Abstract
The introduction of immune checkpoint inhibitors (ICIs) for the treatment of solid cancers dramatically turned the tables in clinical routine. However, therapy success is still limited with up to 70% of non-responders in patients with ICI treatment. Traditionally, most immunotherapy approaches aim at directly stimulating anti-tumor T cell responses. More recently, tumor-associated macrophages have come into focus due to their predominance in solid tumors. Intensive cross-talk with tumor cells and immune as well as stromal cells within the tumor microenvironment can drive either pro- or anti-tumorigenic macrophage phenotypes. In turn, tumor-associated macrophages strongly shape cytokine and metabolite levels in the tumor microenvironment and thus are central players in anti-tumor immunity. Thus, ambivalent macrophage populations exist which raises therapeutic possibilities to either enhance or diminish their functionality. However, molecular signals controlling tumor-associated macrophage polarization are incompletely understood. Gaining in-depth understanding of monocyte/macrophage properties both in circulation and within distinct tumor microenvironments would (i) allow the development of new therapeutic approaches, and (ii) could additionally aid our understanding of underlying mechanisms limiting current therapy with the option of combinatorial therapies to increase efficacy. In this review, we summarize recent data addressing heterogeneity of tumor-associated macrophage populations and we discuss strategies to target macrophages using known molecular pathways with the potential for straight-forward clinical application.
Key words: monocytes, macrophages, tumor-associated macrophages, immunotherapy, immune checkpoint blockade
Highlights
-
•
Single-cell sequencing reveals enormous macrophage complexity.
-
•
Strong functional imprinting of tumor-associated macrophages by tumor microenvironment.
-
•
Suspected active role of monocytes in hyperprogression.
-
•
Several, potential targets on monocytes and tumor-associated macrophages under investigation.
Blood monocytes and tissue macrophages in cancer
Blood monocytes: heterogeneity by redefinition
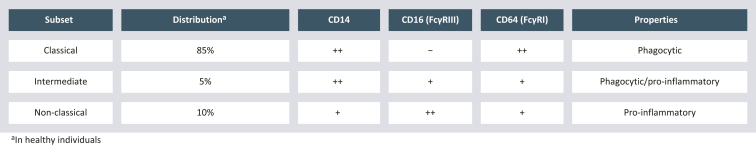
Circulating human blood monocytes were originally characterized by expression of CD14, a lipopolysaccharide co-receptor.1 The discovery of CD16 co-expression led to a redefinition of human monocytes in 20102 and the classification in classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+CD16++) monocytes.3 These three subsets hold distinct functions and differ in expression levels of additional surface markers, e.g. Fcγ receptors (FcγR), human leukocyte antigens (Figure 1), and chemokine receptors.4 As already described for other pathological conditions,3 altered monocyte subset ratios have also been observed in various cancer entities.5, 6, 7, 8, 9 Shifts in monocyte subset frequencies can be due to multiple mechanisms including alterations in monopoiesis and/or direct changes in molecular phenotypes in response to cancer-derived signals. Cytokines regulating monopoiesis, such as colony-stimulating factor (CSF) 1, CSF2, and CSF3, are reported to be altered in the blood of cancer patients which, together with systemic inflammation, reprogram monopoiesis.10 In vitro data suggest that direct contact of monocytes with tumor cells9,11 or tumor cell supernatant7,12 trigger direct responses of circulating monocyte subsets to tumor-derived signals. Soluble factors, as well as extracellular vesicles (membrane-enclosed vesicular structures derived from tumor cells), were attributed an active role in inducing pro-tumorigenic phenotypes in monocytes12,13 and their differentiation into suppressive myeloid cells.14,15 In summary, monocytes adapt to tumor-derived stimuli, which likely drive their pro-tumorigenic properties.
Figure 1.
Monocyte subsets.
Monocyte subset characteristics (based on Wong et al.3 and Ozanska et al.4).
Tissue macrophages: classification by ontogeny
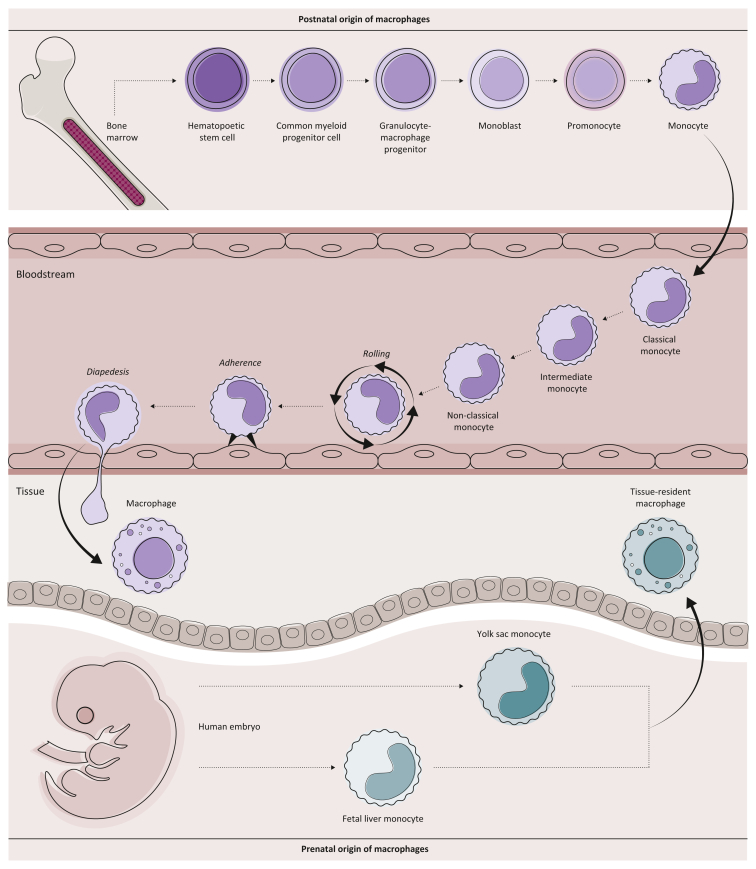
Like blood monocytes, macrophages are characterized by enormous plasticity and cannot only adapt various phenotypes upon sensing inflammatory stimuli but are also strongly imprinted by the tissue microenvironment they interact with at steady state.16, 17, 18 In tissues, we can distinguish two main macrophage lineages with specialized function and ontogeny (Figure 2): tissue-resident macrophages (TRMacs) and monocyte-derived macrophages (MoMacs). TRMacs populate tissues before birth, proliferate locally without contribution from the circulating monocyte pool, and have very tissue-specific functions.19, 20, 21 Damage in the TRMac pool results in tissue pathology, as exemplified by decreased lung function due to an insufficient number of alveolar macrophages leading to accumulation of surfactant protein and reduced air exchange in alveoli.22 In contrast, MoMacs differentiate from blood-derived monocytes and are thus replenished constantly. In homeostasis, MoMacs are dispensable and constitute only a small fraction of total macrophages in many tissues. Nevertheless, MoMacs play a key role in response to inflammatory stimuli and expand dramatically in chronic or acute inflammatory lesions. Taken together, it is now understood that in conditions of chronic inflammation, monocytes are actively recruited into damaged tissue and significantly contribute to pathology upon differentiation into MoMacs.23 Accordingly, large numbers of MoMacs often accumulate in solid tumors.
Figure 2.
Macrophage ontogeny.
Within tissue, two distinct macrophage types, tissue-resident macrophages and monocyte-derived macrophages, can be discriminated. While tissue-resident macrophages populate tissues before birth (prenatal origin) and proliferate locally, monocyte-derived macrophages are replenished from circulating blood monocytes (postnatal origin). Circulating blood monocytes originate from bone marrow-derived hematopoietic stem cells and give rise to classical monocytes, which then can further differentiate to intermediate and non-classical monocytes on request.
Tumor-associated macrophages
Tumor-associated macrophages: the in vitro M1/M2 concept
Within the solid tumor microenvironment (TME), macrophages are termed tumor-associated macrophages (TAMs), comprising both MoMac and TRMac subsets. TAMs present the most abundant immune cell subset in the TME24 and are associated with disease outcome across various cancer types.25 Due to complex signals within the TME, TAMs are polarized and acquire specific molecular signatures that differ from macrophages in healthy tissues. Attempts were made to describe TAM functionality using the ‘M1/M2’ concept, which was initially introduced to describe macrophage plasticity in response to various stimuli observed in vitro.26 Until recently, TAM characterization was mainly carried out by immunohistochemistry, flow cytometry, and bulk gene expression analysis, mostly using only one or a minimal set of markers. Thus, the full cellular diversity and functional markers of TAMs have been poorly captured. Recent advances in single-cell RNA sequencing have shown that M1 and M2 signature genes are co-expressed across different TAM subsets, and do not clearly identify immune-suppressive or stimulatory TAMs, thus highlighting that in vivo macrophage populations do not adhere to the M1/M2 dogma.27,28
Tumor-associated macrophages: polarized by the tumor microenvironment
The TME releases a plethora of extracellular mediators which result in distinct tumor-associated phenotypes of MoMacs and TRMacs29: tumor-infiltrating MoMacs are characterized by immunosuppressive phenotypes associated with several chronic inflammatory conditions,30, 31, 32 whereas TRMacs reduce the expression of pro-inflammatory mediators and up-regulate tissue remodeling factors.33 Several recent studies have focused on the role of TRMacs in the TME, demonstrating tissue specificity, which is consistent with our understanding of very strong imprinting of surrounding tissue niches on TRMac identity and function. In lung cancer, immunosuppressive functions have been reported for TRMacs, whereas in breast cancer, TRMacs were shown to directly fuel protective T cell responses.33, 34, 35 It is also becoming clear that TRMacs preferentially localize in the tumor-surrounding stroma and are depleted from tumor islets. In contrast, MoMacs are strongly recruited by the tumor and are localized in tumor nests directly.33,35,36 Thus, more research is needed to dissect the roles of distinct macrophage populations with respect to the multiple components of the TME. Intriguingly, in these studies, very distinct correlation patterns of two groups of cell types clustering together within patients were described. On the one hand, TRMacs were correlated with multiple memory T cell subsets and B cells; whereas on the other hand several immunosuppressive MoMac populations correlated with regulatory T cells and exhausted/dysfunctional T cells.36 A causal relationship between these cell types accumulating together has yet to be proven, but it is intriguing that MoMacs are part of a suppressive pro-tumorigenic immune cell context, whereas TRMacs are represented in T helper 1 (Th1) polarized, anti-tumorigenic immune environments.
Heterogeneity of tumor-associated macrophages: lessons learned from single-cell RNA sequencing
Due to the high complexity and plasticity of TAMs, unbiased single-cell RNA sequencing of tumor-associated immune cells provides invaluable insights into TAM function for future translational approaches. Two meta studies recently compiled comprehensive single-cell macrophage maps across cancers: Mulder et al. identified several MoMac populations, including triggering receptor expressed on myeloid cells-2 (TREM2)hi MoMacs and interleukin 4 (IL-4)-induced 1 (IL4I1)hi MoMacs, enriched in tumors across tissues.37 TREM2hi MoMacs preferentially accumulated in the tumor core, whereas IL4I1hi MoMacs were enriched in the tumor periphery, likely contributing to the differential transcriptional programs of those MoMac subsets.37 Cheng et al. highlighted a very complex pattern of macrophage transcriptional programs that did not allow consistent classification of macrophages from different tumor types.28 Nevertheless, a secreted phosphoprotein 1 (SPP1)hi MoMac population was found in 8 out of 15 studied cancer types and was characterized by high expression of genes associated with angiogenesis. In addition, a transmembrane glycoprotein NMB (GPNMB)hi MoMac cluster was identified in some of the studied cancers, which is likely consistent with the TREM2hi MoMacs described in Mulder et al.37 TRMacs with strong tissue-specific identity were described, including alveolar macrophages and interstitial macrophages across multiple tissues, which were excluded from tumor lesions, confirming previous findings in non-small cell lung cancer (NSCLC).33,36 Such data integration efforts highlight the enormous complexity of the macrophage compartment and its tissue-specific regulatory patterns, but we are still far from understanding the functional implications of these different macrophage subsets in the TME and identifying universal macrophage targets for immunotherapy.
Response to immune checkpoint blockade: do tumor-associated macrophages play a role?
TAMs have also been shown to contribute to response to ICIs in in vivo models of colon adenocarcinoma, mammary carcinoma, melanoma, and osteosarcoma. In these studies, programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) blockade drives an immune-stimulatory phenotype in macrophages that contributes to effector T cell responses and tumor control.38, 39, 40 In patient cohorts of urothelial cancer41 and NSCLC,42 the presence of inflammatory macrophages predicts response to ICI therapy. However, the clinical monitoring is most often too shallow to get sufficient information on specific macrophage subsets. Usually, CD68 is used as a pan-macrophage marker in histology. Nevertheless, the use of this marker is limited due to its expression on other cell types including granulocytes, dendritic cells (DCs), fibroblasts, endothelial cells, and lymphoid subsets and its inability to distinguish between macrophage subsets.43 Additionally, the spatial distribution should be considered during analysis as macrophage subsets reside within different parts of the tumor and its TME.44 Spatial transcriptomics is a novel technique that enables the determination of gene expression throughout the tissue space,45 which would allow to get a global picture of formalin-fixed tissue slides allowing unbiased immune profiling. This approach might allow for future prediction of response to ICI therapy on the basis of not only macrophage but multiple immune cell subsets. However, one caveat is that current spatial transcriptomics approaches do not achieve single-cell resolution. Method development strategies are tackling this problem, thus holding great promise for future applications.
Tumor-associated macrophages: involvement in hyperprogression
With the advent of ICI therapy, an unconventional response pattern termed hyperprogression has been observed in up to 30% of patients receiving PD-1/PD-L1-targeting ICIs.46 In contrast to pseudoprogression, where an increase in the tumor burden is mediated by infiltration of immune cells, patients with hyperprogression show an accelerated tumor growth and an immense deterioration of the patients’ general health condition. Despite increasing case reports,47, 48, 49, 50, 51, 52, 53 the existence of hyperprogression is still questioned: alternatively, this patient subpopulation might suffer from an extremely aggressive cancer type for which chemotherapy might be more efficient than immunotherapy to slow down tumor growth acceleration especially in the first weeks of treatment.54 Some studies suggest an involvement of monocytes in the promotion of hyperprogression.55 Besides well-known immunomodulatory factors influencing monocytes such as cytokines, chemokines, and extracellular vesicles, external factors, such as therapeutic antibodies directed against immune checkpoints, are also hypothesized to play a specific, immunomodulatory role in monocyte modulation, especially with regard to hyperprogression. Despite modifications of ICI antibodies in their Fc part, these antibodies still show a high affinity to FcγRI (CD64) and IIb (CD32B),56 whereas the latter is considered to have inhibitory effects and might therefore limit therapeutic efficacy and promote drug resistance, especially considering FcγRIIB expression on tumor cells.57 Romano et al. reported the ex vivo binding of ipilimumab to non-classical monocytes via FcγRIIIA (CD16A) triggered antibody-dependent cellular cytotoxicity-mediated lysis of regulatory T cells (Tregs). This effect was only observed for CD16-expressing non-classical monocytes.58 Using an in vivo mouse model, Arlauckas et al. showed that PD-1- TAMs can heist T cell-bound anti-PD-1 antibodies, which can be prevented by blockage of Fcγ receptors on TAMs, resulting in enhanced tumor regression.59 These findings highlight the participation of Fc/FcγR interactions in the context of therapy efficacy. For the first time, Lo Russo et al. provided proof for the active role of therapeutic antibodies in hyperprogression in a xenograft model: hyperprogression was abrogated in all mice when treating them with the anti-PD-1 F(ab)2 fragment compared to treatment with the whole antibody nivolumab, which correlated with suppressive macrophage infiltration.50 These data highlight the rationale to develop Fc-optimized antibodies or antibody fragments for clinical use.
Tumor-associated macrophages as a therapeutic target in solid cancers
Since it was recognized that TAMs play a key role in supporting tumor immune evasion and metastasis,60 a strong focus has been put on targeting TAMs. So far, the main strategies for targeting TAMs include macrophage depletion, inhibition of recruitment, and repolarization.61 However, tumor-specific molecular targets are thus far lacking and still need to be explored in (pre-)clinical settings.
Targeting tumor-associated macrophages: the long shot
Ongoing research has already revealed various therapeutic TAM-related targets with new ones constantly emerging. TREM2 is one of the genes selectively and highly expressed on tumor-infiltrating MoMacs and has recently emerged as a cancer immunotherapy target.30,62,63 Multiple studies have shown a role for TREM2-expressing MoMacs in anti-tumor immunity and response to ICI therapy. In patients, TREM2 expression correlates with poor outcomes, poor therapy response, and metastasis.64, 65, 66 Mice deficient for TREM2, or treated with a TREM2-blocking antibody, showed reduced tumor burden as well as improved response to ICIs in various tumor models.30,62,63 It is clear that the loss of TREM2 remodels the TAM compartment. However, the precise mechanisms of how TREM2 modulates macrophage identity, and by which mechanisms TREM2-expressing MoMacs regulate anti-tumor immunity is unknown. Other MoMac targets are less clear. Immune checkpoint ligands expressed on MoMacs include PD-L1, PD-L2, T cell immunoglobulin and mucin-domain containing (TIM-)3, TIM-4, indoleamine-pyrrole 2,3-dioxygenase, and IL4I1, some of which might be controlled by upstream interferon or CD40 signaling.36,37 Targeting those factors increased anti-tumor immunity in preclinical models or in clinical settings.67 However, whether the observed effects are mediated via macrophages is unclear and it is likely that other cell types such as DCs play equally important roles. This underscores the need for in-depth research to identify and study new targets on various immune cell populations for future therapies.
Targeting tumor-associated macrophages: possibilities within reach of clinical practice
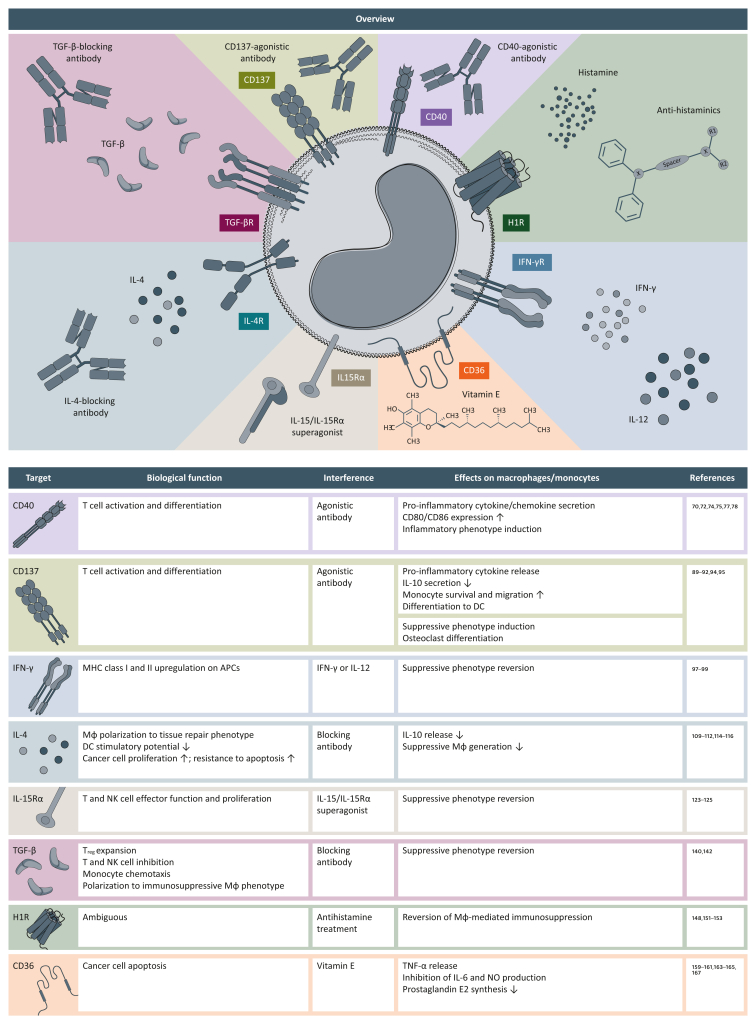
In this section, we will discuss selected potential targets on TAMs that could be adapted for clinical use in the near future (Figure 3).
Figure 3.
New potential therapeutic targets on tumor-associated macrophages.
Potential therapeutic targets currently under investigation are summarized, and their mode of action and the effect on monocytes and/or macrophages (Mφ) are indicated.
APC, antigen-presenting cell; DC, dendritic cell; H1R, histamine receptor H1; IL, interleukin; INF-γ, interferon-γ; MHC, major histocompatibility complex; Mφ, macrophage; NK, natural killer; NO, nitric oxide; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; Treg, regulatory T cell.
CD40
Signaling of CD40 via its ligand CD40L is the major co-stimulatory signal necessary for T cell activation and is involved in various immune functions such as coagulation, infection control, and immune system regulation.68,69 CD40/CD40L signaling leads to DC activation with up-regulation of co-stimulatory molecules68, 69, 70 as well as their migration to the lymph node71 and consequently to T cell activation and differentiation.70 In monocytes, CD40 activation induces pro-inflammatory cytokine and chemokine secretion, as well as up-regulation of CD80 and CD86 expression.68,72,73 Thus, T cell activation is enhanced. In malignant cells, CD40 signaling is able to induce apoptosis both in vitro and in vivo.68 Therefore, agonist-mediated induction of CD40 signaling might promote anti-tumor immunity.70 In various cancer models, CD40 agonists were shown to induce a tumor-specific immune response by triggering the cancer-immunity cycle.73,74 In TAMs, CD40 agonists have been reported to induce an anti-tumorigenic phenotype in vitro.75,76 Long et al. described that these CD40 agonist-redirected macrophages were able to degrade fibrotic tissue in pancreatic carcinoma and thereby enhance chemotherapy efficacy.77 In this context, several reports highlight the importance of macrophages, which can boost an anti-tumor immune response independent of T cells.78,79 Preclinical studies with CD40 agonist monotherapy have shown efficacy; however, mainly immunologically active and immunotherapy-responsive tumor models were used.80 Additionally, CD40 agonist treatment was linked to increased PD-L1 expression on monocytes81 and DCs82 highlighting the potential need for combination with ICIs. Combinatorial therapy with anti-PD-1 has already shown promising results in mouse models.73 Despite these positive effects, the use of CD40 agonists is linked to toxicities including cytokine release syndrome and hepatotoxicity.74 Therefore, there are already attempts of modifying CD40 agonists by bispecific antibodies to ensure specific targeting, e.g. by fibroblast activation protein being expressed to a higher degree in the tumor stroma.74,83 Moreover, F(ab)2 fragments of CD40 agonists (CDX-1140) did not induce cytokine release in vitro with a good safety profile in animal models.84 Additionally, fusions of CD40 and blocking PD-L1 single-chain variable Fcs have been reported.85 Clinical trials showed a certain degree of toxicity upon systemic administration80 and have been mainly tested in combination with other therapies. In pancreatic ductal adenocarcinoma, combination with chemotherapy was well tolerated and associated with anti-tumor activity.79,86 Various clinical trials, including combinations with ICIs and/or chemotherapy, are ongoing (Table 1).73
Table 1.
Selected overview of running clinical trials
| Referencea | Phase | Statusb | Interventionc | Cancer type |
|---|---|---|---|---|
| CD40 | ||||
| NCT03424005 | 1b/2 | ANR | Selicrelumab#1, atezolizumab, bevacizumab | Breast cancer |
| NCT03555149 | 1b/2 | ANR | Selicrelumab#1, bevacizumab, atezolizumab | Colorectal cancer |
| NCT03193190 | 1b/2 | R | Nab-paclitaxel, gemcitabine, oxaliplatin, leucovorin, fluorouracil, atezolizumab, cobimetinib, PEGPH2O, BL-8040, selicrelumab#1, bevacizumab, RO6874281, AB928, tiragolumab, tocilizumab | Pancreatic ductal adenocarcinoma |
| NCT04364230 | 1/2 | R | Peptide vaccine, polyICLC, CDX-1140#2 | Melanoma |
| NCT04536077 | 2 | R | CDX-1140#2; CCX-301, CDX-1140#2 |
Pancreatic cancer |
| NCT04616248 | 1 | NYR | CDX-1140#2, CCX-301, radiotherapy, poly-ICLC | Breast cancer |
| NCT04491084 | 1/2 | R | CDX-1140#2, CCX-301, stereotactic body radiation therapy | NSCLC |
| NCT04520711 | 1/1b | R | TCR-transduced T cells, CDX-1140#2, pembrolizumab | Epithelial cancer |
| NCT03502330 | 1 | ANR | APX005M#3, cabiralizumab; APX005M#3, cabiralizumab, nivolumab |
Melanoma NSCLC Renal cell carcinoma |
| NCT03719430 | 2 | R | APX005M#3, doxorubicin | Soft-tissue carcinoma |
| NCT03165994 | 2 | ANR | APX005M#3, radiation, paclitaxel, carboplatin, surgical resection | Esophageal cancer |
| NCT02706353 | 2 | R | APX005M#3, pembrolizumab | Melanoma |
| NCT03389802 | 1 | R | APX005M#3 | Pediatric CNS tumors |
| NCT04337931 | 2 | ANR | APX005M#3 | Melanoma |
| NCT04130854 | 2 | ANR | mFOLFOX, radiation, APX005M#3 | Rectal adenocarcinoma |
| NCT04495257 | 1 | R | APX005M#3, nivolumab, ipilimumab | Melanoma Renal cell carcinoma |
| NCT02600949 | 1 | R | Imiquimod, pembrolizumab, sotigalimab#3, peptide vaccine | Colorectal adenocarcinoma Pancreatic ductal adenocarcinoma |
| NCT02376699 | 1 | ANR | SEA-CD40#4; SEA-CD40#4, pembrolizumab SEA-CD40#4, pembrolizumab, gemcitabine, nab-paclitaxel |
B Cell lymphoma Follicular lymphoma Hodgkin lymphoma Large B Cell, diffuse lymphoma Non-Hodgkin lymphoma NSCLC Melanoma Squamous cell carcinoma Pancreatic ductal adenocarcinoma |
| CD137 | ||||
|---|---|---|---|---|
| JapicCTI-205153 | 1 | R | STA551#5; STA551#5, atezolizumab |
Advanced/metastatic solid tumors |
| NCT04121676 | 1 | R | AGEN2373#6; AGEN2373#6, botensilimab |
Advanced/metastatic solid tumors |
| NCT04903873 | 1/2 | R | EU101#7 | Colorectal cancer NSCLC |
| NCT04501276 | 1 | R | ADG116, ADG106#8 | Advanced/metastatic solid tumors |
| NCT03792724 | 1/2 | NYR | Urelumab#9, nivolumab | PD-1/PD-L1 sensitive tumors |
| NCT02845323 | 2 | ANR | Nivolumab, urelumab#9 | Urothelial cancer |
| NCT03431948 | 1 | ANR | Nivolumab, urelumab#9, radiation | Advanced solid tumors |
| NCT02451982 | 2 | R | Cyclophosphamide, pancreatic tumor vaccine, nivolumab, urelumab#9 | Pancreatic cancer |
| NCT02652455 | 1 | ANR | Nivolumab, urelumab#9, cyclophosphamide, fludarabine, aldesleukin, autologous tumor-infiltrating lymphocytes | Melanoma |
| NCT02658981 | 1 | ANR | Urelumab#9; Nivolumab, urelumab#9; Anti-LAG-3 monoclonal antibody BMS 986016, nivolumab, urelumab#9 |
Glioblastoma Gliosarcoma Recurrent brain neoplasm |
| NCT03318900 | 1 | ANR | Aldesleukin, CD8-positive T-lymphocytes, cyclophosphamide, utomilumab#10 | Ovarian cancer |
| NCT03414658 | 2 | R | Vinorelbine, trastuzumab, avelumab, utomilumab#10; Trastuzumab, avelumab, utomilumab#10 |
Breast cancer |
| NCT03290937 | 1 | ANR | Cetuximab, irinotecan hydrochloride, utomilumab#10 | Colorectal cancer |
| NCT03217747 | 1/2 | ANR | Avelumab, utomilumab#10; Avelumab, ivuxolimab, utomilumab#10; Avelumab, utomilumab#10, radiation; Avelumab, ivuxolimab, utomilumab#10, radiation |
Castration-resistant prostate cancer |
| NCT02554812 | 1/2 | ANR | Avelumab, utomilumab#10; Avelumab, utomilumab#10, PF-04518600; Avelumab, utomilumab#10; Avelumab, utomilumab#10, vidutolimod |
Bladder cancer Gastric cancer Head and neck squamous cell carcinoma Melanoma NSCLC Ovarian cancer |
| NCT03971409 | 2 | R | Avelumab, utomilumab#10 | Breast cancer |
| NCT05059522 | 3 | R | Avelumab, utomilumab#10, vidutolimod, PF04518600 | NSCLC Ovarian cancer Solid tumors Urothelial cancer |
| Interferon-γ/interleukin 12 | ||||
|---|---|---|---|---|
| NCT03112590 | 1/2 | ANR | INF-γ, paclitaxel, tastuzumab, pertuzumab, post-therapy surgery | Breast cancer |
| NCT03132675 | 2 | R | Tavokinogene telseplasmid#11, pembrolizumab, immunopulse | Melanoma |
| NCT03567720 | 2 | R | Tavokinogene telseplasmid#11, pembrolizumab, immunopulse Tavokinogene telseplasmid#11, pembrolizumab, immunopulse, nab-paclitaxel |
Breast cancer |
| NCT04526730 | 2 | R | Tavokinogene telseplasmid#11, nivolumab, immunopulse | Melanoma |
| NCT02555397 | 1 | ANR | Ad5-yCD/mutTKSR39rep-hIL12#12 | Prostate cancer |
| NCT04911166 | 1 | R | ADV/IL-12 gene therapy#13, atezolizumab | NSCLC |
| NCT03030378 | 1 | R | Edodekin alfa#14, pembrolizumab | Metastatic solid tumors |
| NCT01468896 | 1/2 | ANR | Cetruximab, edodekin alfa#14 | Head and neck squamous cell carcinoma |
| NCT04235777 | 1 | 1 | M7824#30, M9241#15; M7824#30, M9241#15, stereotactic body radiation therapy |
Urogenital cancer Urothelial cancer |
| NCT05361798 | 2 | R | M9241#15, stereotactic body radiation therapy | Prostate cancer |
| NCT04633252 | 1/2 | R | Androgen deprivation therapy, prednisone, M7824#30, docetaxel, M9241#15 | Prostate cancer |
| NCT05286814 | 2 | R | Floxuridine, 5-fluorouracil, irinotecan, oxaliplatin, leucovorin, M9241#15, gemcitabine, dexamethasone | Colorectal cancer Intrahepatic cholangiocarcinoma |
| NCT04708470 | 1/2 | R | Bintrafusp alfa#30, NHS-IL12#15, entinostat | Anal cancer Cervical cancer Colon cancer Neck cancer Oropharyngeal cancer Penile cancer Vaginal cancer Vulvar cancer |
| NCT04303117 | 1/2 | R | NHS-IL12#15, M7824#30 | Kaposi sarcoma |
| NCT04491955 | 2 | R | CV301, MSB0011359C#30, N-803#29; CV301, MSB0011359C#30, N-803#29, NHS-IL12#15 |
Colorectal cancers Small bowel cancers |
| NCT04287868 | 1/2 | R | PDS0101, M7824#30, NHS-IL12#15 | Anal cancer Cervical cancer HPV cancers Oropharyngeal cancer Penile cancer Rectal cancer Vaginal cancer Vulvar cancer |
| NCT02498912 | 1 | ANR | 4H11-28z/fIL-12/EGFRt+ genetically modified T cells#16 | Solid tumors |
| NCT04613492 | 1 | R | MEDI9253#17, durvalumab | Advanced/metastatic solid tumors |
| NCT03439085 | 2 | ANR | MEDI0457#18, durvalumab | HPV cancer |
| NCT04471987 | 1 | R | IL12-L19L19#19 | Advanced/metastatic solid tumors |
| NCT03393884 | 1/2 | R | GEN-1#20, carboplatin, paclitaxel | Ovarian cancer Fallopian tube cancer Peritoneal cancer |
| NCT05352750 | 1 | R | SON-1010#21 | Advanced solid tumors |
| NCT04388033 | 1/2 | R | Dendritic cell/tumor fusion vaccine, IL-12, temozolomide | Glioblastoma |
| NCT05077033 | 1 | R | phIL12 GET#22 | Basal cell carcinoma |
| NCT05095441 | 1 | NYR | C5252#23 | Glioblastoma |
| Interleukin 4 | ||||
|---|---|---|---|---|
| NCT05013450 | 1/2 | R | Dupilumab#24, PD-1/PD-L1 blockade | NSCLC |
| Interleukin 15/interleukin 15 receptor | ||||
|---|---|---|---|---|
| NCT04491955 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04261439 | 1 | R | NIZ985#25, spartalizumab; NIZ985#25, spartalizumab, tislelizumab |
Advanced solid tumors Melanoma NSCLC |
| NCT04234113 | 1 | R | SO-C101#26; SO-C101#26, pembrolizumab |
Anal cancer Thyroid cancer Bladder cancer Biliary tract cancer Cervical cancer Cutaneous squamous cell carcinoma Gastric cancer Head and neck squamous cell carcinoma Hepatocellular carcinoma Melanoma Mesothelioma Merkel cell carcinoma Microsatellite instability high NSCLC Ovarian cancer Renal cell carcinoma Small-cell lung cancer Thymic cancer Triple-negative breast cancer |
| NCT05619172 | 2 | NYR | SOT101#26, cetuximab | Colorectal cancer |
| NCT05256381 | 2 | R | SOT101#26, pembrolizumab | Castration-resistant prostate cancer Colorectal cancer Cutaneous squamous cell carcinoma Hepatocellular carcinoma NSCLC Ovarian cancer |
| NCT04250155 | 1 | R | XmAb24306#27; Atezolizumab+ XmAb24306#27 |
Advanced/metastatic/recurrent solid tumors |
| NCT04616196 | 1/2 | R | NKTR-255#28, cetuximab | Anal squamous cell carcinoma Cervical cancer Colorectal cancer Cutaneous squamous cell carcinoma Head and neck squamous cell carcinoma |
| NCT05327530 | 2 | R | Avelumab+NKTR-255#28 | Urothelial cancer |
| NCT05445882 | 2 | NYR | N-803#29; N-803#29, BN-Brachyury; N-803#29, bintrafusp alfa#30 |
Castration-resistant prostate cancer |
| NCT04847466 | 2 | R | N-803#29, pembrolizumab, PD-L1 t-haNK | Gastroesophageal junction cancer Head and neck squamous cell carcinoma |
| NCT03022825 | 2/3 | R | N-803#29, intravesical Bacillus Calmette–Guerin | Bladder cancer |
| NCT05096663 | 2/3 | R | N-803#29, pembrolizumab | NSCLC |
| NCT02138734 | 1/2 | R | N-803#29, intravesical Bacillus Calmette–Guerin | Bladder cancer |
| NCT04247282 | 1/2 | ANR | M7824#30, N803#29+TriAd vaccine | Head and neck cancer |
| NCT03493945 | 1/2 | R | M7824#30, N803#29; M7824#30, N803#29, MVA-BN-Brachyury, FPV-Brachyury; M7824#30, N803#29, MVA-BN-Brachyury, FPV-Brachyury, epacadostat |
Advanced/metastatic solid tumors Prostate cancer |
| NCT03520686 | 3 | ANR | N-803#29, pembrolizumab; N-803#29, carboplatin, nab-paclitaxel, pembrolizumab; N-803#29, cisplatin or carboplatin, pembrolizumab, pemetrexed |
NSCLC |
| NCT04927884 | 1/2 | ANR | N-803#29, PD-L1 t-haNK, sacituzumab Govitecan-Hziy, cyclophosphamide | Triple-negative breast cancer |
| NCT03228667 | 2 | ANR | N-803#29, pembrolizumab; N-803#29, nivolumab; N-803#29, atezolizumab; N-803#29, avelumab; N-803#29, durvalumab; N-803#29, pembrolizumab, PD-L1 t-haNK; N-803#29, nivolumab, PD-L1 t-haNK; N-803#29, atezolizumab, PD-L1 t-haNK; N-803#29, avelumab, PD-L1 t-haNK; N-803#29, durvalumab, PD-L1 t-haNK |
Cervical cancer Colorectal cancer NSCLC Gastric cancer Head and neck squamous cell carcinoma Hepatocellular carcinoma Melanoma Merkel cell carcinoma Microsatellite instability Mismatch repair deficiency Small-cell lung cancer Renal cell carcinoma Urothelial cancer |
| Transforming growth factor beta | ||||
|---|---|---|---|---|
| NCT04247282 | — | — | Multiple target combination—details on clinical trial see section Interleukin 15/interleukin 15 receptor | — |
| NCT03493945 | — | — | Multiple target combination—details on clinical trial see section Interleukin 15/interleukin 15 receptor | — |
| NCT04491955 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04303117 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04235777 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04287868 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04633252 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT04708470 | — | — | Multiple target combination—details on clinical trial see section Interferon-γ/interleukin 12 | — |
| NCT05445882 | — | — | Multiple target combination—details on clinical trial see section Interleukin 15/interleukin 15 receptor | — |
| NCT04574583 | 1/2 | ANR | SX-682, M7824#30, MVA-BN-CV301, FPV-CV301 | Advanced/metastatic cancer Head and neck squamous cell carcinoma Triple-negative breast cancer |
| NCT04432597 | 1/2 | R | PRGN-2009, M7824#30 | Anal cancer Cervical cancer HPV-positive solid cancer Oropharyngeal cancer Penile cancer Rectal cancer Vaginal cancer Vulvar cancer |
| NCT04297748 | 1/2 | R | 89Zirconium-M7824, M7824#30 | NSCLC |
| NCT04417660 | 2 | R | M7824#30 | Thymic cancer Recurrent thymoma |
| NCT03436563 | 1/2 | ANR | M7824#30 | Colorectal adenocarcinoma |
| NCT04835896 | 1/2 | NYR | M7824#30, paclitaxel | Gastric cancer |
| NCT03427411 | 2 | ANR | M7824#30 | Anal cancer Cervical cancer HPV-positive solid tumors Oropharyngeal cancer Penile cancer Vaginal cancer |
| NCT03631706 | 3 | ANR | M7824#30 | NSCLC |
| NCT05005429 | 2 | R | M7824#30 | Mesothelioma |
| NCT03620201 | 1 | ANR | M7824#30 | Breast cancer |
| NCT03554473 | 1/2 | R | M7824#30, temozolomide; M7824#30, topotecan |
Small-cell lung cancer |
| NCT03840902 | 2 | ANR | M7824#30, etoposide, pemetrexed, carboplatin, paclitaxel, cisplatin, intensity-modulated radiation therapy | NSCLC |
| NCT04066491 | 2/3 | ANR | M7824#30, gemcitabine, cisplatin | Biliary tract cancer |
| NCT05145569 | 1 | NYR | Carboplatin AUC 5 and paclitaxel, M7824#30 | Ovarian cancer |
| NCT03315871 | 2 | R | PROSTVAC-V, PROSTVAC-F, M7824#30, CV301 | Prostate cancer |
| NCT04551950 | 1 | ANR | M7824#30, carboplatin, paclitaxel, bevacizumab, cisplatin; M7824#30, carboplatin, paclitaxel, cisplatin; M7824#30, cisplatin, radiotherapy |
Cervical cancer |
| NCT04481256 | n.a. | R | Radiatin, bintrafusp alfa#30, paclitaxel, carboplatin | Squamous cell carcinoma |
| NCT04246489 | 2 | ANR | Bintrafusp alfa#30 | Cervical cancer |
| NCT05061823 | 3 | R | Bintrafusp alfa#30 | Cancer |
| NCT05012098 | 2 | R | Bintrafusp alfa#30 | Esthesioneuroblastoma |
| NCT04595149 | 2 | R | Bintrafusp alfa#30 | Esophageal cancer |
| NCT04349280 | 1 | ANR | Bintrafusp alfa#30 | Urothelial cancer |
| NCT04396535 | 2 | ANR | Bintrafusp alfa#30, docetaxel | NSCLC |
| NCT04708067 | 1 | R | Bintrafusp alfa#30, hypofractionated radiation therapy | Intrahepatic cholangiocarcinoma |
| NCT04874311 | 2 | R | Bintrafusp alfa#30, doxorubicin | Soft-tissue sarcoma |
| NCT04396886 | 2 | ANR | Bintrafusp alfa#30 | Nasopharyngeal carcinoma Non-keratinizing carcinoma |
| NCT04789668 | 1/2 | ANR | Bintrafusp alfa#30, pimasertib | Breast cancer Hematopoietic and lymphoid cell neoplasm Melanoma Neoplasm in the brain NSCLC |
| NCT04878250 | 2 | NYR | Bintrafusp alfa#30 | Urothelial cancer |
| NCT04457778 | 1 | R | M6223, bintrafusp alfa#30 | Metastatic solid tumors |
| NCT02723955 | 1 | ANR | Feladilimab, bintrafusp alfa#30 | Colorectal cancer Deficient mismatch repair tumor Esophageal cancer Epstein–Barr positive tumor Head and neck carcinoma HPV-positive tumor Melanoma Mesothelioma Microsatellite instability-high tumor NSCLC Prostate cancer Squamous cell carcinoma Urothelial cancer |
| NCT03834662 | 1 | ANR | AVID200#31 | Advanced/metastatic solid tumors |
| NCT05537051 | 1 | NYR | PM1021, PM8001#32 | Advanced solid tumors |
| NCT05028556 | 1 | R | Y101D#33 | Metastatic/advanced solid tumors |
| NCT05381935 | 1 | NYR | ES014#34 | Advanced solid tumors |
| NCT04862767 | 1 | R | TASO-001#35, aldesleukin | Solid tumors |
| Histamine | ||||
|---|---|---|---|---|
| NCT04165096 | 2 | ANR | Pembrolizumab, MK-5890, diphenhydramine#36, acetaminophen | NSCLC |
| NCT04863950 | 2 | R | Lomustine, imipramine hydrochloride#37 | Glioblastoma |
| NCT03253289 | 1 | R | Meclizine#38 | Hepatocellular carcinoma |
| 2022-001284-27 | 2 | ANR | Fexofenadine hydrochloride#39, pembrolizumab | NSCLC |
| Vitamin E | ||||
|---|---|---|---|---|
| NCT04245865 | 2 | R | Fluorouracil, calcium folinate, oxaliplatin, bevacizumab, capecitabine, tocotrienol | Colorectal cancer |
| NCT02705300 | 2 | ANR | FOLFOXIRI, tocotrienol | Colorectal cancer |
| NCT04175470 | 2 | R | Bevacizumab, tocotrienol | Ovarian cancer |
CNS, central nervous system; EGFR, epidermal growth factor receptor; HPV, human papillomavirus; IL, interleukin; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
NCT identifiers refer to trials registered at www.clinicaltrials.gov, https://rctportal.niph.go.jp, or https://www.clinicaltrialsregister.eu/ctr-search/search/.
Status: R, recruiting; ANR, active, not recruiting; NYR, not yet recruiting. Intervention. #1 Selicrelumab—fully human IgG2 agonistic monoclonal antibody (mAb) to CD40. #2 CDX-1140—fully human IgG2 agonistic mAb to CD40. #3 APX005M (alias sotigalimab)—is a humanized rabbit IgG1 agonistic mAb to CD40.#4 SEA-CD40 (alias dacetuzumab)—is a humanized IgG1, non-fucosylated agonistic mAb to CD40. #5 STA551—human IgG1/lambda agonist switch antibody to CD137 with binding only in the presence of ATP. #6 AGEN2373—fully human IgG1 agonistic mAb to CD137. #7 EU101—fully humanized IgG1 agonistic mAb to CD137. #8 ADG106—fully human IgG4 agonistic mAb to CD137. #9 Urelumab—fully human IgG4 agonistic mAb to CD137. #10 Utomilumab—fully human IgG2 agonistic mAb to CD137. #11 Tavokinogene telseplasmid—intratumoral injected plasmid-encoding IL-12 by electroporation using the immunopulse. #12 Ad5-yCD/mutTKSR39rep-hIL12—replication-competent oncolytic adenovirus encoding IL-12 gene, a yeast cytosine deaminase (yCD) and a mutant form of herpes simplex virus type 1 thymidine kinase (HSV-1 TKSR39). #13 ADV/IL-12 gene therapy—adenoviral-mediated interleukin-12. #14 Edodekin alfa—recombinant IL-12. #15 M9241 (alias NHS-IL12)—two IL-12 molecules fused to a human IgG1-recognizing DNA/histone complexes. #16 4H11-28z/fIL-12/EGFRt+ genetically modified T cells—genetically modified autologous T cells transduced with a retroviral vector expressing a chimeric antigen receptor targeting the human tumor-associated antigen MUC16ecto and encoding IL-12 fused to the signaling domain of the zeta chain of the TCR/CD3 complex (28z) and a truncated form of the human epidermal growth factor receptor (EGFRt). #17 MEDI9253—oncolytic viral agent containing the oncolytic, live-attenuated, replication-competent strain of the avian paramyxovirus Newcastle disease virus (NDV) engineered to include a transgene encoding IL-12. #18 MEDI0457—DNA Plasmid-encoding interleukin-12/HPV DNA plasmids therapeutic vaccine. #19 IL12-L19L19—fusion protein of recombinant IL-12 with human mAb L19 specific for the extra-domain B of fibronectin in tandem diabody format. #20 GEN1—IL-12 plasmid with PEG-PEI-cholesterol lipopolymer. #21 SON-1010—single-chain human IL12 linked to a single-chain variable region antibody fragment. #22 phIL12 GET—intratumoral gene transfer of plasmid coding for IL-12. #23 C5252—genetically engineered oncolytic herpes simplex virus type 1 (oHSV-1) expressing the IL-12 and an antibody directed against PD-1.#24 Dupilumab—fully human IgG4 antagonistic dimeric mAb to IL-4 receptor α/γc and IL-4 receptor α/IL-13 receptor α. #25 NIZ985—recombinant IL-15/IL-15Rα heterodimer.#26 SO-C101 (alias SOT101)—fusion protein of recombinant IL-15 linked to IL-15Rα sushi (cytokine-binding) domain. #27 XmAb24306—recombinant IL-15/IL-15Rα cytokine fusion complex. #28 NKTR-255—polyethylene glycol-conjugated recombinant human IL-15 agonist. #29 N-803—mutated form of the cytokine IL-15 (IL-15N72D) and a soluble, dimeric IL-15 receptor alpha (IL-15Ra) Fc fusion protein (IL-15Ra-Fc) (IL-15N72D/IL-15Ra-Fc). #30 M7824 (alias bintrafusp alfa, MSB0011359C)—bifunctional fusion protein of human IgG1 mAb against PD-L1 fused with two extracellular domains of TGF-β receptor type II. #31 AVID200—receptor ectodomain trap. #32 PM8001—bifunctional protein composed of the extracellular domain of the TGF-β receptor type II receptor fused to a humanized anti-PD-L1 IgG1 single-domain antibody. #33 Y101D—recombinant anti-PD-L1 and TGF-β bispecific antibody. #34 ES014—anti-CD39 and TGF-β bispecific antibody. #35 TASO-001—TGF-β2 targeting antisense oligonucleotide. #36 Diphenhydramine—H1-antihistamine antagonist. #37 Imipramine hydrochloride—H1-antihistamine antagonist. #38 Meclizine—H1-antihistamine antagonist. #39 Fexofenadine hydrochloride—H1-antihistamine antagonist.
CD137
CD137 (also known as 4-1BB), a member of the tumor necrosis factor receptor superfamily 9, acts as a co-stimulatory molecule by engaging to its ligand (CD137L), predominantly expressed on activated antigen-presenting cells (APCs).87 Especially, the effects on T cell function have made CD137 a target of interest with promising agonistic anti-tumorigenic effects including long-term activation and survival of T cells in multiple tumor models.87,88 In monocytes, the effect of CD137 agonists can be double-edged89: CD137 agonists are able to create an anti-tumorigenic milieu by inducing pro-inflammatory cytokine secretion while reducing interleukin 10 (IL-10) release and enhancing monocyte survival and migration. Furthermore, monocytes proliferate upon CD137 agonist signaling and differentiate into monocyte-derived DCs, thereby enhancing T cell proliferation.90 Gauttier et al. demonstrated a substantial role for macrophages in the agonistic targeting of CD137. In this study, complete tumor regression was observed in 40%-60% of animals in an in situ model for hepatocellular carcinoma; and therapy efficacy was associated with macrophage recruitment within the tumor nodules.91 Nevertheless, there are conflicting reports indicating pro-tumorigenic effects of CD137 agonistic therapy on macrophages. Geng et al. reported a CD137 agonist-mediated differentiation to a pro-tumorigenic phenotype92 and Jiang et al. showed a promotion of bone metastasis by transformation of monocytes/macrophages into osteoclasts via CD137 activation.93 These reported pro-tumorigenic properties of monocytes/macrophages in vitro and in animal models should at least be considered as potential factors limiting the applicability of CD137 agonists. Despite promising reports in various cancer models, clinical trials have not met expectations.94 Monotherapy studies reported dose-dependent hepatotoxicity with urelumab with limited clinical activity (BMS-663513; NCT00309023, NCT00612664, NCT01471210).95 Another promising candidate, utomilumab (PF-05082566), showed at least a better safety profile (NCT01307267).96 Due to the high frequency of hepatotoxicity, there are novel attempts to improve anti-CD137 agonists as described by Kamata-Sakurai et al. who demonstrated an anti-CD137 switch antibody (STA551) with potent anti-tumor efficacy in vitro and in animal models and now tested in a phase I trial (Table 1) by exerting its agonistic activity only in the presence of extracellular ATP which is highly elevated in solid tumors.94
Interferon gamma
Interferon-gamma (INF-γ) is predominantly secreted by activated T and natural killer (NK) cells and to a lesser extent by APCs and B cells. Its production is up-regulated by inflammatory or immune stimuli including mitogens and cytokines such as interleukin 12 (IL-12) and interleukin (IL-15).97 Via a positive feedback loop, INF-γ triggers the up-regulation of major histocompatibility complex (MHC) class I and II antigen processing and presentation on macrophages via INF-γ receptor 1/2, thereby in turn promoting T cell activation. As a key cytokine-mediating Th1 immunity, INF-γ has a major contribution in cancer beyond its action on macrophages: INF-γ induces tumor cell cycle arrest, tumor cell death (apoptosis, necroptosis), inhibition of angiogenesis, and induces T and NK cell trafficking into the tumor.98 In contrast, INF-γ is attributed with pro-tumorigenic properties including promotion of angiogenesis and epithelial–mesenchymal transition, enhancing the immunosuppressive properties of myeloid cells, and induction of indolamine-2,3-dioxygenase synthesis. In macrophages, INF-γ is shown to re-educate TAMs from a pro- to an anti-tumorigenic phenotype, enhances MHC class I and II expression, and induces IL-12 expression, which in turn activates T and NK cells.99 Mouse models showed that immunotherapy with ICIs was linked with INF-γ production in present T cells97 and Manguso et al. attributed resistance to immunotherapy to defects in INF-γ signaling.100 This observation was in line with patient data: in patients with NSCLC and urothelial cancer under anti-PD-L1 treatment with durvalumab, Higgs et al. described a higher overall response rate and longer median progression-free survival for patients with increased INF-γ gene signature.101 Furthermore, in melanoma patients not responding to anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy, 75% were associated with genomic defects in INF-γ signaling genes in the tumor.102 These findings are in line with our understanding of IFN-γ as a major T cell effector cytokine and Th1 immune mediator. Nevertheless, clinical trials in various cancer entities had no positive effect on the treatment outcome.97,99 Furthermore, endogenous administration of INF-γ led to systemic toxicity and serious side effects which limits the use INF-γ as well. Therefore, development of alternative delivery routes (liposomes, polymers, nanoparticles, gene therapy) are ongoing.97,103 Additionally, administration of IL-12 in various set-ups (e.g. engineered T cells, mRNA, or fusion proteins) is considered to increase INF-γ production104 (Table 1). Daud et al. proofed the concept of gene transfer utilizing in vivo DNA electroporation for IL-12 delivery105 and showed a 41% overall response rate with 36% complete response in melanoma patients in combination with pembrolizumab.106
Interleukin 4
IL-4 is a classical type II cytokine that polarizes macrophages into a specialized phenotype promoting tissue repair mechanisms, which is required for anti-parasite immunity, and contributes to fibrosis and allergy pathology.107 In addition to its effects on macrophages, IL-4 dampens T cell stimulatory mechanisms in DCs,108 and drives cancer cell proliferation and resistance to apoptosis.109, 110, 111, 112 High post-operative IL-4 levels after resection of NSCLC lesions correlated with tumor recurrence, which indicates a potential role of IL-4 in metastasis.113 In preclinical models of lung adenocarcinoma, colon, and mammary carcinoma, IL-4 blockade reduced tumor growth and enhanced anti-tumor immunity. Mechanistically, IL-4 blockade increased immunostimulatory activity of DCs in lung cancer,108 and decreased suppressive IL-10 in TAMs, while also increasing IL-12 and INF-γ levels measured in whole tissue.114 In addition, inhibition of STAT6, which is the downstream signaling target of IL-4, led to a reduction of lung metastases in a mammary carcinoma model.115 A novel strategy allows to target IL-4 signaling specifically in macrophages by the administration of extracellular vesicles carrying STAT6-silencing antisense nucleotides leading to tumor shrinkage in models of colorectal and hepatocellular carcinoma.116 In a mouse model of STAT6 deficiency in CD11b+ myeloid cells, lung cancer growth was significantly inhibited, and the mobilization of suppressive myeloid cells was reduced.117 However, the local effects of IL-4 on different processes of the TME have to be carefully considered. In a breast cancer mouse model, IL-4 had a protective effect by normalizing tumor vasculature and inducing tumor cell death,118 even though human breast cancer progression is also driven by direct IL-4 signaling on cancer cells.109 Thus, in cancers that are contained by a functional Th2 immune response driven by CD4+ T cells, IL-4 signaling plays a controversial role and might not be an ideal target. In contrast, cancers that are dominated by suppressive myeloid cells, CD8+ T cells, and Th1-polarized CD4+ T cells could profit from IL-4 blockade. Currently, in one clinical trial, blocking of IL-4 is being tested in patients with NSCLC using dupilumab (NCT05013450; Table 1) which was initially approved for atopic dermatitis, asthma, and nasal polyps with chronic sinusitis.
Interleukin 15/Interleukin 15 receptor
IL-15 exerts its effect by first binding to the IL-15 receptor alpha (IL-15Rα) on the surface of IL-15R-expressing cells and is then trans-presented and bound to a receptor complex composed of the IL-2 receptor β and common γ chains presented mainly on lymphocytes119,120 inducing T and NK cell proliferation, immunoglobulin synthesis in B cells, survival of memory T and NK cells, and triggering of NK cell effector function.121, 122, 123 Based on the stimulatory role of IL-15 on lymphocytes, it has become a target of interest for cancer therapy. The short in vivo half-life of IL-15121 led to the creation of IL-15/IL-15Rα complexes, often called ‘super-agonists’, to (i) increase their half-life and (ii) avoid the need for trans-presentation and cell–cell contact.119 This approach led to increased effector function in cancer models121 with increased T cell and NK cell-mediated cytotoxicity, recruitment of DCs, and reversed transforming growth factor beta (TGF-β)-mediated NK cell paralysis.120,124 Mattiola et al. described an anti-tumor feedforward loop between NK cells and macrophages.122 In this study, IL-15Rα trans-presented by inflammatory macrophages, enhanced NK cell cytotoxicity, which in turn re-educated suppressive macrophages boosting their anti-tumorigenic activity. Despite the improved action of IL-15/IL-15Rα complexes, monotherapy efficacy in cancer models was limited. The combination with immunotherapy, in contrast, enhanced the anti-tumor efficacy resulting in improved survival, decreased tumor growth, and enhanced cytotoxicity.120,125,126 In clinical trials, IL-15/IL-15Rα was reported to be well tolerated in patients with advanced solid cancers127 and showed an objective response rate in 6 of 21 patients.128 Various trials in patients with advanced or metastatic solid tumors in combination with ICIs are ongoing (Table 1).
Transforming Growth Factor Beta
TGF-β is a pleiotropic cytokine involved in both activating and dampening the immune response and thereby promoting immune tolerance and homeostasis.129 This double-sided behavior can also be observed in cancer, where TGF-β acts as a tumor suppressor in the early phase while with disease progression it switches into a tumor promoter.130, 131, 132, 133, 134 In the initial phase of carcinogenesis, TGF-β inhibits tumor cell growth and proliferation, promotes apoptosis, and blocks growth factor, cytokine, and chemokine production.132,133,135 With disease progression, TGF-β promotes the expansion of Tregs while inhibiting T and NK cell proliferation and effector function.133,135 Furthermore, TGF-β induces monocyte chemotaxis133 and promotes macrophage differentiation toward an immunosuppressive phenotype,135 which can contribute to tumor metastasis.136 Based on this dual role, TGF-β-blocking antibodies were developed which showed efficacy in cancer models.131,133,134,137 The consequences of TGF-β blocking on macrophages are still understudied, but potentially lead to a reversion of their immunosuppressive phenotype.138,139 Combinations of irradiation and TGF-β-blocking antibodies enhanced the anti-tumor response in mice, which could even be boosted by additionally using an anti-PD-1 blocking antibody.133 Feun et al. reported a significant correlation between the poor outcomes of patients with advanced hepatocellular carcinoma treated with pembrolizumab with high baseline plasma TGF-β levels.140 Therefore, a concurrent TGF-β blockade might strengthen the efficacy of ICIs.132 First clinical trials showed that TGF-β-targeting antibodies both in mono- and combination therapies were safe and well tolerated (NCT00356460, NCT00923169, NCT01112293, NCT01401062, NCT01646203)141 with many clinical studies in various phases still ongoing (Table 1).
Histamine
Histamine, a well-known biogenic amine, is involved in various physiological and pathophysiological processes, and increasing evidence suggests an active role of histamine and its receptor antagonists in cancer.142 The effect of histamine on tumor cells, such as their growth, is still controversial; both pro- and anti-tumorigenic effects have been described and seem to be dependent on cell/cancer type, histamine concentration, and involved histamine receptors.143 In monocytes and macrophages, histamine receptor expression is also controversial.144 However, in vitro data indicate that histamine receptor expression changes with the process of macrophage differentiation.145, 146, 147, 148 In line with tumor cells, the effect of histamine on monocytes and macrophages and their contribution to tumorigenesis is still ambiguous.146,149,150 Remarkably, Li et al. showed that histamine polarizes monocytes via histamine receptor H1 to pro-tumorigenic macrophages, which goes in line with an increased expression of the immune checkpoint V-domain Ig suppressor of T cell activation (VISTA).151 However, knockout of histamine receptor H1 or antihistamine treatment was able to revert both macrophage-mediated immunosuppression and revitalized T cell cytotoxic function. So far, less studies focused on the effect of histamine on therapy response, especially with ICIs. In vitro data support the synergistic effect of a histamine receptor H1-targeted antihistamines with platinum drugs in lung and pancreatic cancer cell lines.152 In another study, antihistamine treatment sensitized cells to chemotherapy and reverted multidrug resistance in in vitro models for NSCLC, breast, and prostate cancer.153 Li et al. reported an enhanced efficacy of both anti-PD-1 and anti-CTLA-4 antibodies in combination with a histamine receptor H1 inhibition by fexofenadine hydrochloride, a histamine receptor H1 antagonist, in mouse models.151 Retrospective analysis of cancer patients with antihistamine consumption during ICI therapy showed a significant improvement in survival. Low plasma histamine levels were linked to a tripled objective response rate to anti-PD-1 treatment in line with Faustino-Rocha et al. who reported higher histamine levels linked with faster metastasis formation.142 Grauers Wiktorin et al., in contrast, reported an improved anti-tumor efficacy of anti-PD-1/anti-PD-L1 treatment in combination with histamine dihydrochloride in a colorectal mouse model highlighting the controversies in this topic.154 Currently, the only running trial with antihistamines in cancer patients receiving ICIs uses diphenhydramine (a histamine receptor H1 antagonist) in combination with pembrolizumab, acetaminophen, and MK-5890 (an anti-CD27 antibody) or MK-4830 (an antibody targeting the myeloid-specific anti–immunoglobulin-like transcript 4, ILT4) to assess objective response rate (Table 1).
Vitamin E
The vitamin E group encompasses eight structurally related compounds (α-, β-, γ-, δ-tocopherol and α-, β-, γ-, δ-tocotrienol), which possess antioxidant properties155 and are thus implicated in reduced risk for cardiovascular diseases, immunomodulation, anti-allergic effects, and neurological and hepatic health.156 γ-tocopherol and γ-tocotrienol were described as inducers of apoptosis in mouse and human in vitro models for breast157, 158, 159 and pancreatic cancer.160 Vitamin E might also mediate tumor-associated properties in monocytes and macrophages, as it was reported to induce tumor necrosis factor alpha (TNF-α)-producing macrophages,161 inhibit interleukin 6 (IL-6) and nitric oxide (NO) production,162 and reduce prostaglandin E2 synthesis,163 which can lead to enhanced tumorigenesis, angiogenesis, and metastasis.155 However, Yuan et al. proposed a vitamin E-mediated increase in ICI efficacy, which mainly depended on monocyte-derived DCs.164 The anti-tumorigenic effects were consistent with in vivo studies.165 In contrast, results from clinical trials studying vitamin E for cancer prevention166, 167, 168, 169 or as a therapy supplement170, 171, 172, 173 were inconclusive. A limited number of clinical trials in the context of improved efficacy are ongoing, with only two trials including monoclonal antibody therapies (NCT04245865, NCT04175470; Table 1).
Conclusions
In this review, we discuss the various subsets of monocytes and macrophages, their origin, their properties, and their role in cancer and response to immunotherapy. Both monocytes and macrophages are influenced and primed by their microenvironment, which turns them into either pro- or anti-tumorigenic modulators. Therefore, monocytes and macrophages can contribute actively to cancer progression. Nevertheless, their properties have not been fully understood, especially in the context of immunotherapy success. Monocytes and macrophages present various promising new targets, thus providing novel potential therapy approaches. Furthermore, their contribution to the efficacy of current and future therapies needs to be (re-)evaluated for potential therapy advances. We believe that a thorough molecular understanding of monocyte/macrophage populations is a pre-requisite to unlock their potential as a key target in immunotherapy to improve therapy outcome in combinatorial therapies.
Acknowledgements
We thank Teresa Hatziioannou for the design and creation of the graphical illustrations.
Funding
This work was financially supported by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development, and the Christian Doppler Research Association and is gratefully acknowledged.
Disclosure
ASB has research support from Daiichi Sankyo and Roche, honoraria for lectures, consultation or advisory board participation from Roche, Bristol-Meyers Squibb, Merck, Daiichi Sankyo; as well as travel support from Roche, Amgen, Daiichi Sankyo, and AbbVie. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. All other authors have declared no conflicts of interest.
References
- 1.Ziegler-Heitbrock H.W. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17(9):424–428. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler-Heitbrock L., Ancuta P., Crowe S., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 3.Wong K.L., Yeap W.H., Tai J.J., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53(1-3):41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 4.Ozanska A., Szymczak D., Rybka J. Pattern of human monocyte subpopulations in health and disease. Scand J Immunol. 2020;92(1) doi: 10.1111/sji.12883. [DOI] [PubMed] [Google Scholar]
- 5.Kwiecien I., Rutkowska E., Polubiec-Kownacka M., Raniszewska A., Rzepecki P., Domagala-Kulawik J. Blood monocyte subsets with activation markers in relation with macrophages in non-small cell lung cancer. Cancers (Basel) 2020;12(9):2513. doi: 10.3390/cancers12092513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng A.L., Zhu J.K., Sun J.T., et al. CD16+ monocytes in breast cancer patients: expanded by monocyte chemoattractant protein-1 and may be useful for early diagnosis. Clin Exp Immunol. 2011;164(1):57–65. doi: 10.1111/j.1365-2249.2011.04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauer D., Starlinger P., Reiter C., et al. Intermediate monocytes but not TIE2-expressing monocytes are a sensitive diagnostic indicator for colorectal cancer. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg C., Nowicka M., Guglietta S., et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24(2):144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 9.Sakakura K., Takahashi H., Motegi S.I., Yokobori-Kuwabara Y., Oyama T., Chikamatsu K. Immunological features of circulating monocyte subsets in patients with squamous cell carcinoma of the head and neck. Clin Immunol. 2021;225 doi: 10.1016/j.clim.2021.108677. [DOI] [PubMed] [Google Scholar]
- 10.Kiss M., Caro A.A., Raes G., Laoui D. Systemic reprogramming of monocytes in cancer. Front Oncol. 2020;10:1399. doi: 10.3389/fonc.2020.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szaflarska A., Baj-Krzyworzeka M., Siedlar M., et al. Antitumor response of CD14+/CD16+ monocyte subpopulation. Exp Hematol. 2004;32(8):748–755. doi: 10.1016/j.exphem.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Gartner K., Battke C., Dunzkofer J., et al. Tumor-derived extracellular vesicles activate primary monocytes. Cancer Med. 2018;7(5):2013–2020. doi: 10.1002/cam4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cane S., Ugel S., Trovato R., et al. The endless saga of monocyte diversity. Front Immunol. 2019;10:1786. doi: 10.3389/fimmu.2019.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengos A.E., Gastineau D.A., Gustafson M.P. The CD14(+)HLA-DR(lo/neg) monocyte: an immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front Immunol. 2019;10:1147. doi: 10.3389/fimmu.2019.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Himes B.T., Peterson T.E., de Mooij T., et al. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro Oncol. 2020;22(7):967–978. doi: 10.1093/neuonc/noaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavin Y., Winter D., Blecher-Gonen R., et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosselin D., Link V.M., Romanoski C.E., et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez Conde C., Xu C., Jarvis L.B., et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;376(6594) doi: 10.1126/science.abl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavin Y., Mortha A., Rahman A., Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto D., Chow A., Noizat C., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Dranoff G., Crawford A.D., Sadelain M., et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 23.Park S.H. Regulation of macrophage activation and differentiation in atherosclerosis. J Lipid Atheroscler. 2021;10(3):251–267. doi: 10.12997/jla.2021.10.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentles A.J., Newman A.M., Liu C.L., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruni D., Angell H.K., Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 26.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azizi E., Carr A.J., Plitas G., et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–1308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S., Li Z., Gao R., et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184(3):792–809.e23. doi: 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Ham S., Lima L.G., Lek E., Moller A. The impact of the cancer microenvironment on macrophage phenotypes. Front Immunol. 2020;11:1308. doi: 10.3389/fimmu.2020.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molgora M., Esaulova E., Vermi W., et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182(4):886–900.e17. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoch K.M., Ezerskiy L.A., Morhaus M.M., et al. Acute Trem2 reduction triggers increased microglial phagocytosis, slowing amyloid deposition in mice. Proc Natl Acad Sci U S A. 2021;118(27) doi: 10.1073/pnas.2100356118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaitin D.A., Adlung L., Thaiss C.A., et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178(3):686–698.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casanova-Acebes M., Dalla E., Leader A.M., et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595(7868):578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Usatorre A., Kadioglu E., Boivin G., et al. Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models. Sci Transl Med. 2021;13(606) doi: 10.1126/scitranslmed.abd1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalio Ramos R., Missolo-Koussou Y., Gerber-Ferder Y., et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell. 2022;185(7):1189–1207.e25. doi: 10.1016/j.cell.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Leader A.M., Grout J.A., Maier B.B., et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell. 2021;39(12):1594–1609.e12. doi: 10.1016/j.ccell.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder K., Patel A.A., Kong W.T., et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021;54(8):1883–1900.e5. doi: 10.1016/j.immuni.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Xiong H., Mittman S., Rodriguez R., et al. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79(7):1493–1506. doi: 10.1158/0008-5472.CAN-18-3208. [DOI] [PubMed] [Google Scholar]
- 39.Hartley G.P., Chow L., Ammons D.T., Wheat W.H., Dow S.W. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6(10):1260–1273. doi: 10.1158/2326-6066.CIR-17-0537. [DOI] [PubMed] [Google Scholar]
- 40.Dhupkar P., Gordon N., Stewart J., Kleinerman E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7(6):2654–2664. doi: 10.1002/cam4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng D., Ye Z., Wu J., et al. Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer. Theranostics. 2020;10(15):7002–7014. doi: 10.7150/thno.46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Zugazagoitia J., Ahmed F.S., et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26(4):970–977. doi: 10.1158/1078-0432.CCR-19-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerriero J.L. Macrophages: the road less traveled, changing anticancer therapy. Trends Mol Med. 2018;24(5):472–489. doi: 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frafjord A., Skarshaug R., Hammarstrom C., et al. Antibody combinations for optimized staining of macrophages in human lung tumours. Scand J Immunol. 2020;92(1) doi: 10.1111/sji.12889. [DOI] [PubMed] [Google Scholar]
- 45.Pittet M.J., Michielin O., Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. 2022;19(6):402–421. doi: 10.1038/s41571-022-00620-6. [DOI] [PubMed] [Google Scholar]
- 46.Champiat S., Ferrara R., Massard C., et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 47.Saada-Bouzid E., Defaucheux C., Karabajakian A., et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara R., Mezquita L., Texier M., et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim C.G., Kim K.H., Pyo K.H., et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 50.Lo Russo G., Moro M., Sommariva M., et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989–999. doi: 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki A., Nakamura Y., Mishima S., et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22(4):793–802. doi: 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 52.Arasanz H., Zuazo M., Bocanegra A., et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers (Basel) 2020;12(2):344. doi: 10.3390/cancers12020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim C.G., Kim C., Yoon S.E., et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2021;74(2):350–359. doi: 10.1016/j.jhep.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Frelaut M., Le Tourneau C., Borcoman E. Hyperprogression under immunotherapy. Int J Mol Sci. 2019;20(11):2674. doi: 10.3390/ijms20112674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camelliti S., Le Noci V., Bianchi F., et al. Mechanisms of hyperprogressive disease after immune checkpoint inhibitor therapy: what we (don’t) know. J Exp Clin Cancer Res. 2020;39(1):236. doi: 10.1186/s13046-020-01721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X., Song X., Li K., Zhang T. FcγR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. 2019;10:292. doi: 10.3389/fimmu.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teige I., Martensson L., Frendeus B.L. Targeting the antibody checkpoints to enhance cancer immunotherapy-focus on FcγRIIB. Front Immunol. 2019;10:481. doi: 10.3389/fimmu.2019.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romano E., Kusio-Kobialka M., Foukas P.G., et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arlauckas S.P., Garris C.S., Kohler R.H., et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9(389) doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassetta L., Kitamura T. Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol. 2018;6:38. doi: 10.3389/fcell.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poh A.R., Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binnewies M., Pollack J.L., Rudolph J., et al. Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep. 2021;37(3) doi: 10.1016/j.celrep.2021.109844. [DOI] [PubMed] [Google Scholar]
- 63.Katzenelenbogen Y., Sheban F., Yalin A., et al. Coupled scRNA-seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell. 2020;182(4):872–885.e19. doi: 10.1016/j.cell.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 64.Zhou L., Wang M., Guo H., et al. Integrated analysis highlights the immunosuppressive role of TREM2(+) macrophages in hepatocellular carcinoma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.848367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H., Liu Z., Wen H., et al. Immunosuppressive TREM2(+) macrophages are associated with undesirable prognosis and responses to anti-PD-1 immunotherapy in non-small cell lung cancer. Cancer Immunol Immunother. 2022;71(10):2511–2522. doi: 10.1007/s00262-022-03173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obradovic A., Chowdhury N., Haake S.M., et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell. 2021;184(11):2988–3005.e16. doi: 10.1016/j.cell.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chow A., Schad S., Green M.D., et al. Tim-4(+) cavity-resident macrophages impair anti-tumor CD8(+) T cell immunity. Cancer Cell. 2021;39(7):973–988.e9. doi: 10.1016/j.ccell.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D.K., Wang W. Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies. Oncol Lett. 2020;20(5):176. doi: 10.3892/ol.2020.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vonderheide R.H. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13(4):1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 70.Hassan S.B., Sorensen J.F., Olsen B.N., Pedersen A.E. Anti-CD40-mediated cancer immunotherapy: an update of recent and ongoing clinical trials. Immunopharmacol Immunotoxicol. 2014;36(2):96–104. doi: 10.3109/08923973.2014.890626. [DOI] [PubMed] [Google Scholar]
- 71.Loskog A.S., Eliopoulos A.G. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21(5):301–307. doi: 10.1016/j.smim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Suttles J., Stout R.D. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009;21(5):257–264. doi: 10.1016/j.smim.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 73.Djureinovic D., Wang M., Kluger H.M. Agonistic CD40 antibodies in cancer treatment. Cancers (Basel) 2021;13(6):1302. doi: 10.3390/cancers13061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Labiano S., Roh V., Godfroid C., et al. CD40 agonist targeted to fibroblast activation protein α synergizes with radiotherapy in murine HPV-positive head and neck tumors. Clin Cancer Res. 2021;27(14):4054–4065. doi: 10.1158/1078-0432.CCR-20-4717. [DOI] [PubMed] [Google Scholar]
- 75.Lim C.Y., Chang J.H., Lee W.S., Kim J., Park I.Y. CD40 agonists alter the pancreatic cancer microenvironment by shifting the macrophage phenotype toward M1 and suppress human pancreatic cancer in organotypic slice cultures. Gut Liver. 2022;16(4):645–659. doi: 10.5009/gnl210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumas G., Dufresne M., Asselin E., Girouard J., Carrier C., Reyes-Moreno C. CD40 pathway activation reveals dual function for macrophages in human endometrial cancer cell survival and invasion. Cancer Immunol Immunother. 2013;62(2):273–283. doi: 10.1007/s00262-012-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long K.B., Gladney W.L., Tooker G.M., Graham K., Fraietta J.A., Beatty G.L. IFNγ and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 2016;6(4):400–413. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lum H.D., Buhtoiarov I.N., Schmidt B.E., et al. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79(6):1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 79.Beatty G.L., Chiorean E.G., Fishman M.P., et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bullock T.N.J. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol Immunol. 2022;19(1):14–22. doi: 10.1038/s41423-021-00734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zippelius A., Schreiner J., Herzig P., Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3(3):236–244. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]
- 82.Gu T., Zhu Y.B., Chen C., et al. Fine-tuned expression of programmed death 1 ligands in mature dendritic cells stimulated by CD40 ligand is critical for the induction of an efficient tumor specific immune response. Cell Mol Immunol. 2008;5(1):33–39. doi: 10.1038/cmi.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sum E., Rapp M., Frobel P., et al. Fibroblast activation protein α-targeted CD40 agonism abrogates systemic toxicity and enables administration of high doses to induce effective antitumor immunity. Clin Cancer Res. 2021;27(14):4036–4053. doi: 10.1158/1078-0432.CCR-20-4001. [DOI] [PubMed] [Google Scholar]
- 84.Vitale L.A., Thomas L.J., He L.Z., et al. Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol Immunother. 2019;68(2):233–245. doi: 10.1007/s00262-018-2267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medler J., Kucka K., Melo V., et al. CD40- and 41BB-specific antibody fusion proteins with PDL1 blockade-restricted agonism. Theranostics. 2022;12(4):1486–1499. doi: 10.7150/thno.66119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beatty G.L., Torigian D.A., Chiorean E.G., et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19(22):6286–9295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu D.T., Bac N.D., Nguyen K.H., et al. An update on anti-CD137 antibodies in immunotherapies for cancer. Int J Mol Sci. 2019;20(8):1822. doi: 10.3390/ijms20081822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makkouk A., Chester C., Kohrt H.E. Rationale for anti-CD137 cancer immunotherapy. Eur J Cancer. 2016;54:112–119. doi: 10.1016/j.ejca.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Shao Z., Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89(1):21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 90.Kwajah M.M.S., Schwarz H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol. 2010;40(7):1938–1949. doi: 10.1002/eji.200940105. [DOI] [PubMed] [Google Scholar]
- 91.Gauttier V., Judor J.P., Le Guen V., Cany J., Ferry N., Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer. 2014;135(12):2857–2867. doi: 10.1002/ijc.28943. [DOI] [PubMed] [Google Scholar]
- 92.Geng T., Yan Y., Xu L., et al. CD137 signaling induces macrophage M2 polarization in atherosclerosis through STAT6/PPARdelta pathway. Cell Signal. 2020;72 doi: 10.1016/j.cellsig.2020.109628. [DOI] [PubMed] [Google Scholar]
- 93.Jiang P., Gao W., Ma T., et al. CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics. 2019;9(10):2950–2966. doi: 10.7150/thno.29617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamata-Sakurai M., Narita Y., Hori Y., et al. Antibody to CD137 activated by extracellular adenosine triphosphate is tumor selective and broadly effective in vivo without systemic immune activation. Cancer Discov. 2021;11(1):158–175. doi: 10.1158/2159-8290.CD-20-0328. [DOI] [PubMed] [Google Scholar]
- 95.Segal N.H., Logan T.F., Hodi F.S., et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23(8):1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 96.Segal N.H., He A.R., Doi T., et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res. 2018;24(8):1816–1823. doi: 10.1158/1078-0432.CCR-17-1922. [DOI] [PubMed] [Google Scholar]
- 97.Castro F., Cardoso A.P., Goncalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ni L., Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7(9):4509–4516. doi: 10.1002/cam4.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burke J.D., Young H.A. IFN-γ: a cytokine at the right time, is in the right place. Semin Immunol. 2019;43 doi: 10.1016/j.smim.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manguso R.T., Pope H.W., Zimmer M.D., et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547(7664):413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Higgs B.W., Morehouse C.A., Streicher K., et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res. 2018;24(16):3857–3866. doi: 10.1158/1078-0432.CCR-17-3451. [DOI] [PubMed] [Google Scholar]
- 102.Gao J., Shi L.Z., Zhao H., et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397–404.e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cardoso A.P., Goncalves R.M., Antunes J.C., et al. An interferon-γ-delivery system based on chitosan/poly(γ-glutamic acid) polyelectrolyte complexes modulates macrophage-derived stimulation of cancer cell invasion in vitro. Acta Biomater. 2015;23:157–171. doi: 10.1016/j.actbio.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 104.Berraondo P., Etxeberria I., Ponz-Sarvise M., Melero I. Revisiting interleukin-12 as a cancer immunotherapy agent. Clin Cancer Res. 2018;24(12):2716–2718. doi: 10.1158/1078-0432.CCR-18-0381. [DOI] [PubMed] [Google Scholar]
- 105.Daud A.I., DeConti R.C., Andrews S., et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Algazi A.P., Twitty C.G., Tsai K.K., et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res. 2020;26(12):2827–2837. doi: 10.1158/1078-0432.CCR-19-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minutti C.M., Jackson-Jones L.H., Garcia-Fojeda B., et al. Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science. 2017;356(6342):1076–1080. doi: 10.1126/science.aaj2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maier B., Leader A.M., Chen S.T., et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257–262. doi: 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaggianesi M., Turdo A., Chinnici A., et al. IL4 primes the dynamics of breast cancer progression via DUSP4 inhibition. Cancer Res. 2017;77(12):3268–3279. doi: 10.1158/0008-5472.CAN-16-3126. [DOI] [PubMed] [Google Scholar]
- 110.Nappo G., Handle F., Santer F.R., et al. The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling. Oncogenesis. 2017;6(5):e342. doi: 10.1038/oncsis.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roca H., Craig M.J., Ying C., et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin up-regulation. J Cell Biochem. 2012;113(5):1569–1580. doi: 10.1002/jcb.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todaro M., Lombardo Y., Francipane M.G., et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15(4):762–772. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- 113.Li J., Wang Z., Mao K., Guo X. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol Lett. 2014;8(4):1682–1686. doi: 10.3892/ol.2014.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ito S.E., Shirota H., Kasahara Y., Saijo K., Ishioka C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol Immunother. 2017;66(11):1485–1496. doi: 10.1007/s00262-017-2043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Binnemars-Postma K., Bansal R., Storm G., Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018;32(2):969–978. doi: 10.1096/fj.201700629R. [DOI] [PubMed] [Google Scholar]
- 116.Kamerkar S., Leng C., Burenkova O., et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8(7) doi: 10.1126/sciadv.abj7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu C., Jiang L., Hao S., et al. Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front Immunol. 2019;10:2638. doi: 10.3389/fimmu.2019.02638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu M., Kuo F., Capistrano K.J., et al. TGF-β suppresses type 2 immunity to cancer. Nature. 2020;587(7832):115–120. doi: 10.1038/s41586-020-2836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hangasky J.A., Waldmann T.A., Santi D.V. Interleukin 15 pharmacokinetics and consumption by a dynamic cytokine sink. Front Immunol. 2020;11:1813. doi: 10.3389/fimmu.2020.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bergamaschi C., Stravokefalou V., Stellas D., Karaliota S., Felber B.K., Pavlakis G.N. Heterodimeric IL-15 in cancer immunotherapy. Cancers (Basel) 2021;13(4):837. doi: 10.3390/cancers13040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corbellari R., Stringhini M., Mock J., et al. A novel antibody-IL15 fusion protein selectively localizes to tumors, synergizes with TNF-based immunocytokine, and inhibits metastasis. Mol Cancer Ther. 2021;20(5):859–871. doi: 10.1158/1535-7163.MCT-20-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mattiola I., Pesant M., Tentorio P.F., et al. Priming of human resting NK cells by autologous M1 macrophages via the engagement of IL-1β, IFN-β, and IL-15 pathways. J Immunol. 2015;195(6):2818–2828. doi: 10.4049/jimmunol.1500325. [DOI] [PubMed] [Google Scholar]
- 123.Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33(1):35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fujii R., Jochems C., Tritsch S.R., Wong H.C., Schlom J., Hodge J.W. An IL-15 superagonist/IL-15Rα fusion complex protects and rescues NK cell-cytotoxic function from TGF-β1-mediated immunosuppression. Cancer Immunol Immunother. 2018;67(4):675–689. doi: 10.1007/s00262-018-2121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Y., Wu L., Liu Y., et al. A novel multifunctional anti-PD-L1-CD16a-IL15 induces potent cancer cell killing in PD-L1-positive tumour cells. Transl Oncol. 2022;21 doi: 10.1016/j.tranon.2022.101424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waldmann T.A., Dubois S., Miljkovic M.D., Conlon K.C. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868. doi: 10.3389/fimmu.2020.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Margolin K., Morishima C., Velcheti V., et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res. 2018;24(22):5552–5561. doi: 10.1158/1078-0432.CCR-18-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wrangle J.M., Velcheti V., Patel M.R., et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9(6):a022236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.David C.J., Massague J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19(7):419–435. doi: 10.1038/s41580-018-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie F., Ling L., van Dam H., Zhou F., Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2018;50(1):121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 132.Bai X., Yi M., Jiao Y., Chu Q., Wu K. Blocking TGF-β signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther. 2019;12:9527–9538. doi: 10.2147/OTT.S224013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Batlle E., Massague J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lonning S., Mannick J., McPherson J.M. Antibody targeting of TGF-β in cancer patients. Curr Pharm Biotechnol. 2011;12(12):2176–2189. doi: 10.2174/138920111798808392. [DOI] [PubMed] [Google Scholar]
- 135.Gigante M., Gesualdo L., Ranieri E. TGF-beta: a master switch in tumor immunity. Curr Pharm Des. 2012;18(27):4126–4134. doi: 10.2174/138161212802430378. [DOI] [PubMed] [Google Scholar]
- 136.Zhang S., Che D., Yang F., et al. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget. 2017;8(59):99801–99815. doi: 10.18632/oncotarget.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J., Acharya S., Sahin O., et al. 14-3-3ζ turns TGF-β’s function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell. 2015;27(2):177–192. doi: 10.1016/j.ccell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gneo L., Rizkalla N., Hejmadi R., Mussai F., de Santo C., Middleton G. TGF-β orchestrates the phenotype and function of monocytic myeloid-derived suppressor cells in colorectal cancer. Cancer Immunol Immunother. 2022;71(7):1583–1596. doi: 10.1007/s00262-021-03081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rice L.M., Padilla C.M., McLaughlin S.R., et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125(7):2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feun L.G., Li Y.Y., Wu C., et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125(20):3603–3614. doi: 10.1002/cncr.32339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Derynck R., Turley S.J., Akhurst R.J. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Faustino-Rocha A.I., Ferreira R., Gama A., Oliveira P.A., Ginja M. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017;172:27–41. doi: 10.1016/j.lfs.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 143.Massari N.A., Nicoud M.B., Medina V.A. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2020;177(3):516–538. doi: 10.1111/bph.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarasola M.P., Taquez Delgado M.A., Nicoud M.B., Medina V.A. Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol Res Perspect. 2021;9(5) doi: 10.1002/prp2.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu L., Cheng D., Huang Z., et al. Histamine promotes the differentiation of macrophages from CD11b(+) myeloid cells and formation of foam cells through a Stat6-dependent pathway. Atherosclerosis. 2017;263:42–52. doi: 10.1016/j.atherosclerosis.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 146.Wang K.Y., Arima N., Higuchi S., et al. Switch of histamine receptor expression from H2 to H1 during differentiation of monocytes into macrophages. FEBS Lett. 2000;473(3):345–348. doi: 10.1016/s0014-5793(00)01560-x. [DOI] [PubMed] [Google Scholar]
- 147.Czerner C.P., Klos A., Seifert R., Neumann D. Histamine induces chemotaxis and phagocytosis in murine bone marrow-derived macrophages and RAW 264.7 macrophage-like cells via histamine H4-receptor. Inflamm Res. 2014;63(3):239–247. doi: 10.1007/s00011-013-0694-0. [DOI] [PubMed] [Google Scholar]
- 148.Triggiani M., Petraroli A., Loffredo S., et al. Differentiation of monocytes into macrophages induces the upregulation of histamine H1 receptor. J Allergy Clin Immunol. 2007;119(2):472–481. doi: 10.1016/j.jaci.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 149.Martin R.K., Saleem S.J., Folgosa L., et al. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc Biol. 2014;96(1):151–159. doi: 10.1189/jlb.5A1213-644R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martner A., Wiktorin H.G., Lenox B., et al. Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J Immunol. 2015;194(10):5014–5021. doi: 10.4049/jimmunol.1402991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li H., Xiao Y., Li Q., et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell. 2022;40(1):36–52.e9. doi: 10.1016/j.ccell.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Varbanov H.P., Kuttler F., Banfi D., Turcatti G., Dyson P.J. Screening-based approach to discover effective platinum-based chemotherapies for cancers with poor prognosis. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellegaard A.M., Dehlendorff C., Vind A.C., et al. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–139. doi: 10.1016/j.ebiom.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grauers Wiktorin H., Nilsson M.S., Kiffin R., et al. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol Immunother. 2019;68(2):163–174. doi: 10.1007/s00262-018-2253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ebhohimen I.E., Okanlawon T.S., Osagie A.O., Izevbigie O.N. Vitamin E in human health and oxidative stress related diseases. Vol. 12. Vitamin E in Health and Disease. IntechOpen. 2021 [Google Scholar]
- 156.Galli F., Azzi A., Birringer M., et al. Vitamin E: emerging aspects and new directions. Free Radic Biol Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 157.Yu W., Simmons-Menchaca M., Gapor A., Sanders B.G., Kline K. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr Cancer. 1999;33(1):26–32. doi: 10.1080/01635589909514744. [DOI] [PubMed] [Google Scholar]
- 158.Pierpaoli E., Viola V., Pilolli F., Piroddi M., Galli F., Provinciali M. Gamma- and delta-tocotrienols exert a more potent anticancer effect than alpha-tocopheryl succinate on breast cancer cell lines irrespective of HER-2/neu expression. Life Sci. 2010;86(17-18):668–675. doi: 10.1016/j.lfs.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 159.McIntyre B.S., Briski K.P., Gapor A., Sylvester P.W. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224(4):292–301. doi: 10.1046/j.1525-1373.2000.22434.x. [DOI] [PubMed] [Google Scholar]
- 160.Jiang Q., Rao X., Kim C.Y., et al. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer. 2012;130(3):685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shklar G., Schwartz J. Tumor necrosis factor in experimental cancer regression with alphatocopherol, beta-carotene, canthaxanthin and algae extract. Eur J Cancer Clin Oncol. 1988;24(5):839–850. doi: 10.1016/0277-5379(88)90192-7. [DOI] [PubMed] [Google Scholar]
- 162.Yam M.L., Abdul Hafid S.R., Cheng H.M., Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009;44(9):787–797. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- 163.Jiang Q., Elson-Schwab I., Courtemanche C., Ames B.N. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97(21):11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yuan X., Duan Y., Xiao Y., et al. Vitamin E enhances cancer immunotherapy by reinvigorating dendritic cells via targeting checkpoint SHP1. Cancer Discov. 2022;12(7):1742–1759. doi: 10.1158/2159-8290.CD-21-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang Q. Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms. IUBMB Life. 2019;71(4):495–506. doi: 10.1002/iub.1978. [DOI] [PubMed] [Google Scholar]
- 166.Lee I.M., Cook N.R., Gaziano J.M., et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 167.Lippman S.M., Klein E.A., Goodman P.J., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hall K.T., Buring J.E., Mukamal K.J., et al. COMT and alpha-tocopherol effects in cancer prevention: gene-supplement interactions in two randomized clinical trials. J Natl Cancer Inst. 2019;111(7):684–694. doi: 10.1093/jnci/djy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Klein E.A., Thompson I.M., Jr., Tangen C.M., et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jacobs E.J., Henion A.K., Briggs P.J., et al. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol. 2002;156(11):1002–1010. doi: 10.1093/aje/kwf147. [DOI] [PubMed] [Google Scholar]
- 171.Kunnumakkara A.B., Sung B., Ravindran J., et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70(21):8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pathak A.K., Bhutani M., Guleria R., et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J Am Coll Nutr. 2005;24(1):16–21. doi: 10.1080/07315724.2005.10719438. [DOI] [PubMed] [Google Scholar]
- 173.Retzlaff D., Dorfler J., Kutschan S., Freuding M., Buntzel J., Hubner J. The vitamin E isoform α-tocopherol is not effective as a complementary treatment in cancer treatment: a systematic review. Nutr Cancer. 2022;74(7):2313–2336. doi: 10.1080/01635581.2021.2014905. [DOI] [PubMed] [Google Scholar]