Abstract
Objective
The purpose of this study was to evaluate the time-lapse of periodontal regeneration surgery of combined periodontal-endodontic lesions (PEL) after root canal therapy (RCT) to guide the clinical treatment.
Methods
26 patients (28 teeth) with severe combined PEL were equally divided into 4 groups (n = 7); the control group included patients who underwent periodontal regeneration surgery with no prior RCT and the remaining three experimental groups including patients who received periodontal regeneration surgery post-RCT either immediately or after 3 and 6 months. The probing depth, clinical attachment loss, and periodontal bone density were measured before or after 3, 6, and 12 months post-RCT, respectively.
Results
Periodontal regeneration surgery could improve the PD (Probing Depth), CAL (Clinical Attachment Loss), BD (Bone Mineral Density) values irrespective of whether the RCT was performed within 12 months or not. However, obviously improved PD, CAL and BD were observed when surgery was performed post-RCT. The time lapse between RCT and periodontal regeneration surgery had no obvious effects on the periodontal index in 3 months after the surgery. Moreover, these periodontal indexes tend to stabilize in 3 to 6 months after the surgery with no significant differences.
Conclusion
Although there were no obvious impacts of time lapse between RCT and periodontal regeneration surgery on the severe PEL, an earlier periodontal surgery might contribute to the healing of periodontal lesions.
Keywords: Periodontal-endodontic lesions, Periodontal regeneration surgery, Root canal therapy, Time lapse
Abbreviations: PEL, Combined periodontal-endodontic lesions; RCT, Root canal therapy; PD, Probing Depth; CAL, Clinical Attachment Loss; BD, Bone Mineral Density
1. Introduction
The periodontal-endodontic lesion is one of the most common complications of severe periodontitis and one of the main factors leading to tooth loss (Fang et al., 2021, Gomes et al., 2022). Periodontal tissue and dental pulp tissue are anatomically connected resulting in similar infectious microorganisms between the periodontal pocket and dental pulp (AlJasser et al., 2021). Previous studies reported that toxins and bacteria present in the pulp infect periodontal tissue via the dentinal tubule and lateral canal, thus inducing damage in periodontal tissue (Sharma et al., 2014). Meanwhile, the periodontal tissue lesions in deep periodontal pockets aggravate the formation of combined PEL (Oh et al., 2009). Furthermore, reports suggest that infections from the teeth fold, wedge-shaped defects, and deep caries might also induce inflammation in apical tissue (Rotstein and Simon 2004). The PEL involve pulp tissue, periodontal tissue, and periapical tissue which increases the diversity of clinical manifestations, and difficulties in clinical treatment (Fang et al., 2021). The American Academy of Periodontology and the European Federation of Periodontology, in 2018 classified PEL based on symptoms, treatment, and prognosis (Al-Fouzan 2014). For the PEL patients with periodontitis, the long onset of the cycle, a complication of etiology, and clinical manifestations increased the difficulties in the treatment and affected the quality of life of patients (Papapanou et al., 2018). The treatment mainly included periodontal therapy and root canal therapy (RCT) followed by regenerative surgery in the later stages of treatment. Our previous studies showed that patients with severe PEL receiving RCT significantly promoted the effect of periodontal regeneration surgery(Jia Yan 2018); however, time lapse after RCT remained elusive. Therefore, the present study investigated the time-lapse of periodontal regeneration surgery of PEL after RCT.
2. Materials and methods
2.1. Clinical data
This study was performed in Tianjin Stomatological Hospital, Tianjin, China and approved by the ethics committee of Tianjin Stomatological Hospital, and all patients signed their informed consent. The clinical data of patients with PEL from January 2018 to January 2019 were retrospectively collected from our department. The inclusion and exclusion criteria were listed as follows:
Inclusion criteria: ①Clinically diagnosed as periodontal or real PEL with complete clinical data; ②No obvious dental tissue defect, the pulp vitality test showed the pulp was normal or partial reduction; ③ Periodontal inflammation was stable 6–8 weeks after basic periodontal treatment; ④ The affected tooth with a deep periodontal pockets or furcation lesions, and at least one site has a probing depth (PD) > 6 mm or clinical attachment loss (CAL) > 4 mm; ⑤ The degree of looseness of the affected tooth does not exceed II°; ⑥ X-ray films show that the vertical resorption of the alveolar bone in at least one site of the affected tooth exceeds 1/3 of the root length, and the periodontal ligament were irregularly widen in the apical area.
Exclusion criteria: ① The patients were suffering from systemic diseases; ② The patients were pregnancy or taking contraceptives; ③ The patients were receiving basic periodontal therapy within 12 months; taking antibiotics, corticosteroids, non-steroidal anti-inflammatory drugs or bisphosphonates within 3 months Salt drugs; ④The affected tooth has other dental pulp diseases; ⑤ The patients were smoking over 20 cigarettes per day.
A total of 26 patients with 28 teeth (12 anterior teeth and 16 posterior teeth) were included in our study (Table S1). All the patients were divided into 4 groups based on whether they received RCT prior or not; the control group included patients who underwent periodontal regeneration surgery with no prior RCT and three experimental groups included patients who underwent periodontal bone grafting post-RCT. The experimental groups were divided into three groups based on the timing of periodontal regeneration; The periodontal bone graft group immediately after RCT (group RCT-0), the periodontal bone graft group 3 months after RCT (group RCT-3), and the periodontal bone graft group 6 months after RCT (group RCT-6), with 7 cases in each group. There was no statistical difference in age and gender between the groups.
2.2. Treatment methods
Periodontal regeneration surgery: The tooth was initially anaesthetized with local infiltration anesthesia. The modified Widman flap surgery was performed in our study. An oblique incision was internally made to excise the epithelium of the inner wall of the dental pocket to open the mucoperiosteal flap. Then debrided the granulation tissue and debris in the root surface, apical area, and bone defect area thoroughly. The apical bone defect was implanted with Bio-oss bone powder (Geistlich, Switzerland), and covered it using Bio-gide membrane (Geistlich, Switzerland), with a 3 mm thickness beyond the edge of the bone defect. The gingival flap was coronally restored, and the wound was tightly sutured without tension. Finally, the periodontal plug was placed to protect the wound. Cephalosporin and metronidazole were used to prevent infections after the surgery followed by removal of sutures after 2 weeks post-surgery. The experimental groups were also undergoing conventional root canal preparation and hot gutta-percha filling. The periodontal regenerative surgery and RCT were performed by the same periodontist and dentist, respectively.
2.3. Efficacy evaluation
The periodontal clinical indicators such as PD and CAL were examined by Williams periodontal probe. PD and CAL were used to examine the buccal and lingual mesial, central and distal sites. Bone density measurement: Import the X-ray film into photoshop software to measure the bone density around the root of the tooth (gray value of the bone defect area). Briefly, selected an experimental observation area (region of interest, ROI) of the same area in the postoperative X-ray film, and recorded the gray value. The bone density was represented by gray value.
2.4. Statistical analysis
Statistical analysis of all data was performed using SPSS 17.0. One-way ANOVA was used for the evaluation of clinical indicators such as PD, CAL and bone mineral density, within and between groups, and the statistical results were expressed in the form of mean ± standard deviation, P < 0.05, indicating that the difference was statistically significant.
3. Results
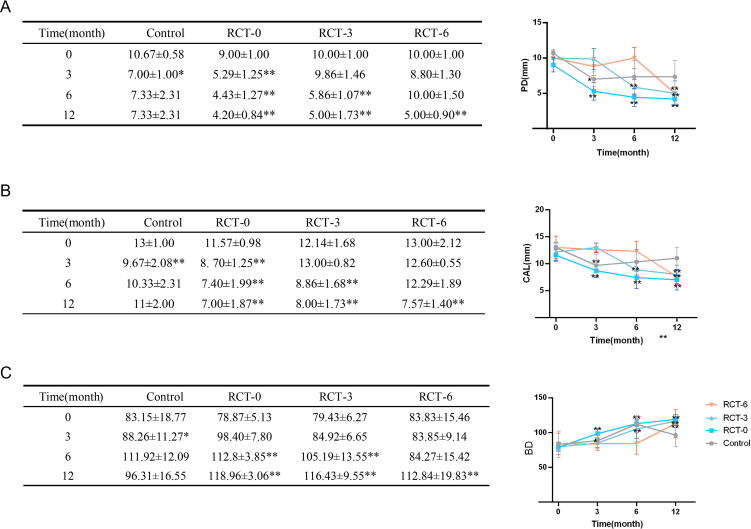
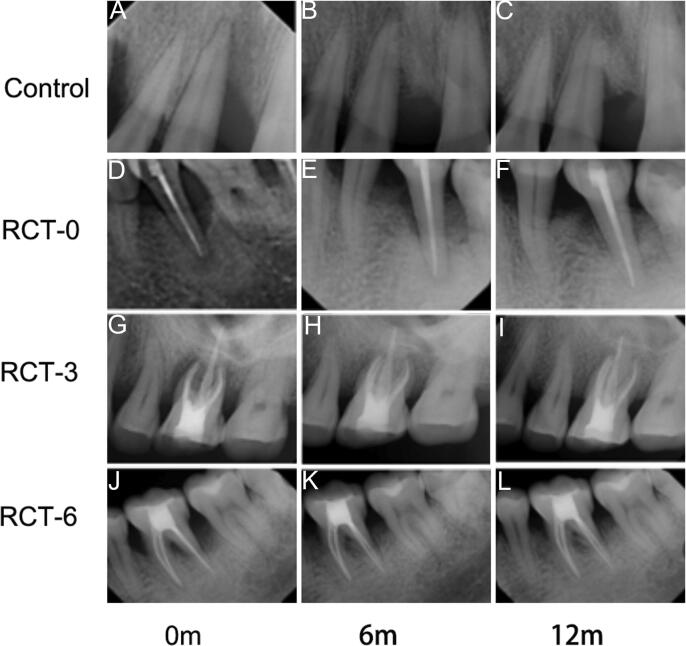
We observed that periodontal surgery significantly reduced the PD (Fig. 1A), CAL (Fig. 1B), and BD (Fig. 1C) values irrespective of whether RCT was performed within the 12-month observation period. Moreover, when periodontal surgery was performed post-RCT, the PD, CAL, and BD values were significantly decreased with greater effect (P < 0.05) and suggested that the RCT treatment could improve the effect of periodontal surgery. However, no significant differences in PD, CAL, and BD values were observed in the experimental groups 3 months after RCT. The groups RCT-0 and RCT-3 were found to be stable with no significant changes between 3 and 6 months after RCT. Furthermore, we noted that the PD values in group RCT-6 decreased during the first 3 months and then increased in the next 3 months, thus indicating that a prolonged duration of RCT might impair the effects of periodontal treatment with no significant differences (Fig. 1). Furthermore, no significant changes were observed in the CAL values in 3 and 6 months after RCT in each group. The periapical radiograph also showed obvious new bone regeneration in RCT-0, RCT-3 and RCT-12 groups while became stable after the surgery (Fig. 2).
Fig. 1.
The periodontal clinical indicators in the patients. Comparison of PD (A), CAL (B) and BD (C) before and after periodontal regeneration (12-month observation period after RCT); * p < 0.05, ** p < 0.01. PD: Probing Depth, CAL: Clinical Attachment Loss, BD: Bone Mineral Density.
Fig. 2.
Representative X-ray pictures before or after RCT in 6 and 12 months (A-C) The control group: This 45-year-old male presented with a vertical bone resorption in teeth #11 (FDI) in the preoperative periapical radiograph. The patient was performed regeneration surgery directly without the RCT treatment. (D-F) The RCT-0 group: This 53-year-old male presented with a vertical bone resorption in teeth #35 (FDI) in the preoperative periapical radiograph. (G-I) The RCT-3 group: This 49-year-old male presented with a vertical bone resorption in teeth #26 (FDI) in the preoperative periapical radiograph. (J-L) The RCT-6 group: This 51-year-old male presented with a vertical bone resorption in teeth #36 (FDI) in the preoperative periapical radiograph.
4. Discussion
Periodontal tissue, pulp tissue, and periapical tissue are anatomically connected (Sun et al., 2021). Therefore, infectious microbes in the periodontal lesions and pulp can interact and spread to each other, accelerating the disease progression and eventually leading to the occurrence of combined lesions (Cho et al., 2017). At present, the treatment of PEL disease mainly includes periodontal therapy and RCT (Vakalis et al., 2005). Perfect root canal treatment could completely remove the infections in the root canal and effectively seal the root canal so that the residual infected microorganisms in the root canal could be prevented from re-infecting the tooth through the apical foramen (Xiong et al., 2022). This blocks the influence of infection and creates a stable environment for periapical and periodontal tissue regeneration (Pourhajibagher et al., 2016). However, root canal therapy alone could not completely remove periodontal lesions, and the prognosis of combined PELs depends largely on the effect of periodontal therapy. A previous study reported that for the severe periodontal origin and coexisting PEL, periodontal inflammation could be not ideally controlled after the basic pulp and periodontal treatment, and periodontal surgery was still required (Sunitha et al., 2008). Surgery treatment could significantly reduce probing depth and attachment loss; increase bone density and bone mass; obtain a good and stable clinical effect, indicating that periodontal regeneration surgery was beneficial to periodontal restoration in combined lesions.
The present study found that PD and CAL were significantly lower in patients with severe PEL than those before surgery, and bone mineral density was also significantly higher. No significant difference in PD and CAL values were found in 1 year after the surgery. This indicated that RCT combined with periodontal regenerative surgery could significantly inhibit the development of combined PEL and promoted the formation of new bone and attachments. Lima et al. reported that RCT after periodontal regenerative surgery hindered the formation of cementum, periodontal attachment, and alveolar bone(Lima et al., 1997). Proper removal of the tissue in the infected root canal and strict root canal filling could prevent the microorganisms in the infected root canal from re-infecting the periodontal tissue. Therefore, RCT should be performed as soon as possible before periodontal regeneration to maintain the effect of periodontal regeneration in a long-term and stable manner. However, at present, research on the timing of periodontal regeneration after RCT is relatively rare. The experimental group of this study analyzed the timing of periodontal regeneration after RCT. The PD and CAL values were significantly decreased, whereas the alveolar bone mineral density was significantly increased. The periodontal regeneration surgery could significantly improve the above indicators and attenuate the progress of periodontal inflammation, thus, finally promoting the regeneration and repair of periodontal tissue. Few studies suggest that RCT should be performed first for affected teeth with severe PEL, and periodontal surgery should be performed at least 3 months after RCT to give sufficient pulp to the teeth. This provided enough time for pulp restoration and avoided the cementum destruction caused by periodontal scaling that entered the root canal and its impact on periodontal healing (Lima et al., 1997). However, a previous study reported that periodontal surgery performed immediately after RCT could hinder the formation of new bone, cementum, and attachments. Besides, Danesh et al. reported that root canal therapy combined with periodontal regeneration surgery to treat PEL could achieve ideal treatment effects (Abbott, 1998, Oh et al., 2009, Danesh et al., 2011, Miao et al., 2015). Although this study found that there was no significant difference in the periodontal clinical indicators (PD, CAL, BD) immediately after RCT in 3 or 6 months after periodontal regenerative surgery. The long-term effects (6 months) were found to be stabilized. However, it was assumed that early periodontal surgery was suggested to obtain periodontal tissue regeneration as soon as possible. The limitations of the present study were the use of a smaller number of cases and limited observation time. The clinical effect of the treatment still needs to expand to clinical samples, and long-term follow-up to evaluate the long-term effect which will provide a basis for the diagnosis and treatment of severe PEL and preserve diseased teeth to a greater extent.
5. Conclusion
Although there were no obvious impacts of time lapse between RCT and periodontal regeneration surgery on the severe PEL, an earlier periodontal surgery might contribute to the healing of periodontal lesions.
Ethical statement
This study was approved by the Ethics Committee of Tianjin Stomatological Hospital, and all patients signed informed consent.
Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. The authors declare no conflict of interest.
Acknowledgment
This research was funded by the Tianjin Stomatological Hospital Key Project (2021KLMS07) and Tianjin Key Medical Discipline (Specialty) Construction Project (WKZKT02).
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2022.12.009.
Contributor Information
Yan Zhang, Email: dentistzy@126.com.
Xinyue Li, Email: lxy_tianjinkqyy@163.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abbott P. Endodontic management of combined endodontic-periodontal lesions. J. N. Z. Soc. Periodontol. 1998:15–28. [PubMed] [Google Scholar]
- Al-Fouzan K.S. A new classification of endodontic-periodontal lesions. Int J Dent. 2014;2014 doi: 10.1155/2014/919173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlJasser R., Bukhary S., AlSarhan M., et al. Regenerative Therapy Modality for Treatment of True Combined Endodontic-Periodontal Lesions: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18126220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.D., Lee J.E., Chung Y., et al. Collaborative Management of Combined Periodontal-endodontic Lesions with a Palatogingival Groove: A Case Series. J Endod. 2017;43:332–337. doi: 10.1016/j.joen.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Danesh F., Karamifar K., Abbott P.V. Management of an extensive invasive root resorptive lesion with mineral trioxide aggregate: a case report. J Oral Sci. 2011;53:397–401. doi: 10.2334/josnusd.53.397. [DOI] [PubMed] [Google Scholar]
- Fang F., Gao B., He T., et al. Efficacy of root canal therapy combined with basic periodontal therapy and its impact on inflammatory responses in patients with combined periodontal-endodontic lesions. Am. J. Transl. Res. 2021;13:14149–14156. [PMC free article] [PubMed] [Google Scholar]
- Gomes B., Berber V., Marinho A., et al. Chemomechanical preparation influences the microbial community and the levels of LPS, LTA and cytokines in combined endodontic-periodontal lesions: A clinical study. J. Periodontal Res. 2022;57:341–356. doi: 10.1111/jre.12964. [DOI] [PubMed] [Google Scholar]
- Jia Yan, L. X., 2018. The effects of endodontic treatment on the regeneration of severe combined periodontal endodontic lesions. Chinese Journal of Conservative Dentistry. 28, 330-335. https://doi.org/10.
- Lima L.A., Anderson G.B., Wang M.M., et al. Healing of intrabony defects and its relationship to root canal therapy. A histologic and histometric study in dogs. J Periodontol. 1997;68:240–248. doi: 10.1902/jop.1997.68.3.240. [DOI] [PubMed] [Google Scholar]
- Miao H., Chen M., Otgonbayar T., et al. Papillary reconstruction and guided tissue regeneration for combined periodontal-endodontic lesions caused by palatogingival groove and additional root: a case report. Clin Case Rep. 2015;3:1042–1049. doi: 10.1002/ccr3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S., Fouad A., Park S. Treatment strategy for guided tissue regeneration in combined endodontic-periodontal lesions: case report and review. J. Endod. 2009;35:1331–1336. doi: 10.1016/j.joen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Papapanou P.N., Sanz M., Buduneli N., et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/jper.17-0721. [DOI] [PubMed] [Google Scholar]
- Pourhajibagher M., Chiniforush N., Raoofian R., et al. Effects of sub-lethal doses of photo-activated disinfection against Porphyromonas gingivalis for pharmaceutical treatment of periodontal-endodontic lesions. Photodiagnosis Photodyn Ther. 2016;16:50–53. doi: 10.1016/j.pdpdt.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Rotstein I., Simon J. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontology. 2004;2000(34):165–203. doi: 10.1046/j.0906-6713.2003.003431.x. [DOI] [PubMed] [Google Scholar]
- Sharma R., Hegde V., Siddharth M., et al. Endodontic-periodontal microsurgery for combined endodontic-periodontal lesions: An overview. Journal of conservative dentistry : JCD. 2014;17:510–516. doi: 10.4103/0972-0707.144571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Guo Z., Guo D., et al. The Microbiota Profile Analysis of Combined Periodontal-Endodontic Lesions Using 16S rRNA Next-Generation Sequencing. J Immunol Res. 2021;2021:2490064. doi: 10.1155/2021/2490064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunitha V.R., Emmadi P., Namasivayam A., et al. The periodontal - endodontic continuum: A review. J Conserv Dent. 2008;11:54–62. doi: 10.4103/0972-0707.44046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalis S.V., Whitworth J.M., Ellwood R.P., et al. A pilot study of treatment of periodontal-endodontic lesions. Int Dent J. 2005;55:313–318. doi: 10.1111/j.1875-595x.2005.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Gu F., Xiang J., et al. Cementodentinal Tear Associated with a Periodontal- Endodontic Combined Lesion: A Case Report with a 14-Month Follow-up. Int J Periodontics Restorative Dent. 2022;42:e27–e32. doi: 10.11607/prd.5555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.