Abstract
Background
Autoimmune IgG response has been described in the pathogenesis of asthma in adults, but IgE autoimmunity has been little explored. Considering high levels of blood eosinophils and immunoglobulin E in asthmatic patients, the possibility of IgE autoantibody response to eosinophil proteins arises.
Objective
To explore the presence of IgE and IgG autoantibodies against Eosinophil peroxidase (EPX) and Eosinophil cationic protein (ECP).
Methods
Three steps were followed: 1) The frequency of IgE and IgG autoantibodies against EPX and ECP was investigated among asthmatic and healthy subjects. 2) The ability of IgE autoantibodies to induce an inflammatory response (basophil activation) was performed. 3) The capacity of autoantibodies to identify patients with severe asthma was evaluated.
Results
Asthmatic and healthy subjects had IgE and IgG autoantibodies against EPX and ECP. Anti-EPX IgE was significantly higher in asthmatic patients. Severe asthmatic patients had a higher frequency and higher levels of IgE and IgG autoantibodies compared to healthy subjects. There was not a correlation between autoantibodies and blood eosinophils. Children younger than 14 years of age had IgE and IgG autoantibodies against to EPX and ECP. IgE autoantibodies to EPX and ECP induced basophil activation in asthmatic patients.
Conclusion
In this study, we identify for the first time IgE autoantibodies against EPX and ECP in adults and children patients with asthma; IgE and IgG autoantibodies against EPX and ECP could serve as a predictive biomarker of the clinical severity.
Keywords: Asthma, Autoreactivity, Autoantibodies, Eosinophils, Immunoglobulin E
Introduction
Allergic asthma is a common respiratory disease with a high impact on quality of life.1 The clinical definition of asthma covers different phenotypes and endotypes that are strongly related to age. Based on the characteristics of the inflammatory process, Th2 cytokines are the most prevalent in patients with asthma (60%–80%),2,3 especially in childhood. In this type of inflammation, specific IgE against environmental allergens and recruitment of eosinophils are important mediators of lung symptoms.4,5 In recent years, new mechanisms have been proposed; IgG reactivity to several autoantigens has been reported in adult patients with asthma,6,7 opening the hypothesis of an autoimmune endotype. IgE autoreactivity against autoantigens and its role in inflammation has been demonstrated in different diseases8, 9, 10, 11, 12 but little is known about asthma and its presence in childhood population.
Mediators of the inflammatory asthma process include basophils, neutrophils, and especially eosinophils.13,14 Eosinophils through their proteins such as major basic protein (MBP), eosinophil peroxidase (EPX) and eosinophil cationic protein (ECP) are capable of generating an inflammatory response.15,16 Recently, sputum IgG response against EPX was detected in asthma and it was associated with severe asthma and corticosteroids resistance.6
Considering the central role of specific IgE and eosinophil cytoplasmic granules in the pathogenesis of asthma, we hypothesized that EPX and ECP may be possibly recognized by IgE autoantibody, and this response could be associated with chronicity and severity of the disease. The aim of this study was to investigate the presence of IgE and IgG autoantibodies against eosinophil proteins EPX and ECP and to explore the possible relationship with asthma inflammation and age.
Methods
Study design and population
A cross-sectional study was performed using 2 groups: asthmatic and healthy subjects. The asthma group included patients with at least 2 years of asthma recruited from 3 medical institutions in Medellín, Colombia. Control subjects were students and workers invited from educational institutions. Severity of asthma was defined according to GINA 2020 recommendations by treatment (https://ginasthma.org/); Mild asthma corresponds to a disease that can be controlled with step 1 or 2 treatment, moderate asthma was step 3 or 4 and severe asthma was step 5 treatment. Patients with clinical indication for the use of a biological therapy for asthma were included if they had not started such therapy. Subjects in both groups consisted of people between 5 and 55 years without a clinical history of autoimmune diseases, atopic dermatitis, chronic obstructive pulmonary disease, or any other chronic pulmonary disease. Smokers were excluded in both groups.
Participants with a diagnosis of systemic diseases (eg, mastocytosis, dermatitis), infection within 4 weeks of participation, or pharmacologic treatment (eg, Cyclosporine), that could affect the interpretation of the laboratory results were not included.
Antigen's production
ECP and EPX were obtained as recombinant proteins according to a previous protocol10,17 using Escherichia coli BL21 (DE3) as an expression vector.
Determination of the total IgE, specific IgE and IgG autoantibodies
Total IgE levels in the serum samples were determined using a fluoroenzyme immunoassay (ImmunoCap System, Thermofisher, Sweden). When the levels were above the reading range of the equipment (>100 KUA/mL), the sample was diluted 1:5 or 1:10 depending on the case, and the total concentration was calculated by conversion.
We explored the presence of IgE autoantibodies against EPX, and ECP by ELISA technique after purification to avoid contamination of vector proteins.10,17 The sera used for the quantification of IgE were previously depleted of IgG by immunoaffinity depletion; For each 500 L of serum and 1X PBS (1:5) were added 80 L of Protein G (Sigma-P3296, Protein G Sepharose®). After 60 min of incubation at room temperature (RT) on a rotating platform, the serum was recovered by centrifugation and stored at −20 °C until use. Residual IgG contents in protein G-adsorbed sera were measured by enzyme-linked immunosorbent assay (ELISA) assays and sera were accepted for anti-EPX/ECP IgE measure when IgG were found to be less than 10 μg/mL. The specificity of the binding was evaluated by inhibition tests.
For ELISA, 100 μL was loaded into wells by duplicated coated with the relevant antigen, and the plates were incubated overnight. Each plate had control and asthma samples. A calibration curve was done (0.001, 0.01, 0.1, 1 u/ml) for each antigen and PBS as negative controls was used. Considering previous studies,18,19 antigens were reduced with SDS. The cut-off value for serum anti-EPX IgE and anti-ECP IgE were defined as the mean plus three standard deviations of absorbance values from 60 healthy controls (IgE-EPX 0.354, IgE-ECP 0.279, IgG-EPX 0.425, IgG-ECP 0.414). The results were expressed in optical density units (OD). The absorbance at 405 nm was determined using a spectrophotometer.
For the determination of anti-TPO IgG, the same protocol was followed, except for the IgG depletion and that the human sera adsorbed with E. coli lysate were diluted 1:50 and the secondary antibody was alkaline phosphatase-conjugated anti-IgG (Pharmigen).
CD203c expression
Peripheral blood basophils were obtained from subjects from each group. We used the CD203c expression protocol previously reported.10,17 Briefly, basophil activation test (BAT) was performed with the whole blood collected in EDTA tubes and red blood cells were lysed with a lysis buffer. The 100 μL of the leukocyte suspension were added along with calcium ionophore, anti-human goat IgE antibodies and anti-IgG4. Basophil activation was stimulated with different concentrations of EPX/ECP antigens (0.01, 0.1 and 1 μg/mL). After washing with PBS, the cells were stained with phycoerythrin (PE)-conjugated antihuman CD203c and fluorescein isothiocyanate (FITC)-conjugated antihuman CD123. CD203c expression was measured by flow cytometry.
The percentage of CD203c expression was defined as the percentage of basophils expressing more CD203c than the critical point, which was ≥10.0% of the basophils incubated with buffer only, similar to previous studies.10,17,20
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM, Inc, Chicago, IL) and GraphPad Prism 8 (La Jolla, CA). The Shapiro–Wilk test was used to check for normality, and our descriptive and statistical analyses were chosen according to the results. The data are presented as the means and standard deviation for variables with a normal distribution and as the median and range when not. The Mann–Whitney U test were used to compare specific IgE levels. Fisher's χ2 test was used to evaluate the differences among groups and proportions. Correlations were assessed with the Pearson or Spearman coefficient (r).
Given the results of previous studies,21, 22, 23, 24 we considered that a sample of at least 30 patients with asthma and 40 healthy subjects would be adequate to ensure a power of 80% and an alpha error of 0.05 for the primary outcome (the presence of IgE autoantibodies).
Results
Patients’ characteristics
Sixty control subjects and 55 asthmatic patients were recruited (Table 1). More sociodemographic characteristics were similar in both groups. The asthmatic group had higher levels of total IgE (mean 181 vs 139 IU/mL) and blood eosinophils (mean 126 vs 106 cells/ml) but these 2 variables were not significantly different. Atopy was present in 70.9% of the asthmatic patients and 20% of control subjects (p < 0.001). Nasal diseases (rhinitis, rhinosinusitis, polyposis), positive anti-nuclear antibodies, and family history of autoimmunity comorbidities were more frequent in asthmatic group, but only rhinitis was significant (p < 0.001).
Table 1.
Sociodemographic characteristics.
| General characteristics | Asthma (n = 55) | Control (n = 60) | P value |
|---|---|---|---|
| Age Median, (Range, min-max) | 206, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44,44, 44, 45, 46, 47, 48, 49, 50 | 236, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44,44, 44, 45, 46, 47, 48, 49, 50 | 0.3 |
| Age ≤ 14 years (%) | 17 (30.9%) | 15 (25%) | 0.3 |
| Sex: male, n (%) | 30 (54.5%) | 26 (43.3%) | 0.2 |
| Atopy (%) | 39 (70.9%) | 12 (20%) | <0.001 |
| Rhinitis (%) | 43 (78.2%) | 11 (18.3%) | <0.001 |
| Sinusitis (%) | 6 (10.9%) | 2 (3.3%) | 0.1 |
| CRS with polyps (%) | 2 (3.6%) | 0 | 0.2 |
| ANAs (%) | 4 (7,2%) | 1 (1.6%) | 0.1 |
| Family history of autoimmunity | 8 (14,4%) | 4 (6.6%) | 0.2 |
| Total IgE (IU/ml), mean ± SD | 181 (174) | 139 (84) | 0.1 |
| Eosinophil serum (cells/ml) mean ± SD | 126 (71) | 10744 | 0.08 |
| Asthma severity according GINA stepsModerateSevere | 42 (76.3%) 13 (23.6%) |
N/A N/A |
N/A N/A |
| Patients with OCS in the last year | 11 (20%) | N/A | N/A |
| Years with Asthma median, (Range) | 38 | N/A | N/A |
| FEV1 (%) median, (Range) | 8930 | N/A | N/A |
| FEV1/FVC ratio median, (Range) | 0,80 (0.5) | N/A | N/A |
All subjects were over 6 years old. N/A: Not applicable. SD: Standard deviation. FVC: Forced vital capacity, FEV1: Forced expiratory volume. Atopy was defined as the presence of specific IgE to an allergenic source (mites, pets, etc.). The patients in the control group were not evaluated for lung function because they did not have respiratory symptoms
IgE against eosinophil autoantigens
Anti-EPX IgE and anti-ECP IgE levels were significantly higher in the asthma group versus control group (Fig. 1). Eight asthmatic patients have anti-EPX IgE and 5 have anti-ECP IgE above the detection threshold (Fig. 1); Three of these patients have both anti-ECP IgE and anti-EPX IgE. Despite the higher number of positive anti-ECP/EPX IgE in the asthma group, only anti-EPX IgE was statistically significant in the asthma group versus control group (anti-ECP IgE 0.04). Patients with IgE autoantibodies against EPX or ECP have a lower FEV1 than asthmatic patients without IgE autoantibodies (91.1% vs 78.3%, p = 0.02) and lower FEV1/FVC (0.82 vs 0.74, p = 0.03).
Fig. 1.
Asthma IgE and IgG against EPX and ECP. In a, patients with IgE or IgG autoantibodies are presented in circles and were compared between groups. In b, concentration levels according to optical density units and eosinophil blood count are presented in mean and standard deviation and were compared between groups. Cut-off; IgE-EPX 0.354, IgE-ECP 0.279, IgG-EPX 0.425, IgG-ECP 0.414
The age minimum and maximum (min-max) of the subjects with IgE autoantibodies was 6–33 years; in the asthma group; most of them had severe asthma (n = 6) (Table 2). Nine asthmatic and 3 subjects in the control groups had IgE autoantibodies and IgG autoantibodies.
Table 2.
Subjects with IgE autoantibodies.
| Group | Age | Sex | Atopy | Rhinosinusitis | Severity | OSC | FEV1 | FEV1/FVC | Blood eosinophils (cell/mL) | Total IgE (IU/mL) | Anti-EPX IgE | Anti-ECP IgE | Anti-EPX IgG | Anti-ECP IgG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asthma | 16 | Female | Yes | No | Severe | 2 | 77 | 0.67 | 132 | 122 | Yes | Yes | Yes | Yes |
| Asthma | 25 | Female | Yes | Yes | Moderate | 0 | 80 | 0.81 | 221 | 300 | Yes | Yes | Yes | Yes |
| Asthma | 12 | Male | Yes | Yes | Severe | 1 | 95 | 0.89 | 140 | 50 | Yes | No | Yes | No |
| Asthma | 6 | Female | Yes | No | Severe | 2 | 55 | 0.6 | 92 | 223 | Yes | No | No | No |
| Asthma | 17 | Male | No | No | Severe | 2 | 70 | 0.61 | 128 | 111 | Yes | Yes | Yes | No |
| Asthma | 28 | Female | Yes | Yes | Severe | 1 | 75 | 0.7 | 314 | 1042 | Yes | No | Yes | No |
| Asthma | 17 | Male | No | No | Severe | 1 | 80 | 0.9 | 57 | 55 | Yes | No | Yes | Yes |
| Asthma | 24 | Male | Yes | Yes | Moderate | 0 | 98 | 0.85 | 315 | 888 | Yes | No | Yes | Yes |
| Asthma | 7 | Female | Yes | No | Severe | 2 | 77 | 0.7 | 205 | 333 | No | Yes | No | Yes |
| Asthma | 33 | Male | Yes | No | Moderate | 0 | 93 | 0.86 | 100 | 128 | No | Yes | No | Yes |
| Control | 10 | Male | Yes | No | N/A | N/A | N/A | N/A | 122 | 169 | Yes | No | Yes | No |
| Control | 30 | Female | No | No | N/A | N/A | N/A | N/A | 130 | 148 | Yes | No | Yes | No |
| Control | 20 | Female | No | No | N/A | N/A | N/A | N/A | 140 | 175 | No | Yes | No | Yes |
| Control | 11 | Female | No | Yes | N/A | N/A | N/A | N/A | 199 | 201 | No | Yes | No | No |
| Control | 23 | Female | No | No | N/A | N/A | N/A | N/A | 134 | 123 | No | Yes | No | No |
Asthma patients in control subjects with anti-EPX IgE or anti-ECP IgE. FVC: Forced vital capacity, FEV1: Forced expiratory volume. N/A: No apply. OCS: Cycles of oral systemic corticosteroid
Asthma and control group showed a low but significant correlation among the total IgE levels and number of eosinophils in serum (Asthma group r = 0.480, CI 0.232 to 0.662, p = 0.002. Control group r = 0.360, CI = 0.109 to 0.568, p = 0.005) (Fig. 3). There was not an association between autoantibody levels and blood eosinophils according to correlation or using different cut-off stratification (>150 cells/ml or >300 cells/ml eosinophils).
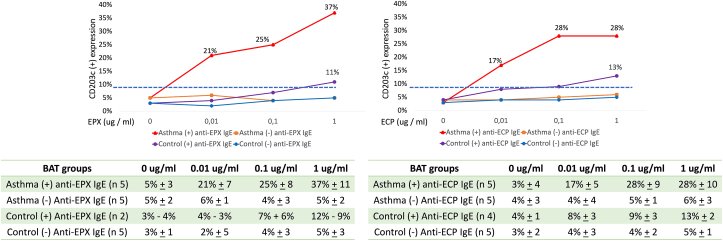
Fig. 3.
Basophil activation test with EPX and ECP. Basophil activation test using different concentrations of EPX and ECP. EPX: Eosinophil peroxidase. ECP: Eosinophil cationic protein. In the table CD203c activation is presented in median and range. “n” number of patients per group. For Control (+) anti-EPX IgE we put the value of CD203 expression for each patient
IgG against eosinophil autoantigens
In the asthma and control group, anti-EPX IgG was the most prevalent autoantibody, the asthma group had a higher frequency of IgG autoantibodies than the control group (18 (32.7%) vs 10 (16.6%) p = 0.04) (Table 3). The age minimum and maximum of the subjects with IgG antibodies was 7–50 years; 7 of them had severe asthma (n = 7) (Table 3).
Table 3.
Subjects with IgG autoantibodies.
| Group | Age | Sex | Atopy | Rhinosinusitis | Severity | OCS | FEV1 | FEV1/FVC | Blood eosinophils (cell/mL) | Total IgE | Anti-EPX IgE | Anti-ECP IgE | Anti-EPX IgG | Anti-ECP IgG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asthma | 16 | Female | Yes | No | Severe | 2 | 77,3 | 0,67 | 132 | 122 | Yes | Yes | Yes | Yes |
| Asthma | 25 | Female | Yes | Yes | Moderate | 0 | 80 | 0,81 | 221 | 300 | Yes | Yes | Yes | Yes |
| Asthma | 17 | Male | No | No | Severe | 2 | 70 | 0,61 | 128 | 111 | Yes | Yes | Yes | No |
| Asthma | 28 | Female | Yes | Yes | Severe | 1 | 75 | 0,7 | 314 | 1042 | Yes | No | Yes | No |
| Asthma | 17 | Male | No | No | Severe | 1 | 80 | 0,9 | 57 | 55 | Yes | No | Yes | Yes |
| Asthma | 24 | Male | Yes | Yes | Moderate | 0 | 98 | 0,85 | 315 | 888 | Yes | No | Yes | Yes |
| Asthma | 7 | Female | Yes | No | Severe | 2 | 77 | 0,7 | 205 | 333 | No | Yes | No | Yes |
| Asthma | 33 | Male | Yes | No | Moderate | 0 | 93 | 0,86 | 100 | 128 | No | Yes | No | Yes |
| Asthma | 12 | Male | Yes | Yes | Severe | 1 | 95 | 0,89 | 140 | 50 | No | No | Yes | No |
| Asthma | 18 | Female | Yes | No | Moderate | 0 | 102 | 0,78 | 156 | 156 | No | No | Yes | No |
| Asthma | 13 | Male | Yes | No | Moderate | 0 | 97 | 0,85 | 125 | 101 | No | No | Yes | No |
| Asthma | 14 | Male | Yes | No | Moderate | 0 | 89 | 0,85 | 126 | 101 | No | No | Yes | No |
| Asthma | 20 | Female | Yes | No | Moderate | 0 | 96 | 0,9 | 130 | 222 | No | No | Yes | No |
| Asthma | 35 | Male | No | No | Moderate | 0 | 93 | 0,71 | 135 | 132 | No | No | Yes | No |
| Asthma | 40 | Male | Yes | No | Moderate | 0 | 95 | 0,95 | 126 | 20 | No | No | Yes | No |
| Asthma | 50 | Female | Yes | No | Severe | 3 | 55 | 0,59 | 130 | 115 | No | No | Yes | Yes |
| Asthma | 45 | Female | Yes | No | Moderate | 0 | 101 | 0,8 | 120 | 113 | No | No | No | Yes |
| Asthma | 29 | Female | No | No | Moderate | 0 | 96 | 0,83 | 90 | 133 | No | No | No | Yes |
| Control | 10 | Male | Yes | No | N/A | N/A | N/A | N/A | 122 | 169 | Yes | No | Yes | No |
| Control | 30 | Female | No | No | N/A | N/A | N/A | N/A | 130 | 148 | Yes | No | Yes | No |
| Control | 20 | Female | No | No | N/A | N/A | N/A | N/A | 140 | 175 | No | Yes | No | Yes |
| Control | 24 | Male | No | No | N/A | N/A | N/A | N/A | 55 | 110 | No | No | Yes | No |
| Control | 33 | Female | No | No | N/A | N/A | N/A | N/A | 110 | 101 | No | No | Yes | No |
| Control | 41 | Male | No | No | N/A | N/A | N/A | N/A | 121 | 88 | No | No | Yes | No |
| Control | 46 | Male | No | No | N/A | N/A | N/A | N/A | 66 | 53 | No | No | Yes | No |
| Control | 30 | Female | No | No | N/A | N/A | N/A | N/A | 160 | 113 | No | No | Yes | Yes |
| Control | 33 | Male | No | No | N/A | N/A | N/A | N/A | 130 | 145 | No | No | No | Yes |
| Control | 34 | Female | No | No | N/A | N/A | N/A | N/A | 109 | 115 | No | No | No | Yes |
Asthma patients in control subjects with anti-EPX IgG or anti-ECP IgG. FVC: Forced vital capacity, FEV1: Forced expiratory volume. N/A: No apply. OCS: Cycles of oral systemic corticosteroid
Asthmatic patients exhibited significantly higher levels of IgG autoantibodies than control subjects (Fig. 1). There was no correlation among autoantibodies levels (Fig. 2).
Fig. 2.
Correlations among autoantibodies and eosinophil count. Concentration of IgE and IgG autoantibodies are presented according OD level. ECP: Eosinophil cationic protein. EPX: Eosinophil peroxidase
Patients with IgG autoantibodies against EPX or ECP have a lower FEV1 than asthmatic patients without IgG autoantibodies (91.1% vs 87.1%, p = 0.06) and lower FEV1/FVV (0.82 vs 0.79, p = 0.1) but it was not significant.
Basophil activation test (BAT)
We observed a similar concentration of basophils in total blood in the 2 groups, being slightly lower in the asthmatic group (28 cells/ul) compared to the control group (40 cells/ul), but without statistical differences.
For the BAT, basophils were obtained from different groups of subjects according to the presence (or not) of asthma, IgE, and IgG autoantibodies (Fig. 3). With the different dilutions of autoantigens used, CD203c expression from patients with asthma and IgE autoantibodies significantly increased in contrast to the other groups.
Basophils of control subjects with IgE autoantibodies have a CD203c expression over 10% when stimulated with the higher concentration of EPX or ECP (1 μg/mL), but it was significantly lower than the asthma group and without significant difference with control subjects with negative IgE autoantibodies (Fig. 3). When analyzing all samples as a group, we observed a moderate correlation between basophil activation and the concentration of anti-EPX IgE (r 0.689 p 0.03) and anti-ECP (r 0.566 p 0.04).
Discussion
In this study, we described for the first time (i) IgE autoantibodies against eosinophil human proteins in children and adults; (ii) Basophil activation mediated by anti-EPX IgE and anti-ECP IgE; (iii) IgE and IgG autoantibodies against eosinophil proteins could be a biomarker of lung function in asthmatic patients.
In childhood, the eosinophilic endotype is frequent and different biomarkers in sputum or bronchopulmonary lavage have been proposed to detect this endotype of T2 response. However, less invasive methods have been used in children and blood eosinophils are the most common biomarker used to indicate the presence of predominantly eosinophilic asthma, but its specificity is low, as we could see in this study where there was no difference in the levels of blood eosinophils between the group with asthma and the control group.25,26 The exploration of autoantibodies may be a starting point for new biomarker options of new biomarkers that allow improving the diagnosis, treatment selection and prognosis of patients. Of the 4 autoantibodies studied, anti-EPX IgE had the highest specificity for the group with asthma, which suggests that it could be useful for the diagnosis of the disease and could be signaling a new endotype with a T2 response but mediated by an autoimmune mechanism. The two IgE autoantibodies (anti-EPX/ECP IgE) were associated with lower lung function, which would indicate their usefulness as possible biomarkers of severity. In the case of IgG autoantibodies, although it was not statistically significant, similar to IgE autoantibodies, there was a tendency to be found more frequently in the group of patients with asthma and also to be present in patients with lower lung function, so that their usefulness as biomarkers cannot be ruled out.
Allergic and autoimmune response are part of a spectrum of immunological diseases and their involvement in the pathogenesis of asthma has been proposed based on presence of immunoglobulins against diverse environmental antigens and self-antigens.21 However, the presence of antibodies or autoantibodies does not necessarily have a clinical impact or relevance in the pathogenesis of a disease. As have been observed with other allergens and auto-allergens, in both groups (asthma and healthy controls) the presence of IgE and IgG antibodies was observed. However, the median levels of these autoantibodies in the asthmatic group was higher than control group and basophil activation even in low concentrations was only present in asthmatic patients, suggesting that these autoantibodies are functional and inflammatory activity could participate in the pathogenesis of the disease.
Compared with other allergic and non-allergic diseases,11,27,28 the role of IgE autoantibodies in asthma has not been studied in detail so far. In general, basophils in the control group presented lower percentages of activation compared to asthmatic patients, however we observed activation using the highest concentration (1 μg/mL). Due to the enzymatic nature of EPX and ECP, a nonspecific degranulation could be the explanation for these results, but this hypothesis needs to be confirmed.
Recently, we described anti-EPEX and Anti-ECP IgE in patients with urticaria and atopic dermatitis,17 so these autoantibodies are no pathognomonic for asthma. These 3 diseases have in common elevated levels of IgE and eosinophils in serum and/or local tissue (Skin or lung respectively). In atopic dermatitis the levels of anti-EPX/ECP were three times higher than in urticaria or asthma. This suggests that the intensity of type 2 inflammation increase the probability of IgE autoimmunity.
Some previous studies suggest that IgG autoimmune response in asthma is present especially in non-atopic asthmatic patients with severe clinical presentations.29, 30, 31, 32, 33, 34 For most of IgG autoantibodies the clinical relevance is unknown and studies evaluating its role in the pathogenesis of asthma are necessary. However, some IgG autoantibodies have been associated with the severity of asthma and the use of high doses of steroids, therefore it could have an important clinical impact.21 Anti-EPX IgG was previously reported in the sputum of 24/65 (37%) asthmatics;6 we observed a similar frequency of anti-EPX IgG (25.4%) in asthmatic patients that was higher than control group but not statistically significant. The higher frequency of IgG autoantibodies, especially in the most severe cases, regardless of age, suggests that their formation is associated with the intensity of the inflammatory process and could potentially serve as biomarkers of severity.
IgE autoantibodies in asthma has been little studied7,35,36 compared with other chronic diseases.11,12,27,28,37, 38, 39 The origin of these autoantibodies is unclear; In some cases, it seems to be secondary to cross-reactivity with environmental proteins,40 in others it seems to be the result of a high exposure of antigens usually hidden in a medium with a high number of inflammatory mediators.41, 42, 43, 44, 45, 46 This may occur with EPX and ECP antigens when they are released by eosinophils during inflammatory process in atopic dermatitis, urticaria, and asthma.17,47, 48, 49, 50, 51
Unlike other studies, we observed that blood eosinophil count and total IgE were high compared with other populations such as Europeans, both in the asthmatic group and in the control group. The study population is located in a tropical area where parasite exposure is endemic; therefore, this stimulus could influence the high levels found of both eosinophils and total IgE.4,52 However, at the time of the study none of the subjects had active parasitosis infection. Despite EPX and ECP are both being produced by eosinophils during the inflammatory response, there was no correlation among the anti-ECP IgE and anti-EPX IgE levels, suggesting that although they are released by the same cell and probably their antigenic presentation occurs at the same time and context; the intensity and frequency of the IgE response to each antigen have different triggers.
The study has some limitations; The sample size was calculated to detect autoantibodies in patients with asthma; therefore, other analyses are only exploratory, for example to assess the association with asthma severity. However, the descriptive data obtained are useful as a starting point for further studies.
Conclusions
We demonstrated IgE autoantibodies against eosinophil proteins EPX and ECP in adults and children. These antibodies can induce an inflammatory response and appear to be related to severe asthma. The detection of autoantibodies could have clinical implication in the diagnosis and therapeutic approach; therefore, more studies are necessary to evaluate its diagnostic performance and its possible usefulness as a predictor of severity.
Abbreviations
ECP, Eosinophil cationic protein; EPX, Eosinophil peroxidase; FVC, Forced vital capacity, FEV1, Forced expiratory volume; OD, optical density units.
Funding
This article is the result of the project "Identification of modifiable risk factors related to the severity of asthma and the development of atopy in the pediatric population of Antioquia” Code "2016-12948, CII-07". We thank the "IPS Universitaria” Clinic, and the University of Antioquia, for their logical support and for financing this project.
Authors’ contribution
JS contributed the central idea. JL and JS evaluated and collected clinical data from patients. JL, MM, and AS organized the databases. JS, AS, EG and MM analyzed the data. JS, AS, and JL wrote the first draft. All authors were involved in writing, reviewing, and editing the final manuscript.
Ethical approval
The Ethics committees of University of Antioquia approved the study (Code CII-07 2016). Written informed consent was obtained from all the participants or in the case of children, it was obtained from their parents.
Authors’ consent for publication
All authors have approved the submission of this manuscript. These results have not been previously published in another journal.
Data availability statement
The datasets generated for this study are available on request to the corresponding author.
Declaration of competing interest
Authors have not conflict of interest to declare.
Acknowledgments
We thank the University of Antioquia and Clinic “IPS Universitaria” for their financial and logistical support.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Adachi M., Hozawa S., Nishikawa M., Yoshida A., Jinnai T., Tamura G. Asthma control and quality of life in a real-life setting: a cross-sectional study of adult asthma patients in Japan (ACQUIRE-2) J Asthma. 2019;56(9):1016–1025. doi: 10.1080/02770903.2018.1514628. [DOI] [PubMed] [Google Scholar]
- 2.Caminati M., Vianello A., Chieco Bianchi F., et al. Relevance of TH2 markers in the assessment and therapeutic management of severe allergic asthma: a real-life perspective. J Investig Allergol Clin Immunol. 2020;30(1):35–41. doi: 10.18176/jiaci.0379. [DOI] [PubMed] [Google Scholar]
- 3.Gurram R.K., Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol. 2019;16(3):225–235. doi: 10.1038/s41423-019-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakzuk J., Acevedo N., Cifuentes L., et al. Early life IgE responses in children living in the tropics: a prospective analysis. Pediatr Allergy Immunol. 2013;24(8):788–797. doi: 10.1111/pai.12161. [DOI] [PubMed] [Google Scholar]
- 5.Acevedo N., Sánchez J., Zakzuk J., et al. Particular characteristics of allergic symptoms in tropical environments: follow up to 24 months in the FRAAT birth cohort study. BMC Pulm Med. 2012;12:13. doi: 10.1186/1471-2466-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee M., Bulir D.C., Radford K., et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;141(4):1269–1279. doi: 10.1016/j.jaci.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Bünder R., Mittermann I., Herz U., et al. Induction of autoallergy with an environmental allergen mimicking a self protein in a murine model of experimental allergic asthma. J Allergy Clin Immunol. 2004;114(2):422–428. doi: 10.1016/j.jaci.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Zeller S., Rhyner C., Meyer N., Schmid-Grendelmeier P., Akdis C.A., Crameri R. Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol. 2009;124(2):278–285. doi: 10.1016/j.jaci.2009.05.015. 85.e1-285. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez J., Munera M., Arango J., Cardona R., Puerta L. IgE auto-antibodies to human fatty acid-binding proteins in atopic dermatitis patients. Curr Trends Immunol. 2020;21:29–39. [Google Scholar]
- 10.Sánchez J., Sánchez A., Cardona R. Causal relationship between anti-TPO IgE and chronic urticaria by. Allergy Asthma Immunol Res. 2019;11(1):29–42. doi: 10.4168/aair.2019.11.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer M., Altrichter S., Schmetzer O., Scheffel J., Church M.K., Metz M. Immunoglobulin E-mediated autoimmunity. Front Immunol. 2018;9:689. doi: 10.3389/fimmu.2018.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dema B., Pellefigues C., Hasni S., et al. Autoreactive IgE is prevalent in systemic lupus erythematosus and is associated with increased disease activity and nephritis. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Wu J., Liu H., et al. Utility of basophil activation test for predicting the outcome of wheezing in children: a pilot study. BMC Immunol. 2021;22(1):4. doi: 10.1186/s12865-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallah N., Rodriguez-Segade S., Gonzalez-Barcala F.J., Takkouche B. Blood eosinophil count as predictor of asthma exacerbation. A meta-analysis. Pediatr Allergy Immunol. Pediatr Allergy Immunol. 2021 Apr;32(3):465–478. doi: 10.1111/pai.13403. [DOI] [PubMed] [Google Scholar]
- 15.Koller D.Y., Wojnarowski C., Herkner K.R., et al. High levels of eosinophil cationic protein in wheezing infants predict the development of asthma. J Allergy Clin Immunol. 1997;99(6 Pt 1):752–756. doi: 10.1016/s0091-6749(97)80007-3. [DOI] [PubMed] [Google Scholar]
- 16.Macedo P., Hew M., Torrego A., et al. Inflammatory biomarkers in airways of patients with severe asthma compared with non-severe asthma. Clin Exp Allergy. 2009;39(11):1668–1676. doi: 10.1111/j.1365-2222.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez J., Sanchez A., Munera M., et al. Presence of IgE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in severe chronic spontaneous urticaria and atopic dermatitis. Allergy Asthma Immunol Res. 2021;13(5):746–761. doi: 10.4168/aair.2021.13.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banga J.P., Tomlinson R.W., Doble N., Odell E., McGregor A.M. Thyroid microsomal/thyroid peroxidase autoantibodies show discrete patterns of cross-reactivity to myeloperoxidase, lactoperoxidase and horseradish peroxidase. Immunology. 1989;67(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- 19.Arscott P.L., Koenig R.J., Kaplan M.M., Glick G.D., Baker J.R. Unique autoantibody epitopes in an immunodominant region of thyroid peroxidase. J Biol Chem. 1996;271(9):4966–4973. doi: 10.1074/jbc.271.9.4966. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y.M., Yang E.M., Yoo H.S., Shin Y.S., Kim S.H., Park H.S. Increased level of basophil CD203c expression predicts severe chronic urticaria. J Kor Med Sci. 2014;29(1):43–47. doi: 10.3346/jkms.2014.29.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee M., Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018;10(5):428–447. doi: 10.4168/aair.2018.10.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee M., Lim H.F., Thomas S., et al. Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017;13:2. doi: 10.1186/s13223-016-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikuls T.R., Payne J.B., Deane K.D., Thiele G.M. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: the spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. 2016;137(1):28–34. doi: 10.1016/j.jaci.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Chan Y.C., Ramadani F., Santos A.F., et al. Auto-anti-IgE": naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol. 2014;134(6):1394–1401.e4. doi: 10.1016/j.jaci.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licari A., Castagnoli R., Brambilla I., et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol. 2018;31(2):44–55. doi: 10.1089/ped.2018.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trivedi M., Denton E. Asthma in children and adults-what are the differences and what can they tell us about asthma? Front Pediatr. 2019;7:256. doi: 10.3389/fped.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez J., Sánchez A., Cardona R. Clinical characterization of patients with chronic spontaneous urticaria according to anti-TPO IgE levels. J Immunol Res. 2019;2019 doi: 10.1155/2019/4202145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grattan C. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;141(3):1165–1166. doi: 10.1016/j.jaci.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Blecher M., Lewis S., Hicks J.M., Josephs S. Beta-blocking autoantibodies in pediatric bronchial asthma. J Allergy Clin Immunol. 1984;74(3 Pt 1):246–251. doi: 10.1016/0091-6749(84)90253-7. [DOI] [PubMed] [Google Scholar]
- 30.Harrison L.C., Callaghan J., Venter J.C., Fraser C.M., Kaliner M.L. Atopy, autonomic function and beta-adrenergic receptor autoantibodies. Ciba Found Symp. 1982;(90):248–262. doi: 10.1002/9780470720721.ch14. [DOI] [PubMed] [Google Scholar]
- 31.Lassalle P., Delneste Y., Gosset P., Gras-Masse H., Wallaert B., Tonnel A.B. T and B cell immune response to a 55-kDa endothelial cell-derived antigen in severe asthma. Eur J Immunol. 1993;23(4):796–803. doi: 10.1002/eji.1830230404. [DOI] [PubMed] [Google Scholar]
- 32.Nahm D.H., Lee K.H., Shin J.Y., Ye Y.M., Kang Y., Park H.S. Identification of alpha-enolase as an autoantigen associated with severe asthma. J Allergy Clin Immunol. 2006;118(2):376–381. doi: 10.1016/j.jaci.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Nahm D.H., Shin M.J., Yim H., et al. Increased levels of circulating autoantibodies to cultured human bronchial epithelial cell in adult patients with nonatopic asthma. J Kor Med Sci. 2001;16(4):407–410. doi: 10.3346/jkms.2001.16.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahm D.H., Lee Y.E., Yim E.J., et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002;165(11):1536–1539. doi: 10.1164/rccm.200201-009OC. [DOI] [PubMed] [Google Scholar]
- 35.Lassalle P., Joseph M., Ramon P., Dracon M., Tonnel A.B., Capron A. Plasmapheresis in a patient with severe asthma associated with auto-antibodies to platelets. Clin Exp Allergy. 1990;20(6):707–712. doi: 10.1111/j.1365-2222.1990.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 36.Garn H., Mittermann I., Valenta R., Renz H. Autosensitization as a pathomechanism in asthma. Ann N Y Acad Sci. 2007;1107:417–425. doi: 10.1196/annals.1381.044. [DOI] [PubMed] [Google Scholar]
- 37.Schmetzer O., Lakin E., Topal F.A., et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018 Sep;142(3):876–882. doi: 10.1016/j.jaci.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q., Zhong H., Chen W.C., et al. Different expression patterns of plasma Th1-, Th2-, Th17- and Th22-related cytokines correlate with serum autoreactivity and allergen sensitivity in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2018;32(3):441–448. doi: 10.1111/jdv.14541. [DOI] [PubMed] [Google Scholar]
- 39.Thorsteinsdottir S., Stokholm J., Thyssen J.P., et al. Genetic, clinical, and environmental factors associated with persistent atopic dermatitis in childhood. JAMA Dermatol. 2019 Jan 1;155(1):50–57. doi: 10.1001/jamadermatol.2018.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Múnera M., Martínez D., Labrada A., Caraballo L., Puerta L. Identification of B Cell epitopes of blo t 13 allergen and cross-reactivity with human adipocytes and heart fatty acid binding proteins. Int J Mol Sci. 2019;20(24) doi: 10.3390/ijms20246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez A., Cardona R., Munera M., Sánchez J. Identification of antigenic epitopes of thyroperoxidase, thyroglobulin and interleukin-24. Exploration of cross-reactivity with environmental allergens and possible role in urticaria and hypothyroidism. Immunol Lett. 2020;220:71–78. doi: 10.1016/j.imlet.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Taillé C., Grootenboer-Mignot S., Estellat C., et al. Perip7lakin is a target for autoimmunity in asthma. Respir Res. 2016;17(1):126. doi: 10.1186/s12931-016-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taillé C., Grootenboer-Mignot S., Boursier C., et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(6):759–766. doi: 10.1164/rccm.201001-0076OC. [DOI] [PubMed] [Google Scholar]
- 44.Gueirard P., Delpech A., Gilbert D., Godin M., Le Loet X., Tron F. Anti-myeloperoxidase antibodies: immunological characteristics and clinical associations. J Autoimmun. 1991;4(3):517–527. doi: 10.1016/0896-8411(91)90163-7. [DOI] [PubMed] [Google Scholar]
- 45.Xu P.C., Chen M., Cui Z., Zhao M.H. Influence of myeloperoxidase by anti-myeloperoxidase antibodies and its association with the disease activity in microscopic polyangiitis. Rheumatology (Oxford) 2010;49(11):2068–2075. doi: 10.1093/rheumatology/keq203. [DOI] [PubMed] [Google Scholar]
- 46.Williams D.E., Le S.N., Hoke D.E., et al. Structural studies of thyroid peroxidase show the monomer interacting with autoantibodies in thyroid autoimmune disease. Endocrinology. 2020;161(2) doi: 10.1210/endocr/bqaa016. [DOI] [PubMed] [Google Scholar]
- 47.Remes S., Korppi M., Remes K., Savolainen K., Mononen I., Pekkanen J. Serum eosinophil cationic protein (ECP) and eosinophil protein X (EPX) in childhood asthma: the influence of atopy. Pediatr Pulmonol. 1998;25(3):167–174. doi: 10.1002/(sici)1099-0496(199803)25:3<167::aid-ppul6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 48.Breuer K., Kapp A., Werfel T. Urine eosinophil protein X (EPX) is an in vitro parameter of inflammation in atopic dermatitis of the adult age. Allergy. 2001;56(8):780–784. doi: 10.1034/j.1398-9995.2001.056008780.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim T.Y., Park H.J., Kim C.W. Eosinophil cationic protein (ECP) level and its correlation with eosinophil number or IgE level of peripheral blood in patients with various skin diseases. J Dermatol Sci. 1997;15(2):89–94. doi: 10.1016/s0923-1811(97)00614-2. [DOI] [PubMed] [Google Scholar]
- 50.Lorenzo G.D., Mansueto P., Melluso M., et al. Blood eosinophils and serum eosinophil cationic protein in patients with acute and chronic urticaria. Mediat Inflamm. 1996;5(2):113–115. doi: 10.1155/S0962935196000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haas N., Motel K., Czarnetzki B.M. Comparative immunoreactivity of the eosinophil constituents MBP and ECP in different types of urticaria. Arch Dermatol Res. 1995;287(2):180–185. doi: 10.1007/BF01262329. [DOI] [PubMed] [Google Scholar]
- 52.Caraballo L., Zakzuk J., Lee B.W., et al. Particularities of allergy in the tropics. World Allergy Organ J. 2016;9:20. doi: 10.1186/s40413-016-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.