Abstract
Traumatic brain injuries (TBIs) affect more than 10 million patients annually worldwide, causing long-term cognitive and psychosocial impairments. Frontal lobe TBIs commonly impair executive function, but laboratory models typically focus primarily on spatial learning and declarative memory. We implemented a multi-modal approach for clinically relevant cognitive-behavioral assessments of frontal lobe function in rats with TBI and assessed treatment benefits of the serotonin-norepinephrine reuptake inhibitor, milnacipran (MLN). Two attentional set-shifting tasks (AST) evaluated cognitive flexibility via the rats' ability to locate food-based rewards by learning, unlearning, and relearning sequential rule sets with shifting salient cues. Adult male rats reached stable pre-injury operant AST (oAST) performance in 3–4 weeks, then were isoflurane-anesthetized, subjected to a unilateral frontal lobe controlled cortical impact (2.4 mm depth, 4 m/sec velocity) or Sham injury, and randomized to treatment conditions. Milnacipran (30 mg/kg/day) or vehicle (VEH; 10% ethanol in saline) was administered intraperitoneally via implanted osmotic minipumps (continuous infusions post-surgery, 60 μL/h). Rats had a 10-day recovery post-TBI/Sham before performing light/location-based oAST for 10 days and, subsequently, odor/media-based digging AST (dAST) on the last test day (26-27 days post-injury) before sacrifice. Both AST tests revealed significant deficits in TBI+VEH rats, seen as elevated total trials and errors (p < 0.05), which generally normalized in MLN-treated rats (p < 0.05). This first simultaneous dual AST assessment demonstrates oAST and dAST are sufficiently sensitive and robust to detect subtle attentional and cognitive flexibility executive impairments after frontal lobe TBI in rats. Chronic MLN administration shows promise for attenuation of post-TBI executive function deficits, thus meriting further investigation.
Keywords: controlled cortical impact (CCI), milnacipran, antidepressants, operant behavior, executive function, attentional set-shifting
Introduction
Because of physical location with proximity to mechanically restrictive structures1–4 (skull compartment and falx cerebri) paired with inherent susceptibilities to metabolic disturbances,5–8 the frontal lobes are often damaged in traumatic brain injury (TBI).9,10 Post-TBI neuropsychological impairments in humans are most commonly reported in the executive, emotional, and attentional cognitive domains, which rely on intact prefrontal cortex (PFC) function.11–13 Despite this, TBI investigations across species have classically explored working memory, spatial memory, and motor function tasks, which do not directly assess the complex PFC-mediated cognitive function.
Executive and attentional impairments frequently observed in patients with TBI are reported to be among the most disabling injury sequelae, predict poor outcomes, and have limited treatment options.9–12,14–16 The Wisconsin Card Sorting Task (WCST) is a validated clinical neuropsychological tool for assessing such higher-level cognitive processes within the executive domain including attention, cognitive flexibility, and set shifting.17 The WCST has been shown to capture TBI severity-dependent executive function impairments18–21 and is particularly sensitive to PFC lesions.22–25
In rats, attentional set-shifting tasks (AST) were designed as a pre-clinical corollary to the WCST with ability to assess PFC-dependent executive function components including attentional set shifting26 and reversal learning,27 collectively measuring cognitive flexibility. The AST involves rats interpreting a variety of rules with shifting cue salience to locate a food reward. The assessment moves rats through stages of advancing difficulty and rule changes including reversals and set shifts.26–29 The PFC subregions are essential for AST and have stage-specific selectivity for subcomponents of the paradigms.26–28,30,31 The AST is sensitive to post-TBI executive dysfunction in rats in a severity-dependent manner32–35; however, direct frontal/prefrontal injuries have yet to be studied.
The AST performance is influenced in a region-specific fashion by the actions of multiple neurotransmitters (principally dopamine [DA], norepinephrine [NE], and serotonin [5-HT]).26–31,35–39 5-HT is thought to mediate the role of orbitofrontal cortex (OFC) in reversal learning,27,30,31,35,40 while NE is reported to have a more medial prefrontal cortex (mPFC)-specific role relevant to set shifting.26,28,37,41,42 Enhancement of noradrenergic tone,28,29 serotonergic tone,29–31,35,36 or both in concert43 effectively improve AST performance.
In the context of the growing literature support for multi-system, multi-neurotransmitter impairments influencing cognitive impairments after TBI32,35,44–52 and the known beneficial effects of increasing serotonergic and noradrenergic tone on AST,28–31,35,36 we hypothesized that a dual-mechanism treatment boosting both 5-HT and NE pathways via chronic, steady-state administration of MLN may provide specific benefits on AST performance in rats subjected to frontal lobe TBI.
Milnacipran is a unique compound among neuropsychiatric medications with a highly selective mechanism of action (e.g., prevention of 5-HT and NE reuptake)53–55 resulting in dynamic increases of both 5-HT and NE levels in multiple brain regions, including the PFC.56 This agent inhibits NE and 5-HT reuptake in a 3:1 ratio, in contrast to other commonly used serotonin and norepinephrine reuptake inhibitors (SNRIs) that preferentially inhibit 5-HT reuptake (e.g., duloxetine 1:5, venlafaxine 1:10, desvenlafaxine 1:11).54,55 Specific to 5-HT reuptake, MLN is less potent than other SNRIs and SSRIs including citalopram, with a half maximal inhibitory concentration (IC50) of approximately 30 nM, while other SSRIs and SNRIs commonly range from 0.5 nM–10 nM.53,57–59
Here, for the first time, we demonstrated that a multi-modal approach using two well-validated AST paradigms in the same brain-injured rats renders both tasks sensitive to PFC-mediated cognitive flexibility impairments after frontal lobe TBI and report the beneficial effects of dual SNRI treatment with MLN on PFC-mediated AST performance.
Methods
Animals
Thirty-four adult male Sprague-Dawley rats (Envigo, Indianapolis, IN) were housed in commercially available clear Plexiglas® individually ventilated cages (Allentown LLC, Allentown, NJ) under a regulated light-controlled cycle (12/12-h light/dark; lights on at 7:00 am) and kept at a constant temperature (21 ± 1°C) with food and water ad libitum. After a week of acclimation to the housing environment, experimental manipulations began during the light phase of the cycle. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh (Pittsburgh, PA) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize pain and discomfort and to minimize the number of rats used.
Operant attentional set-shifting task (oAST)
Rats were food-restricted to 13 g/rat/day with free access to water to maintain 80–85% of their free-feeding weight and to ensure adequate motivation for food reward incentives. Six controlled environment standard operant test chambers (Coulbourn Instruments, Allentown, PA) measuring 12 in (l) × 10 in (w) × 12 in (h) were used for oAST testing with procedures comparable to those of previous publications.60,61
Briefly, rats were sequentially acclimatized to computer-controlled stainless steel operant test chambers (Harvard Apparatus, Holliston, MA), which displayed a reward pellet dispenser and food trough on one wall and three identical integrated, infrared sensor-sensitive nose-poke modules on the opposing wall. Only the left and right nose-poke modules were actively used in this task (Fig. 1). The front and back walls of the chamber were constructed of clear Plexiglas. A house light placed above the food trough provided illumination for the boxes, and the floor was made of a steel-wire mesh grid. The operant boxes were housed in sound-dampening and lightproof wood cubicles, to reduce outside light and noise.
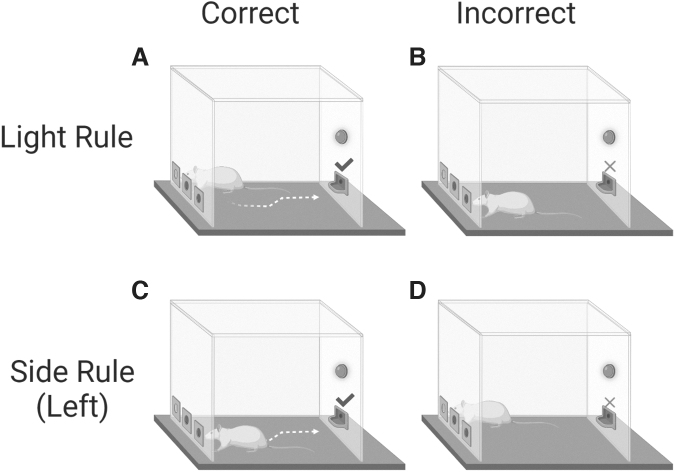
FIG. 1.
The operant-based set-shifting task. Instrumental behavior was guided based on two rules within distinct perceptual dimensions that shifted between each other based on reward delivery or omission feedback. In the ‘‘Light rule,” a nose poke to the illuminated port was correct (A), leading to food reward, whereas a nose poke to the non-illuminated port was incorrect (B), leading to no reward. In the “Side rule,” depicted here on the left side, a nose poke to the valid location (e.g., left port) was correct, regardless of illumination (C). Poking into the illuminated port on the right side, in this case, was incorrect (D), leading to no reward.
A small video camera was mounted inside the cubicle so that the rats' performance could be monitored live by experimenters on a video screen. Rats were first habituated to operant chambers (i.e., 20 min) and reward retrieval (i.e., dispensed at 30-sec intervals into the food trough), which consisted of 45 mg sucrose dustless precision pellets (Bio-Serv, Flemington, NJ), before progressing to set-shifting training.
Subsequently, during training and testing sessions on oAST, rats were challenged to choose correctly between two nose-poke holes according to two discrimination rules (i.e., Light or Side), each incorporating a distinct perceptual dimension, spatial position, and a light cue presented pseudorandomly at one of the two peripheral cue ports (Fig. 2). Pseudorandomization was used to ensure that the same cue light was not illuminated more than twice in a row. For the Light discrimination rule, correct performance for a reward pellet was achieved by a nose poke in the illuminated port, independent of its spatial location (Fig. 2A,B). For the Side rule, a nose poke in the respective valid side (left or right) regardless of illumination location was recorded as correct and rewarded accordingly (Fig. 2C,D).
FIG. 2.
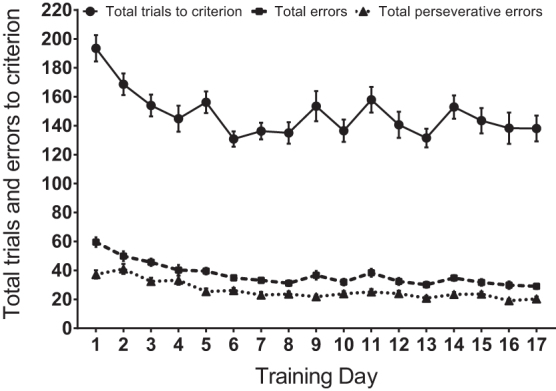
Rats were trained on operant attentional set-shifting tasks (oAST), before surgery and group assignment, for at least 17 days to achieve performance baseline stability (i.e., three consecutive days with <10% variability) as seen via total trials to criterion, total errors, and perseverative errors (daily sets 2–4), which were summed for each rat. Data are expressed as mean (± standard error of the mean). No statistical analyses were necessary prior to group designations, N = 32.
Daily testing consisted of four sets completed in 90 min, with three extradimensional (ED) shifts changing reward contingency with a pseudorandom rule start and counterbalanced set sequence. A rat advanced through dimensional sets by completing a performance criterion of 10 consecutive correct responses. Each daily session included two side rules using left and right cue ports as the correct side, such that left- and right-poking trials were equivalently mixed. After each response, the food trough in the opposite wall of the chamber was illuminated, regardless of the accuracy of response, until rats poked into the trough to end the trial. After a 10-sec intertrial interval, the next trial was initiated as the respective light cue was turned on.60,61
Rats were trained in oAST before surgery to reach stable pre-injury performance baselines (total of 17–21 days; three consecutive days with <10% variability). After 10 days of recovery from surgery and reinstatement of mild food restriction, rats were tested in oAST for 10 days (post-surgical days 11–21, with a break at day 16), followed by training and testing on digging AST (dAST). Total trials to reach performance criterion (10 consecutive correct choices), total errors, and perseverative errors (daily on sets 2–4; choice consistent with immediately preceding set rule) were recorded. Any rats failing to complete four consecutive sets in their entirety during a 3 h allotment on more than three sessions were excluded from the study.
Surgery, drug treatment, and acute neurological evaluation
After pre-surgery operant AST behavioral baselines were reached, rats weighing between 300–325 g were randomly assigned to injury (TBI or Sham) and treatment groups (MLN or vehicle [VEH]). Isoflurane anesthesia was induced and maintained using a 2:1 N2O/O2 gas mixture at concentrations of 4% and 2%, respectively. Rats were intubated endotracheally and placed on mechanical ventilation for anesthesia maintenance.
Osmotic minipumps were implanted intraperitoneally after they were pre-filled with VEH (10% ethanol in saline) or MLN (AK Scientific, Inc., Union City, CA) at concentrations calculated to deliver 30 mg/kg/day (continuous infusion post-surgery, 60 μL/h) of drug freebase via continuous infusion for the duration of the study until sacrifice after dAST. This dose was selected based on previously published work showing that it increases norepinephrine and serotonin in the prefrontal cortex in rats62,63 and has beneficial effects on dAST performance.43
After mini-pump placement, rats were affixed into a stereotaxic frame, and a 6 mm craniectomy was performed over the right hemisphere rostral to bregma and right of midline, overlying the prefrontal cortex.64 A controlled cortical impact (CCI) was performed with a 3 mm impactor diameter, at 2.4 mm injury depth, 4 m/sec impact velocity, and 100 msec dwell time similar to previous publications involving similar surgical procedures over the parietal cortex.32,33,35,65 Sham control rats underwent anesthesia and craniectomy, except for the final impact.
Anesthesia was then discontinued, and the scalp incision was closed with sutures. Rats were extubated, and time to return of hindlimb flexion paw withdrawal (i.e., paw pinch) and righting reflexes (i.e., turn from supine to prone) were assessed every 5 sec. Post-operatively, rats were weighed daily on post-injury days (PID) 1–5 to ensure steady recovery and given ad libitum access to food and water, after which food restriction was resumed from PID 6 onward as above.
dAST
Rats were habituated, trained, and tested in the dAST from PID 23–27. Procedures for AST were adapted from Birrell and Brown26 and have been described previously.28-33,35,66 Briefly, testing occurred in a custom-built, rectangular Plexiglas arena that utilized a discrimination choice of two Terracotta digging pots (internal rim diameter, 7 cm; depth, 6 cm) to present salient and irrelevant stimulus cues across two dimensions (i.e., medium and odor) to signal which pot contained a food reward buried 2 cm below the surface of the digging medium (one quarter of a Honey Nut Cheerio, General Mills Cereals, Minneapolis, MS).
Digging was defined as a vigorous displacement of the medium to retrieve the reward buried in the pot, during which rats explored using their somatosensory, olfactory, and visual senses. The behavioral assessment took place over three consecutive days. Rats were first trained to dig reliably in pots with incrementally larger amounts of sawdust to retrieve the rewards. Subsequently, they were trained to complete simple rule-based discriminations for both odor (e.g., lemon vs. eucalyptus) or media (e.g., felt vs. paper strips) contingencies to a criterion of six consecutive correct responses. The positive and negative cues for each rat were pseudorandomly determined and equally represented. The stimuli implemented during training were not used during testing. Any rat that failed to complete the training procedures was eliminated from further testing.
Ultimately, rats were progressed through a series of seven rule-shifting stages of progressive complexity aiming to measure aspects of cognitive flexibility, specifically via stimulus reversals and an ED shift, and again six consecutive correct trials were required to move from one stage to the next. Each testing stage included a variation between the discriminative stimulus dimension and the positive cue.
Stage 1 involved a single stimulus dimension (odor or medium) and stages 2–7 involved both overlapping dimensions, although only one dimension was relevant in any stage, while the other acted as a distractor. The stages included: (1) simple discrimination (SD); (2) compound discrimination (CD); (3) the first reversal (R1); (4) intradimensional shift (ID); (5) the second reversal (R2); (6) ED shift; and (7) the third reversal (R3). Total trials to reach criterion, total errors, and set loss errors (incorrect choice after ≥3 correct choices) for each stage were recorded. Trials longer than 10 min without choosing a pot were marked as “no choice” and counted as an error. Six consecutive no choice trials or failing to complete a stage within 50 trials resulted in exclusion from the study.
Sacrifice and histology
At five weeks post-surgery after the completion of behavioral testing, using a similar timeline with previous studies,32,33,35 rats were intraperitoneally administered 0.3 mL Fatal-Plus® (Henry Schein Animal Health, Columbus, OH) and transcardially perfused with 200 mL of 0.1 M of phosphate-buffered saline (pH 7.4), followed by 300 mL of 4% paraformaldehyde (PFA). Brains were extracted, post-fixed in 4% PFA for one week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer-thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on Superfrost®/Plus glass microscope slides (Fisher Scientific, Pittsburgh, PA). After drying at room temperature, sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet.
Cortical lesion volumes (mm3) were assessed by an observer blinded to experimental conditions using a Nikon Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan). The area of the lesion (mm2) was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7; Nikon NIS-Elements AR 3.22.14 software; Nikon) and then by summing the lesions obtained, as reported previously.32,33,35,67–69
Statistical analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statistica 64 version 13 Academic software (Dell Inc., Tulsa, OK). Righting reflex, hindlimb reflexive ability, and histological data were analyzed by an unpaired Student t test.
For oAST post-surgery behavior, the total trials to criterion, total errors, and perseverative errors were analyzed by two-way repeated measures ANOVA (Group × Day, with Day as the repeated measure). When significant main effects or interactions were indicated, post hoc comparisons using the Fisher least significant difference (LSD) were implemented. Results from dAST involved total trials to criterion, total response errors, and set loss errors that were recorded for each test stage and analyzed by two-way repeated measures analysis of variance (ANOVA) (Group × Stage, with Stage as the repeated measure). Where necessary after significant main effects of interactions were indicated, post hoc one-way ANOVA analyses were performed for each stage followed by further post hoc comparison within Stage using Fisher LSD tests.
For both oAST and dAST behaviors, the Sham groups (Sham+MLN and Sham+VEH) were not significantly different from each other and were pooled into one Sham group. Results are expressed as the mean ± standard error of the mean (SEM), and significance for all analyses was set at p < 0.05.
Results
One TBI+MLN rat and one Sham+VEH rat were eliminated from all pre- and post-surgery analyses because of persistent oAST timeouts during the 3 h time allotment. Five rats were excluded from statistical analyses for oAST (1 Sham+VEH, 3 Sham+MLN, and 1 TBI+MLN) because of behavior falling outside of two standard deviations from the group mean, rendering them outliers; however, they were included in dAST analyses. Two Sham rats (1 VEH, 1 MLN) completed oAST, but did not complete training or testing on dAST; therefore, they were not included in the latter analysis.
Acute neurological evaluation
Statistically significant differences between TBI and Sham animals in hindlimb reflex paw withdrawal latency were observed for left (TBI range = 153.2 ± 3.7 sec, Sham range = 23.8 ± 1.5 sec; F1,28 = 905.9, p < 0.001), and right (TBI range = 148.5 ± 3.7 sec, Sham range = 18.2 ± 1.6 sec; F1,28 = 904.5, p < 0.001) sides after cessation of anesthesia. No significant differences were observed between groups randomly pre-assigned to start continuous osmotic minipump infusions (MLN or VEH; left paw: F1,28 = 0.159, p = 0.693, right paw: F1,28 = 0.104, p = 0.75). Significant differences in righting reflex were observed between TBI and Sham animals for return to righting ability (TBI range: 262.5 ± 13.5 sec; Sham range: 152.3 ± 12.0 sec, F1,28 = 35.58, p < 0.001) after anesthesia discontinuation, again with no pre-assigned treatment group differences (F1,28 = 0.015, p = 0.902).
oAST
oAST training
Figure 2 represents the performance summary (i.e., total trials to criterion, total errors, and total perseverative errors) before group designation and surgery throughout the first 17 days of operant training, in which performance improved significantly. All rats individually achieved readiness by reaching performance stability for surgery by day 21. The number of trials to reach criterion for each of the four sets, errors per set, and perseverative errors per set (daily sets 2–4) were summed for each rat. No statistical analyses were necessary before group designations.
oAST total trials to criterion
No significant group difference between Sham+VEH and Sham+MLN rats (F1,9 = 0.119, p = 0.738), Day effect (F9,81 = 0.816, p = 0.602), or Group × Day interaction (F9,81 = 1.492, p = 0.165) were observed. Thus, Sham+VEH and Sham+MLN results were pooled into the SHAM group. Figure 3 shows the effects of Group designation on the trials to criterion for each stage of the oAST at PID 11–21 post-surgery (SHAM: n = 11; TBI+VEH: n = 9; TBI+MLN: n = 7). Two-way repeated measures ANOVA for total trials to criterion revealed a significant Group effect (F2,24 = 6.36, p = 0.006) but no significant effect of Day (F9,216 = 0.467, p = 0.895) or Day × Group interaction (F18,216 = 1.006, p = 0.454).
FIG. 3.
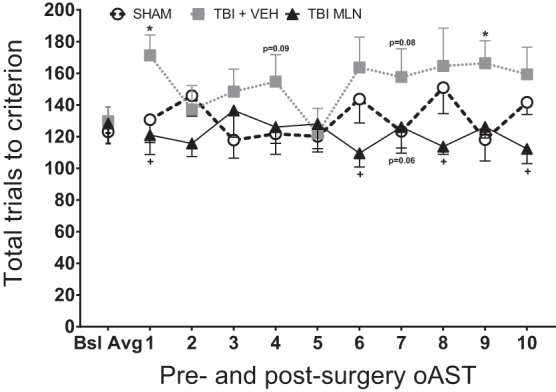
Total operant attentional set-shifting task (oAST) trials to criterion for pre-injury baseline average (last five sessions per rat) and 10 post-injury days of testing ([PID 11–15] followed by a rest day [PID 16] and five more test days [PID 17-21]). Moderate, right hemisphere, frontal lobe injury impaired performance by generally elevating the number of trials to criterion in traumatic brain injury+vehicle (TBI+VEH) compared with SHAM with statistical significance noted on days 1 and 9, as well as trends on days 4 and 7 of post-surgery retrials. Chronic, steady-state drug administration improved behavioral flexibility in TBI+milnacipran (MLN) rats compared with TBI+VEH rats, seen as a reduction in total trials required to complete each session (significant effects on days 1, 6, 8, and 10, as well as a trend on day 7). n = 7–11/group, Mean (± standard error of the mean), *p < 0.05 for TBI+VEH versus SHAM, +p < 0.05 for TBI+MLN versus TBI+VEH.
The Fisher LSD post hoc demonstrated TBI+VEH rats performed worse (higher total number of trials to reach criterion) than SHAM rats on days 1 and 9 (p < 0.05) with similar albeit non-significant trends on days 4 and 7 (p < 0.1). The TBI+MLN rats exhibited restorations in flexible attention (fewer total trials to criterion) than TBI+VEH animals on days 1, 6, 8, and 10 (p < 0.05) with a similar albeit non-significant trend on day 7 (p < 0.1), as seen in Figure 3. There were no differences between TBI+MLN and SHAM on any of the post-surgery test days.
oAST total errors
Total errors for all groups per post-surgery session are depicted in Figure 4. Two-way repeated measures ANOVA revealed a significant Group effect (F2,24 = 6.512, p = 0.006), but no effect of Day (F9,216 = 0.855, p = 0.567) or Day × Group interaction (F18,216 = 1.019, p = 0.439). The Fisher LSD post hoc test demonstrated that TBI+VEH rats performed significantly worse (i.e., engaged in more total errors) than SHAM rats on days 1 (p < 0.01), 4 (p = 0.05), 7 (p < 0.05), and 9 (p < 0.01), as seen in Figure 3. Compared with TBI+VEH rats, TBI+MLN rats had significantly improved performance on days 1 (p < 0.01), 8, and 10 (p < 0.05) with a similar trend on days 6 and 9 (p < 0.1). Fisher LSD post hoc analyses found no differences between TBI+MLN and SHAM animals throughout all post-surgery test sessions.
FIG. 4.
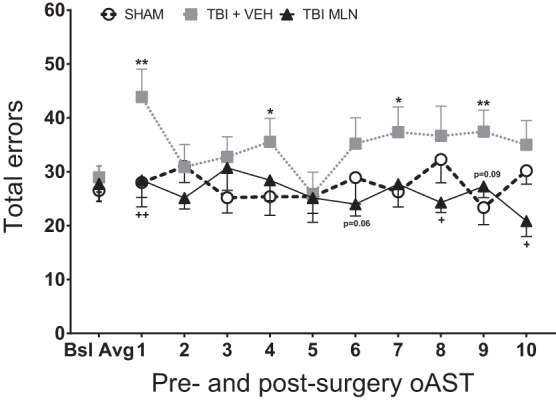
Total operant attetional set-shifting task (oAST) errors for pre-injury baseline average (last five sessions per rat) and 10 post-injury days of testing ([PID 11–15] followed by a rest day [PID 16] and five more test days [PID 17–21]). Frontal lobe-traumatic brain injury (TBI), vehicle (VEH) treated rats committed significantly more errors than SHAM rats on days 1, 4, 7, and 9. Chronic milnacipran (MLN) treatment normalized performance by attenuating the number of errors recorded during testing throughout the 10 post-injury sessions, significantly so on days 1, 8, and 10, with similar trends on days 6 and 9. n = 7-11/group, Mean (± standard error of the mean), **p < 0.01, *p < 0.05 for TBI+VEH versus SHAM, ++p < 0.01, +p < 0.05 for TBI+MLN versus TBI+VEH.
oAST perseverative errors
In Figure 5, cumulative perseverative errors for daily sets 2–4 were analyzed for each group throughout the post-surgery test days. Two-way repeated measures ANOVA for total perseverative errors revealed a significant Group effect (F2,24 = 7.088, p = 0.004), but no effect of Day (F9,216 = 0.929, p = 0.501) or Day × Group interaction (F18,216 = 0.856, p = 0.632).
FIG. 5.
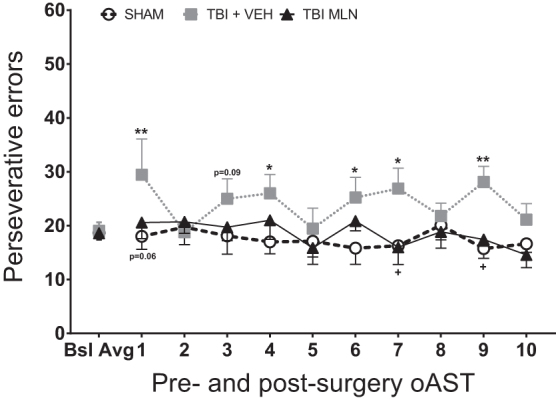
Total operant attentional set-shifting task (oAST) perseverative errors (i.e., responding according to contingency rule from previous set) for pre-injury baseline average (last five sessions per rat) and 10 post-injury days of testing ([PID 11–15] followed by a rest day [PID 16] and five more test days [PID 17–21]). Frontal lobe-TBI rendered a significantly higher number of total perseverative errors compared with the SHAM group, significantly so on days 1, 4, 6, 7, and 9, with a non-significant trend of day 3. Chronic, steady-state milnacipran (MLN) administration attenuated the number of total perseverative errors during testing throughout the 10 post-surgery sessions, thus effectively enhancing behavioral flexibility (significant effects on days 7 and 9, with similar trend on day 1). n = 7–11/group, Mean (± standard error of the mean), *p < 0.05 for TBI+VEH versus SHAM, +p < 0.05 for TBI+MLN versus TBI+VEH.
Fisher LSD post hoc revealed that TBI+VEH rats committed a significantly larger number of total perseverative errors (i.e., attending more to the immediately preceding correct rule rather than switching to the new rule) than SHAM rats on days 1 (p < 0.01), as well as 4, 6, 7 (p < 0.05), and 9 (p < 0.01) with a similar non-significant trend on day 3 (p < 0.1) as seen in Figure 5. Further, TBI+MLN rats committed significantly less total perseverative errors (i.e., restoration of behavioral flexibility) than TBI+VEH rats on days 7 and 9 (p < 0.05), with a similar trend on day 1 (p < 0.1). Post hoc analyses did not reveal further differences between TBI+MLN and SHAM animals.
dAST
Performance on dAST was first analyzed to ensure that there were no drug-related differences between Sham groups. Two-way repeated measures ANOVA (Drug × Stage with Stage as the repeated measure) revealed no effects of Drug on trials to criterion (F1,11 = 0.16, p = 0.697), and no Drug × Stage interaction (F6,66 = 0.221, p = 0.967). Sham+VEH and Sham+MLN rats were thus pooled into a combined SHAM group for subsequent dAST analyses.
dAST training
A two-way repeated measures ANOVA test (Group × Training Stage) for the total number of trials to criterion for training day SD tasks did not reveal effects of Group (F2, 27 = 0.398, p = 0.676) or Group × Training Stage interaction (F2,27 = 0.7264, p = 0.493) before test day. Regardless of Group designation, rats completed odor and medium SDs on training day similarly (data not shown). Simple discrimination is not typically affected by TBI,33,34,70–72 and thus the lack of a Group effect supports test validity.
dAST ED shift: odor-to-medium versus medium-to-odor
Figure 6 shows a comparison between rats subjected to an odor-to-medium versus medium-to-odor ED set-shift on the trials to reach the criterion for each stage of the dAST regardless of Group A two-way repeated ANOVA revealing there was no significant effect of ED shift type on dAST performance across stages (F1,28 = 0.391, p = 0.537); however, there was a significant ED shift type × Stage interaction (F6,168 = 3.183, p < 0.01, Fig. 6A).
FIG. 6.
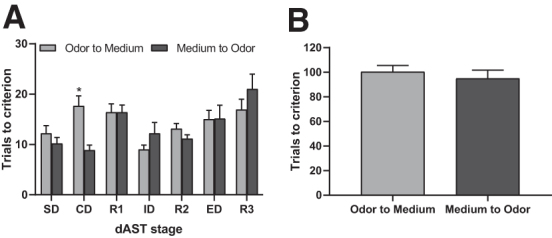
Total trials to reach criterion in digging attentional set-shifting task (dAST, groups collapsed regardless of surgery or treatment) on post-injury day (PID) 25–27. Rats exposed to odor first as the relevant dimension (as opposed to medium) in dAST required significantly more trials to complete the compound dimensional (CD) stage of dAST (A), but total trials to criterion throughout the dAST day were unaffected between the two extradimensional (ED) shifts (B) and were not significantly different. n = 30, mean (± standard error of the mean), *p < 0.01 odor-to-medium versus medium-to-odor.
Fisher LSD post hoc analyses revealed a significant difference on the CD stage (odor first vs. medium first, p < 0.05), suggesting odor-to-medium rats required more trials to criterion than medium-to-odor rats. Odor-based discrimination learning taking more trials than medium-based on CD is likely because of the stimulus pairings used at that stage, rather than a discrepancy between odor- and medium-based learning per se. No other subsequent stage comparisons were significantly different regardless of the dimension to which the contingency belonged, such as odor or medium. A comparison of total trials across the test for rats assigned to either of the two perceptual stimuli set-shifts revealed that no differences were unveiled by means of an unpaired t test (t29 = -0.626, p = 0.537, Fig. 6B).
dAST trials to criterion
Figure 7 shows the effects of Group designation on the trials to criterion for each stage of the dAST at four weeks post-surgery (SHAM: n = 13; TBI+VEH: n = 9; TBI+MLN: n = 8). Two-way repeated measures ANOVA revealed significant main effects of Group (F2,27 = 3.279, p = 0.05) and Stage (F6,162 = 6.918, p < 0.0001), but no significant Group × Stage interaction (F12,162 = 1.56, p = 0.108). For the Stage main effect, post hoc comparisons across all groups collapsed regardless of group designation demonstrating more trials were required to reach criterion during R1 compared with SD and ID, as well as during R3 compared with SD, CD, ID, and R2 (p < 0.05; Fig. 7). As reported previously, this effect validates the inherent difficulty of the stimulus reversals and ED set-shifting stage compared with the other stages of the AST.32,33,35.
FIG. 7.
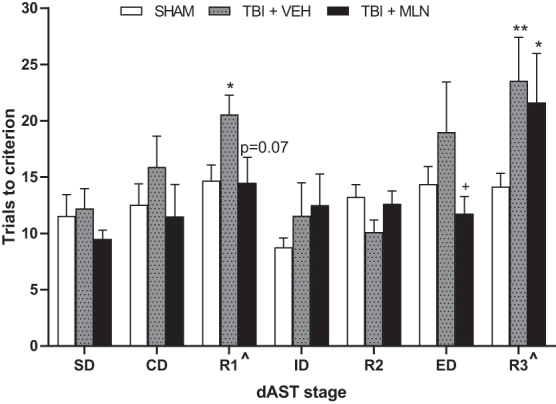
Total trials to reach criterion in digging attentional set-shifting task (dAST) on PID 25–27. The traumatic brain injury+vehicle (TBI+VEH) rats required significantly more trials to criterion in stages R1 and R3 compared with the SHAM group. The TBI+milnacipran (MLN) rats display normalization of performance on some of the dAST stages (significant extradimensional [ED] improvement, non-significant improvement in R1) impacted by injury. The TBI+MLN rats, however, continued to display significant deficits in R3 compared with SHAM). n = 8–13/group. ^p < 0.05 Stage effect confirming task difficulty, **p < 0.01 TBI+VEH versus SHAM, *p < 0.05 TBI+VEH or TBI+MLN versus SHAM, +p < 0.05 TBI+MLN versus TBI+VEH.
Fisher LSD post hoc analyses determined TBI+VEH rats displayed significant cognitive impairments on R1 and R3 (p = 0.05 and p < 0.01, respectively), requiring more trials to criterion compared with SHAM rats (Fig. 7). Moreover, TBI+MLN rats performed significantly better than TBI+VEH on ED (p < 0.05), with a similar non-significant trend on R1 (p = 0.07), suggesting chronic MLN administration was effective, at least in part, at normalizing performance on complex stages of dAST. On R3, performance for TBI+MLN rats remained impaired compared with SHAM (p < 0.05), because more trials to reach criterion were required for the drug-treated injured rats on the last reversal stage of dAST (Fig. 7). No other main effects or interactions were revealed for other test stages.
dAST total errors
A two-way repeated measures ANOVA (Group × Stage with Stage as the repeated measure) revealed a significant main effect of Stage (F6,162 = 11.206, p < 0.0001), but not Group (F2,27 = 2.257, p = 0.124) or Group × Stage interaction (F12,162 = 1.466, p = 0.142). No additional post hoc analyses were performed (data not shown).
dAST set loss errors
A two-way repeated measures ANOVA (Group × Stage with Stage as the repeated measure) revealed no significant main effects of Group (F2,27 = 1.088, p = 0.351), Stage (F6,162 = 2.057, p = 0.06), or Group × Stage interaction (F12,162 = 1.69, p = 0.07). No additional post hoc analyses were performed (data not shown).
Lesion volumes
At five weeks post-surgery, frontal lobe CCI injury induced a mean cortical lesion volume of 10.94 ± 1.68 mm3 in the TBI+VEH group, while the TBI+MLN group had a mean cortical lesion volume of 9.09 ± 0.97 mm3. A two-tailed independent t test found no significant difference between the TBI groups (t8 = -0.884, p = 0.41, n = 4-5/group, data not shown).
Discussion
In this study, we present for the first time a multi-modal, sequential assessment of PFC-mediated oAST and dAST behavioral tasks in rats injured via frontal lobe-targeted TBI administered using a well-established CCI model relevant for executive impairments. Pre-surgery, all rats trained to stable performance baselines in oAST, then post-surgery, they were continuously infused intraperitoneally with MLN (30 mg/kg/day) or VEH. The VEH-treated injured rats exhibited impaired oAST performance evidenced by increased total trials to criterion as well as increased total errors and perseverative errors.
Chronic MLN treatment generally normalized performance, resulting in equivalent TBI+MLN and Sham group performance across all of the above end-points. Subsequent post-injury dAST assessment in the same rats demonstrated marked and significant impairments in reversal learning stages (R1 and R3) for TBI+VEH compared with SHAM controls, while the TBI+MLN group performed comparably to SHAM at all stages except R3, where their performance was similar to TBI+VEH and significantly impaired with respect to SHAMs. Drug-treated injured rats also notably required significantly fewer trials to criterion than TBI+VEH animals to complete ED set-shifting, with a trend on R1, demonstrating benefits of MLN treatment despite a lack of improvement in histological outcomes.
Previous TBI investigations injuring the frontal lobe via CCI have assessed spatial memory, working memory, and/or motor function,73–80 with only a few examining higher order cognitive/executive function.70–72,81 A simpler dig task (comparable to the SD and R1 stages of dAST) has been assessed71,72 in rats with more severe, bilateral frontal TBI where injured rats performed worse than chance in the R1-equivalent phase, demonstrating perseveration on the previously learned association and impaired reversal. Given that lesion volume encompassed the near entirety of the bilateral OFC71,72 (which is critical for reversal learning),27,30,31,40,82–84 this observation is expected. Notably, a parallel cohort of parietal CCI animals in the same studies were not impaired in the dig task.71,72
Within the current frontal CCI literature, the closest report compared with our present experimental design is the 2016 study from Chou and colleagues,70 which investigated the digging-based rule shift assay in mice after unilateral right hemisphere frontal CCI that rendered comparably small lesion volumes and location. They found that reversal learning (comparable to dAST R1, R2, R3 stages) was impaired, but rule shifting (comparable to oAST and dAST ED shifts) was spared.
The findings are concordant with our results, with the exception that oAST ED shifts were sensitive to impairments in our TBI+VEH rats (with a similar trend in dAST ED stage), unlike the spared ED shifts in TBI mice during the digging rule-shift assay.70 This could reflect subtle differences in injury profiles, particularly as it relates to the high degree of mPFC-OFC reciprocal projections, species-specific differences in inter/intraregional connectivity and neurochemistry, or behavioral task design.
Treatments with systemic or local PFC dosing of compounds modulating 5-HT, NE, DA, acetylcholine, and glutamate neurotransmission influence AST performance.29,30,32,35,36,38,39, More specifically, rat mPFC (analogous to primate dorsolateral PFC)85,86 function has been demonstrated to rely on noradrenergic and dopaminergic tone during set shifts.28,37–39,41 Desipramine, a relatively SNRI, has consistently proven beneficial in rescuing dAST ED impairments in chronically stressed rats.29,87 Noradrenergic and dopaminergic function enhancement with methylphenidate also rescued dAST impairments across all stages in a rat model of attention deficit hyperactivity disorder.88
Our TBI+MLN rats demonstrated significant improvements in the ED phase of dAST compared with TBI+VEH group and were not different than Sham. Moreover, we report statistically significant impairments across all outcome measures in oAST (i.e., three ED set-shifting phases per session) suggesting putative impairment of mPFC-mediated set shifting induced by our frontal lobe injury model. Given the aforementioned beneficial roles of NE tone in the mPFC for set-shifting performance, we infer the overall oAST and dAST ED improvements noted in TBI+MLN rats (Figs. 3, 4, 5, 7) were most likely related, at least in part, to enhancement of noradrenergic tone in the mPFC.
While the role of 5-HT is complex, multi-faceted, region-dependent, and intertwined with NE- and DA-responsive systems, numerous investigations suggest 5-HT plays a significant role in OFC-mediated reversal learning relevant to stages R1, R2, and R3 of dAST.27,30,31,40,82,83 Selective serotonergic modulation via chronic daily treatment30,89 or acute pre-task treatment31,89 of the SSRI, citalopram, has proven effective for improving dAST reversal learning deficits after 5-HT depletion or chronic intermittent cold stress, an effect that was blocked by directed infusion of selective 5-HT2A-R blockade into the OFC30 (analogous to ∼⅓ of nonhuman primate caudal orbitomedial PFC).85,86
These effects were not reproduced when treated with a relatively selective noradrenergic agent, desipramine, reinforcing the OFC/5-HT importance for reversal learning.89 The SSRIs, however, such as citalopram, escitalopram, and fluoxetine, can also rescue dAST ED set-shift deficits in models of chronic stress,29,30,36,87,89 suggesting a complicated and intertwined role in which 5-HT modulation may have indirect, downstream influences on DA and/or NE systems in specific pathological states.
One of our recent publications has demonstrated that chronic treatment with the SSRI, citalopram, improved dAST performance in parietal-CCI rats during both ED and R3 stages,35 an effect that was potentiated after a combined treatment with environmental enrichment,35 a robust rodent model intervention recapitulating effects of multi-modal post-injury rehabilitation.65,69,90 Nevertheless, the beneficial effects of chronic MLN administration in our study likely span involvement of both NE and 5-HT enhancement as determined by multi-modal cognitive testing.
Notably, dAST reversal learning (R1 and R3) was impaired in our TBI+VEH animals with significant restoration of performance in R1 enabled by MLN treatment. Interestingly, citalopram rescued R3 deficits in our parietal CCI study,35 while MLN did not achieve this effect in the present investigation using frontal CCI. Given the likely role of 5-HT action in the OFC for reversal learning detailed above, lower MLN potency specific to 5-HT reuptake may underlie differential effects35 between these agents in attenuation of TBI-induced dAST R3 deficits. Alternative possibilities could include a different injury profile (i.e., frontal vs. parietal)35 with disparate effects on interregional connectivity underlying dAST performance or potential unmeasured detrimental influence of noradrenergic enhancement.81
Milnacipran has been reported to produce beneficial effects on dAST ED set-shift impairments induced by chronic unpredictable stress in rats,43 reproducing NE-specific improvements in ED stage performance after desipramine treatment.29,36,87 Similarly, oAST impairments exhibited by TBI+VEH rats as measured by total trials, total errors, and perseverative errors were restored to SHAM levels in TBI+MLN rats in the current study, which represents the first report of MLN beneficial effects in operant set-shifting. The joint positive effect of MLN on both oAST sequential dimensional set shifts as well as dAST set-shifting and reversal learning suggests the dual SNRI mechanism of action may underlie its benefits in frontal lobe CCI animals.
Despite the novelty and robustness of the data presented here, it is important to also highlight limitations. The positive restorative effects of MLN on neurobehavior after TBI would benefit from further exploration throughout additional dosing paradigms and alternative locations, severities, and models of TBI before consideration of enhanced clinical testing. Differential effects on behavioral end-points have been observed when MLN was dosed in morning versus evening in animals,56 thus should be further explored especially considering sleep dysregulation is a major consequence of TBI. In addition, the chronic MLN treatment regimen employed here was not significantly neuroprotective in terms of gross cortical tissue loss.
While statistical differences were not detected, possibly because of a small, but behaviorally critical injury-induced lesion to start with, other work has demonstrated behavioral improvements post-TBI treatment can occur independent of significant tissue rescue,35,91 which could reflect microstructural benefits in lesion-adjacent tissue not readily appreciable by gross ultrastructure histological evaluations.
In terms of behavior, potential influence of a frontal compartment injury on olfaction must be considered as a potential internal confound given proximity of the injury to the olfactory bulb. Animals with TBI were, however, able to successfully reach criterion in all stages, including those in which olfactory cues were salient, suggesting this was not a major factor. Moreover, results are similar across contingencies between operant- and digging- paradigms despite the operant testing not involving odor cues.
Finally, female rats have not yet been studied, an important inclusion given a significant proportion of patients with TBI are female and differences in behavior, injury response, and drug metabolism/treatment effects may exist between sexes. This is an active direction of our ongoing work for future publication.
Conclusions
This work represents the first time that dual AST measures have been assessed in the same animals, demonstrating such behavioral paradigms are sufficiently sensitive and robust to reliably detect attention and cognitive flexibility impairments after frontal/prefrontal TBI in rats. In addition, chronic MLN treatment demonstrates promising attenuation of executive function deficits in rats with TBI, meriting further investigation in the rehabilitation milieu.
Acknowledgments
Figure 1 schematic was created using BioRender.com (accessed on June 12, 2022).
Authors' Contributions
COB, TJC, JPC, DAO, and LAK developed the study. NSR, TJC, EHM, and COB wrote and edited the abstract. TJC, LAK, AP, ALI, CRS, DAO, KOG, AP, NP, IPM, and TNM performed the behavioral assessments. JPC performed surgical procedures. NSR and COB performed statistical analyses. JPC, NSR, TJC, DAO, and LAK performed brain tissue histological assessments. TJC, NSR, EHM, and COB wrote the manuscript. COB, NSR, and EHM revised the manuscript. All authors reviewed and approved the manuscript.
Funding Information
The work presented in this manuscript was supported by the National Institutes of Health (grants NS095950, NS099683, NS110609; PI: COB) and a Rehabilitation Institute Pilot Award from the University of Pittsburgh (PI: COB).
Author Disclosure Statement
Author NSR serves on the board of life science startup company Neuro Vigor, LLC. He receives no direct compensation, and the work presented here is entirely unrelated. For the remaining authors, no competing financial interests exist.
References
- 1. Levin HS, Amparo E, Eisenberg HM, et al. . Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg 1987;66(5):706-713; doi: 10.3171/jns.1987.66.5.0706 [DOI] [PubMed] [Google Scholar]
- 2. Stuss DT. Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Curr Opin Neurol 2011;24(6):584-589; doi: 10.1097/WCO.0b013e32834c7eb9 [DOI] [PubMed] [Google Scholar]
- 3. Bigler ED. Neuropathology of traumatic brain injury. In: Traumatic brain injury. (Bigler ED, ed.) Pro-Ed: Austin, TX; 1990; pp. 13-49. [Google Scholar]
- 4. McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabilitation 2002;17(4):333-344 [PubMed] [Google Scholar]
- 5. Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann NY Acad Sci 2007;1097:84-93; doi: 10.1196/annals.1379.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zlatković J, Todorović N, Bošković M, et al. . Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem 2014;393(1-2):43-57; doi: 10.1007/s11010-014-2045-z [DOI] [PubMed] [Google Scholar]
- 7. Gawryluk JW, Wang JF, Andreazza AC, et al. . Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol 2011;14(8):1069-1074; doi: 10.1017/S1461145711000617 [DOI] [PubMed] [Google Scholar]
- 8. Gawryluk JW, Wang J-F, Andreazza AC, et al. . Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 2011;14(1):123-130; doi: 10.1017/S1461145710000805 [DOI] [PubMed] [Google Scholar]
- 9. McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry 2008;7(1):3-10; doi: 10.1002/j.2051-5545.2008.tb00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 2011;13(3):287-300; doi: 10.31887/DCNS.2011.13.2/tmcallister [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spikman JM, Timmerman ME, Milders MV, et al. . Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J Neurotrauma 2012;29(1):101-111; doi: 10.1089/neu.2011.2084 [DOI] [PubMed] [Google Scholar]
- 12. Spikman JM, Timmerman ME, Coers A, et al. . Early computed tomography frontal abnormalities predict long-term neurobehavioral problems but not affective problems after moderate to severe traumatic brain injury. J Neurotrauma 2016;33(1):22-28; doi: 10.1089/neu.2014.3788 [DOI] [PubMed] [Google Scholar]
- 13. van der Horn HJ, Liemburg EJ, Aleman A, et al. . Brain networks subserving emotion regulation and adaptation after mild traumatic brain injury. J Neurotrauma 2016;33(1):1-9; doi: 10.1089/neu.2015.3905 [DOI] [PubMed] [Google Scholar]
- 14. Milders M, Ietswaart M, Crawford JR, et al. . Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. J Int Neuropsychol Soc 2008;14(2): 318-326; doi: 10.1017/S1355617708080351 [DOI] [PubMed] [Google Scholar]
- 15. Finnanger TG, Skandsen, T, Andersson S, et al. . Differentiated patterns of cognitive impairment 12 months after severe and moderate traumatic brain injury. Brain Inj 2013;27(13-14):1606-1616; doi: 10.3109/02699052.2013.831127 [DOI] [PubMed] [Google Scholar]
- 16. Sorg SF, Delano-Wood L, Luc N, et al. . White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehabil 2014;29(1):21-32; doi: 10.1097/HTR.0b013e31828a1aa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coulacoglou C, Saklofske DH. Executive function, theory of mind, and adaptive behavior. In: Psychometrics and Psychological Assessment; Elsevier: New York; 2017; pp. 91-130; doi: 10.1016/b978-0-12-802219-1.00005-5 [DOI] [Google Scholar]
- 18. Greve KW, Love JM, Sherwin E, et al. . Wisconsin Card Sorting Test in chronic severe traumatic brain injury: factor structure and performance subgroups. Brain Inj 2002;16(1):29-40; doi: 10.1080/0269905011008803 [DOI] [PubMed] [Google Scholar]
- 19. Ord JS, Greve KW, Bianchini KJ, et al. . Executive dysfunction in traumatic brain injury: the effects of injury severity and effort on the Wisconsin Card Sorting Test. J Clin Exp Neuropsychol 2010;32(2):132-140; doi: 10.1080/13803390902858874 [DOI] [PubMed] [Google Scholar]
- 20. Hang R-H, Xu Y-J, Xie H-F, et al. . Evaluating on recognition impairment after traumatic brain injury with WCST. Fa Yi Xue Za Zhi 2011;27(5):346-349. [PubMed] [Google Scholar]
- 21. Benge JF, Caroselli JS, Temple RO. Wisconsin Card Sorting Test: factor structure and relationship to productivity and supervision needs following severe traumatic brain injury. Brain Inj 2007;21(4):395-400; doi: 10.1080/02699050701311091 [DOI] [PubMed] [Google Scholar]
- 22. Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 1996;110(5):872-886; doi: 10.1037//0735-7044.110.5.872 [DOI] [PubMed] [Google Scholar]
- 23. Nyhus E, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn 2009;71(3):437-451; doi: 10.1016/j.bandc.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto K, Uruma G, Abo M. Activation of the prefrontal cortex during the wisconsin card sorting test (Keio Version) as measured by two-channel near-infrared spectroscopy in patients with traumatic brain injury. Eur Neurol 2008;59(1-2):24-30; doi: 10.1159/000109257 [DOI] [PubMed] [Google Scholar]
- 25. Lombardi WJ, Andreason PJ, Sirocco KY, et al. . Wisconsin Card Sorting Test performance following head injury: dorsolateral fronto-striatal circuit activity predicts perseveration. J Clin Exp Neuropsychol 1999;21(1):2-16; doi: 10.1076/jcen.21.1.2.940 [DOI] [PubMed] [Google Scholar]
- 26. Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 2000;20(11):4320-4324; doi: 10.1523/JNEUROSCI.20-11-04320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 2003;146(1-2):97-103; doi: 10.1016/j.bbr.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 28. Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 2006;137(3):1039–1049; doi: 10.1016/j.neuroscience.2005.09.031 [DOI] [PubMed] [Google Scholar]
- 29. Bondi CO, Rodriguez, G., Gould, G.G., Frazer, A., and Morilak, D.A.. (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacol 2008; 33(2):320-331; doi: 10.1038/sj.npp.1301410 [DOI] [PubMed] [Google Scholar]
- 30. Furr A, Lapiz-Bluhm DM, Morilak DA. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. Int J Neuropsychopharmacol 2012;15(9):1295-1305; doi: 10.1017/s1461145711001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, et al. . Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacol 2009;202(1-3):329-341; doi: 10.1007/s00213-008-1224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Njoku I, Radabaugh HL, Nicholas MA, et al. . Chronic treatment with galantamine rescues reversal learning in an attentional set-shifting test after experimental brain trauma. Exp Neurol 2019;315:32-41; doi: 10.1016/j.expneurol.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bondi CO, Cheng JP, Tennant HM, et al. . Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J Neurotrauma 2014;31(10):926-937; doi: 10.1089/neu.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lajud N, Roque A, Cheng JP, et al. . Early life stress preceding mild pediatric traumatic brain injury increases neuroinflammation but does not exacerbate impairment of cognitive flexibility during adolescence. J Neurotrauma 2021;38(4):411-421; doi: 10.1089/neu.2020.7354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Minchew HM, Radabaugh HL, LaPorte ML, et al. . A combined therapeutic regimen of citalopram and environmental enrichment ameliorates attentional set-shifting performance after brain trauma. Eur J Pharmacol 2021;904:174174; doi: 10.1016/j.ejphar.2021.174174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikiforuk A, Popik P. Long-lasting cognitive deficit induced by stress is alleviated by acute administration of antidepressants. Psychoneuroendocrinol 2011;36(1):28-39; doi: 10.1016/j.psyneuen.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 37. McGaughy J, Ross RS, Eichenbaum H.. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting, Neuroscience 2008;153(1):63-71; doi: 10.1016/j.neuroscience.2008.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Floresco SB, Magyar O, Ghods-Sharifi S, et al. . Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacol 2006;31(2):297-309; doi: 10.1038/sj.npp.1300825 [DOI] [PubMed] [Google Scholar]
- 39. Pajkossy P, Szőllősi Á, Demeter G, et al. . Physiological measures of dopaminergic and noradrenergic activity during attentional set shifting and reversal. Front Psychol 2018;9:506; doi: 10.3389/fpsyg.2018.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clarke HF, Walker SC, Dalley JW, et al. . Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 2007;17(1):18-27; doi: 10.1093/cercor/bhj120 [DOI] [PubMed] [Google Scholar]
- 41. Tait DS, Brown VJ, Farovik A, et al. . Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci 2007;25(12):3719-3724; doi: 10.1111/j.1460-9568.2007.05612.x [DOI] [PubMed] [Google Scholar]
- 42. Bulin SE, Hohl KM, Paredes D, et al. . Bidirectional optogenetically-induced plasticity of evoked responses in the rat medial prefrontal cortex can impair or enhance cognitive set-shifting. eNeuro 2020;7(1):ENEURO.0363-19.2019; doi: 10.1523/ENEURO.0363-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naegeli KJ, O'Connor JA, Banerjee P, et al. . Effects of milnacipran on cognitive flexibility following chronic stress in rats. Eur J Pharmacol 2013;703(1-3):62-66; doi: 10.1016/j.ejphar.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 44. Wagner AK, Drewencki LL, Chen X, et al. . Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem 2009;108(4):986-997; doi: 10.1111/j.1471-4159.2008.05840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang T, Huang X-J, Van KC, et al. . Amantadine improves cognitive outcome and increases neuronal survival after fluid percussion traumatic brain injury in rats. J Neurotrauma 2014;31(4):370-377; doi: 10.1089/neu.2013.2917 [DOI] [PubMed] [Google Scholar]
- 46. Prasad MR, Tzigaret CM, Smith D, et al. . Decreased α1-adrenergic receptors after experimental brain injury. J Neurotrauma 1992;9(3):269-279; doi: 10.1089/neu.1992.9.269 [DOI] [PubMed] [Google Scholar]
- 47. Reid WM, Hamm RJ. Post-injury atomoxetine treatment improves cognition following experimental traumatic brain injury. J Neurotrauma 2008;25(3):248-256; doi: 10.1089/neu.2007.0389 [DOI] [PubMed] [Google Scholar]
- 48. Dixon CE, Kochanek PM, Yan HQ, et al. . One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma 1999;16(2):109-122; doi: 10.1089/neu.1999.16.109 [DOI] [PubMed] [Google Scholar]
- 49. Donat CK, Schuhmann MU, Voigt C, et al. . Time-dependent alterations of cholinergic markers after experimental traumatic brain injury. Brain Res 2008;1246:167-177; doi: 10.1016/j.brainres.2008.09.059 [DOI] [PubMed] [Google Scholar]
- 50. Shin SS, Bray ER, Zhang CQ, et al. . Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res 2011;1369:208-215; doi: 10.1016/j.brainres.2010.10.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Olsen AS, Sozda CN, Cheng JP, et al. . Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J Neurotrauma 2012;29(10):1898-1907; doi: 10.1089/neu.2012.2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng JP, Hoffman AN, Zafonte RD, et al. . A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav Brain Res 2008;194(1):79-85; doi: 10.1016/j.bbr.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muneoka K, Shirayama Y, Takigawa M, et al. . Brain region-specific effects of short-term treatment with duloxetine, venlafaxine, milnacipran and sertraline on monoamine metabolism in rats. Neurochem Res 2009;34(3):542-555; doi: 10.1007/s11064-008-9818-2 [DOI] [PubMed] [Google Scholar]
- 54. Vaishnavi SN, Nemeroff CB, Plott SJ, et al. . Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol Psychiatr 2004;55(3):320-322; doi: 10.1016/j.biopsych.2003.07.006 [DOI] [PubMed] [Google Scholar]
- 55. Deecher DC, Beyer CE, Johnston G, et al. . Desvenlafaxine succinate: A new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther 2006;318(2):657-665; doi: 10.1124/jpet.106.103382 [DOI] [PubMed] [Google Scholar]
- 56. Kawai H, Machida M, Ishibashi T, et al. . Chronopharmacological analysis of antidepressant activity of a dual-action serotonin noradrenaline reuptake inhibitor (SNRI), milnacipran, in rats. Biol Pharm Bull 2018;41(2):213-219; doi: 10.1248/bpb.b17-00733 [DOI] [PubMed] [Google Scholar]
- 57. Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clin Psychopharmacol 1994;9 Suppl 1:19-26; doi: 10.1097/00004850-199403001-00004 [DOI] [PubMed] [Google Scholar]
- 58. Baldwin DS. Escitalopram: efficacy and tolerability in the treatment of depression. Hosp Med 2002;63(11):668-671; doi: 10.12968/hosp.2002.63.11.1912 [DOI] [PubMed] [Google Scholar]
- 59. Pollock BG. Citalopram: a comprehensive review. Expert Opin Pharmacother 2001;2(4):681-698; doi: 10.1517/14656566.2.4.681 [DOI] [PubMed] [Google Scholar]
- 60. Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol 2008;19(3):225-234; doi: 10.1097/FBP.0b013e3282feb0ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park J, Wood F, Bondi C, et al. . Anxiety evokes hypofrontality and disrupts rule-relevant encoding by dorsomedial prefrontal cortex neurons. J Neurosci 2016;36(11):3322-3335; doi: 10.1523/JNEUROSCI.4250-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kitaichi Y, Inoue T, Izumi T, et al. . Subchronic milnacipran treatment increases basal extracellular noradrenaline concentrations in the medial prefrontal cortex of rats. Eur J Pharmacol 2005;520(1-3):37-42; doi: 10.1016/j.ejphar.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 63. Mochizuki D, Tsujita R, Yamada S, et al. . Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacol (Berl) 2002;162(3):323-332; doi: 10.1007/s00213-002-1111-5 [DOI] [PubMed] [Google Scholar]
- 64. Paxinos G, Watson C.. The Rat Brain in Stereotaxic Coordinates: hard cover edition. Elsevier: New York; 2006. [Google Scholar]
- 65. Bondi CO, Yelleswarapu NK, Day-Cooney J, et al. . Systemic administration of donepezil attenuates the efficacy of environmental enrichment on neurobehavioral outcome after experimental traumatic brain injury. Restor Neurol Neurosci 2018;36(1):45-57; doi: 10.3233/RNN-170781 [DOI] [PubMed] [Google Scholar]
- 66. Lapiz-Bluhm MDS, Bondi CO, Doyen J, et al. . Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol 2008;20(10):1115-1137; doi: 10.1111/j.1365-2826.2008.01772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kline AE, Massucci JL, Dixon CE, et al. . The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma 2004;21:175-185; doi: 10.1089/089771504322778631 [DOI] [PubMed] [Google Scholar]
- 68. Hoffman AN, Cheng JP, Zafonte RD, et al. . Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci 2008;83(17-18):602-607; doi: 10.1016/j.lfs.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sozda CN, Hoffman AN, Olsen AS, et al. . Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma 2010;27(6):1047-1057; doi: 10.1089/neu.2010.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chou A, Morganti JM, Rosi S. Frontal lobe contusion in mice chronically impairs prefrontal-dependent behavior. PLoS One 2016;11(3):e0151418; doi: 10.1371/journal.pone.0151418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martens KM, Vonder Haar C, Hutsell BA, et al. . The dig task: a simple scent discrimination reveals deficits following frontal brain damage. J Vis Exp 2013;(71):50033; doi: 10.3791/50033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martens KM, Vonder Haar C, Hutsell BA, et al. . A discrimination task used as a novel method of testing decision-making behavior following traumatic brain injury. J Neurotrauma 2012;29(15):2505-2512; doi: 10.1089/neu.2012.2388 [DOI] [PubMed] [Google Scholar]
- 73. Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience 2004;123(2):349-359; doi: 10.1016/j.neuroscience.2003.09.023 [DOI] [PubMed] [Google Scholar]
- 74. Geddes RI, Peterson BL, Stein DG, et al. . Progesterone treatment shows benefit in female rats in a pediatric model of controlled cortical impact injury. PLoS One 2016;11(1); doi: 10.1371/journal.pone.0146419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Geddes RI, Sribnick EA, Sayeed I, et al. . Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PLoS One 2014;9(1:e87252); doi: 10.1371/journal.pone.0087252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoffman SW, Fülöp Z, Stein DG. Bilateral frontal cortical contusion in rats: behavioral and anatomic consequences. J Neurotrauma 1994;11(4):417-431; doi: 10.1089/neu.1994.11.417 [DOI] [PubMed] [Google Scholar]
- 77. Jacqmain J, Nudi ET, Fluharty S, et al. . Pre and post-injury environmental enrichment effects functional recovery following medial frontal cortical contusion injury in rats [Internet]. Behav Brain Res 2014;275:201-211; doi: 10.1016/j.bbr.2014.08.056 [DOI] [PubMed] [Google Scholar]
- 78. Zhang H, Han M, Zhang X, et al. . The effect and mechanism of growth hormone replacement on cognitive function in rats with traumatic brain injury. PLoS One 2014;9(9):e108518; doi: 10.1371/journal.pone.0108518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vonder Haar C, Anderson GD, Hoane MR. Continuous nicotinamide administration improves behavioral recovery and reduces lesion size following bilateral frontal controlled cortical impact injury. Behav Brain Res 2011;224(2):311-317; doi: 10.1016/j.bbr.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vonder Haar C, Martens KM, Riparip L-K, et al. . Frontal traumatic brain injury increases impulsive decision making in rats: A potential role for the inflammatory cytokine interleukin-12. J Neurotrauma 2017;34(19):2790–2800; doi: 10.1089/neu.2016.4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vonder Haar C, Lam FCW, Adams WK, et al. . Frontal traumatic brain injury in rats causes long-lasting impairments in impulse control that are differentially sensitive to pharmacotherapeutics and associated with chronic neuroinflammation. ACS Chem Neurosci 2016;7(11):1531-1542; doi: 10.1021/acschemneuro.6b00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci 2017;37(44):10529-10540; doi: 10.1523/JNEUROSCI.1678-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jennings JH, Kim CK, Marshel JH, et al. . Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 2019;565(7741):645-649; doi: 10.1038/s41586-018-0866-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Adler SM, Girotti M, Morilak DA. Optogenetically-induced long term depression in the rat orbitofrontal cortex ameliorates stress-induced reversal learning impairment. Neurobiol Stress 2020;13:100258; doi: 10.1016/j.ynstr.2020.100258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res 2003;146(1-2):3-17; doi: 10.1016/j.bbr.2003.09.028 [DOI] [PubMed] [Google Scholar]
- 86. Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000;10(3):206-219; doi: 10.1093/cercor/10.3.206 [DOI] [PubMed] [Google Scholar]
- 87. Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatr 2010;34(6):913-923; doi: 10.1016/j.pnpbp.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cao AH, Yu L, Wang YW, et al. . Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behav Brain Funct 2012;8(1):10; doi: 10.1186/1744-9081-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Danet M, Lapiz-Bluhm S, Morilak DA. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. Int J Neuropsychopharmacol 2010;13(8):997-1009; doi: 10.1017/S1461145710000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bondi CO, Klitsch KC, Leary JB, et al. . Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J Neurotrauma 2014;31(10):873-888; doi: 10.1089/neu.2014.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lippert-Gruener M, Maegele M, Garbe J, et al. . Late effects of enriched environment (EE) plus multimodal early onset stimulation (MEOS) after traumatic brain injury in rats: ongoing improvement of neuromotor function despite sustained volume of the CNS lesion. Exp Neurol 2007;203(11):82-94; doi: 10.1016/j.expneurol.2006.07.025 [DOI] [PubMed] [Google Scholar]