Criado Á., Gilabert R., et al. Predicting portal thrombosis in cirrhosis: a prospective study of clinical, ultrasonographic and hemostatic factors. J Hepatol. 2021;75:1367–1376. doi: 10.1016/j.jhep.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 109.Fortea J.I., Carrera I.G., Puente Á., Cuadrado A., Huelin P., Tato C.Á., et al. Portal thrombosis in cirrhosis: role of thrombophilic disorders. J Clin Med. 2020;9:E2822. doi: 10.3390/jcm9092822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ayala R., Grande S., Bustelos R., Ribera C., García-Sesma A., Jimenez C., et al. Obesity is an independent risk factor for pre-transplant portal vein thrombosis in liver recipients. BMC Gastroenterol. 2012;12:114. doi: 10.1186/1471-230X-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdel-Razik A., Mousa N., Elhelaly R., Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol. 2015;27:585–592. doi: 10.1097/MEG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 112.Mangia A., Villani M.R., Cappucci G., Santoro R., Ricciardi R., Facciorusso D., et al. Causes of portal venous thrombosis in cirrhotic patients: the role of genetic and acquired factors. Eur J Gastroenterol Hepatol. 2005;17:745–751. doi: 10.1097/00042737-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Zocco M.A., Di Stasio E., De Cristofaro R., Novi M., Ainora M.E., Ponziani F., et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–689. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Nery F., Correia S., Macedo C., Gandara J., Lopes V., Valadares D., et al. Nonselective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: results of a prospective longitudinal study. Aliment Pharmacol Ther. 2019;49:582–588. doi: 10.1111/apt.15137. [DOI] [PubMed] [Google Scholar]
- 115.Xu X., Guo X., De Stefano V., Silva-Junior G., Goyal H., Bai Z., et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int. 2019;13:468–481. doi: 10.1007/s12072-019-09951-6. [DOI] [PubMed] [Google Scholar]
- 116.Zanetto A., Campello E., Pelizzaro F., Farinati F., Burra P., Simioni P., et al. Haemostatic alterations in patients with cirrhosis and hepatocellular carcinoma: laboratory evidence and clinical implications. Liver Int. 2022;42:1229–1240. doi: 10.1111/liv.15183. [DOI] [PubMed] [Google Scholar]
- 117.Sherman C.B., Behr S., Dodge J.L., Roberts J.P., Yao F.Y., Mehta N. Distinguishing tumor from bland portal vein thrombus in liver transplant candidates with hepatocellular carcinoma: the A-VENA criteria. Liver Transpl. 2019;25:207–216. doi: 10.1002/lt.25345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dodd G.D., Carr B.I. Percutaneous biopsy of portal vein thrombus: a new staging technique for hepatocellular carcinoma. AJR Am J Roentgenol. 1993;161:229–233. doi: 10.2214/ajr.161.2.8392785. [DOI] [PubMed] [Google Scholar]
- 119.Loffredo L., Pastori D., Farcomeni A., Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 120.Qi X., Guo X., Yoshida E.M., Méndez-Sánchez N., De Stefano V., Tacke F., et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med. 2018;16:83. doi: 10.1186/s12916-018-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Francoz C., Belghiti J., Vilgrain V., Sommacale D., Paradis V., Condat B., et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691–697. doi: 10.1136/gut.2004.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amitrano L., Guardascione M.A., Brancaccio V., Margaglione M., Manguso F., Iannaccone L., et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–741. doi: 10.1016/j.jhep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Maruyama H., Okugawa H., Takahashi M., Yokosuka O. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol. 2013;108:568–574. doi: 10.1038/ajg.2012.452. [DOI] [PubMed] [Google Scholar]
- 124.Luca A., Caruso S., Milazzo M., Marrone G., Mamone G., Crinò F., et al. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology. 2012;265:124–132. doi: 10.1148/radiol.12112236. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Xu B.-Y., Wang X.-B., Zheng X., Huang Y., Chen J., et al. Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin Gastroenterol Hepatol. 2020;18:2564–2572.e1. doi: 10.1016/j.cgh.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 126.Englesbe M.J., Schaubel D.E., Cai S., Guidinger M.K., Merion R.M. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16:999–1005. doi: 10.1002/lt.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hibi T., Nishida S., Levi D.M., Selvaggi G., Tekin A., Fan J., et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259:760–766. doi: 10.1097/SLA.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 128.Driever E.G., von Meijenfeldt F.A., Adelmeijer J., de Haas R.J., van den Heuvel M.C., Nagasami C., et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin-rich thrombus. Hepatology. 2022;75:898–911. doi: 10.1002/hep.32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodriguez-Castro K.I., Vitale A., Fadin M., Shalaby S., Zerbinati P., Sartori M.T., et al. A prediction model for successful anticoagulation in cirrhotic portal vein thrombosis. Eur J Gastroenterol Hepatol. 2019;31:34–42. doi: 10.1097/MEG.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 130.Villa E., Cammà C., Marietta M., Luongo M., Critelli R., Colopi S., et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260.e1-4. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 131.Guerrero A., DelCampo L., Piscaglia F., Reiberger T., Han G., Violi F., et al. OS-1544 Anticoagulation improves overall survival through portal vein recanalization in patients with cirrhosis and portal vein thrombosis: individual patient data meta-analysis (IMPORTAL study) J Hepatol. 2021;75(SUPPLEMENT 2):S191–S866. [Google Scholar]
- 132.Delgado M.G., Seijo S., Yepes I., Achécar L., Catalina M.V., García-Criado A., et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. doi: 10.1016/j.cgh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 133.Intagliata N.M., Davitkov P., Allen A.M., Falck-Ytter Y.T., Stine J.G. AGA technical review on coagulation in cirrhosis. Gastroenterology. 2021;161:1630–1656. doi: 10.1053/j.gastro.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 134.La Mura V., Braham S., Tosetti G., Branchi F., Bitto N., Moia M., et al. Harmful and beneficial effects of anticoagulants in patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2018;16:1146–1152.e4. doi: 10.1016/j.cgh.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 135.Senzolo M., Piano S., Shalaby S., Tonon M., Tonello S., Zanetto A., et al. Comparison of fondaparinux and low-molecular-weight heparin in the treatment of portal vein thrombosis in cirrhosis. Am J Med. 2021;134:1278–1285.e2. doi: 10.1016/j.amjmed.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 136.Turco L., de Raucourt E., Valla D.-C., Villa E. Anticoagulation in the cirrhotic patient. JHEP Rep. 2019;1:227–239. doi: 10.1016/j.jhepr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mort J.F., Davis J.P.E., Mahoro G., Stotts M.J., Intagliata N.M., Northup P.G. Rates of bleeding and discontinuation of direct oral anticoagulants in patients with decompensated cirrhosis. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 138.Semmler G., Pomej K., Bauer D.J.M., Balcar L., Simbrunner B., Binter T., et al. Safety of direct oral anticoagulants in patients with advanced liver disease. Liver Int. 2021;41:2159–2170. doi: 10.1111/liv.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. 2022;S0168–8278(21):02033. doi: 10.1016/j.jhep.2021.09.003. -X. [DOI] [PubMed] [Google Scholar]
- 140.Intagliata N.M., Henry Z.H., Maitland H., Shah N.L., Argo C.K., Northup P.G., et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61:1721–1727. doi: 10.1007/s10620-015-4012-2. [DOI] [PubMed] [Google Scholar]
- 141.Nagaoki Y., Aikata H., Daijyo K., Teraoka Y., Shinohara F., Nakamura Y., et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. 2018;48:51–58. doi: 10.1111/hepr.12895. [DOI] [PubMed] [Google Scholar]
- 142.Hanafy A.S., Abd-Elsalam S., Dawoud M.M. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascul Pharmacol. 2019;113:86–91. doi: 10.1016/j.vph.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 143.Perarnau J.-M., Baju A., D’alteroche L., Viguier J., Ayoub J. Feasibility and long-term evolution of TIPS in cirrhotic patients with portal thrombosis. Eur J Gastroenterol Hepatol. 2010;22:1093–1098. doi: 10.1097/MEG.0b013e328338d995. [DOI] [PubMed] [Google Scholar]
- 144.Harding D.J., Perera M.T.P.R., Chen F., Olliff S., Tripathi D. Portal vein thrombosis in cirrhosis: controversies and latest developments. World J Gastroenterol. 2015;21:6769–6784. doi: 10.3748/wjg.v21.i22.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodrigues S.G., Sixt S., Abraldes J.G., De Gottardi A., Klinger C., Bosch J., et al. Systematic review with meta-analysis: portal vein recanalisation and transjugular intrahepatic portosystemic shunt for portal vein thrombosis. Aliment Pharmacol Ther. 2019;49:20–30. doi: 10.1111/apt.15044. [DOI] [PubMed] [Google Scholar]
- 146.Thornburg B., Desai K., Hickey R., Hohlastos E., Kulik L., Ganger D., et al. Pretransplantation portal vein recanalization and transjugular intrahepatic portosystemic shunt creation for chronic portal vein thrombosis: final analysis of a 61-patient cohort. J Vasc Interv Radiol. 2017;28:1714–1721.e2. doi: 10.1016/j.jvir.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 147.Wang Z., Jiang M.-S., Zhang H.-L., Weng N.-N., Luo X.-F., Li X., et al. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? A randomized controlled trial. Radiology. 2016;279:943–951. doi: 10.1148/radiol.2015150369. [DOI] [PubMed] [Google Scholar]
- 148.Azoulay D., Quintini C., Rayar M., Salloum C., Llado L., Diago T., et al. Renoportal anastomosis during liver transplantation in patients with portal vein thrombosis: first long-term results from a multicenter study. Ann Surg. 2021 doi: 10.1097/SLA.0000000000004797. [DOI] [PubMed] [Google Scholar]
- 149.Steffel J., Collins R., Antz M., Cornu P., Desteghe L., Haeusler K.G., et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.