Abstract
Background and Aims
We conducted a systematic literature review to understand the evidence supporting treatment decisions for cholestatic pruritus associated with primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC).
Methods
Studies that enrolled ≥ 75% participants with PBC or PSC and reported ≥ 1 endpoint(s) related to efficacy, safety, health-related quality of life (HRQoL) or other patient-reported outcomes were included. Bias was assessed using the Cochrane risk of bias tool for randomised controlled trials (RCTs) and the Quality of Cohort studies tool for non-RCTs.
Results
Thirty-nine publications were identified, covering 42 studies and six treatment classes (including investigational and approved products): anion-exchange resins, antibiotics (rifampicin/derivatives), opiates, selective serotonin reuptake inhibitors, fibrates, ileal bile acid transporter inhibitors and other agents not categorised in these six classes. Across studies, median sample size was small (n = 18), 20 studies were over 20 years old, 25 followed patients for ≤ 6 weeks, only 25 were RCTs. Pruritus was assessed using several different tools, with inconsistencies in their application. Cholestyramine, considered first-line therapy for moderate-severe cholestatic pruritus, was assessed in six studies (two RCTs) including 56 patients with PBC and 2 with PSC, with evidence of efficacy demonstrated in only three studies, among which, two RCTs were assessed as having a high risk of bias. Findings were similar for other drug classes.
Conclusions
There is a lack of consistent and reproducible evidence available on efficacy, impact on HRQoL, and safety of cholestatic pruritus treatments, leaving physicians to rely on clinical experience rather than evidence-based medicine for treatment selection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-023-07862-z.
Keywords: Cholestatic pruritus, Primary biliary cholangitis, Primary sclerosing cholangitis, Treatment decisions, Quality of life, Evidence-based medicine
Introduction
Cholestatic pruritus is a common and debilitating condition associated with autoimmune liver diseases, such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [1]. While pruritus is experienced by approximately 70% of patients with PBC, its exact prevalence in PSC is unclear and most patients are asymptomatic at diagnosis [2, 3]. In the TARGET-PBC study of 671 patients with PBC, the presence of itching was reported in 81% of patients who had completed a PRO (n = 211). Of the patients with itch, 37% reported clinically significant itch [4]. Pruritus has a profoundly negative impact on health-related quality of life (HRQoL) [5–9]. Patients with clinically significant itch report significantly greater fatigue, worse cognition, and significant impacts on their emotional health, sleep and social life [4].
Current treatment options for the management of cholestatic pruritus are limited, and evidence of efficacy is equivocal [10–12]. The TARGET-PBC study reported that 52% of patients with pruritus had never received treatment for itch. [4] Cholestyramine, a bile acid-binding resin, is the recommended first-line therapy for moderate-severe cholestatic pruritus in several countries, despite a limited evidence base [13–15]. It is the only medication with an indication for the relief of pruritus associated with partial biliary obstruction in the US (approved December 1966), and for the relief of pruritus associated with partial biliary obstruction and primary biliary cirrhosis in Europe (approved July 1988) [16, 17]. Only 25% of patients with clinically significant itch were being treated with bile acid-binding resins (including cholestyramine) in the TARGET-PBC study population [4]. Nalfurafine, a κ-opioid receptor agonist, has been approved in Japan for the treatment of pruritus in chronic liver disease since 2015 [18]. Off-label options that are recommended for the treatment of cholestatic pruritus include the antibiotic, rifampicin, which targets the pregnane X receptor, opioid antagonists (naltrexone, naloxone), and the selective serotonin reuptake inhibitor (SSRI) sertraline [13, 14]. However, as with cholestyramine, there is a limited evidence base to support their use [13, 14]. Oral antihistamines are also frequently prescribed to patients with cholestatic pruritus; however, cholestatic pruritus has a diverse and complex pathogenesis that does not appear to be histamine-mediated [4, 19]. Owing to this, antihistamines are not effective in alleviating pruritus in most cases [18], but may provide some benefit to patients because of their sedative properties [14]. A systematic review of the literature in 2010 concluded that there is little objective evidence to demonstrate oral antihistamines are effective treatment of pruritus and identified only four large, good quality clinical trials to support the use of topical antihistamines. All four studies enrolled patients with pruritus of any etiology and none of the studies included patients with pruritus associated with primary biliary cholangitis. [20] Evidence to conclusively support the use of antihistamines in PBC is lacking and further high-quality clinical studies are needed. Consequently, there is a clear need for new treatments for cholestatic pruritus with supporting efficacy and safety data from well-controlled clinical trials.
More recently, clinical research has focused on the ileal bile acid transporter (IBAT) inhibitors as potential treatment options for cholestatic pruritus in PBC and PSC, including linerixibat (GSK2330672), odevixibat (A4250) maralixibat (SHP625) and volixibat (SHP626), which have all been evaluated in early phase clinical trials [21–24]. In a Phase 2a, double-blind, randomised, placebo-controlled, crossover trial in adults with PBC, 14 days of linerixibat treatment led to significant reductions in pruritus severity versus placebo [23], further supported by evidence of rapid itch improvement observed in the Phase 2b GLIMMER study [25]. Linerixibat is currently being assessed in the ongoing Phase 3 GLISTEN study in PBC (NCT04950127). Odevixibat (approved for the treatment of pruritus in progressive familial intrahepatic cholestasis in the US [26]) has demonstrated improvement in pruritus in adults with PBC [21]; however, this small pilot study was terminated early following a high incidence of abdominal adverse events (AEs) [21]. A 13-week, randomised, double-blind, placebo-controlled Phase 2 trial in adults with PBC, showed that the reductions in pruritus observed with maralixibat (recently approved for treatment of pruritus in Alagille syndrome in the US) were not significantly different compared with placebo [24]; similar to the other IBAT inhibitors, diarrhoea and abdominal pain were the most frequently reported AEs [24].
The peroxisome proliferator-activated receptor (PPAR) agonists seladelpar and elafibranor have been evaluated in Phase 2 trials in patients with PBC and inadequate response to ursodeoxycholic acid (UDCA) [27, 28]. Both were shown to improve pruritus in patients with PBC, although this was not a prespecified evaluation [28, 29]. Although both compounds also reduced levels of disease-related biomarkers, the seladelpar study was terminated early as three patients on masked treatment developed grade 3 aminotransferase elevations (> 5–20 times the upper limit of normal) that were initially deemed to be drug related [27]. Following an expert panel review, it was found that there was no clinical, biochemical or histological evidence to support seladelpar-related liver injury [30]. Both seladelpar and elafibranor are currently being assessed in ongoing Phase 3 studies in PBC (NCT04620733 and NCT04526665, respectively). However, the PPAR agonists were not included in our current literature review, as the associated studies did not meet eligibility criteria for inclusion (see Methods).
The primary objective of this systematic literature review was to evaluate the evidence for efficacy of treatments for cholestatic pruritus associated with PBC and PSC. Impact on HRQoL and safety outcomes were assessed as secondary objectives.
Methods
Identification and Selection of Relevant Studies
A list of relevant studies was created from the bibliographies of prior literature reviews (previously conducted in-house by GSK) and was used to pilot test literature search strategies to ensure these searches returned relevant studies. These literature reviews included a broad review of treatment pathways, disease burden, clinical endpoints, and health technology authority perspectives of pruritus caused by cholestatic liver diseases and reviews focusing on symptom impact, patient-reported outcome (PRO) instruments, and psychometric properties of PROs in cholestatic pruritus.
A pragmatic literature search was also conducted to identify relevant studies using PubMed (including Medline), Embase, Cochrane CENTRAL database (which indexes both publications and trial registry data) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; which indexes the International Standard Randomised Controlled Trial Number [ISRCTN] trial registry and includes non-randomised and observational studies).
Databases, websites and hand searches identified two primary classes of data sources: publications and reports of clinical trials (including journal papers, conference abstracts, papers, posters and presentations); and clinical trial registry records. Each of these data sources was screened to identify relevant studies for inclusion. The two classes were then mapped against each other to associate records from clinical trial registries with corresponding publications and reports. Additional searches were conducted to identify relevant clinical trials from the trial registries, for which either no publication or report were found, or for which associated publications or reports did not provide adequate information to be included in the review.
Searches of PubMed, Embase and the Cochrane CENTRAL database were conducted on 31 March 2020. The WHO ICTRP search was initially conducted on 12 March 2020, but could not be repeated on 31 March 2020, owing to access restrictions imposed during the COVID-19 pandemic, and therefore the earlier search results were used.
Database searches were supplemented by hand searches of the citations of relevant studies and the previous literature reviews conducted by GSK (see above). The final stage of hand searching for reports of clinical trials identified from registries utilised Google Scholar and the Web of Science Core Collection for targeted searches of associated publications and reports. Search strategies are detailed in Supplementary Table 1 provided in Online Resource 1.
For the selection of studies for further analysis, references identified from PubMed were imported first, followed by references identified from Embase; duplicate records were automatically checked and deleted. This was followed by references identified from Cochrane and WHO ICTRP, imported in the same manner. All references were checked to ensure successful export and manually corrected for errors.
Eligibility criteria for study inclusion were as broad as possible to ensure that all relevant articles were captured due to the anticipated paucity of data given the rarity of both diseases (Supplementary Table 2 provided in Online Resource 1). Selected studies were required to include participants over 18 years of age with cholestatic pruritus, with at least 75% of participants with PBC or PSC, and could be either randomised controlled trials (RCTs) or non-randomised studies. Additionally, eligible studies were required to report at least one endpoint related to pruritus efficacy, HRQoL or other PROs, and safety. There were no restrictions on study date, type of comparison among interventions, duration of follow-up, geographical location, clinical setting, publication language, format, or status. Studies with non-pharmacologic treatments and treatments that targeted the underlying liver disease but not pruritus (e.g., UDCA, obeticholic acid, ciclosporine, methotrexate, colchicine, PPAR agonists) were excluded. Preclinical studies, reviews, letters, comments, case reports and editorials were also excluded.
Data Collection and Extraction
Screening of titles/abstracts and full text articles were conducted by two independent researchers. Any discrepancies were resolved by a third researcher as required. Data extraction was performed by one researcher and checked by a second researcher. Discrepancies were resolved by consensus between the two researchers, or by arbitration or unilateral decision by a project co-lead researcher. Key data extracted are shown in Supplementary Table 3 provided in Online Resource 1.
Risk of Bias Assessment
In accordance with recommendations from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), RCTs were assessed using the Cochrane risk of bias tool [31]. For non-randomised studies, the Quality of Cohort studies (Q-Coh) tool was used [32]. For single-arm studies, the risk of bias was assessed using the National Institutes of Health (NIH) before–after (pre–post) study with no control group assessment tool [33]. Further information on each of these bias assessment tools is detailed in the Supplementary Methods provided in Online Resource 1.
Independent risk of bias assessment was made by two senior researchers, with disagreement resolved either by consensus, discussion or unilateral decision of a co-lead researcher. The risk of bias was assessed as unclear if there was insufficient detail reported in the study.
Results
Search Results and Study Selection
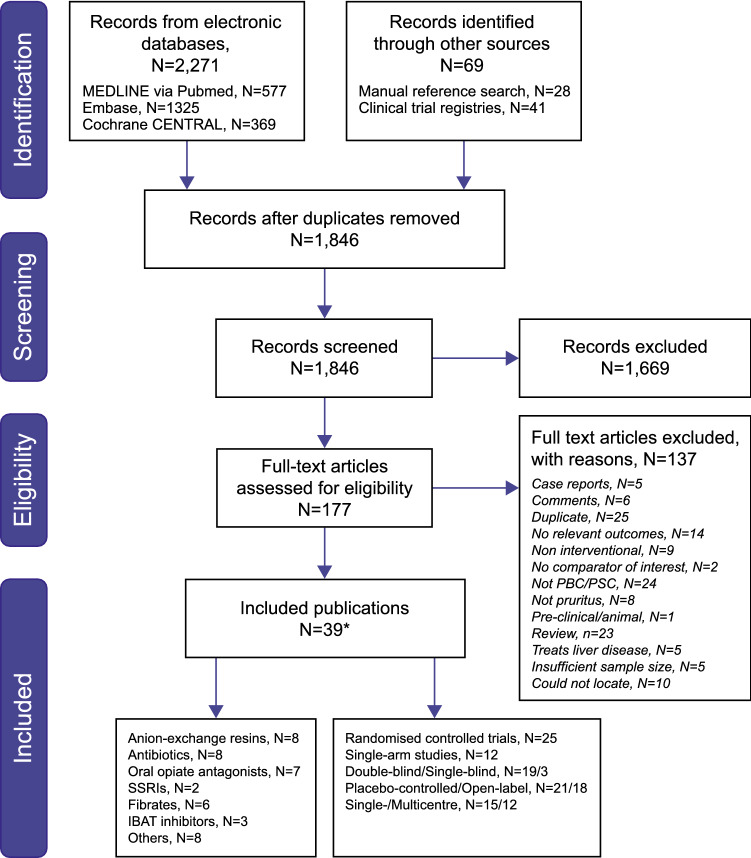
A total of 1846 unique records were identified and screened for inclusion resulting in 39 relevant publications identified for data extraction and further evaluation (Fig. 1) [15, 21, 23, 24, 34–68]. These 39 publications reported on a total of 42 studies: two publications included open-label studies alongside a separate randomised study [53, 58], while one publication included data from two trials [61].
Fig. 1.
PRISMA flow diagram
A total of 23 different treatment interventions were reported for the treatment of cholestatic pruritus from six specific classes: anion-exchange resins (eight studies) [15, 40–42, 45, 57, 62, 67], antibiotics (eight studies, all of which were studies of rifampicin or derivatives) [35, 36, 43, 48, 53, 56, 64], oral opiate agents (seven studies) [37, 55, 58, 59, 65, 66], the SSRI, sertraline (two studies) [34, 49], fibrates (six studies) [39, 44, 46, 47, 54, 68], IBAT inhibitors (three studies) [21, 23, 24] and others (eight studies) [38, 50–52, 60, 61, 63]. Study characteristics for each of the 42 studies eligible for inclusion are summarised in Table 1.
Table 1.
Overview of study characteristics of included studies
| Study | Interventions | Study design | PBC, N (%) PSC, N (%) |
Age, years | Female gender, n (%) |
Treatment duration | Main efficacy endpoint | Concomitant medications |
|---|---|---|---|---|---|---|---|---|
| Anion-exchange resins (n = 8) | ||||||||
| Datta 1966 [40] | Cholestyramine | OL |
20 (100) 0 |
47.6 (mean) | 24 (88.9) of total (n = 27) | 32 months (mean) | Pruritus reduction | NR |
| Van Berge Henegouwen 1974 [62] | Cholestyramine | Prospective, OL |
6 (75) 1 (12.5) |
NR | NR | 5 weeks | NRS; pruritus reduction | Immunosuppressive therapy (n = 6 patients) |
| Duncan 1984 [41] | Cholestyramine vs terfenadine vs chlorpheniramine vs placebo | RCT, SB, CO, PC, SC |
7 (87.5) 1 (12.5) |
NR | NR | 2 weeks | NRS | No antipruritic medication |
| Floreani 1988 [42] | DEAE-dextran vs placebo | RCT, DB, CO, PC |
12 (100) 0 |
NR | 12 (100) | 15 days | Pruritus reduction | NR |
| Zuin 1991 [67] | Cholestyramine vs DEAE-dextran | RCT, OL, PG |
30 (100) 0 |
NR | NR | 2 months | NRS; pruritus reduction | NR |
| Taha 1994 [57] | Cholestyramine 4 g BID vs no treatment vs UDCA vs cholestyramine + UDCA | OL, sequential CO |
8 (100) 0 |
52 (median) | 8 (100) |
2 weeks (4 phases) |
VAS | UDCA |
| Yokomori 2001 [15] | Colestilan vs no treatment | RCT, OL, PG, SC |
11 (100) 0 |
53.6 (mean) | 9 (81.1) | 4 weeks | NRS | No antipruritic medication |
| Kuiper 2010 [45] | Colesevelam vs placebo | RCT, DB, PG, PC, MC |
14 (40) 14 (40) |
52 (mean) | 22 (62.9) of total (n = 35) | 21 days | Pruritus reduction; VAS | UDCA, naltrexone (n = 2 patients) |
| Antibiotics (n = 8) | ||||||||
| Ghent 1988 [43] | Rifampicin vs placebo | RCT, DB, CO, PC, SC |
9 (100) 0 |
57.4 (mean) | 8 (88.9) |
2 weeks (4 cycles) |
VAS | Cholestyramine |
| Bachs 1989 [35] | Rifampicin vs phenobarbitone | RCT, CO, SC |
22 (100) 0 |
49.7 [8.4] (mean [SD]) | 22 (100) | 2 weeks | NRS; pruritus reduction | No antipruritic medication |
| Podesta 1991 [53] | Rifampicin vs placebo | RCT, DB, CO, PC, MC |
14 (100) 0 |
43 (mean) | 13 (92.9) | 1 week | Pruritus reduction; VAS | No antipruritic medication |
| Podesta 1991 [53] | Rifampicin | OL |
18 (100) 0 |
32 − 72 (range) | Pruritus reduction; VAS | No antipruritic medication | ||
| Bachs 1992 [36] | Rifampicin | Prospective, OL, SC |
16 (100) 0 |
49.7 (mean) | 16 (100) | 11.7 months (mean) | NRS; pruritus reduction | No antipruritic medication |
| Loginov 1993 [48] | Rifampicin | OL |
7 (100) 0 |
49.7 ± 2.7 | 7 (100) | 2 weeks | Pruritus reduction | Ion-exchange resins |
| Tabibian 2017 [56] | Rifaximin | Prospective, OL, SC |
0 (0) 16 (100) |
40 (median) | 3 (19) | 12 weeks | 5-D itch scale; fatigue | NR |
| Webb 2018 [64] | Rifampicin | Retrospective, OL, SC |
36 (34.3) 44 (41.9) |
Hepatitis: 37 [28–43] (median [IQR]) No hepatitis: |
Hepatitis: 3 (60) No hepatitis: 62 (62) |
131 days (median) | NR | UDCA, proton pump inhibitors, cholestyramine, antibiotics, 5-aminosalicylic acid preps, azathioprine or 6-mercaptopurine, antihistamines, prednisolone, statins, sertraline, naltrexone |
| Oral opiate agents (n = 7) | ||||||||
| Summerfield 1980 [55] | Naloxone vs placebo | RCT, DB, CO, PC |
NR NR |
n = 20 with cholestatic liver disease (29–71 range) |
13 (65) | 1 night | VAS; scratching activity | Cholestyramine |
| Thornton 1988 [59] | Nalmefene | Prospective, OL, SC |
9 (100) 0 |
57.2 (average) | 8 (88.9) | 6 months | VAS; fatigue | No antipruritic medication |
| Bergasa 1992 [37] | Naloxone vs placebo | SB, CO, PC |
8 (100) 0 |
50 (average) | 8 (100) | 24 h | VAS; scratching activity | No antipruritic medication |
| Wolfhagen 1997 [65] | Naltrexone vs placebo | RCT, DB, PG, PC, SC |
13 (81.3) 3 (18.8) |
52 (median) | 12 (75) | 4 weeks | VAS; interference of itch on sleep; fatigue | No antipruritic medication |
| Terg 2002 [58] | Naltrexone vs placebo | RCT, DB, CO, PC, MC |
15 (75) 1 (5) |
55 (mean) | 17 (85) | 2 weeks | Pruritus reduction; VAS | UDCA, antipruritic medication |
| Terg 2002 [58] | Naltrexone | OL | 9 | 36 − 70 (range) | 2 months | Pruritus reduction; VAS | UDCA, antipruritic medication | |
| Yagi 2018 [66] | Nalfurafine | Prospective, OL, MC |
44 (100) 0 |
66.8 (mean) | 39 (88.6) | 12 weeks |
VAS; PBC-40/ PBC-27 itch |
UDCA, bezafibrate, anti-pruritic medication |
| Selective serotonin reuptake inhibitors (n = 2) | ||||||||
| Mayo 2007 [49] | Sertraline vs placebo | RCT, DB, CO, PC, SC |
18 (75) 4 (16.7) |
NR | 28 (84.5) | 6 weeks | Pruritus reduction; VAS; interference of itch on sleep | UDCA |
| Ataei 2019 [34] | Sertraline vs rifampicin | RCT, SB, PG, SC |
13 (36.1) 23 (63.9) |
41.6 (mean) | 16 (44.4) | 4 weeks | VAS | UDCA |
| Fibrate (n = 6) | ||||||||
| Kanda 2003 [44] |
Bezafibrate vs no treatment |
RCT, OL, PG, SC |
22 (100) 0 |
56 (mean) for total (n = 22) | 19 (86.4) | 6 months | N/A | UDCA |
| Levy 2011 [47] | Fenofibrate | OL, MC |
20 (100) 0 |
56 (median) | 16 (80) | 48 weeks | Pruritus reduction | UDCA |
| Reig 2018 [54] |
Bezafibrate vs no treatment |
Prospective, OL, SC |
48 (100) 0 |
53.1 (mean) | 45 (94) | 38 months (median) | Pruritus reduction; VAS; 5-D itch scale | UDCA |
| Corpechot 2018 [39] | Bezafibrate vs placebo | RCT, DB, PG, PC, MC |
100 (100) 0 |
53 (mean) | 95 (95) | 24 months | VAS; fatigue | UDCA |
| Lemoinne 2018 [46] | Fibrate (bezafibrate and fenofibrate) | Retrospective, MC |
0 (0) 20 (100) |
43.8 (median) | 5 (25) | 4.1 years (median) | NRS; pruritus reduction | UDCA |
| de Vries 2019 [68] | Bezafibrate vs placebo | RCT, DB, PC, PG, MC |
26 (35.1) 46 (62.1) |
46 (median, bezafibrate group) 50 (median, placebo group) |
45 (60.8) | 3 weeks | Pruritus reduction | UDCA, bile acid sequestrants |
| IBAT inhibitors (n = 3) | ||||||||
| Hegade 2017 [23] | Linerixibat vs placebo | RCT, DB, CO, PC, MC |
22 (100) 0 |
52.9 (mean) | 19 (86) | 2 weeks |
NRS; PBC-40/ PBC-27 itch; 5-D itch scale; interference of itch on sleep |
UDCA |
| Al-Dury 2018 [21] | A4250/odevixibat | OL, SC |
10 (100) 0 |
54.9 (mean) | 9 (90) | 4 weeks |
PBC-40/ PBC-27 itch |
UDCA |
| Mayo 2019 [24] | Maralixibat vs placebo | RCT, DB, PG, PC, MC |
66 (100) 0 |
53.1 (mean) | 60 (92.0) | 13 weeks |
Itch RO; PBC-40/PBC-27 itch; 5-D itch scale; interference of itch on sleep |
UDCA |
| Other (n = 8) | ||||||||
| Bloomer 1975 [38] | Phenobarbital | Prospective, OL |
7 (77.8) 2 (22.2) |
32.3 (for n = 15) (average) |
10 (66.7) | 1–5 months | NRS | Cholestyramine |
| Turner 1990 [60] | Flumecinol vs placebo | RCT, DB, PG, PC |
38 (92.7) NR |
NR | NR | 3 weeks | VAS | Cholestyramine |
| Turner 1994 (LD) [61] | Flumecinol vs placebo | RCT, DB, PG, PC |
46 (92) NR |
NR | 47 (94) | 3 weeks | Pruritus reduction; VAS | Antipruritic medication |
| Turner 1994 (HD) [61] | Flumecinol vs placebo | RCT, DB, PG, PC |
19 (100) 0 |
NR | NR | 3 weeks | Pruritus reduction; VAS | Cholestyramine |
| Villamil 2005 [63] | Lidocaine vs placebo | RCT, DB, PG, PC, SC |
13 (72.2) 4 (22.2) |
46.5 (mean) | 14 (77.8) | 7 days | VAS; fatigue | UDCA |
| O'Donohue 2005 [52] | Ondansetron vs placebo | RCT, DB, PG, PC |
17 (89.5) 0 (0) |
55.1 (mean) | 16 (84.2) | 5 days | Pruritus reduction; VAS; scratching activity | No anti-pruritics, no UDCA |
| Mayo 2018 [50] | NGM282 vs placebo | RCT, DB, PG, PC, MC |
45 (100) 0 |
56.2 (mean) | 41 (91.1) | 28 days | Pruritus reduction; VAS; 5-D itch scale | UDCA |
| Mayo 2019 [51] | NGM282 vs placebo | RCT, DB, PG, PC, MC |
0 (0) 62 (100) |
NR | NR | 12 weeks | NRS; 5-D itch scale | NR |
CO, crossover; DB, double-blind; DEAE, diethylaminoethyl; IBAT, ileal bile acid transporter; MC, multicentre; N/A, not applicable; NR, not reported; NRS, numerical rating scale; OL, open-label; PBC, primary biliary cholangitis; PC, placebo-controlled; PG, parallel group; PSC, primary sclerosing cholangitis; RCT, randomised controlled trial; SB, single blind; SC, single centre; UDCA, ursodeoxycholic acid; VAS, visual analogue scale
Risk of Bias Within Studies
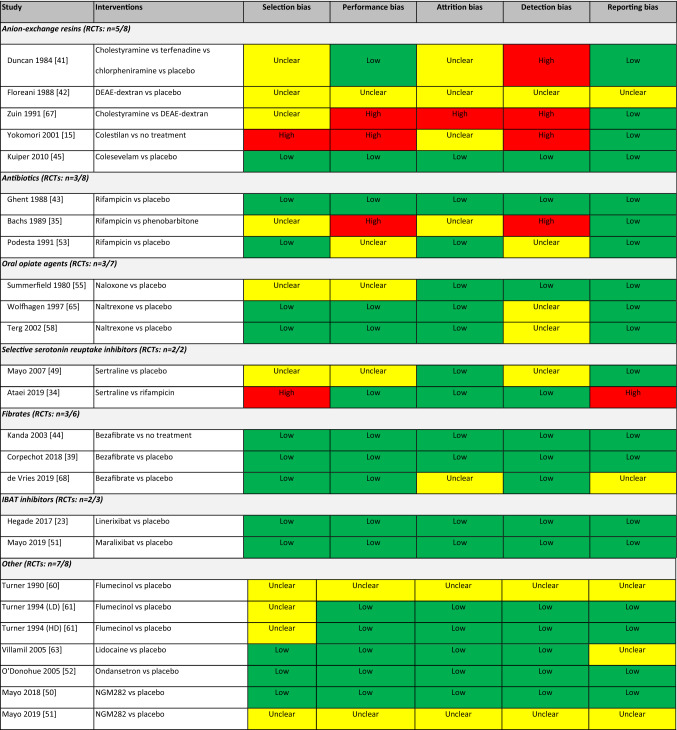
The Cochrane risk of bias tool was used to assess risk of bias in 25 RCTs that met the inclusion criteria. Nearly half of these RCTs did not adequately describe the randomisation methods; however, about two-thirds were judged to be of good quality. Five trials were deemed to have a high risk of bias, while three were rated as unclear in all five domains assessed. Assessment of bias for the individual RCTs is presented in Table 2.
Table 2.
Risk of bias within individual studies (randomised studies only) using the Cochrane risk of bias tool
DEAE diethylaminoethy, HD high dose, IBAT ileal bile acid transporter, LD low dose, RCT randomised controlled trial
The Q-Coh tool was used to assess the risk of bias in three non-randomised cohort studies. The overall quality assessment was rated as acceptable for two of these studies. The remaining study was rated as low quality as 60% of the participants dropped out prior to or shortly after initiating treatment with the investigational drug.
The NIH assessment tool was used to assess the quality of 12 single-arm studies which met the inclusion criteria. Six of these studies [36, 38, 40, 48, 59, 62] were rated as fair and the remainder rated as poor. The latter studies were generally older publications that provided little or no detail on patient selection and had high rates of attrition or poorly defined measures of pruritus.
Patient Characteristics
Patient age (mean and/or median) was reported in 30/42 studies, while three studies reported the age range (Table 1). Gender was reported in 34/42 studies and was predominantly female in 31 of these studies. Overall, 23/42 studies included only patients with PBC while 3/42 studies included only patients with PSC.
Study Characteristics
Of the 42 studies identified, 20 were reported over two decades ago (pre-2000). Studies tended to be short (25 studies followed patients for ≤6 weeks with 12 studies having ≤2 weeks’ follow-up) with a small sample size (median n=18). Study location was reported in 34/42 studies: 18 were in Europe; 5 in North America; 4 in Asia; 5 in South America and 2 in multiple continents. The use of combination medications was reported in 37/42 studies. UDCA was the most common medication used in these studies (19/42 studies). Concomitant treatment of pruritus was not permitted in 10/42 studies.
Pruritus Efficacy Measures
Pruritus efficacy was measured using a number of different assessments (Table 3). Pruritus numerical rating scales (NRS) were used in 10 studies, with two different pruritus NRS employed across the different studies. The first NRS included a categorical scale ranging from 0 (no itching) to 3 (severe itching) and was used in seven studies. The second NRS included a scale from 0 (no itching) to 10 (severe itching) and was used in the remaining three studies. Pruritus reduction and/or improvement was measured using a categorical outcome (e.g., complete resolution, partial resolution or worsened pruritus) in 20 studies. Ten studies provided data for the number of patients experiencing complete (i.e., disappearance) and partial (i.e., improvement) pruritus resolution; four reported data regarding partial resolution only; one reported data on complete resolution only.
Table 3.
Studies and pruritus efficacy measures by medication
| Class | Intervention | N studies (n RCT) | N patients treated (n PBC) | Pruritus efficacy assessment (N studies) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NRS | Pruritus reduction | VAS | PBC-40 / 27 itch | 5-D itch scale | Scratching activity | Sleep interference | N studies with any significant improvement1 (RCTs) | ||||
| Anion-exchange resins | Cholestyramine2 | 5 (2) | 59 (56) | 3 | 3 | 1 | 3 (2) | ||||
| Colesevelam2 | 1 (1) | 17 (4) | 1 | 1 | |||||||
| Colestilan | 1 (1) | 5 (5) | 1 | 1 (1) | |||||||
| DEAE-Dextran | 2 (1) | 31 (31) | 1 | 2 | 1 (1) | ||||||
| Antibiotics | Rifampicin2 | 7 (43) | 191 (110) | 2 | 4 | 3 | 4 (4) | ||||
| Rifaximin | 1 (0) | 16 (0) | 1 | ||||||||
| Opiate agents | Naloxone2 | 2 (1) | 28 (> 8) | 2 | 2 | ||||||
| Naltrexone2 | 3 (23) | 28 (23) | 1 | 2 | 1 | 2 (2) | |||||
| Nalmefene2 | 1 (0) | 9 (9) | 1 | 1 (0) | |||||||
| Nalfurafine4 | 1 (0) | 44 (44) | 1 | 1 | 1 (0) | ||||||
| Selective serotonin reuptake inhibitors | Sertraline1 | 2 (2) | 30 (16) | 1 | 2 | 1 | 1 (1) | ||||
| Fibrates | Bezafibrate | 5 (3) | 167 (120) | 1 | 3 | 2 | 1 | 3 (3) | |||
| Fenofibrate | 2 (0) | 40 (20) | 1 | 2 | 1 (0) | ||||||
| IBAT inhibitors | Maralixibat | 1 (1) | 42 (42) | 1 | 1 | 1 | 1 | ||||
| Linerixibat | 1 (1) | 21 (21) | 1 | 1 | 1 | 1 | 1 (1) | ||||
| A4250/odevixibat | 1 (0) | 10 (10) | 1 | ||||||||
| Other | Lidocaine | 1 (1) | 12 (9) | 1 | 1 (1) | ||||||
| Terfenadine | 1 (1) | 8 (7) | 1 | 1 (1) | |||||||
| Phenobarbital1 | 2 (1) | 31 (29) | 2 | 1 | |||||||
| NGM282 | 2 (2) | 72 (30) | 1 | 1 | 1 | 2 | |||||
| Flumecinol | 3 (3) | 55 (> 51) | 2 | 3 | 2 (2) | ||||||
| Ondansetron | 1 (1) | 9 (> 7) | 1 | 1 | 1 | ||||||
| Chlorpheniramine | 1 (1) | 8 (7) | 1 | ||||||||
1Studies reporting significant improvement in pruritus as defined by the authors of the studies; values in parentheses represent number of RCTs. Blank cells reflect significantly worse, not significant, or not reported
2Recommended treatment included in AASLD guidelines [14]
3One RCT includes an open-label extension reported in the same publication and not counted as a separate study
4A large Phase 3 trial was not included as it did not meet the 75% PBC/PSC inclusion criteria (n = 90/317)
> implies that the number was reported as total and not by study arm and/or studies did not report number of PBC patients
NOTE: table reports available data on all treatments; therefore, the same study may be included more than once
The number of patients refers to the number of patients treated with the specified medication
AASLD American Association for the Study of Liver Diseases, DEAE diethylaminoethyl, NRS numerical rating scale, PBC primary biliary cholangitis, RCT randomised controlled trial, VAS visual analogue scale
A visual analogue scale (VAS) was used to assess pruritus in 20 studies. These studies utilised either a 0–10 or 0–100 VAS, usually defined in units of length (mm or cm). Half of the studies utilised a VAS scale ranging from 0 to 10 and the rest used a scale from 0 to 100.
PBC-40/PBC-27 was used in four studies. The PBC-40/PBC-27 is a questionnaire that investigates the impact of disease across six domains (fatigue, pruritus, cognitive, social, emotional, and other symptoms) and patients rate 40 or 27 items, respectively, on a scale of 0/1 to 5. All four studies reported itch domain scores at follow-up; however, only two studies reported differences versus placebo and only one study reported change from baseline.
The 5-D itch scale was used in six studies. The 5-D itch scale consists of five domains assessing the duration, degree, direction, disability, and distribution of itch with a total score ranging from 5 (no itch) to 25 (severe itch). Four studies reported absolute values, four reported change from baseline and four reported difference versus placebo.
Scratching activity was assessed in three studies. This evaluation involved activity monitoring devices with units of counts per time. Two studies reported percentage reduction in scratching activity, one of which was in terms of change from baseline, while the other was difference versus placebo. The remaining study reported absolute values at follow-up.
Interference of itch on sleep was evaluated in four studies using an NRS, medical outcomes study sleep score or a VAS. Two studies provided change from baseline data and three reported differences versus placebo. The tool for measuring sleep interference was not specified in the study by Mayo and colleagues [49].
Data related to study withdrawals due to a lack of efficacy were sparse, with only 12 of the 42 studies reporting these data. Among the studies that did report these data, most reported zero or low rates of withdrawal for lack of efficacy; there were no reports of excoriations leading to withdrawal in these studies.
Pruritus Efficacy Outcomes
An overview of all pruritus efficacy outcomes reported in the included clinical studies is provided in Table 4 and Supplementary Table 4 provided in Online Resource 1.
Table 4.
Overview of pruritus efficacy measures and outcomes of included clinical trials
| Study | Interventions | Pruritus efficacy assessment(s) | Timepoint of assessment | Key efficacy findings |
|---|---|---|---|---|
| Anion-exchange resins (n = 8) | ||||
| Datta 1966 [40] | Cholestyramine | Pruritus reduction | 13.2 months (mean) | Relieved, n = 14/20; partial relief, n = 3/20; no relief, n = 4/20 |
| Van Berge Henegouwen 1974 [62] | Cholestyramine |
NRS (0–3) Pruritus reduction |
5 weeks |
Mean (SD) reduction in NRS: -1.71 (0.49) No pruritus, n = 7/8; pruritus intensity reduced, n = 1/8 |
| Duncan 1984 [41] |
Cholestyramine Terfenadine Chlorpheniramine Placebo |
NRS (0–3) | 2 weeks | Mean (SD) difference from placebo in NRS: cholestyramine, -7.38 (7.21); terfenadine, -4.50 (4.31); chlorpheniramine, -1.00 (5.04) |
| Floreani 1988 [42] |
DEAE-Dextran Placebo |
Pruritus reduction | 15 days | DEAE-Dextran: no pruritus, n = 5/12; decrease, n = 2/12; no change, n = 5/12; placebo: no change, n = 12/12 |
| Zuin 1991 [67] |
Cholestyramine DEAE-Dextran |
NRS (0–3) Pruritus reduction |
2 months |
Mean NRS: cholestyramine, 0.99 (P < 0.01); DEAE-Dextran, 1.04 (P < 0.01) No pruritus: cholestyramine, n = 5/11; DEAE-Dextran, n = 7/19 |
| Taha 1994 [57] |
Cholestyramine UDCA Cholestyramine + UDCA No treatment |
VAS (0–100) | 2 weeks | Median (IQR) VAS: cholestyramine, 18.43 (5.84, 40.85); UDCA, 28.97 (14.99, 50.13); cholestyramine + UDCA, 27.81 (16.73, 51.86); no treatment, 33.27 (21.56, 75.08) |
| Yokomori 2001 [15] |
UDCA Colestilan + UDCA |
NRS (0–10) | 4 weeks | Mean (SEM) NRS: UDCA, 2.5 (0.3) (NS vs baseline); colestilan + UDCA, 1.2 (0.2) (P < 0.05 vs baseline) |
| Kuiper 2010 [45] |
Colesevelam Placebo |
VAS (0–100) | 3 weeks | ≥ 40% reduction in VAS: colesevelam, n = 6/17; placebo, n = 6/18 |
| Antibiotics (n = 8) | ||||
| Ghent 1988 [43] |
Rifampicin Placebo |
VAS (0–100) | 2 weeks | Mean (SD) VAS: rifampicin, 176.5454 (52.4262); placebo, 346.0124 (97.2144) |
| Bachs 1989 [35] |
Rifampicin Phenobarbitone |
NRS (0–3) Pruritus reduction |
2 weeks |
Mean (SD) change from baseline in VAS: rifampicin, -1.57 (0.81); phenobarbitone, -0.33 (0.69) Pruritus improved: rifampicin, n = 19/21; phenobarbitone, n = 8/18 |
| Podesta 1991 [53] |
Rifampicin Placebo |
VAS (0–100) Pruritus reduction |
1 week |
Mean (SD) change in VAS: rifampicin, -65.93 (23.08); placebo, 16.43 (33.71) Full response (pruritus disappeared): rifampicin, n = 11/14; placebo, n = 0/14 Partial response (50% reduction): rifampicin, n = 3/14; placebo, n = 2/14 |
| Podesta 1991 [53] | Rifampicin | Pruritus reduction | 8.2 months (mean) |
Full response (pruritus disappeared): n = 17/18 Partial response (50% reduction): n = 1/18 |
| Bachs 1992 [36] | Rifampicin | NRS (0–3) |
Multiple timepoints (2 weeks – 24 months) |
Mean (SE) NRS (all assessments P < 0.001 vs baseline) Week 2, 0.6 (0.1); Month 3, 0.2 (0.1); Month 6, 0.4 (0.2); Month 9, 0.3 (0.1); Month 12, 0 (0); Month 15, 0 (0); Month 18, 0 (0); Month 24, 0 (0) |
| Loginov 1993 [48] | Rifampicin | Assessment not reported | 2 weeks | Complete resolution, n = 4/7; significant decrease, n = 3/7 |
| Tabibian 2017 [56] | Rifaximin | 5-D itch scale | 12 weeks | Median (IQR): 7 (6–8) |
| Webb 2018 [64] | Rifampicin | Pruritus reduction | N/A | Pruritus resolution: n = 17/105 |
| Oral opiate agents (n = 7) | ||||
| Summerfield 1980 [55] |
Naloxone Placebo |
VAS (0–10) | 1 night | Change in mean VAS: naloxone, -0.95; placebo, 1.33 |
| Thornton 1988 [59] | Nalmefene | VAS (0–10) | Multiple timepoints (1–6 months) | Mean (SD) VAS: Month 1, 0.5722 (0.8588); Month 3, 0.6474 (0.8247); Month 6, 0.4257 (0.6077) |
| Bergasa 1992 [37] | Naloxone | VAS (0–10); measured by scratching activity monitoring system (counts per time) | 24 h | 19.38% (68.53) |
| Wolfhagen 1997 [65] |
Naltrexone Placebo |
VAS (0–100) | 4 weeks | Mean (SE) change in VAS: naltrexone, -37.3992 (6.6136) (daytime) and -29.9804 (10.6726) (night-time); placebo, 3.1033 (4.2705) (daytime) and 0.3433 (4.1284) (night-time) |
| Terg 2002 [58] |
Naltrexone Placebo |
VAS (0–10) | 2 weeks | Mean (SD) VAS: naltrexone, 3.55 (2.39) (daytime) and 3.55 (2.42) (night-time); placebo, 5.34 (2.41) (daytime) and 5.19 (2.55) (night-time) |
| Terg 2002 [58] | Naltrexone | Ongoing response | NR |
Sustained response: n = 5/7 Exacerbation of pruritus: n = 2/7 |
| Yagi 2018 [66] | Nalfurafine |
VAS (0–100) PBC-40/-27 itch domain |
12 weeks | Mean (SD) VAS: 29.3 (24.64) |
| Selective serotonin reuptake inhibitors (n = 2) | ||||
| Mayo 2007 [49] |
Sertraline Placebo |
VAS (0–10) Pruritus reduction |
6 weeks |
Mean change in VAS: sertraline, -1.86; placebo, 0.38 ≥ 20% reduction pruritus severity: sertraline, n = 8/12; placebo, n = 0/12 |
| Ataei 2019 [34] |
Sertraline Rifampicin |
VAS (0–10) | 4 weeks | Mean (SD) VAS: sertraline, 3.33 (1.68); rifampicin, 3.44 (2.749) |
| Fibrate (n = 6) | ||||
| Kanda 2003 [44] |
Bezafibrate No treatment |
NR | NR | Pruritus decreased significantly in one patient taking UDCA plus bezafibrate 1 month after starting the trial |
| Levy 2011 [47] | Fenofibrate | Pruritus reduction | 3 weeks | Improved, 28%; stable, 44%; deteriorated, 28% |
| Reig 2018 [54] |
Bezafibrate No treatment |
VAS (0–10) Pruritus reduction |
38 months (median) |
Median (IQR) VAS: bezafibrate, 0 (0. 1.4); no treatment, 4.6 (3.7, 6.5) Complete resolution, n = 16/26; partial resolution, n = 7/26; no change, n = 1/26 (for bezafibrate); NR (no treatment) |
| Corpechot 2018 [39] |
Bezafibrate Placebo |
VAS (0–10) | Multiple timepoints (3–24 months) |
Median (IQR) VAS for bezafibrate vs placebo: Month 3: 0.0566 (0–2.9525) vs 2.7376 (0.2036–5.9502) Month 12: 0.0792 (0–1.9570) vs 2.3869 (0.0226–5.9502) Month 24: 0.0566 (0–1.4706) vs 2.3303 (0.9955–4.9434) |
| Lemoinne 2018 [46] | Fibrate: bezafibrate and fenofibrate |
NRS (0–3) Pruritus reduction |
1.56 years (median) | Improved, n = 7/8 |
| de Vries 2019 [68] |
Bezafibrate Placebo |
VAS (0–10) | 3 weeks | ≥ 50% reduction in VAS: bezafibrate (45%) vs placebo (11%); P = 0.003 |
| IBAT inhibitors (n = 3) | ||||
| Hegade 2017 [23] |
Linerixibat Placebo |
NRS (0–10) PBC-40/-27 itch domain 5-D itch scale |
2 weeks |
LS mean (SE) difference vs placebo in NRS: linerixibat 90 mg BID, 1.58 (0.450); linerixibat 90 mg BID – Sequence 1, 2.66 (0.7333); linerixibat 90 mg BID – Sequence 2, 1.58 (0.755); placebo – Sequence 1, 2.80 (0.733); placebo – Sequence 2, 4.60 (0.755) LS mean (SE) PBC-40/-27 itch domain score: linerixibat 90 mg BID, -0.59 (0.176); linerixibat 90 mg BID – Sequence 1, 2.73 (0.343); linerixibat 90 mg BID – Sequence 2, 1.99 (0.349); placebo – Sequence 1, 2.61 (0.343); placebo – Sequence 2, 3.29 (0.349) LS mean (SE) 5 D itch overall domain score: linerixibat 90 mg BID, -4.55 (1.030); linerixibat 90 mg BID – Sequence 1, 13.00 (1.342); linerixibat 90 mg BID – Sequence 2, 10.13 (1.388); placebo – Sequence 1, 15.09 (1.342); placebo – Sequence 2, 17.13 (1.388) |
| Al-Dury 2018 [21] |
A4250/odevixibat Cholestyramine or colestipol |
PBC-40/-27 itch domain | 4 weeks | Mean (SD) overall domain scores: A4250/odevixibat, 4.50 (2.38); cholestyramine or colestipol, 10.50 (3.32) |
| Mayo 2019 [24] |
Maralixibat Placebo |
Adult Itch RO PBC-40/-27 5-D itch scale |
13 weeks |
LS mean (95% CI) difference vs placebo in adult Itch RO score: maralixibat 10 mg, -0.3 (-2.0, 1.5); maralixibat 20 mg, -4.0 (-14.2, 6.2) Mean (SD) PBC-40/-27 scores: maralixibat 10 mg, 6.7 (2.92); maralixibat 20 mg, 6.3 (2.88); placebo. 7.2 (3.76) LS mean (95% CI) difference vs placebo in 5-D itch scale score: maralixibat 10 mg, -0.5 (-3.4, 2.3); maralixibat 20 mg, -0.4 (-3.2, 2.5) |
| Other (n = 8) | ||||
| Bloomer 1975 [38] | Phenobarbital | NRS (0–3) | 2.86 months (mean) | Mean (SD) change from baseline in VAS: -1.11 (0.78) |
| Turner 1990 [60] |
Flumecinol Placebo |
VAS (0–100) | 3 weeks | Mean change from baseline in VAS: flumecinol, 30.7; placebo, 37.3 (P = 0.017) |
| Turner 1994 (LD) [61] |
Flumecinol Placebo |
VAS (0–100) | 3 weeks | Median (95% CI) difference vs placebo in VAS: -8.0 (-20.8, 2.1) |
| Turner 1994 (HD) [61] |
Flumecinol Placebo |
VAS (0–100) | 3 weeks | Median (95% CI) difference vs placebo in VAS: -19.8 (-40.7, -3.3) |
| Villamil 2005 [63] |
Lidocaine Placebo |
VAS (0–100) | 7 days | Mean (SD) VAS: lidocaine, 50.5 (24.5); placebo, 71.7 (8.9) |
| O'Donohue 2005 [52] |
Ondansetron Placebo |
VAS (0–10) Pruritus reduction |
5 days |
Mean percentage reduction in VAS: ondansetron, 21%; placebo, 22% ≥ 50% reduction in VAS: ondansetron, n = 1/9; placebo, n = 2/10 |
| Mayo 2018 [50] | NGM282 vs Placebo | VAS (0–10) | 4 weeks |
LS mean (95%) change in VAS score vs placebo: -4.5 (-13.2, 4.3) Improved (≤ 20% from baseline): NGM282, 3/7; placebo, 3/10 No change: NGM282, 2/7; placebo, 5/10 Worsened (≥ 20% from baseline): NGM282, 0/7; placebo, 1/10 |
| Mayo 2019 [51] |
NGM282 Placebo |
NRS (0–10) | 12 weeks | Mean (95%) difference vs placebo in NRS: NGM282 1 mg, -0.47 (-1.93, 0.99); NGM282 3 mg, -0.52 (-1.96, 0.92) |
BID twice daily, CI confidence interval, CO crossover, DB double blind, IBAT ileal bile acid transporter, IQR interquartile range, LS least squares, MC multicentre, NR not reported, NRS numerical rating scale, OL open label, PBC primary biliary cholangitis, PC placebo controlled; PG parallel group, PSC primary sclerosing cholangitis, RCT randomised controlled trial, RO reported outcome, SB single blind, SC single centre, SD standard deviation, SE standard error, SEM standard error of the mean, UDCA ursodeoxycholic acid, VAS visual analogue scale
Anion-Exchange Resins
Eight studies of anion-exchange resins were identified (Table 1). Cholestyramine was the most assessed intervention among the anion-exchange resins, with five studies evaluating a total of 56 treated patients with PBC (Table 3). Significant improvements in pruritus efficacy with cholestyramine were demonstrated in three of the five studies, two of which were RCTs that were both assessed as having a high risk of bias in at least one domain (Table 2). In one of the RCTs reported by Zuin and colleagues (n = 30), cholestyramine significantly reduced pruritus NRS versus diethylaminoethyl (DEAE)-dextran (P < 0.001), with resolution achieved in 45.5% of patients treated with cholestyramine compared with 36.8% of patients receiving DEAE-dextran [67]. In the Duncan study (n = 8), a pruritus NRS assessed cumulative scores for the last 10 days of each treatment period; mean cumulative scores were significantly lower with cholestyramine (12.9) and terfenadine (15.8) compared with placebo (20.3) (P < 0.05) and chlorpheniramine (19.3) [41].
Antibiotics
Eight studies of antibiotics were identified (Table 1). Among these, rifampicin was the most assessed intervention, with seven studies (three RCTs) including 110 treated patients with PBC (Table 3); an additional study reported by Ataei and colleagues also assessed rifampicin in comparison to the SSRI, sertraline (Table 1; [34]). Among these nine studies, one study (n = 16) reported resolution of pruritus with rifampicin for all 10 patients who completed 12 months of treatment using a pruritus NRS. In another study that included 105 patients, only 17 (16.2%) patients reported resolution of pruritus. Four studies reported some beneficial effects of rifampicin on pruritus; however, data from these studies cannot be used as conclusive evidence for efficacy because of the limited sample size (n = 7─22) and short duration of studies (≤ 2 weeks). In the open-label phase of the study by Podesta and colleagues, complete relief of pruritus symptoms was reported in 17/18 patients and partial relief in 1/18 patients within a few weeks of rifampicin treatment, which was maintained over 8 months (Table 3; [53]).
Oral Opiate Agents
Seven studies of oral opiate agents were identified, three with naltrexone (two RCTs) and two with naloxone (one RCT) (Tables 1 and 3). Two studies (n = 20 and n = 16) showed a significant reduction in day- and night-time pruritus at the end of naltrexone treatment. In the open-label phase of the naltrexone study by Terg and colleagues, 5/7 patients retained a sustained response for the duration of the additional 2-month period with the remaining 2 patients experiencing an exacerbation of pruritus [58]. The two naloxone studies (n = 8 and n = 20) showed a reduction in values on the scratching activity index; however, these studies cannot be used to support efficacy of naloxone as they observed patients for only 24 h and a single night, respectively (Table 4; Supplementary Table 4 provided in Online Resource 1).
Selective Serotonin Reuptake Inhibitors
Two studies of SSRIs were identified both of which were RCTs that evaluated sertraline (Table 1). One of the studies (n = 22) showed improvements in pruritus and improvements in VAS scores with sertraline compared with placebo over 6 weeks, while the other study (n = 36) showed improvements in VAS scores over 4 weeks in patients with PBC and PSC (Table 4; Supplementary Table 4 provided in Online Resource 1).
Fibrates
Six studies of fibrates were identified (Table 1). Bezafibrate was the most assessed intervention among the fibrates, with five studies (three RCTs) including 120 treated patients with PBC (Table 3). One study (n = 22) reported a significant reduction in pruritus in 1 patient taking UDCA plus bezafibrate 1 month after starting the trial. Another study (n = 48) reported complete disappearance of pruritus in 16 patients who reported pruritus prior to initiating bezafibrate therapy. Another study (n = 100) reported improvements in pruritus VAS in patients assigned to bezafibrate compared with placebo over 24 months. In the study reported by de Vries and colleagues (n = 72), bezafibrate treatment led to a significant reduction in VAS scores (≥ 50% reduction of pruritus) in patients with PBC and PSC compared with placebo (45% vs 11%; P = 0.003) after 3 weeks of treatment (Table 4; Supplementary Table 4 provided in Online Resource 1; [68]).
IBAT Inhibitors
Three studies of IBAT inhibitors involving 73 treated patients with PBC were identified: one study (RCT) with linerixibat, one (RCT) with maralixibat and one with odevixibat. Linerixibat was shown to be significantly more effective than placebo in improving itch intensity based on PBC-40, PBC-27 and 5-D itch scale scores. Maralixibat significantly decreased weekly scores on the adult itch reported outcomes tool from baseline to Week 13; however, these reductions, as well as the reductions on the 5-D itch scale, were not significantly different compared with placebo. All 9 patients treated with odevixibat showed an improvement in pruritus based on 5-D itch, VAS and PBC-40 pruritus scores; however, this study was terminated following a high incidence of abdominal AEs.
HRQoL and PROs
The most commonly used PRO among the identified studies was the PBC-40 instrument, yet this was still only applied in 4/42 studies, and generally only in more recent studies [23, 50, 51, 66]. Hegade and colleagues reported a significantly greater reduction following linerixibat treatment compared with placebo in the itch and fatigue domains of PBC-40 [23]. In a Phase 2 study, no clinically meaningful improvements were observed with maralixibat compared with placebo on any domain of the PBC-40 [24]. In another Phase 2 study, treatment with NGM282 did not provide any significant changes in HRQoL assessed by the PBC-40 questionnaire [50]. Another study reported a significant reduction from baseline in the PBC-40 itch domain at Week 12 following nalfurafine treatment [66].
Other PROs assessed included the 36-Item Short Form survey (SF-36) in 2/42 studies [56, 66]. One of these studies reported non-significant reductions ranging from 2 to 20% across all SF-36 domains, except “role-physical” where no change was recorded after 12 weeks of rifaximin treatment [56]. The other study also did not show any significant changes in all domains of the SF-36 following nalfurafine treatment for 12 weeks [66].
Patient impression of change and patient global therapeutic benefit was recorded in only one of the 42 studies (the Phase 2 study of maralixibat) with no significant difference reported for either assessment for maralixibat versus placebo [24].
Satisfaction with study medication was assessed in 2/42 studies [43, 49]. In the study by Ghent and colleagues, 8/9 patients who completed the study reportedly preferred rifampicin to placebo [43]. In the study of Mayo and colleagues, all 12 patients reported a preference for sertraline, and chose to continue participating in an optional 2-year open-label long-term follow-up study [49].
Safety Outcomes
A total of 13 of the 42 studies identified reported treatment-emergent AEs, seven studies reported treatment-related AEs, ten studies reported serious AEs, and approximately a fifth (19%) reported treatment-related serious AEs (Supplementary Table 5 provided in Online Resource 1). Twenty-nine studies reported data relating to withdrawals. Four studies reported a > 40% overall withdrawal rate, whereas nine studies reported no withdrawals in any treatment arm. Data related to withdrawals due to AEs was provided in 28 studies. Of these, two reported > 40% withdrawal due to AEs, whereas nine studies reported no withdrawals due to AEs across all treatment arms.
Anion-Exchange Resins
AEs (occurring in ≥ 5% of patients) were reported in two of the eight studies of anion-exchange resins, both of which assessed cholestyramine. The most frequent reported AEs in patients treated with cholestyramine were diarrhoea (40 − 50%), vomiting (25%), constipation (6.7%) and abdominal pain (6.7%).
Antibiotics
AEs were reported in three of the seven rifampicin studies and the only study identified for rifaximin. The most commonly occurring AEs in patients treated with rifampicin were upper abdominal pain (28.6%), liver transplantation (18.8─20.0%), nausea (14.3%), leukocyte count decrease (14.3%), hepatitis/toxic hepatitis (4.8─12.5%) and peripheral oedema (6.3%).
Oral Opiate Agents
AEs were reported in two of the three studies identified for naltrexone. The most commonly occurring AEs in patients treated with naltrexone were dizziness (37.5─50.0%), opioid withdrawal like symptoms (50.0%), nausea (40.0─50%), vomiting (30.0%), abdominal cramps (25.0─62.5%) and hypertension (5.0%). AEs were not reported for naloxone, and the only study identified for nalmefene reported severe opioid withdrawal in 100% of patients, with increased bilirubin and deepening jaundice reported in 11% of patients.
Selective Serotonin Reuptake Inhibitors
AEs were reported in both studies identified for sertraline. The most commonly occurring AEs in patients were nausea (4.8─16.7%), insomnia (14.3%), fatigue (9.5%), liver transplantation (9.5%), increased bowel frequency (9.5%), visual hallucinations (9.5%) and dizziness (4.8%).
Fibrates
AEs were reported in two of the four studies identified for bezafibrate. The most commonly occurring AEs were myalgia (20.0%), nasopharyngitis (18.0%), abdominal pain (14.0%), arthralgia (14.0%), depressive mood (14.0%), flu-like syndrome (10.0%), polydipsia (9.1%), bronchitis (8.0%) and pruritus (8.0%).
IBAT Inhibitors
AEs were reported in two of the three studies included for IBAT inhibitors. The most commonly occurring AEs in patients treated with linerixibat were diarrhoea (33.0%), headache (29.0%), abdominal distention (14.0%) and abdominal pain (14.0%). The most commonly occurring AEs in patients treated with maralixibat were diarrhoea (52.4─70.0%), nausea (19.0─25.0%), upper abdominal pain (20.0─28.6%), abdominal pain (20.0─23.8%), abdominal distention (15.0%), fatigue (15%) and headache (9.5─15.0%).
Discussion
This systematic review of the literature clearly indicates that the evidence to support current treatment decisions for cholestatic pruritus in PBC and PSC is limited. Given that both are rare cholestatic liver diseases, and pruritus is recognised as a common and burdensome condition of both, patients with PBC and PSC were included in our review to ensure that all potentially informative studies were captured. Despite this approach, only a limited number of studies were identified, with the majority comprising small patient populations and were short in duration. Most studies, including those that assessed guideline-recommended treatments for pruritus, were also judged to be poor in quality. The double-blind RCT is considered to be the ‘gold standard’ method for conducting clinical research; however, only 25 of the 42 studies we identified were RCTs, with fewer than half (n = 18) double-blinded.
Nearly half of the identified studies were conducted more than two decades ago when the treatment paradigm for cholestatic pruritus was markedly different. Thus, findings from these earlier studies may no longer be relevant, owing to updates to clinical guidelines and changes in clinical practice. Social habits and patients’ expectations have also evolved over time, which may have resulted in differences in patient experience and reporting of itch in more recent studies compared with the older investigations.
For pruritus efficacy measures, NRS, pruritus reduction/improvement and VAS were the most frequently reported endpoints; however, there were inconsistencies in the application of these measures between studies, including the use of different numerical scales, differences in the questions asked (e.g., itch intensity, worst itch, overall itch) and the time scale over which patients reported their symptoms (ranging from 12 h to 1 week). This variability in application of itch assessments measures highlights a clear need to establish and adopt universally accepted/gold standard methods in future studies.
Although cholestyramine is recommended as first-line treatment for moderate-severe pruritus associated with PBC and PSC [3, 13, 14], supportive evidence for its use appears limited, owing to a lack of RCTs, small sample sizes, absence of placebo arms, and short treatment duration. Furthermore, evidence to support the use of other treatment classes for cholestatic pruritus is also limited. Antihistamines are commonly prescribed for treatment of pruritus, with the TARGET-PBC study reporting that 73% of patients were prescribed antihistamines for mild itch [4]. Despite the widespread use of antihistamines, this analysis identified only one study that met the criteria for inclusion in this systematic literature review and which concluded that antihistamines were not effective at treating pruritus. Inconsistency between study designs and outcome reporting makes comparisons of efficacy between the different treatment classes problematic and precludes robust statistical analysis. Given the wide variation in reporting standards for several efficacy outcomes and lack of formal statistical analysis to demonstrate differences between treatment arms, there is a clear need for improved study designs to provide quality evidence that can be incorporated into clinical guidelines and to assist physicians when considering the relative benefits and risks of currently available and future treatment options.
Our findings are in agreement with another recently published systemic review and meta-analysis assessing the efficacy of treatments for cholestatic pruritus [69]. The authors of this analysis also concluded that treatments for cholestatic pruritus are supported by data of very low quality and also noted a high degree of inconsistency in treatment approaches across studies. The authors identified a total of 93 studies, while we included 42 studies. This difference can be explained by the broader inclusion criteria in the published review, which included all cholestatic itch conditions, and not limited to PBC and PSC, as was the case in our analysis. Nevertheless, the findings of the reported analysis are consistent with our findings and provide further support for the need for more effective treatment options with good quality supportive evidence to manage this bothersome condition.
With respect to HRQoL and PROs, the paucity of reporting and lack of consistency in applied endpoints across studies that we observed is also consistent with another recent review of the PBC and PSC literature [70], which observed that only 60% of PRO concepts were measured with a PRO instrument, mostly a non-validated VAS or NRS. Development of only 3/83 PRO instruments was based on feedback from the target populations, and psychometric testing was limited to just 6 instruments [70]. For the PBC-40 instrument, significant reductions versus placebo were demonstrated only in the itch and fatigue domains following linerixibat treatment [23]; other studies either did not compare against placebo [66], or failed to show any significant difference between active treatment and placebo arms [24, 50]. It is possible that PRO reporting using PBC-40 is more likely in industry-sponsored research, or that use of this independent, well-validated and robust PRO measure may become more common in future studies.
In addition, to the aforementioned limitations, we noted a substantial risk of bias in many of the identified studies, as assessed using tools consistent with PRISMA-P guidelines [71]. Additional limitations include the earlier access date for the WHO ICTRP database in comparison to the other databases, and exclusion of studies with mixed cholestatic pruritus populations (which led to the exclusion of a large Phase 3 trial of nalfurafine).
With respect to safety, currently available therapies are either not well-tolerated (e.g., cholestyramine) or may be contraindicated in patients with liver disease (e.g., rifampicin). We noted a lack of consistency in defining and/or reporting AEs, particularly in older studies and a substantial variation in withdrawals and withdrawals due to AEs was observed between studies.
In summary, there is a paucity of evidence from high-quality trials for the treatment of pruritus in patients with PBC and PSC. While the primary objective of this systematic literature review was to evaluate evidence of efficacy of treatments for cholestatic pruritus associated with PBC and PSC, no strong evidence base could be identified because of various limitations of the evaluated studies. Owing to the limited data in the literature, there is a clear lack of understanding on the impact and burden of pruritus on patients with PBC and PSC. Additionally, our findings highlight an unmet need for a gold standard assessment for measuring and assessing itch. The lack of consistent and reproducible evidence available on efficacy, safety and impact on HRQoL of different treatments makes it difficult to interpret the relative benefit of currently recommended therapies, leaving physicians to rely on their clinical experience and trial-and-error, rather than evidence-based medicine. Consequently, patients suffering from this burdensome condition may need to suffer for longer duration to gain relief, considering the trial-and-error approach being used to treat pruritus rather than robust clinical evidence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating author comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Hayley Butler, PhD, at Fishawack Indicia Ltd, part of Fishawack Health, and was funded by GSK.
Abbreviations
- AE
Adverse event
- DEAE
Diethylaminoethyl
- HRQoL
Health-related quality of life
- IBAT
Ileal bile acid transporter
- ICTRP
International Clinical Trials Registry Platform
- ISRCTN
International Standard Randomised Controlled Trial Number
- PBC
Primary biliary cholangitis
- NIH
National Institutes of Health
- NRS
Numerical rating scale
- PPAR
Peroxisome proliferator-activated receptor
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRO
Patient-reported outcome
- PSC
Primary sclerosing cholangitis
- Q-Coh
Quality of Cohort Studies
- RCT
Randomised controlled trial
- SF-36
36-Item Short Form survey
- SSRI
Selective serotonin reuptake inhibitor
- UDCA
Ursodeoxycholic acid
- VAS
Visual analogue scale
- WHO
World Health Organization
Author contributions
HTS was involved in study design and data analysis/interpretation. ARdS, AHT and MMM were involved in data analysis/interpretation. JJD, JAM and JVC were involved in study design, study execution and data analysis/interpretation. All authors reviewed and edited the manuscript and approved the final version.
Funding
This study (Study 209985) was funded by GSK.
Data availability
Not applicable as this study does not include individual patient data.
Declarations
Conflict of interest
HTS, ARdS and MMM are employees of GSK and hold stocks/shares in the company. AHT was an employee of GSK at the time of the study and holds GSK stocks/shares. JAM is an employee of Medical Decision Modelling Inc., which is a health economic firm commissioned by GSK to conduct this research. JJD and JVC were employees of Medical Decision Modelling Inc. at the time of the study.
Footnotes
April H. Thompson was affiliated with Specialty Medicines, Global Medical Affairs, GSK, Research Triangle Park, Durham, NC 27701, USA, at time of study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hegade VS, Kendrick SF, Rehman J, Jones DE. Itch and liver: management in primary care. Br J Gen Pract. 2015;65:e418–e420. doi: 10.3399/bjgp15X685477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418–3426. doi: 10.3748/wjg.v23.i19.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindor KD, Kowdley KV, and Harrison ME. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646–659; quiz 660. [DOI] [PubMed]
- 4.Mayo MJ, Carey E, Smith HT, et al. Impact of Pruritus on Quality of Life and Current Treatment Patterns in Patients with Primary Biliary Cholangitis. Dig Dis Sci. 2022. [DOI] [PMC free article] [PubMed]
- 5.Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: A systematic review. J Formos Med Assoc. 2016;115:689–702. doi: 10.1016/j.jfma.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Bergasa NV. Pruritus and fatigue in primary biliary cirrhosis. Clin Liver Dis. 2003;7:879–900. doi: 10.1016/S1089-3261(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 7.Beuers U, Kremer AE, Bolier R, Elferink RP. Pruritus in cholestasis: facts and fiction. Hepatology. 2014;60:399–407. doi: 10.1002/hep.26909. [DOI] [PubMed] [Google Scholar]
- 8.Levy C. Management of pruritus in patients with cholestatic liver disease. Gastroenterol Hepatol (N Y). 2011;7:615–617. [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi HD, Lizaola B, Tapper EB, and Bonder A. Management of pruritus in primary biliary cholangitis: a narrative review. Am J Med. 2017;130:744 e741–744 e747. [DOI] [PubMed]
- 10.Trivella J and Levy C. Safety considerations for the management of cholestatic itch. Expert Opin Drug Saf. 2021:1–10. [DOI] [PubMed]
- 11.Shah RA, Kowdley KV. Mechanisms and treatments of pruritus in primary biliary cholangitis. Semin Liver Dis. 2019;39:209–220. doi: 10.1055/s-0039-1679918. [DOI] [PubMed] [Google Scholar]
- 12.Murray-Brown FL. Naltrexone for cholestatic itch: a systematic review. BMJ Support Palliat Care. 2021;11:217–225. doi: 10.1136/bmjspcare-2020-002801. [DOI] [PubMed] [Google Scholar]
- 13.EASL. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. [DOI] [PubMed]
- 14.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 15.Yokomori H, Oda M, Ishii H. Effects of ursodeoxycholic acid and colestilan versus ursodeoxycholic acid alone on serum bile acids and pruritus: A randomized, open-label study. Curr Ther Res. 2001;62:221–229. doi: 10.1016/S0011-393X(01)80033-3. [DOI] [Google Scholar]
- 16.European Medicines Agency. Questran Light 4g/sachet Powder for Oral Suspension Summary of Product Characteristics Updated 19-Jan-2021 | Neon Healthcare Ltd. Available at: https://www.medicines.org.uk/emc/product/10588/smpc/print. Accessed 15 August, 2022.
- 17.Cerner Multum. Questran Prescribing Information. Available at: https://www.drugs.com/mtm/questran.html. Accessed August 15, 2022.
- 18.Düll MM, Kremer AE. Newer approaches to the management of pruritus in cholestatic liver disease. Curr Hepatol Rep. 2020;19:86–95. doi: 10.1007/s11901-020-00517-x. [DOI] [Google Scholar]
- 19.Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: Emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81:1371–1378. doi: 10.1016/j.jaad.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eschler DC, Klein PA. An evidence-based review of the efficacy of topical antihistamines in the relief of pruritus. J Drugs Dermatol. 2010;9:992–997. [PubMed] [Google Scholar]
- 21.Al-Dury S, Wahlstrom A, Wahlin S, et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci Rep. 2018;8:6658. doi: 10.1038/s41598-018-25214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinicaltrials.gov. A study to evaluate efficacy and safety of an investigational drug named volixibat in patients with itching caused by primary biliary cholangitis (VANTAGE). Available at: https://clinicaltrials.gov/ct2/show/NCT05050136. Accessed August 15, 2022.
- 23.Hegade VS, Kendrick SF, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 24.Mayo MJ, Pockros PJ, Jones D, et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol Commun. 2019;3:365–381. doi: 10.1002/hep4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy C, Kendrick S, Bowlus CL, et al. GLIMMER trial: A randomized, double-blind, placebo-controlled study of linerixibat, an inhibitor of the ileal bile acid transporter, in the treatment of cholestatic pruritus in primary biliary cholangitis. Poster LP38. Presented at American Association for the Study of Liver Diseases. 13–16 November, 2020. Virtual.
- 26.Albireo. Albireo announces FDA approval of Bylvay™ (odevixibat), the first drug treatment for patients with progressive familial intrahepatic cholestasis (PFIC). 2021.
- 27.Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 28.Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74:1344–1354. doi: 10.1016/j.jhep.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Kremer AE, Mayo MJ, Hirschfield G, et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022;42:112–123. doi: 10.1111/liv.15039. [DOI] [PubMed] [Google Scholar]
- 30.CYMABAY T. FDA Lifts All Clinical Holds on Seladelpar. Available at: https://ir.cymabay.com/press-releases/detail/485/fda-lifts-all-clinical-holds-on-seladelpar. Accessed 5 December, 2022.
- 31.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 32.Jarde A, Losilla JM, Vives J, Rodrigo M. Q-Coh: A tool to screen the methodological quality of cohort studies in systematic reviews and meta-analyses. Int J Clin Health Psychol. 2013;13:138–146. doi: 10.1016/S1697-2600(13)70017-6. [DOI] [Google Scholar]
- 33.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataei S, Kord L, Larki A, et al. Comparison of sertraline with rifampin in the treatment of cholestatic pruritus: a randomized clinical trial. Rev Recent Clin Trials. 2019;14:217–223. doi: 10.2174/1574887114666190328130720. [DOI] [PubMed] [Google Scholar]
- 35.Bachs L, Pares A, Elena M, Piera C, Rodes J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;1:574–576. doi: 10.1016/S0140-6736(89)91608-5. [DOI] [PubMed] [Google Scholar]
- 36.Bachs L, Pares A, Elena M, Piera C, Rodes J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102:2077–2080. doi: 10.1016/0016-5085(92)90335-V. [DOI] [PubMed] [Google Scholar]
- 37.Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992;102:544–549. doi: 10.1016/0016-5085(92)90102-5. [DOI] [PubMed] [Google Scholar]
- 38.Bloomer JR, Boyer JL. Phenobarbital effects in cholestatic liver diseases. Ann Intern Med. 1975;82:310–317. doi: 10.7326/0003-4819-82-3-310. [DOI] [PubMed] [Google Scholar]
- 39.Corpechot C, Chazouilleres O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378:2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 40.Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology. 1966;50:323–332. doi: 10.1016/S0016-5085(66)80071-9. [DOI] [PubMed] [Google Scholar]
- 41.Duncan JS, Kennedy HJ, Triger DR. Treatment of pruritus due to chronic obstructive liver disease. Br Med J (Clin Res Ed). 1984;289:22. doi: 10.1136/bmj.289.6436.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floreani ACM, Zagolin M, Naccarato R. Diethylaminoethyldextran (DEAE-Dextran) for itching in primary biliary cirrhosis: a double blind trial. Med Sci Res. 1988;16:731. [Google Scholar]
- 43.Ghent CN and Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology. 1988;94:488–493. [DOI] [PubMed]
- 44.Kanda T, Yokosuka O, Imazeki F, Saisho H. Bezafibrate treatment: a new medical approach for PBC patients? J Gastroenterol. 2003;38:573–578. doi: 10.1007/s00535-002-1102-7. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper EM, van Erpecum KJ, Beuers U, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: results of a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:1334–1340. doi: 10.1002/hep.23821. [DOI] [PubMed] [Google Scholar]
- 46.Lemoinne S, Pares A, Reig A, et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French-Spanish experience. Clin Res Hepatol Gastroenterol. 2018;42:521–528. doi: 10.1016/j.clinre.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Levy C, Peter JA, Nelson DR, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33:235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 48.Loginov AS, Reshetniak VI. and Petrakov AV [The treatment of primary biliary liver cirrhosis with rifampicin] Ter Arkh. 1993;65:57–62. [PubMed] [Google Scholar]
- 49.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 50.Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients With primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayo MJ, Ling L, Hudgens S, et al. LBP-21-Effect of NGM282, an FGF19 analogue, on pruritus in patients with primary sclerosing cholangitis: analysis of a phase, multicenter, randomized, double-blind, placebo-controlled trial. J Hepatol. 2019;70:E151. doi: 10.1016/S0618-8278(19)30267-1. [DOI] [PubMed] [Google Scholar]
- 52.O'Donohue JW, Pereira SP, Ashdown AC, Haigh CG, Wilkinson JR, Williams R. A controlled trial of ondansetron in the pruritus of cholestasis. Aliment Pharmacol Ther. 2005;21:1041–1045. doi: 10.1111/j.1365-2036.2005.02430.x. [DOI] [PubMed] [Google Scholar]
- 53.Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci. 1991;36:216–220. doi: 10.1007/BF01300759. [DOI] [PubMed] [Google Scholar]
- 54.Reig A, Sese P, Pares A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol. 2018;113:49–55. doi: 10.1038/ajg.2017.287. [DOI] [PubMed] [Google Scholar]
- 55.Summerfield JA. Naloxone modulates the perception of itch in man. Br J Clin Pharmacol. 1980;10:180–183. doi: 10.1111/j.1365-2125.1980.tb01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabibian JH, Gossard A, El-Youssef M, et al. Prospective clinical trial of rifaximin therapy for patients with primary sclerosing cholangitis. Am J Ther. 2017;24:e56–e63. doi: 10.1097/MJT.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taha AS, Allison MC, Myara A, Trivin F, Duncan A, Russell RI. Does cholestyramine reduce the efficacy of ursodeoxycholic acid in primary biliary cirrhosis? Eur J Gastroenterol Hepatol. 1994;6:535–538. doi: 10.1097/00042737-199406000-00015. [DOI] [Google Scholar]
- 58.Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37:717–722. doi: 10.1016/S0168-8278(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 59.Thornton JR, Losowsky MS. Opioid peptides and primary biliary cirrhosis. BMJ. 1988;297:1501–1504. doi: 10.1136/bmj.297.6662.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner J, Rawlins M, Nagy J, James O. Flumecinol improves pruritus in cholestatic liver disease - a double blind placebo controlled trial. J Hepatol. 1990;11:S63. doi: 10.1016/0168-8278(90)91587-M. [DOI] [Google Scholar]
- 61.Turner IB, Rawlins MD, Wood P, James OF. Flumecinol for the treatment of pruritus associated with primary biliary cirrhosis. Aliment Pharmacol Ther. 1994;8:337–342. doi: 10.1111/j.1365-2036.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 62.van Berge Henegouwen GP, Brandt KH. Cholestyramine therapy in intrahepatic cholestasis. Ned Tijdschr Geneeskd. 1974;118:1074–1079. [PubMed] [Google Scholar]
- 63.Villamil AG, Bandi JC, Galdame OA, Gerona S, Gadano AC. Efficacy of lidocaine in the treatment of pruritus in patients with chronic cholestatic liver diseases. Am J Med. 2005;118:1160–1163. doi: 10.1016/j.amjmed.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 64.Webb GJ, Rahman SR, Levy C, Hirschfield GM. Low risk of hepatotoxicity from rifampicin when used for cholestatic pruritus: a cross-disease cohort study. Aliment Pharmacol Ther. 2018;47:1213–1219. doi: 10.1111/apt.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113:1264–1269. doi: 10.1053/gast.1997.v113.pm9322521. [DOI] [PubMed] [Google Scholar]
- 66.Yagi M, Tanaka A, Namisaki T, et al. Is patient-reported outcome improved by nalfurafine hydrochloride in patients with primary biliary cholangitis and refractory pruritus? A post-marketing, single-arm, prospective study. J Gastroenterol. 2018;53:1151–1158. doi: 10.1007/s00535-018-1465-z. [DOI] [PubMed] [Google Scholar]
- 67.Zuin M, Grandinetti G, Camisasca M, et al. A comparison of cholestyramine and diethylaminoethyl-dextran for the treatment of hyperlipidemia and pruritus of primary biliary cirrhosis. Curr Ther Res. 1991;49:659–665. [Google Scholar]
- 68.de Vries E, Bolier R, Goet J, et al. Bezafibrate is more effective than placebo in pruritus of chronic cholestasis: the FITCH trial. Hepatology. 2019;70.
- 69.Dervout C, Boulais N, Barnetche T, Nousbaum JB, Brenaut E, and Misery L. Efficacy of Treatments for Cholestatic Pruritus: A Systemic Review and Meta-analysis. Acta Derm Venereol. 2022;102:adv00653. [DOI] [PMC free article] [PubMed]
- 70.Kim HP, Lieber SR, Rogers ME, et al. A systematic review of patient-reported outcomes in primary biliary cholangitis and primary sclerosing cholangitis. Hepatol Commun. 2020;4:1502–1515. doi: 10.1002/hep4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable as this study does not include individual patient data.