Abstract
New concepts and drugs have revolutionized medical treatment for cancers. These drugs, which are very expensive and usually well tolerated, have dramatically improved cancer prognosis. We must use them wisely for patients to fully benefit. Gastric acid antisecretory drugs and particularly proton pump inhibitors (PPIs) revolutionized the treatment of gastroduodenal ulcers and severe gastroesophageal reflux, but are frequently overused for symptomatic treatment of epigastric pain or heartburn. Long-term acid suppression may alter the efficacy of many anticancer drugs, such as tyrosine kinase inhibitors (TKIs), cyclin-dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors (ICIs), by either decreasing gastric acid secretion and thus drug absorption, or by modifying the gut microbiome that modulates the response to ICIs. Oncologists thus need to pay particular attention to the concomitant use of PPIs and anticancer drugs. These interactions translate into major clinical impacts, with demonstrated loss of efficacy for some TKIs (erlotinib, gefitinib, pazopanib), and conflicting results with many other oral drugs, including capecitabine and CDK 4/6 inhibitors. Furthermore, the profound changes in the gut microbiome due to using PPIs have shown that the benefit of using ICIs may be suppressed in patients treated with PPIs. As the use of PPIs is not essential, we must apply the precautionary principle. The first sentence of a recent Comment in Nature was “Every day, millions of people are taking medications that will not help them”. We fear that every day millions of cancer patients are taking medications that harm them. While this may well be only association and not causation, there is enough to make us pause until we reach a clear answer. All these data should encourage medical oncologists to refrain from prescribing PPIs, explaining to patients the risks of interaction in order to prevent inappropriate prescription by another physician.
Key words: proton pump inhibitors, tyrosine kinase inhibitors, immune checkpoint inhibitors, chemotherapy drugs, drug–drug interactions, efficacy
Highlights
-
•
More than one-third of cancer patients under treatment received PPIs.
-
•
Co-administration of PPIs increases toxicity of methotrexate and pemetrexed and decreases efficacy of capecitabine.
-
•
Absorption and efficacy of erlotinib, gefitinib and pazopanib are decreased in case of PPI use.
-
•
Efficacy of ICIs seems to be erased by PPIs.
Introduction
In the past 20 years, the therapeutic landscape in medical oncology has been dramatically modified by the demonstrated efficacy of both targeted therapies, in many tumors with driver molecular alterations [mainly tyrosine kinase inhibitors (TKIs), taken orally], and immune checkpoint inhibitors (ICIs), in different cancers. These efficient new treatments have a totally different safety profile in common, better than conventional systemic chemotherapy, a higher price plus the possibility of therapeutic failures due to unusual drug–drug interactions.1,2
Proton pump inhibitors (PPIs) are some of the most frequently prescribed drugs in the world and frequently prescribed inappropriately to relieve digestive symptoms.3 They are considered very safe, but in the general population, there is an excess of cause-specific mortality related to the use of PPIs. In a longitudinal cohort study (United States Veterans), new users of PPIs experienced excess mortality from cardiovascular and chronic kidney diseases compared to new users of H2 blockers.4 In a recent prospective study we showed that more than a quarter of cancer patients receiving anticancer treatment used PPIs.5 Suppression of gastric acidity can decrease the absorption, and thus the efficacy, of certain targeted therapies, and change the composition of the gut microbiome, which has an impact on the response to immunotherapy.6, 7, 8, 9 It is thus possible that a symptomatic treatment might worsen patients’ prognosis.10,11 Using PPIs in the fragile population of cancer patients is thus a real issue, on its own and because of possible drug–drug interactions.12
In this review, we aim to update these potential interactions between use of PPIs and anticancer treatments.
Literature search strategy
We conducted a search in PubMed database for English language studies published until 15 December 2022 using the following Medical Subjects Headings terms and key words: ‘proton pump inhibitors’, ‘cancers’, ‘chemotherapy’, ‘immunotherapy’, ‘tyrosine kinase inhibitors’, ‘CDK4/6 inhibitor’, ‘microbiome’. This manuscript is not a systematic review as we kept for analysis only articles published in ‘major journals’.
Mechanisms of drug–drug interactions of PPIS with anticancer treatments
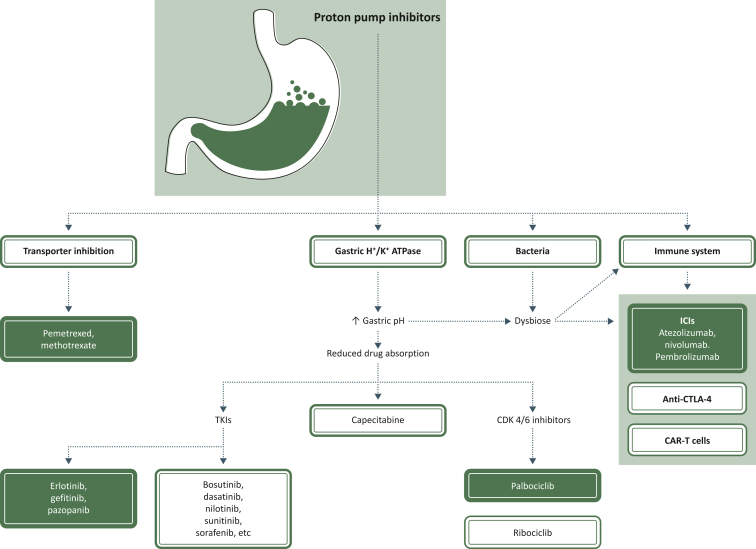
PPIs, substituted benzimidazoles, suppress the secretion of gastric acid via irreversible inhibition of H+/K+ ATPase in the gastric parietal cells. For example, omeprazole 20 mg once daily (results are similar with 40 mg) for 3 months, in healthy subjects, increased median gastric pH from 1.7 to 4.6 and mean percentage of time at pH <4 from 89% to 35%.13 But H+/K+ ATPases are not only located in parietal cells, and non-gastric H+/K+ ATPases can be found in neutrophils, myelomonocytes, osteoclasts, the kidneys, prostate, colon, placenta, pancreas, etc. The effect of PPIs on these ATPases is not well known. PPIs are, directly or indirectly, involved in different processes, such as interactions with certain brain enzymes (impact on dementia), pancreatic secretion, skeletal side-effects (involved in bone fractures), kidney function, sperm motility and virus replication.14, 15, 16 They have antioxidant and anti-inflammatory functions, acting on various cellular types including immune, vascular endothelial and epithelial cells.17 PPIs have a role in the regulation of pro-inflammatory cytokines, explaining how PPIs may have a protective function in idiopathic pulmonary fibrosis.18 PPIs can also affect the transmigration of leukocytes from vessels to inflammatory sites, alter neutrophil–endothelial cell interactions and decrease peripheral blood monocytes.19, 20, 21 The tumor microenvironment is acidic and PPIs, which increase the pH, can affect local immunity.22 Then, contrary to what was initially believed, PPIs do not only target gastric acid secretion (Figure 1).
Figure 1.
Proton pump inhibitor (PPI) interactions with anticancer drugs. Pemetrexed and methotrexate are excreted by renal transporters (hOAT3) inhibited by PPIs, resulting in higher risk of hematological toxicity.35, 36, 37 Inhibition of gastric H+K+ ATPase increases the gastric pH and reduces absorption of many anticancer drugs, particularly tyrosine kinase inhibitors (TKIs), capecitabine and cyclin-dependent kinase (CDK) 4/6 inhibitors.9,23,24,38,39,42,73,75 This increase in gastric pH also eliminates the crucial role in filtering out bacteria and with a direct role of PPIs on bacteria results in a dysbiose.26,27 This dysbiose in association with a direct action of PPIs on immune system may decrease or erase efficacy of immune checkpoint inhibitors and may interact with efficacy of anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and chimeric antigen receptor (CAR)-T cells.31,83,84,89 Colored boxes: drug–drug interactions with clinically demonstrated interactions; white boxes: suspected drug–drug interactions with clinically conflicting results.
But this decrease in gastric acid secretion may be of paramount importance for some drugs administered orally, such as TKIs (Figure 1). Oral administration is flexible and convenient, but requires clear and strict recommendations regarding meals (timing of administration, fat composition, etc.) and drug interactions. The risk of drug–drug interaction is prominent, with an impact on drug toxicity or efficacy in cancer patients, who are usually given many drugs. TKIs are weakly basic, and co-administration with a gastric acid-suppressive drug increasing the gastric pH decreases their bioavailability.9,23 Many (dasatinib, erlotinib, gefitinib, pazopanib, lapatinib, nilotinib, sunitinib and vandetanib) display pH-dependent solubility and absorption.15,23 This decrease in bioavailability can sometimes be significant and associated with decreased efficacy. One review reported a major decrease in the oral absorption of crizotinib, dasatinib, erlotinib, gefitinib, lapatinib and pazopanib, and recommended avoiding concomitant use of PPIs or H2 antagonists.24 A recent systematic review and meta-analysis of the use of gastric acid suppressants and oral anticancer treatments supports the evidence for a possible negative impact of such combinations on survival outcomes.25
On the other hand, gastric acidity plays a crucial role in filtering out bacteria and preventing enteric infections. Using PPIs leads to significant changes in the microbial composition of both gastric and intestinal microbiomes. A decrease in acidity due to PPIs facilitates the growth of upper intestinal bacteria in the gut but also has a direct inhibitory effect on certain bacteria, as some bacteria have H+ ATPases that might be blocked by PPIs.17,26 Drug–microbiome interactions have recently been studied by metagenomic sequencing of fecal samples in large population cohorts; 41 drugs were analyzed, and the overall composition of the gut ecosystem was consistently altered only in cases where PPIs and multiple drugs were used.27 All PPIs had a similar effect on the gut microbiome.27 The functional changes induced by these modifications are not well known.26 There is increasing evidence suggesting that the gut microbiome can modulate the host’s antitumor response and the response to ICIs, anti-cytotoxic T-lymphocyte-associated protein 4 and chimeric antigen receptor-T cells (Figure 1).28, 29, 30, 31 For example, the microbiome of responders to ICIs is enriched in Firmicutes, and in melanoma patients treated with anti-programmed cell death protein 1 (PD-1) there is a difference in the microbiome between treatment responders and non-responders.6,32 It is now clear that antibiotics (ATB) can inhibit the clinical benefits of ICI by modifying the composition of the gut microbiome.2,33,34 PPIs decrease bacterial richness and induce profound changes in the gut microbiome; these alterations are more prominent than the effects of ATB or other commonly used drugs and the impact on immunotherapy efficacy is a major issue.
PPIs and chemotherapy
A systematic review and meta-analysis confirmed previous data showing that the co-administration of PPIs with intravenous methotrexate is associated with delayed high-dose methotrexate elimination (higher plasma concentration at 24 and 48 h) and must be avoided.35 A recent prospective multicentric observational study collected data from patients treated with pemetrexed-based chemotherapy and showed that PPI use (55 patients out of the cohort of 156) was associated with a significantly higher risk of severe hematologic toxicity in a multiparametric analysis [hazard ratio (HR) 2.51; 95% confidence interval (CI) 1.47-4.26].36 This may be related to an inhibitory effect of PPIs on renal human organic anion transporter 3 (Figure 1).37
Capecitabine absorption depends on gastric acidity, and substantial reductions in gastric acidity can lead to less drug dissolution and less absorption. But, in a (very short) series of 12 patients no interaction was found between co-administration of Maalox® and capecitabine. The TRIO-013/LOGIC randomized clinical trial compared capecitabine and oxaliplatin (CapeOx) with or without (placebo) lapatinib, in human epidermal growth factor receptor 2-positive metastatic gastroesophageal cancer. A secondary unplanned analysis compared overall survival (OS) and progression-free survival (PFS) between patients, based on exposure to PPIs. Of the 545 patients, 229 received PPIs; in the placebo group, PPI-treated patients had poorer median PFS (4.2 versus 5.7 months; HR 1.55; 95% CI 1.29-1.81) and median OS (mOS) (9.2 versus 11.3 months; HR 1.34; 95% CI 1.06-1.62); in multivariate analysis (age, race, disease stage, sex), the impact of PPI use remained crucial. In patients also receiving lapatinib, the effect of PPIs on PFS and OS was significant only in multivariate analysis.38 In a retrospective review of 389 colon cancer patients receiving adjuvant FOLFOX or CapeOx regimens, recurrence-free survival was lower in CapeOx-treated PPI users than in non-users. Surprisingly, the opposite (but not statistically significant) was observed in FOLFOX-treated patients. It thus seems that PPIs adversely affected the efficacy of CapeOx but not FOLFOX treatment, thereby affecting the efficacy of capecitabine.39 The same authors reviewed data from capecitabine monotherapy used as an adjuvant for early-stage colorectal cancers. Of the 298 patients, 77 (25.8%) received concurrent PPIs; the 5-year recurrence-free survival was lower in PPI users than in non-users (74% versus 83%; HR 1.89; 95% CI 1.07-3.35). This difference was no longer significant (HR 1.65; 95% CI 0.93-2.94) after adjusting for sex, age, stage and performance status (PS).40 Decreasing efficacy in an adjuvant setting is awful as it translates to patients who will experience disease recurrence and then die from this early-stage cancer. A recent Japanese retrospective observational study of patients with stage II-III colon cancer receiving capecitabine or CapeOx in an adjuvant setting showed that in the 606 patients analyzed, 54 had PPIs; these patients tended to have poorer recurrence-free survival and OS than those treated without PPIs.41 This interaction between PPIs and capecitabine was also shown in a post hoc analysis of the AXEPT phase III trial (trial conducted in Korea and Japan).42 Kichenadasse et al. carried out a secondary analysis of six randomized controlled trials (RCTs) in patients with advanced colorectal cancers using individual patient data.43 Out of 5594 patients, 902 received PPIs at trial entry. PPI use was significantly associated with worse OS (HR 1.20; 95% CI 1.03-1.40) and PFS after adjusting for covariates. Surprisingly, this was observed for intravenous 5-fluorouracil (5-FU) but not for patients treated with capecitabine. No clear explanation was found: alterations in the gut microbiome, altered immune milieu within the tumor, interactions through transporter inhibition by PPIs (OATS, hENT1)?43 A randomized crossover study in 22 patients with cancer compared capecitabine alone, capecitabine with esomeprazole 3 h before and capecitabine plus cola 3 h after esomeprazole. They concluded that capecitabine exposure was not negatively influenced by esomeprazole co-treatment and that altered capecitabine pharmacokinetics did not explain the worse outcome for patients using PPIs concomitantly.44
Future studies are thus warranted as series are accumulating on these possible interactions.45
PPIs and TKIs
TKIs are oral drugs and currently a major weapon in the anticancer arsenal. Many medical oncologists are currently aware of possible drug–drug interactions between PPIs and TKIs, but PPIs may be prescribed by the primary care physician, or purchased over the counter, resulting sometimes in ‘unknown’ drug–drug interactions that can lead to a decrease in efficacy.46,47 It is currently recognized that with many TKIs there are pharmacokinetics/pharmacodynamics relationships, with toxicities in cases of higher drug exposure and inefficacy in cases of lower drug exposure.
Gefitinib and erlotinib showed reduced absorption in cases of concomitant use of PPIs,48,49 resulting in a significant decrease in efficacy in retrospective analyses.50,51 In a large retrospective study of the concomitant use of TKIs and PPIs, nearly one in four older adults with cancer who received TKIs also received PPIs concomitantly, and this was associated with an increased risk of death (+21% for patients receiving erlotinib), a risk not associated with discontinued use of TKIs.52 In this study, no impact was observed in case of co-prescription of PPIs with sunitinib or imatinib, confirming previous results.53 In a large nationwide population-based survey (Taiwan) from 1 January 2010 to 30 December 2018, 4340 newly diagnosed patients with advanced lung adenocarcinoma received gefitinib and 1635 received erlotinib. They compared the outcome of those receiving PPIs or anti-H2 concurrently, or no antisecretory drug; approximately 1/6 received PPIs, and 1/5 anti-H2. The PPI group had the shortest mOS and time to next treatment compared to the anti-H2 group and to non-users. For example, mOS was, in the gefitinib cohort, respectively, 14.35, 17.7 and 21.8 months for PPI, anti-H2 and non-users, and in the erlotinib cohort, 17.0, 20.1 and 23.9 months. The adjusted HR of OS for the PPI group was 1.58 in the gefitinib group and 1.54 in the erlotinib group.54 With first-line sunitinib, in real-world studies, results on the use of PPIs are conflicting.53,55
The effects of esomeprazole on the pharmacokinetics of pazopanib were studied in 13 patients, and the mean area under the curve (AUC) and Cmax decreased by 40% and 42%, respectively, leading the authors to conclude that such concomitant use should be avoided.56 Subtherapeutic exposure was clear even if PPIs were given separately, 1 h after pazopanib.57 In patients over the age of 75 years, a pharmacokinetic study demonstrated decreased oral bioavailability of pazopanib if given with PPIs, confirming that such patients may be underexposed.58 Clinically, in a retrospective analysis of two prospective trials of pazopanib in soft-tissue sarcoma patients, of 333 patients receiving pazopanib, 59 received gastric antisecretory drugs (PPIs or anti-H2) concomitantly. PFS and OS were shorter in pazopanib patients receiving gastric antisecretory drugs (2.8 versus 4.6 months and 8.0 versus 12.6 months, respectively); these effects of PPIs on survival were not observed in the placebo group of patients.59 In shorter series of patients with metastatic renal cell carcinoma (RCC) treated with pazopanib, no impact of PPIs or anti-H2 use on survival parameters was demonstrated.60,61 In a recent retrospective study (147 patients), combining PPIs with pazopanib has a negative impact on OS, in soft-tissue sarcoma and renal cell cancer patients.62
Clinical pharmacology studies consider that exposure to lenvatinib, vandetanib, cabozantinib, alectinib, osimertinib and regorafenib is not significantly modified by PPIs.47,63,64 In a large series of 272 patients treated by regorafenib, 131 used PPIs. In multivariate analysis, no worse outcome was seen among these patients combining regorafenib and PPIs.65 A nationwide cohort study in hepatocellular carcinoma patients from Taiwan showed that patients who took sorafenib, regorafenib, lenvatinib or cabozantinib and were PPI users (n = 2196) had poorer OS than those who were not PPI users (n = 8013); these results were confirmed in a multiparametric analysis.66 Similar results were found in a UK center in sorafenib-treated patients.67 However, we know that PPI use increases the risk of severe complication for patients with cirrhosis, independently of the presence of liver cancer.68 In a secondary analysis of a phase III study in hepatocellular carcinoma patients comparing sorafenib with sunitinib (542 patients receiving sorafenib, 122 also receiving PPIs at baseline), in univariate and adjusted analyses, no significant association between PPI use and either OS or PFS was identified.69
We currently know that PPIs have a clear impact on the bioavailability of certain TKIs (bosutinib, dasatinib, erlotinib, gefitinib, lorlatinib, pazopanib) with a possible impact on outcomes for erlotinib, gefitinib, pazopanib and sunitinib; their concomitant use must be avoided, at least with bosutinib, dasatinib, erlotinib, gefitinib, nilotinib and pazopanib (Figure 1).70
PPIs and other orally given anticancer treatments
No known interaction was demonstrated between PPIs and mechanistic target of rapamycin inhibitors, phosphoinositide 3-kinase inhibitors or poly (ADP-ribose) polymerase inhibitors;71 data regarding BRAF/MEK inhibitors and larotrectinib were scarce but seemed negative.47
The solubility of the CDK 4/6 inhibitor, palbociclib, a weak base, is reduced at a pH >4 and co-administration with PPIs decreased both the AUC and Cmax.72 In a retrospective observational study in metastatic breast cancer patients treated with palbociclib, the concomitant use of PPIs may have had a detrimental effect on PFS. This study compared 56 candidates for first-line treatment with palbociclib with and 56 without concomitant use of PPIs.73 Patients taking PPIs had a shorter PFS (14.0 versus 37.9 months), and that was confirmed in a multivariate analysis.73 On the contrary, gastric pH did not influence the pharmacokinetics of ribociclib.74 But a retrospective analysis conducted in Turkey on 217 patients, receiving, in addition to fulvestrant or letrozole, palbociclib (105 patients) and ribociclib (112 patients), showed that patients receiving PPIs concomitantly (>50% of the population) had a shorter PFS than non-users; this was confirmed for both drugs in a multivariate analysis, showing that PPI use was the only parameter associated with PFS.75
No pharmacokinetic interaction between PPIs and estrogen receptor inhibitors has been described; enzalutamide, an androgen receptor inhibitor, can decrease the PPIs’ plasma levels.76
PPIs and immunotherapy
Taking into account, on the one hand, the possible interactions between the microbiome and PPIs, and between the microbiome and the efficacy of immunotherapies and, on the other hand, the number of cancer patients taking PPIs on a regular basis, it is obvious that one can expect a negative impact of PPI use on treatment.5
Numerous studies have thus addressed the problem of the efficacy of ICIs in PPI users and many meta-analyses have been published on this burning topic.
From a meta-analysis of seven studies (3647 cancer patients), the authors concluded that PPI use had a detrimental effect on the efficacy of ICIs: PPI use increased the risk of death by 39% and the risk of progression by 28%.77 The most recent (February 2022) systematic review and meta-analysis of a correlation between PPI use and the clinical efficacy of ICIs in cancers, collected 17 studies, enrolling 9978 patients treated with ICIs. The authors concluded that PPI use was significantly correlated with worse OS (HR 1.29; 95% CI 1.10-1.50) but without any clear significant impact on PFS (HR 1.19; 95% CI 0.98-1.44). This negative correlation of PPI use with ICI efficacy was clear in patients with non-small-cell lung cancer (NSCLC), patients with urothelial cancers and cohorts with mixed cancers, but not in PFS analysis in patients with melanoma.78
In a cohort of 112 melanoma patients treated with anti-PD-1, significant differences were observed in the microbiomes of responders versus non-responders.79 In a retrospective analysis from CheckMate 069, the objective response rate (and PFS) after immunotherapy (ipilimumab alone or combined with nivolumab, or nivolumab alone) in patients receiving PPIs was lower than in non-users.80 In contrast, a post hoc analysis of three randomized studies in first-line testing of ICIs (CheckMate 066, CheckMate 067 and CheckMate 069) in a total of 1505 patients, including 291 PPI users (19.3%), has recently been published. PPI users were either older or baseline PS1 or higher than non-users. Results from this analysis did not support the idea of a meaningful association between PPI use at baseline and the efficacy of ICIs in advanced melanoma.81
In previously treated NSCLC, retrospective analysis using pooled data from the POPLAR and OAK trials (one phase II and one phase III trial) comparing atezolizumab (n = 757) with docetaxel (n = 755) showed that PPI use was associated with shorter OS and PFS in the atezolizumab population and not in the docetaxel population.82 The most recent (2022) pooled analysis of all five trials testing atezolizumab in NSCLC (IMpower130, IMpower131, IMpower150, OAK and POPLAR) gave similar results. Of the 4458 patients included, 2723 were randomized to treatment with, and 1735 without, atezolizumab; 1225 were using PPIs at treatment initiation (28% and 27%, respectively, in the arms with or without atezolizumab).83 PPI use was associated with worse OS in univariate (HR 1.30; 95% CI 1.17-1.46; P < 0.001) and adjusted (HR 1.23; 95% CI 1.09-1.37; P < 0.001) analysis, and with worse PFS in univariate (HR 1.18; 95% CI 1.07-1.29; P < 0.001) and adjusted (HR 1.15; 95% CI 1.03-1.28; P = 0.01) analysis. In the comparator arm (therapies without atezolizumab), no association between PPI use and OS (HR 1.01; 95% CI 0.88-1.16) or PFS (HR 0.95; 95% CI 0.81-1.12) was observed. This effect was consistent across RCTs. PPI use was associated with 9%, 18% and 9% lower pretreatment counts of, respectively, lymphocytes, CD19+ and CD16+CD56+ peripheral blood immune cells. There was no difference in neutrophils, CD3+, CD4+ or CD8+ immune cells according to PPI use.83
Efficacy of first-line atezolizumab combinations in NSCLC in patients receiving PPIs has been evaluated in a post hoc analysis of IMpower150 (comparing atezolizumab plus carboplatin plus paclitaxel—the ACP regimen—versus bevacizumab plus carboplatin plus paclitaxel—the BCP regimen—versus atezolizumab plus BCP—the ABCP regimen). Of the 1202 patients, 441 (36.7%) received PPIs in a 60-day window. Adjustment variables included age, sex, race, smoking status, Eastern Cooperative Oncology Group PS, histological subtype, effector T-cell gene signature score, programmed death-ligand 1 expression, epidermal growth factor receptor mutation status and the presence of liver metastases. PPI use was associated with a worse OS among participants randomized to an atezolizumab-containing arm in univariate (HR 1.55; 95% CI 1.23-1.94; P < 0.001) and adjusted analysis (HR 1.53; 95% CI 1.21-1.95; P < 0.001); similar results were obtained with PFS. In contrast, in participants randomized to BCP (without atezolizumab), there was no association between PPI use and either OS or PFS. The observed OS treatment effect of the atezolizumab arms (ACP plus ABCP) versus BCP was 1.03 (95% CI 0.77-1.36) for PPI users and 0.68 (95% CI 0.54-0.86) for PPI non-users, showing that PPI use was associated with the OS benefit of atezolizumab disappearing.84
In a Korean cohort study of 2963 NSCLC patients treated with ICIs as second line, 936 were concomitant PPI users. After propensity score matching (1 : 1 ratio), 1646 were analyzed. The use of PPIs was associated with a higher risk of mortality compared to non-use (HR 1.28; 95% CI 1.13-1.46).85
An Italian series evaluated the prognostic impact of concomitant treatments (ATB, PPIs or corticosteroids), quantified by a drug score, in a large series of patients receiving pembrolizumab or chemotherapy for NSCLC. This drug score had a predictive value for response rate, OS and PFS, essentially in the pembrolizumab cohort.86
A systematic review and meta-analysis of 14 studies, including 13 709 patients, explored the relationship between PPI uptake and survival outcomes of patients with advanced NSCLC receiving all kinds of antitumor therapy (chemotherapy, TKI, immunotherapy).87 Subgroup analyses showed that the use of PPIs was correlated with the OS or PFS of all patients (HR for OS 1.35; 95% CI 1.21-1.51; HR for PFS 1.50; 95% CI 1.25-1.80). In the subgroups of each type of treatment, the HRs of OS in the PPI group compared to PPI non-users were 1.47 (95% CI 1.20-1.80) for TKI users (essentially erlotinib and gefitinib), 1.42 (95% CI 1.22-1.65) for ICIs and 1.13 (95% CI 1.04-1.23) for chemotherapy. For PFS, these figures were: 1.71 (95% CI 1.29-2.28) and 1.29 (95% CI 1.16-1.44), respectively, for TKI and ICIs. The authors noted that publication bias and sensitivity analysis confirmed that the results were robust.87
Individual participant data from two urothelial cancer trials (IMvigor210 and 211) testing the efficacy of atezolizumab were analyzed retrospectively with regard to the concomitant use of PPIs (∼30% of patients). In the pooled group of patients receiving atezolizumab (n = 847), PPI use was a negative prognostic marker (for OS, PFS and ORR); in the randomized trial, atezolizumab showed significant efficacy on OS versus chemotherapy (HR 0.69; 95% CI 0.56-0.84) for PPI non-users, and no OS benefit (HR 1.04; 95% CI 0.81-1.34) for PPI users; the same results were observed for PFS and ORR.88 In a recent retrospective series of 227 consecutive Japanese patients with metastatic urothelial cancer treated with pembrolizumab between April 2018 and April 2021, 86 (37.9%) received concomitant PPIs. PPI use was associated with lower (but not significantly so) ORR, worse OS, 9.5 versus 18.8 months (P < 0.0001), and lower immune PFS (based on immune RECIST), 2.5 versus 4.1 months (P < 0.0001).89
In Bordeaux University Hospital, between May 2015 and September 2017, 635 patients received ICIs for cancer. The authors analyzed the influence of diverse co-medications (including PPIs) on the antitumor effect and safety of these ICIs. PPIs were prescribed in 38% of these patients; the mOS of patients receiving PPIs was 9 months versus 26.5 months in those not receiving PPIs (HR 1.70; 95% CI 1.40-2.08). Co-medication with PPIs was also associated with decreased incidence of immune-related adverse events.90
Another survey conducted in a single French center analyzed all consecutive patients with NSCLC, melanoma, RCC and urothelial cancer who received, between January 2018 and December 2019, at least one injection of nivolumab or pembrolizumab.91 Out of this cohort of 212 eligible patients, 58 (27.2%) received ATB and 74 (34.7%) PPIs. The authors built four groups: ATB−/PPI− (n = 107), ATB+/PPI− (n = 31), ATB−/PPI+ (n = 47) and ATB+/PPI+ (n = 27). ATB−/PPI− patients were significantly more frequently PS 0-1 and treated in first line than the others. But patients taking either ATB or PPIs, or both, had a lower probability of an objective response. The same conclusions can be drawn regarding PFS and OS: patients from the ATB−/PPI− group had significantly better figures than those from the other groups, and particularly from the ATB+/PPI+ group. After weighting on the propensity score, the use of ATB alone, PPIs alone or the combination were significant risk factors for death.
Limitations
All these data showing negative interactions between some major anticancer drugs and PPIs came from retrospective analysis and therefore may be discussed. Nevertheless some have been carried out on individual data coming from prospective phase III trials, and interaction was demonstrated in only one arm and confirmed by multiparametric analysis. We do not have randomized phase III trials studying the impact of PPIs and from an ethical point of view, such study will be questionable. But evidences are accumulating and it is time to rethink the use of PPIs as symptomatic treatment in treated cancer patients.
Conclusions
While some of the negative impact seen might be related to unknown confounding factors (PS, symptoms, etc.), we now have a large corpus of prospective and retrospective data to sound the alarm about concomitant use of PPIs and cancer treatments. Interactions are possible with some systemic chemotherapy drugs (5-FU or capecitabine, methotrexate, pemetrexed), but are more than likely with many TKIs and ICIs, and this is a major issue in daily practice for all medical oncologists. It sounds logical to propose a moratorium on using PPIs in cancer patients under treatment: the risk of interaction is high, and the benefit of PPIs is mild in symptomatic treatment in cancer patients. Even if some articles found no clinical interactions, particularly with ICIs, the precautionary principle must be applied until there is demonstration of the absence of clinical interaction.92, 93, 94 Ideally, prescriptions of PPIs should be avoided for heartburn or epigastralgia.95 Some tricks, such as drinking acidic beverages (cola) with erlotinib, could be proposed, but the best way is certainly to replace PPIs, long-lasting drugs, with other therapeutic means.96 If the use of potent acid-suppressive drugs is necessary, H2 antagonists (such as ranitidine) can be used and given 2 h after TKIs, as well as antacids (2 h before or after the anticancer drug).24 In patients treated with ICIs, the interaction is due to the alteration of the gut microbiome, and we can suppose that the negative effect may also be observed after long-term use of H2 antagonists. In such cases, antacids are the best option, although on-demand use of PPIs or H2 antagonists may be proposed.
Harnessing the microbiome to restore the immunotherapy response may be reached in the near future.97 The gut microbiome can easily be modified and modulated (ATB, probiotics, prebiotics, dietary modulations, fecal microbiota transplantation) and may play a major role in next-generation personalized medicine.28
Acknowledgements
The authors thank Prof. Chaitanya Divgi for useful advice.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Yin O.Q., Gallagher N., Li A., Zhou W., Harrell R., Schran H. Effect of grapefruit juice on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol. 2010;50:188–194. doi: 10.1177/0091270009336137. [DOI] [PubMed] [Google Scholar]
- 2.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 3.Vaezi M.F., Yang Y.X., Howden C.W. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y., Bowe B., Yan Y., Xian H., Li T., Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. doi: 10.1136/bmj.l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raoul J.L., Guerin-Charbonnel C., Edeline J., Simmet V., Gilabert M., Frenel J.S. Prevalence of proton pump inhibitor use among patients with cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imhann F., Bonder M.J., Vich Vila A., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reveles K.R., Ryan C.N., Chan L., Cosimi R.A., Haynes W.L. Proton pump inhibitor use associated with changes in gut microbiota composition. Gut. 2018;67:1369–1370. doi: 10.1136/gutjnl-2017-315306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weersma R.K., Zhernakova A., Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Leeuwen R.W.F., Jansman F.G.A., Hunfeld N.G., et al. Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin Pharmacokinet. 2017;56:683–688. doi: 10.1007/s40262-016-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tvingsholm S.A., Dehlendorff C., Osterlind K., Friis S., Jaattela M. Proton pump inhibitor use and cancer mortality. Int J Cancer. 2018;143:1315–1326. doi: 10.1002/ijc.31529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Liu Q., Halfdanarson O.O., et al. Proton pump inhibitors and survival in patients with colorectal cancer: a Swedish population-based cohort study. Br J Cancer. 2021;125:893–900. doi: 10.1038/s41416-021-01480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoul J.L., Edeline J., Gilabert M., Senellart H., Frenel J.S. Proton pump inhibitors and cancers: a hazardous association? Bull Cancer. 2020;107:458–464. doi: 10.1016/j.bulcan.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Delchier J.C., Benamouzig R., Stanescu L., et al. Twenty-four-hour intragastric acidity and plasma gastrin during 3-month treatment with omeprazole in healthy subjects. Aliment Pharmacol Ther. 1997;11:747–753. doi: 10.1046/j.1365-2036.1997.00182.x. [DOI] [PubMed] [Google Scholar]
- 14.Kearns M.D., Boursi B., Yang Y.X. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol. 2017;46:80–84. doi: 10.1016/j.canep.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raoul J.L., Edeline J., Simmet V., Moreau-Bachelard C., Gilabert M., Frenel J.S. Long-term use of proton pump inhibitors in cancer patients: an opinion paper. Cancers (Basel) 2022;14:1156. doi: 10.3390/cancers14051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veettil S.K., Sadoyu S., Bald E.M., et al. Association of proton-pump inhibitor use with adverse health outcomes: a systematic umbrella review of meta-analyses of cohort studies and randomised controlled trials. Br J Clin Pharmacol. 2022;88:1551–1566. doi: 10.1111/bcp.15103. [DOI] [PubMed] [Google Scholar]
- 17.Kedika R.R., Souza R.F., Spechler S.J. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghebremariam Y.T., Cooke J.P., Gerhart W., et al. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J Transl Med. 2015;13:249. doi: 10.1186/s12967-015-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara T., Arakawa T. Lansoprazole decreases peripheral blood monocytes and intercellular adhesion molecule-1-positive mononuclear cells. Dig Dis Sci. 1999;44:1710–1715. doi: 10.1023/a:1026604203237. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida N., Yoshikawa T., Tanaka Y., et al. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14(suppl 1):74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 21.Namazi M.R., Jowkar F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J Clin Pharm Ther. 2008;33:215–217. doi: 10.1111/j.1365-2710.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 22.Corbet C., Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer. 2017;17:577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 23.Budha N.R., Frymoyer A., Smelick G.S., et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92:203–213. doi: 10.1038/clpt.2012.73. [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen R.W., van Gelder T., Mathijssen R.H., Jansman F.G. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15:e315–e326. doi: 10.1016/S1470-2045(13)70579-5. [DOI] [PubMed] [Google Scholar]
- 25.Indini A., Petrelli F., Tomasello G., et al. Impact of use of gastric-acid suppressants and oral anti-cancer agents on survival outcomes: a systematic review and meta-analysis. Cancers (Basel) 2020;12:998. doi: 10.3390/cancers12040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maier L., Pruteanu M., Kuhn M., et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vich Vila A., Collij V., Sanna S., et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting N.L., Lau H.C., Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71:1412–1425. doi: 10.1136/gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vetizou M., Pitt J.M., Daillere R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivan A., Corrales L., Hubert N., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert M.L., Rohrbach R., Schmitt M., Stein-Thoeringer C.K. The potential role of the intestinal micromilieu and individual microbes in the immunobiology of chimeric antigen receptor T-cell therapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.670286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matson V., Fessler J., Bao R., et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinato D.J., Howlett S., Ottaviani D., et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derosa L., Hellmann M.D., Spaziano M., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Song Y., Wang J., et al. Effect of proton pump inhibitors on high-dose methotrexate elimination: a systematic review and meta-analysis. Int J Clin Pharm. 2020;42:23–30. doi: 10.1007/s11096-019-00958-5. [DOI] [PubMed] [Google Scholar]
- 36.Slimano F., Le Bozec A., Cransac A., et al. Association between proton pump inhibitors and severe hematological toxicity in patients receiving pemetrexed-based anticancer treatment: the prospective IPPEM study. Lung Cancer. 2022;166:114–121. doi: 10.1016/j.lungcan.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Ikemura K., Hamada Y., Kaya C., et al. Lansoprazole exacerbates pemetrexed-mediated hematologic toxicity by competitive inhibition of renal basolateral human organic anion transporter 3. Drug Metab Dispos. 2016;44:1543–1549. doi: 10.1124/dmd.116.070722. [DOI] [PubMed] [Google Scholar]
- 38.Chu M.P., Hecht J.R., Slamon D., et al. Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: secondary analysis of the TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. 2017;3:767–773. doi: 10.1001/jamaoncol.2016.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong G.G., Ha V., Chu M.P., et al. Effects of proton pump inhibitors on FOLFOX and CapeOx regimens in colorectal cancer. Clin Colorectal Cancer. 2019;18:72–79. doi: 10.1016/j.clcc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Sun J., Ilich A.I., Kim C.A., et al. Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin Colorectal Cancer. 2016;15:257–263. doi: 10.1016/j.clcc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Kitazume Y., Kawazoe H., Uozumi R., et al. Proton pump inhibitors affect capecitabine efficacy in patients with stage II-III colorectal cancer: a multicenter retrospective study. Sci Rep. 2022;12:6561. doi: 10.1038/s41598-022-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S.Y., Lee J.S., Kang J., et al. Proton pump inhibitor use and the efficacy of chemotherapy in metastatic colorectal cancer: a post hoc analysis of a randomized phase III trial (AXEPT) Oncologist. 2021;26:e954–e962. doi: 10.1002/onco.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kichenadasse G., Miners J.O., Mangoni A.A., Karapetis C.S., Hopkins A.M., Sorich M.J. Proton pump inhibitors and survival in patients with colorectal cancer receiving fluoropyrimidine-based chemotherapy. J Natl Compr Canc Netw. 2021;19:1037–1044. doi: 10.6004/jnccn.2020.7670. [DOI] [PubMed] [Google Scholar]
- 44.van Doorn L., Heersche N., de Man F.M., et al. Effect of the proton pump inhibitor esomeprazole on the systemic exposure of capecitabine: results of a randomized crossover trial. Clin Pharmacol Ther. 2022;111:455–460. doi: 10.1002/cpt.2444. [DOI] [PubMed] [Google Scholar]
- 45.Pouya F.D., Rasmi Y., Camci I.Y., Tutar Y., Nemati M. Performance of capecitabine in novel combination therapies in colorectal cancer. J Chemother. 2021;33:375–389. doi: 10.1080/1120009X.2021.1920247. [DOI] [PubMed] [Google Scholar]
- 46.Parsad S., Ratain M.J. Drug-drug interactions with oral antineoplastic agents. JAMA Oncol. 2017;3:736–738. doi: 10.1001/jamaoncol.2016.3323. [DOI] [PubMed] [Google Scholar]
- 47.Uchiyama A.A.T., Silva P., Lopes M.S.M., et al. Proton pump inhibitors and oncologic treatment efficacy: a practical review of the literature for oncologists. Curr Oncol. 2021;28:783–799. doi: 10.3390/curroncol28010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters S., Zimmermann S., Adjei A.A. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug-drug interactions. Cancer Treat Rev. 2014;40:917–926. doi: 10.1016/j.ctrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Veerman G.D.M., Hussaarts K., Peric R., et al. Influence of cow’s milk and esomeprazole on the absorption of erlotinib: a randomized, crossover pharmacokinetic study in lung cancer patients. Clin Pharmacokinet. 2021;60:69–77. doi: 10.1007/s40262-020-00910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu M.P., Ghosh S., Chambers C.R., et al. Gastric acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clin Lung Cancer. 2015;16:33–39. doi: 10.1016/j.cllc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Fang Y.H., Yang Y.H., Hsieh M.J., Hung M.S., Lin Y.C. Concurrent proton-pump inhibitors increase risk of death for lung cancer patients receiving 1st-line gefitinib treatment – a nationwide population-based study. Cancer Manag Res. 2019;11:8539–8546. doi: 10.2147/CMAR.S222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma M., Holmes H.M., Mehta H.B., et al. The concomitant use of tyrosine kinase inhibitors and proton pump inhibitors: prevalence, predictors, and impact on survival and discontinuation of therapy in older adults with cancer. Cancer. 2019;125:1155–1162. doi: 10.1002/cncr.31917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lalani A.A., McKay R.R., Lin X., Simantov R., Kaymakcalan M.D., Choueiri T.K. Proton pump inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2017;15:724–732. doi: 10.1016/j.clgc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.H., Shen M.C., Tsai M.J., et al. Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib. Sci Rep. 2022;12:7002. doi: 10.1038/s41598-022-10938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boegemann M., Schlack K., Rink M., et al. Effect of comorbidities/comedications on sunitinib outcomes for metastatic renal cell carcinoma: the STAR-TOR registry. Future Oncol. 2020;16:2939–2948. doi: 10.2217/fon-2020-0548. [DOI] [PubMed] [Google Scholar]
- 56.Tan A.R., Gibbon D.G., Stein M.N., et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1635–1643. doi: 10.1007/s00280-013-2164-3. [DOI] [PubMed] [Google Scholar]
- 57.Krens S.D., Lubberman F.J.E., van Egmond M., et al. The impact of a 1-hour time interval between pazopanib and subsequent intake of gastric acid suppressants on pazopanib exposure. Int J Cancer. 2021;148:2799–2806. doi: 10.1002/ijc.33469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mourey L., Le Louedec F., Ravaud A., et al. VOTRAGE study: phase I dose-escalation study of pazopanib in unfit older patients. J Geriatr Oncol. 2021;12:759–764. doi: 10.1016/j.jgo.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Mir O., Touati N., Lia M., et al. Impact of concomitant administration of gastric acid-suppressive agents and pazopanib on outcomes in soft-tissue sarcoma patients treated within the EORTC 62043/62072 trials. Clin Cancer Res. 2019;25:1479–1485. doi: 10.1158/1078-0432.CCR-18-2748. [DOI] [PubMed] [Google Scholar]
- 60.Van De Sijpe G., Beuselinck B., Van Nieuwenhuyse T., et al. Impact of concomitant acid suppressive therapy on pazopanib efficacy and dose reductions in patients with metastatic renal cell carcinoma. Eur J Clin Pharmacol. 2020;76:1273–1280. doi: 10.1007/s00228-020-02902-3. [DOI] [PubMed] [Google Scholar]
- 61.McAlister R.K., Aston J., Pollack M., Du L., Koyama T., Chism D.D. Effect of concomitant pH-elevating medications with pazopanib on progression-free survival and overall survival in patients with metastatic renal cell carcinoma. Oncologist. 2018;23:686–692. doi: 10.1634/theoncologist.2017-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreau-Bachelard C., Letailleur V., Bompas E., Soulie P., Paul J., Raoul J.L. Effect of concomitant proton pump inhibitors with pazopanib on cancer patients: a retrospective analysis. Cancers (Basel) 2022;14:4721. doi: 10.3390/cancers14194721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Man F.M., Hussaarts K., de With M., et al. Influence of the proton pump inhibitor esomeprazole on the bioavailability of regorafenib: a randomized crossover pharmacokinetic study. Clin Pharmacol Ther. 2019;105:1456–1461. doi: 10.1002/cpt.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vishwanathan K., Dickinson P.A., Bui K., et al. The effect of food or omeprazole on the pharmacokinetics of osimertinib in patients with non-small-cell lung cancer and in healthy volunteers. J Clin Pharmacol. 2018;58:474–484. doi: 10.1002/jcph.1035. [DOI] [PubMed] [Google Scholar]
- 65.Yekeduz E., Ozbay M.F., Caglayan D., et al. Clinical outcomes of concomitant use of proton pump inhibitors and regorafenib in patients with metastatic colorectal cancer: a multicenter study. Eur J Clin Pharmacol. 2022;78:1973–1979. doi: 10.1007/s00228-022-03403-1. [DOI] [PubMed] [Google Scholar]
- 66.Wu C.Y., Ho H.J., Wu C.Y., et al. Association between proton pump inhibitor use and mortality in patients with hepatocellular carcinoma receiving tyrosine kinase inhibitor. Gut. 2020 doi: 10.1136/gutjnl-2020-321932. gutjnl-2020-321932. [DOI] [PubMed] [Google Scholar]
- 67.Razak R.A., Fletcher P., Kunene V., Ma Y.T. Association of gastric acid suppression and sorafenib efficacy in advanced hepatocellular carcinoma. J Clin Gastroenterol. 2021;55:169–173. doi: 10.1097/MCG.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 68.Dam G., Vilstrup H., Watson H., Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 69.Ruanglertboon W., Sorich M.J., Logan J.M., Rowland A., Hopkins A.M. The effect of proton pump inhibitors on survival outcomes in advanced hepatocellular carcinoma treated with sorafenib. J Cancer Res Clin Oncol. 2020;146:2693–2697. doi: 10.1007/s00432-020-03261-3. [DOI] [PubMed] [Google Scholar]
- 70.Bridoux M., Simon N., Turpin A. Proton pump inhibitors and cancer: current state of play. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.798272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L., Xiang Y.X., Yang G.P., et al. Pharmacokinetic effects of proton pump inhibitors on the novel PARP inhibitor fluzoparib: a single-arm, fixed-sequence trial in male healthy volunteers. Invest New Drugs. 2021;39:796–802. doi: 10.1007/s10637-020-01034-w. [DOI] [PubMed] [Google Scholar]
- 72.Sun W., Klamerus K.J., Yuhas L.M., et al. Impact of acid-reducing agents on the pharmacokinetics of palbociclib, a weak base with pH-dependent solubility, with different food intake conditions. Clin Pharmacol Drug Dev. 2017;6:614–626. doi: 10.1002/cpdd.356. [DOI] [PubMed] [Google Scholar]
- 73.Del Re M., Omarini C., Diodati L., et al. Drug-drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samant T.S., Dhuria S., Lu Y., et al. Ribociclib bioavailability is not affected by gastric pH changes or food intake: in silico and clinical evaluations. Clin Pharmacol Ther. 2018;104:374–383. doi: 10.1002/cpt.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eser K., Onder A.H., Sezer E., et al. Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study. BMC Cancer. 2022;22:516. doi: 10.1186/s12885-022-09624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibbons J.A., de Vries M., Krauwinkel W., et al. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54:1057–1069. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin B.D., Jiao X.D., Zhou X.C., et al. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1929727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C., Guo H., Mao H., Tong J., Yang M., Yan X. An up-to-date investigation into the correlation between proton pump inhibitor use and the clinical efficacy of immune checkpoint inhibitors in advanced solid cancers: a systematic review and meta-analysis. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.753234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gopalakrishnan V., Spencer C.N., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homicsko K., Richtig G., Tuchmann F., et al. Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann Oncol. 2018;29(suppl 10):X40. [Google Scholar]
- 81.Homicsko K., Dummer R., Hoeller C., et al. Proton pump inhibitor use and efficacy of nivolumab and ipilimumab in advanced melanoma. Cancers (Basel) 2022;14:2300. doi: 10.3390/cancers14092300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chalabi M., Cardona A., Nagarkar D.R., et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Hopkins A.M., Badaoui S., Kichenadasse G., et al. Efficacy of atezolizumab in patients with advanced NSCLC receiving concomitant antibiotic or proton pump inhibitor treatment: pooled analysis of five randomized control trials. J Thorac Oncol. 2022;17:758–767. doi: 10.1016/j.jtho.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Hopkins A.M., Kichenadasse G., McKinnon R.A., et al. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: post hoc analysis of IMpower150. Br J Cancer. 2022;126:42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baek Y.H., Kang E.J., Hong S., Park S.H., Kim J.H., Shin J.Y. Survival outcomes of patients with non-small cell lung cancer concomitantly receiving proton pump inhibitors and immune checkpoint inhibitors. Int J Cancer. 2022;150:1291–1300. doi: 10.1002/ijc.33892. [DOI] [PubMed] [Google Scholar]
- 86.Buti S., Bersanelli M., Perrone F., et al. Predictive ability of a drug-based score in patients with advanced non-small-cell lung cancer receiving first-line immunotherapy. Eur J Cancer. 2021;150:224–231. doi: 10.1016/j.ejca.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 87.Wei N., Zheng B., Que W., Zhang J., Liu M. The association between proton pump inhibitor use and systemic anti-tumour therapy on survival outcomes in patients with advanced non-small cell lung cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88:3052–3063. doi: 10.1111/bcp.15276. [DOI] [PubMed] [Google Scholar]
- 88.Hopkins A.M., Kichenadasse G., Karapetis C.S., Rowland A., Sorich M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin Cancer Res. 2020;26:5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 89.Fukuokaya W., Kimura T., Komura K., et al. Effectiveness of pembrolizumab in patients with urothelial carcinoma receiving proton pump inhibitors. Urol Oncol. 2022;40:346.e1–346.e8. doi: 10.1016/j.urolonc.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 90.Kostine M., Mauric E., Tison A., et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur J Cancer. 2021;157:474–484. doi: 10.1016/j.ejca.2021.08.036. [DOI] [PubMed] [Google Scholar]
- 91.Giordan Q., Salleron J., Vallance C., Moriana C., Clement-Duchene C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng K., Chen K., Teply B.A., Yee G.C., Farazi P.A., Lyden E.R. Impact of proton pump inhibitor use on the effectiveness of immune checkpoint inhibitors in advanced cancer patients. Ann Pharmacother. 2022;56:377–386. doi: 10.1177/10600280211033938. [DOI] [PubMed] [Google Scholar]
- 93.Li C., Xia Z., Li A., Meng J. The effect of proton pump inhibitor uses on outcomes for cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ann Transl Med. 2020;8:1655. doi: 10.21037/atm-20-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mollica V., Santoni M., Matrana M.R., et al. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target Oncol. 2022;17:61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 95.Farrell B., Pottie K., Thompson W., et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354–364. [PMC free article] [PubMed] [Google Scholar]
- 96.van Leeuwen R.W., Peric R., Hussaarts K.G., et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2016;34:1309–1314. doi: 10.1200/JCO.2015.65.2560. [DOI] [PubMed] [Google Scholar]
- 97.Bullman S., Eggermont A., Johnston C.D., Zitvogel L. Harnessing the microbiome to restore immunotherapy response. Nat Cancer. 2021;2:1301–1304. doi: 10.1038/s43018-021-00300-x. [DOI] [PubMed] [Google Scholar]