Abstract
Background:
Parabens are antimicrobial agents prevalently found in daily-use products that can interfere with the endocrine and reproductive systems. In this study, we examined the cross-sectional associations of parabens with hot flashes, hormone concentrations, and ovarian volume in a subsample of 101 nonsmoking, non-Hispanic 45- to 54-year-old women from the Midlife Women's Health Study.
Materials and Methods:
Women self-reported their hot flash history and underwent a transvaginal ultrasound to measure ovarian volume. Participants provided blood for quantification of serum hormones (by enzyme-linked immunosorbent assay or radioimmunoassay) and urine samples for measurements of urinary paraben biomarker levels (by high-performance liquid chromatography negative-ion electrospray ionization-tandem mass spectrometry). Linear or logistic regression models evaluated associations of specific gravity-adjusted paraben biomarker concentrations with hot flashes, hormone concentrations, and ovarian volume.
Results:
We observed marginal associations of propylparaben, methylparaben, and ∑parabens biomarkers (molar sum of four parabens) with hot flashes and follicle-stimulating hormone (FSH) concentrations, and of these paraben biomarkers and ethylparaben with ovarian volume. For example, women tended to have 32% (95% confidence intervals [CI]: 0.9 to 1.81), 40% (95% CI: 1.0 to 1.95), and 40% (95% CI: 0.98 to 2.01) higher odds of having recent, monthly, and mild hot flashes, respectively, for every two-fold increase in ∑parabens. Similarly, women tended to have 14.54% (95% CI: −0.10 to 31.32) higher FSH concentrations, but 5.67% (95% CI: −12.54 to 1.75) reduced ovarian volume for every two-fold increase in ∑parabens
Conclusions:
Overall, our preliminary findings suggest that urinary paraben biomarkers may be associated with menopause-related outcomes in midlife women. Additional studies in larger and diverse populations are needed to expand on these findings.
Keywords: parabens, hot flashes, FSH, ovary, hormone, menopause
Introduction
As women age and approach reproductive senescence, the ovary progressively loses its follicular reserve, leading to a decline in sex-steroid hormone levels. This occurs in conjunction with altered secretion of pituitary hormones such as increased follicle-stimulating hormone (FSH) and reduced anti-Müllerian hormone (AMH).1 The timing of reproductive senescence and its associated physiological effects vary between women. Although women's median age at reproductive senescence/menopause is 50–52 years, others may enter menopause at an earlier age.2,3 This is concerning because early age at menopause increases the risk of various medical conditions such as cognitive impairment, cardiovascular disease, osteoporosis, and menopausal symptoms.4
Symptoms related to reproductive senescence have substantial negative impacts on women's daily function and health, and lead to overall social burden. For example, menopausal hot flashes are reported by millions of women every year and the majority of these women will continue having hot flashes for >7 years.5–9 Hot flashes are associated with sleep disturbances, fatigue, irritability, forgetfulness, mood swings, and substantial physical discomfort.7 Finally, according to a recent report, hot flashes result in an 89% indirect loss in work productivity, substantial yearly losses in wages, and increases in health care expenditures.10 Although early reproductive aging severely impairs women's quality of life, the precipitating factors are not fully known. In particular, little is known about the effects of environmental exposures to endocrine-mimicking chemicals on reproductive senescence and its associated symptoms.
Parabens are a group of p-hydroxybenzoic acid esters that are widely used as antimicrobial preservatives in numerous personal care products, foods, and pharmaceuticals, since the early 1920s (reviewed by Nowak et al.).11 The relatively low costs of production, ease of use, and stability make them industry favorites.11 Methyl-, ethyl-, propyl-, and butyl-parabens are the most widely used parabens. In the body, parabens are rapidly absorbed through the skin and the digestive system.11 Recent findings suggest that parabens are endocrine disrupting chemicals as they exhibit a limited potency as weak androgenic and estrogenic compounds.11 Owing to concerns regarding the safety of use of parabens, some countries restricted or banned their use. For example, in 2014 the European Union banned the use of iso-butylparaben and iso-propylparaben.11
Women are likely at a higher risk of the potential harmful effects of paraben because urinary paraben biomarker levels (biomarkers of paraben exposure) are higher in women compared with men.12,13 This is alarming because parabens are associated with altered sex-steroid hormone levels,14 irregular menstrual cyclicity,15 and decreased odds of successful pregnancy in women undergoing fertility treatments.16 Experimental results also indicate that parabens can target ovarian follicles and alter their steroidogenic capacity.17–22 Yet, the scientific literature about the reprotoxic effects of parabens is extremely limited. Thus, in this pilot study, we hypothesized that higher urinary paraben biomarker concentrations are associated with increased presentation of reproductive aging indicators in midlife women. We aimed to determine if urinary paraben biomarker concentrations are associated with (1) higher risk of hot flashes, (2) lower sex-steroid hormones levels, and (3) diminished ovarian reserve, in generally healthy midlife women.
Materials and Methods
Ethical approval
All participants gave written informed consent according to procedures approved by the University of Illinois at Urbana-Champaign and Johns Hopkins University Institutional Review Boards (File No.: 06741).
Study design
This pilot study is a cross-sectional analysis of data collected as part of the Midlife Women's Health Study (MWHS). The parent study design was previously described in detail.23 In brief, between 2007 and 2015 women residing in Baltimore city (MD, USA) and its surrounding counties were enrolled in the MWHS. The study cohort included women with and without naturally occurring hot flashes between the ages of 45 and 54 years. Eligibility criteria allowed recruitment of women who had intact ovaries and uterus, were not pregnant, not taking hormone therapy, oral contraceptives, or natural agents for treatment of menopausal symptoms, and not being treated for any type of cancer.
In addition, during the initial enrollment to the study (baseline), all women were either late-premenopausal or perimenopausal, but not postmenopausal. Menopausal status was determined using the Stages of Reproductive Aging Workshop +10 (STRAW +10) criteria,24 which determined menopausal status based on the women's responses to questions regarding their menstrual cycles. Specifically, premenopausal women were those who experienced their last menstrual period within the past 3 months and reported 11 or more periods within the past year. Perimenopausal women were those who experienced: (1) their last menstrual period within the past year, but not within the past 3 months or (2) their last menstrual period within the past 3 months and experienced 10 or fewer periods within the past year. Postmenopausal women were considered those who reported not having a menstrual period within the past year. The current pilot study included a subsample of 101 eligible non-Hispanic white women who never smoked and had their urine samples analyzed for paraben biomarker concentrations.
Collection of sociodemographic and lifestyle characteristics
Women completed a self-administered questionnaire at baseline (time of enrollment), which asked questions pertaining to sociodemographic, lifestyle, and health characteristics. The questionnaire collected information about the following sociodemographic and reproductive characteristics: age, educational attainment, employment status, annual household income, marital status, and parity. Women answered “yes” or “no” to the question “In the last 12 months have you had at least 12 drinks of any kind of alcoholic beverage” to determine recent alcohol consumption status. Medication use was determined based on if women reported using at least one medication. In addition, women visited a local clinic where they had their weights and heights recorded to calculate their midlife body mass index (BMI; kg/m2).
Assessment of hot flashes
Hot flash assessment is described in a recent publication by Warner et al.25 In brief, the self-administered questionnaire included several questions on hot flash history. Women's responses at the baseline visit were used for the current analysis. Specifically, women were asked if they had ever experienced hot flashes. Women who responded “no” were categorized as “never had hot flashes” and were prompted to skip the questions related to hot flashes history. Women who responded “yes” were asked (1) if they experienced recent hot flashes in the past 30 days (“yes” or “no”), (2) what was the frequency of hot flashes, and (3) what was the usual severity of hot flashes. Recent hot flashes were categorized as a two-level variable with the categories “had hot flashes in the past 30 days” and “never had hot flashes” by excluding women who “did not have hot flashes in the past 30 days.”
To evaluate hot flash frequency, each woman was asked to report if her hot flashes occur every hour, every 2–5 hours, every 6–11 hours, every 12–23 hours, 1–2 days per week, 5–6 days per week, 2–3 days per month, 1 day per month, less than 1 day per month, or never. We categorized hot flash frequency as a three-level variable with the categories “daily/weekly hot flashes,” “monthly hot flashes,” and “never had hot flashes.” To evaluate hot flash severity, each woman was asked to describe her hot flashes as follows: mild (sensation of heat without sweating), moderate (sensation of heat with sweating), or severe (sensation of heat with sweating that disrupts usual activity). We categorized hot flash severity as a three-level variable with the categories “moderate/severe hot flashes,” “mild hot flashes,” and “never had hot flashes.”
Measurement of hormone concentrations
Measurement of hormones and sex hormone binding globulin (SHBG) is detailed in a recent publication by Chiang et al.26 In brief, serum estradiol, testosterone, progesterone, and SHBG concentrations were measured from up to four blood samples (collected during the first year of the study) by enzyme-linked immunosorbent assays (ELISA; DRG International, NJ). The geometric means of these hormones were used in all subsequent statistical analyses. Serum AMH and FSH were assessed in one blood sample collected at the baseline clinic visit. Measurement of FSH (ELISA) and AMH (radioimmunoassay) levels were conducted at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, United States.26 Because 53% of women had AMH levels below the limit of quantification (LOQ), we categorized AMH as follows: women with levels ≥LOQ versus those with levels <LOQ.
Measurement of ovarian volume
Measurement of ovarian volume is described in Gallicchio et al.27 In brief, during the baseline clinic visit, women also received a transvaginal ultrasound to measure ovarian volume. Ovarian volume was calculated using the formula (length × width × height × 0.526). We used the mean of ovarian volumes for women who had data on both the left and right ovaries, whereas for those who only had data on one ovary, we used the ovarian volume of that one ovary. Ovarian volumes >30 cm3 were excluded from the analysis because these large volumes are indicative of an ovarian cyst.
Measurement of paraben biomarkers in urine samples
Spot urine specimens collected at the initial baseline clinic visit and at subsequent visits during the next three consecutive weeks were stored until used for quantification of urinary paraben biomarkers. Most women provided at least one urine sample with 98% providing multiple urine samples. Urine samples were pooled when a participant provided more than one urine sample during the first month of participation in the study. Paraben biomarker concentrations were measured using isotope dilution high-performance liquid chromatography negative-ion electrospray ionization-tandem mass spectrometry at the Metabolomics Lab of the Roy J. Carver Biotechnology Center (University of Illinois at Urbana-Champaign, IL) as detailed in the Supplemental Data. We Measured: butylparaben, ethylparaben, methylparaben, and propylparaben. These paraben biomarkers were selected because they are commonly incorporated in daily-use products and are frequently measured in women's biological samples. In addition, these paraben biomarkers have been shown to be associated with other adverse reproductive outcomes in experimental models and human studies.11,18,28–31
Statistical analysis
Hormone and paraben biomarker concentrations below the LOQ were converted to LOQ/√2. To account for urine dilution, urinary paraben biomarker concentrations were specific gravity adjusted using the following equation: Pc = P[(1.017 – 1)/(SGi – 1)], where Pc is the specific gravity-adjusted paraben biomarker concentration, 1.017 is the median specific gravity of the pooled samples provided by women in this subsample, P is the measured paraben biomarker concentration (ng/mL), and SGi is the specific gravity of each individual's pooled urine sample.32 We assessed parabens as individual biomarkers (ng/mL) and as a molar sum of all four paraben biomarkers (nmol/mL; ∑parabens). All paraben biomarkers were natural log-transformed in all statistical analyses to meet normality assumptions.
We used logistic regression models to evaluate the associations of urinary paraben biomarker concentrations with AMH and four hot flash outcomes (ever experienced hot flashes, experienced hot flashes in the past 30 days, hot flash frequency, and hot flash severity). Specifically, we used binary logistic regression models to evaluate associations of paraben biomarkers with the odds of ever experiencing or experiencing recent hot flashes compared with never experiencing hot flashes. Similarly, we used binary logistic regression models to evaluate associations of paraben biomarkers with the odds of having AMH concentrations above compared with below the LOQ. We used multinomial logistic regression models to evaluate associations of paraben biomarkers with the odds of experiencing daily/weekly and monthly hot flashes or moderate/severe and mild hot flashes compared with never experiencing hot flashes. The resulting odds ratios and 95% confidence intervals (CIs) from all logistic regression models were back-transformed using the equation [eln(OR)*ln(2.0)], thus results can be interpreted as the odds of each hot flash outcome for every two-fold increase in paraben biomarker concentration.
We used linear regression models to evaluate associations of urinary paraben biomarker concentrations with ovarian volume and serum concentrations of estradiol, testosterone, progesterone, SHBG, and FSH. Estradiol, testosterone, progesterone, and FSH concentrations, as well as ovarian volume were natural log-transformed to fit normality assumptions. The resulting β-estimates and 95% CI from these linear regression models were back-transformed using the equation [(2.00β – 1) × 100] so that results can be interpreted as the percent change (%Δ) in hormone concentrations or ovarian volume for every two-fold increase in paraben biomarker concentration. SHBG already had a normal distribution and was not transformed. The resulting β-estimates and 95% CI from linear regression models evaluating SHBG were back-transformed using the equation [β × ln(2.00)] so that results can be interpreted as the nanomole/liter change in SHBG concentrations for every two-fold increase in paraben biomarker concentration.
For both linear and logistic regression models, we evaluated both unadjusted and adjusted models. Adjusted models controlled for age and midlife BMI because these have been shown to be important determinants of urinary paraben biomarker concentrations,33,34 as well as hot flash risk,27 serum hormone concentrations,35,36 and ovarian volume.27,36–38 Both age and midlife BMI were included as continuous variables, and age was used as a proxy for menopause status because these variables are highly correlated (r = 0.7). All analyses were performed with SAS 9.4 (version 15.1, SAS Institute, Inc., Cary, NC ) using PROC GLM and PROC LOGISTIC for linear and logistic regression analyses, respectively. Owing to the exploratory nature of the study and recommendations from the American Statistical Association and other epidemiologists,39,40 we focused on patterns of associations rather than statistical significance based on P-values. Therefore, we considered results with upper or lower confidence limits close to zero as potentially meaningful. We did not adjust for multiple comparisons.41
Results
Characteristics of MWHS subsample
Baseline characteristics for 101 participants in this pilot study are given in Table 1. Our subsample included only women who were non-Hispanic white and nonsmokers, and who were mostly premenopausal (68%), 45–49 years old (78%), not obese (78%), and had ≥1 live birth (76%). In addition, most women consumed >12 alcoholic drinks in the past year (75%), were college educated (82%), were employed (81%), and were married or living with a partner (77%).
Table 1.
Sociodemographic and Lifestyle Characteristics of Midlife Women's Health Study Subsample (N = 101)
| Characteristic | Median (25th, 75th percentile) or n (%) |
|---|---|
| Age, years | 47.0 (46.0, 49.0) |
| 45–49 | 79 (78.2) |
| 50–54 | 22 (21.8) |
| Menopause status | |
| Premenopausal | 69 (68.3) |
| Perimenopausal | 32 (31.7) |
| Educational attainment | |
| Some college or less | 18 (17.8) |
| College graduate or higher | 83 (82.2) |
| Employment status | |
| Unemployed | 19 (18.8) |
| Employed | 82 (81.2) |
| Annual household income (3 missing)a | |
| <$100,000 | 42 (41.6) |
| $100,000+ | 56 (55.4) |
| Marital status | |
| Single | 12 (11.9) |
| Married/living with partner | 78 (77.2) |
| Widowed/divorced/separated | 11 (10.9) |
| BMI, kg/m2 | 23.0 (21.3, 24.8) |
| <25 | 79 (78.2) |
| ≥25 | 22 (21.8) |
| Parity | |
| Never pregnant | 17 (16.8) |
| No live births | 7 (6.9) |
| One live birth | 9 (8.9) |
| Two or more live births | 68 (67.3) |
| Medication use (1 missing)a | |
| No medications | 55 (54.5) |
| Yes medications | 45 (44.6) |
| Alcohol consumption (1 missing)a | |
| ≤12 alcoholic drinks in past year | 24 (23.8) |
| >12 alcoholic drinks in past year | 76 (75.2) |
Values may not add up to 100% because of missing information.
BMI, body mass index.
Distribution of midlife reproductive outcomes
The distribution of hot flashes, serum hormone and SHBG concentrations, and ovarian volume are given in Table 2. Around 47% of women reported ever experiencing hot flashes, and 29% experienced hot flashes in the past 30 days. Of the women who experienced hot flashes, 51% experienced daily/weekly hot flashes and 64% experienced moderate/severe hot flashes. More than 99% of women had serum concentrations of most hormones above the LOQ, except for AMH, where only 53% of women had concentrations above the LOQ (data not shown).
Table 2.
Distribution of Midlife Reproductive Outcomes in Midlife Women's Health Study Subsample (N = 101)
| n (%) or median (25th, 75th percentiles) | |
|---|---|
| Hot flashes | |
| Ever had hot flashes | |
| Never had hot flashes | 54 (53.5) |
| Ever had hot flashes | 47 (46.5) |
| Had hot flashes in the past 30 days | |
| Never had hot flashes | 54 (53.5) |
| Had hot flashes but not in the past 30 days | 18 (17.8) |
| Had hot flashes in the past 30 days | 29 (28.7) |
| Hot flash frequency (2 missing)a | |
| Never had hot flashes | 54 (53.5) |
| Had daily/weekly hot flashes | 24 (23.8) |
| Had monthly hot flashes | 21 (20.8) |
| Hot flash severity (1 missing)a | |
| Never had hot flashes | 54 (53.5) |
| Had mild hot flashes | 16 (15.8) |
| Had moderate/severe hot flashes | 30 (29.7) |
| Hormonesb | |
| Estradiol, pg/mL | 50.1 (28.9, 72.5) |
| Testosterone, ng/mL | 0.3 (0.2, 0.4) |
| Progesterone, ng/mL | 0.8 (0.3, 1.5) |
| SHBG, nmol/L | 81.7 (56.8, 102.8) |
| FSH, mIU/mL | 8.9 (4.8, 50.2) |
| AMH, ng/mL | 0.1 (0.1, 0.8) |
| Ovarian volume | |
| Ovarian volume, cm3 (10 missing)c | 4.5 (2.3, 6.1) |
Values may not add up to 100% because of missing information.
LOQ of assays are detailed in Chiang et al.26
One woman was excluded owing to having ovarian volume >30 cm3, whereas the remaining nine women were missing ovarian volume values.
AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; LOQ, limit of quantification; SHBG, sex hormone binding globulin.
Urinary paraben biomarker concentrations
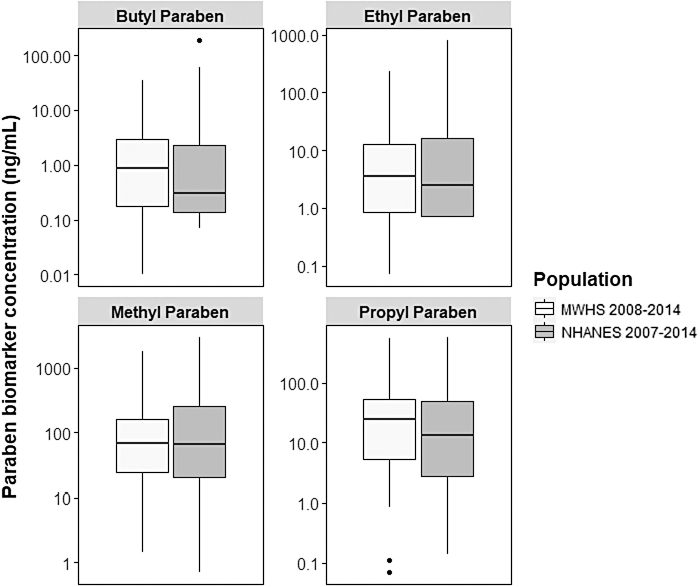
Overall, all women in our subsample had detectable (>LOQ) urinary concentrations of at least one paraben biomarker, and >92% had detectable urinary concentrations of all paraben biomarkers. The order of urinary paraben biomarker concentrations were as follows: butylparaben < ethylparaben < propylparaben < methylparaben. Only methylparaben and propylparaben were strongly correlated with each other (r = 0.9; data not shown), whereas butylparaben and ethylparaben were weakly correlated with the other parabens (r < 0.3; data not shown). Furthermore, the distribution of urinary paraben biomarker concentrations in our subsample were similar to those from U.S. women from the 2007 to 2014 National Health and Nutrition Examination Survey (NHANES) who were 45–54 years old, non-Hispanic white, and never smokers (Fig. 1).42
FIG. 1.
Urinary paraben biomarker concentrations in subsamples of MWHS and NHANES. Box plots display urinary paraben biomarker concentrations (ng/mL) of women in MWHS (2008–2014, N = 101) and same age non-Hispanic white, nonsmoking women from four NHANES survey cycles (2007–2014, n = 107). Box plots include the median (center line in box), the 25th percentile (lower line of box), and the 75th percentile (upper line in box). Dots represent extreme values. MWHS, Midlife Women's Health Study; NHANES, National Health and Nutrition Examination Survey.
Associations of paraben biomarkers with hot flashes
The associations between paraben biomarkers and hot flashes are given in Table 3. Overall, methylparaben, propylparaben, and ∑parabens were marginally associated with at least one hot flash outcome. Methylparaben and ∑parabens were marginally associated with ever having hot flashes, where women had 21% (95% CI: 0.94–1.56) and 23% (95% CI: 0.96–1.59) higher odds of ever experiencing hot flashes with every two-fold increase in methylparaben and ∑parabens, respectively. Methylparaben, propylparaben, and ∑parabens were also borderline associated with experiencing hot flashes in the past 30 days. Specifically, women had 22%–33% higher odds of experiencing recent hot flashes with every two-fold increase in methylparaben (95% CI: 0.97–1.84), propylparaben (95% CI: 0.96–1.55), and ∑parabens (95% CI: 0.96–1.81).
Table 3.
Associations of Urinary Paraben Biomarker Concentrations with Hot Flashes
| Butylparaben |
Ethylparaben |
Methylparaben |
Propylparaben |
∑Parabens |
|
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Odds of ever having hot flashes for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 1.02 (0.87–1.19) | 1.05 (0.91–1.21) | 1.24 (0.99–1.56) | 1.15 (0.97–1.36) | 1.24 (0.99–1.56) |
| Adjusted | 0.98 (0.82–1.16) | 1.06 (0.91–1.25) | 1.21 (0.94–1.56) | 1.14 (0.95–1.38) | 1.23 (0.96–1.59) |
| Odds of experiencing hot flashes in the past 30 days for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 0.98 (0.82–1.17) | 1.01 (0.86–1.19) | 1.36 (1.02–1.81) | 1.21 (0.98–1.50) | 1.31 (0.99–1.74) |
| Adjusted | 0.93 (0.76–1.15) | 1.04 (0.86–1.26) | 1.33 (0.97–1.84) | 1.22 (0.96–1.55) | 1.32 (0.96–1.81) |
| Odds of experiencing daily/weekly hot flashes for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 0.96 (0.80–1.16) | 0.98 (0.82–1.16) | 1.13 (0.87–1.48) | 1.05 (0.86–1.28) | 1.10 (0.84–1.43) |
| Adjusted | 0.91 (0.73–1.14) | 0.99 (0.81–1.21) | 1.07 (0.79–1.46) | 1.01 (0.80–1.27) | 1.05 (0.77–1.44) |
| Odds of experiencing monthly hot flashes for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 1.05 (0.86–1.29) | 1.16 (0.96–1.40) | 1.33 (0.98–1.79) | 1.26 (0.99–1.61) | 1.39 (1.02–1.89) |
| Adjusted | 1.02 (0.83–1.26) | 1.16 (0.95–1.41) | 1.32 (0.95–1.82) | 1.26 (0.98–1.63) | 1.40 (1.00–1.95) |
| Odds of experiencing moderate/severe hot flashes for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 0.98 (0.82–1.16) | 1.07 (0.91–1.25) | 1.12 (0.87–1.44) | 1.08 (0.90–1.30) | 1.13 (0.88–1.45) |
| Adjusted | 0.94 (0.77–1.14) | 1.09 (0.91–1.31) | 1.11 (0.84–1.46) | 1.09 (0.89–1.33) | 1.14 (0.86–1.50) |
| Odds of experiencing mild hot flashes for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 1.11 (0.88–1.38) | 1.04 (0.85–1.27) | 1.51 (1.06–2.16) | 1.30 (0.99–1.72) | 1.49 (1.04–2.13) |
| Adjusted | 1.06 (0.83–1.34) | 1.05 (0.84–1.30) | 1.39 (0.97–2.00) | 1.25 (0.94–1.66) | 1.40 (0.98–2.01) |
Binary logistic regression models evaluated associations of parabens with odds of ever having hot flashes (n = 47) and experiencing hot flashes in the past 30 days (n = 29) compared with never having hot flashes (n = 54). Multinomial logistic regression models evaluated associations of parabens with the odds of experiencing daily/weekly (n = 24) or monthly (n = 21) hot flashes or experiencing moderate/severe (n = 30) or mild (n = 16) hot flashes compared with never having hot flashes (n = 54).
Adjusted models account for age at the baseline visit (continuous) and midlife BMI (continuous).
Bold values indicate potentially meaningful findings.
CI, confidence intervals; OR, odds ratio.
Although parabens were not associated with experiencing daily/weekly hot flashes, methylparaben, propylparaben, and ∑parabens were borderline associated with experiencing monthly hot flashes, such that women had 26%–40% higher odds of experiencing monthly hot flashes with every two-fold increase in methylparaben (95% CI: 0.95–1.82), propylparaben (95% CI: 0.98–1.63), and ∑parabens (95% CI: 1.00–1.95). Finally, methylparaben, propylparaben, and ∑parabens were marginally associated with experiencing mild (but not moderate/severe) hot flashes, such that women had 25%–40% higher odds of experiencing mild hot flashes with every two-fold increase in methylparaben (95% CI: 0.97–2.00), propylparaben (95% CI: 0.94–1.66), and ∑parabens (95% CI: 0.98–2.01).
Associations of paraben biomarkers with serum hormone concentrations
Parabens were not associated with estradiol, testosterone, progesterone, SHBG, or AMH (Table 4). However, we observed marginal positive associations of methylparaben, propylparaben, and ∑parabens, with FSH concentrations. Specifically, FSH concentrations were 10.33%–14.54% higher with every two-fold increase in methylparaben (95% CI: −0.43 to 31.05), propylparaben (95% CI: −0.44 to 22.26), and ∑parabens (95% CI: −0.10 to 31.32).
Table 4.
Associations of Urinary Paraben Biomarker Concentrations with Serum Hormone and Sex Hormone Binding Globulin Concentrations
| Butylparaben, a%Δ (95% CI) | Ethylparaben, a%Δ (95% CI) | Methylparaben, a%Δ (95% CI) | Propylparaben, a%Δ (95% CI) | ∑Parabens, a%Δ (95% CI) | |
|---|---|---|---|---|---|
| % Change in estradiol concentrations for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 2.16 (−2.88 to 7.45) | −0.97 (−5.45 to 3.72) | −2.45 (−9.09 to 4.67) | −1.30 (−6.36 to 4.02) | −2.83 (−9.43 to 4.25) |
| Adjusted | 2.95 (−1.99 to 8.13) | −0.94 (−5.30 to 3.62) | −1.08 (−7.72 to 6.03) | −0.49 (−5.52 to 4.82) | −1.70 (−8.27 to 5.34) |
| % Change in testosterone concentrations for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | −2.40 (−7.06 to 2.50) | 0.21 (−4.19 to 4.82) | −0.08 (−6.69 to 7.01) | 0.77 (−4.25 to 6.04) | 0.02 (−6.60 to 7.11) |
| Adjusted | −2.01 (−6.69 to 2.91) | 0.15 (−4.24 to 4.73) | 0.62 (−6.09 to 7.81) | 1.09 (−3.99 to 6.44) | 0.56 (−6.12 to 7.73) |
| % Change in progesterone concentrations for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 4.44 (−4.85 to 14.65) | −3.11 (−11.03 to 5.52) | −4.58 (−16.21 to 8.68) | −1.70 (−10.8 to 8.32) | −3.60 (−15.36 to 9.79) |
| Adjusted | 7.16 (−1.11 to 16.13) | −3.10 (−10.01 to 4.34) | −0.03 (−10.85 to 12.09) | 0.93 (−7.34 to 9.95) | 0.20 (−10.60 to 12.31) |
| Change (nmol/L) in SHBG concentrations for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | −0.08 (−2.60 to 2.43) | −0.12 (−2.41 to 2.18) | −0.76 (−4.26 to 2.74) | −0.57 (−3.17 to 2.04) | −0.90 (−4.39 to 2.60) |
| Adjusted | −0.12 (−2.57 to 2.33) | −0.33 (−2.55 to 1.90) | −1.18 (−4.61 to 2.26) | −1.12 (−3.68 to 1.44) | −1.35 (−4.76 to 2.07) |
| % Change in FSH concentrations for every two-fold increase in paraben biomarker levels | |||||
| Unadjusted | 5.55 (−5.30 to 17.65) | 3.03 (−6.72 to 13.79) | 19.58 (3.17 to 38.59) | 13.34 (1.51 to 26.56) | 19.06 (2.72 to 38.01) |
| Adjusted | 2.97 (−6.78 to 13.74) | 2.84 (−6.06 to 12.58) | 14.24 (−0.43 to 31.05) | 10.33 (−0.44 to 22.26) | 14.54 (−0.10 to 31.32) |
| Odds of having AMH levels ≥LOD for every two-fold increase in paraben biomarker levels | |||||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Unadjusted |
1.01 (0.87 to 1.18) |
0.99 (0.86 to 1.13) |
1.02 (0.82 to 1.26) |
0.95 (0.81 to 1.12) |
1.01 (0.82 to 1.25) |
| Adjusted | 1.07 (0.90 to 1.27) | 1.00 (0.85 to 1.17) | 1.16 (0.89 to 1.49) | 1.01 (0.84 to 1.22) | 1.13 (0.87 to 1.45) |
Linear regression models evaluating associations of parabens with estradiol, testosterone, progesterone, SHBG, and FSH. Binary logistic regression models evaluated associations of parabens with odds of ever having hot flashes (n = 47) and experiencing hot flashes in the past 30 days (n = 29) compared with never having hot flashes (n = 54). Multinomial logistic regression models evaluated associations of parabens with the odds of experiencing daily/weekly (n = 24) or monthly (n = 21) hot flashes or experiencing moderate/severe (n = 30) or mild (n = 16) hot flashes compared with never having hot flashes (n = 54).
Adjusted models account for age at the baseline visit (continuous) and midlife body mass index (continuous).
Bold values indicate potentially meaningful findings.
SHBG values are expressed as Δ and not %Δ."
Associations of paraben biomarkers with ovarian volume
Although butylparaben was not associated with ovarian volume, we observed marginal inverse associations of ethylparaben, propylparaben, and ∑parabens with ovarian volume (Table 5). Specifically, ovarian volume was 4.09%–5.67% lower with every two-fold increase in ethylparaben (95% CI: −9.04 to 0.16), propylparaben (95% CI: −9.19 to 1.29), and ∑parabens (95% CI: −12.54 to 1.75) biomarker concentrations. In addition, we observed a modest inverse associations between methylparaben and ovarian volume, such that ovarian volume was −4.28% (95% CI: −11.35 to 3.36) smaller with every two-fold increase in methylparaben.
Table 5.
Associations of Urinary Paraben Biomarker Concentrations with Ovarian Volume
| Butylparaben, %Δ (95% CI) | Ethylparaben, %Δ (95% CI) | Methylparaben, %Δ (95% CI) | Propylparaben, %Δ (95% CI) | ∑Parabens, %Δ (95% CI) | |
|---|---|---|---|---|---|
| % Change in ovarian volume for every two-fold increase in paraben biomarker concentrations | |||||
| Unadjusted | 2.48 (−3.14 to 8.43) | −4.10 (−8.86 to 0.92) | −4.01 (−11.40 to 4.00) | −3.67 (−9.00 to 1.97) | −5.23 (−12.45 to 2.58) |
| Adjusted | 2.52 (−2.86 to 8.21) | −4.55 (−9.04 to 0.16) | −4.28 (−11.35 to 3.36) | −4.09 (−9.19 to 1.29) | −5.67 (−12.54 to 1.75) |
Linear regression models evaluating associations of parabens with ovarian volume. Adjusted models account for age at the baseline visit (continuous) and midlife body mass index (continuous).
Bold values indicate potentially meaningful findings.
Discussion
To our knowledge, this is the first study to examine the associations between midlife paraben biomarker concentrations and proxies of women's reproductive aging. Specifically, we observed that methylparaben, propylparaben, and ∑parabens were marginally associated with higher odds of experiencing recent, monthly, and mild hot flashes, and were also marginally positively associated with serum FSH concentrations. We also observed that these three paraben biomarkers along with ethylparaben were marginally inversely associated with ovarian volume. In addition, almost all women had detectable levels of at least methylparaben and propylparaben, which is consistent with the widespread use of these parabens in daily-use products.43–45
Hot flashes are a major hallmark of reproductive senescence experienced by millions of women around the world (reviewed by Ziv-Gal and Flaws).46 Nevertheless, hot flashes' etiology is still unknown. It has been suggested that hormonal imbalances and earlier timing of reproductive senescence are some of the triggering factors.46 Thus, it is possible that hormone-mimicking chemicals in the environment and in daily-use products may increase the risk of hot flashes. In this study, methylparaben, propylparaben, and ∑parabens were marginally significantly positively associated with experiencing recent, monthly, and/or mild hot flashes. Given the small size of our pilot sample, we were likely underpowered to detect some associations and were unable to control for other important confounders that may attenuate the observed marginal associations.
No other studies have examined the associations between paraben biomarkers and hot flashes. However, we previously evaluated cross-sectional associations of phthalates (another class of endocrine disrupting chemicals) with hot flashes in the MWHS cohort.25,47 In the full MWHS population, we generally found that some phthalate metabolite biomarkers were associated with higher risk of experiencing recent and daily/weekly hot flashes.25 Given that personal care products are an important source of phthalates and parabens, these chemicals may also interact to influence hot flashes. Therefore, additional large-scale studies in more diverse populations are needed to not only corroborate our findings pertaining to paraben biomarkers and hot flashes, but also evaluate the impact of a mixture of different chemical classes found in personal care products on hot flashes.
The mechanisms by which environmental exposures may increase the odds of hot flashes are unknown. One potential mechanism of action of parabens is the disruption of hormone secretion. Parabens are considered weak estrogenic compounds that can alter hormone levels and signaling.11 In this pilot study, paraben biomarkers were not associated with levels of estradiol, progesterone, testosterone, SHBG, and AMH. Nevertheless, we preliminarily found that methylparaben, propylparaben, and ∑parabens were marginally associated with elevated levels of FSH. FSH acts on the ovary to promote ovarian follicle growth.1 The mature ovarian follicles are capable of producing estrogen that negatively feeds back on the release of FSH via the hypothalamic–pituitary–ovarian axis. With age, the ovary contains fewer follicles and thus less estrogen is produced. This, eventually, results in increased levels of FSH owing to a lack of a negative feedback on FSH release.1,48
Hence, associations of parabens biomarkers with FSH levels can be owing to an overactivation of the hypothalamic–pituitary–ovarian axis, which can gradually lead to enhanced ovarian aging. Our findings are in partial agreement with other studies. Some experimental data indicate that paraben exposure disrupted steroidogenesis and increased levels of FSH in neonatal and young adult rodents.11,17,20,22,49,50 A study of 25- to 39-year-old women from Poland (n = 500) found that urinary propylparaben biomarker concentrations were inversely associated with serum estradiol levels, but positively associated with serum FSH concentrations.51 In a study by Smith et al. of 18- to 46-year-old U.S. women undergoing infertility evaluation (n = 193), propylparaben biomarker concentrations were marginally associated with serum FSH levels on day 3 of the menstrual cycle, whereas urinary methylparaben, propylparaben, or butylparaben biomarkers were not associated with day 3 serum FSH levels.28
Differences in our findings compared with other observational studies are likely owing to differences in populations because the two previously described studies were conducted in younger women, whereas women in our subsample are older. In addition, we were also underpowered to detect some associations. Therefore, additional larger studies (and with the potential to evaluate longitudinal associations) are needed to identify the impact of parabens on hormone levels, especially in midlife women.
Of interest, we observed marginal inverse associations between most paraben biomarkers and ovarian volume. Our findings are not consistent with those by Smith et al., who reported no association of methylparaben, propylparaben, butylparaben, or ∑parabens with ovarian volume.28 Smith et al. also reported marginal associations of urinary propylparaben biomarker concentrations with lower antral follicle counts.28 In addition, Jurewicz et al. indicated that propylparaben biomarker concentrations were inversely associated with ovarian antral follicle count.51 In experimental studies, paraben exposure resulted in more pronounced effects on the ovary. Specifically, methylparaben exposure at a relatively high dose resulted in a reduced ovarian weight in prepubertal rats,18 and propylparaben exposure resulted in accelerated ovarian aging in mice.50 Finally, propylparaben exposure of mature ovarian follicles resulted in growth inhibition under culture conditions.17 Overall, it is likely that parabens can target the ovary. However, the literature is extremely limited and our preliminary findings warrant further investigation.
One limitation of our preliminary study is that we are unable to generalize our results to a diverse population of women, given that our subsample was restricted to non-Hispanic white, nonsmokers. In addition, the cross-sectional nature of this study also means that there is potential for reverse causation. For example, reproductive aging status could influence women's lifestyles or behaviors to make them more or less likely to use paraben-containing products, which would impact their exposure to parabens. Finally, owing to power, we were unable to control for other potentially important confounding by other lifestyle and sociodemographic characteristics. However, we used earlier literature to inform our short list of potential confounding factors in this homogeneous subset of women. Therefore, additional large-scale prospective studies in diverse populations are needed to corroborate these findings.
This study also has some strengths. All the study participants were recruited and followed in the same clinic using standardized protocols. Hot flashes outcomes and other data were also collected by detailed questionnaires that are accepted by the National Institutes of Health.23,52 Another strength of our study is that paraben biomarker concentrations in our subsample were comparable with those reported in NHANES,42 which highlights the external validity of our study with regard to the exposure. Finally, we evaluated paraben biomarker concentrations in urine samples that were pooled across multiple study visits, which allowed us to reduce misclassification for paraben exposure and to increase accuracy of our estimated exposure.
Conclusion
The results of this pilot study add to the gradually increasing literature on the potential adverse impacts of paraben exposure on female reproductive health. Findings from this pilot study warrant further investigation of the impacts of paraben in the context of hormonally mediated health outcomes in midlife women. Additional experimental studies and larger prospective studies in human populations should further explore these associations and elucidate the specific mechanism of action of parabens on reproductive aging.
Supplementary Material
Acknowledgments
The authors thank the women who participated in the MWHS and the staff members at Johns Hopkins University and the University of Illinois at Urbana-Champaign who helped with the study. The authors also thank Drs. Rebecca Smith and Brandi Smith for their assistance during the early stages of this project.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by start-up funding from the University of Illinois at Urbana-Champaign.
Supplementary Material
References
- 1. Hall JE. Endocrinology of the menopause. Endocrinol Metab Clin North Am 2015;44:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 1992;14:103–115. [DOI] [PubMed] [Google Scholar]
- 3. Stanford JL, Hartge P, Brinton LA, et al. Factors influencing the age at natural menopause. J Chronic Dis 1987;40:995–1002. [DOI] [PubMed] [Google Scholar]
- 4. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015;18:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015;175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Col NF, Guthrie JR, Politi M, et al. Duration of vasomotor symptoms in middle-aged women: A longitudinal study. Menopause 2009;16:453–457. [DOI] [PubMed] [Google Scholar]
- 7. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes 2005;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985;8:261–268. [DOI] [PubMed] [Google Scholar]
- 9. Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J Gen Intern Med 2008;23:1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2015;22:260–266. [DOI] [PubMed] [Google Scholar]
- 11. Nowak K, Ratajczak-Wrona W, Gorska M, et al. Parabens and their effects on the endocrine system. Mol Cell Endocrinol 2018;474:238–251. [DOI] [PubMed] [Google Scholar]
- 12. Calafat AM, Ye X, Wong LY, et al. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect 2010;118:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith KW, Braun JM, Williams PL, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect 2012;120:1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pollack AZ, Mumford SL, Krall JR, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach. Environ Int 2018;120:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishihama Y, Yoshinaga J, Iida A, et al. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol 2016;63:107–113. [DOI] [PubMed] [Google Scholar]
- 16. Smarr MM, Sundaram R, Honda M, et al. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples' fecundity. Environ Health Perspect 2017;125:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gal A, Gedye K, Craig ZR, et al. Propylparaben inhibits mouse cultured antral follicle growth, alters steroidogenesis, and upregulates levels of cell-cycle and apoptosis regulators. Reprod Toxicol 2019;89:100–106. [DOI] [PubMed] [Google Scholar]
- 18. Taxvig C, Vinggaard AM, Hass U, et al. Do parabens have the ability to interfere with steroidogenesis? Toxicol Sci 2008;106:206–213. [DOI] [PubMed] [Google Scholar]
- 19. Guerra MT, Furlong HC, Kempinas WG, et al. Effects of in vitro exposure to butylparaben and di-(2 ethylhexyl) phthalate, alone or in combination, on ovarian function. J Appl Toxicol 2016;36:1235–1245. [DOI] [PubMed] [Google Scholar]
- 20. Boberg J, Axelstad M, Svingen T, et al. Multiple endocrine disrupting effects in rats perinatally exposed to butylparaben. Toxicol Sci 2016;152:244–256. [DOI] [PubMed] [Google Scholar]
- 21. Ahn HJ, An BS, Jung EM, et al. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol Reprod Dev 2012;79:626–636. [DOI] [PubMed] [Google Scholar]
- 22. Vo TT, Yoo YM, Choi KC, et al. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod Toxicol 2010;29:306–316. [DOI] [PubMed] [Google Scholar]
- 23. Ziv-Gal A, Smith RL, Gallicchio L, et al. The Midlife Women's Health Study: A study protocol of a longitudinal prospective study on predictors of menopausal hot flashes. Womens Midlife Health 2017;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop +10: Addressing the unfinished agenda of staging reproductive aging. Climacteric 2012;15:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warner GR, Pacyga DC, Strakovsky RS, et al. Urinary phthalate metabolite concentrations and hot flashes in women from an urban convenience sample of midlife women. Environ Res 2021;197:110891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiang C, Pacyga DC, Strakovsky RS, et al. Urinary phthalate metabolite concentrations and serum hormone levels in pre- and perimenopausal women from the Midlife Women's Health Study. Environ Int 2021;156:106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallicchio L, Miller SR, Kiefer J, et al. Risk factors for hot flashes among women undergoing the menopausal transition: Baseline results from the Midlife Women's Health Study. Menopause 2015;22:1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith KW, Souter I, Dimitriadis I, et al. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ Health Perspect 2013;121:1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bledzka D, Gromadzinska J, Wasowicz W. Parabens. From environmental studies to human health. Environ Int 2014;67:27–42. [DOI] [PubMed] [Google Scholar]
- 30. Honda M, Robinson M, Kannan K. Parabens in human urine from several Asian countries, Greece, and the United States. Chemosphere 2018;201:13–19. [DOI] [PubMed] [Google Scholar]
- 31. Wei F, Mortimer M, Cheng H, et al. Parabens as chemicals of emerging concern in the environment and humans: A review. Sci Total Environ 2021;778:146150. [DOI] [PubMed] [Google Scholar]
- 32. Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect 2009;117:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kiani Feizabadi G, Hajizadeh Y, Feizi A, et al. Urinary concentrations of parabens amongst Iranian adults and their associations with socio-demographic factors. J Environ Health Sci Eng 2020;18:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang HS, Kyung MS, Ko A, et al. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ Res 2016;146:245–251. [DOI] [PubMed] [Google Scholar]
- 35. Gallicchio L, Schilling C, Romani WA, et al. Endogenous hormones, participant characteristics, and symptoms among midlife women. Maturitas 2008;59:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schilling C, Gallicchio L, Miller SR, et al. Relation of body mass and sex steroid hormone levels to hot flushes in a sample of mid-life women. Climacteric 2007;10:27–37. [DOI] [PubMed] [Google Scholar]
- 37. Flaws JA, Rhodes JC, Langenberg P, et al. Ovarian volume and menopausal status. Menopause 2000;7:53–61. [DOI] [PubMed] [Google Scholar]
- 38. Bastos CA, Oppermann K, Fuchs SC, et al. Determinants of ovarian volume in pre-, menopausal transition, and post-menopausal women: A population-based study. Maturitas 2006;53:405–412. [DOI] [PubMed] [Google Scholar]
- 39. Wasserstein RL, Lazar NA. The ASA's Statement on p-values: Context, process, and purpose. Am Stat 2016;70:129–131. [Google Scholar]
- 40. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567:305–307. [DOI] [PubMed] [Google Scholar]
- 41. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2007–2014. Available at: https://wwwn.cdc.gov/nchs/nhanes/NhanesCitation.aspx Accessed March, 2021.
- 43. Wang PG, Zhou W. Rapid determination of parabens in personal care products by stable isotope GC-MS/MS with dynamic selected reaction monitoring. J Sep Sci 2013;36:1781–1787. [DOI] [PubMed] [Google Scholar]
- 44. Rodas M, Portugal LA, Avivar J, et al. Parabens determination in cosmetic and personal care products exploiting a multi-syringe chromatographic (MSC) system and chemiluminescent detection. Talanta 2015;143:254–262. [DOI] [PubMed] [Google Scholar]
- 45. Kaur R, Heena, Kaur R, et al. Trace determination of parabens in cosmetics and personal care products using fabric-phase sorptive extraction and high-performance liquid chromatography with UV detection. J Sep Sci 2020;43:2626–2635. [DOI] [PubMed] [Google Scholar]
- 46. Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy middle-aged women. J Womens Health (Larchmt) 2010;19:1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ziv-Gal A, Gallicchio L, Chiang C, et al. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reprod Toxicol 2016;60:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santoro N. The menopausal transition. Am J Med 2005;118 Suppl 12B:8–13. [DOI] [PubMed] [Google Scholar]
- 49. Lee JH, Lee M, Ahn C, et al. Parabens accelerate ovarian dysfunction in a 4-vinylcyclohexene diepoxide-induced ovarian failure model. Int J Environ Res Public Health 2017;14:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li M, Zhou S, Wu Y, et al. Prenatal exposure to propylparaben at human-relevant doses accelerates ovarian aging in adult mice. Environ Pollut 2021;285:117254. [DOI] [PubMed] [Google Scholar]
- 51. Jurewicz J, Radwan M, Wielgomas B, et al. Parameters of ovarian reserve in relation to urinary concentrations of parabens. Environ Health 2020;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller HG, Li RM. Measuring hot flashes: Summary of a National Institutes of Health workshop. Mayo Clin Proc 2004;79:777–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.