Summary
Background
The emergence of potentially effective new therapies for genetic forms of amyotrophic lateral sclerosis (ALS) necessitates the identification of biomarkers to facilitate early treatment, prior to the onset of motor symptoms. Here, we sought to investigate whether metabolic alterations are detectable in presymptomatic ALS gene mutation carriers, and whether such alterations precede neurofilament light chain (NfL) changes in serum.
Methods
Between 02/2014 and 11/2021, we prospectively studied 60 presymptomatic ALS gene mutation carriers (40% male, age 48.7 ± 14.9; 28 C9orf72, 22 SOD1, 10 other) compared to 73 individuals from the same families (47% male, age 47.4 ± 12.9) without pathogenic mutations as controls. Bioimpedance analysis (BIA) and indirect calorimetry were performed, and Body Mass Index (BMI), Fat Mass (FM), Body Fat Percentage, Body Water (BW), Lean Body Mass (LBM), Extracellular Mass (ECM), Body Cell Mass (BCM), ECM/BCM ratio, Cells Percentage, Phase Angle, Resting Metabolic Rate (RMR), Metabolic Ratio (MR), and NfL were measured. Participants and evaluators were blinded regarding gene carrier status.
Findings
Presymptomatic ALS gene carriers showed reduced LBM (p = 0.02), BCM (p = 0.004), Cells Percentage (p = 0.04), BW (p = 0.02), Phase Angle (p = 0.04), and increased ECM/BCM ratio (p = 0.04), consistently indicating a loss of metabolically active body cells. While in C9orf72 mutation carriers all tissue masses were reduced, only metabolically active tissue was affected in SOD1 mutation carriers. Unexpectedly, RMR (p = 0.009) and MR (p = 0.01) were lower in presymptomatic ALS gene carriers compared to non-carriers. NfL serum levels were similar in mutation carriers and non-carriers (p = 0.60).
Interpretation
The observed metabolic phenomena might reflect reduced physical activity and/or preemptive, insufficient compensatory mechanisms to prepare for the later hypermetabolic state. As pre-symptomatic biomarkers we propose ECM/BCM ratio and Phase Angle for SOD1, and a 4-compartment affection in BIA for C9orf72 mutation carriers.
Funding
This work was an investigator-initiated trial. On the German side, there was no institutional or industrial funding. On the Swedish side, this work was supported by grants from the Swedish Brain Foundation (grants nr. 2013-0279, 2016-0303, 2018-0310, 2020-0353), the Swedish Research Council (grants nr. 2012-3167, 2017-03100), the Knut and Alice Wallenberg Foundation (grants nr. 2012.0091, 2014.0305, 2020.0232), the Ulla-Carin Lindquist Foundation, Umeå University (223-2808-12, 223-1881-13, 2.1.12-1605-14) and the Västerbotten County Council (grants nr 56103-7002829), King Gustaf V:s and Queen Victoria's Freemason's Foundation.
Keywords: Amyotrophic lateral sclerosis, Presymptomatic, Mutations carriers, Metabolism, Metabolic
Research in context.
Evidence before this study
We searched PubMed for reviews, observational studies, clinical trials, and cohort studies in pre-symptomatic gene mutation carriers of amyotrophic lateral sclerosis published up to October 1st, 2022, using the terms “amyotrophic lateral sclerosis” or “ALS” in combination with each of the following terms: “presymptomatic”, “gene carriers”, “mutation carriers”, or “asymptomatic”. After reviewing the abstracts, we identified 38 review articles, 8 clinical trials, and 44 cohort studies dealing with potential early biomarkers. Among the cohort studies, there were 3 investigating neurofilaments, and none investigating metabolic alterations. Among the reviews, there were 4 dealing with neurofilaments, and none dealing with metabolic alterations. Among the clinical studies, none was related to metabolism.
Added value of this study
In this cohort study investigating metabolic alterations in presymptomatic ALS gene mutation carriers, we identified gene-specific biomarkers, which, after further validation in clinical studies, can be used for earlier diagnosis and treatment in ALS gene mutation carriers.
Implications of all the available evidence
Based on pre-existing evidence, neurofilaments are currently explored in clinical studies to define disease onset and treatment start in pre-symptomatic gene mutation carriers of ALS. This study provides evidence that body composition and resting energy expenditure represent earlier and gene-specific biomarkers that should be additionally implemented in clinical trials for this purpose. Furthermore, these biomarkers represent potential targets for disease monitoring and nutritional interventions.
Introduction
In amyotrophic lateral sclerosis (ALS), the onset of disease is usually defined as the occurrence of first paresis. However, according to the current understanding of the disease, ALS pathology does not only affect the motor system,1 but is also associated with profound metabolic changes, including hypermetabolism,2 excessive weight loss,3,4 and abnormalities of lipid5,6 and carbohydrate metabolism.7 These metabolic changes constitute important prognostic factors5,8 and can be targeted by nutritional interventions.9, 10, 11, 12
Accordingly, there is an ongoing discussion whether such alterations precede motor symptoms, thus defining a “presymptomatic phase”. The identification of a presymptomatic phase in ALS could be utilised for earlier diagnosis and, consequently, earlier initiation of treatment. As antisense oligonucleotides (ASOs) and other gene therapies are emerging and may soon become promising therapeutic options,13,14 the identification of suitable early biomarkers to determine disease onset is crucial. Clinical trials targeting mutations in SOD1,13,14 FUS/TLS, C9orf72, and ATXN2 have either been concluded recently or are currently ongoing, and more trials are in the planning phase.
Currently, neurofilament light chain (NfL) blood levels as a marker of axonal damage15 are considered the most promising biomarker to define a presymptomatic stage in ALS, as elevation frequently precedes motor deficits.16 However, NfL serum levels increase relatively close (within 12 months) to the occurrence of first paresis.16,17 Of note, NfL blood levels are the key biomarker in the ongoing tofersen gene therapy intervention in asymptomatic carriers of SOD1 mutations (clinicaltrials.gov identifier NCT04856982).
Here, we aimed to identify additional biomarkers for the presymptomatic phase by investigating metabolic alterations in a cohort of presymptomatic mutation carriers. To that end, we examined body composition, i.e., the ratio of metabolically active and inactive tissue, and resting energy expenditure (REE) in presymptomatic mutation carriers compared to their non-mutation carrier family members by applying bioimpedance analysis (BIA) and indirect calorimetry in a prospective, standardised setting, including participants and evaluators blinded with regard to carrier status.
Methods
Study population and design
Between 02/2014 and 11/2021, presymptomatic carriers of mutations in ALS causing genes and individuals from the same families without mutations were prospectively recruited within a multicenter network (German PreSymptomatic ALS Study, GPS-ALS), including specialised ALS centers from Ulm, Bonn, Mannheim, Rostock (all Germany), and Umeå (Sweden). The study design features a baseline visit and longitudinal follow-up visits in 3-yearly time intervals.
Subjects of any sex were identified by contacting first- or second-degree family members (i.e., parents, siblings, or children) aged >18 years of individuals suffering from definite, laboratory-supported, familial ALS according to the revised El Escorial criteria18 which implies evidence of a proven causative ALS gene mutation. A causative ALS gene mutation was defined as a mutation grade 4 (likely pathogenic) or 5 (pathogenic) according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP).19 As previously defined,16 the term “presymptomatic” required all enrolled participants not to display any signs or symptoms of ALS or cognitive impairment, including a normal neurological examination and cranial MRI. Electromyography was not systematically performed. Participants with concomitant diseases or medication which could potentially influence the results were excluded. Furthermore, included participants did not show any abnormal metabolic profiles in routine laboratory testing.
BIA, indirect calorimetry, and blood drawing for NfL measurement were performed during an outpatient visit in the Study Center of Ulm University in all participants, including those who were recruited in other study centers, and demographic data (including sex as self-reported by study participants) were collected. Subjects as well as study personnel (both clinical and laboratory personnel) were blinded throughout the study with regard to genotype carrier status. However, all participants were informed comprehensively about potential implications of a positive carrier status, including tofersen treatment in case of SOD1 as well as ongoing and upcoming clinical studies for symptomatic and presymptomatic mutation carriers. They were also informed that they may terminate the study at any point of time for the sake of unblinding.
Ethics
The study was approved by the Ethics Committee of Ulm University, application numbers 20/12 and 68/19, and performed fully in accordance with the principles of the Declaration of Helsinki (WMA, 1964) with later amendments. All participants signed an informed consent form prior to inclusion.
Genetic testing
To identify causative ALS mutations, an ALS gene panel was applied, which is continuously updated. The panel currently includes the following genes: ALS2, ANG, ARHGEF28, ATXN2, BSCL2, C9orf72, CCNF, CHCHD10, CHMP2B, DCTN1, ERBB4, FIG4, FUS, GBE1, GLE1, GRN, HNRNPA1, HNRNPA2B1, HSPB1, HSPB8, MAPT, MATR3, MME, NEFH, NEK1, OPTN, PFN1, PRPH, SETX, SIGMAR1, SOD1, SPG11, SPG20, SQSTM1, TAF15, TARDBP, TBK1, TUBA4A, UBQLN2, VAPB, VCP, FEGFA, and VPS54.
DNA was extracted from whole EDTA-containing venous blood tubes. Participants were screened by second-generation sequence methods, and mutations confirmed by Sanger sequencing. For C9orf72, a pathogenic GGGGCC-hexanucleotide repeat expansion was probed by fragment length analysis and repeat-primed PCR (RP-PCR). Samples with a sawtooth pattern in the RP-PCR were confirmed by Southern blot. Subjects with causative ALS mutations grade 4 or 5 according to ACMG-AMP were classified as mutation carriers. The estimated time to disease onset (ETTO) was defined as the difference between the age of onset of the family's index patient and the age of the participant, i.e., ETTO was calculated for each individual based on the age of onset of his/her family member analogous to previous publications.17,20 Accordingly, negative ETTO values signify that the subject has already exceeded the index patient's age of onset at the time of inclusion. The index patients' age of onset was defined based on the occurrence of first paresis.
Bioimpedance analysis
BIA was performed under standardised, fasting conditions in a relaxed, horizontal position at room temperature with a 4-electrode device (Nutribox, Data Input GmbH, Pöcking, Germany). It was performed in the morning between 7:30 and 9:00 a.m. without any preceding physical activity (at least 2 h without moderate exercise and 14 h without heavy exercise) or medication and after a resting period of 20 min. Participants were fasting for at least 5 h as well as abstinent from nicotine (>2 h) and caffeine (>4 h).
BIA constitutes a well-established and validated method to analyze body composition21 based on the varying impedance of tissues caused by the amount of electrolytes (intracellular and extracellular water) and the capacitor effect of cell membranes.
There are four different models of body compartments (Supplementary Fig. S1). The 1-compartment model refers to BMI and does not differentiate body composition. The 2-compartment model includes fat mass (FM) and fat-free mass (Lean Body Mass, LBM). In the 3-compartment model, LBM is further divided into the Body Cell Mass (BCM), which consists of oxygen consuming, glucose oxidating, metabolically active cells, and the Extracellular Mass (ECM). The ECM/BCM quotient is thus the ratio between metabolically inactive and active cells. It has been validated as an indicator of nutritional status and a predictor of mortality in various diseases and conditions,22, 23, 24 with higher values signifying worse prognosis. The 4-compartment model additionally considers intracellular (ICW) and extracellular water (ECW) as components of BCM and ECM, respectively. Phase shift relates to the temporal desynchronization between the sinus curves of the alternating current and the alternating voltage which is measured by the phase angle φ. In general, higher phase angles signify a higher share of healthy cells and muscle mass as well as a higher degree of physical activity. Consistently, the phase angle has been shown do constitute an independent prognostic factor in the symptomatic phase of ALS.25
Indirect calorimetry
Indirect calorimetry was performed under the same standardised conditions as BIA (see above) with the device Quark RMR (COSMED Deutschland GmbH, Fridolfing, Germany). One measurement was performed over 16 min, during which participants were advised to relax without falling asleep.
Indirect calorimetry is a reliable, reproducible, and highly accurate method for measuring energy expenditure26 based on the measurement of gas exchange (consumption of oxygen and production of carbon dioxide). The measured Resting Metabolic Rate (mRMR) can then be calculated based on the modified Weir equation.27 The mRMR was compared with the calculated Resting Metabolic Rate (cRMR) according to the equation of Harris and Benedict28 which estimates energy expenditure based on sex, weight, height, and age. The Metabolic Ratio (MR) refers to the quotient mRMR/cRMR and thus represents the energy expenditure adjusted for all variables included in the cRMR equation.
The first 5 min of each measurement were discarded. Within the following 10 min, a steady state period was identified, which was defined as a period of 5 min displaying a variation of <10% of mRMR, volume of oxygen (VO2), and volume of carbon dioxide (VCO2). The mean values of this steady state period were used for statistical analysis.
Neurofilaments
Neurofilament light chain (NfL) blood levels as a marker of axonal damage are an established diagnostic and prognostic biomarker in symptomatic ALS.15 Serum was obtained from peripheral blood by centrifugation (800 g, 5 min) and stored within 2 h at −80 °C. Blood NfL concentrations were measured with the commercially available kits for the ELLA microfluidic system (Bio-Techne, Minneapolis, USA). The interassay coefficients of variation (CV) were <15%.
Statistics
For descriptive statistics, median and interquartile ranges are given. Distributional assumptions were checked graphically and by means of descriptive methods only. Whenever questionable, a non-parametric analysis approach was chosen in order to account for potentially biased results when using parametric methods. Group comparisons for continuous variables were performed using one-way ANOVA or the Kruskal–Wallis test as appropriate, and pairwise group comparisons were performed with the two-sample t-test or the Wilcoxon rank sum test as appropriate. Changes of continuous variables to baseline were analyzed using the Wilcoxon signed rank test. Two-sided 95% confidence intervals were calculated for median differences. Group comparisons for categorical variables were performed using the chi square test or Fisher's exact test as appropriate. Two-sided 95% confidence intervals were calculated for group differences of proportions. Correlations were examined with scatter plots and correlation coefficients (Spearman).
Multiple regression analysis was used in order to check whether study center was a confounding factor. For reasons of statistical power and since only 15 patients were not recruited in Ulm, study center was included as a binary covariable (Ulm vs. others) in a series of linear regression models.
All statistical tests were performed at a two-sided level of alpha = 0.05 and were interpreted as exploratory. Adjustment of p-values for multiple testing was done with Dunn's test, and unadjusted as well as adjusted p-values are presented. As this was an explorative pilot study, all results were interpreted as hypothesis generating rather than as a verification of a specific hypothesis. Missing values were not replaced.
Statistical analyses were done with GraphPad Prism, version 7.05.
Role of the funders
This work was an investigator-initiated trial. On the German side, there was no institutional or industrial funding. On the Swedish side, this work was supported by grants from the Swedish Brain Foundation (grants nr. 2013-0279, 2016-0303, 2018-0310, 2020-0353), the Swedish Research Council (grants nr. 2012-3167, 2017-03100), the Knut and Alice Wallenberg Foundation (grants nr. 2012.0091, 2014.0305, 2020.0232), the Ulla-Carin Lindquist Foundation, Umeå University (223-2808-12, 223-1881-13, 2.1.12-1605-14) and the Västerbotten County Council (grants nr 56103-7002829), King Gustaf V:s and Queen Victoria's Freemason's Foundation. These funders were not involved in any aspects regarding conduction, analysis, interpretation, or publication of the study.
Results
Between 02/2014 and 11/2021, 133 participants were recruited, of which 60 were presymptomatic ALS gene carriers (40% male, age 48.7 ± 14.9; 28 C9orf72, 22 SOD1, 10 other, genotypes are given in Supplementary Table S1) and 73 were individuals from the same families (47% male, age 47.4 ± 12.9) who were not carrying any known ALS-related gene mutations and were serving as controls. Baseline characteristics (Table 1) did not show any significant differences between ALS mutation carriers and non-carriers with regard to age and sex ratio. Follow-up data were available for 14 subjects (10 carriers and 4 non-carriers) after a median time interval of 36 (30.5–45.3) months.
Table 1.
Baseline characteristics.
| ALS mutation carriers (n = 60) |
Non-carriers (n = 73) |
p-value (carrier vs. non-carrier) | |||||
|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | ||
| Age (years) | 48.7 ± 14.9 | 53.1 ± 23.3 | 45.8 ± 21.2 | 47.2 ± 12.9 | 47.1 ± 38.2 | 47.7 ± 29.7 | 0.72 |
| Sex | 24 (40%) | 36 (60%) | 34 (46.6%) | 39 (53.4%) | 0.49 | ||
| NfL blood levels (pg/ml) | 14 (10–19) | 15 (10–31) | 13 (10–18) | 15 (10–21) | 15 (11–21) | 15 (10–21) | 0.60 |
| Mutations | |||||||
| C9orf72 | 28 | 7 | 21 | ||||
| SOD1 | 22 | 13 | 9 | ||||
| FUS | 4 | 1 | 3 | ||||
| KIF5A | 3 | 1 | 2 | ||||
| SETX | 1 | 1 | 0 | ||||
| TBK1 | 1 | 1 | 0 | ||||
| TDP43 | 1 | 0 | 1 | ||||
| Expected time to onset (years) | 4.0 (−6.0 to 14.0) | −3.0 (−13.5 to 13.5) | 7.0 (−3.0 to 17.0) | ||||
| Disease onset (paresis within observation period) | 12 (20%) | 9 (37.5%) | 3 (8.3%) | ||||
| Deceased (within observation period) | 4 (7%) | 3 (12.5%) | 1 (2.7%) | ||||
Data are n (%), mean ± SD, or median (IQR).
NfL: Neurofilament light chain.
Metabolic data were available for all participants. In the linear regression models, no differences between study centers (Ulm vs. others) were detected with the exception of MR (see below).
NfL blood levels were obtained from 60/60 ALS mutation carriers and 71/73 mutation-negative relatives. We found no differences between carriers (14 (10–19) pg/ml) and non-carriers (15 (10–21) pg/ml; p = 0.60, Wilcoxon rank sum). Similarly, NfL levels were not different between presymptomatic SOD1 and C9orf72 mutation carriers (p = 0.61, Wilcoxon rank sum).
Body composition
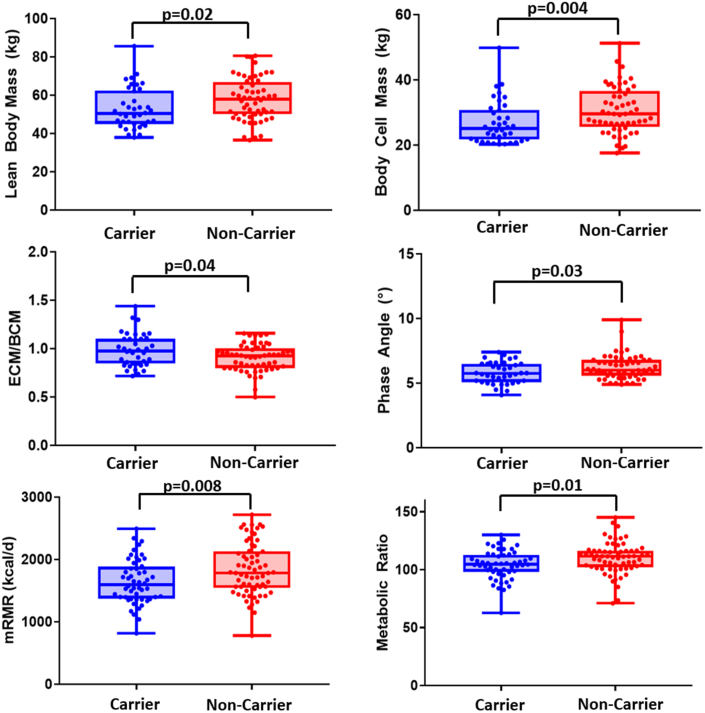
Compared to non-carriers, ALS mutation carriers showed lower LBM (median difference −7.5 kg (95% CI −9.5 to −0.9); p = 0.004, Wilcoxon rank sum), lower cell percentages (−1.2% (−3.5 to −0.1); p = 0.04, Wilcoxon rank sum), higher ECM/BCM ratios (0.05 (0–0.14); p = 0.04, Wilcoxon rank sum), lower BW (−5.5 kg (−7.0 to −0.7); p = 0.02, Wilcoxon rank sum), and lower Phase Angles (−0.2 (−0.7 to 0.0); p = 0.04, Wilcoxon rank sum), which consistently point towards a loss of metabolically active, healthy cells (body water is mainly stored in muscle cells; Fig. 1, Table 2, Supplementary Table S2). On the other hand, there were no significant differences between ALS mutation carriers and non-carriers with regard to Fat Mass (p = 0.45, Wilcoxon rank sum), Fat Percentage (p = 0.61, Wilcoxon rank sum), and ECM (p = 0.28, Wilcoxon rank sum), which comprise metabolically inactive tissue, as well as BMI (p = 0.31, Wilcoxon rank sum).
Fig. 1.
Body composition and resting energy expenditure in carriers vs. non-carriers. Boxplots show Lean Body Mass (upper left), Body Cell Mass (BCM; upper right), ratio between Extracellular Mass (ECM) and BCM (center left), Phase Angle (center right), measured Resting Metabolic Rate (mRMR; bottom left), and Metabolic Ratio (bottom right) in ALS mutation carriers (blue) and non-carriers (red). p-values based on Wilcoxon rank sum test.
Table 2.
Body composition and resting energy expenditure in carriers vs. non-carriers.
| ALS mutation carriers (n = 60) | Non-carriers (n = 73) | Median difference (95% CI) | p-value | |
|---|---|---|---|---|
| BMI (kg/m2) | 24.7 (21.1–27.8) | 24.9 (22.8–29.0) | −0.2 (−2.9 to 1.0) | 0.31 |
| FM (kg) | 19.7 (14.8–30.6) | 21.3 (16.8–30.0) | −1.6 (−5.1 to 2.8) | 0.49 |
| Fat (%) | 28.8 (23.9–34.8) | 27.6 (21.3–34.0) | 1.2 (−2.9 to 5.3) | 0.62 |
| LBM (kg) | 50.6 (45.0–62.5) | 58.0 (50.1–66.9) | −7.5 (−9.5 to −0.9) | 0.02 |
| ECM (kg) | 25.1 (22.6–28.9) | 26.9 (23.4–31.0) | −1.8 (−3.4 to 0.9) | 0.28 |
| BCM (kg) | 25.2 (21.8–30.9) | 29.6 (25.6–36.6) | −4.4 (−6.3 to −1.3) | 0.004 |
| Cells (%) | 50.7 (47.5–54.2) | 51.9 (50.0–55.5) | −1.2 (−3.5 to −0.1) | 0.04 |
| ECM/BCM | 0.98 (0.85–1.10) | 0.93 (0.80–1.00) | 0.05 (0.00–0.14) | 0.04 |
| BW (kg) | 37.0 (32.9–45.7) | 42.5 (36.7–49.0) | −5.5 (−7.0 to −0.7) | 0.02 |
| Phase Angle (°) | 5.8 (5.1–6.5) | 6.0 (5.6–6.8) | −0.2 (−0.7 to 0.0) | 0.04 |
| mRMR (kcal/d) | 1598 (1376–1885) | 1785 (1548–2133) | −187 (−337 to −50) | 0.008 |
| MR | 1.04 (0.98–1.13) | 1.11 (1.02–1.16) | −0.07 (−0.10 to −0.01) | 0.01 |
| NfL (pg/ml) | 14 (10–19) | 15 (10–21) n = 71 | −1 (−3 to 2) | 0.60 |
Data are median (IQR), Wilcoxon Rank Sum test.
BMI: Body Mass Index. FM: Fat Mass. LBM: Lean Body Mass. ECM: Extracellular Mass. BCM: Body Cell Mass. BW: Body Water. mRMR: measured Resting Metabolic Rate. MR: Metabolic Ratio. NfL: Neurofilament light Chain blood levels.
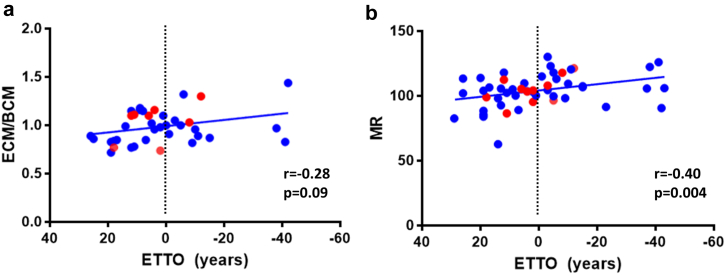
The ECM/BCM ratio is independent of BMI and already established as a prognostic biomarker in other diseases.22, 23, 24 We found a trend towards a negative correlation between ECM/BCM ratio and ETTO (r = −0.28, p = 0.09, Spearman; Fig. 2a), i.e., presymptomatic gene carriers who approached their expected onset of motor symptoms generally showed higher values. Differences of ECM/BCM ratios between carriers and non-carriers were greater in females (carriers: 1.00 (0.89–1.16) vs. non-carriers: 0.94 (0.85–1.03); p = 0.06, Wilcoxon rank sum) compared to males (carriers: 0.86 (0.77–1.04) vs. non-carriers: 0.83 (0.78–0.97); p = 0.66, Wilcoxon rank sum).
Fig. 2.
Correlation of ECM/BCM ratio and MR with ETTO in mutation carriers. Scatter plots show the correlation of Extracellular Mass (ECM)/Body Cell Mass (BCM) with the Expected Time to Onset (ETTO; a) and the correlation of the Metabolic Ratio (MR) with ETTO (b). Dotted vertical lines mark the expected time of disease onset. Negative ETTO values relate to subjects who have already passed their expected age of onset. Red dots indicate subjects who had disease onset during the observation period. p-values based on Spearman.
We found that C9orf72 mutation carriers, in contrast to SOD1, showed massively reduced masses of all body compartments. Compared to SOD1, C9orf72 mutation carriers had lower BMI (median difference −5.5 kg/m2 (95% CI −8.7 to −2.6); p = 0.004, Wilcoxon rank sum), lower FM (−6.7 kg (−15.8 to −0.8); p = 0.02, Wilcoxon rank sum), lower LBM (−17.3 kg (−19.8 to −3.9); p = 0.004, Wilcoxon rank sum), lower ECM (−5.3 kg (−9.8 to −2.5); p < 0.001, Wilcoxon rank sum), lower BCM (−7.0 kg (−10.5 to −0.1); p = 0.04, Wilcoxon rank sum), and lower BW (−12.6 kg (−14.5 to −2.8); p = 0.004, Wilcoxon rank sum; Supplementary Fig. S2, Table 3, Supplementary Table S3). Thus, all tissue types, including metabolically inactive tissue, were reduced in C9orf72 mutation carriers, while in SOD1, only metabolically active tissue was affected.
Table 3.
Body composition and resting energy expenditure in SOD1 vs. C9orf72 vs. controls.
| SOD1 (n = 22) | C9orf72 (n = 28) | NC (n = 73) | 3-group comparison | Median difference (95% CI); SOD1vs. NC | Median difference (95% CI); C9orf72vs. NC | Median difference (95% CI); C9orf72 vs. SOD1 | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 28.0 (25.8–32.4) | 22.5 (20.7–25.9) | 24.9 (22.8–29.0) | p = 0.004 | 3.1 (−1.0 to 5.7) p = 0.12 (0.45) | −2.4 (−4.9 to −0.9) p = 0.007 (0.02) | −5.5 (−8.7 to −2.6) p = 0.004 (0.005) |
| FM (kg) | 25.8 (16.7–36.3) | 19.1 (13.0–21.3) | 21.4 (16.8–30.0) | p = 0.06 | 4.4 (−2.9 to 10.9) p = 0.25 (0.70) | −2.3 (−8.3 to 0.8) p = 0.09 (0.28) | −6.7 (−15.8 to −0.8) p = 0.02 (0.06) |
| Fat (%) | 32.2 (25.0–38.5) | 29.0 (21.5–33.3) | 27.6 (21.3–34.0) | 0.48 | 4.6 (−2.6 to 9.1) p = 0.26 (0.70) | 1.4 (−4.5 to 5.3) p = 0.93 (>0.99) | −3.2 (−8.9 to 3.0) p = 0.29 (0.99) |
| LBM (kg) | 64.1 (48.0–67.5) | 46.8 (43.6–52.0) | 58.0 (50.1–66.9) | p < 0.001 | 6.1 (−5.1 to 10.6) p = 0.57 (>0.99) | −11.2 (−14.5 to −4.8) p < 0.001 (0.001) | −17.3 (−19.8 to −3.9) p = 0.003 (0.007) |
| ECM (kg) | 28.9 (25.1–35.0) | 23.6 (20.5–26.3) | 26.9 (23.4–31.0) | p = 0.002 | 2.0 (–0.7 to 5.9) p = 0.11 (0.33) | −3.3 (−5.9 to −1.1) p = 0.005 (0.02) | −5.3 (−9.8 to −2.5) p < 0.001 (0.002) |
| BCM (kg) | 30.7 (22.3–36.6) | 23.7 (21.5–25.8) | 29.6 (25.6–36.6) | p = 0.001 | 1.1 (−5.4 to 4.6) p = 0.91 (>0.99) | −5.9 (−8.7 to −2.8) p < 0.001 (<0.001) | −7.0 (−10.5 to −0.1) p = 0.04 (0.04) |
| Cells (%) | 48.6 (46.4–55.6) | 51.8 (47.6–54.3) | 51.9 (50.0–55.5) | p = 0.16 | −3.3 (−5.5 to 0.4) p = 0.08 (0.26) | −0.1 (−3.5 to 0.9) p = 0.25 (0.70) | 3.2 (−2.8 to 5.4) p = 0.60 (>0.99) |
| ECM/BCM | 1.05 (0.80–1.16) | 0.93 (0.84–1.10) | 0.93 (0.80–1.00) | p = 0.16 | 0.12 (−0.02 to 0.22) p = 0.08 (0.26) | −0.00 (−0.13 to 0.03) p = 0.23 (0.66) | −0.12 (−0.20 to 0.11) p = 0.63 (>0.99) |
| BW (kg) | 46.9 (35.2–49.4) | 34.3 (31.9–38.1) | 42.5 (36.7–49.0) | p < 0.001 | 4.4 (−3.8 to 7.7) p = 0.57 (>0.99) | −8.2 (−10.6 to −3.5) p < 0.001 (0.001) | −12.6 (−14.5 to −2.8) p = 0.004 (0.008) |
| Phase Angle (°) | 5.4 (5.0–6.8) | 6.0 (5.1–6.5) | 6.0 (5.6–6.8) | p = 0.17 | −0.6 (−1.1 to 0.1) p = 0.09 (0.28) | 0.0 (−0.2 to 0.7) p = 0.22 (0.64) | 0.6 (−0.6 to 1.0) p = 0.62 (>0.99) |
| mRMR (kcal/d) | 1830 (1401–2080) | 1507 (1326–1670) | 1785 (1548–2133) | p = 0.001 | 45 (−249 to 184) p = 0.72 (>0.99) | −278 (−504 to −147) p < 0.001 (<0.001) | −323 (−532 to −69) p = 0.008 (0.04) |
| MR | 1.04 (0.95–1.12) | 1.05 (0.98–1.13) | 1.11 (1.02–1.16) | p = 0.02 | −0.07 (−0.12 to 0.00) p = 0.06 (0.08) | −0.06 (0.00 to –0.12) p = 0.05(0.07) | 0.01 (−0.08 to 0.07) p = 0.99 (>0.99) |
| NfL (pg/ml) | 12.5 (10.0–23.5) | 14.0 (10.5–19.0) | 15.0 (10.0–21.0) | p = 0.72 | −2.5 (−5.0 to 2.0) p = 0.44 (>0.99) | −1.0 (−4.0 to 3.0) p = 0.75 (>0.99) | 1.5 (−3.0 to 5.0) p = 0.61 (>0.99) |
Data are median (IQR), Wilcoxon Rank Sum test (2-group comparisons), Kruskal–Wallis (3-group comparisons).
NC: Non-Carriers. BMI: Body Mass Index. FM: Fat Mass. LBM: Lean Body Mass. ECM: Extracellular Mass. BCM: Body Cell Mass. BW: Body Water. mRMR: measured Resting Metabolic Rate. MR: Metabolic Ratio. NfL: Neurofilament light chain blood levels.
Bold values indicate statistical significance. Underlined values indicate p-values between 0.05 and 0.10. p-values adjusted for multiple testing (Dunn's test) are given in brackets.
Resting energy expenditure
Unexpectedly, we found that the measured Resting Metabolic Rate (mRMR) was lower in mutation carriers compared to mutation-negative subjects (median difference −188 kcal/d (95% CI −337 to −50); p = 0.008, Wilcoxon rank sum). Mutation carriers also displayed a lower Metabolic Ratio (MR = mRMR/cRMR), which accounts for age, weight, height, and sex (−0.07 (−0.10 to −0.01); p = 0.01, Wilcoxon rank sum; Fig. 1). As multiple linear regression models indicated a potential center-related effect with regard to MR (Supplementary Table S2), we repeated our analysis for the subgroup of patients from Ulm, but obtained similar results (−0.06 (−0.10 to 0.10); p = 0.05, Wilcoxon rank sum). There were no differences between male and female mutation carriers (p = 0.78, Wilcoxon rank sum) or between SOD1 and C9orf72 mutation carriers (p = 0.99, Wilcoxon rank sum).
As these findings differ from the symptomatic phase, we analyzed whether MR increased over time. Indeed, we found a negative correlation between MR and expected time to onset (r = −0.40, p = 0.004; Spearman; Fig. 2b), indicating that REE increased as the expected onset approached. We also checked whether follow-up MR values increased, but found no significant differences compared to baseline (p = 0.27, Wilcoxon signed rank), possibly because the number of gene carriers with available follow-up data was too small (n = 10) and/or these subjects were too far from their expected disease onset (ETTO: 3.0 (−5.3 to 15.3) years).
Discussion
In this study, we investigated metabolic alterations in a cohort of 60 presymptomatic ALS mutation carriers using 73 non-carriers from the same families as controls, aiming to identify potential novel early biomarkers for disease onset. The term “presymptomatic” was defined as the absence of any clinical signs or symptoms of manifest disease, consistent with previous publications.16
Strengths of the presented data are the prospective setting featuring standardised conditions for metabolic examinations, blinding of participants and evaluators with regard to carrier status, the number of subjects representing a large cohort of pre-symptomatic ALS gene mutation carriers, and the direct comparison with mutation-negative family members as optimal controls. The genetic heterogeneity and the lack of available follow-up data are limitations of the study. Thus, as the results are mainly based on cross-sectional data, the temporal course of the metabolic findings remains largely unknown. Furthermore, the concept of ETTO as applied in this study must be further validated.
With these limitations in mind, we were still able to identify highly distinct metabolic features in pre-symptomatic ALS gene carriers. Overall, results obtained with BIA consistently point towards a loss of metabolically active cells compared to metabolically inactive tissue, as reflected by an increased ECM/BCM ratio in mutation carriers. These observations might reflect a presymptomatic reduction of physical activity, potentially corroborating a recent report that physical activity in ALS patients decreases significantly several years prior to disease onset.29 Consistent with this hypothesis, we found normal NfL blood values in carriers and non-carriers. Therefore, alterations in body composition might constitute an earlier and, potentially, more sensitive biomarker of the presymptomatic phase.
Although catabolic alterations were prevalent across all studied mutations, we observed specific features in the two largest genetic subgroups, SOD1 and C9orf72. Compared to both presymptomatic SOD1 mutation carriers and controls, C9orf72 mutation carriers showed significantly lower masses of all body compartments. Thus, in contrast to SOD1 mutation carriers, not only metabolically active, but also metabolically inactive tissue was affected in C9orf72 mutation carriers. These mutation-specific findings are consistent with a recent publication, reporting that presymptomatic C9orf72 mutation carriers, compared to healthy controls, showed a lower metabolic cardiovascular risk profile, including lower BMI, lower fasting serum glucose, and higher HDL, while SOD1 mutation carriers had an opposite profile.6 Therefore, different metabolic biomarkers have to be considered for the presymptomatic phase for both genes:
-
(1)
In SOD1 presymptomatic mutation carriers, the ECM/BCM ratio stands out as a potential stand-alone biomarker for several reasons: First, it is independent of BMI and can thus be used universally; second, it compares the amount of metabolically inactive tissue (which is not affected in the presymptomatic phase) with metabolically active tissue (which is heavily affected), thus encompassing the underlying pathophysiological changes in one value; third, it has been validated as a prognostic biomarker in various chronic diseases associated with catabolism22, 23, 24; and fourth, our data indicate a potential correlation with ETTO. Furthermore, the Phase Angle also relates to metabolically active tissue and may constitute another useful parameter.
-
(2)
In C9orf72 presymptomatic mutation carriers, all types of tissue masses measured by BIA are affected. In this context, BMI constitutes a screening parameter, which is easy to apply and comprises all body compartments (one-compartment model), while measurement of FM and LBM (two-compartment model), ECM and BCM (three-compartment model), and BW (four-compartment model) can verify the involvement of all tissue types.
Most unexpected was the finding that presymptomatic mutation carriers, as opposed to symptomatic ALS patients,2 did not show an increased, but rather a significantly reduced REE, which, however, increased with approaching expected age of onset. This feature was observed in both SOD1 and C9orf72 mutation carriers. Assuming that hypermetabolism occurs only in the motor-symptomatic stage, one possible explanation is that this finding reflects compensatory measures, i.e., the organism's efforts to reduce energy consumption to compensate for an imminent deficit, and to build up triglyceride stores for future needs. This effort would be in line and work synergistically with the reduction of physical activity discussed above. Moreover, both the capability to store energy prior to the hypermetabolic phase and the amount of energy incorporated during the hypermetabolic phase might affect prognosis, as it has been shown repeatedly that weight loss constitutes an independent negative prognostic factor,30 and, consistently, high-caloric interventions yield beneficial effects in ALS.9,10,31 Considering the SOD1 vs. C9orf72 differences described above and the fact that this European cohort of presymptomatic SOD1 mutation carriers features a significant share of mutations associated with comparatively mild disease courses, as the respective mutations (D90A, L117V) result in a mutant protein with biophysical properties comparable to native wtSOD1, presymptomatic SOD1 patients might feature superior preemptive energy-storing capabilities, which later translate to a more benign course of disease. In this context, it would be interesting to obtain similar data from US cohorts with more aggressive SOD1 mutations like A4V. This hypothesis would be in line with the finding that, in contrast to C9orf72 mutation carriers, fat mass as the main energy store is not reduced in SOD1 mutation carriers.
In summary, we conclude that presymptomatic ALS gene carriers feature alterations in body composition, which indicate catabolism and a loss of metabolically active cells, resembling reduced physical activity, as well as reduced REE. We propose that the ECM/BCM ratio as well as the Phase Angle should be further explored as potential biomarkers for disease onset in presymptomatic SOD1 mutation carriers, while measuring BMI as a screening parameter and confirming a combined 4-compartment affection by BIA might serve the same purpose for C9orf72 mutation carriers. Furthermore, the MR may be used in both genetic subgroups to detect a presymptomatic hypometabolic state. These parameters may constitute valuable outcome measures in intervention studies and potentially guide earlier treatment decisions. As this was an exploratory study, further studies are needed to confirm our results, explore inter-device variability, further analyze longitudinal changes, and define adequate thresholds.
Contributors
Conceptualization: JD, PW, JHW, PMA, JS, FR, LD, HT, JK, ACL; Data Curation: JD, AH, AK, KG, KM, KF, PMA, FR, LD, HT; Formal Analysis: JD, BM; Investigation: JD, PW, DB, SW, KK, AH, CH, MW, AK, JP; Methodology: JD, BM, PW, JHW, PMA, JS, FR, LD, HT, JK, ACL; Project Administration: JD, PW, JK, ACL; Resources: PW, JHW, JP, KF, PMA, JS, HT, ACL; Supervision: JD, PW, JK, ACL; Visualization: JD, DB; Writing – original draft: JD; Writing – review and editing: JD, PW, DB, SW, KK, AH, CH, MW, AK, KG, KM, JHW, JP, KF, PMA, AR, JS, FR, LD, TH, BM, JK, ACL. JD and BM have directly accessed and verified the underlying data reported in the manuscript. All authors confirm that they read and approved the final version of the manuscript.
Data sharing statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, and figures) as well as the study protocol will be available. Data will be available beginning 3 months and ending 5 years following article publication. Data will be shared with researchers who provide a methodologically sound proposal. Data will be shared for analyses to achieve the aims in the approved proposal. Proposals should be directed to johannes.dorst@uni-ulm.de; to gain access, data requestors will need to sign a data access agreement. Data are available for 5 years at https://www.uniklinik-ulm.de/neurologie.html.
Declaration of interests
JD reports honoraria for presentations from Biogen and ITF Pharma.
PW reports grants or contracts from Alle Lieben Schmidt e.V., Boris Canessa Stiftung, and EHDN; consulting fees from Biogen, ITF Pharma, and Novartis.
DB reports stocks from Ionis pharmaceuticals.
PMA reports consultancies or advisory boards for Biogen, Roche, Avrion, Regeneron, uniQure and Orphazyme; clinical trial site investigator for Biogen, Alexion, Sanofi, Lilly AL-S Pharma, Amylyx, PTC Pharmaceuticals, Orion Pharma and Orphazyme; since 1993 Director of the ALS-genetic laboratory at Umeå University Hospital that performs clinical and research genetic testing; member of the ClinGen ALS Gene Curation Expert panel.
LD reports grants from Cytokinetics and Lecture Fees from Cytokinetics.
ACL reports grants or contracts from European Union (Horizon), BMBF, Deutsche Forschungsgemeinschaft (DFG), and Deutscher Akademischer Austauschdienst (DAAD) as well as sponsored trials by Amylyx, Ferrer International, Novartis Research and Development, Mitsubishi Tanabe, Apellis Pharmaceuticals, Alexion, Orion Pharma, Biogen, and Orphazyme; payment for talks from Biologix, the German Society of Neurology, Biogen, Springer Medicine, Amylyx, and Streamed Up! GmbH; support for attending meetings and/or travel from Biogen; participation on advisory boards from Roche Pharme, Biogen, Alextor, and Amylyx; President of Deutsche Neurowissenschaftliche Gesellschaft (NWG).
All other authors report no conflicts of interests.
Acknowledgements
We thank the study participants and their families, and the participating staff at the study center.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104521.
Appendix A. Supplementary data
References
- 1.Brettschneider J., Del Tredici K., Toledo J.B., et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38. doi: 10.1002/ana.23937. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desport J.C., Preux P.M., Magy L., et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2001;74(3):328–334. doi: 10.1093/ajcn/74.3.328. Epub 2001/08/28. [DOI] [PubMed] [Google Scholar]
- 3.Janse van Mantgem M.R., van Eijk R.P.A., van der Burgh H.K., et al. Prognostic value of weight loss in patients with amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2020;91(8):867–875. doi: 10.1136/jnnp-2020-322909. [DOI] [PubMed] [Google Scholar]
- 4.Peter R.S., Rosenbohm A., Dupuis L., et al. Life course body mass index and risk and prognosis of amyotrophic lateral sclerosis: results from the ALS registry Swabia. Eur J Epidemiol. 2017;32(10):901–908. doi: 10.1007/s10654-017-0318-z. [DOI] [PubMed] [Google Scholar]
- 5.Dupuis L., Corcia P., Fergani A., et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70(13):1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. Epub 2008/01/18. [DOI] [PubMed] [Google Scholar]
- 6.Xia K., Witzel S., Witzel C., et al. Mutation-specific metabolic profiles in presymptomatic amyotrophic lateral sclerosis. Eur J Neurol. 2022;28(10) doi: 10.1111/ene.15584. [DOI] [PubMed] [Google Scholar]
- 7.Pradat P.F., Bruneteau G., Gordon P.H., et al. Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11(1-2):166–171. doi: 10.3109/17482960902822960. Epub 2010/02/27. [DOI] [PubMed] [Google Scholar]
- 8.Dorst J., Kuhnlein P., Hendrich C., Kassubek J., Sperfeld A.D., Ludolph A.C. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. 2011;258(4):613–617. doi: 10.1007/s00415-010-5805-z. Epub 2010/12/04. [DOI] [PubMed] [Google Scholar]
- 9.Ludolph A.C., Dorst J., Dreyhaupt J., et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann Neurol. 2019;17(10) doi: 10.1002/ana.25661. [DOI] [PubMed] [Google Scholar]
- 10.Wills A.M., Hubbard J., Macklin E.A., et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;383(9934):2065–2072. doi: 10.1016/S0140-6736(14)60222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorst J., Doenz J., Kandler K., et al. Fat-rich versus carbohydrate-rich nutrition in ALS: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2022;93(3):298–302. doi: 10.1136/jnnp-2021-328331. [DOI] [PubMed] [Google Scholar]
- 12.Dorst J., Schuster J., Dreyhaupt J., et al. Effect of high-caloric nutrition on serum neurofilament light chain levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(9):1007–1009. doi: 10.1136/jnnp-2020-323372. Epub 2020 Aug 11. [DOI] [PubMed] [Google Scholar]
- 13.Miller T., Cudkowicz M., Shaw P.J., et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109–119. doi: 10.1056/NEJMoa2003715. [DOI] [PubMed] [Google Scholar]
- 14.Miller T., Cudkowicz M. American Neurological Association Annual Meeting (virtual); 2021. Results from the phase 3 VALOR study and its open-label extension: evaluating the clinical efficacy and safety of tofersen in adults with ALS and confirmed SOD1 mutation. [Google Scholar]
- 15.Steinacker P., Feneberg E., Weishaupt J., et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry. 2016;87(1):12–20. doi: 10.1136/jnnp-2015-311387. Epub 2015/08/25. [DOI] [PubMed] [Google Scholar]
- 16.Benatar M., Wuu J., Andersen P.M., Lombardi V., Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. 2018;84(1):130–139. doi: 10.1002/ana.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weydt P., Oeckl P., Huss A., et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79(1):152–158. doi: 10.1002/ana.24552. Epub 2015/11/04. [DOI] [PubMed] [Google Scholar]
- 18.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. Research Group on Motor Neuron Diseases. Epub 2001/07/24. [DOI] [PubMed] [Google Scholar]
- 19.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller S., Preische O., Sohrabi H.R., et al. Decreased body mass index in the preclinical stage of autosomal dominant Alzheimer's disease. Sci Rep. 2017;7(1):1225. doi: 10.1038/s41598-017-01327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchholz A.C., Bartok C., Schoeller D.A. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19(5):433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 22.Ruperto M., Barril G. The extracellular mass to body cell mass ratio as a predictor of mortality risk in hemodialysis patients. Nutrients. 2022;14(8):1659. doi: 10.3390/nu14081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staufer K., Halilbasic E., Hillebrand P., et al. Impact of nutritional status on pulmonary function after lung transplantation for cystic fibrosis. United European Gastroenterol J. 2018;6(7):1049–1055. doi: 10.1177/2050640618778381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott M., Fischer H., Polat H., et al. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9(1):20–25. [PubMed] [Google Scholar]
- 25.Desport J.C., Marin B., Funalot B., Preux P.M., Couratier P. Phase angle is a prognostic factor for survival in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9(5):273–278. doi: 10.1080/17482960801925039. [DOI] [PubMed] [Google Scholar]
- 26.Haugen H.A., Chan L.N., Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22(4):377–388. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 27.Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6(3):213–221. [PubMed] [Google Scholar]
- 28.Harris J.A., Benedict F.G. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbohm A., Peter R., Dorst J., et al. Life course of physical activity and risk and prognosis of amyotrophic lateral sclerosis in a German ALS registry. Neurology. 2021;97(19):e1955–e1963. doi: 10.1212/WNL.0000000000012829. [DOI] [PubMed] [Google Scholar]
- 30.Desport J.C., Preux P.M., Truong T.C., Vallat J.M., Sautereau D., Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53(5):1059–1063. doi: 10.1212/wnl.53.5.1059. Epub 1999/09/25. [DOI] [PubMed] [Google Scholar]
- 31.Dorst J., Cypionka J., Ludolph A.C. High-caloric food supplements in the treatment of amyotrophic lateral sclerosis: a prospective interventional study. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7-8):533–536. doi: 10.3109/21678421.2013.823999. Epub 2013/08/16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.