Dear Editors,
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory autoimmune disease of the central nervous system in which the main characteristics are visual impairment due to optic neuritis and neurological symptoms due to transverse myelitis. NMOSD may cause severe visual impairment that results in blindness and transverse spinal symptoms at a level that requires a wheelchair. As with early diagnosis and treatment in the acute stage, treatment interventions to prevent exacerbations in the chronic stage are important. Prednisolone monotherapy is reportedly effective for preventing relapses of NMOSD, but relapses are known to be common with prednisolone treatment ≤ 10 mg/day [1]. Hence, to prevent NMOSD relapse, long-term use of prednisolone at ≥ 10 mg/day is considered necessary. With long-term use of such doses of prednisolone, however, adverse effects including diabetes, hyperlipidemia, susceptibility to infection, osteoporosis, mental disorders, and central obesity become a problem.
Since 2019, the drugs eculizumab, satralizumab, inebilizumab, and rituximab have been available in Japan to target molecules related to the pathology of NMOSD, after approval by the national health insurance system. Here we report three cases of anti-aquaporin-4 (AQP-4) antibody-positive NMOSD in which steroids were successfully tapered after the introduction of satralizumab [2], an interleukin-6 receptor inhibitor. Steroid tapering protocols have included a trial in which steroids were discontinued simultaneously with the start of satralizumab [3] and a report on tapering [4]. Currently, the method of steroid tapering and the speed of tapering after the introduction of satralizumab are decided on a case-by-case basis. We had three patients in whom prednisolone tapering after the start of satralizumab was conducted at a faster pace than previously reported. An effective prednisolone tapering method is discussed.
Cases
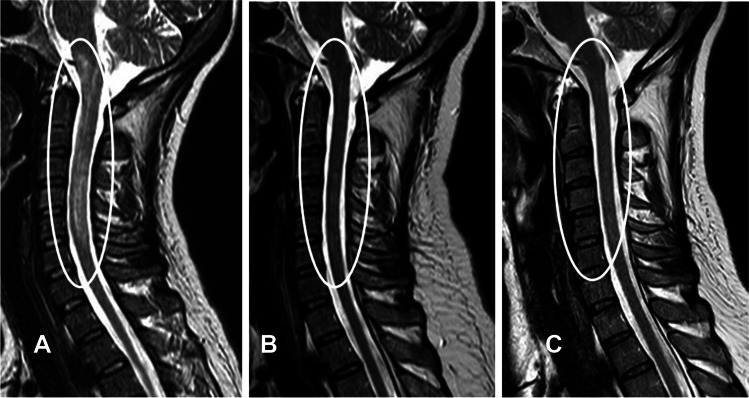
Patient 1 was a 40-year-old woman. In this woman, loss of muscle strength in bilateral limbs with left dominance and sensory disturbance occurred in 2018. In this case, a long, large lesion was seen from the medulla oblongata to C5 on magnetic resonance imaging (MRI) (Fig. 1A). At onset, anti-AQP-4 antibody titer (enzyme-linked immunosorbent assay; ELISA) was > 40.0 U/ml (normal range < 1.5), and cerebrospinal fluid showed positive results for an oligoclonal band. After three courses of steroid pulse therapy, prednisolone administration was started at 50 mg/day and tapered to 10 mg/day within about 5 months. Together with improvements in both limb muscle strength and sensory disturbance, this patient improved from requiring a wheelchair for mobility to being able to walk with a cane. However, symptoms did not change afterwards and no further improvements were observed. She experienced steroid side effects, so satralizumab was introduced 22 months after initial onset. At the time of satralizumab introduction, anti-AQP-4 antibody titer (ELISA) was 5.5 U/ml. Eight weeks after the introduction of satralizumab, the prednisolone dose was tapered from 10 mg/day at a pace of 1 mg/week, and prednisolone was able to be tapered and discontinued over 40 weeks. At the time satralizumab was introduced, the patient was able to walk 100 m with a cane (Expanded Disability Status Scale [EDSS], 6.0; pyramidal FS 3, cerebellar FS 3.0, sensory 4.0, bladder FS 3.0), and symptoms remained stable for more than 6 months. After the start of satralizumab, gradual improvements were seen in leg spastic paralysis and the extent and severity of sensory abnormalities. In the end, the patient improved to being able to walk for 1 km without a cane (EDSS 3.5; pyramidal FS 2.0, cerebellar FS 2.0, sensory 2.0, bladder FS 3.0). No relapse has been seen for more than 2 years since starting satralizumab (Fig. 1B, C). The most recent EDSS score, in December 2022, was 3.5 (pyramidal FS 2.0, cerebellar FS 2.0, sensory 2.0, bladder FS 3.0), and anti-AQP-4 antibody titer (ELISA) was 5.7 U/ml.
Fig. 1.
Results from MRI. A MRI at onset. A long, large lesion from the medulla oblongata to C5 and swelling of the spinal cord are seen. TR = 3449 ms; TE = 100 ms. B MRI just before starting satralizumab. Shrinkage of the lesion from the time of onset is evident. TR = 3729 ms; TE = 120 ms. C MRI 2 years after starting satralizumab. The lesion has shrunk from before the start of satralizumab, and the lesion is difficult to identify. TR = 3729 ms; TE = 120 ms
Patient 2 was a 63-year-old woman. Initial onset in this woman took the form of nausea and vomiting in 2017, and a relapse with decreased right visual acuity in 2020. Onset of NMOSD occurred with a long, large spinal lesion from C1 to Th1. Anti-AQP-4 antibody titer (ELISA) was 9.3 U/ml, and cerebrospinal fluid showed a positive result for an oligoclonal band. Two courses of steroid pulse therapy had little effect, and plasma exchange (total 4 times) was conducted. Loss of muscle strength from transverse spinal symptoms, sensory disturbance, and spastic paralysis were alleviated, and gait disorder improved from requiring a wheelchair for mobility to walking with a cane. She was transferred to a rehabilitation hospital, and ultimately daily living activities without a cane were again possible and she was able to return to work. She experienced a relapse with right optic neuritis 2 years after initial onset. At the time of relapse, anti-AQP-4 antibody titer (ELISA) was 3.5 U/ml. One course of steroid pulse therapy had little effect, and plasma exchange (total 2 times) was performed. With treatment, vision improved from being able to see hand motions to a corrected vision of 0.8 with mild central scotoma remaining. Daily prednisolone administration at 30 mg was subsequently continued for about 8 months. Steroid side effects were seen, so the decision was made to administer satralizumab about 9 months after the relapse of optic neuritis. Eight weeks after starting satralizumab, prednisolone was tapered at a pace of 1 mg/week, and prednisolone was discontinued after 100 weeks. Three months after the discontinuation of steroids, anti-AQP-antibody titer (ELISA) rose to 12.4 U/ml. This result was a shock to the patient, and prednisolone was restarted at 1 mg/day on her insistence, but was ultimately discontinued. No relapse of NMOSD has been seen in this patient for more than 2 years since starting satralizumab. The most recent EDSS score, in December 2022, was 2.5 (pyramidal FS 2.0, sensory 2.0, visual FS 1.0), and anti-AQP-4 antibody titer (ELISA) was 5.4 U/ml.
Patient 3 was a 65-year-old woman with left optic neuritis. Initial onset in this patient involved a decrease in left visual acuity in 2021. At the time of onset, anti-AQP-4 antibody titer (ELISA) was > 40.0 U/ml. Cerebrospinal fluid was oligoclonal band-negative. Two courses of steroid pulse therapy had little effect, and plasma exchange (total 3 times) was performed. With treatment, vision improved from being able to see hand motions to a corrected vision of 0.8 with mild central scotoma remaining. Satralizumab was introduced 9 months after initial symptom onset. Prednisolone was tapered from 10 mg/day at a pace of 1 mg/week, to 3 mg/day over 28 weeks. In this patient as well, no relapse was seen for 7 months after starting satralizumab. The most recent EDSS score, in December 2022, was 1.0 (visual FS 1.0), and anti-AQP-4 antibody titer (ELISA) was > 40.0 U/ml.
All three patients were anti-AQP-4 antibody-positive on ELISA. Patient 1 also showed positive results with a cell-based assay (CBA). All three patients were diagnosed with anti-AQP-4 antibody-positive NMOSD based on international diagnostic criteria [5]. In terms of steroid pulse therapy, one course was taken to be methylprednisolone at 1 g/day for 3 consecutive days. Blood purification therapy was simple plasma exchange. Satralizumab was introduced at a dose of 120 mg/day, administered by intravenous injection the first time, after 2 weeks, after 4 weeks, after 8 weeks, and at 4-week intervals thereafter. In all three patients, prednisolone was tapered at a pace of 1 mg/4 weeks from 8 weeks after starting satralizumab. In patients 1 and 2, steroid side effects including central obesity, hyperlipidemia, and diabetes were seen. In all three patients, assessment of relapse after the start of satralizumab has been conducted based on clinical symptoms and MRI until now. None of the three patients have experienced serious side effects, such as infection, while they were using satralizumab, and no problems with COVID-19 vaccination were seen after starting satralizumab.
In these cases, we were able to taper prednisolone after the start of satralizumab, an interleukin-6 receptor inhibitor, adopting an effective schedule for starting and tapering steroid dose. The level of soluble interleukin-6 receptors in serum is an important indicator of the effective expression of satralizumab on the immune system, but is known to plateau at around 8 weeks after starting satralizumab [2]. Therefore, the effect of satralizumab on NMOSD pathology may be expected to become constant from 8 weeks after the start of satralizumab. Based on this, we set the timing for the start of prednisolone tapering as 8 weeks after starting satralizumab.
Considering the patient burden in coming to the hospital, satralizumab tapering was conducted during outpatient visits for subcutaneous injection of satralizumab at 4-week intervals. The pace of prednisolone tapering was thus 1 mg/4 weeks. In a past article, the reduction rate for prednisolone tapering was reported as 0.24 mg/30 days, or 0.008 mg/day [4]. For the patients in this study, the speed of tapering was about 4.5 times faster, at 1 mg/4 weeks or 0.036 mg/day. We should note here that no exacerbation of NMOSD was seen even with this rapid pace of tapering.
With NMOSD, relapse within 1 year of initial onset or relapse is termed “clustered attacks”, and relapses are common. Patients 1 and 2 received satralizumab in this cluster period. Neither of these patients experienced relapses, so administration of satralizumab is thought to have contributed to the prevention of relapse. When tapering prednisolone, a fast pace may present a risk of relapse, but the present results indicate that the timing for the start of tapering may be more important than the pace. In a multicenter, double-blinded trial of satralizumab among NMOSD patients, a significant effect in preventing relapse was seen with the introduction of satralizumab alone [3]. Prednisolone had been decreased and discontinued in patients prior to this trial, and in an anti-AQP-4 antibody-positive group, relapse rates of 17% after 1 year and 23% after 2 years were seen. While tapering prednisolone before starting satralizumab reduced the risk of relapse compared with placebo, but further decreases might have been achievable using our tapering protocol. Although an effect in preventing NMOSD relapse was shown during this satralizumab cluster period, more cases need to be studied in the future.
Due to problems associated with treatment under the healthcare insurance system, treating NMOSD with disease-modifying drugs such as Imuran or other immunosuppressants and satralizumab was initially difficult. Evidence for the use of prednisolone was obtained from reports that found an inhibition in recurrence with long-term use. With regard to the amount of prednisolone used for one year from initial onset or relapse, recurrence was seen to be inhibited with doses of 10–15 mg/day or more, whereas relapse was common with doses ≤ 10 mg/day [1]. Based on this, long-term steroid therapy was conducted in our patients with the purpose of inhibiting recurrence.
Treatment with satralizumab later became possible in our hospital with approval for the use of this drug under health insurance. As a result, satralizumab was introduced in three cases. Treatment with eculizumab also became possible, but the schedule requiring outpatient infusion once every two weeks was difficult for all three patients, and this agent was therefore not used. The advantages of satralizumab from the perspective of the patient are that the drug is administered by subcutaneous injection once every 4 weeks, self-injection is possible, and administration is relatively easy to start on an outpatient basis rather than as an inpatient. In all three of these cases, satralizumab was started after being authorized for insurance coverage and approved for use in our hospital. Fortunately, no relapses were encountered during the use of prednisolone in any of our three patients, and oral steroids themselves were effective in preventing relapses.
In our 3 patients, prednisolone was ultimately reduced to ≤ 3 mg/day after starting administration of satralizumab, and continued with no relapses of NMOSD. A large-scale study examined the effects of satralizumab monotherapy without steroid administration on the prevention of NMOSD relapse [3]. In that report, satralizumab monotherapy was shown to be effective. On that basis, we attempted a rapid tapering of prednisolone in the present patients, and all three have continued without relapse. These results are also thought to support the effectiveness of satralizumab monotherapy. This rapid decrease to a dose with few steroid side effects or other effects suggests that the tapering method described here is effective.
Funding
This work was supported by JSPS KAKENHI Grant No. JP 20K07777.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Highlights
• A case series of AQP-4-positive NMOSD after satralizumab treatment is described.
• Prednisolone tapering was started 8 weeks after starting satralizumab.
• Prednisolone was tapered by 1 mg/4 weeks.
• Patients with NMOSD can taper prednisolone to less than 3 mg/day.
• We discuss the timing and speed of prednisolone tapering with satralizumab.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takai Y, Kuroda H, Misu T, Akaishi T, Nakashima I, Takahashi T, Nishiyama S, Fujihara K, Aoki M. Optimal management of neuromyelitis optica spectrum disorder with aquaporin-4 antibody by oral prednisolone maintenance therapy. Mult Scler Relat Disord. 2021;49:102750. doi: 10.1016/j.msard.2021.102750. [DOI] [PubMed] [Google Scholar]
- 2.Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, Patti F, Tsai CP, Saiz A, Yamazaki H, Kawata Y, Wright P, De Seze J. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381:2114–2124. doi: 10.1056/NEJMoa1901747. [DOI] [PubMed] [Google Scholar]
- 3.Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, Yamamura T, Terada Y, Kawata Y, Wright P, Gianella-Borradori A, Garren H, Weinshenker BG. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19:402–412. doi: 10.1016/S1474-4422(20)30078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamura T, Weinshenker B, Yeaman MR, De Seze J, Patti F, Lobo P, von Büdingen HC, Kou X, Weber K, Greenberg B. Long-term safety of satralizumab in neuromyelitis optica spectrum disorder (NMOSD) from SAkuraSky and SAkuraStar. Mult Scler Relat Disord. 2022;66:104025. doi: 10.1016/j.msard.2022.104025. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG, International Panel for NMO Diagnosis International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]