Key Points
Question
What is the prevalence of diabetic retinopathy (DR) in children with type 2 diabetes (T2D)?
Findings
In this systematic review and meta-analysis of 27 observational studies including 5924 unique patients with pediatric T2D, 6.99% of participants with T2D had DR; the prevalence increased significantly more than 5 years after T2D diagnosis. The heterogeneity was high across studies.
Meaning
These results suggest that the increasing risk of DR in children with T2D warrants the implementation of global screening programs at diagnosis and annually to ensure early detection and treatment to preserve vision in this population.
Abstract
Importance
Type 2 diabetes (T2D) is increasing globally. Diabetic retinopathy (DR) is a leading cause of blindness in adults with T2D; however, the global burden of DR in pediatric T2D is unknown. This knowledge can inform retinopathy screening and treatments to preserve vision in this population.
Objective
To estimate the global prevalence of DR in pediatric T2D.
Data Sources
MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Library, the Web of Science, and the gray literature (ie, literature containing information that is not available through traditional publishing and distribution channels) were searched for relevant records from the date of database inception to April 4, 2021, with updated searches conducted on May 17, 2022. Searches were limited to human studies. No language restrictions were applied. Search terms included diabetic retinopathy; diabetes mellitus, type 2; prevalence studies; and child, adolescent, teenage, youth, and pediatric.
Study Selection
Three teams, each with 2 reviewers, independently screened for observational studies with 10 or more participants that reported the prevalence of DR. Among 1989 screened articles, 27 studies met the inclusion criteria for the pooled analysis.
Data Extraction and Synthesis
This systematic review and meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for systematic reviews and meta-analyses. Two independent reviewers performed the risk of bias and level of evidence analyses. The results were pooled using a random-effects model, and heterogeneity was reported using χ2 and I2 statistics.
Main Outcomes and Measures
The main outcome was the estimated pooled global prevalence of DR in pediatric T2D. Other outcomes included DR severity and current DR assessment methods. The association of diabetes duration, sex, race, age, and obesity with DR prevalence was also assessed.
Results
Among the 27 studies included in the pooled analysis (5924 unique patients; age range at T2D diagnosis, 6.5-21.0 years), the global prevalence of DR in pediatric T2D was 6.99% (95% CI, 3.75%-11.00%; I2 = 95%; 615 patients). Fundoscopy was less sensitive than 7-field stereoscopic fundus photography in detecting retinopathy (0.47% [95% CI, 0%-3.30%; I2 = 0%] vs 13.55% [95% CI, 5.43%-24.29%; I2 = 92%]). The prevalence of DR increased over time and was 1.11% (95% CI, 0.04%-3.06%; I2 = 5%) at less than 2.5 years after T2D diagnosis, 9.04% (95% CI, 2.24%-19.55%; I2 = 88%) at 2.5 to 5.0 years after T2D diagnosis, and 28.14% (95% CI, 12.84%-46.45%; I2 = 96%) at more than 5 years after T2D diagnosis. The prevalence of DR increased with age, and no differences were noted based on sex, race, or obesity. Heterogeneity was high among studies.
Conclusions and Relevance
In this study, DR prevalence in pediatric T2D increased significantly at more than 5 years after diagnosis. These findings suggest that retinal microvasculature is an early target of T2D in children and adolescents, and annual screening with fundus photography beginning at diagnosis offers the best assessment method for early detection of DR in pediatric patients.
This systematic review and meta-analysis uses data from observational studies to estimate the global prevalence of diabetic retinopathy among patients with pediatric type 2 diabetes.
Introduction
The obesity epidemic has been the primary factor in the increase in pediatric type 2 diabetes (T2D) case numbers globally.1,2,3,4,5,6,7,8 Type 2 diabetes is a more aggressive disorder in youths than it is in adults, with early comorbidities and complications including hypertension, nephropathy, polycystic ovary syndrome, and dyslipidemia.9,10,11,12,13,14,15
Diabetic retinopathy (DR) is the leading cause of blindness in adults with T2D and has several subtypes.16,17 Hyperglycemia increases vascular permeability and can lead to capillary occlusion, which results in nonproliferative DR (NPDR). This phase may be followed by a proliferative phase of DR with the formation of new blood vessels. Macular edema with fluid accumulation can also develop and may impact central vision.18
Children are developing T2D early in life and will live with their diabetes for several decades, which may increase their lifetime risk of developing DR and progress to blindness if undetected and untreated.19,20 While current guidelines recommend screening for DR in youths with T2D at diagnosis and annually thereafter, the global burden of DR is still not fully quantified.21,22 Understanding the scale of DR will help define its natural history and support the development of personalized clinical practice guidelines dedicated to children with T2D.
The main goal of this systematic review and meta-analysis was to assess the global prevalence of DR in pediatric patients with T2D. We also aimed to assess the severity profile of DR and the current diagnostic assessment methods. Other outcomes included the association of diabetes duration, sex, race, age, obesity, hypertension, and hemoglobin A1c (HbA1c) level with DR prevalence.
Methods
Systematic Review Protocol and Registration
This systematic review and meta-analysis has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018091127).23 The study was exempt from the need for review and approval by an ethics review board because we used only aggregated deanonymized data that were already published. This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE)24 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)25 reporting guidelines for systematic reviews and meta-analyses (eTable 1 in Supplement 1).
Search Strategies
A senior health sciences librarian (L.B.) developed the search strategies in MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Web of Science: Conference Proceedings Citation Index–Science. We searched the gray literature (ie, literature containing information produced by government agencies, academic institutions, and for-profit organizations that is not available through traditional publishing and distribution channels) through access to ClinicalTrials.gov, the Cochrane Central Registry of Controlled Trials, the Web of Science: Conference Proceedings Citation Index–Science, and article references screened at the full-text stage to retrieve potentially eligible articles (eTables 2-6 in Supplement 1). Concepts of pediatrics and T2D were combined with terms referencing observational study design and DR. Search terms included diabetic retinopathy; diabetes mellitus, type 2; prevalence studies; and child, adolescent, teenage, youth, and pediatric. No language restrictions were applied, and the searches were limited to human studies. If a conference abstract was suitable for inclusion, we searched for full-text articles in the included databases. We also contacted the principal investigators to determine whether the studies were published and to obtain data as needed. The initial database searches were from the date of database inception to April 4, 2021, with updated searches conducted on May 17, 2022.
Eligibility Criteria
The T2D diagnostic criteria used by different studies are reported in eTable 7 in Supplement 1. In our original protocol, eligible studies included patients with T2D who were diagnosed at 18 years or younger, reported findings for 10 or more patients, had an observational study design (including cross-sectional and cohort studies), and reported the prevalence of DR. During screening, we identified a substantial number of studies that defined pediatric populations as 21 years or younger, so this age cutoff was adopted for study inclusion. We excluded articles reporting on patients with gestational diabetes or other types of diabetes. We planned to include the largest reported sample size for large studies with serial data reporting.
Study Selection, Data Abstraction, and Quality Appraisal
Three teams of 2 independent reviewers (including M.C., J.D., A.N., M.H., Y.Q., S.S.J.C., A.R., and P.P.T.) screened titles, abstracts, and full-text articles, completed data abstraction, and assessed the risk of bias and level of evidence. Disagreements were resolved through discussion, and a third reviewer (M.C.S.) was available for consultation and resolution of ongoing disputes. The extracted data included age at diagnosis and study inclusion, sex, race, diabetes duration, sample size, obesity rates, method of DR diagnosis, DR classification, total prevalence of DR, and sex- and race-specific prevalence proportions (if reported). Race-based data were collected because race-based differences in T2D prevalence and DR rates were previously reported.26,27 Race-specific data from the studies were obtained either from medical records or self-reported information from participants. If a longitudinal study reported the prevalence of DR at multiple time points, we extracted the values closest to the time of diabetes diagnosis. We also contacted the principal investigators of the studies to retrieve missing data when needed.
The risk of bias was assessed using a validated instrument developed for prevalence studies that evaluates the internal and external validity of studies.28 The level of evidence was evaluated using the Oxford Centre for Evidence-Based Medicine criteria.29 Local and current random sample surveys were given a level of 1 and nonrandom surveys a level of 3 (corresponding to the highest and lowest levels of evidence used in this systematic review and meta-analysis); studies were also rated lower based on imprecision, indirectness, and inconsistency.29
Statistical Analysis
We performed a random-effects meta-analysis if 2 or more studies reported on the prevalence of DR in similar populations using identical study designs, methods, and outcomes.30,31 Otherwise, we tabulated the results and presented a narrative review of the studies. The primary outcome was the global pooled prevalence (with 95% CI) of DR. We conducted the meta-analysis with prevalence estimates transformed using the Freeman-Tukey double arcsine method31 to prevent the need to stabilize variances because some studies reported prevalence rates of 0%, and we transformed the results back to prevalence estimates for interpretation.32,33 To verify the results of the Freeman-Tukey double arcsine analysis and to control for sampling error and bias, an exploratory analysis was also conducted using the random intercept mixed-effects logistic regression model, recognizing that the model does not account for study weights.34 In addition to 95% CIs, we also estimated 95% prediction intervals (PIs) to assess the possible range of new values in the present study.35 Both inconsistency index (I2 statistic) and χ2 P values were used to quantify heterogeneity. An I2 greater than 75% and P < .10 were indicators of heterogeneity.36
Subgroup analyses, meta-regression analysis, sensitivity analysis, and publication bias evaluations were performed only if more than 10 studies were identified for a given outcome. Subgroup meta-analyses were performed when 2 or more studies reported the prevalence of DR by sex or race, with the latter classified using National Institutes of Health definitions.37 We also performed subgroup analyses by DR severity classification (minimal to moderate NPDR, severe NPDR, proliferative DR, and macular edema), assessment method (fundoscopy or fundus photography), and diabetes duration (<2.5 years, 2.5-5.0 years, or >5.0 years). We also added a random-effects meta-regression analysis to assess the separate associations of obesity, hypertension, HbA1c level, age at diagnosis, age at study inclusion, and diabetes duration with DR prevalence.36 We reported the statistical significance of the regression coefficient for the association between each variable and DR prevalence. We calculated the mean difference in HbA1c level for patients with vs without DR. Sensitivity analysis was performed by removing studies reported only in conference abstracts, studies with a sample size of 50 patients or less, studies that included some participants older than 18 years, or studies with a high risk of bias. Publication bias assessment was conducted with a contour-enhanced funnel plot and Egger test, and visual inspection was used to assess asymmetry.38 The meta-analysis of prevalence was performed using the metafor package in RStudio software, version 1.1.383, using R language version 3.4.3 (R Foundation for Statistical Computing).39,40,41
Results
Study Selection and Characteristics
We screened 1989 deduplicated titles and abstracts, and 190 abstracts were chosen for full-text screening. A total of 29 studies12,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69 met the inclusion criteria (eFigure 1 in Supplement 1). Of those, 6 studies42,43,44,45,46,47 (20.7%) had a cross-sectional design, 13 studies48,49,50,51,52,53,54,55,56,57,58,59,60 (44.8%) had a retrospective cohort design, and 10 studies12,61,62,63,64,65,66,67,68,69 (34.5%) had a prospective cohort design. Additional details about the included studies are reported in the Table and eTable 8 in Supplement 1.
Table. Studies Included in the Systemic Review and Meta-analysis.
| Source | Study location | Study design | Age, y | Duration of diabetes, y | Prevalence of DR, No. (%) | Sample size | Diabetic retinopathy classification: No. (%) of participants | Method of assessment | Definition | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of T2D | Study enrollment | |||||||||
| Eppens et al,42 2006 | Western Pacific | Cross-sectional | Median (IQR), 12.0 (10.7 to 13.5)a | Median (IQR), 14.9 (13.2 to 16.4)a | Median (IQR), 2.3 (1.4 to 3.6)a | 2 (0.6) | 284 | NR | NR | NR |
| Farah et al,43 2006 | US | Cross-sectional | <21.0 | Range, 10.0 to 21.0a | Median (range), 1.8 (<2.0 to 15.0)a | 1 (2.5) | 40 | Minimal NPDR: 1 (2.5) | Fundoscopy | Modified Airlie House |
| Unnikrishnan et al,44 2008 | India | Cross-sectional | Mean (SD), 16.2 (2.9) | Mean (SD), 18.9 (4.9) | NR | 0 | 36 | NR | Fundoscopy | Background or proliferative retinopathy |
| Aulich et al,45 2019 | Australia | Cross-sectional | NR | Mean (SD), 15.1 (1.9)a | Median (IQR), 1.8 (0.3 to 3.3)a | 2 (6.7) | 30 | NR | 7-Field stereoscopic fundus photography | ETDRS |
| Khalil et al,46 2019 | Egypt | Cross-sectional | Mean (SD), 18.0 (2.0) | Mean (SD), 19.8 (1.1) | Mean (SD), 2.5 (2.0) | 0 | 13 | NR | Fundoscopy | Normal fundus, nonproliferative retinopathy, or proliferative retinopathy |
| Ferm et al,47 2021 | US | Cross-sectional | NR | NR | NR | 13 (3.1) | 416 | NR | Nonmydriatic fundus photography | NR |
| Scott et al,48 2006 | New Zealand | Retrospective cohort | NR | Mean (SD), 20.0 (0.4) | Mean (SD), 3.0 (0.3) | 8 (7.6) | 105 | Mild to moderate NPDR: 4 (3.8); severe NPDR, PDR, or macular edema: 4 (3.8) | Either fundoscopy or fundus photography (type NR) | NR |
| Lee et al,49 2007 | Japan | Retrospective cohort | NR | NR | NR | 10 (27.8) | 36 | NR | NR | NR |
| Osman et al,50 2013 | Sudan | Retrospective cohort | <10 y: 3 participants; 11 to 18 y: 35 participants | NR | NR | 0 | 38 | NR | NR | Medical records |
| Dart et al,51 2014 | Canada | Retrospective cohort | Mean (SD), 13.5 (2.2) | Mean (SD), 16.5 (2.3) | Median (range), 4.4 (0 to 27.4) | 40 (11.7) | 342 | NR | NR | Medical records |
| Geloneck et al,52 2015 | US | Retrospective cohort | Mean (SD), 11.8 (2.7) | Mean (SD), 14.5 (2.1) | Mean (SD), 2.8 (2.3) | 0 | 32 | NR | Fundoscopy | Medical records |
| Newton et al,53 2015b | New Zealand | Retrospective cohort | Range, 6.5 to 17.0c | <17.0 | 0 | 1 (4.3) | 23 | NR | NR | Medical records |
| Wang et al,54 2017 | US | Retrospective cohort | Median (IQR), 18.0 (16.0 to 21.0) | NR | Median (IQR), 3.1 (1.9 to 4.9) | 127 (7.2) | 1768 | NPDR: 6 (0.3); PDR: 1 (0.1); unspecified: 120 (6.8) | NR | Medical records |
| Yeh and Bernardo,55 2017b | US | Retrospective cohort | Mean, 13.8 | NR | NR | 1 (7.1) | 14 | NR | Fundoscopy | Medical records |
| Koziol et al,56 2020 | Poland | Retrospective cohort | NR | <18.0 | NR | 79 (1.8) | 4291 | NR | NR | Medical records |
| Ek et al,57 2020 | Sweden | Retrospective cohort | Mean (SD), 15.0 (1.9)a | Mean (SD), 22.2 (3.7) | Mean (SD), 6.7 (2.8) | 32 (31.1) | 103 | NR | Fundus photography (type NR) | NR |
| Porter et al,58 2020 | US | Retrospective cohort | Mean (SD), 17.0 (3.0) | Mean (SD), 18.1 (2.6) | Mean (SD), 1.1 (1.3) | 3 (6.0) | 50 | Mild NPDR: 3 (6.0) | NR | ETDRS |
| Amutha et al,59 2021 | India | Retrospective cohort | Mean (SD), 16.6 ( 2.5)a | Mean (SD), 23.2 ( 9.7)a | Median (IQR), 5.7 (NR to NR)a | 118 (27.5) | 429 | NR | Both fundoscopy (for initial screening) and 7-field stereoscopic fundus photography (for confirmation) | ETDRS |
| Bai et al,60 2022 | US | Retrospective cohort | Mean (SD), 17.3 (3.4) | <22.0 | Range, 0 to 15.0 | 17 (26.6) | 64 | NPDR: 11 (64.7); PDR: 4 (6.3); macular edema: 2 (3.2) | NR | Medical records |
| Eppens et al,12 2006 | Australia | Prospective cohort | Median (IQR), 13.2 (11.6 to 15.0)a | Median (IQR), 15.3 (13.6 to 16.4)a | Median (IQR), 1.3 (0.6 to 3.1)a | 1 (4.0) | 25 | NR | 7-Field stereoscopic fundus photography | Modified Airlie House |
| Shield et al,61 2009 | Ireland; UK | Prospective cohort | Median (IQR), 13.6 (9.9 to 16.8)a | Median (IQR), 14.5 (10.8 to 17.8)a | Median, 1.0a | 0 | 55 | NR | NR | NR |
| Ruhayel et al,62 2010 | Australia | Prospective cohort | Mean (SD), 11.6 (1.9)a | Mean (SD), 16.8 (1.7)a | Mean (SD), 5.2 (2.0)a | 4 (25.0) | 16 | NPDR: 4 (25.0); 3 with unilateral hard exudates and 1 with dot hemorrhages | NR | Medical records |
| Jefferies et al,63 2012 | New Zealand | Prospective cohort | Median (IQR), 12.9 (7.1 to 15.5) | NR | NR | 0 | 52 | NR | NR | NR |
| Schmidt et al,64 2012 | Austria; Germany | Prospective cohort | Mean (SD), 13.5 (3.4) | Mean (SD), 15.3 (3.0) | NR | 12 (1.8) | 684 | NR | NR | NR |
| Jensen et al,66 2021b | US | Prospective cohort | <20.0 | NR | Mean (SD), 7.5 (2.1) | 140 (31.3) | 447 | NR | Fundus photography (type NR) | NR |
| Mean (SD), 12.4 (2.1) | 126 (55.0) | 229 | Mild NPDR: 91 (39.7); moderate NPDR: 26 (11.4); PDR: 9 (4.0) | |||||||
| Preechasuk et al,67 2022 | Thailand | Prospective cohort | Mean (SD), 16.9 (6.4) | Mean (SD), 23.4 (8.5) | Median (IQR), 5.2 (1.6-9.4) | 8 (9.0) | 89 | Mild to moderate NPDR: 4 (4.5); severe NPDR: 4 (4.5) | NR | Presence of any severity of DR, macular edema, vitreous hemorrhage, or tractional retinal detachment |
| TODAY Study Group,68 2013 | US | Prospective cohort | NR | Mean (SD), 18.1 (2.5) | Mean (SD), 4.9 (1.5) | 71 (13.7) | 517 | Minimal NPDR: 64 (12.3); mild NPDR: 7 (1.4) | 7-Field stereoscopic fundus photography | ETDRS |
| TODAY Study Group,65 2021 | US | Prospective cohort | NR | Mean (SD), 25.4 (2.5) | Mean (SD), 12.0 (1.5) | 210 (50.0) | 420 | Minimal NPDR: 95 (22.6); mild NPDR: 68 (16.2); moderate NPDR: 16 (3.8); moderately severe NPDR: 3 (0.7); severe NPDR: 5 (1.2); early or stable, treated PDR: 10 (2.4); high-risk PDR: 5 (1.2); macular edema: 14 (3.3) | 7-Field stereoscopic fundus photography | ETDRS |
| Zuckerman Levin et al,69 2022 | Israel | Prospective cohort | Mean (SD), 14.7 (1.9) | Mean (SD), 14.7 (1.9) | At presentation: 0 | At presentation: 4 (1.9) | At presentation: 216 | NR | NR | Progressive retinal changes (nonproliferative or proliferative) |
| At follow-up: mean (SD), 2.9 (2.1) | At follow-up: 5 (4.6) | At follow-up: 108 | ||||||||
Abbreviations: DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; NPDR, nonproliferative diabetic retinopathy; NR, not reported; PDR, proliferative diabetic retinopathy; T2D, type 2 diabetes.
Represents value for whole cohort in study, not just patients screened for retinopathy.
Abstract only.
In this abstract, there was 1 participant with Prader-Willi syndrome who was diagnosed with T2D at age 6.5 years.
All patients were diagnosed with T2D between ages 6.5 years and 21.0 years, with 1 patient diagnosed at age 6.5 years and having a background diagnosis of Prader-Willi syndrome. The diabetes duration ranged from inclusion at diabetes diagnosis to 15.0 years after diagnosis.
Pooled Global Prevalence of DR
The number of DR cases was small, and heterogeneity was high across studies. Among 29 eligible studies,12,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69 1 study65 was excluded from the pooled analysis because it provided follow-up data on the same patient set included in another study,68 and 1 study56 was excluded because it provided the number of observations of DR, but it was unclear whether the data included unique patients. In this article, we report the prevalence values without and with the inclusion of the latter study56 because the results did not change substantially. The pooled global prevalence of DR across 27 studies12,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57,58,59,60,61,62,63,64,66,67,68,69 involving 5924 unique patients was 6.99% (95% CI, 3.75%-11.00%; I2 = 95%; P < .001; 615 patients) (Figure 1). The DR prevalence was 1.14% (95% CI, 0.05%-3.07%; I2 = 41%; P = .13; 18 of 819 patients) in cross-sectional studies,42,43,44,45,46,47 11.29% (95% CI, 5.82%-18.10%; I2 = 94%; P < .001; 357 of 3004 patients) in retrospective cohort studies,48,49,50,51,52,53,54,55,57,58,59,60 and 6.52% (95% CI, 0.95%-15.66%; I2 = 97%; P < .001; 240 of 2101 patients) in prospective cohort studies.12,61,62,63,64,66,67,68,69 When including the study that reported the number of observations of patients with T2D and DR,56 the pooled global prevalence was 6.69% (95% CI, 3.64%-10.47%; I2 = 97%; P < .001; 4291 observations; 10 215 total unique patients plus observations).
Figure 1. Prevalence of Diabetic Retinopathy in Youths With Type 2 Diabetes by Study Design.
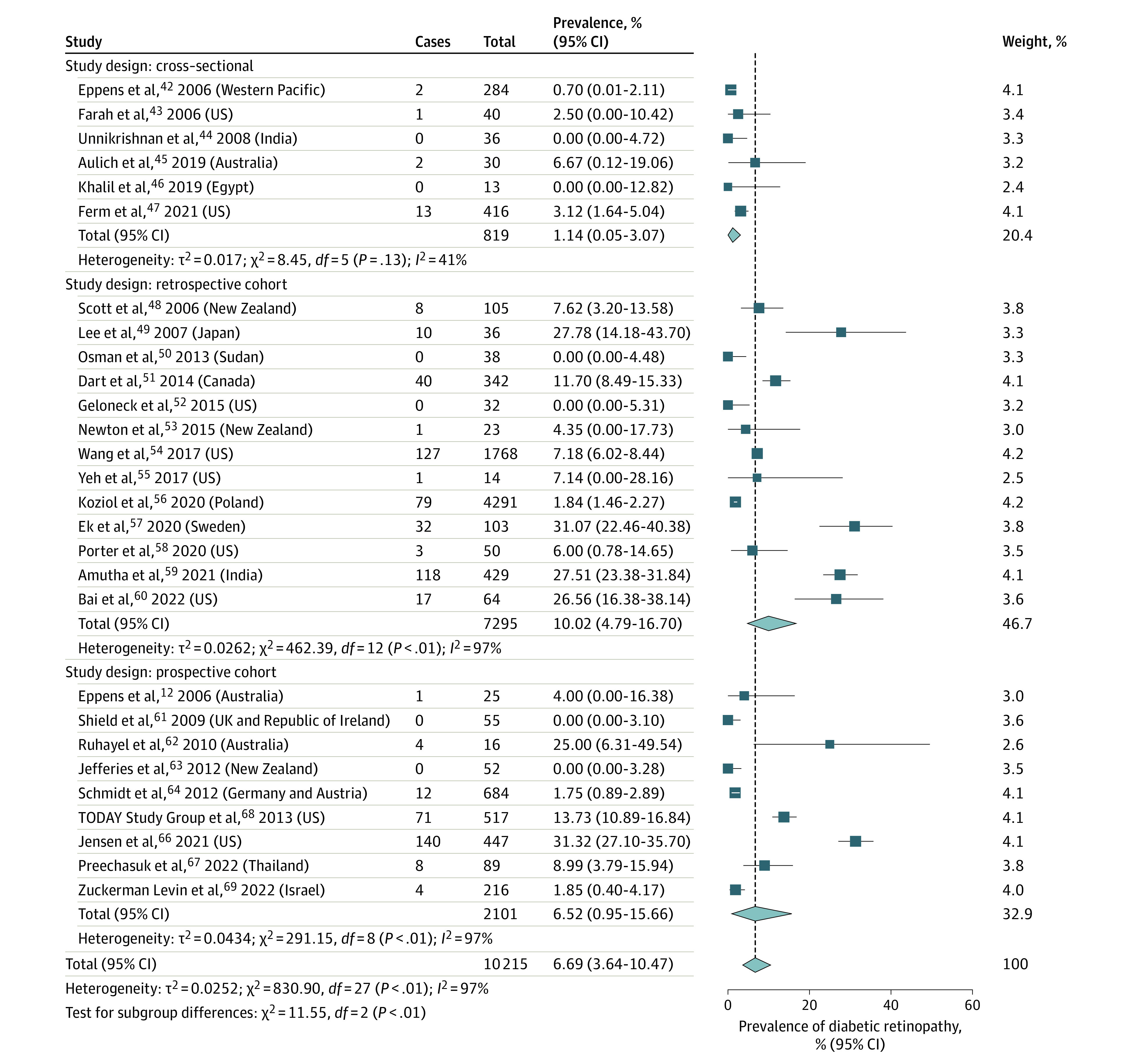
The exploratory analysis using the random intercept mixed-effects logistic regression model, in which the results were compared with the Freeman-Tukey double arcsine analysis, had consistent results with overlapping 95% CIs. All outcomes, with the exception of DR prevalence by race, had results somewhat close to each other using the 2 methods; for example, DR prevalence was 6.99% vs 5.03% when comparing the generalized linear mixed-effects method vs the Freeman-Tukey double arcsine transformation method. However, the data were limited. The 95% PIs were broad (including a range of 0.30%-50.75% in the generalized linear mixed-effects model vs 0%-33.99% in the Freeman-Tukey double arcsine transformation model), primarily due to the high heterogeneity among the studies (eTable 9 in Supplement 1).
Prevalence of DR Based on Severity
Only 9 studies43,48,54,58,60,65,66,67,68 reported DR classification using Early Treatment for Diabetic Retinopathy Study criteria or modified Airlie House criteria, which both classify DR severity based on the presence and extent of retinal thickening, microaneurysms, cotton wool spots, dot blot hemorrhages, venous beading, intraretinal microvascular anomalies, and neovascularization. The prevalence of minimal to moderate NPDR was 11.16% (95% CI, 1.52%-27.21%; I2 = 97%; P < .001; 200 of 1030 patients),43,48,58,66,67,68 the prevalence of severe NPDR was 2.57% (95% CI, 0.58%-5.69%; I2 = 50%; P = .16; 12 of 509 patients),65,67 the prevalence of proliferative DR was 2.43% (95% CI, 0.04%-7.47%; I2 = 95%; P < .001; 28 of 2481 patients),54,60,65,66 and the prevalence of macular edema was 3.09% (95% CI, 1.64%-4.91%; I2 = 0%; P = .88; 16 of 484 patients) (Figure 2).60,65
Figure 2. Prevalence of Diabetic Retinopathy in Youths With Type 2 Diabetes by Severity.
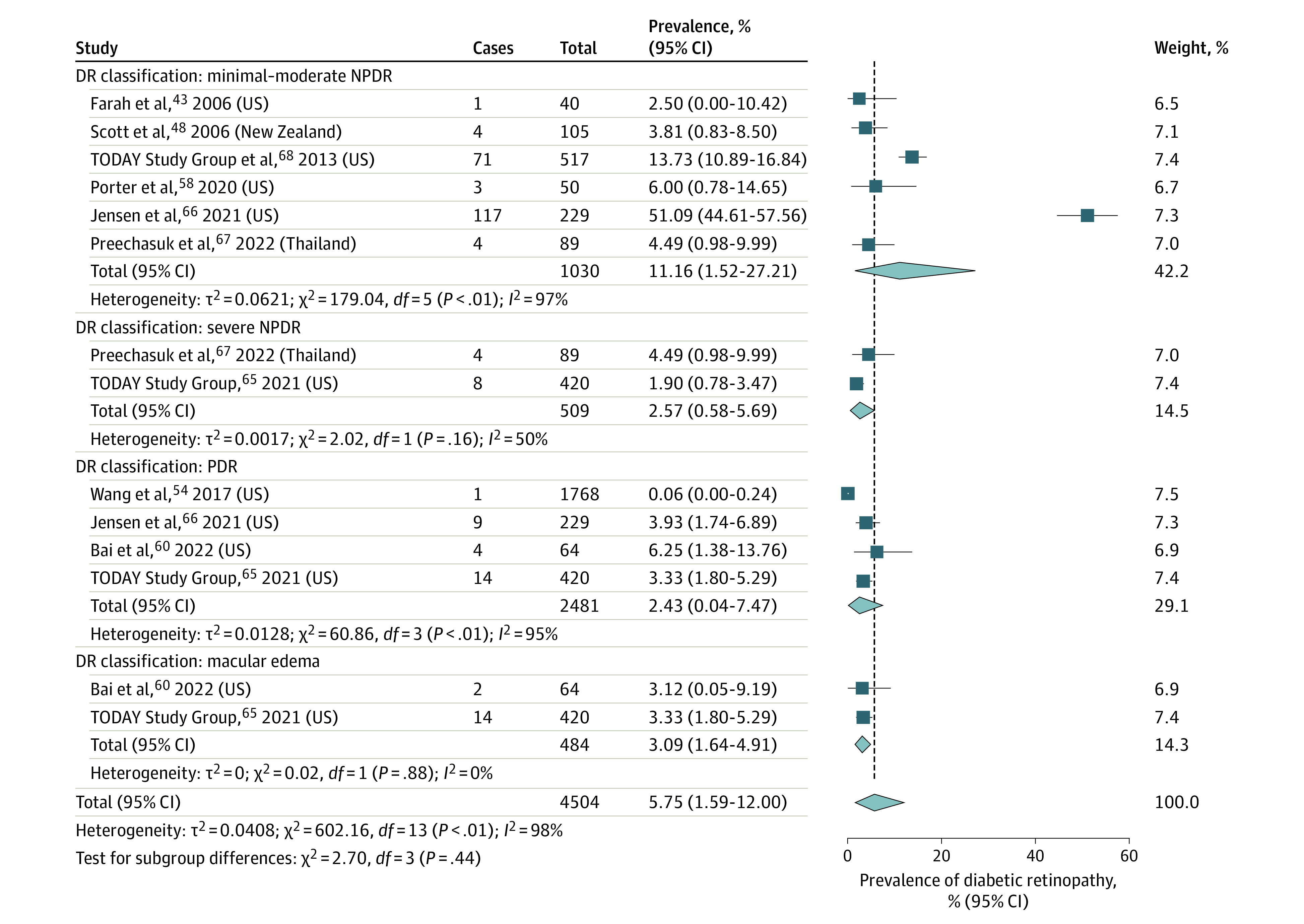
DR indicates diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; and PDR, proliferative diabetic retinopathy.
Prevalence of DR Based on Method of Retinopathy Assessment
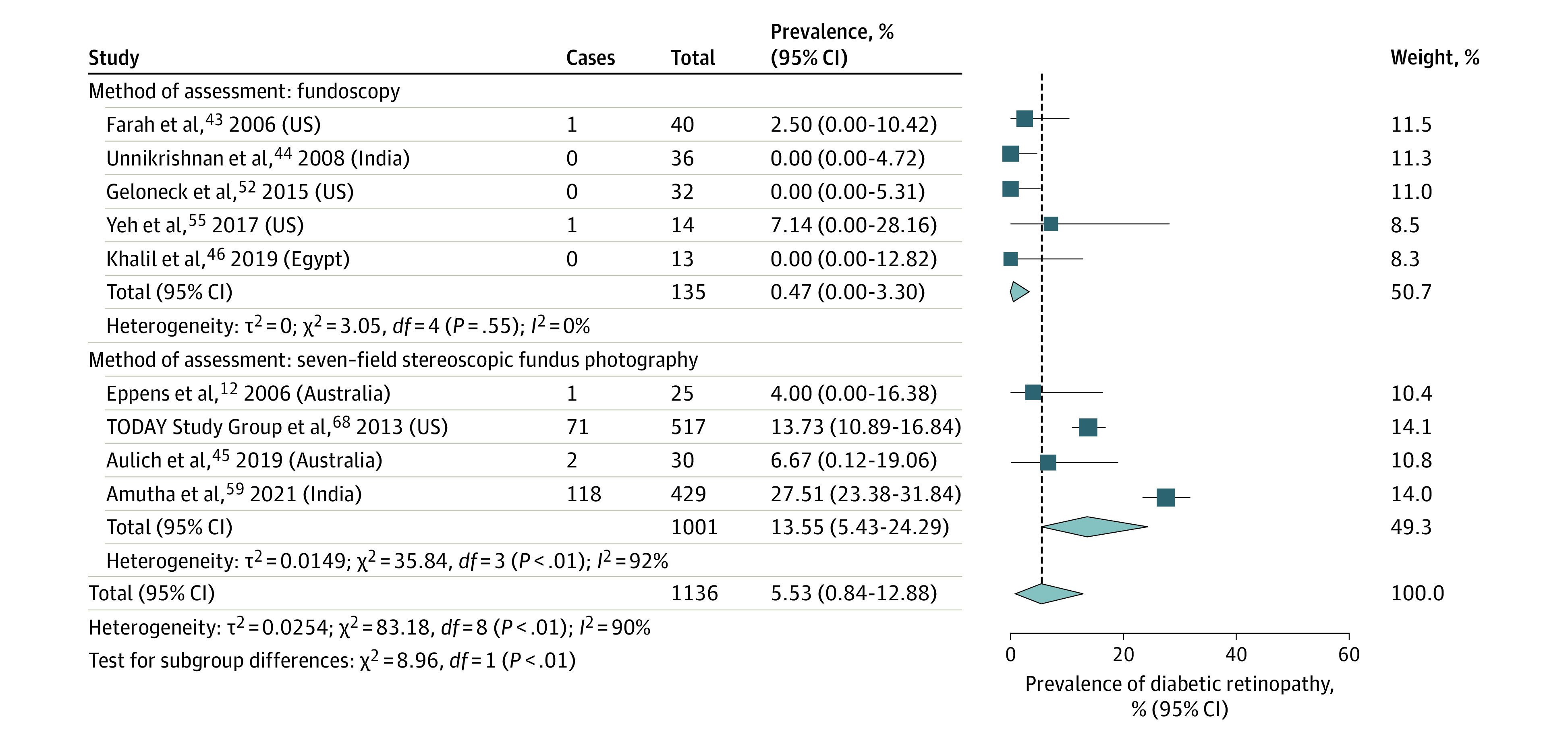
The prevalence of DR in 5 studies43,44,46,52,55 using fundoscopy to diagnose DR was 0.47% (95% CI, 0%-3.30%; I2 = 0%; P = .55; 2 of 135 patients). The prevalence of DR in 4 studies12,45,59,68 using 7-field stereoscopic fundus photography to diagnose DR was 13.55% (95% CI, 5.43%-24.29%; I2 = 92%; P < .001; 192 of 1001 patients) (Figure 3). Other assessment methods included nonmydriatic fundus photography in 1 study,47 and an unspecified form of fundus photography in 3 studies.48,57,66 A total of 15 studies42,49,50,51,53,54,56,58,60,61,62,63,64,67,69 did not report the DR assessment method used.
Figure 3. Prevalence of Diabetic Retinopathy in Youths With Type 2 Diabetes by Method of Assessment.
Global Prevalence of DR Based on Diabetes Duration
Analyzing only prospective cohort studies that reported mean T2D duration,61,62,65,66,67,68,69 the prevalence of DR with T2D duration of less than 2.5 years was 1.11% (95% CI, 0.04%-3.06%; I2 = 5%; P = .30; 4 of 271 patients)61,69 (eFigure 2 in Supplement 1). For T2D duration of 2.5 to 5.0 years, the prevalence was 9.04% (95% CI, 2.24%-19.55%; I2 = 88%; P < .001; 76 of 625 patients)68,69; for T2D duration of greater than 5.0 years, the prevalence was 28.14% (95% CI, 12.84%-46.45%; I2 = 96%; P < .001; 362 of 972 patients).62,65,66,67 When analyzing only retrospective cohort studies that reported mean T2D duration,48,52,53,58 DR prevalence was similar for T2D duration of less than 2.5 years (5.30%; 95% CI, 0.87%-12.19%; I2 = 0%; P = .90; 4 of 73 patients)53,58 and T2D duration of 2.5 to 5.0 years (3.09%; 95% CI, 0%-14.06%; I2 = 75%; P = .05; 8 of 137 patients)48,52 (eFigure 3 in Supplement 1). Analyzing only cross-sectional studies42,43,44,45,46,47 was not possible because these studies did not report all ranges of T2D duration. When all study designs12,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69 were included in the analysis, the prevalence of DR at less than 2.5 years after diagnosis of diabetes was 1.78% (95% CI, 0.25%-4.20%; I2 = 23%; P = .26; 9 of 384 patients).43,53,58,61,69 The prevalence increased sharply at 2.5 to 5.0 years after diagnosis to 5.08% (95% CI, 1.04%-11.22%; I2 = 80%; P < .001; 84 of 775 patients),46,48,52,68,69 with a further increase to 28.83% (95% CI, 15.97%-43.63%; I2 = 95%; P < .001; 394 of 1075 patients)57,62,65,66,67 at 5.0 years after diagnosis (eFigure 4 in Supplement 1).
Global Prevalence of DR Based on Sex
The odds ratio of DR prevalence was lower in male vs female patients (0.40; 95% CI, 0.02-7.21; I2 = 75%; P = .53; 45 of 88 male patients vs 91 of 177 female patients)49,66 (eFigure 5 in Supplement 1), with a wide 95% CI that prevented reaching a definite conclusion on sex differences in DR prevalence. Two studies54,57 did not report a sex-specific prevalence value but found that male patients had a higher risk of DR than female patients when examining hazard ratios.
Prevalence of DR Based on Race
Racial classifications were based on medical records48,50,52,53,60,69 or self-reporting60,63,66 by participants in the studies. The overall pooled prevalence of DR in Middle Eastern or White patients was 24.07% (95% CI, 6.26%-47.91%; I2 = 88%; P < .001; 57 of 171 patients).46,57,66 Asian patients had a prevalence of 13.31% (95% CI, 2.49%-30.05%; I2 = 93%; P < .001; 136 of 590 patients)44,49,59,67 (eFigure 6 in Supplement 1). There were insufficient data to assess the pooled prevalence in other racial groups.
Association of Age, Diabetes Duration, Obesity, HbA1c Level, and Hypertension With DR Prevalence
Age (P < .001; 15 patients), diabetes duration (P = .02; 13 patients), and hypertension prevalence (P = .03; 17 patients) were positively associated with DR prevalence. Meta-regression analysis revealed no associations between obesity prevalence (P = .93; 13 patients) or mean age at diabetes diagnosis (P = .26; 14 patients) and DR prevalence. In addition, there was no association between glycemic control (P = .60; 18 patients) and DR prevalence after examining the data in aggregate and by study design. However, patients with T2D who developed DR had a higher HbA1c level when compared with patients without retinopathy (mean HbA1c difference, 1.37 (95% CI, 0.95-1.79; I2 = 0%; P < .001)47,68 (eFigure 7 in Supplement 1).
Risk of Bias, Level of Evidence, and Publication Bias
Most studies had a low risk of bias (8 studies44,47,52,54,56,57,58,60 [27.6%]) or a moderate risk of bias (20 studies12,42,43,45,46,48,50,51,53,55,59,61,62,63,64,65,66,67,68,69 [69.0%]), with only 1 study49 (3.4%) having a high risk of bias (eTable 10 in Supplement 1). The risk of bias was higher if the patients were from a single clinic or city and not a nationally representative sample; data from those studies12,43,45,46,48,49,50,51,52,53,55,58,59,60,62,63,67 may therefore have limited generalizability. Some studies42,43,49 did not use representative sampling frameworks, and others43,45,49,65,66,68 did not take a census or randomly select patients. Some studies12,46,53,55,59,62,64,65,66,68,69 also had missing data of greater than 25%, potentially leading to nonresponse bias.
The risk of bias was also present if the definition of DR or the assessment method was not described, which occurred in 7 studies.42,49,61,63,64,67,69 In some studies,44,48,54,56,61,64 it was unclear whether all participants were examined using the same methods. A total of 14 studies12,47,51,54,56,57,58,59,60,61,63,64,67,69 (48.3%) had the highest level of evidence (level 1), while 9 studies42,44,46,48,50,52,53,55,62 (31.0%) had level 2 evidence, and 6 studies43,45,49,65,66,68 (20.7%) had level 3 evidence. No publication bias was identified for the prevalence of DR outcome (eFigure 8 in Supplement 1) based on the Egger test (P = .52).
Sensitivity Analysis
Results of the sensitivity analyses are shown in eTable 11 in Supplement 1. Of note, excluding the studies that involved patients older than 18 years decreased the pooled estimate of DR prevalence to 3.03% (95% CI, 1.02%-5.81%) (Figure 1; eTable 11 in Supplement 1), suggesting that DR risk increased with age.
Discussion
This systematic review and meta-analysis found that the global prevalence of DR was 6.99% among children with T2D, and DR prevalence increased significantly at more than 5 years after T2D diagnosis. The current data suggest that approximately 1 in 14 children and adolescents with T2D will have DR within a few years after diabetes diagnosis. While most patients included in this review had minimal or mild NPDR, a substantial minority had more severe disease, such as proliferative DR or macular edema, that can lead to visual impairment and potentially irreversible vision loss. Notably, there was some evidence to suggest that the prevalence of DR rapidly increased with age and diabetes duration; almost 1 in 4 children with T2D for 5 years or more developed DR. The analysis of the associations of sex and race with DR prevalence was inconclusive due to limited data. The scale of DR prevalence in youths with T2D supports the recommendations for periodic patient screening.21,22
When the data from pediatric patients with T2D are compared with those of pediatric patients with type 1 diabetes (T1D), only 2% of children with T1D develop mild NPDR, irrespective of their age at diabetes onset, and none develop proliferative DR or macular edema.70 However, DR prevalence increases sharply at 5 years after diagnosis, to approximately 25%.70 Clinical practice guidelines for T1D currently recommend screening for DR at puberty or beginning at age 11 years if the child has had diabetes for 2 to 5 years.71 The early years of transition from pediatric to adult care among patients aged 18 to 21 years are also associated with an increase in DR among patients with T1D.72 Surveillance for DR is important during this transitional stage, when many patients also have difficulty maintaining glycemic control.72
Among adults with T2D, 21% to 39% of patients have DR at diagnosis, and the rates increase thereafter.19,73 Our results suggested that diabetes duration was positively associated with DR in pediatric T2D. Notably, the findings of the current review suggest that this increase is emerging decades earlier among these children compared with adults with T2D. The long-term outcomes of DR are not yet known due to the relative novelty of the condition. Longitudinal studies are warranted to assess these outcomes.
Hyperglycemia can result in structural and functional retinal abnormalities in pediatric patients with T2D as early as 2 years after diagnosis.74 Adolescents with T2D have substantial multifocal electroretinographic implicit time delays compared with youths with T1D and youths without diabetes.74 In adults with T2D, similar findings have suggested impairment of neural retinal function, future vascular lesions, and increased risk of DR.74,75,76,77,78 In addition, adolescents with T2D had substantially lower retinal thickness and retinal venular dilation when compared with patients without diabetes.74 These findings suggest that retinal abnormalities are present early in pediatric T2D, so it is important that screening be undertaken to detect DR early in this population to prevent impaired vision and blindness.
While the current pediatric T2D clinical practice guidelines recommend regular screening of DR at baseline and annually thereafter, screening guidelines are not routinely followed.21,22 Only 22% to 54% of pediatric patients with T2D have had dilated eye examinations.79,80 Because the findings of this review suggest the prevalence of DR increases rapidly with diabetes duration, there is an immediate need for regular screening to be performed consistently. The benefits of early identification of DR include increased focus on improving glycemic control to minimize microvascular disease, maintaining blood pressure, and streamlining the monitoring of DR progression.19,54 Intensive glycemic control in adolescents with T1D reduced DR by 53% in the Diabetes Control and Complications Trial.81,82 Similarly, the UK Prospective Diabetes Study in adults with T2D showed that strict glycemic and blood pressure control reduced DR progression by 34%.83,84 In our study, hypertension prevalence was associated with DR prevalence, suggesting that hypertension may also be associated with DR in pediatric T2D. However, although patients with higher HbA1c values had higher DR prevalence, this finding did not reach statistical significance. The analysis of the association of DR with HbA1c levels yielded variable results, with some studies47,49,54,57,62,65,68 reporting an association and other studies52,58,67 finding no association. When pooling studies that reported mean HbA1c values in patients with and without DR,47,68 the mean difference suggested significantly higher HbA1c levels in patients with vs without DR. It is possible that our analysis of mean HbA1c levels did not reveal an association with DR prevalence because it was based on mean HbA1c values extracted from cross-sectional data, which may not account for longitudinal fluctuations in glycemic control over time. In addition, there is some evidence to suggest that there are racial and ethnic differences in HbA1c levels, with Asian, Black, Hispanic, and Indigenous individuals having higher HbA1c values than White individuals.85 While maintaining adequate glycemic control is an important step in preventing microvascular complications, any association between glycemic control and DR is likely polygenic.19
The screening method had implications for DR prevalence and explained some of the heterogeneity among studies included in this review, and 7-field stereoscopic fundus photography identified more cases of DR than fundoscopy. Compared with the gold standard of 7-field stereoscopic photography, indirect fundoscopy has a sensitivity of 76% and a specificity of 95%.86 In contrast, 4-field wide-angle stereoscopic photography has a sensitivity of 94% and a specificity of 96%.86 Digital nonmydriatic imaging has a sensitivity of 98% and a specificity of 86% for detecting DR, and direct fundoscopy with pupil dilation has a sensitivity of 65% and a specificity of 97%.86 It is important to note that all but 143 of the included studies12,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57,58,59,60,61,62,63,64,65,66,67,68,69 did not specify whether direct or indirect fundoscopy was performed. Fundus photography is more sensitive than fundoscopy for diagnosing mild cases of DR, while fundoscopy is better at detecting retinal thickening from macular edema and early neovascularization.19,52,87 Although both methods are accepted in screening guidelines, it has been suggested that fundus photography be used in the pediatric population because fundoscopy is challenging to perform in children.52 Fundus photography requires specialized equipment and trained staff to acquire the images and perform the analysis.19,88 These technologies may not be accessible, especially in low- and middle-income countries where pediatric T2D rates are rapidly increasing.89 The disparity in global access to health care resources impacts adherence to and generalizability of screening guidelines, and advocacy for access to retinal imaging and training personnel in different health care settings is important.90 Furthermore, emerging technologies, such as low-cost cameras, automated grading of retinal images, and virtual access to specialist assessments and care, will likely offer more equitable access to care and improve outcomes.91,92 For example, autonomous artificial intelligence systems have been developed for the early detection of DR. These systems have been reported to have a sensitivity of 85.7% and a specificity of 79.3% in diagnosing more than mild severity of DR in youths when compared with consensus grading by retinal specialists.93,94 While these systems are not yet approved for use in children, they offer a promising screening solution because they do not require supervision by eye care professionals and can be used in settings in which specialists are not available.93
Data on DR prevalence in pediatric T2D by sex or race were scarce. No conclusions about DR prevalence by sex or race could be reached due to the limited data. National estimates of DR among US and UK adults suggest that Black, Hispanic, and South Asian individuals have a 5% to 10% higher prevalence of DR compared with White individuals.95,96,97 In the SEARCH for Diabetes in Youth Study,98 youths with T2D from non-White racial groups had a higher prevalence of DR (odds ratio, 2.05; 95% CI, 0.97-4.33). Differences in HbA1c level or diabetes duration did not explain this finding.98 An extensive study of ophthalmic screening patterns in the US80 found that Black and Hispanic children, especially those with low socioeconomic status, had 11% to 18% lower rates of DR screening than White children. These findings highlight the need for the creation of equitable screening strategies for DR that can reach all pediatric patients with T2D.
Limitations
This study has several limitations. The heterogeneity was high across studies. Some studies did not report the DR assessment method42,49,50,51,53,54,56,58,60,61,62,63,64,67,69 or the type of fundus photography48,57,66 used. Data on DR prevalence by sex and race were limited, so we could not reach any conclusions.
In addition, the small number of DR cases in the included studies can have implications for the reliability of the variance and pooled estimates.34 For this reason, we used the Freeman-Tukey double arcsine transformation99,100 to stabilize variance in our meta-analysis. We also conducted a generalized linear mixed analysis for each outcome to assess the robustness of the results99,100 (eTable 11 in Supplement 1). We ultimately presented the results of our Freeman-Tukey transformation analysis because generalized linear mixed models do not provide a weight for each study, which is important information for clinicians to have access to when making practice recommendations.99,100 We calculated 95% PIs alongside 95% CIs to provide information on the estimated range of true case rates in this study (eTable 11 in Supplement 1). Given the high heterogeneity across studies included in this review, the 95% PIs were broad for some outcomes and suggested the need for more high-quality, adequately powered longitudinal studies.
Conclusions
The findings of this systematic review and meta-analysis suggest that the retinal microvasculature is an early target of T2D in children and that the risk of DR continues to increase over time. Mechanistic insights into the pathogenesis of DR in children with T2D remain limited, and this area warrants prioritized investigation. Increasing the number of children with T2D who undergo regular DR screening is important to meet current clinical practice guideline standards. Fundus photography is more sensitive in diagnosing early DR than fundoscopy. These assessments will likely maintain vision and quality of life and improve long-term outcomes. Equitable access to health care resources to detect and treat DR is a global priority that needs increased attention.
eTable 1. MOOSE Checklist
eTable 2. Search Strategy—MEDLINE
eTable 3. Search Strategy—Embase
eTable 4. Search Strategy—CINAHL
eTable 5. Search Strategy—Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 6. Search Strategy—Web of Science: Conference Proceedings Citation Index–Science
eTable 7. Type 2 Diabetes Criteria Used Across the Included Studies
eTable 8. Characteristics of Included Studies
eTable 9. Comparison of Meta-analysis Results Using Freeman-Tukey Double Arcsine Transformation and Generalized Linear Mixed-Effects Model With Confidence and Prediction Intervals
eTable 10. Risk of Bias of Included Studies
eTable 11. Results of Sensitivity Analysis
eFigure 1. PRISMA Flow Diagram Illustrating Study Selection
eFigure 2. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in Prospective Cohort Studies
eFigure 3. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in Retrospective Cohort Studies
eFigure 4. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in All Studies
eFigure 5. Forest Plot of the Odds Ratio of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Sex
eFigure 6. Forest Plot Illustrating Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Race
eFigure 7. Forest Plot Showing Mean Difference in HbA1c in Participants With vs Without Diabetic Retinopathy
eFigure 8. Funnel Plot Examining Publication Bias for Diabetic Retinopathy Prevalence Outcome
eReferences.
Data Sharing Statement
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693-700. doi: 10.1016/j.jpeds.2004.12.042 [DOI] [PubMed] [Google Scholar]
- 2.Amed S, Hamilton JK, Sellers EAC, et al. Differing clinical features in Aboriginal vs. non-Aboriginal children presenting with type 2 diabetes. Pediatr Diabetes. 2012;13(6):470-475. doi: 10.1111/j.1399-5448.2012.00859.x [DOI] [PubMed] [Google Scholar]
- 3.Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35(6):737-744. doi: 10.1111/dme.13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tajima N, Morimoto A. Epidemiology of childhood diabetes mellitus in Japan. Pediatr Endocrinol Rev. 2012;10(suppl 1):44-50. [PubMed] [Google Scholar]
- 5.Cioana M, Deng J, Nadarajah A, et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(12):e2247186. doi: 10.1001/jamanetworkopen.2022.47186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carino M, Elia Y, Sellers E, et al. Comparison of clinical and social characteristics of Canadian youth living with type 1 and type 2 diabetes. Can J Diabetes. 2021;45(5):428-435. doi: 10.1016/j.jcjd.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Lecoutre S, Maqdasy S, Breton C. Maternal obesity as a risk factor for developing diabetes in offspring: an epigenetic point of view. World J Diabetes. 2021;12(4):366-382. doi: 10.4239/wjd.v12.i4.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill J, Nielsen M, Fox MH. Understanding the social factors that contribute to diabetes: a means to informing health care and social policies for the chronically ill. Perm J. 2013;17(2):67-72. doi: 10.7812/TPP/12-099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26(11):2999-3005. doi: 10.2337/diacare.26.11.2999 [DOI] [PubMed] [Google Scholar]
- 10.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823-1831. doi: 10.1016/S0140-6736(07)60821-6 [DOI] [PubMed] [Google Scholar]
- 11.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years—clinical observation from a secondary care cohort. QJM. 2009;102(11):799-806. doi: 10.1093/qjmed/hcp121 [DOI] [PubMed] [Google Scholar]
- 12.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300-1306. doi: 10.2337/dc05-2470 [DOI] [PubMed] [Google Scholar]
- 13.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. ; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825-835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cioana M, Deng J, Hou M, et al. Prevalence of hypertension and albuminuria in pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(4):e216069. doi: 10.1001/jamanetworkopen.2021.6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cioana M, Deng J, Nadarajah A, et al. Prevalence of polycystic ovary syndrome in patients with pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(2):e2147454. doi: 10.1001/jamanetworkopen.2021.47454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Dept of Health & Human Services. 2011. Accessed February 22, 2023. https://stacks.cdc.gov/view/cdc/13329
- 17.Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580-1591. doi: 10.1016/j.ophtha.2021.04.027 [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6):1816. doi: 10.3390/ijms19061816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong DS, Aiello L, Gardner TW, et al. ; American Diabetes Association . Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87. doi: 10.2337/diacare.27.2007.S84 [DOI] [PubMed] [Google Scholar]
- 20.Willis JR, Doan QV, Gleeson M, et al. Vision-related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135(9):926-932. doi: 10.1001/jamaophthalmol.2017.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(suppl 27):28-46. doi: 10.1111/pedi.12719 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42(suppl 1):S148-S164. doi: 10.2337/dc19-S013 [DOI] [PubMed] [Google Scholar]
- 23.Samaan MC, Cioana M, Banfield L, et al. The prevalence of comorbidities in pediatric type 2 diabetes mellitus: a systematic review. International Prospective Register of Systematic Reviews (PROSPERO) identifier: CRD42018091127. March 19, 2018. Accessed January 30, 2023. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018091127
- 24.Stroup DF, Berlin JA, Morton SC, et al; Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KJ, Dunn EC, Koru-Sengul T. Association of race/ethnicity with specific glycemic thresholds for predicting diabetic retinopathy. JAMA Ophthalmol. 2019;137(4):463-464. doi: 10.1001/jamaophthalmol.2019.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabelea D, Mayer-Davis EJ, Saydah S, et al; SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;11(17):1778-1786. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 29.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM levels of evidence. Oxford Centre for Evidence-Based Medicine. 2011. Accessed February 9, 2023. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 30.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 31.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 32.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals of Mathematical Statistics. 1950;21(4):607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 33.Miller JJ. The inverse of the Freeman-Tukey double arcsine transformation. Am Stat. 1978;32(4):138. doi: 10.1080/00031305.1978.10479283 [DOI] [Google Scholar]
- 34.Zhou S, Shen C. Statistical considerations for meta-analysis of diabetic retinopathy prevalence. JAMA Ophthalmol. 2022;140(11):1141. doi: 10.1001/jamaophthalmol.2022.3470 [DOI] [PubMed] [Google Scholar]
- 35.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 36.Deeks JJ, Higgins JPT, Altman DG, eds. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0. Cochrane; 2019:chap 10. [Google Scholar]
- 37.Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. National Institutes of Health. April 8, 2015. Notice No. NOT-OD-15-089. Accessed May 7, 2019. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html
- 38.Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0. Cochrane; 2019:chap 13. [Google Scholar]
- 39.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 40.R: a language and environment for statistical computing. Version 1.1.383. RStudio. 2017. Accessed February 9, 2023. https://www.npackd.org/p/rstudio/1.1.383
- 41.R: a language and environment for statistical computing. Version 3.4.3. R Foundation for Statistical Computing. 2017. Accessed February 9, 2023. https://anaconda.org/r/r/files?version=3.4
- 42.Eppens MC, Craig ME, Jones TW, Silink M, Ong S, Ping YJ; International Diabetes Federation Western Pacific Region Steering Committee . Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin. 2006;22(5):1013-1020. doi: 10.1185/030079906X104795 [DOI] [PubMed] [Google Scholar]
- 43.Farah SE, Wals KT, Friedman IB, Pisacano MA, DiMartino-Nardi J. Prevalence of retinopathy and microalbuminuria in pediatric type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2006;19(7):937-942. doi: 10.1515/JPEM.2006.19.7.937 [DOI] [PubMed] [Google Scholar]
- 44.Unnikrishnan AG, Bhatia E, Bhatia V, et al. Type 1 diabetes versus type 2 diabetes with onset in persons younger than 20 years of age. Ann N Y Acad Sci. 2008;1150:239-244. doi: 10.1196/annals.1447.056 [DOI] [PubMed] [Google Scholar]
- 45.Aulich J, Cho YH, Januszewski AS, et al. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatr Diabetes. 2019;20(8):1118-1127. doi: 10.1111/pedi.12913 [DOI] [PubMed] [Google Scholar]
- 46.Khalil SA, Megallaa MH, Rohoma KH, et al. Prevalence of chronic diabetic complications in newly diagnosed versus known type 2 diabetic subjects in a sample of Alexandria population, Egypt. Curr Diabetes Rev. 2019;15(1):74-83. doi: 10.2174/1573399814666180125100917 [DOI] [PubMed] [Google Scholar]
- 47.Ferm ML, DeSalvo DJ, Prichett LM, Sickler JK, Wolf RM, Channa R. Clinical and demographic factors associated with diabetic retinopathy among young patients with diabetes. JAMA Netw Open. 2021;4(9):e2126126. doi: 10.1001/jamanetworkopen.2021.26126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott A, Toomath R, Bouchier D, et al. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J. 2006;119(1235):U2015. [PubMed] [Google Scholar]
- 49.Lee Z, Sato Y, Urakami T. Relationship between retinopathy development and systemic factors in type 2 childhood diabetes. ARticle in Japanese. Nippon Ganka Gakkai Zasshi. 2007;111(5):397-400. [PubMed] [Google Scholar]
- 50.Osman HAM, Elsadek N, Abdullah MA. Type 2 diabetes in Sudanese children and adolescents. Sudan J Paediatr. 2013;13(2):17-23. [PMC free article] [PubMed] [Google Scholar]
- 51.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37(2):436-443. doi: 10.2337/dc13-0954 [DOI] [PubMed] [Google Scholar]
- 52.Geloneck MM, Forbes BJ, Shaffer J, Ying G, Binenbaum G. Ocular complications in children with diabetes mellitus. Ophthalmology. 2015;122(12):2457-2464. doi: 10.1016/j.ophtha.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newton K, Stanley J, Wiltshire E. Audit of type 2 diabetes in youth in Wellington, New Zealand 2001–2013. Pediatr Diabetes. 2015;16(suppl 21):147. doi: 10.1111/pedi.12309 [DOI] [Google Scholar]
- 54.Wang SY, Andrews CA, Herman WH, Gardner TW, Stein JD. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology. 2017;124(4):424-430. doi: 10.1016/j.ophtha.2016.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh T, Bernardo J. Complications of type 2 diabetes in adolescent patients: the Rhode Island Hospital experience compared to the TODAY study. Horm Res Paediatr. 2017;88(suppl 1):626. doi: 10.1159/000481424 [DOI] [Google Scholar]
- 56.Koziol M, Nowak MS, Udziela M, Piątkiewicz P, Grabska-Liberek I, Szaflik JP. First nation-wide study of diabetic retinopathy in Poland in the years 2013-2017. Acta Diabetol. 2020;57(10):1255-1264. doi: 10.1007/s00592-020-01540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ek AE, Samuelsson U, Janson A, Carlsson A, Elimam A, Marcus C. Microalbuminuria and retinopathy in adolescents and young adults with type 1 and type 2 diabetes. Pediatr Diabetes. 2020;21(7):1310-1321. doi: 10.1111/pedi.13074 [DOI] [PubMed] [Google Scholar]
- 58.Porter M, Channa R, Wagner J, Prichett L, Liu TYA, Wolf RM. Prevalence of diabetic retinopathy in children and adolescents at an urban tertiary eye care center. Pediatr Diabetes. 2020;21(5):856-862. doi: 10.1111/pedi.13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amutha A, Ranjit U, Anjana RM, et al. Clinical profile and incidence of microvascular complications of childhood and adolescent onset type 1 and type 2 diabetes seen at a tertiary diabetes center in India. Pediatr Diabetes. 2021;22(1):67-74. doi: 10.1111/pedi.13033 [DOI] [PubMed] [Google Scholar]
- 60.Bai P, Barkmeier AJ, Hodge DO, Mohney BG. Ocular sequelae in a population-based cohort of youth diagnosed with diabetes during a 50-year period. JAMA Ophthalmol. 2022;140(1):51-57. doi: 10.1001/jamaophthalmol.2021.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shield JPH, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94(3):206-209. doi: 10.1136/adc.2008.143313 [DOI] [PubMed] [Google Scholar]
- 62.Ruhayel SD, James RA, Ehtisham S, Cameron FJ, Werther GA, Sabin MA. An observational study of type 2 diabetes within a large Australian tertiary hospital pediatric diabetes service. Pediatr Diabetes. 2010;11(8):544-551. doi: 10.1111/j.1399-5448.2010.00647.x [DOI] [PubMed] [Google Scholar]
- 63.Jefferies C, Carter P, Reed PW, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995-2007. Pediatr Diabetes. 2012;13(4):294-300. doi: 10.1111/j.1399-5448.2011.00851.x [DOI] [PubMed] [Google Scholar]
- 64.Schmidt F, Kapellen TM, Wiegand S, et al. ; DPV-Wiss Study Group; BMBF Competence Network Diabetes . Diabetes mellitus in children and adolescents with genetic syndromes. Exp Clin Endocrinol Diabetes. 2012;120(10):579-585. doi: 10.1055/s-0032-1306330 [DOI] [PubMed] [Google Scholar]
- 65.TODAY Study Group . Development and progression of diabetic retinopathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care. 2021;45(5):1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen ET, Rigdon J, Rezaei K, et al. Prevalence of diabetic retinopathy in youth-onset type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth study. Diabetes. 2021;70(suppl 1):1032-P. doi: 10.2337/db21-1032-P [DOI] [Google Scholar]
- 67.Preechasuk L, Tantasuwan S, Likitmaskul S, et al. Clinical characteristics, glycemic control, and microvascular complications compared between young-onset type 1 and type 2 diabetes patients at Siriraj Hospital–a tertiary referral center. Diabetes Metab Syndr Obes. 2022;15:1375-1387. doi: 10.2147/DMSO.S354787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36(6):1772-1774. doi: 10.2337/dc12-2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuckerman Levin N, Cohen M, Phillip M, et al. Youth-onset type 2 diabetes in Israel: a national cohort. Pediatr Diabetes. 2022;23(6):649-659. doi: 10.1111/pedi.13351 [DOI] [PubMed] [Google Scholar]
- 70.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520-526. doi: 10.1001/archopht.1984.01040030398010 [DOI] [PubMed] [Google Scholar]
- 71.Bjornstad P, Dart A, Donaghue KC, et al. ISPAD Clinical Practice Consensus Guidelines 2022: microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr Diabetes. 2022;23(8):1432-1450. doi: 10.1111/pedi.13444 [DOI] [PubMed] [Google Scholar]
- 72.Gubitosi-Klug RA, Bebu I, White NH, et al. ; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Screening eye exams in youth with type 1 diabetes under 18 years of age: once may be enough? Pediatr Diabetes. 2019;20(6):743-749. doi: 10.1111/pedi.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohner EM, Aldington SJ, Stratton IM, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non–insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116(3):297-303. doi: 10.1001/archopht.116.3.297 [DOI] [PubMed] [Google Scholar]
- 74.Bronson-Castain KW, Bearse MA Jr, Neuville J, et al. Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina. 2012;32(1):92-102. doi: 10.1097/IAE.0b013e318219deac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bearse MA Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25(5):425-448. doi: 10.1016/j.preteyeres.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han Y, Schneck ME, Bearse MA Jr, et al. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(11):4106-4112. doi: 10.1167/iovs.04-0405 [DOI] [PubMed] [Google Scholar]
- 77.Ng JS, Bearse MA Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49(4):1622-1628. doi: 10.1167/iovs.07-1157 [DOI] [PubMed] [Google Scholar]
- 78.Han Y, Bearse MA Jr, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(3):948-954. doi: 10.1167/iovs.03-1101 [DOI] [PubMed] [Google Scholar]
- 79.Valent D, Pestak K, Otis M, Shubrook J. Type 2 diabetes in the pediatric population: are we meeting ADA clinical guidelines in Ohio? Clin Pediatr (Phila). 2010;49(4):316-322. doi: 10.1177/0009922809344424 [DOI] [PubMed] [Google Scholar]
- 80.Wang SY, Andrews CA, Gardner TW, Wood M, Singer K, Stein JD. Ophthalmic screening patterns among youths with diabetes enrolled in a large US managed care network. JAMA Ophthalmol. 2017;135(5):432-438. doi: 10.1001/jamaophthalmol.2017.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 82.Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381-389. doi: 10.1056/NEJM200002103420603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 84.UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703-713. doi: 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herman WH, Ma Y, Uwaifo G, et al. ; Diabetes Prevention Program Research Group . Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457. doi: 10.2337/dc06-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li KX, Lovell M, Evans K, Gallego PH. Reviewing guidelines on diabetic retinopathy screening in children and adolescents with type 1 diabetes: is there consistency amongst practitioners? Canadian Journal of Optometry. 2015;77(4):13-18. doi: 10.15353/cjo.77.485 [DOI] [Google Scholar]
- 87.Palmberg P, Smith M, Waltman S, et al. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology. 1981;88(7):613-618. doi: 10.1016/S0161-6420(81)34975-6 [DOI] [PubMed] [Google Scholar]
- 88.Friedman DS, Ali F, Kourgialis N. Diabetic retinopathy in the developing world: how to approach identifying and treating underserved populations. Am J Ophthalmol. 2011;151(2):192-194. doi: 10.1016/j.ajo.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 89.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228-236. doi: 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 90.Piyasena MMPN, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One. 2019;14(4):e0198979. doi: 10.1371/journal.pone.0198979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajalakshmi R, Prathiba V, Arulmalar S, Usha M. Review of retinal cameras for global coverage of diabetic retinopathy screening. Eye (Lond). 2021;35(1):162-172. doi: 10.1038/s41433-020-01262-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruamviboonsuk P, Tiwari R, Sayres R, et al. Real-time diabetic retinopathy screening by deep learning in a multisite national screening programme: a prospective interventional cohort study. Lancet Digit Health. 2022;4(4):e235-e244. doi: 10.1016/S2589-7500(22)00017-6 [DOI] [PubMed] [Google Scholar]
- 93.Wolf RM, Liu TYA, Thomas C, et al. The SEE study: safety, efficacy, and equity of implementing autonomous artificial intelligence for diagnosing diabetic retinopathy in youth. Diabetes Care. 2021;44(3):781-787. doi: 10.2337/dc20-1671 [DOI] [PubMed] [Google Scholar]
- 94.Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649-656. doi: 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57(4):347-370. doi: 10.1016/j.survophthal.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 97.Sivaprasad S, Gupta B, Gulliford MC, et al. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdom (DRIVE UK). PLoS One. 2012;7(3):e32182. doi: 10.1371/journal.pone.0032182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayer-Davis EJ, Davis C, Saadine J, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetic retinopathy in the SEARCH for Diabetes in Youth cohort: a pilot study. Diabet Med. 2012;29(9):1148-1152. doi: 10.1111/j.1464-5491.2012.03591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Widyaputri F, Rogers S, Lim L. Statistical considerations for meta-analysis of diabetic retinopathy prevalence—reply. JAMA Ophthalmol. 2022;140(11):1141-1142. doi: 10.1001/jamaophthalmol.2022.3473 [DOI] [PubMed] [Google Scholar]
- 100.Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3(3):e178. doi: 10.1002/hsr2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. MOOSE Checklist
eTable 2. Search Strategy—MEDLINE
eTable 3. Search Strategy—Embase
eTable 4. Search Strategy—CINAHL
eTable 5. Search Strategy—Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 6. Search Strategy—Web of Science: Conference Proceedings Citation Index–Science
eTable 7. Type 2 Diabetes Criteria Used Across the Included Studies
eTable 8. Characteristics of Included Studies
eTable 9. Comparison of Meta-analysis Results Using Freeman-Tukey Double Arcsine Transformation and Generalized Linear Mixed-Effects Model With Confidence and Prediction Intervals
eTable 10. Risk of Bias of Included Studies
eTable 11. Results of Sensitivity Analysis
eFigure 1. PRISMA Flow Diagram Illustrating Study Selection
eFigure 2. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in Prospective Cohort Studies
eFigure 3. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in Retrospective Cohort Studies
eFigure 4. Forest Plot of Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Diabetes Duration in All Studies
eFigure 5. Forest Plot of the Odds Ratio of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Sex
eFigure 6. Forest Plot Illustrating Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes by Race
eFigure 7. Forest Plot Showing Mean Difference in HbA1c in Participants With vs Without Diabetic Retinopathy
eFigure 8. Funnel Plot Examining Publication Bias for Diabetic Retinopathy Prevalence Outcome
eReferences.
Data Sharing Statement


