Abstract
Autonomy support is a concept that is derived from self-determination theory. Autonomy refers to the freedom to act as one chooses. The current study aimed to examine if autonomy support was associated with dried blood spot validated pre-exposure prophylaxis (PrEP) adherence, and whether the association was mediated by PrEP adherence goal setting and progress toward PrEP adherence goals. Our sample was drawn from Black men who have sex with men (MSM) from across three cities (Chapel Hill, NC; Los Angeles, CA; and Washington, DC) in the United States between February 2013 and September 2014. We used logistic regression to evaluate associations between study variables and path analysis to test mediation effects. Participants were, on average, 28 [standard deviation (SD) = 1.12] years old and 25% were unemployed. We found that MSM who experienced high autonomy support were more likely to adhere to PrEP [odds ratio (OR) = 1.17; 95% confidence interval: 1.00–1.38]. MSM who set PrEP adherence goals were more likely to adhere to PrEP. Moreover, MSM who reported making progress toward their goals were also more likely to adhere to PrEP. Finally, client perception of coordination quality enhanced the magnitude of the association between goal setting and goal progress and the effect size of goal progress on PrEP adherence. Autonomy support, goal setting, goal monitoring/evaluation, and care coordination quality influenced PrEP adherence among Black MSM. Our findings indicate that while it is important to set goals for PrEP adherence, goal setting may need to be accompanied by progress monitoring to achieve the maximal effect.
Keywords: HIV prevention, Black MSM, autonomy support, PrEP adherence
Introduction
Black men who have sex with men (MSM) continue to have disproportionately high rates of HIV infection in the United States.1 Between 2010 and 2018, the annual HIV incidence among Black MSM remained relatively unchanged from 10,000 to 9100.2–4 However, during the same time period, the HIV incidence among White MSM decreased significantly from 8200 to 5400.2–4 Thus, while declines in HIV incidence among Black MSM have plateaued, the racial disparity in HIV incidence has widened over time. This racial gap is occurring despite the advent of highly effective biobehavioral prevention tools such as HIV pre-exposure prophylaxis (PrEP).5,6 Racial inequities in PrEP use have been observed since the beginning of its introduction into the national prevention toolkit, with the use among Black MSM markedly lower than that of White MSM.1,7,8
To date, a great deal of attention has been given to the individual-level motivational (e.g., perceived low risk), structural (e.g., costs, unstable housing) and lack of health insurance9,10 and sociocontextual (e.g., anti-Black racism and heterosexism)11–13 factors influencing PrEP adherence among Black MSM. More recently, there has been a growing interest in examining the role of health care environments as one specific type of social context that either facilitates or undermines PrEP use and adherence among Black MSM.14,15
Autonomy support is a concept that is derived from self-determination theory (SDT).16 Autonomy refers to the freedom to act as one chooses.16,17 For example, allowing Black MSM to make decisions about their health care without their providers trying to steer the decision. Individual's autonomy can be constrained through oppressive social processes that compel, coerce, or otherwise function to control an individual's behavior.16–19 An individual's autonomy can also be supported through liberating social processes that promote informed decision making and nurture an individual's capacity to enact whatever decision they make or goal they set.16–20 Health care settings are social environments composed of an array of individuals (e.g., counselors, nurses, physicians, social workers) who collectively influence the climate within which individuals make health behavior decisions—including decisions about the use of PrEP.16,21 Evidence from meta-analyses indicates that interventions that use autonomy-supportive approaches positively affected behavioral and clinical outcomes across a wide range of health domains.22,23
For example, the results of a randomized controlled trial of an online SDT-based intervention among adolescents in a primary care setting indicated that it produced significant increased cardiorespiratory fitness and health-related quality of life, as well as exhibited a preventive effect against increased body mass index.24 Another RCT found that, higher self-reported smoking quit rates, lower levels of lung expiratory carbon monoxide and saliva cotinine at 6-month follow-up, among participants receiving an SDT-based smoking cessation intervention compared with those who only received educational leaflets.25 There are fewer studies of autonomy support in HIV prevention.14,26 This is an important limitation of the current HIV prevention research evidence base, especially because it is already well established that health care workers can play a gatekeeper role by tacitly enacting and/or supporting approaches that restrict Black MSM's access to PrEP12,27,28—nullifying possibilities for PrEP use goal attainment.
Goal setting is a collaborative strategy by which health care providers and their clients outline shorter term objectives that are useful for attaining long-term HIV prevention objectives. Goal setting has been shown to have a statistically significant positive effect on a wide array of health behaviors29 [d = 0.44; 95% confidence interval (CI): 0.31–0.56] and among diverse populations and cultural contexts.29 Moreover, goal setting can also be autonomy supportive if it is grounded in informed decision making, centers the client's preferences, and minimizes external pressure. Goal setting, with progress monitoring and feedback, also helps to ensure both congruence and sufficiency between the goal and the steps one plans to take to accomplish it.29 Nonetheless, to date, limited research has studied whether goal setting and goal progress monitoring affects PrEP adherence among Black MSM. Additionally, there can be economic, legal, social, material, and logistical challenges to Black MSM attaining PrEP use goals.30
Overcoming these challenges may require a constellation of services and resources that, if not available or not well coordinated, could result in service discontinuities that then become de facto structural impediments to PrEP use for Black MSM.31
Care coordination models were widely used in the first few decades of the domestic HIV epidemic.32–36 Their focus was on reducing disease progression, disability, and mortality among people living with HIV who also experienced economic, legal, and social hardships that complicated their medical care.32–36 Today, it is understood that economic, legal, and social hardships also complicate the paths that Black MSM take to attaining their HIV prevention goals.37 Care coordination models have recently begun to be used as a tool for HIV prevention with Black MSM.26 High-quality care coordination can potentially buffer the negative impact of hardships on Black MSM's ability to make progress toward their PrEP use goals; however, this proposition has not been empirically tested.
Building on prior research,14,15,26,38,39 the purposes of this study were to test our hypotheses that (H1) health care provider autonomy support was associated with PrEP adherence, (H2) health care provider autonomy support was associated with PrEP through a mediating pathway of PrEP adherence goal setting (AGS) and making progress toward achieving the PrEP adherence goal, (H3) care coordination quality moderates the relationship between AGS and progress toward the PrEP adherence goal, and (H4) care coordination quality moderates the relationship between progress toward the PrEP adherence goal and PrEP adherence.
Methods
Design
This was a secondary analysis of data collected in a parent study: HPTN 073, which was an open-label, vanguard, clinical demonstration study to assess feasibility and acceptability of PrEP in a sample of Black MSM in three US cities: Chapel Hill, NC; Los Angeles, CA; and Washington D.C. Full details of the parent study are published elsewhere.26 The parent study consisted of PrEP-eligible, Black MSM (N = 226) who were offered oral PrEP and a Client-Centered Care Coordination (C4™) intervention over the course of 52 weeks. C4™ was developed using SDT whereby participants in the study guided their engagement with service providers in accessing HIV prevention and other health-related services.15,26 HPTN 073 was approved by the Institutional Review Boards of the University of California, Los Angeles; the University of North Carolina at Chapel Hill; and George Washington University. The current study was exempt from IRB review because it only utilized deidentified data from HPTN 073 following the HIPAA Safe Harbor guidelines.
Measures
Clinical outcome
PrEP adherence
Adherence to PrEP was assessed at 26 weeks and defined as meeting the 90% sensitivity threshold for ≥4 oral doses per week of combination emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) in a dried blood spot (DBS). This threshold was measured by concentrations of tenofovir diphosphate (TFV; a byproduct of TDF metabolism) and FTC: ≥4.2 ng/mL for TFV and ≥4.6 ng/mL for FTC in plasma; 9.9 fmol/106 for TFV diphosphate and 0.4 fmol/106 for FTC triphosphate in PBMCs.40,41 Levels ≥either of these thresholds were classified as adherent and those below the thresholds on both TFV and FTC were categorized as nonadherent.26
Predictors
Autonomy support
The Healthcare Climate Questionnaire (HCCQ)14,18,42 is a 15-item measure, with strong internal consistency (α = 0.96) that assessed the extent to which a client experienced support in the health care environment across three theoretically grounded21,43 domains: autonomy (freedom and choice), competence (individual agency), and relatedness (closeness).14,18,19 The HCCQ was administered through self-report computer-assisted self-interview (CASI) survey starting at week 4, and then at weeks 8, 13, and 26. Participants were asked to indicate the degree to which they agreed with a series of statements regarding interactions with individuals in the health care environment. Sample scale items included: “I feel that the team accepts me,” “The team listens to how I would like to do things,” and “The healthcare team encourages me to ask questions.” Response options were on a 7-point Likert-type scale that ranged from 1 = strongly disagree to 7 = strongly agree. Higher mean scores correspond with a higher perception of autonomy support.
PrEP AGS
This item was documented using a case report form (CRF) completed by the C4™ counselor.15 The CRF instructed the counselor to provide a binary response (yes/no) to the following item, “Did the participant set a PrEP adherence goal at this visit?” This item was assessed at all study visits. AGS was assessed at each study visit; however, the current analysis does not include AGS documentation from weeks 39 to 52 because those are after the 26-week PrEP adherence outcome measurement time point.
PrEP adherence goal progress
Progress toward a PrEP adherence goal was also measured using a CRF completed by the C4™ counselor. The CRF instructed the counselor to provide a binary (yes/no) response to the following item: “Did the participant make progress toward his PrEP adherence goal(s) set at last study visit?” This was an external assessment made by the counselor that included the client's subjective understanding of whether they have made progress and the counselor's evaluation of any evidence that the client has made steps toward any goals that were set. Adherence goal progress (AGP) was assessed only if the participant set a PrEP adherence goal at the previous study visit. AGP was assessed at each study visit; however, the current analysis does not include AGP documentation from weeks 39 to 52 because those are after the 26-week PrEP adherence outcome time point.
Care coordination quality
The Client Perception of Coordination Quality (CPCQ) assessed the extent to which the client perceived that their care services were well coordinated in four domains: quality, coherence, satisfaction, and impact.44 The 15-item measure had good internal consistency (0.84) in the current sample and was administered through CASI starting at week 13, and then at weeks 26, 39, and 52.15 However, the current analysis does not include CPCQ ratings from weeks 39 to 52 because those are after the 26-week PrEP adherence outcome time point. Sample items included: “How often have service providers responded appropriately to changes in your needs?” “How often did providers seem to be unnecessarily repeating tests or assessments?” and “How often were you confused about the roles of different providers?”44 Response options were on a 5-point Likert-type scale that ranged from “1 = never to 5 = always.” Items indicating poor coordination quality were reverse coded. The CPCQ was scored using the mean, with higher mean scores corresponding with greater quality.
Covariates
Several covariates were included in the multiple regression analysis. Age was used as a continuous variable. Both education attainment, and income were entered in the model as continuous variables.
Data analyses
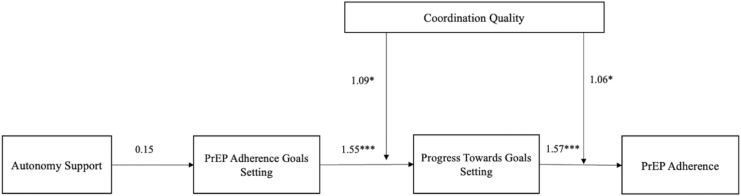
All analyses were conducted on observations that included nonmissing data for the outcome, PrEP adherence. Table 1 presents sample characteristics of Black MSM who initiated PrEP. Next, we conducted a logistic regression analysis to evaluate the association between independent variables and PrEP adherence (Table 2). We also evaluated a moderation effect of CPCQ on the associations between AGS and AGP on PrEP adherence (Table 3). The moderator was mean centered. Lastly, we conducted a mediation (path) analysis to examine direct and indirect pathways to PrEP adherence and to test our hypotheses that autonomy support effects PrEP adherence through goal setting, goal progress, and care coordination quality (Table 4). Figure 1 shows the direct and indirect pathways to PrEP adherence. The mean and variance-adjusted weighted least squares estimator was used instead of maximum likelihood estimation because this is the preferred estimator when the dependent variable is categorical and not normally distributed.45
Table 1.
Demographics of Participant Who Initiated Pre-Exposure Prophylaxis (N = 179)
| Frequency (%) | |
|---|---|
| Age | |
| Range | 18–69 |
| Mean (SD) | 29.4 (9.9) |
| Ethnicity | |
| Black non-Latino (e.g., African American, African Caribbean) | 161 (90%) |
| Black Latino | 14 (8%) |
| Other | 4 (2%) |
| Education | |
| Some high school | 8 (4.42) |
| High school graduate or equivalent | 36 (20.3) |
| Vocational/trade/technical school | 8 (4.42) |
| AA or other 2-year degree | 8 (4.4) |
| BA/BS degree | 35 (19.9) |
| Masters or other advanced degree | 17 (9.7) |
| Annual income | |
| <$20,000 | 82 (46%) |
| $20,000 to $40,000 | 45 (25%) |
| ≥$40,000 | 49 (27%) |
| No response | 4 (1%) |
| Employment status | |
| Employed full time | 67 (37.6) |
| Employed part-time | 53 (30.1) |
| Self-employed | 9 (5.3) |
| Disabled | 4 (2) |
| Unemployed or in between jobs | 38 (21.2) |
| Other | 7 (4) |
| Marital status | |
| Single/divorced/widowed | 149 (83) |
| Married | 30 (17) |
| Study location | |
| Washington, DC | 59 (33) |
| Los Angeles, CA | 61 (34) |
| Chapel Hill/Durham, NC | 59 (33) |
| CAI with HIV+ or unknown causal male partner | |
| No | 102 (57%) |
| Yes | 77 (43%) |
| STI prevalence at 6 months | |
| No | 146 (82%) |
| Yes | 33 (18%) |
SD, standard deviation; STI, sexually transmitted infection.
Table 2.
Logistic Regression on Individuals Who Initiated Pre-Exposure Prophylaxis and Adherence (N = 179)
| PrEP adherence | OR | SE | 95% CI |
|---|---|---|---|
| Autonomy support | 1.17* | 0.06 | 1.00 to 1.38 |
| Age | 0.61*** | 0.07 | 0.47 to 0.78 |
| Income | 1.24*** | 0.09 | 1.07 to 1.44 |
| Education | 1.17* | 0.09 | 1.00 to 1.37 |
*p < 0.05, **p < 0.01, ***p < 0.001.
CI, confidence interval; OR, odds ratio; PrEP, pre-exposure prophylaxis; SE, standard error.
Table 3.
Associations Between Pre-Exposure Prophylaxis Adherence Goal Setting and Progress Toward Pre-Exposure Prophylaxis Adherence Goal of Black Men Who Have Sex with Men Who Initiated Pre-Exposure Prophylaxis (N = 179)
| Main effects |
Moderating effects |
|||
|---|---|---|---|---|
| OR | SE | OR | SE | |
| CPCQ | 0.87* | 0.57 | ||
| AGS | 0.43** | 0.12 | ||
| AGP | 0.60* | 0.12 | ||
| CPCQ x AGS | 1.09* | 0.04 | ||
| CPCQ x AGP | 1.06* | 0.03 | ||
p < 0.05, **p < 0.01.
AGS = setting a PrEP adherence goal.
AGP = progress toward PrEP adherence goal.
AGP, adherence goal progress; AGS, adherence goal setting; CPCQ, Client Perception of Coordination Quality.
Table 4.
Direct and Indirect Effects on Individuals Who Initiated Pre-Exposure Prophylaxis (N = 179)
| B | SE | 95% CI | |
|---|---|---|---|
| Direct effects | |||
| AGS | |||
| Autonomy support | 0.15 | 0.01 | −0.01 to 0.04 |
| Progress toward adherence goal | |||
| AGS | 1.55*** | 0.03 | 1.50 to 1.61 |
| PrEP adherence | |||
| Progress toward adherence goal | 1.57*** | 0.03 | 1.51 to 1.68 |
| Indirect effects | |||
| Progress toward adherence goal | |||
| Autonomy support | −0.24 | 0.27 | −0.78 to 0.28 |
| PrEP adherence | |||
| AGS | 0.00 | 0.00 | −0.01 to 0.00 |
| Autonomy support | 0.00 | 0.00 | 0.00 to 0.00 |
p < 0.001.
AGS = setting a PrEP adherence goal; AGP = progress toward PrEP adherence goal.
FIG. 1.
Pathways to PrEP adherence, indirect and direct effects, moderating effects, and betas reported. PrEP, pre-exposure prophylaxis.
The percentage of missing data was <5%.45 Full information maximum likelihood was used to handle missing data.46 Model fit indices were not included in the report, as the models were just identified.45 Instead, beta coefficients and p-values are included and used to examine associations among key study variables. All analyses were done in M-plus 8.4.
Results
Table 1 describes the characteristics of the sample of Black MSM who initiated PrEP (N = 179). The average age of the participants was 29.4 (SD = 9.9); ∼46% reported having income below $20,000 in the previous 12 months. Thirty-seven percent reported being employed full-time and ∼20% of the sample reported having a BS/BA degree. Most (69%) reported having health insurance in the past 12 months. The majority (83%) of the sample reported being single, divorced, or widowed. Eighteen percent of the sample reported receiving an sexually transmitted infection (STI) diagnosis in the 6 months before enrollment.
Logistic regression
Autonomy support was associated with PrEP adherence [odds ratio (OR) = 1.17; 95% CI: 1.00–1.38], indicating that individuals who reported higher autonomy support from their health care providers were more likely to have DBS levels that were consistent with PrEP adherence. Care coordination quality was associated with PrEP adherence (OR = 0.88; 95% CI: 0.76–0.98). Age was statistically significant and associated with PrEP adherence (OR = 0.61; 95% CI: 0.47–0.78). Younger Black MSM were more likely to adhere to PrEP than older Black MSM. Black MSM with a higher income were more likely to have DBS levels that were consistent with PrEP adherence than lower income individuals (OR = 1.24; 95% CI: 1.07–1.44). Lastly, participants with a higher education were more likely to have DBS levels that were consistent with PrEP adherence compared with individuals with lower education (OR = 1.17; 95% CI: 1.00–1.37).
Moderation
We tested CPCQ as an interaction term. The variable CPCQ was entered into the model to examine if it exerted a moderating effect on our two goal-related variables: AGS and AGP. The results are displayed in Table 2. Black MSM who experienced high quality in the coordination of their care services were more likely to set a PrEP adherence goal at their study visit. Similarly, Black MSM who experienced high-quality care coordination were also more likely to make progress toward their PrEP adherence goals (Table 2).
Mediation
The hypothesized path model for how autonomy support effects PrEP adherence is displayed in Fig. 1. The coefficients for the path model are presented in Table 3. Setting a PrEP adherence goal was significantly and directly associated to making progress toward achieving a PrEP adherence goal (β = 1.55; p < 0.001). Making progress toward achieving a PrEP adherence goal was significant and directly associated with DBS levels consistent with PrEP adherence (β = 1.57; p < 0.001).
Discussion
The support MSM receive in health care facilities has been shown to influence their sexual health behaviors, including condom use, HIV testing, and HIV PrEP adherence.47–50 Among Black MSM in the United States, the underlying mechanism by which a supportive health care environment is associated with HIV PrEP adherence is not fully known. This is particularly important given the fact that PrEP use remains low among the general Black racialized populations in the United States.51 This study investigated the hypotheses that autonomy support was associated with greater odds of PrEP adherence, and that the association was mediated by setting a PrEP adherence goal and making progress toward the PrEP adherence goal. We also investigated the hypotheses that high-quality care coordination increases the magnitude of the association between setting a PrEP adherence goal and making progress toward the goal and of the association between making progress toward the adherence goal and observed biomarker-validated PrEP adherence. The results of the analyses provided support for all the hypotheses that we tested.
The finding that autonomy support was associated with increased odds of PrEP adherence is consistent with results from other cross-sectional studies among MSM, which found that autonomy supportive health care environments increased the odds of sexual health promotive behaviors, including PrEP use, condom use, and linkage to care.14,48 Provision of autonomy support means that health care providers do not impose their ideas on MSM but rather engage in open discourse, provide MSM with useful information and support them to make informed decisions on their own volition.15 SDT suggests that people's innate propensity to make healthy life decisions (including PrEP adherence) can be stimulated and nurtured by supporting their need for autonomy. Autonomy support enhances client's intrinsic motivation and helps to sustain behavior change.21,52 The influence of autonomy support on health behaviors and health outcomes has been demonstrated across a range of health domains and demographic groups.22,53–55 For example, our results in this study are also corroborated by a meta-analysis that found an overall medium effect size (d+ = 0.47; 95% CI = 0.44–0.83) of autonomous motivation on health behavior change across 67 intervention studies.56
In a qualitative study among Black cisgender women living with HIV, autonomy support was identified as a key element for long-term engagement in HIV testing and care, including medication adherence.57 Autonomy support is an ideal approach to implement in health care environments because its effects are robust and not likely to be isolated to only MSM, but have residual impact on other patient groups and health conditions that were not the original intervention target.
The result supported our hypotheses that the association between autonomy support and PrEP adherence was explained, at least in part, by setting a PrEP adherence goal and making progress toward that adherence goal. In the current study, clients were allowed to determine whether they wanted to set adherence goals. The clients continued to receive support and services from the counselor and health care providers regardless of their decision to set a goal or not. This noncontingent support meant that clients “owned” the goals that they set and thus were likely to commit to them. Goal setting when done out of client's volition, and without the pressure of health care provider-imposed contingencies, is likely to lead to goal progress.15 Further, goal setting in HPTN 073 was a collaborative endeavor between client and C4™ counselor. There was attention to set goals that were feasible and always centering the client's autonomy. Goal progress was determined by the external appraisal of the trained C4™ counselor, which was informed by the MSM client's self-report but not solely determined by their self-report. Goal setting, when accompanied with progress appraisal and provision of relevant feedback, helps to ensure congruence between the goal and action plan.29
Goal setting and monitoring of progress also promotes positive behavior change.29 Goal progress appraisal provides an avenue to remind clients of their commitments and to identify and mitigate barriers impeding progress as well as optimize facilitators that are accelerating progress. Appraisal of goal progress has been linked to goal attainment.58 Even though appraising the progress of client's goals is useful, the dichotomous grading (yes/no) of PrEP adherence progress does not capture the extent of the progress made. This highlights the need for more methodological innovation in the development of measures to assess how much progress clients make toward achieving their PrEP adherence goals.
Finally, we found that care coordination quality increased the association of AGS on goal progress, and it also increased the association of AGP on PrEP adherence. These findings supported our study hypotheses. While there is very little research to date that has investigated associations between care coordination and antiretroviral medication for HIV PrEP, there is existing research that examined care coordination and adherence to antiretroviral medication adherence for HIV treatment. Care coordination has been shown to improve medication adherence among people living with HIV.59 In a retrospective cohort study in the United States, participants living with HIV who engaged in an HIV care coordination program had 11% higher likelihood of having suppressed viral load compared with those who did not participate (Relative Risk = 1.11; 95% CI: 1.08–1.14).60
Finally, it is our proposition that the enhancing effects of high-quality care coordination functions by removing social and structural barriers to PrEP adherence among MSM.11 An example of this is the barrier that financial costs pose to PrEP access for many Black MSM. Our finding that income was associated with more PrEP adherence highlights the potential impact of this known social determinant of health on the sexual health behaviors of Black MSM.26 Consistent with our finding, in another study among young MSM in California, the odds of PrEP adherence were four times higher for participants with higher income [adjusted odds ratio (aOR) = 4.13; 95% CI: 1.87–9.12].61 This raises concerns about the affordability of PrEP, which costs an average of $8000 a year.62 For MSM who either have no or insufficient insurance coverage (69% of study participants had health insurance), the cost of PrEP may serve as an economic barrier for adherence. Even among MSM with income, the cost of PrEP may still be prohibitive and/or a lower relative priority compared with more immediate day-to-day needs that also require a share of an individual's finite income.
It may also indicate a need for greater awareness of the available programs that are designed to expand access to PrEP for individuals who have limited incomes.63 Care coordination interventions approach these issues through a tripartite focus on case management, resource utilization management, and brokering services between a network of local agencies based on the client's needs. For example, if a client did not have access to insurance to cover payments for required medication, laboratory monitoring (e.g., kidney function tests), or clinic visits, care coordination activates to arrange a solution with other service providers to bridge the service gap caused by insurance insufficiency. This could result in linkage to a clinic that provide services at no cost to the client or identifying and registering the client with programs for which they may be eligible, such as manufacturer drug assistance/discount programs, Veteran's Affairs benefits, or Medicaid. In this study, we showed the care coordination quality is a key service metric that has an important impact on PrEP adherence for Black MSM.
Limitations
The study has some limitations that should be considered in the interpretation of our findings. First, AGP was assessed using a binary measure (yes or no), which likely obfuscates variability in the degree of progress made among Black MSM in the study; however, this limitation is overcome by several design features. The measurement of PrEP AGP was informed both by the self-assessment of the study participant and the external appraisal of the C4™ counselor. The C4™ counselor made the final determination regarding whether or not progress was made toward a PrEP adherence goal. Additionally, we collected biomarker data on PrEP adherence by measuring serum concentrations in DBSs. The results of our mediation analysis indicated a positive relationship between DBS-measured PrEP adherence and report of progress toward PrEP adherence goals. These strengthen our confidence in the validity of our measure of the progress toward PrEP adherence goals. Second, the study was conducted with a nonprobability-based sample thereby limiting the external validity of the findings from this study.
Nonetheless, HPTN 073 was a vanguard study designed to determine the implementation feasibility and acceptability of a PrEP program among Black MSM. While the results cannot be generalized to the entire national US population of Black MSM it provides important proof-of-concept evidence regarding a model for PrEP services and the pathway by which the activities in the model may lead to increased PrEP adherence. Last, the observational design used for this study limits our ability to assert causal relationship between study variables. This limitation notwithstanding, our results provide important preliminary data to support the scientific premise of future studies and the generation of hypotheses that can be investigated in longitudinal randomized controlled trials with probability-based samples of Black MSM.
Conclusions
Autonomy support, goal setting, goal progress, and care coordination quality influence PrEP adherence among Black MSM. Our findings indicate that while it is important to set goals for PrEP adherence, high-quality care coordination can enhance its impact on goal progress and ultimately on PrEP adherence. The C4™ intervention is an evidence-based service platform that integrates all these important elements that contribute to the enhancement of PrEP adherence among Black MSM.
Data Access Statement
Data are publicly available from the HIV Prevention Trials Network at https://www.hptn.org/resources/concept_ancillarystudy.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Overall support for the HIV Prevention Trials Network (HPTN) is provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068619 (HPTN Leadership and Operations Center), UM1AI068617 (HPTN Statistical and Data Management Center), and UM1AI068613 (HPTN Laboratory Center). Additional support was provided by the National Institute on Drug Abuse and the National Institute of Mental Health, of the National Institutes of Health, US Department of Health and Human Services. This was also made possible by core services and support provided by the Yale University Center for Interdisciplinary Research on AIDS (P30 MH062294), the Yale Research Education Institute for Diverse Scholars (R25 MH087271), and the District of Columbia Center for AIDS Research, (P30 AI117970)— NIH-funded programs. The study product, tenofovir disoproxil fumarate/emtricitabine, was donated by Gilead Sciences, Inc.
References
- 1. Mayer KH, Nelson L, Hightow-Weidman L, et al. . The persistent and evolving HIV epidemic in American men who have sex with men. Lancet 2021;397(10279):1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou J, Yamanaka Y, John M, et al. . Religion and HIV in Tanzania: Influence of religious beliefs on HIV stigma, disclosure, and treatment attitudes. BMC Public Health 2009;9:75; doi: 10.1186/1471-2458-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC. Diagnoses of HIV infection in the United States and dependent areas, 2020. HIV Surveillance Report 2020. 2022;33. Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Last accessed: October 1, 2022]. [Google Scholar]
- 4. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021;26. Available from: www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Last accessed: October 1, 2022]. [Google Scholar]
- 5. Grant RM, Lama JR, Anderson PL, et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587–2599; doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landovitz RJ, Donnell D, Clement ME, et al. . Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021;385(7):595–608; doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang YA, Zhu W, Smith DK, et al. . HIV preexposure prophylaxis, by race and ethnicity—United States, 2014–2016. MMWR Morbid Mortal Wkly Rep 2018;67(41):1147–1150; doi: 10.15585/mmwr.mm6741a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoots BE, Finlayson T, Nerlander L, et al. . Willingness to take, use of, and indications for pre-exposure prophylaxis among men who have sex with men-20 US cities, 2014. Clin Infect Dis 2016;63(5):672–677. doi: 10.1093/cid/ciw367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okafor CN, Gorbach PM, Ragsdale A, et al. . Correlates of preexposure prophylaxis (PrEP) use among men who have sex with men (MSM) in Los Angeles, California. J Urban Health 2017;94(5):710–715; doi: 10.1007/s11524-017-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ojikutu BO, Bogart LM, Higgins-Biddle M, et al. . Facilitators and barriers to pre-exposure prophylaxis (PrEP) use among Black individuals in the United States: Results from the National Survey on HIV in the Black Community (NSHBC). AIDS Behav 2018;22(11):3576–3587; doi: 10.1007/s10461-018-2067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilton L. Men Who Have Sex with Men of Color in the Age of AIDS: The Sociocultural Contexts of Stigma, Marginalization, and Structural Inequalities. In: HIV/AIDS in Communities of Color. (Stone V, Ojikutu B, Rawlings K, Smith K. eds.) Springer: Berlin, Germany; 2009: pp. 179–211. [Google Scholar]
- 12. Calabrese SK, Earnshaw VA, Krakower DS, et al. . A closer look at racism and heterosexism in medical students' clinical decision-making related to HIV pre-exposure prophylaxis (PrEP): Implications for PrEP education. AIDS Behav 2018;22(4):1122–1138; doi: 10.1007/s10461-017-1979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lelutiu-Weinberger C, Golub SA. Enhancing PrEP access for Black and Latino men who have sex with men. J Acquir Immune Defic Syndr 2016;73(5):547–555; doi: 10.1097/QAI.0000000000001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson LE, Wilton L, Agyarko-Poku T, et al. . Predictors of condom use among peer social networks of men who have sex with men in Ghana, West Africa. PLoS One 2015;10(1):e0115504; doi: 10.1371/journal.pone.0115504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson LE, Wilton L, Williams GC, et al. . Client-centered care coordination for HIV/STI prevention: A theoretical, conceptual and methodological overview—HIV Prevention Trials Network (HPTN) 073. Sexual Res Soc Policy 2022;19:1365–1382; doi: 10.1007/s13178-022-00687-x. [DOI] [Google Scholar]
- 16. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55(1):68–78. [DOI] [PubMed] [Google Scholar]
- 17. Williams GC, Niemiec CP, Patrick H, et al. . The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med 2009;37(3):315–324; doi: 10.1007/s12160-009-9090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams GC, Cox EM, Kouides R, et al. . Presenting the facts about smoking to adolescents: Effects of an autonomy-supportive style. Arch Pediatr Adolesc Med 1999;153(9):959–964. [DOI] [PubMed] [Google Scholar]
- 19. Williams GC, Rodin GC, Ryan RM, et al. . Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol 1998;17(3):269–276. [DOI] [PubMed] [Google Scholar]
- 20. Reeve J, Hyungshim J, Hardré P, et al. Providing a rationale in an autonomy supportive way as a strategy to motivate others during an uninteresting task. Motiv Emot 2002;26(3):183–207. [Google Scholar]
- 21. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychol Inquiry 2000;11(4):227–268. [Google Scholar]
- 22. Ntoumanis N, Ng JYY, Prestwich A, et al. . A meta-analysis of self-determination theory-informed intervention studies in the health domain: Effects on motivation, health behavior, physical, and psychological health. Health Psychol Rev 2020:1–31; doi: 10.1080/17437199.2020.1718529. [DOI] [PubMed] [Google Scholar]
- 23. Sheeran P, Wright CE, Avishai A, et al. . Self-determination theory interventions for health behavior change: Meta-analysis and meta-analytic structural equation modeling of randomized controlled trials. J Consult Clin Psychol 2020;88(8):726–737; doi: 10.1037/ccp0000501. [DOI] [PubMed] [Google Scholar]
- 24. Riiser K, Londal K, Ommundsen Y, et al. . The outcomes of a 12-week Internet intervention aimed at improving fitness and health-related quality of life in overweight adolescents: The Young & Active controlled trial. PLoS One 2014;9(12):e114732; doi: 10.1371/journal.pone.0114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li WHC, Ho KY, Wang MP, et al. . Effectiveness of a brief self-determination theory-based smoking cessation intervention for smokers at emergency departments in Hong Kong: A randomized clinical trial. JAMA Intern Med 2020;180(2):206–214; doi: 10.1001/jamainternmed.2019.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wheeler DP, Fields SD, Beauchamp G, et al. . Pre-exposure prophylaxis initiation and adherence among Black men who have sex with men (MSM) in three US cities: Results from the HPTN 073 study. J Int AIDS Soc 2019;22(2):e25223; doi: 10.1002/jia2.25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calabrese SK, Earnshaw VA, Underhill K, et al. . Prevention paradox: Medical students are less inclined to prescribe HIV pre-exposure prophylaxis for patients in highest need. J Int AIDS Soc 2018;21(6):e25147; doi: 10.1002/jia2.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Ryn M, Burgess DJ, Dovidio JF, et al. . The impact of racism on clinician cognition, behavior, and clinical decision making. Du Bois Rev 2011;8(1):199–218; doi: 10.1017/S1742058X11000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Epton T, Currie S, Armitage CJ. Unique effects of setting goals on behavior change: Systematic review and meta-analysis. J Consult Clin Psychol 2017;85(12):1182–1198; doi: 10.1037/ccp0000260. [DOI] [PubMed] [Google Scholar]
- 30. Levy ME, Wilton L, Phillips G, 2nd, et al. Understanding structural barriers to accessing HIV testing and prevention services among Black men who have sex with men (BMSM) in the United States. AIDS Behav 2014;18(5):972–996; doi: 10.1007/s10461-014-0719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lelutiu-Weinberger C, Wilton L, Koblin BA, et al. . The role of social support in HIV testing and PrEP awareness among young Black men and transgender women who have sex with men or transgender women. J Urban Health 2020;97(5):715–727; doi: 10.1007/s11524-019-00396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson MM, Penrose K, Nash D, et al. . Impact of an HIV care coordination program on the timeliness of viral suppression and immune recovery among clients newly diagnosed with HIV. AIDS Behav 2020;24(4):1237–1242; doi: 10.1007/s10461-019-02732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robertson MM, Penrose K, Irvine MK, et al. . Impact of an HIV care coordination program on durable viral suppression. J Acquir Immune Defic Syndr 2019;80(1):46–55; doi: 10.1097/QAI.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Donnell L, Irvine MK, Wilkes AL, et al. . STEPS to care: Translating an evidence-informed HIV care coordination program into a field-tested online practice improvement toolkit. AIDS Educ Prev 2020;32(4):296–310; doi: 10.1521/aeap.2020.32.4.296. [DOI] [PubMed] [Google Scholar]
- 35. Jordan AO, Cohen LR, Harriman G, et al. . Transitional care coordination in New York City jails: Facilitating linkages to care for people with HIV returning home from Rikers Island. AIDS Behav 2013;17 Suppl 2:S212–S219; doi: 10.1007/s10461-012-0352-5. [DOI] [PubMed] [Google Scholar]
- 36. Irvine MK, Chamberlin SA, Robbins RS, et al. . Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis 2015;60(2):298–310. doi: 10.1093/cid/ciu783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nelson LE, Wilton L, Moineddin R, et al. . Economic, legal, and social hardships associated with HIV risk among Black men who have sex with men in six US cities. J Urban Health 2016;93(1):170–188; doi: 10.1007/s11524-015-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos SR, Beauchamp G, Wheeler DP, et al. . Optimizing PrEP continuance: A secondary analysis examining perceived autonomy support and care coordination quality among Black MSM in HPTN 073. Int J Environ Res Public Health 2022;19(8):4489; doi: 10.3390/ijerph19084489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whitfield DL, Nelson LE, Komarek A, et al. . Implementation of client-centered care coordination for HIV prevention with Black men who have sex with men: Activities, personnel costs, and outcomes-HPTN 073. J Racial Ethn Health Disparities 2022:1–10; doi: 10.1007/s40615-021-01209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Clarke W, Marzinke MA, et al. . Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV prevention trials network 073. Antimicrob Agents Chemother 2017;61(7):e02743–16; doi: 10.1128/AAC.02743-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hendrix CW, Andrade A, Bumpus NN, et al. . Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32(1):32–43; doi: 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: A test of self-determination theory. J Pers Soc Psychol 1996;70(4):767–779; doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 43. Ryan RM, Patrick H, Deci EL, et al. . Facilitating health behaviour change and its maintenance: Interventions based on self-determination theory. Eur Health Psychol 2008;10(1):2–5. [Google Scholar]
- 44. McGuiness C, Sibthorpe B. Development and initial validation of a measure of coordination of health care. Int J Qual Health Care 2003;15(4):309–318; doi: 10.1093/intqhc/mzg043. [DOI] [PubMed] [Google Scholar]
- 45. Kline RB. Principles and Practice of Structural Equation Modeling. 4th ed. The Guilford Press: New York, NY; 2015. [Google Scholar]
- 46. Muthén LK, Muthén BO. Statistical Analysis with Latent Variables: User's Guide. 7th ed. Muthén & Muthén: Los Angeles, CA; 2010. [Google Scholar]
- 47. Katz IT, Ryu AE, Onuegbu AG, et al. . Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. J Int AIDS Soc 2013;16(3 Suppl 2):18640; doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gu LY, Zhang N, Mayer KH, et al. . Autonomy-supportive healthcare climate and HIV-related stigma predict linkage to HIV care in men who have sex with men in Ghana, West Africa. J Int Assoc Provid AIDS Care 2021;20:2325958220978113; doi: 10.1177/2325958220978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Musheke M, Ntalasha H, Gari S, et al. . A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC Public Health 2013;13:220; doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shangani S, Naanyu V, Operario D, et al. . Stigma and healthcare-seeking practices of men who have sex with men in Western Kenya: A mixed-methods approach for scale validation. AIDS Patient Care STDS 2018;32(11):477–486; doi: 10.1089/apc.2018.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mounzer KC, Fusco JS, Hsu RK, et al. . Are we hitting the target? HIV pre-exposure prophylaxis from 2012 to 2020 in the OPERA cohort. AIDS Patient Care STDS 2021;35(11):419–427; doi: 10.1089/apc.2021.0064. [DOI] [PubMed] [Google Scholar]
- 52. Patrick H, Williams GC. Self-determination theory: Its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act 2012;9:18; doi: 10.1186/1479-5868-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chirkov V, Ryan RM, Kim Y, et al. . Differentiating autonomy from individualism and independence: A self-determination theory perspective on internalization of cultural orientations and well-being. J Pers Soc Psychol 2003;84(1):97–110. [PubMed] [Google Scholar]
- 54. Ng JYY, Ntoumanis N, Thogersen-Ntoumani C, et al. . Self-determination theory applied to health contexts: A meta-analysis. Perspect Psychol Sci 2012;7(4):325–340. [DOI] [PubMed] [Google Scholar]
- 55. Chirkov VI, Ryan RM, Willness C. Cultural context and psychological needs in Canada and Brazil: Testing a self-determination approach to the internalization of cultural practices, identity, and well-being. J Cross Cult Psychol 2005;36:423–443. [Google Scholar]
- 56. Sheeran P, Wright CE, Avishai A, Villegas ME, Rothman AJ, Klein WMP. Does increasing autonomous motivation or perceived competence lead to health behavior change? A meta-analysis. Health Psychol 2021;40(10):706–716; doi: 10.1037/hea0001111. [DOI] [PubMed] [Google Scholar]
- 57. Quinlivan EB, Messer LC, Adimora AA, et al. . Experiences with HIV testing, entry, and engagement in care by HIV-infected women of color, and the need for autonomy, competency, and relatedness. AIDS Patient Care STDS 2013;27(7):408–415; doi: 10.1089/apc.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harkin B, Webb TL, Chang BP, et al. . Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychol Bull 2016;142(2):198–229; doi: 10.1037/bul0000025. [DOI] [PubMed] [Google Scholar]
- 59. Westergaard RP, Genz A, Panico K, et al. . Acceptability of a mobile health intervention to enhance HIV care coordination for patients with substance use disorders. Addict Sci Clin Pract 2017;12(1):11; doi: 10.1186/s13722-017-0076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nash D, Robertson MM, Penrose K, et al. . Short-term effectiveness of HIV care coordination among persons with recent HIV diagnosis or history of poor HIV outcomes. PLoS One 2018;13(9):e0204017; doi: 10.1371/journal.pone.0204017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holloway IW, Dougherty R, Gildner J, et al. . Brief report: PrEP uptake, adherence, and discontinuation among California YMSM using geosocial networking applications. J Acquir Immune Defic Syndr 2017;74(1):15–20; doi: 10.1097/QAI.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kay ES, Pinto RM. Is insurance a barrier to HIV preexposure prophylaxis? Clarifying the Issue. Am J Public Health 2020;110(1):61–64; doi: 10.2105/AJPH.2019.305389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zajacova A, Lawrence EM. The relationship between education and health: Reducing disparities through a contextual approach. Ann Rev Public Health 2018;39:273–289; doi: 10.1146/annurev-publhealth-031816-044628. [DOI] [PMC free article] [PubMed] [Google Scholar]