Abstract
Purpose:
Dysfunctional breathing behaviors are prevalent in chronic obstructive pulmonary disease (COPD). Although these behaviors contribute to dyspnea, abnormal carbon dioxide (CO2) levels, and COPD exacerbations, they are modifiable. Current dyspnea treatments for COPD are suboptimal, because they do not adequately address dysfunctional breathing behaviors and anxiety together. We developed a complementary mind–body breathlessness therapy, called capnography-assisted respiratory therapy (CART), that uses real-time CO2 biofeedback at the end of exhalation (end-tidal CO2 or ETCO2), to target dysfunctional breathing habits and improve dyspnea treatment and pulmonary rehabilitation (PR) adherence in COPD. The study aim was to test the feasibility of integrating CART with a traditional, clinic-based PR program in an urban setting.
Methods:
We used a feasibility pre- and post-test design, with 2:1 randomization to CART+PR or control (PR-alone) groups, to test and refine CART. Multi-component CART consisted of six, 1-h weekly sessions of slow breathing and mindfulness exercises, ETCO2 biofeedback, motivational counseling, and a home program. All participants were offered twice weekly, 1-h sessions of PR over 10 weeks (up to 20 sessions).
Results:
Thirty-one participants with COPD were enrolled in the study. Approximately a third of participants had symptoms of psychological distress. Results showed that CART was feasible and acceptable based on 74% session completion and 91.7% homework exercise completion (n = 22). Within-group effect sizes for CART+PR were moderate to large (Cohen's d = 0.51–1.22) for reduction in resting Borg dyspnea (anticipatory anxiety) and respiratory rate, St. George's Respiratory Questionnaire (SGRQ) respiratory symptoms; and increase in Patient-Reported Outcomes Measurement Information System (PROMIS) physical function and physical activity; all p < 0.05.
Conclusions:
CART is a new mind–body breathing therapy that targets eucapnic breathing, interoceptive function, and self-regulated breathing to relieve dyspnea and anxiety symptoms in COPD. Study findings supported the feasibility of CART and showed preliminary signals that CART may improve exercise tolerance, reduce dyspnea, and enhance PR completion by targeting reduced dysfunctional breathing patterns (CTR No. NCT03457103).
Keywords: breathing therapy, capnography biofeedback, dyspnea anxiety, pulmonary rehabilitation, interoception
Introduction
Chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD), are the third leading cause of death1 and a significant cause of years lived with disability.2 About 14% of Americans have COPD.3 The prevalence and burden of COPD on patients, their families, and society is high. COPD is characterized by airflow limitation, which leads to dyspnea (breathlessness), and abnormal levels of carbon dioxide (CO2) and oxygen. Dysfunctional or irregular breathing patterns in COPD (e.g., a rapid, upper thoracic dominant breathing pattern and breath holds) are also very prevalent,4,5 which increase dyspnea and related anxiety symptoms, and increase the risk of physical activity avoidance and COPD exacerbations. Currently, there are limited dyspnea and anxiety treatment options, and management of dyspnea is suboptimal in COPD.6
Evidence-based pulmonary rehabilitation (PR) is the standard of care for COPD, consisting of aerobic conditioning, upper body strengthening, and self-management education. Nonetheless, only 1%–2% of individuals who need PR receive it and dropout rates are greater than 31%–40%.7,8 Risk factors for PR dropouts include higher psychological distress and depression9,10 and greater dyspnea.10 Anxiety is further associated with worse PR adherence, greater dyspnea and perceived functional impairment post-PR, and worse quality of life and 6-minute walk distance (6MWD).11,12 There is, therefore, an urgent call for new approaches to improve dyspnea and related anxiety symptom management and PR utilization in COPD.13
Capnography-assisted respiratory therapy (CART) has been shown to be feasible and efficacious in adults with asthma and panic disorder,14,15 both common comorbidities of COPD. However, the feasibility of CART in adults with COPD has not yet been investigated. The aim of this study was to test the feasibility of CART, a new mind–body breathing therapy and adjunct to outpatient PR, and share lessons learned, in preparation for a future efficacy trial.
Investigators studying complementary interventions in different chronic disease populations may be able to apply the lessons learned for combining a mind–body intervention with a traditional outpatient intervention. The overarching hypothesis is that CART's mind–body breathing exercises with biofeedback (targeting improved dyspnea and anxiety symptom management and PR participation) are feasible in patients with COPD.16
This study was approved by New York University Langone Health's Institutional Review Board.
Materials and Methods
Design and procedures
Using the Obesity Related Behavioral Intervention Trails Model, we implemented a Phase II proof-of-concept preliminary feasibility study of multi-component CART to optimize the intervention and identify early signals for readiness to conduct an efficacy trial.17,18 Participants referred to an urban outpatient PR program were randomized to CART+PR versus PR alone (usual care), using a 2:1 ratio, to gain more experience with the CART intervention.19,20 All participants completed an informed consent form before study participation.
Recruitment was over a 10-month period from August 2018 through July 2019. To improve study internal validity, subjects met the following a priori inclusion criteria: (1) over 40 years of age; (2) had COPD as defined by Forced Expiratory Volume in 1 second (FEV1)/Forced Vital Capacity (FVC) of <0.70 on spirometry; (3) were medically cleared to participate in PR; and (4) spoke English. Exclusion criteria were: (1) required 24-h supplemental oxygen, SpO2 (because SpO2 can increase CO2 in stable COPD21); (2) scored ≤23/30 on the Mini Mental State Examination (MMSE);22 (3) were being actively treated for cancer; (4) had morbid obesity (body mass index >40) that negatively impacts the mechanics of the chest wall and lungs;23 (5) were current smokers as tobacco smoke increases upper airway symptoms such as rhinosinusitis);24,25 and (6) had unstable cardiac disease limiting exercise safety.
Random allocation to treatment group was concealed for exercise stress tests (cardiopulmonary exercise testing [CPET]) and physiological measures to avoid bias.26 Participants were paid $25 to complete each of two assessments for a total of $50. This study was registered at Clinicaltrials.gov (NCT03457103).
Acceptability of CART was also evaluated through semi-structured exit interviews, and these results were reported elsewhere.27 Acceptability, which is a component of feasibility,28 was defined as the “perception among patients that a given treatment, was agreeable, palatable, or satisfactory.”29,p.67 Qualitative data supported the acceptability of CART based on patient perceptions and themes generated.27
Breathing intervention (CART)
CART is described in detail elsewhere.27 CART consisted of six, 1 h weekly sessions over 6 weeks. Its essential components included 10 core breathing exercises, capnography biofeedback for entire session duration including interview (Fig. 1), brief motivational counseling,30 and a home program (Table 1). Brief motivational interviewing included open-ended questions, affirmations, reflections, and summaries as time permitted in sessions to promote change talk and a collaborative therapeutic relationship (especially to conduct the initial interview, set session agendas, and homework goals, and to discuss discharge planning).
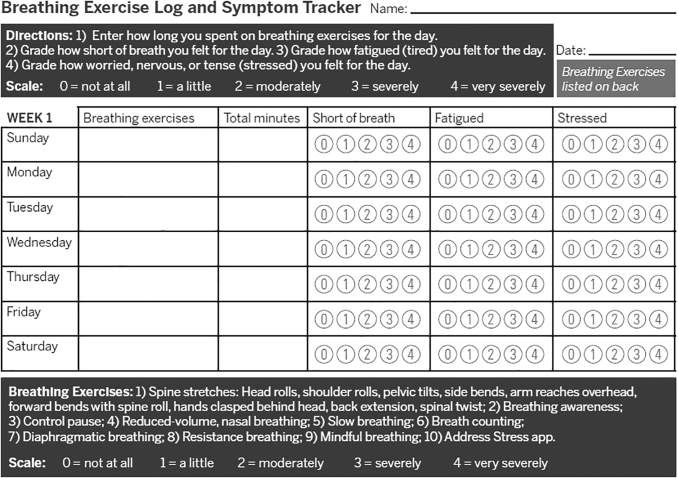
FIG. 1.
CART breathing exercise log and symptom tracker. CART, capnography-assisted respiratory therapy.
Table 1.
Capnography-Assisted Respiratory Therapy Core Breathing Exercises in Combination with Capnography Biofeedback
| Session titles | Core exercises introduced in each session |
|---|---|
| (1) Introducing the Mind–Body Connection and Biofeedback | Ribcage stretches coordinated with the exhale (in all six sessions).71 |
| (2) Completing the Out-Breath | Tongue maneuver to promote closed-mouth, nasal breathing.72 Breath counting. Participant is instructed to breathe “in, out, and pause” to count one breath, and so on, until they count five breaths. Slow, nasal breathing awareness in relief (recovery) postures (e.g., beach pose; forward leaning, first in standing and then in sitting, with arms supported).73 Brief (≤3 min) mindful breathing exercises using scripts and Address Stress app.74,75 Mindfulness exercises involved guiding participants to “pay attention to their breath and breathing mechanics on purpose, in the present moment, nonjudgmentally”76, p.91 without forcing the breath, while in recovery (semi-reclined) postures. |
| (3) Pausing After the Out-Breath | Control pause.31 Gentle breaths in and out through the nose at rest, followed by a pause at the end of the exhalation. Participant counts the length of the pause between breaths to become aware of the transition between breaths. Diaphragmatic breathing.77 Participant notices “pump handle” and “buckle handle” movements of the ribcage at rest in recovery postures that optimize breathing mechanics. Manual touch and TheraBand may be used to facilitate awareness. |
| (4) Becoming Aware of Breath Volume & More Rhythmical Breathing | Volume-regulated breathing to promote eucapnic breathing to address overbreathing or underbreathing.33 Reduced-volume breathing involves taking lighter, softer, quieter breaths in and out through the nose (or in through the nose and out through pursed lips). Increased-volume breathing involves taking slower breaths, prolonging both the inhalation and exhalation, and optimizing breathing mechanics and posture. Pursed-lips breathing with physical challenge (≤5 min).78 |
| (5) Expanding Breath Awareness & Releasing Tension | Humming (resistance breathing) at rest and then with physical exertion to self-selected music.79,80 Slow, nasal breathing awareness in recovery posture(s)—with or without a cognitive challenge.73 |
| (6) Self-regulating the Breath in Daily Life | Further practice and review of core breathing exercises introduced in previous sessions. |
Participants recorded their tailored home exercises and symptoms on an Exercise Log and Symptom Tracker and measured their heart rate (HR) and oxygen saturation with a pulse oximeter, to record and track their progress (Fig. 2); at least 5 min of homework breathing exercises were encouraged, a minimum of 4 days per week to promote lifestyle change. After the evaluation session, each session began by reviewing progress with homework. CART was delivered on the same day and in coordination with standard PR visits.
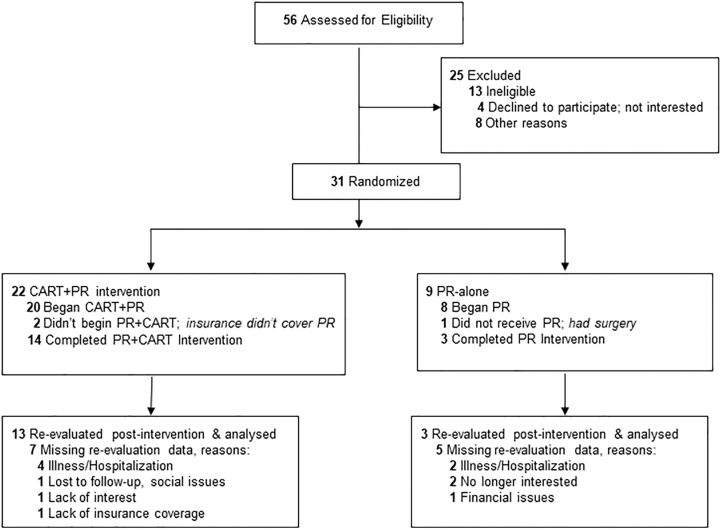
FIG. 2.
CONSORT flow diagram. CART, capnography-assisted respiratory therapy; CONSORT, Consolidated Standards of Reporting Trials; PR, pulmonary rehabilitation.
Author A.M.N., a PR clinician (an occupational therapist trained in mindfulness-based stress reduction and breathing behavioral analysis and capnography) developed and implemented CART. In-person, individual therapy targeted slower, more eucapnic, and self-regulated breathing, efficient recovery from physical challenges,31–33 improved interoceptive function34,35 (“the sense of the internal physiological condition of the body),”36,p.971 and physical activity engagement. Core breathing exercises targeted identifying and reducing dysfunctional breathing habits and abnormal ETCO2 levels (biomedical risk factor).
In particular, exercises emphasized learning more functional breathing habits, extending expiratory time, optimizing respiratory pump biomechanics (e.g., correcting asynchronous motion of the chest wall), mindful awareness of breathing, and awareness of symptoms of abnormal ETCO2 levels. Tailoring focused on addressing each participant's specific dysfunctional breathing habits and dyspnea triggers. CART capnography biofeedback (CapnoTrainer; Better Physiology, Cheyenne, WY) in-session provided continuous, real-time visual biofeedback of ETCO2, respiratory rate (RR), and airflow (e.g., evenness of volume and breath duration) to guide tailoring of exercises, monitor exercise response and progress, and promote positive reinforcement and targeted nudging of more self-regulated breathing.
Biofeedback also provided a simplified visual of breathing physiology to promote learning. Slow, self-regulated, eucapnic breathing was promoted first at rest before introducing tailored, ≤5-min physical activity challenges in later sessions. Fundamental to CART was motivational interviewing, a patient-centered counseling style, to collaboratively set goals and motivate behavior change.37 The home program consisted of tailored, slow breathing, and mindful exercises, facilitated by audio files of therapist-guided breathing exercises and an exercise log; the Breathing Well™ app was also introduced.
CART fidelity (“the extent to which a new treatment is successfully carried out within a given setting”)38,p.69 was optimized by the development of a standardized protocol (manual), which included a session outline and timeline to minimize intervention drift.39 The therapist's motivational interviewing training entailed 10 h of training (an 8-h online course and a 2-h live workshop) with Motivational Interviewing Network of Trainers (MINT)-certified trainers. Author L.E.-J. also monitored motivational interviewing fidelity using the Motivational Interviewing Treatment Integrity-440 by reviewing and scoring three random session transcripts.
CART therapist's monitoring of participants' breathing behaviors and exercise performance (i.e., in-session observations of exercise response with biofeedback and review of homework logs each session) helped to ensure optimum receipt of treatment dose.39 Exercise logs and motivational interviewing also helped to optimize and monitor enactment of new breathing behaviors and exercises into participants' daily routines.39
Pulmonary rehabilitation
All participants received a 10-week (16–20 sessions) center-based PR program, consisting of twice weekly 1-h exercise training (aerobic conditioning and upper body strengthening) sessions using gym exercise equipment, pursed lips breathing instruction (without biofeedback), and tailored self-management education implemented by physical therapists as part of routine, standard care. Initial exercise intensity was tailored based on baseline six-minute walk test and CPET. Supplemental oxygen was provided as prescribed and needed to optimize exercise training. Participants were enrolled into PR on a rolling basis.
Measures
The primary outcome was feasibility. We defined feasibility as “the extent to which a new treatment can be successfully used or carried out within a given setting as reflected in recruitment, retention, and participation rates.”29,p.69 Our a priori benchmarks for feasibility were a mean CART session completion of 70% and mean CART homework exercise completion of 70% of days on CART program. CART homework completion feasibility benchmark was calculated based on expectation of 4 days per week and a 6-week duration.
Secondary outcomes included CPET minutes walked using the Naughton protocol and a treadmill,41 six minute walk test distance (6MWD),42 St. George's Respiratory Questionnaire (SGRQ),43,44 Chronic Respiratory Disease Questionnaire (CRQ) Mastery,45,46 Dyspnea Management Questionnaire (DMQ)–Dyspnea Anxiety,47 isotime RR and ETCO2 in mmHg with CPET, and Borg rate of perceived exertion (RPE) Scale.48 Borg dyspnea-CPET was used as a measure of anticipatory anxiety.
Secondary measures also included Patient-Reported Outcomes Measurement Information System (PROMIS)-3649,50 physical function, anxiety, depression, sleep disturbance, fatigue, and satisfaction with participation in social roles; moderate to vigorous physical activity (MVPA),51,52 Generalized Anxiety Disorder Scale (GAD-7) symptoms,53 Self-Evaluation of Breathing Questionnaire (SEBQ)54—a measure of dysfunctional breathing symptoms, and PR program adherence (number of sessions completed).
Statistical analysis
We first described descriptive data. We then estimated the mean change and variances for primary and secondary outcomes. The paired t test was used to evaluate within-group intervention differences (pre-intervention to post-PR intervention) at 10 weeks. The Cohen's d effect sizes for within-group differences were also reported. Analyses were performed using R 4.0.3 with significance set at p < 0.05. Consistent with a feasibility trial, between-group comparisons were not calculated.55,56
Results
Thirty-one participants with mild-very severe COPD enrolled in the study (Fig. 2). The demographics of the sample were 58.1%, were female with a mean (standard deviation [SD]) age of 72 (9.5); the sample was 77.4% Caucasian, 22.6% African American, and 9.7% Hispanic (Table 2). Overall, 67.7% had >high school education. A majority (79%) of our sample had symptomatic, moderate-severe COPD.57 GAD-7 anxiety screening at baseline showed that 32.3% of participants had anxiety symptoms (score of >5); 30.8% were taking anti-anxiety or antidepressant medications for psychological distress.
Table 2.
Baseline Characteristics of Participants
| PR alone | CART+PR | Overall | |
|---|---|---|---|
| N | 9 | 22 | 31 |
| Gender—female (%) | 4 (44.4) | 14 (63.6) | 18 (58.1) |
| Age, mean (SD) | 71.44 (7.26) | 72.82 (10.45) | 72.42 (9.54) |
| Home oxygen use—yes (%) | 2 (22.2) | 4 (18.2) | 6 (19.4) |
| Post-FEV1/FVC ratio, mean (SD) | 0.52 (0.12) | 0.52 (0.14) | 0.52 (0.13) |
| Post-FEV1, L, mean (SD) | 1.21 (0.38) | 1.44 (0.71) | 1.37 (0.64) |
| Post-FEV1, % predicted, mean (SD) | 47.42 (13.44) | 55.50 (24.51) | 53.27 (22.09) |
| Post-FVC, L, mean (SD) | 2.31 (0.52) | 2.62 (0.90) | 2.54 (0.82) |
| COPD severity (%) | |||
| Mild 1 | 0 (0.0) | 4 (19.0) | 4 (13.8) |
| Moderate 2 | 3 (37.5) | 8 (38.1) | 11 (37.9) |
| Severe 3 | 4 (50.0) | 8 (38.1) | 12 (41.4) |
| Very severe 4 | 1 (12.5) | 1 (4.8) | 2 (6.9) |
| mMRC dyspnea, mean (SD) | 4.11 (1.05) | 3.50 (1.01) | 3.68 (1.05) |
| Smoking pack-years, mean (SD) | 57.34 (37.79) | 38.09 (26.76) | 43.59 (30.87) |
| Currently smoking—yes (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI, kg/m2, mean (SD) | 25.30 (6.71) | 27.19 (5.46) | 26.64 (5.80) |
| Exacerbation in previous year—yes (%) | 5 (55.6) | 8 (36.4) | 13 (41.9) |
| Exacerbation in previous year, n, mean (SD) | 2.60 (1.52) | 4.00 (3.70) | 3.46 (3.04) |
| Congestive heart failure comorbidity—yes (%) | 0 (0.0) | 4 (18.2) | 4 (12.9) |
| Married (%) | 4 (44.4) | 8 (36.4) | 12 (38.7) |
| Hispanic (%) | 0 (0.0) | 3 (13.6) | 3 (9.7) |
| African American | 2 (22.2) | 5 (22.7) | 7 (22.6) |
| Caucasian (%) | 7 (77.8) | 17 (77.3) | 24 (77.4) |
| >High school (%) | 7 (77.8) | 14 (63.6) | 21 (67.7) |
| Taking anxiety or depression medication—yes (%) | 3 (37.5) | 5 (27.8) | 8 (30.8) |
Means (SDs) are presented for continuous variables; frequencies (%) are presented for categorical variables.
BMI, body mass index; CART, capnography-assisted respiratory therapy; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; mMRC, modified Medical Research Council; PR, pulmonary rehabilitation; SD, standard deviation.
Completion of CART sessions was 73.5% overall (n = 22), and attendance was 100% for participants who also completed all PR sessions (n = 13): Table 3. Mean (SD) number of days of completing CART homework exercises per participant was 22.08 (8.01), which represented a homework adherence of 91.7%. These CART implementation results exceeded our pre-established feasibility benchmarks.
Table 3.
Intervention Adherence
| PR | n | CART+PR | n | p | |
|---|---|---|---|---|---|
| N | 9 | 22 | |||
| Time to complete PR (weeks) | 13.25 (4.57) | 4 | 11.08 (1.98) | 13 | 0.18 |
| Time to complete PR (days) | 94.75 (31.06) | 4 | 79.15 (14.52) | 13 | 0.172 |
| Number of PR sessions | 11.44 (9.81) | 9 | 14.14 (7.37) | 22 | 0.409 |
| Drop-out (%) | 6 (66.6) | 9 | 9 (40.9) | 22 | 0.365 |
| Drop-out before PR assessment (%) | 0 (0.0) | 9 | 0 (0.0) | 22 | 1.000 |
| Drop-out after PR assessment (%) | 2 (22.2) | 9 | 1 (4.5) | 22 | 0.184 |
| Drop-out after commencing PR (%) | 5 (55.6) | 9 | 6 (27.3) | 22 | 0.218 |
| Drop-out after commencing CART (%) | — | 0 | 4 (18.2) | 22 | — |
| CART sessions completed, n | — | 0 | 4.41 (2.42) | 22 | — |
| CART sessions, % | — | 0 | 73.48 (40.39) | 22 | — |
| CART HEP (days of home exercise), n | — | 0 | 22.08 (8.01) | 12 | — |
Means (SDs) are presented; n is the number of patients with available data. The two-sample t test was used.
CART, capnography-assisted respiratory therapy; HEP, home exercise program; PR, pulmonary rehabilitation; SD, standard deviation.
The mean number of PR sessions completed was 14.14 (7.37) for CART+PR and 11.44 (9.81) for PR-alone (n = 31). Participants completed 22 days of homework exercises while on CART program (an adherence of 91.7%). Participants were willing to be randomized; there were no dropouts based on group assignment. PR dropout rate (for treatment starters) was 27.3% for PR+CART versus 55.6% for PR-alone, p = 0.218. PR dropout was due to illness (n = 2), hospitalizations (n = 2), family emergency (n = 1), PR adverse events (n = 2), and insufficient interest (n = 3). Seventeen of 31 (55%) participants completed PR overall in both groups (i.e., they completed all prescribed 17–20 PR sessions); n = 31.
CART+PR within-group effect sizes for exercise and respiratory outcome measures were moderate to large (0.51–1.22) for reduction in Borg dyspnea-CPET, exercise duration (total minutes walked, CPET), and isotime RR (submaximal CPET exercise, Naughton protocol, Phase I only); p < 0.05: Table 4. The 6MWD within-group mean improvement of 39.4 m for CART+PR was clinically significant (>30 m),58 and it represented a small–moderate effect (0.43), p = 0.01.
Table 4.
Exercise and Respiratory Physiological Measures
| CART+PR |
|
PR alone |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N a | Mean (SD)-pre | Mean (SD)-post | Within-group difference (CI) | p | Cohen's d effect size | N | Mean (SD)-pre | Mean (SD)-post | Within-group difference | |
| 6MWD meters | 12 | 358.27 ± 87.85 | 397.64 ± 92.2 | 39.37 (14.13 to 64.61) | 0.01 | 0.43 | 4 | 322.94 ± 72.66 | 347.93 ± 84.71 | 24.99 |
| Post 6MWT Borg RPE | 12 | 12.75 ± 2.42 | 11.25 ± 2.99 | −1.5 (−3.87 to 0.87) | 0.191 | 0.55 | 4 | 14 ± 2 | 14.25 ± 2.22 | 0.25 |
| Borg Dyspnea (CPET) sitting | 9 | 4.89 ± 4.78 | 0.78 ± 1.09 | −4.11 (−7.96 to −0.26) | 0.039 | 1.22 | — | — | — | — |
| Exercise duration CPET Total minutes | 10 | 6.69 ± 3.72 | 10.08 ± 4.34 | 3.39 (1.7 to 5.08) | 0.001 | 0.81 | 2 | 6.06 ± 0.14 | 7.45 ± 0.07 | 1.39 |
| ETCO2 at rest, mean (SD) | 10 | 27.6 ± 3.59 | 28.74 ± 3.07 | 1.14 (−0.71 to 2.99) | 0.196 | 0.34 | 2 | 29.81 ± 5.11 | 25.26 ± 2.25 | −4.55 |
| ETCO2 I | 10 | 29.42 ± 3.83 | 30.78 ± 3.06 | 1.37 (−1.08 to 3.81) | 0.239 | 0.39 | 2 | 33.81 ± 6.77 | 30.27 ± 1.83 | −3.55 |
| ETCO2 II | 9 | 31.16 ± 3.25 | 32.09 ± 3.15 | 0.92 (−1.02 to 2.87) | 0.305 | 0.29 | 2 | 34.11 ± 7.79 | 30.95 ± 1.75 | −3.16 |
| ETCO2 III | 7 | 31.48 ± 4.38 | 32.06 ± 3.06 | 0.58 (−1.15 to 2.31) | 0.444 | 0.11 | 2 | 35.64 ± 5.42 | 33.56 ± 1.81 | −2.08 |
| HRmax | 10 | 121.5 ± 15.04 | 127.7 ± 15.17 | 6.2 (−1.32 to 13.72) | 0.095 | 0.41 | 2 | 110.5 ± 2.12 | 113.5 ± 2.12 | 3 |
| RR sitting | 10 | 21.3 ± 5.49 | 18.55 ± 5.2 | −2.75 (−7.56 to 2.05) | 0.227 | 0.51 | 2 | 22.1 ± 3.68 | 17.9 ± 6.13 | −4.2 |
| RR I | 10 | 25.99 ± 5.47 | 22.46 ± 5.27 | −3.53 (−6.16 to −0.91) | 0.014 | 0.66 | 2 | 26.15 ± 6.01 | 22.15 ± 6.43 | −4 |
| RR III | 7 | 32.01 ± 5.19 | 26.3 ± 7.48 | −5.71 (−12.72 to 1.29) | 0.093 | 0.87 | 2 | 39.85 ± 0.21 | 32.35 ± 0.07 | −7.5 |
The paired two-sample t test was used.
N is the number of subjects who have both pre- and post-data.
6MWD, six minute walk distance; CART, capnography-assisted respiratory therapy; CI, confidence interval; CPET, cardiopulmonary exercise testing; ETCO2, end-tidal CO2; HR, heart rate; PR, pulmonary rehabilitation; RPE, rate of perceived exertion; RR, respiratory rate; SD, standard deviation.
Small CART+PR effect sizes (0.29–0.41) were also obtained for improved (higher) ETCO2 at rest during sub-maximal exercise phases I–II during CPET and HRmax (fitness), p > 0.05. Effect sizes were moderate to large for reducing RR with submaximal exercise (phases I and III) in CART+PR; p = 0.014–0.09. Similar reductions in RR were seen in the PR-alone group (n = 2).
Within-group CART+PR effect sizes and improvement were small to large (0.35–0.76) for clinical outcomes that included reduced respiratory symptoms and improved quality of life (SGRQ symptoms, impacts and total); reduced Dyspnea Management questionnaire Computer Adaptive Test dyspnea anxiety; and improved generic PROMIS quality of life (less PROMIS fatigue, sleep disturbance, and improved physical function), and increased MVPA, p < 0.05 (Table 5).
Table 5.
Main Clinical Symptoms, Physical Activity, and Quality of Life Outcomes
| |
CART+PR |
|
PR alone |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mean (SD)-pre | Mean (SD)-post | Within-group difference (CI) | p | Cohen's d effect size | N | Mean (SD)-pre | Mean (SD)-post | Within-group difference (CI) | |
| DMQ Dyspnea Anxiety | 13 | 56.02 ± 7.22 | 58.45 ± 6.27 | 2.43 (0.09 to 4.77) | 0.04 | 0.35 | 3 | 56.54 ± 3.41 | 53.38 ± 3.59 | −3.16 |
| SGRQ Symptoms | 13 | 53.69 ± 18.1 | 37.93 ± 24.61 | −15.76 (−28.06 to −3.47) | 0.02 | 0.71 | 3 | 46.85 ± 22.27 | 45.37 ± 6.62 | −1.48 |
| SGRQ Activity | 13 | 68.16 ± 17.74 | 60.56 ± 18.39 | −7.6 (−16.85 to 1.65) | 0.1 | 0.42 | 3 | 82.02 ± 10.4 | 71.23 ± 8.74 | −10.79 |
| SGRQ Impacts | 12 | 41.93 ± 22.11 | 29.79 ± 17.85 | −12.14 (−19.88 to −4.4) | 0.01 | 0.57 | 3 | 44.05 ± 14.03 | 32.77 ± 1.74 | −11.28 |
| SGRQ Total | 12 | 52.47 ± 18.08 | 40.72 ± 16.48 | −11.76 (−19.68 to −3.83) | 0.01 | 0.68 | 3 | 56.65 ± 11.79 | 46.69 ± 0.85 | −9.96 |
| CRQ Mastery | 13 | 5.15 ± 1.53 | 5.58 ± 1.08 | 0.42 (−0.34 to 1.18) | 0.25 | 0.31 | 3 | 5.33 ± 1.04 | 5.33 ± 1.15 | 0 |
| PROMIS Anxiety | 13 | 53.18 ± 9.89 | 51.26 ± 9.22 | −1.92 (−6.24 to 2.41) | 0.35 | 0.2 | 3 | 63.97 ± 13.38 | 63.07 ± 14.16 | −0.9 |
| PROMIS Depression | 13 | 51.54 ± 10.11 | 48.77 ± 9.1 | −2.77 (−6.39 to 0.85) | 0.12 | 0.28 | 3 | 62.03 ± 8.71 | 58.6 ± 14.2 | −3.43 |
| PROMIS Fatigue | 13 | 53.64 ± 9.14 | 49.45 ± 8.41 | −4.18 (−8.16 to −0.21) | 0.04 | 0.47 | 3 | 54.3 ± 5.77 | 59.5 ± 10.05 | 5.2 |
| PROMIS Sleep Disturbance | 13 | 53.17 ± 7.22 | 51 ± 6.24 | −2.17 (−4 to −0.34) | 0.02 | 0.31 | 3 | 53.03 ± 7.7 | 54.07 ± 12.4 | 1.03 |
| PROMIS Social Roles | 13 | 49.6 ± 10.37 | 50.95 ± 9.52 | 1.35 (−2.28 to 4.97) | 0.43 | 0.13 | 3 | 40.63 ± 14.65 | 45.23 ± 5.3 | 4.6 |
| PROMIS Physical Function | 13 | 38.53 ± 5.22 | 41.28 ± 5.11 | 2.75 (0.1 to 5.39) | 0.04 | 0.53 | 3 | 33.1 ± 2.63 | 36.13 ± 3.06 | 3.03 |
| SEBQ Total (lower is better) | 13 | 12.08 ± 6.78 | 8.92 ± 5.56 | −3.15 (−6.42 to 0.11) | 0.06 | 0.5 | 3 | 12.67 ± 2.31 | 11.67 ± 1.15 | −1 |
| MVPA | 13 | 63.85 ± 81.6 | 150.77 ± 130.8 | 86.92 (17.96 to 155.88) | 0.02 | 0.76 | 3 | 26.67 ± 30.55 | 140 ± 192.87 | 113.33 |
| GAD-7 | 13 | 4.15 ± 4.14 | 3.62 ± 2.87 | −0.54 (−1.97 to 0.89) | 0.43 | 0.13 | 3 | 8.67 ± 6.03 | 8.67 ± 7.23 | 0 (−6.57 to 6.57) |
The paired two-sample t test was used.
N is the number of subjects who have both pre- and post-data.
CART, capnography-assisted respiratory therapy; CI, confidence interval; CRQ, Chronic Respiratory Disease Questionnaire. DMQ, Dyspnea Management Questionnaire; GAD-7, Generalized Anxiety Disorder Scale; MVPA, moderate to vigorous physical activity; PR, pulmonary rehabilitation; PROMIS, Patient-Reported Outcomes Measurement Information System; SD, standard deviation; SEBQ, Self-Evaluation of Breathing Questionnaire; SGRQ, St. George's Respiratory Questionnaire.
The CART+PR's effect sizes for CRQ Mastery, and PROMIS Anxiety and Depression were small (0.2–0.31), p > 0.05. The within-group difference for the SEBQ post-intervention for CART+PR approached significance, p = 0.06, signaling that CART may improve dyspnea by improving breathing mechanics and awareness.
Discussion
This is the first study to investigate the feasibility and explore preliminary empirical support for multi-component CART in adults with COPD. A strength of our study was that it included physiological measurements of ETCO2 and RR as endpoints to measure change in ventilation post-intervention. A main finding from this pilot feasibility study was that CART attendance rate was high at 74%, exceeding our a priori feasibility benchmark of 70% (n = 22). Although too preliminary to reliably test, a higher percent of patients in the CART group (72.7%) completed PR versus 44.4% of those in the PR only group, suggesting that a larger powered study may find that CART enhances engagement in PR and reduces PR attrition.
Participants in CART+PR completed PR 2 weeks faster on average than PR alone (n = 17), which may be clinically significant for improved PR cost-effectiveness and capacity, and increased exercise training intensity. CART may improve the ability to persevere with standard PR. Consistent with prior literature demonstrating challenges with PR use and low completion,13,59 less PR dropout in CART+PR is notable. Although a 55.6% PR dropout after commencing the program seemed unexpectedly high in the PR-alone group, it is reflective of a clinically significant problem of low rate of PR use and poor patient compliance in COPD.60
Our study findings provided preliminary signals that CART may successfully augment PR clinical outcomes, especially increased CPET exercise walking minutes and decreased respiratory (SGRQ) symptoms and anticipatory dyspnea-anxiety (Borg dyspnea-CPET at rest), with large within-group Cohen d effect sizes, p < 0.05. Similarly, a systematic review of 16 breathing studies for COPD found that breathing exercises of 4–15 weeks improved exercise capacity compared with no intervention.61 CART+PR also showed a large effect for increased self-reported physical activity (MVPA) post-intervention. Preliminary feasibility and efficacy signals support progression to future research to further investigate CART in a Phase II randomized trial.62
The CART+PR within-group effect size of 1.22 for reduction in resting Borg dyspnea-CPET (a measure of anticipatory anxiety) was the greatest level of improvement in clinical outcomes. CPET, which is a treadmill-paced exercise stress test, appeared to be a sensitive provocative test of anticipatory anxiety in COPD. Considering approximately a third of the participants reported symptoms of psychological distress, CART may address worse PR outcomes and limited anxiety treatment options for adults with COPD and comorbid anxiety.
Similarly, a systematic review of 13 randomized clinical trials (RCTs) of group-based mind–body exercises (Tai Chi, qigong, and yoga), with a focus on the breath, found that these exercises reduced anxiety and depression in adults with COPD compared to usual care.63 Results of subgroup analyses for anxiety found 24 weeks of group yoga and qigong sessions an optimal dose to improve symptoms, which is a significantly longer intervention and greater “dose” than CART's 6-week duration. This meta-analysis found effect sizes of 0.60–0.91 for anxiety and depression reduction.
Compared with other mind–body exercises, CART is innovative because it: (1) uses an individual (rather than a group) format, (2) combines biomechanical strategies with tailored breathing exercises that can be easily performed at home, (3) uses biofeedback technology to display psychophysiology to reinforce breathing regularity and confidence for greater appeal, and (4) incorporates motivational strategies37 that synergistically optimize long-term behavior change.
The preliminary findings add support for the conceptual framework of CART. Underlying CART is the hypothesis that dyspnea and anxiety are linked by irregular levels of CO2 and dysfunctional breathing habits. Unlike the PR-alone group, a trend and small 0.29–0.39 effect size of improved (increased) mean ETCO2 (at rest and with submaximal exercise) was seen in the CART+PR group, p > 0.05. We also found a 3.15 unit improvement in the SEBQ, indicating a 0.5 (moderate) effect size and improvements in symptoms associated with dysfunctional breathing behaviors (p = 0.06).
These signals perhaps indicate that participants in CART unlearned dysfunctional breathing behaviors and improved the efficiency of their breathing when challenged. Reductions in RR with submaximal CPET exercise were seen in both treatment groups, indicating improved exercise tolerance (physical fitness). A slower RR may decrease dynamic hyperinflation of the lungs in COPD to relieve dyspnea.64 RR reductions in the CART+PR group were moderate to large effect sizes (0.66–0.87) with submaximal exercise; p = 0.014–0.093.
A significant body of literature supports that CO2 is a biomarker of a dysfunctional, inefficient breathing pattern.32 CO2 hypersensitivity is also a known biomarker (trait) of anxiety disorders.65 Consistent with the literature, participants with COPD in the sample had lower resting and exertional ETCO2 (hypocapnic) values.33
CART may potentially augment a PR training effect by improving breathing efficiency and self-efficacy, and relieving dyspnea anxiety. CART may alleviate symptoms of hypocapnia by motivating patients to unlearn underlying dysregulated breathing habits (e.g., hyperventilation, open-mouth breathing, breath holding) and recover more quickly from physical exertion to relieve breathing discomfort and distress.
These potential benefits of CART may help participants persevere with exercise training (PR) and dyspnea-provoking activities.27 A more functional, efficient breathing patterns learned in CART may have downstream effects on physical activity levels by reducing activity avoidance.
Consistent with preliminary study findings, complementary benefits of mind–body breathing exercises integrated with PR are supported in the literature. A systematic review of 10 mind–body therapies (e.g., controlled breathing, focused attention, and/or meditation interventions) provided preliminary support for mind–body adjunctive interventions augmenting short form health survey-36 quality of life general health and mental health scores in COPD compared with PR alone.66 Given the low-to-very low quality of available evidence, inconsistencies in definition of PR, however, augmentative effects of mind–body therapies to PR still remain uncertain.
Lessons learned
There were several lessons learned from this feasibility study. The assessment battery, which took ∼1–3/4 h to complete, was well tolerated by participants. Recruitment was impacted by lower-than-expected referral of patients with COPD to PR. Therefore, future studies will need to improve partnerships with referring physicians to increase the pool of eligible patients. This study provided empirical support for the importance and value of using CPET for measuring dyspnea, anxiety, and ETCO2 in breathing therapy clinical trials. Based on comparative effect sizes, total exercise duration with CPET may be a better primary clinical outcome than 6MWT in a future efficacy trial of CART.
We found a high prevalence of anxiety symptoms (31%–32%) based on the GAD-7 and use of prescribed anti-anxiety or antidepressant medications in our sample. Given the prevalence of dyspnea-related anxiety in COPD, it may be valuable to include additional measures of dyspnea-related anxiety (such as dyspnea-related distress67 and Anxiety Sensitivity Index68) to better characterize and target this patient group and comorbidity in a future study. Given that interoceptive dysfunction is a hypothesized behavioral risk factor targeted by CART, a specific measure of breathing interoception such as the Multidimensional Assessment of Interoceptive Awareness34 could be included in a future pilot study of CART and considered as a potential stratification variable.69
PR dropout was a barrier to CART completion. To improve feasibility of a future trial of CART, a phased intervention approach (i.e., offering CART as a transitional program before PR) could be considered. A phased approach may facilitate the study of individual CART completion rates and effects on clinical endpoints and PR engagement. Offering CART before PR may be a more critical time point for delivering this intervention. We also recommend including anxiety symptoms as an inclusion criterion to improve the potential impact of CART.
Participants were inconsistent with filling out the paper log. Therefore, we plan to use an electronic device and app to measure RR for objective home exercise program adherence monitoring and quality checks in a future CART study. Participants found attending CART and standard PR sessions on the same day convenient. They expressed preferring CART sessions to be scheduled just before their PR appointments, when they had more energy. CART adherence was optimized by offering flexibility in scheduling; the six CART sessions were not always scheduled on consecutive weeks, or the first part of standard PR. CART was feasibly implemented using existing outpatient PR space, and it was optimized by using a separate, private, and relatively quiet space.
Limitations
We did not control for additional attention given in the CART+PR treatment group compared with the usual care PR-alone group. Because of the small sample size, caution should be used in interpreting Cohen's d effect sizes. The individual benefits of CART alone could not be estimated. Although randomization was 2:1, because of the small sample size, more participants were randomized to the intervention group creating a greater imbalance in groups.
However, the purpose of this feasibility trial was not to compare the efficacy of CART or engagement in PR between groups55 but to test proof-of-concept and identify early signals of CART to determine whether further pilot testing and a future efficacy trial are indicated.17 A limitation of the study was high study dropout rates and missing re-evaluation data. To reduce study dropout rates in a future study, employment of more retention strategies, greater staff time focused on optimizing participant engagement (e.g., offering flexibility in study visits and sending monthly newsletters), and a higher monetary incentive could be helpful to improve feasibility of a future RCT.70
Although CART was standardized, its fidelity was not formally monitored. More studies of the fidelity and optimal timing, dose, value of booster sessions of CART (separate and in combination with PR), as well as closer monitoring of any changes in medication (e.g., rescue medication or anti-depressant) use over the course of the study are needed.
Conclusion
CART adherence success benchmarks (i.e., ≥70% session completion and days of homework exercises logged) were met, further strengthening available qualitative evidence, to support progression of CART research in adults with COPD. The results also showed signals of efficacy of CART (especially for 6MWD meters, CPET total minutes, and Borg dyspnea pre-CPET), supporting further optimization and testing of CART. CART addresses an important need to optimize dyspnea treatment and reduce risk of PR non-adherence.
CART is an innovative, patient-centered, breathing therapy that targets self-regulated, eucapnic breathing for relief of dyspnea and related anxiety in COPD. By promoting unlearning of dysfunctional breathing habits and optimizing self-efficacy for managing symptoms, CART may improve dyspnea treatment, prevent PR dropouts, and address disparities in PR outcomes for those with higher anxiety. Lessons learned in this feasibility study may assist with designing a full-scale efficacy trial of CART and other mind–body interventions.
Additional research of the optimal timing (during vs. before a PR program) and dose of CART is needed. Timing CART as a steppingstone and transitional intervention for PR may enable both individual and augmentative effects of CART to be evaluated in a future study.
Authors' Contributions
A.M.N. conceptualized, designed, and implemented the study and intervention. Y.W. and A.T. performed the statistical analyses. J.H.W. and F.H. assisted with implementing the study and informed selection of outcome assessments. A.S. informed the development of the mindfulness component of the study intervention. E.C., G.J.-L., and N.S. critically reviewed and edited article drafts. J.R. assisted with developing the recruitment strategy. R.G. assisted with refining the intervention and provided feedback on the assessments. L.E.-J. implemented fidelity testing of the motivational interviewing component of the study intervention. All authors meet ICMJE's criteria for authorship.
Author Disclosure Statement
The authors have no conflicts of interest or disclosures.
Funding Information
This work was supported in part by grant 1R34AT010673-01A1 from National Institutes of Health, National Center for Complementary & Integrative Health. This work was also funded under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant No. 90SFGE0003). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this article do not necessarily represent the policy of NIDILRR, ACL, or HHS.
References
- 1. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020;8(6):585–596; doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–1858; doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siu AL, Bibbins-Domingo K, Grossman DC, et al. . Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA 2016;315(13):1372–1377; doi: 10.1001/jama.2016.2638 [DOI] [PubMed] [Google Scholar]
- 4. Boulding R, Stacey R, Niven R, et al. . Dysfunctional breathing: A review of the literature and proposal for classification. Eur Respir Rev 2016;25(141):287–294; doi: 10.1183/16000617.0088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Law N, Ruane LE, Low K, et al. . Dysfunctional breathing is more frequent in chronic obstructive pulmonary disease than in asthma and in health. Respir Physiol Neurobiol 2018;247:20–23; doi: 10.1016/j.resp.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 6. Mahler DA, Selecky PA, Harrod CG, et al. . American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137(3):674–691; doi: 10.1378/chest.09-1543 [DOI] [PubMed] [Google Scholar]
- 7. Almadana Pacheco V, Pavon Masa M, Gomez-Bastero Fernandez AP, et al. . Patient profile of drop-outs from a pulmonary rehabilitation program. Arch Bronconeumol 2017;53(5):257–262; doi: 10.1016/j.arbres.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 8. Garrod R, Marshall J, Barley E, et al. . Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 2006;27(4):788–794; doi: 10.1183/09031936.06.00130605 [DOI] [PubMed] [Google Scholar]
- 9. Tselebis A, Kosmas E, Bratis D, et al. . Contribution of psychological factors in dropping out from chronic obstructive pulmonary disease rehabilitation programs. Biomed Res Int 2014;2014:401326; doi: 10.1155/2014/401326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahin H, Naz I. Why are COPD patients unable to complete the outpatient pulmonary rehabilitation program? Chron Respir Dis 2018;15(4):411–418; doi: 10.1177/1479972318767206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Leupoldt A, Taube K, Lehmann K, et al. . The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest 2011;140(3):730–736; doi: 10.1378/chest.10-2917 [DOI] [PubMed] [Google Scholar]
- 12. Janssens T, De Peuter S, Stans L, et al. . Dyspnea perception in COPD: Association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest 2011;140(3):618–625; doi: 10.1378/chest.10-3257 [DOI] [PubMed] [Google Scholar]
- 13. Man WD, Puhan MA, Harrison SL, et al. . Pulmonary rehabilitation and severe exacerbations of COPD: Solution or white elephant? ERJ Open Res 2015;1(2); doi: 10.1183/23120541.00050-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ritz T, Rosenfield D, Steele AM, et al. . Controlling asthma by training of Capnometry-Assisted Hypoventilation (CATCH) vs slow breathing: A randomized controlled trial. Chest 2014;146(5):1237–1247; doi: 10.1378/chest.14-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meuret AE, Wilhelm FH, Ritz T, et al. . Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res 2008;42(7):560–568; doi: 10.1016/j.jpsychires.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox NS, Oliveira CC, Lahham A, et al. . Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: A systematic review using the Theoretical Domains Framework. J Physiother 2017;63(2):84–93; doi: 10.1016/j.jphys.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 17. Czajkowski SM, Powell LH, Adler N, et al. . From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol 2015;34(10):971–982; doi: 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig P, Dieppe P, Macintyre S, et al. . Developing and evaluating complex interventions: The new Medical Research Council guidance. Int J Nurs Stud 2013;50(5):587–592; doi: 10.1016/j.ijnurstu.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Moore CG, Carter RE, Nietert PJ, et al. . Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4(5):332–337; doi: 10.1111/j.1752-8062.2011.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dibao-Dina C, Caille A, Sautenet B, et al. . Rationale for unequal randomization in clinical trials is rarely reported: A systematic review. J Clin Epidemiol 2014;67(10):1070–1075; doi: 10.1016/j.jclinepi.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 21. Pilcher J, Perrin K, Beasley R. The effect of high concentration oxygen therapy on PaCO2 in acute and chronic respiratory disorders. Transl Respir Med 2013;1(1):8; doi: 10.1186/2213-0802-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–198; doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018;12(9):755–767; doi: 10.1080/17476348.2018.1506331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caminha GP, Pizzichini E, Lubianca Neto JF, et al. . Rhinosinusitis symptoms, smoking and COPD: Prevalence and associations. Clin Otolaryngol 2018;43(6):1560–1565; doi: 10.1111/coa.13215 [DOI] [PubMed] [Google Scholar]
- 25. Kim JS, Rubin BK. Nasal and sinus involvement in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2008;14(2):101–104; doi: 10.1097/MCP.0b013e3282f4efc9 [DOI] [PubMed] [Google Scholar]
- 26. Kao LS, Tyson JE, Blakely ML, et al. . Clinical research methodology I: Introduction to randomized trials. J Am Coll Surg 2008;206(2):361–369; doi: 10.1016/j.jamcollsurg.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norweg AM, Skamai A, Kwon SC, et al. . Acceptability of capnography-assisted respiratory therapy: A new mind-body intervention for COPD. ERJ Open Res 2021;7(4):1–14; doi: 10.1183/23120541.00256-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowen DJ, Kreuter M, Spring B, et al. . How we design feasibility studies. American journal of preventive medicine 2009;36(5):452–457; doi: 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proctor E, Silmere H, Raghavan R, et al. . Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Administration and policy in mental health 2011;38(2):65–76; doi: 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med 2001;20(1):68–74; doi: 10.1016/s0749-3797(00)00254-3 [DOI] [PubMed] [Google Scholar]
- 31. Courtney R, Cohen M. Investigating the claims of Konstantin Buteyko, M.D., Ph.D.: The relationship of breath holding time to end tidal CO2 and other proposed measures of dysfunctional breathing. J Altern Complement Med 2008;14(2):115–123; doi: 10.1089/acm.2007.7204 [DOI] [PubMed] [Google Scholar]
- 32. O'Donnell DE, James MD, Milne KM, et al. . The pathophysiology of dyspnea and exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med 2019;40(2):343–366; doi: 10.1016/j.ccm.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 33. Rocha A, Arbex FF, Sperandio PA, et al. . Excess ventilation in chronic obstructive pulmonary disease-heart failure overlap. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 2017;196(10):1264–1274; doi: 10.1164/rccm.201704-0675OC [DOI] [PubMed] [Google Scholar]
- 34. Mehling WE, Acree M, Stewart A, et al. . The Multidimensional Assessment of Interoceptive Awareness, version 2 (MAIA-2). PLoS One 2018;13(12):e0208034; doi: 10.1371/journal.pone.0208034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khalsa SS, Adolphs R, Cameron OG, et al. . Interoception and mental health: A roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3(6):501–513; doi: 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seth AK, Tsakiris M. Being a beast machine: The somatic basis of selfhood. Trends Cogn Sci 2018;22(11):969–981; doi: 10.1016/j.tics.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 37. Miller WR, Rollnick S.. Motivational Interviewing: Helping People Change. The Guilford Press: New York, NY; 2013. [Google Scholar]
- 38. McHugh RK, Whitton SW, Peckham AD, et al. . Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: A meta-analytic review. J Clin Psychiatry 2013;74(6):595–602; doi: 10.4088/JCP.12r07757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bellg AJ, Borrelli B, Resnick B, et al. . Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23(5):443–451; doi: 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 40. Moyers TB, Rowell LN, Manuel JK, et al. . The Motivational Interviewing Treatment Integrity Code (MITI 4): Rationale, preliminary reliability and validity. J Subst Abuse Treat 2016;65:36–42; doi: 10.1016/j.jsat.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ATS/ACCP statement on cardiopulmonary exercise testing. American journal of respiratory and critical care medicine 2003;167(2):211–277; doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 42. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117; doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 43. Jones PW, Quirk FH, Baveystock CM, et al. . A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145(6):1321–1327; doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 44. Barr JT, Schumacher GE, Freeman S, et al. . American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther 2000;22(9):1121–1145; doi: 10.1016/S0149-2918(00)80089-2 [DOI] [PubMed] [Google Scholar]
- 45. Guyatt GH, Berman LB, Townsend M, et al. . A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773–778; doi: 10.1136/thx.42.10.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guyatt GH, King DR, Feeny DH, et al. . Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol 1999;52(3):187–192; doi: 10.1016/s0895-4356(98)00157-7 [DOI] [PubMed] [Google Scholar]
- 47. Norweg A, Ni P, Garshick E, et al. . A multidimensional computer adaptive test approach to dyspnea assessment. Arch Phys Med Rehabil 2011;92(10):1561–1569; doi: 10.1016/j.apmr.2011.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–381. [PubMed] [Google Scholar]
- 49. Hays RD, Spritzer KL, Fries JF, et al. . Responsiveness and minimally important difference for the Patient-Reported Outcomes Measurement Information System (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis 2015;74(1):104–107; doi: 10.1136/annrheumdis-2013-204053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin FJ, Pickard AS, Krishnan JA, et al. . Measuring health-related quality of life in chronic obstructive pulmonary disease: Properties of the EQ-5D-5L and PROMIS-43 short form. BMC Med Res Methodol 2014;14:78; doi: 10.1186/1471-2288-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moy ML, Gould MK, Liu IA, et al. . Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res 2016;2(1):1–12; doi: 10.1183/23120541.00062-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coleman KJ, Ngor E, Reynolds K, et al. . Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc 2012;44(11):2071–2076; doi: 10.1249/MSS.0b013e3182630ec1 [DOI] [PubMed] [Google Scholar]
- 53. Lowe B, Decker O, Muller S, et al. . Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 2008;46(3):266–274; doi: 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 54. Courtney R, Greenwood KM. Preliminary investigation of a measure of dysfunctional breathing symptoms: The self evaluation of breathing questionnaire (SEBQ). Int J Osteopath Med 2009;12:121–127; doi: 10.1016/j.ijosm.2009.02.001 [DOI] [Google Scholar]
- 55. Abbott JH. The distinction between randomized clinical trials (RCTs) and preliminary feasibility and pilot studies: What they are and are not. J Orthop Sports Phys Ther 2014;44(8):555–558; doi: 10.2519/jospt.2014.0110 [DOI] [PubMed] [Google Scholar]
- 56. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res 2011;45(5):626–629; doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017;195(5):557–582; doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 58. Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur Respir J 2014;44(6):1428–1446; doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 59. Rochester CL, Vogiatzis I, Holland AE, et al. . An official American Thoracic Society/European Respiratory Society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015;192(11):1373–1386; doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 60. Han MK, Martinez CH, Au DH, et al. . Meeting the challenge of COPD care delivery in the USA: A multiprovider perspective. Lancet Respir Med 2016;4(6):473–526; doi: 10.1016/S2213-S2600(16)00094-1 [DOI] [PubMed] [Google Scholar]
- 61. Holland AE, Hill CJ, Jones AY, et al. . Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;10:CD008250; doi: 10.1002/14651858.CD008250.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mellor K, Dutton SJ, Hopewell S, et al. . How are progression decisions made following external randomised pilot trials? A qualitative interview study and framework analysis. Trials 2022;23(1):132; doi: 10.1186/s13063-022-06063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li Z, Liu S, Wang L, et al. . Mind-body exercise for anxiety and depression in COPD patients: A systematic review and meta-analysis. Int J Environ Res Public Health 2019;17(1):22; doi: 10.3390/ijerph17010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Collins EG, Langbein WE, Fehr L, et al. . Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2008;177(8):844–852; doi: 10.1164/rccm.200703-477OC [DOI] [PubMed] [Google Scholar]
- 65. Bandelow B, Baldwin D, Abelli M, et al. . Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry 2017;18(3):162–214; doi: 10.1080/15622975.2016.1190867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gendron LM, Nyberg A, Saey D, et al. . Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;10:CD012290; doi: 10.1002/14651858.CD012290.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Donesky D, Nguyen HQ, Paul SM, et al. . The affective dimension of dyspnea improves in a dyspnea self-management program with exercise training. J Pain Symptom Manage 2014;47(4):757–771; doi: 10.1016/j.jpainsymman.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 68. Vujanovic AA, Arrindell WA, Bernstein A, et al. . Sixteen-item Anxiety Sensitivity Index: Confirmatory factor analytic evidence, internal consistency, and construct validity in a young adult sample from the Netherlands. Assessment 2007;14(2):129–143; doi: 10.1177/1073191106295053 [DOI] [PubMed] [Google Scholar]
- 69. Lenze EJ, Nicol GE, Barbour DL, et al. . Precision clinical trials: A framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci 2021;46(1):E97–E110; doi: 10.1503/jpn.200042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ejiogu N, Norbeck JH, Mason MA, et al. . Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist 2011;51(Suppl 1):S33–S45; doi: 10.1093/geront/gnr027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Sa RB, Pessoa MF, Cavalcanti AGL, et al. . Immediate effects of respiratory muscle stretching on chest wall kinematics and electromyography in COPD patients. Respir Physiol Neurobiol 2017;242:1–7; doi: 10.1016/j.resp.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 72. Kim EJ, Choi JH, Kim KW, et al. . The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur Arch Otorhinolaryngol 2011;268(4):533–539; doi: 10.1007/s00405-010-1397-6 [DOI] [PubMed] [Google Scholar]
- 73. Migliore A. Management of dyspnea guidelines for practice for adults with chronic obstructive pulmonary disease. Occup Ther Health Care 2004;18(3):1–20; doi: 10.1080/J003v18n03_01 [DOI] [PubMed] [Google Scholar]
- 74. Williams M, Penman D.. Mindfulness: An Eight-Week Plan for Finding Peace in a Frantic World. Rodale, Inc.: New York, NY; 2011. [Google Scholar]
- 75. McCown D, Reibel D, Micozzi M.. Teaching Mindfulness: A Practical Guide for Clinicians and Educators. Springer: New York, NY; 2011. [Google Scholar]
- 76. Paulson S, Davidson R, Jha A, et al. . Becoming conscious: The science of mindfulness. Ann N Y Acad Sci 2013;1303:87–104; doi: 10.1111/nyas.12203 [DOI] [PubMed] [Google Scholar]
- 77. Chaitow L, Bradley D, Gilbert C. Recognizing and Treating Breathing Disorders: A Multidisciplinary Approach. 2nd ed. Churchill Livingstone Elsevier: New York; 2014. [Google Scholar]
- 78. Spruit MA, Singh SJ, Garvey C, et al. . An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Critical Care Med 2013;188(8):e13–e64; doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 79. Weitzberg E, Lundberg JO. Humming greatly increases nasal nitric oxide. Am J Respir Crit Care Med 2002;166(2):144–145; doi: 10.1164/rccm.200202-138BC [DOI] [PubMed] [Google Scholar]
- 80. Maniscalco M, Pelaia G, Sofia M. Exhaled nasal nitric oxide during humming: Potential clinical tool in sinonasal disease? Biomark Med 2013;7(2):261–266; doi: 10.2217/bmm.13.11 [DOI] [PubMed] [Google Scholar]