Summary
Damage to the atrioventricular conduction system (AVCS), the main electrical connection between the atrial and ventricular chambers, can result in a variety of cardiac conduction disorders. Here, we provide a protocol for selective damage of the mouse AVCS to study its response during injury. We describe tamoxifen-induced cellular ablation, detection of AV block through electrocardiography, and quantification of histological and immunofluorescence markers to analyze the AVCS. This protocol can be used to study mechanisms associated with AVCS injury repair and regeneration.
For complete details on the use and execution of this protocol, please refer to Wang et al. (2021).1
Subject areas: Cell Biology, Cell Differentiation, Health Sciences, Microscopy, Model Organisms
Graphical abstract
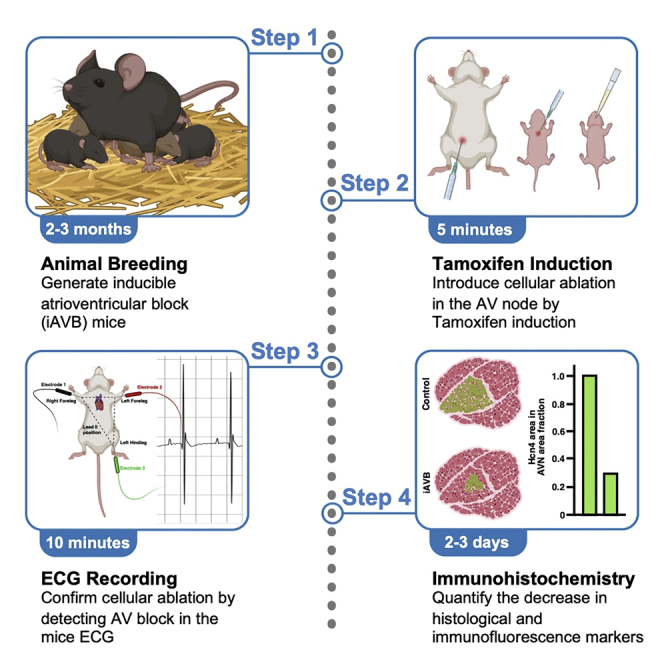
Highlights
-
•
Reproducible ablation injury model of the mouse atrioventricular conduction system
-
•
Different routes of tamoxifen induction among neonatal and adult mice
-
•
Simplified instructions on neonatal and adult mice electrocardiography
-
•
Detailed walkthrough of immunohistochemical techniques for AVCS tissue
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Damage to the atrioventricular conduction system (AVCS), the main electrical connection between the atrial and ventricular chambers, can result in a variety of cardiac conduction disorders. Here, we provide a protocol for selective damage of the mouse AVCS to study its response during injury. We describe tamoxifen-induced cellular ablation, detection of AV block through electrocardiography, and quantification of histological and immunofluorescence markers to analyze the AVCS. This protocol can be used to study mechanisms associated with AVCS injury repair and regeneration.
Before you begin
Institutional permissions
All animal experiments were performed in an American Association for the Accreditation of Laboratory Animal Care-accredited and specific pathogen-free animal facility at the University of Texas Southwestern Medical Center. All mice were bred, maintained, and housed in accordance with procedures outlined in the Guide for the Care and Use of Laboratory Animals under a study proposal (protocol no. 2017-102162) approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center.
Animal breeding
Timing: 2–3 months
-
1.
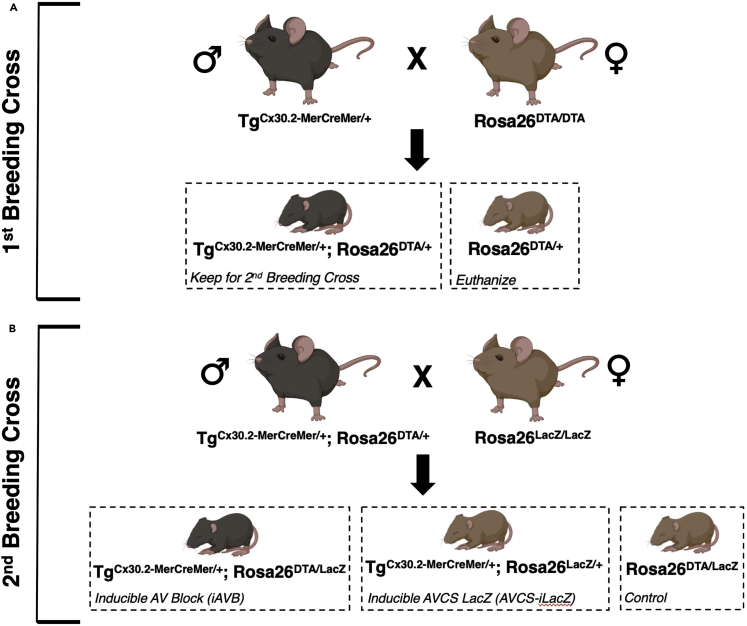
Prepare mutant mice carrying the Connexin 30.2 (Cx30.2)-MerCreMer transgene (TgCx30.2-MerCreMer/+) and breed with Diphtheria Toxin A (DTA) reporter mice (Rosa26DTA/DTA) to generate TgCx30.2-MerCreMer/+; Rosa26DTA/+ mice (Figure 1A).
Note: Male TgCx30.2-MerCreMer/+ mice and female reporter mice (i.e., Rosa26DTA/DTA & Rosa26LacZ/LacZ) should be used in the breeding crosses.
-
2.
Breed TgCx30.2-MerCreMer/+; Rosa26DTA/+ mice with LacZ reporter mice (Rosa26LacZ/LacZ) to generate TgCx30.2-MerCreMer/+; Rosa26DTA/LacZ (i.e., inducible AV block or iAVB), TgCx30.2-MerCreMer/+; Rosa26LacZ/+ (i.e., inducible AVCS LacZ or AVCS-iLacZ), and Rosa26DTA/LacZ (i.e., control) mice (Figure 1B).
Note: 6- to 8-week-old mice should be used in the breeding crosses.
-
3.
Check for the presence of LacZ, DTA, and Cre recombinase genes by genotyping – use the polymerase chain reaction (PCR) protocol provided in (Tables 1, 2, 3) and the primers listed in the key resources table.
Figure 1.
Animal breeding schematic to generate the inducible AV block (iAVB) mouse model and appropriate mouse controls
(A) First breeding cross.
(B) Second breeding cross.
Table 1.
PCR cycling conditions for Cx30.2-MerCreMer
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 93°C | 1 min | 1 |
| Denaturation | 93°C | 20 s | 29 cycles |
| Annealing | 68°C | 3 min | |
| Hold | 4°C | ∞ | |
Table 2.
PCR cycling conditions for DTA and LacZ
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 95°C | 3 min | 1 |
| Denaturation | 94°C | 30 s | 35 cycles |
| Annealing | 65°C | 1 min | |
| Extension | 72°C | 1 min | |
| Final extension | 72°C | 2 min | 1 |
| Hold | 4°C | ∞ | |
Table 3.
PCR reaction master mix
| Reagent | Amount (10 μL) |
|---|---|
| DNA template | 1.0 μL |
| Apex Taq Master Mix 2X | 5.0 μL |
| Primer 1 | 0.4 μL |
| Primer 2 | 0.4 μL |
| ddH2O | 3.2 μL |
Set up electrocardiography (ECG) and anesthesia machine
Timing: 5 min
-
4.
Turn on Powerlab 16/30 (ADInstruments, CO, USA) connected to a Bio Amp (Figure 2).
-
5.
Respectively connect the anesthesia induction chamber and rodent facemask with F/AIR Canister (Bickford, NY, USA).
Note: Weigh the F/AIR before and after experiment.
-
6.
Open oxygen tank.
-
7.
Open Sigma Delta Vaporizer (Penlon, UK) by turning the knob for oxygen flow to 1.5–2 L per minute (lpm) and isoflurane to 2%. Direct the oxygen and isoflurane to the induction chamber.
Note: Make sure there is enough volume of isoflurane (i.e., between the minimum and maximum mark) in the vaporizer.
-
8.
Open laptop and LabChart 8 (ADInstruments, CO, USA).
-
9.
Go to Channel Settings (under Setup) and change the following: Range = 10 mV, Input Amplifier = Bio Amp, Units = mV, and Calculation = Smoothing.
Figure 2.
Components of ECG and anesthesia equipment
(1) Bio Amp, (2) PowerLab 16/30, (3) Laptop w/ LabChart8, (4) Mouse pad, (5) ECG needle electrodes, (6) Alcohol pad, (7) Rodent facemask, (8) Anesthesia induction chamber, (9) Sigma Delta Vaporizer, (10) F/AIR Canister, (11) Oxygen tank.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Polyclonal anti-HCN4 guinea pig antibody (1:200 dilution) | Alomone Labs | Cat#AGP-004 |
| Polyclonal anti-Nppa rabbit antibody (1:200 dilution) | Abgent | Cat#AP8534a |
| Alexa Fluor 488 polyclonal goat anti-guinea pig IgG (H+L) (1:400 dilution) | Invitrogen | Cat#A11073 |
| Alexa Fluor 555 polyclonal goat anti-rabbit IgG (H+L) (1:400 dilution) | Invitrogen | Cat#A21422 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
| Ethanol | Pharmco | Cat#111000200 |
| Sesame oil | Thermo Fisher Scientific | Cat#241002500 |
| Atropine | Sigma-Aldrich | Cat#A0132 |
| Isoproterenol | Sigma-Aldrich | Cat#I5752 |
| (Para-)Formaldehyde solution 4% | Thermo Fisher Scientific | Cat#J19943.K2 |
| Glutaraldehyde 25% | Thermo Fisher Scientific | Cat#A17876.0F |
| X-gal | Thermo Fisher Scientific | Cat#B1690 |
| Paraffin | Thermo Fisher Scientific | Cat#171400025 |
| Tissue Freezing Medium | Electron Microscopy Sciences | Cat#72592 |
| Universal blocking buffer 10× | Biogenex Laboratories | Cat#HK0855K |
| Phosphate-buffered saline (PBS) 1× | Thermo Fisher Scientific | Cat#20012027 |
| Deoxycholic acid | MilliporeSigma | Cat#D2510 |
| NP-40 detergent solution | Thermo Fisher Scientific | Cat#85124 |
| Potassium hexacyanoferrate(III) (K3Fe(CN)6) | MilliporeSigma | Cat#60299 |
| Potassium hexacyanoferrate(II) (K4Fe(CN)6) | MilliporeSigma | Cat#60279 |
| Acetylthiocholine iodide | MilliporeSigma | Cat#A5751 |
| Sodium acetate | MilliporeSigma | Cat#241245 |
| Acetic acid | MilliporeSigma | Cat#W200611 |
| Sodium citrate | MilliporeSigma | Cat#PHR1416 |
| Cupric sulfate (CuSO4) | MilliporeSigma | Cat#931071 |
| Tetraisopropyl pyrophosphoramide (iso-OMPA) | Santa Cruz Biotechnology | Cat#sc-215956 |
| Cedarwood oil | Spectrum | Cat#CE107 |
| Eosin B | MilliporeSigma | Cat#45260 |
| Xylene | MilliporeSigma | Cat#214736 |
| Nuclear fast red | MilliporeSigma | Cat#N3020 |
| Harris hematoxylin | MilliporeSigma | Cat#HHS32 |
| Triton X-100 | MilliporeSigma | Cat#X100 |
| VECTASHIELD® Antifade Mounting Medium w/ DAPI | Vector Laboratories | Cat#H-1200-10 |
| Isoflurane | Thermo Fisher Scientific | Cat#AAAL17315-14 |
| Apex Taq Master Mix 2X | Genesee Scientific, Identifier | Cat#42-138 |
| Critical commercial assays | ||
| Trichrome One-Step Blue & Red Stain Kit (includes: Bouin’s Fluid, Modified Mayer’s Hematoxylin, One-Step Trichrome Stain Blue/Red) | StatLab | Cat#KTTRBPT |
| Experimental models: Organisms/strains | ||
| Mouse: Tg(Cx30.2-MerCreMer); 6–8-week-old; male | Wang et al.1 | N/A |
| Mouse: B6;129S4-Gt(ROSA)26Sortm1Sor/J (LacZ reporter mice); 6–8-week-old; female |
The Jackson Laboratory | Stock no. 003309; RRID: IMSR_JAX:003309 |
| Mouse: B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J (DTA reporter mice); 6–8-week-old; female |
The Jackson Laboratory | Stock no. 009669; RRID: IMSR_JAX:009669 |
| Oligonucleotides | ||
| Primers: Tg(Cx30.2-MerCreMer) Forward: | Wang et al.1 | 5′-GCATTACCGGTCGATGCAACGAGTGATGAG-3′ |
| Primers: Tg(Cx30.2-MerCreMer) Reverse: | Wang et al.1 | 5′- GAGTGAACGAACCTGGTCGAAATCAGTGCG -3′ |
| Primers: Rosa26-LacZ Forward: | Integrated DNA Technologies (IDT) | 5′-AAAGTCGCTCTGAGTTGTTAT-3′ |
| Primers: Rosa26-LacZ Reverse: | Integrated DNA Technologies (IDT) | 5′-GCGAAGAGTTTGTCCTCAACC-3′ |
| Primers: Rosa26-DTA Forward: | Integrated DNA Technologies (IDT) | 5′-CGACCTGCAGGTCCTCG-3′ |
| Primers: Rosa26-DTA Reverse: | Integrated DNA Technologies (IDT) | 5-CTCGAGTTTGTCCAATTATGTCAC-3′ |
| Software and algorithms | ||
| LabChart8 | ADInstruments | https://www.adinstruments.com/support/downloads/windows/labchart |
| Others | ||
| PowerLab 16/30 | ADInstruments | https://www.adinstruments.com/products/powerlab-daq-hardware |
| Bio Amp | ADInstruments | https://www.adinstruments.com/research/animal/autonomic/ecg |
| Anesthesia induction chamber | Harvard Apparatus | https://www.harvardapparatus.com/induction-chambers.html |
| F/AIR Canister | Vetamac, Inc. | Cat#80120 |
| Sigma Delta vaporizer | Penlon Limited | https://www.penlon.com/Product-Groups/Vaporizer/Sigma-Delta |
| Confocal laser-scanning microscope | Nikon Instruments, Inc. | https://www.microscope.healthcare.nikon.com/products/confocal-microscopes/a1hd25-a1rhd25 |
| Mouse pad | E-Z Systems | https://www.ezsystemsinc.com/product/hb-103-bed-breather/ |
| mouse pad alternative | Styrofoam board wrapped in surgical drape | N/A |
| BD Insulin Syringe | ADW Diabetes | Cat#SY8290328291 |
| CM1850 Cryostat | Leica Biosystems | https://www2.leicabiosystems.com |
| ECG needle electrodes | ADInstruments | https://www.adinstruments.com/products/needle-electrodes |
| Alcohol pad | Med Lab Supply | https://www.medical-and-lab-supplies.com/syringes-and-needles/alcohol-prep-pads.html |
| Rodent facemask | E-Z Systems | https://www.ezsystemsinc.com/product/hb-103-bed-breather/ |
| Rodent facemask alternative | Nitrile glove cut-out | N/A |
| Oxygen tank | Parkland Scientific, Inc. | Cat#PX-8703-1T |
Materials and equipment
Tamoxifen Solution (100 μg/μL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Tamoxifen | 100 μg/μL | 100 mg |
| Ethanol, 100% | 9:1 sesame oil-ethanol | 100 μL |
| Sesame Oil | 900 μL | |
| Total | – | 1,000 μL |
Store at –20°C for 6 months.
Note: If Tamoxifen is not dissolving in 37°C water bath, the tube can be placed in a 42°C or 67°C water bath.
Atropine solution (1 mg/mL)
-
•
Dissolve 50 mg of Atropine in 1,000 μL of 1× PBS to make 50 mg/mL Atropine stock solution. In a 15-mL tube, mix 100 μL of 50 mg/mL Atropine and 4,900 μL of 1× PBS.
50 mg/mL Atropine can be stored at –20°C for 1 year.
Isoproterenol solution (10 μg/mL)
-
•
Dissolve 10 mg of Isoproterenol in 1,000 μL of 1× PBS to make 10 mg/mL Isoproterenol stock solution. In a 15-mL tube, mix 5 μL of 10 mg/mL Isoproterenol and 4,995 μL of 1× PBS.
10 mg/mL Isoproterenol can be stored as stock solution at –20°C for 1 year.
Washing buffer for X-Gal staining
| Reagent | Final concentration | Amount |
|---|---|---|
| Deoxycholic acid, 10% | 0.01% | 50 μL |
| NP-40, 10% | 0.02% | 100 μL |
| 1× PBS | – | 49.85 mL |
| Total | – | 50 mL |
Store at 4°C for 6 months.
X-Gal staining solution
| Reagent | Final concentration | Amount |
|---|---|---|
| MgCl2, 100 mM | 2.0 mM | 1.0 mL |
| K3Fe(CN)6, 100 mM | 5.0 mM | 2.5 mL |
| K4Fe(CN)6, 100 mM | 5.0 mM | 2.5 mL |
| Deoxycholic acid, 10% | 0.01% | 50 μL |
| NP-40, 10% | 0.02% | 100 μL |
| X-Gal, 40 mg/mL | 1 mg/mL | 1.25 mL |
| 1× PBS | – | 42.6 mL |
| Total | – | 50 mL |
Note: Always use freshly prepared X-gal staining solution. Wrap in foil to avoid light exposure.
Acetylthiocholine staining buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Acetylthiocholine iodide | 1.7 mM | 0.58 mg |
| Sodium acetate, 0.06 N | 0.2 mM | 633 μL |
| Acetic acid, 0.1 N | 0.2 mM | 20 μL |
| Sodium citrate, 0.1 M | 4.8 mM | 48 μL |
| Cupric sulfate, 30 mM | 3.0 mM | 100 μL |
| iso-OMPA, 4 mM | 0.08 mM | 20 μL |
| K3Fe(CN)6, 0.5 M | 0.5 mM | 1 μL |
| Total | – | 1,000 μL |
Note: Always use freshly prepared acetylthiocholine staining buffer.
Preparation of primary antibody
| Reagent | Final concentration | Amount |
|---|---|---|
| Anti-HCN4 guinea pig antibody (1:200) | 1:200 | 2 μL |
| Anti-Nppa rabbit antibody (1:200) | 1:200 | 2 μL |
| 1× Universal blocking buffer | – | 396 μL |
| Total | – | 400 μL |
CRITICAL: If a sample requires staining with multiple antibodies targeting different proteins, it is essential to use antibodies raised in different hosts.
Preparation of secondary antibody
| Reagent | Final concentration | Amount |
|---|---|---|
| Alexa Fluor 488 goat anti-guinea pig IgG (H+L) antibody (1:400) | 1:400 | 1 μL |
| Alexa Fluor 555 goat anti-rabbit IgG (H+L) antibody (1:400) | 1:400 | 1 μL |
| 1× Universal blocking buffer | – | 398 μL |
| Total | – | 400 μL |
Note: Multiple secondary antibodies from different hosts could be added. In that case, the amount of universal blocking buffer is adjusted according to the amount of secondary antibody added.
Step-by-step method details
Induction of AVCS cellular ablation by Tamoxifen
Timing: 1 min/adult mouse, 5 min/neonatal mouse
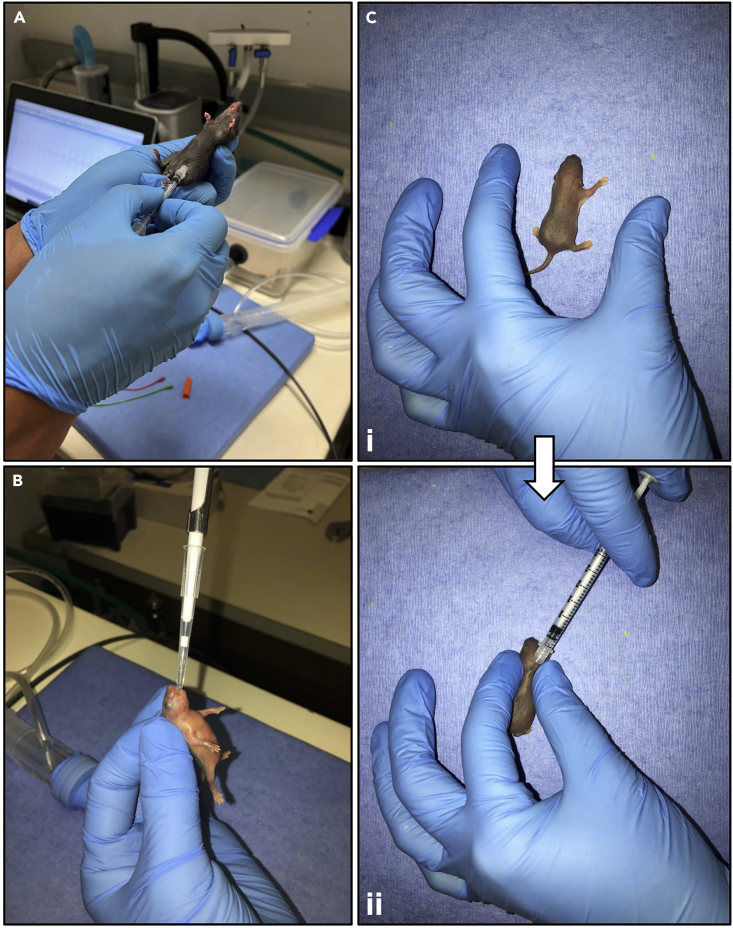
In this step, we describe the intraperitoneal (i.p.), per orem (p.o.), and subcutaneous (s.c.) administration of Tamoxifen on adult (P42) and neonatal (P0) mice. Unlike adult mice given Tamoxifen for 7 days, we decided to do a single-day administration of Tamoxifen on neonatal mice to minimize maternal cannibalization.
-
1.Intraperitoneal (i.p.) administration of Tamoxifen on adult mice (Figure 3A).
-
a.On day 0, place adult (P42) mouse on metal grid and grab loose skin at the back of the neck between your thumb and forefinger.
-
b.Hold tail with little and ring finger.
-
c.Perform injection of a single dose of 1 mg Tamoxifen.
-
i.Aim for the lower right abdomen with the needle directed towards the thorax in a 30°–40° angle to minimize risk of puncturing intestinal organs.
-
ii.Perforate skin and slowly aspirate.
-
iii.If there is no aspirated liquid (i.e., blood or green liquid), administer Tamoxifen solution.Note: Insulin syringe is preferred (e.g., BD Insulin Syringe, NK, USA), as these diameters are less likely to perforate intestinal organs and hurt the mouse, compared to larger needles.
-
i.
-
d.Repeat steps a.-b. for day 1–7 of Tamoxifen induction on adult mice.
-
a.
-
2.Per orem (p.o.) administration of Tamoxifen on neonatal mice (Figure 3B).
-
a.On day 0, collect entire litter of neonatal (P0) mice and place in a separate cage.
-
b.Gently grab a neonatal mouse at the back of the neck and visualize the animal’s mouth.
-
c.Using a pipettor, get 8–10 μL of Tamoxifen equivalent to a single 0.8–1 mg dose.
-
d.Position the pipette tip in the animal’s mouth and slowly administer the Tamoxifen solution.Note: Give the neonatal mouse time to swallow the solution and hold the animal upright to ensure ingestion of the Tamoxifen.
-
e.Put the neonatal mouse in an extra cage to allow recovery until other neonatal mice are given Tamoxifen.
-
f.Relocate all neonatal mice (i.e., entire litter) to their original cage after induction.
-
a.
-
3.Subcutaneous (s.c.) administration of Tamoxifen on neonatal mice (Figure 3C).
-
a.On day 0, collect entire litter of neonatal (P0) mice and place in a separate cage.
-
b.Gently put your thumb and forefinger to the middle of the thoracic spine to immobilize the animal.
-
c.Perform injection of a single dose of 1 mg Tamoxifen.
-
i.Aim for the skin at the back of the neck with the needle directed towards the tail in a 20°–30° angle.
-
ii.Perforate skin and slowly administer Tamoxifen solution.
-
i.
-
d.Put the neonatal mouse in an extra cage to allow recovery until other neonatal mice are given Tamoxifen.
-
e.Relocate all mice to their original cage after induction.
-
a.
Figure 3.
Administration of Tamoxifen solution to adult and neonatal mice
(A) Intraperitoneal (i.p.) administration on adult mice.
(B) Per orem (p.o.) administration on neonatal mice.
(C) Subcutaneous (s.c.) administration on neonatal mice.
ECG recording of adult and neonatal mice
Timing: 10 min/mouse
In this step, we show how to perform electrocardiography (ECG) on adult and neonatal mice to check for the effect of AVCS cellular ablation after Tamoxifen induction.
-
4.Anesthetize mice for ECG with 2% isoflurane in 2.0 L/min O2.
-
a.Adult anesthesia:
-
i.Place adult mouse in anesthesia chamber.
-
ii.Once anesthetized, place the animal at the mouse pad and hook to rodent facemask.
-
i.
-
b.Neonatal anesthesia:
-
i.Place neonatal mouse in the mouse pad.
-
ii.Hook animal directly to rodent facemask.
-
i.
-
a.
Note: Assess depth of anesthesia by checking loss of righting reflex or response to pain stimulation (e.g., toe pinch).
-
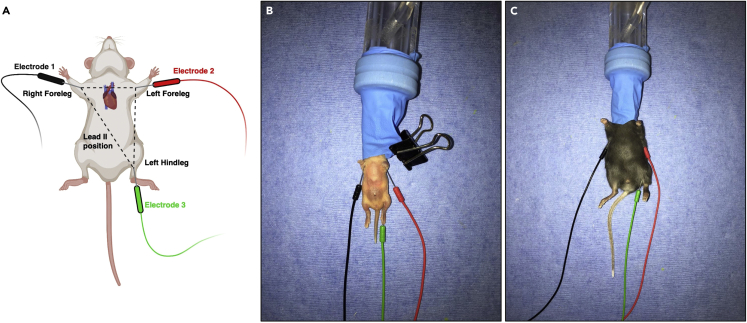
5.Place subcutaneous ECG electrodes in the conventional lead II position (Figure 4).
-
a.Place positive (red) electrode at the left forelimb.
-
b.Place negative (black) electrode at the right forelimb.
-
c.Place reference (green) electrode at the left hindlimb.
-
a.
Note: Clean mouse limbs and ECG electrodes with alcohol pads before inserting them into the mouse.
-
6.
Once ECG electrodes are in place, click start at the LabChart 8 program to record the animal’s ECG.
-
7.
Record ECG for at least one to one-and-a-half minutes.
-
8.
Remove ECG electrodes once recording is finished and return the animal to its cage.
-
9.
Repeat on subsequent days as necessary.
Note: Minimize the influence of isoflurane on the heart rate by doing the following: (1) Minimize isoflurane induction time (neonatal mice induction time is longer compared to adult mice); (2) Calculate ECG measurements (i.e., PR interval, QRS interval, etc.) on the first 30 s of an ECG tracing.
Optional: Since mice PR interval shortens as they grow, perform ECG on normal mice of different ages (e.g., P7, P14, p21, p28) to determine normal PR interval values for each age group. Normal PR interval range was set as Mean ± 2 Standard Deviation in Wang et al.1 (Table 4).
Figure 4.
Electrocardiography (ECG) conventional lead II placement in neonatal and adult mice
(A) Schematic of conventional lead II placement of ECG electrodes.
(B) Actual conventional lead II placement of ECG electrodes in neonatal mouse.
(C) Actual conventional lead II placement of ECG electrodes in adult mouse.
Table 4.
Normal range of PR intervals across different mice ages with 2% isoflurane/200 mL/min O2
| P7 (n = 27) | P14 (n = 17) | P21 (n = 16) | P28 (n = 35) | |
|---|---|---|---|---|
| Average PR interval (ms) | 51 | 39 | 38 | 37 |
| Mean ± 2SD (ms) | 38–63 | 30–48 | 28–47 | 39–44 |
| Number of mice | 27 | 17 | 22 | 35 |
Pharmacological challenge post-Tamoxifen induction
Timing: 10 min/mouse
In this step, we describe how to perform pharmacological studies using Atropine and Isoproterenol injection to further check the effect of AVCS cellular ablation after Tamoxifen induction.
-
10.
Freshly prepare pharmacological agents, i.e., Atropine and Isoproterenol.
-
11.
After recording ECG for 1 min, perform IP injection of pharmacological agents at a dose of 2 μg/g or 50 ng/g of Atropine or Isoproterenol, respectively.
-
12.
Continue recording the animal’s ECG for 5 min following drug administration.
Mice preparation for heart removal
Timing: 30 min
In the following steps, we show how to collect and process mice hearts for immunohistological staining and analysis.
-
13.
Place mice in an empty cage and slowly fill with CO2 at 3L/min.
-
14.Wait until euthanasia is complete (i.e., no more breathing can be observed).
-
a.Perform cervical dislocation as secondary method of euthanasia.
-
a.
-
15.
Place euthanized mice in a clean dissection pad.
-
16.
Cut the chest open and expose the chest cavity.
-
17.
Cut the major blood vessels connecting the heart to the cardiac base.
-
18.
Place harvested heart in cold 1× PBS.
-
19.
Gently pump the harvested heart with forceps to remove excess blood.
Preparation and X-Gal staining of mouse heart for paraffin block and sliced sections
Timing: 2–3 days
-
20.
Fix the harvested heart in 0.2% glutaraldehyde for one (1) hour at 4°C.
-
21.
Wash with washing buffer for 10 min twice.
-
22.
Incubate and shake the harvested heart in X-gal staining buffer at room temperature (20°C–22°C) overnight (12–16 h), covered with foil.
-
23.
Post-fix the harvested heart in neutral formalin overnight (12–16 h) for paraffin embedding.
CRITICAL: (Para-)Formaldehyde solution is a harmful material for humans. Handling (para-)formaldehyde solution should be done inside a chemical fume hood and wearing protective gear such as glasses, lab coat, and gloves.
-
24.
Place the harvested heart in a mold in the appropriate orientation.
-
25.
Embed harvested heart in paraffin.
Pause point: Paraffin blocks can be stored at room temperature (20°C–22°C) for years.
-
26.
Section the mouse heart paraffin block in a posterior to anterior (PA) direction.
-
27.
Collect sections where mitral valve and tricuspid valve are present.
Preparation of mouse heart for frozen block and sliced sections
Timing: 1–2 days
-
28.
Fix the harvested heart in 4% paraformaldehyde (PFA) for one (1) hour at 4°C.
-
29.
Sequentially incubate the harvested heart in 10% and 20% sucrose/PBS at 4°C for 12–24 h.
-
30.
Fill a cryomold with Tissue Freezing Medium (TFM).
-
31.
Place the harvested heart in the TFM-filled cryomold.
-
32.
Orient the harvested heart into the four-chamber orientation.
-
33.
Snap-freeze the harvested heart with TFM using dry ice or liquid nitrogen.
-
34.
Store mouse heart frozen block at –80°C prior to sectioning.
-
35.
Section the mouse heart frozen block into 8-micron slices in a posterior to anterior (PA) direction, using a Leica CM3050S cryostat.
-
36.
Collect sections where septal leaflet of the tricuspid valve is present.
Pause point: Frozen sections can be stored at –80°C for years.
Histological staining protocol
Timing: 24–48 h
-
37.For paraffin sections:
-
a.Wash paraffin sections with 100% Cedarwood Oil (CWO) for 5 min three times to deparaffinize sections.
-
b.Wash sections with 1:1 CWO and 100% ethanol for 2 min.
-
c.Rinse sections three times with 100% ethanol for 5 min.
-
d.Rinse sections three times with running water for 5 min.
-
a.
-
38.For cryosections:
-
a.Air-dry tissue sections for 15–20 min at room temperature (20°C–22°C).
-
b.Wash thoroughly with PBS for 2 min twice.
-
a.
Note: Be careful of washing/rinsing cryosections as they come off the slide easier.
-
39.Hematoxylin and eosin (H&E) staining.
-
a.Incubate sections in Harris hematoxylin for 5 s.
-
b.Wash thoroughly in running water for 5 min.
-
c.Incubate sections in 100% alcohol for 10 s.
-
d.Incubate sections in eosin for 5 s.
-
e.Wash sections in alcohol for 5 s three times, with one final wash in 100% alcohol for 10 s.
-
f.Wash sections in xylene for 10 s, and another two washes in xylene for 5 s each.
-
g.Seal sections with cover slips and mounting medium.
-
a.
-
40.Masson’s Trichrome (MTC) staining.
-
a.Immerse sections in Bouin’s Fluid (preheated to 56°C) for 60 min or overnight (12–16 h) at room temperature (20°C–22°C).Note: All reagents are obtained from Trichrome One-Step Blue & Red Stain kit (#KTTRBPT, American MasterTech Scientific) unless otherwise noted.
-
b.Rinse sections with running water for 3 min or until sections are colorless.
-
c.Immerse sections in Modified Mayer’s Hematoxylin for 7 min.
-
d.Rinse sections with running water for 3 min.
-
e.Immerse sections in One-Step Trichrome Stain (Blue & Red) for 3–8 min.
-
f.Rinse sections with running water for 5 s.
-
g.Rinse sections three times with absolute alcohol for 1 min.
-
h.Rinse sections three times with Xylene for 1 min.
-
i.Seal sections with cover slips and mounting medium.
-
a.
-
41.Nuclear-fast red (NFR) staining.
-
a.Incubate sections in Nuclear Fast Red solution for 5 min.
-
b.Rinse sections with running water for 5 min.
-
c.Wash sections three times with 100% ethanol for 2 min.
-
d.Rinse sections three times with Xylene for 1 min.
-
e.Seal sections with cover slips and mounting medium.
-
a.
-
42.Acetylcholinesterase activity staining.
-
a.Incubate sections in Acetylthiocholine staining buffer at 37°C overnight (12–16 h).
-
b.Rinse section slides with distilled water.
-
c.Counterstain section slides with eosin (steps 39d–g).
-
d.Seal sections with cover slips and mounting medium.
-
a.
Immunostaining protocol for cryosections
Timing: 2–3 days
-
43.
Air-dry fixed tissue sections for 15–20 min at room temperature (20°C–22°C).
-
44.
Wash sections three times with PBS for 5 min.
-
45.
Add 0.3% Triton-X100 in PBS to the fixed sections and allow permeabilization for 20 min at room temperature (20°C–22°C).
-
46.
Wash sections three times with PBS for 5 min.
-
47.
Block fixed sections with Universal Blocking buffer for 10 min at room temperature (20°C–22°C).
-
48.
Dilute primary antibodies in the appropriate amount and apply 30 μL to each section.
-
49.
Incubate overnight (12–16 h) at 4°C.
-
50.
Wash sections three times with PBS for 5 min.
-
51.
Dilute secondary antibodies in the appropriate amount and apply 30 μL to each section.
-
52.
Incubate for 1 h at room temperature (20°C–22°C).
-
53.
Wash sections with PBS.
-
54.
Mount tissue sections with Vectashield Mounting Medium with DAPI.
Expected outcomes
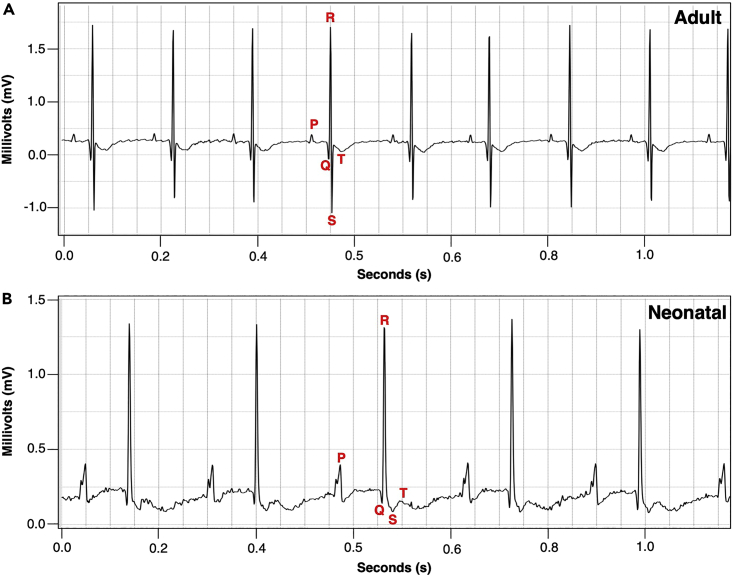
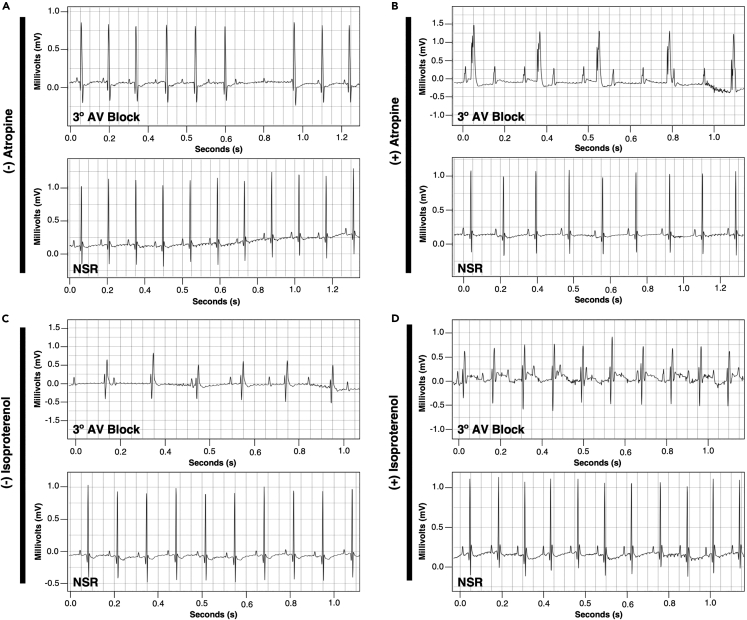
In this protocol, we showed how the previously characterized AVCS-specific Cx30.2 enhancer2 can be utilized to introduce cellular ablation in the mouse AVCS. After crossing TgCx30.2-MerCreMer/+ mouse with DTA and LacZ reporter mice (Rosa26DTA/LacZ) and induction with Tamoxifen, both adult and neonatal iAVB mice (TgCx30.2-MerCreMer/+; Rosa26DTA/LacZ) exhibit atrioventricular (AV) block, as detected by electrocardiography (ECG) (Figure 5). Wang et al.1 presented the classification and descriptions of different degrees of atrioventricular (AV) blocks observed among iAVB mice (Table 5; Figure 6). Pharmacological challenge with Atropine or Isoproterenol can further show the presence of AV block among adult and neonatal iAVB mice (Figure 7).
Figure 5.
Examples of ECG tracing
(A) ECG tracing of an adult mouse.
(B) ECG tracing of a neonatal mouse.
Table 5.
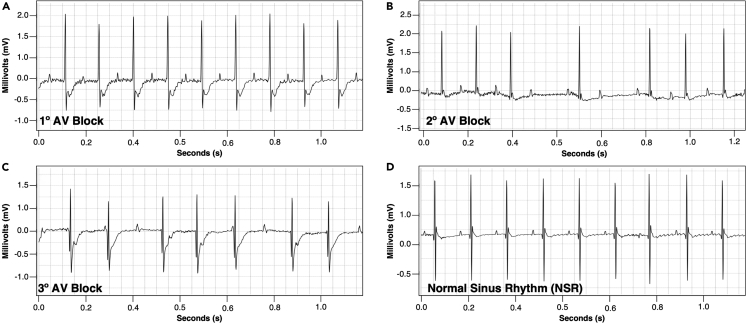
Classification and description of atrioventricular block in mice
| Atrioventricular (AV) block classification | Description | |
|---|---|---|
| 1° Degree AV Block | PR prolongation without dissociation from QRS complex | |
| 2° Degree AV Block | Mobitz Type I | Partial dissociation between P wave and QRS complex as evidenced by intermittent absence of QRS complex following a P wave with gradual PR interval prolongation |
| Mobitz Type II | Partial dissociation between P wave and QRS complex as evidenced by intermittent absence of QRS complex following a P wave with consistent PR interval | |
| 3° Degree AV Block | Complete dissociation between P wave and QRS complex most evidenced by distinct PP and RR intervals | |
Figure 6.
ECG tracings of different degrees of AV block in the murine heart
(A) ECG tracing of 1° AV block.
(B) ECG tracing of 2° AV block.
(C) ECG tracing of 3° AV block.
(D) ECG tracing of a normal sinus rhythm.
Figure 7.
Pharmacological challenge with Atropine and Isoproterenol
(A and B) ECG tracings of iAVB and control mice challenged with Atropine.
(C and D) ECG tracings of iAVB and control mice challenged with Isoproterenol.
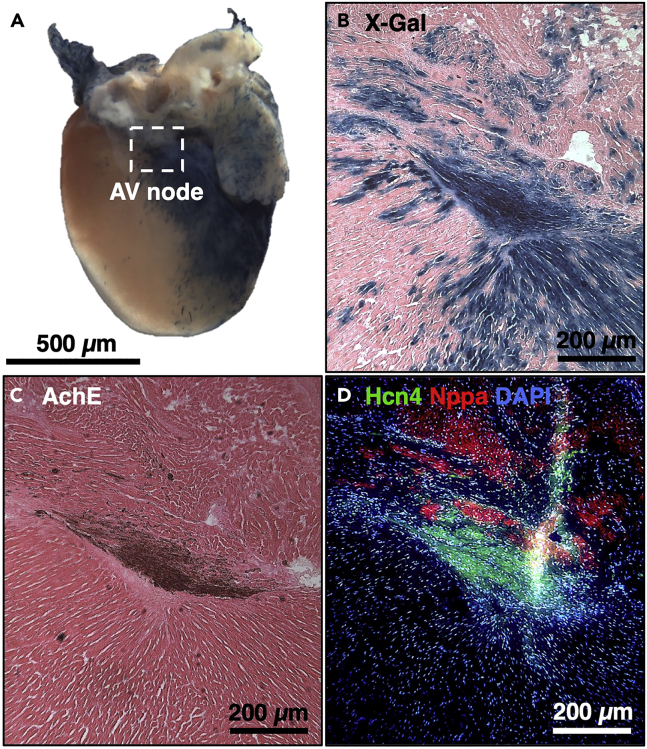
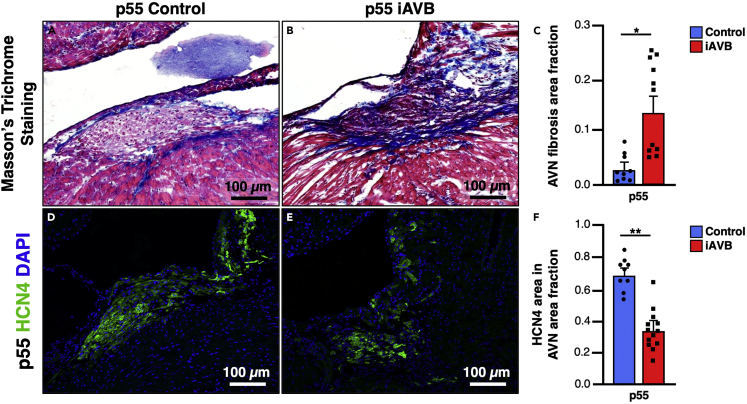
The AV node of adult and neonatal AVCS-iLacZ mice (TgCx30.2-MerCreMer/+; Rosa26LacZ/+) highly stains for X-gal, acetylcholine esterase, and HCN4 (Figure 8). Masson’s trichrome staining of the AV node of iAVB mice should show an increase in the area of fibrosis compared to control mice AV node (Figure 9). The AV node of iAVB mice should also show decreased HCN4 positive cells compared to control mice AV node, pointing to AVCS cellular ablation (Figure 9).
Figure 8.
Immunohistochemical staining of the AV node
(A) X-Gal staining of AVCS-iLacZ (TgCx30.2-MerCreMer/+; Rosa26LacZ/+) mouse heart, induced at P0 and harvested at P28, scale bar: 500 μm.
(B) X-Gal staining of the AV node of the AVCS-iLacZ mouse, scale bar: 200 μm.
(C) Acetylcholine esterase staining of the AV node of the AVCS-iLacZ, scale bar: 200 μm.
(D) Immunofluorescence staining of the AV node of the AVCS-iLacZ mouse using HCN4 antibody, scale bar: 200 μm.
Figure 9.
Cellular ablation of the AV node of iAVB (TgCx30.2-MerCreMer/+; Rosa26DTA/LacZ) mouse heart after Tamoxifen induction
(A and B) Masson’s trichrome staining of the AV node of iAVB mouse compared to control, scale bar: 100 μm.
(C) Quantification of the AVN fibrosis of iAVB mouse compared to control, ∗p-value <0.05. Error bars represent SEM.
(D and E) HCN4 immunofluorescence staining of the AV node of iAVB mouse compared to control, scale bar: 100 μm.
(F) Quantification of HCN4 positive cell dropout in the AVN of iAVB mouse compared to control, ∗∗p-value <0.01. Error bars represent SEM.
Quantification and statistical analysis
The fibrotic area in the AV node was quantified as the relative area of positive Masson’s trichrome-stained area (blue fibrosis) normalized to the total section area using ImageJ/Fiji. HCN4 positive cell drop out was calculated as the ratio of HCN4 positive area to the area of atrioventricular node. All data are shown as mean ± standard error of mean (s.e.m). P values are determined using the Fisher’s Exact Test or Student’s two-tailed t-test. A p value less than 0.05 is considered statistically significant.
Limitations
The protocol only includes surface ECG since we only needed to detect AV block phenotypes for this protocol. Further characterization of the cardiac dysrhythmia phenotype is outside the scope of the current protocol, but can be pursued with other techniques (e.g., optical mapping and intracardiac ECG).
Masson’s trichrome staining was the only method used to evaluate fibrosis coverage in this protocol. Other methods should be considered.
Troubleshooting
Problem 1
Maternal cannibalization of neonatal mice post-Tamoxifen induction (steps 2 & 3).
Potential solution
The following can decrease the chance of maternal cannibalization of neonatal mice post-Tamoxifen induction: (1) rub hands with cage beddings before handling the neonatal mice; (2) always remove and return entire litter; (3) administer Tamoxifen to neonatal mice at almost P1 of age (<24 h from birth; (4) make sure milk spots are present in neonatal mice before induction with Tamoxifen.
Problem 2
High amount of noise in the ECG recordings (step 5).
Potential solution
In order to decrease the amount of noise in the ECG recordings, avoid inserting the ECG electrodes deeply into the muscle layer and ensure subcutaneous placement. From our experience, needle electrodes are better than micro-hook electrodes. Avoid unnecessary movement of the ECG electrodes while recording (e.g., placing the wirings of the ECG electrodes over the animal’s chest and abdomen; moving hands above the mouse while recording). Take particular care and avoid damaging the insulators in the electrode wirings. Damaged wiring insulators can also introduce noise in the ECG recordings. We highly recommend using a Bio Amp to enhance the ECG signal and reduce noise. If noise persists, ensure that electrode wirings are well connected to the Bio Amp or replace faulty ECG electrodes if necessary.
Problem 3
No AV block phenotype after Tamoxifen induction (steps 1–3).
Potential solution
Check if the Tamoxifen-induced animals have the correct genotype. Remove from the experiment any mice that have the wrong genotype or any mice that did not exhibit AV block after Tamoxifen induction. If entire litter did not exhibit AV block, genotype Tamoxifen-induced mice and their parents. If both parents and offspring have the correct genotype, prepare a new batch of Tamoxifen solution for another round of induction. If AV block is too severe with low survivability of mice, Tamoxifen dosage can be decreased up to 0.5 mg.
Problem 4
Inadequate cryopreservation and poor sectioning of tissue samples (steps 25 & 26).
Potential solution
Make sure no air bubbles are formed when adding TFM during mounting of heart samples into the cryomold. The presence of bubbles indicates that TFM is not in contact with the heart sample which leads to inadequate cryopreservation. Air bubbles can lead to suboptimal cryosections due to poor transferring of heart sections to microscope slides (e.g., rolling/curling of sections in the slides). Air bubbles can be removed using forceps. Excess sucrose solution around the heart sample (“watery heart”) can also lead to suboptimal cryosections due to poor transferring as well. This can be prevented by bathing the heart sample in TFM placed in a foil prior to mounting in the cryomold. Maintaining the cutting chamber temperature at –23°C and ensuring a sharp cutting knife can give optimal heart sections.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nikhil V. Munshi (Email: Nikhil.Munshi@UTSouthwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets/code.
Acknowledgments
This work was supported by the NIH (HL136604, HL133642, and HL135217, to N.V.M.), the Burroughs Wellcome Fund (1009838, to N.V.M.), and the March of Dimes Foundation (5-FY13-203, to N.V.M.). We thank John Shelton and the Histopathology Core Facility for sharing critical reagents and advice.
Author contributions
L.W. developed the study protocol. J.G.L.D.R. wrote the study protocol. N.V.M. obtained funding and supervised the study project.
Declaration of interests
The authors declare no competing interests.
References
- 1.Wang L., Bhakta M., Fernandez-Perez A., Munshi N.V. Inducible cardiomyocyte injury within the atrioventricular conduction system uncovers latent regenerative capacity in the mice. J. Clin. Invest. 2021;131:e138637. doi: 10.1172/JCI138637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munshi N.V., McAnally J., Bezprozvannaya S., Berry J.M., Richardson J.A., Hill J.A., Olson E.N. Cx30.2 enhancer analysis identifies Gata4 as a novel regulator of atrioventricular delay. Development. 2009;136:2665–2674. doi: 10.1242/dev.038562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.