Abstract
Background
Information about the adherence to scientific societies guidelines in the ‘real-world’ therapeutic management of oncological patients are lacking. This multicenter, prospective survey was aimed to improve the knowledge relative to 2017-2018 recommendations of the Italian Association of Medical Oncology (AIOM).
Patients and methods
Treatment-naive adult patients with pancreatic adenocarcinoma were enrolled. Group A received adjuvant therapy, group B received primary chemotherapy, and group C had metastatic disease. The results on patients accrued until 31 October 2019 with a mature follow-up were presented.
Results
Since July 2017, 833 eligible patients of 923 (90%) were enrolled in 44 Italian centers. The median age was 69 years (range 36-89 years; 24% >75 years); 48% were female; 93% had Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 or 1; group A: 16%, group B: 30%; group C: 54%; 72% Nord, 13% Center, 15% South. In group A, guidelines adherence was 68% [95% confidence interval (CI) 59% to 76%]; 53% of patients received gemcitabine and 15% gemcitabine + capecitabine; median CA19.9 was 29 (range 0-7300; not reported 15%); median survival was 36.4 months (95% CI 27.5-47.3 months). In group B, guidelines adherence was 96% (95% CI 92% to 98%); 55% of patients received nab-paclitaxel + gemcitabine, 27% FOLFIRINOX, 12% gemcitabine, and 3% clinical trial; median CA19.9 was 337 (range 0-20220; not reported 9%); median survival was 18.1 months (95% CI 15.6-19.9 months). In group C, guidelines adherence was 96% (95% CI 94% to 98%); 71% of patients received nab-paclitaxel + gemcitabine, 16% gemcitabine, 8% FOLFIRINOX, and 4% clinical trial; liver and lung metastases were reported in 76% and 23% of patients, respectively; median CA19.9 value was 760 (range 0-1374500; not reported 9%); median survival was 10.0 months (95% CI 9.1-11.1 months).
Conclusions
The GARIBALDI survey shows a very high rate of adherence to guidelines and survival outcome in line with the literature. CA19.9 testing should be enhanced; nutritional and psychological counseling represent an unmet need. Enrollment to assess adherence to updated AIOM guidelines is ongoing.
Key words: pancreatic adenocarcinoma, adjuvant, first line, prospective survey, adherence to guidelines
Highlights
-
•
This survey evaluates the agreement with national recommendations included in 2017-2018 AIOM guidelines.
-
•
A total of 833 eligible patients with pancreatic adenocarcinoma were included; grouped in 3 settings, according to the type of treatment.
-
•
The study shows a very high rate of adherence to guidelines and survival outcome in line with the literature.
Introduction
Pancreatic adenocarcinoma is a rare, poorly understood, and difficult-to-manage disease for a number of reasons, including genetic heterogeneity, location of the tumor, imaging drawbacks, delayed diagnosis, early metastatization, lack of biomarkers, inherited or rapidly acquired resistance to therapies, high rate of tumor- and treatment-related complications requiring a complex multidisciplinary approach, and, last but not least, therapeutic nihilism.
In the past 15 years, improvements in systemic chemotherapy have encouraged interest in the field and expanded the therapeutic armamentarium. Accordingly, a constant update of medical knowledge, a critical analysis of trials design and results, and a continuous revision of therapeutic guidelines are crucial to handover progress in the clinical practice in real time.
The Italian Association of Medical Oncology (AIOM) Pancreatic Adenocarcinoma Working Group, involving oncologists, pancreatic surgeons, radiologists, gastroenterologists, pathologists, oncologist nurses, and patients with cancer, is committed in a meticulous and careful process of development of clinical guidelines.1 This process includes a literature research on MEDLINE and EMBASE databases, a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 flow selection of manuscripts, and a Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to classify the quality of each kind of evidence. Guidelines also take into account the indications of the Italian Drug Agency (AIFA) that regulates the prescription of drugs and include a peer and methodological review and an annual update at the end of October. Noteworthy, guidelines analyze only fully published manuscripts to warrant a more thorough and exhaustive assessment process of trial results according to the GRADE methodology.
Information about the level of acknowledgement and application of expert recommendations out in the country is lacking. The present survey was aimed to collect and analyze data on the therapeutic management of patients with pancreatic adenocarcinoma in a wide patient and center population to inform the Society about the need for educational and training programs, health policy interventions, and outcome figures in a real-world context as compared with the clinical trials population.
Patients and methods
The primary aim of this Italian, multicenter, prospective, noninterventional survey was to describe the pattern of clinical management for treatment-naive patients with pancreatic adenocarcinoma undergoing either a first-line of medical therapy or observation and evaluate the agreement with national recommendations included in 2017-2018 AIOM guidelines. As secondary aims, to describe the overall survival (OS), characteristics of medical centers, and types of medical practices. The survey was divided prospectively in two periods because national recommendations statements changed in 2019. The first period is presented here and includes patients treated until 31 October 2019, referring to 2017 and 2018 AIOM guidelines; the second period, following 2019-2021 AIOM guidelines, was closed on November 2022.
Institutions that were invited to participate into the survey were selected to adequately represent different geographical and expertise areas, namely, the number of invited centers per geographic region was proportional to the local population. Centers were also selected based on the number of attending oncologists per center to warrant a balanced coverage of small- (<10 oncologists; n = 13), medium- (10-20 oncologists; n = 18), and large-sized (>20 oncologists; n = 14) institutions. Furthermore, institutions were classified based on self-declared annual number of patients with pancreatic adenocarcinoma managed in the center as ‘high volume’ (>50 treated patients/year), ‘medium volume’ (25-50 treated patients/year), and ‘low volume’ (<25 treated patients/year).
According to patients’ inclusion criteria, all chemotherapy and radiotherapy-naive patients aged ≥18 years who signed the study informed consent and had a pathological diagnosis of pancreatic ductal adenocarcinoma, receiving active follow-up or treatment in the participating institutions, irrespective of stage, therapeutic management, and performance status, were eligible for this survey. Patients with prior surgery or other previous or concomitant malignancies were eligible.
The GARIBALDI study complied with the Declaration of Helsinki and was conducted per good clinical practice guidelines; it was approved by the Ethics Committees of all study sites. All patients provided written informed consent before enrollment.
For the purpose of the primary analysis, because (i) the surgical classification distinguishing patients with ‘resectable’, ‘borderline resectable’, and unresectable’ disease, apart from lacking prognostic validation, is irreproducible among selected high-volume centers and even more so among the oncological centers involved in this study2; (ii) it was unrealistic to centrally review all the CT scans to verify the correct surgical category; (iii) the purpose of the GARIBALDI study was to assess guideline adherence rather than to compare groups’ outcome; (iv) guidelines allow either surgery or chemotherapy upfront in nonmetastatic disease; and (v) the oncological centers involved in the study also enrolled patients who were addressed after potential surgery, patients were pragmatically categorized into three different subgroups of populations: (i) those receiving adjuvant therapy after resection (group A); (ii) those receiving primary chemotherapy for nonmetastatic disease (group B); and (iii) metastatic patients (group C). Because of the noninterventional design of the study, the treatment choice was related only to the attending oncologist’s decision.
As per primary endpoint, the percentage of patients managed according to 2017-2018 AIOM guidelines, was assessed separately for the three subgroups, namely, enrollment into a clinical trial, gemcitabine3 and gemcitabine–capecitabine4 adjuvant therapy being the options recommended by guidelines in group A, while mFOLFIRINOX5 was included only in 2019 AIOM guidelines; enrollment into a clinical trial, gemcitabine,6 FOLFIRINOX,7 nab-paclitaxel–gemcitabine combination,8 and PEXG (cisplatin, epirubicin, capecitabine, gemcitabine) regimen9,10 being the options recommended by guidelines for patients in groups B and C, while the nab-paclitaxel in combination with cisplatin, capecitabine, and gemcitabine (PAXG) regimen11,12 was included only in 2019 AIOM guidelines.
Patients’ characteristics and journey (age, sex, PS, previous cancer history, home-to-hospital distance, first consulted physician), diagnostic process (diagnosis–treatment interval, percentage of patients with baseline CA19.9 testing), tumor characteristics (stage and site of disease, CA19.9 value, site of metastasis), center characteristics (size, self-declared volume, geographical region, academic/community, multidisciplinary team presence and composition), and treatment characteristics (percentage of patients receiving nutritional and psychologic counseling, percentage of patients enrolled into a clinical interventional trial) were assessed as secondary outcomes.
Sample size calculation was not based on formal hypothesis testing, rather on consideration related to timing and representativeness of centers. We planned to enroll ∼1000 patients for each period of the survey to provide narrow confidence intervals around the estimation of the primary endpoint (i.e. adherence to guideline recommendations). We expected that the prevalence of each subgroup varied from 20% to 60% of the overall population. Therefore estimating a guideline adherence between 50% and 90%, the width of exact 95% confidence interval of agreement in each group was expected to vary from 5% to 14%.
Adherence to therapeutic guidelines was estimated by exact methods. Baseline covariate distributions were summarized using descriptive statistics (median and range for continuous variables, and absolute and percentage frequencies for categorical variables). Survival distributions were estimated by the Kaplan–Meier method. OS times were calculated from the date of first administration of medical therapy to death from any cause. The survival status was updated in June 2022. For patients alive at the update of survival status, survival data were right-censored to the date of last information available.
After obtaining the informed consent to study participation and data processing, eligible patients were centrally registered by a web system, accessible 24 h a day at http://GARIBALDI.aiom.it. The complete electronic case report form (eCRF) is presented in Supplementary Material S1, available at https://doi.org/10.1016/j.esmoop.2022.100777.
All registered patients received a unique identification number before any study specific procedures was performed.
Data collected were pseudonymized to guarantee the protection of privacy as for D.Lgs. 196/2003 and Del n. 52, 24 July 2008 and for the GDPR 679/16—‘European regulation on the protection of personal data’. Data collection was electronically carried out throughout using a remote data entry process and complied with good clinical practice procedures, allowing integrity and transparency of data and maintaining memory of the changes done. Most monitoring activities were centralized by systematically checking each reported information for consistency, completeness, and accuracy by the coordinating data center that, if appropriate, issued data clarification forms (DCFs).
This survey was sponsored by AIOM that played the role of not-for-profit sponsor. It was supported by Celgene Italia with an unrestricted economical support for costs related to data collection and management, generation of eCRF for remote data entry, data quality control, central and local monitoring, and statistical analysis. Celgene Italia had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication.
Results
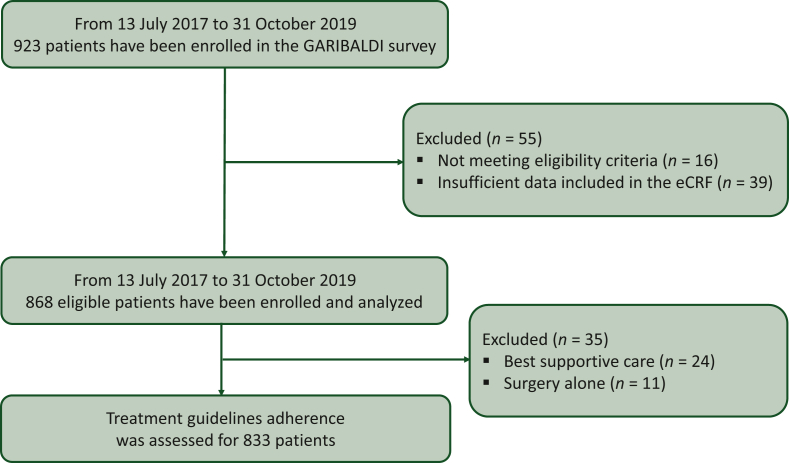
Between 13 July 2017 and 31 October 2019, 923 patients were enrolled by 45 Italian centers. A total of 55 patients were excluded because they were ineligible (n = 16, 2%) or had insufficient data included in eCRF (n = 39, 4%). Previous cancer history was reported for 147 patients (17.0%; 2 missing) mainly comprising those with breast cancer (n = 38, 25.9%), prostate cancer (n = 19, 12.9%), and colon cancer (n = 10, 6.8%). Prior cancer preceded the diagnosis of pancreatic cancer by a median of 8.1 years (n = 129; Q1–Q3 = 2.1-16.4 years). The median home to hospital distance was 15.0 km (Q1–Q3 = 6-32 km; 34 missing). The first consulted physician was the general practitioner in 30% (254/850), the ward physician in 22% (184/850), the surgeon in 16% (140/850), and the gastroenterologist in 13% (108/850) of cases. The oncologist was consulted upfront by only 2% (17/850) of patients. The median interval between first physician consultation and pathologic diagnosis was 21.0 days (Q1–Q3 = 8-51/745 patients) and that between pathologic diagnosis and treatment start (only for groups B and C) was 26.0 days (Q1–Q3 = 16-38/647 patients). Most institutions (n = 26; 58%) were located in the Northern part of the country, 11 (24%) in the Center, and 8 (18%) in the South. The relative resident population in the three regions is 46.5%, 19.9%, and 33.6%, respectively, of the national population (https://www.istat.it). Fourteen (31%) centers were classified as large, 18 (40%) as medium, and 13 (29%) as small size. Self-defined high-volume institutions were 18 (43%), medium volume 16 (38%), and low volume 8 (19%; self-definition was not provided by three centers). Academic centers were 36% (16/45), while community centers were 64% (N = 29/45). A multidisciplinary team was present in 86% of institutions (n = 38; 1 missing).
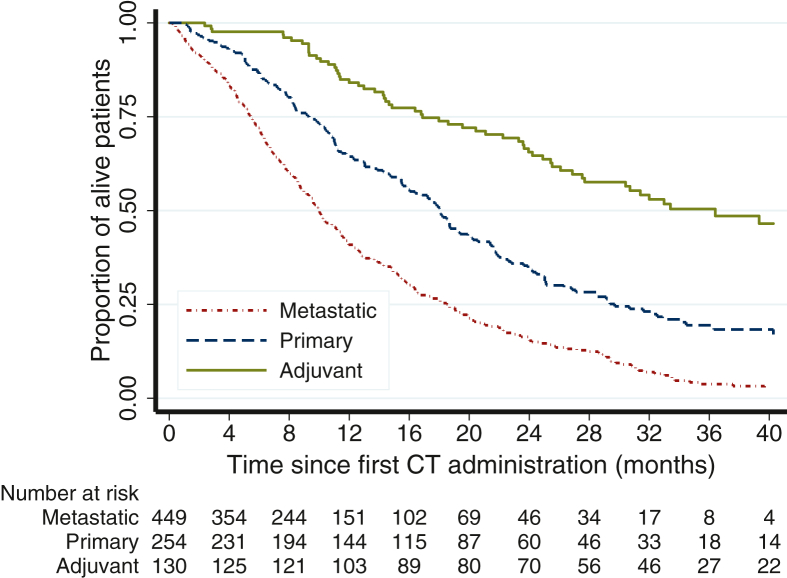
Twenty-four patients (3%) who were addressed to best supportive care and 11 (1%) who received surgery alone were excluded from further analysis due to the small uninformative subset. Altogether, 833 patients (90%) were considered eligible for primary analysis (Figure 1). Patients’ and tumor characteristics are summarized in Table 1. Treatment characteristics by group are reported in Table 2. Participation into a clinical interventional trial was 0% (0/129 patients; 1 missing) for group A, 2.8% (7/253 patients; 1 missing) for group B, and 4.0% (18/448 patients; 1 missing) for group C. Treatment guidelines adherence was 88/129 [68%; 95% confidence interval (CI) 59% to 76%; 1 missing] in group A, 243/254 (96%; 95% CI 92% to 98%) in group B, 432/448 (96%; 95% CI 94% to 98%; 1 missing) in group C. The likelihood of guideline violation was higher in high-volume centers [>50 pancreatic patients/year: 52/498 patients (10.4%); 25-50 pancreatic patients/year: 9/204 patients (4.4%); <25 pancreatic patients/year: 5/92 patients (5.4%); Cochran–Armitage test for trend, P = 0.014], in central and southern Italy [North: 42/605 patients (6.9%); Center: 11/107 patients (10.3%); South: 15/119 (12.6%); Cochran-Armitage test for trend, P = 0.026], and in academic centers [Academy: 43/392 patients (11.0%); Community: 25/439 patients (5.7%); chi-square test, P = 0.006]. Nutritional counseling was recommended to 24/126 (19.0%) patients in group A, 56/249 (22.5%) in group B, and 77/426 (18.1%) in group C. Nutritional counseling was lower in southern Italy [North: 125/595 patients (21.0%); Center: 19/93 patients (20.4%); South: 13/115 patients (11.3%); Cochran–Armitage test for trend, P = 0.026]. Nutritional counseling was not statistically different for self-declared volume and academic/community centers (data not shown). Psychological counseling was recommended to 11/125 (8.8%) patients in group A, 16/250 (6.4%) patients in group B, and 40/427 (9.4%) patients in group C. Psychological counseling was lower in central and southern Italy [North: 60/595 patients (10.1%); Center: 4/93 patients (4.3%); South: 3/116 (2.6%); Cochran–Armitage test for trend, P =0.003] and in community centers [Academy: 43/390 patients (11.0%); Community: 24/414 patients (5.8%); chi-square test, P = 0.007]. Psychological counseling was not statistically different for self-declared volume (data not shown). After a median [interquartile range (IQR)] of 34.5 (27.5-44.5) months, 60/130 (46.2%) were deceased in group A. The median OS was 36.4 (27.5-47.3) months. After a median (IQR) of 34.5 (22.3-43.8) months, 173/254 (68.1%) were deceased in group B. The median OS was 18.1 (15.6-19.9) months. After a median (IQR) of 33.3 (16.9-41.6) months, 361/449 (80.4%) were deceased in group C. The median OS was 10.0 (9.1-11.1) months (Figure 2).
Figure 1.
GARIBALDI flow diagram.
eCRF, electronic case report form.
Table 1.
Characteristics of the patients and tumors
| Group |
||||
|---|---|---|---|---|
| Metastatic | Primary | Adjuvant | ||
| Age | N | 449 | 253 | 130 |
| Median | 68.8 | 68.6 | 68.2 | |
| Range | 35.9-89.1 | 35.6-88.8 | 44.5-87.5 | |
| Missing | 0 | 1 (0.4) | 0 | |
| >75 years | n (%) | 93 (21) | 72 (28) | 33 (25) |
| Sex | ||||
| Female | n (%) | 204 (45) | 133 (52) | 61 (47) |
| Male | n (%) | 245 (55) | 121 (48) | 69 (53) |
| ECOG PS | ||||
| 0 | n (%) | 222 (51) | 134 (53) | 80 (62) |
| 1 | n (%) | 177 (41) | 106 (42) | 37 (29) |
| 2 | n (%) | 36 (8) | 10 (4) | 12 (9) |
| 3 | n (%) | 2 (<1) | 1 (<1) | 0 |
| Missing | 12 (2.7) | 3 (1.2) | 1 (0.8) | |
| CA19.9 | n | 408 | 232 | 110 |
| Median | 759.5 | 336.5 | 28.5 | |
| Q1–Q3 | 80.5-6880.0 | 62.5-1348.5 | 10.0-192.0 | |
| Missing | 41 (9.1) | 22 (8.7) | 20 (15.4) | |
| Stage | ||||
| I-II | n (%) | 0 | 96 (43) | 96 (74) |
| III | n (%) | 0 | 126 (57) | 34 (26) |
| IV | n (%) | 449 (100) | 0 (0) | 0 (0) |
| Missing | 0 | 32 (12.6) | 0 (0) | |
| Site of disease | ||||
| Head | n (%) | 201 (59) | 173 (74) | 73 (76) |
| Body | n (%) | 137 (34) | 64 (27) | 16 (17) |
| Tail | n (%) | 132 (33) | 21 (9) | 16 (17) |
| Missing | 50 (11.1) | 19 (7.5) | 34 (26.2) | |
| Site of metastasis | ||||
| Liver | n (%) | 301 (76) | NA | NA |
| Lymph nodes | n (%) | 128 (32) | NA | NA |
| Lung | n (%) | 92 (23) | NA | NA |
| Other site | n (%) | 84 (21) | NA | NA |
| Missing | 54 (12.0) | 0 | 0 | |
ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not applicable.
Table 2.
Treatment characteristics for group
| Group | Values, n (%) |
|---|---|
| A | |
| Gemcitabine | 69 (53.5) |
| FOLFIRINOX | 29 (22.5) |
| Capecitabine + gemcitabine | 19 (14.7) |
| Nab-paclitaxel + gemcitabine | 5 (3.9) |
| Capecitabine | 5 (3.9) |
| Gem + paclitaxel | 1 (0.8) |
| FOLFOX | 1 (0.8) |
| Missing | 1 (0.8) |
| Total patients, n | 130 |
| B | |
| Nab-paclitaxel + gemcitabine | 139 (54.7) |
| FOLFIRINOX | 69 (27.2) |
| Gemcitabine | 30 (11.8) |
| PAXG | 5 (2.0) |
| PEXG | 3 (1.2) |
| GemOx | 2 (0.8) |
| Capecitabine/5-FU | 2 (0.8) |
| Gemcitabine + irinotecan | 1 (0.4) |
| Gemcitabine + capecitabine | 1 (0.4) |
| Irinotecan + oxaliplatin | 1 (0.4) |
| Gemcitabine + anetumab ravtansine | 1 (0.4) |
| Total patients, n | 254 |
| C | |
| Nab-paclitaxel + gemcitabine | 316 (70.5) |
| Gemcitabine | 71 (15.8) |
| FOLFIRINOX | 37 (8.3) |
| GemOx | 5 (1.1) |
| CAPOX | 2 (0.4) |
| PAXG | 2 (0.4) |
| 5-FU | 2 (0.4) |
| Nab-paclitaxel + gemcitabine + LY3200882 | 2 (0.4) |
| GemOx + olaparib | 2 (0.4) |
| Gemcitabine + anetumab ravtansine | 2 (0.4) |
| PEXG | 1 (0.2) |
| Nab-paclitaxel + gemcitabine + BBI-608 | 1 (0.2) |
| Irinotecan + nab-paclitaxel + gemcitabine | 1 (0.2) |
| FOLFIRINOX → olaparib | 1 (0.2) |
| Gem + cisplatin | 1 (0.2) |
| FOLFOX | 1 (0.2) |
| IRINOX | 1 (0.2) |
| Missing | 1 (0.2) |
| Total patients, n | 449 |
5-FU, fluorouracil; Gem, gemcitabine.
Figure 2.
Overall survival by subgroup population.
CT, chemotherapy.
Discussion
Adherence to chemotherapy regimen recommended by AIOM guidelines was 96% in metastatic disease and in the primary chemotherapy setting, and 69% in resected patients in this National prospective survey involving 45 centers enrolling >900 patients in 2 years.
Pancreatic cancer treatment adherence13, 14, 15 was previously reported to be in the range observed for other cancers such as head and neck (74%),16 ovarian (30%),17 prostate (54%),18 or gastric cancer (32%).19 Namely, a 34.5% adherence to stage-specific National Comprehensive Cancer Network (NCCN) guidelines on pancreatic ductal adenocarcinoma was reported in 3706 patients treated between 2001 and 2006 in 50 large Californian hospitals.13 A similarly low compliance rate with national guidelines 6 years after its publication was observed by a Dutch group showing that adjuvant chemotherapy was administered to 57.2% of patients and chemotherapy for metastatic disease to 36.6%, with little to no improvement over time for three subsequent periods (2012-2013 versus 2014-2015 versus 2016-2017).14 Conversely, an 86%-99% acceptance of the guideline recommendation for adjuvant chemotherapy in pancreatic ductal adenocarcinoma in two separate time intervals (2003-2007 and 2007-2014) was reported in 49 and 94 patients, respectively, treated at a single German institution.15
Overall, compliance rate is difficult to interpret in these studies due to the small sample size,15 to the dated period,13 to the use of chemotherapy as a binary variable (yes/no),13, 14, 15 to the inclusion of selected institutions (either single15 or large13). Furthermore, there are several reasons for not recommending chemotherapy (e.g. age, clinical condition, late recovery or early metastases after surgery, burden of disease, comorbidities, socioeconomic status) that may hamper guidelines application in real-life. The GARIBALDI survey provides a more dynamic scenario because guidelines are annually updated and the assessment was carried out immediately after publication, and includes institutions of any size, experience, and geographical region. Another strength of the GARIBALDI survey is the focus on the use of evidence-based chemotherapy regimens that is more informative from the oncological perspective.
We cannot rule out that the active participation in this prospective survey and, even more, its specific topic may represent a potential selection bias. However, while the first theme is common to any single trial, the 69% recommendations compliance in the adjuvant setting suggests that participating physicians did not enroll patients on the basis of guideline adherence. In addition, this survey population was selected without stringent eligibility criteria and is therefore more representative of the real-world scenario when compared with a typical trial population in which patients that are younger (e.g. aged 60-61 years in7,8 versus >68 years in the GARIBALDI survey) and frail subpopulations (e.g. patients aged >75 years or with moderate organ function impairment or with prior cancer history) are, at best, underrepresented. In this context, divergence from recommendation may be tempting and even justified. Accordingly, we reckon that the collected data provide inestimable information and a realistic picture of the national landscape, despite a small proportion (6%) of ineligible patients and a slight underrepresentation of the southern part of the country and of small-sized institutions.
Other prior studies in several cancer types correlated guideline adherence with improved survival.13,16, 17, 18, 19 Because of the high compliance rate observed in this survey, a comparison of the outcome of patients who did or did not receive a treatment as per guideline recommendation is unsuitable. This may be due to a more limited therapeutic armamentarium that does not offer alternative options or to a more ‘scholastic’ approach of oncologist dealing with a challenging disease. Another potential reason of this success may be the inclusive and influential AIOM’s policy in drawing guidelines that are conflict of interest free, peer reviewed, and rigorously evidence based. Endorsing this hypothesis is the lower rate (15%) of adjuvant capecitabine + gemcitabine regimen use as opposed to gemcitabine (54%), paralleling the level of evidence ranking as per a panel of expert assessment. Similarly, FOLFIRINOX was recommended in 8% of metastatic patients compared with 71% receiving nab-paclitaxel and gemcitabine combination.
A weakness of the guideline process was highlighted by the lower compliance rate observed in the adjuvant setting. In fact, to fulfill the GRADE process, the mFOLFIRINOX regimen was included among the adjuvant therapy recommendations only in the October 2019 update because guidelines are published once in a year while the full manuscript was published in January 2019.5 To avoid potential delays in releasing recommendations derived from pivotal trials, AIOM should therefore consider to allow either a late breaking update or a preliminary conditional assessment based on congress presentations.
This survey also allowed to identify weaknesses in the patients’ journey including a low rate of psychologic and nutritional counseling, a low rate of patients included into prospective interventional clinical trials, a long median time interval between first physician referral and treatment start, and a low rate of patients calling on the oncologist upfront. Based on these observations, AIOM may consider tailored educational interventions.
Overall, the observed figures endorse AIOM’s effort to produce up-to-date, evidence-based recommendations by a meticulous and methodologically robust peer-reviewed process and provide valuable data to inform the society’s educational plan aimed to improving patients’ engagement plan and diagnostic–therapeutic pathway.
Acknowledgements
The authors thank all patients involved, all participating sites, and all figures involved in the conduction and management of this study. A full list of the GARIBALDI Study Group can be found in Appendix 1.
Funding
This study was partially funded by Bristol-Myers Squibb (no grant number).
Disclosure
RM declares grants or contracts from AstraZeneca and participation on a Data Safety Monitoring Board or Advisory Board for Eli Lilly, PANAVANCE, Celgene, AstraZeneca, Viatris, Merck Sharp & Dohme, Servier, SOTIO, and Baxter. BF declares payment for consulting fees from Servier and AAA Novartis and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Eli Lilly, MSD, EISAI, and Bayer. MM declares grants or contracts from Roche and Novartis, payment for consulting fees from Viatris, AstraZeneca, MSD, and Merck; payment for participation on a Data Safety Monitoring Board or Advisory Board from Novartis. CL declares payment for consulting fees from Astrazeneca e Merck and support for attending meetings and/or travel from Pfizer, Ipsen and Celgene. DMMC declares payment for participation on a Data Safety Monitoring Board or Advisory Board from OncoSil and Olaparib and for Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Editorial Board Future Oncology. SM declares payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Eli Lilly, Roche, Pfizer, Janssen, Italfarmaco, and BMS and for participation on a Data Safety Monitoring Board or Advisory Board from Eli Lilly, Merck, Pfizer, Janssen, and BMS; payment or has a leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from AIOM Sicilia. GE declares payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Viatris, Amgen, and AstraZeneca and support for attending meetings and/or travel from Ipsen and Viatris. SC declares payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, Merk, Servier, Bayer, and Sanofi and support for attending meetings and/or travel from Amgen, Merk, and Servier. GGC declares payment for attending meetings and/or travel from Incyte, Eli Lilly, Amgen, and MSD and for the participation on a Data Safety Monitoring Board or Advisory Board for Eli Lilly, MSD, and AstraZeneca. ME declares payment for consulting fees from MSD. GM declares consulting fees from Italfarmaco, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Roche, Merck, Eli Lilly, Servier, and Amgen. PL declares consulting fees from Ipsen and Italfarmaco. CP declares institutional grants from Ely Lilly, Bayer, and Roche; consulting fees from BMS, Ely Lilly, Novartis, and Merck and for the participation on a Data Safety Monitoring Board or Advisory Board for BMS, Amgen, and Novartis. All other authors have declared no conflicts of interest.
Contributor Information
M. Reni, Email: reni.michele@hsr.it.
GARIBALDI Study Group:
Michele Reni, Marina Macchini, Giulia Orsi, Umberto Peretti, Mariamaddalena Valente, Elisa Giommoni, Lorenzo Antonuzzo, Francesco Di Costanzo, Francesca Bergamo, Vittorina Zagonel, Sara Lonardi, Federica Buggin, Michele Milella, Silvia Palmerio, Luigi Cavanna, Camilla Di Nunzio, Maria Cristina Di Marco, Elisa Grassi, Massimiliano Spada, Marco Messina, Stefano Cordio, Francesco Avola, Giuseppe Aprile, Salvatore Pagano, Francesca Simionato, Giovanni Gerardo Cardellino, Federica Majer, Evaristo Maiello, Tiziana Pia Latiano, Cinzia Chiarazzo, Fabrizio Artioli, Giorgia Razzini, Antonella Pasqualini, Michele Ghidini, Elisa Binda, Silvia Lazzarelli, Silvia Bozzarelli, Simona Sala, Gabriele Luppi, Elisa Pettorelli, Andrea Spallanzani, Giovanni Vicario, Flavia Salmaso, Marco Basso, Nicola Silvestris, Sabina Del Curatolo, Fable Zustovich, Francesca Bongiovanni, Ciro Longobardi, Ilenia Sandi, Caterina Fontanella, Silvia Montelatici, Monica Giordano, Giovanna Luchena, Micol Gilardoni, Emiliano Tamburini, Britt Rudnas, Barbara Venturini, Barbara Merelli, Giorgia Negrini, Elio Maria Vici, Alessandra Marabese, Cristina Garetto, Paola Curcio, Saverio Cinieri, Margherita Cinefra, Pasqualinda Ferrara, Maurizio Cantore, Patrizia Morselli, Guglielmo Fumi, Agnese Isidori, Giovanni Ciccarese, Giovanni Luca Paolo Frassineti, Flavia Pagan, Vanja Vaccaro, Chiara Spoto, Marianna Ferrara, Carlo Garufi, Marta Caporale, Enrico Vasile, Francesca Salani, Elisa Barone, Rossana Berardi, Azzurra Onofri, Zelmira Ballatore, Alessandra Lucarelli, Alessandra Barucca, Amedeo Pancotti, Teresa Scipioni, Katia Bencardino, Giovanna Marrapese, Laura Idotta, Fausto Petrelli, Veronica Lonati, Anna Ceribelli, Angelo Giuli, Cristina Zannori, Maria Bassanelli, Andrea Mambrini, Laura Ginocchi, Massimo Orlandi, Luigi Celio, Monica Niger, Lavinia Biamonte, Stefano Tamberi, Alessandra Piancastelli, Giorgio Papiani, Irene Valli, Paolo Allione, Maria Giovanna Boe, Mario Scartozzi, Eleonora Lai, Annagrazia Pireddu, Pina Ziranu, Laura Demurtas, Marco Puzzoni, Stefano Mariani, Andrea Pretta, Nicole Liscia, Clementina Savastano, Valentina Malaspina, Giuseppe Tonini, Teresa Grassani, Barbara Barco, Tagliaferri Pierosandro, Domenico Ciliberto, Antonella Ierardi, Natale Daniele Calandruccio, Vincenzo Minotti, Roberta Matocci, Valter Torri, Luca Porcu, Erica Rulli, Irene De Simone, Luciano Carlucci, Eliana Rulli, Davide Poli, Paola Tonto, Francesca Scellato, and Carmine Pinto
Appendix 1: GARIBALDI Study Group
List of participating institutions and coauthors:
Vita e Salute University, IRCCS San Raffaele Scientific Institute: Michele Reni, Marina Macchini, Giulia Orsi, Umberto Peretti, Mariamaddalena Valente
Azienda Ospedaliero-Universitaria Careggi, Florence: Elisa Giommoni, Lorenzo Antonuzzo, Francesco Di Costanzo
Istituto Oncologico Veneto, IRCCS: Francesca Bergamo, Vittorina Zagonel, Sara Lonardi, Federica Buggin
Policlinico Universitario G.B. Rossi Borgo Roma, Verona: Michele Milella, Silvia Palmerio
Piacenza General Hospital, Piacenza: Luigi Cavanna, Camilla Di Nunzio
Policlinico S.Orsola-Malpighi, Bologna: Maria Cristina Di Marco, Elisa Grassi
Fondazione Istituto G. Giglio di Cefalù: Massimiliano Spada, Marco Messina
ARNAS Garibaldi Nesima – Catania: Stefano Cordio, Francesco Avola
San Bortolo General Hospital, ULSS8 Berica, Vicenza, Italy– Vicenza: Giuseppe Aprile, Salvatore Pagano, Francesca Simionato
University & General Hospital, Udine: Giovanni Gerardo Cardellino, Federica Majer
Hospital Casa Sollievo Della Sofferenza-San Giovanni Rotondo (Foggia): Evaristo Maiello, Tiziana Pia Latiano, Cinzia Chiarazzo
Carpi and Mirandola Hospitals, Carpi e Mirandola: Fabrizio Artioli, Giorgia Razzini, Antonella Pasqualini
ASST of Cremona, Hospital of Cremona, Italy: Michele Ghidini, Elisa Binda, Silvia Lazzarelli
Clinical Institute Humanitas – Rozzano (Milan): Silvia Bozzarelli, Simona Sala
A.O. Universitario Policlinico-Modena: Gabriele Luppi, Elisa Pettorelli, Andrea Spallanzani
Azienda U.L.S.S. 8-Castelfranco Veneto: Giovanni Vicario, Flavia Salmaso, Marco Basso
I.R.C.C.S. Ospedale Oncologico-Bari: Nicola Silvestris, Sabina Del Curatolo
Ospedale di Belluno Oncologico Medica-Belluno: Fable Zustovich, Francesca Bongiovanni, Ciro Longobardi, Ilenia Sandi, Caterina Fontanella, Silvia Montelatici
ASST-lariana-San Fermo della Battaglia (CO): Monica Giordano, Giovanna Luchena, Micol Gilardoni
Ospedale Infermi-Rimini: Emiliano Tamburini, Britt Rudnas, Barbara Venturini
ASST Papa Giovanni XXIII-Bergamo: Barbara Merelli, Giorgia Negrini
Ospedale centrale-Bolzano: Elio Maria Vici, Alessandra Marabese
A.S.O. Santa Croce e Carle Ospedale d’insegnamento-Cuneo: Cristina Garetto, Paola Curcio
ASL Brindisi Presidio Osp. Sen. Antonio Perrino-Brindisi: Saverio Cinieri, Margherita Cinefra, Pasqualinda Ferrara
A.O. Carlo Poma-Mantova: Maurizio Cantore, Patrizia Morselli
Azienda Ospedaliera S.Maria-Terni; Guglielmo Fumi, Agnese Isidori, Giovanni Ciccarese
Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST)-Meldola; Giovanni Luca Paolo Frassineti, Flavia Pagan
IFO - IRCCS istituto Nazionale Tumori Regina Elena-Roma; Vanja Vaccaro, Chiara Spoto, Marianna Ferrara
ASL Pescara: Carlo Garufi, Marta Caporale
Polo Oncologico - U.O. Oncologia 1 e Oncologia 2-Pisa: Enrico Vasile, Francesca Salani, Elisa Barone
A.O.U. Ospedali Riuniti-Ancona: Rossana Berardi, Azzurra Onofri, Zelmira Ballatore, Alessandra Lucarelli, Alessandra Barucca
Oncologia ASL-Teramo: Amedeo Pancotti, Teresa Scipioni
Niguarda Cancer Center Grande Ospedale Metropolitano Niguarda-Milano: Katia Bencardino, Giovanna Marrapese, Laura Idotta
ASST Bergamo OVEST-Treviglio: Fausto Petrelli, Veronica Lonati
Ospedale San Camillo De Lellis-Rieti: Anna Ceribelli, Angelo Giuli, Cristina Zannori, Maria Bassanelli
A.S.L. 1 Massa Carrara: Andrea Mambrini, Laura Ginocchi, Massimo Orlandi
Istituto Nazionale Tumori(INT)-Milano: Luigi Celio, Monica Niger, Lavinia Biamonte
Ospedale U.O degli Infermi-Faenza: Stefano Tamberi, Alessandra Piancastelli, Giorgio Papiani, Irene Valli
ASLCN2 Alba-Bra-Alba: Paolo Allione, Maria Giovanna Boe
A.O.U. di Cagliari S.C. Oncologia Medica-Monserrato: Mario Scartozzi, Eleonora Lai, Annagrazia Pireddu, Pina Ziranu, Laura Demurtas, Marco Puzzoni, Stefano Mariani, Andrea Pretta, Nicole Liscia
AOU San Giovanni di Dio E Ruggi D’Aragona-Salerno: Clementina Savastano, Valentina Malaspina
Policlinico Universitario Campus Bio-Medico-Roma: Giuseppe Tonini, Teresa Grassani
ASST Valle Olona – PO-Saronno: Barbara Barco
Unità Operativa di Oncologia Medica-Catanzaro: Tagliaferri Pierosandro, Domenico Ciliberto, Antonella Ierardi, Natale Daniele Calandruccio
A.O. di Perugia: Vincenzo Minotti, Roberta Matocci
Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milano: Valter Torri, Luca Porcu, Erica Rulli, Irene De Simone, Luciano Carlucci, Eliana Rulli, Davide Poli, Paola Tonto, Francesca Scellato
Medical Oncology, Comprehensive Cancer Centre, AUSL-IRCCS di Reggio Emilia, Italy: Carmine Pinto
Supplementary data
References
- 1.Silvestris N., Brunetti O., Bittoni A., et al. Clinical practice guidelines for diagnosis, treatment and follow-up of exocrine pancreatic ductal adenocarcinoma: evidence evaluation and recommendations by the Italian Association of Medical Oncology (AIOM) Cancers (Basel) 2020;12:1681. doi: 10.3390/cancers12061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reni M., Zanon S., Balzano G., et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol. 2017;28:2786–2792. doi: 10.1093/annonc/mdx495. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H., Post S., Neuhaus P., et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos J.P., Palmer D.H., Ghaneh P., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T., Hammel P., Hebbar M., et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.Burris H.A., 3rd, Moore M.J., Andersen J., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Desseigne F., Ychou M., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Von Hoff D.D., Ervin T., Arena F.P., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reni M., Cereda S., Rognone A., et al. A randomized phase II trial of two different 4-drug combinations in advanced pancreatic adenocarcinoma: cisplatin, capecitabine, gemcitabine plus either epirubicin or docetaxel (PEXG or PDXG regimen) Cancer Chemother Pharmacol. 2012;69:115–123. doi: 10.1007/s00280-011-1680-2. [DOI] [PubMed] [Google Scholar]
- 10.Reni M., Balzano G., Zanon S., et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):413–423. doi: 10.1016/S2468-1253(18)30081-5. [DOI] [PubMed] [Google Scholar]
- 11.Reni M., Zanon S., Balzano G., et al. A randomised phase 2 trial of nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in locally advanced or borderline resectable pancreatic adenocarcinoma. Eur J Cancer. 2018;102:95–102. doi: 10.1016/j.ejca.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Reni M., Zanon S., Peretti U., et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(10):691–697. doi: 10.1016/S2468-1253(18)30196-1. [DOI] [PubMed] [Google Scholar]
- 13.Visser B.C., Ma Y., Zak Y., et al. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB. 2012;14(8):539–547. doi: 10.1111/j.1477-2574.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay T.M., Latenstein A.E.J., Bonsing B.A., et al. Nationwide compliance with a multidisciplinary guideline on pancreatic cancer during 6-year follow-up. Pancreatology. 2020;20(8):1723–1731. doi: 10.1016/j.pan.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Weinrich M., Bochow J., Kutsch L., et al. High compliance with guideline recommendations but low completion rates of adjuvant chemotherapy in resected pancreatic cancer: a cohort study. Ann Med Surg. 2018;32:32–37. doi: 10.1016/j.amsu.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen O., Brauer P.R., Judson B.L., et al. Guideline – adherence in advanced stage head and neck cancer is associated with improved survival – a national study. Oral Oncol. 2022;125 doi: 10.1016/j.oraloncology.2021.105694. [DOI] [PubMed] [Google Scholar]
- 17.Jochum F., De Rozario T., Lecointre L., et al. Adherence to European ovarian cancer guidelines and impact on survival: a French multicenter study (FRANCOGYN) Int J Gynecol Cancer. 2021;31(11):1443–1452. doi: 10.1136/ijgc-2021-002934. [DOI] [PubMed] [Google Scholar]
- 18.González Serrano A., Martínez Tapia C., de la Taille A., et al. Adherence to treatment guidelines and associated survival in older patients with prostate cancer: a prospective multicentre cohort study. Cancers (Basel) 2021;13(18):4694. doi: 10.3390/cancers13184694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow S.R., Ma Z., Hani L., et al. Adherence to guidelines at the patient- and hospital-levels is associated with improved overall survival in patients with gastric cancer. J Surg Oncol. 2022;126:479–489. doi: 10.1002/jso.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.