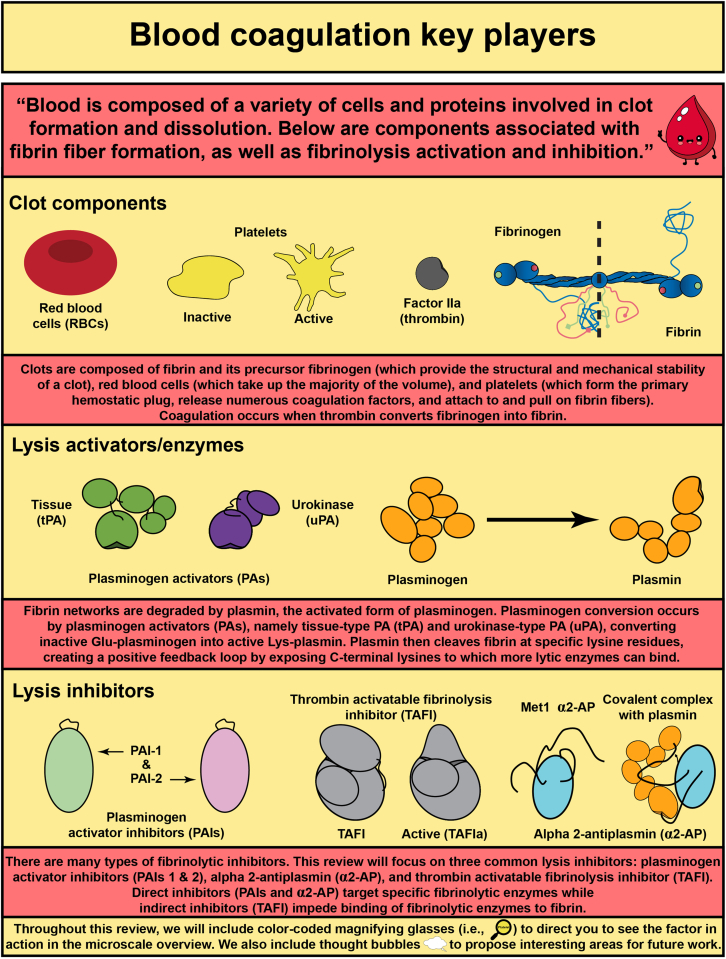
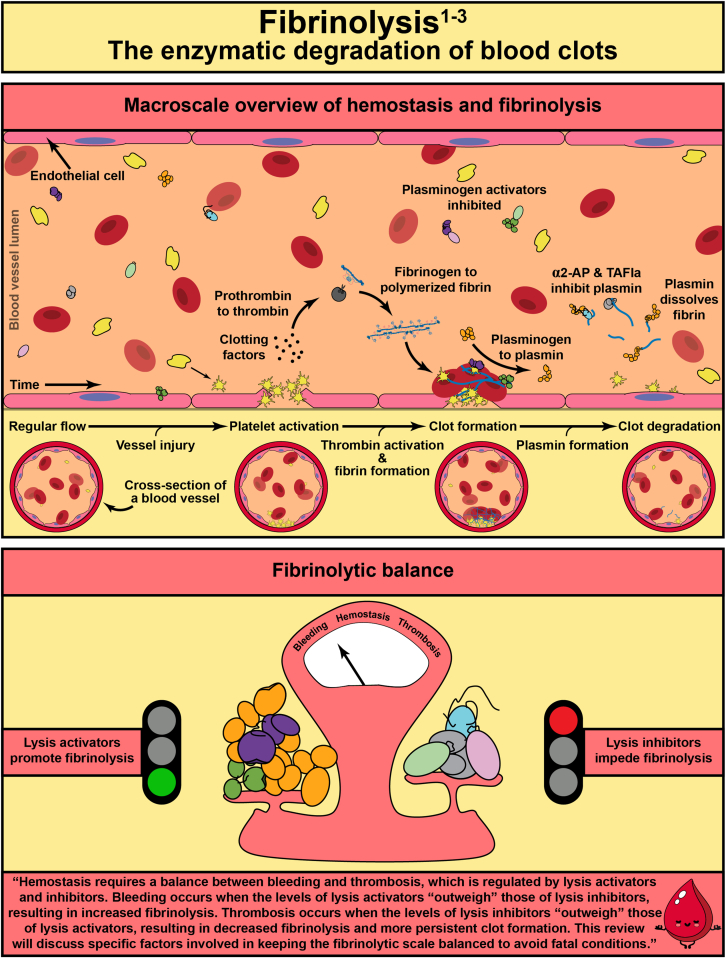
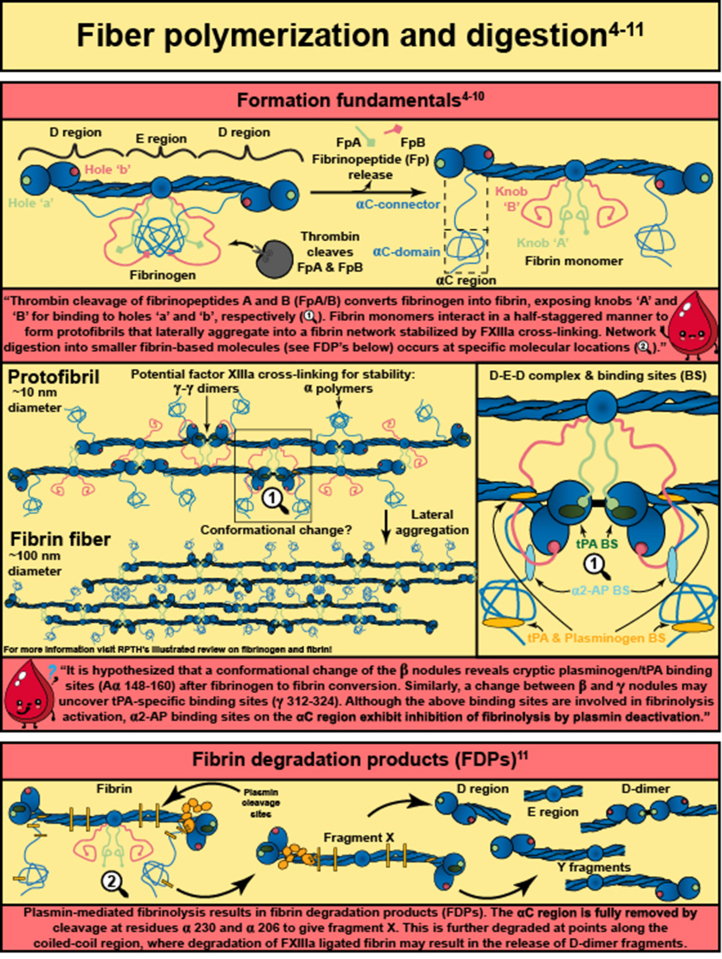
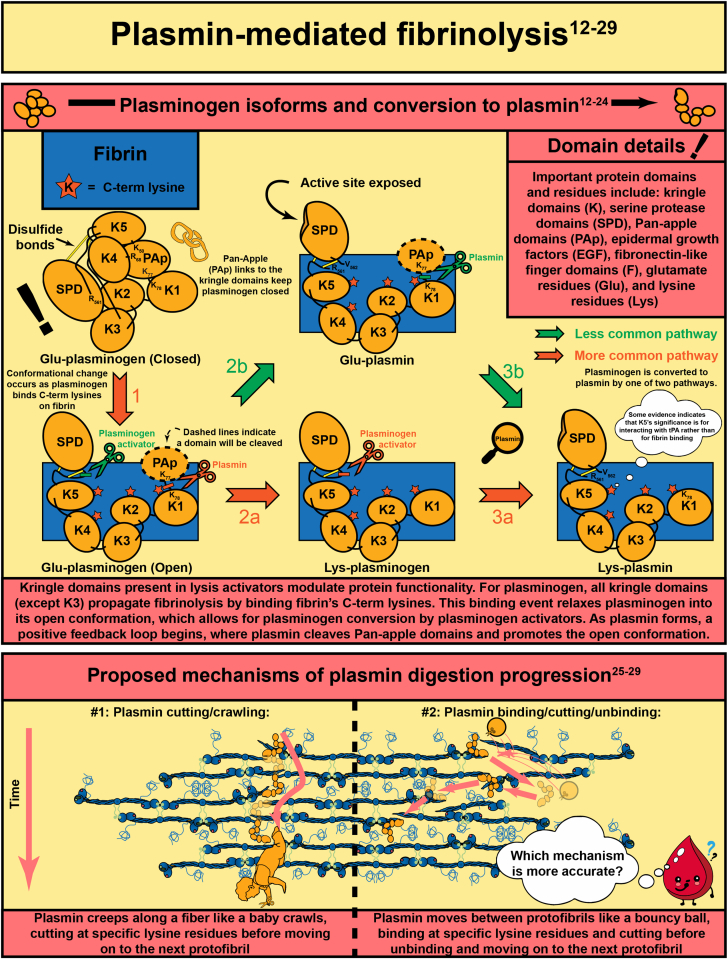
Abstract
In response to vessel injury (or other pathological conditions), the hemostatic process is activated, resulting in a fibrous, cellular-rich structure commonly referred to as a blood clot. Succeeding the clot’s function in wound healing, it must be resolved. This illustrated review focuses on fibrinolysis—the degradation of blood clots or thrombi. Fibrin is the main mechanical and structural component of a blood clot, which encases the cellular components of the clot, including platelets and red blood cells. Fibrinolysis is the proteolytic degradation of the fibrin network that results in the release of the cellular components into the bloodstream. In the case of thrombosis, fibrinolysis is required for restoration of blood flow, which is accomplished clinically through exogenously delivered lytic factors in a process called external lysis. Fibrinolysis is regulated by plasminogen activators (tissue-type and urokinase-type) that convert plasminogen into plasmin to initiate fiber lysis and lytic inhibitors that impede this lysis (plasminogen activator inhibitors, alpha 2-antiplasmin, and thrombin activatable fibrinolysis inhibitor). Furthermore, the network structure has been shown to regulate lysis: thinner fibers and coarser clots lyse faster than thicker fibers and finer clots. Clot contraction, a result of platelets pulling on fibers, results in densely packed red blood cells (polyhedrocytes), reduced permeability to fibrinolytic factors, and increased fiber tension. Extensive research in the field has allowed for critical advancements leading to improved thrombolytic agents. In this review, we summarize the state of the field, highlight gaps in knowledge, and propose future research questions.
Keywords: blood, blood coagulation, blood clot, clot lysis time, fibrinolysis, fibrin
Essentials
-
•
Fibrinolysis is the degradation of the fibrin network of a blood clot.
-
•
Fibrinolysis is required to achieve hemostatic balance.
-
•
Fibrinolysis can occur naturally in the body or with delivery of pharmacological agents.
-
•
Rate of fibrinolysis is affected by the fibrin structure and the clot’s cellular components.
Acknowledgments
NIH R00HL148646-01 (V.T.), New Jersey Commission for Cancer Research COCR22PRF010
(R.R.), NIH T32 GM135141 (R.R.), NIH R15HL148842 (N.E.H.), and R15HL150666 (N.E.H.)
Author contributions
R.A.R., N.C.K., B.B., N.H., and V.T. contributed to creating all illustrations and writing the manuscript. All authors read and approved the final version of the paper.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Funding information NIH R00HL148646-01 (V.T.), New Jersey Commission for Cancer Research COCR22PRF010 (R.R.), NIH T32 GM135141 (R.R.), NIH R15HL148842 (N.E.H.), and R15HL150666 (N.E.H.)
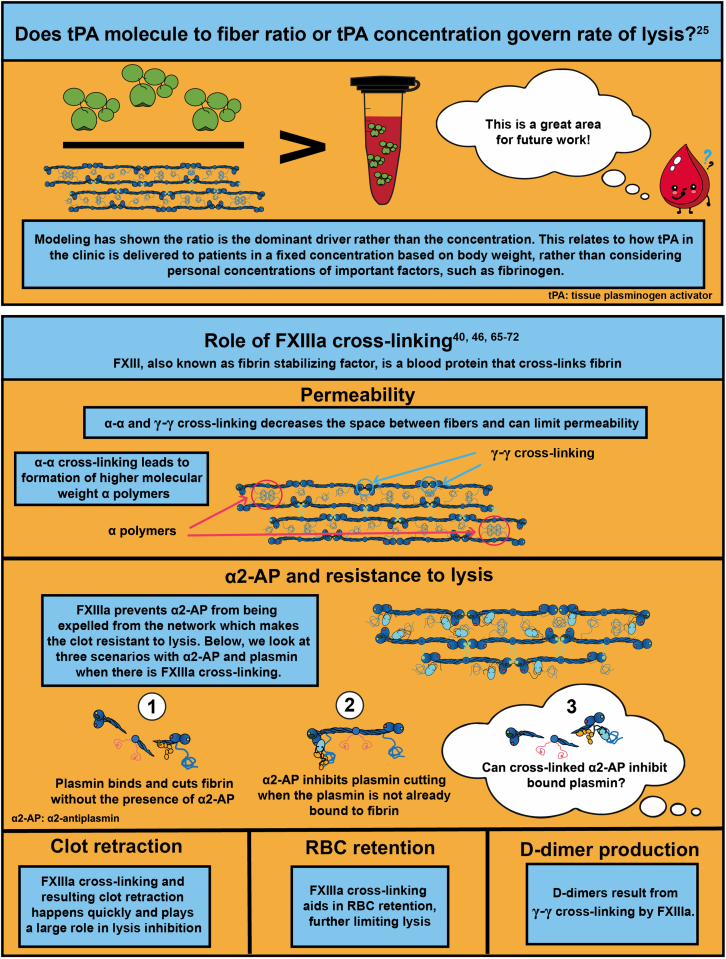
Handling Editor: M Sholzberg
Rebecca A. Risman and Nicholas C. Kirby contributed equally to this study.
References
- 1.Medved L., Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost. 2003;89:409–419. [PubMed] [Google Scholar]
- 2.Hudson N.E. Biophysical mechanisms mediating fibrin fiber lysis. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2748340. [DOI] [PMC free article] [PubMed] [Google Scholar]
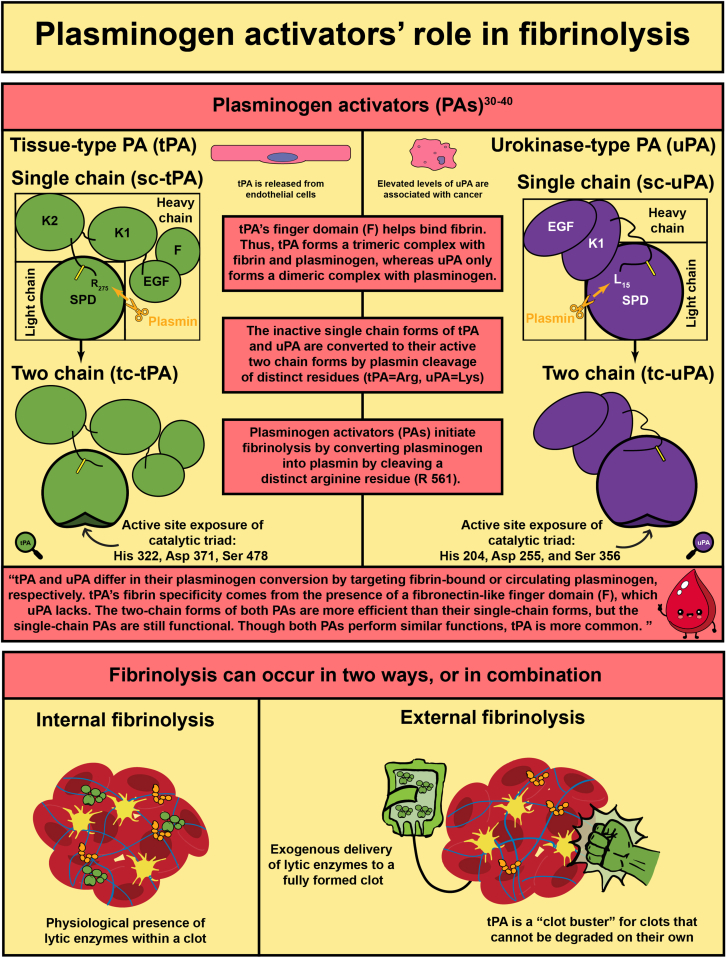
- 3.Longstaff C., Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost. 2015;13(Suppl 1):S98–S105. doi: 10.1111/jth.12935. [DOI] [PubMed] [Google Scholar]
- 4.Weisel J.W. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 5.Wolberg A.S. Plasma and cellular contributions to fibrin network formation, structure and stability. Haemophilia. 2010;16(Suppl 3):7–12. doi: 10.1111/j.1365-2516.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 6.Hasumi K., Yamamichi S., Harada T. Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems. FEBS J. 2010;277:3675–3687. doi: 10.1111/j.1742-4658.2010.07783.x. [DOI] [PubMed] [Google Scholar]
- 7.Lynch S.R., Laverty S.M., Bannish B.E., Hudson N.E. Microscale structural changes of individual fibrin fibers during fibrinolysis. Acta Biomater. 2022;141:114–122. doi: 10.1016/j.actbio.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakovlev S., Makogonenko E., Kurochkina N., Nieuwenhuizen W., Ingham K., Medved L. Conversion of fibrinogen to fibrin: mechanism of exposure of tPA- and plasminogen-binding sites. Biochemistry. 2000;39:15730–15741. doi: 10.1021/bi001847a. [DOI] [PubMed] [Google Scholar]
- 9.Pieters M., Wolberg A.S. Fibrinogen and fibrin: an illustrated review. Res Pract Thromb Haemost. 2019;3:161–172. doi: 10.1002/rth2.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker J.B., Nesheim M.E. The molecular weights, mass distribution, chain composition, and structure of soluble fibrin degradation products released from a fibrin clot perfused with plasmin. J Biol Chem. 1999;274:5201–5212. doi: 10.1074/jbc.274.8.5201. [DOI] [PubMed] [Google Scholar]
- 11.Henschen A., McDonagh J. In: Neuberger A., van Deenen L.L.M., editors. Vol. 13. Elsevier; 1986. Chapter 7 Fibrinogen, fibrin and factor XIII. (New Comprehensive Biochemistry). p. 171–241. [Google Scholar]
- 12.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mican J., Toul M., Bednar D., Damborsky J. Structural biology and protein engineering of thrombolytics. Comput Struct Biotechnol J. 2019;17:917–938. doi: 10.1016/j.csbj.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolev K., Tenekedjiev K., Komorowicz E., Machovich R. Functional evaluation of the structural features of proteases and their substrate in fibrin surface degradation. J Biol Chem. 1997;272:13666–13675. doi: 10.1074/jbc.272.21.13666. [DOI] [PubMed] [Google Scholar]
- 16.Lucas M.A., Fretto L.J., McKee P.A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983;258:4249–4256. [PubMed] [Google Scholar]
- 17.Wu G., Quek A.J., Caradoc-Davies T.T., Ekkel S.M., Mazzitelli B., Whisstock J.C., et al. Structural studies of plasmin inhibition. Biochem Soc Trans. 2019;47:541–557. doi: 10.1042/BST20180211. [DOI] [PubMed] [Google Scholar]
- 18.Miszta A., Huskens D., Donkervoort D., Roberts M.J.M., Wolberg A.S., de Laat B. Assessing plasmin generation in health and disease. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22052758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law R.H., Caradoc-Davies T., Cowieson N., Horvath A.J., Quek A.J., Encarnacao J.A., et al. The X-ray crystal structure of full-length human plasminogen. Cell Rep. 2012;1:185–190. doi: 10.1016/j.celrep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Urano T., Castellino F.J., Suzuki Y. Regulation of plasminogen activation on cell surfaces and fibrin. J Thromb Haemost. 2018;16:1487–1497. doi: 10.1111/jth.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva M.M., Thelwell C., Williams S.C., Longstaff C. Regulation of fibrinolysis by C-terminal lysines operates through plasminogen and plasmin but not tissue-type plasminogen activator. J Thromb Haemost. 2012;10:2354–2360. doi: 10.1111/j.1538-7836.2012.04925.x. [DOI] [PubMed] [Google Scholar]
- 22.Motta A., Laursen R.A., Llinas M., Tulinsky A., Park C.H. Complete assignment of the aromatic proton magnetic resonance spectrum of the kringle 1 domain from human plasminogen: structure of the ligand-binding site. Biochemistry. 1987;26:3827–3836. doi: 10.1021/bi00387a014. [DOI] [PubMed] [Google Scholar]
- 23.Petros A.M., Ramesh V., Llinas M. 1H NMR studies of aliphatic ligand binding to human plasminogen kringle 4. Biochemistry. 1989;28:1368–1376. doi: 10.1021/bi00429a064. [DOI] [PubMed] [Google Scholar]
- 24.Kim P.Y., Tieu L.D., Stafford A.R., Fredenburgh J.C., Weitz J.I. A high affinity interaction of plasminogen with fibrin is not essential for efficient activation by tissue-type plasminogen activator. J Biol Chem. 2012;287:4652–4661. doi: 10.1074/jbc.M111.317719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannish B.E., Chernysh I.N., Keener J.P., Fogelson A.L., Weisel J.W. Molecular and physical mechanisms of fibrinolysis and thrombolysis from mathematical modeling and experiments. Sci Rep. 2017;7:6914. doi: 10.1038/s41598-017-06383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
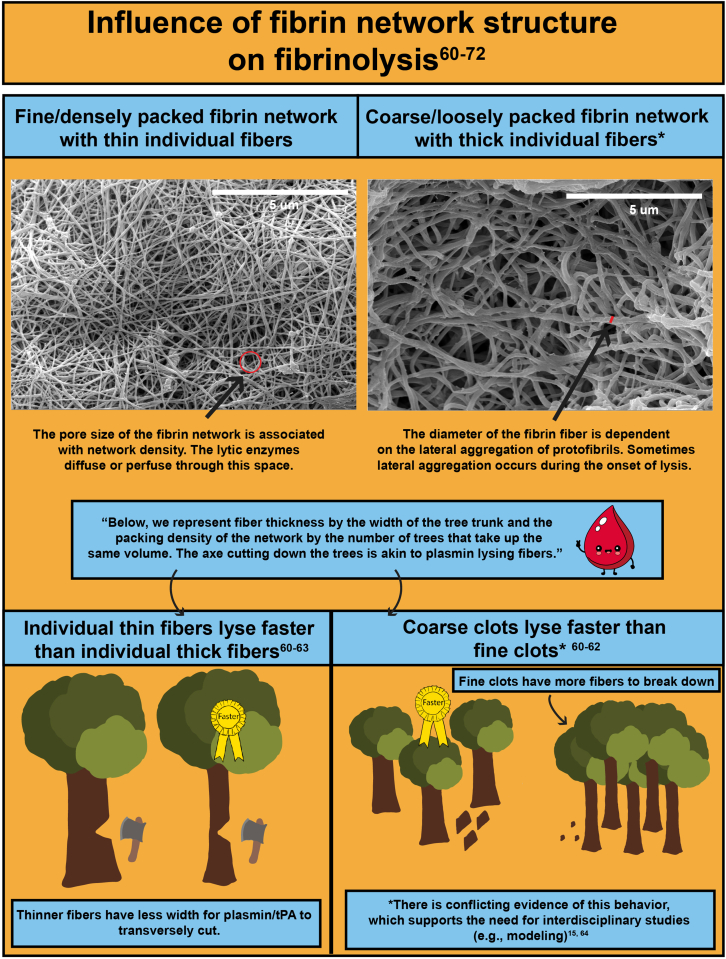
- 26.Gabriel D.A., Muga K., Boothroyd E.M. The effect of fibrin structure on fibrinolysis. J Biol Chem. 1992;267:24259–24263. [PubMed] [Google Scholar]
- 27.Weisel J.W., Litvinov R.I. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 28.Weisel J.W., Veklich Y., Collet J.P., Francis C.W. Structural studies of fibrinolysis by electron and light microscopy. Thromb Haemost. 1999;82:277–282. [PubMed] [Google Scholar]
- 29.Diamond S.L., Anand S. Inner clot diffusion and permeation during fibrinolysis. Biophys J. 1993;65:2622–2643. doi: 10.1016/S0006-3495(93)81314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voskuilen M., Vermond A., Veeneman G.H., van Boom J.H., Klasen E.A., Zegers N.D., et al. Fibrinogen lysine residue A alpha 157 plays a crucial role in the fibrin-induced acceleration of plasminogen activation, catalyzed by tissue-type plasminogen activator. J Biol Chem. 1987;262:5944–5946. [PubMed] [Google Scholar]
- 31.Chevilley A., Lesept F., Lenoir S., Ali C., Parcq J., Vivien D. Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front Cell Neurosci. 2015;9:415. doi: 10.3389/fncel.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoylaerts M., Rijken D.C., Lijnen H.R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- 33.Carr M.E., Alving B.M. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul Fibrinolysis. 1995;6:567–573. doi: 10.1097/00001721-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Zeslawska E., Schweinitz A., Karcher A., Sondermann P., Sperl S., Sturzebecher J., et al. Crystals of the urokinase type plasminogen activator variant beta(c)-uPAin complex with small molecule inhibitors open the way towards structure-based drug design. J Mol Biol. 2000;301:465–475. doi: 10.1006/jmbi.2000.3966. [DOI] [PubMed] [Google Scholar]
- 35.Renatus M., Bode W., Huber R., Sturzebecher J., Prasa D., Fischer S., et al. Structural mapping of the active site specificity determinants of human tissue-type plasminogen activator. Implications for the design of low molecular weight substrates and inhibitors. J Biol Chem. 1997;272:21713–21719. doi: 10.1074/jbc.272.35.21713. [DOI] [PubMed] [Google Scholar]
- 36.Renatus M., Engh R.A., Stubbs M.T., Huber R., Fischer S., Kohnert U., et al. Lysine 156 promotes the anomalous proenzyme activity of tPA: X-ray crystal structure of single-chain human tPA. EMBO J. 1997;16:4797–4805. doi: 10.1093/emboj/16.16.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamba D., Bauer M., Huber R., Fischer S., Rudolph R., Kohnert U., et al. The 2.3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J Mol Biol. 1996;258:117–135. doi: 10.1006/jmbi.1996.0238. [DOI] [PubMed] [Google Scholar]
- 38.Hébert M., Lesept F., Vivien D., Macrez R. The story of an exceptional serine protease, tissue-type plasminogen activator (tPA) Rev Neurol (Paris) 2016;172:186–197. doi: 10.1016/j.neurol.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Madunić J. The urokinase plasminogen activator system in human cancers: an overview of its prognostic and predictive role. Thromb Haemost. 2018;118:2020–2036. doi: 10.1055/s-0038-1675399. [DOI] [PubMed] [Google Scholar]
- 40.Mutch N.J., Thomas L., Moore N.R., Lisiak K.M., Booth N.A. TAFIa, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812–817. doi: 10.1111/j.1538-7836.2007.02430.x. [DOI] [PubMed] [Google Scholar]
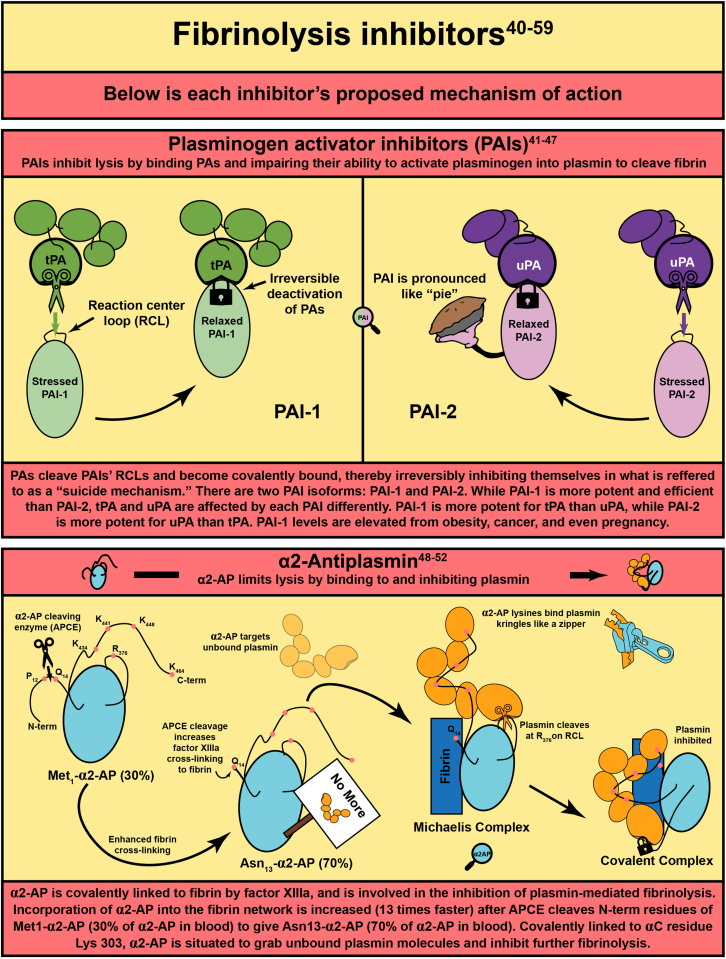
- 41.Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–2966. doi: 10.2741/2285. [DOI] [PubMed] [Google Scholar]
- 42.Cesari M., Pahor M., Incalzi R.A. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrop S.J., Jankova L., Coles M., Jardine D., Whittaker J.S., Gould A.R., et al. The crystal structure of plasminogen activator inhibitor 2 at 2.0 A resolution: implications for serpin function. Structure. 1999;7:43–54. doi: 10.1016/s0969-2126(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 44.Gong L., Liu M., Zeng T., Shi X., Yuan C., Andreasen P.A., et al. Crystal structure of the Michaelis complex between tissue-type plasminogen activator and plasminogen activators inhibitor-1. J Biol Chem. 2015;290:25795–25804. doi: 10.1074/jbc.M115.677567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruithof E.K., Tran-Thang C., Gudinchet A., Hauert J., Nicoloso G., Genton C., et al. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- 46.Fraser S.R., Booth N.A., Mutch N.J. The antifibrinolytic function of factor XIII is exclusively expressed through alpha(2)-antiplasmin cross-linking. Blood. 2011;117:6371–6374. doi: 10.1182/blood-2011-02-333203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thelwell C., Longstaff C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J Thromb Haemost. 2007;5:804–811. doi: 10.1111/j.1538-7836.2007.02422.x. [DOI] [PubMed] [Google Scholar]
- 48.Alshehri F.S.M., Whyte C.S., Mutch N.J. Factor XIII-A: an indispensable "factor" in haemostasis and wound healing. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22063055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Law R.H., Sofian T., Kan W.T., Horvath A.J., Hitchen C.R., Langendorf C.G., et al. X-ray crystal structure of the fibrinolysis inhibitor alpha2-antiplasmin. Blood. 2008;111:2049–2052. doi: 10.1182/blood-2007-09-114215. [DOI] [PubMed] [Google Scholar]
- 50.Muszbek L., Yee V.C., Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999;94:271–305. doi: 10.1016/s0049-3848(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 51.Schneider M., Nesheim M. A study of the protection of plasmin from antiplasmin inhibition within an intact fibrin clot during the course of clot lysis. J Biol Chem. 2004;279:13333–13339. doi: 10.1074/jbc.M313164200. [DOI] [PubMed] [Google Scholar]
- 52.Singh S., Saleem S., Reed G.L. Alpha2-antiplasmin: the devil you don't know in cerebrovascular and cardiovascular disease. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.608899. [DOI] [PMC free article] [PubMed] [Google Scholar]
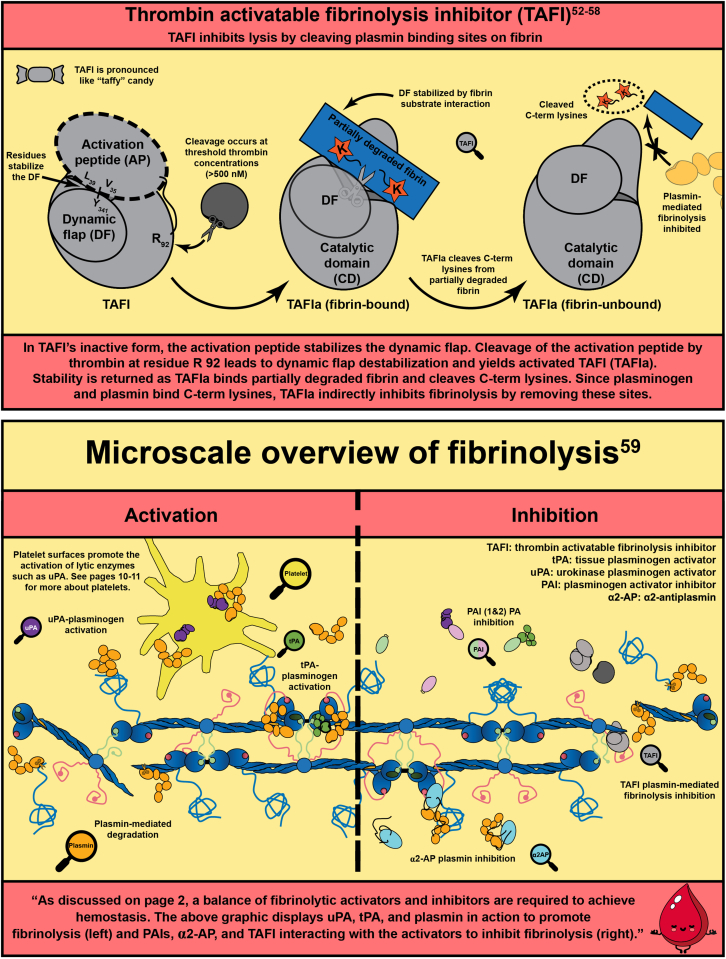
- 53.Marx P.F., Brondijk T.H., Plug T., Romijn R.A., Hemrika W., Meijers J.C., et al. Crystal structures of TAFI elucidate the inactivation mechanism of activated TAFI: a novel mechanism for enzyme autoregulation. Blood. 2008;112:2803–2809. doi: 10.1182/blood-2008-03-146001. [DOI] [PubMed] [Google Scholar]
- 54.Acquasaliente L., Pelc L.A., Di Cera E. Probing prothrombin structure by limited proteolysis. Sci Rep. 2019;9:6125. doi: 10.1038/s41598-019-42524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davie E.W., Kulman J.D. An overview of the structure and function of thrombin. Semin Thromb Hemost. 2006;32(Suppl 1):3–15. doi: 10.1055/s-2006-939550. [DOI] [PubMed] [Google Scholar]
- 56.Bouma B.N., Mosnier L.O. Thrombin activatable fibrinolysis inhibitor (TAFI)--how does thrombin regulate fibrinolysis? Ann Med. 2006;38:378–388. doi: 10.1080/07853890600852898. [DOI] [PubMed] [Google Scholar]
- 57.Bajzar L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler Thromb Vasc Biol. 2000;20:2511–2518. doi: 10.1161/01.atv.20.12.2511. [DOI] [PubMed] [Google Scholar]
- 58.Mutch N.J., Moore N.R., Wang E., Booth N.A. Thrombus lysis by uPA, scuPA and tPA is regulated by plasma TAFI. J Thromb Haemost. 2003;1:2000. doi: 10.1046/j.1538-7836.2003.00383.x. 7. [DOI] [PubMed] [Google Scholar]
- 59.Chapin J.C., Hajjar K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17–24. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collet J.P., Park D., Lesty C., Soria J., Soria C., Montalescot G., et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 61.Collet J.P., Lesty C., Montalescot G., Weisel J.W. Dynamic changes of fibrin architecture during fibrin formation and intrinsic fibrinolysis of fibrin-rich clots. J Biol Chem. 2003;278:21331–21335. doi: 10.1074/jbc.M212734200. [DOI] [PubMed] [Google Scholar]
- 62.Collet J.P., Soria J., Mirshahi M., Hirsch M., Dagonnet F.B., Caen J., et al. Dusart syndrome: a new concept of the relationship between fibrin clot architecture and fibrin clot degradability: hypofibrinolysis related to an abnormal clot structure. Blood. 1993;82:2462–2469. [PubMed] [Google Scholar]
- 63.Carr M.E. Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38:55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 64.Wu J.H., Siddiqui K., Diamond S.L. Transport phenomena and clot dissolving therapy: an experimental investigation of diffusion-controlled and permeation-enhanced fibrinolysis. Thromb Haemost. 1994;72:105–112. [PubMed] [Google Scholar]
- 65.Hethershaw E.L., Cilia La Corte A.L., Duval C., Ali M., Grant P.J., Ariens R.A., et al. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J Thromb Haemost. 2014;12:197–205. doi: 10.1111/jth.12455. [DOI] [PubMed] [Google Scholar]
- 66.Walton B.L., Byrnes J.R., Wolberg A.S. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015;13(Suppl 1):S208–S215. doi: 10.1111/jth.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantero M., Rojas H., Angles-Cano E., Marchi R. Fibrin gamma/gamma' influences the secretion of fibrinolytic components and clot structure. BMC Mol Cell Biol. 2019;20:47. doi: 10.1186/s12860-019-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aoki N. Clot retraction increases clot resistance to fibrinolysis by condensing alpha 2-plasmin inhibitor crosslinked to fibrin. Thromb Haemost. 1993;70:376. [PubMed] [Google Scholar]
- 69.Rijken D.C., Uitte de Willige S. Inhibition of fibrinolysis by coagulation factor XIII. Biomed Res Int. 2017;2017 doi: 10.1155/2017/1209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrnes J.R., Wolberg A.S. Newly-recognized roles of factor XIII in thrombosis. Semin Thromb Hemost. 2016;42:445–454. doi: 10.1055/s-0036-1571343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrnes J.R., Duval C., Wang Y., Hansen C.E., Ahn B., Mooberry M.J., et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood. 2015;126:1940–1948. doi: 10.1182/blood-2015-06-652263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutch N.J., Engel R., Uitte de Willige S., Philippou H., Ariens R.A. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115:3980–3988. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- 73.Kim O.V., Litvinov R.I., Alber M.S., Weisel J.W. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat Commun. 2017;8:1274. doi: 10.1038/s41467-017-00885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki Y., Sano H., Mochizuki L., Honkura N., Urano T. Activated platelet-based inhibition of fibrinolysis via thrombin-activatable fibrinolysis inhibitor activation system. Blood Adv. 2020;4:5501–5511. doi: 10.1182/bloodadvances.2020002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
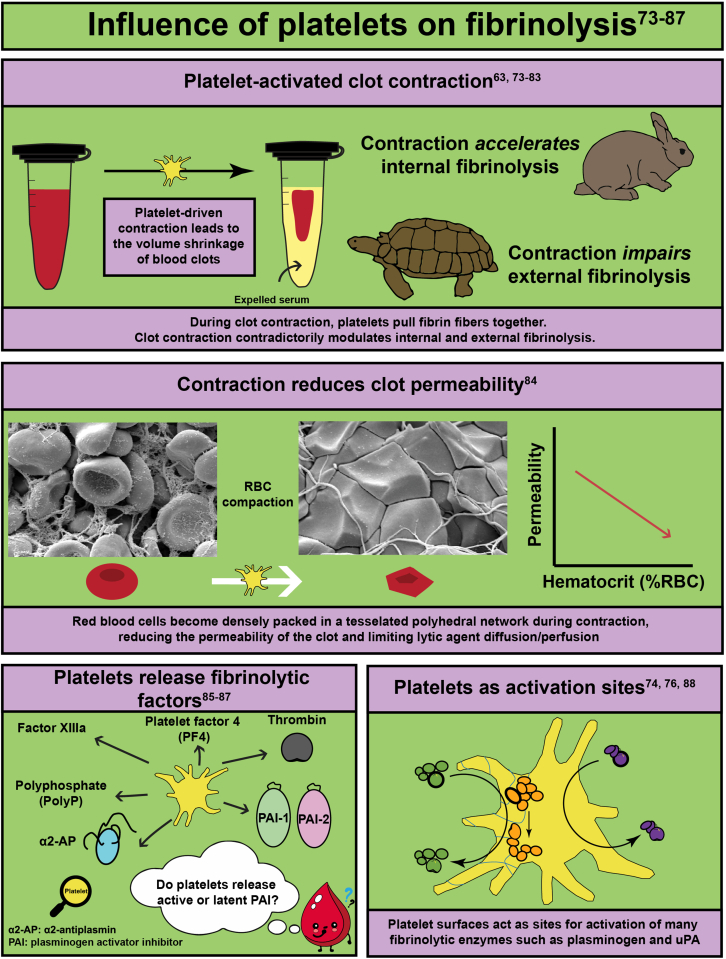
- 75.Carroll R.C., Gerrard J.M., Gilliam J.M. Clot retraction facilitates clot lysis. Blood. 1981;57:44–48. [PubMed] [Google Scholar]
- 76.Whyte C.S., Mitchell J.L., Mutch N.J. Platelet-mediated modulation of fibrinolysis. Semin Thromb Hemost. 2017;43:115–128. doi: 10.1055/s-0036-1597283. [DOI] [PubMed] [Google Scholar]
- 77.Cines D.B., Lebedeva T., Nagaswami C., Hayes V., Massefski W., Litvinov R.I., et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–1603. doi: 10.1182/blood-2013-08-523860. [DOI] [PMC free article] [PubMed] [Google Scholar]
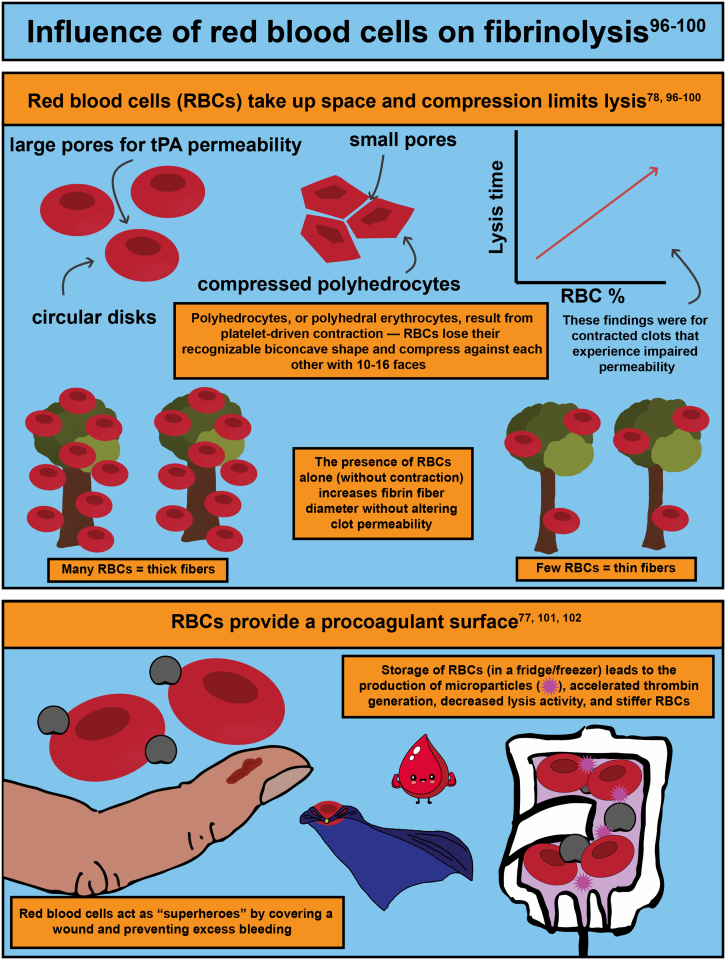
- 78.Tutwiler V., Mukhitov A.R., Peshkova A.D., Le Minh G., Khismatullin R.R., Vicksman J., et al. Shape changes of erythrocytes during blood clot contraction and the structure of polyhedrocytes. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tutwiler V., Peshkova A.D., Le Minh G., Zaitsev S., Litvinov R.I., Cines D.B., et al. Blood clot contraction differentially modulates internal and external fibrinolysis. J Thromb Haemost. 2019;17:361–370. doi: 10.1111/jth.14370. [DOI] [PubMed] [Google Scholar]
- 80.Sabovic M., Lijnen H.R., Keber D., Collen D. Effect of retraction on the lysis of human clots with fibrin specific and non-fibrin specific plasminogen activators. Thromb Haemost. 1989;62:1083–1087. [PubMed] [Google Scholar]
- 81.Blinc A., Keber D., Lahajnar G., Zupancic I., Zorec-Karlovsek M., Demsar F. Magnetic resonance imaging of retracted and nonretracted blood clots during fibrinolysis in vitro. Haemostasis. 1992;22:195–201. doi: 10.1159/000216319. [DOI] [PubMed] [Google Scholar]
- 82.Matveyev M., Domogatsky S.P. Penetration of macromolecules into contracted blood clot. Biophys J. 1992;63:862–863. doi: 10.1016/S0006-3495(92)81656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samson A.L., Alwis I., Maclean J.A.A., Priyananda P., Hawkett B., Schoenwaelder S.M., et al. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood. 2017;130:2453–2462. doi: 10.1182/blood-2017-06-789032. [DOI] [PubMed] [Google Scholar]
- 84.Kunitada S., FitzGerald G.A., Fitzgerald D.J. Inhibition of clot lysis and decreased binding of tissue-type plasminogen activator as a consequence of clot retraction. Blood. 1992;79:1420–1427. [PubMed] [Google Scholar]
- 85.Plow E.F., Collen D. The presence and release of alpha 2-antiplasmin from human platelets. Blood. 1981;58:1069–1074. [PubMed] [Google Scholar]
- 86.Brogren H., Karlsson L., Andersson M., Wang L., Erlinge D., Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–3948. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
- 87.Deguchi K., Shirakawa S. Plasminogen activation by tissue plasminogen activator in the presence of platelets. Thromb Res Suppl. 1988;8:65–72. doi: 10.1016/0049-3848(88)90155-7. [DOI] [PubMed] [Google Scholar]
- 88.Ni R., Neves M.A.D., Wu C., Cerroni S.E., Flick M.J., Ni H., et al. Activated thrombin-activatable fibrinolysis inhibitor (TAFIa) attenuates fibrin-dependent plasmin generation on thrombin-activated platelets. J Thromb Haemost. 2020;18:2364–2376. doi: 10.1111/jth.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
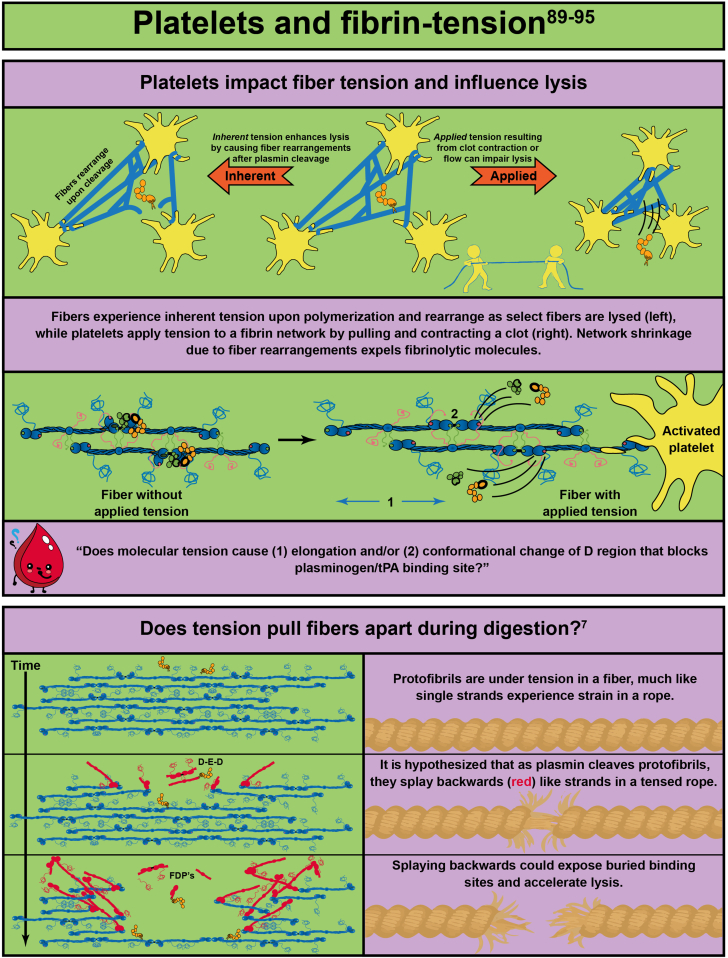
- 89.Varju I., Sotonyi P., Machovich R., Szabo L., Tenekedjiev K., Silva M.M., et al. Hindered dissolution of fibrin formed under mechanical stress. J Thromb Haemost. 2011;9:979–986. doi: 10.1111/j.1538-7836.2011.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown A.E., Litvinov R.I., Discher D.E., Purohit P.K., Weisel J.W. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325:741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam W.A., Chaudhuri O., Crow A., Webster K.D., Li T.D., Kita A., et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collet J.P., Montalescot G., Lesty C., Weisel J.W. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–434. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- 93.Cone S.J., Fuquay A.T., Litofsky J.M., Dement T.C., Carolan C.A., Hudson N.E. Inherent fibrin fiber tension propels mechanisms of network clearance during fibrinolysis. Acta Biomater. 2020;107:164–177. doi: 10.1016/j.actbio.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiewak R., Gosselin A., Merinov D., Litvinov R.I., Weisel J.W., Tutwiler V., et al. Biomechanical origins of inherent tension in fibrin networks. J Mech Behav Biomed Mater. 2022;133 doi: 10.1016/j.jmbbm.2022.105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feller T., Harsfalvi J., Csanyi C., Kiss B., Kellermayer M. Plasmin-driven fibrinolysis in a quasi-two-dimensional nanoscale fibrin matrix. J Struct Biol. 2018;203:273–280. doi: 10.1016/j.jsb.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Choi M.H., Park G.H., Lee J.S., Lee S.E., Lee S.J., Kim J.H., et al. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke. 2018;49:652–659. doi: 10.1161/STROKEAHA.117.019138. [DOI] [PubMed] [Google Scholar]
- 97.Gersh K.C., Nagaswami C., Weisel J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wohner N., Sotonyi P., Machovich R., Szabo L., Tenekedjiev K., Silva M.M., et al. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31:2306–2313. doi: 10.1161/ATVBAHA.111.229088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wautier M.P., Heron E., Picot J., Colin Y., Hermine O., Wautier J.L. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J Thromb Haemost. 2011;9:1049–1055. doi: 10.1111/j.1538-7836.2011.04251.x. [DOI] [PubMed] [Google Scholar]
- 100.Whelihan M.F., Zachary V., Orfeo T., Mann K.G. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weisel J.W., Litvinov R.I. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271–282. doi: 10.1111/jth.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levin G., Sukhareva E., Lavrentieva A. Impact of microparticles derived from erythrocytes on fibrinolysis. J Thromb Thrombolysis. 2016;41:452–458. doi: 10.1007/s11239-015-1299-y. [DOI] [PubMed] [Google Scholar]
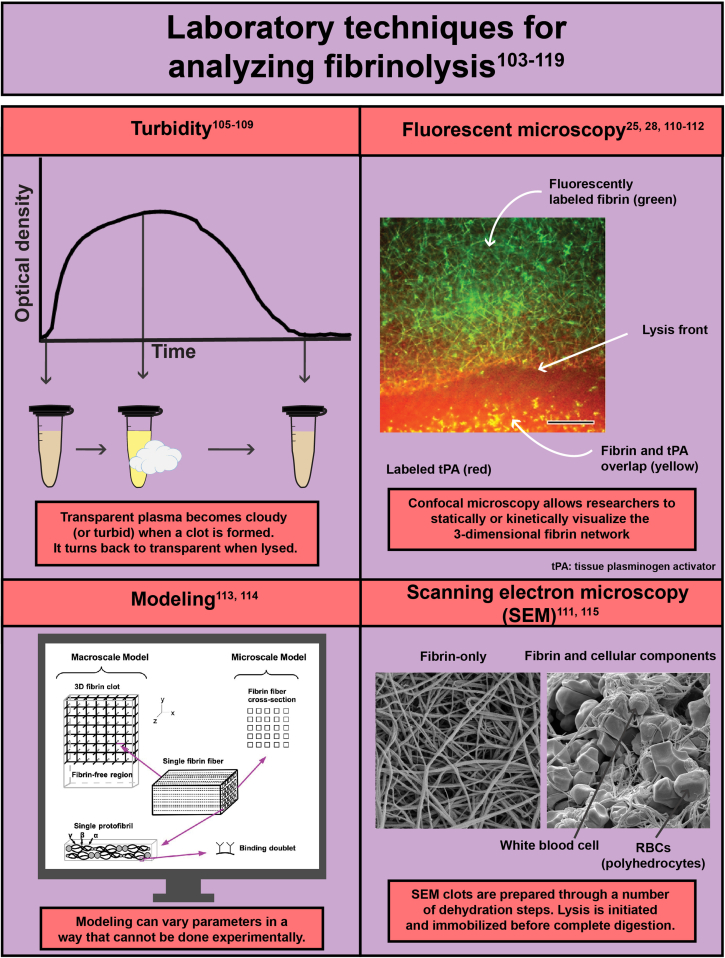
- 103.Longstaff C. Measuring fibrinolysis. Hamostaseologie. 2021;41:69–75. doi: 10.1055/a-1325-0268. [DOI] [PubMed] [Google Scholar]
- 104.Ilich A., Bokarev I., Key N.S. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39:441–447. doi: 10.1111/ijlh.12688. [DOI] [PubMed] [Google Scholar]
- 105.Pieters M., Philippou H., Undas A., de Lange Z., Rijken D.C., Mutch N.J., et al. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1007–1012. doi: 10.1111/jth.14002. [DOI] [PubMed] [Google Scholar]
- 106.Risman R.A., Abdelhamid A., Weisel J.W., Bannish B.E., Tutwiler V. Effects of clot contraction on clot degradation: a mathematical and experimental approach. Biophys J. 2022;121:3271–3285. doi: 10.1016/j.bpj.2022.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carter A.M., Cymbalista C.M., Spector T.D., Grant P.J., EuroCLOT Investigators Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb Vasc Biol. 2007;27:2783–2789. doi: 10.1161/ATVBAHA.107.153221. [DOI] [PubMed] [Google Scholar]
- 108.Lisman T., de Groot P.G., Meijers J.C., Rosendaal F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 109.Zeng Z., Nallan Chakravarthula T., Muralidharan C., Hall A., Linnemann A.K., Alves N.J. Fluorescently conjugated annular fibrin clot for multiplexed real-time digestion analysis. J Mater Chem B. 2021;9:9295–9307. doi: 10.1039/d1tb02088a. [DOI] [PubMed] [Google Scholar]
- 110.Bannish B.E., Hudson N.E. The utility and potential of mathematical models in predicting fibrinolytic outcomes. Curr Opin Biomed Eng. 2021:20. doi: 10.1016/j.cobme.2021.100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Longstaff C., Thelwell C., Williams S.C., Silva M.M., Szabo L., Kolev K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: kinetic and microscopic studies. Blood. 2011;117:661–668. doi: 10.1182/blood-2010-06-290338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kocevar K., Blinc R., Musevic Atomic force microscope evidence for the existence of smecticlike surface layers in the isotropic phase of a nematic liquid crystal. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;62:R3055–R3058. doi: 10.1103/physreve.62.r3055. [DOI] [PubMed] [Google Scholar]
- 113.Bannish B.E., Keener J.P., Fogelson A.L. Modelling fibrinolysis: a 3D stochastic multiscale model. Math Med Biol. 2014;31:17–44. doi: 10.1093/imammb/dqs029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anand M., Rajagopal K., Rajagopal K.R. A model for the formation and lysis of blood clots. Pathophysiol Haemost Thromb. 2005;34:109–120. doi: 10.1159/000089931. [DOI] [PubMed] [Google Scholar]
- 115.Veklich Y., Francis C.W., White J., Weisel J.W. Structural studies of fibrinolysis by electron microscopy. Blood. 1998;92:4721–4729. [PubMed] [Google Scholar]
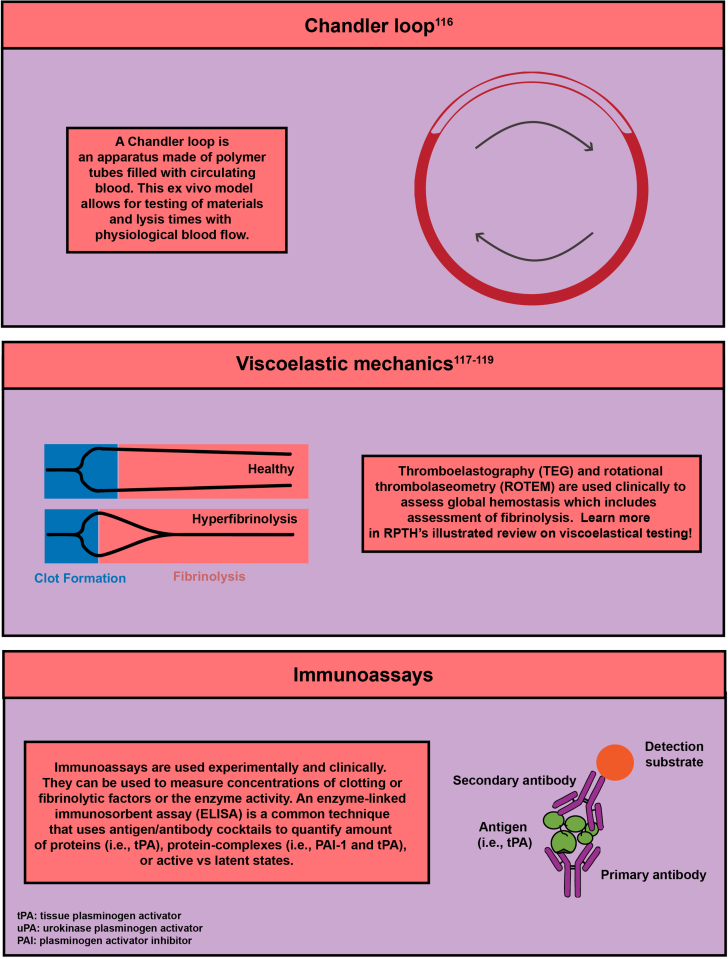
- 116.Whyte C.S., Mostefai H.A., Baeten K.M., Lucking A.J., Newby D.E., Booth N.A., et al. Role of shear stress and tPA concentration in the fibrinolytic potential of thrombi. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22042115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harr J.N., Moore E.E., Chin T.L., Chapman M.P., Ghasabyan A., Stringham J.R., et al. Viscoelastic hemostatic fibrinogen assays detect fibrinolysis early. Eur J Trauma Emerg Surg. 2015;41:49–56. doi: 10.1007/s00068-014-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ryan E.A., Mockros L.F., Weisel J.W., Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hartmann J., Hermelin D., Levy J.H. Viscoelastic testing an illustrated review of technology and clinical applications. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2022.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
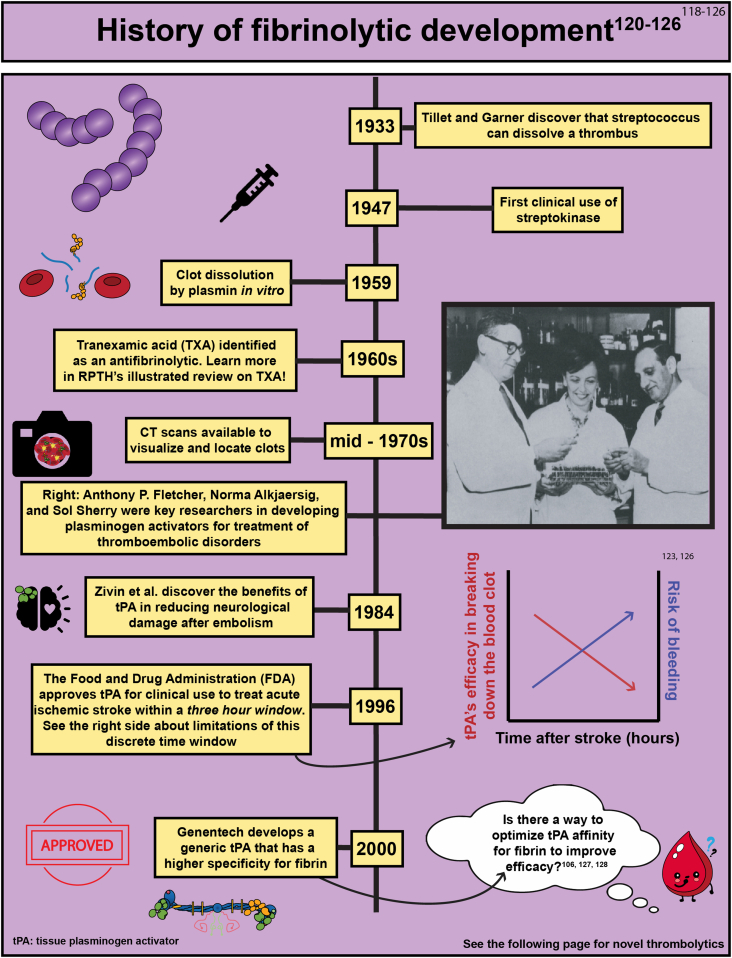
- 120.Tillett W.S., Garner R.L. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933;58:485–502. doi: 10.1084/jem.58.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alkjaersig N., Fletcher A.P., Sherry S. The mechanism of clot dissolution by plasmin. J Clin Invest. 1959;38:1086–1095. doi: 10.1172/JCI103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rother J., Ford G.A., Thijs V.N. Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities. Cerebrovasc Dis. 2013;35:313–319. doi: 10.1159/000348705. [DOI] [PubMed] [Google Scholar]
- 123.Zivin J.A., Fisher M., DeGirolami U., Hemenway C.C., Stashak J.A. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 124.Relke N., Chornenki N.L.J., Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sherry S. The origin of thrombolytic therapy. J Am Coll Cardiol. 1989;14:1085–1092. doi: 10.1016/0735-1097(89)90493-2. [DOI] [PubMed] [Google Scholar]
- 126.Liu X. Beyond the time window of intravenous thrombolysis: standing by or by stenting? Interv Neurol. 2012;1:3–15. doi: 10.1159/000338389. [DOI] [PMC free article] [PubMed] [Google Scholar]
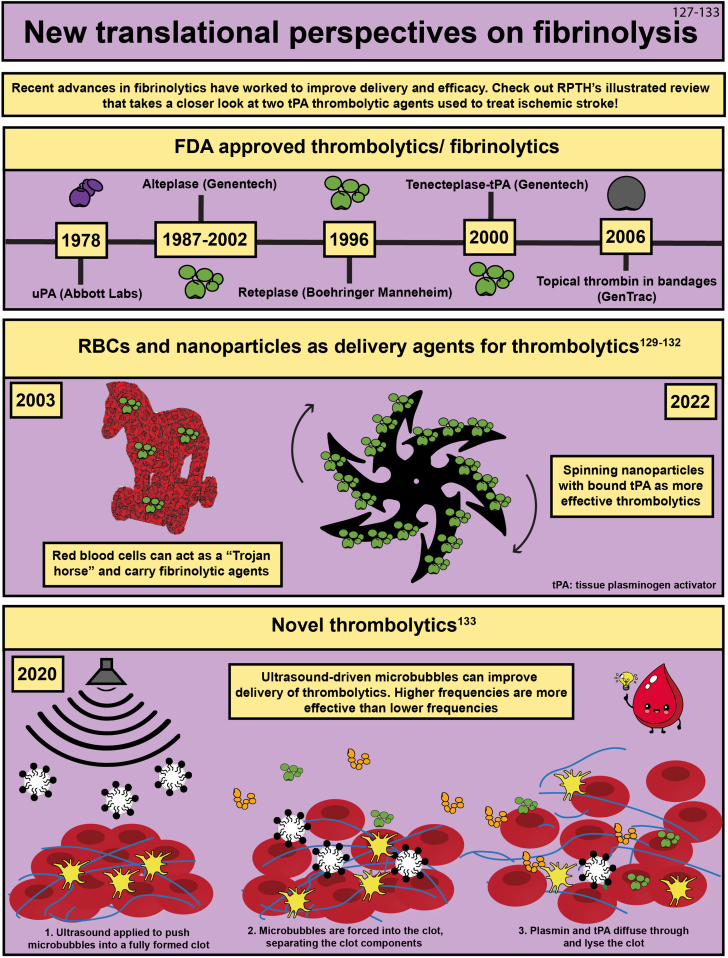
- 127.Gurewich V., Pannell R., Simmons-Byrd A., Sarmientos P., Liu J.N., Badylak S.F. Thrombolysis vs. bleeding from hemostatic sites by a prourokinase mutant compared with tissue plasminogen activator. J Thromb Haemost. 2006;4:1559–1565. doi: 10.1111/j.1538-7836.2006.01993.x. [DOI] [PubMed] [Google Scholar]
- 128.Cook S.M., Skora A., Gillen C.M., Walker M.J., McArthur J.D. Streptokinase variants from Streptococcus pyogenes isolates display altered plasminogen activation characteristics - implications for pathogenesis. Mol Microbiol. 2012;86:1052–1062. doi: 10.1111/mmi.12037. [DOI] [PubMed] [Google Scholar]
- 129.Zhu A., Rajendram P., Tseng E., Coutts S.B., Yu A.Y.X. Alteplase or tenecteplase for thrombolysis in ischemic stroke: an illustrated review. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Villa C.H., Pan D.C., Johnston I.H., Greineder C.F., Walsh L.R., Hood E.D., et al. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2018;2:165–176. doi: 10.1182/bloodadvances.2017011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murciano J.C., Medinilla S., Eslin D., Atochina E., Cines D.B., Muzykantov V.R. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21:891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 132.Disharoon D., Trewyn B.G., Herson P.S., Marr D.W.M., Neeves K.B. Breaking the fibrinolytic speed limit with microwheel co-delivery of tissue plasminogen activator and plasminogen. J Thromb Haemost. 2022;20:486–497. doi: 10.1111/jth.15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S., Guo X., Xiu W., Liu Y., Ren L., Xiao H., et al. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz8204. [DOI] [PMC free article] [PubMed] [Google Scholar]