Abstract
Precision biotics (PBs) are chemically synthesized complex glycans that modulate specific microbiome metabolic functions. The objective of the present study was to evaluate the effect of the supplementation of PB on the growth performance, and cecal microbiome modulation of broiler chickens raised under commercial conditions. A total of 190,000-day-old Ross 308 straight-run broilers were randomly assigned to 2 dietary treatments. There were 5 houses per treatment with 19,000 birds per house. In each house, there were 6 rows of battery cages with 3 tiers. The 2 dietary treatments included a control diet (a commercial broiler diet) and a PB supplemented diet at 0.9 kg/MT. On a weekly basis, 380 birds were randomly selected for body weight (BW) determination. At 42 d of age, the BW and feed intake (FI) of each house were recorded, the feed conversion ratio (cFCR) was calculated and corrected with the final BW, and the European production index (EPI) was calculated. Additionally, 8 birds per house (40 birds/experimental group) were randomly selected to collect cecal content for microbiome analysis. The supplementation of PB significantly improved (P < 0.05) the BW of the birds at 7, 14, and 21 d and numerically improved the BW of the birds by 64 and 70 g at 28 and 35 d of age, respectively. At 42 d, the PB numerically improved BW by 52 g, and significantly improved (P < 0.05) the cFCR by 2.2 points and the EPI by 13 points. The functional profile analysis showed a clear and significant difference in the cecal microbiome metabolism between control vs. PB supplemented birds. A higher abundance of pathways was modulated by PB which were associated with amino acid fermentation and putrefaction, particularly from lysine, arginine, proline, histidine, and tryptophane which led to a significant increase (P = 0.0025) in the Microbiome Protein Metabolism Index (MPMI) compared to nonsupplemented birds. In conclusion, the supplementation of PB efficiently modulated pathways related to protein fermentation and putrefaction, resulting in higher MPMI and improved growth performance of broilers.
Key words: broiler, microbiome metabolic modulators, precision biotic, growth performance
INTRODUCTION
The increased recognition of antimicrobial resistance as a public health risk and, therefore, the imposed restrictions in the use of antimicrobial growth promoters (AGP) has driven the search for novel nutritional strategies for broiler chickens. In addition, the advances in molecular biology, analytics, and data science in the past years have enhanced our understating on the gastrointestinal tract (GIT) microbiome of humans and chickens (Oakley et al., 2014; Proctor et al., 2019; Glendinning et al., 2020; Sun et al., 2021). These novel approaches have led to the development of precision biotics (PBs) that are able to specifically modulate microbiome pathways of the GIT of chickens (Walsh et al., 2021; Bortoluzzi et al., 2023). It has been found that the targeted modulation of microbiome pathways by the PB, mainly related to protein metabolism and utilization, and short-chain fatty acid (SCFA) production, improve the growth performance of chickens (Walsh et al., 2021; Jacquier et al., 2022), and increase the resistance against enteric stress (Crhanova et al., 2011; Clavijo and Flórez, 2018; Blokker et al., 2022).
PBs are complex glycans with specific glycosidic linkages (Jacquier et al., 2022) and varying chain size, that can redirect the functions of the microbiome, beyond the changes in the taxonomic composition of the microbial community, toward increased beneficial outputs, such as higher propionate production (Walsh et al., 2021). Additionally, it is known that in certain conditions, for instance, enteric challenges or diets containing low quality protein, the amount of undigested protein that reaches the distal portions of the GIT of chickens may increase. In such circumstances, protein may be fermented by the cecal microbiome and generate metabolites that are detrimental for the health and welfare of the birds (Apajalahti and Vienola, 2016). Bortoluzzi et al. (2023) demonstrated that this PB can, in vivo, shift the microbiome metabolic pathways toward better utilization of protein, which resulted in a higher Microbiome Protein Metabolism Index (MPMI), defined as the ratio between the abundance of beneficial by detrimental genes related to protein metabolism. It was also reported, ex vivo, that the PB can change the microbial pathways and increase the MPMI regardless of the BW (low or high) of the chickens from which the samples were collected (Bortoluzzi et al., 2023). Hence, the ability to redirect specific metabolic functions of the microbiome with precision nutritional ingredients is essential to minimize the impact of undigested protein that accumulates in the ceca, aiming to improve growth performance and welfare of chickens.
By shifting microbiome pathways of the GIT of chickens with the use of PB creates possibilities to modulate host physiology. The host-microbiome interface as a single organism offers the idea of a holobiont organism (Bordenstein and Theis, 2015). The immunomodulation as an indirect effect of the changed microbiome is a notable example of this symbiotic relationship. As indicated by Blokker et al. (2022), the PB improved the resilience of broiler chickens against enteric stress and improved the growth performance. Therefore, the objective of the present study was to evaluate the effect of the supplementation of PB on the growth performance, and cecal microbiome modulation of broiler chickens raised under field conditions.
MATERIALS AND METHODS
Birds, Experimental Design and Diets
A commercial trial was carried out on a farm in Weifang City, Shandong Province, China. The animal care and use procedures were in accordance with the Guidelines of Farm Animal Welfare Requirements: meat-type chicken (CAS, 2017), and followed by qualified personnel. A total of 190,000-day-old Ross 308 straight-run broilers were randomly assigned to 2 dietary treatments. There were 5 houses per treatment with 19,000 birds per house. In each house, there were 6 rows of battery cages with 3 tiers, and approximately 1,200 cages per house, with 16 birds per cage. The cage unit was divided into 1.4 m × 0.8 m with approximately 16 birds per unit. All birds were reared in an environmentally controlled room with a lighting program of 23L: 1D during the first week and 20L: 4D afterward until the end of the trial. The temperature started at 31°C on the first day and dropped gradually to 27.5°C by the end of the first week, 26°C by the second week, 25.5°C by the third week, 23.5°C by the fourth week, 22.5°C by the fifth week and maintained at 22°C during the sixth week. The vaccination program was as follows: in the hatchery, a combined vaccine against Newcastle disease (ND) and infectious bronchitis (IB) was applied by spray and a quadruple vaccine against ND, IB, avian influenza and infectious bursal disease was applied by injection; at 7 d, a combined vaccine against ND and IB was applied by nasal/eye drops; at 21 d, a combined vaccine against ND and IB was applied by water. The trial lasted for 42 d comprising of a 14-day starter diet, a 14-day grower diet, and a 14-day finisher diet. Birds had ad libitum access to feed and water.
The 2 dietary treatments included a control diet (a commercial broiler diet) and a PB supplemented diet at 0.9 kg/MT (Symphiome, DSM Nutritional Products). The PB is a complex glycan mixture technically defined as a PB, from DSM Nutritional Products, Kaiseraugst, Switzerland (Jacquier et al., 2022). The diet composition and nutrient content of control diets for the 3 phases are shown in Table 1. All the diets were pelleted at 70°C to 75°C.
Table 1.
Diet composition and nutrient content of the experimental control diets, as-fed.
| Ingredient, % | Starter | Grower | Finisher |
|---|---|---|---|
| 0–14 d | 14–28 d | 28–42 d | |
| Corn | 26.97 | 24.56 | 22.89 |
| Wheat | 12.00 | 15.00 | 15.00 |
| Brown rice | 10.00 | 12.00 | 15.00 |
| Soybean meal, 46% crude protein | 30.34 | 26.07 | 21.27 |
| Wheat shorts | 10.00 | 10.00 | 10.00 |
| Corn gluten meal | 3.00 | 4.00 | 5.00 |
| Peanut meal | 2.00 | 2.00 | 3.00 |
| Soya oil | 1.35 | 2.51 | 4.43 |
| Limestone | 1.51 | 1.41 | 1.28 |
| Dicalcium phosphate | 1.35 | 1.03 | 0.78 |
| L-Lysine sulfate | 0.45 | 0.43 | 0.42 |
| DL-Methionine | 0.24 | 0.18 | 0.13 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vit-min premix1 | 0.50 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 | 100.00 |
| Formulated nutrients, % | |||
| Crude protein, % | 23.00 | 22.00 | 21.00 |
| AME, kcal/kg | 2869 | 2999 | 3179 |
| Total Ca, % | 0.90 | 0.80 | 0.70 |
| Total P, % | 0.65 | 0.57 | 0.51 |
Vitamin-mineral premix provided (per kg of diet): vitamin A 9,000 IU, vitamin D3 2,000 IU, vitamin E 11 IU, vitamin K3 1.0 mg, vitamin B1 1.2 mg, vitamin B2 5.8 mg, vitamin B6 2.6 mg, vitamin B12 0.012 mg, niacin 66 mg, pantothenic acid 10 mg, biotin 0.10 mg, folic acid 0.7 mg, I 0.65 mg, Se 0.35 mg.
Sampling and Measurements
On a weekly basis, 380 birds (2%) per house were randomly selected for BW. At 42 d of age, bird weight (BW) and feed consumption of each house were recorded, the feed conversion ratio (FCR) was calculated as feed consumption divided by body weight gain (BWG), and the FCR corrected with the final body weight (cFCR) was also reported. The dead birds were recorded per house. The European production index (EPI) was calculated with the formula: EPI = (Average grams gained/day × % survival rate)/feed conversion ratio × 10.
At 42 d of age, 8 birds per house (40 birds/experimental group and 80 birds in total) were randomly selected, the cecal content was aseptically collected (disinfecting surfaces and utensils, and changing gloves after every sampled bird), and immediately put in dry ice. The samples were then frozen at −80°C until further processing (DNA isolation and sequencing).
Microbiome Analysis—DNA Extraction, Sequencing, and Functional Mapping
The microbial DNA from the cecal content sample was extracted using MagPure Stool DNA KF Kit B (Magen, Guangzhou, China) following the manufacturer's instructions. DNA was quantified with Qubit Fluorometer using Qubit dsDNA BR Assay kit (Invitrogen, Shanghai, China) and the quality was checked by running an aliquot of DNA on 1% agarose gel.
After DNA extraction, 1 µg of genomic DNA was randomly fragmented with the use of a Covaris focused-ultrasonicator, followed by purification with AxyPrep Mag PCR Clean Up kit. The fragmented DNA was selected by Agencourt AMPure XPMedium kit to an average size of 200 to 400 bp. The fragments were end repaired by End Repair Mix and purified afterward. The repaired DNA was combined with A-Tailing Mix, then the Illumina adaptors were ligated to the Adenylate 3′Ends DNA and followed by purification. The products were selected based on the insert size. Several rounds of PCR amplification with PCR Primer Cocktail and PCR Master Mix were performed to enrich the Adapter-ligated DNA fragments. After purification, the library was qualified by the Agilent 2100 bioanalyzer (Agilent, Shanghai, China) and ABI StepOnePlus Realtime PCR System. Finally, the qualified libraries were sequenced on the Illumina Hiseq platform (BGI, Wuhan, China). Quality control of the sequences and the functional mapping was done according to Bortoluzzi et al. (2023) and is fully described in the Supplementary Material.
Functional Metagenomic Profiling and Microbiome Protein Metabolism Index
Computed functional metagenomic profiles and the MPMI calculation was done according to Bortoluzzi et al. (2023) and is fully described in the Supplementary Material. Briefly, top microbial metabolic reactions and KEGG pathways were identified by sorting EC Numbers by “Mean Decrease in Accuracy” using the truncated random forest classifier. Functional metagenomic clustering was performed by local Fisher discriminant analysis (LFDA), and a graphical representation of metagenomic similarity was obtained by plotting each microbiome on a 2-dimensional plane.
The MPMI corresponds to metabolic processes associated with desirable microbial protein assimilation, while reactions appearing in the denominator correspond to undesirable microbial protein putrefaction pathways. A higher value of the MPMI is associated with more beneficial microbial protein metabolism.
Statistical Analysis
Growth performance data were subjected to a Student t test using JMP Pro v. 16.0 (SAS Institute, Cary, NC). House served as the experimental unit. Statistical significance was considered at P ≤ 0.05. Functional metagenomics and metabolism index data were analyzed for statistical significance using either the Kruskal-Wallis test by ranks or Mann-Whitney U test (Wilcoxon rank-sum test).
RESULTS
Growth Performance
The random selection of approximately 380 birds per house on a weekly basis showed that the PB significantly improved (P < 0.05) BW of birds at 7, 14, and 21 d of age and numerically improved BW of the birds by 64 and 70 g at 28 and 35 d of age, respectively (Table 2). Additionally, the PB numerically improved BW of birds by 52 g on d 42, and significantly improved (P < 0.05) the cFCR by 2.2 points and the EPI by 13 points (Table 3).
Table 2.
Efficacy of a precision biotic on the body weight of broilers measured on a weekly basis1.
| Treatments | Body weight |
||||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | 28 d | 35 d | |
| Control | 193b | 502b | 927b | 1,396 | 2,076 |
| Precision biotic | 203a | 529a | 1,001a | 1,460 | 2,146 |
| SEM | 2 | 6 | 17 | 18 | 24 |
| P value | 0.03 | 0.007 | 0.02 | 0.07 | 0.15 |
Data were collected from 2% of birds of each house, with 5 replicate houses per treatment.
Means with different superscript are statistically different (P < 0.05).
Table 3.
Efficacy of a precision biotic on the growth performance of broilers from 1 to 42 d of age1.
| Treatment | BW, g/bird | FI, g/bird | FCR | cFCR | Mortality, % | EPI |
|---|---|---|---|---|---|---|
| Control | 2,735 | 3,986 | 1.457 | 1.471a | 1.62 | 450b |
| Precision biotic | 2,787 | 4,029 | 1.446 | 1.449b | 1.56 | 463a |
| SEM | 28.1 | 53.7 | 0.01 | 0.01 | 0.09 | 4.3 |
| P value | 0.23 | 0.62 | 0.36 | 0.04 | 0.70 | 0.04 |
Data were collected from all the birds of each house, with 5 replicate houses per treatment.
Abbreviations: BW, body weight; cFCR, FCR corrected with body weight; EPI, European production index; FCR, feed conversion ratio; FI, feed intake.
Means with different superscript are statistically different (P < 0.05).
Functional Microbiome Profile, Microbiome Protein Metabolism Index, and Pathobionts
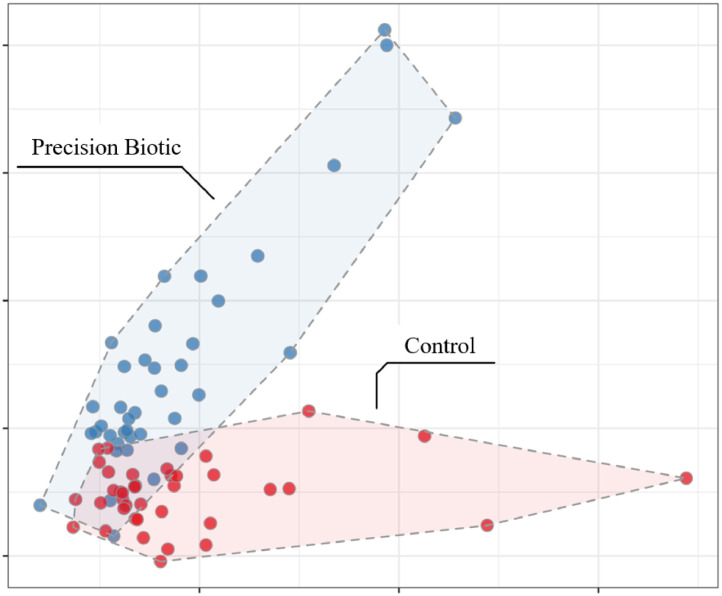
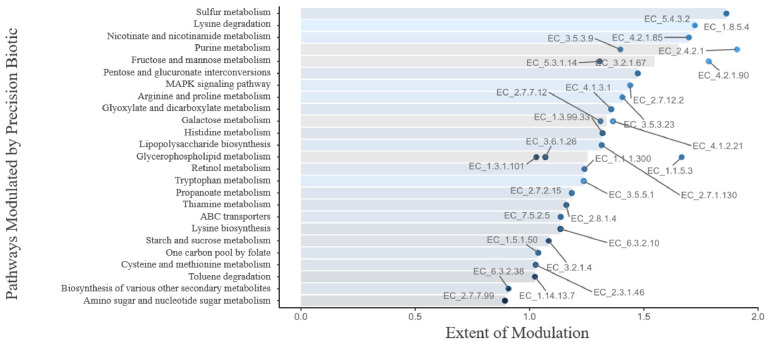
The functional changes in the cecal microbiome of broiler chickens are represented in Figure 1, Figure 2. The LFDA of functional profiles (Figure 1) shows a clear and significant difference in the cecal microbiome metabolism profile between control vs. PB supplemented birds. The abundance of pathways modulated by PB involves those associated with amino acid fermentation and putrefaction, particularly from lysine, arginine, proline, histidine, and tryptophane (Figure 2). Other pathways of importance related to purine, vitamins, carbohydrates, and ABC transporters were also modulated by the supplementation of PB. The enzyme numbers shown in Figure 2 represent the enzyme that was most responsible to the change within that specific pathway.
Figure 1.
Local Fisher discriminant analysis (LFDA) of functional profiles demonstrating distinct clusters for control and precision biotic fed birds (n = 40 samples/treatment group).
Figure 2.
KEGG pathway importance in the microbiome of broiler chickens supplemented with precision biotic relative to the microbiome of chickens fed the control diet (set as 0). Enzymes (labeled points) were mapped back to KEGG pathways (horizontal bars; n = 40 samples/treatment group).
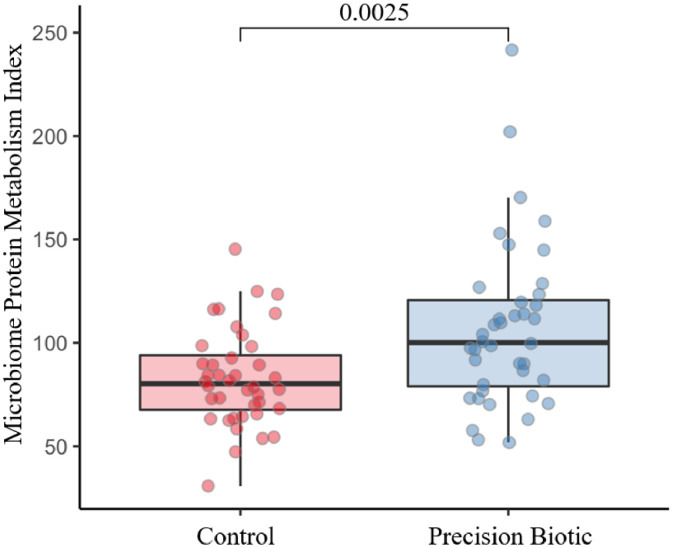
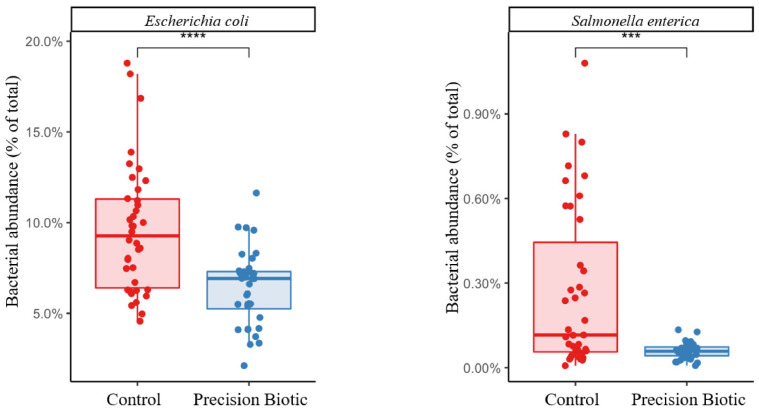
The MPMI was calculated for each bird/sample and values were plotted to compare between control vs. PB supplemented birds (Figure 3). It was observed that the supplementation of PB significantly increased (P = 0.0025) the MPMI showing that there was an enrichment in the abundance of beneficial and reduction of putrefactive pathways that led to a higher MPMI in supplemented birds. Lastly, regarding the abundance of pathogens (Escherichia coli and Salmonella enterica), it was observed that the supplementation of PB significantly reduced their relative abundances in the cecal microbiome (Figure 4).
Figure 3.
Microbiome Protein Metabolic Index (MPMI) of the cecal microbiome of broiler chickens supplemented or not with precision biotic (PB). The index is increased with the supplementation of PB (n = 50; P = 0.0025; n = 40 samples/treatment group).
Figure 4.
Relative abundance (% of total) of Escherichia coli and Salmonella enterica in the cecal microbiome of broiler chickens supplemented or not with precision biotic (PB; P < 0.01; n = 40 samples/treatment group).
DISCUSSION
The PB used in the present study has been previously shown, in experimental research settings, to modulate the pathways of the cecal microbiome (Walsh et al., 2021; Bortoluzzi et al., 2023), resulting in improved growth performance and welfare (Jacquier et al., 2022) and increased resilience of the chickens against enteric stress (Blokker et al., 2022). This is the first study, however, demonstrating that the outputs obtained in research trials with the PB supplementation in broiler chickens were, indeed, translated into commercial conditions. The magnitude of this study shows its paramount importance wherein 190,000 broilers were used. Additionally, the number of samples used for sequencing of the cecal microbiome highlights the robustness and representativeness of the results.
In a previous meta-analysis by Walsh et al. (2021) it has been reported how the PB used herein consistently improved the growth performance of broiler chickens. Also, Jacquier et al. (2022) demonstrated that this PB not only improved the growth performance of chickens, but also had positive effects on the litter quality, which translated into enhanced gait score. In the present study, it was observed that the supplementation of PB significantly increased the BW of the birds with the cFCR also being improved by 2.2 points at 42 d. It its known that when chickens are supplemented with AGPs or its alternatives, the absence of sanitary challenge (i.e., intestinal stress and challenge created by experimental models or natural infections) may negatively impact the outcomes and the tested products may not be as efficacious as they would be during increased sanitary pressure (Adedokun and Olojede, 2019). Even though the current study was carried out under commercial conditions, the overall growth performance of the control group was satisfactory (final cFCR of 1.471) suggesting a good overall health of the birds. However, the cFCR improved by 2.2 extra points with the PB, which shows that by harnessing the full potential of the intestinal microbiome, precision nutritional ingredients may improve the performance of the chickens to reach or to go beyond their genetic potential.
The precise modulation of microbiome metabolic functions by PB has the advantage of promoting changes beyond the taxonomic composition of the microbial communities in the GIT since it was selected to target specific core microbiome pathways. The pivotal observation made by the Human Microbiome Project Consortium (2012) that the microbiome pathway profile is stable among microorganisms despite their taxonomic variation, endorses that assumption. It has already been documented the presence of common core enzyme related to amino acid, energy, and nucleotide metabolism in a broadly diverse range of organisms with varying taxonomic composition (Jiang et al., 2016). The development of PB can target many different outcomes where the microbiome of the animals exerts a function, which includes but is not limited to digestion, absorption, immune homeostasis, pathogen barrier, fluid absorption, gut-brain axis control, and environmental emissions. It was demonstrated herein that the inclusion of PB to diets of broiler chickens reduced the load of some pathogens, that is, E. coli and S. enterica, but more importantly, it clearly increased the abundance of beneficial protein metabolism pathways in the microbiome, leading to higher MPMI, as also previously demonstrated by Bortoluzzi et al. (2023).
It has been reported that glycans are transported by ABC transporters, transmembrane or membrane-associated proteins, and degraded by hydrolases to be up taken by bacteria (Koropatkin et al., 2012). Also, as suggested by Hao et al. (2021), glycans are transported into the periplasmic space, and hydrolyzed into simple sugars to signal HTCS-like regulator to induce expression of polysaccharide utilization genes. The beneficial modulation of the microbiome pathways will benefit the host and increase the resilience against enteric challenges, especially in broiler chickens raised under commercial conditions. It has been demonstrated that the PB tested herein ameliorated the negative impact of coccidiosis in chickens by improving the intestinal morphology and modulating the expression of immune, SCFA transporters, and cell-cycling related genes (Blokker et al., 2022). Furthermore, it enhanced the growth performance and intestinal mucosa health of chickens to the same extent as an AGP (Blokker et al., 2022).
There are many other aspects that still need to be evaluated, such as the impact of PB depending on the diet type, vaccination programs, interactions with other additives (feed enzymes and probiotics, for instance), to fully understand the mechanism of action of PB. However, we have confirmed that the PB can precisely shift microbiome functions and significantly increase the productivity and health of broiler flocks. In conclusion, the results presented herein prove that the PB can efficiently harness the full potential of the intestinal microbiome by modulating protein metabolism and utilization with beneficial effects on the growth performance as noted with improved BW and cFCR.
ACKNOWLEDGMENTS
This study was supported by the collaborative project between DSM Nutritional Products to evaluate the efficacy of eubiotics on white feather broilers and Qingdao Technology Huimin demonstration project (21-1-4-ny-16-nsh).
DISCLOSURES
T. C., Q. Z., B. B., J. G., and C. B. are employed by DSM Nutritional Products. L. Y. and Z. L. are employed by Shangdong New Hope Liuhe Group and declare no conflict of interest. The studies were funded by DSM Nutritional Products, and supported by the Qingdao Technology Huimin demonstration project.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102596.
Appendix. Supplementary materials
REFERENCES
- Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. Sci. 2019;5:348. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016;221:323–330. [Google Scholar]
- Blokker B., Bortoluzzi C., Iaconis C., Perez-Calvo E., Walsh M.C., Schyns G., Tamburini I., Geremia J.M. Evaluation of a novel precision biotic on enterohepatic health markers and growth performance of broiler chickens under enteric challenge. Animals. 2022;12:2502. doi: 10.3390/ani12192502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein S.R., Theis K.R. Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLOS Biol. 2015;13 doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Tamburini I., Geremia J. Microbiome modulation, microbiome protein metabolism index, and growth performance of broilers supplemented with a precision biotic. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAS . Standards of China Association for Standardization; Beijing, China: 2017. Farm Animal Welfare Requirements: Meat-Type Chicken. T/CAS 267-2017. [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning L., Stewart R.D., Pallen M.J., Watson K.A., Watson M. Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biol. 2020;21:34. doi: 10.1186/s13059-020-1947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z., Wang X., Yang H., Tu T., Zhang J., Luo H., Huang H., Su X. PUL-mediated plant cell wall polysaccharide utilization in the gut bacteroidetes. Int. J. Mol. Sci. 2021;22:3077. doi: 10.3390/ijms22063077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier V., Walsh M.C., Schyns G., Claypool J., Blokker B., Bortoluzzi C., Geremia J. Evaluation of a precision biotic on the growth performance, welfare indicators, ammonia output, and litter quality of broiler chickens. Anim. Open Access J. MDPI. 2022;12:231. doi: 10.3390/ani12030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xiong X., Danska J., Parkinson J. Metatranscriptomic analysis of diverse microbial communities reveals core metabolic pathways and microbiome-specific functionality. Microbiome. 2016;4:2. doi: 10.1186/s40168-015-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Proctor L.M., Creasy H.H., Fettweis J.M., Lloyd-Price J., Mahurkar A., Zhou W., Buck G.A., Snyder M.P., Strauss J.F., Weinstock G.M., White O., Huttenhower C., The Integrative HMP (iHMP) Research Network Consortium The integrative human microbiome project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Hou L., Yang Y. The development of the gut microbiota and short-chain fatty acids of layer chickens in different growth periods. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.666535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.C., Jacquier V., Schyns G., Claypool J., Tamburini I., Blokker B., Geremia J.M. A novel microbiome metabolic modulator improves the growth performance of broiler chickens in multiple trials and modulates targeted energy and amino acid metabolic pathways in the cecal metagenome. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.