Abstract
Introduction
LPIN1 deficiency is an autosomal recessive form of early childhood recurrent severe rhabdomyolysis. Although not completely lucid yet, LPIN1 has been shown to modulate endosomal-related pro-inflammatory responses via peroxisome proliferator-activated receptor α (PPARα) and PPARγ coactivator 1α (PGC-1α). Treatment with anti-inflammatory agents such as dexamethasone has been proposed to improve the outcome.
Case
We report a male toddler with recurrent episodes of complicated rhabdomyolysis, requiring prolonged intensive care unit admissions. Whole exome sequencing revealed a common homozygous 1.7 kb intragenic deletion in LPIN1. Despite optimal metabolic cares, the patient presented with an extremely high CK level where he benefited from intravenous dexamethasone (0.6 mg/Kg/day) for a period of 6 days.
Results
Dexamethasone administration shortened the course of active rhabdomyolysis, intensive care admission and rehabilitation. It also prevented rhabdomyolysis-related complications such as kidney injury and compartment syndrome.
Conclusion
Our patient showed a favorable response to parenteral dexamethasone, in addition to hyperhydration with IV fluids, sufficient calorie intake, and restricted dietary fat. The improvement with corticosteroids suggests an uncontrolled inflammatory response as the pathophysiology of LPIN1 deficiency.
Keywords: Hereditary rhabdomyolysis, LPIN1, Dexamethasone
1. Introduction
Lipin family consists of three phosphatidate phosphatase (PAP) enzymes that catalyze production of diacylglycerol (DAG) which, in turn, serves as the precursor of triacylglycerol (TAG), phosphatidylcholine (PC) and phosphatidylethanolamine (PE) [12]. LPIN1 is known as a transcriptional regulator of fatty acid oxidation (FAO)-related genes via interaction with peroxisome proliferator-activated receptor α (PPARα) and PPARγ coactivator 1α (PGC-1α) [3,12]. Moreover, LPIN1 has anti-inflammatory roles by downregulating nuclear factor of activated T-cells (NFATc4) [6], promoting autophagy clearance via mTOR signaling [16], and suppressing Toll-like receptor 9 (TLR9) induced pro-inflammatory responses induced by endosomal accumulation of mitochondrial DNA [5].
Patients with LPIN1 deficiency (OMIM# 268200) present with early childhood recurrent episodes of severe rhabdomyolysis with plasma creatine kinase (CK) levels more than 6000 IU/L [4,14,15]. In addition to skeletal muscle, LPIN1 is also abundantly expressed in the heart [11] and despite normal cardiac function at intervals, patients are at risk of cardiac arrest during crises [1,7]. Nevertheless, in contrast to FAO disorders or other mitochondrial conditions, patients have minimal extra-muscular manifestations, which could imply a tissue-specific function for LPIN1 [2].
The inflammatory nature of LPIN1 deficiency has been further endorsed by favorable response to dexamethasone [8,14] and hydroxychloroquine sulfate [5]. Here, we report a case of LPIN1 deficiency presenting with episodes of severe rhabdomyolysis who benefited from intravenous dexamethasone. Our report contributes to the compelling evidence of implication of anti-inflammatory agents in management of LPIN1 deficiency.
1.1. Case
Our patient, now a 5-year boy, presented first at the age of 3 years with a history of 48-h progressive myalgia and emesis following a viral gastroenteritis. His pain did not respond to regular analgesics and his plasma CK level was elevated at 170,000 IU/L (refence range: 39–308 IU/L). Rhabdomyolysis management was initiated with IV 10% dextrose-normal saline at 1.5 times maintenance rate in a local hospital. He was, however, later admitted to the intensive care unit of our tertiary hospital in the context of extreme loss of energy, delirium, drooling, respiratory distress, hyperkalemia, and hyperphosphatemia. He required mechanical ventilation and insulin infusion for hyperkalemia. He developed stage 3 acute kidney injury (AKI) and hypertension requiring renal replacement therapy. His kidney function and hypertension improved progressively on amlodipine and furosemide. CK levels were stabilized at a suboptimal level after 20 days (Fig. 1). Meanwhile, his admission was complicated by bilateral leg compartment syndrome for which he underwent fasciotomy and prolonged rehabilitation prior to discharge.
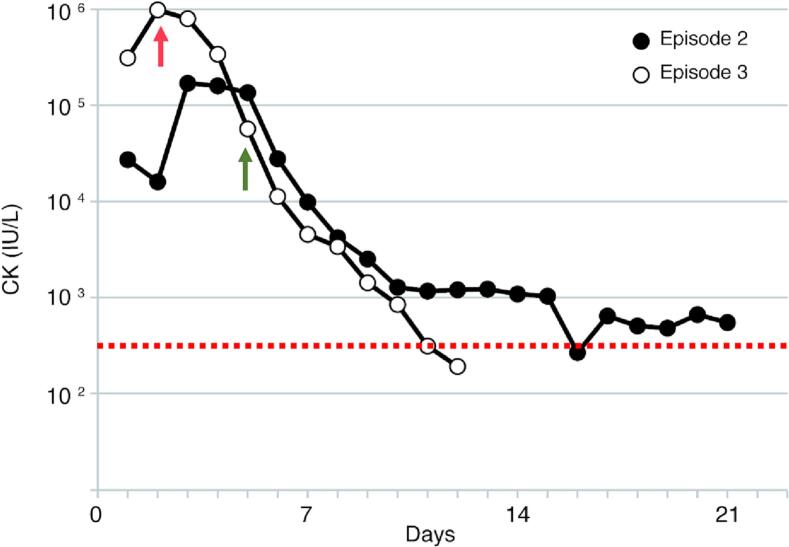
Fig. 1.
Serial plasma creatine kinase (CK) levels during the second and third episodes of acute rhabdomyolysis treated without (episode 2) and with (episode 3) IV dexamethasone (0.6 mg/Kg/day) for six days. Red arrow indicates introduction of dexamethsone and green arrow indicates switch to non-invasive ventilation. Dotted line indicates normal upper limit of serum CK (300 IU/L).
Upon interview, parents reported a previous one-week admission for rhabdomyolysis at the age of 2 years in the context of upper respiratory tract infection; decreased oral intake, myalgia, and muscle weakness. He had been only treated with intravenous fluids (dextrose 10% and normal saline), but his muscle strength had not recovered to its baseline before 6 weeks after the crisis. He was a picky eater with lower caloric intake upon diet evaluation. He was, otherwise, healthy with normal physical activity at the interval and his prenatal and past medical history was unremarkable. Parents were non-consanguineous of Caucasian descent and his three older half-siblings were all healthy.
Extensive metabolic workup, including plasma acylcarnitines and lipid profile was unremarkable. Brain MRI, ECG and echocardiogram were normal. Whole exome sequencing (WES) revealed a common homozygous 1.7 kb intragenic deletion in LPIN1 [9]. Microarray confirmed the breakpoints as arr[GRCh37] 2p25.1(11958745-11960507)x0, consistent with molecular diagnosis of LPIN1 deficiency. We started him on a low-fat diet with a maximum of 30% of total daily calories from fat. Moreover, because his plasma free carnitine level was suboptimal, we started him on oral l-carnitine supplementation (25 mg/Kg/day).
Six months later, he presented with a third episode of severe rhabdomyolysis with plasma CK level at 1 × 106 IU/L on admission (Fig. 1), extreme muscle weakness, delirium, and respiratory failure. The trigger was not recognized despite thorough investigations, and he tested negative for COVID-19 by nucleotide amplification. Following our previous experience [8], we started him on IV dexamethasone at 0.6 mg/Kg/day for a period of 6 days along with IV 10% dextrose-normal saline, and enteral feeding with Pediasure Fibre 1.0 kcal enriched with Polycal to reduce total fat intake to 30%. This provided 110% dietary reference intake (DRI) calories and 130% total fluid intake (TFI) when given in full amount. His clinical status promptly improved and within three days he was switched from mechanical ventilation onto BiPAP for three more days before breathing normally on room air. Notably, although his plasma CK level on admission was extremely high compared to the previous episode, it was normalized in a shorter duration (Fig. 1). Dexamethasone was tapered at a rate of 1.0 mg every 24 h until 1 mg /day, at which point he was switched to oral hydrocortisone. Unfortunately, prior to discharge, he developed nosocomial-necrotizing pneumonia with methicillin-susceptible Staphylococcus aureus for which he was re-admitted to the intensive care unit and covered with broad-spectrum antibiotics. Although total duration of admission was prolonged for another 20 days, during which he was on stress dose of hydrocortisone, his CK levels remained stable and he did not develop any complications. Of note, his immunity was not compromised secondary to dexamethsone, nor was identified any primary immunodeficiency. Furthermore, he developed food aversion and required nasogastric (NG) feeding tube for almost 9 months. Also, his mobility improved by physiotherapy and orthotics following the prolonged ICU admission.
A dietary plan was provided at discharge: when well, diet management involved a heart healthy diet with focus on fruits, vegetables, whole grains and lean protein sources. When ill and still able to eat, focus was on eating very low-fat foods and high sugar containing fluids, topping up with Pediasure to ensure adequate calorie intake. If not able to eat, Pediasure Fibre and Polycal was given continuously via NG tube, to provide calories at 115% DRI (with fat less than 30% of total calories) and fluids at 130% TFI.
Table 1 summarizes the clinical findings and management of the patient in his three episodes of rhabdomyolysis. Serum CK was not measured during asymptomatic intervals. The only change in management of the third episode was addition of dexamethasone. Plasma CK levels were not measured during asymptomatic intervals.
Table 1.
Summary of the clinical and laboratory findings in three episodes of rhabdomyolysis.
| Clinical finding | Episode 1 | Episode 2 | Episode 3 |
|---|---|---|---|
| Peak CK (IU/L) | Unknown | 170 × 103 | 1 × 106 |
| Trigger | Respiratory infection | Viral gastroenteritis | Unknown |
| Length of admission (days) | 7 days | 20 days | 7 days |
| Acute kidney injury | No | Yes | No |
| Electrolyte imbalance | Unknown | Hyperkalemia, Hyperphosphatemia |
No |
| Renal replacement therapy | No | Yes | No |
| Hypertension | No | Yes (resolved) | No |
| Arrhythmia | No | No | No |
| Other complications | Prolonged fatigue Muscle weakness | Compartment syndrome Prolonged fatigue Muscle weakness |
None |
| Management | IV fluids only | IV fluids Dietary fat restriction l-Carnitine |
IV fluids Dietary fat restriction l-Carnitine IV Dexamethasone |
2. Discussion
Here we report a male toddler with LPIN1 deficiency and recurrent episodes of severe rhabdomyolysis. In his first two admissions, our patient was treated conventionally with IV fluids and supportive measures. However, in the third crisis he showed a drastic response to the combination of IV fluids, adequate calories, and IV dexamethasone and despite extremely elevated levels on admission, serum CK was normalized in almost 10 days (Fig. 1). Moreover, despite prolonged admission for nosocomial-necrotizing pneumonia, rhabdomyolysis did not recur and he had a shorter period of rehabilitation. This observation is consistent with our previous report as well as others, where administration of IV dexamethasone during rhabdomyolysis crisis was associated with a better clinical outcome [8,14]. The dose and duration of IV dexamethasone was determined based on our previous experience [8] and recommended pediatric dose to impose a minimal risk of adrenal suppression. ACTH stimulation test can assess need for stress doses post hospitalization. The peak of the CK levels occurs during the first 3 days, therefore, weaning steroids could be considered after 3 days depending on patient's clinical status.
Our patient has a homozygous intragenic deletion encompassing exon 18, known to be common in Caucasians [9]. There is no established phenotype-genotype correlation in LPIN1 deficiency and pathogenic variants could affect any of the three domains of the protein [9].
Corticosteroids has been used by others to treat acute episodes of rhabdomyolysis in LPIN1 deficiency with variable outcomes [13,14]. In fact, even lower equivalent doses of corticosteroids were beneficial in a cohort of LPIN1 deficient patients who were treated with oral prednisone (1–2 mg/kg) at home and IV methylprednisolone (1–2 mg/kg/day for 3 days) during hospitalization, with decreased mortality and longer survival. However, corticosteroids did not reduce the number of episodes [14].
Pathophysiology of LPIN1 deficiency is not completely understood yet, but compelling evidence implies dysregulated pro-inflammatory responses as a core mechanism. LPIN1 deficiency-related DAG depletion impairs protein kinase D (PKD)-Vps34 phosphatidylinositol 3-kinase signaling cascade, which is required for endo-lysosomal maturation and regulation of autophagy and TLR9 induced responses [5,16]. Anti-inflammatory agents such as hydroxychloroquine sulfate and dexamethasone were shown, both in vitro and in vivo, to rescue endosomal maturation [5,8].
Accumulated lipid droplets in myocytes have been proposed as a source of inflammatory percusses [13,14]. Muscle histology in patients is consistent with lipid depositions in type I fibers, atrophy of type II fibers, and ragged-red fibers [9,16]. Notably, lipid accumulation could be prevented, in vitro in human primary myoblasts treated with dexamethasone [10]. In very long-chain acyl-CoA dehydrogenase deficiency (VLCADD) the slow oxidative type I muscle fibers are replaced by the fast glycolytic type II fibers, an adaption via PGC-1α [17]. It seems this protective measure is absent in LPIN1 deficient skeletal muscle, which could be related to abrogated function of PGC-1α, and which could explain episodic presentation of rhabdomyolysis caused by inflammatory triggers.
In summary, rhabdomyolysis in LPIN1 deficiency stems from uncontrolled inflammatory responses secondary to impaired endosomal autophagy, in parallel to energy depletion. Our report and others' [8,14] provide evidence for a role of dexamethsone in treatment of patients. Meanwhile, oral corticosteroids could be considered, early in the course of a flare up, to be started at home before presenting to a care facility. Acute crises can be severe, but respond well to a combination of IV fluids, adequate caloric intake with fat restriction and IV dexamethasone. Meanwhile, given that so far all the observations have been retrospective, findings should be interpreted with caution and further clinical trials are prompted. Furthermore, a possible genotype-related therapeutic response should be studied.
Data availability
The data that has been used is confidential.
References
- 1.Bergounioux J., Brassier A., Rambaud C., Bustarret O., Michot C., Hubert L., Arnoux J.B., Laquerriere A., Bekri S., Galene-Gromez S., Bonnet D., Hubert P., de Lonlay P. Fatal rhabdomyolysis in 2 children with LPIN1 mutations. J. Pediatr. 2012;160(6):1052–1054. doi: 10.1016/j.jpeds.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Donkor J., Sariahmetoglu M., Dewald J., Brindley D.N., Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 2007;282(6):3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 3.Finck B.N., Gropler M.C., Chen Z., Leone T.C., Croce M.A., Harris T.E., Lawrence J.C., Jr., Kelly D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4(3):199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Hamel Y., Mamoune A., Mauvais F.X., Habarou F., Lallement L., Romero N.B., Ottolenghi C., de Lonlay P. Acute rhabdomyolysis and inflammation. J. Inherit. Metab. Dis. 2015;38(4):621–628. doi: 10.1007/s10545-015-9827-7. [DOI] [PubMed] [Google Scholar]
- 5.Hamel Y., Mauvais F.X., Madrange M., Renard P., Lebreton C., Nemazanyy I., Pelle O., Goudin N., Tang X., Rodero M.P., Tuchmann-Durand C., Nusbaum P., Brindley D.N., van Endert P., de Lonlay P. Compromised mitochondrial quality control triggers lipin1-related rhabdomyolysis. Cell Rep. Med. 2021;2(8) doi: 10.1016/j.xcrm.2021.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.B., Kumar A., Wang L., Liu G.H., Keller S.R., Lawrence J.C., Jr., Finck B.N., Harris T.E. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol. Cell. Biol. 2010;30(12):3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legendre A., Khraiche D., Ou P., Mauvais F.X., Madrange M., Guemann A.S., Jais J.P., Bonnet D., Hamel Y., de Lonlay P. Cardiac function and exercise adaptation in 8 children with LPIN1 mutations. Mol. Genet. Metab. 2018;123(3):375–381. doi: 10.1016/j.ymgme.2017.12.429. [DOI] [PubMed] [Google Scholar]
- 8.Meijer I.A., Sasarman F., Maftei C., Rossignol E., Vanasse M., Major P., Mitchell G.A., Brunel-Guitton C. LPIN1 deficiency with severe recurrent rhabdomyolysis and persistent elevation of creatine kinase levels due to chromosome 2 maternal isodisomy. Mol. Genet. Metab. Rep. 2015;5:85–88. doi: 10.1016/j.ymgmr.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michot C., Hubert L., Brivet M., De Meirleir L., Valayannopoulos V., Muller-Felber W., Venkateswaran R., Ogier H., Desguerre I., Altuzarra C., Thompson E., Smitka M., Huebner A., Husson M., Horvath R., Chinnery P., Vaz F.M., Munnich A., Elpeleg O., Delahodde A., de Keyzer Y., de Lonlay P. LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Hum. Mutat. 2010;31(7):E1564–E1573. doi: 10.1002/humu.21282. [DOI] [PubMed] [Google Scholar]
- 10.Michot C., Mamoune A., Vamecq J., Viou M.T., Hsieh L.S., Testet E., Laine J., Hubert L., Dessein A.F., Fontaine M., Ottolenghi C., Fouillen L., Nadra K., Blanc E., Bastin J., Candon S., Pende M., Munnich A., Smahi A., Djouadi F., Carman G.M., Romero N., de Keyzer Y., de Lonlay P. Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim. Biophys. Acta. 2013;1832(12):2103–2114. doi: 10.1016/j.bbadis.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra M.S., Schilling J.D., Wang X., Jay P.Y., Huss J.M., Su X., Finck B.N. Cardiac lipin 1 expression is regulated by the peroxisome proliferator activated receptor gamma coactivator 1alpha/estrogen related receptor axis. J. Mol. Cell. Cardiol. 2011;51(1):120–128. doi: 10.1016/j.yjmcc.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reue K. The lipin family: mutations and metabolism. Curr. Opin. Lipidol. 2009;20(3):165–170. doi: 10.1097/MOL.0b013e32832adee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepien K.M., Schmidt W.M., Bittner R.E., O’Toole O., McNamara B., Treacy E.P. Long-term outcomes in a 25-year-old female affected with lipin-1 deficiency. JIMD Rep. 2019;46(1):4–10. doi: 10.1002/jmd2.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuchmann-Durand C., Roda C., Renard P., Mortamet G., Berat C.M., Altenburger L., de Larauz M.H., Thevenet E., Cottart C.H., Moulin F., Bouchereau J., Brassier A., Arnoux J.B., Schiff M., Bednarek N., Lamireau D., Garros A., Mention K., Cano A., Finger L., Pelosi M., Brochet C.S., Caccavelli L., Raphalen J.H., Renolleau S., Oualha M., de Lonlay P. Systemic corticosteroids for the treatment of acute episodes of rhabdomyolysis in lipin-1-deficient patients. J. Inherit. Metab. Dis. 2023 doi: 10.1002/jimd.12592. [DOI] [PubMed] [Google Scholar]
- 15.Zeharia A., Shaag A., Houtkooper R.H., Hindi T., de Lonlay P., Erez G., Hubert L., Saada A., de Keyzer Y., Eshel G., Vaz F.M., Pines O., Elpeleg O. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 2008;83(4):489–494. doi: 10.1016/j.ajhg.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P., Verity M.A., Reue K. Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 2014;20(2):267–279. doi: 10.1016/j.cmet.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.S. Tucci, N. Mingirulli, Z. Wehbe, V. I. Dumit, J. Kirschner, Mitochondrial fatty acid biosynthesis and muscle fiber plasticity in very long-chain acyl-CoA dehydrogenase-deficient mice, FEBS Lett 592 (2) 219–232, doi:10.1002/1873-3468.12940. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.